
A Medical Research Assistant (MRA) is an individual who supports and conducts research in the medical field, assisting in the design, implementation and analysis of medical studies. They work alongside researchers and scientists to collect, organize, and interpret data, and may also be responsible for recruiting participants and conducting interviews and surveys. MRAs are also involved in laboratory work, such as preparing samples and operating equipment, and may assist in the preparation of reports, publications and presentations. They play a crucial role in the advancement of medical research and the development of new treatments and therapies.

What Does A Medical Research Assistant Do?
How to become a medical research assistant.
To become a Medical Research Assistant, an individual typically needs a bachelor's degree in a related field such as biology, chemistry, or psychology. Some employers may also consider candidates with a degree in a related field or relevant laboratory experience. On-the-job training is also provided to new MRAs.
Avg. Experience
Get Medical Research Assistant Jobs Emailed to You
By signing up, you agree to the terms of use and privacy policy
Medical Research Assistant Jobs
Senior cat risk analyst.
September 25, 2024
Associate Product Analyst - Personal Lines
May 10, 2024
Lead Accounting Analyst (Flex Home/Office)
September 5, 2024
Insurance Defense Trial Attorney (Primarily Home in ...
August 6, 2024
Senior Executive Compensation Analyst
October 10, 2024
Operations Technician Senior - Commercial Lines
October 9, 2024
Average Salary for Medical Research Assistant
Medical research assistant education.
The most common degrees for a Medical Research Assistant are Master's degree (66.67% of jobs require this), High school (33.33%).
Medical Research Assistant Degrees
Search for medical research assistant jobs, upload your resume.
In our recent survey, recruiters told us that resume search is the top tool they use to find the best candidates. Post your resume today to ensure recruiters and hiring managers can easily find you.
Powered by Web Scribble Solutions , Inc.
Welcome to the Kaplan Community Career Center
Salary data is provided by Web Scribble and comes from the Department of Labor and thousands of companies’ job postings.
Upload Your Resume - Increase your visibility with employers
Explore Career Guides - Empower and shape your professional future
By clicking "Sign me up", you are signing up with WebScribble Solutions, Inc. and agree to WebScribble's Terms of Use and Privacy Policy .
By clicking "Create Account", you are creating an account with Web Scribble Solutions, Inc. and agree to Web Scribble's Terms of Use and Privacy Policy .
Create Your Kaplan Community Career Center Job Seeker Account
By clicking "Register", you are registering for services with Web Scribble Solutions, Inc. and agree to Web Scribble's Terms of Use and Privacy Policy .
Get discovered by our corporate partners!
- Elevate your visibility with a Job Seeker account.
- Kaplan recruiters will vet your resume and connect you with partner employers.
- Get one step closer to the role of your dreams.

How to Become a Clinical Research Assistant: A Complete Guide to Becoming A CTA with No Experience on Resume

How To Become A Clinical Research Assistant
A complete guide to becoming a clinical trial assistant with no experience on resume.
Clinical Research Assistant
The work of a clinical trial/research assistant (CTA) in clinical research can never be overstated. It is an important career that requires a lot of interest and dedication in order to be successful. If you have developed interest in becoming a CTA, there are certain questions that you must ask yourself. Are you really cut out for this career path? Are you eager to take up more responsibilities in a work place? Can you monitor the trial subject and ensure that the trial is conducted in a safe and ethical manner? If your answers to this questions are yes, then you might just be cut out for the job of a clinical trial assistant.
CCRPS offers the only accredited 5-day, on-demand advanced clinical research assistant certification (ACRAC) course available to help your learn and apply knowledge and increase your chances of 1) getting a job 2) being efficient and successful in your career.
Responsibilities of a Clinical Research Assistant
A clinical trial assistant have a lot of responsibilities and roles to fulfill within a clinical research institute to ensure the success of a project. Some of these responsibilities include:
Maintaining the standard operating procedures (SOP).
Provide regular report updates of the progress of clinical studies to the appropriate personnel.
Planning and conducting of pre-study site evaluation.
Conduct clinical site feasibility and are as well involved in study visibility.
Assess the study subjects to ensure that the appropriate clinical protocols are observed and the trial is in sync with laid down regulations.
Research Assistant Job Description
Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives.
May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related area. Knowledge of FDA regulatory requirements is required. Has knowledge of commonly-used concepts, practices and procedures within a particular field. Rely on instructions and pre-established guidelines to perform the functions of the job. Work under immediate supervision. Primary job functions do not typically require exercising independent judgment. Typically reports to a supervisor or manager.
