
- Bipolar Disorder

Bipolar Disorder and Schizophrenia—Similar Yet Different
Distinct brain systems are involved in bipolar disorder and schizophrenia..
Posted March 5, 2013 | Reviewed by Abigail Fagan
- What Is Bipolar Disorder?
- Take our Bipolar Disorder Test
- Find a therapist to treat bipolar disorder
Bipolar disorder is a psychiatric illness that is characterized by episodes of “mania.” Symptoms include euphoria, distractibility, irritability, and grandiosity. During a manic episode , people often have remarkable energy and move, think, and talk rapidly. They sleep little but do not appear to be tired.
They may also experience delusions such as the belief that they are able to fly or are Jesus or another famous person. They may be suspicious that people are out to harm them. During a manic episode, some people hear voices or see visions. Severe depressive episodes are also often part of bipolar disorder.
Schizophrenia is an illness characterized by a group of so-called “positive” symptoms that may include hallucinations (hearing voices, seeing visions), delusions (fixed false beliefs), and/or a thought disorder (speech that makes little sense). Words are spoken, but the connections between sentences or paragraphs are illogical. (This is called a formal thought disorder.)
Also, people with schizophrenia often exhibit “negative” symptoms, where they become uninterested in interacting with others, lose the ability to take pleasure in previously enjoyed activities, talk less, and exhibit a demeanor that is rather flat or without much expressiveness.
Psychiatrists often see patients with a mixture of the above symptoms, and it may be difficult to determine whether a patient has schizophrenia or bipolar disorder even after following him or her for many years. Such patients may receive a diagnostic label of schizoaffective disorder, bipolar type.
Genes and environment both contribute to the causes of bipolar disorder and schizophrenia. In terms of genetic factors, it appears that small changes in a large number of genes can add up and increase the risk of someone developing one of these disorders. Some genes are more related to the development of schizophrenia, and other genes are more related to bipolar disorder. However, some genes seem to be related to both disorders. In other words, some abnormalities in the same genes may be shared by schizophrenia and bipolar disorder.
S. A. Meda and colleagues recently published a study in Biological Psychiatry that compared the interactions between specific brain systems in several groups of people: those without psychiatric illness, those with clear-cut bipolar disorder, and those with clear-cut schizophrenia. This research team used functional brain imaging to examine the interrelationships among five brain networks. A brain network is composed of brain regions that function together and are responsible for activities of the mind such as motivation , emotion , and cognition ( memory , attention , planning, etc). Networks interact with each other, and various behaviors are likely related to these interactions.
The five networks that were examined in this study involved 1) visual processing, 2) social awareness, 3) recognizing and interpreting emotions, 4) integrating language with emotions and planning, and 5) control and regulation of movement and timing of responses. Each of these networks involves a number of specific brain structures that are increasingly well understood.
How do these five networks interact in persons with schizophrenia or bipolar disorder compared to healthy controls? It turns out that patients with schizophrenia had a diminished interaction between two of these specific networks when compared to either controls or persons with bipolar disorder. Patients with bipolar disorder demonstrated increased interactions between a different pair of networks when compared to patients with schizophrenia or controls. However, there were two networks that showed diminished interactions in both schizophrenia and bipolar disorder when compared to controls.
The researchers interpret these results as indicating that there are certain brain network interactions that are uniquely out of balance in schizophrenia. Other network interactions are uniquely out of balance in bipolar disorder. However, there are some interactions that are similarly out of balance in both schizophrenia and bipolar disorder. They suggest that the networks that are similarly out of balance in both illnesses may be related to certain psychotic symptoms, such as delusions.

Furthermore, they suggest that the pattern of connections specific to schizophrenia involves brain regions that may be related to the negative symptoms described above and that the pattern of abnormal interactions unique to bipolar disorder may be related to brain regions involved in mood regulation.
Interestingly, these authors also examined brain network interactions in psychiatrically healthy, first-degree relatives of persons with these two illnesses. Healthy relatives of persons with bipolar disorder exhibited some of the same out-of-balance network interactions as their symptomatic relatives.
What should we make of this type of research? The bottom line is that we are beginning to be able to relate symptoms of illnesses to abnormalities in the interactions between specific brain networks. The more we understand symptoms at a brain level, the better our chances of pinpointing the cause(s) of abnormal brain system interactions. It is likely that this sort of work will help us develop imaging procedures to recognize patterns of connections that might predict risk for developing specific psychiatric illnesses. Eventually, as we elucidate causes, we should be more equipped to develop increasingly specific forms of treatment.
Years ago, this would be considered science fiction. Now, it is a matter of “when” not “if.”
This column was co-written by Eugene Rubin MD, PhD and Charles Zorumski MD

Eugene Ru bin , M.D., Ph.D. , is Professor Emeritus in the Department of Psychiatry at Washington University School of Medicine in St. Louis.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

Sticking up for yourself is no easy task. But there are concrete skills you can use to hone your assertiveness and advocate for yourself.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Bipolar Disorder vs. Schizophrenia: What’s the Difference?
They can have overlapping features
Bipolar disorder and schizophrenia have some similarities, but they are different disorders. Schizophrenia is characterized by continuous or relapsing episodes of psychosis, while bipolar disorder is a mood disorder that can sometimes manifest with psychotic symptoms. Since they can appear alike, bipolar disorder and schizophrenia can be mistaken for each other.
This article will compare bipolar disorder vs. schizophrenia, including symptoms, causes, and treatment.
Laura Porter / Verywell
Bipolar Disorder vs. Schizophrenia: Symptoms
Some symptoms of bipolar disorder, schizophrenia, or schizoaffective disorder can overlap. The symptoms of schizophrenia spectrum disorders and bipolar disorder vary by person, and no two cases are exactly alike.
Bipolar Disorder
The symptoms of bipolar disorder include clinically significant episodes of depression, as well as hypomania and/or mania.
Symptoms of depression in bipolar disorder include:
- Lack of interest in things previously enjoyed
- Feelings of worthlessness
- Changes in appetite
- A sense of hopelessness and thoughts of suicide
Symptoms of mania in bipolar disorder include:
- Restlessness, inability to fall asleep
- Racing thoughts
- Elevated or irritable moods
- Unrealistic planning
- Overestimation of personal abilities
- Risk-taking
Bipolar disorder can also cause overwhelming paranoia or an exaggerated sense of self-importance with an extreme detachment from reality. These symptoms may look like schizophrenia.
Mixed-affective episodes occur when mania and depression happen at the same time. For example, a person may feel hopeless but also have racing thoughts or risk-taking behavior.
Schizophrenia
Schizophrenia includes positive, negative, and cognitive symptoms. Positive symptoms refer to behaviors or thoughts that are not usually present but come up during an episode. Negative symptoms are when behaviors or thinking patterns that are typically present go away during an episode.
Positive symptoms of schizophrenia include:
- Delusions and/or hallucinations
- Disorganized speech
- Disorganized behavior
Negative symptoms of schizophrenia include:
- Apathy (lack of interest)
- Withdrawal from others
- Lack of emotional expression
- Excessive sleeping
Cognitive symptoms of schizophrenia include:
- Diminished attention
- Impaired memory and learning
- Difficulty thinking and problem-solving
Cognitive symptoms of schizophrenia, such as memory impairment, can affect a person’s ability to take care of themselves.
Schizophrenia is characterized by psychosis. Between 20% and 60% of people with bipolar disorder will experience a psychotic episode.
Schizoaffective disorder includes the symptoms of schizophrenia, but a person living with the disorder will also have prolonged and persistent mood symptoms.
What Causes Bipolar Disorder vs. Schizophrenia?
Bipolar disorder and schizophrenia are each believed to stem from genetic, biological, and environmental causes.
Bipolar disorder affects around 2% of the population. Schizophrenia affects around 1% of the population. Schizoaffective disorder is much less common, only affecting about 0.3% of the population.
The average age of onset of symptoms for each of these conditions is in the early 20s, though the age range of symptom onset is wider for bipolar disorder.
There appears to be a strong genetic component to both schizophrenia and bipolar disorder.
Twin studies have shown that identical twins are more likely to share a diagnosis of schizophrenia than non-identical (fraternal) twins. These findings support other research suggesting that altered brain connectivity could also be strongly influenced by genes.
Bipolar disorder risk is also related to genetic factors. Twin studies have shown similar findings for shared diagnoses between identical and nonidentical twins. The findings were associated with lower volume in certain parts of the brain that researchers think could be linked to the disorder.
Factors that occur before someone is born (prenatally) may also contribute to the development of schizophrenia. Researchers are also exploring whether prenatal factors also contribute to the development of bipolar disorder.
Parental emotional stress, infections, birth complications, low oxygen levels, and fetal distress are associated with a higher risk of developing schizophrenia.
Environment
A person’s community and environment play a role in the risk of bipolar disorder and schizophrenia. Environmental factors do not cause these conditions, but researchers think they may contribute to symptoms in people who are genetically more likely to get them
Researchers think that exposure to substance abuse and/or excessive stress can cause metabolic changes in the body that cause more expression of the genetic factors that contribute to these disorders. It’s also possible that they lead to brain changes that could be involved in the symptoms of the disorders.
Living in an urban area is one environmental factor that has been associated with mental health conditions like bipolar disorder and schizophrenia. Research has shown that pollution, noise, disrupted sleep, and social stress could all play a role in the development of these conditions.
Contributing environmental factors include childhood trauma, social isolation, and substance abuse.
Are Bipolar Disorder and Schizophrenia Diagnosed in the Same Way?
Bipolar disorder, schizophrenia, and schizoaffective disorder are diagnosed based on criteria outlined in the "Diagnostic and Statistical Manual of Disorder of Mental Disorders" (DSM-5), which is the disease classification system used by mental health professionals.
Other causes of symptoms, such as substance use, brain injury, a medical illness like brain inflammation ( encephalitis ), or another mental health condition, all have to be ruled out before a person can be diagnosed with any of these conditions.
A bipolar disorder diagnosis requires a person to have at least one manic or hypomanic episode, as well as at least one major depressive episode.
The criteria include having at least two of the following symptoms for at least one month, and at least one needs to be one of the first three symptoms:
- Hallucinations
- Severely disorganized behavior
- Negative symptoms, such as catatonic behavior, apathy, and lack of expression
The symptoms must be associated with a deterioration in self-care, relationships, or work.
To be diagnosed with schizophrenia, the DSM-5 specifies that a person must have had ongoing signs of schizophrenia for at least six months and had the specific symptoms from the above list for at least one month within that period. The exception is if they have started treatment that has successfully managed their symptoms.
A diagnosis of schizoaffective disorder requires symptoms of schizophrenia. The mood symptoms must be present most of the time, and the symptoms of psychosis must be present for over two weeks without mood symptoms.
There is some debate about whether schizoaffective disorder should be its own diagnosis. Some experts believe schizoaffective disorder should be a category of schizophrenia, severe depression, or bipolar disorder.
Treatment Options
Bipolar disorder and schizophrenia are not curable, but there are treatments such as medication and therapy.
Treatments for schizophrenia include antipsychotic medications, which are taken daily to prevent symptoms. Treatments for bipolar disorder include lithium and other mood stabilizers, usually along with antipsychotic medications.
Counseling can also be an important component of management. Certain types of psychosocial therapy can be beneficial for schizophrenia and bipolar disorder. These are often used in addition to medication. Therapy can help a person learn skills to better manage symptoms and navigate their everyday life.
Therapies that can be beneficial for schizophrenia include cognitive behavioral therapy , behavioral skills training, supported employment, and cognitive remediation interventions. Therapies for bipolar disorder include cognitive behavioral therapy, interpersonal and social rhythm therapy (IPSRT), and family-focused therapy .
Treatment with electroconvulsive (ECT) therapy can be beneficial for people who have depressive episodes and/or manic episodes of bipolar disorder. ECT has been studied as a potential therapy for the treatment of schizophrenia, and it can relieve some symptoms in the short term. However, it is not considered a routine treatment for schizophrenia.
Schizophrenia and bipolar disorder are both lifelong conditions, but treatment can improve the outlook for them. Both conditions can increase a person’s risk of substance abuse and suicide .
Between 4% and 19% of people with bipolar disorder die by suicide, a rate that is 10 to 30 times higher than the general population. A 2020 study found that the suicide rate for people with schizophrenia spectrum disorders is over 20 times higher than it is for the general population.
These disorders are also associated with a risk of health problems related to neglecting physical symptoms as well as a lack of motivation or ability to get medical attention. The effects of some treatments for these conditions may also contribute to disease risk.
Both conditions can get worse quickly, with severe dissociation from reality, thoughts of suicide, and/or self-harm. Episodes of acute worsening may need to be addressed with inpatient hospitalization to ensure a person’s safety.
Both bipolar disorder and schizophrenia spectrum disorders lead to severe distress and relationship challenges. While these conditions cannot be cured, they can be treated with therapy and medication.
Once a diagnosis is made and treatment is started, a person’s symptoms can often be managed, and their quality of life and safety can get better.
Maintaining a consistent routine, reducing stress, eating a nutritious diet, and staying active can all contribute to a better overall outcome for people with bipolar disorder or schizophrenia.
National Institute of Mental Health. Schizophrenia .
APA. Bipolar I and bipolar II disorders .
National Institute of Mental Health. Bipolar disorder .
Chakrabarti S, Singh N. Psychotic symptoms in bipolar disorder and their impact on the illness: A systematic review. World journal of psychiatry . 2022;12(9):1204-1232. doi:10.5498/wjp.v12.i9.1204
Ather Muneer. Mixed states in bipolar disorder: etiology, pathogenesis and treatment . Chonnam medical journal . 2017;53(1):1-1. doi:10.4068/cmj.2017.53.1.1
APA. What is schizophrenia? .
McCutcheon R, Richard S.E. Keefe, McGuire P. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment . Molecular Psychiatry . 2023;28(5):1902-1918. doi:10.1038/s41380-023-01949-9
National Institutes of Mental Health. Schizoaffective disorder.
Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options . P T . 2014;39(9):638-645.
Cao H, Ingvar M, Hultman CM, Cannon T. Evidence for cerebello-thalamo-cortical hyperconnectivity as a heritable trait for schizophrenia . Transl Psychiatry. 2019 Aug 20;9(1):192. doi:10.1038/s41398-019-0531-5
Squarcina L, Fagnani C, Bellani M, Altamura CA, Brambilla P. Twin studies for the investigation of the relationships between genetic factors and brain abnormalities in bipolar disorder . Epidemiol Psychiatr Sci . 2016 Dec;25(6):515-520. doi:10.1017/S2045796016000615
Stilo SA, Murray RM. Non-genetic factors in schizophrenia . Curr Psychiatry Rep . 2019 Sep 14;21(10):100. doi:10.1007/s11920-019-1091-3
NIMH. Schizophrenia .
Misiak B, Stramecki F, Gawęda Ł, Prochwicz K, Sąsiadek MM, Moustafa AA, Frydecka D. Interactions between variation in candidate genes and environmental factors in the etiology of schizophrenia and bipolar disorder: a systematic review . Mol Neurobiol. 2018 Jun;55(6):5075-5100. doi:10.1007/s12035-017-0708-y
Substance Abuse and Mental Health Services Administration. Table 3.20, DSM-IV to DSM-5 Psychotic Disorders . Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health [Internet]. Rockville (MD) (US); 2016 Jun.
Parker G. How well does the DSM-5 capture schizoaffective disorder? . Can J Psychiatry . 2019;64(9):607-610. doi:10.1177/0706743719856845
Laws KR, Darlington N, Kondel TK, McKenna PJ, Jauhar S. Cognitive behavioural therapy for schizophrenia - outcomes for functioning, distress and quality of life: a meta-analysis . BMC Psychol . 2018;6(1):32. doi:10.1186/s40359-018-0243-2
Perugi G, Medda P, Toni C, Mariani MG, Socci C, Mauri M. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania, and catatonic features . Curr Neuropharmacol . 2017;15(3):359-371. doi:10.2174/1570159X14666161017233642
Sinclair DJM, Zhao S, Qi F, Nyakyoma K, Kwong JSW, Adams CE. Electroconvulsive therapy for treatment-resistant schizophrenia . Schizophr Bull. 2019 Jun 18;45(4):730-732. doi:10.1093/schbul/sbz037
Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: A brief review . Medicina (Kaunas) . 2019;55(8):403. doi:10.3390/medicina55080403
Zaheer J, Olfson M, Mallia E, Lam JSH, de Oliveira C, Rudoler D, Carvalho AF, Jacob BJ, Juda A, Kurdyak P. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: A 20-year total population study in Ontario, Canada . Schizophr Res . 2020 Aug;222:382-388. doi:10.1016/j.schres.2020.04.025
By Heidi Moawad, MD Dr. Moawad is a neurologist and expert in brain health. She regularly writes and edits health content for medical books and publications.

Patient Case #1: 27-Year-Old Woman With Bipolar Disorder
- Theresa Cerulli, MD
- Tina Matthews-Hayes, DNP, FNP, PMHNP
Custom Around the Practice Video Series
Experts in psychiatry review the case of a 27-year-old woman who presents for evaluation of a complex depressive disorder.
EP: 1 . Patient Case #1: 27-Year-Old Woman With Bipolar Disorder
Ep: 2 . clinical significance of bipolar disorder, ep: 3 . clinical impressions from patient case #1, ep: 4 . diagnosis of bipolar disorder, ep: 5 . treatment options for bipolar disorder, ep: 6 . patient case #2: 47-year-old man with treatment resistant depression (trd), ep: 7 . patient case #2 continued: novel second-generation antipsychotics, ep: 8 . role of telemedicine in bipolar disorder.
Michael E. Thase, MD : Hello and welcome to this Psychiatric Times™ Around the Practice , “Identification and Management of Bipolar Disorder. ”I’m Michael Thase, professor of psychiatry at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, Pennsylvania.
Joining me today are: Dr Gustavo Alva, the medical director of ATP Clinical Research in Costa Mesa, California; Dr Theresa Cerulli, the medical director of Cerulli and Associates in North Andover, Massachusetts; and Dr Tina Matthew-Hayes, a dual-certified nurse practitioner at Western PA Behavioral Health Resources in West Mifflin, Pennsylvania.
Today we are going to highlight challenges with identifying bipolar disorder, discuss strategies for optimizing treatment, comment on telehealth utilization, and walk through 2 interesting patient cases. We’ll also involve our audience by using several polling questions, and these results will be shared after the program.
Without further ado, welcome and let’s begin. Here’s our first polling question. What percentage of your patients with bipolar disorder have 1 or more co-occurring psychiatric condition? a. 10%, b. 10%-30%, c. 30%-50%, d. 50%-70%, or e. more than 70%.
Now, here’s our second polling question. What percentage of your referred patients with bipolar disorder were initially misdiagnosed? Would you say a. less than 10%, b. 10%-30%, c. 30%-50%, d. more than 50%, up to 70%, or e. greater than 70%.
We’re going to go ahead to patient case No. 1. This is a 27-year-old woman who’s presented for evaluation of a complex depressive syndrome. She has not benefitted from 2 recent trials of antidepressants—sertraline and escitalopram. This is her third lifetime depressive episode. It began back in the fall, and she described the episode as occurring right “out of the blue.” Further discussion revealed, however, that she had talked with several confidantes about her problems and that she realized she had been disappointed and frustrated for being passed over unfairly for a promotion at work. She had also been saddened by the unusually early death of her favorite aunt.
Now, our patient has a past history of ADHD [attention-deficit/hyperactivity disorder], which was recognized when she was in middle school and for which she took methylphenidate for adolescence and much of her young adult life. As she was wrapping up with college, she decided that this medication sometimes disrupted her sleep and gave her an irritable edge, and decided that she might be better off not taking it. Her medical history was unremarkable. She is taking escitalopram at the time of our initial evaluation, and the dose was just reduced by her PCP [primary care physician]from 20 mg to 10 mg because she subjectively thought the medicine might actually be making her worse.
On the day of her first visit, we get a PHQ-9 [9-item Patient Health Questionnaire]. The score is 16, which is in the moderate depression range. She filled out the MDQ [Mood Disorder Questionnaire] and scored a whopping 10, which is not the highest possible score but it is higher than 95% of people who take this inventory.
At the time of our interview, our patient tells us that her No. 1 symptom is her low mood and her ease to tears. In fact, she was tearful during the interview. She also reports that her normal trouble concentrating, attributable to the ADHD, is actually substantially worse. Additionally, in contrast to her usual diet, she has a tendency to overeat and may have gained as much as 5 kg over the last 4 months. She reports an irregular sleep cycle and tends to have periods of hypersomnolence, especially on the weekends, and then days on end where she might sleep only 4 hours a night despite feeling tired.
Upon examination, her mood is positively reactive, and by that I mean she can lift her spirits in conversation, show some preserved sense of humor, and does not appear as severely depressed as she subjectively describes. Furthermore, she would say that in contrast to other times in her life when she’s been depressed, that she’s actually had no loss of libido, and in fact her libido might even be somewhat increased. Over the last month or so, she’s had several uncharacteristic casual hook-ups.
So the differential diagnosis for this patient included major depressive disorder, recurrent unipolar with mixed features, versus bipolar II disorder, with an antecedent history of ADHD. I think the high MDQ score and recurrent threshold level of mixed symptoms within a diagnosable depressive episode certainly increase the chances that this patient’s illness should be thought of on the bipolar spectrum. Of course, this formulation is strengthened by the fact that she has an early age of onset of recurrent depression, that her current episode, despite having mixed features, has reverse vegetative features as well. We also have the observation that antidepressant therapy has seemed to make her condition worse, not better.
Transcript Edited for Clarity
Dr. Thase is a professor of psychiatry at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, Pennsylvania.
Dr. Alva is the medical director of ATP Clinical Research in Costa Mesa, California.
Dr. Cerulli is the medical director of Cerulli and Associates in Andover, Massachusetts.
Dr. Tina Matthew-Hayes is a dual certified nurse practitioner at Western PA Behavioral Health Resources in West Mifflin, Pennsylvania.

Evaluating the Efficacy of Lumateperone for MDD and Bipolar Depression With Mixed Features

Blue Light, Depression, and Bipolar Disorder

Efficacy of Modafinil for Treatment of Neurocognitive Impairment in Bipolar Disorder

Four Myths About Lamotrigine

Securing the Future of Lithium Research

An Update on Early Intervention in Psychotic Disorders
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- DOI: 10.1016/j.jpsychires.2024.07.056
- Corpus ID: 271697174
Verbal fluency in schizophrenia and bipolar disorder - A longitudinal, family study.
- Sussy C. Luperdi , Patricia Correa-Ghisays , +4 authors V. Balanzá-Martínez
- Published in Journal of Psychiatric… 1 August 2024
81 References
Micemi: a method to identify cognitive endophenotypes of mental illnesses, a systematic review and meta-analysis of global and social functioning among people at risk of bipolar disorder., evaluating cognitive function in unaffected relatives of individuals with bipolar disorders: a meta-analysis., differential trajectory of cognitive functions in neurocognitive subgroups of newly diagnosed patients with bipolar disorder and their unaffected first-degree relatives - a longitudinal study., a meta-analysis of social cognitive deficits in schizophrenia: does world region matter, specific metabolic syndrome components predict cognition and social functioning in people with type 2 diabetes mellitus and severe mental disorders, deficits of social cognition in bipolar disorder: systematic review and meta‐analysis, the long-term course of cognition in bipolar disorder: a systematic review and meta-analysis of patient-control differences in test-score changes, associations between cognition and subsequent mood episodes in patients with bipolar disorder and their unaffected relatives: a systematic review., a comparison of cognitive performance in the suffolk county cohort and their unaffected siblings, related papers.
Showing 1 through 3 of 0 Related Papers
Our Most Troubling Madness: Case Studies in Schizophrenia Across Cultures
Information & authors, metrics & citations, view options.

Information
Published in.

