Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 11 June 2020

Key steps for effective breast cancer prevention
- Kara L. Britt ORCID: orcid.org/0000-0001-6069-7856 1 , 2 ,
- Jack Cuzick ORCID: orcid.org/0000-0001-7420-7512 3 &
- Kelly-Anne Phillips ORCID: orcid.org/0000-0002-0475-1771 2 , 4 , 5
Nature Reviews Cancer volume 20 , pages 417–436 ( 2020 ) Cite this article
20k Accesses
362 Citations
66 Altmetric
Metrics details
- Breast cancer
- Cancer prevention
Despite decades of laboratory, epidemiological and clinical research, breast cancer incidence continues to rise. Breast cancer remains the leading cancer-related cause of disease burden for women, affecting one in 20 globally and as many as one in eight in high-income countries. Reducing breast cancer incidence will likely require both a population-based approach of reducing exposure to modifiable risk factors and a precision-prevention approach of identifying women at increased risk and targeting them for specific interventions, such as risk-reducing medication. We already have the capacity to estimate an individual woman’s breast cancer risk using validated risk assessment models, and the accuracy of these models is likely to continue to improve over time, particularly with inclusion of newer risk factors, such as polygenic risk and mammographic density. Evidence-based risk-reducing medications are cheap, widely available and recommended by professional health bodies; however, widespread implementation of these has proven challenging. The barriers to uptake of, and adherence to, current medications will need to be considered as we deepen our understanding of breast cancer initiation and begin developing and testing novel preventives.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Causal machine learning for predicting treatment outcomes

Refining the impact of genetic evidence on clinical success

Utility of polygenic scores across diverse diseases in a hospital cohort for predictive modeling
Narod, S. A., Iqbal, J. & Miller, A. B. Why have breast cancer mortality rates declined? J. Cancer Policy 5 , 8–17 (2015).
Article Google Scholar
Althuis, M. D., Dozier, J. M., Anderson, W. F., Devesa, S. S. & Brinton, L. A. Global trends in breast cancer incidence and mortality 1973–1997. Int. J. Epidemiol. 34 , 405–412 (2005).
Article PubMed Google Scholar
Boffetta, P. & Parkin, D. M. Cancer in developing countries. CA Cancer J. Clin. 44 , 81–90 (1994).
Article CAS PubMed Google Scholar
Glass, A. G. & Hoover, R. N. Rising incidence of breast cancer: relationship to stage and receptor status. J. Natl Cancer Inst. 82 , 693–696 (1990).
Li, C. I., Daling, J. R. & Malone, K. E. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J. Clin. Oncol. 21 , 28–34 (2003). This paper uses data from the NCI SEER Program to show that the proportion of hormone receptor-positive tumours rose in the 1990s .
Parkin, D. M. & Fernandez, L. M. Use of statistics to assess the global burden of breast cancer. Breast J. 12 (Suppl. 1), S70–S80 (2006).
Ries, L. A. G., Melbert, D. & Krapcho, M. SEER Cancer Statistics Review , 1975–2004 (NIH, 2006).
Global Burden of Disease Cancer Collaboration. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 4 , 1553–1568 (2018).
Bigaard, J., Stahlberg, C., Jensen, M. B., Ewertz, M. & Kroman, N. Breast cancer incidence by estrogen receptor status in Denmark from 1996 to 2007. Breast Cancer Res. Treat. 136 , 559–564 (2012).
Coughlin, S. S. Epidemiology of Breast Cancer in Women Vol. 1152 (ed. Ahmad, A.) (Springer, 2019).
He, C. et al. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum. Genet. 128 , 515–527 (2010).
Article CAS PubMed PubMed Central Google Scholar
Stolk, L. et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet. 44 , 260–268 (2012).
Stone, J. et al. The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol. Biomarkers Prev. 15 , 612–617 (2006).
Broca, P. Taite des tumeurs Vol. 13 (Libraire De La Faculte De Medecine, 1866).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1 . Science 266 , 66–71 (1994). This study uses positional cloning to identify the chromosome 17q-linked BRCA1 gene as a tumour suppressor, which if mutated predisposes individuals to both breast cancer and ovarian cancer .
Wooster, R. et al. Identification of the breast cancer susceptibility gene BRCA2 . Nature 378 , 789–792 (1995). This study identifies the breast cancer susceptibility gene BRCA2 on chromosome 13q12-q13 with mutations detected in this gene in families with breast cancer .
Ligtenberg, M. J. et al. Characteristics of small breast and/or ovarian cancer families with germline mutations in BRCA1 and BRCA2. Br. J. Cancer 79 , 1475–1478 (1999).
Kuchenbaecker, K. B. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317 , 2402–2416 (2017). This prospective cohort study of BRCA1 and BRCA2 female carriers shows that the risk of breast cancer by 80 years of age was 72% and 69%, respectively .
Easton, D. F. et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 372 , 2243–2257 (2015). This special report on multigene or panel testing looks for the presence of genetic variants that may be associated with a risk of breast cancer .
National Comprehensive Cancer Network. Genetic/Familial High-risk Assessment: Breast and Ovarian (National Comprehensive Cancer Network, 2019).
Michailidou, K. et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 47 , 373–380 (2015).
Mavaddat, N. et al. Prediction of breast cancer risk based on profiling with common genetic variants. J. Natl Cancer Inst. 107 , djv036 (2015).
Article PubMed PubMed Central CAS Google Scholar
Mavaddat, N. et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am. J. Hum. Genet. 104 , 21–34 (2019). This paper develops a PRS for predicting breast cancer using the largest available genome-wide association data set .
Rudolph, A. et al. Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the Breast Cancer Association Consortium. Int. J. Epidemiol. 47 , 526–536 (2018).
Article PubMed PubMed Central Google Scholar
Muranen, T. A. et al. Genetic modifiers of CHEK2*1100delC-associated breast cancer risk. Genet. Med. 19 , 599–603 (2017).
Kuchenbaecker, K. B. et al. Associations of common breast cancer susceptibility alleles with risk of breast cancer subtypes in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 16 , 3416 (2014).
Esserman, L. J., Study, W. & Athena, I. The WISDOM study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 3 , 34 (2017).
Evans, D. G. R. et al. Breast cancer pathology and stage are better predicted by risk stratification models that include mammographic density and common genetic variants. Breast Cancer Res. Treat. 176 , 141–148 (2019).
Gabrielson, M. et al. Cohort profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int. J. Epidemiol. 46 , 1740–1741g (2017).
French, J. D. et al. Functional variants at the 11q13 risk locus for breast cancer regulate cyclin D1 expression through long-range enhancers. Am. J. Hum. Genet. 92 , 489–503 (2013).
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet 360 , 187–195 (2002). This reanalysis of 47 epidemiological studies shows that the RR of breast cancer is reduced by 4.3% for each year a woman breastfeeds, in addition to a reduction of 7% for each birth .
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 13 , 1141–1151 (2012). This meta-analysis of data from 117 epidemiological studies shows that each year younger at menarche or older at menopause is associated with a 5% and 2.9% increased risk of breast cancer, respectively .
Article PubMed Central Google Scholar
Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 394 , 1159–1168 (2019). This meta-analysis of MHT use shows that all therapy types except vaginal oestrogens are associated with increased breast cancer risk .
Dall, G. V. & Britt, K. L. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Front. Oncol. 7 , 110 (2017).
MacMahon, B. et al. Age at first birth and breast cancer risk. Bull. World Health Organ. 43 , 209–221 (1970). This landmark international collaborative study shows that a young age at first childbirth significantly decreases breast cancer risk .
CAS PubMed PubMed Central Google Scholar
Morris, D. H., Jones, M. E., Schoemaker, M. J., Ashworth, A. & Swerdlow, A. J. Secular trends in age at menarche in women in the UK born 1908–93: results from the breakthrough generations study. Paediatr. Perinat. Epidemiol. 25 , 394–400 (2011).
Braithwaite, D. et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 20 , 713–720 (2009).
Sisti, J. S. et al. Reproductive risk factors in relation to molecular subtypes of breast cancer: results from the Nurses’ Health Studies. Int. J. Cancer 138 , 2346–2356 (2016).
Silva, C. A. et al. Gonadal function in adolescents and young women with juvenile systemic lupus erythematosus. Lupus 11 , 419–425 (2002).
Harris, M. A., Prior, J. C. & Koehoorn, M. Age at menarche in the Canadian population: secular trends and relationship to adulthood BMI. J. Adolesc. Health 43 , 548–554 (2008).
Fernandez-Rhodes, L. et al. Association of adiposity genetic variants with menarche timing in 92,105 women of European descent. Am. J. Epidemiol. 178 , 451–460 (2013).
Chisholm, J. S., Quinlivan, J. A., Petersen, R. W. & Coall, D. A. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum. Nat. 16 , 233–265 (2005).
Carwile, J. L. et al. Sugar-sweetened beverage consumption and age at menarche in a prospective study of US girls. Hum. Reprod. 30 , 675–683 (2015).
Belsky, J., Steinberg, L. & Draper, P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child. Dev. 62 , 647–670 (1991).
