
- Previous Article
- Next Article

Research Design and Methods
Article information, literature review of type 2 diabetes management and health literacy.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Rulla Alsaedi , Kimberly McKeirnan; Literature Review of Type 2 Diabetes Management and Health Literacy. Diabetes Spectr 1 November 2021; 34 (4): 399–406. https://doi.org/10.2337/ds21-0014
Download citation file:
- Ris (Zotero)
- Reference Manager
The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs. These challenges can be even more profound among minority populations and non-English speakers in the United States.
A literature search and standard data extraction were performed using PubMed, Medline, and EMBASE databases. A total of 1,914 articles were identified, of which 1,858 were excluded based on the inclusion criteria, and 46 were excluded because of a lack of relevance to both diabetes management and health literacy. The remaining 10 articles were reviewed in detail.
Patients, including ethnic minorities and non-English speakers, who are engaged in diabetes education and health literacy improvement initiatives and ongoing follow-up showed significant improvement in A1C, medication adherence, medication knowledge, and treatment satisfaction. Clinicians considering implementing new interventions to address diabetes care for patients with low health literacy can use culturally tailored approaches, consider ways to create materials for different learning styles and in different languages, engage community health workers and pharmacists to help with patient education, use patient-centered medication labels, and engage instructors who share cultural and linguistic similarities with patients to provide educational sessions.
This literature review identified a variety of interventions that had a positive impact on provider-patient communication, medication adherence, and glycemic control by promoting diabetes self-management through educational efforts to address low health literacy.
Diabetes is the seventh leading cause of death in the United States, and 30.3 million Americans, or 9.4% of the U.S. population, are living with diabetes ( 1 , 2 ). For successful management of a complicated condition such as diabetes, health literacy may play an important role. Low health literacy is a well-documented barrier to diabetes management and can lead to poor management of medical conditions, low engagement with health care providers (HCPs), increased hospitalizations, and, consequently, higher health care costs ( 3 – 5 ).
The Healthy People 2010 report ( 6 ) defined health literacy as the “degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.” Diabetes health literacy also encompasses a wide range of skills, including basic knowledge of the disease state, self-efficacy, glycemic control, and self-care behaviors, which are all important components of diabetes management ( 3 – 5 , 7 ). According to the Institute of Medicine’s Committee on Health Literacy, patients with poor health literacy are twice as likely to have poor glycemic control and were found to be twice as likely to be hospitalized as those with adequate health literacy ( 8 ). Associations between health literacy and health outcomes have been reported in many studies, the first of which was conducted in 1995 in two public hospitals and found that many patients had inadequate health literacy and could not perform the basic reading tasks necessary to understand their treatments and diagnoses ( 9 ).
Evaluation of health literacy is vital to the management and understanding of diabetes. Several tools for assessing health literacy have been evaluated, and the choice of which to use depends on the length of the patient encounter and the desired depth of the assessment. One widely used literacy assessment tool, the Test of Functional Health Literacy in Adults (TOFHLA), consists of 36 comprehension questions and four numeric calculations ( 10 ). Additional tools that assess patients’ reading ability include the Rapid Estimate of Adult Literacy in Medicine (REALM) and the Literacy Assessment for Diabetes. Tests that assess diabetes numeracy skills include the Diabetes Numeracy Test, the Newest Vital Sign (NVS), and the Single-Item Literacy Screener (SILS) ( 11 ).
Rates of both diabetes and low health literacy are higher in populations from low socioeconomic backgrounds ( 5 , 7 , 12 ). People living in disadvantaged communities face many barriers when seeking health care, including inconsistent housing, lack of transportation, financial difficulties, differing cultural beliefs about health care, and mistrust of the medical professions ( 13 , 14 ). People with high rates of medical mistrust tend to be less engaged in their care and to have poor communication with HCPs, which is another factor HCPs need to address when working with their patients with diabetes ( 15 ).
The cost of medical care for people with diabetes was $327 billion in 2017, a 26% increase since 2012 ( 1 , 16 ). Many of these medical expenditures are related to hospitalization and inpatient care, which accounts for 30% of total medical costs for people with diabetes ( 16 ).
People with diabetes also may neglect self-management tasks for various reasons, including low health literacy, lack of diabetes knowledge, and mistrust between patients and HCPs ( 7 , 15 ).
These challenges can be even more pronounced in vulnerable populations because of language barriers and patient-provider mistrust ( 17 – 19 ). Rates of diabetes are higher among racial and ethnic minority groups; 15.1% of American Indians and Alaskan Natives, 12.7% of Non-Hispanic Blacks, 12.1% of Hispanics, and 8% of Asian Americans have diagnosed diabetes, compared with 7.4% of non-Hispanic Whites ( 1 ). Additionally, patient-provider relationship deficits can be attributed to challenges with communication, including HCPs’ lack of attention to speaking slowly and clearly and checking for patients’ understanding when providing education or gathering information from people who speak English as a second language ( 15 ). White et al. ( 15 ) demonstrated that patients with higher provider mistrust felt that their provider’s communication style was less interpersonal and did not feel welcome as part of the decision-making process.
To the authors’ knowledge, there is no current literature review evaluating interventions focused on health literacy and diabetes management. There is a pressing need for such a comprehensive review to provide a framework for future intervention design. The objective of this literature review was to gather and summarize studies of health literacy–based diabetes management interventions and their effects on overall diabetes management. Medication adherence and glycemic control were considered secondary outcomes.
Search Strategy
A literature review was conducted using the PubMed, Medline, and EMBASE databases. Search criteria included articles published between 2015 and 2020 to identify the most recent studies on this topic. The search included the phrases “diabetes” and “health literacy” to specifically focus on health literacy and diabetes management interventions and was limited to original research conducted in humans and published in English within the defined 5-year period. Search results were exported to Microsoft Excel for evaluation.
Study Selection
Initial screening of the articles’ abstracts was conducted using the selection criteria to determine which articles to include or exclude ( Figure 1 ). The initial search results were reviewed for the following inclusion criteria: original research (clinical trials, cohort studies, and cross-sectional studies) conducted in human subjects with type 2 diabetes in the United States, and published in English between 2015 and 2020. Articles were considered to be relevant if diabetes was included as a medical condition in the study and an intervention was made to assess or improve health literacy. Studies involving type 1 diabetes or gestational diabetes and articles that were viewpoints, population surveys, commentaries, case reports, reviews, or reports of interventions conducted outside of the United States were excluded from further review. The criteria requiring articles to be from the past 5 years and from the United States were used because of the unique and quickly evolving nature of the U.S. health care system. Articles published more than 5 years ago or from other health care systems may have contributed information that was not applicable to or no longer relevant for HCPs in the United States. Articles were screened and reviewed independently by both authors. Disagreements were resolved through discussion to create the final list of articles for inclusion.

PRISMA diagram of the article selection process.
Data Extraction
A standard data extraction was performed for each included article to obtain information including author names, year of publication, journal, study design, type of intervention, primary outcome, tools used to assess health literacy or type 2 diabetes knowledge, and effects of intervention on overall diabetes management, glycemic control, and medication adherence.
A total of 1,914 articles were collected from a search of the PubMed, MEDLINE, and EMBASE databases, of which 1,858 were excluded based on the inclusion and exclusion criteria. Of the 56 articles that met criteria for abstract review, 46 were excluded because of a lack of relevance to both diabetes management and health literacy. The remaining 10 studies identified various diabetes management interventions, including diabetes education tools such as electronic medication instructions and text message–based interventions, technology-based education videos, enhanced prescription labels, learner-based education materials, and culturally tailored interventions ( 15 , 20 – 28 ). Figure 1 shows the PRISMA diagram of the article selection process, and Table 1 summarizes the findings of the article reviews ( 15 , 20 – 28 ).
Findings of the Article Reviews (15,20–28)
SAHLSA, Short Assessment of Health Literacy for Spanish Adults.
Medical mistrust and poor communication are challenging variables in diabetes education. White et al. ( 15 ) examined the association between communication quality and medical mistrust in patients with type 2 diabetes. HCPs at five health department clinics received training in effective health communication and use of the PRIDE (Partnership to Improve Diabetes Education) toolkit in both English and Spanish, whereas control sites were only exposed to National Diabetes Education Program materials without training in effective communication. The study evaluated participant communication using several tools, including the Communication Assessment Tool (CAT), Interpersonal Processes of Care (IPC-18), and the Short Test of Functional Health Literacy in Adults (s-TOFHLA). The authors found that higher levels of mistrust were associated with lower CAT and IPC-18 scores.
Patients with type 2 diabetes are also likely to benefit from personalized education delivery tools such as patient-centered labeling (PCL) of prescription drugs, learning style–based education materials, and tailored text messages ( 24 , 25 , 27 ). Wolf et al. ( 27 ) investigated the use of PCL in patients with type 2 diabetes and found that patients with low health literacy who take medication two or more times per day have higher rates of proper medication use when using PCL (85.9 vs. 77.4%, P = 0.03). The objective of the PCL intervention was to make medication instructions and other information on the labels easier to read to improve medication use and adherence rates. The labels incorporated best-practice strategies introduced by the Institute of Medicine for the Universal Medication Schedule. These strategies prioritize medication information, use of larger font sizes, and increased white space. Of note, the benefits of PCL were largely seen with English speakers. Spanish speakers did not have substantial improvement in medication use or adherence, which could be attributed to language barriers ( 27 ).
Nelson et al. ( 25 ) analyzed patients’ engagement with an automated text message approach to supporting diabetes self-care activities in a 12-month randomized controlled trial (RCT) called REACH (Rapid Education/Encouragement and Communications for Health) ( 25 ). Messages were tailored based on patients’ medication adherence, the Information-Motivation-Behavioral Skills model of health behavior change, and self-care behaviors such as diet, exercise, and self-monitoring of blood glucose. Patients in this trial were native English speakers, so further research to evaluate the impact of the text message intervention in patients with limited English language skills is still needed. However, participants in the intervention group reported higher engagement with the text messages over the 12-month period ( 25 ).
Patients who receive educational materials based on their learning style also show significant improvement in their diabetes knowledge and health literacy. Koonce et al. ( 24 ) developed and evaluated educational materials based on patients’ learning style to improve health literacy in both English and Spanish languages. The materials were made available in multiple formats to target four different learning styles, including materials for visual learners, read/write learners, auditory learners, and kinesthetic learners. Spanish-language versions were also available. Researchers were primarily interested in measuring patients’ health literacy and knowledge of diabetes. The intervention group received materials in their preferred learning style and language, whereas the control group received standard of care education materials. The intervention group showed significant improvement in diabetes knowledge and health literacy, as indicated by Diabetes Knowledge Test (DKT) scores. More participants in the intervention group reported looking up information about their condition during week 2 of the intervention and showed an overall improvement in understanding symptoms of nerve damage and types of food used to treat hypoglycemic events. However, the study had limited enrollment of Spanish speakers, making the applicability of the results to Spanish-speaking patients highly variable.
Additionally, findings by Hofer et al. ( 22 ) suggest that patients with high A1C levels may benefit from interventions led by community health workers (CHWs) to bridge gaps in health literacy and equip patients with the tools to make health decisions. In this study, Hispanic and African American patients with low health literacy and diabetes not controlled by oral therapy benefited from education sessions led by CHWs. The CHWs led culturally tailored support groups to compare the effects of educational materials provided in an electronic format (via iDecide) and printed format on medication adherence and self-efficacy. The study found increased adherence with both formats, and women, specifically, had a significant increase in medication adherence and self-efficacy. One of the important aspects of this study was that the CHWs shared cultural and linguistic characteristics with the patients and HCPs, leading to increased trust and satisfaction with the information presented ( 22 ).
Kim et al. ( 23 ) found that Korean-American participants benefited greatly from group education sessions that provided integrated counseling led by a team of nurses and CHW educators. The intervention also had a health literacy component that focused on enhancing skills such as reading food package labels, understanding medical terminology, and accessing health care services. This intervention led to a significant reduction of 1–1.3% in A1C levels in the intervention group. The intervention established the value of collaboration between CHW educators and nurses to improve health information delivery and disease management.
A collaboration between CHW educators and pharmacists was also shown to reinforce diabetes knowledge and improve health literacy. Sharp et al. ( 26 ) conducted a cross-over study in four primary care ambulatory clinics that provided care for low-income patients. The study found that patients with low health literacy had more visits with pharmacists and CHWs than those with high health literacy. The CHWs provided individualized support to reinforce diabetes self-management education and referrals to resources such as food, shelter, and translation services. The translation services in this study were especially important for building trust with non-English speakers and helping patients understand their therapy. Similar to other studies, the CHWs shared cultural and linguistic characteristics with their populations, which helped to overcome communication-related and cultural barriers ( 23 , 26 ).
The use of electronic tools or educational videos yielded inconclusive results with regard to medication adherence. Graumlich et al. ( 20 ) implemented a new medication planning tool called Medtable within an electronic medical record system in several outpatient clinics serving patients with type 2 diabetes. The tool was designed to organize medication review and patient education. Providers can use this tool to search for medication instructions and actionable language that are appropriate for each patient’s health literacy level. The authors found no changes in medication knowledge or adherence, but the intervention group reported higher satisfaction. On the other hand, Yeung et al. ( 28 ) showed that pharmacist-led online education videos accessed using QR codes affixed to the patients’ medication bottles and health literacy flashcards increased patients’ medication adherence in an academic medical hospital.
Goessl et al. ( 21 ) found that patients with low health literacy had significantly higher retention of information when receiving evidence-based diabetes education through a DVD recording than through an in-person group class. This 18-month RCT randomized participants to either the DVD or in-person group education and assessed their information retention through a teach-back strategy. The curriculum consisted of diabetes prevention topics such as physical exercise, food portions, and food choices. Participants in the DVD group had significantly higher retention of information than those in the control (in-person) group. The authors suggested this may have been because participants in the DVD group have multiple opportunities to review the education material.
Management of type 2 diabetes remains a challenge for HCPs and patients, in part because of the challenges discussed in this review, including communication barriers between patients and HCPs and knowledge deficits about medications and disease states ( 29 ). HCPs can have a positive impact on the health outcomes of their patients with diabetes by improving patients’ disease state and medication knowledge.
One of the common themes identified in this literature review was the prevalence of culturally tailored diabetes education interventions. This is an important strategy that could improve diabetes outcomes and provide an alternative approach to diabetes self-management education when working with patients from culturally diverse backgrounds. HCPs might benefit from using culturally tailored educational approaches to improve communication with patients and overcome the medical mistrust many patients feel. Although such mistrust was not directly correlated with diabetes management, it was noted that patients who feel mistrustful tend to have poor communication with HCPs ( 20 ). Additionally, Latino/Hispanic patients who have language barriers tend to have poor glycemic control ( 19 ). Having CHWs work with HCPs might mitigate some patient-provider communication barriers. As noted earlier, CHWs who share cultural and linguistic characteristics with their patient populations have ongoing interactions and more frequent one-on-one encounters ( 12 ).
Medication adherence and glycemic control are important components of diabetes self-management, and we noted that the integration of CHWs into the diabetes health care team and the use of simplified medication label interventions were both successful in improving medication adherence ( 23 , 24 ). The use of culturally tailored education sessions and the integration of pharmacists and CHWs into the management of diabetes appear to be successful in reducing A1C levels ( 12 , 26 ). Electronic education tools and educational videos alone did not have an impact on medication knowledge or information retention in patients with low health literacy, but a combination of education tools and individualized sessions has the potential to improve diabetes medication knowledge and overall self-management ( 20 , 22 , 30 ).
There were several limitations to our literature review. We restricted our search criteria to articles published in English and studies conducted within the United States to ensure that the results would be relevant to U.S. HCPs. However, these limitations may have excluded important work on this topic. Additional research expanding this search beyond the United States and including articles published in other languages may demonstrate different outcomes. Additionally, this literature review did not focus on A1C as the primary outcome, although A1C is an important indicator of diabetes self-management. A1C was chosen as the method of evaluating the impact of health literacy interventions in patients with diabetes, but other considerations such as medication adherence, impact on comorbid conditions, and quality of life are also important factors.
The results of this work show that implementing health literacy interventions to help patients manage type 2 diabetes can have beneficial results. However, such interventions can have significant time and monetary costs. The potential financial and time costs of diabetes education interventions were not evaluated in this review and should be taken into account when designing interventions. The American Diabetes Association estimated the cost of medical care for people with diabetes to be $327 billion in 2017, with the majority of the expenditure related to hospitalizations and nursing home facilities ( 16 ). Another substantial cost of diabetes that can be difficult to measure is treatment for comorbid conditions and complications such as cardiovascular and renal diseases.
Interventions designed to address low health literacy and provide education about type 2 diabetes could be a valuable asset in preventing complications and reducing medical expenditures. Results of this work show that clinicians who are considering implementing new interventions may benefit from the following strategies: using culturally tailored approaches, creating materials for different learning styles and in patients’ languages, engaging CHWs and pharmacists to help with patient education, using PCLs for medications, and engaging education session instructors who share patients’ cultural and linguistic characteristics.
Diabetes self-management is crucial to improving health outcomes and reducing medical costs. This literature review identified interventions that had a positive impact on provider-patient communication, medication adherence, and glycemic control by promoting diabetes self-management through educational efforts to address low health literacy. Clinicians seeking to implement diabetes care and education interventions for patients with low health literacy may want to consider drawing on the strategies described in this article. Providing culturally sensitive education that is tailored to patients’ individual learning styles, spoken language, and individual needs can improve patient outcomes and build patients’ trust.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors conceptualized the literature review, developed the methodology, analyzed the data, and wrote, reviewed, and edited the manuscript. R.A. collected the data. K.M. supervised the review. K.M. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Portions of this research were presented at the Washington State University College of Pharmacy and Pharmaceutical Sciences Honors Research Day in April 2019.
Email alerts
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 08 November 2019
Type 2 diabetes and pre-diabetes mellitus: a systematic review and meta-analysis of prevalence studies in women of childbearing age in the Middle East and North Africa, 2000–2018
- Rami H. Al-Rifai ORCID: orcid.org/0000-0001-6102-0353 1 ,
- Maria Majeed 1 ,
- Maryam A. Qambar 2 ,
- Ayesha Ibrahim 2 ,
- Khawla M. AlYammahi 2 &
- Faisal Aziz 1
Systematic Reviews volume 8 , Article number: 268 ( 2019 ) Cite this article
11k Accesses
18 Citations
Metrics details
Investing in women’s health is an inevitable investment in our future. We systematically reviewed the available evidence and summarized the weighted prevalence of type 2 diabetes (T2DM) and pre-diabetes mellitus (pre-DM) in women of childbearing age (15–49 years) in the Middle East and North African (MENA) region.
We comprehensively searched six electronic databases to retrieve published literature and prevalence studies on T2DM and pre-DM in women of childbearing age in the MENA. Retrieved citations were screened and data were extracted by at least two independent reviewers. Weighted T2DM and pre-DM prevalence was estimated using the random-effects model.
Of the 10,010 screened citations, 48 research reports were eligible. Respectively, 46 and 24 research reports on T2DM and pre-DM prevalence estimates, from 14 and 10 countries, were included. Overall, the weighted T2DM and pre-DM prevalence in 14 and 10 MENA countries, respectively, were 7.5% (95% confidence interval [CI], 6.1–9.0) and 7.6% (95% CI, 5.2–10.4). In women sampled from general populations, T2DM prevalence ranged from 0.0 to 35.2% (pooled, 7.7%; 95% CI, 6.1–9.4%) and pre-DM prevalence ranged from 0.0 to 40.0% (pooled, 7.9%; 95% CI, 5.3–11.0%). T2DM was more common in the Fertile Crescent countries (10.7%, 95% CI, 5.2–17.7%), followed by the Arab Peninsula countries (7.6%, 95% CI, 5.9–9.5%) and North African countries and Iran (6.5%, 95% CI, 4.3–9.1%). Pre-DM prevalence was highest in the Fertile Crescent countries (22.7%, 95% CI, 14.2–32.4%), followed by the Arab Peninsula countries (8.6%, 95% CI, 5.5–12.1%) and North Africa and Iran (3.3%, 95% CI, 1.0–6.7%).
Conclusions
T2DM and pre-DM are common in women of childbearing age in MENA countries. The high DM burden in this vital population group could lead to adverse pregnancy outcomes and acceleration of the intergenerational risk of DM. Our review presented data and highlighted gaps in the evidence of the DM burden in women of childbearing age, to inform policy-makers and researchers.
Systematic review registration
PROSPERO CRD42017069231
Peer Review reports
The global burden of type 2 diabetes mellitus (T2DM) is rapidly increasing, affecting individuals of all ages. The global T2DM prevalence nearly doubled in the adult population over the past decade from 4.7% in 1980 to 8.5% in 2014 [ 1 ]. The global burden of T2DM in people 20–79 years is further projected to increase to 629 million in 2045 compared to 425 million in 2017 [ 1 ]. Low- and middle-income countries will be the most affected with the rise in the burden of T2DM. For the period between 2017 and 2045, the projected increase in the prevalence of T2DM in the Middle East and North Africa (MENA) region is 110% compared to 16% in Europe, 35% in North Africa and the Caribbean, and 62% in South and Central America [ 1 ]. Pre-diabetes (pre-DM) or intermediate hyperglycaemia is defined as blood glucose levels above the normal range, but lower than DM thresholds [ 1 ]. The burden of pre-DM is increasing worldwide. By 2045, the number of people aged between 20 and 79 years old with pre-DM is projected to increase to 587 million (8.3% of the adult population) compared to 352.1 million people worldwide in 2017 (i.e., 7.3% of the adult population of adults aged 20 to 79 years) [ 1 ]. About three quarters (72.3%) of people with pre-DM live in low- and middle-income countries [ 1 ].
Pre-DM or T2DM are associated with various unfavorable health outcomes. People with pre-DM are at high risk of developing T2DM [ 1 ]. Annually, it is estimated that 5–10% of people with pre-DM will develop T2DM [ 2 , 3 ]. Pre-DM and T2DM are also associated with early onset of nephropathy and chronic kidney disease [ 4 , 5 , 6 , 7 ], diabetic retinopathy [ 6 , 8 , 9 ], and increased risk of macrovascular disease [ 10 , 11 ]. T2DM is also reported to increase the risk of developing active [ 12 ] and latent tuberculosis [ 13 ]. The rising levels of different modifiable key risk factors, mainly body overweight and obesity, driven by key changes in lifestyle, are the attributes behind the continued burgeoning epidemics of pre-DM and T2DM [ 14 , 15 , 16 ]. Women of childbearing age (15–49 years) [ 17 ] are also affected by the global rise in pre-DM and T2DM epidemics. Rising blood glucose levels in women of childbearing age has pre-gestational, gestational, and postpartum consequences, including increased intergenerational risk of DM [ 18 ].
The total population in 20 countries (Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Malta, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Syria, Tunisia, the United Arab Emirates, and Yemen) in the Middle East and North Africa region comprises almost 6.7% (~ 421 million people) of the world’s population, with about 200 million females as of July 1, 2015 [ 19 ]. In adults ≥ 18 years, T2DM prevalence rose sharply by 2.3 times in each of the Eastern Mediterranean regions and the African region, between 1980 and 2014 [ 20 ]. This sharp increase in these two regions is higher than that reported in the region of the Americas (1.7 times), the European region (1.4 times), and the Western Pacific Region (1.9 times) [ 20 ].
Key pre-DM and T2DM risk factors, body overweight and obesity, are highly prevalent in people in the MENA countries. In 2013, the age-standardized prevalence of overweight and obesity among women ≥ 20 years was 65.5% (obese 33.9%) [ 21 ]. The high burden of overweight and obesity in several MENA countries attributed to the interrelated economic, dietary, lifestyle behavioral factors. The nutrition transitions and changes in the food consumption habits were supported by the witnessed economic development in most of the MENA countries. For instance, in the past five decades, the economic development in the Arab Gulf countries linked to the discovery of oil and gas reserves led to changes in eating habits towards the consumption of foods rich in fat and calories as well as increasing behavioral habits towards a sedentary lifestyle [ 22 , 23 ]. This is particularly true with the significant shift from the consumption of traditional low-fat food to fat-rich foods, as well as with a major change from an agricultural lifestyle to an urbanized lifestyle that is often accompanied by decreased levels of physical activity. The urbanized lifestyle increases exposure to fast foods through the high penetration of fast food restaurants serving fat-rich foods, the reliance on automobiles for transport, and the increasing penetration of cell phones, all of which facilitate low levels of physical activity. Globally, physical inactivity is estimated to cause around 27% of diabetes cases [ 24 ]. In eight Arab countries, based on national samples, low levels of physical activity in adults ranged from 32.1% of the population in Egypt in 2011–2012 to as high as 67% of the population in Saudi Arabia in 2005 [ 25 ]. Furthermore, fruit and vegetable consumption is inversely associated with weight gain [ 26 ]. Studies indicated a low intake of fruit and vegetables in some of the MENA countries [ 27 , 28 ]. The growing burden of the possible risk factors of body overweight and obesity in women may further affect and exacerbate the burden of DM and its associated complications in the MENA countries.
To develop effective prevention and control interventions, there is a need for understanding the actual burden of pre-DM and T2DM epidemics in vital population groups, such as women of childbearing age (15–49 years), in the MENA region. Thus, individual studies need to be compiled and summarized. According to our previously published protocol (with a slight deviation) [ 29 ], here, we present the results of the systematically reviewed published quantitative literature (systematic review “1”), to assess the burden (prevalence) of T2DM and pre-DM in women of childbearing age in the MENA region, from 2000 to 2018.
Investing in women’s health paves the way for healthier families and stronger economies. Societies that prioritize women’s health are likely to have better population health overall and to remain more productive for generations to come [ 30 ]. Against this background, our review was aimed at characterizing the epidemiology of T2DM and pre-DM in population groups of women of childbearing age in the MENA through (1) systematically reviewing and synthesizing all available published records of T2DM and pre-DM and (2) estimating the mean T2DM and pre-DM prevalence at national, sub-regional, and regional levels, from January 2000 to July 2018. The findings of the review fill an evidence gap to inform policy-makers on the epidemiologic burden of T2DM and pre-DM in women of childbearing age.
Following our published protocol [ 29 ] that is registered with the International Prospective Registry of Systematic Reviews (PROSPERO registration number “CRD42017069231” dated 12/06/2017), we reported here systematic review “1”. This review adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 guidelines [ 31 , 32 , 33 ]. The PRISMA checklist is provided in the Additional file 1 .
Data source and search strategy
To identify eligible studies on T2DM and pre-DM prevalence measures in MENA countries, we implemented a comprehensive computerized search of six electronic databases (MEDLINE, EMBASE, Web of Science, SCOPUS, Cochrane library, and Academic Search Complete) from January 1, 2000, to July 12, 2018, using variant Medical Subject Headings (MeSH) and free-text (Text) terms. The detailed search strategy is presented in an additional box file (see Additional file 2 ). We also hand-searched the reference lists of eligible studies for further studies that might have been missed.
We defined the participants, exposure, comparator, outcome(s), and type of study “PECO(T)”. The PECO(T) statement provides the framework for the identification and selection of studies for inclusion [ 34 ]. As we were looking for prevalence studies, we only considered participants and the outcomes.
Inclusion and exclusion criteria
Participants : Women of childbearing age were defined according to the World Health Organization (WHO) as women aged between 15 and 49 years (thereafter, women of childbearing age) [ 35 ]. Pregnant women were also considered in this review as long as they were tested for T2DM and/or pre-DM according to what was reported in the individual studies.
Outcomes : T2DM and pre-DM. The included studies should have reported quantitative or calculable pre-DM or T2DM prevalence estimate(s) in women of childbearing age regardless of the sample size, pregnancy status, or pre-DM/T2DM ascertainment methodology, in any of the 20 MENA region countries [ 36 ]. We excluded studies of self-reported pre-DM/T2DM not supported with either anti-DM medications or a documented diagnosis. We also excluded studies on metabolic syndrome as long as there was no clear information on the proportion of women of childbearing age with pre-DM or T2DM. Studies were also excluded if they pooled women of childbearing age with pre-DM/T2DM with other non-communicable diseases in the same category, or together with males, or for each gender separately but without age stratification. We excluded studies with incalculable pre-DM/T2DM prevalence after attempting to contact the authors at least twice with no response.
Types of studies : We included observational studies if they were cross-sectional, comparative cross-sectional, case-control (not comparing T2DM/pre-DM vs. no T2DM/pre-DM), or cohort study designs. We excluded observational studies of other study designs.
Detailed eligibility criteria are available in the published protocol [ 29 ]. The PRISMA flow chart for the selection of studies is shown in Fig. 1 .
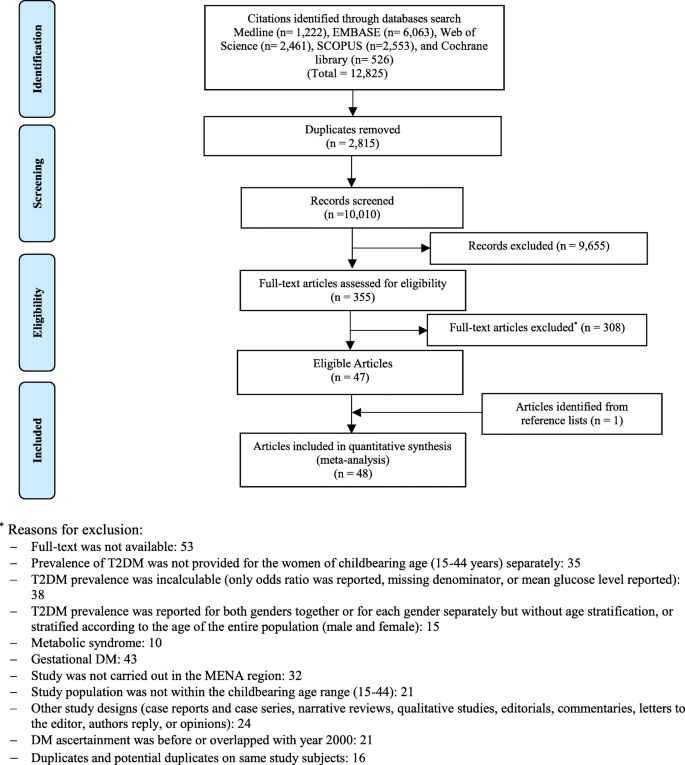
PRISMA flow chart
Identifying eligible studies
Titles and abstracts of the remaining citations were screened independently by four reviewers (AI, KA, MM, and MQ) for any potential study on pre-DM/T2DM in childbearing age women. Full-texts of the identified potentially eligible studies were thoroughly screened and independently assessed by the four reviewers. The qualities of the extracted studies were independently assessed by two other reviewers (RHA and FA). Discrepancies in data extraction were discussed and resolved.
Data extraction
Data from fully eligible studies were extracted into a pre-defined data extraction excel file using a pre-defined list of variables [ 29 ]. Our outcome of interest was the national/regional weighted pooled prevalence of T2DM and pre-DM in women of childbearing age in the MENA. We extracted the following data on the baseline characteristics of the eligible research reports (author names, year of publication, country, city, and study setting), study methodology (design, time period, sampling strategy, and T2DM/pre-DM ascertainment methodology), and study population (age, pregnancy status, co-morbidity, and number of women with the outcomes of interest).
In research reports which provided stratified T2DM/pre-DM prevalence estimates, the prevalence of the total sample was replaced with the stratified estimates keeping the rule of having at least 10 tested subjects per strata, otherwise we extracted information on the whole tested sample. We followed a pre-defined sequential order when extracting stratified prevalence estimates. Outcome measures stratified according to body mass index (BMI) were prioritized, followed by age and year. This prioritization scheme was used to identify the strata with more information on the tested women. When the strata were not prioritized, the overall outcome prevalence measured was extracted. For a research report that stratified the prevalence of the outcome of interest at these different levels (i.e., age and BMI), one stratum per research report was considered and included to avoid double counting. If the outcome measure was ascertained by more than one ascertainment guideline, we extracted relevant information based on the most sensitive and reliable ascertainment assay (i.e., prioritizing fasting blood glucose “FBG” over self-reported DM status), or the most recent and updated criteria (i.e., prioritizing WHO 2006 over WHO 1999 criteria).
We generated a funnel plot to explore the small-study effect on the pooled prevalence estimates. The funnel plot was created by plotting each prevalence measure against its standard error. The asymmetry of the funnel plot was tested using the Egger’s test [ 37 ] (see Additional files 3 and 4 ).
Quality appraisal and risk of bias
We assessed the methodological quality and risk of bias (ROB) of the studies on T2DM or pre-DM prevalence measures using six-quality items adapted from the National Heart, Lung, and Blood Institute (NIH) tool [ 38 ]. Of the 14 items proposed for observational studies on the NIH tool, eight items were not used as they are relevant only for cohort studies assessing the relationship between an exposure and an outcome [ 38 ]. We also assessed the robustness of the implemented sampling methodology and the ascertainment methodology of the measured outcome(s) using three additional quality criteria (sampling methodology, ascertainment methodology, and precision of the estimate). Studies were considered as having “high” precision if at least 100 women tested for T2DM/pre-DM; a reasonable precision, given a pooled prevalence of 7.2% for T2DM or 7.6% for pre-DM estimated in this study, was obtained. We computed the overall proportion of research reports with potentially low risk of bias across each of the nine quality criteria. We also computed the proportion (out of nine) of quality items with potentially a low risk of bias for each of the included research reports.
Quantitative synthesis: meta-analysis
Meta-analyses of the extracted data to estimate the weighted pooled prevalence of T2DM and pre-DM and the corresponding 95% confidence interval (CI) were executed. The variances of prevalence measures were stabilized by the Freeman-Tukey double arcsine transformation method [ 39 , 40 ]. The estimated pooled prevalence measures were weighted using the inverse variance method [ 40 ], and an overall pooled prevalence estimate was generated using a Dersimonian–Laird random-effects model [ 41 ]. Heterogeneity measures were also calculated using the Cochran’s Q statistic and the inconsistency index; I –squared ( I 2 ) [ 42 ]. In addition to the pooled estimates, the prevalence measures were summarized using ranges and medians. The prediction interval, which estimated the 95% interval in which the true effect size in a new prevalence study will lie, was also reported [ 42 , 43 ].
Country-level pooled estimates were generated according to the population group of tested women (general population, pregnant, non-pregnant with history of gestational DM (GDM), and patients with co-morbidity), and the overall country-level pooled prevalence, regardless of the tested population and study period. To assess if the prevalence of T2DM and pre-DM is changing over time, we stratified studies into two time periods: 2000–2009 and 2010–2018. In order not to miss any important data when estimating country-level, sub-regional, and regional prevalence, the period for studies that overlapped these two periods was defined as “overlapping”. In studies with an unclear data collection period, we used the median (~ 2 years) that was obtained from subtracting the year of publication from the year of data collection to estimate the year of data collection in those studies. The “patients with co-morbidity” included women of childbearing age with organ transplant, kidney dialysis, cancer, HIV, chronic obstructive pulmonary disease, polycystic ovarian syndrome (PCOS), or schizophrenia. Categorization of the study period was arbitrary with an aim to estimate the change in T2DM and pre-DM at the country-level and overall, over time.
We also estimated the weighted pooled prevalence, regardless of country, according to the tested women’s population group, study period, T2DM/pre-DM ascertainment guidelines (WHO guidelines, American DM Association (ADA) guidelines, International DM Association (IDF) guidelines, or medical records/anti-DM medications/self-reported), and sample size (< 100 or ≥ 100). The overall weighted pooled prevalence of T2DM and pre-DM regardless of the country, tested population, study period, ascertainment guidelines, and sample size was also generated. Providing pooled estimates regardless of the ascertainment guidelines was justified by the fact that the subject women were defined and treated as T2DM or pre-DM patients following each specific ascertainment guidelines.
To provide prevalence estimates at a more sub-regional level, countries in the MENA region were re-grouped into three sub-regions, namely, “Arab Peninsula, Fertile crescent, and North Africa and Iran.” The pooled prevalence in these three sub-regions was estimated according to the tested population group, study period, ascertainment guidelines, and sample size, as well as overall for each sub-region.
We also estimated the weighted pooled prevalence of T2DM and pre-DM according to age group. We categorized women of childbearing age into three age groups (15–29 years, 30–49 years) and not specified/overlapping. The “not specified/overlapping” category covers women who did fell in the other two age groups. For example, women with an age range of 25–34 years or 18–40 years. The age group weighted pooled prevalence produced regardless of the country, sub-region, and tested population as well as study period.
All meta-analyses were performed using the metaprop package [ 33 ] in Stata/SE v15 [ 44 ].
Sources of heterogeneity: meta-regression
Random-effects univariate and multivariable meta-regression models were implemented to identify sources of between-study heterogeneity and to quantify their contribution to variability in the T2DM and pre-DM prevalence. In univariate meta-regression models, analysis was performed by country, tested population, study period, ascertainment guidelines, and sample size. All variables with a p < 0.1, in the univariate models, were included in the multivariable model. In the final multivariable model, a p value ≤ 0.05 was considered statistically significant, contributing to heterogeneity in prevalence estimates.
All meta-regression analyses were performed using the metareg package in Stata/SE v15 [ 44 ].
Search and eligible research reports
Of the 12,825 citations retrieved from the six databases, 48 research reports were found eligible (Fig. 1 ); 46 reported T2DM prevalence [ 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 ] while 24 reported pre-DM prevalence [ 48 , 49 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 60 , 62 , 63 , 66 , 67 , 70 , 73 , 75 , 81 , 85 , 88 , 89 , 90 ].
Scope of reviewed T2DM reports
The 46 research reports on T2DM prevalence yielded 102 T2DM prevalence studies. The 46 reports were from 14 countries (Algeria, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Morocco, Oman, Qatar, Saudi Arabia, Tunisia, the United Arab Emirates [UAE], and Yemen); ranging by year between 2000 in Saudi Arabia [ 79 ] and 2018 in UAE [ 81 ]. Sixteen (34.9%) research reports were reported in Saudi Arabia [ 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 ], followed by 19.6% in the UAE [ 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 ], and 15.2% in Iran [ 47 , 48 , 49 , 50 , 51 , 52 , 53 ]. Over one third (37.3%) of the yielded 102 T2DM prevalence studies were in Saudi Arabia. Of the 102 T2DM prevalence studies, 79.4% were in women sampled from general populations and 11.8% in pregnant women. Over two thirds (69.6%) of the T2DM prevalence studies were in or before 2009 and 82.4% tested ≥ 100 women (Table 1 ).
Pooled T2DM prevalence
In the 14 countries, the weighted T2DM prevalence in women of childbearing age estimated at 7.5% (95% CI, 6.1–9.0%, I 2 , 98.2%) (Table 2 , Fig. 2 ). The weighted T2DM prevalence was not significantly different ( p = 0.4) in studies reported between 2000 and 2009 (7.9%, 95% CI, 6.2–9.7%, I 2 , 97.9%) and studies reported between 2010 and 2018 (5.8%, 95% CI, 3.4–8.7%, I 2 , 95.4%) (Table 2 ). The weighted T2DM prevalence was higher in women with an age range of 15–19 years (10.9%, 95% CI, 8.8–13.3%, I 2 , 97.9%) than women with an age range of 30–49 years (2.5%, 95% CI, 1.8–3.2%, I 2 , 83.6%) (see Additional file 5 ).
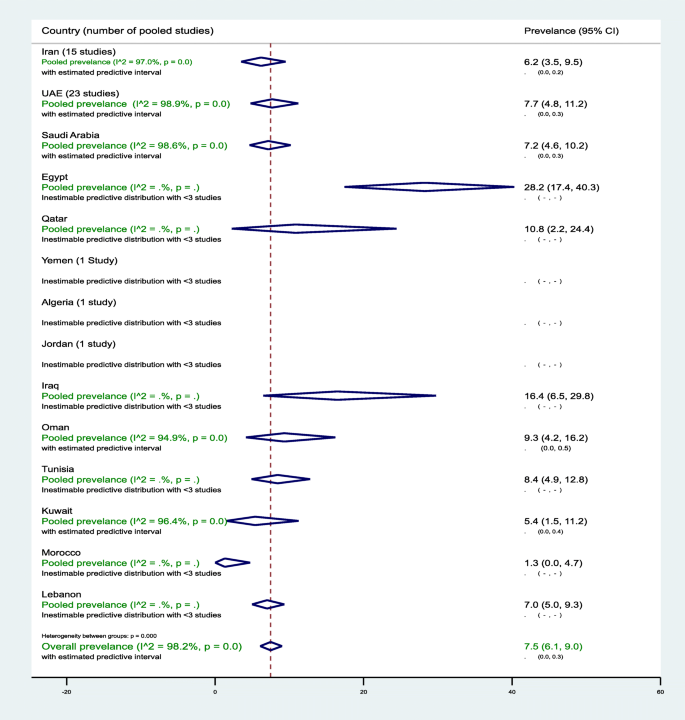
Forest plot of the meta-analyses for the 14 MENA countries’ studies on T2DM
Pooled findings of 102 T2DM prevalence estimates reported in 14 countries in the MENA region. The individual 102 estimates and their 95% confidence interval (CI) omitted to fit the plot. The diamond is centered on the summary effect estimate, and the width indicates the corresponding 95% CI. UAE, United Arab Emirates; T2DM, type 2 diabetes mellitus; MENA, Middle East and Northern Africa
The highest two weighted T2DM estimates were observed in infertile women of childbearing age in Egypt (28.2%, 95% CI, 17.4–40.3%) and in non-pregnant women with a history of GDM in Iran (24.7%, 95% CI, 18.5–31.5%) (Table 2 ). In general populations, the weighted T2DM prevalence ranged between 1.3% (95% CI, 0.0–4.7%) in 2001–2002 in Morocco [ 60 ] and 16.4% (95% CI, 6.5–29.8%, I 2 , 96.5%) in Iraq in 2007 [ 55 ] and in 2011–2012 [ 54 ]. In Saudi Arabia, in women of childbearing age sampled from general populations, the pooled T2DM prevalence estimated at 8.0% (95% CI, 5.3–11.3%, I 2 , 96.5%) (Table 1 ). In Saudi Arabia, the weighted T2DM prevalence in women of childbearing age, regardless of source of population and timeline, estimated at 7.2% (95% CI, 4.6–10.2%, I 2 , 98.6%) (Table 2 ). In Oman, the weighted T2DM prevalence in women of childbearing age sampled from general populations estimated at 8.0% (95% CI, 2.9–15.4%, I 2 , 95.9%) in 2000. In Qatar, the weighted T2DM was prevalence in women of childbearing age sampled from general populations 10.7% (95% CI, 2.2–24.4%, I 2 , 93.7%) between 2007 and 2008. In the UAE, in women of childbearing age sampled from general populations, the pooled T2DM prevalence estimated at 8.0% (95% CI, 4.8–11.9%, I 2 , 98.9%) that declined from 9.4% (95% CI, 5.6–14.1%, I 2 , 95.1%) between 2000 and 2009 to 6.0% (95% CI, 3.3–6.5%, I 2 , 90.5%) between 2010 and 2018 (Table 2 ).
Sub-regional pooled T2DM prevalence
The pooled T2DM prevalence measures estimated at 6.5% (95% CI, 4.3–9.1%, I 2 , 96.0%) in North African countries including Iran, 10.7% (95% CI 5.2–17.7%, I 2 , 90.7%) in the Fertile Crescent countries, and 7.6% (95% CI, 5.9–9.5%, I 2 , 98.5%) in the Arabian Peninsula countries (see Additional file 6 ).
Additional file 7 shows figures presenting the sub-regional-weighted prevalence of T2DM (Fig. 1 ) in women of childbearing age from 2000 to 2009 and from 2010 to 2018. Additional file 8 shows figures presenting timeline view of the weighted prevalence of T2DM (Fig. 1 ) by publication year.
Meta-bias in T2DM prevalence
The asymmetry in the funnel plot examining the small-study effects on the pooled T2DM prevalence among women of childbearing age indicates evidence for the presence of a small-study effect (Egger’s test p < 0.0001). The funnel plot is presented in an additional figure file (see Additional file 3 ).
Predictors of heterogeneity in T2DM prevalence
In the univariate meta-regression models, all variables except study period, T2DM ascertainment criteria, and sample size were associated with T2DM prevalence at p value < 0.1. In the adjusted meta-regression model, none of the included variables was significantly associated with T2DM prevalence at p value < 0.05. In two studies in infertile women of childbearing age in Egypt, the T2DM prevalence was higher (adjusted odds ratio (aOR), 5.26, 95% CI, 0.87–32.1) compared to women of childbearing age in Saudi Arabia. Overall, compared to women of childbearing age sampled from general populations, T2DM prevalence in non-pregnant women of childbearing age with a history of GDM was 234% higher (aOR, 3.34%, 95% CI, 0.90–12.41) (see Additional file 9 ).
Scope of reviewed pre-DM reports
The 24 research reports on pre-DM prevalence yielded 52 pre-DM prevalence studies and were from 10 countries (Iran, Iraq, Jordan, Kuwait, Morocco, Oman, Qatar, Saudi Arabia, UAE, and Yemen); ranging by year between 2002 in Oman [ 62 ] and 2018 in Saudi Arabia [ 81 ]. Thirteen (25.0%), 11 (21.2%), and 11 (21.2%) of the pre-DM prevalence studies were from Iran, Saudi Arabia, and UAE, respectively. Approximately 87.0% of the pre-DM prevalence studies tested women of childbearing age sampled from general populations. The pre-DM prevalence estimates ranged from 0.0% in various age groups in multiple countries [ 51 , 60 , 70 ] to 40.0% in Iraq in women aged 20–39 years, recruited from the general population [ 55 ] (Table 1 ).
Pooled pre-DM prevalence
In the 10 countries, the weighted pre-DM prevalence in women of childbearing age was estimated at 7.6% (95% CI, 5.2–10.4%, I 2 , 99.0%) (Table 3 , Fig. 3 ). The weighted pre-DM prevalence in studies reported between 2000 and 2009 (4.8%, 95% CI 4.0–7.8%, I 2 , 97.1%) was significantly lower ( p < 0.001) than the weighted prevalence estimated in studies reported between 2010 and 2018 (9.3%, 95%, 4.7–15.2%, I 2 , 93.9%) (Table 3 ). Weighted pre-DM prevalence was 1.70 times higher in women with an age range of 15–19 years (9.0%, 95% CI, 4.9–14.1%, I 2 , 99.2%) than women with an age range of 30–49 years (5.3%, 95% CI, 1.8–10.3%, I 2 , 99.0%) (see Additional file 5 ).
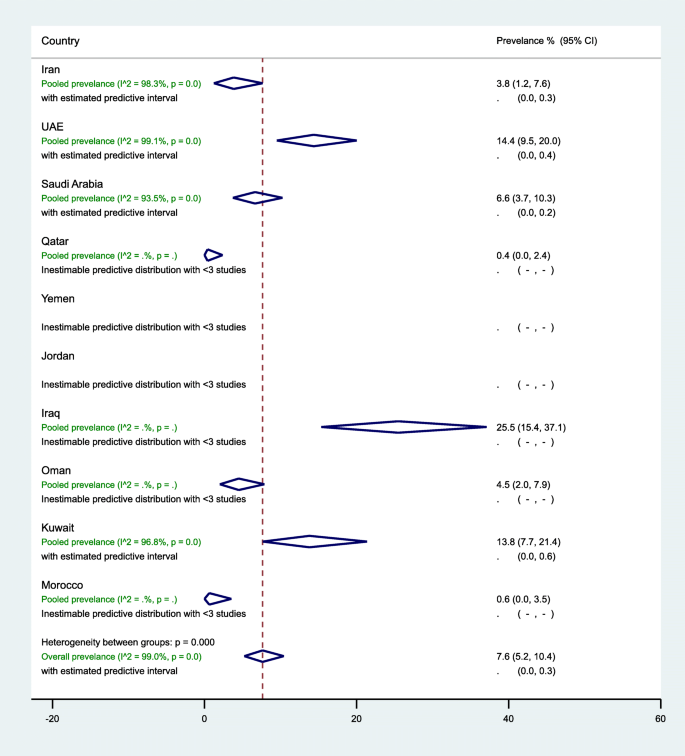
Forest plot of the meta-analyses for the 10 MENA countries’ studies on pre-DM pooled findings of 52 pre-DM prevalence estimates reported in 10 countries in the MENA region. The individual 52 estimates and their 95% confidence interval (CI) omitted to fit the plot. The diamond is centered on the summary effect estimate, and the width indicates the corresponding 95% CI. UAE, United Arab Emirates; pre-DM, pre-diabetes mellitus; MENA, Middle East and Northern Africa
In general populations, the highest three weighted pre-DM prevalence estimates were observed in women of childbearing age in Iraq (25.5%, 95% CI, 15.4–37.1%, I 2 , 92.2%), followed by UAE (15.5%, 95% CI, 10.5–21.2%, I 2 , 99.0%), and Kuwait (13.8%, 95% CI, 7.7–21.4%, I 2 , 96.8%) (Table 3 ). In 13 studies in Iran (7 from the general population), the prevalence of pre-DM ranged from 0.0 to 21.4% with an overall weighted prevalence of 3.8% (95% CI, 1.2–7.6%, I 2 , 98.3%). The 11 pre-DM studies in Saudi Arabia were in women of childbearing age sampled from the general population, with an overall weighted pre-DM prevalence of 6.6% (95% CI, 3.7–10.3%, I 2 , 93.5%) (2000–2009: 9.4% vs. 2010–2018: 4.4%). Regardless of the tested population in UAE, the weighted pre-DM prevalence was 6.6% (95% CI, 5.1–8.3%, I 2 , 65.6%) in studies reported between 2000 and 2009, and 12.0% (95% CI, 8.9–15.5%) in studies reported between 2010 and 2018 with an overall pre-DM prevalence of 14.4% (95% CI, 9.5–20.0%, I 2 , 99.1%) (Table 3 ).