- Schizophrenia
- Case Studies
- Clinical Anthropology
- Social Defeat
- Serious Psychotic Disorder
- Diagnostic Neutrality
Funding Information
Export citations.
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. For more information or tips please see 'Downloading to a citation manager' in the Help menu .
| Format | |
|---|---|
| Citation style | |
| Style | |
To download the citation to this article, select your reference manager software.
There are no citations for this item
View options
Login options.
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Purchase Options
Purchase this article to access the full text.
PPV Articles - American Journal of Psychiatry
Not a subscriber?
Subscribe Now / Learn More
PsychiatryOnline subscription options offer access to the DSM-5-TR ® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
Share article link
Copying failed.
PREVIOUS ARTICLE
Next article, request username.
Can't sign in? Forgot your username? Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
Create a new account
Change password, password changed successfully.
Your password has been changed
Reset password
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password.
Your Phone has been verified
As described within the American Psychiatric Association (APA)'s Privacy Policy and Terms of Use , this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences. Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
- Search the site GO Please fill out this field.
- Newsletters
- Health Conditions A-Z
- Mental Illness
- Bipolar Disorder
What's the Difference Between Bipolar Disorder and Schizophrenia?
:max_bytes(150000):strip_icc():format(webp)/CarrieMadormo-dda3990886f14acba66f8063e9e915cc.jpg)
Differences
Similarities, can you have both.
- Seeking Care
Kseniya Ovchinnikova / Getty Images
Bipolar disorder and schizophrenia are both mental health conditions that significantly affect a person’s life and relationships. Bipolar disorder is a mood disorder that causes mood cycles of manic highs, depressive lows, and stable periods. Schizophrenia is a personality disorder that causes a person to lose touch with reality through psychosis, hallucinations, and delusions.
Both bipolar disorder and schizophrenia affect how a person feels, acts, and perceives the world. However, treatment options and the overall outlook for each condition differ.
Symptoms of Bipolar Disorder vs. Schizophrenia
Bipolar disorder and schizophrenia share some similar symptoms, as they both affect how a person thinks, feels, and behaves. Here's a breakdown of each condition's symptoms:
| Paranoia | Paranoia |
| An exaggerated sense of self-importance | An exaggerated sense of self-importance |
| Detachment from reality | Detachment from reality |
| Disorganized thinking | Disorganized thinking |
| Agitation | Agitation |
| Depression | Depression |
| Sleep disturbances | Sleep disturbances |
| Withdrawal from others | Withdrawal from others |
| Racing thoughts | Delusions |
| Restlessness | Hallucinations |
| Risk-taking behaviors | Disorganized speech and behavior |
| Sadness | Lack of emotional expression |
| Lack of joy or pleasure in favorite activities | Isolation |
| Changes in appetite | Impaired memory and processing |
| Thoughts of self-harm or suicide | Difficulty with problem-solving |
As you may have noticed in the chart above, many of the symptoms of bipolar disorder and schizophrenia are similar.
However, schizophrenia causes a person to lose touch with reality and experience hallucinations or delusions:
- Hallucinations are false experiences that may cause you to see, hear, feel, taste, or smell things that are not there. A common example of a schizophrenia hallucination is hearing voices.
- Delusions are false beliefs that cause a person to believe things that are not true. Common delusions include believing oneself is famous, being harassed, or being stalked.
People with bipolar disorder, on the other hand, experience severe mood changes from mania to depression . A person with bipolar disorder cycles through episodes of mania, depression, and stable moods:
- Manic episodes make you feel elated, restless, wired, and irritable.
- Depressive episodes cause you to feel sad, hopeless, and anxious.
Bipolar disorder and schizophrenia are different conditions. While they share some common symptoms, these conditions have differences in prevalence rates, treatments, and outlooks.
Bipolar Disorder Is More Common
Bipolar disorder is more common than schizophrenia. In 2019, 40 million people were living with bipolar disorder worldwide. In the same year, there were 24 million living with schizophrenia.
The National Institute of Mental Health estimates that 4.4% of adults in the United States experience bipolar disorder at some point in their lives. It is more difficult to identify how many people in the United States experience schizophrenia because the symptoms often overlap with other mental health conditions.
Different Treatment Options
Both bipolar disorder and schizophrenia require lifelong treatment. There is no cure for either condition at this time. However, the treatment options for both conditions are different.
Mental health professionals (such as psychiatrists, prescribing psychologists, and psychiatric nurse practitioners) often recommend mood-stabilizing medications for bipolar disorder. Medications like lithium can help to reduce the number and severity of mood episodes. A person with bipolar disorder also benefits from regular psychotherapy .
However, a psychiatrist will likely recommend several types of medications for schizophrenia. These include:
- Antipsychotic medications to treat hallucinations and delusions
- Antidepressants to stabilize moods
- Anti-anxiety medications to reduce anxiety and paranoia
In addition to medication therapy, someone with schizophrenia may benefit from psychotherapy, social skills training, employment help, and daily support.
Different Prognosis
Bipolar disorder and schizophrenia are serious mental health conditions that require lifelong treatment. While both conditions can significantly affect your life, you can typically manage symptoms of bipolar disorder with medications and therapy.
However, the outlook for schizophrenia isn't always as promising. Some people with schizophrenia may experience difficulty taking care of themselves on their own. They may require help with personal hygiene, finding housing, daily activities, and employment. Schizophrenia causes you to act in unpredictable ways, and this can be distressing to those around them.
It is critical for people with schizophrenia to work with their psychiatrist, rely on a good group of loved ones, and adhere to treatment to manage the condition well.
Bipolar disorder and schizophrenia share common symptoms, but that's not their only similarity. Both conditions also see a similar diagnostic process, have common ages of onset, and are caused by similar factors.
Similar Diagnostic Process
If you or a loved one may have symptoms of a mental health condition, seeking support can be worrisome or anxiety-inducing—but it's important. Knowing what to expect can help, and fortunately, the diagnostic process for most mental health conditions looks similar. For these conditions specifically, the diagnostic process may involve a:
- Physical exam: Blood tests, brain scans, drug and alcohol screenings
- Psychiatric exam: Questions about family history, lifestyle habits, and symptoms and close observation or clinical interview
- Symptoms diary: Tracks your symptom frequency and severity
To be diagnosed with bipolar disorder, you must experience at least one manic or hypomanic episode and one major depressive episode.
To be diagnosed with schizophrenia, you must experience two of the following symptoms (delusions, hallucinations, disorganized speech, catatonic behavior, and avolition) for at least one month.
Similar Age of Diagnosis
Young adults are most likely to be diagnosed with both bipolar disorder and schizophrenia. The average age of diagnosis with bipolar disorder is 25. This is the age when bipolar symptoms are most likely to occur. Schizophrenia symptoms usually develop between the ages of 13 and 29.
While these conditions are lifelong, many people might experience fewer symptoms as they age.
Bipolar Disorder and Schizophrenia Have Similar Causes
Both bipolar disorder and schizophrenia have complex causes and risk factors that involve genetics and environmental factors. Both conditions also tend to run in families. In fact, research suggests that a person with an identical twin with bipolar disorder or schizophrenia is more likely to develop the condition, too.
However, your genetics don't always tell the whole story. Your environment also plays a role. While environmental factors alone do not cause mental health conditions, they can affect symptom severity. For example, people who have experienced substance use disorder (SUD) or excessive stress and trauma may be more likely to develop symptoms of bipolar disorder or schizophrenia.
It is possible to experience both bipolar disorder and schizophrenia at the same time.
People who have symptoms of both conditions may be diagnosed with schizoaffective disorder, which causes a combination of schizophrenia and mood disorder symptoms. Someone living with schizoaffective disorder may experience both psychosis and extreme mood changes.
Medications and treatment can help keep symptoms at bay.
When To Seek Care and Who to Contact
Bipolar disorder and schizophrenia are serious mental health conditions that require lifelong treatment. It is important to seek help as soon as you (or someone you love) develop symptoms of either condition.
If you are concerned about your mood fluctuations or believe that you are experiencing hallucinations or delusions, reach out to your primary healthcare provider. This provider will then refer you to a mental health professional for diagnosis and treatment.
If you do not currently have a regular healthcare provider, contact your insurance company or local health department for recommendations. Sometimes, mental health conditions can raise your risk of suicidal thoughts.
If you are in a crisis and need support, please know that help is available. You can call the National Suicide and Crisis Lifeline at 988 or visit this website for additional resources.
A Quick Review
Bipolar disorder and schizophrenia are serious mental health conditions that affect how a person thinks, feels, and behaves. Bipolar disorder causes significant mood changes, including mania and depression, while schizophrenia causes a person to lose touch with reality through hallucinations and delusions.
While researchers are still working to find a cure, treatment options like medications, therapy, and social support can help you manage either condition well.
:max_bytes(150000):strip_icc():format(webp)/sr-a21a100afbf343f481150ff49b3a733e.jpeg)
National Institute of Mental Health. Bipolar disorder .
Bowie CR, Best MW, Depp C, et al. Cognitive and functional deficits in bipolar disorder and schizophrenia as a function of the presence and history of psychosis . Bipolar Disord . 2018;20(7):604-613. doi:10.1111/bdi.12654
National Institute of Mental Health. Bipolar disorder in children and teens .
Chakrabarti S, Singh N. Psychotic symptoms in bipolar disorder and their impact on the illness: A systematic review. World journal of psychiatry . 2022;12(9):1204-1232. doi:10.5498/wjp.v12.i9.1204
McCutcheon R, Richard S.E. Keefe, McGuire P. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment . Molecular Psychiatry . 2023;28(5):1902-1918. doi:10.1038/s41380-023-01949-9
National Institute of Mental Health. Schizophrenia .
World Health Organization. Mental disorders .
National Alliance on Mental Illness. Different types of therapy for bipolar disorder .
Musket CW, Kuo SS, Rupert PE, et al. Why does age of onset predict clinical severity in schizophrenia? A multiplex extended pedigree study . Am J Med Genet B Neuropsychiatr Genet . 2020;183(7):403-411. doi:10.1002/ajmg.b.32814
Cao H, Ingvar M, Hultman CM, Cannon T. Evidence for cerebello-thalamo-cortical hyperconnectivity as a heritable trait for schizophrenia . Transl Psychiatry. 2019;9(1):192. doi:10.1038/s41398-019-0531-5
Squarcina L, Fagnani C, Bellani M, Altamura CA, Brambilla P. Twin studies for the investigation of the relationships between genetic factors and brain abnormalities in bipolar disorder . Epidemiol Psychiatr Sci . 2016;25(6):515-520. doi:10.1017/S2045796016000615
Stilo SA, Murray RM. Non-genetic factors in schizophrenia . Curr Psychiatry Rep . 2019;21(10):100. doi:10.1007/s11920-019-1091-3
Misiak B, Stramecki F, Gawęda Ł, et al. Interactions between variation in candidate genes and environmental factors in the etiology of schizophrenia and bipolar disorder: a systematic review . Mol Neurobiol. 2018;55(6):5075-5100. doi:10.1007/s12035-017-0708-y
National Institutes of Mental Health. Schizoaffective disorder.
Léger M, Wolff V, Kabuth B, Albuisson E, Ligier F. The mood disorder spectrum vs. schizophrenia decision tree: EDIPHAS research into the childhood and adolescence of 205 patients . BMC Psychiatry . 2022;22(1):194. doi:10.1186/s12888-022-03835-0
Related Articles
- Research article
- Open access
- Published: 02 October 2020
Association of ocular diseases with schizophrenia, bipolar disorder, and major depressive disorder: a retrospective case-control, population-based study
- Chun-Hao Liu 1 , 2 , 3 , 4 na1 ,
- Eugene Yu-Chuan Kang 3 , 5 na1 ,
- Yu-Hsiang Lin 3 , 6 , 7 ,
- Wei-Chi Wu 3 , 5 ,
- Zhuo-Hao Liu 3 , 8 ,
- Chang-Fu Kuo 3 , 9 ,
- Chi-Chun Lai 3 , 5 &
- Yih-Shiou Hwang 3 , 5
BMC Psychiatry volume 20 , Article number: 486 ( 2020 ) Cite this article
6842 Accesses
15 Citations
3 Altmetric
Metrics details
Psychiatric disorders and ocular neurovascular diseases may share a similar pathophysiological route of vascular structures or neurological changes. The aim of this study is to investigate the association between ocular neurovascular diseases and the risk of major psychiatric disorders.
This was a retrospective case–control, population-based study including patients aged ≥20 and were diagnosed between 1997 and 2013. Ocular neurovascular diseases diagnosed between 1997 and 2006 and newly diagnosed psychiatric disorders including bipolar disorder (BD), major depressive disorder (MDD), and schizophrenia between 2007 and 2013 were registered. Patients were propensity-score matched with control groups without psychiatric disorders in each cohort based on selected covariates.
A total of one million sampled patients in the database were categorized based on their diagnoses; 2243 (37.4% men) were categorized into the BD group, 10,110 (35.2% men) into the MDD group, and 1623 (43.1% men) into the schizophrenia group. In the BD group, all glaucoma (OR 1.49, [1.18–1.89]), open-angle glaucoma (OR 2.08, [1.34–3.24]), and closed-angle glaucoma (OR 2.12, [1.36–3.33]) showed statistical significance of risk. In the MDD group, age-related macular degeneration (OR 1.33, [1.13–1.57]), all glaucoma (OR 1.24, [1.11–1.37]), open-angle glaucoma (OR 1.47, [1.21–1.80]), and dry eye syndrome (OR 1.22, [1.13–1.31]) were associated with a significantly higher risk. In the schizophrenia group, only all glaucoma (OR 1.47, [1.02–2.11]), glaucoma suspect (OR 1.88, [1.01–3.49]), and open-angle glaucoma (OR 2.19, [1.13–4.26]) showed statistical significance.
Conclusions
In this population-based study, ocular neurovascular diseases, especially glaucoma, were associated with increased risks of BD, MDD, and schizophrenia.
Peer Review reports
Psychiatric disorders, including schizophrenia, bipolar disorder (BD), and major depressive disorder (MDD), can cause significant global disease burden, disability, and even premature mortality [ 1 , 2 ]. Although many studies have focused on the pathophysiology of psychiatric disorders, the association between psychiatric disorders and systemic physical conditions is still under investigation. In 2015, a study reported that patients diagnosed with BD or MDD had a tier II moderate risk of cardiovascular disorders in later life [ 3 ]. Additionally, BD and MDD were associated with vascular diseases through pathophysiological factors (such as inflammation or endothelial dysfunction), behavioral and environmental factors, and medication-related factors [ 3 , 4 ].
Microvasculature of the retina is easily observed and shares the same morphological, physiological, and pathological properties as the cerebral vasculature, making the eyes ideal “windows” for evaluating central nervous system disorders [ 5 ]. According to previous investigations, retinal vascular change or degeneration was associated with cerebral diseases such as Alzheimer’s and Parkinson’s disease [ 6 , 7 ]. These associations indicate that we can monitor or screen cerebral diseases through certain ocular conditions. Understanding any association between psychiatric disorders and ocular diseases may lead to the discovery that they also share similar pathophysiological routes of vascular structure or neurological changes.
Previous studies have found some significant retinal changes in patients with psychiatric disorders, especially BD and schizophrenia; both had a higher tortuosity index of retinal arterioles [ 8 ] and increased complexity of vascular branching [ 9 ]. Another study found that a lower arteriovenular ratio was associated with higher diastolic blood pressure, and a higher arterio-venular ratio was associated with better endothelial function in patients with BD but not in healthy controls [ 10 ]. In addition to the microvasculature, the retinal ganglion cell layer also showed some differences in patients with BD and schizophrenia. The retinal ganglion cell layers were thinner in patients with BD, [ 11 ] whereas the retinal nerve fiber and ganglion cell layer were both thinner in patients with BD and schizophrenia compared with healthy controls [ 12 , 13 ]. There has only been a limited study on retinal structure abnormalities in patients with MDD.
The association between psychiatric disorders and ocular diseases is still under investigation; only a few studies have focused on this issue, and the majority of them were cross sectional studies that lacked a large sample size. Because these studies were unable to clarify the association between psychiatric disorders and ocular diseases, we conducted a population-based study to investigate the association between psychiatric disorders and ocular diseases by using a longitudinal design.
Study population
This retrospective case–control study examined the association between ophthalmology diseases and psychiatric disorders. This study was conducted based on the Longitudinal Health Insurance Database 2010 (LHID 2010), which is a subset of the database from the Taiwan National Health Insurance Research Database (NHIRD). The LHID 2010 includes data relating to the insurance claims of one million randomly sampled people from 1997 to 2013. The single-payer Taiwan National Health Insurance (NHI) covered most of the medical expenditure, including inpatient and outpatient services in Taiwan. Because of NHI’s mandatory enrollment and affordability in Taiwan, long-term follow-up is nearly complete. Further information regarding the NHI program and the NHIRD has been reported in previous publications [ 14 , 15 , 16 ]. To ensure patient privacy, all identifiable data were encrypted before release; thus, researchers cannot identify individuals from the data. The study was approved by the Chang Gung Memorial Hospital Institutional Review Board (201900967B0).
Study design
In this study, we investigated whether exposure to ocular neurovascular disease in a psychiatric disorder–free cohort increases the risk of psychiatric disorder. We established three study cohorts based on three psychiatric disorders: (1) BD, (2) MDD, and (3) schizophrenia. Patients were respectively identified based on their diagnosis of BD, MDD, or schizophrenia, made between January 1, 2007, and December 31, 2013. Cases were ascertained through three or more diagnoses by a psychiatrist during outpatient visits. The date of the first diagnosis of BD, MDD, or schizophrenia was the index date for the case group. The control group included patients without any diagnosis of BD, MDD, or schizophrenia during the period of our database (1997 to 2013), respectively, in each cohort. The index date of the control group was assigned from that of their counterpart case group. Patients with BD, MDD, or schizophrenia diagnosed between 1997 and 2006; aged less than 20 years; or with a history of substance use or alcoholism were excluded (Fig. 1 ). Finally, patients who had received a new diagnosis of psychiatric disorders were propensity-score matched with control patients based on selected covariates.