Behie, A. M. & O’Donnell, M. H. Prenatal smoking and age at menarche: influence of the prenatal environment on the timing of puberty. Hum. Reprod. 30 , 957–962 (2015).
Elks, C. E. et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 42 , 1077–1085 (2010).
Boynton-Jarrett, R. et al. Gestational weight gain and daughter’s age at menarche. J. Womens Health 20 , 1193–1200 (2011).
Deardorff, J. et al. Maternal pre-pregnancy BMI, gestational weight gain, and age at menarche in daughters. Matern. Child. Health J. 17 , 1391–1398 (2013).
Gao, Y. T. et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int. J. Cancer 87 , 295–300 (2000).
O’Brien, K. M., Sun, J., Sandler, D. P., DeRoo, L. A. & Weinberg, C. R. Risk factors for young-onset invasive and in situ breast cancer. Cancer Causes Control. 26 , 1771–1778 (2015).
Reeves, G. K. et al. Reproductive factors and specific histological types of breast cancer: prospective study and meta-analysis. Br. J. Cancer 100 , 538–544 (2009).
Rodstrom, K. et al. Evidence for a secular trend in menopausal age: a population study of women in Gothenburg. Menopause 10 , 538–543 (2003).
Gold, E. B. et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am. J. Epidemiol. 178 , 70–83 (2013).
Gold, E. B. et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am. J. Epidemiol. 153 , 865–874 (2001).
Gold, E. B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. North. Am. 38 , 425–440 (2011).
He, L. N. et al. Association study of the oestrogen signalling pathway genes in relation to age at natural menopause. J. Genet. 86 , 269–276 (2007).
Weel, A. E. et al. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J. Clin. Endocrinol. Metab. 84 , 3146–3150 (1999).
CAS PubMed Google Scholar
den Tonkelaar, I., te Velde, E. R. & Looman, C. W. Menstrual cycle length preceding menopause in relation to age at menopause. Maturitas 29 , 115–123 (1998).
Ramazzini, B. De Morbis Artificum (Diseases of Workers) (University of Chicago Press, 1940).
Albrektsen, G., Heuch, I., Hansen, S. & Kvale, G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br. J. Cancer 92 , 167–175 (2005).
Ursin, G. et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br. J. Cancer 93 , 364–371 (2005).
Ma, H., Bernstein, L., Pike, M. C. & Ursin, G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 8 , R43 (2006). This meta-analysis of epidemiological studies investigates parity, age at first birth, breastfeeding and age at menarche in relation to ER + PR + and ER – PR – breast cancer risk .
Anderson, K. N., Schwab, R. B. & Martinez, M. E. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res. Treat. 144 , 1–10 (2014).
Tamimi, R. M. et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res. Treat. 131 , 159–167 (2012).
Gaudet, M. M. et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 130 , 587–597 (2011).
Australian Birth Statistics. Births, Australia, 2017 Cat. No. 3301.0 (Australian Bureau of Statistics, 2018).
Hamilton, B. E., Martin, J. A., Osterman, M. J. K. & Rossen, L. M. Births: Provisional data for 2018 (NCHS, 2019).
Office for National Statistics. Births in England and Wales 2018, Statistical Bulletin (2019).
Australian Institute of Health and Welfare. Reproductive Health Indicators Australia 2002 Cat. No. PER 20 (Pew Research Center, 2003).
Livingstone, G. & Cohn, D. Childlessness Up Among all Women; Down Among Women with Advanced Degrees (Pew Research Center, 2010).
Australian Institute of Health and Welfare. Cancer in Australia 2017 Cat. No. CAN 100 (Australian Institute of Health and Welfare, 2017).
Martin, J. A., Hamilton, B. E., Osterman, M. J. K., Driscoll, A. K. & Drake, P. Final data for 2017 National Vital Statistics Reports Vol. 67 No. 8 Report No. 1568–7856 (Electronic) 1568–7856 (Linking), 844–855 (National Center for Health Statistics, 2018).
Huo, D. et al. Parity and breastfeeding are protective against breast cancer in Nigerian women. Br. J. Cancer 98 , 992–996 (2008).
Khalis, M. et al. Menstrual and reproductive factors and risk of breast cancer: a case–control study in the Fez region, Morocco. PLoS One 13 , e0191333 (2018).
Schedin, P. Pregnancy-associated breast cancer and metastasis. Nat. Rev. Cancer 6 , 281–291 (2006). This comprehensive Review discusses the role of the pro-inflammatory immune microenvironment of the post-partum breast in the development of pregnancy-associated breast cancer .
Britt, K., Ashworth, A. & Smalley, M. Pregnancy and the risk of breast cancer. Endocr. Relat. Cancer 14 , 907–933 (2007).
Dall, G. V. et al. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 237 , 323–336 (2018).
Chang, C. C. et al. A human breast epithelial cell type with stem cell characteristics as target cells for carcinogenesis. Radiat. Res. 155 , 201–207 (2001).
Russo, J., Tay, L. K. & Russo, I. H. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res. Treat. 2 , 5–73 (1982).
Russo, I. H. & Russo, J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J. Natl Cancer Inst. 61 , 1439–1449 (1978).
Land, C. E. et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat. Res. 160 , 707–717 (2003).
Tokunaga, M. et al. Incidence of female breast cancer among atomic bomb survivors, 1950–1985. Radiat. Res. 138 , 209–223 (1994).
Britt, K. L. et al. Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells. Breast Cancer Res. 11 , R20 (2009).
Article PubMed CAS PubMed Central Google Scholar
Meier-Abt, F. et al. Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium. Breast Cancer Res. 15 , R36 (2013).
Siwko, S. K. et al. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells—implications for pregnancy-induced protection against breast cancer. Stem Cell 26 , 3205–3209 (2008).
Dall, G. V. et al. SCA-1 labels a subset of estrogen-responsive bipotential repopulating cells within the CD24 + CD49f hi mammary stem cell-enriched compartment. Stem Cell Rep. 8 , 417–431 (2017).
Article CAS Google Scholar
Jindal, S. et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res. 16 , R31 (2014).
Martinson, H. A., Jindal, S., Durand-Rougely, C., Borges, V. F. & Schedin, P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int. J. Cancer 136 , 1803–1813 (2015). This study finds that the post-partum mammary gland of mice is in an immunosuppressed state immediately after pregnancy, which can drive tumour formation .
Santucci-Pereira, J. et al. Genomic signature of parity in the breast of premenopausal women. Breast Cancer Res. 21 , 46 (2019).
Balogh, G. A. et al. Genomic signature induced by pregnancy in the human breast. Int. J. Oncol. 28 , 399–410 (2006).
Lambertini, M. et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 49 , 65–76 (2016).
Hadjisavvas, A. et al. An investigation of breast cancer risk factors in Cyprus: a case control study. BMC Cancer 10 , 447 (2010).
Fortner, R. T. et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses’ Health Studies. Breast Cancer Res. 21 , 40 (2019).
Islami, F. et al. Breastfeeding and breast cancer risk by receptor status — a systematic review and meta-analysis. Ann. Oncol. 26 , 2398–2407 (2015).
Takabatake, Y. et al. Lactation opposes pappalysin-1-driven pregnancy-associated breast cancer. EMBO Mol. Med. 8 , 388–406 (2016).
World Heath Organization. Global Strategy on Infant and Young Child Feeding: Infant and Young Child Nutrition (WHO, 2002).
Australian Institute of Health and Welfare. 2010 Australian National Infant Feeding Survey: Indicator Results (AIHW, 2011).
Ayton, J., van der Mei, I., Wills, K., Hansen, E. & Nelson, M. Cumulative risks and cessation of exclusive breast feeding: Australian cross-sectional survey. Arch. Dis. Child. 100 , 863–868 (2015).
McAndrew, F. et al. Infant Feeding Survey 2010 (Health and Social Care Information Centre, IFF Research, 2012).
Victora, C. G. et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387 , 475–490 (2016).
Rollins, N. C. et al. Why invest, and what it will take to improve breastfeeding practices? Lancet 387 , 491–504 (2016).
Sickles, E. A., D’Orsi, C. J., Bassett, L. W. ACR BI-RADS Mammography Vol. 5 134–136 (American College of Radiology, 2013).
Spak, D. A., Plaxco, J. S., Santiago, L., Dryden, M. J. & Dogan, B. E. BI-RADS ® fifth edition: a summary of changes. Diagn. Interv. Imaging 98 , 179–190 (2017).
McCormack, V. A. & dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15 , 1159–1169 (2006). This meta-analysis shows that increasing breast density is associated with an increased risk of breast cancer .
Krishnan, K. et al. Mammographic density and risk of breast cancer by mode of detection and tumor size: a case–control study. Breast Cancer Res. 18 , 63 (2016).
Hopper, J. L. Odds per adjusted standard deviation: comparing strengths of associations for risk factors measured on different scales and across diseases and populations. Am. J. Epidemiol. 182 , 863–867 (2015).
Sprague, B. L. et al. Prevalence of mammographically dense breasts in the United States. J. Natl Cancer Inst. 106 , dju255 (2014).
