

Introduction
Case report, statement of ethics, disclosure statement, funding sources, author contributions, a case of acute kidney injury in a patient with renal hypouricemia without intense exercise.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Daiki Aomura , Kosuke Sonoda , Makoto Harada , Koji Hashimoto , Yuji Kamijo; A Case of Acute Kidney Injury in a Patient with Renal Hypouricemia without Intense Exercise. Case Rep Nephrol Dial 12 May 2020; 10 (1): 26–34. https://doi.org/10.1159/000506673
Download citation file:
- Ris (Zotero)
- Reference Manager
Exercise-induced acute kidney injury (EIAKI) frequently develops in patients with renal hypouricemia (RHUC). However, several cases of RHUC with acute kidney injury (AKI) but without intense exercise have been reported. We encountered a 15-year-old male with RHUC who experienced AKI. He reported no episodes of intense exercise and displayed no other representative risk factors of EIAKI, although a vasopressor had been administered for orthostatic dysregulation before AKI onset. His kidney dysfunction improved with discontinuation of the vasopressor and conservative treatment. Thus, AKI can develop in patients with RHUC in the absence of intense exercise, for which vasopressors may be a risk factor.
Exercise-induced acute kidney injury (EIAKI) is a major complication in patients with renal hypouricemia (RHUC). EIAKI usually develops after intense exercise, such as anaerobic exertion, and is not accompanied by rhabdomyolysis [ 1 ]. However, there are several case reports of patients experiencing EIAKI without intense exercise [ 2-4 ]. Although the pathomechanism and risk factors of EIAKI remain unclear, many reports suggest that an oxidation-reduction imbalance is associated with EIAKI onset [ 5 ]. We herein report a case of acute kidney injury (AKI) in a patient with RHUC in the absence of intense exercise, which may have been caused by an oral vasopressor.
A 15-year-old male complained of strong fatigue after intense exercise since childhood. He had no remarkable medical history apart from allergic rhinitis. After entering high school, he often felt unwell, especially in the morning, and frequently missed classes. He was diagnosed as having orthostatic dysregulation and prescribed amezinium metilsulfate 10 mg/day, but his symptoms persisted. Eight days after the start of treatment he was switched to etilefrine 5 mg/day. However, his fatigue progressively worsened. He was found vomiting and unresponsive after collapsing in the bathroom on the eighth night following the prescription change and taken to the hospital by his family. In the emergency room he exhibited mild consciousness disturbance (Glasgow Coma Scale: E4V4M6) and complained of right lower abdominal pain. Laboratory tests (blood and urine), whole-body computed tomography, and head magnetic resonance imaging did not indicate any abnormalities (serum creatinine level 1.0 mg/dL, uric acid level 7.2 mg/dL). His conscious state and abdominal pain were improved on the next day, but his blood pressure gradually increased from 100/60 to 180/80 mm Hg and his serum creatinine level rose from 1.0 to 5.5 mg/dL during 5 days of admission. He was then transferred to our institution for the treatment of AKI and severe hypertension.
At the time of admission to our hospital the patient was fully conscious and alert. His body temperature was 37.2°C, blood pressure was 161/98 mm Hg, heart rate was 83 beats/min, and respiratory rate was 17 breaths/min. His height was 174 cm and his body weight was 54 kg. Physical examination detected no signs of dehydration, rash, or other abnormalities of the neck, chest, abdomen, or extremities. He had been taking loratadine 10 mg/day for his allergic rhinitis for several months. Both loratadine and etilefrine had been discontinued upon admission to the previous hospital. There was no family history of kidney dysfunction, and he reported no episodes of intense exercise other than daily commuting by bicycle to school. No alcohol consumption, smoking, or illegal drug use were reported. His laboratory data at the time of transfer to our hospital are summarized in Table 1 . Urinalysis showed mild proteinuria (0.66 g/gCr) and elevation of the tubulointerstitial injury marker β2 microglobulin (1,498 μg/L). Hematuria was not observed. His serum level of uric acid was low at 3.2 mg/dL and his fractional excretion of uric acid was high at 49.7%. Laboratory markers of rhabdomyolysis, diabetes mellitus, infection, and collagen diseases such as creatine phosphokinase, hemoglobin A1c, C-reactive protein, and autoimmune antibodies were all within normal range. An electrocardiogram disclosed left anterior hemiblock and nonspecific intraventricular conduction delay that had been