Sub-regional pooled pre-DM prevalence
The pooled pre-DM prevalence estimated at 3.3% (95% CI, 1.0–6.7%, I 2 , 98.1%) in North African countries including Iran, 22.7% (95% CI, 14.2–32.4%, I 2 , 90.0%) in the Fertile crescent countries, and 8.6% (95% CI, 5.5–12.1%, I 2 , 99.1%) in the Arabian Peninsula countries (see Additional files 10 ). Additional file 7 shows figures presenting the sub-regional weighted prevalence of pre-DM (Fig. 2 ) in women of childbearing age from 2000 to 2009 and from 2010 to 2018. Additional file 8 shows figures presenting timeline view of the weighted prevalence of pre-DM (Fig. 2 ) by publication year.
Meta-bias in pre-DM prevalence measures
The asymmetry in the funnel plot examining the small-study effects on the pooled pre-DM prevalence among women of childbearing age indicates evidence for the presence of a small-study effect (Egger’s test p < 0.0001). The funnel plot is presented in an additional figure file (Additional file 4 ).
Predictors of heterogeneity in pre-DM prevalence
Country, study period, and pre-DM ascertainment criteria were associated with a difference in the pre-DM prevalence in the univariate meta-regression models at p value < 0.1. In the univariate meta-regression models, pre-DM prevalence in women of childbearing age in Iraq was 424% higher compared to such women in Saudi Arabia (OR, 5.24, 95% CI, 1.45–18.94%). This significant association turned insignificant in the multivariable model (aOR, 2.20, 95% CI, 0.52–10.82%). In the multivariable model, compared to Saudi Arabia, pre-DM prevalence in women of childbearing age was 70% lower in Iran (aOR, 0.30, 95% CI, 0.11–0.79%) and 88% lower in Morocco (aOR, 0.12, 95% CI, 0.01–0.91%) (see Additional file 11 ).
Quality assessment of the T2DM/pre-DM research reports
Findings of our summarized and research report-specific quality assessments for relevant DM prevalence studies can be found in Additional file 12 . Briefly, all the 48 research reports clearly stated their research questions or objectives, clearly specified and defined their study populations, and selected or recruited the study subjects from the same or similar populations. There was a clear gap in the reporting or justifying of the sample size calculation in 79.2% of the research reports. The majority (87.5%) of the research reports tested ≥ 100 women of childbearing age, and they were classified as having high precision.
Overall, the 48 research reports were of reasonable quality with potentially low ROB in an average of 7.2 items (range, 6–9). Four (8.3%) of the 48 reports had potentially low ROB in all the measured nine quality items [ 66 , 82 , 83 , 86 ] (see Additional file 12 ).
We provided, to our knowledge, the first regional study that comprehensively reviewed and estimated the regional, sub-regional, and country-level burden of T2DM and pre-DM in various populations of women of childbearing age in the MENA. Based on the available data from 14 and 10 studies in MENA countries, the present findings document the comparable burden of T2DM (7.5%, 95% CI 6.9–9.0%) and pre-DM (7.6%, 95% CI 5.2–10.4%) in women of childbearing age. The estimated prevalence of T2DM and pre-DM in 14 countries in the MENA is similar to the estimated worldwide crude diabetes prevalence of 8.2% (95% credible interval (CI) 6.6–9.9%) in adult women in 2014 (age-standardized 7.9%, 95% CI 6.4–9.7%) [ 91 ]. The T2DM and pre-DM prevalence in women of childbearing age varied across the three sub-regions in the MENA, by population group, time period, DM ascertainment criteria, and sample size. The obvious common prevalence of T2DM and pre-DM in women of childbearing age in the MENA countries reflects the highest prevalence of adult diabetes estimated for the MENA [ 91 ]. In this region, the crude diabetes prevalence in adult women increased from 5.0% in 1980 to 9.0% in 2014 [ 91 ]. This increase in diabetes prevalence among adult populations in the MENA over time is higher than many other regions including Europe and Central and West Africa [ 91 ]. The highest national adult diabetes prevalence estimates documented in the MENA is 5–10 times greater than the lowest national prevalence estimates documented in Western European countries [ 91 ].
T2DM is a significant public health problem in both developed and developing countries that can lead to various health complications including increased overall risk of dying prematurely [ 20 ]. The common burden of T2DM and pre-DM in women of childbearing age, which is reflected in the high burden of adult diabetes in this region [ 91 ], might be mainly driven by the sociodemographic changes in this region. In recent decades, there was an increase in median age, sedentary lifestyle, and physical inactivity in the MENA [ 92 ]. These lifestyle changes are linked to an increase in the burden of body overweight and obesity that are shared predisposing factors for pre-DM and T2DM [ 20 ]. At the population level, physical inactivity was very common in many MENA countries (Saudi Arabia 67.6% in 2005; Kuwait 62.6% in 2014; Qatar 45.9% in 2012; Egypt 32.1% in 2011–2012; Iraq 47.0% in 2015) [ 25 ]. The burden of body overweight and obesity is higher in many low-income and middle-income countries in the MENA than in Europe and Asia Pacific countries [ 93 ]. Obesity in women in several Middle Eastern countries was 40–50% [ 93 ]. The age-standardized prevalence of obesity was 32.0% in Egypt, 35.5% in Jordan, 30.4% in Iraq, 32.5% in Libya, and 35.4% in Saudi Arabia [ 94 ]. In Tunisia, 43.7% and 24.1% of 35–70-year-old females in urban and rural areas, respectively, were obese [ 95 ]. In 2016, in almost all of the countries in MENA, the mean BMI for people aged ≥ 18 years was ≥ 25.0 [ 96 ].
To curb the burden of DM and its associated complications in women of childbearing age in the MENA countries, our results suggest three main implications for care. First, based on the estimated 5–10% progression rate from pre-DM to T2DM [ 3 , 10 ], out of the 47,958 tested women of childbearing age for pre-DM (Table 3 ), we estimate that 2398 to 4796 women are expected to progress to T2DM. This risk of progression to T2DM could be reduced through lifestyle and drug-based interventions as it was reported elsewhere [ 97 , 98 , 99 ]. In England, 55–80% of participants with hyperglycemia at baseline had normal glycaemia at 10 year follow-up [ 3 ]. The high burden of DM along with pre-DM in women of childbearing age could accelerate maternal complications including GDM leading to increased intergenerational risk of DM. Programs to halt the growing epidemic of DM among different population groups could start by addressing the key risk factors including sedentary lifestyle and increased body weight. Addressing this problem would require social and public policies and efforts to reduce the national and regional burden of increased body weight and obesity through enhancing healthy eating behaviors and physical activity. Second, there is a critical need for strengthened surveillance systems that match the scale and nature of the DM epidemic in women of childbearing age in the MENA. Enhancing early detection and management of high-risk individuals requires accessible and affordable health care systems, outreach campaigns to raise public awareness, and social and medical support to induce and maintain a healthy lifestyle. Adult people at increased risk of T2DM and pre-DM can be predicted based on good screening tools from the Centers for Disease Control and Prevention (CDC) [ 100 ] and the American Diabetes Association (T2DM Risk Test) [ 101 ]. Early screening and detection will require government-funded prevention programs. Third, controlling the burden of T2DM and pre-DM in MENA countries requires strong and successful partnerships between public health and clinical departments. Physicians have a fundamental role in the care of individual patients to screen, diagnose, and treat both pre-DM and T2DM in clinical settings. In addition, physicians have a fundamental role in working to raise awareness and participating in developing prevention programs and engaging communities. Concerted efforts and partnership between physicians, health departments, and community agencies are needed to strengthen health care services, encouraging and facilitating early screening and detection, and promoting healthy diets and physical activity.
Providing summary estimates and up-to-date mapping gaps-in-evidence of T2DM and pre-DM prevalence in women of childbearing age in different MENA countries provides the opportunities for future public health interventions and research to better characterize the T2DM and pre-DM epidemiology nationally and regionally. Nevertheless, present review findings suggest that the DM burden in women of childbearing age in MENA countries is capturing only the tip of the iceberg. Identifying gaps-in-evidence through systematically reviewing and summarizing the literature has public health research implications. Our review shows that in many countries, the estimation of the burden of T2DM or pre-DM in women of childbearing age in general populations occurred more than a decade ago (Table 1 ). Additionally, the review shows that there was no data on the burden of T2DM and pre-DM in women of childbearing age in several countries in the MENA region. This lack of evidence on a key public heath outcome requires a strongly resourced research capacity and research funding schemes. There is evidence that federally funded research can impact important health issues that affect a large segment of the population [ 102 ].
This robust approach to the literature search and review as well as in retrieving and extracting relevant data from the published literature allowed us to provide summary estimates on the burden of T2DM and pre-DM in women of childbearing age from the 14 and 10 countries in the MENA, respectively. Once the diagnosis was established, regardless of the ascertainment criteria, patients were treated as having diabetes or pre-diabetes. Thus, generating pooled estimates, regardless of the DM ascertainment criteria, stratified according to various population groups, provided more insights into the actual burden of T2DM and pre-DM in various populations of women of childbearing age. The meta-regression analysis identified sources of variations in T2DM and pre-DM prevalence and sources of between-study heterogeneity in prevalence estimates. (Additional files 9 and 11 show these in more detail). The country-stratified and population-stratified T2DM and pre-DM prevalence reports revealed gaps in evidence that can help strengthen research and DM control programs in the most affected countries and populations. The use of probability sampling was very common in the studies included, which may provide broader insights on the representation of our findings to the general or specific group of women of childbearing age at the national, but not at the regional, level.
Limitations
There are important but unavoidable limitations when interpreting the results of our review. Despite the estimated DM prevalence, the actual DM burden could have been underestimated, at country, sub-regional, or regional level, due to several reasons. The inaccessibility of data on pre-DM or T2DM in women of childbearing age from several countries in the MENA may not necessarily mean an actual lack of data. To meet the aim of our review of estimating the burden of pre-DM and T2DM in women of childbearing age, in several published studies reviewed, women of childbearing age were found to have been combined with those of other age groups or with men. The presented overall pooled estimates, regardless of the tested population group, should not be interpreted as the total burden of the outcome at the population level. Utilizing data on T2DM and pre-DM from only 14 and 10 countries may limit the findings from being generalizable to the entire MENA region. Although we followed a thorough and well-defined search strategy, there is a potential of publication bias as shown in funnel plots (Additional files 3 and 4 ). The estimated T2DM and pre-DM prevalence suggest that only the tip of the iceberg was captured. The presented estimates may not be representative of the true prevalence for each population. This underestimation may be particularly true in low-resource settings where necessary resources and capacity in investigating pre-DM at the community level are lacking. The wide array of blood glucose cut-off points and criteria used for T2DM and pre-DM ascertainment also suggests that overestimation and underestimation bias cannot be excluded. Unless estimated from individual population-based studies only, the presented weighted pooled estimates at the country, sub-regional, or regional level should not be interpreted as the burden of the measured outcomes at the population level. Also, the presented pooled estimates according to the two time periods, from 2000 to 2009 and from 2010 to 2018, should not be interpreted as an over-time change in the burden of the measured outcomes. While our meta-analyses revealed substantial heterogeneity across studies, the meta-regression analyses identified the potential sources of between-study heterogeneity within the framework of the present study and the level of detail that can be used in describing these sources (Tables 1 and 2 ). Thus, much of the variability in T2DM and pre-DM prevalence across studies might remain unexplained.
Despite these potential limitations, our study provided a characterization of the scale of T2DM and pre-DM among women of childbearing age in several MENA countries based on the best available evidence. Data presented in this review can be used to (a) understand the burden of T2DM and pre-DM among a vital population group and to identify at high-risk populations within this specific population group; (b) guide the planning, implementation, and evaluation of programs to prevent and control DM; (c) implement immediate public health actions to prioritize the allocation of public health resources; and (d) formulate research hypotheses and provide a basis for epidemiologic studies. Future research opportunities should prioritize large country-level and multicenter comparable studies, to determine the prevalence of T2DM and pre-DM in various population groups of women of childbearing age. A definitive characterization of the burden of DM in women of childbearing age at the regional and sub-regional level would require comparable and empirical studies using standardized methodology and comparable DM ascertainment assays.
In conclusion, women of childbearing age in the MENA region bear an appreciable burden of T2DM and pre-DM. The estimated burden of T2DM and pre-DM was higher in the Arabian Peninsula and Fertile Crescent countries compared to the rest of the MENA countries identified with prevalence estimates in this review. Although both T2DM (7.5%) and pre-DM (7.6%) had similar overall estimated prevalence, there is need for a more focused attention on early detection and control by public health authorities to avoid DM-associated pre-gestational, gestational, and post-gestational complications. Country-level early DM detection and control programs should consider the key risk factors of DM, mainly the growing burden of body overweight and obesity. Furthermore, facilitating high-quality research and surveillance programs in countries with limited data on DM prevalence and reporting of DM prevalence estimates in women of childbearing age warrant focus.
Availability of data and materials
The datasets used and/or analyzed during the current study and its supplementary information files are available from the corresponding author on reasonable request.
Abbreviations
American DM association
Adjusted odds ratio
Confidence interval
Diabetes mellitus
Gestational diabetes mellitus
International Diabetes Mellitus Association
Middle East and North Africa
Medical Subject Headings
National Heart, Lung, and Blood Institute
Participants, exposure, comparator, and outcome
- Pre-diabetes mellitus
Preferred Reporting Items for Systematic Review and Meta-Analysis
Risk of bias
- Type 2 diabetes
United Arab Emirates
World Health Organization
International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels: International Diabetes Federation, 2017. https://diabetesatlas.org/resources/2017-atlas.html Accessed 5 Nov 2018.
Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–9.
Article CAS PubMed Google Scholar
Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med. 2007;24(2):200–7.
Metcalf PA, Baker JR, Scragg RK, Dryson E, Scott AJ, Wild CJ. Microalbuminuria in a middle-aged workforce. Effect of hyperglycemia and ethnicity. Diabetes Care. 1993;16(11):1485–93.
Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The inter-tribal Heart project. J Am Soc Nephrol. 2002;13(6):1626–34.
Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23(8):1113–8.
Plantinga LC, Crews DC, Coresh J, Miller ER 3rd, Saran R, Yee J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5(4):673–82.
Article PubMed PubMed Central Google Scholar
Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care. 2007;30(10):2708–15.
Article PubMed Google Scholar
Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287(19):2528–33.
Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, obesity, and lifestyle study (AusDiab). Circulation. 2007;116(2):151–7.
Brunner EJ, Shipley MJ, Witte DR, Fuller JH, Marmot MG. Relation between blood glucose and coronary mortality over 33 years in the Whitehall study. Diabetes Care. 2006;29(1):26–31.
Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One. 2017;12(11):e0187967. https://doi.org/10.1371/journal.pone.0187967 .
Article CAS PubMed PubMed Central Google Scholar
Lee MR, Huang YP, Kuo YT, Luo CH, Shih YJ, Shu CC, et al. Diabetes mellitus and latent tuberculosis infection: a systematic review and metaanalysis. Clin Infect Dis. 2017;64(6):719–27.
PubMed Google Scholar
Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol. 2014;176:149–52.
InterAct C, Romaguera D, Norat T, Wark PA, Vergnaud AC, Schulze MB, et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56:1520–30.
Article CAS Google Scholar
World Health Organisation. Sexual and reproductive health. 2015. http://www.who.int/reproductivehealth/topics/infertility/definitions/en/ . Accessed 5 Feb 2019.
Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–6.
United Nations. Total Population - Both Sexes. World Population Prospects: The; 2015. pp. Revision 2016. https://esa.un.org/unpd/wpp/Download/Standard/Population/ . .
World Health Organization. Global report on diabetes. 2016. https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=B27DA6B4FB2DCD29CA71FB7C373A17FA?sequence=1 . Accessed 30 Jan 2019.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–81.
Galal O. Nutrition-related health patterns in the Middle East. Asia Pac J Clin Nutr. 2003;12(3):337–43 PubMed PMID: 14505998 .
Ng SW, Zaghloul S, Ali HI, Harrison G, Popkin BM. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12(1):1–13.
World Health Organization. Health education and promotion. Physical activity. Available at: http://www.emro.who.int/health-education/physical-activity/background.html . Accessed 15 Jan 2019.
Sharara E, Akik C, Ghattas H, Makhlouf OC. Physical inactivity, gender and culture in Arab countries: a systematic assessment of the literature. BMC Public Health. 2018;18(1):639.
Bes-Rastrollo M, Martinez-Gonzalez MA, Sanchez-Villegas A, de la Fuente AC, Martinez JA. Association of fiber intake and fruit/vegetable consumption with weight gain in a Mediterranean population. Nutrition. 2006;22(5):504–11.
Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN study. Bull World Health Organ. 2007;85(1):19–26.
Nasreddine L, Mehio-Sibai A, Mrayati M, Adra N, Hwalla N. Adolescent obesity in Syria: prevalence and associated factors. Child Care Health Dev. 2010;36(3):404–13.
Al-Rifai RH, Aziz F. Prevalence of type 2 diabetes, prediabetes, and gestational diabetes mellitus in women of childbearing age in Middle East and North Africa, 2000-2017: protocol for two systematic reviews and meta-analyses. Syst Rev. 2018;1:96.
Article Google Scholar
Onarheim KH, Iversen JH, Bloom DE. Economic benefits of investing in women's health: a systematic review. PLoS One. 2016;11(3):e0150120.
Article PubMed PubMed Central CAS Google Scholar
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39.
Woodruff TJ, Sutton P. The navigation guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect. 2014;122(10):1007–14.
http://www.who.int/reproductivehealth/topics/infertility/definitions/en/ . .
The World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lendinggroups . Accessed 10 Nov 2017.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55.
National Heart, Lung, and blood institute. Quality assessment tool for observational cohort and cross-sectional studies https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools . .
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607–11.
Miller JJ. The inverse of the freeman –Tukey double arcsine transformation. The American Statistician. 1978;32(4):138
Google Scholar
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Borenstein MHL, Higgins JP, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
Book Google Scholar
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
StataCorp. Stata statistical software: release 15. College Station: StataCorp LLC; 2017.
Salima T, Mounira K, Nadjia D. Assessment of nutritional status of pregnant women attending the City Tebessa PMI (Algeria). Natl J Physiol Pharm Pharmacol. 2011;1(2):97–105.
Eldesoky A-EE, Gad YZ, Ahmed N. Nonalcoholic fatty liver disease in young adult Egyptian women with polycystic ovary syndrome. Egyptian Liver J. 2013;3:15–9.
Ebrahimi H, Emamian MH, Hashemi H, Fotouhi A. High incidence of diabetes mellitus among a middle-aged population in Iran: a longitudinal study. Can J Diabetes. 2016;40(6):570–5.
Valizadeh M, Alavi N, Mazloomzadeh S, Piri Z, Amirmoghadami H. The risk factors and incidence of type 2 diabetes mellitus and metabolic syndrome in women with previous gestational diabetes. Int J Endocrinol Metab. 2015;13(2):e21696.
Hossein-Nezhad A, Mirzaei K, Maghbooli Z, Larijani B. Maternal glycemic status in GDM patients after delivery. Iran J Diabetes Lipid Disorders. 2009;8(1):95–104.
Azimi-Nezhad M, Ghayour-Mobarhan M, Safarian M, Esmailee H, Parizadeh SM, Rajabi-Moghadam M, et al. Anthropometric indices of obesity and the prediction of cardiovascular risk factors in an Iranian population. ScientificWorld J. 2009;9:424–30.
Azimi-Nezhad M, Ghayour-Mobarhan M, Parizadeh MR, Safarian M, Esmaeili H, Parizadeh SM, et al. Prevalence of type 2 diabetes mellitus in Iran and its relationship with gender, urbanisation, education, marital status and occupation. Singap Med J. 2008;49(7):571–6.
CAS Google Scholar
Hadaegh F, Bozorgmanesh MR, Ghasemi A, Harati H, Saadat N, Azizi F. High prevalence of undiagnosed diabetes and abnormal glucose tolerance in the Iranian urban population: Tehran Lipid and Glucose Study. BMC Public Health. 2008;8:176.
Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract. 2005;69(3):279–86.
Mansour AA, Al-Maliky AA, Kasem B, Jabar A, Mosbeh KA. Prevalence of diagnosed and undiagnosed diabetes mellitus in adults aged 19 years and older in Basrah. Iraq Diabetes Metab Syndr Obes. 2014;7:139–44.
Mansour AA, Wanoose HL, Hani I, Abed-Alzahrea A, Wanoose HL. Diabetes screening in Basrah, Iraq: a population-based cross-sectional study. Diabetes Res Clin Pract. 2008;79(1):147–50.
Abu-Zaiton A, Al-Fawwaz A. Prevalence of diabetes, obesity, hypertension and associated factors among students of Al-albayt University, Jordan. World J Med Sci. 2013;9(1):49–54.
Ahmed F, Waslien C, Al-Sumaie MA, Prakash P, Allafi A. Trends and risk factors of hyperglycemia and diabetes among Kuwaiti adults: National Nutrition Surveillance Data from 2002 to 2009. BMC Public Health. 2013;13:103.
Diejomaoh M, Jirous J, Al-Azemi M, Gupta M, Al-Jaber M, Farhat R, et al. Insulin resistance in women with recurrent spontaneous miscarriage of unknown aetiology. Med Princ Pract. 2007;16(2):114–8.
Tohme RA, Jurjus AR, Estephan A. The prevalence of hypertension and its association with other cardiovascular disease risk factors in a representative sample of the Lebanese population. J Hum Hypertens. 2005;19(11):861–8.
Rguibi M, Belahsen R. Prevalence and associated risk factors of undiagnosed diabetes among adult Moroccan Sahraoui women. Public Health Nutr. 2006;9(6):722–7.
Gowri V, Mathew M, Gravell D, AlFalahi K, Zakwani I, Ganguly SS, et al. Protein Z levels in pregnant Omani women: correlation with pregnancy outcome. J Thromb Thrombolysis. 2011;32(4):453–8.
Al-Lawati JA, Al Riyami AM, Mohammed AJ, Jousilahti P. Increasing prevalence of diabetes mellitus in Oman. Diabet Med. 2002;19(11):954–7.
Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009;84(1):99–106.
Al-Nazhan SA, Alsaeed SA, Al-Attas HA, Dohaithem AJ, Al-Serhan MS, Al-Maflehi NS. Prevalence of apical periodontitis and quality of root canal treatment in an adult Saudi population. Saudi Med J. 2017;38(4):413–21.
Saeed AAW. Combined systolic diastolic hypertension among adults in Saudi Arabia: prevalence, risk factors and predictors: results of a national survey. Int J Med Res Health Sci. 2017;6(6):171–6.
Bahijri SM, Jambi HA, Al Raddadi RM, Ferns G, Tuomilehto J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi Arabia--A Community-Based Survey. PLoS One. 2016;11(4):e0152559. https://doi.org/10.1371/journal.pone.0152559 .
Al-Rubeaan K, Al-Manaa HA, Khoja TA, Ahmad NA, Al-Sharqawi AH, Siddiqui K, et al. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2015;7(5):622–32.
Serehi AA, Ahmed AM, Shakeel F, Alkhatani K, El-Bakri NK, Buhari BA, et al. A comparison on the prevalence and outcomes of gestational versus type 2 diabetes mellitus in 1718 Saudi pregnancies. Int J Clin Exp Med. 2015;8(7):11502–7.
PubMed PubMed Central Google Scholar
Al-Rubeaan K, Al-Manaa HA, Khoja TA, Youssef AM, Al-Sharqawi AH, Siddiqui K, et al. A community-based survey for different abnormal glucose metabolism among pregnant women in a random household study (SAUDI-DM). BMJ Open. 2014;4(8):e005906.
Amin TT, Al Sultan AI, Mostafa OA, Darwish AA, Al-Naboli MR. Profile of non-communicable disease risk factors among employees at a Saudi university. Asian Pac J Cancer Prev. 2014;15(18):7897–907.
Wahabi HA, Esmaeil SA, Fayed A, Al-Shaikh G, Alzeidan RA. Pre-existing diabetes mellitus and adverse pregnancy outcomes. BMC Res Notes. 2012;5:496.
Saeed AA. Association of tobacco products use and diabetes mellitus-results of a national survey among adults in Saudi Arabia. Balkan Med J. 2012;29(3):247–51. https://doi.org/10.5152/balkanmedj.2012.035 .
Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9:76.
Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31(1):19–23.
Al-Baghli NA, Al-Ghamdi AJ, Al-Turki KA, Al Elq AH, El-Zubaier AG, Bahnassy A. Prevalence of diabetes mellitus and impaired fasting glucose levels in the Eastern Province of Saudi Arabia: results of a screening campaign. Singap Med J. 2010;51(12):923–30.
Al-Qahtani DA, Imtiaz ML, Saad OS, Hussein NM. A comparison of the prevalence of metabolic syndrome in Saudi adult females using two definitions. Metab Syndr Relat Disord. 2006;4(3):204–14.
Shaaban LA, Al-Saleh RA, Alwafi BM, Al-Raddadi RM. Associated risk factors with ante-partum intra-uterine fetal death. Saudi Med J. 2006;27(1):76–9 PubMed PMID: 16432598.
Habib FA. Incidence of post cesarean section wound infection in a tertiary hospital, Riyadh, Saudi Arabia. Saudi Med J. 2002;23(9):1059–63.
Karim A, Ogbeide DO, Siddiqui S, Al-Khalifa IM. Prevalence of diabetes mellitus in a Saudi community. Saudi Med J. 2000;21(5):438–42.
CAS PubMed Google Scholar
Ben Romdhane H, Ben Ali S, Aissi W, Traissac P, Aounallah-Skhiri H, Bougatef S, et al. Prevalence of diabetes in Northern African countries: the case of Tunisia. BMC Public Health. 2014;14:86.
Sulaiman N, Albadawi S, Abusnana S, Mairghani M, Hussein A, Al Awadi F, et al. High prevalence of diabetes among migrants in the United Arab Emirates using a cross-sectional survey. Sci Rep. 2018;8(1):6862.
Shah SM, Ali R, Loney T, Aziz F, ElBarazi I, Al Dhaheri S, et al. Prevalence of Diabetes among migrant women and duration of residence in the United Arab Emirates: a cross sectional study. PLoS One. 2017;12(1):e0169949.
Al Dhaheri AS, Mohamad MN, Jarrar AH, Ohuma EO, Ismail LC, Al Meqbaali FT, et al. A cross-sectional study of the prevalence of metabolic syndrome among young female Emirati adults. PLoS One. 2016;11(7):e0159378.
Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes mellitus prevalence: effect of the laboratory analytical variation. Diabetes Res Clin Pract. 2015;109(3):493–9.
Hajat C, Harrison O, Al SZ. Weqaya: a population-wide cardiovascular screening program in Abu Dhabi, United Arab Emirates. Am J Public Health. 2012;102(5):909–14.
Baynouna LM, Revel AD, Nagelkerke NJ, Jaber TM, Omar AO, Ahmed NM, et al. High prevalence of the cardiovascular risk factors in Al-Ain, United Arab Emirates. An emerging health care priority. Saudi Med J. 2008;29(8):1173–8.
Saadi H, Carruthers SG, Nagelkerke N, Al-Maskari F, Afandi B, Reed R, et al. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract. 2007;78(3):369–77.
Malik M, Bakir A, Saab BA, King H. Glucose intolerance and associated factors in the multi-ethnic population of the United Arab Emirates: results of a national survey. Diabetes Res Clin Pract. 2005;69(2):188–95.
Agarwal MM, Punnose J, Dhatt GS. Gestational diabetes: implications of variation in post-partum follow-up criteria. Eur J Obstet Gynecol Reprod Biol. 2004;113(2):149–53.
Gunaid AA, Assabri AM. Prevalence of type 2 diabetes and other cardiovascular risk factors in a semirural area in Yemen. East Mediterr Health J. 2008;14(1):42–56.
Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30.
Engelhardt H, Schulz F, Büyükkeçeci Z. Demographic and Human Development in the Middle East and North Africa. https://doi.org/10.20378/irbo-50993 . Bamberg: University of Bamberg Press, Universitätsbibliothek Bamberg; 2018. 88 Seiten : Illustrationen, Diagramme p.
Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96.
World health Organization. Global Health Observatory (GHO) data. Overweight and obesity. Noncommunicable diseases. Obesity among adults. 2016. Available at: https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/.1-8 Accessed 10 Dec 2018.
El Ati J, Traissac P, Delpeuch F, Aounallah-Skhiri H, Beji C, Eymard-Duvernay S, et al. Gender obesity inequities are huge but differ greatly according to environment and socio-economics in a North African setting: a national cross-sectional study in Tunisia. PLoS One. 2012;7(10):e48153.
World health Organization. Global Health Observatory (GHO) data. Overweight and obesity. Noncommunicable diseases. Mean body mass index (BMI) trends among adults. Available at https://www.who.int/gho/ncd/risk_factors/overweight_obesity/bmi_trends_adults/en/ Accessed 10 Dec 2018.
Diabetes Prevention Program Research G, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97.
Centers for Disease Control and Prevention. National Diabetes Prevention Program. https://www.cdc.gov/diabetes/prevention/index.html Accessed 10 Mar 2019.
American Diabetes Association. Type 2 Diabetes Risk Test. http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/?loc=atrisk-slabnav Accessed 2 Mar 2019.
Drees BM, Yun S. Reducing the burden of diabetes mellitus in the state of Missouri: a call to action. Mo Med. 2016;113(5):352–7.
Download references
Acknowledgments
Authors are grateful to the Institute of Public Health, College of Medicine and Health Sciences at the United Arab Emirates University for the infrastructure provided.
This systematic review was funded by the Summer Undergraduate Research Experience (SURE) PLUS-Grant of the United Arab Emirates University, 2017 (Research grant: 31M348). The funder had no role in the study design, collection, analysis, or interpretation of the data, nor in writing and the decision to submit this article for publication.
Author information
Authors and affiliations.
Institute of Public Health, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 15551, Al Ain, United Arab Emirates
Rami H. Al-Rifai, Maria Majeed & Faisal Aziz
Department of Biology, College of Sciences, United Arab Emirates University, P.O. Box 15551, Al Ain, United Arab Emirates
Maryam A. Qambar, Ayesha Ibrahim & Khawla M. AlYammahi
You can also search for this author in PubMed Google Scholar
Contributions
RHA conceptualized and designed the study. AI, MM, MQ, KA, and FA assessed the eligibility of the retrieved citations in the titles/abstracts and full-text screening phases. RHA, MM, and FA critically assessed the eligible studies and extracted data. RHA analyzed and interpreted the data. RHA drafted the manuscript. All authors critically reviewed the manuscript. RHA read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rami H. Al-Rifai .
Ethics declarations
Ethics approval and consent to participate.
There are no primary data used in this review. There is no need for any ethical approval or an exemption letter according to the United Arab Emirates University-Human Research Ethics Committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA checklist.
Additional file 2.
Search strategies for the six databases, from January 1, 2000 to July 12, 2018.
Additional file 3
Funnel plots examining small-study effects on the pooled T2DM prevalence among women of childbearing age. Egger’s test p <0.0001.
Additional file 4
Funnel plots examining small-study effects on the pooled pre-DM prevalence among women of childbearing age. Egger’s test p <0.0001.
Additional file 5.
Weighted prevalence of T2DM and pre-DM in childbearing age women in MENA countries according to age group.
Additional file 6.
Sub-regional weighted prevalence of T2DM in women of childbearing age according to the tested population, data collection period, T2DM ascertainment, sample size, and overall, in 14 MENA countries.
Additional file 7.
Sub-regional weighted prevalence of T2DM (Figure 1 ) and pre-DM (Figure 2 ) in women of childbearing age from 2000 to 2009 and from 2010 to 2018. Square represents the estimated prevalence and lines around the square represent the upper and lower limit of the 95% confidence interval of the prevalence.
Additional file 8.
Timeline view of the weighted prevalence of T2DM (Figure 1 ) and pre-DM (Figure 2 ) in women of childbearing age, by publication year.
Additional file 9.
Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on T2DM prevalence in women of childbearing age by the different measured characteristics.
Additional file 10.
Sub-regional weighted prevalence of pre-DM in childbearing age women according to the tested population, data collection period, Pre-DM ascertainment, sample size, and overall, in the four sub regions of the 10 MENA countries.
Additional file 11.
Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on pre-DM prevalence in women of childbearing age by the different measured characteristics.
Additional file 12.
Quality assessment of the 48 research reports included in the analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Al-Rifai, R.H., Majeed, M., Qambar, M. et al. Type 2 diabetes and pre-diabetes mellitus: a systematic review and meta-analysis of prevalence studies in women of childbearing age in the Middle East and North Africa, 2000–2018. Syst Rev 8 , 268 (2019). https://doi.org/10.1186/s13643-019-1187-1
Download citation
Received : 17 March 2019
Accepted : 07 October 2019
Published : 08 November 2019
DOI : https://doi.org/10.1186/s13643-019-1187-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Women of childbearing age
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Risk models and scores...
Risk models and scores for type 2 diabetes: systematic review
- Related content
- Peer review
- Douglas Noble , lecturer 1 ,
- Rohini Mathur , research fellow 1 ,
- Tom Dent , consultant 2 ,
- Catherine Meads , senior lecturer 1 ,
- Trisha Greenhalgh , professor 1
- 1 Centre for Primary Care and Public Health, Barts and the London School of Medicine and Dentistry, London E1 2AT, UK
- 2 PHG Foundation, Cambridge, UK
- Correspondence to: D Noble d.noble{at}qmul.ac.uk
- Accepted 5 October 2011
Objective To evaluate current risk models and scores for type 2 diabetes and inform selection and implementation of these in practice.
Design Systematic review using standard (quantitative) and realist (mainly qualitative) methodology.
Inclusion criteria Papers in any language describing the development or external validation, or both, of models and scores to predict the risk of an adult developing type 2 diabetes.
Data sources Medline, PreMedline, Embase, and Cochrane databases were searched. Included studies were citation tracked in Google Scholar to identify follow-on studies of usability or impact.
Data extraction Data were extracted on statistical properties of models, details of internal or external validation, and use of risk scores beyond the studies that developed them. Quantitative data were tabulated to compare model components and statistical properties. Qualitative data were analysed thematically to identify mechanisms by which use of the risk model or score might improve patient outcomes.
Results 8864 titles were scanned, 115 full text papers considered, and 43 papers included in the final sample. These described the prospective development or validation, or both, of 145 risk prediction models and scores, 94 of which were studied in detail here. They had been tested on 6.88 million participants followed for up to 28 years. Heterogeneity of primary studies precluded meta-analysis. Some but not all risk models or scores had robust statistical properties (for example, good discrimination and calibration) and had been externally validated on a different population. Genetic markers added nothing to models over clinical and sociodemographic factors. Most authors described their score as “simple” or “easily implemented,” although few were specific about the intended users and under what circumstances. Ten mechanisms were identified by which measuring diabetes risk might improve outcomes. Follow-on studies that applied a risk score as part of an intervention aimed at reducing actual risk in people were sparse.
Conclusion Much work has been done to develop diabetes risk models and scores, but most are rarely used because they require tests not routinely available or they were developed without a specific user or clear use in mind. Encouragingly, recent research has begun to tackle usability and the impact of diabetes risk scores. Two promising areas for further research are interventions that prompt lay people to check their own diabetes risk and use of risk scores on population datasets to identify high risk “hotspots” for targeted public health interventions.
Introduction
The prevalence of diabetes is rising rapidly throughout the world. 1 By 2010 its prevalence in the adult populations of the United Kingdom, the United States, mainland China, and the United Arab Emirates had exceeded 7%, 2 11%, 3 15%, 4 and 17%, 5 respectively. Americans born in 2000 or later have a lifetime risk of more than one in three of developing diabetes. 6 Type 2 diabetes (which accounts for over 95% of diabetes worldwide) results from a complex gene-environment interaction for which several risk factors, such as age, sex, ethnicity, family history, obesity, and hypertension, are well documented. The precise interaction of these and other risk factors with one another is, however, a complex process that varies both within and across populations. 7 8 9 10 11 Epidemiologists and statisticians are striving to produce weighted models that can be presented as scores to reflect this complexity but which at the same time are perceived as sufficiently simple, plausible, affordable, and widely implementable in clinical practice. 12 13
Cohort studies have shown that early detection of established diabetes improves outcome, although the evidence base for screening the entire population is weak. 14 15 The proportion of cases of incident type 2 diabetes in people with impaired glucose tolerance or impaired fasting glucose levels was reduced in landmark trials from China, 16 Finland, 17 and the United States 18 by up to 33%, 50%, and 58%, respectively, through lifestyle changes (increased exercise, weight loss) or pharmacotherapy, or both, although changes may be more modest in a non-trial population. Some have argued that because combining known risk factors predicts incident diabetes at least as effectively as impaired glucose metabolism, a diabetes risk score may be a better and more practical means of identifying people for preventive interventions than either a glucose tolerance test or a fasting blood glucose level. 19 Others favour targeting the assessment of diabetes risk in those with established impaired glucose metabolism on the basis that interventions in this group are particularly effective. 20
Risk models and scores first emerged for cardiovascular disease, and these are widely used in clinical and public health practice. In the United Kingdom, for example, all electronic patient record systems in general practice offer the facility to calculate the Framingham score, a patient’s risk of a cardiovascular event within 10 years. This risk score features in many guidelines and decision pathways (such as the cut-off for statin therapy 21 ), and general practitioners receive financial rewards for calculating it. 22 In contrast, although numerous models and scores have been developed for diabetes risk, we found limited evidence for use of these as part of a formal health policy, guideline, or incentive scheme for practitioners in any country (one Australian scheme incentivises general practitioners’ measurement of the risk of diabetes in adults aged 40-49 23 ). This is perhaps surprising, given that morbidity and mortality from cardiovascular disease has been decreasing in many countries since the 1970s, 24 whereas those from diabetes continue to increase. 3
A diabetes risk score is an example of a prognostic model. 25 Such scores should ideally be developed by taking a large, age defined population cohort of people without diabetes, measuring baseline risk factors, and following the cohort for a sufficiently long time to see who develops diabetes. 26 Although prospective longitudinal designs in specially assembled cohorts are expensive, difficult, and time consuming to execute, cross sectional designs in which risk factors are measured in a population including people both with and without diabetes are methodologically inferior. They use prevalence as a proxy for incidence and conflate characteristics of people with diabetes with risk factors in those without diabetes, and thus are incapable of showing that a putative risk factor predated the development of diabetes. In practice, researchers tend to take one of two approaches: they either study a cohort of people without diabetes, which was assembled some years previously with relevant baseline metrics for some other purpose (for example, the British Regional Heart Study 27 ), or they analyse routinely available data, such as electronic patient records. 8 Both approaches are potentially susceptible to bias.
Some diabetes risk scores are intended to be self administered using questions such as “have you ever been told you have high blood pressure?” Scores that rely entirely on such questions may be hosted on the internet (see for example www.diabetes.org.uk/riskscore ). Some researchers have used self completion postal questionnaires as the first part of a stepwise detection programme. 28 To the extent that these instruments are valid, they can identify two types of people: those who already have diabetes whether or not they know it (hence the questionnaire may serve as a self administered screening tool for undiagnosed diabetes) and those at high risk of developing diabetes—that is, it may also serve as a prediction tool for future diabetes. Prevalence rates for diabetes derived from self assessment studies thus cannot be compared directly with the rate of incident diabetes in a prospective longitudinal sample from which those testing positive for diabetes at baseline have been excluded.
A good risk score is usually defined as one that accurately estimates individuals’ risk—that is, predictions based on the score closely match what is observed (calibration); the score distinguishes reliably between high risk people, who are likely to go on to develop the condition, and low risk people, who are less likely to develop the condition (discrimination); and it performs well in new populations (generalisability). Validating a risk model or score means testing its calibration and discrimination either internally (by splitting the original sample, developing the score on one part and testing it on another), temporally (re-running the score on the same or a similar sample after a time period), or, preferably, externally (running the score on a new population with similar but not identical characteristics from the one on which it was developed). 26 29 Caution is needed when extrapolating a risk model or score developed in one population or setting to a different one—for example, secondary to primary care, adults to children, or one ethnic group to another. 30
Risk scores and other prognostic models should be subject to “impact studies”—that is, studies of the extent to which the score is actually used and leads to improved outcomes. Although most authors emphasise quantitative evaluation of impact such as through cluster randomised controlled trials, 30 much might also be learnt from qualitative studies of the process of using the score, either alone or as an adjunct to experimental trials. One such methodology is realist evaluation, which considers the interplay between context, mechanism (how the intervention is perceived and taken up by practitioners), and outcome. 31 In practice, however, neither quantitative nor qualitative studies of impact are common in the assessment of risk scores. 30
We sought to identify, classify, and evaluate risk models and scores for diabetes and inform their selection and implementation in practice. We wanted to determine the key statistical properties of published scores for predicting type 2 diabetes in adults and how they perform in practice. Hence we were particularly interested in highlighting those characteristics of a risk score that would make it fit for purpose in different situations and settings. To that end we reviewed the literature on development, validation, and use of such scores, using both quantitative data on demographics of populations and statistical properties of models and qualitative data on how risk scores were perceived and used by practitioners, policy makers, and others in a range of contexts and systems.
Theoretical and methodological approach
We followed standard methodology for systematic reviews, summarised in guidance from a previous study and the York Centre for Reviews and Dissemination. 32 33 The process was later extended by drawing on the principles of realist review, an established form of systematic literature review that uses mainly qualitative methods to produce insights into the interaction between context, mechanism, and outcome, hence explaining instances of both success and failure. 34 Our team is leading an international collaborative study, the Realist and Meta-narrative Evidence Synthesis: Evolving Standards (RAMESES) to develop methodological guidance and publication standards for realist review. 35
Search strategy
We identified all peer reviewed cohort studies in adults over age 18 that had derived or validated, or both, a statistically weighted risk model for type 2 diabetes in a population not preselected for known risk factors or disease, and which could be applied to another population. Studies were included that had developed a new risk model based on risk factors and that used regression techniques to weight risk factors appropriately, or validated an existing model on a new population, or did both. Exclusion criteria were cross sectional designs, studies that had not finished recruiting, studies on populations preselected for risk factors or disease, studies that did not link multiple risk factors to form a scoring system or weighted model, screening or early detection studies, genetic studies, case series, studies on under 18s, animal studies, and studies that applied a known risk model or score to a population but did not evaluate its statistical potential.
In January 2011 we undertook a scoping search, beginning with sources known to the research team and those recommended by colleagues. We used the 29 papers from this search to develop the definitive protocol, including search terms and inclusion and exclusion criteria. In February 2011 a specialist librarian designed a search strategy (see web extra) with assistance from the research team. Key words were predict, screen, risk, score, [type two] diabetes, model, regression, risk assessment, risk factor, calculator, analysis, sensitivity and specificity, ROC and odds ratio. Both MESH terms and text words were used. Titles and abstracts were searched in Medline, PreMedline, Embase, and relevant databases in the Cochrane Library from inception to February 2011, with no language restrictions.
Search results from the different databases were combined in an endnote file and duplicates removed electronically and manually. In February and March 2011 two researchers independently scanned titles and abstracts and flagged potentially relevant papers for full text analysis.
Two researchers independently read the interim dataset of full text papers and reduced this to a final dataset of studies, resolving disagreements by discussion. Bilingual academic colleagues translated non-English papers and extracted data in collaboration with one of the research team. To identify recently published papers two researchers independently citation tracked the final dataset of studies in Google Scholar. Reference lists of the final dataset and other key references were also scanned.
Quantitative data extraction and analysis
Properties of included studies were tabulated on an Excel spreadsheet. A second researcher independently double checked the extraction of primary data from every study. Discrepancies were resolved by discussion. Where studies trialled multiple models with minimal difference in the number of risk factors, a judgment was made to extract data from the authors’ preferred models or (if no preferences were stated in the paper) the ones judged by two researchers to be the most complete in presentation of data or statistical robustness. Data extraction covered characteristics of the population (age, sex, ethnicity, etc), size and duration of study, completeness of follow-up, method of diagnosing diabetes, details of internal or external validation, or both, and the components and metrics used by authors of these studies to express the properties of the score, especially their calibration and discrimination—for example, observed to predicted ratios, sensitivity and specificity, area under the receiver operating characteristic curve. We aimed to use statistical meta-analysis where appropriate and presented heterogeneous data in disaggregated form.
Qualitative data extraction and analysis
For the realist component of the review we extracted data and entered these on a spreadsheet under seven headings (box 1).
Box 1: Categories for data entry
Intended users.
Authors’ assumptions (if any) about who would use the risk score, on which subgroups or populations
Proposed action based on the score result
Authors’ assumptions (if any) on what would be offered to people who score above the designated cut-off for high risk
Authors’ hypothesised (or implied) mechanism by which use of the score might improve outcomes for patients
Authors’ adjectives to describe their risk model or score
Relative advantage
Authors’ claims for how and in what circumstances their model or score outperforms previous ones
Authors’ stated concerns about their model or score
Real world use, including citation tracking
Actual data in this paper or papers citing it on use of the score in the real world
One researcher extracted these data from our final sample of papers and another checked a one third sample of these. Our research team discussed context-mechanism-outcome interactions hypothesised or implied by authors and reread the full sample of papers with all emerging mechanisms in mind to explore these further.
Impact analysis
We assessed the impact of each risk score in our final sample using three criteria: any description in the paper of use of the score beyond the population for whom it was developed and validated; number of citations of the paper in Google Scholar and number of these that described use of the score in an impact study; and critical appraisal of any impact studies identified on this citation track. In this phase we were guided by the question: what is the evidence that this risk score has been used in an intervention which improved (or sought to improve) outcomes for individuals at high risk of diabetes?
Prioritising papers for reporting
Given the large number of papers, statistical models, and risk scores in our final sample, we decided for clarity to highlight a small number of scores that might be useful to practising clinicians, public health specialists, or lay people. Adapting previous quality criteria for risk scores, 26 we favoured those that had external validation by a separate research team on a different population (generalisability), statistically significant calibration, a discrimination greater than 0.70, and 10 or fewer components (usability).
Figure 1 ⇓ shows the flow of studies through the review. One hundred and fifteen papers were analysed in detail to produce a final sample of 43. Of these 43 papers, 18 described the development of one or more risk models or scores, 8 27 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 17 described external validation of one or more models or scores on new populations, 9 10 19 52 53 54 55 56 57 58 59 60 61 62 63 64 65 and eight did both. 7 66 67 68 69 70 71 72 In all, the 43 papers described 145 risk models and scores, of which 94 were selected for extraction of full data (the other 51 were minimally different, were not the authors’ preferred model, or lacked detail or statistical robustness). Of the final sample of 94 risk models, 55 were derivations of risk models on a base population and 39 were external validations (of 14 different models) on new populations. Studies were published between 1993 and 2011, but most appeared in 2008-11 (fig 2 ⇓ ). Indeed, even given that weaker cross sectional designs had been excluded, the findings suggest that new risk models and scores for diabetes are currently being published at a rate of about one every three weeks.
Fig 1 Flow of studies through review
- Download figure
- Open in new tab
- Download powerpoint
Fig 2 Publication of diabetes risk models and scores 1990-2010. Eleven new risk models and scores had been published in the first five months of 2011
Table 1 ⇓ gives full details of the studies in the sample, including the origin of the study, setting, population, methodological approach, duration, and how diabetes was diagnosed. The studies were highly heterogeneous. Models were developed and validated in 17 countries representing six continents (30 in Europe, 25 in North America, 21 in Asia, 8 in Australasia, 8 in the Middle East, 1 in South America, and 1 in Africa).
Summary of 43 papers from which 94 diabetes risk models or scores were identified for systematic review
- View inline
Comparisons across studies were problematic owing to heterogeneity of data and highly variable methodology, presentation techniques, and missing data. Cohorts ranged in size from 399 to 2.54 million. The same data and participants were often included in several different models in the same paper. Ten research populations were used more than once in different papers. 9 10 27 37 41 42 44 46 47 48 49 51 52 53 54 55 56 63 64 65 66 70 71 In total, risk models were tested on 6.88 million participants, although this figure includes duplicate tests on the same dataset. Participants aged 18 to 98 were studied for periods ranging from 3.15 to 28 years. Completeness of follow-up ranged from 54% to 99% and incidence of diabetes across the time periods studied ranged from 1.3% to 20.9%.
None of the models in the sample was developed on a cohort recruited prospectively for the express purpose of devising it. Rather, all authors used the more pragmatic approach of retrospectively studying a research dataset that had been assembled some years previously for a different purpose. Forty two studies excluded known diabetes in the inception cohort. Diagnosis of diabetes in a cohort at inception and completion of the study was done in different ways, including self report, patient questionnaires, clinician diagnosis, electronic code, codes from the International Classification of Diseases , disease or drug registers, diabetes drugs, dietary treatment, fasting plasma glucose levels, oral glucose tolerance test, and measurement of haemoglobin A 1c . In some studies the method was not stated. Half the studies used different diagnostic tests at inception and completion of the study.