Huo, C. W. et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 17 , 79 (2015).
Huo, C. W. et al. High mammographic density in women is associated with protumor inflammation. Breast Cancer Res. 20 , 92 (2018).
Cuzick, J. et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case–control study. J. Natl Cancer Inst. 103 , 744–752 (2011). This nested case–control study within the IBIS-1 prevention trial shows that women in the tamoxifen group who experienced a 10% or greater reduction in breast density had a 63% reduction in breast cancer risk .
Shawky, M. S. et al. Mammographic density: a potential monitoring biomarker for adjuvant and preventative breast cancer endocrine therapies. Oncotarget 8 , 5578–5591 (2017).
Wolfe, J. N. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 37 , 2486–2492 (1976).
Boyd, N. F. et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J. Natl Cancer Inst. 87 , 670–675 (1995).
Suzuki, R., Orsini, N., Saji, S., Key, T. J. & Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status-a meta-analysis. Int. J. Cancer 124 , 698–712 (2009). This meta-analysis shows that postmenopausal women in the highest body weight categories had an 80% increased RR for breast cancer compared with those in the lowest weight categories .
Renehan, A. G., Zwahlen, M. & Egger, M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer 15 , 484–498 (2015).
Travis, R. C. & Key, T. J. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 5 , 239–247 (2003).
Brinton, L. A. et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J. Natl Cancer Inst. 106 , djt465 (2014).
Eliassen, A. H., Colditz, G. A., Rosner, B., Willett, W. C. & Hankinson, S. E. Adult weight change and risk of postmenopausal breast cancer. JAMA 296 , 193–201 (2006). This study uses data from the Nurses’ Health Study to show that women who maintained or lost weight as they got older had a reduced RR of postmenopausal breast cancer compared with those who gained weight .
Harvie, M. et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa Women’s Health Study. Cancer Epidemiol. Biomarkers Prev. 14 , 656–661 (2005).
Mannisto, S. et al. Body-size indicators and risk of breast cancer according to menopause and estrogen-receptor status. Int. J. Cancer 68 , 8–13 (1996).
Keum, N. et al. Adult weight gain and adiposity-related cancers: a dose–response meta-analysis of prospective observational studies. J. Natl Cancer Inst . 107 , djv088 (2015).
Renehan, A. G. et al. Young adulthood body mass index, adult weight gain and breast cancer risk: the PROCAS study (United Kingdom). Br. J. Cancer 122 , 1552–1561 (2020).
Lynch, B. M., Neilson, H. K. & Friedenreich, C. M. Physical activity and breast cancer prevention. Recent. Results Cancer Res. 186 , 13–42 (2011).
Neilson, H. K. et al. Moderate–vigorous recreational physical activity and breast cancer risk, stratified by menopause status: a systematic review and meta-analysis. Menopause 24 , 322–344 (2017).
World Cancer Research Fund/American Institute for Cancer Research. Physical Activity and the Risk of Cancer Report No. 0022-2623 (Print) 0022-2623 (Linking) https://www.wcrf.org/sites/default/files/Physical-activity.pdf (2018).
Wu, Y., Zhang, D. & Kang, S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res. Treat. 137 , 869–882 (2013).
World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Breast Cancer https://www.wcrf.org/sites/default/files/Summary-of-Third-Expert-Report-2018.pdf (2018).
Lammert, J. et al. Physical activity during adolescence and young adulthood and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 169 , 561–571 (2018).
Wang, M. et al. Prepubertal physical activity up-regulates estrogen receptor β, BRCA1 and p53 mRNA expression in the rat mammary gland. Breast Cancer Res. Treat. 115 , 213–220 (2009).
Kurgan, N. et al. Inhibition of human lung cancer cell proliferation and survival by post-exercise serum is associated with the inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers (Basel) 9 , 46 (2017).
Rundqvist, H. et al. Effect of acute exercise on prostate cancer cell growth. PLoS One 8 , e67579 (2013).
Dethlefsen, C. et al. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 77 , 4894–4904 (2017).
National Statistics NHS. Health Survey for England 2012 (NHS, 2013).
Chen, W. Y., Rosner, B., Hankinson, S. E., Colditz, G. A. & Willett, W. C. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306 , 1884–1890 (2011). This paper uses data from the Nurses’ Health Study to show that women consuming 3–6 glasses of wine per week or two drinks per day were 15% or 50% more likely, respectively, to develop breast cancer than non-drinkers .
Allen, N. E. et al. Moderate alcohol intake and cancer incidence in women. J. Natl Cancer Inst. 101 , 296–305 (2009).
Arriaga, M. E. et al. The preventable burden of breast cancers for premenopausal and postmenopausal women in Australia: a pooled cohort study. Int. J. Cancer 145 , 2383–2394 (2019).
Singletary, K. W. & Gapstur, S. M. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 286 , 2143–2151 (2001).
Dorgan, J. F. et al. Serum hormones and the alcohol–breast cancer association in postmenopausal women. J. Natl Cancer Inst. 93 , 710–715 (2001).
Triano, E. A. et al. Class I alcohol dehydrogenase is highly expressed in normal human mammary epithelium but not in invasive breast cancer: implications for breast carcinogenesis. Cancer Res. 63 , 3092–3100 (2003).
Seitz, H. K. & Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 5 , 121–128 (2010).
Meadows, G. G. & Zhang, H. Effects of alcohol on tumor growth, metastasis, immune response, and host survival. Alcohol. Res. 37 , 311–322 (2015).
PubMed PubMed Central Google Scholar
World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective (American Institute for Cancer Research, 2007).
Ronksley, P. E., Brien, S. E., Turner, B. J., Mukamal, K. J. & Ghali, W. A. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 342 , d671 (2011).
GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392 , 1015–1035 (2018).
Hopper, J. L. et al. Age-specific breast cancer risk by body mass index and familial risk: prospective family study cohort (ProF-SC). Breast Cancer Res. 20 , 132 (2018).
Kehm, R. et al. Recreational physical activity and breast cancer risk: a cohort study of women selected for familial and genetic risk. Cancer Res. 80 , 116–125 (2020).
Chlebowski, R. T. et al. Dietary modification and breast cancer mortality: long-term follow-up of the women’s health initiative randomized trial. J. Clin. Oncol. 38 , 1419–1428 (2020).
Chlebowski, R. T. et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J. Natl Cancer Inst. 97 , 439–448 (2005).
Li, N. et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J. Hematol. Oncol. 12 , 140 (2019).
Li, C. I., Malone, K. E. & Daling, J. R. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol. Biomarkers Prev. 11 , 601–607 (2002).
PubMed Google Scholar
Millikan, R. C. et al. Epidemiology of basal-like breast cancer. Breast Cancer Res. Treat. 109 , 123–139 (2008).
Pharoah, P. & Ponder, B. in Genes and Common Diseases: Genetics in Modern Medicine (eds A. Wright & N. Hastie) 224–232 (Cambridge Univ. Press, 2007).
Banegas, M. P. et al. Projecting individualized absolute invasive breast cancer risk in US Hispanic women. J. Natl Cancer Inst. 109 , djw215 (2017).
Gail, M. H. et al. Projecting individualized absolute invasive breast cancer risk in African American women. J. Natl Cancer Inst. 99 , 1782–1792 (2007).
Matsuno, R. K. et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J. Natl Cancer Inst. 103 , 951–961 (2011).
Cintolo-Gonzalez, J. A. et al. Breast cancer risk models: a comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res. Treat. 164 , 263–284 (2017).
Antoniou, A. C. et al. A comprehensive model for familial breast cancer incorporating BRCA1 , BRCA2 and other genes. Br. J. Cancer 86 , 76–83 (2002).
Antoniou, A. C., Pharoah, P. P., Smith, P. & Easton, D. F. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br. J. Cancer 91 , 1580–1590 (2004).
Berry, D. A., Parmigiani, G., Sanchez, J., Schildkraut, J. & Winer, E. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J. Natl Cancer Inst. 89 , 227–238 (1997).
Gail, M. H. et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl Cancer Inst. 81 , 1879–1886 (1989).
Parmigiani, G., Berry, D. & Aguilar, O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2 . Am. J. Hum. Genet. 62 , 145–158 (1998).
Rosner, B. & Colditz, G. A. Nurses’ Health Study: log-incidence mathematical model of breast cancer incidence. J. Natl Cancer Inst. 88 , 359–364 (1996).
Terry, M. B. et al. 10-Year performance of four models of breast cancer risk: a validation study. Lancet Oncol. 20 , 504–517 (2019). This study uses data from a large international prospective cohort study to validate commonly used breast cancer risk prediction models and shows that models that include multigenerational family cancer history, such as IBIS and BOADICEA, perform best, even for women at average risk .
Tice, J. A. et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann. Intern. Med. 148 , 337–347 (2008).
Tyrer, J., Duffy, S. W. & Cuzick, J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 23 , 1111–1130 (2004).
Petracci, E. et al. Risk factor modification and projections of absolute breast cancer risk. J. Natl Cancer Inst. 103 , 1037–1048 (2011).