a Flow chart of subject selection of bipolar disorder (BD) from the NHIRD. b Flow chart of subject selection of major depressive disorder (MDD) from the NHIRD. c Flow chart of subject selection of schizophrenia from the NHIRD
Exposure to ocular disease and covariates
Within the three cohorts, we recorded ocular neurovascular diseases, including age-related macular degeneration, central serous retinopathy, retinal vascular occlusion, diabetic retinopathy, glaucoma, dry eye syndrome, and optic neuritis, diagnosed between January 1, 1997, and December 31, 2006. To detect the possibility of unmeasured confounding factors, we also identified several negative control exposures to ocular diseases, including retinal detachment, uveitis, and blepharitis [ 17 ]. Exposure was ascertained through three or more diagnoses by an ophthalmologist during outpatient visits, and all exposures to ocular disease were censored and counted for the analysis. The covariates included age at the index date, sex, urbanization level, monthly income, comorbidities (anxiety disorder, hypertension, dyslipidemia, diabetes, coronary heart disease, chronic obstructive pulmonary disease, chronic kidney disease, and stroke), and the Charlson Comorbidity Index (CCI) score. Comorbidity was considered based on a minimum of three outpatient diagnoses or one inpatient diagnosis performed between 1997 and the index date. Comorbidities were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, including outcomes, exposure to ocular disease, and comorbidities (Supplementary Table 1 ).
Statistical analysis
To reduce possible confounding factors caused by selection bias, a propensity score matching (PSM) method was used in this study. The propensity score was the predicted probability to be in the case group given the values of covariates and using multivariable logistic regression without considering interaction effects. The variables selected to calculate the propensity score were baseline characteristics (Table 1 ). Each patient in the case group was matched with four control patients. The matching was processed using a greedy nearest neighbor algorithm with a caliper of 0.2 times the standard deviation of the logit of the propensity score, with random matching order and without replacement. Three separate PSMs were conducted for each of the three psychiatric disorders. The quality of matching was checked using the absolute value of the standardized difference (STD) between the groups, where a value less than 0.1 was considered a negligible difference. The association of each ocular disease and psychiatric disorder was investigated using the generalized estimating equation (GEE), in which the within-pair clustering of outcomes after PSM was accounted for by using a robust standard error and exchangeable working correlation. The link function was logit, and the distribution was binomial in the GEE model. A two-sided P value of <.05 was considered statistically significant, and no adjustment for multiple testing (multiplicity) was made in this study. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), including the procedures of “psmatch” for PSM and “genmod” for GEE.
Study cohorts and characteristics
Among one million patients in the LHID 2010, between 2007 and 2013, a total of 4226 patients were diagnosed with BD, 17281 were diagnosed with MDD, and 6073 were diagnosed with schizophrenia. After applying excluding criteria, there were 2325 newly diagnosed BD cases; 10,970 newly diagnosed MDD cases, and 1645 newly diagnosed patients with schizophrenia eligible for analysis. After PSM, 2243 cases and 8972 controls, 10,110 cases and 40,440 controls, and 1623 cases and 6492 controls remained for the BD, MDD, and schizophrenia cohorts, respectively ( Fig. 1 ) . After PSM, the mean age of cases was 43.5 ± 16.1 years for the BD cohort, 47.7 ± 16.9 years for the MDD cohort, and 41.9 ± 14.3 years for the schizophrenia cohort. All the characteristics were well-balanced between the case and control groups in the 3 study cohorts (Table 1 ).
Bipolar disorder cohort
The result of the GEE model showed that previous glaucoma was associated with a higher risk of BD (OR 1.49, 95% CI 1.18–1.89). Among the different types of glaucoma, glaucoma suspect (OR 1.63, 95% CI 1.09–2.43), open-angle glaucoma (OR 2.08, 95% CI 1.34–3.24), closed-angle glaucoma (OR 2.12, 95% CI 1.36–3.33), and undetermined glaucoma (OR 1.46, 95% CI 1.04–2.04) showed significant associations with BD. In addition, there were no associations between the negative control ocular diseases and risk of BD (Table 2 ).
Major depressive disorder cohort
Among the ophthalmologic diseases, the GEE model showed that age-related macular degeneration (OR 1.33, 95% CI 1.13–1.57), glaucoma (OR 1.24, 95% CI 1.11–1.37), and dry eye syndrome (OR 1.22, 95% CI 1.13–1.31) were significantly associated with a higher risk of MDD. Among the different types of glaucoma, glaucoma suspect (OR 1.47, 95% CI 1.22–1.76), open-angle glaucoma (OR 1.47, 95% CI 1.21–1.80), and undetermined glaucoma (OR 1.21, 95% CI 1.05–1.40) were significantly associated with MDD. With respect to the negative control diseases, blepharitis was associated with a lower risk of MDD (OR 0.87, 95% CI 0.77–0.98; Table 3 ).
Schizophrenia cohort
The results demonstrated that a presence of previous glaucoma was significantly associated with a higher risk of schizophrenia (OR 1.47, 95% CI 1.02–2.11). Among the different types of glaucoma, glaucoma suspect (OR 1.88, 95% CI 1.01–3.49) and open-angle glaucoma (OR 2.19, 95% CI 1.13–4.26) showed significant associations with schizophrenia. However, no significant associations were observed between other ocular neurovascular diseases or negative control and schizophrenia (Table 4 ).
In our study, we found that ocular neurovascular diseases were associated with psychiatric disorders. Glaucoma had the strongest association in all BD, MDD, and schizophrenia cohorts. Additionally, age-related macular degeneration and dry eye syndrome were associated with MDD.
Glaucoma was once considered to be a disease related to elevated intraocular pressure but is now viewed as a neurodegenerative disease [ 18 ]. It was also associated with ocular perfusion pressure and vascular dysfunction [ 19 ]. A previous study reported that glaucoma was associated with anxiety, depression, and sleep disturbance, and the severity of glaucoma was a predictor of psychiatric disorder [ 20 ]. Another large-scale population-based study showed a significant association between glaucoma and anxiety/depression [ 21 ]. However, most of those studies consisted of a cross-sectional design and failed to clarify temporality. A previous retrospective study found that veterans with severe mental illness (schizophrenia, schizoaffective disorder, BD, and other psychosis) had an elevated risk of ocular diseases, including cataracts and glaucoma [ 22 ]. However, the study did not discuss the pattern of different ocular diseases in each mental illness.
In the present study, we found associations between glaucoma and BD, MDD, and schizophrenia. Among the different types of glaucoma, glaucoma suspect and open-angle glaucoma were associated with all the three target psychiatric disorders. Closed-angle glaucoma was associated with BD and positively correlated with MDD but without statistical significance. Undetermined glaucoma was associated with BD and MDD and also positively correlated with schizophrenia, although this was not statistically significant. Other than glaucoma, age-related macular degeneration and dry eye syndrome were associated with MDD, but this trend was not observed in the BD and schizophrenia groups.
Loss of vision, social, and daily life function has been reported to be associated with increased risk of MDD in glaucoma [ 23 , 24 ]. In our study, we analyzed additional ocular disorders with potential loss of vision and social function in addition to glaucoma. For example, retinal vascular occlusion is associated with severe vision loss and visual field defect [ 25 ]; diabetic retinopathy is among the leading causes of legal blindness in working-age adults and the leading cause of vision-threatening retinopathy [ 26 , 27 ]. In non-neurovascular exposures, the visual outcome of retinal detachment is uncertain, and surgical interventions for retinal detachment (with attendant postoperative complications) are usually required [ 28 , 29 ]; uveitis, which causes several vision-threatening complications such as macular edema and cataract, was reported to account for up to 10% of legal blindness in the United States [ 30 , 31 ]. For these ocular disorders, however, we did not find a significant association with MDD. Thus, psychosocial factors or vision loss may not explain the association between glaucoma and psychiatric disorders identified in our study.
Neurodegenerative process may support the association between glaucoma and psychiatric disorders. One previous study has found that BD and MDD were associated with neurological deficits [ 3 ]. Furthermore, glaucoma is characterized by progressive optic nerve degeneration [ 32 ] and was recently considered to be a neurodegenerative disease [ 33 ]. It has been suggested that the link between mental disorders and glaucoma is attributed to their similar disease pathophysiology. Although the pathogenesis of glaucoma is related to retinal ganglion cell death, caused by intraocular pressure, different types of glaucoma have a slightly different pathophysiology [ 34 ]. For example, closed-angle glaucoma is related to ocular structural abnormalities, which leads to elevated intraocular pressure, whereas open-angle glaucoma is more likely to occur in primary neural pathological processes [ 34 ]. This may indicate a stronger association between open-angle glaucoma and metal disorders found in our study. As for glaucoma suspect, it is dependent on a normal open angle upon ocular examination and is sometimes defined as an early open-angle glaucoma. In addition to the neurodegenerative hypothesis, glaucoma treatment and its chronic asymptomatic, but potentially blinding nature, may also increase the risk of MDD [ 21 , 35 , 36 , 37 ].
Regarding dry eye syndrome and age-related macular degeneration, which were both shown to be associated with MDD, there is supporting evidence to our findings. The connection between dry eye syndrome and affective disorders, especially anxiety and MDD, has been discussed [ 38 ]. Although the cause and effect relationship remains unknown, similar etiopathogenic and neuropathogenic mechanisms were suggested [ 38 ]. As for age-related macular degeneration, it is a potentially distressing medical condition because of the vision loss, financial burden of treatment, and long-term need of intraocular injection [ 39 ]. Although the association between age-related macular degeneration and MDD at the neurological level has not been confirmed, one previous study has indicated a possible physiological connection between the diseases [ 40 ].
We chose three common ocular diseases, other than neurovascular disease, as our negative controls for exposure, which included retinal detachment, uveitis, and blepharitis. None of the three diseases showed any significant association with any psychiatric disorders, except blepharitis in MDD. Unlike dry eye syndrome or other ocular neurovascular diseases, blepharitis showed a negative association with MDD. As with MDD, blepharitis has been confirmed to be a risk factor for dry eye syndrome and could accelerate the development of the syndrome [ 38 ]. In addition, blepharitis has been suggested to be an early form and manifestation of dry eye syndrome [ 41 ]. We hypothesize that patients with MDD and blepharitis, both being risk factors for dry eye syndrome, experience early development of dry eye syndrome. Patients may be diagnosed as having and be treated more predominantly for dry eye syndrome than blepharitis in ophthalmology clinics. This may result in a relatively lower prevalence of blepharitis among MDD group compared with its control group.
To the best of our knowledge, this is the first population-based study with long-term follow-ups to test the association between ocular neurovascular diseases and psychiatric disorders. We not only demonstrated the association but also revealed the temporality between the two groups of diseases. We used a large nationwide, population-based sample as our study population and tried to minimize recall bias and selection bias.
There are still some limitations to this study. First, we identified our study groups based on the ICD codes. We attempted to validate the diagnosis by using three similar diagnoses within our study period, which were made by experts (ophthalmologists or psychiatrists). Without a chart review, we were missing each patient’s raw data or other clinical manifestation. Second, psychiatric disorders result from the interactions of bio-psycho-social factors. Our study focused on the biological aspect but cannot evaluate psycho-social confounders between ocular diseases and psychiatric disorders. Third, because the study period included the transition from the ICD-9 and ICD-10 coding system in the Taiwan NHI, we included both ICD-9 and ICD-10 diagnoses, despite the two systems were not perfectly matched for each diagnosis. Fourth, we suggest that patients do not use psychotropic medication before the diagnosis of a psychiatric disorder to rule out the effect of psychotropic medication on the eye; however, in very rare circumstances, they may still use psychotropic medication for other purposes. Finally, we cannot rule out patients who were diagnosed with a psychiatric disorder before our study period but then returned to the NHI system after a long time period without treatment or follow-up. An additional limitation of our study is that patients with prodromal schizophrenia or with untreated psychosis were not be enrolled because the study was based on data collected from a health insurance database. Moreover, the onset of psychiatric disorders, especially schizophrenia, is typically during adolescence or early adulthood. The mean age of our cohort was in the 40s, which means the study enrolled mostly late onset schizophrenia and excluded early onset cases, and thus, the study population cannot represent all the cases of psychiatric disorders. We examined the demographic data of both excluded and included samples of each major psychiatric disorder group and identified no significant difference between the two (Supplementary Table 2 ). The most common comorbid ocular neurovascular disease among the excluded samples was glaucoma in all groups (BD, MDD, and schizophrenia) (3.02, 4.08, and 1.96%, respectively; Supplementary Table 3 ).
Under the hypothesis of the shared pathophysiology of neurovascular dysfunction, our study established the temporality and association between ocular neurovascular diseases and certain psychiatric diseases. Glaucoma, among other ocular neurovascular diseases, had the most significant association of an increased risk for BD, MDD, and schizophrenia. Among the different types of glaucoma, open-angle glaucoma was associated with all three psychiatric disorders, but closed-angle glaucoma was only associated with an increased risk of BD. Additionally, age-related macular degeneration and dry eye syndrome was associated with an increased risk of MDD. Based on the results, we suggest raising awareness of psychiatric disorder during ophthalmology follow-up for glaucoma and of ocular problems during psychiatry follow-up. Appropriate early screening or consultation with another specialty may be indicated. The actual pathophysiology between glaucoma and psychiatric disorder need further investigation. Knowing more about the pathophysiology, may contribute to more knowledge about the mechanism of psychiatric disorder.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due legal restriction but are available from the corresponding author on reasonable request.
Abbreviations
Bipolar disorder
Charlson Comorbidity Index
Generalized estimating eq.
International Classification of Diseases, Ninth Revision, Clinical Modification
Longitudinal Health Insurance Database 2010
Major depressive disorder
National Health Insurance Research Database
National Health Insurance
Propensity score matching
Standardized difference
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382(9904):1575–86.
Article PubMed Google Scholar
Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–41.
Article PubMed PubMed Central Google Scholar
Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(10):965–86.
Goldstein BI. Bipolar disorder and the vascular system: mechanisms and new prevention opportunities. Can J Cardiol. 2017;33(12):1565–76.
London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53.
Article CAS PubMed Google Scholar
Shariflou S, Georgevsky D, Mansour H, Rezaeian M, Hosseini N, Gani F, et al. Diagnostic and prognostic potential of retinal biomarkers in early on-set Alzheimer's disease. Curr Alzheimer Res. 2017;14(9):1000–7.
Choi S, Jahng WJ, Park SM, Jee D. Association of age-Related Macular Degeneration on Alzheimer or Parkinson disease: a retrospective cohort study. Am J Ophthalmol. 2020;210:41–7.
Appaji A, Nagendra B, Chako DM, Padmanabha A, Jacob A, Hiremath CV, et al. Retinal vascular tortuosity in schizophrenia and bipolar disorder. Schizophr Res. 2019;212:26–32.
Appaji A, Nagendra B, Chako DM, Padmanabha A, Hiremath CV, Jacob A, et al. Retinal vascular fractal dimension in bipolar disorder and schizophrenia. J Affect Disord. 2019;259:98–103.
Naiberg MR, Hatch JK, Selkirk B, Fiksenbaum L, Yang V, Black S, et al. Retinal photography: a window into the cardiovascular-brain link in adolescent bipolar disorder. J Affect Disord. 2017;218:227–37.
Kalenderoglu A, Sevgi-Karadag A, Celik M, Egilmez OB, Han-Almis B, Ozen ME. Can the retinal ganglion cell layer (GCL) volume be a new marker to detect neurodegeneration in bipolar disorder? Compr Psychiatry. 2016;67:66–72.
Khalil DH, Said MM, Abdelraouf MA. Peripapillary retinal nerve fiber layer and ganglion cell complex degeneration in Egyptian patients with bipolar disorder. Eye (London, England). 2019;33(12):1852–8.
Article Google Scholar
Lizano P, Bannai D, Lutz O, Kim LA, Miller J, Keshavan M. A meta-analysis of retinal Cytoarchitectural abnormalities in schizophrenia and bipolar disorder. Schizophr Bull. 2019;4;46(1):43-53.
Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527–9.
Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health. 2018;40:e2018062.
Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349–58.
Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology (Cambridge, Mass). 2010;21(3):383–8.
Chang EE, Goldberg JL. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology. 2012;119(5):979–86.
Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Cur Opin Pharmacol. 2013;13(1):36–42.
Article CAS Google Scholar
Agorastos A, Skevas C, Matthaei M, Otte C, Klemm M, Richard G, et al. Depression, anxiety, and disturbed sleep in glaucoma. J Neuropsychiatry Clin Neurosci. 2013;25(3):205–13.
Zhang X, Olson DJ, Le P, Lin F-C, Fleischman D, Davis RM. The association between Glaucoma, anxiety, and depression in a large population. Am J Ophthalmol. 2017;183:37–41.
Saeedi O, Ashraf H, Malouf M, Slade EP, Medoff DR, Li L, et al. Prevalence of diagnosed ocular disease in veterans with serious mental illness. Gen Hosp Psychiatry. 2016;43:1–5.
Wang SY, Singh K, Lin SC. Prevalence and predictors of depression among participants with Glaucoma in a nationally representative population sample. Am J Ophthamol. 2012;154(3):436–44.
Jampel HD, Frick KD, Janz NK, Wren PA, Musch DC, Rimal R, Lichter PR, et al. Depression and mood indicators in newly diagnosed Glaucoma patients. Am J Ophthamol. 2007;144(2):238–44.
Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25.
Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–39.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36.
Williamson TH, Shunmugam M, Rodrigues I, Dogramaci M, Lee E. Characteristics of rhegmatogenous retinal detachment and their relationship to visual outcome. Eye (Lond). 2013;27(9):1063–9.
Lv Z, Li Y, Wu Y, Qu Y. Surgical complications of primary rhegmatogenous retinal detachment: a meta-analysis. PLoS One. 2015;10(3):e0116493.
Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844–8.
Article CAS PubMed PubMed Central Google Scholar
Tomkins-Netzer O, Talat L, Bar A, Lula A, Taylor SR, Joshi L, Lightman S. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmol. 2014;121(12):2387–92.
Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–81.
Lawlor M, Danesh-Meyer H, Levin LA, Davagnanam I, De Vita E, Plant GT. Glaucoma and the brain: trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv Ophthalmol. 2018;63(3):296–306.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–11.
Mabuchi F, Yoshimura K, Kashiwagi K, Shioe K, Yamagata Z, Kanba S, et al. High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J Glaucoma. 2008;17(7):552–7.
Jampel HD, Frick KD, Janz NK, Wren PA, Musch DC, Rimal R, et al. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144(2):238–44.
Janz NK, Wren PA, Guire KE, Musch DC, Gillespie BW, Lichter PR. Fear of blindness in the collaborative initial Glaucoma treatment study: patterns and correlates over time. Ophthalmology. 2007;114(12):2213–20.
Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–65.
Senra H, Macedo AF, Nunes N, Balaskas K, Aslam T, Costa E. Psychological and psychosocial interventions for depression and anxiety in patients with age-related macular degeneration: a systematic review. Am J Geriatr Psychiatry. 2019;27(8):755–73.
Maynard ML, Zele AJ, Kwan AS, Feigl B. Intrinsically photosensitive retinal ganglion cell function, sleep efficiency and depression in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(2):990–6.
Rynerson JM, Perry HD. DEBS - a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016;10:2455–67.
Download references
Acknowledgements
The study was supported by Chang Gung Memorial Hospital, Taoyuan, Taiwan (CMRPG3C0171, CMRPG3B0441, CORPG3C0081) and National Science Council Research Grants, Taipei, Taiwan (MOST 105–2314-B-182A-076, MOST 106–2314-B-182A-045 -MY3). The funding organization had no role in the design and conduct of this study, including data collection, analysis, interpretation of the data, approval of the manuscript, or decision to submit the work for publication.
Author information
Chun-Hao Liu and Eugene Yu-Chuan Kang contributed equally to this work.
Authors and Affiliations
Department of Psychiatry, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
Chun-Hao Liu
Department of Psychiatry, New Taipei Municipal Tu-Cheng Hospital, New Taipei, Taiwan
College of Medicine, Chang Gung University, Taoyuan, Taiwan
Chun-Hao Liu, Eugene Yu-Chuan Kang, Yu-Hsiang Lin, Wei-Chi Wu, Zhuo-Hao Liu, Chang-Fu Kuo, Chi-Chun Lai & Yih-Shiou Hwang
Department of Sinophone Literatures, National Dong Hwa University, Hualien, Taiwan
Department of Ophthalmology, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
Eugene Yu-Chuan Kang, Wei-Chi Wu, Chi-Chun Lai & Yih-Shiou Hwang
Department of Urology, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
Yu-Hsiang Lin
Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Department of Neurosurgery, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
Zhuo-Hao Liu
Department of Rheumatology, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
Chang-Fu Kuo
You can also search for this author in PubMed Google Scholar
Contributions
Dr. YSH has full access to the data and takes overall responsibility. Conception and design: CHL, YCK, YSH; Data collection and collation: YHL, ZHL; Data analysis and interpretation: WCW, CCL, CFK; Writing: CHL, YCK. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yih-Shiou Hwang .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Chang Gung Memorial Hospital Institutional Review Board (201900967B0). Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: supplementary table 1.
. ICD-9 CM diagnostic codes used in this study.
Additional file 2: Supplementary Table 2.
Characteristics of cases according to exclude or not. Supplementary Table 3. Ocular disease according to bipolar disorder (BD), major depressive disorder (MDD) and schizophrenia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, CH., Kang, E.YC., Lin, YH. et al. Association of ocular diseases with schizophrenia, bipolar disorder, and major depressive disorder: a retrospective case-control, population-based study. BMC Psychiatry 20 , 486 (2020). https://doi.org/10.1186/s12888-020-02881-w
Download citation
Received : 22 June 2020
Accepted : 19 September 2020
Published : 02 October 2020
DOI : https://doi.org/10.1186/s12888-020-02881-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ocular neurovascular diseases
- Psychiatric disorders
- Mental disorders
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Expert Review
- Open access
- Published: 18 October 2023
Genomic findings in schizophrenia and their implications
- Michael J. Owen ORCID: orcid.org/0000-0003-4798-0862 1 ,
- Sophie E. Legge ORCID: orcid.org/0000-0001-6617-0289 1 ,
- Elliott Rees ORCID: orcid.org/0000-0002-6168-9222 1 ,
- James T. R. Walters 1 &
- Michael C. O’Donovan ORCID: orcid.org/0000-0001-7073-2379 1
Molecular Psychiatry volume 28 , pages 3638–3647 ( 2023 ) Cite this article
23k Accesses
11 Citations
145 Altmetric
Metrics details
- Schizophrenia
There has been substantial progress in understanding the genetics of schizophrenia over the past 15 years. This has revealed a highly polygenic condition with the majority of the currently explained heritability coming from common alleles of small effect but with additional contributions from rare copy number and coding variants. Many specific genes and loci have been implicated that provide a firm basis upon which mechanistic research can proceed. These point to disturbances in neuronal, and particularly synaptic, functions that are not confined to a small number of brain regions and circuits. Genetic findings have also revealed the nature of schizophrenia’s close relationship to other conditions, particularly bipolar disorder and childhood neurodevelopmental disorders, and provided an explanation for how common risk alleles persist in the population in the face of reduced fecundity. Current genomic approaches only potentially explain around 40% of heritability, but only a small proportion of this is attributable to robustly identified loci. The extreme polygenicity poses challenges for understanding biological mechanisms. The high degree of pleiotropy points to the need for more transdiagnostic research and the shortcomings of current diagnostic criteria as means of delineating biologically distinct strata. It also poses challenges for inferring causality in observational and experimental studies in both humans and model systems. Finally, the Eurocentric bias of genomic studies needs to be rectified to maximise benefits and ensure these are felt across diverse communities. Further advances are likely to come through the application of new and emerging technologies, such as whole-genome and long-read sequencing, to large and diverse samples. Substantive progress in biological understanding will require parallel advances in functional genomics and proteomics applied to the brain across developmental stages. For these efforts to succeed in identifying disease mechanisms and defining novel strata they will need to be combined with sufficiently granular phenotypic data.
Similar content being viewed by others

Schizophrenia genomics: genetic complexity and functional insights

Mapping genomic loci implicates genes and synaptic biology in schizophrenia

The molecular pathology of schizophrenia: an overview of existing knowledge and new directions for future research
Introduction.
Schizophrenia is a highly heritable psychiatric condition with a lifetime prevalence of around 1%. It is a highly complex, multi-domain syndrome which is associated with perturbations in many aspects of brain function [ 1 ]. Its core features, around which modern diagnostic criteria have been built, consist of a combination of positive, negative and disorganised symptoms as well as certain exclusion criteria [ 2 , 3 ]. However, these central attributes are frequently accompanied by a wide range of other features, including impairments of most aspects of cognitive function [ 4 ], affective symptoms [ 3 ], movement disorders [ 5 ] and sensory abnormalities [ 6 ]. Among those who meet diagnostic criteria, there is considerable heterogeneity in individual symptoms, mode of onset, course, and outcome [ 3 , 7 ]. The boundaries between schizophrenia and other psychiatric syndromes are indistinct, as are the boundaries with wellness [ 3 ]. For instance, there is overlap in symptoms with schizoaffective disorder, bipolar disorder and childhood neurodevelopmental disorders [ 8 , 9 ]. Antipsychotic drugs form the mainstay of current pharmacotherapy, but these are largely ineffective in treating negative and disorganised symptoms, are ineffective in treating psychosis in around 30% of cases and are associated with a significant number of adverse effects [ 3 ]. Therapeutic advances are badly needed, but these have proved elusive with progress impeded by a poor understanding of pathophysiology, clinical heterogeneity and a lack of valid biomarkers and model systems [ 10 ].
The high heritability of schizophrenia together with advances in genomic technology and the complexity and inaccessibility of the human brain have driven a substantial effort to understand the genetics of the condition in the hope that this will illuminate pathogenesis and provide novel approaches to prediction and stratification. This intense and highly collaborative endeavour has, over the past 15 years, resulted in considerable progress. In this article, we will review recent findings, summarise the key insights these have revealed, and consider important remaining challenges and how these can be met.