detected when he was an elementary school student. A chest X-ray revealed no abnormalities. Ultrasound echography showed bilateral mild kidney swelling with increased renal cortical echogenicity (Fig. 1 ). No stenotic lesions were detected in the aorta or renal arteries, although the resistance index of the intrarenal arteries was slightly high (left 0.69, right 0.69), indicating a circulatory disturbance in the renal microvessels. Hydronephrosis and renal calcification were absent. An ultrasound-guided kidney biopsy performed 3 days after arrival at our hospital showed mild interstitial edema, vascular endothelial cell swelling in the renal interlobular arterioles, and no obvious signs of acute tubular necrosis (ATN) (Fig. 2 ). Treatment with continuous intravenous infusion of extracellular fluids and nicardipine gradually improved his kidney function and hypertension. His serum uric acid level decreased to 1.0 mg/dL (Fig. 3 ), and his fractional excretion of uric acid was at 55.9% at 10 days after admission. He was ultimately diagnosed as having AKI with RHUC and discharged 12 days after transfer to our hospital. Hypouricemia was found in his parents and a sister, indicating a hereditary condition. However, genetic screening did not detect any known causative RHUC mutations on URAT1/SLC22A12 or GLUT9/SLC2A9 .
Main laboratory data on admission to our hospital

Renal ultrasound showed mild kidney swelling with increased renal cortical echogenicity. Hydronephrosis and renal calcification were not observed. Renal imaging findings were similar bilaterally (left 105 × 62 mm, right 115 × 63 mm).

Kidney biopsy specimen findings. Mild interstitial edema and vascular lumen narrowing by endothelial cell swelling (arrow) were detected (periodic acid-methenamine silver stain). No other abnormalities were found, including signs of acute tubular necrosis.

Clinical course of the present case. Vasopressors that had been administered for 15 days were discontinued on admission. After transfer to our hospital, his renal function improved gradually with continuous intravenous infusion of extracellular fluids and nicardipine. The serum uric acid level decreased steadily to 1.0 mg/dL during hospitalization.
Ishikawa et al. [ 6 ] first described EIAKI as AKI with accompanying abdominal or lower back pain after intense exercise, such as a 100-meter dash. EIAKI is differentiated from AKI with rhabdomyolysis by normal or slightly elevated serum myoglobin and creatine phosphokinase levels. EIAKI typically occurs in young males, with more than half having RHUC. Enhanced computed tomography often displays a wedge-shaped contrast defect in the kidneys. As for the clinical course of EIAKI, kidney dysfunction improves naturally without any special treatment [ 1, 7 ]. Although the reported patient had no intense episodes of exercise, EIAKI was diagnosed because he had RHUC, his kidney function recovered naturally, and he was young and male.
Blood pressure and serum creatinine level in our patient increased gradually following admission to the former hospital. As high blood pressure alone might cause AKI, we could not exclude the possible involvement of hypertension in AKI development. However, his serum creatinine level ultimately improved to 0.7 mg/dL after the final discharge despite having been 1.0 mg/dL on first admission, indicating that it had already been elevated by 0.3 mg/dL at the former hospital. Considering the fact that his blood pressure was normal on admission, AKI was thought to have developed before blood pressure elevation. Furthermore, his serum uric acid level was much higher on first admission (7.2 mg/dL) than at discharge (1.0 mg/dL), suggesting AKI onset prior to the former hospital visit. We suspected that AKI caused hypertension, which in turn worsened AKI. The elevation of blood pressure was assumed to be an exacerbation factor of EIAKI rather than its main cause.
The reported patient had no intense episodes of exercise. Lee et al. [ 3 ] described 17 AKI patients with abdominal or lower back pain who exhibited the characteristic patchy kidney sign on enhanced computed tomography. Among them, 5 patients reported no episodes of intense exercise. To the best of our knowledge, there have been 8 patients with EIAKI who did not have any episodes of intense exertion [ 2-4 ], with 5 experiencing infection or analgesic usage before EIAKI onset (Table 2 ), thought to be risk factors of EIAKI in addition to RHUC [ 3, 8, 9 ]. These reports support the notion that EIAKI can develop without intense exercise and the existence of risk factors other than strong exertion. However, to date no reports have focused on the relationship between lack of intense exercise and the etiology and development mechanism of EIAKI.