Minimum Education Requirements For Clinical Trial Assistant
Requirements:
Completed degree(s) from an accredited institution that are above the minimum education requirement may be substituted for experience on a year for year basis
High school diploma or equivalent; college degree preferred
The educational requirement for a clinical research assistant is at the very least a high school diploma or associate degree in a health science. That's the least requirement, although more employers now prefer a B.Sc degree. Even if you don’t have a health science degree. if you took sciences related courses like nursing, life sciences, medical science, biotechnology, you should absolutely let the companies you are applying to know.
Another avenue you can become a clinical trial assistant is through certification. This is possible and is most common for people without formal education in the fields mentioned. Certification can be very demanding as it requires a lot of administrative knowledge in the area of clinical research. Many CTAs move on to become CRCs, CRAs, and administrators.

Skills You Need To Show On Your Research Assistant Resume
To be successful as a clinical research assistant, there are certain skill sets that are required.
A knowledge of the challenges and restrictions involved in the implementation and retention of databases.
A complete understanding of the responsibilities and liabilities involved in the use of humans for trial tests.
An ability to make excellent clinical development plan.
Must be able to ensure that data gotten from clinical trials are accurate and reliable and the legal rights and privacy of the subjects are protected.
Having these above listed skills and being efficient in them make the job of a clinical trial assistant easier and more interesting. Responsibilities:
Conduct literature reviews
Collect and analyze data
Prepare materials for submission to granting agencies and foundations
Prepare interview questions
Recruit and/or interview subjects
Maintain accurate records of interviews, safeguarding the confidentiality of subjects, as necessary
Summarize interviews
Provide ready access to all experimental data for the faculty researcher and/or supervisor
Request or acquire equipment or supplies necessary for the project
Manage and respond to project related email
Prepare, maintain and update website materials
Supervise undergraduate students working on the research project (maintaining records on assignment completion, acting as liaison/mediator between the undergraduate students and the faculty researcher)
Attend project meetings
Attend area seminars and other meetings as necessary
Summarize project results
Prepare progress reports
Prepare other articles, reports and presentations
Monitor the project budget
Travel to field sites to collect and record data and/or samples as appropriate to the specific objectives of the study
As appropriate to the specified position, code and verify data in accordance with specified research protocol and coding procedures and enter data into a computer database and/or spreadsheet application for subsequent analysis
Develop or assist in the development of interview schedules; contact potential subjects to introduce and explain study objectives and protocol and to arrange interviews, either in person or by telephone
Identify and compile lists of potential research subjects in accordance with study objectives and parameters, as appropriate to the individual position
Conduct and record face-to-face and/or telephone interviews with subjects, in accordance with predetermined interview protocol, data collection procedures and documentation standards
Review and edit data to ensure completeness and accuracy of information; follow up with subjects to resolve problems or clarify data collected
May set up, calibrate and maintain laboratory and/or field research equipment, as specified by the requirements of the study
May lead or guide the work of student employees
Perform miscellaneous job-related duties as assigned
Prepare findings for publication and assist in laboratory analysis, quality control, or data management
Write and contribute to publications
Develop research protocols
Track progress over time
Assist with preparation of all educational and training workshops and evaluation strategies
Engage clinical and community partners in research
Market training and technical assistance resources to clinical partners and academic investigators
Develop assessment and evaluation tools
Compile data for progress reports
Where To Reach Out For Trial Assistant Experiences And Internships
Landing that first trial assistant experience or internship can be a stepping stone to a rewarding career in clinical research. In this blog, we'll explore various avenues to find these valuable opportunities and launch your journey in this dynamic field.
Education and Certification:
While not always mandatory, a degree in a life sciences field like biology, health sciences, or nursing can be beneficial. However, even without a degree, you can break into the field. Consider pursuing a certification program offered by organizations like the Association of Clinical Research Professionals (ACRP) to demonstrate your knowledge and commitment.
Finding Trial Assistant Opportunities:
Industry-Specific Platforms: Leverage job boards frequented by the clinical research community. Look for platforms like Society for Clinical Research Associates (SOCRA) or ACRP job boards. These boards often list internship and entry-level positions specifically for Clinical Trial Assistants (CTAs).
General Job Boards: Don't neglect popular job boards like Indeed or LinkedIn. Utilize relevant keywords like "clinical trial assistant internship" or "research assistant" to filter your search and uncover a wider range of opportunities.
University Resources:
Career Services Departments: Many universities have dedicated career centers that assist students in finding internships. Connect with your career advisor to discuss your interest in clinical research and explore internship opportunities within the university or with partnering institutions.