Genetic architecture
Common variants.
GWAS have identified an important role for common variants (minor allele frequency >1%). Following the first successful GWAS study of schizophrenia, which identified a single locus containing the gene ZNF804A [ 11 ], multiple waves of GWAS have been reported, each building on and both confirming and extending the findings of earlier iterations. The largest published GWAS, to date, which included 76 755 individuals with schizophrenia and 243 649 controls, identified 287 associations, 5 of which map to the X-chromosome, meeting standard criteria for genome-wide significance [ 12 ] (Fig. 1 ). Typical of common variant associations to traits associated with low fecundity, the effect sizes are small (mean OR 1.06; range 1.04–1.23) and together the genome-wide significant loci explain only around 2–3% of variance in liability to the disorder, or about 10% of the total variance estimated to be conferred by common alleles (Fig. 2 ). This study also found that the common variant genetic architecture of schizophrenia did not differ between males and females, the inference being this class of alleles is unlikely to explain reported sex differences in the epidemiology and course of the disorder [ 13 ]. Fine mapping of associated loci to identify credible causal SNPs identified a subset of 120 genes that were prioritized as likely to mediate the associations at some of the loci, only a small minority of which ( N = 16) were implicated by associated variants that change the sequence of the encoded proteins.
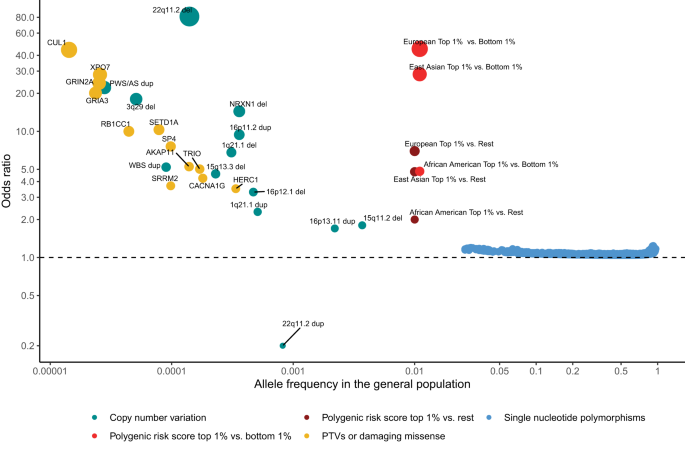
Effect sizes expressed as odds ratios (OR) versus general population frequencies for copy number variants (CNVs), damaging rare coding variants (protein-truncating variants (PTVs) or missense variants), common single nucleotide polymorphisms (SNPs), and polygenic risk scores (PRS). The OR less than 1 of 22q11dup CNV denotes a protective effect. CNVs effect sizes are from [ 20 ]. To constrain the ORs for 22q11.2 del and PWS/AS dup below infinity, a single carrier for each CNV was added to the controls. Dup and del refer to duplication and deletions. PTV and missense associated genes are from references [ 29 , 30 ]. Population frequencies and ORs for RCV associated genes are from [ 29 ]. RCV effect sizes refer to the excess burden of RCVs in the named gene. CNV and RCV effect sizes are imprecise due to the small number of observations. SNP and PRS data are from [ 12 ]. PRS ORs are given for individuals in the top centile relative to all other individuals, and top centile versus the bottom centile. Data for European, East Asian and African American genetic ancestry are given separately. The effect size in the Latino population is not plotted as it has not yet been estimated in sufficient samples. It should be noted that most rare alleles are not expected to confer large effects. The shape of the curve provides an indication of the maximum frequency that selection pressures permit alleles of a given effect size to attain, not the expected effect size for an allele of a particular frequency.
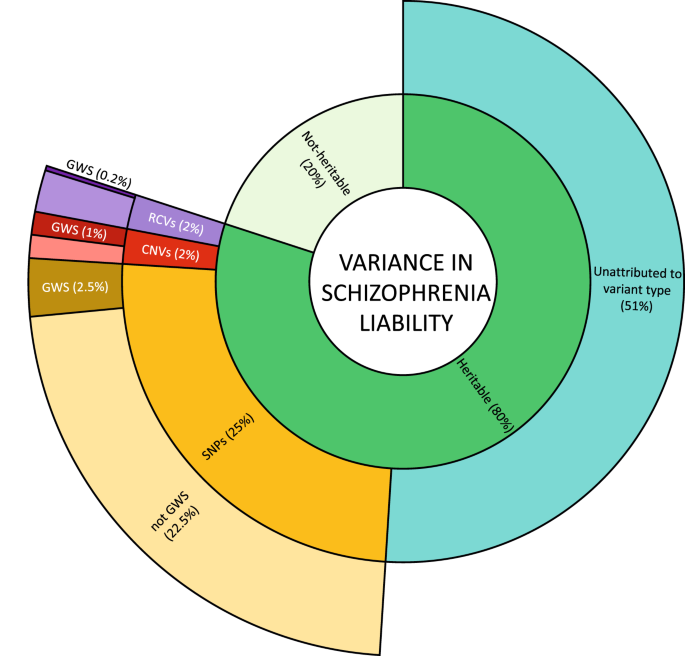
Inner ring: heritability is estimated from twin studies at around 80%. The remaining 20% attributed to non-heritable risk factors include environmental risk factors, stochastic effects, and de novo mutations. Middle ring: estimates of the contribution to variance in liability from currently known classes of heritable risk alleles. Outer ring: variance in liability assigned to specific risk alleles, or in the case of RCVs, a burden test of RCVs in associated genes. Percentages refer to variance in total liability and are based on studies of people largely of European biogeographic ancestry. Values are approximations (see text). SNP single nucleotide polymorphisms and small insertion/deletion polymorphisms with minor allele frequencies greater than 0.01. CNV large copy number variants with population frequencies less than 0.01. RCV rare coding variants with frequencies typically less than 0.0001. GWS significance surpassing the relevant thresholds allowing for multiple testing for SNPs, CNVs, and burden tests of RCVs.
Given the small fraction of common variant liability explained by alleles achieving genome-wide significance, large numbers of common variants remain to be discovered. How many is not resolved, but schizophrenia and other psychiatric, cognitive and behavioural traits are amongst the most polygenic of all human traits [ 14 ] with a lower bound from recent estimates of around 10,000 causal variants [ 15 ], although other estimates are considerably higher [ 14 ]. Consistent with high polygenicity, common risk variants are found in proximity to a very large number of genes, but this is not random. Thus, associations are enriched around genes that are conserved across species, and which are relatively intolerant of mutations in humans [ 16 ]. They are also enriched in genes that are expressed in the brain, in neurons, both excitatory and inhibitory, and in genes encoding proteins involved in fundamental biological processes related to neuronal function, in particular gene-sets related to synaptic structure and function (Fig. 3 ) [ 12 ]. Finally, they are also enriched in genes implicated by rare variant studies in neurodevelopmental disorders including schizophrenia [ 12 ]. The implications of these patterns of biological enrichment are discussed further below.
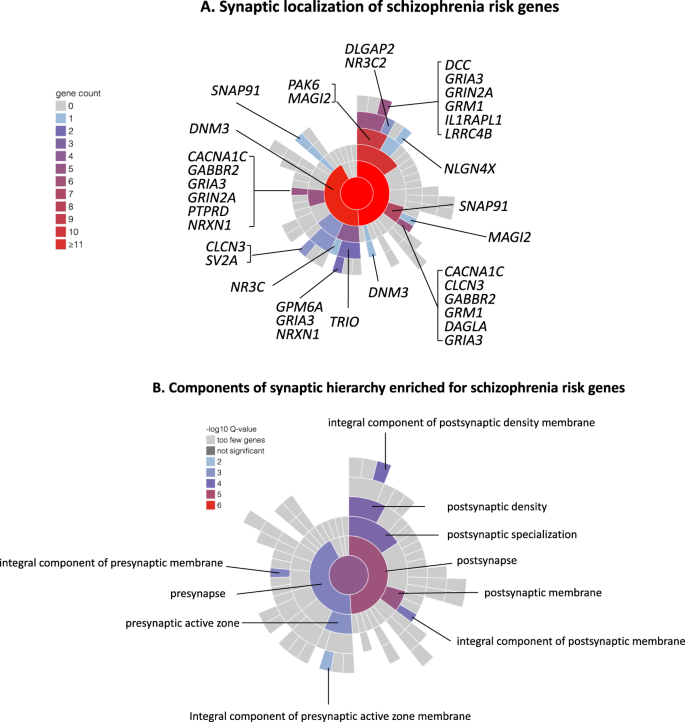
A Synaptic location of prioritized protein coding genes from Schizophrenia Working group of the Psychiatric Genomics Consortium [ 12 ], genes attaining FDR < 0.05 for enrichment for rare coding variants in the study of the Schizophrenia Exome Meta-analysis Consortium [ 29 ], and neurexin 1, the only schizophrenia associated CNV to implicate a single gene [ 20 ]. Plot and locations were generated and defined according to the Synaptic Gene Ontologies (SYNGO) Consortium Website ( https://syngoportal.org ). The data required to generate the plot and obtain granular detail of, and the evidence for, the locations and synaptic functions for each gene are available as Supplementary Table 1 . Colours denote the number of genes in each cellular component. Numbers are cumulative from the periphery to the centre of the plot respectively depicting the lowest and highest levels of the hierarchy of the ontology. B Components of the SYNGO ontology hierarchy are denoted as significantly enriched for genes as in A. Colour denotes the significance of enrichment as determined by SYNGO. Enrichment is calculated relative to a background of all brain expressed genes.
Rare copy number variants
Numerous studies have consistently demonstrated that rare copy number variations (CNVs), defined as deletions or duplications of DNA segments greater than 1 kilobases (KB) in size, are risk factors for schizophrenia. Across the genome, people with schizophrenia are enriched for rare (<1% frequency) CNVs larger than 20 KB compared with controls, with deletions that overlap genes having the strongest effects on risk [ 17 ]. Additionally, the genome-wide rate of de novo CNVs is significantly higher in schizophrenia cases compared with controls [ 18 ].
The first specific genetic risk factor to be robustly associated with schizophrenia was a 1.5–3 megabase (MB) deletion of 22q11.2 [ 19 ], which had previously been found to cause DiGeorge and Velocardiofacial Syndromes. Following this discovery, CNV studies using SNP genotyping array data from over 20,000 cases and 20,000 controls have identified 12 specific CNVs as risk factors for schizophrenia [ 17 , 20 ] (Fig. 1 ). 11 of these CNVs affect multiple genes and are recurrent events formed by non-allelic homologous recombination between low copy repeats, which results in similar CNV breakpoints across carriers. The only single gene disrupting CNV that is currently implicated in schizophrenia involves non-recurrent exonic deletions of NRXN1 . Individually, the 12 schizophrenia-associated CNVs occur in 0.015%–0.64% of cases [ 21 ] but confer strong risks for schizophrenia in individual carriers, with estimated odds ratios ranging between 1.8 and 81.2 [ 20 , 21 ] (Fig. 1 ). Being rare events, the confidence intervals for these estimates are wide, and there is some evidence from population studies that the point estimates may be overestimated, although it should be noted that even the largest population study [ 22 ] includes relatively few schizophrenia cases ( N = 1704–2590) and is also unable to provide accurate estimates of effect size. It is clear [ 17 , 20 ] that additional risk CNVs are identifiable through SNP genotyping arrays, but they are likely to be rarer, smaller in size than can be resolved by arrays, or have smaller effect sizes than those currently implicated, and therefore require larger samples for their discovery.
A duplication of 22q11.2, the reciprocal of the risk deletion at this locus, is the only replicated CNV that is enriched in controls compared with cases [ 17 , 23 , 24 ], suggesting a protective effect against schizophrenia (Fig. 1 ). The protective effects do not, however, extend to other neurodevelopmental disorders, as it is a risk factor for developmental delay and autism spectrum disorders [ 25 ]. From the perspective of exploiting the finding for therapeutics, it is clearly important to determine if duplication of the same or distinct specific gene(s) protects against schizophrenia and increases the risk of other neurodevelopmental disorders.
Rare coding variants
Exome-sequencing studies have demonstrated that very rare single-nucleotide variants (SNVs) and small insertions and deletions (indels) that alter the amino acid sequences of genes, collectively termed rare coding variants (RCVs), also contribute to schizophrenia liability. While the exome-wide rate of de novo damaging coding variants in schizophrenia is only modestly higher than expected, there is a stronger enrichment of such variants in cases within genes that are intolerant to protein truncating variants (PTVs) in humans, in genes implicated in early-onset neurodevelopmental disorders, and in genes related to glutamatergic postsynaptic proteins [ 10 , 26 , 27 ], Case-control studies have also shown that in people with schizophrenia, these sets of genes are enriched for ultra-rare (occurring in less than 1 in 10,000 people) damaging coding variants [ 28 , 29 ].
Sequencing studies are currently underpowered to implicate specific schizophrenia RCVs, but they have begun to identify specific genes where the total burden of any such RCV is significantly greater than in controls. The largest exome-sequencing study of schizophrenia to date was performed by the Schizophrenia Exome Sequencing Meta-Analysis (SCHEMA) Consortium; 10 genes were identified as having an exome-wide significant excess of ultra-rare damaging coding variants through meta-analysis of data from 24,248 schizophrenia cases, 97,322 controls, and 3,402 proband-parent trios [ 29 ] (Fig. 1 ). As a group, these genes were enriched in cases for both PTVs and damaging missense variants, with the gene-specific ORs ranging from 3–50, albeit with large confidence intervals. A subsequent study that meta-analysed targeted sequencing data from 161 genes in 11,580 cases and 10,555 controls with data from the SCHEMA consortium identified two additional risk genes at exome -wide significance [ 30 ] (Fig. 1 ).
Heritability explained and unexplained
Twin studies suggest inherited alleles (as distinct from de novo or somatic mutations) account for about 60–80% of within population variance in liability to schizophrenia [ 31 , 32 ] but how this heritability is distributed across alleles of the various frequencies, effect sizes, and types has not been precisely delineated. Current understanding ( Fig. 2 ) suggests alleles detectable by GWAS (i.e. SNP heritability) make the biggest single contribution, estimated at around 25% [ 12 ]. RCVs are estimated to have a burden heritability of around 2%, primarily from ultra-rare PTVs but also to a degree from damaging missense mutations [ 33 ]. Large rare CNVs may contribute a similar amount to that for RCVs [ 34 ]. Thus, assuming additivity, the classes of variation that have been studied most intensively collectively explain around 30% of total variance in liability, or around 40% of the expected heritability. It should be stressed the vast majority of explained heritability is attributable to GWAS loci, RCVs, and CNVs that do not meet stringent criteria for significant association, indeed, only around 10% (but see also ancestry section) of explained heritability is attributable to such findings [ 12 , 17 , 33 ]. Thus, while there has been substantial progress in identifying risk alleles for schizophrenia, there is scope for a great deal more, even using the tools widely in use today.
In schizophrenia as in other common disorders and traits, it is unclear what accounts for the substantial gap between the heritability potentially explained by the current genomic data and that expected from classical genetic epidemiology. It seems certain that some of it will be attributable to classes of allele that are not adequately interrogated by current technology, for example rare non-coding alleles, structural variants other than large CNVs, and polymorphic repetitive sequences, both common and rare, which are difficult to tag by linkage disequilibrium (the phenomenon that makes GWAS possible). It is expected that the potential contribution of these classes of variant will be resolved soon with the increasing use of whole genome and long read sequencing technologies. It is also possible that the heritability captured by SNPs and other types of known risk allele is underestimated relative to family studies due to the higher phenotypic and ancestry heterogeneity in large case control studies. Conversely, the narrow sense (additive model) heritability estimated by genetic epidemiology might be inflated by, for example, statistical gene-gene interactions and inadequate control for shared environments [ 35 , 36 , 37 ].
Ancestry effects
Genomic studies of schizophrenia are based predominantly on participants classified as of European biogeographic ancestry. However, large studies of other ancestries have begun to emerge, of which the most informative was a study of 22,778 cases and 35,362 controls of East Asian ancestry [ 38 ]. While novel loci were identified, perhaps the most important finding was that the common variant genetic architecture of schizophrenia is essentially identical in East Asians and Europeans. Similar findings have been noted for other complex traits, including ones that, unlike schizophrenia, show very substantial geographical differences in prevalence [ 39 ]. Although the findings suggest that the common genetic architecture, and therefore presumably the fundamental biology, is essentially identical in East Asians and Europeans [ 38 ], there is a clear imperative to increase diversity in genomic studies as not all the fruits of genomic studies are likely to generalize across populations. For example, polygenic risk score analysis captures around 8% of variance in liability in people classified as (white) European, 7% as Latino, 6% as East Asian, but only 1.5% those considered African American [ 12 ]. Given that polygenic risk scoring is likely to play many roles in healthcare [ 40 ], it is critical that the Eurocentric bias of studies be rectified if genomics is not to contribute to further widening inequalities in care provision. At the same time, the inclusion of more diverse samples will increase discovery [ 38 , 41 ], partly because of increased sample sizes but also because, even if risk loci have similar effects in all populations, they are likely to have higher minor allele frequencies in some populations than in others, increasing the power for their detection [ 39 ]. Moreover, the inclusion of haplotypes with diverse patterns of LD is expected to improve the precision of localizing GWAS signals to specific causal alleles [ 38 ].
Pleiotropy, heterogeneity and transdiagnostic effects
A striking finding has been the demonstration of moderate to extensive overlap in common risk alleles between psychiatric disorders suggesting significant biological pleiotropy [ 42 , 43 , 44 ], albeit estimates of shared risk might be inflated in some instances by assortative mating [ 45 ]. There are various reasons why overlapping genetic effects were not unexpected, including evidence from many large-scale family studies that psychiatric phenotypes do not “breed true” (e.g. [ 32 ]). It is important to note that the common allele genetic correlation between two cohorts of people with the same psychiatric diagnosis is typically greater than it is between cohorts with different diagnoses [ 42 , 46 ] suggesting that, while current diagnostic criteria may not define biologically distinct conditions, they do identify groups of cases whose members have, on average, more in common with each other than they do with groups with other psychiatric disorders.
Pleiotropic associations to schizophrenia PRS have been confirmed in large-scale phenome-wide analyses in population-based samples that assessed hundreds of phenotypes [ 47 , 48 , 49 ]. Whilst the strongest associations were for other psychiatric conditions, associations were also found for cognitive, psychosocial, and physical health phenotypes.
Relationship to other psychiatric and neurodevelopmental disorders
The extent to which common variant liability to schizophrenia is shared with another diagnosis is greatest for bipolar disorder, with a genetic correlation of around 0.7 [ 44 ], the overlap being stronger between SZ and BDI than BDII [ 50 ]. Given the two disorders share many clinical features, it is important to note the genetic correlation between SZ and BD is substantially higher than can be plausibly attributed to diagnostic misclassification [ 42 ] or assortative mating [ 45 ]. The strong phenotypic and genetic overlaps (amongst other things) between schizophrenia and bipolar disorder argue against regarding the two as entirely distinct syndromes [ 8 , 9 ], although the imperfect overlaps in liabilities nevertheless suggest there is some biological validity in distinguishing between them.
The pattern of overlapping genetic risk seen for rare alleles is somewhat different, the evidence suggesting schizophrenia has greatest overlaps with childhood onset neurodevelopmental disorders (NDDs), particularly intellectual disability (ID), autism and attention deficit hyperactivity disorder (ADHD), rather than adult-onset psychiatric disorders. Overlaps occur at the level of genes containing rare disruptive mutations [ 29 , 51 , 52 ], as well as at the level of specific risk alleles, including both CNVs and rare disruptive mutations [ 51 , 53 ]. Finally, genes implicated by GWAS in schizophrenia are enriched for genes associated with rare disruptive mutations in NDDs [ 12 , 29 ].
Symptomatic heterogeneity and transdiagnostic effects
The latent structure of symptoms in schizophrenia consists of positive, negative disorganised and affective symptoms as well as cognitive ability [ 54 , 55 , 56 , 57 ]. Within cases, the severity of negative and of disorganised symptoms is associated with higher PRS for schizophrenia, and with greater familial aggregation of the disorder [ 55 , 57 , 58 , 59 , 60 ]. Perhaps surprisingly, neither PRS nor familial risk for schizophrenia appear to be associated with positive symptoms in individuals with established illness [ 55 , 57 , 58 , 60 ]. One possibility is that samples with established schizophrenia show insufficient variance in positive symptoms to detect the effects, and consistent with this, there is evidence positive symptoms are associated with PRS for schizophrenia in people with bipolar disorder [ 50 , 61 , 62 ] and with positive symptoms in a first-episode psychosis sample which included a broad range of psychosis diagnoses, only around a third meeting criteria for schizophrenia [ 63 ].
There is evidence that in people with schizophrenia, the presence of manic symptoms is associated with the burden of bipolar risk alleles carried by an individual rather than liability to schizophrenia per se [ 56 , 59 , 64 ], and it seems likely by extension that similar considerations will apply to depressive symptoms [ 65 ]. However, dissecting the molecular genetic underpinnings of affective symptoms in schizophrenia is not straightforward given that some of the liability to those disorders also confers liability to schizophrenia, making it difficult to separate modifier and causal effects. One approach to this is to apply structural equation modelling to try to distinguish between liability that is shared across disorders and liability that that is relatively specific to one disorder [ 66 ]. Such methods are yet to be applied to large well-phenotyped schizophrenia samples, but when applied to bipolar disorder, the findings suggest that manic, psychotic (independent of mood), and depressive symptoms are respectively associated with the specific components of liability to BD, schizophrenia and MDD in individual carriers [ 67 ]. A picture is therefore beginning to emerge supporting the notion that clinical heterogeneity at least in part reflects aetiological heterogeneity, and that the clinical picture expressed by an individual is the result of a confluence of partly orthogonal symptom dimensions and their underlying genetic risk factors.
Cognitive impairment
Cognitive impairment is a variable feature of schizophrenia but is strongly and consistently associated with poor functional outcomes [ 68 ]. There is a negative genetic correlation between common alleles associated with schizophrenia and those associated with cognitive ability (r g = −0.21) [ 69 ], which, given the similar SNP heritabilities of schizophrenia and cognitive ability of 20–25%, implies about 5% of variance in liability to schizophrenia is potentially explained by the shared effects of common alleles on cognition. Schizophrenia PRS have been shown to predict lower cognitive ability in population samples [ 49 , 70 ]. Within individuals with schizophrenia the evidence is less consistent, some studies finding a negative association between cognitive ability and schizophrenia polygenic risk score [ 55 , 60 , 71 , 72 ] but others not [ 73 , 74 , 75 ]. CNVs previously associated with schizophrenia have been associated with poorer cognitive ability in population-based samples [ 76 , 77 ] and in those with schizophrenia [ 78 ], as have ultra-rare coding variants [ 79 ].
The evidence with respect to premorbid cognitive impairment suggests that, while only a small proportion (10%) of variance is explained by identified genetic risk factors, the majority of this is accounted for by IQ PRS [ 55 , 79 , 80 ]. In contrast, there is little or no effect of schizophrenia common allele liability [ 55 , 81 ], whereas rare risk alleles are associated with poorer performance [ 79 , 80 ]. Whether schizophrenia genetic liability is associated with a poor cognitive trajectory or decline after the onset of psychosis is still unclear [ 55 , 60 , 82 , 83 ].
Course and treatment resistance
Phenotypes indicating a more chronic or severe illness course in schizophrenia such as greater number and length of hospital admissions are highly correlated among affected sibling pairs [ 58 ] and have been associated with higher schizophrenia polygenic risk scores [ 12 , 84 ]. Studies have not provided a decisive answer as to whether common variant liability to schizophrenia is elevated in people with treatment resistant schizophrenia (TRS), perhaps due to small samples and heterogeneity in the definition of TRS [ 85 , 86 , 87 , 88 , 89 ], but if it does, the inconsistent findings probably indicate such a link is likely to be fairly small. This conclusion is also supported by what is by far the largest study, which found that, with respect to schizophrenia liability, the common variant genetic architectures of TRS and non-TRS schizophrenia are qualitatively and quantitatively indistinguishable, but that there was an additional contribution of common risk alleles that was relatively specific to people with TRS [ 90 ]. Moreover, that relatively specific contribution showed moderately strong genetic correlations with intelligence and cognitive traits, tentatively suggesting TRS might represent a form of the disorder particularly enriched for neurodevelopmental aetiology. Discussed below, rare variants have provided the strongest genetic evidence for a link between schizophrenia and neurodevelopment, but the relationship between these classes of variant and TRS is not yet clear, likely due to the low power of the published CNV and sequencing studies to date. Thus, some have reported a particularly high burden of CNVs in people with TRS [ 87 , 91 ], but others found no differences [ 88 , 89 ]. Sequencing studies similarly are not yet conclusive, although in one study, cases with TRS have been reported to be enriched for RCVs in gene sets related to antipsychotic function and to agents involved in the treatment of amoebiasis and other protozoan diseases [ 92 ], while in another study, damaging RCVs were enriched in 112 TRS individuals compared with 218 individuals with typical schizophrenia [ 93 ]. Neither finding has to our knowledge been replicated in a published manuscript.
Implications of genetic findings
The evolutionary paradox of schizophrenia.
Schizophrenia is associated with markedly reduced fecundity [ 94 ] from which it has been postulated that risk alleles should segregate in the population at very low frequencies due to purifying selection. As expected under a purifying selection model, there is indeed an (approximately) inverse relationship between the effect sizes of risk alleles and their population frequencies (Fig. 1 ). However, as we have seen, much of the heritability nevertheless comes from common alleles.
Recent studies have cast some light on this “evolutionary paradox” [ 95 ]. The population frequency of high penetrance fitness-reducing mutations seems to be determined by mutation-selection-balance whereby risk alleles are selected against, but this is offset by de novo mutations [ 96 ]. Evidence to support this has come from schizophrenia risk CNVs [ 97 ] which are maintained at higher frequencies than expected from strong purifying selection because they are in mutation hotspots, and therefore rapidly replenished. Individual high penetrance coding variants are, as expected, extremely rare, but the large number of genes involved in schizophrenia offers a sizable genomic target for pathogenic de novo mutation, allowing this class of variant collectively to occur at a higher frequency than might be expected given purifying selection pressures on individual mutations. Finally, regarding common alleles, one popular hypothesis is that these might attain or persist at high frequencies due to pleiotropic effects on traits that confer reproductive advantages to unaffected carriers, a form of balancing positive selection. However, the current evidence suggests that when background selection effects are controlled for, alleles under positive selection are actually depleted rather than enriched for association with schizophrenia [ 98 ]. Moreover, while there is evidence that common variant liability to schizophrenia might indeed be associated with pleiotropic effects on increased fecundity, at least in contemporary European environmental contexts, the effects are too small in unaffected carriers to offset the negative impact on the fecundity of cases [ 99 ].
Overall, the evidence suggests that purifying rather than positive or balancing selection is the rule for schizophrenia risk variants [ 98 , 100 ], but at those detected by GWAS, the effects are weak, allowing risk alleles to achieve high frequencies under a mutation-selection-drift model, higher frequencies being facilitated at some loci by a reduction in haplotype diversity due to background selection [ 96 , 98 ]. These findings do not, however, exclude a role for positive or balancing selection at some loci [ 101 ].
Pleiotropy and trans-diagnostic genetic effects
There are many important implications of the pattern of overlapping genetic effects observed between psychiatric disorders. We highlight four. First, the genomic data support the widespread view that our systems of diagnostic classification are not optimal for basic or clinical research. This does not mean diagnosis-based research should be completely abandoned as there are no generally agreed alternatives of demonstrable superiority for research or for treatment. However, attempts to reduce heterogeneity, define the corresponding underlying biology, and identify novel strata of clinical utility, will only succeed through the pursuit of complementary approaches that cut across or divide current diagnostic groups (or both). Findings referred to above support approaches based on stratifying patients based on patterns of symptoms or cognitive ability, but there are other credible approaches including stratification by a particular aetiological factor such as a rare mutation, or a particular environmental exposure. However, progress in stratification will require access to genomic data from large samples in which the phenotypes have been measured with greater granularity than has hitherto been the norm for genomic studies [ 58 , 102 ]. Secondly, the pleiotropy seen for both common and rare risk variants for schizophrenia argues against there being a simple one to one mapping of risk alleles onto psychiatric conditions and related traits. This conclusion is strengthened by emerging evidence that, while genetic overlaps between psychiatric disorders and related traits are extensive, there are few disorder-specific variants, and most risk alleles show mixed direction effects on susceptibility to different outcomes [ 15 ]. This suggests that individual susceptibility to specific disorders may reflect the specific constellation and effect sizes of highly pleiotropic variants that contribute generally to the development of psychiatric conditions and related traits rather than a set of disorder specific risk variants. It follows that researchers should be extremely cautious interpreting observational or mechanistic studies, whether in humans or model systems, that seek to infer causal relationships between possible underlying genetics or neurobiology and specific diagnoses or other phenotypic outcomes [ 43 ]. Finally, the overlaps in genetic risk between schizophrenia and NDDs point to the need to consider the relationship between them, and it is to this that we will turn next.
The neurodevelopmental continuum
Schizophrenia has long been considered to result at least in part from disturbances of neurodevelopment [ 103 , 104 ]. Indeed, there is a modest common variant genetic correlation with both autism (rG = 0.21) and ADHD (rG = 0.17) [ 105 , 106 ]. However, the findings from rare variant studies for overlap at the genic and mutational level with childhood NDDs reviewed above point to a stronger relationship and an extension of the neurodevelopmental hypothesis of schizophrenia [ 51 ]. Further supporting the hypothesis implicating shared aetiology between schizophrenia and neurodevelopmental disorders, as reviewed elsewhere, there is evidence that many of the environmental risk factors for schizophrenia impact on the developing brain, and are shared with childhood NDDs [ 3 ].
It is important to note that the enrichment of rare risk mutations is not equal across neurodevelopmental disorders, but is greatest in ID, followed respectively by autism and ADHD where the burdens are equivalent [ 107 ] and then by by schizophrenia [ 26 , 51 ]. Moreover, as we discuss above for schizophrenia, but also in ASD and ADHD [ 107 ], the burden is higher in those with pre-morbid cognitive impairment. These findings suggest that neurodevelopmental disorders may be conceptualized as lying on a continuum of neurodevelopmental impairment reflecting the relative burden of rare damaging mutations, the magnitude of their effects, and perhaps the timing of their impacts on brain development and resulting functional outcomes [ 51 ]. There is also evidence that the phenotype expressed by carriers of the rare mutations that impact on neurodevelopment is influenced by the burden of disorder associated common genetic variants, for example CNV carriers who present with schizophrenia also have an elevated burden of common risk alleles for the disorder [ 108 , 109 , 110 ] while rare coding variant carriers with ASD have elevated burdens of common risk alleles for ASD and this associates differentially with the observed phenotypic features [ 111 ]. The evidence for a neurodevelopmental continuum points to the need for more transdiagnostic research across neurodevelopmental disorders, which are still largely studied separately, and has implications for nosology and clinical practice [ 51 ] as well as basic gene discovery [ 30 , 52 , 107 ]
What neurobiological mechanisms are implicated by genetics?
It is often assumed that schizophrenia results from pathophysiological perturbations to specific brain regions or circuits. There is strong evidence implicating disturbances of dopaminergic neurotransmission in the genesis of psychotic symptoms, but these are unlikely to explain all the clinical features of the disorder [ 112 ]. Moreover, rather than highlighting circumscribed anatomical or functional abnormalities, the hundreds of neuropathological and neuroimaging studies to date point to widespread and variable involvement of many brain regions and circuits [ 3 ]. This lack of neuroanatomical specificity has been supported by genomic studies of all classes of genetic variation, both common and rare which, while providing some support for the involvement of dopaminergic neurotransmission [ 113 ], have found that genes with high relative expression in most regions of the human brain are enriched for risk variants [ 12 , 29 ]. Associations are enriched particularly in CNS neurons, both excitatory and inhibitory, and in genes encoding proteins involved in fundamental biological processes related to neuronal function, in particular gene-sets related to synaptic development, maturation, structure and function [ 27 , 29 , 114 , 115 , 116 ] (Fig. 3 ).
These findings must be interpreted in the context of current limitations in understanding the human brain transcriptome and proteome, regionally and developmentally, the fact that very few GWAS and CNV associations can be robustly linked to specific genes, and that there are errors of omission and commission in assigning biological functions to genes. The latter point is well illustrated by the finding that, even among the genes implicated with high certainty through the precision of exome sequencing, only a minority can be confidently assigned to functions likely to be relevant to schizophrenia. However, as they stand, the recent findings pose an alternative to the view that schizophrenia is the result of dysfunction in a limited set of circumscribed brain regions and circuits. Rather they suggest that fundamentally the condition may best be understood as resulting from disturbances in neuronal, and particularly synaptic, function that are not confined to a small number of brain regions and circuits. Thus, the clinical features of schizophrenia may reflect altered neuronal function across many brain regions and functions, a hypothesis in line with the extreme diversity of psychopathology associated with the disorder and its association with a broad range of cognitive, sensory, perceptual, motor, and other impairments [ 4 , 5 , 6 ]. It is also supported by large-scale structural brain imaging studies which have demonstrated reduced brain size and widespread reductions in cortical thickness, surface area, and size of subcortical structures [ 43 , 117 ] to be associated with schizophrenia, and that a morphometric score representing deviations from the norm from 75 different brain regions could predict both schizophrenia and common variant liability to the disorder [ 118 ].
While schizophrenia as a syndrome may result from widely distributed neuronal pathology, it is likely that individual symptoms, cognitive impairments, and other features of schizophrenia are associated with dysfunction in specific brain regions or circuits with the extensive heterogeneity reflecting regional and circuit level variability in the downstream impact of these disturbances in neuronal function. If this is the case, there are important implications for research aiming to identify neurobiological endophenotypes that mediate the effects of genetic risk on behavioural or symptomatic outcomes, and drawing causal inferences may ultimately require experimental validation [ 43 ].