One third of the papers focused almost exclusively on the statistical properties of the models. Many of the remainder had a clinician (diabetologist or general practitioner) as coauthor and included an (often short and speculative) discussion on how the findings might be applied in clinical practice. Three described their score as a “clinical prediction rule.” 45 51 59
Quantitative findings
Table 2 ⇓ gives details of the components of the 94 risk models included in the final sample and their statistical properties—including (where reported) their discrimination, calibration, sensitivity, specificity, positive and negative predictive value, and area under the receiver operating characteristic curve. Many papers offered additional sophisticated statistical analysis, although there was no consistency in the approach used or statistical tests. Heterogeneity of data (especially demographic and ethnic diversity of validation cohorts and different score components) in the primary studies precluded formal meta-analysis.
Key characteristics of 94 diabetes risk models or scores included in systematic review
All 94 models presented a combination of risk factors as significant in the final model, and different models weighted different components differently. The number of components in a single risk score varied from 3 to 14 (n=84, mean 7.8, SD 2.6). The seven risk scores that were classified as having high potential for use in practice offered broadly similar components and had similar discriminatory properties (area under receiver operating characteristic curve 0.74-0.85, table 4). Overall, the areas under the receiver operating characteristic curve ranged from 0.60 to 0.91. Certain components used in some models (for example, biomarkers) are rarely available in some pathology laboratories and potentially too expensive for routine use. Some models that exhibited good calibration and discrimination on the internal validation cohort performed much less well when tested on an external cohort, 62 67 suggesting that the initial model may have been over-fitted by inclusion of too many variables that had only minor contributions to the total risk. 73 Although this study did not seek out genetic components, those studies that had included genetic markers alongside sociodemographic and clinical data all found that the genetic markers added little or nothing to the overall model. 9 10 36 50
Reporting of statistical data in some studies was incomplete—for example, only 40 of the 94 models quantified any form of calibration statistic. Forty three presented sensitivity and specificity, 27 justified the rationale for cut-off points, 22 presented a positive predictive value, 19 presented a negative predictive value, and 26 made some attempt to indicate the percentage of the population that would need clinical follow-up or testing if they scored as “high risk.” Some models performed poorly—for example, there was a substantial gap between expected and observed numbers of participants who developed diabetes over the follow-up period. The false positive and false negative rates in many risk scores raised questions about their utility in clinical practice (for example, positive predictive values ranged from 5% to 42%, negative predictive values from 88% to 99%). However, some scores were designed as non-invasive preliminary instruments, with a recommended second phase involving a blood test. 7 43 52 53 55 58 65
Risk models and scores tended to “morph” when they were externally validated because research teams dropped components from the original (for example, if data on these were not available), added additional components (for example, to compensate for missing categories), or modified what counted in a particular category (for example, changing how ethnicity was classified); in some cases these modifications were not clarified. A key dimension of implementation is appropriate adaptation to a new context. It was considered that this did not negate the external validation.
Qualitative findings
Table 3 ⇓ provides the qualitative findings from the risk scores. Of the 43 papers in the full sample, three did not recommend use of the model tested because the authors believed it had no advantage over existing ones. 50 56 60 Authors of the other 40 papers considered that at least one of their scores should be adopted and used, and to justify this made various claims. The commonest adjective used by authors to describe their score was “simple” (26 of 43); others mentioned “low cost,” “easily implemented,” “feasible,” and “convenient.”
Summary of authors’ assumptions and claims about their diabetes risk models or scores
Sixteen of the 43 studies that recommended use of a particular risk model or score did not designate an intended user for it. Some authors assigned agency to a risk score—that is, they stated, perhaps inadvertently, that the score itself had the potential to prevent diabetes, change behaviour, or reduce health inequalities. Although most authors did state an intended target group, this was usually given in vague terms, such as “the general population” or “individuals who are likely to develop diabetes.” Eleven of the 43 papers gave a clear statement of what intervention might be offered, by whom, to people who scored above the cut-off for high risk; the other papers made no comment on this or used vague terms such as “preventive measures,” without specifying by whom these would be delivered.
In all, authors of the papers in the full sample either explicitly identified or appeared to presume 10 mechanisms (box 2) by which, singly or in combination, use of the diabetes risk score might lead to improved patient outcomes (see table 3).
Box 2: 10 suggested mechanisms by which diabetes risk scores could help improve patient outcomes
Direct impact —clinicians will pick up high risk patients during consultations and offer advice that leads to change in patients’ behaviour and lifestyle
Indirect impact —routine use of the score increases clinicians’ awareness of risk for diabetes and motivation to manage it
Self assessment
Direct impact —people are alerted by assessing their own risk (for example, using an online tool), directly leading to change in lifestyle
Indirect impact —people, having assessed their own risk, are prompted to consult a clinician to seek further tests or advice on prevention
Technological
Individual impact —a risk model programmed into the electronic patient record generates a point of care prompt in the clinical encounter
Population impact —a risk model programmed into the electronic patient record generates aggregated data on risk groups, which will inform a public health intervention
Public health
Planners and commissioners use patterns of risk to direct resources into preventive healthcare for certain subgroups
Administrative
An administrator or healthcare assistant collects data on risk and enters these onto the patients’ records, which subsequently triggers the technological, clinical, or public health mechanisms
Research into practice
Use of the risk score leads to improved understanding of risk for diabetes or its management by academics, leading indirectly to changes in clinical practice and hence to benefits for patients
Future research
Use of the risk score identifies focused subpopulations for further research (with the possibility of benefit to patients in later years)
Risk models and scores had been developed in a range of health systems. Differences in components could be explained partly in terms of their intended context of use. For example, the QDScore, intended for use by general practitioners, was developed using a database of electronic records of a nationally representative sample of the UK general practice population comprising 2.5 million people. The QDScore is composed entirely of data items that are routinely recorded on general practice electronic records (including self assigned ethnicity, a deprivation score derived from the patient’s postcode, and clinical and laboratory values). 8 Another score, also intended to be derived from electronic records but in a US health maintenance organisation (covering people of working age who are in work), has similar components to the QDScore except that ethnicity and socioeconomic deprivation are not included. In contrast, the FINDRISC score was developed as a population screening tool intended for use directly by lay people; it consists of questions on sociodemographic factors and personal history along with waist circumference but does not include clinical or laboratory data; high scorers are prompted to seek further advice from a clinician. 52 Such a score makes sense in many parts of Finland and also in the Netherlands where health and information literacy rates are high, but would be less fit for purpose in a setting where these were low.
Prioritising scores for practising clinicians
Table 4 ⇓ summarises the properties of seven validated diabetes risk scores which we judged to be the most promising for use in clinical or public health practice. The judgments on which this selection was based were pragmatic; other scores not listed in table 4 (also see tables 1 and 2) will prove more fit for purpose in certain situations and settings. One score that has not yet been externally validated was included in table 4 because it is the only score already being incentivised in a national diabetes prevention policy. 23
Components of seven diabetes risk models or scores with potential for adaptation for use in routine clinical practice
Studies of impact of risk scores on patient outcomes
None of the 43 papers that validated one or more risk scores described the actual use of that score in an intervention phase. Furthermore, although these papers had been cited by a total of 1883 (range 0-343, median 12) subsequent papers, only nine of those 1883 papers (table 5 ⇓ ) described application and use of the risk score as part of an impact study aimed at changing patient outcomes. These covered seven studies, of which (to date) three have reported definitive results. All three reported positive changes in individual risk factors, but surprisingly none recalculated participants’ risk scores after the intervention period to see if they had changed. While one report on the ongoing FIN-D2D study suggests that incident diabetes has been reduced in “real world” (non-trial) participants who were picked up using a diabetes risk score and offered a package of preventive care, 74 this is a preliminary and indirect finding based on drug reimbursement claims, and no actual data are given in the paper. With that exception, no published impact study on a diabetes risk score has yet shown a reduction in incident diabetes.
Results of impact citation search (studies using diabetes risk models or scores as part of an intervention to improve outcomes)
Numerous diabetes risk scores now exist based on readily available data and provide a good but not perfect estimate of the chance of an adult developing diabetes in the medium term future. A few research teams have undertaken exemplary development and validation of a robust model, reported its statistical properties thoroughly, and followed through with studies of impact in the real world.
Limitations of included studies
We excluded less robust designs (especially cross sectional studies). Nevertheless, included studies were not entirely free from bias and confounding. This is because the “pragmatic” use of a previously assembled database or cohort brings an inherent selection bias (for example, the British Regional Heart Study cohort was selected to meet the inclusion criteria for age and comorbidity defined by its original research team and oriented to research questions around cardiovascular disease; the population for the QDScore is drawn from general practice records and hence excludes those not registered with a general practitioner).
Most papers in our sample had one or more additional limitations. They reported models or scores that required collection of data not routinely available in the relevant health system; omitted key statistical properties such as calibration and positive and negative predictive values that would allow a clinician or public health commissioner to judge the practical value of the score; or omitted to consider who would use the score, on whom, and in what circumstances. We identified a mismatch between the common assumption of authors who develop a risk model (that their “simple” model can now be taken up and used) and the actual uptake and use of such models (which seems to happen very rarely). However, there has recently been an encouraging—if limited—shift in emphasis from the exclusive pursuit of statistical elegance (for example, maximising area under the receiver operating curve) to undertaking applied research on the practicalities and outcomes of using diabetes risk scores in real world prevention programmes.
Strengths and limitations of the review
The strengths of this review are our use of mixed methodology, orientation to patient relevant outcomes, extraction and double checking of data by five researchers, and inclusion of a citation track to identify recently published studies and studies of impact. We applied both standard systematic review methods (to undertake a systematic and comprehensive search, translate all non-English texts, and extract and analyse quantitative data) and realist methods (to consider the relation between the components of the risk score, the context in which it was intended to be used, and the mechanism by which it might improve outcomes for patients).
The main limitation of this review is that data techniques and presentation in the primary studies varied so much that it was problematic to determine reasonable numerators and denominators for many of the calculations. This required us to make pragmatic decisions to collate and present data as fairly and robustly as possible while also seeking to make sense of the vast array of available risk scores to the general medical reader. We recognise that the final judgment on which risk scores are, in reality, easy to use will lie with the end user in any particular setting. Secondly, authors of some of the primary studies included in this review were developing a local tool for local use and made few or no claims that their score should be generalised elsewhere. Yet, the pioneers of early well known risk scores 49 68 have occasionally found their score being applied to other populations (perhaps ethnically and demographically different from the original validation cohort), their selection of risk factors being altered to fit the available categories in other datasets, and their models being recalibrated to provide better goodness of fit. All this revision and recalibration to produce “new” scores makes the systematic review of such scores at best an inexact science.
Why did we not recommend a “best” risk score?
We have deliberately not selected a single, preferred diabetes risk score. There is no universal ideal risk score, as the utility of any score depends not merely on its statistical properties but also on its context of use, which will also determine which types of data are available to be included. 75 76 Even when a risk model has excellent discrimination (and especially when it does not) the trade-off between sensitivity and specificity plays out differently depending on context. Box 3 provides some questions to ask when selecting a diabetes risk score.
Box 3: Questions to ask when selecting a diabetes risk score, and examples of intended use
What is the intended use case for the score.
If intended for use:
In clinical consultations, score should be based on data on the medical record
For self assessment by lay people, score should be based on things a layperson would know or be able to measure
In prevention planning, score should be based on public health data
What is the target population?
If intended for use in high ethnic and social diversity, a score that includes these variables may be more discriminatory
What is expected of the user of the score?
If for opportunistic use in clinical encounters, the score must align with the structure and timeframe of such encounters and competencies of the clinician, and (ideally) be linked to an appropriate point of care prompt. Work expected from the intended user of the score may need to be incentivised or remunerated, or both
What is expected of the participants?
If to be completed by laypeople, the score must reflect the functional health literacy of the target population
What are the consequences of false positive and false negative classifications?
In self completion scores, low sensitivity may falsely reassure large numbers of people at risk and deter them from seeking further advice
What is the completeness and accuracy of the data from which the score will be derived?
A score based on automated analysis of electronic patient records may include multiple components but must be composed entirely of data that are routinely and reliably entered on the record in coded form, and readily searchable (thus, such scores are only likely to be useful in areas where data quality in general practice records is high)
What resource implications are there?
If the budget for implementing the score and analysing data is fixed, the cost of use must fall within this budget
Given the above, what would be the ideal statistical and other properties of the score in this context of use?
What trade-offs should be made (sensitivity v specificity, brevity v comprehensiveness, one stage v two stage process)?
Risk scores as complex interventions
Our finding that diabetes risk scores seem to be used rarely can be considered in the light of the theoretical literature on diffusion of innovation. As well as being a statistical model, a risk score can be thought of as a complex, technology based innovation, the incorporation of which into business as usual (or not) is influenced by multiple contextual factors including the attributes of the risk score in the eyes of potential adopters (relative advantage, simplicity, and ease of use); adopters’ concerns (including implications for personal workload and how to manage a positive score); their skills (ability to use and interpret the technology); communication and influence (for example, whether key opinion leaders endorse it); system antecedents (including a healthcare organisation’s capacity to embrace new technologies, workflows, and ways of working); and external influences (including policy drivers, incentive structures, and competing priorities). 77 78
Challenges associated with risk scores in use
While the developers of most diabetes risk scores are in little doubt about their score’s positive attributes, this confidence seems not to be shared by practitioners, who may doubt the accuracy of the score or the efficacy of risk modification strategies, or both. Measuring diabetes risk competes for practitioners’ attention with a host of other tasks, some of which bring financial and other rewards. At the time of writing, few opinion leaders in diabetes seem to be promoting particular scores or the estimation of diabetes risk generally—perhaps because, cognisant of the limited impacts shown to date (summarised in table 5), they are waiting for further evidence of whether and how use of the risk score improves outcomes. Indeed, the utility of measuring diabetes risk in addition to cardiovascular risk is contested within the diabetes research community. 79 In the United Kingdom, the imminent inclusion of an application for calculating QDScore on EMIS, the country’s most widely used general practice computer system, may encourage its use in the clinical encounter. But unless the assessment of diabetes risk becomes part of the UK Quality and Outcomes Framework, this task may continue to be perceived as low priority by most general practitioners. Given current evidence, perhaps this judgment is correct. Furthermore, the low positive predictive values may spell trouble for commissioners. Identifying someone as “[possibly] high risk” will inevitably entail a significant cost in clinical review, blood tests, and (possibly) intervention and follow-up. Pending the results of ongoing impact studies, this may not be the best use of scarce resources.
Delivering diabetes prevention in people without any disease requires skills that traditionally trained clinicians may not possess. 80 We know almost nothing about the reach, uptake, practical challenges, acceptability, and cost of preventive interventions in high risk groups in different settings. 12 The relative benefit of detecting and targeting high risk people rather than implementing population-wide diabetes prevention strategies is unknown. 13 Effective prevention and early detection of diabetes are likely to require strengthening of health systems and development of new partnerships among the clinicians, community based lifestyle programmes, and healthcare funders. 81
Mechanisms by which risk scores might have impact
Although most authors of papers describing diabetes risk scores have hypothesised (or seem to have assumed) a clinical mechanism of action (that the score would be used by the individual’s clinician to target individual assessment and advice), the limited data available on impact studies (see table 5) suggest that a particularly promising area for further research is interventions that prompt self assessment—that is, laypeople measuring their own risk of diabetes. The preliminary findings from the impact studies covered in this review also suggest that not everyone at high risk is interested in coming forward for individual preventive input, nor will they necessarily stay the course of such input. It follows that in areas where aggregated data from electronic patient records are available, the diabetes risk scores may be used as a population prediction tool—for example, to produce small area statistics (perhaps as pictorial maps) of diabetes risk across a population, thereby allowing targeted design and implementation of community level public health interventions. 82 Small area mapping of diabetes risk may be a way of operationalising the recently published guidance on diabetes prevention from the National Institute for Health and Clinical Excellence, which recommends the use of “local and national tools . . . to identify local communities at high risk of developing diabetes to assess their specific needs.” 83
Towards an impact oriented research agenda for risk scores
We recommend that funding bodies and journal editors help take this agenda forward by viewing the risk score in use as a complex intervention and encouraging more applied research studies in which real people identified as at “high risk” using a particular risk score are offered real interventions; success in risk score development is measured in terms of patient relevant intermediate outcomes (for example, change in risk score) and final outcomes (incident diabetes and related morbidity) rather than in terms of the statistical properties of the tool; a qualitative component (for example, process evaluation, organisational case study, patient’s experience of lifestyle modification) explores both facilitators and barriers of using the score in a real world setting; and an economic component evaluates cost and cost effectiveness.
Millions of participants across the world have already participated in epidemiological studies aimed at developing a diabetes risk score. An extensive menu of possible scores are now available to those who seek to use them clinically or to validate them in new populations, none of which is perfect but all of which have strengths. Nevertheless, despite the growing public health importance of type 2 diabetes and the enticing possibility of prevention for those at high risk of developing it, questions remain about how best to undertake risk prediction and what to do with the results. Appropriately, the balance of research effort is now shifting from devising new risk scores to exploring how best to use those we already have.
What is already known on this topic
The many known risk factors for type 2 diabetes can be combined in statistical models to produce risk scores

What this study adds
Dozens of risk models and scores for diabetes have been developed and validated in different settings
Sociodemographic and clinical data were much better predictors of diabetes risk than genetic markers
Research on this topic is beginning to shift from developing new statistical risk models to considering the use and impact of risk scores in the real world
Cite this as: BMJ 2011;343:d7163
We thank Helen Elwell, librarian at the British Medical Association Library, for help with the literature search; Samuel Rigby for manually removing duplicates; and Sietse Wieringa, Kaveh Memarzadeh, and Nicholas Swetenham for help with translation of non-English papers. BMJ reviewers Wendy Hu and John Furler provided helpful comments on an earlier draft.
Contributors: DN conceptualised the study, managed the project, briefed and supported all researchers, assisted with developing the search strategy and ran the search, scanned all titles and abstracts, extracted quantitative data on half the papers, citation tracked all papers, checked a one third sample of the qualitative data extraction, and cowrote the paper. TG conceptualised the qualitative component of the study, extracted qualitative data on all papers, independently citation tracked all papers, and led on writing the paper. RM independently scanned all titles and abstracts of the electronic search, extracted quantitative data from some papers, assisted with other double checking, and helped revise drafts of the paper. TD helped revise and refine the study aims, independently double checked quantitative data extraction from all papers, and helped revise drafts of the paper. CM advised on systematic review methodology, helped develop the search strategy, extracted quantitative data from some papers, and helped revise drafts of the paper. TG acts as guarantor.
Funding: This study was funded by grants from Tower Hamlets, Newham, and City and Hackney primary care trusts, by a National Institute of Health Research senior investigator award for TG, and by internal funding for staff time from Barts and the London School of Medicine and Dentistry. The funders had no input into the selection or analysis of data or the content of the final manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode .
- ↵ Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010 ; 87 : 4 -14. OpenUrl CrossRef PubMed Web of Science
- ↵ Holman N, Forouhi NG, Goyder E, Wild SH. The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: estimates of total diabetes prevalence for England, 2010-2030. Diabetic Med 2011 ; 28 : 575 -82. OpenUrl CrossRef PubMed
- ↵ National Diabetes Clearinghouse. National Diabetes Statistics 2011. National Institute of Health, USA. http://diabetes.niddk.nih.gov/dm/pubs/statistics/#Diagnosed20 .
- ↵ Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med 2010 ; 362 : 2425 -6. OpenUrl CrossRef PubMed
- ↵ Saadi H, Carruthers SG, Nagelkerke N, Al-Maskari F, Afandi B, Reed R, et al. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract 2007 ; 78 : 369 -77. OpenUrl CrossRef PubMed Web of Science
- ↵ Williamson DF, Vinicor F, Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004 ; 140 : 951 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care 2006 ; 29 : 1872 -7. OpenUrl Abstract / FREE Full Text
- ↵ Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 2009 ; 338 : b880 . OpenUrl Abstract / FREE Full Text
- ↵ Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008 ; 359 : 2208 -9. OpenUrl CrossRef PubMed Web of Science
- ↵ Talmud PJ, Hingorani AD, Cooper JA, Marmot MG, Brunner EJ, Kumari M, et al. Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. BMJ 2010 ; 340 : b4838 . OpenUrl Abstract / FREE Full Text
- ↵ Park KS. The search for genetic risk factors of type 2 diabetes mellitus. Diabetes Metab J 2011 ; 35 : 12 -22. OpenUrl CrossRef PubMed
- ↵ Simmons RK, Unwin N, Griffin SJ. International Diabetes Federation: an update of the evidence concerning the prevention of type 2 diabetes. Diabetes Res Clin Pract 2010 ; 87 : 143 -9. OpenUrl CrossRef PubMed Web of Science
- ↵ Yates T, Davies M, Khunti K. Preventing type 2 diabetes: can we make the evidence work? Postgrad Med J 2009 ; 85 : 475 -80. OpenUrl Abstract / FREE Full Text
- ↵ Simmons RK, Echouffo-Tcheugui JB, Griffin SJ. Screening for type 2 diabetes: an update of the evidence. Diabetes Obes Metab 2010 ; 12 : 838 -44. OpenUrl CrossRef PubMed Web of Science
- ↵ Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008 ; 336 : 1180 -5. OpenUrl Abstract / FREE Full Text
- ↵ Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997 ; 20 : 537 -44. OpenUrl Abstract / FREE Full Text
- ↵ Tuomilehto J, Lindstrom J. The major diabetes prevention trials. Curr Diab Rep 2003 ; 3 : 115 -22. OpenUrl PubMed
- ↵ Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002 ; 346 : 393 -403. OpenUrl CrossRef PubMed Web of Science
- ↵ Mann DM, Bertoni AG, Shimbo D, Carnethon MR, Chen H, Jenny NS, et al. Comparative validity of 3 diabetes mellitus risk prediction scoring models in a multiethnic US cohort: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010 ; 171 : 980 -8. OpenUrl Abstract / FREE Full Text
- ↵ Tuomilehto J, Lindstrom J, Hellmich M, Lehmacher W, Westermeier T, Evers T, et al. Development and validation of a risk-score model for subjects with impaired glucose tolerance for the assessment of the risk of type 2 diabetes mellitus-The STOP-NIDDM risk-score. Diabetes Res Clin Pract 2010 ; 87 : 267 -74. OpenUrl CrossRef PubMed Web of Science
- ↵ National Institute for Health and Clinical Excellence. Lipid modification. 2011. www.nice.org.uk/nicemedia/live/11982/40675/40675.pdf .
- ↵ McElduff P, Lyratzopoulos G, Edwards R, Heller RF, Shekelle P, Roland M. Will changes in primary care improve health outcomes? Modelling the impact of financial incentives introduced to improve quality of care in the UK. Qual Saf Health Care 2004 ; 13 : 191 -7. OpenUrl Abstract / FREE Full Text
- ↵ Anon. Type 2 diabetes risk evaluation for men and women aged 40-49 years at high risk of type 2 diabetes. MBS item 713. 2011. www.agpn.com.au/__data/assets/pdf_file/0015/15009/20081126_brf_practice-detailing-card-item-713.pdf .
- ↵ Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med 1990 ; 322 : 1635 -41. OpenUrl CrossRef PubMed Web of Science
- ↵ Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ 2009 ; 338 : b375 . OpenUrl FREE Full Text
- ↵ Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ 2009 ; 338 : b605 . OpenUrl FREE Full Text
- ↵ Wannamethee SG, Papacosta O, Whincup PH, Thomas MC, Carson C, Lawlor DA, et al. The potential for a two-stage diabetes risk algorithm combining non-laboratory-based scores with subsequent routine non-fasting blood tests: results from prospective studies in older men and women. Diabet Med 2011 ; 28 : 23 -30. OpenUrl PubMed
- ↵ Wareham NJ, Griffin SJ. Risk scores for predicting type 2 diabetes: comparing axes and spades. Diabetologia 2011 ; 54 : 994 -5. OpenUrl CrossRef PubMed Web of Science
- ↵ Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009 ; 338 : b604 . OpenUrl FREE Full Text
- ↵ Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009 ; 338 : b606 . OpenUrl FREE Full Text
- ↵ Pawson R, Tilley N. Realistic evaluation . Sage, 1997 .
- ↵ Kahn KS, Kunz R, Kleijnen J, Antes G. Systematic reviews to support evidence-based medicine , 2nd ed. Royal Society of Medicine, 2011 .
- ↵ Centre for Reviews and Dissemination. Systematic Reviews: centre for reviews and dissemination guidance for undertaking reviews in health care. York Publishing Services. 2011. www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf .
- ↵ Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005 ; 10 (suppl 1): 21 -34. OpenUrl Abstract / FREE Full Text
- ↵ Greenhalgh T, Wong G, Westhorp G, Pawson R, on behalf of the RAMESES study group. Protocol-realist and meta-narrative evidence synthesis: evolving standards (RAMESES). BMC Med Res Methodol 2011 ; 11 : 115 . OpenUrl CrossRef PubMed
- ↵ Balkau B, Lange C, Fezeu L, Tichet J, de Lauzon-Guillain B, Czernichow S, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2008 ; 31 : 2056 -61. OpenUrl Abstract / FREE Full Text
- ↵ Chen L, Magliano DJ, Balkau B, Colagiuri S, Zimmet PZ, Tonkin AM, et al. AUSDRISK: an Australian type 2 diabetes risk assessment tool based on demographic, lifestyle and simple anthropometric measures. Med J Aust 2010 ; 192 : 197 -202. OpenUrl PubMed Web of Science
- ↵ Chuang SY, Yeh WT, Wu YL, Chang HY, Pan WH, Tsao CK. Prediction equations and point system derived from large-scale health check-up data for estimating diabetic risk in the Chinese population of Taiwan. Diabetes Res Clin Pract 2011 ; 92 : 128 -36. OpenUrl CrossRef PubMed
- ↵ Gao WG, Qiao Q, Pitkaniemi J, Wild S, Magliano D, Shaw J, et al. Risk prediction models for the development of diabetes in Mauritian Indians. Diabet Med 2009 ; 26 : 996 -1002. OpenUrl CrossRef PubMed Web of Science
- ↵ Joseph J, Svartberg J, Njolstad I, Schirmer H. Incidence of and risk factors for type-2 diabetes in a general population: the Tromso Study. Scand J Public Health 2010 ; 38 : 768 -75. OpenUrl Abstract / FREE Full Text
- ↵ Kahn HS, Cheng YJ, Thompson TJ, Imperatore G, Gregg EW. Two risk-scoring systems for predicting incident diabetes mellitus in US adults age 45 to 64 years. Ann Intern Med 2009 ; 150 : 741 -51. OpenUrl CrossRef PubMed Web of Science
- ↵ Kolberg JA, Jorgensen T, Gerwien RW, Hamren S, McKenna MP, Moler E, et al. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care 2009 ; 32 : 1207 -12. OpenUrl Abstract / FREE Full Text
- ↵ Liu M, Pan C, Jin M. A Chinese diabetes risk score for screening of undiagnosed diabetes and abnormal glucose tolerance. Diabetes Technol Ther 2011 ; 13 : 501 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Mehrabi Y, Sarbakhsh P, Hadaegh F, Khadem-Maboudi A. Prediction of diabetes using logic regression. Iran J Endocrinol Metab 2010 ; 12 : 16 -24. OpenUrl
- ↵ Rathmann W, Kowall B, Heier M, Herder C, Holle R, Thorand B, et al. Prediction models for incident type 2 diabetes mellitus in the older population: KORA S4/F4 cohort study. Diabet Med 2010 ; 27 : 1116 -23. OpenUrl CrossRef PubMed
- ↵ Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, et al. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2005 ; 28 : 2013 -8. OpenUrl Abstract / FREE Full Text
- ↵ Schulze MB, Weikert C, Pischon T, Bergmann MM, Al-Hasani H, Schleicher E, et al. Use of multiple metabolic and genetic markers to improve the prediction of type 2 diabetes: the EPIC-Potsdam Study. Diabetes Care 2009 ; 32 : 2116 -9. OpenUrl Abstract / FREE Full Text
- ↵ Stern MP, Morales PA, Valdez RA, Monterrosa A, Haffner SM, Mitchell BD, et al. Predicting diabetes. Moving beyond impaired glucose tolerance. Diabetes 1993 ; 42 : 706 -14. OpenUrl Abstract / FREE Full Text
- ↵ Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 2002 ; 136 : 575 -81. OpenUrl CrossRef PubMed Web of Science
- ↵ von Eckardstein A, Schulte H, Assmann G. Risk for diabetes mellitus in middle-aged Caucasian male participants of the PROCAM study: implications for the definition of impaired fasting glucose by the American Diabetes Association. Prospective Cardiovascular Munster. J Clin Endocrinol Metab 2000 ; 85 : 3101 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007 ; 167 : 1068 -74. OpenUrl CrossRef PubMed Web of Science
- ↵ Alssema M, Feskens EJ, Bakker SJ, Gansevoort RT, Boer JM, Heine RJ, et al. [Finnish questionnaire reasonably good predictor of the incidence of diabetes in The Netherlands]. Ned Tijdschr Geneeskd 2008 ; 152 : 2418 -24. OpenUrl PubMed
- ↵ Alssema M, Vistisen D, Heymans MW, Nijpels G, Glumer C, Zimmet PZ, et al. The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance (DETECT-2) update of the Finnish diabetes risk score for prediction of incident type 2 diabetes. Diabetologia 2011 ; 54 : 1004 -12. OpenUrl CrossRef PubMed Web of Science
- ↵ Bozorgmanesh M, Hadaegh F, Azizi F. Transportability of the updated diabetes prediction model from Atherosclerosis Risk in Communities Study to a Middle Eastern adult population: community-based cohort study. Acta Diabetol 2010 ; doi: 10.1007/s00592-010-0241-1 .
- ↵ Bozorgmanesh M, Hadaegh F, Zabetian A, Azizi F. San Antonio heart study diabetes prediction model applicable to a Middle Eastern population? Tehran glucose and lipid study. Int J Public Health 2010 ; 55 : 315 -23. OpenUrl CrossRef PubMed Web of Science
- ↵ Cameron AJ, Magliano DJ, Zimmet PZ, Welborn TA, Colagiuri S, Tonkin AM, et al. The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med 2008 ; 264 : 177 -86. OpenUrl CrossRef PubMed Web of Science
- ↵ Collins GS, Altman DG. External validation of QDSCORE((R)) for predicting the 10-year risk of developing type 2 diabetes. Diabet Med 2011 ; 28 : 599 -607. OpenUrl CrossRef PubMed
- ↵ Guerrero-Romero F, Rodriguez-Moran M. [Validation of an instrument for screening cases of type 2 diabetes and monitoring at-risk individuals in Mexico]. Rev Panam Salud Publica 2010 ; 27 : 181 -6. OpenUrl CrossRef PubMed
- ↵ Kanaya AM, Wassel Fyr CL, de Rekeneire N, Shorr RI, Schwartz AV, Goodpaster BH, et al. Predicting the development of diabetes in older adults: the derivation and validation of a prediction rule. Diabetes Care 2005 ; 28 : 404 -8. OpenUrl Abstract / FREE Full Text
- ↵ Mainous AG, III, Diaz VA, Everett CJ. Assessing risk for development of diabetes in young adults. Ann Fam Med 2007 ; 5 : 425 -9. OpenUrl Abstract / FREE Full Text
- ↵ McNeely MJ, Boyko EJ, Leonetti DL, Kahn SE, Fujimoto WY. Comparison of a clinical model, the oral glucose tolerance test, and fasting glucose for prediction of type 2 diabetes risk in Japanese Americans. Diabetes Care 2003 ; 26 : 758 -63. OpenUrl Abstract / FREE Full Text
- ↵ Nichols GA, Brown JB. Validating the Framingham Offspring Study equations for predicting incident diabetes mellitus. Am J Manag Care 2008 ; 14 : 574 -80. OpenUrl PubMed Web of Science
- ↵ Rahman M, Simmons RK, Harding AH, Wareham NJ, Griffin SJ. A simple risk score identifies individuals at high risk of developing type 2 diabetes: a prospective cohort study. Fam Pract 2008 ; 25 : 191 -6. OpenUrl Abstract / FREE Full Text
- ↵ Urdea M, Kolberg J, Wilber J, Gerwien R, Moler E, Rowe M, et al. Validation of a multimarker model for assessing risk of type 2 diabetes from a five-year prospective study of 6784 Danish people (Inter99). J Diabetes Sci Technol 2009 ; 3 : 748 -55. OpenUrl Abstract / FREE Full Text
- ↵ Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 2005 ; 165 : 2644 -50. OpenUrl CrossRef PubMed Web of Science
- ↵ Bozorgmanesh M, Hadaegh F, Ghaffari S, Harati H, Azizi F. A simple risk score effectively predicted type 2 diabetes in Iranian adult population: population-based cohort study. Eur J Public Health 2011 ; 21 : 554 -9. OpenUrl Abstract / FREE Full Text
- ↵ Chien K, Cai T, Hsu H, Su T, Chang W, Chen M, et al. A prediction model for type 2 diabetes risk among Chinese people. Diabetologia 2009 ; 52 : 443 -50. OpenUrl CrossRef PubMed Web of Science
- ↵ Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003 ; 26 : 725 -31. OpenUrl Abstract / FREE Full Text
- ↵ Rosella LC, Manuel DG, Burchill C, Stukel TA. A population-based risk algorithm for the development of diabetes: development and validation of the Diabetes Population Risk Tool (DPoRT). J Epidemiol Community Health 2011 ; 65 : 613 -20. OpenUrl Abstract / FREE Full Text
- ↵ Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 2007 ; 30 : 510 -5. OpenUrl Abstract / FREE Full Text
- ↵ Simmons RK, Harding AH, Wareham NJ, Griffin SJ. Do simple questions about diet and physical activity help to identify those at risk of type 2 diabetes? Diabet Med 2007 ; 24 : 830 -5. OpenUrl CrossRef PubMed Web of Science
- ↵ Sun F, Tao Q, Zhan S. An accurate risk score for estimation 5-year risk of type 2 diabetes based on a health screening population in Taiwan. Diabetes Res Clin Pract 2009 ; 85 : 228 -34. OpenUrl CrossRef PubMed Web of Science
- ↵ Harrell F. Regression modelling strategies with applications to linear models, logistic regression and survival analysis . Springer-Verlag, 2001 .
- ↵ Lindstrom J, Absetz P, Hemio K, Peltomaki P, Peltonen M. Reducing the risk of type 2 diabetes with nutrition and physical activity—efficacy and implementation of lifestyle interventions in Finland. Public Health Nutr 2010 ; 13 (6A): 993 -9. OpenUrl CrossRef PubMed
- ↵ Herman WH. Predicting risk for diabetes: choosing (or building) the right model. Ann Intern Med 2009 ; 150 : 812 -4. OpenUrl PubMed Web of Science
- ↵ Wright C, Dent T. Quality standards in risk prediction: summary of an expert meeting. PHG Foundation, 2011. www.phgfoundation.org .
- ↵ Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Peacock R. Diffusion of innovations in service organisations: systematic literature review and recommendations for future research. Milbank Q 2004 ; 82 : 581 -629. OpenUrl CrossRef PubMed Web of Science
- ↵ Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly 1989 ; 13 : 319 -40. OpenUrl CrossRef Web of Science
- ↵ Chamnan P, Simmons RK, Jackson R, Khaw KT, Wareham NJ, Griffin SJ. Non-diabetic hyperglycaemia and cardiovascular risk: moving beyond categorisation to individual interpretation of absolute risk. Diabetologia 2011 ; 54 : 291 -9. OpenUrl CrossRef PubMed Web of Science
- ↵ Saaristo T, Peltonen M, Keinanen-Kiukaanniemi S, Vanhala M, Saltevo J, Niskanen L, et al. National type 2 diabetes prevention programme in Finland: FIN-D2D. Int J Circumpolar Health 2007 ; 66 : 101 -12. OpenUrl PubMed Web of Science
- ↵ Narayan KM, Williamson DF. Prevention of type 2 diabetes: risk status, clinic, and community. J Gen Intern Med 2010 ; 25 : 154 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Stewart JE, Battersby SE, Lopez-De FA, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011 ; 10 : 18 . OpenUrl CrossRef PubMed
- ↵ National Institute for Health and Clinical Excellence. Preventing type 2 diabetes—population and community interventions. 2011. www.nice.org.uk/guidance/PH35 .
- Stone MA, Camosso-Stefinovic J, Wilkinson J, de Lusignan S, Hattersley AT, Khunti K. Incorrect and incomplete coding and classification of diabetes: a systematic review. Diabet Med 2010 ; 27 : 491 -7. OpenUrl CrossRef PubMed Web of Science
- Rathmann W, Kowall B, Schulze MB. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort: response to Kolberg et al. Diabetes Care 2010 ; 33 : e28 . OpenUrl FREE Full Text
- Stern M, Williams K, Eddy D, Kahn R. Validation of prediction of diabetes by the Archimedes model and comparison with other predicting models. Diabetes Care 2008 ; 31 : 1670 -1. OpenUrl Abstract / FREE Full Text
- Colagiuri S, Vita P, Cardona-Morrell M, Singh MF, Farrell L, Milat A, et al. The Sydney Diabetes Prevention Program: a community-based translational study. BMC Public Health 2010 ; 10 : 328 . OpenUrl CrossRef PubMed
- Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care 2009 ; 32 : 1418 -20. OpenUrl Abstract / FREE Full Text
- Jallinoja P, Pajari P, Absetz P. Repertoires of lifestyle change and self-responsibility among participants in an intervention to prevent type 2 diabetes. Scand J Caring Sci 2008 ; 22 : 455 -62. OpenUrl CrossRef PubMed Web of Science
- Kulzer B, Hermanns N, Gorges D, Schwarz P, Haak T. Prevention of diabetes self-management program (PREDIAS): effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care 2009 ; 32 : 1143 -6. OpenUrl Abstract / FREE Full Text
- Laatikainen T, Dunbar JA, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health 2007 ; 7 : 249 . OpenUrl CrossRef PubMed
- Vermunt PW, Milder IE, Wielaard F, van Oers JA, Westert GP. An active strategy to identify individuals eligible for type 2 diabetes prevention by lifestyle intervention in Dutch primary care: the APHRODITE study. Fam Pract 2010 ; 27 : 312 -9. OpenUrl Abstract / FREE Full Text
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 October 2023
Precision subclassification of type 2 diabetes: a systematic review
- Shivani Misra ORCID: orcid.org/0000-0003-2886-0726 1 , 2 na1 ,
- Robert Wagner ORCID: orcid.org/0000-0002-6120-0191 3 , 4 , 5 na1 ,
- Bige Ozkan ORCID: orcid.org/0000-0003-2745-1189 6 , 7 ,
- Martin Schön ORCID: orcid.org/0000-0002-9224-4189 4 , 5 , 8 ,
- Magdalena Sevilla-Gonzalez ORCID: orcid.org/0000-0001-6135-9998 9 , 10 , 11 ,
- Katsiaryna Prystupa ORCID: orcid.org/0000-0003-3368-1028 4 , 5 ,
- Caroline C. Wang 6 ,
- Raymond J. Kreienkamp ORCID: orcid.org/0000-0002-1683-323X 10 , 12 , 13 , 14 ,
- Sara J. Cromer 10 , 11 , 12 , 13 ,
- Mary R. Rooney ORCID: orcid.org/0000-0002-5607-4848 6 , 15 ,
- Daisy Duan ORCID: orcid.org/0000-0002-4392-3206 16 ,
- Anne Cathrine Baun Thuesen ORCID: orcid.org/0000-0002-8639-9117 17 ,
- Amelia S. Wallace ORCID: orcid.org/0000-0002-1466-3791 6 , 15 ,
- Aaron Leong 10 , 11 , 12 , 18 ,
- Aaron J. Deutsch ORCID: orcid.org/0000-0001-6750-5335 10 , 11 , 12 , 13 ,
- Mette K. Andersen ORCID: orcid.org/0000-0001-8227-1469 17 ,
- Liana K. Billings ORCID: orcid.org/0000-0001-7991-3010 19 , 20 ,
- Robert H. Eckel 21 ,
- Wayne Huey-Herng Sheu 22 , 23 , 24 ,
- Torben Hansen ORCID: orcid.org/0000-0001-8748-3831 17 ,
- Norbert Stefan ORCID: orcid.org/0000-0002-2186-9595 5 , 25 , 26 ,
- Mark O. Goodarzi ORCID: orcid.org/0000-0001-6364-5103 27 ,
- Debashree Ray ORCID: orcid.org/0000-0002-0979-2935 15 , 28 ,
- Elizabeth Selvin ORCID: orcid.org/0000-0001-6923-7151 6 , 15 ,
- Jose C. Florez 10 , 11 , 12 , 13 ,
- ADA/EASD PMDI ,
- James B. Meigs 10 , 11 , 18 na2 &
- Miriam S. Udler ORCID: orcid.org/0000-0003-3824-9162 10 , 11 , 12 , 13 na2
Communications Medicine volume 3 , Article number: 138 ( 2023 ) Cite this article
5919 Accesses
7 Citations
19 Altmetric
Metrics details
- Body mass index
- Type 2 diabetes
Heterogeneity in type 2 diabetes presentation and progression suggests that precision medicine interventions could improve clinical outcomes. We undertook a systematic review to determine whether strategies to subclassify type 2 diabetes were associated with high quality evidence, reproducible results and improved outcomes for patients.
We searched PubMed and Embase for publications that used ‘simple subclassification’ approaches using simple categorisation of clinical characteristics, or ‘complex subclassification’ approaches which used machine learning or ‘omics approaches in people with established type 2 diabetes. We excluded other diabetes subtypes and those predicting incident type 2 diabetes. We assessed quality, reproducibility and clinical relevance of extracted full-text articles and qualitatively synthesised a summary of subclassification approaches.
Here we show data from 51 studies that demonstrate many simple stratification approaches, but none have been replicated and many are not associated with meaningful clinical outcomes. Complex stratification was reviewed in 62 studies and produced reproducible subtypes of type 2 diabetes that are associated with outcomes. Both approaches require a higher grade of evidence but support the premise that type 2 diabetes can be subclassified into clinically meaningful subtypes.
Critical next steps toward clinical implementation are to test whether subtypes exist in more diverse ancestries and whether tailoring interventions to subtypes will improve outcomes.
Plain language summary
In people with type 2 diabetes there may be differences in the way people present, including for example, their symptoms, body weight or how much insulin they make. We looked at recent publications describing research in this area to see whether it is possible to separate people with type 2 diabetes into different subgroups and, if so, whether these groupings were useful for patients. We found that it is possible to group people with type 2 diabetes into different subgroups and being in one subgroup can be more strongly linked to the likelihood of developing complications over others. This might mean that in the future we can treat people in different subgroups differently in ways that improves their treatment and their health but it requires further study.
Similar content being viewed by others
Heterogeneity and endotypes in type 1 diabetes mellitus
Cluster analysis of patient characteristics, treatment modalities, renal impairments, and inflammatory markers in diabetes mellitus
Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine
Introduction.
Type 2 diabetes is a global health problem posing substantial burdens on human health 1 . The diagnosis of type 2 diabetes is based on elevated blood glucose coupled with the absence of clinical features indicating alternative subtypes, such as type 1, monogenic, pancreatic or medication-induced diabetes 2 . A diagnosis of type 2 diabetes is generally the default or can be arrived at through exclusion of other types. Traditionally, most type 2 diabetes care guidelines have advocated treatment choice based on cost-effectiveness and side effects of specific medications, which have no relationship to underlying pathophysiology in the individual. More recent guidelines have suggested differential glucose-lowering therapies on the basis of higher body mass index (BMI) (favouring use of glucagon-like peptide analogue, GLP-1) or presence or absence of cardiovascular and/or renal disease and/or heart failure (favouring GLP-1 and/or sodium-glucose co-transporter 2, SGLT-2 inhibitors) 3 .
There is considerable heterogeneity in the clinical characteristics of patients with type 2 diabetes. Clinicians recognise that differences in degree of obesity or body fat distribution, age, dyslipidaemia or presence of metabolic syndrome can influence prognosis in diabetes and can be important considerations in treatment and management 4 , 5 , 6 . There is increasing awareness that type 2 diabetes heterogeneity may reflect differences in the underlying pathophysiology, environmental contributors, and the genetic risk of affected individuals. The mechanisms leading to the development of type 2 diabetes may differ from one individual to another and this could impact treatment and outcome.
Accurate characterisation of the heterogeneity in type 2 diabetes may help individualise care and improve outcomes. This goal has been realised in part for monogenic diabetes, where treatments can be tailored to genetic subtype to deliver precision care achieving better outcomes than standard care 7 . Given the complex pathophysiology and genetics of type 2 diabetes, applying precision medicine approaches is challenging. Critical to this endeavour is a better understanding of specific subtypes.
There are many studies of type 2 diabetes subtypes. The literature reflects diverse approaches based on the presence or absence of one or more simple clinical features or biomarkers and, more recently, sophisticated methods that deploy machine learning (ML) or use omics data. Classification approaches such as clustering methods to categorise this heterogeneity show inter-cluster differences in progression to complications or need for insulin treatment. These approaches consider clinical features at diagnosis 8 or clinical information combined with genetic data to characterise disease heterogeneity 9 , 10 . Simpler approaches are more easily implemented across all resource settings, while complex approaches may have greater precision in classifying heterogeneity. The breadth and scope of the evidence in favour of type 2 diabetes subclassification have not to date been thoroughly examined.
The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine 11 . This Systematic Review is written with the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine 12 .
In this systematic review for the PMDI we aimed to provide a critical assessment of the evidence to date for type 2 diabetes subclassification using (i) simple approaches based on categorisation of clinical features, biomarkers, imaging, or other parameters, and (ii) complex subclassification approaches that use ML incorporating clinical data and/or genomic data. We aimed to identify areas where further research is needed with the goal to improve patient and health system outcomes in type 2 diabetes care.
Our analysis shows that many simple approaches to subclassification have been tried but none have been replicated and most are not associated with meaningful clinical outcomes. However, a more complex stratification, using machine learning applied to clinical variables, yielded reproducible subtypes of type 2 diabetes that are associated with outcomes. Both approaches, however, require a higher grade of evidence but support the premise that type 2 diabetes can be subclassified into clinically meaningful subtypes.
This systematic review was written and conducted in accordance with our pre-established protocol (PROSPERO ID CRD42022310539) and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) 13 . We systematically reviewed papers to address two research questions devised by an expert working group: 1) What are the main subtypes of type 2 diabetes defined using simple clinical criteria and/or routinely available laboratory tests (simple approaches), and 2) What subphenotypes of type 2 diabetes can be reproducibly identified using ML and/or genomics approaches (complex approaches)? Subsequently, we refer to the first question as simple approaches and the second question as complex approaches . The quality of each paper was reported, and the aggregate of data evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system 14 .
Study eligibility criteria
We included English-language original research studies of all design types that analysed populations with prevalent or new-onset type 2 diabetes and attempted in some way to stratify or subgroup patients with type 2 diabetes. We used broad terms to identify stratification studies and all approaches to stratification (the exposure) were included (supplementary table 1 ). We excluded studies examining risk for the development of type 2 diabetes, use of glycaemic control (e.g. HbA1c strata) alone to stratify, studies of stratification in types of diabetes other than type 2 diabetes, and review articles or case reports.
For simple approaches the exposure was defined as any of the following; a routine blood or urine biomarker that was widely available in most clinic settings; a blood or urine biomarker that might not be routinely available now but could have the potential to become easily accessible; any routinely available imaging modality; any physiological assessment that could be undertaken in an outpatient setting or results from routinely available dynamic tests. The stratification approach was either a cut-off or categorisation based on one or more of the above or if an index, ratio, trend or other analysis was undertaken, it could be calculated without complex mathematics. Finally, all outcomes were accepted for example clinical characterisation of subgroups, association with specific biomarkers and association with complications or mortality.
For complex approaches, the exposure used was defined as any of the inputs for the simple approach outlined above and/or any form of genetic data. However, unlike the simple approach, the stratification approach either deployed ML approaches or used other complex statistical approaches for stratification. All outcomes were accepted, as above, for simple.
Literature search and selection strategy
PUBMED and EMBASE databases were searched from inception to May 2022 for relevant articles using a strategy devised by expert health sciences librarians ( supplementary methods ). We undertook independent searches for each systematic review question. From both searches, each abstract and subsequently, full text paper, was screened by two independent team members for eligibility. In addition to the initial exclusion criteria, at the full-text review stage, we further excluded studies where exposures were not clearly defined and/or if the data on outcomes of the stratification were not available in results or supplementary material. We also excluded studies where the only stratification modality was a measure of glycaemic control, as this itself provides the diagnosis of type 2 diabetes. In cases of disagreement between two reviewers, a third reviewer made the final decision. The process involved group-based discussions to resolve disagreements to ensure all decisions were made on the same grounds.
Data extraction
Data were manually extracted from each full-text paper by individual team members and cross-checked by an independent team member at the data synthesis stage. We extracted relevant data on study design (observational or clinical trial), analysis design (cross-sectional or prospective), study population characteristics, stratification method and results (exposure), outcomes, and study quality assessment. For population characteristics, we extracted data on whether the type 2 diabetes population was new-onset or prevalent, the sample size, ethnicity and gender, the duration of diabetes (for cross-sectional analysis) and duration of follow-up (for longitudinal follow-up). For exposures, we extracted the approach to stratification and the number and nature of subgroups identified. For outcomes, we documented the type of outcome studied and the findings according to stratified subgroup.