Research Departments: Universities often conduct their own clinical trials. Reach out to professors or research departments to inquire about potential research assistant positions. This can provide valuable hands-on experience.
Government Websites:
Regulatory Agencies: The US Food and Drug Administration (FDA) offers student volunteer programs ([resources for getting experience in clinical research]).
Networking:
Professional Associations: Join associations like ACRP or SOCRA. Attend industry conferences or webinars to connect with professionals, learn about the field, and discover potential internship or job openings.
LinkedIn: Build your professional profile on LinkedIn and connect with individuals working in clinical research. Reach out to them politely and express your interest in gaining experience. Show genuine curiosity and highlight your transferable skills.
Local Directories:
CTAs can leverage online directories to target their search. After obtaining your certification, reach out to request experiences or internships at:
Clinical research organizations (CROs)
Pharmaceutical companies
Biotechnology companies
These directories can be found through professional association websites or a simple online search using terms like "USA clinical trial directory" or "USA CRO directory". Here are some examples:
ClinicalTrials.gov (a comprehensive listing of clinical trials registered in the US)
CRO Directory (searchable directory of contract research organizations)
BioPharmCatalyst (industry resource with listings of clinical trials and CROs)
Volunteering:
Hospitals and Research Institutions: Volunteer at hospitals or research institutions involved in clinical trials. This can provide valuable firsthand experience and build connections with professionals in the field.
Additional Tips:
Tailor Your Resume and Cover Letter: Highlight relevant coursework, volunteer experiences, and any transferable skills that demonstrate your aptitude for the role. Research the specific company or institution you're applying to and tailor your application to their needs.
Be Proactive: Don't wait for opportunities to come to you. Research companies conducting trials in your area and directly contact their clinical research departments. Express your enthusiasm and willingness to learn.
By exploring these avenues and demonstrating your enthusiasm, you'll increase your chances of landing a trial assistant experience or internship and taking that crucial first step towards a fulfilling career in clinical research.
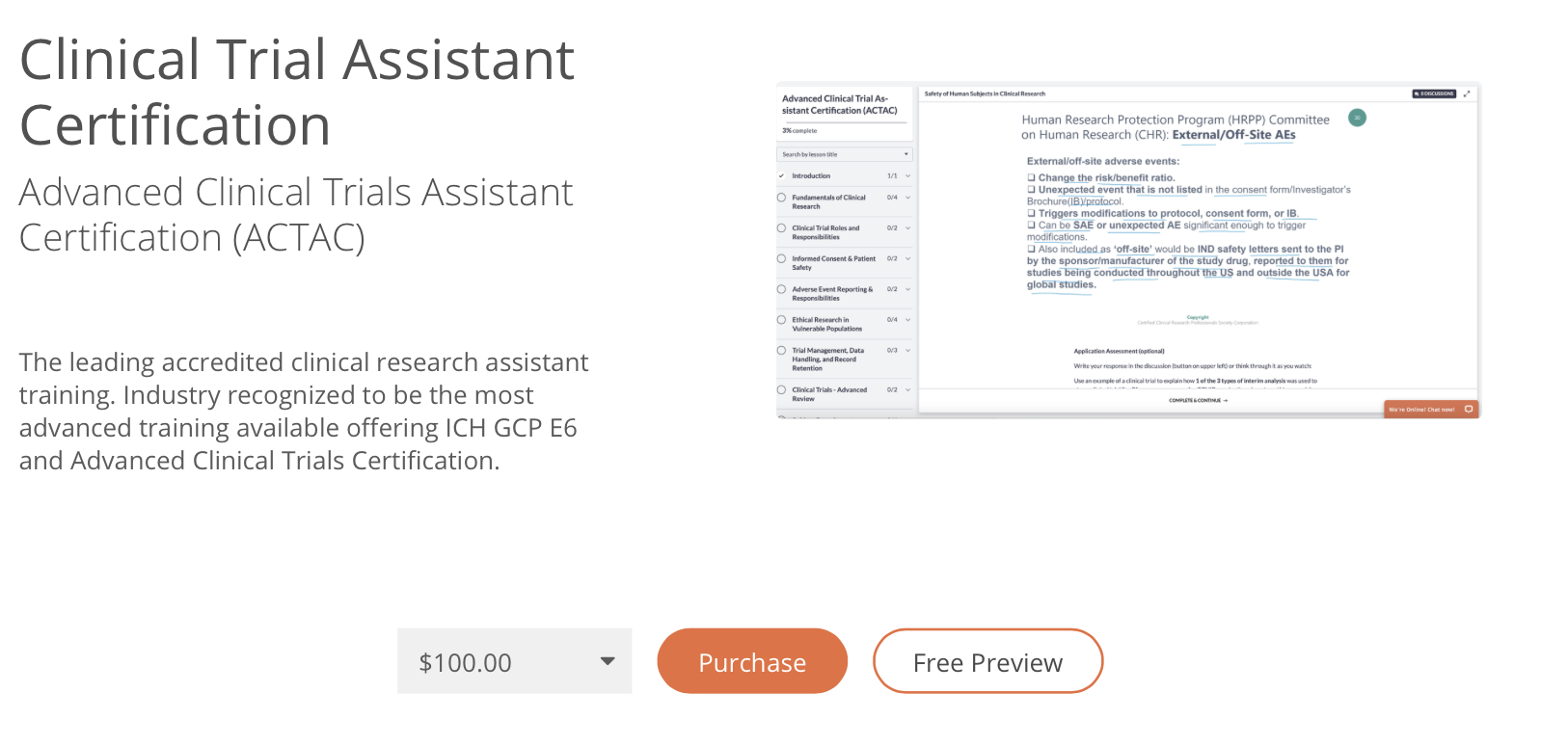
Clinical Trial Assistant Training
Unlike the hundreds of CTAs who apply to a position, you can give your resume and interview a huge advantage by having certification. CCRPS' offers complete clinical trial assistant training and certification by the ACCRE through our clinical trial assistant training course. Certification as a CTA can help you show competency to work and apply for roles; many students use the course as a way to update their resume and land the interview at the site they desire. If you plan to continue a career in clinical research, ask our 24/7 chat and phone advisors for partial scholarships. We also offer up to 4 month payment plans ($100 per month).
Advanced Clinical Trial Assistant Training: CTA Syllabus CCRPS