On a more positive note, if schizophrenia is essentially a disorder of neuronal, and particularly synaptic, function that is not confined to a specific brain regions and circuits, then it should be possible to gain mechanistic insights from animal and human cellular model systems based on genomic findings. Since models based on high-risk mutations offer the most robust starting points [ 10 ], such studies are likely to become a major focus of research efforts over the coming decade. However, researchers will need to keep in mind that the extensive pleiotropy of that class of mutation implies that what is being modelled is not a specific diagnostic entity, and that just as clinical diagnosis in a carrier depends on the common (and no doubt rare) variant liability to a range of other disorders, so will some of the model system phenotypic readouts. Moreover, from the schizophrenia perspective, the use of such variants is likely to bias towards modelling aberrant neurodevelopment as opposed to the other pathophysiological processes that are undoubtedly in play, and that potentially , may be more open to remediation.
Conclusions
In many ways, the past 15 years have seen substantial progress in understanding the genetics of schizophrenia that has yielded more insights than any other area of biological psychiatry. Many specific genes and loci have been implicated and this has begun to point towards some of the neurobiological mechanisms likely to be involved and provided a firm basis upon which mechanistic research can proceed. Genetic findings have revealed the nature of schizophrenia’s close relationship to other conditions, particularly BD and childhood NDDs and provided an explanation for how common risk alleles persist in the population in the face of reduced fecundity.
Yet, as is so often the case in science, with advance has come a greater appreciation of the challenges ahead. Current genomic strategies only potentially explain around 40% of the heritability with a much smaller proportion explained by robustly identified loci. The extreme polygenicity of schizophrenia, together with the implication of many alleles of small effect, pose challenges for attempts to understand biological mechanisms. The high degree of pleiotropy points to the need for more transdiagnostic research and the shortcomings of current diagnostic criteria as a means of delineating biologically distinct strata. It also poses challenges for inferring causality in observational and experimental studies in both humans and model systems. Finally, the Eurocentric bias of genomic studies needs to be rectified to maximise benefits and ensure these are felt across diverse communities.
Many of these challenges can be overcome by the application of new and emerging technologies, such as whole-genome and long-read sequencing, to large and diverse samples. Substantive progress in biological understanding of schizophrenia will require parallel advances in functional genomics and proteomics applied to the brain across developmental stages. However, it is our view that these efforts will only succeed in their ultimate aim of identifying disease mechanisms and defining novel strata if the increased granularity of genomic data can be combined with sufficiently granular phenotypic data. Such phenotypic data should include measures of symptom domains, as well as markers of clinical and functional outcome, all of which have the benefit that they can be applied in transdiagnostic analyses. In addition, the inclusion of candidate biomarkers, such as cognition, neuroimaging or blood-based assays, will help elucidate aetiological pathways between genetic risk and these phenotypic outcomes and have the potential for use in mechanistically informed stratification.
Data availability
No new data were generated for this article.
Owen MJ, Legge SE. The nature of schizophrenia: as broad as it is long. Schizophr Res. 2022;242:109–12.
PubMed Google Scholar
Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150:3–10.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97.
PubMed PubMed Central Google Scholar
Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18:146–61.
Perju-Dumbrava L, Kempster P. Movement disorders in psychiatric patients. BMJ Neurol Open. 2020;2:e000057.
Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16:535–50.
CAS PubMed PubMed Central Google Scholar
van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45.
Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–6.
Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going… but still not gone. Br J Psychiatry. 2010;196:92–5.
Rees E, Owen MJ. Translating insights from neuropsychiatric genetics and genomics for precision psychiatry. Genome Med. 2020;12:43.
O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–5.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–28.
O’Connor LJ, Schoech AP, Hormozdiari F, Gazal S, Patterson N, Price AL. Extreme polygenicity of complex traits is explained by negative selection. Am J Hum Genet. 2019;105:456–76.
Hindley G, Frei O, Shadrin AA, Cheng W, O’Connell KS, Icick R, et al. Charting the landscape of genetic overlap between mental disorders and related traits beyond genetic correlation. Am J Psychiatry. 2022;179:833–43.
Mangan RJ, Alsina FC, Mosti F, Sotelo-Fonseca JE, Snellings DA, Au EH, et al. Adaptive sequence divergence forged new neurodevelopmental enhancers in humans. Cell. 2022;185:4587–603.e4523.
Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–35.
CAS PubMed Google Scholar
Georgieva L, Rees E, Moran JL, Chambert KD, Milanova V, Craddock N, et al. De novo CNVs in bipolar affective disorder and schizophrenia. Hum Mol Genet. 2014;23:6677–83.
Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–5.
Rees E, Kendall K, Pardinas AF, Legge SE, Pocklington A, Escott-Price V, et al. Analysis of intellectual disability copy number variants for association with schizophrenia. JAMA Psychiatry. 2016;73:963–9.
Rees E, Kirov G. Copy number variation and neuropsychiatric illness. Curr Opin Genet Dev. 2021;68:57–63.
Calle Sanchez X, Helenius D, Bybjerg-Grauholm J, Pedersen C, Hougaard DM, Borglum AD, et al. Comparing copy number variations in a danish case cohort of individuals with psychiatric disorders. JAMA Psychiatry. 2022;79:59–69.
Rees E, Kirov G, Sanders A, Walters JT, Chambert KD, Shi J, et al. Evidence that duplications of 22q11.2 protect against schizophrenia. Mol Psychiatry. 2014;19:37–40.
Li Z, Chen J, Xu Y, Yi Q, Ji W, Wang P, et al. Genome-wide analysis of the role of copy number variation in schizophrenia risk in Chinese. Biol Psychiatry. 2016;80:331–7.
Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–41.
Howrigan DP, Rose SA, Samocha KE, Fromer M, Cerrato F, Chen WJ, et al. Exome sequencing in schizophrenia-affected parent-offspring trios reveals risk conferred by protein-coding de novo mutations. Nat Neurosci. 2020;23:185–93.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84.
Genovese G, Fromer M, Stahl EA, Ruderfer DM, Chambert K, Landen M, et al. Increased burden of ultra-rare protein-altering variants among 4877 individuals with schizophrenia. Nat Neurosci. 2016;19:1433–41.
Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–16.
Liu D, Meyer D, Fennessy B, Feng C, Cheng E, Johnson JS, et al. Schizophrenia risk conferred by rare protein-truncating variants is conserved across diverse human populations. Nat Genet. 2023;55:369–76.
Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92.
Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9.
Weiner DJ, Nadig A, Jagadeesh KA, Dey KK, Neale BM, Robinson EB, et al. Polygenic architecture of rare coding variation across 394,783 exomes. Nature. 2023;614:492–9.
Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90.
Wainschtein P, Jain D, Zheng Z, Group TOAW, Consortium NT-OfPM, Cupples LA. et al. Assessing the contribution of rare variants to complex trait heritability from whole-genome sequence data. Nat Genet. 2022;54:263–73.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9.
Jang SK, Evans L, Fialkowski A, Arnett DK, Ashley-Koch AE, Barnes KC, et al. Rare genetic variants explain missing heritability in smoking. Nat Hum Behav. 2022;6:1577–86.
Lam M, Chen CY, Li Z, Martin AR, Bryois J, Ma X, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51:1670–8.
Tsuo K, Zhou W, Wang Y, Kanai M, Namba S, Gupta R, et al. Multi-ancestry meta-analysis of asthma identifies novel associations and highlights the value of increased power and diversity. Cell Genom. 2022;2:100212.
Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51:584–91.
Bigdeli TB, Fanous AH, Li Y, Rajeevan N, Sayward F, Genovese G, et al. Genome-wide association studies of schizophrenia and bipolar disorder in a diverse cohort of US veterans. Schizophr Bull. 2021;47:517–29.
Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94.
PubMed Central Google Scholar
O’Donovan MC, Owen MJ. The implications of the shared genetics of psychiatric disorders. Nat Med. 2016;22:1214–9.
Brainstorm Consortium, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757.
Google Scholar
Border R, Athanasiadis G, Buil A, Schork AJ, Cai N, Young AI, et al. Cross-trait assortative mating is widespread and inflates genetic correlation estimates. Science. 2022;378:754–61.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Bigdeli TB, Voloudakis G, Barr PB, Gorman BR, Genovese G, Peterson RE, et al. Penetrance and pleiotropy of polygenic risk scores for schizophrenia, bipolar disorder, and depression among adults in the US veterans affairs health care system. JAMA Psychiatry. 2022;79:1092–101.
Zheutlin AB, Dennis J, Karlsson Linner R, Moscati A, Restrepo N, Straub P, et al. Penetrance and pleiotropy of polygenic risk scores for schizophrenia in 106,160 patients across four health care systems. Am J Psychiatry. 2019;176:846–55.
Leppert B, Millard LAC, Riglin L, Davey Smith G, Thapar A, Tilling K, et al. A cross-disorder PRS-pheWAS of 5 major psychiatric disorders in UK Biobank. PLoS Genet. 2020;16:e1008185.
Allardyce J, Leonenko G, Hamshere M, Pardinas AF, Forty L, Knott S, et al. Association Between Schizophrenia-Related Polygenic Liability and the Occurrence and Level of Mood-Incongruent Psychotic Symptoms in Bipolar Disorder. JAMA Psychiatry. 2018;75:28–35.
Owen MJ, O’Donovan MC. Schizophrenia and the neurodevelopmental continuum:evidence from genomics. World Psychiatry. 2017;16:227–35.
Rees E, Han J, Morgan J, Carrera N, Escott-Price V, Pocklington AJ, et al. De novo mutations identified by exome sequencing implicate rare missense variants in SLC6A1 in schizophrenia. Nat Neurosci. 2020;23:179–84.
Rees E, Creeth HDJ, Hwu HG, Chen WJ, Tsuang M, Glatt SJ, et al. Schizophrenia, autism spectrum disorders and developmental disorders share specific disruptive coding mutations. Nat Commun. 2021;12:5353.
Rijsdijk FV, Gottesman II, McGuffin P, Cardno AG. Heritability estimates for psychotic symptom dimensions in twins with psychotic disorders. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:89–98.
Legge SE, Cardno AG, Allardyce J, Dennison C, Hubbard L, Pardinas AF, et al. Associations between schizophrenia polygenic liability, symptom dimensions, and cognitive ability in schizophrenia. JAMA Psychiatry. 2021;78:1143–51.
Ruderfer DM, Fanous AH, Ripke S, McQuillin A, Amdur RL. Schizophrenia Working Group of the Psychiatric Genomics C et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19:1017–24.
Fanous AH, Zhou B, Aggen SH, Bergen SE, Amdur RL, Duan J, et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am J Psychiatry. 2012;169:1309–17.
Taylor J, de Vries YA, van Loo HM, Kendler KS. Clinical characteristics indexing genetic differences in schizophrenia: a systematic review. Mol Psychiatry. 2023;28:883–90.
Ahangari M, Bustamante D, Kirkpatrick R, Nguyen TH, Verrelli BC, Fanous A, et al. Relationship between polygenic risk scores and symptom dimensions of schizophrenia and schizotypy in multiplex families with schizophrenia. Br J Psychiatry. 2022;223:1–8.
Jonas KG, Lencz T, Li K, Malhotra AK, Perlman G, Fochtmann LJ, et al. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl Psychiatry. 2019;9:300.
Hamshere ML, O’Donovan MC, Jones IR, Jones L, Kirov G, Green EK, et al. Polygenic dissection of the bipolar phenotype. Br J Psychiatry. 2011;198:284–8.
Coombes BJ, Markota M, Mann JJ, Colby C, Stahl E, Talati A, et al. Dissecting clinical heterogeneity of bipolar disorder using multiple polygenic risk scores. Transl Psychiatry. 2020;10:314.
Quattrone D, Reininghaus U, Richards AL, Tripoli G, Ferraro L, Quattrone A, et al. The continuity of effect of schizophrenia polygenic risk score and patterns of cannabis use on transdiagnostic symptom dimensions at first-episode psychosis: findings from the EU-GEI study. Transl Psychiatry. 2021;11:423.
Bipolar Disorder Working Group of the Psychiatric Genomics Consortium. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173:1705–15.e1716.
Dennison CA, Legge SE, Hubbard L, Lynham AJ, Zammit S, Holmans P, et al. Risk factors, clinical features, and polygenic risk scores in schizophrenia and schizoaffective disorder depressive-type. Schizophr Bull. 2021;47:1375–84.
Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav. 2019;3:513–25.
Richards AL, Cardno A, Harold G, Craddock NJ, Di Florio A, Jones L, et al. Genetic liabilities differentiating bipolar disorder, schizophrenia, and major depressive disorder, and phenotypic heterogeneity in bipolar disorder. JAMA Psychiatry. 2022;79:1032–9.
McCutcheon RA, Keefe RSE, McGuire PK Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-01949-9 . Online ahead of print.
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912–9.
Hubbard L, Tansey KE, Rai D, Jones P, Ripke S, Chambert KD, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2016;42:832–42.
Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the cognitive genomics consortium (COGENT). Mol Psychiatry. 2014;19:168–74.
Wang SH, Hsiao PC, Yeh LL, Liu CM, Liu CC, Hwang TJ, et al. Polygenic risk for schizophrenia and neurocognitive performance in patients with schizophrenia. Genes Brain Behav. 2018;17:49–55.
Richards AL, Pardinas AF, Frizzati A, Tansey KE, Lynham AJ, Holmans P, et al. The Relationship Between Polygenic Risk Scores and Cognition in Schizophrenia. Schizophr Bull. 2020;46:336–44.
Mallet J, Le Strat Y, Dubertret C, Gorwood P. Polygenic risk scores shed light on the relationship between schizophrenia and cognitive functioning: review and meta-analysis. J Clin Med. 2020;9:341.
Shafee R, Nanda P, Padmanabhan JL, Tandon N, Alliey-Rodriguez N, Kalapurakkel S, et al. Polygenic risk for schizophrenia and measured domains of cognition in individuals with psychosis and controls. Transl Psychiatry. 2018;8:78.
Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–6.
Kendall KM, Bracher-Smith M, Fitzpatrick H, Lynham A, Rees E, Escott-Price V, et al. Cognitive performance and functional outcomes of carriers of pathogenic copy number variants: analysis of the UK Biobank. Br J Psychiatry. 2019;214:297–304.
Hubbard L, Rees E, Morris DW, Lynham AJ, Richards AL, Pardinas AF, et al. Rare copy number variants are associated with poorer cognition in schizophrenia. Biol Psychiatry. 2021;90:28–34.
Creeth HDJ, Rees E, Legge SE, Dennison CA, Holmans P, Walters JTR, et al. Ultrarare coding variants and cognitive function in schizophrenia. JAMA Psychiatry. 2022;79:963–70.
Rammos A, Kirov G, Hubbard L, Walters JTR, Holmans P, Owen MJ et al. Family-based analysis of the contribution of rare and common genetic variants to school performance in schizophrenia. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-02013-2 . Online ahead of print.
Song J, Yao S, Kowalec K, Lu Y, Sariaslan A, Szatkiewicz JP, et al. The impact of educational attainment, intelligence and intellectual disability on schizophrenia: a Swedish population-based register and genetic study. Mol Psychiatry. 2022;27:2439–47.
Habtewold TD, Liemburg EJ, Islam MA, de Zwarte SMC, Boezen HM, Investigators G, et al. Association of schizophrenia polygenic risk score with data-driven cognitive subtypes: A six-year longitudinal study in patients, siblings and controls. Schizophr Res. 2020;223:135–47.
Kepinska AP, MacCabe JH, Cadar D, Steptoe A, Murray RM, Ajnakina O. Schizophrenia polygenic risk predicts general cognitive deficit but not cognitive decline in healthy older adults. Transl Psychiatry. 2020;10:422.
Meier SM, Agerbo E, Maier R, Pedersen CB, Lang M, Grove J, et al. High loading of polygenic risk in cases with chronic schizophrenia. Mol Psychiatry. 2016;21:969–74.
Frank J, Lang M, Witt SH, Strohmaier J, Rujescu D, Cichon S, et al. Identification of increased genetic risk scores for schizophrenia in treatment-resistant patients. Mol Psychiatry. 2015;20:150–1.
Wimberley T, Gasse C, Meier SM, Agerbo E, MacCabe JH, Horsdal HT. Polygenic risk score for schizophrenia and treatment-resistant Schizophrenia. Schizophr Bull. 2017;43:1064–9.
Martin AK, Mowry B. Increased rare duplication burden genomewide in patients with treatment-resistant schizophrenia. Psychol Med. 2016;46:469–76.
Legge SE, Dennison CA, Pardinas AF, Rees E, Lynham AJ, Hopkins L, et al. Clinical indicators of treatment-resistant psychosis. Br J Psychiatry. 2020;216:259–66.
Kowalec K, Lu Y, Sariaslan A, Song J, Ploner A, Dalman C, et al. Increased schizophrenia family history burden and reduced premorbid IQ in treatment-resistant schizophrenia: a Swedish National Register and Genomic Study. Mol Psychiatry. 2021;26:4487–95.
Pardinas AF, Smart SE, Willcocks IR, Holmans PA, Dennison CA, Lynham AJ, et al. Interaction testing and polygenic risk scoring to estimate the association of common genetic variants with treatment resistance in schizophrenia. JAMA Psychiatry. 2022;79:260–9.
Farrell M, Dietterich TE, Harner MK, Bruno LM, Filmyer DM, Shaughnessy RA, et al. Increased prevalence of rare copy number variants in treatment-resistant psychosis. Schizophr Bull. 2022;49:881–92.
Ruderfer DM, Charney AW, Readhead B, Kidd BA, Kahler AK, Kenny PJ, et al. Polygenic overlap between schizophrenia risk and antipsychotic response: a genomic medicine approach. Lancet Psychiatry. 2016;3:350–7.
Zoghbi AW, Dhindsa RS, Goldberg TE, Mehralizade A, Motelow JE, Wang X, et al. High-impact rare genetic variants in severe schizophrenia. Proc Natl Acad Sci USA. 2021;118:e2112560118.
Power RA, Kyaga S, Uher R, MacCabe JH, Langstrom N, Landen M, et al. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry. 2013;70:22–30.
Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? Behav Brain Sci. 2006;29:385–404.
Keller MC. Evolutionary perspectives on genetic and environmental risk factors for psychiatric disorders. Annu Rev Clin Psychol. 2018;14:471–93.
Rees E, Moskvina V, Owen MJ, O’Donovan MC, Kirov G. De novo rates and selection of schizophrenia-associated copy number variants. Biol Psychiatry. 2011;70:1109–14.
Pardinas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50:381–9.
Escott-Price V, Pardinas AF, Santiago E, Walters J, Kirov G, Owen MJ, et al. The relationship between common variant schizophrenia liability and number of offspring in the UK Biobank. Am J Psychiatry. 2019;176:661–6.
Gazal S, Finucane HK, Furlotte NA, Loh PR, Palamara PF, Liu X, et al. Linkage disequilibrium-dependent architecture of human complex traits shows action of negative selection. Nat Genet. 2017;49:1421–7.
Li M, Wu DD, Yao YG, Huo YX, Liu JW, Su B, et al. Recent positive selection drives the expansion of a schizophrenia risk nonsynonymous variant at SLC39A8 in Europeans. Schizophr Bull. 2016;42:178–90.
Owen MJ, O’Donovan MC. Large-scale genomics: a paradigm shift in psychiatry? Biol Psychiatry. 2021;89:5–7.
Weinberger DR. The pathogenesis of schizophrenia: A neurodevelopmental theory. In Nasrallah HA, Weinberger DR (eds) The Neurology of Schizophrenia. Amsterdam: Elsevier Science Publishers; 1986, pp 397–406.
Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295:681–2.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44.
Demontis D, Walters GB, Athanasiadis G, Walters R, Therrien K, Nielsen TT, et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet. 2023;55:198–208.
Satterstrom FK, Walters RK, Singh T, Wigdor EM, Lescai F, Demontis D, et al. Autism spectrum disorder and attention deficit hyperactivity disorder have a similar burden of rare protein-truncating variants. Nat Neurosci. 2019;22:1961–5.
Tansey KE, Rees E, Linden DE, Ripke S, Chambert KD, Moran JL, et al. Common alleles contribute to schizophrenia in CNV carriers. Mol Psychiatry. 2016;21:1153.
Cleynen I, Engchuan W, Hestand MS, Heung T, Holleman AM, Johnston HR, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion. Mol Psychiatry. 2021;26:4496–510.
Bergen SE, Ploner A, Howrigan D, Group CNVA, the Schizophrenia Working Group of the Psychiatric Genomics C, O’Donovan MC, et al. Joint contributions of rare copy number variants and common SNPs to risk for schizophrenia. Am J Psychiatry. 2019;176:29–35.
Warrier V, Zhang X, Reed P, Havdahl A, Moore TM, Cliquet F, et al. Genetic correlates of phenotypic heterogeneity in autism. Nat Genet. 2022;54:1293–304.
Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–87.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry. 2015;77:52–58.
Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–53.
Pocklington AJ, Rees E, Walters JT, Han J, Kavanagh DH, Chambert KD, et al. Novel findings from CNVs implicate inhibitory and excitatory signaling complexes in schizophrenia. Neuron. 2015;86:1203–14.
van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53.
Lancaster TM, Dimitriadis SI, Perry G, Zammit S, O’Donovan MC, Linden DE. Morphometric analysis of structural MRI using schizophrenia meta-analytic priors distinguish patients from controls in two independent samples and in a sample of individuals with high polygenic risk. Schizophr Bull. 2022;48:524–32.
Download references
The work was supported by a Medical Research Council Centre grant MR/L010305/1 and programme grant MR/P005748/1, a UKRI Future Leaders Fellowship Grant MR/T018712/1 (to ER) and a grant from NIMH (Award U01MH109514). The content is the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Author information
Authors and affiliations.
Centre for Neuropsychiatric Genetics and Genomics, Division of Psychological Medicine and Clinical Neurosciences, Cardiff University, Cardiff, UK
Michael J. Owen, Sophie E. Legge, Elliott Rees, James T. R. Walters & Michael C. O’Donovan
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to writing this paper.
Corresponding authors
Correspondence to Michael J. Owen or Michael C. O’Donovan .
Ethics declarations
Competing interests.
ER, JTRW, MCO and MJO reported receiving grants from Akrivia Health outside the submitted work. JTRW, MJO and MCO reported receiving grants from Takeda Pharmaceutical Company Ltd outside the submitted work. Takeda and Akrivia played no part in the conception, design, implementation, or interpretation of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary table 1, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Owen, M.J., Legge, S.E., Rees, E. et al. Genomic findings in schizophrenia and their implications. Mol Psychiatry 28 , 3638–3647 (2023). https://doi.org/10.1038/s41380-023-02293-8
Download citation
Received : 28 April 2023
Revised : 02 October 2023
Accepted : 03 October 2023
Published : 18 October 2023
Issue Date : September 2023
DOI : https://doi.org/10.1038/s41380-023-02293-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genomic insights into the shared and distinct genetic architecture of cognitive function and schizophrenia.
- Olivia Wootton
- Alexey A. Shadrin
- Shareefa Dalvie
Scientific Reports (2024)
Blunted brain responses to neutral faces in healthy first-degree relatives of patients with schizophrenia: an image-based fMRI meta-analysis
- Anna M. Fiorito
- Giuseppe Blasi
- Guillaume Sescousse
Schizophrenia (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals
You are here
- Volume 11, Issue 10
- Occupational therapy interventions for adults with severe mental illness: a scoping review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- María Rocamora-Montenegro 1 ,
- http://orcid.org/0000-0001-5324-1535 Laura-María Compañ-Gabucio 1 , 2 ,
- http://orcid.org/0000-0001-5742-2704 Manuela Garcia de la Hera 1 , 2 , 3
- 1 Department of Public Health History of Science and Gynaecology , Universidad Miguel Hernandez de Elche , Sant Joan d'Alacant , Alicante , Spain
- 2 ISABIAL , Instituto de Investigación Sanitaria y Biomédica de Alicante , Alicante , Comunidad Valenciana , Spain
- 3 Consortium for Biomedical Research in Epidemiology and Public Health (CIBERESP) , Madrid , Spain
- Correspondence to Laura-María Compañ-Gabucio; lcompan{at}umh.es
Objective To identify the occupational therapy (OT) interventions in adults with severe mental illness (SMI) most investigated in intervention studies and to describe their characteristics.
Design Scoping review.
Data sources On 17 January 2020, we searched the following electronic databases: MEDLINE, Scopus, Web of Science and EMBASE. We also performed a manual search of TESEO doctoral thesis database and of the journals indexed in the first quartile of OT according to the SCImago Journal Rank. We updated our search on 10 March 2021, performing a complementary search on ProQuest database and repeating the search in all sources. The terms included in the search strategy were: schizophrenia, schizotypal personality, delusional, schizoaffective, psychotic, bipolar, major depression, obsessive–compulsive, severe mental, OT and intervention.
Study selection The study screening was peer-reviewed. Inclusion criteria were: (1) OT intervention studies in SMI: experimental, randomised, non-randomised and pilot/exploratory studies; (2) adult population with SMI: schizophrenia, schizotypal personality disorder, delusional disorder, obsessive–compulsive disorder, schizoaffective disorder, psychotic disorder, bipolar disorder, major depressive disorder; (3) OT identified as a discipline involved in the intervention; (4) English or Spanish language and (5) studies with full text available.
Results Thirty-five studies met the inclusion criteria. OT interventions were classified in psychosocial, psychoeducational, cognitive and exercise interventions. The most used OT intervention was psychosocial intervention.
Conclusion Psychosocial intervention was the most investigated OT intervention in SMI, followed by psychoeducational, cognitive and exercise interventions. These interventions are usually group interventions in patients with schizophrenia, performed by a multidisciplinary team (in which an occupational therapist collaborates), with 2–3 weekly 60 min sessions and a duration of 3–6 months.
- mental health
- schizophrenia & psychotic disorders
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. No additional data available, all data relevant to the study are included in the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2020-047467
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
There is little evidence regarding occupational therapy intervention in severe mental illness.
We gave a detailed description of four types of occupational therapy intervention in severe mental illness.
We conducted a peer-reviewed database search to ensure comprehensiveness.
We did not assess the quality of the studies included.
We did not include studies on addiction, anxiety or eating disorders.
Introduction
Mental disorders represent a major issue, constituting the most frequent cause of disease burden in Europe. 1 In Spain, it is estimated that at least 9% of the population is affected by a mental disorder, apart from those caused by substance abuse; and slightly more than 15% will suffer from one throughout their lives. 2 Severe mental illnesses (SMIs) are the most limiting mental disorders, and those with these conditions, according to the National Institute of Mental Health of the USA, are defined as ‘a group of heterogeneous people, who suffer from serious psychiatric disorders that present with long-lasting mental disorders, which carry a variable degree of disability and social dysfunction, and which must be cared for through various social and health resources of the psychiatric and social care network’. 3
The disorders that are included in SMI are schizophrenia, schizotypal personality disorder, delusional disorder, schizoaffective disorder, psychotic disorder, bipolar disorder, major depressive disorder and obsessive–compulsive disorder. 4 Among the most frequent limitations that people with SMI experience is a lower participation in healthy activity patterns, including active and significant participation in the community, unemployment, self-care and sleep disturbances. 5 6
Treatment for people with SMI requires, therefore, the integration of different levels of care and different interventions that include, in addition to pharmacological treatment, rehabilitation and social support programmes that allow them to participate in the community in a more independent and integrated way. 7 One of these non-pharmacological interventions is occupational therapy (OT), which can support recovery as a significant treatment component of these patients through meaningful activities, influencing aspects such as autonomy in activities of daily living (ADL), quality of life and personal well-being. 8–10 In fact, a recent scoping review showed that different factors such as employment, may influence the recovery process of people with SMI. 11 OT through vocational rehabilitation such as supported employment intervention could improve SMI patients’ social functioning and hospitalisation, although not all SMI patients are motivated to work. 12
Although scientific evidence regarding the OT interventions in patients with SMI is scarce, some studies suggest that these interventions have a beneficial effect. Arbesman and Logsdon 13 carried out a systematic review in which they described a greater involvement in education and employment of people with SMI who were intervened with OT focused on social participation. Similarly, Conn et al 14 showed OT to be a key intervention for weight loss in people with SMI, improving their motivation and helping them to acquire healthy lifestyles.
Currently, SMI constitute a significant health problem that imposes daily limitations on those who suffer from them. In the field of OT, although there are various interventions to increase the autonomy of people with SMI and decrease their everyday restrictions, these interventions are very diverse and supported by little scientific evidence. In this sense, this scoping review is necessary to provide a detailed summary of the different OT interventions in SMI to facilitate the elaboration of evidence-based intervention programmes. Thus, we seek to answer the following research question: Which OT interventions in adults with SMI have been most investigated in intervention studies and how they are? The objective of this review was to identify the OT interventions in adults with SMI most investigated in intervention studies and to describe their characteristics.
Methodology
We performed a peer scoping review whose content was reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for Scoping Reviews. 15 In addition, it was conducted following the indications of the Cochrane Manual 16 and previously developed guidelines. 17 18 Specifically, we used the Cochrane Manual to elaborate the results section and the main tables of this scoping review. We consulted the ‘3.4.1 Description of studies’ section of chapter 3 to know how to present the main characteristics of the included studies, and the ‘3.4.3 Effects of interventions’ section of chapter 3 to know how to present the characteristics of the OT interventions in SMI described in the included studies, adequately. As the Cochrane Manual recommendations are specific to systematic reviews, we contrasted these recommendations with those indicated in other specific scoping reviews guidelines/frameworks. 17 18 We did not prepare a draft or publish a protocol for this scoping review.
Search strategy and review criteria
On 17 January 2020, we consulted the databases MEDLINE (PubMed), Scopus, Web of Science and EMBASE. These databases are widely used in review studies and the majority of them are included in the optimal database combination search 19 which guarantee an adequate and efficient coverage of the scientific literature. This was supplemented by manual searching of journals indexed in the first quartile of OT according to the SCImago Journal Rank in 2018: American Journal of Occupational Therapy, Journal of Occupational Rehabilitation and Occupational Therapy Journal of Research . We excluded the Journal of Physical and Occupational Therapy in Pediatrics (POTP) as it belongs to the paediatric community, a criterion for exclusion from this review. In addition, grey literature was hand searched in TESEO which is a Spanish doctoral thesis database. We used the same search strategy in all databases and journals consulted, using all the disorders included in SMI, ‘OT’, and ‘intervention’ as search terms, with Boolean operators ‘OR’ and ‘AND’ ( table 1 ).
- View inline
Database and search strategies
In order to update and complement our search process, we consulted the Psychology Database from ProQuest on 10 March 2021. This database provides abstracts and articles from key Psychology journals, many of which are indexed in PsycINFO. In addition, we reran our search strategy in all databases and journals to identify articles published from January 2020 to March 2021.
The inclusion criteria in this review were: (1) OT intervention studies in SMI: experimental, randomised, non-randomised and pilot/exploratory studies; (2) adult population with SMI: schizophrenia, schizotypal personality disorder, delusional disorder, obsessive–compulsive disorder, schizoaffective disorder, psychotic disorder, bipolar disorder, major depressive disorder; (3) OT identified as a discipline involved in the intervention; (4) English or Spanish language and (5) studies with full text available. Those studies that did not meet the established inclusion criteria were excluded.
Study selection, data extraction and synthesis
We downloaded all titles and abstracts retrieved from all searches using Microsoft Excel. Two reviewers screened and selected the articles independently. One of them (MR-M) identified and removed duplicate records, and then two review authors (L-MC-G and MR-M) independently examined titles and abstracts and removed any irrelevant papers. Finally, L-MC-G and MR-M examined the full texts for study compliance with review eligibility criteria. A third review author (MGdIH) resolved discrepancies between these authors regarding study inclusion. We did not critically assess the quality of the included studies because it is not required in scoping review 15 17 18 and also because our objective was not to evaluate the efficacy or effectiveness of the OT interventions in SMI. However, the main limitations found in each included study are described in online supplemental table 1 and discussed in the results section.
Supplemental material
A data charting model and item definitions were drafted a priori by all authors. We used Microsoft Excel to create an ‘Excel data form’. We conducted data extraction independently using the Excel data form and presented the characteristics of included studies following the Cochrane Manual, 16 detailing author/s and year of publication, type of study, sample, OT interventions carried out, results and limitations.
We carried out a descriptive synthesis of the results. Tables and figures were used (where possible) to present the flow of study selection process and the characteristics of the included studies. In addition, as a multidisciplinary research team, we discussed the categories to classify the different types of OT interventions in SMI that are used in the included studies.
Patient and public involvement
No patients or public were involved in this review.
The initial search retrieved 1217 published articles on OT intervention in SMI, which resulted in 790 after removing duplicate articles. Fifty-four studies met the inclusion criteria in abstract peer review and went on to full-text review. In this initial search, we extracted data from 12 articles which fulfilled the inclusion criteria for this scoping review. The complementary search on ProQuest retrieved 2068 published articles and the updated search on initial databases and journals retrieved 149 published articles, 23 of which fulfilled the inclusion criteria. In total, we extracted data from 35 published articles on OT intervention in SMI. The study selection flowchart is shown in figure 1 .