Clinical findings of current and previous reported cases of EIAKI without strenuous exercise

The pathomechanism of EIAKI is unclear, but renal circulatory disturbance by reactive oxygen species (ROS) is thought to be a main cause [ 5 ]. Intense exercise, such as anaerobic exertion, produces large amounts of ROS, which are rapidly removed by uric acid and other scavengers in the healthy population [ 8 ]. Patients with RHUC have insufficient scavengers, resulting in inadequate ROS removal and the subsequent activation of vasoconstrictive factors, vasoconstriction, and renal ischemia [ 2 ]. Since renal vasoconstriction is known to trigger further vasoconstriction and oxidative stress via activation of the renin-angiotensin system and blood pressure elevation [ 10 ], EIAKI patients are thought to show a vicious cycle between oxidative stress and vasoconstriction – oxidative stress causes stronger vasoconstriction and vasoconstriction causes more oxidative stress – culminating in acute and severe renal injury. In the present case, the patient had been taking vasopressors for orthostatic dysregulation for 15 days prior to the onset of AKI. Amezinium metilsulfate inhibits monoamine oxidase activity and suppresses the uptake of noradrenaline, while etilefrine activates type α1 and β1 adrenaline receptors. Thus, both vasopressors increased cardiac output and the constriction of peripheral vessels [ 11, 12 ]. Bellomo et al. [ 13 ] reported that activation of type α1 adrenaline receptors could cause excessive renal vasoconstriction and decreased renal blood flow in models of healthy renal hemodynamics. Radaković et al. [ 14 ] described that adrenaline induction increased ROS and caused a disruption in oxidant/antioxidant balance. Considering these results and the developmental mechanism of EIAKI (i.e., ROS and renal ischemia), we suspect that the vasopressors may have affected the onset or worsening of EIAKI by increasing ROS, exacerbating vasoconstriction, and forming a vicious cycle of diminished renal hemodynamics. Karasawa et al. [ 15 ] reported a case of EIAKI who was given midodrine, another vasopressor, before the onset of EIAKI, and Saito et al. [ 16 ] described that vasoexpansion by low-dose dopamine improved the resistance index of renal arterioles in 2 cases of EIAKI, implying the relation between vasopressors and EIAKI in clinical settings. Although no studies have directly addressed the relationship between vasopressors and EIAKI, past reports and our own results indicate an importance of catecholamine level homeostasis in the pathogenesis of EIAKI. We suspect that vasopressors may be associated with AKI onset in RHUC patients and may be a risk factor of EIAKI.
Renal biopsy showed no significant abnormalities in the present case. Although patients with EIAKI generally exhibit ATN, Ohta et al. [ 2 ] reported no abnormalities in 6 of 28 renal biopsies from EIAKI patients, which implied that EIAKI could develop without ATN. AKI with renal ischemia often causes ATN. However, tubular necrosis is sometimes absent without a sufficient degree or duration of ischemia, and early treatment for renal ischemia leads to a rapid improvement in renal function in such cases [ 17 ]. In the present patient, vasopressors, which might be a risk factor for EIAKI, were discontinued and intravenous antihypertensive medication was induced just after the first admission. The serum uric acid level was temporarily elevated on admission by AKI, and the patient’s scavenging ability with serum uric acid was thought to be temporarily improved. These factors could have mitigated the vicious cycle between renal vasoconstriction and oxidative stress, reduced the severity of renal ischemia, and prevented ATN development. However, as no studies have addressed the cause or meaning of a lack of ATN in some EIAKI patients, a greater number of studies are needed.
In conclusion, AKI can develop in patients with RHUC without intense exercise, possibly through the use of vasopressors. Further related case reports are needed to clarify the association between vasopressor use and AKI in patients with RHUC.
The present case report adhered to the Declaration of Helsinki. Informed consent for publication was obtained from the patient.
The authors declare no conflicts of interest.
The authors received no specific funding for this work.
D. Aomura drafted the article. K. Sonoda, M. Harada, and K. Hashimoto revised the article critically for important intellectual content. Y. Kamijo revised the article critically for important intellectual content and gave final approval of the version to be submitted.

Email alerts
Citing articles via, suggested reading.