Data synthesis
Following full-text data extraction, we undertook a qualitative analysis of exposures (measures used to stratify individuals) for each systematic review question. For simple sub-classification approaches, we extracted the details of stratification criteria in each paper ( supplementary methods ), then categorised the exposure as blood/urine test, imaging, age). After data extraction, these exposures were further refined into subcategories based on common emerging themes (e.g., use of pancreatic autoantibodies, BMI categories, measures of beta-cell function, use of lipid profiles). For complex approaches, the exposure included both the input clinical and/or genetic data used and the ML approach to analysis (e.g., k-means, hierarchical clustering, latent-class analysis), deployed. In both reviews, outcomes were heterogeneous, so we broadly categorised them where possible. Due to the variability in exposures and outcomes, it was not possible to undertake formal meta-analyses of any outcome. All coding, categorisation and thematic synthesis was undertaken and agreed upon by at least three members of the research team.
Quality assessment
The GRADE system was used to assess the quality of the studies extracted 13 . At least two members assessed whether study exposures and outcomes were clearly defined, valid and reliable, and whether confounders were appropriately accounted and adjusted for. Disagreements were resolved by discussion between the joint first and senior authors during group discussion. Assessors evaluated study limitations, consistency of results, imprecision, and reporting bias to assign study-specific and overall GRADE certainty ratings as very low, low, moderate and high 15 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Search and screening for simple and complex systematic review questions
The first question examined simple stratification approaches using clinical variables that may reveal type 2 diabetes heterogeneity. A total of 6097 studies met the inclusion criteria and were screened (Fig. 1A ). Of these, 183 studies were included for full text data review, of which 132 studies were subsequently excluded. The most common reasons for exclusion at the full-text review stage were studies conducted in populations without prevalent or incident type 2 diabetes, study designs that used ML approaches or stratification approaches that used HbA1c or diabetes medications. In total, 51 “simple approach” studies underwent full-text data extraction.
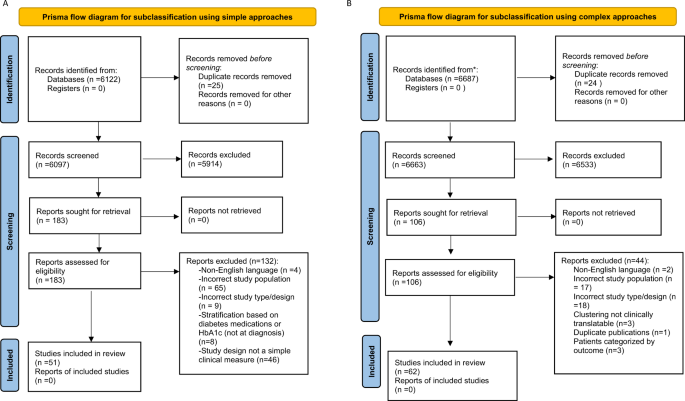
A This shows the flow diagram for simple approaches to subclassification and B Complex approaches.
The second question aimed to identify papers with complex approaches, mostly ML-based strategies, to identify subgroups of patients with type 2 diabetes (Fig. 1B ). A total of 6639 studies were screened, of which 106 were found eligible for full-text review. The most common reasons for exclusion were study populations not comprising participants with type 2 diabetes or classification approaches not using ML. In total, 62 ‘complex’ studies underwent full-text data extraction.
Use of simple approaches to subclassify type 2 diabetes
Description of extracted studies.
The 51 studies using simple type 2 diabetes subclassification approaches incorporated 1,751,350 participants with prevalent or new-onset type 2 diabetes. Among them, 39% (20/51) of studies included participants of white European ancestry, 43% (22/51) incorporated exclusively participants from non-white European ancestries and 17% (9/51) included mixed ancestry groups (Supplementary Data 1 ). The majority of the studies (78%, 40/51) were conducted in populations with prevalent type 2 diabetes, and 22% (11/51) in new-onset type 2 diabetes. Approximately half the studies had a prospective design (25/51), the remaining half had a cross-sectional (26/51) design. For longitudinal studies, study follow-up periods ranged from <1 year to 22 years.
Studies included a wide range of exposures (Fig. 2 ) based on routine clinical measurements with standard cut-offs or groupings. These included assessment of individual routine clinic-based measurements (e.g., levels of BMI, or biomarker variability over time) or composite stratification incorporating two or more tiers of criteria (e.g. groupings combining one or more biomarkers or anthropometric measurements) including both routine and non-routine but clinically available tests, including oral glucose tolerance tests (OGTT) which, while a glycaemic test, also indirectly measures insulin resistance. The associations of stratified exposure characteristics were investigated with various outcomes: 1) measures of glycaemia, 2) clinical characteristics, 3) measures of diabetes progression such as time-to-insulin treatment or development of microvascular complications and 4) cardiovascular outcomes and/or mortality.
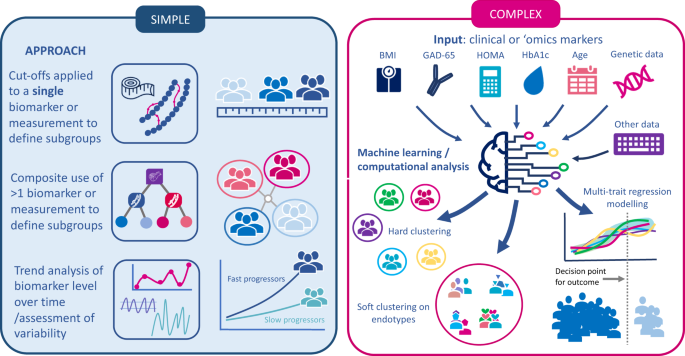
The figure summarises simple approaches that have been taken to subclassify type 2 diabetes and complex approaches. HbA1c glycated haemoglobin, BMI body mass index, GAD-65 glutamic acid decarboxylase-65 antibodies.
Description of categorised subgroups
Simple approaches to classification included use of lipid profiles ( n = 8), BMI ( n = 6), pancreatic beta-cell related measures ( n = 6), pancreatic autoantibodies ( n = 6), age at diagnosis ( n = 2), OGTT data ( n = 4), cardiovascular measures ( n = 3), other biomarkers in urine or blood and alternative approaches ( n = 5) (Table 1 ).
Different categories of triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, atherogenic small dense lipoproteins with and without features of metabolic syndrome were used to stratify type 2 diabetes in eight studies. Cardiovascular disease (CVD) outcomes were assessed in 3/8 of the studies 16 , 17 , 18 which showed that a more atherogenic metric of the specific lipid exposure (e.g., higher LDL cholesterol) was associated with a greater frequency of CVD outcomes. Other outcomes included pulse wave velocity 19 or clinical characteristics; age, BMI, presence of metabolic syndrome in specific subgroups.
The six studies assessing pancreatic autoantibodies focused on glutamic acid decarboxylase 65 (GAD-65) levels. Studies used positive versus negative status or high versus low titre, and one study sub-stratified by age. Outcomes included time-to-insulin treatment 20 , 21 , associations with other clinical characteristics such as lipid profiles, BMI and blood pressure 22 , 23 , 24 and measures of beta-cell function. There was no consistency in study design and most were observational with low to moderate evidence grade; two studies showed that GAD-65 positivity was associated with faster time-to-insulin treatment 20 , 21 .
Patients with type 2 diabetes were stratified according to their BMI in six studies, either by BMI alone ( n = 5) or BMI in combination with HbA1c. The number of BMI categories varied between two and six in the identified studies. The association between BMI and glycaemic outcomes (change in HbA1c from baseline) was assessed in four studies either as primary or secondary outcomes 6 , 25 , 26 . We graded the quality of evidence as very low to moderate, and no consistency of effect was observed across all studies. In one secondary analysis of a randomised control trial, higher BMI at baseline was associated with faster progression to adverse renal outcomes, however, this was not replicated in any other study 27 .
Age at diagnosis was assessed as a stratification tool in two studies; younger age (mean age 33 years) was associated with higher rates of proliferative retinopathy in an observational study with 12 months follow-up versus older age (mean 50 years) 4 . In a second study, patients aged 60–75 versus those >75 years had a high risk of CVD and mortality when stratified by cholesterol levels 6 . Neither study was replicated to confirm findings.
Four studies used results from oral glucose tolerance tests (OGTT) as exposures. The specific stratification approach applied to OGTT profiles was different in each study and based on cut-offs of fasting glucose levels, glucose gradients after stimulation and responses to different drug treatments. Outcomes included clamp-derived insulin sensitivity and differences in the shape of glucose profiles between youths and adults 28 .
Measures of estimated beta-cell function were assessed in six studies including C-peptide levels and homoeostasis model assessment-2 indices for beta-cell function (HOMA2-B) or insulin resistance (HOMA2-IR), which require measurement of fasting insulin and glucose levels. C-peptide was defined using variable cut-offs. Outcomes included clinical phenotype data, response to medication, and microvascular or macrovascular complications. For example, hyperinsulinaemia and higher urine C-peptide were independently associated with cardiovascular disease.
Other exposure variables included less routine biomarkers, pulse wave velocity, ketosis/ketoacidosis and other disease indices, but these were each single studies precluding grouping. All data are summarised in Table 1 .
Use of complex approaches to subclassify type 2 diabetes
There were 62 studies of complex/ML approaches to type 2 diabetes subclassification in a total of 793,291 participants (Table 2 ). Over half of the studies included non-European ancestry in relevant proportions (>20%). Only ~30% (19 out of 62) of the studies analysed participants with new-onset diabetes. Mean diabetes duration ranged from recent onset (within 1 year) to over 36 years. Most data were from observational studies (46 out of 62), with some post-hoc analyses of clinical trials (10), survey data (4) and mixed study types (2). Half of the studies had prospective design (31 out of 62) with a mean follow-up duration ranging from 1 year to 11.6 years. K-means clustering was the most applied ML approach (30 out of 62). Eight studies used established centroids 8 to assign participants to clusters. Two studies decomposed combinations of genetic variants and their association with clinical and laboratory phenotypes into genotype-phenotype clusters by using Bayesian non-negative matrix factorisation.
Description of the categorised subgroups
Following the seminal work by Ahlqvist et al. 8 , multiple studies used the variables derived at time of diabetes diagnosis: age, HbA1c, BMI, HOMA2-B, HOMA2-IR and GAD-65 antibody (Table 2 ). The majority of these studies employed C-peptide-based homoeostasis model assessment indices (HOMA, or its updated variant, HOMA2, using fasting insulin and glucose), as surrogates for insulin resistance (HOMA2-IR) and insulin secretion (HOMA2-B). In different contexts and populations, 22 studies replicated identification of the four non-autoimmune diabetes subtypes first described by Ahlqvist et al. 8 : severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD), and mild age-related diabetes (MARD). The subset of studies including measurements of GAD antibody also identified the fifth cluster, severe autoimmune diabetes (SAID). Associations of these subtypes with clinical outcomes, including glycaemia, microvascular and macrovascular outcomes, and death, were replicated in 12 studies (Table 3 ).
Thirteen additional papers used variations of the original set of variables from Ahlqvist et al. 8 by substituting HOMA with C-peptide, adding lipid traits, e.g. HDL-cholesterol, or approximating the clusters from different/simplified variable sets by applying advanced statistical learning approaches such as self-normalising neural networks. These approaches identified some type 2 diabetes subgroups resembling the clusters from Ahlqvist et al. and also novel subgroups related to the additional variables (Fig. 3 ). Several of the novel subgroups were associated with clinical outcomes. However, these findings have not been replicated in other studies (Table 2 ).
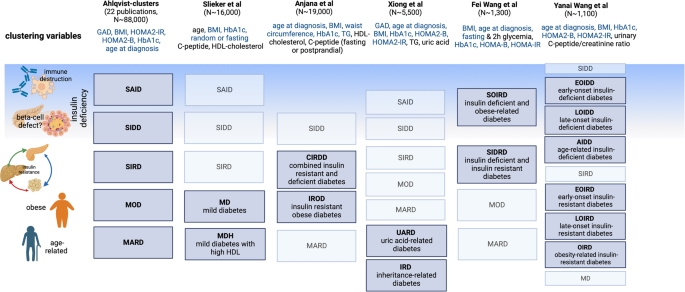
Clustering variables denoted in blue are consistent across the different studies, those in black are unique to the particular study outlined. A greyed-out box indicates that the indicated diabetes cluster was replicated from the Ahlqvist study, a dark blue box indicates a new diabetes cluster. GAD, glutamic decarboxylase antibody; BMI, body mass index; HDL, high-density lipoprotein cholesterol; HOMA2-IR/B, homoeostasis model assessment-2 insulin resistance/beta cell function. SAID, severe autoimmune diabetes; SIDD, severe insulin-deficient diabetes; SIRD, severe insulin resistant diabetes; MOD, mild obesity-related diabetes; MARD, mild age-related diabetes.
Additional papers ( n = 27) assessed various sets of phenotypic inputs for ML approaches. Grouped into five categories of inputs, studies identified many subtypes and associations with clinical outcomes, however, they all lacked replication (Table 2 ). Four papers applied complex ML methods to a set of less than ten clinical variables such as systolic blood pressure, waist circumference, BMI, fasting plasma glucose, and age at diabetes diagnosis, and resulting subgroups were variably associated with outcomes, such as mortality. Eleven studies used a larger set of more than ten clinical features as inputs for classification, including data from electronic health records 29 , 30 , and identified subgroups variably associated with clinical outcomes, including risk of cardiovascular disease. Two other studies specifically employed cardiovascular traits, including ECG 31 and echocardiographic 32 for ML algorithm inputs, and each identified subgroups with different associations with risk of cardiovascular disease. Finally, four studies involved inputs of change of glycaemic variables (HbA1c trajectories, glycaemia during a mixed meal test, continuous glucose monitoring features) 33 , 34 , 35 , one study focused on fasting GLP-1, GIP and ghrelin levels 36 , and two studies focused on behavioural traits such as novelty seeking, harm avoidance, and hospital anxiety and depression scale.
Human genetic risk information is rapidly penetrating clinical medicine. Two sets of papers utilised genomic data to identify diabetes subtypes, either in the form of inherited common genetic variation 10 , 37 or gene expression data from muscle biopsies 38 (Table 2 ). The first approach clustered genetic variants with clinical traits associated with type 2 diabetes to identify subsets of variants predicted to act in shared mechanistic processes. Using these sets of genetic variants, process-specific or partitioned polygenic scores were constructed in individuals with type 2 diabetes and were associated with differences in clinical features and prevalence of metabolic outcomes, with replication across multiple cohorts. The muscle gene expression study has not been replicated. Overall, half of the studies had cross-sectional designs, and the other half involved prospective follow-up (Table 2 ).
For simple approaches, of the 51 studies assessed, 55% were quality graded as very low-, or low-GRADE certainty, 45% had moderate certainty and none achieved high certainty. For complex approaches, around 70% of the studies had moderate evidence certainty. In both approaches, the majority of the studies had moderate or lower GRADE certainty on account of the (1) study design not addressing precision medicine objectives (not an RCT testing differential treatment effects in subclassified type 2 diabetes groups), (2) lack of a meaningful clinical outcome (i.e. although subgroups of type 2 diabetes were found, the measured outcome had little clinical significance because the study was not designed to study this) (3) Confidence in the findings were low due to small sample sizes, lack of replication or lack of diversity of studied subgroups and (4) the potential for bias was large due to lack of adjustment for possible confounders.
Summary of findings
This systematic review analysed two broad approaches to the subclassification of type 2 diabetes to identify clinically meaningful subtypes that may advance precision diagnostics. We found many simple stratification approaches using, for example, clinical features such as BMI, age at diagnosis, and lipid levels, but none had been replicated and many lacked associations with clinical outcomes. Complex stratification models using ML approaches with and without genetic data showed reproducible subtypes of type 2 diabetes associated with outcomes. Both approaches require a higher grade of evidence but support the premise that type 2 diabetes can be subclassified into clinically meaningful subtypes.
Simple approaches to subclassification included urine and blood biomarkers, anthropometric measures, clinical data such as age at diagnosis, surrogate beta-cell metrics derived from blood C-peptide or insulin along with other less diabetes-related biomarkers such as bilirubin levels or pulse wave velocity. Approaches to subclassification were diverse. Some studies dichotomised continuous variables based on clinical cut-points. Other studies used a composite exposure (two or more criteria each with cut-points) or analysed changes in continuous variables over time e.g. change in eGFR over time.
The study designs, specific cut-offs and outcomes were heterogenous, and no studies met high-quality GRADE certainty. No study evaluating a simple approach to type 2 diabetes subtyping has been adequately reproduced, although some studies identified biologically plausible subgroups. For example, subclassifications derived using BMI, beta-cell function, lipid profiles and age appeared to be associated with some outcomes which could be helpful in clinical practice. These potential subclassifications need to be replicated in better-designed studies (see section on additional supporting literature). Other evidence not included in our systematic review (either due to the study population including people without diabetes or the analysis was only performed in people with the exposure without a comparison group), support the role of simple variables in stratifying diabetes; for example, younger age at diagnosis is reproducibly associated with worse cardiorenal outcomes in a number of studies 39 .
Machine learning approaches yielded some reproducible subtypes of type 2 diabetes using a variety of clinical and genetic variables. The best-replicated subtypes were the clusters first described by Ahlqvist et al. 8 , which were replicated in 22 studies, including ~88,000 individuals of diverse ancestry. There also was replication of genetic subtypes of type 2 diabetes from Udler et al. 10 with associations with clinical features seen in multiple cohorts across almost 454,000 individuals 36 . However, the latter associations involved small absolute effects with unclear clinical utility for individual patient management, and studies were restricted to individuals of European ancestry. While there was replication of the clusters from Ahlqvist et al. across studies, the generated clusters appeared to be dependent on the characteristics of the underlying populations, especially factors such as distribution of ancestry, age, duration of diabetes, anthropometric trait variability as in BMI, and the variety of variable terms included in learning models. Nevertheless, at least some of the resulting subtypes appeared to be robust to differences in specific ML method, input variables, and populations (Fig. 3 ).
Many of the input variables for the complex ML subtyping approaches were also used in studies involving simple approaches to subclassification, recapitulating the biological plausibility of specific clustering variables in defining type 2 diabetes subtypes. One study directly compared a simple clinical approach to the clustering approach from Ahlqvist et al. 8 and found that simple single clinical measures analysed in a quantitative (rather than categorical) framework could better predict relevant clinical outcomes, such as incidence of chronic kidney disease and glycaemic response to medications 40 . Thus, further research is needed to determine whether assigning a patient to one of the clusters from Ahlqvist et al. 8 offers additional clinical benefit beyond evaluation of simple clinical measures and also beyond current standard of care. For example, high quality randomised controlled trial evidence is needed to demonstrate that knowledge of a patient’s clinical or genetic cluster membership could meaningfully guide treatment and/or clinical care and improve outcomes.
Study quality
No studies included in our systematic review had above moderate certainty of evidence. Some strengths of included studies were the large sample sizes, the diversity of variables considered, and inclusion of both prevalent and new-onset cases of type 2 diabetes. However, the varied study designs and lack of replication limits our ability to draw firm conclusions about the most effective approaches to subclassification. Most variables used for subclassification capture momentary metabolic states, which limits their long-term utility as cluster assignment is likely to change over time 41 , 42 . Most studies were retrospective analyses of established cohorts, and there were, at the time of the search, no data available involving subtype-stratified clinical trials or real world implementation of approaches. Finally, most studies focused on European-ancestry populations, and the clinical value of these approaches may vary across different ancestries. While East Asian ancestries had representation in some studies, research in Black, South Asian and Hispanic populations remains sparse. This is particularly important, as four out of five people with type 2 diabetes come from marginalised groups or live in low- or middle-income countries. Future precision diagnostic interventions should address and narrow inequalities.
Additional supporting literature
Since our literature search was conducted, four new publications have advanced our understanding of type 2 diabetes subclassification.
Two recent studies applied ML approaches to stratify diabetes heterogeneity, both considering continuous approaches rather than with discrete clusters 43 , 44 . Nair et al. used a non-linear transformation and visualisation of nine variables onto a tree-like structure 44 and with replication in two large datasets. This approach linked underlying disease heterogeneity to risk of complications; those at risk of cardiovascular disease had a different phenotype to those with microvascular complications and to drug response and demonstrated associations of gradients across the tree using genetic process-specific scores from Udler et al. 10 Wesolowska-Andersen et al. performed soft-clustering from 32 clinical variables which yielded 4 diabetes archetypes comprising a third of the study population. The remaining study population was deemed as mixed-phenotype. This study has not been replicated 43 . A third study re-identified the genetic subtypes and their clinical associations from Udler et al. 45 .
Additionally, one of the first clinical trials to assess precision medicine approaches for diabetes management was published after our literature search. The TriMaster Study tested dichotomised BMI and eGFR strata in a three-period crossover trial using three pharmacologic interventions with the primary hypothesis being stratum-specific differences in HbA1c 46 . Participants with obesity (BMI > 30 kg/m 2 ) showed a glycaemic benefit on pioglitazone versus sitagliptin and participants with lower eGFR (60–90 ml/min/1.73 m 2 ) responded with lower HbA1c to sitagliptin as compared to canagliflozin. In a secondary analysis, drug-choice corresponding to patient preferences yielded lower glycemia than a random allocation, suggesting that listening to patients is critical in informing therapeutic decisions 47 . Ramifications of this study are limited by the non-comparable pharmacologic doses used, and the primary focus on glycaemia which may not be indicative of long-term therapeutic success and/or prevention of complications. Yet these studies have generated higher quality evidence linking type 2 diabetes heterogeneity to treatment and disease outcomes. It remains to be seen if these can be replicated in other ancestries and translated into ‘usable products’ for healthcare professionals.
It is worth noting that ketosis-prone type 2 diabetes, an established type 2 diabetes subtype, was not captured adequately in our systematic review: only one study included ketosis-prone type 2 diabetes as an exposure 48 . Study designs for ketosis-prone type 2 diabetes were usually analyses of cohorts with diabetic ketoacidosis at presentation with type 1 diabetes as the outcome, rather than as an exposure in people with type 2 diabetes. Since our search was designed to identify studies stratifying type 2 diabetes, this literature was not captured. Like many other ‘simple’ criteria for classification, the characteristics of people with diabetic ketoacidosis at presentation of type 2 diabetes have been studied, but with few prospective studies that have been replicated 49 .
Age at diagnosis as a simple approach to stratification also did not feature strongly in our search results. The body of literature that outlines higher risk of microvascular or macrovascular complications in early-onset type 2 diabetes has focussed on comparing people with type 2 diabetes to those without diabetes in different age groups 39 , 50 or studied cohorts of early-onset cases in isolation 51 and, thus, would not have been captured in our search strategy. Recent epidemiological studies have compared outcomes between early and late age onset strata 52 , 53 showcasing higher risks of cardiorenal outcomes with early age at onset, but these were retrospective analyses of health record databases, potentially confounded by age-related risk of complications and duration of diabetes. To move forward, prospective studies stratifying different interventions (e.g., tighter treatment targets or better cardiovascular risk reduction) in those diagnosed at younger age, are needed.
Findings in context
We found that simple features have not been precisely and reproducibly evaluated to a high enough standard to subclassify type 2 diabetes into subtypes. This is not surprising, as many studies were not necessarily conducted for the purpose of ‘precision diagnosis’, but rather as studies of clinical phenotypes spanning a time period that preceded the current research focus on precision medicine. It is important to re-emphasise that many of the simple clinical criteria studied, do have other bodies of evidence supporting associations with outcomes, like age -at -diagnosis. While these studies have set the scene, the field needs more robust evidence.
‘Complex’ methods for diabetes subclassification have shown better reproducibility, have been linked to a variety of meaningful clinical outcomes more consistently, and more recently have been able to demonstrate differential treatment responses related to stratification.
What do these findings mean for a precision medicine approach to type 2 diabetes diagnosis? Ideally, subclassification strategies should be deployed at diagnosis of type 2 diabetes on the basis of measured clinical characteristics such that people in different subgroups of type 2 diabetes could be treated differently. One key question is whether such efforts would cost-effectively improve clinical outcomes, compared to the current standard of care. However, another more fundamental question is whether subclassification approaches at diagnosis alone are enough? For example, another approach may be to iteratively subclassify longitudinal disease trajectories. Such an approach is supported by studies that have shown cluster-based assignments of type 2 diabetes at diagnosis are not robust and may change over time 54 . It may be argued that subclassification at one-time point is overly simplistic and should be regularly reviewed based on trajectory.
Irrespective of the subclassification approach studied, they need replication in independent datasets, assessment in diverse populations, in people with both new-onset and prevalent diabetes, and investigation using prospective data, ideally in the form of randomised clinical trials. Clinical trials of treatment approaches tailored to diabetes subtypes will be necessary to understand the clinical benefits of clinical subtyping. Ideally, sub-phenotyping should lead to benefits for patients in real-world clinical settings. Conducting these studies will be challenging due to the necessity for extensive follow-up, large sample sizes, and substantial resource requirements. There is a pressing need for innovative strategies to generate high-quality evidence on treatment options tailored to specific diabetes subtypes in diverse populations. These data will be critical to determine generalisability of findings and amenability for clinical translation including in resource-constrained settings.
Clinical applicability
The current evidence supports distinguishable subtypes of type 2 diabetes and that these subtypes are associated with variation in clinical outcomes. However, the very low to moderate quality of existing studies and the need for replication in ancestry-diverse studies make it difficult to identify a strongly evidence-based, universally applicable approach.
The most clinically valuable methods are likely to be those that are easy and inexpensive to implement. For more complex approaches, computer decision support tools will need to be developed and assessed for feasibility and utility. Although the evidence supporting complex approaches has leap-frogged the evidence in favour of more simplified approaches, there is still likely a place for simple approaches that can be more accessible at diverse clinical interfaces. Meanwhile how cluster assignment could be translated into actionable data for the individual remains unclear; will for example, a given person with type 2 diabetes exist in a distinct subgroup with associated outcomes or will the subtype of type 2 diabetes have associated probabilities or risks of certain outcomes? While stratifying people with type 2 diabetes into discrete subtypes might result in information loss, compared to continuous risk modelling 40 , discrete clusters might inform clinical decisions 42 .
Limitations
The limitations of this review reflect the limitations of the literature. To manage the breadth of literature analysed in this systematic review, focussed on genomic data and did not include proteomic or metabolomic data as these are potentially more premature for clinical use. We also did not include studies on participants at risk of type 2 diabetes, although we recognise that a body of evidence is emerging to stratify type 2 diabetes incidence risk using multiple approaches that are similar to those for established type 2 diabetes. Since we focused on studies that attempted to subgroup type 2 diabetes, we also did not capture analyses of independent cohorts with a particular type 2 diabetes phenotype at baseline, for example, studies of young people with type 2 diabetes or those with ketosis-prone type 2 diabetes, as outlined.
Next steps and recommendations
Future research should aim to identify and validate clinically useful and cost-effective methods for type 2 diabetes subclassification that can be applied across diverse populations. Such research will involve replication of a given approach in independent datasets, including from diverse ancestral populations, to ensure generalisability that doesn’t widen health disparities. For simple stratification approaches, there is still much that can be done—agreement on standardised study designs for precision diagnostics studies could be a first step. For ML requiring real-time computation, the development of strategies to overcome local resource constraints in implementing these methods could be explored.
In this first systematic review of the evidence underpinning type 2 diabetes diagnostic subclassification, multiple approaches were identified. Among them are strategies that used simple criteria based on fundamental categorisation of mostly routine measures, and complex approaches with multi-trait or genetic inputs that required ML or other computation. While simple approaches are more easily deployed, the study designs and level of evidence currently limits any firm conclusions regarding the utility of such approaches. The clinical variables and data incorporated into ‘complex’ approaches have yielded reproducible subclassifications and a growing body of evidence supports clinically meaningful associations of subtypes with outcomes and treatment responses. This is a rapidly evolving field with higher quality evidence emerging. It will be crucial to develop interventions that target diverse populations and be feasible in all resource settings to prevent widening existing inequalities in the precision medicine era of diabetes care.
Data availability
The extracted data from full-text articles included in this systematic review are available in Supplementary Data 1 .
Diabetes Atlas 10th Edition, World Health Organisation. https://diabetesatlas.org/atlas/tenth-edition/ , accessed 6 Jun 2023 (2021).
ElSayed, N. A. et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 46 , S19–S40 (2023).
CAS PubMed Google Scholar
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45 , 2753–2786 (2022).
CAS PubMed PubMed Central Google Scholar
Parameswarappa, D. C. et al. Severity of diabetic retinopathy and its relationship with age at onset of diabetes mellitus in India: a multicentric study. Indian J. Ophthalmol. 69 , 3255–3261 (2021).
PubMed PubMed Central Google Scholar
Prando, R. et al. Is type 2 diabetes a different disease in obese and nonobese patients? Diabetes Care 21 , 1680–1685 (1998).
van Hateren, K. J. J. et al. The lipid profile and mortality risk in elderly type 2 diabetic patients: a ten-year follow-up study (ZODIAC-13). PLoS One 4 , e8464 (2009).
Shepherd, M. H. et al. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia 61 , 2520–2527 (2018).
Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6 , 361–369 (2018).
PubMed Google Scholar
Mahajan, A. et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 50 , 559 (2018).
Udler, M. S. et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 15 , e1002654 (2018).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care 45 , 261–266 (2022).
Tobias, D. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. https://doi.org/10.1038/s41591-023-02502-5 . (2023).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372 , n71 (2021).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 , 924–926 (2008).
What is GRADE? | BMJ Best Practice. https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade/ .
Valensi, P. et al. Atherogenic dyslipidemia and risk of silent coronary artery disease in asymptomatic patients with type 2 diabetes: a cross-sectional study. Cardiovasc. Diabetol. 15 , 104 (2016).
Huang, J. et al. Elevated serum small dense low-density lipoprotein cholesterol may increase the risk and severity of coronary heart disease and predict cardiovascular events in patients with type 2 diabetes mellitus. Dis. Mark. 2021 , 5597028 (2021).
Google Scholar
Zhao, Q. et al. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc. Diabetol. 20 , 190 (2021).
Wang, S. et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc. Diabetol. 20 , 82 (2021).
Turner, R. et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 350 , 1288–1293 (1997).
Lee, S. A. et al. Progression to insulin deficiency in Korean patients with Type 2 diabetes mellitus positive for anti-GAD antibody. Diabet. Med. 28 , 319–324 (2011).
Tuomi, T. et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 48 , 150–157 (1999).
Buzzetti, R. et al. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 30 , 932–938 (2007).
Ipadeola, A., Adeleye, J. O. & Akinlade, K. S. Latent autoimmune diabetes amongst adults with type 2 diabetes in a Nigerian tertiary hospital. Prim. Care Diabetes 9 , 231–236 (2015).
Dennis, J. M. et al. Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diabetes Care 41 , 705–712 (2018).
Faraz, A., Ashraf, H. & Ahmad, J. Clinical features, biochemical profile, and response to standard treatment in lean, normal-weight, and overweight/obese Indian type 2 diabetes patients. Rev. Diabet. Stud. 17 , 68–74 (2021).
Mohammedi, K. et al. Associations between body mass index and the risk of renal events in patients with type 2 diabetes. Nutr. Diabetes 8 , 7 (2018).
Arslanian, S. et al. The shape of the glucose response curve during an oral glucose tolerance test: forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care 42 , 164–172 (2019).
Li, L. et al. Identification of type 2 diabetes subgroups through topological analysis of patient similarity. Sci. Transl. Med. 7 , 311ra174 (2015).
Kirk, I. K. et al. Linking glycemic dysregulation in diabetes to symptoms, comorbidities, and genetics through EHR data mining. eLife 8 , e44941 (2019).
Kuusisto, J., Lempiäinen, P., Mykkänen, L. & Laakso, M. Insulin resistance syndrome predicts coronary heart disease events in elderly type 2 diabetic men. Diabetes Care 24 , 1629–1633 (2001).
Ernande, L. et al. Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J. Am. Coll. Cardiol. 70 , 1704–1716 (2017).
Karpati, T. et al. Patient clusters based on HbA1c trajectories: a step toward individualized medicine in type 2 diabetes. PLoS ONE 13 , e0207096 (2018).
Tao, R. et al. Multilevel clustering approach driven by continuous glucose monitoring data for further classification of type 2 diabetes. BMJ Open Diabetes Res. Care 9 , e001869 (2021).
Mariam, A. et al. Type 2 diabetes subtype responsive to ACCORD intensive glycemia treatment. Diabetes Care https://doi.org/10.2337/dc20-2700 (2021).
Amato, M. C., Pizzolanti, G., Torregrossa, V., Pantò, F. & Giordano, C. Phenotyping of type 2 diabetes mellitus at onset on the basis of fasting incretin tone: results of a two‐step cluster analysis. J. Diabetes Investig. 7 , 219–225 (2016).
DiCorpo, D. et al. Type 2 diabetes partitioned polygenic scores associate with disease outcomes in 454,193 individuals across 13 cohorts. Diabetes Care 45 , 674–683 (2022).
Khoshnejat, M., Kavousi, K., Banaei-Moghaddam, A. M. & Moosavi-Movahedi, A. A. Unraveling the molecular heterogeneity in type 2 diabetes: a potential subtype discovery followed by metabolic modeling. BMC Med. Genom. 13 , 119 (2020).
CAS Google Scholar
Sattar, N. et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 139 , 2228–2237 (2019).
Dennis, J. M., Shields, B. M., Henley, W. E., Jones, A. G. & Hattersley, A. T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 7 , 442–451 (2019).
Lugner, M. et al. Comparison between data-driven clusters and models based on clinical features to predict outcomes in type 2 diabetes: nationwide observational study. Diabetologia 64 , 1973–1981 (2021).
Herder, C. & Roden, M. A novel diabetes typology: towards precision diabetology from pathogenesis to treatment. Diabetologia https://doi.org/10.1007/s00125-021-05625-x (2022).
Wesolowska-Andersen, A. et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: an IMI DIRECT study. Cell Rep. Med. 3 , 100477 (2022).
Nair, A. T. N. et al. Heterogeneity in phenotype, disease progression and drug response in type 2 diabetes. Nat. Med. 28 , 982–988 (2022).
Kim, H. et al. High-throughput genetic clustering of type 2 diabetes loci reveals heterogeneous mechanistic pathways of metabolic disease. Diabetologia 66 , 495–507 (2023).
Shields, B. M. et al. Patient stratification for determining optimal second-line and third-line therapy for type 2 diabetes: the TriMaster study. Nat. Med. 1–8 https://doi.org/10.1038/s41591-022-02120-7 (2022).
Shields, B. M. et al. Patient preference for second- and third-line therapies in type 2 diabetes: a prespecified secondary endpoint of the TriMaster study. Nat. Med. 1–8 https://doi.org/10.1038/s41591-022-02121-6 (2022).
Lontchi-Yimagou, E. et al. Ketosis-prone atypical diabetes in Cameroonian people with hyperglycaemic crisis: frequency, clinical and metabolic phenotypes. Diabet. Med. 34 , 426–431 (2017).
Mauvais-Jarvis, F. et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes 53 , 645–653 (2004).
Wright, A. K. et al. Age-, sex- and ethnicity-related differences in body weight, blood pressure, HbA1c and lipid levels at the diagnosis of type 2 diabetes relative to people without diabetes. Diabetologia 63 , 1542–1553 (2020).
TODAY Study Group et al. Long-term complications in youth-onset type 2 diabetes. N. Engl. J. Med. 385 , 416–426 (2021).
Paul, S. K., Shaw, J. E., Fenici, P. & Montvida, O. Cardiorenal complications in young-onset type 2 diabetes compared between white Americans and African Americans. Diabetes Care 45 , 1873–1881 (2022).
Dibato, J. E. et al. Association of cardiometabolic multimorbidity and depression with cardiovascular events in early-onset adult type 2 diabetes: a multiethnic study in the U.S. Diabetes Care 44 , 231–239 (2021).
Bello-Chavolla, O. Y. et al. Clinical characterization of data-driven diabetes subgroups in Mexicans using a reproducible machine learning approach. BMJ Open Diabetes Res. Care 8 , e001550 (2020).
O’Gorman, D. J. et al. In vivo and in vitro studies of GAD-antibody positive subjects with Type 2 diabetes: a distinct sub-phenotype. Diabetes Res. Clin. Pract. 80 , 365–370 (2008).
Tajiri, Y., Kimura, M., Mimura, K. & Umeda, F. Variation of fasting serum C-peptide level after admission in Japanese patients with type 2 diabetes mellitus. Diabetes Technol. Ther. 11 , 593–599 (2009).
Stidsen, J. V. et al. Pathophysiology-based phenotyping in type 2 diabetes: a clinical classification tool. Diabetes/Metab. Res. Rev. 0 , e3005 (2018).
Anoop, S., Misra, A., Bhatt, S. P., Gulati, S. & Mahajan, H. High fasting C-peptide levels and insulin resistance in non-lean & non-obese (BMI > 19 to <25 kg/m 2 ) Asian Indians with type 2 diabetes are independently associated with high intra-abdominal fat and liver span. Diabetes Metab. Syndr. 13 , 708–715 (2019).
Wang, Y. et al. Urinary C‐peptide/creatinine ratio: a useful biomarker of insulin resistance and refined classification of type 2 diabetes mellitus. J. Diabetes 13 , 893–904 (2021).
Saxena, T., Maheshwari, S. & Goyal, R. K. Serum insulin assay: an important therapeutic tool in management of freshly diagnosed type 2 diabetes mellitus. J. Assoc. Physicians India 48 , 815–817 (2000).
Selvaraj, R., Rajappa, M. & Shamanna, S. B. Phenotypic characterization of patients with type 2 diabetes mellitus. Int. J. Diabetes Dev. Ctries 40 , 242–247 (2020).
Dennis, J. M. et al. Sex and BMI alter the benefits and risks of sulfonylureas and thiazolidinediones in type 2 diabetes: a framework for evaluating stratification using routine clinical and individual trial data. Diabetes Care 41 , 1844–1853 (2018).
Feng, W. et al. Lixisenatide is effective and safe as add-on treatment to basal insulin in Asian individuals with type 2 diabetes and different body mass indices: a pooled analysis of data from the GetGoal Studies. BMJ Open Diabetes Res. Care 9 , e002290 (2021).
Miñambres, I. et al. Characterization of the hypertriglyceridemic waist phenotype in patients with type 2 diabetes mellitus in Spain: an epidemiological study. Rev. Clin. Esp. 221 , 576–581 (2021).
Kompoti, M. et al. Elevated serum triglycerides is the strongest single indicator for the presence of metabolic syndrome in patients with type 2 diabetes. Cardiovasc. Diabetol. 5 , 21 (2006).
Radenković, S. P. et al. The hypertriglyceridemic waist phenotype and metabolic syndrome by differing criteria in type 2 diabetic patients and their relation to lipids and blood glucose control. Endokrynol. Pol. 62 , 316–323 (2011).
Fu, L. et al. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 20 , 201 (2021).
Færch, K. et al. Trajectories of cardiometabolic risk factors before diagnosis of three subtypes of type 2 diabetes: a post-hoc analysis of the longitudinal Whitehall II cohort study. Lancet Diabetes Endocrinol. 1 , 43–51 (2013).
Arslanian, S. A. et al. OGTT glucose response curves, insulin sensitivity, and β-cell function in rise: comparison between youth and adults at randomization and in response to interventions to preserve β-cell function. Diabetes Care 44 , 817–825 (2021).
Mitsui, R. et al. Factors responsible for deteriorating glucose tolerance in newly diagnosed type 2 diabetes in Japanese men. Metabolism 55 , 53–58 (2006).
Greenfield, S. et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann. Intern. Med. 151 , 854–860 (2009).
Roy Chowdhury, S. et al. Diabetic retinopathy in newly diagnosed subjects with type 2 diabetes mellitus: contribution of β-cell function. J. Clin. Endocrinol. Metab. 101 , 572–580 (2016).
Yun, J.-S. et al. Severe hypoglycemia and the risk of cardiovascular disease and mortality in type 2 diabetes: a nationwide population-based cohort study. Cardiovasc. Diabetol. 18 , 103 (2019).
Echouffo-Tcheugui, J. B. et al. Duration of diabetes and incident heart failure: the ARIC (Atherosclerosis Risk In Communities) study. JACC Heart Fail. 9 , 594–603 (2021).
Januszewicz, A. et al. Office and ambulatory pulse pressure–association with clinical characteristics and cardiovascular risk factors in normoalbuminuric patients with type 2 diabetes (ROADMAP study). J. Hum. Hypertens. 25 , 679–685 (2011).
Liu, J.-J. et al. Aortic pulse wave velocity, central pulse pressure, augmentation index and chronic kidney disease progression in individuals with type 2 diabetes: a 3- year prospective study. BMC Nephrol. 21 , 359 (2020).
Segar, M. W. et al. Prevalence and prognostic implications of diabetes with cardiomyopathy in community-dwelling adults. J. Am. Coll Cardiol. 78 , 1587–1598 (2021).
Ihara, K. et al. Profibrotic circulating proteins and risk of early progressive renal decline in patients with type 2 diabetes with and without albuminuria. Diabetes Care 43 , 2760–2767 (2020).
Linnemann, B., Voigt, W., Nobel, W. & Janka, H. U. C-reactive protein is a strong independent predictor of death in type 2 diabetes: association with multiple facets of the metabolic syndrome. Exp. Clin. Endocrinol. Diabetes 114 , 127–134 (2006).
Fukui, M. et al. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int. 74 , 1197–1201 (2008).
Jian, W. et al. Role of serum vaspin in progression of type 2 diabetes: a 2-year cohort study. PLoS One 9 , e94763 (2014).
Gong, S. et al. Clinical and genetic features of patients with type 2 diabetes and renal glycosuria. J. Clin. Endocrinol. Metab. 102 , 1548–1556 (2017).
Saleem, T. et al. The profile of plasma free amino acids in type 2 diabetes mellitus with insulin resistance: association with microalbuminuria and macroalbuminuria. Appl. Biochem. Biotechnol. 188 , 854–867 (2019).
Usman, M., Khunti, K., Davies, M. J. & Gillies, C. L. Association and relative importance of multiple risk factor control on cardiovascular disease, end-stage renal disease and mortality in people with type 2 diabetes: a population-based retrospective cohort study. Prim. Care Diabetes 15 , 218–226 (2021).
Feng, X. et al. Myocardial infarction and coronary artery disease in menopausal women with type 2 diabetes mellitus negatively correlate with total serum bile acids. Front. Endocrinol. 12 , 754006 (2021).
Warren, R. A. et al. Haptoglobin phenotype modifies the effect of fenofibrate on risk of coronary event: ACCORD lipid trial. Diabetes Care 45 , 241–250 (2022).
Yu, H. et al. Serum chromogranin A correlated with albuminuria in diabetic patients and is associated with early diabetic nephropathy. BMC Nephrol. 23 , 41 (2022).
Chang, Y.-S., Li, Y.-H. & Lee, I.-T. A synergistic effect of variability in estimated glomerular filtration rate with chronic kidney disease on all-cause mortality prediction in patients with type 2 diabetes: a retrospective cohort study. Cardiovasc. Diabetol. 20 , 209 (2021).
Mansour Aly, D. et al. Genome-wide association analyses highlight etiological differences underlying newly defined subtypes of diabetes. Nat. Genet. 53 , 1534–1542 (2021).
Zaharia, O. P. et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 7 , 684–694 (2019).
Saito, K. et al. Usefulness of subclassification of adult diabetes mellitus among inpatients in Japan. J. Diabetes Investig. 13 , 706–713 (2022).
Saatmann, N. et al. Physical fitness and cardiovascular risk factors in the novel diabetes subgroups. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgab810 (2021).
Maalmi, H. et al. Differences in the prevalence of erectile dysfunction between novel subgroups of recent-onset diabetes. Diabetologia 65 , 552–562 (2022).
Zou, X., Zhou, X., Zhu, Z. & Ji, L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 7 , 9–11 (2019).
Pigeyre, M. et al. Validation of the classification for type 2 diabetes into five subgroups: a report from the ORIGIN trial. Diabetologia 65 , 206–215 (2022).
Herder, C. et al. Differences in biomarkers of inflammation between novel subgroups of recent-onset diabetes. Diabetes https://doi.org/10.2337/db20-1054 (2021).
Prasad, R. B. et al. Subgroups of patients with young-onset type 2 diabetes in India reveal insulin deficiency as a major driver. Diabetologia https://doi.org/10.1007/s00125-021-05543-y (2021).
Peng, X. et al. Roles of plasma leptin and resistin in novel subgroups of type 2 diabetes driven by cluster analysis. Lipids Health Dis. 21 , 7 (2022).
Xie, J. et al. Validation of type 2 diabetes subgroups by simple clinical parameters: a retrospective cohort study of NHANES data from 1999 to 2014. BMJ Open 12 , e055647 (2022).
Christensen, D. H. et al. Type 2 diabetes classification: a data-driven cluster study of the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort. BMJ Open Diabetes Res. Care 10 , e002731 (2022).
Gao, H. et al. Cardiorenal risk profiles among data-driven type 2 diabetes sub-phenotypes: a post-hoc analysis of the China health and nutrition survey. Front. Endocrinol. 13 , 828403 (2022).
Li, P.-F. & Chen, W.-L. Are the different diabetes subgroups correlated with all-cause, cancer-related, and cardiovascular-related mortality? J. Clin. Endocrinol. Metab. 105 , e4240–e4251 (2020).
Xing, L. et al. Clinical characteristics and risk of diabetic complications in data-driven clusters among type 2 diabetes. Front. Endocrinol. 12 , 617628 (2021).
Wang, W. et al. Application of new international classification of adult‐onset diabetes in Chinese inpatients with diabetes mellitus. Diabetes Metab. Res. 37 , e3427 (2021).
Ratter-Rieck, J. et al. Leukocyte counts and T-cell frequencies differ between novel subgroups of diabetes and are associated with metabolic parameters and biomarkers of inflammation. Diabetes 70 , 2652–2662 (2021).
Aoki, Y. et al. Assessing reproducibility and utility of clustering of patients with type 2 diabetes and established CV disease (SAVOR -TIMI 53 trial). PLoS One 16 , e0259372 (2021).
Tanabe, H. et al. Factors associated with risk of diabetic complications in novel cluster-based diabetes subgroups: a Japanese retrospective cohort study. JCM 9 , 2083 (2020).
Fedotkina, O. et al. Novel reclassification of adult diabetes is useful to distinguish stages of β-cell function linked to the risk of vascular complications: the DOLCE study from Northern Ukraine. Front. Genet. 12 , 637945 (2021).
Wang, F. et al. Novel subgroups and chronic complications of diabetes in middle-aged and elderly Chinese: a prospective cohort study. Front. Endocrinol. 12 , 802114 (2022).
Antonio-Villa, N. E. et al. Prevalence trends of diabetes subgroups in the United States: a data-driven analysis spanning three decades from NHANES (1988-2018). J. Clin. Endocrinol. Metab. dgab762 https://doi.org/10.1210/clinem/dgab762 (2021).
Safai, N., Ali, A., Rossing, P. & Ridderstråle, M. Stratification of type 2 diabetes based on routine clinical markers. Diabetes Res. Clin. Pract. 141 , 275–283 (2018).
Slieker, R. C. et al. Distinct molecular signatures of clinical clusters in people with type 2 diabetes: an IMI-RHAPSODY study. Diabetes 70 , 2683–2693 (2021).
Slieker, R. C. et al. Replication and cross-validation of type 2 diabetes subtypes based on clinical variables: an IMI-RHAPSODY study. Diabetologia 64 , 1982–1989 (2021).
Article PubMed PubMed Central Google Scholar
Bancks, M. P. et al. Association of diabetes subgroups with race/ethnicity, risk factor burden and complications: the MASALA and MESA studies. J. Clin. Endocrinol. Metab. 106 , e2106–e2115 (2021).
Xiong, X. et al. Identification of two novel subgroups in patients with diabetes mellitus and their association with clinical outcomes: A two‐step cluster analysis. J. Diabetes Investig. 12 , 1346–1358 (2021).
Anjana, R. M. et al. Novel subgroups of type 2 diabetes and their association with microvascular outcomes in an Asian Indian population: a data-driven cluster analysis: the INSPIRED study. BMJ Open Diabet. Res. Care 8 , e001506 (2020).
Kahkoska, A. R. et al. Validation of distinct type 2 diabetes clusters and their association with diabetes complications in the DEVOTE, LEADER and SUSTAIN-6 cardiovascular outcomes trials. Diabetes Obes. Metab. 22 , 1537–1547 (2020).
Lin, C.-C. et al. Three-year trajectories of metabolic risk factors predict subsequent long-term mortality in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 179 , 108995 (2021).
Bancks, M. P. et al. Type 2 diabetes subgroups, risk for complications, and differential effects due to an intensive lifestyle intervention. Diabetes Care 44 , 1203–1210 (2021).
Gunaid, A. A., Al-Kebsi, M. M., Bamashmus, M. A., Al-Akily, S. A. & Al-Radaei, A. N. Clinical phenotyping of newly diagnosed type 2 diabetes in Yemen. BMJ Open Diabetes Res. Care 6 , e000587 (2018).
Basu, S., Raghavan, S., Wexler, D. J. & Berkowitz, S. A. Characteristics associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the ACCORD trial. Diabetes Care 41 , 604–612 (2017).
Sharma, A. et al. Cluster analysis of cardiovascular phenotypes in patients with type 2 diabetes and established atherosclerotic cardiovascular disease: a potential approach to precision medicine. Diabetes Care 45 , 204–212 (2022).