- Download figure
- Open in new tab
- Download powerpoint
Flowchart of the study selection process. AJOT, American Journal of Occupational Therapy; JOR, Journal of Occupational Rehabilitation; OTJR, Occupational Therapy Journal of Research.
Below we present the results regarding the characteristics of the studies included in this scoping review ( online supplemental table 1 ) in addition to the characteristics of the OT interventions in SMI studied in the included articles ( online supplemental table 2 ).
Main characteristics of included studies
The main characteristics of included studies are summarised in online supplemental table 1 . Fifteen of the studies were carried out in Asia, 11 in Europe, 6 in North America, 1 in South America, 1 in Oceania and 1 in South Africa. Fifteen of the articles included are randomised controlled trials, 20–34 10 are quasi-experimental studies, 35–44 5 are a non-randomised experimental study 45–49 and 5 are pilot studies. 50–54
OT interventions in SMI
We present below the specific characteristics of the OT interventions in SMI to answer our research question in detail. The specific characteristics of included studies, such as the type of intervention, the duration of the intervention, the number of sessions performed or the measurement instruments used, are shown in online supplemental table 2 .
First, we explored if the investigators used the same type of intervention in both the control and intervention groups ( online supplemental table 2 ). In most studies (n=18), standard OT intervention or pharmacological treatment was performed in both the control and intervention groups. 20 23 24 26–28 30 32 33 40 42 44 47 48 50–53 However, in the intervention group, treatment was reinforced by specific OT interventions in SMI, including: a home-based rehabilitation programme, 20 a social cognition enhancement programme, 23 an OT programme focused on work reintegration, 24 a collaborative journal, 26 a computerised cognitive programme, 27 an emotion regulation skills programme, 28 a group programme for balance in ADL, 30 individualised OT, 32 dance therapy, 33 training in shopping skills, 40 activity-based OT, 42 OT narrative medicine, 44 weight loss psychiatric rehabilitation, 47 metacognitive training, 48 a prevocational programme, 50 a programme to reconnect patients with a significant activity, 51 an OT programme focused on expressive activities 52 and an early OT intervention. 53
In the remaining studies, both the control and intervention groups participated in a different programme (n=10), 21 25 29 31 34 36 38 41 46 54 all participants received the same intervention (n=4) 37 39 40 43 or participants were divided in three different study groups (n=3) ( online supplemental table 2 ). 22 45 49 In studies with both the control and intervention groups we found interventions such as an instrumental enrichment programme versus standard OT, 21 a programme focused on the management of the disease versus traditional OT, 25 a physical exercise programme versus traditional OT, 29 a metacognitive programme versus traditional OT, 31 a home-visit OT programme versus a management tool for daily life performance programme, 34 a recovery education programme versus traditional mental health treatment, 36 an aerobic dance programme versus a manual activities programme, 38 a balancing everyday life programme versus traditional OT, 41 a motivational intervention versus traditional OT 46 and a programme focused on executive functions versus a programme based on handmade activities. 54 In the included studies with a unique study group in which all participants were treated, we found interventions such as indoor and outdoor exercise programme, 37 a ‘therapeutic package’, 39 care as usual and cognitive–behavioural therapy 40 and a psychoeducation for schizophrenia programme. 43 Finally, three studies included three study groups to compare two different interventions with a control group: cognitive remediation therapy versus intensive OT versus healthy patients, 22 project activity group versus discussion group versus no treatment, 45 and OT at the community mental health centre (CMHC) versus OT at CMHC+psychosocial skill training versus outpatient follow-up. 49
Second, we analysed what type of SMI was treated in each study and which was the role of the occupational therapist in the intervention team ( online supplemental table 2 ). Schizophrenia was the most frequent object of study among the selected studies (n=25), 20–23 25 27 31–36 38 40 43 46 48–54 followed by schizoaffective disorder (n=10), 23 25 27 31 32 34 45 50 51 53 major depression (n=6), 24 28 29 34 36 42 a broad spectrum of disorders or non-specific SMI (n=5) 30 37 41 44 47 and bipolar disorder (n=3). 26 36 49 In all the articles included, an occupational therapist formed part of the professional team, mainly as part of a multidisciplinary team composed of psychologists, nurses, dieticians, physicians, sports therapists, psychiatrics, physiotherapists, informal caregivers, pharmacists or social workers (n=18), 20 23 24 26–33 36 37 43 47 50 54 and secondarily alone (n=17). 21 22 25 34 38–42 44–46 48 49 51–53
Third, as we have shown in online supplemental table 2 and as described below, the articles analysed were classified into four clearly differentiated interventions, except one study. 53 In this study the intervention used was conventional OT in schizophrenia and schizoaffective disorder. This intervention was led exclusively by an occupational therapist, the programme lasted 12 weeks with 2–5 weekly 30-min sessions and it included exercise, craft and daily life skills activities.
We classified the included studies in the following four interventions:
Psychosocial intervention
Psychosocial intervention was the most used OT intervention in the included studies (n=14). 20 26 30 32 34 40–42 45 46 49 50 52 54 In general, these interventions are performed exclusively by occupational therapists, but in five studies this intervention was performed by a multidisciplinary team made up of occupational therapists, psychologists, social workers, informal caregivers, psychiatrists or nurses. 20 26 30 50 54 The main objectives of psychosocial intervention were to improve the symptoms of the disorders and occupational balance, as well as the social and work reintegration of patients with SMI. Among the different SMI treated with this intervention, psychosocial intervention was applied mainly in schizophrenia (n=8), 20 34 40 45 46 49 52 55 schizoaffective disorder (n=4), 32 34 45 50 bipolar disorder (n=2), 26 49 in a broad spectrum of disorders (n=2) 30 41 and major depressive disorder (n=2). 25 34
The intervention programmes lasted between 3 and 9 months, and the sessions were mainly between 60 and 90 min long, although in three articles 40 42 45 the duration of the programme was notably shorter, lasting only 2 and 4 weeks. In turn, it should be noted that in three of the studies 26 30 41 only 1 weekly session was applied, while the rest 32 34 40 42 45 46 49 50 52 54 varied between 2 and 5 sessions per week. In one of the studies the number of session was not specify. 20 This intervention was generally carried out in a group (n=8), 26 30 40–42 45 50 55 only in six studies was it carried out individually. 20 32 34 46 49 52
Psychoeducational intervention
Psychoeducational intervention was the second most used intervention in the studies included in this review (n=9). 24 25 27 35 36 39 43 44 51 Only in four studies, the intervention was performed exclusively by an occupational therapist. 25 39 44 51 The main objectives of this intervention were to improve disease management, to increase social abilities such as non-verbal techniques, and for the patient to acquire a significant activity, such as reading. The principal disorder treated in these interventions was schizophrenia, although in three studies were schizoaffective disorder, 25 51 in one major depression 24 and in one a broad spectrum of disorders. 44
The intervention programmes lasted between 3 and 9 months, and the sessions were mainly between 50 and 90 min long, although in one article the duration of the session was 120 min 24 and in other two articles the duration of the sessions was not specify. 43 51 In two articles the duration of the programme was notably shorter, lasting only 2 25 and 4 weeks, 43 while in one article the duration of the programme was notably longer, lasting 12 months. 36 In four of the studies 33 43 44 51 only 1 weekly session was applied, while the rest 24 25 27 36 39 varied between 2 and 5 sessions per week. This intervention was generally carried out in a group (n=7), 24 27 35 36 39 43 44 only in two studies was it carried out individually. 25 51
Cognitive intervention
Cognitive intervention was the third most used intervention in the studies included in this review (n=7). 21–23 28 31 38 48 In four articles the intervention was carried out exclusively by an occupational therapist. 21 22 38 48 The main objective of cognitive intervention was to improve cognitive functions and processing strategies. The principal disorder treated with these interventions was schizophrenia, although in one study was it major depression. 28
The duration of the intervention programmes was from 1 to 3 months, although in one of the studies the duration was 6 months. 23 The sessions lasted between 45–60 min, but in one study 26 they lasted for up to 2 hours, in other they lasted ninety minutes, 23 and in other the duration of the intervention programme was not specify. 31 In general, in all the interventions, the sessions were carried out 2–5 times a week, except in one study 31 where only 1 weekly session was applied. This intervention was generally carried out in a group (n=5), 23 28 31 38 48 only in two studies was it carried out individually. 21 22
Exercise intervention
Less frequently, an exercise intervention was used (n=4). 29 33 37 47 In all of these studies the intervention was carried out exclusively by a multidisciplinary team made up of occupational therapists, sport therapists, physicians, sport psychologists, psychiatrics or dieticians. The main objectives of exercise interventions were to compensate cognitive impairment common in psychiatric disabilities, to increase the knowledge and understanding of rules and to strengthen participants’ ability to work as part of a team. In two studies the SMI treated was not specify, 37 47 in one schizophrenia was treated, 33 and in one major depression was treated. 29
The duration of the intervention programmes was 3 months, 37 47 although in one of the studies the duration was 2 months, 33 and in another the duration was only 1 month. 29 The sessions lasted 30, 29 40–50, 33 60 37 and 120 min. 47 In general, the sessions were carried out 2–3 times a week, except in one study 47 where only 1 weekly session was applied. This intervention was carried out in a group in all four studies. 29 33 37 47
Finally, we explored the measurement instruments used to assess the effect of the interventions performed in each study to facilitate the elaboration of evidence-based intervention programmes. As we have shown in online supplemental table 2 , different questionnaires and scales were used. Among the included studies, the use of measuring instruments on the symptoms of the disease, mood and executive functions stands out.
Symptoms of the disease
Ten studies used Positive and Negative Symptoms Scale (PANSS) to assess the symptoms of the disease, 27 28 31–33 43 48–50 54 one used Andreasen’s scale for assessment of negative symptoms and Andreasen’s scale for assessment of positive symptoms, 52 and one used The Young Mania Rating Scale to asses maniac symptoms. 26
To assess mood, that is, depression, authors used several measurement instruments and scales, such as the Montgomery Asberg Depression rating scale, the Calgary Depression Scale for Schizophrenia, the Brief Psychiatric Rating Scale, the Beck Depression Inventory or the Hamilton Depression Rating Scale. 24 26–29 31 36 53
Executive function
In addition, investigators used a variety of measurement instruments to assess executive functions, including the Trail Making Test Parts A and B, the Brief Assessment of Cognition in Schizophrenia, the Behavioural Assessment of the Dysexecutive Syndrome, the N-Back Task and the Executive Function Performance Test. 20 22 32 38 40 50 54
Other outcomes
To a lesser extent, other questionnaires were used to evaluate memory 20–22 27 29 38 45 50 such as Wechsler Adult Intelligence Scale, the General Aptitude Test Battery, Rey Auditory Verbal Learning Test, the Rey’s Complex Figure or Mini-mental state examination; psychosocial functioning 21 30 32 34 39 49 such as the Global Assessment of Functioning, the Personal and Social Performance or the Social Functioning Scale and quality of life 25 27 30 34 39 41 42 such as the 36-Item Short-Form Health Survey (SF-36) questionnaire, the General Health Questionnaire and the Manchester Short Assessment of Quality of Life.
Main results of included studies
We summarised the main results of OT interventions in SMI in online supplemental table 1 . In general, intervention groups obtained better results than control groups in all the studies, although in five of the studies included both intervention and control groups presented better results after intervention. 24 27 34 35 37 40 43 Authors showed that the interventions carried out in their studies resulted in significant improvements in aspects such as participation and social functioning (n=19), 20 21 23–25 30 32–34 39 40 42 44–46 48 49 51 54 cognitive functioning (n=11), 21 22 29 31 32 35 36 40 47 50 53 that is, executive function and memory; general symptoms (n=8) 27–30 33 49 50 52 and well-being (n=5), 28 36 37 43 47 although, in three studies, these improvements were no longer presented during follow-up. 24 30 36 In fact, it should be noted that in only three of the included studies, 26 38 41 the improvements found were not statistically significant.
Main limitations reported in included studies
All the studies reported limitations ( online supplemental table 1 ). Most of the studies included in this review have a small sample size (n=22), 20 22–25 29 31 33 37–40 44 45 47 49–54 have not evaluated the long-term effects of the intervention (n=11), 20 24 32 33 35 39 42 45 49 51 53 are non-blinding studies (n=10), 26 27 30 32 34 38 40 42 49 53 have results which are not generalisable (n=7), 22 33 37 40 42 47 53 have a lack of randomisation (n=5) 36 38 47 48 50 or they do not have a comparison group (n=6). 33 35 37 39 43 50
The present scoping review aimed to identify the most investigated OT interventions in adults with SMI in intervention studies and to describe their characteristics. We explored the scientific evidence available in this regard in several databases and journals, in which we found 35 articles with different types of interventions in which occupational therapists collaborated. We found four clear types of OT intervention in SMI: psychosocial, psychoeducational, cognitive and exercise interventions. The articles included in this review provide insight into the current characteristics of OT interventions in people with SMI and could provide occupational therapists with new ideas and perspectives for the implementation, development and evaluation of their interventions.
In this review, more than half (60%) of the selected articles were published in the last decade. These results may show that although recent evidence regarding OT interventions in a mental health setting is limited, there has been an increasing number of publications related to SMI over recent years. Moreover, the oldest articles included in this review are from the year 1999 20 45 which appears to show that OT is not a relatively new healthcare discipline, but that scientific research in the field of OT has been carried out for several years. This research started very early, in fact The World Federation of Occupational Therapists meetings began in 1951, 55 and in some countries, like Spain, the first health department including an OT service was set up in 1969. 56
In general, the included articles showed that OT intervention had beneficial results in several SMI patients’ health outcomes such as cognition, social skills or mood. These positive results could be the consequence of publication bias or the consequence of the study limitations such as small sample size, lack of randomisation or non-blinded researchers, which could compromise their validity. However, the significance of the associations found in the included articles should not be influenced by these limitations. In fact, some reviews have pointed out the effectiveness of OT interventions in SMI aimed at facilitating work, 13 community integration 57 or weight loss. 58 Moreover, OT has been identified as a non-pharmacological approach that can be an important adjunct to other psychiatric treatments. 8
In this review, the most widely described OT intervention in SMI among the included studies was the psychosocial intervention followed by psychoeducational, cognitive and exercise intervention. One reason could be that psychosocial impairments should rather be seen as a consequence of chronic mental illness. 59 Their improvement and a patient’s greater ability to participate socially are the central treatment goals. How well this can be achieved and through which intervention must be investigated in scientific studies. Another reason could be the fact that we only included those articles where occupational therapists were one of the professionals who performed the interventions in SMI. In this sense, OT is a discipline that rehabilitates the patient through the use of occupation and meaningful activities so that they can acquire the greatest level of autonomy and daily life functioning. 60 Thus, it is possible that occupational therapists use psychosocial and psychoeducational interventions more frequently than other professionals, since social limitations are not only one of the most relevant symptoms of SMI but are also closely related to an impairment in daily life functioning. 61 Cognitive or exercise interventions, on the other hand, are probably performed more frequently by other professionals such as psychologist or physicians. In fact, in this review, the intervention was led exclusively by an occupational therapist in seventeen articles, nine of which were psychosocial interventions, 32 34 40–42 45 46 49 52 and four psychoeducational interventions. 25 39 44 51
Psychosocial, psychoeducational, cognitive and exercise interventions were the main interventions that we found based on our search strategy and inclusion criteria. However, there are other interventions that can be used in SMI from OT such as vocational, individual placement and support (IPS) and place first then train interventions. 62 63 These interventions are usually aimed at helping people with SMI to find and maintain competitive employment as well as promote recovery and overcome barriers to participation in their jobs . 62 64 An explanation for the non-inclusion of these types of interventions may be the fact that we only included those articles in which the occupational therapist was involved in the intervention and this was clearly specified. It would be interesting to conduct more review studies that specifically address this type of interventions.
Based on the synthesis of information on the characteristics of the interventions carried out in the articles included, we could say that a ‘typical’ OT programme intervention in SMI can include the following characteristics: group intervention in patients with schizophrenia, performed by a multidisciplinary team (in which an occupational therapist collaborates), with 2–3 weekly 60 min sessions, and a duration of between 3 and 6 months. None of the articles contained an explanation as to why they chose these characteristics for their intervention programmes, but most of the articles mentioned that the interventions were carried out in private mental health centres, so these characteristics may be influenced by the regulations/policy of each centre. SMI symptomatology, that is, social difficulties, represents another possible factor that may influence the characteristics of the interventions; carrying out a group intervention could favour the patient’s opportunities for peer contact and emotional, practical and peer support, within a safe environment for them. 65
In general, regardless of the type of intervention performed in each study, the results of the articles included in this review showed positive effects of OT interventions. Psychosocial interventions resulted in improvements in the symptoms, occupational balance and sociooccupational reintegration of the patients. Other studies supported these improvements, especially of psychosocial interventions based on activity and lifestyle, and those focused on vocational and occupational rehabilitation. 66–68 Psychoeducational intervention showed favourable results in these people’s self-perceived health and social participation. Similarly showed Doroud et al 69 and Petersen et al , 70 who pointed out that participating in meaningful activities is experienced as a break from the discomfort caused by symptoms and as a means to rediscover forgotten resources and reconnect with daily life. Cognitive interventions led to improvements in memory and executive functions and consequently in SMI patients’ functionality and participation. These results are in line with those found by Wykes et al 71 which showed that an intervention based on cognitive remediation could reduce cognitive deficits, achieving a short-term impact on social functioning. Exercise interventions improved well-being, alertness and depression symptoms. Similar results were found in recent published studies 72–74 and additionally, was found a relationship between exercise interventions and healthy lifestyles acquisition 74
The measurement instruments used in the included articles to assess these outcomes varied widely between studies. Therefore, providing a synthesis of the information regarding this characteristic of the OT intervention in SMI was practically impossible for us. In general terms, PANSS was the most widely used scale among the included studies. This is consistent with the rest of the results of this scoping review if we consider that it is a specific instrument widely used to assess the presence of symptoms in schizophrenia, 75 which is this the most studied type of SMI in this scoping review. Moreover, this was not the only test used to assess the illness symptoms, which were the main health outcome assessed among the included studies. Considering that psychosocial intervention was the most used intervention, we expected to find social skills as the second main health result assessed in the included studies but, instead, it was mood, that is to say, depression, followed by executive. Interestingly, mood assessment was generally performed on articles retrieved from the ProQuest psychology database (information not shown). We found that mood was one of the most studied outcomes in the included studies, and it may be partly explained by the fact that people with SMI often experience stigma which can produce consequences that can be related to low mood, such as burden, feelings of embarrassment or shame and poor quality of life . 76 In addition, people with SMI often present other chronic conditions that coexist with the SMI, 77 which can also be related to mood impairment.
We highlight the implications of this review for the practice of OT and similar professionals. This scoping review provides occupational therapists with tools that facilitate the development of OT intervention sessions in SMI by knowing in advance some characteristics of these four types of intervention: psychosocial, psychoeducational, cognitive and exercise. Somehow, this updated summary of the scientific evidence that exists on SMI interventions could be useful for occupational therapists to perform evidence-based OT, although the information presented in this review should be interpreted with caution because we did not assess the quality of the included studies.
Strengths and limitations
This scoping review presents some limitations that may influence the results obtained. Although a systematic peer review was used to ensure scientific rigour, the lack of completeness of the information reported, the publication bias limiting null results intervention and selection bias are limitations for the majority of reviews. Regarding the inclusion criteria, we only included those studies published in Spanish or English and with full text available, we may, therefore, not have included significant articles because they were published in another language, this may be a potential source of bias. In addition, it was difficult to establish the search strategy because the disorders included in SMI spectrum were not clearly defined in published articles. Thus, we decided to use the WHO definition of SMI, which includes schizophrenia and related conditions, bipolar disorder and moderate and severe depression. 78 This could lead to the non-inclusion of other relevant articles whose study population was other mental illnesses that could also be serious such as anxiety, addiction, personality disorders or eating disorders. Moreover, we only included in this review those articles where occupational therapists were one of the professionals who performed the interventions in SMI. Thus, we may not have included some articles in which occupational therapist was involved in the intervention but this was not clearly specified in the study, which favoured the selection bias. In this sense, we have not included studies in which IPS, vocational or first place then train interventions were used, which may lead to an incomplete overview of current OT interventions in SMI. Regarding the studies included in this review, it is possible that they contained biases associated with the experimental study design, which was the only type of study included in this review. In addition, we did not assess the quality of the final selected articles, so we could have included some articles with low methodological quality. However, we collected and presented the main limitations reported in included studies in an attempt to provide readers with information closely related the quality of the studies. Furthermore, not all the articles included measure the same variables or use the same measurement instruments. Although our objective was not to statistically analyse the numerical results, the great variety of measurement instruments used made difficult to compare the results between studies and to draw conclusions. Thus, the results of this scoping review must be interpreted with caution.
However, this review also has several strengths. This is a necessary and original review, because to our knowledge, there is no other review whose aim was to describe the OT interventions which are most often used in intervention studies. In addition, scoping reviews stands out for their ability to identify knowledge gaps on the subject of study, which provides opportunity for future research. This review highlights the following knowledge gaps: (1) to our knowledge, there are no OT intervention studies in SMI in Spain; (2) most of the studies had limitations that could compromises the validity of their results, such as: small sample size and lack of randomisation, (3) most of the included studies are supported by little evidence of the effects of long-term interventions; (4) there is a wide variety of measurement instruments that differ between studies and (5) there is a low representation of IPS, vocational and place first then train interventions studies in which the role of the occupational therapist was clearly specified. The results of this scoping review may provide a useful theoretical basis on which to develop new OT interventions in SMI. Especially for researchers developing interventions based on The Medical Research Council (MRC) Framework, 79 who can use the results presented in this review to complete the first stage of this framework: ‘Developing complex intervention’, specifically the stage 1.1 ‘Identifying evidence base by reviewing published literature and existing systematic reviews’. However, it would be necessary to supplement this information with the results of some systematic reviews, as indicated by the recommendations of the MRC framework.
In conclusion, the most investigated OT interventions in SMI were psychosocial, psychoeducational, cognitive and exercise interventions. These interventions are usually group interventions in patients with schizophrenia, performed by a multidisciplinary team (in which an occupational therapist collaborates), with 2–3 weekly 60 min sessions, and a duration of between 3 and 6 months. Moreover, although there are different interventions and each one covers different aspects, they all have a common objective: to reduce, through occupation, the limitations that SMI cause in patients, thus improving their quality of life. Although previous studies have shown beneficial effects of the interventions described in this review, further research is required to clearly define parameters such as optimal dose and frequency of sessions, and to understand the long-term effects of the interventions. In the case of the MRC framework, further studies are needed to continue with the stage 2 ‘Assessing feasibility and piloting methods’.
Ethics statements
Patient consent for publication.
Not applicable.
Acknowledgments
We would like to acknowledge the English revision made by Jessica Gorlin.
- Instituto Nacional de Epidemiología de Salud Carlos III
- World Health Organization
- National Institute of Mental Health (NIMH)
- Lipskaya-Velikovsky L ,
- Elgerisi D ,
- Easterbrook A , et al
- Swarbrick M ,
- Diez-Carral B ,
- Montoya-del-Corte P ,
- Solana-Álvarez A
- Lindström M ,
- Hariz G-M ,
- Bernspång B
- van Nieuwenhuizen C , et al
- Yokoyama N , et al
- Arbesman M ,
- James C , et al
- Tricco AC ,
- Zarin W , et al
- Higgins J ,
- Colquhoun H ,
- Bramer WM ,
- Rethlefsen ML ,
- Kleijnen J , et al
- Sellwood W ,
- Thomas CS ,
- Tarrier N , et al
- Hadas-Lidor N ,
- Tyano S , et al
- Brammer M ,
- Mellers J , et al
- Schene AH ,
- Koeter MWJ ,
- Kikkert MJ , et al
- Chan SH-W ,
- Chamberlain J , et al
- Pitschel-Walz G ,
- Gsottschneider A , et al
- Berking M ,
- Cuijpers P , et al
- Buschert V ,
- Prochazka D ,
- Bartl H , et al
- Tjörnstrand C ,
- Sandlund M , et al
- Meijer CJ ,
- Verkerk O , et al
- Shimada T ,
- Inagaki Y , et al
- Abaoğlu H , et al
- Mashimo I ,
- Yotsumoto K ,
- Fujimoto H , et al
- Mclnnis E ,
- Sally Rogers E ,
- Hutchinson DS , et al
- Eik-Nes N ,
- Palmstierna T , et al
- Chang Y-C , et al
- Singh B , et al
- Argentzell E ,
- Bäckström M ,
- Lund K , et al
- Ramano EM ,
- Shinozaki A ,
- Hayashi T ,
- Wasmuth S ,
- Wilburn VG ,
- Hamm JA , et al
- Schindler VP
- Van Sciver A , et al
- Kaizerman-Dinerman A ,
- Karaman İmran Gökçen Yilmaz ,
- Kasal Meltem İzcı ,
- İngeç C , et al
- Rouleau S ,
- Saint-Jean M ,
- Stip E , et al
- Edgelow M ,
- Foruzandeh N ,
- Tatsumi E , et al
- Vizzotto ADB ,
- Celestino DL ,
- Buchain PC , et al
- World Federation of Occupational Therapists (WFOT)
- Triviñó Juárez JM ,
- Romero Ayuso DM
- Gibson RW ,
- D'Amico M ,
- Jaffe L , et al
- Naslund JA ,
- Whiteman KL ,
- McHugo GJ , et al
- Weinmann S ,
- Riedel-Heller SG , et al
- Anthony WA , et al
- Viertiö S ,
- Tuulio-Henriksson A ,
- Perälä J , et al
- de Winter L ,
- Bergmans C , et al
- Maciver D ,
- Aas RW , et al
- Akasyah W ,
- Santosa WRB
- Davidson M ,
- Wackett J , et al
- Lambert RA ,
- Lorgelly P ,
- Harvey I , et al
- Petersen KS ,
- Bjørkedal STB ,
- Torsting AM , et al
- Corner J , et al
- Leung TKS ,
- Ng PPK , et al
- Ringen PA ,
- Antonsen B , et al
- Skinner T , et al
- Peralta V ,
- Zakaria H , et al
- Gureje O , et al
- Macintyre S , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
Contributors All authors contributed to the conception or design of the review and to the data analysis and interpretation. MGdIH coordinated the scoping review. L-MC-G and MR-M conducted a peer-reviewed search and screening study. MR-M wrote the first draft of the paper and MGdIH and L-MC-G provided critical revision of the article. MR-M is the guarantor of this work. All authors gave final approval of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- News and Features
- Conferences
- Clinical Tools
- Special Collections
Retinal Abnormalities May Be Biomarkers for Schizophrenia, Bipolar Disorder
Relative to healthy controls, individuals with schizophrenia and bipolar disorder (BD) display peripapillary and macular structural retinal abnormalities, according to results from a systematic review and meta-analysis published in Schizophrenia Bulletin .
Patients with schizophrenia and BD often report visual processing deficits, including impaired contrast sensitivity, visual acuity, and motion discrimination. Accordingly, some researchers have proposed that optical coherence tomography (OCT) may be a useful noninvasive, high-resolution tool to evaluate neurodegenerative pathologies and potential biomarkers of schizophrenia and BD.
To assess retinal and macular changes in schizophrenia and BD, investigators from Beth Israel Deaconess Medical Center conducted a systematic review and meta-analysis. The investigators searched publication databases for OCT-based studies of schizophrenia and BD (confirmed using Diagnostic and Statistical Manual of Mental Disorders [DSM] criteria) published between 2012 and 2022.
A total of 39 studies were included in the analysis, which comprised 1802 healthy controls, 1084 patients with schizophrenia, and 770 patients with BD.
The investigators found that the probands of the patient groups showed thinner peripapillary retinal nerve fiber layer (RNFL) thickness (standardized mean difference [SMD], -0.43; P <.001) and thinner subregional thickness in the superior (SMD, -0.28; P =.0012), inferior (SMD, -0.31; P <.0001), nasal (SMD, -0.18; P =.0130), and temporal (SMD, -0.19; P =.0022) regions relative to controls.
The funnel plots for the comparisons showed asymmetry. After trim-and-fill analyses, results remained significant for peripapillary RNFL in probands among both patient groups, superior regional thickness in probands among patients with BD, inferior regional thickness in probands among both patient groups, and temporal regional thickness in probands.
In the macula, patient probands displayed thinning in the retina (SMD, -0.60; P =.0009); fovea (SMD, -0.36; P =.0370); inner superior (SMD, -0.40; P <.0001), inner inferior (SMD, -0.38; P =.0002), inner nasal (SMD, -0.40; p =.0013), inner temporal (SMD, -0.47; P =.0003), outer superior (SMD, -0.48; P =.0002), outer inferior (SMD, -0.29; P =.0095), outer nasal (SMD, -0.39; P =.0039), and outer temporal (SMD, -0.28; P =.0099) regions; ganglion cell layer-inner plexiform layer (SMD, -0.28; P <.0001); ganglion cell complex (SMD, -0.42; P =.0349); inner plexiform layer (IPL; SMD, -0.26; P =.0032); outer nuclear layer (ONL; SMD, -0.52; P =.0372); and retinal pigmented epithelium (RPE; SMD, -0.37; P =.0018) thicknesses as well as a decreased macular volume (SMD, -0.34; P =.0060) relative to controls.
These comparisons also exhibited asymmetry. After trim-and-fill analyses, group differences remained significant for inner superior and inferior macular regions in probands among both patient groups, inner nasal and temporal regions in probands among patients with BD, outer inferior region in probands among patients with schizophrenia, and outer nasal and temporal regions, IPL, ONL, and RPE in probands.
The investigators observed significant moderating effects with illness duration, Newcastle Ottawa Scale (NOS) score, smoking status, age, cardiometabolic disease, symptom scores, OCT device, and/or gender for multiple peripapillary and macular outcomes.
The investigators concluded, “This study builds upon existing literature on the pathology of [schizophrenia] and BD patients, identifies potential biomarkers for diagnosis and patient screening and monitoring for neurodegeneration (including cognitive decline), and serves as a foundation for further clinical research to develop more targeted treatments for [ schizophrenia ] and BD .”
The major limitation of the present analysis was that OCT outcomes were averaged between the left and right eyes or came from only one eye, depending on the study.
References:
Sheehan N, Bannai D, Silverstein SM, Lizano P. Neuroretinal alterations in schizophrenia and bipolar disorder: an updated meta-analysis. Schizophr Bull . 2024:sbae102. doi:10.1093/schbul/sbae102
Picked For You
Latest News