Gail, M. H., Anderson, W. F., Garcia-Closas, M. & Sherman, M. E. Absolute risk models for subtypes of breast cancer. J. Natl Cancer Inst. 99 , 1657–1659 (2007).
Colditz, G. A. & Rosner, B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am. J. Epidemiol. 152 , 950–964 (2000).
Brentnall, A. R., Cuzick, J., Buist, D. S. M. & Bowles, E. J. A. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 4 , e180174 (2018).
Bodian, C. A., Perzin, K. H. & Lattes, R. Lobular neoplasia. Long term risk of breast cancer and relation to other factors. Cancer 78 , 1024–1034 (1996).
Claus, E. B., Risch, N. & Thompson, W. D. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73 , 643–651 (1994).
Amir, E. et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J. Med. Genet. 40 , 807–814 (2003).
Brentnall, A. R. et al. A case–control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int. J. Cancer 146 , 2122–2129 (2020).
Quante, A. S., Whittemore, A. S., Shriver, T., Strauch, K. & Terry, M. B. Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res. 14 , R144 (2012).
van Veen, E. M. et al. Use of single-nucleotide polymorphisms and mammographic density plus classic risk factors for breast cancer risk prediction. JAMA Oncol. 4 , 476–482 (2018).
Cuzick, J. et al. Impact of a panel of 88 single nucleotide polymorphisms on the risk of breast cancer in high-risk women: results from two randomized tamoxifen prevention trials. J. Clin. Oncol. 35 , 743–750 (2017).
Steyerberg, E. W. et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21 , 128–138 (2010).
Louro, J. et al. A systematic review and quality assessment of individualised breast cancer risk prediction models. Br. J. Cancer 121 , 76–85 (2019).
Endogenous Hormones and Breast Cancer Collaborative Group. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 14 , 1009–1019 (2013).
Article PubMed Central CAS Google Scholar
Terry, M. B., McDonald, J. A., Wu, H. C., Eng, S. & Santella, R. M. Epigenetic biomarkers of breast cancer risk: across the breast cancer prevention continuum. Adv. Exp. Med. Biol. 882 , 33–68 (2016).
Machella, N. et al. Double-strand breaks repair in lymphoblastoid cell lines from sisters discordant for breast cancer from the New York site of the BCFR. Carcinogenesis 29 , 1367–1372 (2008).
Phillips, K. A. et al. Transitioning to routine breast cancer risk assessment and management in primary care: what can we learn from cardiovascular disease? Aust. J. Prim. Health 22 , 255–261 (2016).
Collins, I. M. et al. iPrevent®: a tailored, web-based, decision support tool for breast cancer risk assessment and management. Breast Cancer Res. Treat. 156 , 171–182 (2016).
Phillips, K. A. et al. Accuracy of estimates from the iPrevent breast cancer risk assessment and risk management tool. JNCI – Cancer Spec. 3 , pkz066 (2019).
Lo, L. L. et al. The iPrevent online breast cancer risk assessment and risk management tool: usability and acceptability testing. JMIR Form. Res. 2 , e24 (2018).
Keogh, L. A. et al. Consumer and clinician perspectives on personalising breast cancer prevention information. Breast 43 , 39–47 (2019).
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 69 , 438–451 (2019).
National Comprehensive Cancer Network. Breast cancer risk reduction. NCCN https://www2.tri-kobe.org/nccn/guideline/breast/english/breast_risk.pdf (2019).
National Institute for Health and Care Excellence. Familial breast cancer: Classification and care of people at risk of familial breast cancer and management of breast cancer and related risks in people with a family history of breast cancer. NICE https://www.nice.org.uk/guidance/cg164 (2020).
Visvanathan, K. et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J. Clin. Oncol. 37 , 3152–3165 (2019).
Li, X. et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta-analysis and systematic review. Clin. Cancer Res. 22 , 3971–3981 (2016).
Hartmann, L. C. et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N. Engl. J. Med. 340 , 77–84 (1999).
Carbine, N. E., Lostumbo, L., Wallace, J. & Ko, H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 4 , CD002748 (2018).
Jakub, J. W. et al. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with BRCA mutations: a multi-institutional study. JAMA Surg. 153 , 123–129 (2018).
Metcalfe, K. et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br. J. Cancer 121 , 15–21 (2019).
Rebbeck, T. R., Kauff, N. D. & Domchek, S. M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl Cancer Inst. 101 , 80–87 (2009).
Heemskerk-Gerritsen, B. A. et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J. Natl Cancer Inst. 107 , djv033 (2015).
Article PubMed CAS Google Scholar
Terry, M. B. et al. Risk-reducing oophorectomy and breast cancer risk across the spectrum of familial risk. J. Natl Cancer Inst. 111 , 331–334 (2019).
Kotsopoulos, J. et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J. Natl Cancer Inst. 109 , djw177 (2017).
Google Scholar
Mai, P. L. et al. Risk-reducing salpingo-oophorectomy and breast cancer risk reduction in the Gynecologic Oncology Group Protocol-0199 (GOG-0199). JNCI Cancer Spectr. 4 , pkz075 (2019).
Milne, R. L. & Antoniou, A. C. Modifiers of breast and ovarian cancer risks for BRCA1 and BRCA2 mutation carriers. Endocr. Relat. Cancer 23 , T69–T84 (2016).
Cuzick, J. et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381 , 1827–1834 (2013).
Cuzick, J. et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 16 , 67–75 (2015). This paper describes the long-term follow-up of the IBIS-I trial participants who received 5 years of preventive tamoxifen and shows that the protection against breast cancer is maintained for at least 20 years .
Nelson, H. D., Smith, M. E., Griffin, J. C. & Fu, R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 158 , 604–614 (2013).
Skandarajah, A. R. et al. Patient and medical barriers preclude uptake of tamoxifen preventative therapy in women with a strong family history. Breast 32 , 93–97 (2017).
Vogel, V. G. et al. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev. Res. 3 , 696–706 (2010).
Freedman, A. N. et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J. Clin. Oncol. 29 , 2327–2333 (2011).
Cuzick, J. et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet 395 , 117–122 (2020). This paper describes the long-term follow-up of a randomized controlled trial of anastrozole or placebo for breast cancer prevention and shows that 5 years of anastrozole confers at least 10 years of preventive benefit .
Cuzick, J. et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383 , 1041–1048 (2014).
Goss, P. E. et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364 , 2381–2391 (2011).
Noonan, S. et al. A survey among breast cancer specialists on the low uptake of therapeutic prevention with tamoxifen or raloxifene. Cancer Prev. Res. 11 , 38–43 (2018).
Collins, I. M. et al. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med. J. Aust. 199 , 680–683 (2013).
Collins, I. M. et al. Assessing and managing breast cancer risk: clinicians’ current practice and future needs. Breast 23 , 644–650 (2014).
Keogh, L. A., Hopper, J. L., Rosenthal, D. & Phillips, K. A. Australian clinicians and chemoprevention for women at high familial risk for breast cancer. Hered. Cancer Clin. Pract. 7 , 9 (2009).
Smith, S. G. et al. Prescribing tamoxifen in primary care for the prevention of breast cancer: a national online survey of GPs’ attitudes. Br. J. Gen. Pract. 67 , e414–e427 (2017).
Smith, S. G. et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann. Oncol. 27 , 575–590 (2016). This meta-analysis shows that uptake of therapeutic agents for the prevention of breast cancer is low and persistent long-term use is often insufficient, meaning that women do not experience the full preventive effect .
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl Cancer Inst. 90 , 1371–1388 (1998).
Donnelly, L. S. et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br. J. Cancer 110 , 1681–1687 (2014).
Bober, S. L., Hoke, L. A., Duda, R. B., Regan, M. M. & Tung, N. M. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J. Clin. Oncol. 22 , 4951–4957 (2004).
Cuzick, J. et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360 , 817–824 (2002).
Heisey, R., Pimlott, N., Clemons, M., Cummings, S. & Drummond, N. Women’s views on chemoprevention of breast cancer: qualitative study. Can. Fam. Physician 52 , 624–625 (2006).
Kuderer, N. M. & Peppercorn, J. CYP2D6 testing in breast cancer: ready for prime time? Oncology 23 , 1223–1232 (2009).
DeCensi, A. et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J. Natl Cancer Inst. 95 , 779–790 (2003).
DeCensi, A. et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J. Clin. Oncol. 37 , 1629–1637 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00952731 (2009).
Rojas, L. B. & Gomes, M. B. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 5 , 6 (2013).
Bodmer, M., Meier, C., Krahenbuhl, S., Jick, S. S. & Meier, C. R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 33 , 1304–1308 (2010).
EU Clinical Trials Register. ClinicalTrialsRegister.eu https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2009-009921-28 (2010).
Boissier, S. et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 60 , 2949–2954 (2000).
Chlebowski, R. T. et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J. Clin. Oncol. 28 , 3582–3590 (2010).
Daubine, F., Le Gall, C., Gasser, J., Green, J. & Clezardin, P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J. Natl Cancer Inst. 99 , 322–330 (2007).
van der Pluijm, G. et al. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J. Clin. Invest. 98 , 698–705 (1996).