Aravindakshan, M. R. et al. Distinct pathoclinical clusters among patients with uncontrolled type 2 diabetes: results from a prospective study in rural India. BMJ Open Diabetes Res. Care 10 , e002654 (2022).
Raghavan, S. et al. Generalizability of heterogeneous treatment effects based on causal forests applied to two randomized clinical trials of intensive glycemic control. Ann. Epidemiol. 65 , 101–108 (2022).
Landi, I. et al. Deep representation learning of electronic health records to unlock patient stratification at scale. NPJ Digit. Med. 3 , 96 (2020).
Eby, E. L. et al. Evaluating the relationship between clinical and demographic characteristics of insulin-using people with diabetes and their health outcomes: a cluster analysis application. BMC Health Serv. Res. 21 , 669 (2021).
Segar, M. W. et al. Development and validation of optimal phenomapping methods to estimate long-term atherosclerotic cardiovascular disease risk in patients with type 2 diabetes. Diabetologia 64 , 1583–1594 (2021).
Seng, J. J. B. et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care 43 , 1048–1056 (2020).
Nowakowska, M. et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med. 17 , 145 (2019).
Hertroijs, D. F. L. et al. A risk score including body mass index, glycated haemoglobin and triglycerides predicts future glycaemic control in people with type 2 diabetes. Diabetes Obes. Metab. 20 , 681–688 (2018).
Yoda, N. et al. Classification of adult patients with type 2 diabetes using the Temperament and Character Inventory. Psychiatry Clin. Neurosci. 62 , 279–285 (2008).
Skinner, T. C. et al. Comparison of illness representations dimensions and illness representation clusters in predicting outcomes in the first year following diagnosis of type 2 diabetes: results from the DESMOND trial. Psychol. Health 26 , 321–335 (2011).
Obura, M. et al. Post-load glucose subgroups and associated metabolic traits in individuals with type 2 diabetes: an IMI-DIRECT study. PLoS One 15 , e0242360 (2020).
Download references
Acknowledgements
No specific funding was received to undertake this body of work. The authors acknowledge individual and institutional funding as follows: S.M. has a personal award from Wellcome Trust Career Development scheme (223024/Z/21/Z) and holds Institutional funds from the NIHR Biomedical Research Centre Funding Scheme; B.O. is supported by American Heart Association grant (20SFRN35120152); M.S.G. is supported by the American Diabetes Association (9-22-PDFPM-04) and NIH (5UM1DK078616-14); R.J.K. is supported by NIGMS T32GM774844 and Pediatric Endocrine Society Rising Star Award; SJC is supported by a Junior Faculty Development Award from the American Diabetes Association (7-21-JDFM-005); D.D. is supported by NIH grant K23DK133690; A.C.B.T., M.A. and T.H. acknowledge that The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by and unrestricted grant from the Novo Nordisk Foundation (NNF18CC0034900); A.W. is supported by NIH/NHLBI grant T32HL007024; A.L. is supported by grant 2020096 from the Doris Duke Foundation and the American Diabetes Association Grant 7-22-ICTSPM-23; A.J.D. is supported by NIH/NIDDK grant T32DK007028; W.H.H.S. obtained funding from MOST, Taiwan (MOST 107-2314-B-075A-001 -MY3 and by MOST 109-2321-B-075A-001). M.G. is supported by the Eris M. Field Chair in Diabetes Research and NIH grant P30-DK063491; D.R. is supported by NIH/NIDDK grant R21DK125888, and other grants from the NIH; E.S. is supported by NIH/NHLBI grant K24 HL152440 and other grants from the NIH; J.C.F. is supported by NIH K24 HL157960; J.B.M. reports funding from NIH U01 DK078616, R01 HL151855; M.U. is supported by an NIH K23DK114551. The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence licence was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL).
Author information
These authors contributed equally: Shivani Misra, Robert Wagner.
These authors jointly supervised this work: James B. Meigs, Miriam S. Udler.
Authors and Affiliations
Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Shivani Misra
Department of Diabetes and Endocrinology, Imperial College Healthcare NHS Trust, London, UK
Department of Endocrinology and Diabetology, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Moorenstr. 5, 40225, Düsseldorf, Germany
Robert Wagner
Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf, Auf’m Hennekamp 65, 40225, Düsseldorf, Germany
Robert Wagner, Martin Schön, Katsiaryna Prystupa & Martin Schön
German Center for Diabetes Research (DZD), Ingolstädter Landstraße 1, 85764, Neuherberg, Germany
Robert Wagner, Martin Schön, Katsiaryna Prystupa, Norbert Stefan, Martin Schön & Norbert Stefan
Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Bige Ozkan, Caroline C. Wang, Mary R. Rooney, Amelia S. Wallace, Elizabeth Selvin & Bige Ozkan
Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins School of Medicine, Baltimore, MD, USA
Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia
Martin Schön
Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA
Magdalena Sevilla-Gonzalez & Magdalena Sevilla-Gonzalez
Programs in Metabolism and Medical & Population Genetics, The Broad Institute of MIT and Harvard, Cambridge, MA, USA
Magdalena Sevilla-Gonzalez, Raymond J. Kreienkamp, Sara J. Cromer, Aaron Leong, Aaron J. Deutsch, Jose C. Florez, James B. Meigs & Miriam S. Udler
Department of Medicine, Harvard Medical School, Boston, MA, USA
Magdalena Sevilla-Gonzalez, Sara J. Cromer, Aaron Leong, Aaron J. Deutsch, Jose C. Florez, Sara J. Cromer, Magdalena Sevilla-Gonzalez, Tinashe Chikowore, Aaron J. Deutsch, Aaron Leong, Camille E. Powe, Jose C. Florez, James B. Meigs, Miriam S. Udler, James B. Meigs & Miriam S. Udler
Diabetes Unit, Division of Endocrinology, Massachusetts General Hospital, Boston, MA, USA
Raymond J. Kreienkamp, Sara J. Cromer, Aaron Leong, Aaron J. Deutsch, Jose C. Florez & Miriam S. Udler
Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA
Raymond J. Kreienkamp, Sara J. Cromer, Aaron J. Deutsch, Jose C. Florez, Jordi Merino, Raymond J. Kreienkamp, Aaron J. Deutsch, Jose C. Florez, Miriam S. Udler & Miriam S. Udler
Department of Pediatrics, Division of Endocrinology, Boston Children’s Hospital, Boston, MA, USA
Raymond J. Kreienkamp & Raymond J. Kreienkamp
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Mary R. Rooney, Amelia S. Wallace, Debashree Ray, Elizabeth Selvin & Caroline C. Wang
Division of Endocrinology, Diabetes and Metabolism, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Anne Cathrine Baun Thuesen, Mette K. Andersen, Torben Hansen, Jordi Merino, Anne Cathrine B. Thuesen, Christoffer Clemmensen, Mariam Nakabuye & Ruth J. F. Loos
Division of General Internal Medicine, Massachusetts General Hospital, 100 Cambridge St 16th Floor, Boston, MA, USA
Aaron Leong & James B. Meigs
Division of Endocrinology, Diabetes and Metabolism, NorthShore University Health System, Skokie, IL, USA
Liana K. Billings
Department of Medicine, Pritzker School of Medicine, University of Chicago, Chicago, IL, USA
Division of Endocrinology, Metabolism and Diabetes, University of Colorado School of Medicine, Aurora, CO, USA
Robert H. Eckel
Institute of Molecular and Genomic Medicine, National Health Research Institute, Miaoli County, Taiwan, ROC
Wayne Huey-Herng Sheu
Division of Endocrinology and Metabolism, Taichung Veterans General Hospital, Taichung, Taiwan, ROC
Division of Endocrinology and Metabolism, Taipei Veterans General Hospital, Taipei, Taiwan, ROC
Wayne Huey-Herng Sheu & Wayne Huey-Herng Sheu
University Hospital of Tübingen, Tübingen, Germany
Norbert Stefan & Norbert Stefan
Institute of Diabetes Research and Metabolic Diseases (IDM), Helmholtz Center Munich, Neuherberg, Germany
Norbert Stefan
Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Mark O. Goodarzi
Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Debashree Ray
Division of Preventative Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Deirdre K. Tobias & Vanessa Santhakumar
Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Deirdre K. Tobias, Zhila Semnani-Azad, Marta Guasch-Ferré & Paul W. Franks
Diabetes Unit, Endocrine Division, Massachusetts General Hospital, Boston, MA, USA
Jordi Merino, Sara J. Cromer, Raymond J. Kreienkamp, Aaron Leong, Camille E. Powe, Jose C. Florez, Marie-France Hivert & Miriam S. Udler
Department of Clinical Sciences, Lund University Diabetes Centre, Lund University, Malmö, Sweden
Abrar Ahmad, Monika Dudenhöffer-Pfeifer, Hugo Fitipaldi, Hugo Pomares-Millan, Maria F. Gomez & Paul W. Franks
Department of Obstetrics and Gynaecology, the Rosie Hospital, Cambridge, UK
Catherine Aiken
NIHR Cambridge Biomedical Research Centre, University of Cambridge, Cambridge, UK
Departments of Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Jamie L. Benham
Department of Molecular Genetics, Madras Diabetes Research Foundation, Chennai, India
Dhanasekaran Bodhini
Division of Pediatric Endocrinology, Department of Pediatrics, Saint Louis University School of Medicine, SSM Health Cardinal Glennon Children’s Hospital, St. Louis, MO, USA
Amy L. Clark
Department of Clinical and Biomedical Sciences, University of Exeter Medical School, Exeter, Devon, UK
Kevin Colclough, Alice Hughes, Kashyap Amratlal Patel, Katherine Young, Angus G. Jones, Elisa de Franco, Sarah E. Flanagan, Andrew McGovern, John M. Dennis, Andrew T. Hattersley & Richard Oram
CIBER-BBN, ISCIII, Madrid, Spain
Rosa Corcoy
Institut d’Investigació Biomèdica Sant Pau (IIB SANT PAU), Barcelona, Spain
Departament de Medicina, Universitat Autònoma de Barcelona, Bellaterra, Spain
Programs in Metabolism and Medical & Population Genetics, Broad Institute, Cambridge, MA, USA
Sara J. Cromer, Raymond J. Kreienkamp, Magdalena Sevilla-Gonzalez, Aaron J. Deutsch, Camille E. Powe, Jose C. Florez & Miriam S. Udler
Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA
Jamie L. Felton, Linda A. DiMeglio, Carmella Evans-Molina, Arianna Harris-Kawano, Heba M. Ismail, Dianna Perez, Gabriela S. F. Monaco & Emily K. Sims
Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN, USA
Center for Diabetes and Metabolic Diseases, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Biostatistics and Epidemiology, Rutgers School of Public Health, Piscataway, NJ, USA
Ellen C. Francis
University Hospital Leuven, Leuven, Belgium
Pieter Gillard & Chantal Mathieu
Department of Nutrition, Université de Montréal, Montreal, QC, Canada
Véronique Gingras
Research Center, Sainte-Justine University Hospital Center, Montreal, QC, Canada
Department of Pediatrics, Erasmus Medical Center, Rotterdam, The Netherlands
Romy Gaillard
Division of Population Health & Genomics, School of Medicine, University of Dundee, Dundee, UK
Eram Haider, Robert Massey, Adem Y. Dawed & Ewan R. Pearson
Department of Pediatrics, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Jennifer M. Ikle & Anna L. Gloyn
Stanford Diabetes Research Center, Stanford School of Medicine, Stanford University, Stanford, CA, USA
University of Florida, Gainesville, FL, USA
Laura M. Jacobsen
Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Anna R. Kahkoska
Helsinki University Hospital, Abdominal Centre/Endocrinology, Helsinki, Finland
Jarno L. T. Kettunen & Tiinamaija Tuomi
Folkhalsan Research Center, Helsinki, Finland
Jarno L. T. Kettunen
Institute for Molecular Medicine Finland FIMM, University of Helsinki, Helsinki, Finland
Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Lee-Ling Lim
Asia Diabetes Foundation, Hong Kong SAR, China
Department of Medicine & Therapeutics, Chinese University of Hong Kong, Hong Kong SAR, China
Lee-Ling Lim, Claudia Ha-ting Tam, Chuiguo Huang, Gechang Yu, Yingchai Zhang & Ronald C. W. Ma
Departments of Pediatrics and Clinical Genetics, Kuopio University Hospital, Kuopio, Finland
Jonna M. E. Männistö
Department of Medicine, University of Eastern Finland, Kuopio, Finland
Centre for Cardiovascular Science, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Niamh-Maire Mclennan, Rebecca M. Reynolds & Robert K. Semple
Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA, USA
Rachel G. Miller & Tina Costacou
Metabolic Disease Unit, University Hospital of Padova, Padova, Italy
Mario Luca Morieri
Department of Medicine, University of Padova, Padova, Italy
Department of Orthopedics, Zuyderland Medical Center, Sittard-Geleen, The Netherlands
Jasper Most
Departments of Pediatrics and Medicine, University of Chicago, Chicago, IL, USA
Rochelle N. Naylor
Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
Scott J. Pilla, Sarah Kanbour, Sudipa Sarkar & Nestoras Mathioudakis
Department of Health Policy and Management, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA
Scott J. Pilla
Section of Academic Primary Care, US Department of Veterans Affairs Eastern Colorado Health Care System, Aurora, CO, USA
Sridaran Raghaven
Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA
Mohn Center for Diabetes Precision Medicine, Department of Clinical Science, University of Bergen, Bergen, Norway
Pernille Svalastoga, Ingvild Aukrust, Janne Molnes & Pål Rasmus Njølstad
Children and Youth Clinic, Haukeland University Hospital, Bergen, Norway
Pernille Svalastoga & Pål Rasmus Njølstad
Eastern Health Clinical School, Monash University, Melbourne, VIC, Australia
Wubet Worku Takele, Gebresilasea Gendisha Ukke & Siew S. Lim
Laboratory for Molecular Epidemiology in Diabetes, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China
Claudia Ha-ting Tam, Chuiguo Huang, Gechang Yu, Yingchai Zhang & Ronald C. W. Ma
Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong, China
Claudia Ha-ting Tam & Ronald C. W. Ma
Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA
Mustafa Tosur & Maria J. Redondo
Division of Pediatric Diabetes and Endocrinology, Texas Children’s Hospital, Houston, TX, USA
Mustafa Tosur, Marzhan Urazbayeva & Maria J. Redondo
Children’s Nutrition Research Center, USDA/ARS, Houston, TX, USA
Mustafa Tosur
Stanford University School of Medicine, Stanford, CA, USA
Jessie J. Wong & Korey K. Hood
Internal Medicine, University of Manitoba, Winnipeg, MB, Canada
Jennifer M. Yamamoto
Department of Diabetology, APHP, Paris, France
Chloé Amouyal
Sorbonne Université, INSERM, NutriOmic team, Paris, France
Department of Nutrition, Dietetics and Food, Monash University, Melbourne, VIC, Australia
Maxine P. Bonham & Gloria K. W. Leung
Monash Centre for Health Research and Implementation, Monash University, Clayton, VIC, Australia
Mingling Chen
Health Management Center, The Second Affiliated Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
Feifei Cheng
MRC/Wits Developmental Pathways for Health Research Unit, Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Tinashe Chikowore
Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Sydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Department of Women and Children’s health, King’s College London, London, UK
Sian C. Chivers & Sara L. White
Lifecourse Epidemiology of Adiposity and Diabetes (LEAD) Center, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Dana Dabelea, Kristen Boyle & Wei Perng
Section of Adult and Pediatric Endocrinology, Diabetes and Metabolism, Kovler Diabetes Center, University of Chicago, Chicago, IL, USA
Laura T. Dickens
Department of Pediatrics, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN, USA
Linda A. DiMeglio
Richard L. Roudebush VAMC, Indianapolis, IN, USA
Carmella Evans-Molina
Biomedical Research Institute Girona, IdIBGi, Girona, Spain
María Mercè Fernández-Balsells
Diabetes, Endocrinology and Nutrition Unit Girona, University Hospital Dr Josep Trueta, Girona, Spain
Institute of Health System Science, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY, USA
Stephanie L. Fitzpatrick
University of California at San Francisco, Department of Pediatrics, Diabetes Center, San Francisco, CA, USA
Stephen E. Gitelman
Division of Endocrinology, Diabetes and Metabolism, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Adelaide Medical School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia
Jessica A. Grieger, Nahal Habibi, Kai Liu, Maleesa Pathirana & Alejandra Quinteros
Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia
Jessica A. Grieger, Nahal Habibi, Maleesa Pathirana & Shao J. Zhou
Department of Public Health and Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, 1014, Copenhagen, Denmark
Marta Guasch-Ferré
Division of Endocrinology and Diabetes, Department of Pediatrics, Sanford Children’s Hospital, Sioux Falls, SD, USA
Benjamin Hoag
University of South Dakota, School of Medicine, E Clark St, Vermillion, SD, USA
Department of Biomedical Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Randi K. Johnson & Maggie A. Stanislawski
Department of Epidemiology, Colorado School of Public Health, Aurora, CO, USA
Randi K. Johnson
Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK
Angus G. Jones, Andrew T. Hattersley & Richard Oram
Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, Oxford, UK
Robert W. Koivula, Katharine R. Owen & Paul W. Franks
Division of General Internal Medicine, Massachusetts General Hospital, Boston, MA, USA
UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Ingrid M. Libman
Center for Translational Immunology, Benaroya Research Institute, Seattle, WA, USA
S. Alice Long
Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
William L. Lowe Jr.
Department of Pathology & Molecular Medicine, McMaster University, Hamilton, ON, Canada
Robert W. Morton
Population Health Research Institute, Hamilton, ON, Canada
Robert W. Morton, Russell de Souza & Diana Sherifali
Department of Translational Medicine, Medical Science, Novo Nordisk Foundation, Tuborg Havnevej 19, 2900, Hellerup, Denmark
Robert W. Morton & Paul W. Franks
Department of Diabetes and Endocrinology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
Ayesha A. Motala
Center for Public Health Genomics, Department of Public Health Sciences, University of Virginia, Charlottesville, VA, USA
Suna Onengut-Gumuscu & Stephen S. Rich
Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA
James S. Pankow
Department of Chronic Diseases and Metabolism, Clinical and Experimental Endocrinology, KU Leuven, Leuven, Belgium
Sofia Pazmino, Nele Steenackers & Bart Van der Schueren
School of Health and Wellbeing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK
John R. Petrie
Department of Obstetrics, Gynecology, and Reproductive Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Camille E. Powe
Sanford Children’s Specialty Clinic, Sioux Falls, SD, USA
Rashmi Jain
Department of Pediatrics, Sanford School of Medicine, University of South Dakota, Sioux Falls, SD, USA
Rashmi Jain & Kurt Griffin
Centre for Physical Activity Research, Rigshospitalet, Copenhagen, Denmark
Mathias Ried-Larsen
Institute for Sports and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark
Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, Indiana University School of Medicine, Indianapolis, IN, USA
AMAN Hospital, Doha, Qatar
Sarah Kanbour
Department of Preventive Medicine, Division of Biostatistics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Denise M. Scholtens
Institute of Molecular and Genomic Medicine, National Health Research Institutes, Taipei City, Taiwan, ROC
Divsion of Endocrinology and Metabolism, Taichung Veterans General Hospital, Taichung, Taiwan, ROC
Center for Interventional Immunology, Benaroya Research Institute, Seattle, WA, USA
Cate Speake
Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Andrea K. Steck & Peter A. Gottlieb
Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, Denmark
University of Newcastle, Newcastle upon Tyne, UK
Rachael Taylor
Section of Genetics and Epidemiology, Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
Sok Cin Tye
Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands
Department of Gastroenterology, Baylor College of Medicine, Houston, TX, USA
Marzhan Urazbayeva
Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium
Bart Van der Schueren
Sorbonne University, Inserm U938, Saint-Antoine Research Centre, Institute of Cardiometabolism and Nutrition, Paris, 75012, France
Camille Vatier
Department of Endocrinology, Diabetology and Reproductive Endocrinology, Assistance Publique-Hôpitaux de Paris, Saint-Antoine University Hospital, National Reference Center for Rare Diseases of Insulin Secretion and Insulin Sensitivity (PRISIS), Paris, France
Department of Diabetes and Endocrinology, Royal Melbourne Hospital, Parkville, VIC, Australia
John M. Wentworth
Walter and Eliza Hall Institute, Parkville, VIC, Australia
John M. Wentworth & Tiinamaija Tuomi
Department of Medicine, University of Melbourne, Parkville, VIC, Australia
Deakin University, Melbourne, VIC, Australia
Wesley Hannah
Department of Epidemiology, Madras Diabetes Research Foundation, Chennai, India
Department of Diabetes and Endocrinology, Guy’s and St Thomas’ Hospitals NHS Foundation Trust, London, UK
Sara L. White
School of Agriculture, Food and Wine, University of Adelaide, Adelaide, Australia
Shao J. Zhou
Institut Cochin, Inserm U, 10116, Paris, France
Jacques Beltrand & Michel Polak
Pediatric Endocrinology and Diabetes, Hopital Necker Enfants Malades, APHP Centre, Université de Paris, Paris, France
Department of Medical Genetics, Haukeland University Hospital, Bergen, Norway
Ingvild Aukrust & Janne Molnes
Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
Kristin A. Maloney & Toni I. Pollin
Department of Epidemiology, Geisel School of Medicine at Dartmouth, Hanover, NH, USA
Hugo Pomares-Millan
Nephrology, Dialysis and Renal Transplant Unit, IRCCS—Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Bologna, Italy
Michele Provenzano
Department of Medical Genetics, AP-HP Pitié-Salpêtrière Hospital, Sorbonne University, Paris, France
Cécile Saint-Martin
Global Center for Asian Women’s Health, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Cuilin Zhang
Department of Obstetrics and Gynecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA
Department of Epidemiology and Biostatistics, University of California San Francisco, California, USA
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Sungyoung Auh & Rebecca J. Brown
Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
Russell de Souza
Ann & Robert H. Lurie Children’s Hospital of Chicago, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Andrea J. Fawcett & Jami L. Josefson
Department of Clinical and Organizational Development, Children’s Memorial Hospital, Chicago, IL, USA
Andrea J. Fawcett
American Diabetes Association, Arlington, VA, USA
Chandra Gruber
College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Eskedar Getie Mekonnen
Faculty of Medicine and Health Sciences, Global Health Institute, University of Antwerp, 2160, Antwerp, Belgium
Department of Medicine and Kovler Diabetes Center, University of Chicago, Chicago, IL, USA
Emily Mixter & Louis H. Philipson
School of Nursing, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
Diana Sherifali
Division of Endocrinology, Metabolism, Diabetes, University of Colorado, Boulder, CO, USA
Department of Clinical Medicine, School of Medicine, Trinity College Dublin, Dublin, Ireland
John J. Nolan
Department of Endocrinology, Wexford General Hospital, Wexford, Ireland
Division of Endocrinology, NorthShore University HealthSystem, Skokie, IL, USA
Department of Medicine, Prtizker School of Medicine, University of Chicago, Chicago, IL, USA
Department of Genetics, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Anna L. Gloyn
Faculty of Health, Aarhus University, Aarhus, Denmark
Maria F. Gomez
Departments of Pediatrics and Medicine and Kovler Diabetes Center, University of Chicago, Chicago, USA
Siri Atma W. Greeley
Sanford Research, Sioux Falls, SD, USA
Kurt Griffin
University of Washington School of Medicine, Seattle, WA, USA
Irl B. Hirsch
Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA, USA
Marie-France Hivert
Department of Medicine, Universite de Sherbrooke, Sherbrooke, QC, Canada
Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Republic of Korea
Soo Heon Kwak
Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
Lori M. Laffel
Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Ruth J. F. Loos
Broad Institute, Cambridge, MA, USA
James B. Meigs
Division of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Department of Diabetes & Endocrinology, Imperial College Healthcare NHS Trust, London, UK
Department of Diabetology, Madras Diabetes Research Foundation & Dr. Mohan’s Diabetes Specialities Centre, Chennai, India
Viswanathan Mohan
Department of Medicine, Faculty of Medicine and Health Sciences, University of Auckland, Auckland, New Zealand
Rinki Murphy
Auckland Diabetes Centre, Te Whatu Ora Health New Zealand, Auckland, New Zealand
Medical Bariatric Service, Te Whatu Ora Counties, Health New Zealand, Auckland, New Zealand
Oxford NIHR Biomedical Research Centre, University of Oxford, Oxford, UK
Katharine R. Owen
Metabolic Research Laboratories and MRC Metabolic Diseases Unit, University of Cambridge, Wellcome-MRC Institute of Metabolic Science, Cambridge, UK
Susan E. Ozanne
Department of Epidemiology & Public Health, University of Maryland School of Medicine, Baltimore, MD, USA
Toni I. Pollin
Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI, USA
Rodica Pop-Busui
AdventHealth Translational Research Institute, Orlando, FL, USA
Richard E. Pratley
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Leanne M. Redman
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK
Robert K. Semple
Yale School of Medicine, New Haven, CT, USA
Jennifer L. Sherr
Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Arianne Sweeting
Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, NSW, Australia
Kaiser Permanente Northwest, Kaiser Permanente Center for Health Research, Portland, OR, USA
Kimberly K. Vesco
Clinial Research, Steno Diabetes Center Copenhagen, Herlev, Denmark
Tina Vilsbøll
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
You can also search for this author in PubMed Google Scholar
ADA/EASD PMDI
- Deirdre K. Tobias
- , Jordi Merino
- , Abrar Ahmad
- , Catherine Aiken
- , Jamie L. Benham
- , Dhanasekaran Bodhini
- , Amy L. Clark
- , Kevin Colclough
- , Rosa Corcoy
- , Sara J. Cromer
- , Daisy Duan
- , Jamie L. Felton
- , Ellen C. Francis
- , Pieter Gillard
- , Véronique Gingras
- , Romy Gaillard
- , Eram Haider
- , Alice Hughes
- , Jennifer M. Ikle
- , Laura M. Jacobsen
- , Anna R. Kahkoska
- , Jarno L. T. Kettunen
- , Raymond J. Kreienkamp
- , Lee-Ling Lim
- , Jonna M. E. Männistö
- , Robert Massey
- , Niamh-Maire Mclennan
- , Rachel G. Miller
- , Mario Luca Morieri
- , Jasper Most
- , Rochelle N. Naylor
- , Bige Ozkan
- , Kashyap Amratlal Patel
- , Scott J. Pilla
- , Katsiaryna Prystupa
- , Sridaran Raghaven
- , Mary R. Rooney
- , Martin Schön
- , Zhila Semnani-Azad
- , Magdalena Sevilla-Gonzalez
- , Pernille Svalastoga
- , Wubet Worku Takele
- , Claudia Ha-ting Tam
- , Anne Cathrine B. Thuesen
- , Mustafa Tosur
- , Amelia S. Wallace
- , Caroline C. Wang
- , Jessie J. Wong
- , Jennifer M. Yamamoto
- , Katherine Young
- , Chloé Amouyal
- , Mette K. Andersen
- , Maxine P. Bonham
- , Mingling Chen
- , Feifei Cheng
- , Tinashe Chikowore
- , Sian C. Chivers
- , Christoffer Clemmensen
- , Dana Dabelea
- , Adem Y. Dawed
- , Aaron J. Deutsch
- , Laura T. Dickens
- , Linda A. DiMeglio
- , Monika Dudenhöffer-Pfeifer
- , Carmella Evans-Molina
- , María Mercè Fernández-Balsells
- , Hugo Fitipaldi
- , Stephanie L. Fitzpatrick
- , Stephen E. Gitelman
- , Mark O. Goodarzi
- , Jessica A. Grieger
- , Marta Guasch-Ferré
- , Nahal Habibi
- , Torben Hansen
- , Chuiguo Huang
- , Arianna Harris-Kawano
- , Heba M. Ismail
- , Benjamin Hoag
- , Randi K. Johnson
- , Angus G. Jones
- , Robert W. Koivula
- , Aaron Leong
- , Gloria K. W. Leung
- , Ingrid M. Libman
- , S. Alice Long
- , William L. Lowe Jr.
- , Robert W. Morton
- , Ayesha A. Motala
- , Suna Onengut-Gumuscu
- , James S. Pankow
- , Maleesa Pathirana
- , Sofia Pazmino
- , Dianna Perez
- , John R. Petrie
- , Camille E. Powe
- , Alejandra Quinteros
- , Rashmi Jain
- , Debashree Ray
- , Mathias Ried-Larsen
- , Zeb Saeed
- , Vanessa Santhakumar
- , Sarah Kanbour
- , Sudipa Sarkar
- , Gabriela S. F. Monaco
- , Denise M. Scholtens
- , Elizabeth Selvin
- , Wayne Huey-Herng Sheu
- , Cate Speake
- , Maggie A. Stanislawski
- , Nele Steenackers
- , Andrea K. Steck
- , Norbert Stefan
- , Julie Støy
- , Rachael Taylor
- , Sok Cin Tye
- , Gebresilasea Gendisha Ukke
- , Marzhan Urazbayeva
- , Bart Van der Schueren
- , Camille Vatier
- , John M. Wentworth
- , Wesley Hannah
- , Sara L. White
- , Gechang Yu
- , Yingchai Zhang
- , Shao J. Zhou
- , Jacques Beltrand
- , Michel Polak
- , Ingvild Aukrust
- , Elisa de Franco
- , Sarah E. Flanagan
- , Kristin A. Maloney
- , Andrew McGovern
- , Janne Molnes
- , Mariam Nakabuye
- , Pål Rasmus Njølstad
- , Hugo Pomares-Millan
- , Michele Provenzano
- , Cécile Saint-Martin
- , Cuilin Zhang
- , Sungyoung Auh
- , Russell de Souza
- , Andrea J. Fawcett
- , Chandra Gruber
- , Eskedar Getie Mekonnen
- , Emily Mixter
- , Diana Sherifali
- , Robert H. Eckel
- , John J. Nolan
- , Louis H. Philipson
- , Rebecca J. Brown
- , Liana K. Billings
- , Kristen Boyle
- , Tina Costacou
- , John M. Dennis
- , Jose C. Florez
- , Anna L. Gloyn
- , Maria F. Gomez
- , Peter A. Gottlieb
- , Siri Atma W. Greeley
- , Kurt Griffin
- , Andrew T. Hattersley
- , Irl B. Hirsch
- , Marie-France Hivert
- , Korey K. Hood
- , Jami L. Josefson
- , Soo Heon Kwak
- , Lori M. Laffel
- , Siew S. Lim
- , Ruth J. F. Loos
- , Ronald C. W. Ma
- , Chantal Mathieu
- , Nestoras Mathioudakis
- , James B. Meigs
- , Shivani Misra
- , Viswanathan Mohan
- , Rinki Murphy
- , Richard Oram
- , Katharine R. Owen
- , Susan E. Ozanne
- , Ewan R. Pearson
- , Wei Perng
- , Toni I. Pollin
- , Rodica Pop-Busui
- , Richard E. Pratley
- , Leanne M. Redman
- , Maria J. Redondo
- , Rebecca M. Reynolds
- , Robert K. Semple
- , Jennifer L. Sherr
- , Emily K. Sims
- , Arianne Sweeting
- , Tiinamaija Tuomi
- , Miriam S. Udler
- , Kimberly K. Vesco
- , Tina Vilsbøll
- , Robert Wagner
- , Stephen S. Rich
- & Paul W. Franks
Contributions
In this manuscript, SM and RW contributed equally as first authors. J.B.M. and MSU jointly supervised the work. B.O., M.S. and M.S.-G. contributed equally as second authors. Review Design: S.M., R.W., B.O., M.S., M.S.G., K.P., C.C.W., R.J.K., S.J.C., M.R.R., D.D., A.C.B.T., A.S.W., A.L., A.J.D., M.K.A., L.K.B., R.H.E., W.H.H.S., T.H., N.S., M.O.G., D.R., E.S., J.C.F., A.D.A./E.A.S.D. P.M.D.I. J.B.M. and M.S.U. Systematic Review Implementation: S.M., R.W., B.O., M.S., M.S.G., K.P., C.C.W., R.J.K., S.J.C., M.R.R., D.D., A.C.B.T., A.S.W., A.L., A.J.D., M.K.A., L.K.B., R.H.E., W.H.H.S., T.H., N.S., M.O.G., D.R., E.S., J.C.F., J.B.M. and M.S.U. Full-text data extraction: S.M., R.W., B.O., M.S., M.S.G., K.P., C.C.W., R.J.K., S.J.C., M.R.R., D.D., A.C.B.T., A.S.W., A.L., A.J.D., M.K.A., L.K.B., R.H.E., W.H.H.S., T.H., N.S., M.O.G., D.R., E.S., J.C.F., J.B.M. and M.S.U. Data synthesis: S.M., R.W., B.O., J.B.M., M.U., M.S., K.P., C.W., M.S.G., J.C.F., R.K., M.R.R., A.S.W. Manuscript writing: S.M., R.W., J.B.M. and M.U. Manuscript Review: S.M., R.W., B.O., M.S., M.S.G., K.P., C.C.W., R.J.K., S.J.C., M.R.R., D.D., A.C.B.T., A.S.W., A.L., A.J.D., M.K.A., L.K.B., R.H.E., W.H.H.S., T.H., N.S., M.O.G., D.R., E.S., J.C.F., J.B.M. and M.S.U. Project Management: S.M., R.W., A.D.A./E.A.S.D. P.M.D.I., J.B.M. and M.U.
Corresponding author
Correspondence to Shivani Misra .
Ethics declarations
Competing interests.
The authors declare the following conflicts of interest. S.M. has investigator-initiated funding from DexCom, has received speaker fees (donated to institution) from Sanofi for a scientific talk over which she had full control of content and serves on the Board of Trustees for the Diabetes Research & Wellness Foundation (UK); R.W. declares lecture fees from Novo Nordisk, Sanofi and Eli Lilly. He served on an advisory board for Akcea Therapeutics, Daiichi Sankyo, Sanofi, Eli Lilly, and NovoNordisk; S.J.C. reports a close family member employed by a Johnson & Johnson company; R.H.S. reports fees from Novo Nordisk and Amgen; L.K.B. has received consulting honoraria from Bayer, Novo Nordisk, Sanofi, Lilly, and Xeris; W.H.H.S. reported as Advisor and/or Speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals., Daiichi-Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi-Aventis, Takeda Pharmaceutical Company; N.S. is Senior Associate Editor of Diabetes and has received speaking honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer and Sanofi for scientific talks over which he had full control of content; M.G. has served on an advisory board for Nestle Health Science; E.S. is a Deputy Editor of Diabetes Care and a member of the editorial board of Diabetologia and receives payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate on measurements of glycemic control and screening tests for type 2 diabetes; J.C.F. has received speaking honoraria from AstraZeneca and Novo Nordisk for scientific talks over which he had full control of content; J.B.M. is an Academic Associate for Quest Inc. Diagnostics R&D; M.U. reports an unpaid collaborator with AstraZeneca. All other authors have no disclosures. A.L. reports a close family member employed by Merck & Co., Inc.
Peer review
Peer review information.
Communications Medicine thanks Albert Salas-Huetos, Peter Rossing and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary data 1, description of additional supplementary files, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Misra, S., Wagner, R., Ozkan, B. et al. Precision subclassification of type 2 diabetes: a systematic review. Commun Med 3 , 138 (2023). https://doi.org/10.1038/s43856-023-00360-3
Download citation
Received : 09 May 2023
Accepted : 15 September 2023
Published : 05 October 2023
DOI : https://doi.org/10.1038/s43856-023-00360-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Multi-ancestry polygenic mechanisms of type 2 diabetes.
- Aaron J. Deutsch
- Miriam S. Udler
Nature Medicine (2024)
- Jordi Merino
- Paul W. Franks
Nature Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Registered Report Protocol
Registered Report Protocols describe a study’s rationale and methods for which the planned work was peer-reviewed prior to data collection.
See all article types »
Type 1 and type 2 diabetes mellitus: Clinical outcomes due to COVID-19. Protocol of a systematic literature review
Contributed equally to this work with: Juan Pablo Pérez Bedoya, Alejandro Mejía Muñoz
Roles Conceptualization, Investigation, Methodology, Project administration, Writing – original draft
* E-mail: [email protected]
Current address: National Faculty of Public Health, University of Antioquia, Medellin, Antioquia, Colombia
Affiliation Epidemiology Group, National Faculty of Public Health, University of Antioquia, Medellín, Colombia
Affiliation Biology and Control of Infectious Diseases Group, Faculty of Exact and Natural Sciences, University of Antioquia, Medellín, Colombia
Roles Supervision, Validation, Writing – review & editing
¶ ‡ NCB and PADV also contributed equally to this work.
Affiliation Department of Translational Medicine, Herbert Wertheim College of Medicine & Department of Global Health, Robert Stempel College of Public Health and Social Work, Florida International University, Miami, FL, United States of America
- Juan Pablo Pérez Bedoya,
- Alejandro Mejía Muñoz,
- Noël Christopher Barengo,
- Paula Andrea Diaz Valencia

- Published: September 9, 2022
- https://doi.org/10.1371/journal.pone.0271851
- See the preprint
- Peer Review
- Reader Comments
Introduction
Diabetes has been associated with an increased risk of complications in patients with COVID-19. Most studies do not differentiate between patients with type 1 and type 2 diabetes, which correspond to two pathophysiological distinct diseases that could represent different degrees of clinical compromise.
To identify if there are differences in the clinical outcomes of patients with COVID-19 and diabetes (type 1 and type 2) compared to patients with COVID-19 without diabetes.
Observational studies of patients with COVID-19 and diabetes (both type 1 and type 2) will be included without restriction of geographic region, gender or age, whose outcome is hospitalization, admission to intensive care unit or mortality compared to patients without diabetes. Two authors will independently perform selection, data extraction, and quality assessment, and a third reviewer will resolve discrepancies. The data will be synthesized regarding the sociodemographic and clinical characteristics of patients with diabetes and without diabetes accompanied by the measure of association for the outcomes. The data will be synthesized regarding the sociodemographic and clinical characteristics of patients with diabetes and without diabetes accompanied by the measure of association for the outcomes.
Expected results
Update the evidence regarding the risk of complications in diabetic patients with COVID-19 and in turn synthesize the information available regarding type 1 and type 2 diabetes mellitus, to provide keys to a better understanding of the pathophysiology of diabetics.
Systematic review registry
This study was registered at the International Prospective Registry for Systematic Reviews (PROSPERO)— CRD42021231942 .
Citation: Pérez Bedoya JP, Mejía Muñoz A, Barengo NC, Diaz Valencia PA (2022) Type 1 and type 2 diabetes mellitus: Clinical outcomes due to COVID-19. Protocol of a systematic literature review. PLoS ONE 17(9): e0271851. https://doi.org/10.1371/journal.pone.0271851
Editor: Alok Raghav, GSVM Medical College, INDIA
Received: July 7, 2022; Accepted: August 23, 2022; Published: September 9, 2022
Copyright: © 2022 Pérez Bedoya et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding: This research was developed within the framework of the project "Repository for the surveillance of risk factors for chronic diseases in Colombia, the Caribbean and the Americas" and has the financial support of the Ministry of Science, Technology and Innovation of Colombia—Minciencias 844 (grant number 111584467754). The opinions expressed are those of the authors and not necessarily of Minciencias.
Competing interests: The authors have declared that no competing interests exist.
The Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2), the causal viral agent of coronavirus disease 2019 (COVID-19), currently has the world in one of the greatest public health crises of recent times since its appearance at the end of 2019 in the city of Wuhan, China [ 1 ]. The infection has a mild or even asymptomatic course in most cases, but in elderly patients (over 60 years-of-age) and in those with pre-existing chronic comorbidities, it can progress severe complications such as pneumonia, acute respiratory distress (ARDS) with hyperinflammatory involvement and multi-organ failure, leading in some cases to death [ 2 ].
Different studies have reported that patients diagnosed with diabetes who suffer from COVID-19 disease have higher morbidity and mortality compared with people without diabetes [ 3 ]. An analysis by Gude Sampedro et al. using prognostic models found that diabetic patients had greater odds of being hospitalized (OR 1.43; 95% CI: 1.18 to 1.73), admitted to the intensive care unit (OR 1.61; 95% CI: 1.12 to 2.31) and dying from COVID-19 (OR 1.79; 95% CI %: 1.38 to 2.32) compared with patients without diabetes [ 4 ]. However, it is difficult to establish whether diabetes alone directly contributed to the increase likelihood of complications.
Several studies using secondary data have emerged during the course of the pandemic that seek to determine the association of diabetes with mortality and other clinical outcomes in patients with COVID-19, such as, for example, a meta-analysis carried out by Shang et al. of severe infection and mortality from COVID-19 in diabetic patients compared with those without diabetes. They reported that patients with COVID-19 and diabetes had higher odds of serious infection (OR = 2.38, 95% CI: 2.05 to 2.78) and mortality (OR = 2, 21, 95% CI: 1.83 to 2.66) than patients without diabetes [ 5 ]. Despite the fact that there are several primary studies that attempt to explain the association between diabetes and COVID-19, most studies lack epidemiological rigor in the design and methodology used [ 6 ]. In addition, many of them did not distinguish between type 1 and type 2 diabetes, which are two very different conditions with different clinical development and pathophysiological mechanisms [ 7 ]. This may lead to different degrees of clinical complications from COVID-19. Currently, there is a gap in knowledge about the complications in patients with COVID-19 according to the type of diabetes. Moreover, only limited information exist how COVID-19 affects type 1 patients [ 8 , 9 ].
The objective of this systematic literature review will be to identify whether there are differences in the clinical outcomes of both type 1 and type 2 diabetes patients diagnosed with COVID-19 compared with patients with COVID-19 without a diagnosis of diabetes. This study will provide scientific evidence regarding the risk of complications in diabetic patients with COVID-19 and, in turn, synthesize the available information regarding to type 1 and type 2 diabetes.
Study design
This systematic literature review protocol was prepared according to the Preferred Reporting Elements for Systematic Review and Meta-Analysis Protocols (PRISMA-P) [ 10 ] ( S1 Appendix ). The results of the final systematic review will be reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) [ 11 , 12 ]. In the event of significant deviations from this protocol, they will be reported and published with the results of the review.
Eligibility criteria
Participants (population)..
Patients with a confirmed diagnosis of COVID-19 without restriction of geographic region, sex, or age. For the diagnosis of COVID-19, the operational definition of confirmed case of the World Health Organization in its latest update will be used as a reference. Confirmed case of SARS-CoV-2 infection: a person with a positive Nucleic Acid Amplification Test (NAAT), regardless of clinical criteria OR epidemiological criteria or a person meeting clinical criteria AND/OR epidemiological criteria (suspect case A) with a positive professional- use or self-test SARS-CoV-2 Antigen RDT [ 13 ].
Patients with COVID-19 and concomitant diagnosis of unspecified diabetes mellitus, differentiated into type 1 diabetes mellitus or type 2 diabetes mellitus, without restriction of geographic region, gender, or age of the patients, who present definition of clinical criteria and /or paraclinical tests used by researchers to classify patients according to their diabetes status.
The operational definition of a confirmed case of diabetes mellitus provided by the American Diabetes Association will be used as a guide. The reference diagnostic criteria for diabetes are fasting plasma glucose ≥126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 h or 2-h plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during OGTT or hemoglobin A1C ≥6.5% (48 mmol/mol) or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, at random plasma glucose ≥200 mg/dL [ 14 ].
In selected primary studies, identification of diabetes status may be based on medical history and International Classification of Diseases codes for type 1 or type 2 diabetes, use of antidiabetic medications, or previously defined diagnostic criteria.
Comparator.
Patients with COVID-19 who do not have a concomitant diagnosis of diabetes mellitus.
The main endpoint is all-cause mortality (according to the definitions of each primary study) and the secondary outcomes are hospitalization and admission to the ICU, where the authors specify a clear definition based on clinical practice guidelines and provide a well-defined criteria for patient outcomes.
Type of study.
Primary observational original research studies (prospective or retrospective cohort, case-control design, and cross-sectional studies) will be included in this systematic review.
Exclusion criteria
Clinical trials, editorials, letters to the editor, reviews, case reports, case series, narrative reviews or systematic reviews and meta-analyses, as well as research in the field of basic sciences based on experimental laboratory models, will be excluded. Original research articles that only include other types of diabetes, such as monogenic diabetes, gestational diabetes, latent autoimmune diabetes in adults, ketosis-prone diabetes, among others, or articles with publication status prior to publication will not be considered. In addition, articles whose main hypothesis is not diabetes and do not have the established outcomes will be excluded.
Information sources and search strategy
Electronic bibliographic databases..
For the preparation of the search strategy, the recommendations of the PRISMA-S guide [ 15 ] will be adopted. Relevant articles will be identified by electronic search applying the equation previously developed by the researchers and validated by an expert librarian ( S2 Appendix ). The following electronic bibliographic databases will be used: MEDLINE, EMBASE, LILACS, OVID MEDLINE, WHO (COVID-19 Global literature on coronavirus disease) and SCOPUS with a publication date from December 2019 to August 15, 2022, without language restriction.
The search for potential primary studies published in gray literature will be performed through the World Health Organization database for COVID-19 (WHO COVID-19 Global literature on coronavirus disease). This database contains different electronic bibliographic databases incorporated into its browser, including Web of Science, EuropePMC and Gray literature, among others.
Unlike electronic bibliographic databases.
To identify other potentially eligible studies, the references of relevant publications will be reviewed to perform a snowball manual search. This technique consists of searching for new articles from the primary studies already selected in order to guarantee exhaustiveness in the search.
Study selection process
Two researchers will independently evaluate all the titles and abstracts of the retrieved articles, using the free access Rayyan® software [ 16 ] with previously established selection criteria. Disagreements will be resolved in first instance through discussion and in the second instance through a third reviewer. Subsequently, the full text of the articles selected in the eligibility phase will be read independently by two researchers, both using the same instrument previously validated in Excel according to predefined criteria. Discrepancies will be resolved by discussion or a third reviewer. The process of identification, selection and inclusion of primary studies will be described and presented using the flowchart recommended by the PRISMA statement in its latest version 2020 [ 11 , 12 ].
Data collection and extraction
Standardized and validated forms will be used to collect the data extracted from the primary studies, accompanied by a detailed instruction manual to specify the guiding questions, and avoid the introduction of bias. Data will be extracted from those articles in full text format. If the full text is not available, contact the author or search for the manuscript with the help of the library system. This process will be carried out by two researchers independently. A third investigator will verify the extracted data to ensure the accuracy of the records. The authors of the primary studies will be contacted to resolve any questions that may arise. The reviewers will resolve the disagreements through discussion and one of the two referees will adjudicate the discrepancies presented through discussion and consensus.
In specific terms, the following data will be collected both for the primary studies that report diabetes and COVID-19 and for those that differentiate between DMT1 and DMT2: author, year and country where the study was carried out; study design; general characteristics of the population, sample size, demographic data of the participants (sex, age, ethnicity), percentage of patients with diabetes, percentage of patients with type 1 and/or type 2 diabetes, percentage of patients without diabetes, frequency of comorbidities in diabetics and non-diabetics, percentage of diabetic and non-diabetic patients who presented the outcomes (hospitalization, ICU admission and mortality) and association measures reported for the outcomes. Data extraction will be done using a Microsoft Excel 365 ® spreadsheets.
Quality evaluation
The study quality assessment tool provided by the National Institutes of Health (NIH) [ 17 ] will be used for observational studies such as cohort, case-control, and cross-sectional. Two tools will be sued: one for cohort and cross-sectional studies (14 questions/domains) and one for case-control studies (12 questions/domains). These tools are aimed at detecting elements that allow evaluation of possible methodological problems, including sources of bias (for example, patient selection, performance, attrition and detection), confounding, study power, the strength of causality in the association between interventions and outcomes, among other factors. The different tools that will be used reflect a score of "1" or "0" depending on the answer "yes" or "no", respectively for each question or domain evaluated, or failing that, the indeterminate criterion option. For observational cohort studies, which consist of 14 risk of bias assessment domains, the studies will be classified as having good quality if they obtain ≥10 points, of fair quality if they obtain 8 to 9 points, and of poor quality if they obtain less than 8 points. On the other hand, in the case of case-control studies that consist of 12 bias risk assessment domains, the studies will be classified as good quality if they obtained ≥8 points, regular quality if they obtained 6 to 7 points and of poor quality if they obtained less than 6 points. However, the internal discussion between the research team will always be considered as the primary quality criterion.
Data synthesis
A narrative synthesis with summary tables will be carried out according to the recommendations adapted from the Synthesis Without Meta-analysis (SWiM) guide to describe in a structured way the methods used, and the findings found in the primary studies, as well as the criteria for grouping of the studies [ 18 ]. A narrative synthesis will be presented in two sections, one for patients with COVID-19 and diabetes and another for patients with COVID-19 and type 1 or type 2 diabetes.
Assessment of clinical and methodological heterogeneity will determine the feasibility of the meta-analysis. Possible sources of heterogeneity identified are the clinical characteristics of the study population, the criteria used to define the outcomes in the groups of patients, the time period of the pandemic in which the study was carried out, and the availability of measurement and control for potential confounding factors. For this reason, it is established a priori that this diversity of findings will make it difficult to carry out an adequate meta-analysis [ 19 ]. However, if meta-analysis is considered feasible, the random effects model will be used due to the high probability of heterogeneity between studies. Statistical heterogeneity will be assessed using the X 2 test and the I 2 statistic, and publication bias assessed using funnel plots if there are sufficient (>10) studies [ 20 ].