Spotlight Course
Want to read more.
Please login or register first to view this content.
Login Register
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Immune-based Machine learning prediction of diagnosis and illness state in schizophrenia and bipolar disorder
Affiliations.
- 1 Scientific Initiative for Neuropsychiatric and Psychopharmacological Studies (SINAPS), University Psychiatric Hospital Campus Duffel (UPCD), Rooienberg 19, 2570 Duffel, Belgium; Collaborative Antwerp Psychiatric Research Institute (CAPRI), University of Antwerp, Campus Drie Eiken, S.003, Universiteitsplein 1, 2610 Wilrijk, Belgium. Electronic address: [email protected].
- 2 Scientific Initiative for Neuropsychiatric and Psychopharmacological Studies (SINAPS), University Psychiatric Hospital Campus Duffel (UPCD), Rooienberg 19, 2570 Duffel, Belgium; Collaborative Antwerp Psychiatric Research Institute (CAPRI), University of Antwerp, Campus Drie Eiken, S.003, Universiteitsplein 1, 2610 Wilrijk, Belgium.
- 3 Université Paris Est Créteil (UPEC), Inserm U955, IMRB Translational Neuropsychiatry Laboratory, AP-HP, Hôpitaux Universitaires H Mondor, DMU IMPACT, FHU ADAPT, Fondation FondaMental, Créteil, France.
- 4 Biomedical Informatics Research Center Antwerp (BIOMINA), University of Antwerp, Campus Middelheim, M.G.111, Middelheimlaan 1, 2020 Antwerp, Belgium; Department of Mathematics and Computer Science, University of Antwerp, Campus Middelheim, M.G.105, Antwerp, Belgium.
- 5 Inserm, Centre d'Investigation Clinique 1430, AP-HP, Hôpital Henri Mondor, Université Paris Est Créteil, Faculté de Médecine de Créteil 8, Rue Du Général Sarrail 94010, Créteil, France.
- 6 Plateforme de Ressources Biologiques, Hôpital Henri Mondor, 51 Avenue due Maréchal de Lattre de Tassigny, 94010 Créteil, France.
- PMID: 39151650
- DOI: 10.1016/j.bbi.2024.08.013
Background: Schizophrenia and bipolar disorder frequently face significant delay in diagnosis, leading to being missed or misdiagnosed in early stages. Both disorders have also been associated with trait and state immune abnormalities. Recent machine learning-based studies have shown encouraging results using diagnostic biomarkers in predictive models, but few have focused on immune-based markers. Our main objective was to develop supervised machine learning models to predict diagnosis and illness state in schizophrenia and bipolar disorder using only a panel of peripheral kynurenine metabolites and cytokines.
Methods: The cross-sectional I-GIVE cohort included hospitalized acute bipolar patients (n = 205), stable bipolar outpatients (n = 116), hospitalized acute schizophrenia patients (n = 111), stable schizophrenia outpatients (n = 75) and healthy controls (n = 185). Serum kynurenine metabolites, namely tryptophan (TRP), kynurenine (KYN), kynurenic acid (KA), quinaldic acid (QUINA), xanthurenic acid (XA), quinolinic acid (QUINO) and picolinic acid (PICO) were quantified using liquid chromatography-tandem mass spectrometry (LC-MS/MS), while V-plex Human Cytokine Assays were used to measure cytokines (interleukin-6 (IL-6), IL-8, IL-17, IL-12/IL23-P40, tumor necrosis factor-alpha (TNF-ɑ), interferon-gamma (IFN-γ)). Supervised machine learning models were performed using JMP Pro 17.0.0. We compared a primary analysis using nested cross-validation to a split set as sensitivity analysis. Post-hoc, we re-ran the models using only the significant features to obtain the key markers.
Results: The models yielded a good Area Under the Curve (AUC) (0.804, Positive Prediction Value (PPV) = 86.95; Negative Prediction Value (NPV) = 54.61) for distinguishing all patients from controls. This implies that a positive test is highly accurate in identifying the patients, but a negative test is inconclusive. Both schizophrenia patients and bipolar patients could each be separated from controls with a good accuracy (SCZ AUC 0.824; BD AUC 0.802). Overall, increased levels of IL-6, TNF-ɑ and PICO and decreased levels of IFN-γ and QUINO were predictive for an individual being classified as a patient. Classification of acute versus stable patients reached a fair AUC of 0.713. The differentiation between schizophrenia and bipolar disorder yielded a poor AUC of 0.627.
Conclusions: This study highlights the potential of using immune-based measures to build predictive classification models in schizophrenia and bipolar disorder, with IL-6, TNF-ɑ, IFN-γ, QUINO and PICO as key candidates. While machine learning models successfully distinguished schizophrenia and bipolar disorder from controls, the challenges in differentiating schizophrenic from bipolar patients likely reflect shared immunological pathways by the both disorders and confounding by a larger state-specific effect. Larger multi-centric studies and multi-domain models are needed to enhance reliability and translation into clinic.
Keywords: Bipolar; Cytokines; Kynurenines; Machine learning; Schizophrenia.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Unrelated to the submitted work, LDP reports grants from Boehringer-Ingelheim and Janssen R&D, MM reports grants from Janssen R&D, Boehringer Ingelheim Pharma GmbH & Co. and Takeda Pharmaceutical Company. RT, LDP and ML are members of the ECNP Immuno-NeuroPsychiatry Network. The I-GIVE study received funding from Agence Nationale de la Recherche (I-GIVE ANR-13-SAMA-0004-01), INSERM (Institut National de la Santé et de la Recherche Médicale) and Fondation FondaMental in France.
Similar articles
- Kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Skorobogatov K, Autier V, Foiselle M, Richard JR, Boukouaci W, Wu CL, Raynal S, Carbonne C, Laukens K, Meysman P, Coppens V, le Corvoisier P, Barau C, De Picker L, Morrens M, Tamouza R, Leboyer M. Skorobogatov K, et al. Brain Behav Immun Health. 2023 Jan 2;27:100584. doi: 10.1016/j.bbih.2022.100584. eCollection 2023 Feb. Brain Behav Immun Health. 2023. PMID: 36685639 Free PMC article.
- Activation of kynurenine pathway in ex vivo fibroblasts from patients with bipolar disorder or schizophrenia: cytokine challenge increases production of 3-hydroxykynurenine. Johansson AS, Owe-Larsson B, Asp L, Kocki T, Adler M, Hetta J, Gardner R, Lundkvist GB, Urbanska EM, Karlsson H. Johansson AS, et al. J Psychiatr Res. 2013 Nov;47(11):1815-23. doi: 10.1016/j.jpsychires.2013.08.008. Epub 2013 Aug 23. J Psychiatr Res. 2013. PMID: 24012176
- Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Halstead S, Siskind D, Amft M, Wagner E, Yakimov V, Shih-Jung Liu Z, Walder K, Warren N. Halstead S, et al. Lancet Psychiatry. 2023 Apr;10(4):260-271. doi: 10.1016/S2215-0366(23)00025-1. Epub 2023 Feb 27. Lancet Psychiatry. 2023. PMID: 36863384
- The kynurenine pathway in schizophrenia and bipolar disorder. Erhardt S, Schwieler L, Imbeault S, Engberg G. Erhardt S, et al. Neuropharmacology. 2017 Jan;112(Pt B):297-306. doi: 10.1016/j.neuropharm.2016.05.020. Epub 2016 May 28. Neuropharmacology. 2017. PMID: 27245499 Review.
- Dysregulation of kynurenine pathway and potential dynamic changes of kynurenine in schizophrenia: A systematic review and meta-analysis. Cao B, Chen Y, Ren Z, Pan Z, McIntyre RS, Wang D. Cao B, et al. Neurosci Biobehav Rev. 2021 Apr;123:203-214. doi: 10.1016/j.neubiorev.2021.01.018. Epub 2021 Jan 26. Neurosci Biobehav Rev. 2021. PMID: 33513412 Review.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neuropsychiatr Dis Treat
- v.4(1); 2008 Feb
Differential diagnoses and management strategies in patients with schizophrenia and bipolar disorder
A carlo altamura.
1 Department of Psychiatry, University of Milan, IRCCS Fondazione Ospedale Maggiore Policlinico Mangiagalli e Regina Elena, Milan, Italy
Jose M Goikolea
2 Bipolar Disorder Program, Hospital Clinic i Universitari, Barcelona, Spain
Successful treatment of psychiatric disorders, including bipolar disorder and schizophrenia, is complicated and is affected by a broad range of factors associated with the diagnosis, choice of treatment and social factors. In these patients, treatment management must focus on accurate and early diagnosis, to ensure that correct treatment is administered as soon as possible. In both disorders, the treatment of the disease in the acute phase must be maintained long term to provide continuous relief and normal function; the treatment choice in the early stages of the disease may impact on long-term outcomes. In schizophrenia, treatment non-compliance is an important issue, with up to 50% of patients discontinuing treatment for reasons as diverse as efficacy failure, social barriers, and more commonly, adverse events. Treatment non-compliance also remains an issue in bipolar disorder, as tolerability of mood stabilizers, especially lithium, is not always good, and combination treatments are frequent. In order to achieve an optimal outcome in which the patient continues with their medication life-long, treatment should be tailored to each individual, taking into account treatment and family history, and balancing efficacy with tolerability to maximize patient benefit and minimize the risk of discontinuation. These case studies illustrate how treatment should be monitored, tailored and often changed over time to meet these needs.
Introduction
Successful treatment of psychiatric disorders, such as bipolar disorder and schizophrenia, should ideally result in long-term control in which the disease is managed effectively. However, successful treatment is complicated by a number of compounding factors, such as compliance and drug-resistance phenomena ( Altamura 1990 ). Of these, time to accurate diagnosis is a particular issue as this can delay initiation of the correct treatment for the patient ( Berk et al 2007 ). Administration of effective treatment is essential both in the acute phase to manage the disease rapidly, but also in the long term to ensure that treatment benefits are maintained ( Altamura et al 2000 ). Successful treatment can be considered to be one that provides the most effective balance between efficacy and tolerability ( Bowden 1995 ; Thomas 2007 ). While the initial treatment may be effective in managing acute symptoms of schizophrenia and manic or depressive episodes of bipolar disorder, continuous assessment of the patient is required to ensure optimal tolerability and efficacy ( Altamura 1992 ). It is recognized that maintaining the initial treatment is ideal; however, alternative or additional treatments may be required at particular stages of the disease, as the course develops, to address any imbalance between efficacy and tolerability. The need for such continuous assessment and flexibility in treatment is demonstrated by the high incidence of treatment non-compliance in psychiatric disorders, which is a major factor in determining the outcomes of treatment ( Altamura et al 2000 ; Dolder et al 2002 ; Thomas 2007 ). Sociodemographic factors, such as family history and employment status, are associated with poor treatment compliance, while treatment-related factors, such as lack of efficacy and adverse events and, particularly in bipolar disorder, disease symptoms (eg, poor insight and cognitive deficits) may lead directly to discontinuation of therapy ( Gray et al 2002 ; Rettenbacher et al 2004 ; Linden et al 2006 ; Clatworthy et al 2007 ). The importance of adverse events is particularly true for schizophrenia, in which patients frequently receive high doses of antipsychotic drugs which affects compliance, particularly with first generation antipsychotics ( Altamura 1990 ; Altamura et al 2000 , 2007 ).
A further consideration is the presentation of pediatric and adolescent forms of psychiatric disorders which may differ somewhat in terms of symptoms, disease course, and treatment options from the corresponding adult disorders. This can be seen in pediatric bipolar disorder which is often characterized by the presence of mixed-mania and rapid cycling and a high degree of comorbidity with behavioral and attention disorders ( Chang and Ketter 2001 ) – factors that contribute to difficulties in diagnosis. Similar problems are encountered in childhood onset schizophrenia which may be difficult to differentiate from affective and personality disorders due to the predominance of negative symptoms ( Masi et al 2006 ). Treatment of early and very early-onset schizophrenia is further complicated by differences in response to pharmacological therapy compared with adults, a problem exacerbated by a lack of clinical trials in this patient sub-population ( Young and Findling 2004 ).
This article explores some of the challenges of successfully treating patients with bipolar disorder and schizophrenia. In both of these conditions successful treatment is often hindered by misdiagnosis, poor treatment compliance and inter-patient variability in treatment efficacy and tolerability. These issues are highlighted through the use of case studies giving an insight into how to maximize clinical success in difficult-to-treat patients with diverse psychiatric disorders.
Managing treatment options in bipolar disorder
Mania is the basis for the diagnosis of bipolar disorder. Pure euphoric mania is usually easy to identify; but other types of presentation, such as psychotic mania, or when symptoms of mania and depression occur together as a mixed episode, are frequently misdiagnosed. Mixed hypomania is more frequent in patients with type I bipolar disorder and has been reported in 57% of patients ( Suppes et al 2005 ). Such co-occurring symptoms can make diagnosis of bipolar disorder difficult, such that there is often a delay between the onset of symptoms and correct diagnosis ( Hantouche et al 2006 ). Inaccurate diagnosis commonly results in patients receiving incorrect therapies, which may delay successful treatment of the initial episodes, and in some cases may even exacerbate them, for example causing rapid-cycling states ( Sachs et al 2007 ). The following case study illustrates the difficulties associated with the treatment of a patient presenting with mixed symptoms of bipolar disorder.
Clinical case study
A 36-year-old female with no previous psychiatric history was first hospitalized due to an acute episode with psychotic symptoms (reference and grandiosity delusions), high anxiety, irritability, global insomnia and agitation. She was diagnosed with brief psychotic disorder which was treated with thioridazine up to 200 mg/day. Four months later, she suffered a major depression that was treated by a private psychiatrist with fluoxetine up to 40 mg/day. After 1 month, the depression progressed to a mixed state with high irritability, anxiety, demanding behavior, insomnia, and depressive thoughts. Fluoxetine was stopped and the patient was treated with low doses of haloperidol (up to 5 mg/day). During the following weeks, the patient progressively went to a new depressive episode with hypothymia (low mood), tiredness, hypersomnia, and psychomotor inhibition. Haloperidol was tapered off but major depression lasted a further 3 months. The patient visited another psychiatrist who started imipramine up to 150 mg/day to treat the depression, this resulted in a fast improvement of the depressive episode. About 2 months later, the patient experienced another acute episode, very similar to the first one, that required hospitalization. At this time, approximately 2 years after the first episode, she was diagnosed with bipolar disorder type I, manic episode with congruent psychotic symptoms. Following the diagnosis, the patient was treated with lithium (1000 mg/day; serum level 0.87 meq/L) for the following 1.5 years; however, she still experienced both depressive and hypomanic episodes of an irritable nature. Combined treatment with lithium and antidepressants (first fluoxetine up to 40 mg/day; then imipramine up to 150 mg/day) improved the severity of the episodes, removing psychotic tendencies, but the frequency of episodes increased to 2-week cycles of depression following by 1 week of hypomania and 1–2 weeks of euthymia. To address this rapid cycling, carbamazepine up to 600 mg/day was added to the treatment regimen; however, this caused skin rashes and was discontinued after 2 weeks of treatment.
At this point, the patient first came to our clinic. Subclinical hypothyroidism was detected and was treated with thyroxine 100 μg/day, antidepressants were discontinued and the patient was switched from lithium to sodium valproate 2000 mg/day over a period of 3 weeks. Lithium was tapered off and valproate was started at 1000 mg/day, resulting in serum levels of 27.1 μg/mL. Valproate was increased to 2000 mg/day, resulting in serum levels of 75 μg/mL. After two milder cycles, the patient was stabilized and began to recover pre-morbid functioning.
The patient maintained this treatment regimen for 5 years during which time she experienced weight gain. Patient dissatisfaction led to the gradual replacement of valproate with lamotrigine, up to 200 mg/day. Three months after switching the medication, the patient suffered a mixed depression with hypothymia, apathy, depressive thoughts, mood lability, irritability and insomnia. The previous regimen with valproate was restarted; the patient stabilized and was euthymic with only occasional anxiety in response to family problems. This good global functioning currently allows the patient to care for her mother, who suffers from an advanced mixed dementia, and her father. Other than weight gain, valproate was generally well tolerated and no other adverse events were experienced.
The patient described provides a clear example of the complications surrounding successful treatment of bipolar disorder. Correct diagnosis of bipolar disorder is often delayed by over 5 years due to the presence of mixed symptoms ( Hantouche et al 2006 ; Berk et al 2007 ). This prevents correct treatment from being administered and can affect the long-term outcomes of the disease ( Berk et al 2007 ). The diagnosis of bipolar disorder type I in this patient was delayed by 2 years due to previous misdiagnoses of brief psychiatric disorder and depression, during which time the patient was mostly suffering an affective episode which resulted in two hospitalizations and complete dysfunction. Therefore, although the time of misdiagnosis was relatively short, the clinical consequences of the misdiagnosis were severe.
Mood stabilizers are the mainstay of treatment for bipolar disorder; these include lithium and sodium valproate ( Bowden et al 2005 , 2006 ). Sodium valproate has demonstrated improved efficacy over lithium in treating mixed episodes in bipolar disorder, with better outcomes reported for depressive episodes and overall function ( Bowden et al 2005 ). Other anticonvulsants used in the treatment of bipolar disorder include carbamazepine and lamotrigine.
Rapid cycling is a common observation in bipolar disorder, and is associated with reduced treatment responses, poorer long-term prognosis and a probable higher suicide risk than patients who do not display rapid cycling ( Schneck 2006 ). Furthermore, these patients often experience more depressive than manic episodes. As observed in this patient, subclinical hypothyroidism can be related to rapid cycling ( Papadimitriou et al 2005 ). This finding and the substitute hormone treatment helped the patient to stabilize. Rapid cycling is estimated to occur in 14%–53% of patients ( Maj et al 1994 ; Tondo and Baldessarini 1998 ; Suppes et al 2001 ), of which over 70% have been attributed to a poor response to lithium. ( Dunner and Fieve 1974 ; Bowden 1995 ; Calabrese et al 2005 ). Long-term open clinical trials with sodium valproate in patients with rapid cycling have demonstrated initial good responses, with few recurrent episodes ( McElroy et al 1988 ; Calabrese and Delucchi 1990 ). This supports suggestions that sodium valproate has a broader spectrum of use than lithium ( Bowden 1995 ). Although the most recent data from a long-term study by Calabrese and colleagues did not support the hypothesis that sodium valproate was more effective than lithium for the treatment of rapid cycling in bipolar disorder, there was a trend towards a greater advantage with sodium valproate in terms of mood symptoms and adverse events ( Calabrese et al 2005 ). Furthermore, sodium valproate has demonstrated antidepressive effects in bipolar disorder, preventing or delaying the onset of depressive episodes ( Ghaemi and Goodwin 2001 ; Bowden et al 2005 ).
Carbamazepine has been reported to be effective in some patients with rapid cycling bipolar disorder ( Post et al 1986 ; Joyce 1988 ; Tondo et al 2003 ), especially when used in combination with lithium ( Baethge et al 2005 ). However, this combination caused a rash in the patient presented here and was subsequently discontinued; this is in agreement with studies showing an increase in adverse events with combined carbamazapine/lithium therapy ( Baethge et al 2005 ). A small number of reports have demonstrated effectiveness of lamotrigine in rapid-cycling, especially when combined with valproate ( da Rocha et al 2007 ). This was the rationale for converting the patient from valproate to lamotrigine following the onset of weight gain. However, lamotrigine monotherapy was associated with a regression of symptoms in this patient. The reintroduction of valproate improved the severity of the episodes, leading to good global functioning and stabilization, but problems with weight gain continued. Referral to a dietician and subsequent life-style changes regarding diet and exercise regimens may improve weight gain in patients with bipolar disorder ( Chue and Kovacs 2003 ).
Outcomes of bipolar disorder can also be negatively influenced by incorrect administration of mood stabilizers and antidepressants ( Goldberg et al 2007 ), and rapid cycling can be induced or worsened by antidepressants in the absence of a mood stabilizer ( Sachs et al 2007 ). It is possible that both the use of a high dose of fluoxetine (40 mg/day vs the standard 20 mg/day) and the combination of lithium and antidepressants in the patient described here exacerbated the hypomanic episodes and led to the presentation of the rapid cycling state.
Early and accurate diagnosis of bipolar disorder is essential to ensure that correct treatment is received at the earliest opportunity since incorrect treatment can negatively affect outcomes. As demonstrated in the case presented, correct treatment of bipolar disorder can be successful in the long term once this is achieved.
Non-compliance and treatment choices in schizophrenia
Treatment compliance is essential for the long-term control of schizophrenia. Despite advances in the newer atypical (second-generation) antipsychotic therapies, treatment non-compliance is reported to occur in up to 50% of patients with schizophrenia ( Fenton et al 1997 ; Dolder et al 2002 ; Gray et al 2002 ; Rettenbacher et al 2004 ; Thomas 2007 ), with discontinuation rates of 74% within 18 months reported in some studies ( Lieberman et al 2005 ). The impact of non-compliance is far reaching in maintenance therapy, with non-compliant patients often experiencing impaired long-term outcomes including higher relapse rates and the need for rehospitalization ( Fenton et al 1997 ; Ascher-Svanum et al 2006 ). Factors associated with treatment non-compliance are multifactoral including sociodemographic variables (age, occupation, social status or level of education), attitudes of patients and carers/family towards the illness and treatment, illness-related issues such as psychopathology and comorbidities, and treatment issues such as adverse events ( Rettenbacher et al 2004 ; Linden et al 2006 ). One of the most common reasons cited for non-compliance of treatment in schizophrenia are adverse events ( Naber and Karow 2001 ). The spectrum of adverse events with antipsychotic therapy is varied and is dependent on the choice of drug. Among the most common adverse events with typical antipsychotics are extrapyramidal symptoms (EPS), sexual dysfunction, and weight gain. The newer atypical antipsychotic drugs have been associated with fewer EPS ( Geddes et al 2000 ; Davis et al 2003 ; Lieberman et al 2005 ); however, there have been increasing concerns that these drugs lead to metabolic side effects including diabetes, dyslipidemia, and obesity ( Mortimer et al 2003 ; Meyer and Koro 2004 ; Peuskens et al 2007 ; Spurling et al 2007 ). Switching non-compliant patients to alternative antipsychotics may improve compliance by better balancing efficacy and tolerability ( Peuskens 2002 ; Linden et al 2006 ; Linden et al 2007 ). The following case study illustrates the issues surrounding the treatment of schizophrenia, demonstrating that many changes in therapy may be required to determine the most effective treatment for any individual.
A 40-year-old male presented with the appearance of persecutory delusions with sub-threshold depressive symptoms. At the time of presentation, the patient was receiving aripiprazole 15 mg/day, which had a good impact on negative symptoms and social abilities. However, the aripiprazole was causing gastrointestinal side effects including severe nausea and constipation leading to occasional dissatisfaction and a lack of treatment compliance. The patient was also experiencing concomitant obesity with a body weight of 96 kg and glucose intolerance (156 mg/dL). The patient was diagnosed with paranoid schizophrenia according to DSM-IV criteria and structured clinical interview. This was episodic in nature with residual symptoms occurring between acute episodes. This diagnosis was based on the prevalence of organized delusions and hallucinations, and the separation of several acute psychotic episodes with periods of negative and depressive symptoms.
Treatment history revealed that the patient was predisposed to develop EPS and had a variable response to prior antipsychotic therapy. From the age of onset at 30-years, the patient’s initial treatment with haloperidol up to 10 mg/day for 2 years managed positive symptoms and was used in maintenance therapy. However, the development of negative symptoms and emergence of EPS led to a change in therapy to risperidone (8 mg/day). After 1 year, the risperidone dose was reduced to 4 mg/day because of further EPS, but this resulted in the reappearance of positive symptoms and treatment was subsequently switched to olanzapine 20 mg/day. The patient received olanzapine for 1.5 years during which time positive symptoms regressed. However, during this time the patient experienced a significant weight gain of approximately 10 kg. This weight gain led to the patient switching treatment from olanzapine to aripiprazole up to 15 mg/day.
After 2 years of aripiprazole therapy, the patient presented at our clinic as described above. The patient was converted to amisulpride at a starting dose of 400 mg/day, because of psychotic recurrences and low tolerability to other antipsychotic drugs. Amisulpride was gradually increased to an effective dose of 800 mg/day. This approach was undertaken to avoid any risk of treating abruptly at the optimal effective dose in the acute phase of the illness given the patient’s very complex medical history. There was regular monitoring of blood pressure (weekly), electrocardiogram and prolactin plasma levels (at baseline, at month 1, and every 3 months). All the parameters remained within normal levels during treatment, with the exception of prolactin which showed an increase at month 3 (43 ng/mL), that persisted at the other timepoints. The patient was not converted to clozapine due to therecent significant weight gain with olanzapine and his current obesity status, since the potentialrisk of developing ametabolic syndrome with clozapineis similar to that with olanzapine in terms of weight gain. Given that the patient had previously shownthe reappearance of positive symptoms with doses <4 mg/day of risperidone, quetiapine was not considered useful since there was the possibility of worsening positive symptoms because ofthe weaker blockade of dopamine (D2) receptors.
Treatment with amisulpride led to remission of psychotic episodes with an improvement in depressive symptoms and somatizations. The patient is still being monitored and will remain on this treatment until complete remission of psychotic episodes, depressive symptoms and somatizations. Globally, the treatment with amisulpride well tolerated. There was an improvement in the patient’s glucose intolerance (156 mg/dL vs 102 mg/dL at baseline and month 6, respectively). The patient was referred to a dietology specialist in order to manage obesity, after 6 months receiving amisulpride the patient’s body weight had reduced to 92 kg.
Treatment non-compliance has a significant impact on the outcomes of schizophrenia. Many of the currently available antipsychotic therapies have comparable efficacy in the treatment of symptoms in the acute phase; however, their tolerability profiles can vary ( Davis et al 2003 ; Mortimer et al 2003 ). The presented case study demonstrates such variability, with the patient experiencing a range of complications under different therapies. This was further complicated by the emergence of different symptoms of the disease during long-term therapy. At the time of presentation, the patient was receiving aripiprazole, having already previously received therapy with haloperidol, risperidone and olanzapine. While poor compliance to aripiprazole at presentation was attributed to the gastrointestinal side effects, a large number of external factors are associated with treatment non-compliance in patients with schizophrenia, including social status, occupation and social support ( Linden et al 2006 ). This patient was unemployed with no social support with a family history of mental health issues; his father suffered from alcohol dependence and his sister had probable schizoaffective disorder, bipolar type. These additional contributing factors to compliance should be assessed on an individual basis when considering antipsychotic therapy.
Amisulpride was considered to be the best choice of antipsychotic therapy for the patient, based on the low tolerability to other antipsychotic therapies and the presentation of depressive symptoms. Switching to amisulpride has previously been demonstrated to improve treatment compliance ( Linden et al 2006 ; Kim et al 2007 ; Linden et al 2007 ; Spurling et al 2007 ). This may be related to the improved tolerability profile associated with amisulpride when comparing weight gain, metabolic control and EPS, which were particular issues for the patient illustrated here. As with most atypical antipsychotics, amisulpride is associated with a low incidence of EPS ( Wetzel et al 1998 ; Herrera-Estrella et al 2005 ; Nuss et al 2007 ). Furthermore, clinical studies with amisulpride and olanzapine have demonstrated that patients receiving amisulpride have less weight gain and less increase in blood glucose than those receiving olanzapine ( Mortimer 2004 ; Mortimer et al 2004 ; Peuskens et al 2007 ). Amisulpride has clear benefits over risperidone with regard to weight gain as well ( Sechter et al 2002 ). Importantly, these studies clearly demonstrate comparable efficacy between the different agents, in agreement with an earlier meta-analysis of the efficacy of atypical antipsychotics ( Davis et al 2003 ).
Although amisulpride has demonstrated good efficacy in the treatment of positive symptoms ( Sechter et al 2002 ; Mortimer et al 2004 ; Herrera-Estrella et al 2005 ; Nuss et al 2007 ), it is also effective for the treatment of negative and depressive symptoms of schizophrenia ( Speller et al 1997 ; Muller et al 2002 ; Peuskens et al 2002 ; Mortimer et al 2004 ; Herrera-Estrella et al 2005 ; Murphy et al 2006 ; Kim et al 2007 ; Nuss et al 2007 ), having clear benefits over olanzapine and risperidone with improved symptom control and longer time to depressive episodes ( Peuskens et al 2002 ; Mortimer 2004 ; Kim et al 2007 ). However, olanzapine has also been attributed to have treatment effects on negative symptoms ( Lecrubier et al 2006 ).
The prolactin-elevating effects of amisulpride are well recognized, therefore prolactin plasma levels were measured regularly in this patient. In this case, no clinical symptoms of hyperprolactinemia were detected. Nevertheless, patients treated with amisulpride should be observed and asked about symptoms such as sexual dysfunction, galactorrhea and amenorrhea.
The balance between efficacy and tolerability is an essential aspect when considering antipsychotic treatment and the establishment of a positive tolerability/efficacy ratio should be an integral determinant in the choice of treatment. Whilst the efficacy of treatment is important, maintaining good long-term tolerability is essential to ensure that the treatment is continued and be of benefit. There are considerable differences in the tolerability of different antipsychotic therapies, and there may also be considerable difference in the response of different patients to these drugs. The tolerability/efficacy ratio therefore provides a measure to help to tailor antipsychotic therapy individually; it is essential that both aspects be considered when starting or switching therapies in schizophrenia. This case illustrates the benefit of continuously monitoring and changing treatment of schizophrenia to achieve this optimal treatment ratio ( Altamura et al 2007 ).
Conclusions
Early and accurate diagnosis is essential for the successful treatment of psychiatric disorders. As well as having a beneficial impact on treatment of the acute phase of the disease, it can also affect long-term outcomes of treatment. For example, a high incidence of treatment non-compliance is reported across a range of psychiatric disorders, many of which are attributed to treatment effects such as lack of efficacy or adverse events. This is highlighted by the two cases reported here, where misdiagnosis and inappropriate drug therapy resulted in poor treatment outcomes in both bipolar disorder and schizophrenia. Although the specific factors contributing to successful treatment may vary between psychiatric disorders, the approach to treatment should be very similar. Diagnosis and treatment should be tailored to the individual patient, taking into account a detailed family and treatment history, and ensuring that the tolerability is adequate to maximize long-term compliance of medication.
- Altamura AC. Drug-resistance phenomena in major psychoses:their discrimination and causal mechanisms. Clin Neuropharmacol. 1990; 13 :S1–15. [ PubMed ] [ Google Scholar ]
- Altamura AC. A multidimentional (pharmacokinetic and clinical-biological) approach to neuroleptic response in Schizophrenia. Schizophrenia Res. 1992; 8 :187–98. [ PubMed ] [ Google Scholar ]
- Altamura AC, Bobes J, Cunningham D, et al. Schizophrenia: diagnosis and continuing treatment. Principles of practice from the European Expert Panel on the Contemporary Treatment of Schizophrenia. Int J Psych Clin Pract. 2000;(Suppl 1):1–11. [ PubMed ] [ Google Scholar ]
- Altamura AC, Bobo VB, Meltzer HY. Factors affecting outcome in Schizophrenia and their relevance for psychopharmacological treatment. Int Clin Psychopharmacol. 2007; 22 :249–67. [ PubMed ] [ Google Scholar ]
- Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006; 67 :453–60. [ PubMed ] [ Google Scholar ]
- Baethge C, Baldessarini RJ, Mathiske-Schmidt K, et al. Long-term combination therapy versus monotherapy with lithium and carbamazepine in 46 bipolar I patients. J Clin Psychiatry. 2005; 66 :174–82. [ PubMed ] [ Google Scholar ]
- Berk M, Dodd S, Callaly P, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J Affect Disord. 2007; 23 :23. [ PubMed ] [ Google Scholar ]
- Bowden CL. Predictors of response to divalproex and lithium. J Clin Psychiatry. 1995; 56 :25–30. [ PubMed ] [ Google Scholar ]
- Bowden CL, Collins MA, McElroy SL, et al. Relationship of mania symptomatology to maintenance treatment response with divalproex, lithium, or placebo. Neuropsychopharmacology. 2005; 30 :1932–9. [ PubMed ] [ Google Scholar ]
- Bowden CL, Swann AC, Calabrese JR, et al. A randomized, placebo-controlled, multicenter study of divalproex sodium extended release in the treatment of acute mania. J Clin Psychiatry. 2006; 67 :1501–10. [ PubMed ] [ Google Scholar ]
- Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 patients with rapid-cycling bipolar disorder. Am J Psychiatry. 1990; 147 :431–4. [ PubMed ] [ Google Scholar ]
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005; 162 :2152–61. [ PubMed ] [ Google Scholar ]
- Chang KD, Ketter TA. Special issues in the treatment of paediatric bipolar disorder. Expert Opin Pharmacother. 2001; 2 :613–22. [ PubMed ] [ Google Scholar ]
- Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord. 2003; 5 :62–79. [ PubMed ] [ Google Scholar ]
- Clatworthy J, Bowskill R, Rank T, et al. Adherence to medication in bipolar disorder: a qualitative study exploring the role of patients’ beliefs about the condition and its treatment. Bipolar Disord. 2007; 9 :656–64. [ PubMed ] [ Google Scholar ]
- da Rocha FF, Soares FM, Correa H, et al. Addition of lamotrigine to valproic acid: A successful outcome in a case of rapid-cycling bipolar affective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31 :1548–9. Epub 2007 Jun 22. [ PubMed ] [ Google Scholar ]
- Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003; 60 :553–64. [ PubMed ] [ Google Scholar ]
- Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002; 159 :103–8. [ PubMed ] [ Google Scholar ]
- Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974; 30 :229–33. [ PubMed ] [ Google Scholar ]
- Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997; 23 :637–51. [ PubMed ] [ Google Scholar ]
- Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000; 321 :1371–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ghaemi SN, Goodwin FK. Long-term naturalistic treatment of depressive symptoms in bipolar illness with divalproex vs. lithium in the setting of minimal antidepressant use. J Affect Disord. 2001; 65 :281–7. [ PubMed ] [ Google Scholar ]
- Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007; 164 :1348–55. [ PubMed ] [ Google Scholar ]
- Gray R, Wykes T, Gournay K. From compliance to concordance:a review of the literature on interventions to enhance compliance with antipsychotic medication. J Psychiatr Ment Health Nurs. 2002; 9 :277–84. [ PubMed ] [ Google Scholar ]
- Hantouche EG, Akiskal HS, Azorin JM, et al. Clinical and psychometric characterization of depression in mixed mania: a report from the French National Cohort of 1090 manic patients. J Affect Disord. 2006; 96 :225–32. [ PubMed ] [ Google Scholar ]
- Herrera-Estrella M, Apiquian R, Fresan A, et al. The effects of amisulpride on five dimensions of psychopathology in patients with schizophrenia: a prospective open-label study. BMC Psychiatry. 2005; 5 :22. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Joyce PR. Carbamazepine in rapid cycling bipolar affective disorder. Int Clin Psychopharmacol. 1988; 3 :123–9. [ PubMed ] [ Google Scholar ]
- Kim SW, Shin IS, Kim JM, et al. Amisulpride versus risperidone in the treatment of depression in patients with schizophrenia: A randomized, open-label, controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31 :1504–9. Epub 2007 Jul 13. [ PubMed ] [ Google Scholar ]
- Lecrubier Y, Quintin P, Bouhassira M, et al. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta Psychiatr Scand. 2006; 114 :319–27. [ PubMed ] [ Google Scholar ]
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005; 353 :1209–23. Epub 2005 Sep 19. [ PubMed ] [ Google Scholar ]
- Linden M, Eich FX, Pyrkosch L. Do differences in atypical antipsychotics matter in routine practice? Medication switch from olanzapine and risperidone to amisulpride. Int Clin Psychopharmacol. 2007; 22 :175–8. [ PubMed ] [ Google Scholar ]
- Linden M, Scheel T, Eich FX. Improvement of patient compliance after switching from conventional neuroleptics to the atypical neuroleptic amisulpride. J Psychopharmacol. 2006; 20 :815–23. Epub 2006 Jan 9. [ PubMed ] [ Google Scholar ]
- McElroy SL, Keck PE Jr, Pope HG Jr, et al. Valproate in the treatment of rapid-cycling bipolar disorder. J Clin Psychopharmacol. 1988; 8 :275–9. [ PubMed ] [ Google Scholar ]
- Maj M, Magliano L, Pirozzi R, et al. Validity of rapid cycling as a course specifier for bipolar disorder. Am J Psychiatry. 1994; 151 :1015–9. [ PubMed ] [ Google Scholar ]
- Masi G, Mucci M, Pari C. Children with schizophrenia: clinical picture and pharmacological treatment. CNS Drugs. 2006; 20 :841–66. [ PubMed ] [ Google Scholar ]
- Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004; 70 :1–17. [ PubMed ] [ Google Scholar ]
- Mortimer AM. How do we choose between atypical antipsychotics? The advantages of amisulpride. Int J Neuropsychopharmacol. 2004; 7 :S21–5. [ PubMed ] [ Google Scholar ]
- Mortimer A, Martin S, Loo H, et al. A double-blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int Clin Psychopharmacol. 2004; 19 :63–9. [ PubMed ] [ Google Scholar ]
- Mortimer A, Williams P, Meddis D. Impact of side-effects of atypical antipsychotics on non-compliance, relapse and cost. J Int Med Res. 2003; 31 :188–96. [ PubMed ] [ Google Scholar ]
- Muller MJ, Wetzel H, Benkert O. Differential effects of high-dose amisulpride versus flupentixol on latent dimensions of depressive and negative symptomatology in acute schizophrenia: an evaluation using confirmatory factor analysis. Int Clin Psychopharmacol. 2002; 17 :249–61. [ PubMed ] [ Google Scholar ]
- Murphy BP, Stuart AH, Wade D, et al. Efficacy of amisulpride in treating primary negative symptoms in first-episode psychosis: a pilot study. Hum Psychopharmacol. 2006; 21 :511–7. [ PubMed ] [ Google Scholar ]
- Naber D, Karow A. Good tolerability equals good results: the patient’s perspective. Eur Neuropsychopharmacol. 2001; 11 :S391–6. [ PubMed ] [ Google Scholar ]
- Nuss P, Hummer M, Tessier C. The use of amisulpride in the treatment of acute psychosis. Therapeutics and Clin Management. 2007; 3 :3–11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Papadimitriou GN, Calabrese JR, Dikeos DG, et al. Rapid cycling bipolar disorder: biology and pathogenesis. Int J Neuropsychopharmacol. 2005; 8 :281–92. Epub 2005 Mar 1. [ PubMed ] [ Google Scholar ]
- Peuskens J. Switching to amisulpride. Curr Med Res Opin. 2002; 18 :s23–8. [ PubMed ] [ Google Scholar ]
- Peuskens J, De Hert M, Mortimer A. Metabolic control in patients with schizophrenia treated with amisulpride or olanzapine. Int Clin Psychopharmacol. 2007; 22 :145–52. [ PubMed ] [ Google Scholar ]
- Peuskens J, Moller HJ, Puech A. Amisulpride improves depressive symptoms in acute exacerbations of schizophrenia: comparison with haloperidol and risperidone. Eur Neuropsychopharmacol. 2002; 12 :305–10. [ PubMed ] [ Google Scholar ]
- Post R, Uhde T, Kramlinger K, et al. Carbamazepine treatment of mania: clinical and biochemical aspects. Clin Neuropharmacol. 1986; 9 :547–9. [ PubMed ] [ Google Scholar ]
- Rettenbacher MA, Burns T, Kemmler G, et al. Schizophrenia: attitudes of patients and professional carers towards the illness and antipsychotic medication. Pharmacopsychiatry. 2004; 37 :103–9. [ PubMed ] [ Google Scholar ]
- Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007; 356 :1711–22. Epub 2007 Mar 28. [ PubMed ] [ Google Scholar ]
- Schneck CD. Treatment of rapid-cycling bipolar disorder. J Clin Psychiatry. 2006; 67 :22–7. [ PubMed ] [ Google Scholar ]
- Sechter D, Peuskens J, Fleurot O, et al. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology. 2002; 27 :1071–81. [ PubMed ] [ Google Scholar ]
- Speller JC, Barnes TR, Curson DA, et al. One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms. Amisulpride v. haloperidol. Br J Psychiatry. 1997; 171 :564–8. [ PubMed ] [ Google Scholar ]
- Spurling RD, Lamberti JS, Olsen D, et al. Changes in metabolic parameters with switching to aripiprazole from another second-generation antipsychotic: a retrospective chart review. J Clin Psychiatry. 2007; 68 :406–9. [ PubMed ] [ Google Scholar ]
- Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network II. Demographics and illness characteristics of the first 261 patients. J Affect Disord. 2001; 67 :45–59. [ PubMed ] [ Google Scholar ]
- Suppes T, Mintz J, McElroy SL, et al. Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network: a sex-specific phenomenon. Arch Gen Psychiatry. 2005; 62 :1089–96. [ PubMed ] [ Google Scholar ]
- Thomas P. The stable patient with schizophrenia – from antipsychotic effectiveness to adherence. Eur Neuropsychopharmacol. 2007; 17 :S115–22. [ PubMed ] [ Google Scholar ]
- Tondo L, Baldessarini RJ. Rapid cycling in women and men with bipolar manic-depressive disorders. Am J Psychiatry. 1998; 155 :1434–6. [ PubMed ] [ Google Scholar ]
- Tondo L, Hennen J, Baldessarini RJ. Rapid-cycling bipolar disorder:effects of long-term treatments. Acta Psychiatr Scand. 2003; 108 :4–14. [ PubMed ] [ Google Scholar ]
- Wetzel H, Grunder G, Hillert A, et al. Amisulpride versus flupentixol in schizophrenia with predominantly positive symptomatology – a double-blind controlled study comparing a selective D2-like antagonist to a mixed D1-/D2-like antagonist. The Amisulpride Study Group. Psychopharmacology (Berl) 1998; 137 :223–32. [ PubMed ] [ Google Scholar ]
- Young CM, Findling RL. Pharmacologic treatment of adolescent and child schizophrenia. Expert Rev Neurother. 2004; 4 :53–60. [ PubMed ] [ Google Scholar ]