Early Breast Cancer Trialists’ Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386 , 1353–1361 (2015).
Dhesy-Thind, S. et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 35 , 2062–2081 (2017).
Gnant, M., Harbeck, N. & St. Thomssen, C. Gallen/Vienna 2017: a brief summary of the consensus discussion about escalation and de-escalation of primary breast cancer treatment. Breast Care (Basel) 12 , 102–107 (2017).
Vestergaard, P., Fischer, L., Mele, M., Mosekilde, L. & Christiansen, P. Use of bisphosphonates and risk of breast cancer. Calcif. Tissue Int. 88 , 255–262 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02781805 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03323658 (2017).
EU Clinical Trials Register. ClinicalTrialsRegister.eu https://www.clinicaltrialsregister.eu/ctr-search/search?query=2009-010260-41 (2009).
Moon, R. C. et al. N -(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 39 , 1339–1346 (1979).
Veronesi, U. et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J. Natl Cancer Inst. 91 , 1847–1856 (1999).
Xu, L. et al. Tamoxifen and risk of contralateral breast cancer among women with inherited mutations in BRCA1 and BRCA2 : a meta-analysis. Breast Cancer 22 , 327–334 (2015).
Phillips, K. A. et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 31 , 3091–3099 (2013). This large international study of pooled cohort data shows that tamoxifen use for first breast cancer is associated with a marked reduction in contralateral breast cancer risk in both BRCA1 and BRCA2 mutation carriers .
Nolan, E. et al. RANK ligand as a potential target for breast cancer prevention in BRCA1 -mutation carriers. Nat. Med. 22 , 933–939 (2016). This study identifies RANK + luminal progenitors as the cancer cell of origin for breast cancer that develops in BRCA1 mutation carriers and shows that inhibition of RANKL signalling can reduce proliferation in patient-derived organoids and tumour development in transgenic mice .
Nolan, E., Lindeman, G. J. & Visvader, J. E. Out-RANKing BRCA1 in mutation carriers. Cancer Res. 77 , 595–600 (2017).
EU Clinical Trials Register. ClinicalTrialsRegister.eu https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-002505-35/AT (2018).
Poole, A. J. et al. Prevention of Brca1 -mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314 , 1467–1470 (2006).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02408770 (2015).
Lu, L., Shi, L., Zeng, J. & Wen, Z. Aspirin as a potential modality for the chemoprevention of breast cancer: a dose–response meta-analysis of cohort studies from 857,831 participants. Oncotarget 8 , 40389–40401 (2017).
de Pedro, M. et al. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res. Treat. 149 , 525–536 (2015).
Kehm, R. D. et al. Regular use of aspirin and other non-steroidal anti-inflammatory drugs and breast cancer risk for women at familial or genetic risk: a cohort study. Breast Cancer Res. 21 , 52 (2019).
Gierach, G. L. et al. Nonsteroidal anti-inflammatory drugs and breast cancer risk in the National Institutes of Health–AARP Diet and Health Study. Breast Cancer Res. 10 , R38 (2008).
Marshall, S. F. et al. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J. Natl Cancer Inst. 97 , 805–812 (2005).
Terry, M. B. et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA 291 , 2433–2440 (2004).
Unsworth, A., Anderson, R. & Britt, K. Stromal fibroblasts and the immune microenvironment: partners in mammary gland biology and pathology? J. Mammary Gland. Biol. Neoplasia 19 , 169–182 (2014).
Visvader, J. E. & Stingl, J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 28 , 1143–1158 (2014).
Gil Del Alcazar, C. R. et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 7 , 1098–1115 (2017). This paper assesses normal breast, DCIS and invasive ductal carcinomas to reveal co-evolution of cancer cells and the immune microenvironment .
Cossart, Y. E. The rise and fall of infectious diseases: Australian perspectives, 1914–2014. Med. J. Aust. 201 , S11–S14 (2014).
Harvard University. BayesMendel R Package https://projects.iq.harvard.edu/bayesmendel/brcapro (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00078832 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03063619 (2017).
EU Clinical Trials Register. ClinicalTrialsRegister.eu https://www.clinicaltrialsregister.eu/ctr-search/search?query=2016-001087-11 (2016).
Australia NewZealand Clinical Trials registry. ANZCTR https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000694617 (2014).
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 347 , 1713–1727 (1996). This study uses a large data set of epidemiological data spanning 25 countries to show that women who are currently taking the OCP (or those still within 10 years of stopping) are at an increased risk of breast cancer.
Morch, L. S. et al. Contemporary hormonal contraception and the risk of breast cancer. N. Engl. J. Med. 377 , 2228–2239 (2017).
Australia Institute of Health and Welfare. AIHW https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity-an-interactive-insight/contents/prevalence (2019).
Provenzano, P. P. et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 6 , 11 (2008).
Alowami, S., Troup, S., Al-Haddad, S., Kirkpatrick, I. & Watson, P. H. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 5 , R129–R135 (2003).
Cho, A., Howell, V. M. & Colvin, E. K. The extracellular matrix in epithelial ovarian cancer—a piece of a puzzle. Front. Oncol. 5 , 245 (2015).
Provenzano, P. P. & Keely, P. J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 124 , 1195–1205 (2011).
Lisanti, M. P. et al. JNK1 stress signaling is hyper-activated in high breast density and the tumor stroma: connecting fibrosis, inflammation, and stemness for cancer prevention. Cell Cycle 13 , 580–599 (2014).
Dushyanthen, S. et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 13 , 202 (2015).
Tower, H., Ruppert, M. & Britt, K. The immune microenvironment of breast cancer progression. Cancers 11 , 1375 (2019).
Article CAS PubMed Central Google Scholar
Simon, T. & Bromberg, J. S. Regulation of the immune system by laminins. Trends Immunol. 38 , 858–871 (2017).
Lu, P., Takai, K., Weaver, V. M. & Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3 , a005058 (2011).
Hallmann, R. et al. The regulation of immune cell trafficking by the extracellular matrix. Curr. Opin. Cell Biol. 36 , 54–61 (2015).
Kajita, M. & Fujita, Y. EDAC: epithelial defence against cancer-cell competition between normal and transformed epithelial cells in mammals. J. Biochem. 158 , 15–23 (2015).
Hunter, D. J. et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol. Biomarkers Prev. 19 , 2496–2502 (2010).
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 350 , 1047–1059 (1997).
Althuis, M. D. et al. Hormonal content and potency of oral contraceptives and breast cancer risk among young women. Br. J. Cancer 88 , 50–57 (2003).
Beral, V., Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362 , 419–427 (2003).
Jones, J., Mosher, W. & Daniels, K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Health Stat Report 60 , 1–25 (2012).
Daniels, K. & Abma, J. C. Current contraceptive status among women aged 15–49: United States, 2015–2017 CDC https://www.cdc.gov/nchs/data/databriefs/db327-h.pdf (2018). US statistics indicate that as much as 5% of OCP users are older premenopausal women aged 40–49 years.
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288 , 321–333 (2002).
Chlebowski, R. T. et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 1 , 296–305 (2015).
Chlebowski, R. T. et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA 289 , 3243–3253 (2003).
Chlebowski, R. T. et al. Long-term influence of estrogen plus progestin and estrogen alone use on breast cancer incidence: The Women's Health Initiative randomized trials [abstract]. Cancer Res. 80 , GS5-00 (2020).
Download references
Acknowledgements
K.L.B. is a Victorian Cancer Agency mid-career fellow and is also supported by the Peter MacCallum Research Foundation and Harold Homes Equity Trustees grant. K.-A.P is an Australian National Breast Cancer Foundation Fellow.
Author information
Authors and affiliations.
Breast Cancer Risk and Prevention Laboratory, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
Kara L. Britt
The Sir Peter MacCallum Department of Oncology, The University of Melbourne, Parkville, VIC, Australia
Kara L. Britt & Kelly-Anne Phillips
Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary University of London, London, UK
Jack Cuzick
Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
Kelly-Anne Phillips
Centre for Epidemiology and Biostatistics, School of Population and Global Health, The University of Melbourne, Parkville, VIC, Australia
You can also search for this author in PubMed Google Scholar
Contributions
K.L.B and K.-A.P researched data for the article, made substantial contributions to discussions of the content and wrote the article. J.C. provided vital input to the article and insight into preventives that are currently being trialled. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Kara L. Britt .
Ethics declarations
Competing interests.
The authors wish to disclose that Cancer Research UK (CRUK) licences the International Breast Cancer Intervention Study (IBIS; also known as Tyrer–Cuzick) model for commercial use and J.C. receives some benefit. K.A.-P has a patent, System and Process of Cancer Risk Estimation (Australian Innovation Patent), issued regarding iPrevent. K.L.B has no competing interests.
Additional information
Peer review information.