- Online ISSN 2296-9705
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account

MICHAEL G. MERCADO, MD, DUSTIN K. SMITH, DO, AND ESTHER L. GUARD, DO
Am Fam Physician. 2019;100(11):687-694
Author disclosure: No relevant financial affiliations.
Acute kidney injury is a clinical syndrome characterized by a rapid decline in glomerular filtration rate and resultant accumulation of metabolic waste products. Acute kidney injury is associated with an increased risk of mortality, cardiovascular events, and progression to chronic kidney disease. Severity of acute kidney injury is classified according to urine output and elevations in creatinine level. Etiologies of acute kidney injury are categorized as prerenal, intrinsic renal, and postrenal. Accurate diagnosis of the underlying cause is key to successful management and includes a focused history and physical examination, serum and urine electrolyte measurements, and renal ultrasonography when risk factors for a postrenal cause are present (e.g., older male with prostatic hypertrophy). General management principles for acute kidney injury include determination of volume status, fluid resuscitation with isotonic crystalloid, treatment of volume overload with diuretics, discontinuation of nephrotoxic medications, and adjustment of prescribed drugs according to renal function. Additional supportive care measures may include optimizing nutritional status and glycemic control. Pharmacist-led quality-improvement programs reduce nephrotoxic exposures and rates of acute kidney injury in the hospital setting. Acute kidney injury care bundles are associated with improved in-hospital mortality rates and reduced risk of progression. Nephrology consultation should be considered when there is inadequate response to supportive treatment and for acute kidney injury without a clear cause, stage 3 or higher acute kidney injury, preexisting stage 4 or higher chronic kidney disease, renal replacement therapy, and other situations requiring subspecialist expertise.
Acute kidney injury is defined as the sudden loss of kidney function over hours to days resulting in the inability to maintain electrolyte, acid-base, and water balance. Because of an aging population and increasing prevalence of hypertension and diabetes mellitus, from 2005 to 2014, the number of hospitalizations with a principal diagnosis of acute kidney injury increased from 281,500 to 504,600, and the number of hospitalizations with a secondary diagnosis of acute kidney injury increased from 1 million to 2.3 million. 1 Patients with acute kidney injury requiring renal dialysis and other forms of renal replacement therapy are 50 times more likely to progress to chronic kidney disease than those not requiring renal replacement therapy. 2 Risk factors for acute kidney injury are listed in Table 1 . 3 – 6
A universal definition and staging system for acute kidney injury proposed by the Kidney Disease: Improving Global Outcomes (KDIGO) group merges the earlier RIFLE (risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, end-stage renal disease) and Acute Kidney Injury Network definitions. 7 – 9 The KDIGO system ( Table 2 7 ) is used in this article.
Acute kidney injury is a complex clinical syndrome with prerenal, intrinsic renal, and postrenal etiologies. 10 Table 3 summarizes these etiologies. 10 – 13
PRERENAL CAUSES
Prerenal acute kidney injury is associated with decreased renal perfusion and glomerular filtration rate (GFR) caused by intravascular volume depletion secondary to hypovolemia, peripheral vasodilation, decreased arterial pressures, and impaired cardiac function resulting in decreased cardiac output. 14 Sepsis is the most common cause of acute kidney injury seen in the intensive care unit (ICU). 15 Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs are the most common medications that lower renal perfusion.
The kidneys activate mechanisms to compensate for the reduced renal perfusion in an attempt to maintain the GFR. 14 However, patients with impairment to these mechanisms, such as those with chronic kidney disease, have an elevated risk of acute kidney injury. 3
INTRINSIC RENAL CAUSES
Intrinsic renal causes of acute kidney injury are categorized by the location of the injury, most commonly the glomerulus or tubule, and include the interstitial or vascular portions of the kidney. 11 Intrinsic acute kidney injury requires early identification and prompt subspecialty consultation.
Immune complexes from systemic illness (e.g., membranoproliferative glomerulonephritis, polyarteritis nodosa) cause acute inflammation and structural damage to the glomeruli. Acute tubular necrosis, the most common intrinsic kidney injury, is damage to the tubular cells of the kidney from ischemic or nephrotoxic causes. Ischemic causes include prolonged periods of severe hypotension, hypovolemia, or hypoperfusion to the kidneys (e.g., from hemorrhage, shock, sepsis, cirrhosis, peritonitis, or infarcts) that do not improve with rehydration. 11 Nephrotoxic causes include endogenous and exogenous toxins.