Exploratory ecological analysis
An exploratory ecological analysis of the association between the frequency of clinical outcomes of diabetic patients with COVID-19 and the indicators related to the health care dimension, reported for the different countries analyzed by means of the correlation coefficient, will be carried out. The open public databases of the World Bank (WB) [ 21 ], the World Health Organization (WHO) [ 22 ] and Our World In Data [ 23 ] will be used to extract population indicators related to health care, among those prioritized, universal health coverage, hospital beds per 1,000 people, doctors per 1,000 people, current health spending as a percentage of gross domestic product (GDP), percentage of complete vaccination coverage for COVID-19.
Since the first epidemiological and clinical reports were released from the city of Wuhan regarding the clinical characteristics of patients with COVID-19, a high incidence of chronic non-communicable diseases has been observed in Covid-19 patients. Current scientific evidence has shown that certain comorbidities increase the risk for hospitalization, severity of illness or death from COVID-19, such as hypertension, cardiovascular disease, chronic kidney disease, chronic respiratory disease, diabetes, among others [ 24 ].
One of the main chronic comorbidities affected by the COVID-19 pandemic is diabetes. Multivariate analysis of several observational epidemiological studies have revealed that COVID-19 patients with diabetes were at increased risk of hospitalization, ICU admission, and mortality compared with patients without diabetes [ 4 ].
For this reason, it is expected that this systematic literature review will provide scientific support regarding the outcomes and complications that patients diagnosed with COVID-19 with type 1 or type 2 diabetes present compared with patients without diabetes. This information will be useful for healthcare personnel, public health professionals and epidemiologists involved in patient care or decision making, generating epidemiological evidence. Thus, highlighting the decisive role of epidemiological research in the context of the pandemic, especially in the field of diabetes epidemiology may improve comprehensive management and care of diabetic patients. This study may also provide important information that can be used to update of clinical practice guidelines.
Limitations
There are some potential limitations to the proposed systematic review. Firstly, both type 1 and type 2 diabetes may have different key search terms and some studies may be missed. To minimize this limitation, different search equations have been designed for each database in an exhaustive and sensitive manner. In addition to reading references and level ball as an additional strategy. Another limitation is that observational studies evaluating the effect of an intervention may be susceptible to significant confounding bias and may present high heterogeneity in the findings. To report these possible biases, an adequate quality assessment will be carried out, with highly sensitive and previously validated tools, exclusive for each type of observational design. The review is intended for publication in a peer-reviewed journal.
The status of the study
The study is in the selection phase of the records by applying the eligibility criteria to the titles and abstracts. Completion of the project is expected in September 2022 with the publication of the results.
Conclusions
This report describes the systematic review protocol that will be utilized to update the evidence regarding the risk of complications in diabetic patients with COVID-19 and in turn synthesize the information available regarding DM1 and DM2, to provide keys to a better understanding of the pathophysiology of diabetics.
Supporting information
S1 appendix. prisma-p (preferred reporting items for systematic review and meta-analysis protocols) 2015 checklist: recommended items to address in a systematic review protocol..
https://doi.org/10.1371/journal.pone.0271851.s001
S2 Appendix. Search string details for each database.
https://doi.org/10.1371/journal.pone.0271851.s002
- View Article
- PubMed/NCBI
- Google Scholar
- 13. World Health Organization. WHO COVID-19 Case definition Updated in Public health surveillance for COVID-19. 2022 July 22. [cited 18 August 2022]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1
- 17. National Institutes of Health (NIH) [Internet]. Study Quality Assessment Tools; 2021. [cited 16 June 2022]. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 19. Cochrane Handbook for Systematic Reviews of Interventions; 2022. [cited 16 June 2022]. Available at: https://training.cochrane.org/handbook/current
- 21. World Bank Group [Internet]. World Bank Indicators; 2022. [cited 16 June 2022]. Available at: https://datos.bancomundial.org/indicador
- 22. World Health Organization (WHO) [Internet]. Global Health Observatory Data Repository; 2021. [cited 16 June 2022]. Available at: https://apps.who.int/gho/data/node.home
- 23. Our World In Data [Internet]. Statistics and Research Coronavirus Pandemic (COVID-19); 2022. [cited 16 June 2022]. Available at: https://ourworldindata.org/coronavirus
- 24. Centers for Disease Control and Prevention [Internet]. COVID-19. People with Certain Medical Conditions; 2021. [cited 16 June 2022]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=ht
- Reference Manager
- Simple TEXT file
People also looked at
Review article, a systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: time to establish new norms.

- 1 Baycrest Centre, Rotman Research Institute, Toronto, ON, Canada
- 2 Sunnybrook Research Institute, Heart and Stroke Foundation Canadian Partnership for Stroke Recovery, Toronto, ON, Canada
- 3 Department of Medical Biophysics, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 4 Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 5 Departments of Psychology and Psychiatry, University of Toronto, Toronto, ON, Canada
The rising prevalence of type 2 diabetes (T2DM) and hypertension in older adults, and the deleterious effect of these conditions on cerebrovascular and brain health, is creating a growing discrepancy between the “typical” cognitive aging trajectory and a “healthy” cognitive aging trajectory. These changing health demographics make T2DM and hypertension important topics of study in their own right, and warrant attention from the perspective of cognitive aging neuroimaging research. Specifically, interpretation of individual or group differences in blood oxygenation level dependent magnetic resonance imaging (BOLD MRI) or positron emission tomography (PET H 2 O 15 ) signals as reflective of differences in neural activation underlying a cognitive operation of interest requires assumptions of intact vascular health amongst the study participants. Without adequate screening, inclusion of individuals with T2DM or hypertension in “healthy” samples may introduce unwanted variability and bias to brain and/or cognitive measures, and increase potential for error. We conducted a systematic review of the cognitive aging neuroimaging literature to document the extent to which researchers account for these conditions. Of the 232 studies selected for review, few explicitly excluded individuals with T2DM (9%) or hypertension (13%). A large portion had exclusion criteria that made it difficult to determine whether T2DM or hypertension were excluded (44 and 37%), and many did not mention any selection criteria related to T2DM or hypertension (34 and 22%). Of all the surveyed studies, only 29% acknowledged or addressed the potential influence of intersubject vascular variability on the measured BOLD or PET signals. To reinforce the notion that individuals with T2DM and hypertension should not be overlooked as a potential source of bias, we also provide an overview of metabolic and vascular changes associated with T2DM and hypertension, as they relate to cerebrovascular and brain health.
Introduction
Amongst middle-aged and older adults, the rising prevalence of T2DM, hypertension, and other conditions that comprise the metabolic syndrome is a global health epidemic, attributed largely to sedentary lifestyles, poor diet, and lack of exercise. In 2008, it was estimated that 347 million adults worldwide had T2DM, up from 153 million in 1980 ( Danaei et al., 2011 ). Over the next two decades, it is expected that these numbers will continue to rise, by as much as 38% by 2030 ( Shaw et al., 2010 ). Prevalence rates of hypertension are even higher. In 2000, the global prevalence of hypertension was 26.4%, affecting an estimated 972 million people worldwide. Again, these numbers are expected to increase by approximately 60% by 2025, to a total of 1.56 billion people ( Kearney et al., 2005 ). Critically, hypertension is present in up to 75% of individuals with T2DM ( Colosia et al., 2013 ). The growing number of middle-aged and older adults living with T2DM and/or hypertension makes these conditions important topics of study in their own right.
Better long-term health care and disease management allow middle-aged and older adults to live with T2DM and hypertension for many years; however, both of these conditions have long-term deleterious effects on cerebrovascular and brain health, and contribute to cognitive impairment and decline ( Gorelick et al., 2011 ). T2DM and midlife hypertension confer a high risk for development of mild cognitive impairment (MCI) and dementia ( Launer et al., 2000 ; Kloppenborg et al., 2008 ; Creavin et al., 2012 ; Crane et al., 2013 ; Roberts et al., 2014 ), and older individuals with T2DM progress to dementia at faster rates ( Xu et al., 2010 ; Morris et al., 2014 ). These changing health demographics have created a discrepancy: what we define as “normal” or “typical” cognitive aging is becoming farther and farther removed from what would be considered optimal, or “healthy” cognitive aging.
This trend warrants attention from the perspective of cognitive aging research. Without adequate screening procedures in place, inclusion of individuals with T2DM and hypertension in otherwise healthy study samples may introduce unwanted variability and bias to brain and/or cognitive measures, and increase the potential for type 1 and type 2 errors. Functional neuroimaging studies may be particularly vulnerable in this regard. Blood oxygenation level dependent magnetic resonance imaging (BOLD MRI) and positron emission tomography (PET H 2 O 15 ) measure hemodynamic changes associated with neural activity, and thus provide an indirect measure of neural function ( Logothetis et al., 2001 ). To interpret individual or group differences in BOLD or PET signaling as reflective of individual or group differences in neural activation underlying a cognitive operation of interest, we rely on assumptions of intact neurovascular signaling, cerebrovascular reactivity, and vascular health amongst the study participants. These assumptions may be true in young and healthy individuals, but do not hold in older adults with conditions that affect vascular health ( D'Esposito et al., 2003 ). Even normal, age-related changes in the integrity of the cerebrovascular system can undermine these assumptions ( D'Esposito et al., 1999 ).
Yet, it was our impression that relatively few studies in the cognitive aging neuroimaging literature consider T2DM or hypertension during recruitment, or control for potential confounds associated with these conditions during analysis. To clarify the extent to which current research practices consider T2DM and hypertension in study design, we present the results of a systematic review of the cognitive aging neuroimaging literature, looking at study inclusion/exclusion criteria and methodology related to T2DM and hypertension. Then, to reinforce the notion that individuals with T2DM and hypertension should not be overlooked as a potential source of bias, we provide an overview of metabolic and vascular changes associated with T2DM and hypertension, as they relate to vascular health, structural brain atrophy, and functional integrity. The final section discusses best practices moving forward.
Systematic Review
This review focuses on the cognitive aging neuroimaging literature, however the issues associated with inclusion of individuals with T2DM and hypertension in study samples are by no means limited to this area of research. Any research study whose population of interest has high prevalence rates of T2DM or hypertension should be cognizant of these issues. For example, psychiatric populations have a higher incidence of metabolic disruption and T2DM that is mediated, at least partially, by the use of mood stabilizers, anticonvulsants, and antipsychotic medications ( Regenold et al., 2002 ; Newcomer and Haupt, 2006 ).
It should also be noted that the purpose of this review is not to quantitatively compare the results of studies that have excluded T2DM and/or hypertension with those that have not. This type of comparison is not feasible for numerous reasons, the primary one being that the extent to which individuals with T2DM or hypertension were present in study samples that did not screen for either condition is unknown. Rather, the aim of this review is to highlight the proportion of studies in the cognitive aging neuroimaging literature that consider T2DM and/or hypertension in their inclusion/exclusion criteria, or attempt to account for the potential bias introduced by inclusion of these individuals in their study groups.
We searched PsychInfo, MedLine, and PubMed between 1995 and February, 2013 using the search terms [“functional magnetic resonance imaging” or “positron emission tomography”], [“geriatrics” or “aging” or “age differences”], and [“cognit*” or “neuropsych*” or “memory” or “attention”]. Across the three databases, these search terms produced 704 unique empirical studies. From these results, we excluded studies that did not include a “healthy” or “normal” older adult sample ( n = 125), included a clinical sample other than MCI or Alzheimer disease (AD)/dementia (e.g., psychiatric; n = 46), did not use BOLD or PET H 2 O 15 imaging ( n = 227), and did not scan during a cognitive or resting state task ( n = 74; Figure 1 ).
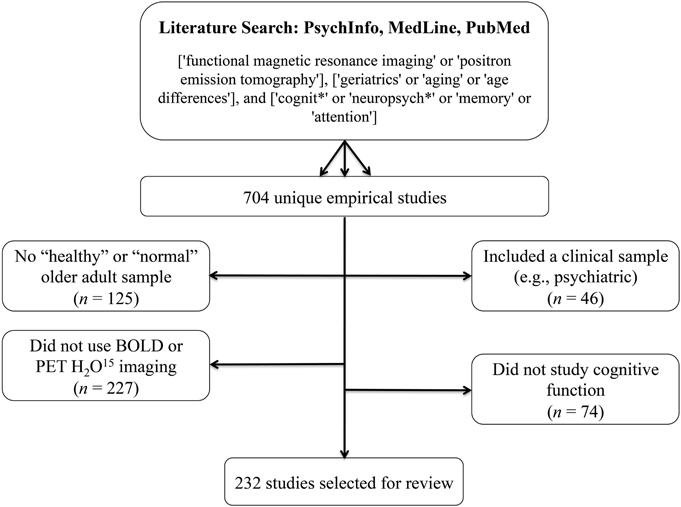
Figure 1. Literature search terms and exclusion criteria . Based on these criteria, 232 studies were selected for review.
Based on these criteria, 232 studies were selected for review. These studies are identified with an asterisk (*) in the reference section. Two hundred and nineteen of these used BOLD imaging, one used both BOLD and PET H 2 O 15 , and 12 used PET H 2 O 15 only. One hundred and sixty five of these studies compared a “healthy” older group with a group of young participants, 34 studies compared a “healthy” older sample to an MCI and/or AD group (two of which also included a young adult comparison group), and the remaining 33 studies looked only at a “healthy” older sample. The majority of surveyed studies employed a memory paradigm during imaging (e.g., encoding/recognition of words, pictures, scenes, faces, autobiographical memory, spatial memory, associative memory, implicit learning). Working memory and executive processes were also well-studied (e.g., cognitive control, inhibition, decision making, mental rotation, task-switching, attention, judgment, processing speed, naming, imagery, verb generation, fluency). We also included resting-state studies in the sample.
Our primary concern was how sample selection was reported to have occurred. In particular, we were interested to learn how many studies specifically screened for T2DM and/or hypertension in their healthy older adult samples. For each of the 232 identified studies, the inclusion/exclusion criteria were examined according to the following criteria: (i) explicit exclusion of T2DM and/or hypertension, or exclusion of medical disorders/physical illnesses/systemic illnesses (implying that all medical conditions, including T2DM and hypertension, were excluded); (ii) exclusion of “significant,” “major,” or “severe” medical/physical/systemic disorders; or (iii) no screening criteria related to T2DM and/or hypertension provided. We also surveyed each of the 232 studies to determine how subjects were screened (e.g., self-report questionnaire, clinical assessment with a medical doctor, laboratory testing), and how—if at all—the potential influence of intersubject vascular variability on the measured BOLD or PET signals was addressed.
In each section below, superscript numbers, letters, and symbols are used to represent the extent to which studies screened for T2DM and hypertension, the screening method, and the degree to which studies attempted to account for intersubject vascular variability, respectively. The identified studies are denoted in the reference section according to these superscript classifiers.
Inclusion/exclusion of T2DM and hypertension
Of the 232 studies surveyed, only 22 (9.5%) explicitly excluded individuals with T2DM( 1 ), and only 29 (12.5%) explicitly excluded individuals with hypertension( 2 ). Thirteen studies—approximately 6%—excluded both T2DM and hypertension. Fourteen studies (6.0%) excluded individuals on antihypertensive medication( 3 ), however few of these studies also clarified whether individuals were assessed for untreated hypertension and excluded, if present. Nineteen studies (8.2%) excluded medical illnesses, systemic illnesses, medical disorders or physical illnesses( 4 ). This criterion implies that all medical conditions, including T2DM and hypertension, were excluded.
In contrast, almost half of the included studies (102; 44.0%) had exclusion criteria that made it difficult to determine whether T2DM was excluded( 5 ), and 85 studies (36.6%) had exclusion criteria that made it difficult to determine whether hypertension and/or antihypertensive medications were excluded( 6 ). These studies listed “major medical illnesses,” “significant medical conditions,” “serious systemic illnesses,” “conditions/medications interfering with cognitive and/or brain function,” “vascular disease,” “cardiovascular disease,” and/or “conditions/medications interfering with the fMRI signal” as exclusion criteria, or simply described their sample as “healthy.” There were also many studies that did not mention any selection criteria related to T2DM (80; 34.5%)( 7 ) or hypertension (51; 22.0%)( 8 ).
In addition, 26 studies (11.2%) included individuals with controlled hypertension( 9 ), 8 studies (3.5%) included controlled and uncontrolled hypertension( 10 ), 3 studies (1.3%) included individuals with controlled T2DM( 11 ), and 6 studies (2.5%) included individuals with controlled and uncontrolled T2DM in their healthy cohort( 12 ). Figure 2 provides a visual depiction of these results.
Figure 2. The extent to which T2DM and hypertension were accounted for in the inclusion/exclusion criteria of the healthy samples that were surveyed .
Screening method
The majority of studies (173; 75%) did not report how they conducted their medical screening( a ). Only 28 studies (12%) reported having screened subjects with physician-conducted medical examinations and/or laboratory testing( b ). Sixteen studies (7%) screened participants with telephone interviews, in-person clinical interviews, medical history, chart reviews, or a combination of these methods( c ). The remaining 15 studies (6%) used a self-report questionnaire to assess medical status( d ).
Accounting for intersubject vascular variability
A survey of the 232 included studies found that just under one third (29%) acknowledged and/or addressed the potential influence of intersubject vascular variability on the reported results. Many excluded subjects with a high vascular burden by screening for white matter hyperintensities in the imaging data( ■ ). Others compared groups on vascular risk factors( + ), compared outcome measures on hypertension status or antihypertensive treatment status( ♦ ), or attempted to control for health, blood pressure, and/or white matter hyperintensities in the reported associations ( ❖ ). Several studies noted in their discussion the possibility that the reported results were influenced by vascular factors, or explained why they did not think this was an issue( • ). A few studies used the measured BOLD or PET signals to examine and account for individual differences in vascular health( □ ); for example, by ensuring that groups were equated on BOLD signal variability, by comparing the temporal characteristics of the hemodynamic response curve across groups, with proportional scaling of the BOLD or PET signal, or by focusing on group by task interactions (instead of group main effects) or comparing within-subject task contrasts across individuals or groups to minimize any individual or group differences in vascular integrity.
There are rigorous ways to account for intersubject vascular variability, such as additional task data or an additional imaging contrast. Several studies included in the present review used arterial spin labeling (ASL) MRI ( ▴ ) or PET ( ▾ ) to measure resting cerebral blood flow and control for individual differences in perfusion. Three studies used a breath-hold task to index individual differences in cerebrovascular reactivity ( ❍ ), and two studies included a low-level motor or baseline task to ensure that participants demonstrated an adequate hemodynamic response ( × ).
Our results found that fewer than 10% of the selected functional imaging studies on cognitive aging explicitly excluded individuals with T2DM from their normative samples, and fewer than 15% explicitly excluded individuals with hypertension. A number of studies reported selection criteria that were insufficient to determine whether T2DM or hypertension were screened. Critically, one third of included studies had no reported inclusion or exclusion criteria related to T2DM, while almost a quarter had no reported inclusion or exclusion criteria related to hypertension. Only 67 of the 232 selected studies (29%) acknowledged or addressed the potential influence of intersubject vascular variability on the measured BOLD or PET signals.
Moreover, the large majority of studies did not include information about the medical screening process itself (e.g., laboratory testing vs. clinical interview vs. self-report questionnaire). This is not ideal when established tests for T2DM and hypertension are available (for example, 24-h ambulatory blood pressure monitoring would be the gold-standard for determining hypertension status, and an oral glucose tolerance test for determining T2DM status). Furthermore, we posit that participants may be less likely to volunteer T2DM or hypertension status as a “significant” medical illness without specific probing (i.e., compared to cancer, HIV, multiple sclerosis, or heart disease), because when these conditions are well-controlled they can have a minimal impact on day-to-day functioning, and, in the case of T2DM, can be controlled by diet alone. Collectively, these observations point to a lack of awareness that T2DM and hypertension are major medical illnesses that interfere significantly with cognitive and brain function in older adults.
Overview: Metabolic and Vascular Complications of Type 2 Diabetes Mellitus and Hypertension
To reinforce the position that T2DM and hypertension are conditions that can have a major effect on brain health and cognitive aging, this next section reviews evidence on the cognitive deficits, structural changes, and functional consequences associated with T2DM and hypertension, and describes some of the mechanisms that mediate these changes.
Type 2 Diabetes Mellitus
T2DM is the result of peripheral insulin resistance, which leads to insulin dysregulation and hyperglycemia. These metabolic changes affect cerebrovascular health, structural integrity, and brain function, and underlie the associations between T2DM, cognitive decline, and dementia risk.
Insulin dysregulation
Insulin is a peptide hormone that is critical for regulation of blood glucose levels. Binding of insulin to its receptors, found on nearly all cells throughout the body, facilitates the cellular uptake of glucose from the blood. When bound, insulin and insulin-like growth factor also activate complex intracellular signaling pathways that promote cell growth and survival, regulate glucose metabolism, and inhibit oxidative stress and apoptosis (for a review, see Nakae et al., 2001 ).
The defining characteristic of T2DM is peripheral insulin resistance, which occurs when cells in the body decrease their response to insulin stimulation. In the developing stage of this disease, the pancreas is able to produce enough insulin to overcome this resistance. This results in peripheral hyperinsulinemia, and blood glucose levels remain within the normal range. As the disease progresses, however, the pancreas can no longer keep up, and blood glucose levels begin to rise. When blood glucose levels are high even in the fasting state, T2DM is diagnosed.
Peripheral insulin resistance and hyperinsulinemia have a counterintuitive impact on insulin levels within the central nervous system. In the face of peripheral hyperinsulinemia, insulin transport across the blood brain barrier is effectively reduced, resulting in a brain hypo -insulinemic state (e.g., Heni et al., 2013 ). Low brain insulin levels and disrupted insulin signaling contribute to cognitive impairments directly, particularly in medial temporal lobe regions where insulin receptors are abundant ( Convit, 2005 ; Craft, 2006 ). Indirectly, low brain insulin levels exacerbate amyloid beta (Aβ) and tau pathology, hallmarks of Alzheimer disease (AD). It is here that we see the link between T2DM and Alzheimer disease pathology: brain insulin deficiency results in the down-regulation of insulin degrading enzyme (IDE; Luchsinger, 2008 ), which also has a role in degrading Aβ ( Carlsson, 2010 ). As a result, Aβ degradation is effectively reduced, contributing to its aggregation and amyloid plaque formation. Decreased brain insulin levels also suppress the enzymes involved in tau phosphorylation, contributing to the formation of neurofibrillary tangles ( Akter et al., 2011 ). While the downstream impact of T2DM-mediated brain insulin deficiency and insulin resistance is more moderate than that associated with AD, the underlying pathogenic mechanisms are similar ( Steen et al., 2005 ), and it has been proposed that AD is a form of diabetes mellitus that selectively affects the brain (T3DM; for discussion, see de la Monte and Wands, 2008 ). Given this, is not surprising that individuals with T2DM show a pattern of memory impairment, medial temporal lobe atrophy, and reduced hippocampal connectivity that is similar to the classic pattern of memory deficits, neurodegeneration, and network disruption in AD (e.g., Gold et al., 2007 ; Zhou et al., 2010 ; Baker et al., 2011 ; Cui et al., 2014 ).
Hyperglycemia
When cells in the body become resistant to the effects of insulin, blood glucose levels rise, resulting in hyperglycemia. Endothelial cells are particularly vulnerable to the effects of hyperglycemia, because they are less efficient at reducing glucose uptake in the face of high blood glucose levels ( Kaiser et al., 1993 ). Under such conditions, the resultant intracellular hyperglycemia induces an overproduction of reactive oxygen species in the mitochondria, which increases oxidative stress within the cell. This initiates a cascade of biochemical events that mediate much of the microvascular and macrovascular damage associated with T2DM including, but not limited to, increased intracellular formation of advanced glycation end-products (AGEs) and protein kinase C activation ( Du et al., 2000 ; Nishikawa et al., 2000 ; Brownlee, 2005 ; Giacco and Brownlee, 2010 ; Johnson, 2012 ).
AGEs are formed during normal metabolism on proteins with slower rates of turnover, in almost all cells throughout the body. AGE accumulation over time is a major factor in normal aging; however, under hyperglycemic conditions, AGE production is exacerbated beyond normal levels. AGEs cause intracellular damage and induce apoptosis through a process called cross-linking ( Shaikh and Nicholson, 2008 ). AGEs also contribute to oxidative stress, and themselves activate inflammatory signaling cascades (for a review, see Yan et al., 2008 ). Critically, under hyperglycemic conditions, the Aβ protein itself can act as an AGE ( Granic et al., 2009 ), which enhances its own aggregation and further increases amyloid plaque formation.
Protein kinase C activation, on the other hand, affects a variety of changes in gene expression that culminate in vascular dysfunction. Production of nitric oxide (NO), a vasodilator, is decreased, and production of endothelin-1, a vasoconstrictor, is increased. As a result, blood vessels are less able to dilate to accommodate increased blood flow demand. Over time, chronic exposure to high concentrations of endothelin-1 and decreased concentrations of NO contribute to diminished vessel elasticity, and structural changes in the vessel wall that result in atherosclerotic plaque formation ( Kalani, 2008 ).
In the brain, hyperglycemia-mediated macro- and microvascular damage reduces the delivery of nutrients and oxygen required to meet metabolic demands. Altered cerebral autoregulation has been observed in middle-aged adults with T2DM ( Brown et al., 2008 ), and may be an early manifestation of microvascular disease ( Kim et al., 2008 ). Older adults with T2DM show decreased blood flow velocity, increased cerebrovascular resistance, and impaired vasoreactivity ( Novak et al., 2006 ). Over time, declines in cerebrovascular health and reduced perfusion of brain tissue lead to structural atrophy and altered brain function.
Cognitive effects
The cognitive profile of individuals with T2DM includes deficits in attention, processing speed, learning and memory, and executive function (e.g., Reaven et al., 1990 ; Brands et al., 2007 ; Yeung et al., 2009 ; Whitehead et al., 2011 ). Moreover, these individuals, and individuals with pre-diabetes (impaired glucose tolerance), show an accelerated trajectory of cognitive decline relative to that associated with healthy aging ( Gregg et al., 2000 ; Fontbonne et al., 2001 ; Arvanitakis et al., 2004 ; Yaffe et al., 2004 ; Fischer et al., 2009 ; Nooyens et al., 2010 ; Espeland et al., 2011 ; for conflicting results, see van den Berg et al., 2010 ).
Cognitive deficits in T2DM have been linked to multiple disease-related processes, including: (i) poor glucose control (i.e., hemoglobin A1c [HbA1c]; Ryan and Geckle, 2000 ; Kanaya et al., 2004 ; Cukierman-Yaffe et al., 2009 ; Maggi et al., 2009 ; Luchsinger et al., 2011 ; Tuligenga et al., 2014 ; for conflicting results, see Christman et al., 2011 ), (ii) glucose intolerance ( Rizzo et al., 2010 ; Zhong et al., 2012b ), (iii) high peripheral AGE levels ( Yaffe et al., 2011 ), (iv) high levels of inflammatory cytokines ( Marioni et al., 2010 ), and (v) peripheral hyperinsulinemia and insulin resistance ( Bruehl et al., 2010 ; Zhong et al., 2012a ). Even in non-diabetic adults, poorer glucoregulation has been associated with deficits and/or declines in verbal memory, working memory, processing speed, and executive function ( Dahle et al., 2009 ; Bruehl et al., 2010 ; Messier et al., 2010 , 2011 ; Ravona-Springer et al., 2012 ).
The link between cognitive impairment and poor metabolic control may be largely mediated by the structural and functional brain changes that occur in the presence of chronic insulin dysregulation and hyperglycemia. Associations between glucoregulation, hypoperfusion in temporal regions, hippocampal atrophy, and memory impairment have been observed in T2DM ( Gold et al., 2007 ; Last et al., 2007 ), and in non-diabetic adults with decreased peripheral glucose regulation ( Convit et al., 2003 ), or high fasting plasma glucose levels within the normal range ( Cherbuin et al., 2012 ; Kerti et al., 2013 ). In other studies of T2DM, cognitive deficits and structural brain atrophy were linked to cerebral hypoperfusion and altered vascular reactivity ( Last et al., 2007 ; Brundel et al., 2012 ), and disrupted default-mode network connectivity was associated with peripheral hyperinsulinemia, insulin resistance, and white matter integrity ( Musen et al., 2012 ; Hoogenboom et al., 2014 ). Regardless of the underlying cause, brain atrophy in T2DM is associated with poor cognition ( Moran et al., 2013 ), and cognitive declines have been associated with progression of brain atrophy over time ( van Elderen et al., 2010 ; Reijmer et al., 2011 ). Some studies suggest that structural changes may occur early in the course of T2DM; enlarged lateral ventricles, particularly within the frontal horns, have been observed less than a year after diagnosis ( Lee et al., 2013 ), and middle-aged, as well as older adults with T2DM, show reduced prefrontal volumes ( Bruehl et al., 2009 ) and generalized global atrophy ( de Bresser et al., 2010 ; Kamiyama et al., 2010 ; Espeland et al., 2013 ).
Hypertension
The brain is one of the most highly perfused organs. The cerebral hemispheres are supplied by capillary beds connected to the pial vasculature by penetrating arterioles, and the pial vasculature stems from a system of arteries branching off the anterior, middle, and posterior cerebral arteries. Maintenance of brain function depends on a constant blood supply through this network. Hypertension causes changes to the structure and function of these blood vessels, which impacts perfusion in affected areas. Hypoperfusion, for example, can interfere with the delivery of oxygen and nutrients required to meet metabolic demands, and makes hypertension a major risk factor for vascular cognitive impairment, stroke, and dementia.
Cerebrovascular changes
Hypertension places enormous stress on the cerebral circulation (for a comprehensive review, see Pires et al., 2013 ). A hallmark of chronic hypertension is increased vascular resistance, particularly in the small blood vessels that perfuse the brain. Vascular resistance increases as vessel walls thicken. This remodeling is an adaptive response required to maintain chronically increased blood pressure, but it decreases the interior space of the blood vessels (the lumen). Vascular resistance also increases as the number of blood vessels decrease. Rat models of hypertension have shown both of these effects: reductions in lumen diameter and in the number of capillaries making up capillary beds in the cerebral vasculature ( Sokolova et al., 1985 ).
Blood flow is reduced when vascular resistance is high, and chronic hypertension-mediated hypoperfusion has been linked to white matter degradation, gray matter atrophy, and cognitive deficits. Studies of older adults with hypertension show reduced blood flow, particularly in occipito-temporal, prefrontal, and medial temporal lobe regions ( Beason-Held et al., 2007 ), positive correlations between blood pressure and white matter burden ( White et al., 2011 ; Raji et al., 2012 ), and negative correlations between blood pressure and total brain volume ( Nagai et al., 2008 ). Blood vessel function is also impacted by hypertension. Cerebral autoregulation (i.e., the ability to maintain a constant perfusion rate over a range of arterial pressures) is impaired, as is cerebrovascular reactivity, the ability of blood vessels to dilate to accommodate increased blood flow demand ( Last et al., 2007 ; Hajjar et al., 2010 ).
The cognitive profile of older adults with hypertension includes poorer performance on tests of executive function, including verbal fluency, Trails B-A switching score, Stroop interference scores ( Bucur and Madden, 2010 ), slowed processing speed ( Dahle et al., 2009 ), and deficits in attention and memory (see Gifford et al., 2013 for a meta-analysis). Prospective cohort studies show that midlife cardiovascular risk factors like hypertension predict cognitive impairment in later life (e.g., Virta et al., 2013 ), and, similarly, cross-sectional studies show a relation between higher systolic blood pressure and poorer cognitive performance, even within the normotensive range, a relation that is particularly strong in midlife (e.g., Knecht et al., 2008 , 2009 ). Hypertension is associated with decreases in cognitive reserve ( Giordano et al., 2012 ), and older adults with MCI and cardiovascular risk factors like hypertension are twice as likely to develop dementia compared to those without such risk factors ( Johnson et al., 2010 ; Ettorre et al., 2012 ). Moreover, cognitive declines may be faster in those with MCI and hypertension, compared to those without hypertension ( Li et al., 2011 ; Goldstein et al., 2013 ).
The association between hypertension and cognitive decline appears to be strongest in executive and processing speed domains, and weakest in memory and language domains. Hypertension increased the risk of non-amnestic MCI, but not amnestic MCI, regardless of APOEε 4 genotype or hypertensive medication status ( Reitz et al., 2007 ), and predicted progression to dementia in non-amnestic MCI, but not amnestic or multi-domain MCI ( Oveisgharan and Hachinski, 2010 ). The impact of hypertension on executive and processing speed domains is consistent with studies that show a positive relation between hypertension and white matter changes ( Kennedy and Raz, 2009 ; Raz et al., 2012 ), and between white matter changes and deficits in processing speed, executive function, and attention, but not memory (e.g., Debette et al., 2011 ).
Cognitive deficits in hypertensive adults are linked to various indicators of vascular and brain health. There are correlations between white matter integrity and performance on tests of executive function and attention ( Hannesdottir et al., 2009 ), and between decreased flow-mediated dilation and poorer executive function ( Smith et al., 2011 ). Deficits in attention and psychomotor speed in late middle-aged adults with hypertension are associated with reductions in global brain perfusion, reductions that were not fully ameliorated following 6-months of antihypertensive treatment ( Efimova et al., 2008 ). Global cognitive decline has been linked to reduced cerebral blood flow in the face of white matter lesions and lacunar infarcts ( Kitagawa et al., 2009 ), to higher pulse pressure and arterial stiffness ( Scuteri et al., 2007 ; Waldstein et al., 2008 ; Triantafyllidi et al., 2009 ), and to hypertension-mediated deep-brain vascular pathology ( Yakushiji et al., 2012 ). In another large study of patients with MCI, those with hypertension and deep white matter lesions were at higher risk of dementia ( Clerici et al., 2012 ).
Conclusions
Taken together, these studies provide abundant evidence that middle-aged and older adults with T2DM and hypertension, relative to healthy older adults, are more likely to show signs of cognitive dysfunction, widespread structural atrophy, vascular damage, and functional changes. In light of their rising prevalence amongst older adults, there is an increasing likelihood that, without adequate screening at recruitment, individuals with T2DM and/or hypertension will be included in healthy older adult samples. This may introduce unwanted variability and bias to brain and/or cognitive measures, and increase the potential for type 1 and type 2 errors. Given the state of the neuroimaging literature on this topic and the need to advance our understanding, we view T2DM and hypertension as important new frontiers in cognitive neuroscience.
Moving forward, there is an opportunity to develop best practices when it comes to cognitive neuroscience research in older adult populations. Reconciling the vascular risk component in T2DM and hypertension may be the most tractable option since there are myriad approaches one can take to do this. The most rigorous approach in this respect may be inclusion of a breath-hold task, or a measure of cerebral blood flow (e.g., ASL) in the functional imaging protocol, as this allows for a direct estimate of each subject's vascular health. Breath-hold tasks can be used to index cerebrovascular reactivity in response to non-neuronal signals. The breath-hold period induces hypercapnia, which stimulates vasodilation and increases blood flow and blood volume in the brain, a signal change that occurs independently of neuronal activation. ASL or resting-state PET scans provide a direct measure of blood flow, and can be used to account for individual differences in perfusion. As noted above, these methods have already been used in some studies of cognitive aging to account for individual differences in cerebrovascular health. Whether other means of equating vascular risk across participants or across groups (e.g., screening participants for excessive white matter hyperintensities, post-hoc comparison of outcome measures or study groups on vascular risk factors, or statistical analyses aimed at controlling for the effects of vascular variability in the reported results) are similarly effective requires further study.
It may also be important for investigators to acknowledge a distinction between “healthy” and “typical” brain aging. Studies characterizing healthy aging should adopt T2DM and hypertension as exclusion criteria. Conversely, given the high prevalence of T2DM and hypertension in older adults, community- or population-based studies characterizing the typical trajectory of cognitive aging would benefit by including these participants to maximize the generalizability of results, and reconciling the heterogeneity through study design groups (e.g., stratifying based on diagnosis of T2DM and hypertension) or covariates in their analysis.
As the proportion of older adults living with T2DM and hypertension increase, it is imperative that functional imaging studies are designed to account for these population trends. The current state of the cognitive aging neuroimaging literature suggests that there is limited appreciation and/or awareness that T2DM and hypertension are significant medical illnesses that disrupt brain vasculature, brain structure, and brain function. By adopting best practices that take T2DM and hypertension into account, we can advance our understanding of these conditions, and of cognitive aging in general.
Author Contributions
Liesel-Ann C. Meusel selecting, indexing, and reviewing articles, writing of drafts; Nisha Kansal selecting articles, editing of drafts; Ekaterina Tchistiakova contributing to the first draft, editing of drafts; William Yuen selecting articles, contributing to the first draft, editing of drafts; Bradley J. MacIntosh provided conceptual foundation for paper, editing of drafts; Carol E. Greenwood provided conceptual foundation for paper, editing of drafts; Nicole D. Anderson provided conceptual foundation for paper, editing of drafts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported in part by postdoctoral fellowships from the Centre for Stroke Recovery and the Alzheimer Society of Canada awarded to Liesel-Ann C. Meusel, and grant funds from CIHR (MOP111244).
Akter, K., Lanza, E. A., Martin, S. A., Myronyuk, N., Rua, M., and Raffa, R. B. (2011). Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br. J. Clin. Pharmacol . 71, 365–376. doi: 10.1111/j.1365-2125.2010.03830.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
* 5,6,a Anderson, K. E., Lynch, K., Zarahn, E., Scarmeas, N., Van Heertum, R., Sackeim, H. et al. (2005). H215O PET study of impairment of nonverbal recognition with normal aging. J. Neuropsychiatry Clin. Neurosci . 17, 192–200. doi: 10.1176/appi.neuropsych.17.2.192
* 7,8,d Anguera, J. A., Reuter-Lorenz, P. A., Willingham, D. T., and Seidler, R. D. (2011). Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J. Cogn. Neurosci . 23, 11–25. doi: 10.1162/jocn.2010.21451
* 5,6,a Ansado, J., Monchi, O., Ennabil, N., Faure, S., and Joanette, Y. (2012). Load-dependent posterior-anterior shift in aging in complex visual selective attention situations. Brain Res . 1454, 14–22. doi: 10.1016/j.brainres.2012.02.061
* 5,6,a,□ Antonova, E., Parslow, D., Brammer, M., Dawson, G. R., Jackson, S. H. D., and Morris, R. G. (2009). Age-related neural activity during allocentric spatial memory. Memory 17, 125–143. doi: 10.1080/09658210802077348
Arvanitakis, Z., Wilson, R. S., Bienias, J. L., Evans, D. A., and Bennett, D. A. (2004). Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol . 61, 661–666. doi: 10.1001/archneur.61.5.661
* 5,6,b Bäckman, L., Karlsson, S., Fischer, H., Karlsson, P., Brehmer, Y., Rieckmann, A. et al. (2011). Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol. Aging 32, 1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018
* 5,6,a Bagurdes, L. A., Mesulam, M. M., Gitelman, D. R., Weintraub, S., and Small, D. M. (2008). Modulation of the spatial attention network by incentives in healthy aging and mild cognitive impairment. Neuropsychologia 46, 2943–2948. doi: 10.1016/j.neuropsychologia.2008.06.005
* 5,6,c Bai, F., Liao, W., Watson, D. R., Shi, Y., Wang, Y., Yue, C. et al. (2011). Abnormal whole-brain functional connection in amnestic mild cognitive impairment patients. Behav. Brain Res . 216, 666–672. doi: 10.1016/j.bbr.2010.09.010
* 5,6,a Bai, F., Zhang, Z., Yu, H., Shi, Y., Yuan, Y., Zhu, W. et al. (2008). Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci. Lett . 438, 111–115. doi: 10.1016/j.neulet.2008.04.021
Baker, L. D., Cross, D. J., Minoshima, S., Belongia, D., Watson, G. S., and Craft, S. (2011). Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol . 68, 51–57. doi: 10.1001/archneurol.2010.225
* 5,6,c,▴ Bangen, K. J., Kaup, A. R., Mirzakhanian, H., Wierenga, C. E., Jeste, D. V., and Eyler, L. T. (2012). Compensatory brain activity during encoding among older adults with better recognition memory for face-name pairs: an integrative functional, structural, and perfusion imaging study. J. Int. Neuropsychol. Soc . 18, 402–413. doi: 10.1017/S1355617712000197
* 9,12,b,▴ Bangen, K. J., Restom, K., Liu, T. T., Jak, A. J., Wierenga, C. E., Salmon, D. P. et al. (2009). Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol. Aging 30, 1276–1287. doi: 10.1016/j.neurobiolaging.2007.11.012
* 7,8,a,▾ Beason-Held, L. L., Kraut, M. A., and Resnick, S. M. (2008). I. Longitudinal changes in aging brain function. Neurobiol. Aging 29, 483–496. doi: 10.1016/j.neurobiolaging.2006.10.031
Beason-Held, L. L., Moghekar, A., Zonderman, A. B., Kraut, M. A., and Resnick, S. M. (2007). Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke 38, 1766–1773. doi: 10.1161/STROKEAHA.106.477109
* 9,12,c,+ Beeri, M. S., Lee, H., Cheng, H., Wollman, D., Silverman, J. M., and Prohovnik, I. (2011). Memory activation in healthy nonagenarians. Neurobiol. Aging 32, 515–523. doi: 10.1016/j.neurobiolaging.2009.02.022
* 1,2,a Berlingeri, M., Bottini, G., Danelli, L., Ferri, F., Traficante, D., Sacheli, L. et al. (2010). With time on our side? Task-dependent compensatory processes in graceful aging. Exp. Brain Res . 205, 307–324. doi: 10.1007/s00221-010-2363-7
* 4,a Bernard, F. A., Desgranges, B., Eustache, F., and Baron, J.-C. (2007). Neural correlates of age-related verbal episodic memory decline: a PET study with combined subtraction/correlation analysis. Neurobiol. Aging 28, 1568–1576. doi: 10.1016/j.neurobiolaging.2006.07.004
* 5,6,a Bollinger, J., Rubens, M. T., Masangkay, E., Kalkstein, J., and Gazzaley, A. (2011). An expectation-based memory deficit in aging. Neuropsychologia 49, 1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021
Brands, A. M. A., Van den Berg, E., Manschot, S. M., Biessels, G. J., Kappelle, L. J., De Haan, E. H. F. et al. (2007). A detailed profile of cognitive dysfunction and its relation to psychological distress in patients with type 2 diabetes mellitus. J. Int. Neuropsychol. Soc . 13, 288–297. doi: 10.1017/S1355617707070312
* 2,5,a Braskie, M. N., Landau, S. M., Wilcox, C. E., Taylor, S. D., O'Neil, J. P., Baker, S. L. et al. (2011). Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum. Brain Mapp . 32, 947–961. doi: 10.1002/hbm.21081
* 5,6,a Braskie, M. N., Small, G. W., and Bookheimer, S. Y. (2009). Entorhinal cortex structure and functional MRI response during an associative verbal memory task. Hum. Brain Mapp . 30, 3981–3992. doi: 10.1002/hbm.20823
* 1,10,b,❖ Braskie, M. N., Small, G. W., and Bookheimer, S. Y. (2010). Vascular health risks and fMRI activation during a memory task in older adults. Neurobiol. Aging 31, 1532–1542. doi: 10.1016/j.neurobiolaging.2008.08.016
Brown, C. M., Marthol, H., Zikeli, U., Ziegler, D., and Hilz, M. J. (2008). A simple deep breathing test reveals altered cerebral autoregulation in type 2 diabetic patients. Diabetologia 51, 756–761. doi: 10.1007/s00125-008-0958-3
Brownlee, M. (2005). The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625. doi: 10.2337/diabetes.54.6.1615
Bruehl, H., Sweat, V., Hassenstab, J., Polyakov, V., and Convit, A. (2010). Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J. Clin. Exp. Neuropsychol . 32, 487–493. doi: 10.1080/13803390903224928
Bruehl, H., Wolf, O. T., Sweat, V., Tirsi, A., Richardson, S., and Convit, A. (2009). Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res . 1280, 186–194. doi: 10.1016/j.brainres.2009.05.032
Brundel, M., van den Berg, E., Reijmer, Y. D., de Bresser, J., Kappelle, L. J., and Biessels, G. J. (2012). Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J. Diabetes Complicat . 26, 205–209. doi: 10.1016/j.jdiacomp.2012.03.021
Bucur, B., and Madden, D. J. (2010). Effects of adult age and blood pressure on executive function and speed of processing. Exp. Aging Res . 36, 153–168. doi: 10.1080/03610731003613482
* 7,9,a Burgmans, S., van Boxtel, M. P. J., Vuurman, E. F. P. M., Evers, E. A. T., and Jolles, J. (2010). Increased neural activation during picture encoding and retrieval in 60-year-olds compared to 20-year-olds. Neuropsychologia 48, 2188–2197. doi: 10.1016/j.neuropsychologia.2010.04.011
* 2,5,a Cabeza, R., Anderson, N. D., Locantore, J. K., and McIntosh, A. R. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–1402. doi: 10.1006/nimg.2002.1280
* 2,5,d Cabeza, R., Daselaar, S. M., Dolcos, F., Prince, S. E., Budde, M., and Nyberg, L. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex 14, 364–375. doi: 10.1093/cercor/bhg133
* 2,5,a Cabeza, R., Grady, C. L., Nyberg, L., McIntosh, A. R., Tulving, E., Kapur, S. et al. (1997). Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J. Neurosci . 17, 391–400.
Pubmed Abstract | Pubmed Full Text
* 7,8,a Campbell, K. L., Grady, C. L., Ng, C., and Hasher, L. (2012). Age differences in the frontoparietal cognitive control network: implications for distractibility. Neuropsychologia 50, 2212–2223. doi: 10.1016/j.neuropsychologia.2012.05.025
* 7,8,a Cappell, K. A., Gmeindl, L., and Reuter-Lorenz, P. A. (2010). Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 46, 462–473. doi: 10.1016/j.cortex.2009.11.009
* 7,8,a Carlson, M. C., Erickson, K. I., Kramer, A. F., Voss, M. W., Bolea, N., Mielke, M. et al. (2009). Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J. Gerontol. A Biol. Sci. Med. Sci . 64, 1275–1282. doi: 10.1093/gerona/glp117
Carlsson, C. M. (2010). Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J. Alzheimers. Dis . 20, 711–722. doi: 10.3233/JAD-2010-100012
* 7,8,a Celone, K. A., Calhoun, V. D., Dickerson, B. C., Atri, A., Chua, E. F., Miller, S. L. et al. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci . 26, 10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006
* 2,5,a Chen, N., Chou, Y., Song, A. W., and Madden, D. J. (2009). Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Struct. Funct . 213, 571–585. doi: 10.1007/s00429-009-0218-4
Cherbuin, N., Sachdev, P., and Anstey, K. J. (2012). Higher normal fasting plasma glucose is associated with hippocampal atrophy: the PATH Study. Neurology 79, 1019–1026. doi: 10.1212/WNL.0b013e31826846de
Christman, A. L., Matsushita, K., Gottesman, R. F., Mosley, T., Alonso, A., Coresh, J. et al. (2011). Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 54, 1645–1652. doi: 10.1007/s00125-011-2095-7
* 5,6,a Chua, E. F., Schacter, D. L., and Sperling, R. A. (2009). Neural basis for recognition confidence in younger and older adults. Psychol. Aging 24, 139–153. doi: 10.1037/a0014029
* 5,6,b Clément, F., and Belleville, S. (2009). Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum. Brain Mapp . 30, 4033–4047. doi: 10.1002/hbm.20827
Clerici, F., Caracciolo, B., Cova, I., Fusari Imperatori, S., Maggiore, L., Galimberti, D. et al. (2012). Does vascular burden contribute to the progression of mild cognitive impairment to dementia? Dement. Geriatr. Cogn. Disord . 34, 235–243. doi: 10.1159/000343776
Colosia, A. D., Palencia, R., and Khan, S. (2013). Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab. Syndr. Obes . 6, 327–338. doi: 10.2147/DMSO.S51325
Convit, A. (2005). Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol. Aging 26(Suppl. 1), 31–35. doi: 10.1016/j.neurobiolaging.2005.09.018
Convit, A., Wolf, O. T., Tarshish, C., and de Leon, M. J. (2003). Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc. Natl. Acad. Sci. U.S.A . 100, 2019–2022. doi: 10.1073/pnas.0336073100
* 7,8,b Cook, I. A., Bookheimer, S. Y., Mickes, L., Leuchter, A. F., and Kumar, A. (2007). Aging and brain activation with working memory tasks: an fMRI study of connectivity. Int. J. Geriatr. Psychiatry 22, 332–342. doi: 10.1002/gps.1678
Craft, S. (2006). Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis. Assoc. Disord . 20, 298–301. doi: 10.1097/01.wad.0000213866.86934.7e
Crane, P. K., Walker, R., Hubbard, R. A., Li, G., Nathan, D. M., Zheng, H. et al. (2013). Glucose levels and risk of dementia. N. Engl. J. Med . 369, 540–548. doi: 10.1056/NEJMoa1215740
Creavin, S. T., Gallacher, J., Bayer, A., Fish, M., Ebrahim, S., and Ben-Shlomo, Y. (2012). Metabolic syndrome, diabetes, poor cognition, and dementia in the Caerphilly prospective study. J. Alzheimers Dis . 28, 931–939. doi: 10.3233/JAD-2011-111550
Cui, Y., Jiao, Y., Chen, Y.-C., Wang, K., Gao, B., Wen, S. et al. (2014). Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 63, 749–760. doi: 10.2337/db13-0519
Cukierman-Yaffe, T., Gerstein, H. C., Williamson, J. D., Lazar, R. M., Lovato, L., Miller, M. E. et al. (2009). Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 32, 221–226. doi: 10.2337/dc08-1153
Dahle, C. L., Jacobs, B. S., and Raz, N. (2009). Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol. Aging 24, 154–162. doi: 10.1037/a0014283
Danaei, G., Finucane, M. M., Lu, Y., Singh, G. M., Cowan, M. J., Paciorek, C. J. et al. (2011). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378, 31–40. doi: 10.1016/S0140-6736(11)60679-X
* 2,5,d Daselaar, S. M., Fleck, M. S., Dobbins, I. G., Madden, D. J., and Cabeza, R. (2006). Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb. Cortex 16, 1771–1782. doi: 10.1093/cercor/bhj112
* 7,8,d Daselaar, S. M., Veltman, D. J., Rombouts, S. A. R. B., Raaijmakers, J. G. W., and Jonker, C. (2003). Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 126, 43–56. doi: 10.1093/brain/awg005
* 2,5,a Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., and Cabeza, R. (2008). Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. doi: 10.1093/cercor/bhm155
Debette, S., Seshadri, S., Beiser, A., Au, R., Himali, J. J., Palumbo, C. et al. (2011). Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468. doi: 10.1212/WNL.0b013e318227b227
de Bresser, J., Tiehuis, A. M., van den Berg, E., Reijmer, Y. D., Jongen, C., Kappelle, L. J. et al. (2010). Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 33, 1309–1314. doi: 10.2337/dc09-1923
* 7,9,a de Chastelaine, M., Wang, T. H., Minton, B., Muftuler, L. T., and Rugg, M. D. (2011). The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb. Cortex 21, 2166–2176. doi: 10.1093/cercor/bhq294
de la Monte, S. M., and Wands, J. R. (2008). Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol . 2, 1101–1113. doi: 10.1177/193229680800200619
* 5,6,a Dennis, N. A., and Cabeza, R. (2011). Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol. Aging 32, 2318.e17–30. doi: 10.1016/j.neurobiolaging.2010.04.004
* 5,6,a Dennis, N. A., Daselaar, S., and Cabeza, R. (2007a). Effects of aging on transient and sustained successful memory encoding activity. Neurobiol. Aging 28, 1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006
* 5,6,a Dennis, N. A., Hayes, S. M., Prince, S. E., Madden, D. J., Huettel, S. A., and Cabeza, R. (2008a). Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn. Mem. Cogn . 34, 791–808. doi: 10.1037/0278-7393.34.4.791
* 5,6,a Dennis, N. A., Kim, H., and Cabeza, R. (2008b). Age-related differences in brain activity during true and false memory retrieval. J. Cogn. Neurosci . 20, 1390–1402. doi: 10.1162/jocn.2008.20096
* 5,6,a Dennis, N. A., Kim, H., and Cabeza, R. (2007b). Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia 45, 3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003.