Nature Reviews Cancer thanks O. Olopade, S. Narod and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related Links
Breast Cancer Surveillance Consortium: https://tools.bcsc-scc.org/BC5yearRisk/intro.htm
BOADICEA: https://ccge.medschl.cam.ac.uk/boadicea/
BCRAT: https://bcrisktool.cancer.gov/calculator.html
IBIS: http://www.ems-trials.org/riskevaluator/
Confluence project: https://dceg.cancer.gov/research/cancer-types/breast-cancer/confluence-project
Press release of results from Topical Endoxifen trial: https://www.globenewswire.com/news-release/2019/06/27/1875229/0/en/Atossa-Genetics-Preliminary-Phase-2-Study-Achieves-Primary-Endpoint-Topical-Endoxifen-Rapidly-Reduces-Breast-Density.html
(MD). The extent of white or radio-opaque tissue (dense area) shown on a mammogram. Per cent MD is used to represent this dense area as a proportion of the total tissue area of the breast on a mammogram.
(MHT). Sex hormones given to treat symptoms or prevent long-term morbidities associated with female menopause. Also known as hormone replacement therapy.
The time in a girl’s life when her first menstrual bleeding or period begins.
The state of having borne offspring (liveborn or stillborn). Also used to indicate the number of pregnancies reaching viable gestational age (liveborn or stillborn — pregnancies resulting in multiple births, such as twins, count as one).
The exchange of nucleotide sequences between two similar or identical molecules of DNA. It is used by cells to accurately repair damage that occurs on both strands of DNA, such as double-strand breaks or inter-strand DNA cross-links.
(RR). The ratio of the probability of an event occurring in the group exposed to the modifier of interest versus the probability of the event occurring in the non-exposed group. A relative risk of 1.5 means people exposed to the risk modifier, on average, have a 50% higher risk than those not exposed.
(OCP). A birth control pill taken orally. Most contain oestrogen and progesterone, which when given at certain times in the menstrual cycle at defined doses can prevent the ovary from releasing the egg for fertilization.
A cell death-mediated process by which the lactating breast returns to the pre-pregnant state after weaning (or after childbirth if lactation is not initiated). It is characterized by robust tissue remodelling.
(MaSCs). Cells within the mammary gland that have the capacity to form a new mammary tree when transplanted into a cleared mammary fat pad. MaSCs reside within the basal/myoepithelial compartment and can be identified with CD24/EpCAM and either CD29 or CD49f.
(OR). A statistic that quantifies the strength of the association between an exposure and an outcome. OR = 1 means that the exposure does not affect the odds of outcome, OR >1 means that the exposure is associated with higher odds of outcome, and OR <1 means that the exposure is associated with lower odds of outcome.
A cell signalling protein secreted by adipose (fat) cells.
A genetic condition, affecting about 1 in every 550 men, in which a male is born with an extra copy of the X chromosome. This results in higher levels of female hormones.
Excessive enlargement of the male breast. May be unilateral (one side) or bilateral (both sides).
The risk of developing a disease over a time period, for example, a person may have a one in ten risk (that is, a 10% risk) of a certain disease in their life. Absolute risk is one of the most easily understood ways of communicating health risks to the general public.
(HR). A measure of how often a particular event happens in one group compared with another group, over time. HR = 1.0 means that there is no difference in survival between the two groups. HR >1.0 or HR <1.0 means that survival was better in one of the groups.
A breast cancer subtype that is more prevalent in African-American women, characterized by high histological grade, high mitotic indices and lack of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) protein overexpression.
A genetic disorder that is caused by the combined action of more than one gene.
Tools that estimate a person’s likelihood of developing breast cancer within a specific time frame.
The ability of a risk model to separate individuals who will get breast cancer from those who will not. A value of 1.0 represents perfect discrimination, a value of 0.5 means that the model performance is no better than chance alone, values of 0.6–0.7 are considered good and values of 0.5–0.6 are considered sufficient.
The ratio of the observed number of breast cancer cases to the expected number; values of one indicate optimal calibration.
The removal of as much breast tissue as possible to reduce the breast cancer risk.
A surgical procedure to remove both ovaries and fallopian tubes.
A route of drug administration wherein the drug is delivered across the skin, via patches or creams, for systemic distribution.
A type of luminal epithelial cell within the mammary epithelium that has both luminal differentiation markers and progenitor activity (colony-forming and repopulating activity in vivo).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Britt, K.L., Cuzick, J. & Phillips, KA. Key steps for effective breast cancer prevention. Nat Rev Cancer 20 , 417–436 (2020). https://doi.org/10.1038/s41568-020-0266-x
Download citation
Accepted : 23 April 2020
Published : 11 June 2020
Issue Date : August 2020
DOI : https://doi.org/10.1038/s41568-020-0266-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Changes in blood metabolomes as potential markers for severity and prognosis in doxorubicin-induced cardiotoxicity: a study in her2-positive and her2-negative breast cancer patients.
- Chanisa Thonusin
- Nichanan Osataphan
- Nipon Chattipakorn
Journal of Translational Medicine (2024)
Healthy lifestyles, systemic inflammation and breast cancer risk: a mediation analysis
- Yanyu Zhang
- Mengjie Song
- Haomin Yang
BMC Cancer (2024)
Assessment of breast cancer awareness among female pharmacy students at a university in Turkey
- Aslınur Albayrak
- Kayhan Nuri Cengiz
BMC Medical Education (2024)
Triglyceride-glucose index is a risk factor for breast cancer in China: a cross-sectional study
- Jinghua Zhang
- Yongying Bai
Lipids in Health and Disease (2024)
The integration of multidisciplinary approaches revealed PTGES3 as a novel drug target for breast cancer treatment
- Xuewei Zheng
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

Appointments at Mayo Clinic
- Women's health
Breast cancer prevention: How to reduce your risk
Breast cancer prevention starts with healthy habits — such as limiting alcohol and staying physically active. Learn what you can do to lower your breast cancer risk.
If you're concerned about getting breast cancer, you might wonder what you can do to help prevent it. You can't change some risk factors, such as family history. But you can make lifestyle changes to lower your risk.
What can I do to lower my risk of breast cancer?
Research shows that lifestyle changes can lower the chances of getting breast cancer, even in people at high risk. To lower your risk:
- Limit or stay away from alcohol. It's safest not to drink alcohol. But if you do drink it, enjoy it in moderation. The more alcohol you have, the greater your risk of getting breast cancer. In general, women should have no more than one drink a day. Even small amounts raise the risk of breast cancer. One drink is about 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of 80-proof distilled spirits.
- Stay at a healthy weight. Ask a member of your health care team whether your weight is healthy. If it is, work to maintain that weight. If you need to lose weight, ask your health care professional how to do so. Simple steps may help. Watch your portion sizes. Try to eat fewer calories. And slowly build up the amount of exercise you do.
- Get active. Physical activity can help you stay at a healthy weight, which helps prevent breast cancer. So try to move more and sit less. Most healthy adults should aim for at least 150 minutes a week of moderate aerobic exercise. Or try to get at least 75 minutes of vigorous aerobic exercise a week. Aerobic exercise gets your heart pumping. Some examples are walking, biking, running and swimming. Also aim to do strength training at least twice a week.
- Breastfeed. If you have a baby, breastfeeding might play a role in helping prevent breast cancer. The longer you breastfeed, the greater the protective effect.
Limit hormone therapy after menopause. Combination hormone therapy uses estrogen and progestin. It may raise the risk of breast cancer. Talk with your health care professional about the risks and benefits of hormone therapy. You might be able to manage your symptoms with treatments and medicines that don't use hormones. If you decide that the benefits of short-term hormone therapy outweigh the risks, use the lowest amount that works for you. Have your health care team track the length of time you take hormones.
Studies show that estrogen alone in people who have had hysterectomies does not raise breast cancer risk. Estrogen is linked with a small increase in blood clot and stroke risk.
- If you smoke, quit. Some research suggests that smoking tobacco raises the risk of breast cancer. Breathing in another person's cigarette smoke also may raise the risk. If you or a loved one needs help quitting, talk with a member of your health care team.
Can a healthy diet help prevent breast cancer?
Eating a healthy diet might lower your risk of some types of cancer. It also might lower the odds of getting diabetes and heart disease or having a stroke.
Some research suggests that people who eat a Mediterranean diet might have a lower risk of breast cancer, especially after menopause. The Mediterranean diet focuses mostly on plant foods. It includes fruits and vegetables, whole grains, legumes and nuts. People who follow the Mediterranean diet choose healthy fats such as extra-virgin olive oil over butter. And they eat fish instead of red meat.
A balanced diet can help you stay at a healthy weight. And healthy weight is a key factor in helping prevent breast cancer.
Is there a link between birth control pills and breast cancer?
There's some evidence that hormonal types of birth control raise the risk of breast cancer. These include birth control pills and intrauterine devices (IUDs) that release hormones. But the risk is very small. And it drops after you stop using hormonal birth control.
Talk with a member of your health care team about your birth control options. Your health care professional can help you weigh the benefits and risks. The benefits of birth control pills include:
- Controlling menstrual bleeding.
- Preventing unwanted pregnancy.
- Lowering the risk of other cancers, such as endometrial cancer and ovarian cancer.
What else can I do?
If you notice any changes in how your breasts look or feel, tell a member of your health care team right away. For example, get a checkup if you feel a new lump or see skin changes. And ask your health care professional when to start mammograms and other screening tests based on your medical history.