DEMO COURSE
Introduction
Accreditation Council For Clinical Research & Education for CCRPS
Fundamentals Of Clinical Research
An Introduction to Clinical Research
An Overview of ICH GCP
Code of Federal Regulations
CFR 21 Part 11
Clinical Trial Roles And Responsibilities
Sponsor/CRO Responsibilities
13 Principles, IRB, & Investigator Roles
Informed Consent & Patient Safety
Informed Consent FREE PREVIEW
Safety of Human Subjects in Clinical Research FREE PREVIEW
Adverse Event Reporting & Responsibilities
Reporting Responsibilities of the Investigators
Adverse Events
Ethical Research In Vulnerable Populations
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated FREE PREVIEW
Ethics of Research Involving Pregnant Women and Fetuses
Ethics of Research Involving Prisoners
Trial Management, Data Handling, And Record Retention
Trial Management – Data Handling and Record Retention
a) Common Terminology Used In Clinical Research
b) Commonly Used Abbreviations and Terms in Clinical Research
Clinical Trials - Advanced Review
Advanced Designs of Clinical Trials
Advanced Review of Phases of Clinical Trials (Preclinical & Phase 0-4)
FREE PREVIEW
Subject Recruitment, Retention, And Compliance
Patient Recruitment in Clinical Trials
Patient Engagement and Retention in Clinical Trials
Patient Adherence and Compliance in Clinical Trials
Misconduct And Fraud
Scientific Misconduct and Fraud
Detecting Falsification
Clinical Trial Assistant Certification Exam
ICH GCP Clinical Trials Assistant Exam (30 Questions)
What To Know For Clinical Trial Assistant Interview Questions
The work of a clinical research assistant is one of extreme importance to the clinical research institute, and employers will to testing to see if you understand what position entails.
Clinical research assistants is to test new medications, therapies and types of treatment and new medical devices to be sure of the safety of their use and the efficacy or efficiency of their work. These clinical trials are very much regulated and seriously monitored to ensure that they comply with the laid down regulations. The need to keep various records, in order to meet up with compliance requirements can be a burden. That is where clinical research assistants come in.
Clinical research assistant are responsible for performing the different safety and quality checks within their unit. Some of these checks are routinely carried out daily, weekly or monthly. The daily checks are usually the first thing they carry out on resuming to work everyday. This is to ensure the safety of all the staffs and volunteers within the clinical research institute and as well improve the quality of the data collected and the results.
The job of a clinical research assistant is to help in finding subjects that can be used for clinical trials, they are responsible for collecting and analyzing the data gotten from clinical tests and trials and they also evaluate the result. They are the ones that keep all the record of activities in the clinical research institute for the purpose of references. They practically ensure that the clinical trial activities are in line with laid down regulations. The amount of data to be collected, evaluated and stored form the trials make this job an important one. That means it is a job in high demand.