IMAGES
COMMENTS
A cross-sectional study was held with a majority of patients suffering from psychiatric problems such as bipolar, schizophrenia, schizoaffective, and other depressive disorders.
This article provides an overview of the specificity and continuity of schizophrenia and mood disorders on the basis of biomarkers, such as genes, molecules, cells, circuits, physiology and clinical phenomenology. Overall, the discussions herein provided support for the view that schizophrenia, schizoaffective disorder and bipolar disorder are ...
For this purpose, it focused on four groups of studies including those of BD [type I (BP I) and type II (BP II) disorders], studies of mania, bipolar depression, and mixed episodes. Four types of psychotic symptoms were examined including delusions, hallucinations, mood-congruent and mood-incongruent symptoms, and FRS.
Diagnostic instability of bipolar disorder and schizophrenia is not rare and can be predicted using clinical and demographic factors.
Patients with bipolar disorder demonstrated increased interactions between a different pair of networks when compared to patients with schizophrenia or controls.
Here, we focus on 'late' first onsets, because studies on the effect of reproductive aging on first-onset severe mental illness (that is, schizophrenia spectrum disorders and bipolar disorder ...
Introduction Bipolar disorder and schizophrenia exhibit different patterns of cognitive impairment, with schizophrenia demonstrating more profound deficiencies in verbal memory and bipolar disorder in social cognition. Understanding these patterns may guide the development of interventions to enhance cognition in these disorders.
Psychotic disorders, like schizophrenia and bipolar disorder, disturb particularly human aspects of perception and cognition. The overall burden of suffering for patients, their families, and society is huge.
Methods: This was a retrospective case-control, population-based study including patients aged ≥20 and were diagnosed between 1997 and 2013. Ocular neurovascular diseases diagnosed between 1997 and 2006 and newly diagnosed psychiatric disorders including bipolar disorder (BD), major depressive disorder (MDD), and schizophrenia between 2007 ...
BACKGROUND AND AIM[|]Shared genetic risk between schizophrenia (SCZ) and bipolar disorder (BD) is well-established, yet the extent to which they share environmental risk factors remains unclear. We compare associations between environmental exposures from birth to diagnosis with the risk of SCZ and BD, adjusting for genetic risk.[¤]METHOD[|]We conducted a Swedish register-based nested case ...
Abstract Background: Shared genetic risk between schizophrenia (SCZ) and bipolar disorder (BD) is well-established, yet the extent to which they share environmental risk factors remains unclear. We compare the associations between environmental exposures during childhood/prior to disorder onset with the risk of developing SCZ and BD.
Bipolar disorder and schizophrenia have some similarities, but they are different disorders. Schizophrenia is characterized by continuous or relapsing episodes of psychosis, while bipolar disorder is a mood disorder that can sometimes manifest with psychotic symptoms. Since they can appear alike, bipolar disorder and schizophrenia can be mistaken for each other.
Psychotic disorders/schizophrenia are further known to have overlapping symptoms, and increased frequency in comorbidity can be a diagnostic and therapeutic challenge in clinical practice.
Experts in psychiatry review the case of a 27-year-old woman who presents for evaluation of a complex depressive disorder.
Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia O Agid1,2, B Shapira2,3, J Zislin3 ...
Background Bipolar disorder is a mental illness with a high misdiagnosis rate and commonly misdiagnosed as other mental disorders including depression, schizophrenia, anxiety disorders, obsessive-compulsive disorders, and personality disorders, resulting in the mistreatment of clinical symptoms and increasing of recurrent episodes.
Semantic Scholar extracted view of "Verbal fluency in schizophrenia and bipolar disorder - A longitudinal, family study." by Sussy C. Luperdi et al.
The second section consists of 12 case studies, which are presented in a detailed and articulate manner, spanning four continents. Each case study illustrates in detail a particular sociocultural context that affects the healing process for schizophrenia. Readers can select which case studies to read based on their interest.
Bipolar disorder and schizophrenia both affect your behavior, emotions, and thoughts. But, these mental health conditions have distinct differences.
PDF | On Apr 1, 2008, A. M. Zaharie and others published A family case study on schizophrenia and bipolar disorder: Genotype and Cognition | Find, read and cite all the research you need on ...
Background Psychiatric disorders and ocular neurovascular diseases may share a similar pathophysiological route of vascular structures or neurological changes. The aim of this study is to investigate the association between ocular neurovascular diseases and the risk of major psychiatric disorders. Methods This was a retrospective case-control, population-based study including patients aged ...
We report a case control study in which rates of EPL, due to parental death or permanent separation before the age of 17 years were evaluated in patients with major depression (MD), bipolar disorder (BPD) and schizophrenia (SCZ), compared to individually matched, healthy control subjects (MD-Control, 79 pairs; BPD-Control, 79 pairs; SCZ-Control ...
Methods This was a retrospective case-control, population-based study including patients aged ≥20 and were diagnosed between 1997 and 2013. Ocular neurovascular diseases diagnosed between 1997 and 2006 and newly diagnosed psychiatric disorders including bipolar disorder (BD), major depressive disorder (MDD), and schizophrenia between 2007 and 2013 were registered. Patients were propensity ...
Genetic findings have also revealed the nature of schizophrenia's close relationship to other conditions, particularly bipolar disorder and childhood neurodevelopmental disorders, and provided ...
The terms included in the search strategy were: schizophrenia, schizotypal personality, delusional, schizoaffective, psychotic, bipolar, major depression, obsessive-compulsive, severe mental, OT and intervention. Study selection The study screening was peer-reviewed.
Optical coherence tomography studies identified retinal abnormalities among people with schizophrenia and bipolar disorder.
Case study: Organizing outpatient pharmacological treatment of bipolar disorder in autism, intellectual disability and Phelan-McDermid syndrome (22q13.3 deletion syndrome). ... Differences in daily life executive functioning between people with autism and people with schizophrenia. Journal of Autism and Developmental Disorders, 53(7), 2773-2785.
The differentiation between schizophrenia and bipolar disorder yielded a poor AUC of 0.627. Conclusions: This study highlights the potential of using immune-based measures to build predictive classification models in schizophrenia and bipolar disorder, with IL-6, TNF-ɑ, IFN-γ, QUINO and PICO as key candidates. While machine learning models ...
To determine what type of bipolar disorder a person has, mental health care professionals assess the pattern of symptoms and how impaired the person is during their most severe episodes. Four Types Of Bipolar Disorder. Bipolar I Disorder is an illness in which people have experienced one or more episodes of mania. Most people diagnosed with ...
Successful treatment of psychiatric disorders, including bipolar disorder and schizophrenia, is complicated and is affected by a broad range of factors associated with the diagnosis, choice of treatment and social factors. In these patients, treatment ...