D'Esposito, M., Deouell, L. Y., and Gazzaley, A. (2003). Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci . 4, 863–872. doi: 10.1038/nrn1246
D'Esposito, M., Zarahn, E., Aguirre, G. K., and Rypma, B. (1999). The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 10, 6–14. doi: 10.1006/nimg.1999.0444
* 5,6,b,• Dickerson, B. C., Salat, D. H., Greve, D. N., Chua, E. F., Rand-Giovannetti, E., Rentz, D. M. et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. doi: 10.1212/01.wnl.0000171450.97464.49
* 5,6,a,× DiGirolamo, G. J., Kramer, A. F., Barad, V., Cepeda, N. J., Weissman, D. H., Milham, M. P. et al. (2001). General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport 12, 2065–2071. doi: 10.1097/00001756-200107030-00054
* 1,6,b,■ Donix, M., Poettrich, K., Weiss, P. H., Werner, A., von Kummer, R., Fink, G. R. et al. (2010). Age-dependent differences in the neural mechanisms supporting long-term declarative memories. Arch. Clin. Neuropsychol . 25, 383–395. doi: 10.1093/arclin/acq037
* 5,6,a Drobyshevsky, A., Baumann, S. B., and Schneider, W. (2006). A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage 31, 732–744. doi: 10.1016/j.neuroimage.2005.12.016
Du, X. L., Edelstein, D., Rossetti, L., Fantus, I. G., Goldberg, H., Ziyadeh, F. et al. (2000). Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. U.S.A . 97, 12222–12226. doi: 10.1073/pnas.97.22.12222
* 1,2,a,■ Duarte, A., Graham, K. S., and Henson, R. N. (2010). Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiol. Aging 31, 1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014
* 1,2,a,■ Duarte, A., Henson, R. N., and Graham, K. S. (2008). The effects of aging on the neural correlates of subjective and objective recollection. Cereb. Cortex 18, 2169–2180. doi: 10.1093/cercor/bhm243
* 3,5,a Dulas, M. R., and Duarte, A. (2011). The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage 57, 1192–1204. doi: 10.1016/j.neuroimage.2011.05.036
* 3,5,a Dulas, M. R., and Duarte, A. (2012). The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cereb. Cortex 22, 37–50. doi: 10.1093/cercor/bhr056
* 7,9,a Duverne, S., Motamedinia, S., and Rugg, M. D. (2009). The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb. Cortex 19, 733–744. doi: 10.1093/cercor/bhn122
Efimova, I. Y., Efimova, N. Y., Triss, S. V., and Lishmanov, Y. B. (2008). Brain perfusion and cognitive function changes in hypertensive patients. Hypertens. Res . 31, 673–678. doi: 10.1291/hypres.31.673
* 1,2,a Emery, L., Heaven, T. J., Paxton, J. L., and Braver, T. S. (2008). Age-related changes in neural activity during performance matched working memory manipulation. Neuroimage 42, 1577–1586. doi: 10.1016/j.neuroimage.2008.06.021
* 7,8,a Erickson, K. I., Colcombe, S. J., Wadhwa, R., Bherer, L., Peterson, M. S., Scalf, P. E. et al. (2007). Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol. Aging 28, 272–283. doi: 10.1016/j.neurobiolaging.2005.12.012
Espeland, M. A., Bryan, R. N., Goveas, J. S., Robinson, J. G., Siddiqui, M. S., Liu, S. et al. (2013). Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women's Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 36, 90–97. doi: 10.2337/dc12-0555
Espeland, M. A., Miller, M. E., Goveas, J. S., Hogan, P. E., Coker, L. H., Williamson, J. et al. (2011). Cognitive function and fine motor speed in older women with diabetes mellitus: results from the women's health initiative study of cognitive aging. J. Womens. Health (Larchmt) . 20, 1435–1443. doi: 10.1089/jwh.2011.2812
Ettorre, E., Cerra, E., Marigliano, B., Vigliotta, M., Vulcano, A., Fossati, C. et al. (2012). Role of cardiovascular risk factors (CRF) in the patients with mild cognitive impairment (MCI). Arch. Gerontol. Geriatr . 54, 330–332. doi: 10.1016/j.archger.2011.04.025
* 5,6,a Fakhri, M., Sikaroodi, H., Maleki, F., Ali Oghabian, M., and Ghanaati, H. (2012). Age-related frontal hyperactivation observed across different working memory tasks: an fMRI study. Behav. Neurol . 25, 351–361. doi: 10.3233/BEN-2012-120280
* 4,a,• Fera, F., Weickert, T. W., Goldberg, T. E., Tessitore, A., Hariri, A., Das, S. et al. (2005). Neural mechanisms underlying probabilistic category learning in normal aging. J. Neurosci . 25, 11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005
* 7,9,a Fernandes, M. A., Pacurar, A., Moscovitch, M., and Grady, C. (2006). Neural correlates of auditory recognition under full and divided attention in younger and older adults. Neuropsychologia 44, 2452–2464. doi: 10.1016/j.neuropsychologia.2006.04.020
* 2,11,a,■,▴ Filippini, N., Ebmeier, K. P., MacIntosh, B. J., Trachtenberg, A. J., Frisoni, G. B., Wilcock, G. K. et al. (2011). Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 54, 602–610. doi: 10.1016/j.neuroimage.2010.08.009
* 2,12,a,■,• Filippini, N., Nickerson, L. D., Beckmann, C. F., Ebmeier, K. P., Frisoni, G. B., Matthews, P. M. et al. (2012). Age-related adaptations of brain function during a memory task are also present at rest. Neuroimage 59, 3821–3828. doi: 10.1016/j.neuroimage.2011.11.063
Fischer, A. L., de Frias, C. M., Yeung, S. E., and Dixon, R. A. (2009). Short-term longitudinal trends in cognitive performance in older adults with type 2 diabetes. J. Clin. Exp. Neuropsychol . 31, 809–822. doi: 10.1080/13803390802537636
Fontbonne, A., Berr, C., Ducimetière, P., and Alpérovitch, A. (2001). Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 24, 366–370. doi: 10.2337/diacare.24.2.366
* 3,5,a Gandini, D., Lemaire, P., Anton, J.-L., and Nazarian, B. (2008). Neural correlates of approximate quantification strategies in young and older adults: an fMRI study. Brain Res . 1246, 144–157. doi: 10.1016/j.brainres.2008.09.096
* 5,6,d,■ Garrett, D. D., Kovacevic, N., McIntosh, A. R., and Grady, C. L. (2011). The importance of being variable. J. Neurosci . 31, 4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011
* 3,5,a,□ Gazzaley, A., Cooney, J. W., Rissman, J., and D'Esposito, M. (2005). Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci . 8, 1298–1300. doi: 10.1038/nn1543
Giacco, F., and Brownlee, M. (2010). Oxidative stress and diabetic complications. Circ. Res . 107, 1058–1070. doi: 10.1161/CIRCRESAHA.110.223545
Gifford, K. A., Badaracco, M., Liu, D., Tripodis, Y., Gentile, A., Lu, Z. et al. (2013). Blood pressure and cognition among older adults: a meta-analysis. Arch. Clin. Neuropsychol . 28, 649–664. doi: 10.1093/arclin/act046
* 7,8,a,■ Gigi, A., Babai, R., Penker, A., Hendler, T., and Korczyn, A. D. (2010). Prefrontal compensatory mechanism may enable normal semantic memory performance in mild cognitive impairment (MCI). J. Neuroimaging 20, 163–168. doi: 10.1111/j.1552-6569.2009.00386.x
Giordano, N., Tikhonoff, V., Palatini, P., Bascelli, A., Boschetti, G., De Lazzari, F. et al. (2012). Cognitive functions and cognitive reserve in relation to blood pressure components in a population-based cohort aged 53 to 94 years. Int. J. Hypertens . 2012:274851. doi: 10.1155/2012/274851
* 5,6,a,+ Giovanello, K. S., De Brigard, F., Hennessey Ford, J., Kaufer, D. I., Burke, J. R., Browndyke, J. N. et al. (2012). Event-related functional magnetic resonance imaging changes during relational retrieval in normal aging and amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc . 18, 886–897. doi: 10.1017/S1355617712000689
* 5,6,a Giovanello, K. S., Kensinger, E. A., Wong, A. T., and Schacter, D. L. (2010). Age-related neural changes during memory conjunction errors. J. Cogn. Neurosci . 22, 1348–1361. doi: 10.1162/jocn.2009.21274
Gold, S. M., Dziobek, I., Sweat, V., Tirsi, A., Rogers, K., Bruehl, H. et al. (2007). Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50, 711–719. doi: 10.1007/s00125-007-0602-7
* 5,6,b Gold, B. T., Jiang, Y., Jicha, G. A., and Smith, C. D. (2010a). Functional response in ventral temporal cortex differentiates mild cognitive impairment from normal aging. Hum. Brain Mapp . 31, 1249–1259. doi: 10.1002/hbm.20932
* 2,7,d,■ Gold, B. T., Powell, D. K., Xuan, L., Jicha, G. A., and Smith, C. D. (2010b). Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol. Aging 31, 512–522. doi: 10.1016/j.neurobiolaging.2008.04.005
Goldstein, F. C., Levey, A. I., and Steenland, N. K. (2013). High blood pressure and cognitive decline in mild cognitive impairment. J. Am. Geriatr. Soc . 61, 67–73. doi: 10.1111/jgs.12067
Gorelick, P. B., Scuteri, A., Black, S. E., DeCarli, C., Greenberg, S. M., Iadecola, C. et al. (2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. doi: 10.1161/STR.0b013e3182299496
* 4, a,• Gould, R. L., Brown, R. G., Owen, A. M., Ffytche, D. H., and Howard, R. J. (2003). fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage 20, 1006–1019. doi: 10.1016/S1053-8119(03)00365-3
* 1,9,a Grady, C. L., Grigg, O., and Ng, C. (2012). Age differences in default and reward networks during processing of personally relevant information. Neuropsychologia 50, 1682–1697. doi: 10.1016/j.neuropsychologia.2012.03.024
* 5,6,d,■,• Grady, C. L., Protzner, A. B., Kovacevic, N., Strother, S. C., Afshin-Pour, B., Wojtowicz, M. et al. (2010). A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb. Cortex 20, 1432–1447. doi: 10.1093/cercor/bhp207
* 7,9,a,■,• Grady, C. L., Springer, M. V., Hongwanishkul, D., McIntosh, A. R., and Winocur, G. (2006). Age-related changes in brain activity across the adult lifespan. J. Cogn. Neurosci . 18, 227–241. doi: 10.1162/089892906775783705
Granic, I., Dolga, A. M., Nijholt, I. M., van Dijk, G., and Eisel, U. L. M. (2009). Inflammation and NF-kappaB in Alzheimer's disease and diabetes. J. Alzheimers Dis . 16, 809–821. doi: 10.3233/JAD-2009-0976
Gregg, E. W., Yaffe, K., Cauley, J. A., Rolka, D. B., Blackwell, T. L., Narayan, K. M. et al. (2000). Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch. Intern. Med . 160, 174–180. doi: 10.1001/archinte.160.2.174
* 5,6,c Grön, G., Bittner, D., Schmitz, B., Wunderlich, A. P., Tomczak, R., and Riepe, M. W. (2003). Variability in memory performance in aged healthy individuals: an fMRI study. Neurobiol. Aging 24, 453–462. doi: 10.1016/S0197-4580(02)00128-8
* 6,7,a,□ Grossman, M., Cooke, A., DeVita, C., Alsop, D., Detre, J., Chen, W. et al. (2002). Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage 15, 302–317. doi: 10.1006/nimg.2001.0971
Hajjar, I., Zhao, P., Alsop, D., and Novak, V. (2010). Hypertension and cerebral vasoreactivity: a continuous arterial spin labeling magnetic resonance imaging study. Hypertension 56, 859–864. doi: 10.1161/HYPERTENSIONAHA.110.160002
* 5,6,a Hampstead, B. M., Stringer, A. Y., Stilla, R. F., Amaraneni, A., and Sathian, K. (2011). Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia 49, 2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008
* 5,6,a Hampstead, B. M., Stringer, A. Y., Stilla, R. F., Giddens, M., and Sathian, K. (2012). Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus 22, 1652–1658. doi: 10.1002/hipo.22006
* 7,8,a Han, S. D., Arfanakis, K., Fleischman, D. A., Leurgans, S. E., Tuminello, E. R., Edmonds, E. C. et al. (2012a). Functional connectivity variations in mild cognitive impairment: associations with cognitive function. J. Int. Neuropsychol. Soc . 18, 39–48. doi: 10.1017/S1355617711001299
* 4, b Han, Y., Lui, S., Kuang, W., Lang, Q., Zou, L., and Jia, J. (2012b). Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS ONE 7:e28664. doi: 10.1371/journal.pone.0028664
Hannesdottir, K., Nitkunan, A., Charlton, R. A., Barrick, T. R., MacGregor, G. A., and Markus, H. S. (2009). Cognitive impairment and white matter damage in hypertension: a pilot study. Acta Neurol. Scand . 119, 261–268. doi: 10.1111/j.1600-0404.2008.01098.x
* 7,8,a Hartley, A. A., Jonides, J., and Sylvester, C.-Y. C. (2011). Dual-task processing in younger and older adults: similarities and differences revealed by fMRI. Brain Cogn . 75, 281–291. doi: 10.1016/j.bandc.2011.01.004
* 3,7,a,• Hedden, T., Van Dijk, K. R. A., Shire, E. H., Sperling, R. A., Johnson, K. A., and Buckner, R. L. (2012). Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb. Cortex 22, 1038–1051. doi: 10.1093/cercor/bhr172
Heni, M., Schöpfer, P., Peter, A., Sartorius, T., Fritsche, A., Synofzik, M. et al. (2013). Evidence for altered transport of insulin across the blood–brain barrier in insulin-resistant humans. Acta Diabetol . doi: 10.1007/s00592-013-0546-y. [Epub ahead of print].
* 5,9,b Holtzer, R., Rakitin, B. C., Steffener, J., Flynn, J., Kumar, A., and Stern, Y. (2009). Age effects on load-dependent brain activations in working memory for novel material. Brain Res . 1249, 148–161. doi: 10.1016/j.brainres.2008.10.009
Hoogenboom, W. S., Marder, T. J., Flores, V. L., Huisman, S., Eaton, H. P., Schneiderman, J. S. et al. (2014). Cerebral white matter integrity and resting-state functional connectivity in middle-aged patients with type 2 diabetes. Diabetes 63, 728–738. doi: 10.2337/db13-1219
* 5,6,a Hosseini, S. M. H., Rostami, M., Yomogida, Y., Takahashi, M., Tsukiura, T., and Kawashima, R. (2010). Aging and decision making under uncertainty: behavioral and neural evidence for the preservation of decision making in the absence of learning in old age. Neuroimage 52, 1514–1520. doi: 10.1016/j.neuroimage.2010.05.008
* 5,6,a Huang, C.-M., Polk, T. A., Goh, J. O., and Park, D. C. (2012). Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia 50, 55–66. doi: 10.1016/j.neuropsychologia.2011.10.022
* 5,6,a Hubert, V., Beaunieux, H., Chételat, G., Platel, H., Landeau, B., Viader, F. et al. (2009). Age-related changes in the cerebral substrates of cognitive procedural learning. Hum. Brain Mapp . 30, 1374–1386. doi: 10.1002/hbm.20605
* 4, c, • Iidaka, T., Sadato, N., Yamada, H., Murata, T., Omori, M., and Yonekura, Y. (2001). An fMRI study of the functional neuroanatomy of picture encoding in younger and older adults. Brain Res. Cogn. Brain Res . 11, 1–11. doi: 10.1016/S0926-6410(00)00058-6
* 1,2,b,♦ Jennings, J. R., van der Veen, F. M., and Meltzer, C. C. (2006). Verbal and spatial working memory in older individuals: a positron emission tomography study. Brain Res . 1092, 177–189. doi: 10.1016/j.brainres.2006.03.077
* 1,2,a Jimura, K., and Braver, T. S. (2010). Age-related shifts in brain activity dynamics during task switching. Cereb. Cortex 20, 1420–1431. doi: 10.1093/cercor/bhp206
* 5,6,a Jin, M., Pelak, V. S., and Cordes, D. (2012). Aberrant default mode network in subjects with amnestic mild cognitive impairment using resting-state functional MRI. Magn. Reson. Imaging 30, 48–61. doi: 10.1016/j.mri.2011.07.007
Johnson, E. L. (2012). Glycemic variability in type 2 diabetes mellitus: oxidative stress and macrovascular complications. Adv. Exp. Med. Biol . 771, 139–154.
* 5,6,a Johnson, M. K., Mitchell, K. J., Raye, C. L., and Greene, E. J. (2004). An age-related deficit in prefrontal cortical function associated with refreshing information. Psychol. Sci . 15, 127–132. doi: 10.1111/j.0963-7214.2004.01502009.x
Johnson, J. K., Pa, J., Boxer, A. L., Kramer, J. H., Freeman, K., and Yaffe, K. (2010). Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dement. Geriatr. Cogn. Disord . 30, 344–351. doi: 10.1159/000318836
* 9,11,a,■ Johnson, S. C., Schmitz, T. W., Asthana, S., Gluck, M. A., and Myers, C. (2008). Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn . 15, 129–145. doi: 10.1080/13825580601139444
* 10,12,c Jones, D. T., Machulda, M. M., Vemuri, P., McDade, E. M., Zeng, G., Senjem, M. L. et al. (2011). Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77, 1524–1531. doi: 10.1212/WNL.0b013e318233b33d
Kaiser, N., Sasson, S., Feener, E. P., Boukobza-Vardi, N., Higashi, S., Moller, D. E. et al. (1993). Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42, 80–89. doi: 10.2337/diab.42.1.80
Kalani, M. (2008). The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc. Health Risk Manag . 4, 1061–1068. doi: 10.2147/VHRM.S3920
* 5,6,a Kalkstein, J., Checksfield, K., Bollinger, J., and Gazzaley, A. (2011). Diminished top-down control underlies a visual imagery deficit in normal aging. J. Neurosci . 31, 15768–15774. doi: 10.1523/JNEUROSCI.3209-11.2011
Kamiyama, K., Wada, A., Sugihara, M., Kurioka, S., Hayashi, K., Hayashi, T. et al. (2010). Potential hippocampal region atrophy in diabetes mellitus type 2: a voxel-based morphometry VSRAD study. Jpn. J. Radiol . 28, 266–272. doi: 10.1007/s11604-009-0416-2
Kanaya, A. M., Barrett-Connor, E., Gildengorin, G., and Yaffe, K. (2004). Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch. Intern. Med . 164, 1327–1333. doi: 10.1001/archinte.164.12.1327
* 5,6,a,° Kannurpatti, S. S., Motes, M. A., Rypma, B., and Biswal, B. B. (2010). Neural and vascular variability and the fMRI-BOLD response in normal aging. Magn. Reson. Imaging 28, 466–476. doi: 10.1016/j.mri.2009.12.007
* 5,6,a,° Kannurpatti, S. S., Motes, M. A., Rypma, B., and Biswal, B. B. (2011). Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Hum. Brain Mapp . 32, 1125–1140. doi: 10.1002/hbm.21097
* 5,6,a Kaufmann, L., Ischebeck, A., Weiss, E., Koppelstaetter, F., Siedentopf, C., Vogel, S. E. et al. (2008). An fMRI study of the numerical Stroop task in individuals with and without minimal cognitive impairment. Cortex 44, 1248–1255. doi: 10.1016/j.cortex.2007.11.009
Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., and He, J. (2005). Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223. doi: 10.1016/S0140-6736(05)17741-1
Kennedy, K. M., and Raz, N. (2009). Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res . 1297, 41–56. doi: 10.1016/j.brainres.2009.08.058
* 7,8,a Kennedy, K. M., Rodrigue, K. M., Devous, M. D. Sr., Hebrank, A. C., Bischof, G. N., and Park, D. C. (2012). Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage 62, 1–8. doi: 10.1016/j.neuroimage.2012.03.077
Kerti, L., Witte, A. V., Winkler, A., Grittner, U., Rujescu, D., and Floel, A. (2013). Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 81, 1746–1752. doi: 10.1212/01.wnl.0000435561.00234.ee
* 9,11,a,■,× Kikuchi, M., Hirosawa, T., Yokokura, M., Yagi, S., Mori, N., Yoshikawa, E. et al. (2011). Effects of brain amyloid deposition and reduced glucose metabolism on the default mode of brain function in normal aging. J. Neurosci . 31, 11193–11199. doi: 10.1523/JNEUROSCI.2535-11.2011
Kim, E., Cho, M. H., Cha, K. R., Park, J. S., Ahn, C.-W., Oh, B. H. et al. (2008). Interactive effect of central obesity and hypertension on cognitive function in older out-patients with Type 2 diabetes. Diabet. Med . 25, 1440–1446. doi: 10.1111/j.1464-5491.2008.02612.x
* 6,7,a Kim, S.-Y., and Giovanello, K. S. (2011). The effects of attention on age-related relational memory deficits: fMRI evidence from a novel attentional manipulation. J. Cogn. Neurosci . 23, 3637–3656. doi: 10.1162/jocn_a_00058
* 4,b Kircher, T., Weis, S., Leube, D., Freymann, K., Erb, M., Jessen, F. et al. (2008). Anterior hippocampus orchestrates successful encoding and retrieval of non-relational memory: an event-related fMRI study. Eur. Arch. Psychiatry Clin. Neurosci . 258, 363–372. doi: 10.1007/s00406-008-0805-z
* 1,9,a Kirchhoff, B. A., Anderson, B. A., Barch, D. M., and Jacoby, L. L. (2012). Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb. Cortex 22, 788–799. doi: 10.1093/cercor/bhr129
Kitagawa, K., Oku, N., Kimura, Y., Yagita, Y., Sakaguchi, M., Hatazawa, J. et al. (2009). Relationship between cerebral blood flow and later cognitive decline in hypertensive patients with cerebral small vessel disease. Hypertens. Res . 32, 816–820. doi: 10.1038/hr.2009.100
Kloppenborg, R. P., van den Berg, E., Kappelle, L. J., and Biessels, G. J. (2008). Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur. J. Pharmacol . 585, 97–108. doi: 10.1016/j.ejphar.2008.02.049
* 2,5,a Klostermann, E. C., Braskie, M. N., Landau, S. M., O'Neil, J. P., and Jagust, W. J. (2012). Dopamine and frontostriatal networks in cognitive aging. Neurobiol. Aging 33, 623.e15–24. doi: 10.1016/j.neurobiolaging.2011.03.002
Knecht, S., Wersching, H., Lohmann, H., Berger, K., and Ringelstein, E. B. (2009). How much does hypertension affect cognition? Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J. Neurol. Sci . 283, 149–152. doi: 10.1016/j.jns.2009.02.362
Knecht, S., Wersching, H., Lohmann, H., Bruchmann, M., Duning, T., Dziewas, R. et al. (2008). High-normal blood pressure is associated with poor cognitive performance. Hypertension 51, 663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577
* 7,8,b,• Koch, W., Teipel, S., Mueller, S., Buerger, K., Bokde, A. L. W., Hampel, H. et al. (2010). Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage 51, 280–287. doi: 10.1016/j.neuroimage.2009.12.008
* 7,8,a Krause, J. B., Taylor, J. G., Schmidt, D., Hautzel, H., Mottaghy, F. M., and Müller-Gärtner, H. W. (2000). Imaging and neural modelling in episodic and working memory processes. Neural Netw . 13, 847–859
* 1,9,a Kühn, S., Schmiedek, F., Schott, B., Ratcliff, R., Heinze, H.-J., Düzel, E. et al. (2011). Brain areas consistently linked to individual differences in perceptual decision-making in younger as well as older adults before and after training. J. Cogn. Neurosci . 23, 2147–2158. doi: 10.1162/jocn.2010.21564
* 7,8,a Kukolja, J., Thiel, C. M., Wilms, M., Mirzazade, S., and Fink, G. R. (2009). Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiol. Aging 30, 630–645. doi: 10.1016/j.neurobiolaging.2007.08.015
* 6,7,a Kukolja, J., Thiel, C. M., Wolf, O. T., and Fink, G. R. (2008). Increased cortisol levels in cognitively challenging situations are beneficial in young but not older subjects. Psychopharmacology (Berl.) 201, 293–304. doi: 10.1007/s00213-008-1275-8
* 1,2,a,• Lamar, M., Yousem, D. M., and Resnick, S. M. (2004). Age differences in orbitofrontal activation: an fMRI investigation of delayed match and nonmatch to sample. Neuroimage 21, 1368–1376. doi: 10.1016/j.neuroimage.2003.11.018
* 7,8,a Langenecker, S. A., Briceno, E. M., Hamid, N. M., and Nielson, K. A. (2007). An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Res . 1135, 58–68. doi: 10.1016/j.brainres.2006.11.068
* 5,6,a Langenecker, S. A., and Nielson, K. A. (2003). Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage 20, 1384–1392. doi: 10.1016/S1053-8119(03)00372-0
* 5,6,a Langenecker, S. A., Nielson, K. A., and Rao, S. M. (2004). fMRI of healthy older adults during Stroop interference. Neuroimage 21, 192–200. doi: 10.1016/j.neuroimage.2003.08.027
Last, D., Alsop, D. C., Abduljalil, A. M., Marquis, R. P., de Bazelaire, C., Hu, K. et al. (2007). Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 30, 1193–1199. doi: 10.2337/dc06-2052
Launer, L. J., Ross, G. W., Petrovitch, H., Masaki, K., Foley, D., White, L. R. et al. (2000). Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol. Aging 21, 49–55. doi: 10.1016/S0197-4580(00)00096-8
Lee, J. H., Yoon, S., Renshaw, P. F., Kim, T.-S., Jung, J. J., Choi, Y. et al. (2013). Morphometric changes in lateral ventricles of patients with recent-onset type 2 diabetes mellitus. PLoS ONE 8:e60515. doi: 10.1371/journal.pone.0060515
* 5,6,a Leshikar, E. D., Gutchess, A. H., Hebrank, A. C., Sutton, B. P., and Park, D. C. (2010). The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex 46, 507–521. doi: 10.1016/j.cortex.2009.07.011
* 5,6,a Li, C., Zheng, J., Wang, J., Gui, L., and Li, C. (2009a). An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer's disease. Curr. Alzheimer Res . 6, 525–530. doi: 10.2174/156720509790147142
Li, J., Wang, Y. J., Zhang, M., Xu, Z. Q., Gao, C. Y., Fang, C. Q. et al. (2011). Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76, 1485–1491. doi: 10.1212/WNL.0b013e318217e7a4
* 7,8,a Li, Z., Moore, A. B., Tyner, C., and Hu, X. (2009b). Asymmetric connectivity reduction and its relationship to “HAROLD” in aging brain. Brain Res . 1295, 149–158. doi: 10.1016/j.brainres.2009.08.004
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., and Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. doi: 10.1038/35084005
Luchsinger, J. A. (2008). Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur. J. Pharmacol . 585, 119–129. doi: 10.1016/j.ejphar.2008.02.048
Luchsinger, J. A., Palmas, W., Teresi, J. A., Silver, S., Kong, J., Eimicke, J. P. et al. (2011). Improved diabetes control in the elderly delays global cognitive decline. J. Nutr. Health Aging 15, 445–449. doi: 10.1007/s12603-011-0057-x
* 3,5,a MacDonald, S. W. S., Nyberg, L., Sandblom, J., Fischer, H., and Bäckman, L. (2008). Increased response-time variability is associated with reduced inferior parietal activation during episodic recognition in aging. J. Cogn. Neurosci . 20, 779–786. doi: 10.1162/jocn.2008.20502
* 2,5,a,■ Madden, D. J., Costello, M. C., Dennis, N. A., Davis, S. W., Shepler, A. M., Spaniol, J. et al. (2010). Adult age differences in functional connectivity during executive control. Neuroimage 52, 643–657. doi: 10.1016/j.neuroimage.2010.04.249
* 1,2,d,■ Madden, D. J., Langley, L. K., Denny, L. L., Turkington, T. G., Provenzale, J. M., Hawk, T. C. et al. (2002a). Adult age differences in visual word identification: functional neuroanatomy by positron emission tomography. Brain Cogn . 49, 297–321. doi: 10.1006/brcg.2001.1502
* 2,5,d,■ Madden, D. J., Spaniol, J., Whiting, W. L., Bucur, B., Provenzale, J. M., Cabeza, R. et al. (2007). Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol. Aging 28, 459–476. doi: 10.1016/j.neurobiolaging.2006.01.005
* 1,2,d,■ Madden, D. J., Turkington, T. G., Provenzale, J. M., Denny, L. L., Langley, L. K., Hawk, T. C. et al. (2002b). Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol. Aging 17, 24–43. doi: 10.1037/0882-7974.17.1.24
Maggi, S., Limongi, F., Noale, M., Romanato, G., Tonin, P., Rozzini, R. et al. (2009). Diabetes as a risk factor for cognitive decline in older patients. Dement. Geriatr. Cogn. Disord . 27, 24–33. doi: 10.1159/000183842
* 1,9,a Maillet, D., and Rajah, M. N. (2011). Age-related changes in the three-way correlation between anterior hippocampus volume, whole-brain patterns of encoding activity and subsequent context retrieval. Brain Res . 1420, 68–79. doi: 10.1016/j.brainres.2011.08.071
Marioni, R. E., Strachan, M. W. J., Reynolds, R. M., Lowe, G. D. O., Mitchell, R. J., Fowkes, F. G. R. et al. (2010). Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 59, 710–713. doi: 10.2337/db09-1163
* 3,5,b Mathis, A., Schunck, T., Erb, G., Namer, I. J., and Luthringer, R. (2009). The effect of aging on the inhibitory function in middle-aged subjects: a functional MRI study coupled with a color-matched Stroop task. Int. J. Geriatr. Psychiatry 24, 1062–1071. doi: 10.1002/gps.2222
* 4,a Mattay, V. S., Fera, F., Tessitore, A., Hariri, A. R., Berman, K. F., Das, S. et al. (2006). Neurophysiological correlates of age-related changes in working memory capacity. Neurosci. Lett . 392, 32–37. doi: 10.1016/j.neulet.2005.09.025
* 1,2,a,• Matthäus, F., Schmidt, J.-P., Banerjee, A., Schulze, T. G., Demirakca, T., and Diener, C. (2012). Effects of age on the structure of functional connectivity networks during episodic and working memory demand. Brain Connect . 2, 113–124. doi: 10.1089/brain.2012.0077
* 7,8,a,° Mayhew, S. D., Li, S., Storrar, J. K., Tsvetanov, K. A., and Kourtzi, Z. (2010). Learning shapes the representation of visual categories in the aging human brain. J. Cogn. Neurosci . 22, 2899–2912. doi: 10.1162/jocn.2010.21415
* 7,8,a,□ McGeown, W. J., Shanks, M. F., Forbes-McKay, K. E., and Venneri, A. (2009). Patterns of brain activity during a semantic task differentiate normal aging from early Alzheimer's disease. Psychiatry Res . 173, 218–227. doi: 10.1016/j.pscychresns.2008.10.005
* 5,6,a Meier, T. B., Desphande, A. S., Vergun, S., Nair, V. A., Song, J., Biswal, B. B. et al. (2012). Support vector machine classification and characterization of age-related reorganization of functional brain networks. Neuroimage 60, 601–613. doi: 10.1016/j.neuroimage.2011.12.052
* 7,9,c Meinzer, M., Flaisch, T., Seeds, L., Harnish, S., Antonenko, D., Witte, V. et al. (2012a). Same modulation but different starting points: performance modulates age differences in inferior frontal cortex activity during word-retrieval. PLoS ONE 7:e33631. doi: 10.1371/journal.pone.0033631
* 5,6,d Meinzer, M., Flaisch, T., Wilser, L., Eulitz, C., Rockstroh, B., Conway, T. et al. (2009). Neural signatures of semantic and phonemic fluency in young and old adults. J. Cogn. Neurosci . 21, 2007–2018. doi: 10.1162/jocn.2009.21219
* 7,9,c Meinzer, M., Seeds, L., Flaisch, T., Harnish, S., Cohen, M. L., McGregor, K. et al. (2012b). Impact of changed positive and negative task-related brain activity on word-retrieval in aging. Neurobiol. Aging 33, 656–669. doi: 10.1016/j.neurobiolaging.2010.06.020
Messier, C., Awad-Shimoon, N., Gagnon, M., Desrochers, A., and Tsiakas, M. (2011). Glucose regulation is associated with cognitive performance in young nondiabetic adults. Behav. Brain Res . 222, 81–88. doi: 10.1016/j.bbr.2011.03.023
Messier, C., Tsiakas, M., Gagnon, M., and Desrochers, A. (2010). Effect of age and glucoregulation on cognitive performance. J. Clin. Exp. Neuropsychol . 32, 809–821. doi: 10.1080/13803390903540323
* 7,9,a Meulenbroek, O., Kessels, R. P. C., de Rover, M., Petersson, K. M., Rikkert, M. G. M. O., Rijpkema, M. et al. (2010a). Age-effects on associative object-location memory. Brain Res . 1315, 100–110. doi: 10.1016/j.brainres.2009.12.011
* 1,2,a Meulenbroek, O., Petersson, K. M., Voermans, N., Weber, B., and Fernández, G. (2004). Age differences in neural correlates of route encoding and route recognition. Neuroimage 22, 1503–1514. doi: 10.1016/j.neuroimage.2004.04.007
* 7,8,a Meulenbroek, O., Rijpkema, M., Kessels, R. P. C., Rikkert, M. G. M. O., and Fernández, G. (2010b). Autobiographical memory retrieval in patients with Alzheimer's disease. Neuroimage 53, 331–340. doi: 10.1016/j.neuroimage.2010.05.082
* 7,8,b,■ Miettinen, P. S., Pihlajamäki, M., Jauhiainen, A. M., Niskanen, E., Hänninen, T., Vanninen, R. et al. (2011). Structure and function of medial temporal and posteromedial cortices in early Alzheimer's disease. Eur. J. Neurosci . 34, 320–330. doi: 10.1111/j.1460-9568.2011.07745.x
* 3,7,a,• Milham, M. P., Erickson, K. I., Banich, M. T., Kramer, A. F., Webb, A., Wszalek, T. et al. (2002). Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn . 49, 277–296. doi: 10.1006/brcg.2001.1501
* 5,6,a Mitchell, K. J., Johnson, M. K., Raye, C. L., and D'Esposito, M. (2000). fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res. Cogn. Brain Res . 10, 197–206. doi: 10.1016/S0926-6410(00)00029-X
* 7,8,a Mitchell, K. J., Johnson, M. R., Higgins, J. A., and Johnson, M. K. (2010). Age differences in brain activity during perceptual versus reflective attention. Neuroreport 21, 293–297. doi: 10.1097/WNR.0b013e32833730d6
* 7,8,a Mitchell, K. J., Raye, C. L., Johnson, M. K., and Greene, E. J. (2006). An fMRI investigation of short-term source memory in young and older adults. Neuroimage 30, 627–633. doi: 10.1016/j.neuroimage.2005.09.039
* 1,2,a,▴ Mohtasib, R. S., Lumley, G., Goodwin, J. A., Emsley, H. C. A., Sluming, V., and Parkes, L. M. (2012). Calibrated fMRI during a cognitive Stroop task reveals reduced metabolic response with increasing age. Neuroimage 59, 1143–1151. doi: 10.1016/j.neuroimage.2011.07.092
Moran, C., Phan, T. G., Chen, J., Blizzard, L., Beare, R., Venn, A. et al. (2013). Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36, 4036–4042. doi: 10.2337/dc13-0143
* 3,5,a Morcom, A. M., and Friston, K. J. (2012). Decoding episodic memory in ageing: a Bayesian analysis of activity patterns predicting memory. Neuroimage 59, 1772–1782. doi: 10.1016/j.neuroimage.2011.08.071
* 3,5,a,□ Morcom, A. M., Good, C. D., Frackowiak, R. S. J., and Rugg, M. D. (2003). Age effects on the neural correlates of successful memory encoding. Brain 126, 213–229. doi: 10.1093/brain/awg020
* 5,6,a Mormino, E. C., Brandel, M. G., Madison, C. M., Marks, S., Baker, S. L., and Jagust, W. J. (2012). Aβ Deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb. Cortex 22, 1813–1823. doi: 10.1093/cercor/bhr255
* 5,6,a Mormino, E. C., Smiljic, A., Hayenga, A. O., Onami, S. H., Greicius, M. D., Rabinovici, G. D. et al. (2011). Relationships between β-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex 21, 2399–2407. doi: 10.1093/cercor/bhr025
Morris, J. K., Vidoni, E. D., Honea, R. A., and Burns, J. M. (2014). Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol. Aging 35, 585–589. doi: 10.1016/j.neurobiolaging.2013.09.033
* 5,6,a Mowinckel, A. M., Espeseth, T., and Westlye, L. T. (2012). Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. Neuroimage 63, 1364–1373. doi: 10.1016/j.neuroimage.2012.08.004
* 5,6,a Murphy, K., and Garavan, H. (2004). Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage 21, 219–228. doi: 10.1016/j.neuroimage.2003.09.016
* 3,7,b Murty, V. P., Sambataro, F., Das, S., Tan, H.-Y., Callicott, J. H., Goldberg, T. E. et al. (2009). Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J. Cogn. Neurosci . 21, 1920–1933. doi: 10.1162/jocn.2009.21130
Musen, G., Jacobson, A. M., Bolo, N. R., Simonson, D. C., Shenton, M. E., McCartney, R. L. et al. (2012). Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61, 2375–2379. doi: 10.2337/db11-1669
Nagai, M., Hoshide, S., Ishikawa, J., Shimada, K., and Kario, K. (2008). Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J. Hypertens . 26, 1636–1641. doi: 10.1097/HJH.0b013e3283018333
* 7,8,a Nagel, I. E., Preuschhof, C., Li, S.-C., Nyberg, L., Bäckman, L., Lindenberger, U. et al. (2009). Performance level modulates adult age differences in brain activation during spatial working memory. Proc. Natl. Acad. Sci. U.S.A . 106, 22552–22557. doi: 10.1073/pnas.0908238106
* 10,12,a Nagel, I. E., Preuschhof, C., Li, S.-C., Nyberg, L., Bäckman, L., Lindenberger, U. et al. (2011). Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J. Cogn. Neurosci . 23, 2030–2045. doi: 10.1162/jocn.2010.21560
Nakae, J., Kido, Y., and Accili, D. (2001). Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev . 22, 818–835. doi: 10.1210/edrv.22.6.0452
Newcomer, J. W., and Haupt, D. W. (2006). The metabolic effects of antipsychotic medications. Can. J. Psychiatry 51, 480–491.