Their importance means that there are a variety of places where they can work. Clinical research assistants can work at clinical research institutes, medical centers, pharmaceutical companies, biotech companies and a whole host of other medically and clinically inclined organizations.
The standard equipment like freezers and fridges are checked at least twice daily. This is important because they are used for storing specific research samples and other medications that needs to be kept in controlled temperature and a slight deviation from that can affect the validity of the result and the research. The emergency equipment are also checked regularly on a daily basis.
Part of a clinical research assistant's job is to assist all members of the team and deal with different queries from members of the public. It is also their duty to control all medical stock used in their unit, prepare materials for screening visits, prepare consent forms, questionnaires and information sheets and keeping study files while archiving the files for completed studies.
In the midst of these many duties, it is very important that the clinical research assistant is very capable of multitasking. A good communication skill (both written and verbal) is very important to do this work successfully. One thing that is a must for anyone aspiring to take up this job is to have a keen eye for details. It is also important to be able to ask the right question and develop your knowledge base as much as possible. If you can demonstrate that you have these skills in your interview, you should be all set to go.
Clinical Research Assistant Salary

Per Payscale
The salary of a clinical research assistant can vary depending on different factors like location, institution or employer etc. However, the average yearly salary is $41,000 at an hourly average of $17. It can rise as high as $55,000 or as low as $32,000.
If you'll like to apply for the post of a clinical research assistant, it makes it easier for you if you have B.Sc in life science or social science related courses. If you don't, go enroll for a bachelor's degree and get experiences by volunteering in clinical trials
The purpose of clinical research is to test new medications, therapies, and new medical devices to be sure of the safety of their use and their efficiency. These clinical trials are very much regulated and seriously monitored to ensure that they comply with the laid down regulations. The need to keep various records, in order to meet up with compliance requirements can be a burden.
That is where clinical research assistants come in….
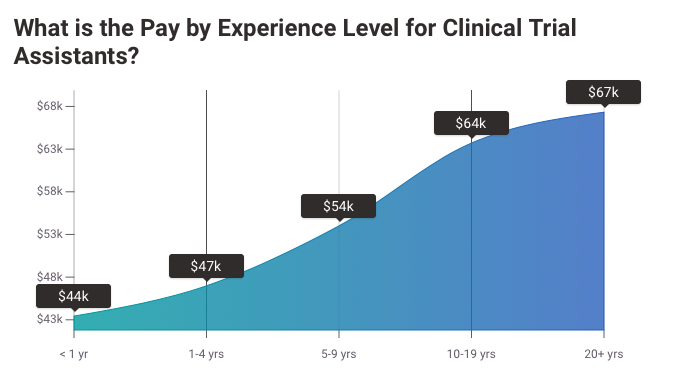
CTA Salary Prediction Provided by Payscale
CCRPS offers the only clinical research assistant certification (ACRAC) course available
The job of a clinical research assistant is to help in finding subjects that can be used for clinical trials, collecting and analyzing the data from clinical tests, and they also evaluate the result.
They are the ones who keep all the record of activities in the clinical research institute for future references. They practically ensure that the clinical trial activities are in line with laid down regulations. The amount of data to be collected, evaluated and stored form the trials make this job an important one. That means it is a job in high demand.
Their importance means that there are a variety of places where they can work. Clinical research assistants can work at clinical research institutes, medical centers, pharmaceutical companies, biotech companies and a whole host of other medically and clinically inclined organizations.
The educational requirements required to work as a clinical research assistant includes a bachelor’s degree, master’s degree or a doctorate degree in life sciences or other medical related sciences.
These are just basic educational requirements, if you are interested in getting into clinical research, you need more than just degrees in life science. Not because they are not important but because they do not offer you the core knowledge and experience needed to be successful in this career. Based on your chosen discipline in clinical research, you can choose to offer courses related to your discipline and you will be taught by seasoned and experience lecturers in the industry keen to pass on their knowledge and experience. You can also register to be a member of clinical research based associations at CCRPS and find more expert information on the clinical research field. All you need to have a rapid career is right here.
CCRPS offers the only accredited 5-day, on-demand clinical research assistant certification (ACRAC) course available to help your learn and apply knowledge and increase your chances of 1) getting a job 2) being efficient and successful in your career.
Principal Investigator Training - Role of Principal Investigator in Clinical Research
Pharmacovigilance: a complete guide to pharmacovigilance and drug safety training.