Some people have a higher risk of breast cancer. This can be due to things such as having a family history of the disease or certain gene changes. If your health care professional tells you that your risk is higher, you may be advised to take steps such as:
- Genetic counseling and testing.
- More-frequent breast exams.
- Breast cancer screening tests at an earlier age.
- Medicines or surgery to prevent breast cancer.
- Breast cancer prevention (PDQ) — Professional Version. National Cancer Institute. https://www.cancer.gov/types/breast/hp/breast-prevention-pdq. Accessed June 29, 2023.
- What can I do to reduce my risk of breast cancer? Centers for Disease Control and Prevention. https://www.cdc.gov/cancer/breast/basic_info/prevention.htm. Accessed June 7, 2023.
- Colditz GA. Overview of cancer prevention. https://www.uptodate.com/contents/search. Accessed June 7, 2023.
- Diet and physical activity: What's the cancer connection? American Cancer Society. https://www.cancer.org/cancer/risk-prevention/diet-physical-activity/diet-and-physical-activity.html. Accessed June 7, 2023.
- Physical activity and cancer. American Cancer Society. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet. Accessed June 7, 2023.
- Can I lower my risk of breast cancer? American Cancer Society. https://www.cancer.org/cancer/breast-cancer/risk-and-prevention/can-i-lower-my-risk.html. Accessed June 7, 2023.
- Chlebowski RT. Factors that modify breast cancer risk in women. https://www.uptodate.com/contents/search. Accessed June 7, 2023.
- Oral contraceptives and cancer risk. National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/oral-contraceptives-fact-sheet#q3. Accessed June 7, 2023.
- Menopausal hormone therapy and cancer risk. American Cancer Society. https://www.cancer.org/cancer/cancer-causes/medical-treatments/menopausal-hormone-replacement-therapy-and-cancer-risk.html. Accessed June 7, 2023.
- Frequently asked questions about the American Cancer Society's breast cancer screening guideline. American Cancer Society. https://www.cancer.org/cancer/types/breast-cancer/frequently-asked-questions-about-the-american-cancer-society-new-breast-cancer-screening-guideline.html. Accessed June 8, 2023.
- Torres CGP, et al. Mediterranean diet and risk of breast cancer: An umbrella review. Clinical Nutrition. 2023; doi:10.1016/j.clnu.2023.02.012.
- Secondhand tobacco smoke (environmental tobacco smoke). National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/secondhand-smoke. Accessed June 8, 2023.
- Menopausal hormone therapy and cancer risk. American Cancer Society. https://www.cancer.org/cancer/risk-prevention/medical-treatments/menopausal-hormone-replacement-therapy-and-cancer-risk.html. Accessed June 20, 2023.
Products and Services
- A Book: Mayo Clinic Book of Home Remedies
- A Book: Beyond Breast Cancer
- Breast implants: Saline vs. silicone
- Breast implants and cancer
- Evaluating breast lumps
- COVID-19 vaccine: Should I reschedule my mammogram?
- Dense breast tissue
- Does soy really affect breast cancer risk?
- Natural breast enhancement
- Silicone breast implants: What happens if they rupture?
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
- Breast cancer prevention How to reduce your risk
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Advances in Breast Cancer Research
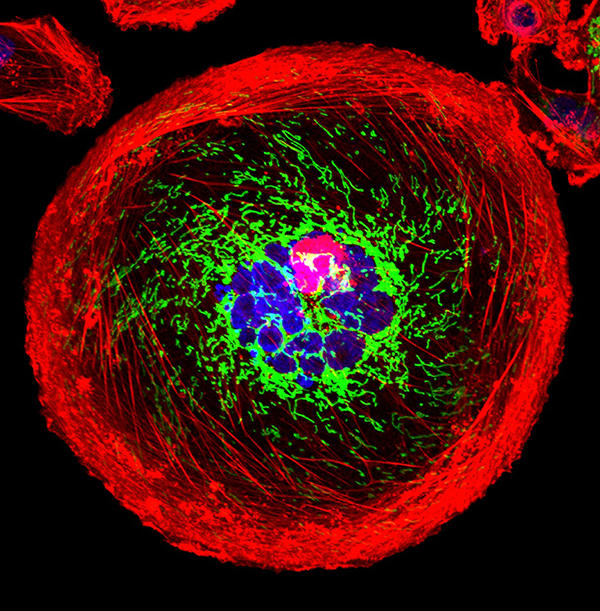
A polyploid giant cancer cell (PGCC) from triple-negative breast cancer.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat breast cancer. They are also looking at how to address disparities and improve quality of life for survivors of the disease.
This page highlights some of what's new in the latest research for breast cancer, including new clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Early Detection of Breast Cancer
Breast cancer is one of a few cancers for which an effective screening test, mammography , is available. MRI ( magnetic resonance imaging ) and ultrasound are also used to detect breast cancer, but not as routine screening tools for people with average risk.
Ongoing studies are looking at ways to enhance current breast cancer screening options. Technological advances in imaging are creating new opportunities for improvements in both screening and early detection.
One technology advance is 3-D mammography , also called breast tomosynthesis . This procedure takes images from different angles around the breast and builds them into a 3-D-like image. Although this technology is increasingly available in the clinic, it isn’t known whether it is better than standard 2-D mammography , for detecting cancer at a less advanced stage.
NCI is funding a large-scale randomized breast screening trial, the Tomosynthesis Mammographic Imaging Screening Trial (TMIST) , to compare the number of advanced cancers detected in women screened for 5 years with 3-D mammography with the number detected in women screened with 2-D mammography.
Two concerns in breast cancer screening, as in all cancer screening, are:
- the potential for diagnosing tumors that would not have become life-threatening ( overdiagnosis )
- the possibility of receiving false-positive test results, and the anxiety that comes with follow-up tests or procedures
As cancer treatment is becoming more individualized, researchers are looking at ways to personalize breast cancer screening. They are studying screening methods that are appropriate for each woman’s level of risk and limit the possibility of overdiagnosis.
For example, the Women Informed to Screen Depending on Measures of Risk (WISDOM) study aims to determine if risk-based screening—that is, screening at intervals that are based on each woman’s risk as determined by her genetic makeup, family history , and other risk factors—is as safe, effective, and accepted as standard annual screening mammography.
WISDOM is also making a focused effort to enroll Black women in the trial. Past studies tended to contain a majority of White women and therefore, there is less data on how screening can benefit Black women. Researchers are taking a number of steps to include as many Black women as possible in the study while also increasing the diversity of all women enrolled.
Breast Cancer Treatment
The mainstays of breast cancer treatment are surgery , radiation , chemotherapy , hormone therapy , and targeted therapy . But scientists continue to study novel treatments and drugs, along with new combinations of existing treatments.
It is now known that breast cancer can be divided into subtypes based on whether they:
- are hormone receptor (HR) positive which means they express estrogen and/or progesterone receptors ( ER , PR )
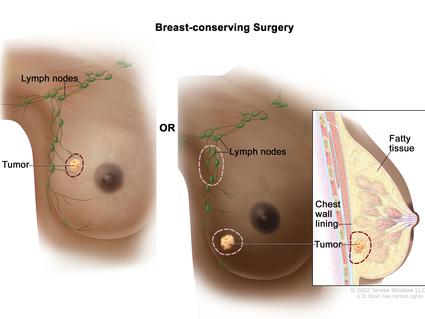
Shortening Radiation Therapy for Some with Early Breast Cancer
A condensed course was as effective and safe as the standard course for women with higher-risk early-stage breast cancer who had a lumpectomy.
As we learn more about the subtypes of breast cancer and their behavior, we can use this information to guide treatment decisions. For example:
- The NCI-sponsored TAILORx clinical trial. The study, which included patients with ER-positive, lymph node-negative breast cancer, found that a test that looks at the expression of certain genes can predict which women can safely avoid chemotherapy.
- The RxPONDER trial found that the same gene expression test can also be used to determine treatment options in women with more advanced breast cancer. The study found that some postmenopausal women with HR positive, HER-2 negative breast cancer that has spread to several lymph nodes and has a low risk of recurrence do not benefit from chemotherapy when added to their hormone therapy.
- The OFSET trial is comparing the addition of chemotherapy to usual treatment ( ovarian function suppression plus hormone therapy) to usual treatment alone in treating premenopausal estrogen receptor (ER)-positive/HER2-negative breast cancer patients who are at high risk of their cancer returning. This will help determine whether or not adding chemotherapy helps prevent the cancer from returning.
Genomic analyses, such as those carried out through The Cancer Genome Atlas (TCGA) , have provided more insights into the molecular diversity of breast cancer and eventually could help identify even more breast cancer subtypes. That knowledge, in turn, may lead to the development of therapies that target the genetic alterations that drive those cancer subtypes.
HR-Positive Breast Cancer Treatment
Hormone therapies have been a mainstay of treatment for HR-positive cancer. However, there is a new focus on adding targeted therapies to hormone therapy for advanced or metastatic HR-positive cancers. These treatments could prolong the time until chemotherapy is needed and ideally, extend survival. Approved drugs include:

Drug Combo Effective for Metastatic Breast Cancer in Younger Women
Ribociclib plus hormone therapy were superior to standard chemotherapy combos in a recent trial.