* 4, b, ■ Nichols, L. M., Masdeu, J. C., Mattay, V. S., Kohn, P., Emery, M., Sambataro, F. et al. (2012). Interactive effect of apolipoprotein e genotype and age on hippocampal activation during memory processing in healthy adults. Arch. Gen. Psychiatry 69, 804–813. doi: 10.1001/archgenpsychiatry.2011.1893
* 7,8,a,• Nielson, K. A., Douville, K. L., Seidenberg, M., Woodard, J. L., Miller, S. K., Franczak, M. et al. (2006). Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol. Aging 27, 1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022
* 5,6,a Nielson, K. A., Langenecker, S. A., and Garavan, H. (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol. Aging 17, 56–71. doi: 10.1037/0882-7974.17.1.56
* 3,5,a,□ Nielson, K. A., Langenecker, S. A., Ross, T. J., Garavan, H., Rao, S. M., and Stein, E. A. (2004). Comparability of functional MRI response in young and old during inhibition. Neuroreport 15, 129–133. doi: 10.1097/00001756-200401190-00025
Nishikawa, T., Edelstein, D., Du, X. L., Yamagishi, S., Matsumura, T., Kaneda, Y. et al. (2000). Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790. doi: 10.1038/35008121
Nooyens, A. C. J., Baan, C. A., Spijkerman, A. M. W., and Verschuren, W. M. M. (2010). Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care 33, 1964–1969. doi: 10.2337/dc09-2038
* 7,10,c,❖ Nordahl, C. W., Ranganath, C., Yonelinas, A. P., Decarli, C., Fletcher, E., and Jagust, W. J. (2006). White matter changes compromise prefrontal cortex function in healthy elderly individuals. J. Cogn. Neurosci . 18, 418–429. doi: 10.1162/089892906775990552
Novak, V., Last, D., Alsop, D. C., Abduljalil, A. M., Hu, K., Lepicovsky, L. et al. (2006). Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 29, 1529–1534. doi: 10.2337/dc06-0261
* 5,6,a Nyberg, L., Dahlin, E., Stigsdotter Neely, A., and Bäckman, L. (2009). Neural correlates of variable working memory load across adult age and skill: dissociative patterns within the fronto-parietal network. Scand. J. Psychol . 50, 41–46. doi: 10.1111/j.1467-9450.2008.00678.x
* 7,8,a,• O'Brien, J. L., O'Keefe, K. M., LaViolette, P. S., DeLuca, A. N., Blacker, D., Dickerson, B. C. et al. (2010). Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74, 1969–1976. doi: 10.1212/WNL.0b013e3181e3966e
* 7,8,a Osaka, M., Otsuka, Y., and Osaka, N. (2012a). Verbal to visual code switching improves working memory in older adults: an fMRI study. Front. Hum. Neurosci . 6:24. doi: 10.3389/fnhum.2012.00024
* 7,8,a Osaka, M., Yaoi, K., Otsuka, Y., Katsuhara, M., and Osaka, N. (2012b). Practice on conflict tasks promotes executive function of working memory in the elderly. Behav. Brain Res . 233, 90–98. doi: 10.1016/j.bbr.2012.04.044
* 7,8,a Otsuka, Y., Osaka, N., Morishita, M., Kondo, H., and Osaka, M. (2006). Decreased activation of anterior cingulate cortex in the working memory of the elderly. Neuroreport 17, 1479–1482. doi: 10.1097/01.wnr.0000236852.63092.9f
Oveisgharan, S., and Hachinski, V. (2010). Hypertension, executive dysfunction, and progression to dementia: the canadian study of health and aging. Arch. Neurol . 67, 187–192. doi: 10.1001/archneurol.2009.312
* 6,7,a Pacheco, J., Beevers, C. G., McGeary, J. E., and Schnyer, D. M. (2012). Memory monitoring performance and PFC activity are associated with 5-HTTLPR genotype in older adults. Neuropsychologia 50, 2257–2270. doi: 10.1016/j.neuropsychologia.2012.05.030
* 7,8,a Park, D. C., Welsh, R. C., Marshuetz, C., Gutchess, A. H., Mikels, J., Polk, T. A. et al. (2003). Working memory for complex scenes: age differences in frontal and hippocampal activations. J. Cogn. Neurosci . 15, 1122–1134. doi: 10.1162/089892903322598094
* 4, a, □ Park, J., Carp, J., Hebrank, A., Park, D. C., and Polk, T. A. (2010). Neural specificity predicts fluid processing ability in older adults. J. Neurosci . 30, 9253–9259. doi: 10.1523/JNEUROSCI.0853-10.2010
* 1,2,a Paxton, J. L., Barch, D. M., Racine, C. A., and Braver, T. S. (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb. Cortex 18, 1010–1028. doi: 10.1093/cercor/bhm135
* 1,9,b Persson, J., Kalpouzos, G., Nilsson, L.-G., Ryberg, M., and Nyberg, L. (2011). Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus 21, 753–766. doi: 10.1002/hipo.20794
* 7,10,a,+ Persson, J., Nyberg, L., Lind, J., Larsson, A., Nilsson, L.-G., Ingvar, M. et al. (2006). Structure-function correlates of cognitive decline in aging. Cereb. Cortex 16, 907–915. doi: 10.1093/cercor/bhj036
* 7,10,c,♦ Persson, J., Pudas, S., Lind, J., Kauppi, K., Nilsson, L.-G., and Nyberg, L. (2012). Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb. Cortex 22, 2297–2304. doi: 10.1093/cercor/bhr306
* 2,5,a Persson, J., Sylvester, C.-Y. C., Nelson, J. K., Welsh, K. M., Jonides, J., and Reuter-Lorenz, P. A. (2004). Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage 23, 1382–1390. doi: 10.1016/j.neuroimage.2004.08.004
* 7,8,a Petrella, J. R., Townsend, B. A., Jha, A. P., Ziajko, L. A., Slavin, M. J., Lustig, C. et al. (2005). Increasing memory load modulates regional brain activity in older adults as measured by fMRI. J. Neuropsychiatry Clin. Neurosci . 17, 75–83. doi: 10.1176/appi.neuropsych.17.1.75
* 5,6,a Piefke, M., Onur, Ö. A., and Fink, G. R. (2012). Aging-related changes of neural mechanisms underlying visual-spatial working memory. Neurobiol. Aging 33, 1284–1297. doi: 10.1016/j.neurobiolaging.2010.10.014
* 7,8,a Pihlajamäki, M., and Sperling, R. A. (2009). Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer's disease and at-risk older individuals. Behav. Neurol . 21, 77–91. doi: 10.3233/BEN-2009-0231
Pires, P. W., Dams Ramos, C. M., Matin, N., and Dorrance, A. M. (2013). The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol . 304, H1598–H1614. doi: 10.1152/ajpheart.00490.2012
* 4, c Podell, J. E., Sambataro, F., Murty, V. P., Emery, M. R., Tong, Y., Das, S. et al. (2012). Neurophysiological correlates of age-related changes in working memory updating. Neuroimage 62, 2151–2160. doi: 10.1016/j.neuroimage.2012.05.066
* 7,8,a Prakash, R. S., Erickson, K. I., Colcombe, S. J., Kim, J. S., Voss, M. W., and Kramer, A. F. (2009). Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn . 71, 328–335. doi: 10.1016/j.bandc.2009.07.005
* 7,8,a Protzner, A. B., Mandzia, J. L., Black, S. E., and McAndrews, M. P. (2011). Network interactions explain effective encoding in the context of medial temporal damage in MCI. Hum. Brain Mapp . 32, 1277–1289. doi: 10.1002/hbm.21107
* 7,8,a,• Putcha, D., O'Keefe, K., LaViolette, P., O'Brien, J., Greve, D., Rentz, D. M. et al. (2011). Reliability of functional magnetic resonance imaging associative encoding memory paradigms in non-demented elderly adults. Hum. Brain Mapp . 32, 2027–2044. doi: 10.1002/hbm.21166
* 1,9,d Rajah, M. N., Languay, R., and Grady, C. L. (2011). Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J. Neurosci . 31, 17941–17954. doi: 10.1523/JNEUROSCI.1690-11.2011
* 7,8,a,• Rajah, M. N., Languay, R., and Valiquette, L. (2010). Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex 46, 535–549. doi: 10.1016/j.cortex.2009.07.006
* 5,6,a,□ Rajah, M. N., and McIntosh, A. R. (2008). Age-related differences in brain activity during verbal recency memory. Brain Res . 1199, 111–125. doi: 10.1016/j.brainres.2007.12.051
Raji, C. A., Lopez, O. L., Kuller, L. H., Carmichael, O. T., Longstreth, W. T. Jr., Gach, H. M. et al. (2012). White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol. Aging 33, 834.e7–16. doi: 10.1016/j.neurobiolaging.2011.08.010
* 2,7,d,▴ Ramsøy, T. Z., Liptrot, M. G., Skimminge, A., Lund, T. E., Sidaros, K., Christensen, M. S. et al. (2012). Healthy aging attenuates task-related specialization in the human medial temporal lobe. Neurobiol. Aging 33, 1874–1889. doi: 10.1016/j.neurobiolaging.2011.09.032
* 6,7,a Rand-Giovannetti, E., Chua, E. F., Driscoll, A. E., Schacter, D. L., Albert, M. S., and Sperling, R. A. (2006). Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol. Aging 27, 173–182. doi: 10.1016/j.neurobiolaging.2004.12.013
Ravona-Springer, R., Moshier, E., Schmeidler, J., Godbold, J., Akrivos, J., Rapp, M. et al. (2012). Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J. Alzheimers Dis . 30, 299–309. doi: 10.3233/JAD-2012-120106
* 5,6,a Raye, C. L., Mitchell, K. J., Reeder, J. A., Greene, E. J., and Johnson, M. K. (2008). Refreshing one of several active representations: behavioral and functional magnetic resonance imaging differences between young and older adults. J. Cogn. Neurosci . 20, 852–862. doi: 10.1162/jocn.2008.20508
Raz, N., Yang, Y., Dahle, C. L., and Land, S. (2012). Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim. Biophys. Acta 1822, 361–369. doi: 10.1016/j.bbadis.2011.08.007
Reaven, G. M., Thompson, L. W., Nahum, D., and Haskins, E. (1990). Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care 13, 16–21. doi: 10.2337/diacare.13.1.16
Regenold, W. T., Thapar, R. K., Marano, C., Gavirneni, S., and Kondapavuluru, P. V. (2002). Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J. Affect. Disord . 70, 19–26. doi: 10.1016/S0165-0327(01)00456-6
Reijmer, Y. D., van den Berg, E., de Bresser, J., Kessels, R. P. C., Kappelle, L. J., Algra, A. et al. (2011). Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab. Res. Rev . 27, 195–202. doi: 10.1002/dmrr.1163
Reitz, C., Tang, M.-X., Manly, J., Mayeux, R., and Luchsinger, J. A. (2007). Hypertension and the risk of mild cognitive impairment. Arch. Neurol . 64, 1734–1740. doi: 10.1001/archneur.64.12.1734
* 5,6,a,▴ Restom, K., Bangen, K. J., Bondi, M. W., Perthen, J. E., and Liu, T. T. (2007). Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage 37, 430–439. doi: 10.1016/j.neuroimage.2007.05.024
* 7,8,a Rieckmann, A., Fischer, H., and Bäckman, L. (2010). Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. Neuroimage 50, 1303–1312. doi: 10.1016/j.neuroimage.2010.01.015
* 7,8,a Rieckmann, A., Karlsson, S., Fischer, H., and Bäckman, L. (2011). Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J. Neurosci . 31, 14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011
Rizzo, M. R., Marfella, R., Barbieri, M., Boccardi, V., Vestini, F., Lettieri, B. et al. (2010). Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 33, 2169–2174. doi: 10.2337/dc10-0389
Roberts, R. O., Knopman, D. S., Geda, Y. E., Cha, R. H., Pankratz, V. S., Baertlein, L. et al. (2014). Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers. Dement . 10, 18–26. doi: 10.1016/j.jalz.2013.01.001
* 5,6,a Rombouts, S. A. R. B., Barkhof, F., Goekoop, R., Stam, C. J., and Scheltens, P. (2005a). Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp . 26, 231–239. doi: 10.1002/hbm.20160
* 5,6,a,• Rombouts, S. A. R. B., Goekoop, R., Stam, C. J., Barkhof, F., and Scheltens, P. (2005b). Delayed rather than decreased BOLD response as a marker for early Alzheimer's disease. Neuroimage 26, 1078–1085. doi: 10.1016/j.neuroimage.2005.03.022
* 7,10,b,❖ Rosano, C., Venkatraman, V. K., Guralnik, J., Newman, A. B., Glynn, N. W., Launer, L. et al. (2010). Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J. Gerontol. A Biol. Sci. Med. Sci . 65, 639–647. doi: 10.1093/gerona/glq038
* 3,5,a Rosen, A. C., Gabrieli, J. D. E., Stoub, T., Prull, M. W., O'Hara, R., Yesavage, J. et al. (2005). Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex 41, 595–602. doi: 10.1016/S0010-9452(08)70199-0
Ryan, C. M., and Geckle, M. O. (2000). Circumscribed cognitive dysfunction in middle-aged adults with type 2 diabetes. Diabetes Care 23, 1486–1493. doi: 10.2337/diacare.23.10.1486
* 4, b Rypma, B., Berger, J. S., Genova, H. M., Rebbechi, D., and D'Esposito, M. (2005). Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex 41, 582–594. doi: 10.1016/S0010-9452(08)70198-9
* 4, b Rypma, B., Eldreth, D. A., and Rebbechi, D. (2007). Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex 43, 65–76. doi: 10.1016/S0010-9452(08)70446-5
* 5,6,a Sala-Llonch, R., Arenaza-Urquijo, E. M., Valls-Pedret, C., Vidal-Piñeiro, D., Bargall,ó, N., Junqu,é, C. et al. (2012). Dynamic functional reorganizations and relationship with working memory performance in healthy aging. Front. Hum. Neurosci . 6, 152. doi: 10.3389/fnhum.2012.00152
* 5,6,b Salami, A., Eriksson, J., and Nyberg, L. (2012). Opposing effects of aging on large-scale brain systems for memory encoding and cognitive control. J. Neurosci . 32, 10749–10757. doi: 10.1523/JNEUROSCI.0278-12.2012
* 4, a, • Sambataro, F., Murty, V. P., Callicott, J. H., Tan, H.-Y., Das, S., Weinberger, D. R. et al. (2010). Age-related alterations in default mode network: impact on working memory performance. Neurobiol. Aging 31, 839–852. doi: 10.1016/j.neurobiolaging.2008.05.022
* 3,5,a Schneider-Garces, N. J., Gordon, B. A., Brumback-Peltz, C. R., Shin, E., Lee, Y., Sutton, B. P. et al. (2010). Span, CRUNCH, and beyond: working memory capacity and the aging brain. J. Cogn. Neurosci . 22, 655–669. doi: 10.1162/jocn.2009.21230
* 7,9,c Schulte, T., Müller-Oehring, E. M., Chanraud, S., Rosenbloom, M. J., Pfefferbaum, A., and Sullivan, E. V. (2011). Age-related reorganization of functional networks for successful conflict resolution: a combined functional and structural MRI study. Neurobiol. Aging 32, 2075–2090. doi: 10.1016/j.neurobiolaging.2009.12.002
Scuteri, A., Tesauro, M., Appolloni, S., Preziosi, F., Brancati, A. M., and Volpe, M. (2007). Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J. Hypertens . 25, 1035–1040. doi: 10.1097/01.hjh.0000170384.38708.b7
* 7,8,a Shafto, M. A., Stamatakis, E. A., Tam, P. P., and Tyler, L. K. (2010). Word retrieval failures in old age: the relationship between structure and function. J. Cogn. Neurosci . 22, 1530–1540. doi: 10.1162/jocn.2009.21321
Shaikh, S., and Nicholson, L. F. B. (2008). Advanced glycation end products induce in vitro cross-linking of alpha-synuclein and accelerate the process of intracellular inclusion body formation. J. Neurosci. Res . 86, 2071–2082. doi: 10.1002/jnr.21644
Shaw, J. E., Sicree, R. A., and Zimmet, P. Z. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract . 87, 4–14. doi: 10.1016/j.diabres.2009.10.007
* 5,6,b,• Siedlecki, K. L., Habeck, C. G., Brickman, A. M., Gazes, Y., and Stern, Y. (2009). Examining the multifactorial nature of cognitive aging with covariance analysis of positron emission tomography data. J. Int. Neuropsychol. Soc . 15, 973–981. doi: 10.1017/S1355617709990592
* 7,8,a Simon, J. R., Vaidya, C. J., Howard, J. H., and Howard, D. V. (2012). The effects of aging on the neural basis of implicit associative learning in a probabilistic triplets learning task. J. Cogn. Neurosci . 24, 451–463. doi: 10.1162/jocn_a_00116
Smith, P. J., Blumenthal, J. A., Babyak, M. A., Hinderliter, A., and Sherwood, A. (2011). Association of vascular health and neurocognitive performance in overweight adults with high blood pressure. J. Clin. Exp. Neuropsychol . 33, 559–566. doi: 10.1080/13803395.2010.537648
Sokolova, I. A., Manukhina, E. B., Blinkov, S. M., Koshelev, V. B., Pinelis, V. G., and Rodionov, I. M. (1985). Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc. Res . 30, 1–9. doi: 10.1016/0026-2862(85)90032-9
* 2,5,d Solbakk, A.-K., Fuhrmann Alpert, G., Furst, A. J., Hale, L. A., Oga, T., Chetty, S. et al. (2008). Altered prefrontal function with aging: insights into age-associated performance decline. Brain Res . 1232, 30–47. doi: 10.1016/j.brainres.2008.07.060
* 5,6,a Solé-Padullés, C., Bartrés-Faz, D., Junqué, C., Vendrell, P., Rami, L., Clemente, I. C. et al. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 30, 1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008
* 5,6,d,■ Spaniol, J., and Grady, C. (2012). Aging and the neural correlates of source memory: over-recruitment and functional reorganization. Neurobiol. Aging 33, 425.e3–18. doi: 10.1016/j.neurobiolaging.2010.10.005
* 6,7,a Sperling, R. A., Bates, J. F., Chua, E. F., Cocchiarella, A. J., Rentz, D. M., Rosen, B. R. et al. (2003). fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr . 74, 44–50. doi: 10.1136/jnnp.74.1.44
* 10,12,b Stebbins, G. T., Carrillo, M. C., Dorfman, J., Dirksen, C., Desmond, J. E., Turner, D. A. et al. (2002). Aging effects on memory encoding in the frontal lobes. Psychol. Aging 17, 44–55. doi: 10.1037/0882-7974.17.1.44
Steen, E., Terry, B. M., Rivera, E. J., Cannon, J. L., Neely, T. R., Tavares, R. et al. (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease—is this type 3 diabetes? J. Alzheimers Dis . 7, 63–80.
* 5,6,b Steffener, J., Brickman, A. M., Rakitin, B. C., Gazes, Y., and Stern, Y. (2009). The impact of age-related changes on working memory functional activity. Brain Imaging Behav . 3, 142–153. doi: 10.1007/s11682-008-9056-x
* 5,6,a Stern, Y., Habeck, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J. et al. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 15, 394–402. doi: 10.1093/cercor/bhh142
* 4, a Stern, Y., Rakitin, B. C., Habeck, C., Gazes, Y., Steffener, J., Kumar, A. et al. (2012). Task difficulty modulates young-old differences in network expression. Brain Res . 1435, 130–145. doi: 10.1016/j.brainres.2011.11.061
* 7,8,a Stern, Y., Zarahn, E., Habeck, C., Holtzer, R., Rakitin, B. C., Kumar, A. et al. (2008). A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb. Cortex 18, 959–967. doi: 10.1093/cercor/bhm134
* 4, a Stevens, W. D., Hasher, L., Chiew, K. S., and Grady, C. L. (2008). A neural mechanism underlying memory failure in older adults. J. Neurosci . 28, 12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008
* 7,9,a St Jacques, P. L., Rubin, D. C., and Cabeza, R. (2012). Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol. Aging 33, 1298–1310. doi: 10.1016/j.neurobiolaging.2010.11.007
* 5,6,a Thiyagesh, S. N., Farrow, T. F. D., Parks, R. W., Accosta-Mesa, H., Young, C., Wilkinson, I. D. et al. (2009). The neural basis of visuospatial perception in Alzheimer's disease and healthy elderly comparison subjects: an fMRI study. Psychiatry Res . 172, 109–116. doi: 10.1016/j.pscychresns.2008.11.002
* 7,8,a Thomsen, T., Specht, K., Rimol, L. M., Hammar, A., Nyttingnes, J., Ersland, L. et al. (2004). Brain localization of attentional control in different age groups by combining functional and structural MRI. Neuroimage 22, 912–919. doi: 10.1016/j.neuroimage.2004.02.015
* 5,9,a Townsend, J., Adamo, M., and Haist, F. (2006). Changing channels: an fMRI study of aging and cross-modal attention shifts. Neuroimage 31, 1682–1692. doi: 10.1016/j.neuroimage.2006.01.045
Triantafyllidi, H., Arvaniti, C., Lekakis, J., Ikonomidis, I., Siafakas, N., Tzortzis, S. et al. (2009). Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am. J. Hypertens . 22, 525–530. doi: 10.1038/ajh.2009.35
* 5,6,b Trivedi, M. A., Murphy, C. M., Goetz, C., Shah, R. C., Gabrieli, J. D. E., Whitfield-Gabrieli, S. et al. (2008a). fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement. Geriatr. Cogn. Disord . 26, 123–137. doi: 10.1159/000148190
* 1,6,a Trivedi, M. A., Schmitz, T. W., Ries, M. L., Hess, T. M., Fitzgerald, M. E., Atwood, C. S. et al. (2008b). fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer's disease. Neuropsychologia 46, 1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035
* 4, b Trivedi, M. A., Stoub, T. R., Murphy, C. M., George, S., deToledo-Morrell, L., Shah, R. C. et al. (2011). Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging Behav . 5, 126–136. doi: 10.1007/s11682-011-9117-4
* 5,6,a Tsukiura, T., Sekiguchi, A., Yomogida, Y., Nakagawa, S., Shigemune, Y., Kambara, T. et al. (2011). Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face-name associations. J. Cogn. Neurosci . 23, 200–213. doi: 10.1162/jocn.2010.21476
Tuligenga, R. H., Dugravot, A., Tabák, A. G., Elbaz, A., Brunner, E. J., Kivimäki, M. et al. (2014). Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol . 2, 228–235. doi: 10.1016/S2213-8587(13)70192-X.
* 7,8,a Tyler, L. K., Shafto, M. A., Randall, B., Wright, P., Marslen-Wilson, W. D., and Stamatakis, E. A. (2010). Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb. Cortex 20, 352–364. doi: 10.1093/cercor/bhp105
* 7,8,a Vallesi, A., McIntosh, A. R., and Stuss, D. T. (2009). Temporal preparation in aging: a functional MRI study. Neuropsychologia 47, 2876–2881. doi: 10.1016/j.neuropsychologia.2009.06.013
* 5,6,a,■ Vallesi, A., McIntosh, A. R., and Stuss, D. T. (2011). Overrecruitment in the aging brain as a function of task demands: evidence for a compensatory view. J. Cogn. Neurosci . 23, 801–815. doi: 10.1162/jocn.2010.21490
van den Berg, E., Reijmer, Y. D., de Bresser, J., Kessels, R. P. C., Kappelle, L. J., and Biessels, G. J. (2010). A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 53, 58–65. doi: 10.1007/s00125-009-1571-9
* 4, a Vandenbroucke, M. W. G., Goekoop, R., Duschek, E. J. J., Netelenbos, J. C., Kuijer, J. P. A., Barkhof, F. et al. (2004). Interindividual differences of medial temporal lobe activation during encoding in an elderly population studied by fMRI. Neuroimage 21, 173–180. doi: 10.1016/j.neuroimage.2003.09.043
* 4, c van der Veen, F. M., Nijhuis, F. A. P., Tisserand, D. J., Backes, W. H., and Jolles, J. (2006). Effects of aging on recognition of intentionally and incidentally stored words: an fMRI study. Neuropsychologia 44, 2477–2486. doi: 10.1016/j.neuropsychologia.2006.04.023
van Elderen, S. G. C., de Roos, A., de Craen, A. J. M., Westendorp, R. G. J., Blauw, G. J., Jukema, J. W. et al. (2010). Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 75, 997–1002. doi: 10.1212/WNL.0b013e3181f25f06
* 7,8,a van Impe, A., Coxon, J. P., Goble, D. J., Wenderoth, N., and Swinnen, S. P. (2011). Age-related changes in brain activation underlying single- and dual-task performance: visuomanual drawing and mental arithmetic. Neuropsychologia 49, 2400–2409. doi: 10.1016/j.neuropsychologia.2011.04.016
* 5,6,a,• Velanova, K., Lustig, C., Jacoby, L. L., and Buckner, R. L. (2007). Evidence for frontally mediated controlled processing differences in older adults. Cereb. Cortex 17, 1033–1046. doi: 10.1093/cercor/bhl013
* 7,8,a,❖ Venkatraman, V. K., Aizenstein, H., Guralnik, J., Newman, A. B., Glynn, N. W., Taylor, C. et al. (2010). Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage 49, 3436–3442. doi: 10.1016/j.neuroimage.2009.11.019
Virta, J. J., Heikkilä, K., Perola, M., Koskenvuo, M., Räihä, I., Rinne, J. O. et al. (2013). Midlife cardiovascular risk factors and late cognitive impairment. Eur. J. Epidemiol . 28, 405–416. doi: 10.1007/s10654-013-9794-y
* 7,8,a Voelcker-Rehage, C., Godde, B., and Staudinger, U. M. (2010). Physical and motor fitness are both related to cognition in old age. Eur. J. Neurosci . 31, 167–176. doi: 10.1111/j.1460-9568.2009.07014.x
* 7,8,a Waiter, G. D., Fox, H. C., Murray, A. D., Starr, J. M., Staff, R. T., Bourne, V. J. et al. (2008). Is retaining the youthful functional anatomy underlying speed of information processing a signature of successful cognitive ageing? An event-related fMRI study of inspection time performance. Neuroimage 41, 581–595. doi: 10.1016/j.neuroimage.2008.02.045
Waldstein, S. R., Carrington, S., Thayer, J. F., Najjar, S. S., Scuteri, A., and Zonderman, A. B. (2008). Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51, 99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674
* 5,6,a Wang, L., Laviolette, P., O'Keefe, K., Putcha, D., Bakkour, A., Van Dijk, K. R. A. et al. (2010a). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage 51, 910–917. doi: 10.1016/j.neuroimage.2010.02.046
* 7,9,a Wang, L., Li, Y., Metzak, P., He, Y., and Woodward, T. S. (2010b). Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage 50, 862–872. doi: 10.1016/j.neuroimage.2010.01.044
* 7,9,a Wang, T. H., Kruggel, F., and Rugg, M. D. (2009). Effects of advanced aging on the neural correlates of successful recognition memory. Neuropsychologia 47, 1352–1361. doi: 10.1016/j.neuropsychologia.2009.01.030
Weis, S., Leube, D., Erb, M., Heun, R., Grodd, W., and Kircher, T. (2011). Functional neuroanatomy of sustained memory encoding performance in healthy aging and in Alzheimer's disease. Int. J. Neurosci . 121, 384–392. doi: 10.3109/00207454.2011.565892
White, W. B., Wolfson, L., Wakefield, D. B., Hall, C. B., Campbell, P., Moscufo, N. et al. (2011). Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 124, 2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036
Whitehead, B. P., Dixon, R. A., Hultsch, D. F., and MacDonald, S. W. S. (2011). Are neurocognitive speed and inconsistency similarly affected in type 2 diabetes? J. Clin. Exp. Neuropsychol . 33, 647–657. doi: 10.1080/13803395.2010.547845
* 7,9,a,× Wierenga, C. E., Benjamin, M., Gopinath, K., Perlstein, W. M., Leonard, C. M., Rothi, L. J. G. et al. (2008). Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol. Aging 29, 436–451. doi: 10.1016/j.neurobiolaging.2006.10.024
* 7,9,b,■,□ Wierenga, C. E., Stricker, N. H., McCauley, A., Simmons, A., Jak, A. J., Chang, Y.-L. et al. (2010). Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer's disease. Neuroimage 51, 1222–1233. doi: 10.1016/j.neuroimage.2010.03.021
* 5,6,a Wood, G., Ischebeck, A., Koppelstaetter, F., Gotwald, T., and Kaufmann, L. (2009). Developmental trajectories of magnitude processing and interference control: an FMRI study. Cereb. Cortex 19, 2755–2765. doi: 10.1093/cercor/bhp056
* 7,8,a Woodard, J. L., Seidenberg, M., Nielson, K. A., Antuono, P., Guidotti, L., Durgerian, S. et al. (2009). Semantic memory activation in amnestic mild cognitive impairment. Brain 132, 2068–2078. doi: 10.1093/brain/awp157
* 5,6,a Woodard, J. L., Seidenberg, M., Nielson, K. A., Miller, S. K., Franczak, M., Antuono, P. et al. (2007). Temporally graded activation of neocortical regions in response to memories of different ages. J. Cogn. Neurosci . 19, 1113–1124. doi: 10.1162/jocn.2007.19.7.1113
* 5,6,c Woodard, J. L., Seidenberg, M., Nielson, K. A., Smith, J. C., Antuono, P., Durgerian, S. et al. (2010). Prediction of cognitive decline in healthy older adults using fMRI. J. Alzheimers Dis . 21, 871–885. doi: 10.3233/JAD-2010-091693
* 5,6,c Woodard, J. L., Sugarman, M. A., Nielson, K. A., Smith, J. C., Seidenberg, M., Durgerian, S. et al. (2012). Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Curr. Alzheimer Res . 9, 436–446. doi: 10.2174/156720512800492477
* 5,6,a,• Wu, J.-T., Wu, H.-Z., Yan, C.-G., Chen, W.-X., Zhang, H.-Y., He, Y. et al. (2011). Aging-related changes in the default mode network and its anti-correlated networks: a resting-state fMRI study. Neurosci. Lett . 504, 62–67. doi: 10.1016/j.neulet.2011.08.059
Xu, W., Caracciolo, B., Wang, H.-X., Winblad, B., Bäckman, L., Qiu, C. et al. (2010). Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 59, 2928–2935. doi: 10.2337/db10-0539
Yaffe, K., Blackwell, T., Kanaya, A. M., Davidowitz, N., Barrett-Connor, E., and Krueger, K. (2004). Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63, 658–663. doi: 10.1212/01.WNL.0000134666.64593.BA
Yaffe, K., Lindquist, K., Schwartz, A. V., Vitartas, C., Vittinghoff, E., Satterfield, S. et al. (2011). Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 77, 1351–1356. doi: 10.1212/WNL.0b013e3182315a56
Yakushiji, Y., Noguchi, T., Hara, M., Nishihara, M., Eriguchi, M., Nanri, Y. et al. (2012). Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke 43, 1800–1805. doi: 10.1161/STROKEAHA.111.647065
Yan, S. F., Ramasamy, R., and Schmidt, A. M. (2008). Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab . 4, 285–293. doi: 10.1038/ncpendmet0786
Yeung, S. E., Fischer, A. L., and Dixon, R. A. (2009). Exploring effects of type 2 diabetes on cognitive functioning in older adults. Neuropsychology 23, 1–9. doi: 10.1037/a0013849
* 5,6,c Ystad, M., Eichele, T., Lundervold, A. J., and Lundervold, A. (2010). Subcortical functional connectivity and verbal episodic memory in healthy elderly—a resting state fMRI study. Neuroimage 52, 379–388. doi: 10.1016/j.neuroimage.2010.03.062
* 5,6,c Ystad, M., Hodneland, E., Adolfsdottir, S., Haász, J., Lundervold, A. J., Eichele, T. et al. (2011). Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage 55, 24–31. doi: 10.1016/j.neuroimage.2010.11.016
Zhong, Y., Miao, Y., Jia, W. P., Yan, H., Wang, B. Y., and Jin, J. (2012a). Hyperinsulinemia, insulin resistance and cognitive decline in older cohort. Biomed. Environ. Sci . 25, 8–14. doi: 10.3967/0895-3988.2012.01.002
Zhong, Y., Zhang, X. Y., Miao, Y., Zhu, J. H., Yan, H., Wang, B. Y. et al. (2012b). The relationship between glucose excursion and cognitive function in aged type 2 diabetes patients. Biomed. Environ. Sci . 25, 1–7. doi: 10.3967/0895-3988.2012.01.001
Zhou, H., Lu, W., Shi, Y., Bai, F., Chang, J., Yuan, Y. et al. (2010). Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci. Lett . 473, 5–10. doi: 10.1016/j.neulet.2009.12.057
* 7,8,a,• Zhu, D. C., Zacks, R. T., and Slade, J. M. (2010). Brain activation during interference resolution in young and older adults: an fMRI study. Neuroimage 50, 810–817. doi: 10.1016/j.neuroimage.2009.12.087
Keywords: type 2 diabetes mellitus, hypertension, cognition, aging, imaging
Citation: Meusel L-AC, Kansal N, Tchistiakova E, Yuen W, MacIntosh BJ, Greenwood CE and Anderson ND (2014) A systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: time to establish new norms. Front. Aging Neurosci . 6 :148. doi: 10.3389/fnagi.2014.00148
Received: 25 January 2014; Accepted: 17 June 2014; Published online: 08 July 2014.
Reviewed by:
Copyright © 2014 Meusel, Kansal, Tchistiakova, Yuen, MacIntosh, Greenwood and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole D. Anderson, Rotman Research Institute, Baycrest, 3560 Bathurst Street, Toronto, ON M6A 2E1, Canada e-mail: [email protected]
Self-care and type 2 diabetes mellitus (T2DM): a literature review in sex-related differences
Affiliations.
- 1 . [email protected].
- 2 . [email protected].
- 3 . [email protected].
- 4 . [email protected].
- 5 . [email protected].
- 6 . [email protected].
- 7 . [email protected].
- 8 . [email protected].
- 9 . [email protected].
- 10 . [email protected].
- 11 . [email protected].
- PMID: 36043961
- PMCID: PMC9534249
- DOI: 10.23750/abm.v93i4.13324
T2DM is a multifactorial disease, and it is considered a worldwide challenge for its increasing prevalence and its negative impact on patients' wellbeing. Even if it is known that self-care is a key factor in reaching optimal outcomes, and males and females implement different self-care behaviors, sex-related differences in self-care of patients with T2DM have been poorly investigated. Especially, an overall view of the available evidence has not yet been done. Accordingly, this review aims to summarize, critically review, and interpret the available evidence related to the sex-related differences in self-care behaviors of patients with T2DM. An extensive literature review was performed with a narrative synthesis following the PRISMA statement and flowchart through four databases: PubMed, CINAHL, Scopus, and Embase. From the 5776 identified records by the queries, only 29 articles were included, having a high-quality evaluation. Both females and males with T2DM must improve their self-care: more males reported performing better behaviors aimed at maintaining health and clinical stability (i.e., self-care maintenance) than females, but mainly in relation to physical activity. On the other hand, more females reported performing adequate behaviors aimed at monitoring their signs and symptoms (i.e., self-care monitoring) but with worse glycemic control and diabetic complications (i.e., self-care management). This review firstly provides an overall view of different self-care behaviors implemented by males and females with T2DM, showing that self-care management should be improved in both sexes. Health education must include the problems related to the diabetic pathology and the patient's own characteristics, such as sex.
Publication types
- Diabetes Complications* / complications
- Diabetes Mellitus, Type 2* / epidemiology
- Diabetes Mellitus, Type 2* / therapy
The Effect of Thiazolidinediones in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
- Published: 29 April 2024
Cite this article

- Mohammed A. Abdalla ORCID: orcid.org/0000-0002-6016-3157 1 , 8 ,
- Najeeb Shah 1 ,
- Harshal Deshmukh 1 ,
- Amirhossein Sahebkar 2 , 3 , 4 ,
- Linda Östlundh 5 ,
- Rami H. Al-Rifai 6 ,
- Stephen L. Atkin 7 &
- Thozhukat Sathyapalan 1
66 Accesses
1 Altmetric
Explore all metrics
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine condition affecting women of reproductive age. It is characterised by insulin resistance and is a risk for type 2 diabetes mellitus (T2DM). The aim of this study was to review the literature on the effect of pioglitazone and rosiglitazone in women with PCOS.
We searched PubMed, MEDLINE, Scopus, Embase, Cochrane Library and the Web of Science in April 2020 and updated in March 2023. Studies were deemed eligible if they were randomised controlled trials (RCTs) reporting the effect of pioglitazone and rosiglitazone in PCOS. The study follows the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Two reviewers independently extracted data and assessed the risk of bias using the Cochrane risk of bias tool.
Out of 814 initially retrieved citations, 24 randomised clinical trials (RCTs) involving 976 participants were deemed eligible. Among women with PCOS, treatment with rosiglitazone compared to metformin resulted in a significant increase in the mean body weight (mean difference (MD) 1.95 kg; 95% CI 0.03–3.87, p = 0.05). Metformin treatment was associated with a reduction in mean body mass index (BMI) compared to pioglitazone (MD 0.85 kg/m 2 ; 95% CI 0.13–1.57, p = 0.02). Both pioglitazone compared to placebo (MD 2.56 kg/m 2 ; 95% CI 1.77–3.34, p < 0.00001) and rosiglitazone compared to metformin (MD 0.74 kg/m 2 ; 95% CI 0.07–1.41, p = 0.03) were associated with a significant increase in BMI. Treatment with pioglitazone compared to placebo showed a significant reduction in triglycerides (MD − 0.20 mmol/L; 95% CI − 0.38 to − 0.03, p = 0.02) and fasting insulin levels (MD − 11.47 mmol/L; 95% CI − 20.20, − 2.27, p = 0.01). Rosiglitazone compared to metformin was marginally significantly associated with a reduction in the luteinising hormone (LH) (MD − 0.62; 95% CI − 1.25–0.00, p = 0.05).
Both pioglitazone and rosiglitazone were associated with significant increases in body weight and BMI when compared with metformin or placebo. Pioglitazone significantly reduced triglycerides and fasting insulin when compared with placebo while rosiglitazone showed a modest reduction of LH when compared with metformin.
PROSPERO Registration No.
CRD42020178783.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
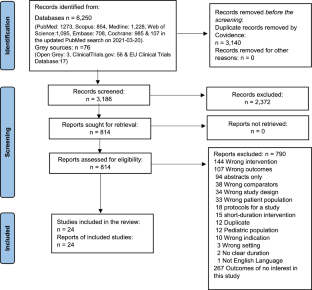
Similar content being viewed by others
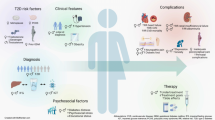
Sex differences in type 2 diabetes
How Food Choices Impact on Male Fertility
Current Medical Therapy for Adenomyosis: From Bench to Bedside
Data availability.
The datasets generated and analysed for this review are available upon compelling request to the authors.
Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. 2007;22(8):2279–86.
Article CAS PubMed Google Scholar
Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther Adv Endocrinol Metab. 2021;12:2042018821989238.
Article CAS PubMed PubMed Central Google Scholar
Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T. 2013;38(6):336–55.
PubMed PubMed Central Google Scholar
Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3(8):1545–73.
Abdalla M, Deshmukh H, Atkin SL, Sathyapalan T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): a review. Life Sci. 2020;259: 118174.
Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2012;61(1):114–23.
Azziz R, Ehrmann DA, Legro RS, Fereshetian AG, O’Keefe M, Ghazzi MN. Troglitazone decreases adrenal androgen levels in women with polycystic ovary syndrome. Fertil Steril. 2003;79(4):932–7.
Article PubMed Google Scholar
Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007;92(12):4546–56.
Garruti G, Depalo R, Vita MG, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online. 2009;19(4):552–63.
Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50(1):205–25.
Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20.
Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2020;11:2042018820938305.
Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2002;18(Suppl 2):S10-15.
Psaty BM, Furberg CD. Rosiglitazone and cardiovascular risk. N Engl J Med. 2007;356(24):2522–4.
Ajjan RA, Grant PJ. The cardiovascular safety of rosiglitazone. Expert Opin Drug Saf. 2008;7(4):367–76.
Bailey CJ. Thiazolidinediones. In: Enna SJ, Bylund DB, editors. xPharm: the comprehensive pharmacology reference. Elsevier; 2007. https://doi.org/10.1016/B978-008055232-3.61047-5 .
Valsamakis G, Lois K, Kumar S, Mastorakos G. Metabolic and other effects of pioglitazone as an add-on therapy to metformin in the treatment of polycystic ovary syndrome (PCOS). Hormones (Athens). 2013;12(3):363–78.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Article PubMed PubMed Central Google Scholar
Babineau J. Product review: Covidence (systematic review software). J Can Health Libr Assoc. 2014;35(2):68–71.
Article Google Scholar
Das S, Chatterjee SS. Cabell’s blacklist: a new way to tackle predatory journals. Indian J Psychol Med. 2018;40(2):197–8.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Wiley; 2019.
Book Google Scholar
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Shahebrahimi K, Jalilian N, Bazgir N, Rezaei M. Comparison clinical and metabolic effects of metformin and pioglitazone in polycystic ovary syndrome. Indian J Endocrinol Metab. 2016;20(6):805–9.
Sohrevardi SM, Nosouhi F, Hossein Khalilzade S, et al. Evaluating the effect of insulin sensitizers metformin and pioglitazone alone and in combination on women with polycystic ovary syndrome: an RCT. Int J Reprod Biomed. 2016;14(12):743–54.
CAS PubMed PubMed Central Google Scholar
Cetinkalp S, Karadeniz M, Erdogan M, Ozgen G, Saygl F, Ylmaz C. The effects of rosiglitazone, metformin, and estradiol-cyproterone acetate on lean patients with polycystic ovary syndrome. Endocrinologist. 2009;19(3):94–7.
Article CAS Google Scholar
Kilicdag EB, Bagis T, Zeyneloglu HB, et al. Homocysteine levels in women with polycystic ovary syndrome treated with metformin versus rosiglitazone: a randomized study. Hum Reprod. 2005;20(4):894–9.
Lam PM, Tam WH, Ma RC, et al. The reproductive and metabolic effect of rosiglitazone on Chinese women with polycystic ovarian syndrome—a double-blind randomized placebo-controlled study. Fertil Steril. 2011;96(2):445–451.e441.
Cho LW, Kilpatrick ES, Keevil BG, Coady AM, Atkin SL. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol. 2009;70(2):233–7.
Li Y, Tan J, Wang Q, Duan C, Hu Y, Huang W. Comparing the individual effects of metformin and rosiglitazone and their combination in obese women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2020;113(1):197–204.
Ziaee A, Oveisi S, Abedini A, Hashemipour S, Karimzadeh T, Ghorbani A. Effect of metformin and pioglitazone treatment on cardiovascular risk profile in polycystic ovary syndrome. Acta Med Indones. 2012;44(1):16–22.
PubMed Google Scholar
Brettenthaler N, De Geyter C, Huber PR, Keller U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(8):3835–40.
Mohiyiddeen L, Watson AJ, Apostolopoulos NV, Berry R, Alexandraki KI, Jude EB. Effects of low-dose metformin and rosiglitazone on biochemical, clinical, metabolic and biophysical outcomes in polycystic ovary syndrome. J Obstet Gynaecol. 2013;33(2):165–70.
Yilmaz M, Karakoç A, Törüner FB, et al. The effects of rosiglitazone and metformin on menstrual cyclicity and hirsutism in polycystic ovary syndrome. Gynecol Endocrinol. 2005;21(3):154–60.
Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Aroda VR, Ciaraldi TP, Burke P, et al. Metabolic and hormonal changes induced by pioglitazone in polycystic ovary syndrome: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2009;94(2):469–76.
Cataldo NA, Abbasi F, McLaughlin TL, et al. Metabolic and ovarian effects of rosiglitazone treatment for 12 weeks in insulin-resistant women with polycystic ovary syndrome. Hum Reprod. 2006;21(1):109–20.
Jensterle M, Janez A, Mlinar B, Marc J, Prezelj J, Pfeifer M. Impact of metformin and rosiglitazone treatment on glucose transporter 4 mRNA expression in women with polycystic ovary syndrome. Eur J Endocrinol. 2008;158(6):793–801.
Jensterle M, Sebestjen M, Janez A, et al. Improvement of endothelial function with metformin and rosiglitazone treatment in women with polycystic ovary syndrome. Eur J Endocrinol. 2008;159(4):399–406.
Steiner CA, Janez A, Jensterle M, Reisinger K, Forst T, Pfützner A. Impact of treatment with rosiglitazone or metformin on biomarkers for insulin resistance and metabolic syndrome in patients with polycystic ovary syndrome. J Diabetes Sci Technol. 2007;1(2):211–7.
Dereli D, Dereli T, Bayraktar F, Ozgen AG, Yilmaz C. Endocrine and metabolic effects of rosiglitazone in non-obese women with polycystic ovary disease. Endocr J. 2005;52(3):299–308.
Anaforoglu I, Algun E, Incecayir O, Ersoy K. Higher metabolic risk with National Institutes of Health versus Rotterdam diagnostic criteria for polycystic ovarian syndrome in Turkish women. Metab Syndr Relat Disord. 2011;9(5):375–80.
Du Q, Wang YJ, Yang S, Wu B, Han P, Zhao YY. A systematic review and meta-analysis of randomized controlled trials comparing pioglitazone versus metformin in the treatment of polycystic ovary syndrome. Curr Med Res Opin. 2012;28(5):723–30.
Xu Y, Wu Y, Huang Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS: a meta-analysis. Arch Gynecol Obstet. 2017;296(4):661–77.
Huang R, Zhao PF, Xu JH, Liu DD, Luo FD, Dai YH. Effects of placebo-controlled insulin-sensitizing drugs on hormonal parameters in polycystic ovary syndrome patients: a network meta-analysis. J Cell Biochem. 2018;119(3):2501–11.
Zhao H, Xing C, Zhang J, He B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18(1):171.
Juurlink DN, Gomes T, Lipscombe LL, Austin PC, Hux JE, Mamdani MM. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ. 2009;339: b2942.
Froment P, Touraine P. Thiazolidinediones and fertility in polycystic ovary syndrome (PCOS). PPAR Res. 2006;2006:73986.
Naka KK, Kalantaridou SN, Kravariti M, et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: a prospective randomized study. Fertil Steril. 2011;95(1):203–9.
Ortega-González C, Luna S, Hernández L, et al. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin-resistant women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(3):1360–5.
Batista JG, Soares JM, Maganhin CC, Simões RS, Tomaz G, Baracat EC. Assessing the benefits of rosiglitazone in women with polycystic ovary syndrome through its effects on insulin-like growth factor 1, insulin-like growth factor-binding protein-3 and insulin resistance: a pilot study. Clinics (Sao Paulo). 2012;67(3):283–7.
Li T, Zhang T, Cui T, et al. Involvement of endogenous testosterone in hepatic steatosis in women with polycystic ovarian syndrome. J Steroid Biochem Mol Biol. 2020;204:105752.
Glintborg D, Støving RK, Hagen C, et al. Pioglitazone treatment increases spontaneous growth hormone (GH) secretion and stimulated GH levels in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(10):5605–12.
Glintborg D, Hermann AP, Andersen M, et al. Effect of pioglitazone on glucose metabolism and luteinizing hormone secretion in women with polycystic ovary syndrome. Fertil Steril. 2006;86(2):385–97.
Glintborg D, Frystyk J, Højlund K, et al. Total and high molecular weight (HMW) adiponectin levels and measures of glucose and lipid metabolism following pioglitazone treatment in a randomized placebo-controlled study in polycystic ovary syndrome. Clin Endocrinol. 2008;68(2):165–74.
Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC. Endocrine and metabolic effects of rosiglitazone in overweight women with PCOS: a randomized placebo-controlled study. Hum Reprod. 2006;21(6):1400–7.
Download references
The rapid service fee and open access fee were funded by the authors.
Author information
Authors and affiliations.
Allam Diabetes Centre, Academic Diabetes, Endocrinology and Metabolism, The University of Hull, Hull York Medical School (HYMS), Hull, UK
Mohammed A. Abdalla, Najeeb Shah, Harshal Deshmukh & Thozhukat Sathyapalan
Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran
Amirhossein Sahebkar
Applied Biomedical Research Centre, Mashhad University of Medical Sciences, Mashhad, Iran
School of Medicine, The University of Western Australia, Perth, Australia
College of Medicine and Health Sciences, The National Medical Library, United Arab Emirates University, Al Ain, United Arab Emirates
Linda Östlundh
College of Medicine and Health Sciences, Institute of Public Health, United Arab Emirates University, Al Ain, United Arab Emirates
Rami H. Al-Rifai
School of Postgraduate Studies and Research, RCSI Medical University of Bahrain, Al Sayh, Kingdom of Bahrain
Stephen L. Atkin
Dasman Diabetes Institute, Department of Translational Research, State of Kuwait, Kuwait City, Kuwait
Mohammed A. Abdalla
You can also search for this author in PubMed Google Scholar
Contributions
Mohammed Altigani Abdalla Ahmed; designed the review, completed the screening, assessed the quality, extracted, collected and analysed the data, wrote, revised and edited the final manuscript. Najeeb Shah; assessed the quality, extracted and collected the data, and revised and edited the final manuscript. Harshal Deshmukh; revised and edited the final manuscript. Amirhossein Sahebkar; revised and edited the final manuscript; Linda Östlundh; developed and performed the systematic search, assessed for predatory journals, and revised and edited the final manuscript. Rami H. Al-Rifai; participated in the critical discussion and revised and edited the final manuscript. Stephen L. Atkin: participated in the critical discussion and revised the final draft of the manuscript. Finally, Thozhukat Sathyapalan; acted as a mediator for assessing the quality of the evidence, supervised the study, participated in the critical discussion, and revised and edited the final manuscript.
Corresponding author
Correspondence to Mohammed A. Abdalla .
Ethics declarations
Conflict of interest.
Dr Mohammed A Abdalla is currently affiliated with Dasman Diabetes Institute, Department of Translational Research, State of Kuwait, Kuwait. Ms Linda Östlundh is now affiliated with Örebro University, Sweden. Najeeb Shah, Harshal Deshmukh, Amirhossein Sahebkar, Rami Al-Rifai, Stephen Atkin and Thozhukat Sathyapalen have nothing to declare.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Thus, no ethical approval was required.
Additional information
Prior Publication: This work was part of Dr Mohammed A Abdalla’s PhD thesis which was deposited at the University of Hull repository website https://hull-repository.worktribe.com .
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 217 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Abdalla, M.A., Shah, N., Deshmukh, H. et al. The Effect of Thiazolidinediones in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02848-3
Download citation
Received : 16 January 2024
Accepted : 18 March 2024
Published : 29 April 2024
DOI : https://doi.org/10.1007/s12325-024-02848-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Polycystic ovary syndrome
- Pioglitazone
- Rosiglitazone
- Pharmacological therapy
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs.
Introduction. Diabetes mellitus (DM) is probably one of the oldest diseases known to man. It was first reported in Egyptian manuscript about 3000 years ago. 1 In 1936, the distinction between type 1 and type 2 DM was clearly made. 2 Type 2 DM was first described as a component of metabolic syndrome in 1988. 3 Type 2 DM (formerly known as non-insulin dependent DM) is the most common form of DM ...
Type 2 diabetes mellitus (T2DM) accounts for >90% of the cases of diabetes in adults. Resistance to insulin action is the major cause that leads to chronic hyperglycemia in diabetic patients. ... Arora T, Taheri S. Sleep optimization and diabetes control: a review of the literature. Diabetes Ther. 2015 Dec; 6 ((4)):425-68. [PMC free article ...
In this paper, we present a systematic review of the literature on the association of these risk factors with the incidence/prevalence of type 2 diabetes. We give insights on the contribution of independent risk factors in the development of type 2 diabetes along with possible solutions towards a preventive approach.
Abstract. Objective: The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs. These challenges ...
Type 2 diabetes mellitus is a chronic metabolic disorder associated with hyperglycaemia caused by impaired insulin secretion and insulin resistance. In this Primer, DeFronzo et al. discuss the ...
Objective To assess what proportions of studies reported increasing, stable, or declining trends in the incidence of diagnosed diabetes. Design Systematic review of studies reporting trends of diabetes incidence in adults from 1980 to 2017 according to PRISMA guidelines. Data sources Medline, Embase, CINAHL, and reference lists of relevant publications. Eligibility criteria Studies of open ...
This Review summarizes information from systematic reviews and major cohort studies regarding emerging complications of type 1 and type 2 diabetes mellitus to identify and quantify associations ...
The global burden of type 2 diabetes mellitus (T2DM) is rapidly increasing, affecting individuals of all ages. The global T2DM prevalence nearly doubled in the adult population over the past decade from 4.7% in 1980 to 8.5% in 2014 [].The global burden of T2DM in people 20-79 years is further projected to increase to 629 million in 2045 compared to 425 million in 2017 [].
Objective To evaluate current risk models and scores for type 2 diabetes and inform selection and implementation of these in practice. Design Systematic review using standard (quantitative) and realist (mainly qualitative) methodology. Inclusion criteria Papers in any language describing the development or external validation, or both, of models and scores to predict the risk of an adult ...
The body of literature that outlines higher risk of microvascular or macrovascular complications in early-onset type 2 diabetes has focussed on comparing people with type 2 diabetes to those ...
Most studies do not differentiate between patients with type 1 and type 2 diabetes, which correspond to two pathophysiological distinct diseases that could represent different degrees of clinical compromise. ... The objective of this systematic literature review will be to identify whether there are differences in the clinical outcomes of both ...
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycemia. It may be due to impaired insulin secretion, resistance to peripheral actions of insulin, or both. According to the International Diabetes Federation (IDF), approximately 415 million adults between the ages of 20 to 79 years had diabetes mellitus in 2015.[1] DM is proving to be a global public ...
Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies 1 2. Author links open overlay panel Jannasch Franziska 4 5, Kröger Janine 4 5, ... Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of ...
Living with diabetes: literature review and secondary analysis of qualitative data. H. Stuckey, Corresponding Author. H. Stuckey ... Upon diagnosis of type 2 diabetes, friends and family sympathized with the respondent and normalized diabetes because 'this happens' and 'is common' [S22]. Respondents looked to close friends for encouragement 'to ...
Objective: We summarized evidence from prospective studies that examined associations of dietary patterns with type 2 diabetes by considering different methodologic approaches. Methods: The literature search (MEDLINE and Web of Science) identified prospective studies (cohorts or trials) that associated dietary patterns with diabetes incidence ...
Literature focused on youth with type 2 diabetes is also reviewed. Data from the National Health and Nutrition Examination Survey (NHANES) 2015-2018 showed that only 2.3% of U.S. adults with diagnosed diabetes had Healthy Eating Index (HEI) scores ≥80 indicating a "good" diet, while 44.6% had HEI scores <50 indicating a "poor" diet.
This review explores the current conventional drugs used in the treatment of type 2 DM, the associated limitations related to their usage and the cutting edge novel nanoformulations that are under continual research for circumventing the stated drawbacks of the conventional drug use. 2. Pathophysiology of diabetes.
The rising prevalence of type 2 diabetes (T2DM) and hypertension in older adults, and the deleterious effect of these conditions on cerebrovascular and brain health, is creating a growing discrepancy between the "typical" cognitive aging trajectory and a "healthy" cognitive aging trajectory. These changing health demographics make T2DM and hypertension important topics of study in ...
Prevention of type 2 diabetes (T2D) is a great challenge worldwide. The aim of this evidence synthesis was to summarize the available evidence in order to update the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy. We conducted a systematic review and, where appropriate, meta-analyses of ...
This systematic review and meta-analysis evaluated the combined effects of clinician-led and community-based group exercise interventions on a range of health outcomes in adults with type 2 diabetes mellitus. Our literature search spanned Medline, Scopus, PubMed, Embase, and CINAHL databases, focusing on peer-reviewed studies published between January 2003 and January 2023.
Non-autoimmune forms of T1D correspond to approximately 4 to 7% of newly diagnosed T1D and include T1DB, as well as other types of atypical diabetes, for example fulminant type 1 diabetes and ...
Self-care and type 2 diabetes mellitus (T2DM): a literature review in sex-related differences Acta Biomed. 2022 Aug ... This review firstly provides an overall view of different self-care behaviors implemented by males and females with T2DM, showing that self-care management should be improved in both sexes. Health education must include the ...
Introduction Polycystic ovary syndrome (PCOS) is a complex endocrine condition affecting women of reproductive age. It is characterised by insulin resistance and is a risk for type 2 diabetes mellitus (T2DM). The aim of this study was to review the literature on the effect of pioglitazone and rosiglitazone in women with PCOS. Methods We searched PubMed, MEDLINE, Scopus, Embase, Cochrane ...
Abstract. Diabetes Mellitus (DM) is a multi-factorial chronic health condition that affects a large part of population and according to the World Health Organization (WHO) the number of adults living with diabetes is expected to increase. Since type 2 diabetes mellitus (T2DM) is suffered by the majority of diabetic patients (around 90-95% ...
Methods: A literature review had been chosen in this study with PRISMA approach to sort the articles from EBSCOHost, Google Scholar, Science Direct, Wiley Online and ProQuest. ... Widyaningsih, D. S., Prasetyowati, A. T., Harwanto, A., & Goenka, S. (2022). Newspaper Leg Exercise to Reduce the Type 2 Diabetes Mellitus Physical Symptoms. Journal ...