- Palbociclib (Ibrance) , ribociclib (Kisqali) , and everolimus (Afinitor) have all been approved by the FDA for use with hormone therapy for treatment of advanced or metastatic breast cancer. Ribociclib has been shown to increase the survival of patients with metastatic breast cancer . It has also shown to slow the growth of metastatic cancer in younger women when combined with hormone therapy.
- Elacestrant (Orserdu) is approved for HR-positive and HER2-negative breast cancer that has a mutation in the ESR1 gene, and has spread. It is used in postmenopausal women and in men whose cancer has gotten worse after at least one type of hormone therapy.
- Abemaciclib (Verzenio) can be used with or after hormone therapy to treat advanced or metastatic HR-positive, HER2-negative breast cancer. In October 2021, the Food and Drug Administration ( FDA ) approved abemaciclib in combination with hormone therapy to treat some people who have had surgery for early-stage HR-positive, HER2-negative breast cancer.
- Alpelisib (Piqray) is approved to be used in combination with hormone therapy to treat advanced or metastatic HR-positive, HER2-negative breast cancers that have a mutation in the PIK3CA gene .
- Sacituzumab govitecan-hziy (Trodelvy) is used for HR-positive and HER2-negative breast cancer that has spread or can't be removed with surgery. It is used in those who have received hormone therapy and at least two previous treatments. It has shown to extend the amount of time that the disease doesn't get worse ( progression-free survival ) and also shown to improve overall survival .
HER2-Positive Breast Cancer Treatment
The FDA has approved a number of targeted therapies to treat HER2-positive breast cancer , including:
- Trastuzumab (Herceptin) has been approved to be used to prevent a relapse in patients with early-stage HER2-positive breast cancer.
- Pertuzumab (Perjeta) is used to treat metastatic HER2-positive breast cancer, and also both before surgery ( neoadjuvant ) and after surgery ( adjuvant therapy ).
- Trastuzumab and pertuzumab together can be used in combination with chemotherapy to prevent relapse in people with early-stage HER2-positive breast cancer. Both are also used together in metastatic disease, where they delay progression and improve overall survival.
- Trastuzumab deruxtecan (Enhertu) is approved for patients with advanced or metastatic HER2-positive breast cancer who have previously received a HER2-targeted treatment. A 2021 clinical trial showed that the drug lengthened the time that people with metastatic HER2-positive breast cancer lived without their cancer progressing. The trial also showed that it was better at shrinking tumors than another targeted drug, trastuzumab emtansine (Kadcyla).
- Tucatinib (Tukysa) is approved to be used in combination with trastuzumab and capecitabine (Xeloda) for HER2-positive breast cancer that cannot be removed with surgery or is metastatic. Tucatinib is able to cross the blood–brain barrier, which makes it especially useful for HER2-positive metastatic breast cancer, which tends to spread to the brain.
- Lapatinib (Tykerb) has been approved for treatment of some patients with HER2-positive advanced or metastatic breast cancer, together with capecitabine or letrozole.
- Neratinib Maleate (Nerlynx) can be used in patients with early-stage HER2-positive breast cancer and can also be used together with capecitabine (Xeloda) in some patients with advanced or metastatic disease.
- Ado-trastuzumab emtansine (Kadcyla) is approved to treat patients with metastatic HER2-positive breast cancer who have previously received trastuzumab and a taxane . It's also used in some patients with early-stage HER2-positive breast cancer who have completed therapy before surgery ( neoadjuvant ) and have residual disease at the time of surgery.
HER2-Low Breast Cancer
A newly defined subtype, HER2-low, accounts for more than half of all metastatic breast cancers. HER2-low tumors are defined as those whose cells contain lower levels of the HER2 protein on their surface. Such tumors have traditionally been classified as HER2-negative because they did not respond to drugs that target HER2.
However, in a clinical trial, trastuzumab deruxtecan (Enhertu) improved the survival of patients with HER2-low breast cancer compared with chemotherapy , and the drug is approved for use in such patients.

Immunotherapy Improves Survival in Triple-Negative Breast Cancer
For patients whose tumors had high PD-L1 levels, pembrolizumab with chemo helped them live longer.
Triple-Negative Breast Cancer Treatment
Triple-negative breast cancers (TNBC) are the hardest to treat because they lack both hormone receptors and HER2 overexpression , so they do not respond to therapies directed at these targets. Therefore, chemotherapy is the mainstay for treatment of TNBC. However, new treatments are starting to become available. These include:
- Sacituzumab govitecan-hziy (Trodelvy) is approved to treat patients with TNBC that has spread to other parts of the body . Patients must have received at least two prior therapies before receiving the drug.
- Pembrolizumab (Keytruda) is an immunotherapy drug that is approved to be used in combination with chemotherapy for patients with locally advanced or metastatic TNBC that has the PD-L1 protein. It may also be used before surgery (called neoadjuvant ) for patients with early-stage TNBC, regardless of their PD-L1 status.
- PARP inhibitors, which include olaparib (Lynparza) and talazoparib (Talzenna) , are approved to treat metastatic HER2-negative or triple-negative breast cancers in patients who have inherited a harmful BRCA gene mutation. Olaparib is also approved for use in certain patients with early-stage HER2-negative or triple-negative breast cancer.
- Drugs that block the androgen receptors or prevent androgen production are being tested in a subset of TNBC that express the androgen receptor.
For a complete list of drugs for breast cancer, see Drugs Approved for Breast Cancer .
NCI-Supported Breast Cancer Research Programs
Many NCI-funded researchers working at the NIH campus, as well as across the United States and world, are seeking ways to address breast cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some are more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in breast cancer.
TMIST is a randomized breast screening trial that compares two Food and Drug Administration (FDA)-approved types of digital mammography, standard digital mammography (2-D) with a newer technology called tomosynthesis mammography (3-D).
The Breast Specialized Programs of Research Excellence (Breast SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Breast SPOREs support the development of new therapies and technologies, and studies to better understand tumor resistance, diagnosis, prognosis, screening, prevention, and treatment of breast cancer.
The NCI Cancer Intervention and Surveillance Modeling Network (CISNET) focuses on using modeling to improve our understanding of how prevention, early detection, screening, and treatment affect breast cancer outcomes.
The Confluence Project , from NCI's Division of Cancer Epidemiology and Genetics (DCEG) , is developing a research resource that includes data from thousands of breast cancer patients and controls of different races and ethnicities. This resource will be used to identify genes that are associated with breast cancer risk, prognosis, subtypes, response to treatment, and second breast cancers. (DCEG conducts other breast cancer research as well.)
The Black Women’s Health Study (BWHS) Breast Cancer Risk Calculator allows health professionals to estimate a woman’s risk of developing invasive breast cancer over the next 5 years. With the NCI-funded effort, researchers developed a tool to estimate the risk of breast cancer in US Black women. The team that developed the tool hopes it will help guide more personalized decisions on when Black women—especially younger women—should begin breast cancer screening.
The goal of the Breast Cancer Surveillance Consortium (BCSC) , an NCI-funded program launched in 1994, is to enhance the understanding of breast cancer screening practices in the United States and their impact on the breast cancer's stage at diagnosis, survival rates, and mortality.
There are ongoing programs at NCI that support prevention and early detection research in different cancers, including breast cancer. Examples include:
- The Cancer Biomarkers Research Group , which promotes research in cancer biomarkers and manages the Early Detection Research Network (EDRN) . EDRN is a network of NCI-funded institutions that are collaborating to discover and validate early detection biomarkers. Within the EDRN, the Breast and Gynecologic Cancers Collaborative Group conducts research on breast and ovarian cancers.
- NCI's Division of Cancer Prevention houses the Breast and Gynecologic Cancer Research Group which conducts and fosters the development of research on the prevention and early detection of breast and gynecologic cancers.
Breast Cancer Survivorship Research
NCI’s Office of Cancer Survivorship, part of the Division of Cancer Control and Population Sciences (DCCPS), supports research projects throughout the country that study many issues related to breast cancer survivorship. Examples of studies funded include the impact of cancer and its treatment on physical functioning, emotional well-being, cognitive impairment , sleep disturbances, and cardiovascular health. Other studies focus on financial impacts, the effects on caregivers, models of care for survivors, and issues such as racial disparities and communication.
Breast Cancer Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for breast cancer prevention , screening , and treatment .
Breast Cancer Research Results
The following are some of our latest news articles on breast cancer research and study updates:
- Can Some People with Breast Cancer Safely Skip Lymph Node Radiation?
- Study Adds to Debate about Mammography in Older Women
- Pausing Long-Term Breast Cancer Therapy to Become Pregnant Appears to Be Safe
- A Safer, Better Treatment Option for Some Younger Women with Breast Cancer
- Shorter Course of Radiation Is Effective, Safe for Some with Early-Stage Breast Cancer
- Pembrolizumab Improves Survival in Advanced Triple-Negative Breast Cancer
View the full list of Breast Cancer Research Results and Study Updates .

IMAGES
VIDEO