Acute interstitial nephritis, a common cause of acute kidney injury, is most often due to a hypersensitivity reaction to medications, usually an antibiotic or nonsteroidal anti-inflammatory drug. 16 Acute interstitial nephritis related to proton pump inhibitors is increasingly common, especially in older people. 17 , 18 Infections cause 5% to 10% of acute interstitial nephritis cases. 16 Vascular causes of acute kidney injury include large vessel diseases, such as renal artery thrombosis; embolism; stenosis; and operative renal arterial clamping. 11
POSTRENAL CAUSES
Postrenal acute kidney injury is due to extrarenal obstruction of urinary flow. Causes include neurogenic bladder; retroperitoneal fibrosis; and the tumor burden of bladder, prostate, or cervical cancer. Prostatic hypertrophy is the most common cause in older men. 11
The history and physical examination are important in determining the etiology of acute kidney injury. The history can identify nephrotoxic medications or a systemic illness contributing to impaired renal function. The physical examination should focus on evaluating intravascular volume status. Skin rashes may indicate an underlying condition (e.g., systemic lupus erythematosus, atheroembolism/vasculitis) or exposure (e.g., drug rash suggesting acute interstitial necrosis) leading to acute kidney injury. 11
SERUM CREATININE LEVEL
The serum creatinine level, which is part of the diagnostic criteria for acute kidney injury, is easily obtained. However, it is not an ideal marker, because creatinine concentration is influenced by age, sex, race, muscle mass, and protein catabolic rate. Additionally, serum creatinine is a slow changing surrogate for decreased GFR and may take 24 to 72 hours to reach a new steady state following acute kidney injury. 6

URINE OUTPUT
Urine output can be difficult to accurately assess because of collection and documentation errors. Serum creatinine or urine output can be used for diagnosis of acute kidney injury, although patients who meet diagnostic criteria for both are at increased risk of mortality from renal replacement therapy and hospitalization. 7 , 19
CREATININE CLEARANCE
Creatinine clearance is a direct measure of GFR, and serial creatinine clearance testing provides a more efficient and accurate assessment of renal function than serum creatinine testing. 20 Creatinine clearance can be performed in collection periods of one to 24 hours, although longer collection times increase the likelihood of errors related to inaccurate time recording and incomplete collection. 6 A cohort study of 484 patients in the ICU found that four-hour creatinine clearance testing is a valid measurement of acute kidney injury (defined as an increase in serum creatinine greater than 50% in the control group or a decrease in creatinine clearance greater than 33% in the intervention group). A decrease of greater than 33% in the first 12 hours conferred a twofold elevated risk of dialysis or death. 20
URINALYSIS AND URINE MICROSCOPY
Urinalysis in combination with urine microscopy provides insight into the location and cause of acute kidney injury. Table 4 summarizes common findings and associated diagnoses based on urine evaluation. 21
URINE ELECTROLYTES
The fractional excretion of sodium and the fractional excretion of urea are used to identify prerenal azotemia. Online tools for calculating fractional excretion of sodium and urea are available at https://www.mdcalc.com/fractional-excretion-sodium-fena and https://www.mdcalc.com/fractional-excretion-urea-feurea .
A fractional excretion of sodium less than 1% suggests a prerenal cause of acute kidney injury, whereas a value greater than 2% suggests an intrinsic cause. A fractional excretion of urea less than 35% suggests a prerenal cause, whereas a value greater than 50% suggests an intrinsic cause. Interpretation of urine electrolytes is limited because it is a single measure in time, and the results are confounded by acute volume changes. Fractional excretion of urea is more sensitive in patients with increased sodium excretion caused by diuretic therapy. 22
OTHER TESTS
Renal ultrasonography may show evidence of a postrenal cause of acute kidney injury but should be performed only when the history suggests the presence of urinary tract obstruction. 23 Renal biopsy is reserved for patients with intrinsic acute kidney injury of unclear etiology or when diagnostic confirmation is necessary before initiating disease-specific therapy.
Management of acute kidney injury is primarily supportive, with the goals of preventing further damage and promoting recovery of renal function. 7 Figure 1 is a suggested approach to the management of acute kidney injury based primarily on expert opinion. 11 , 24 The prompt diagnosis and treatment of the underlying cause is critical. 12
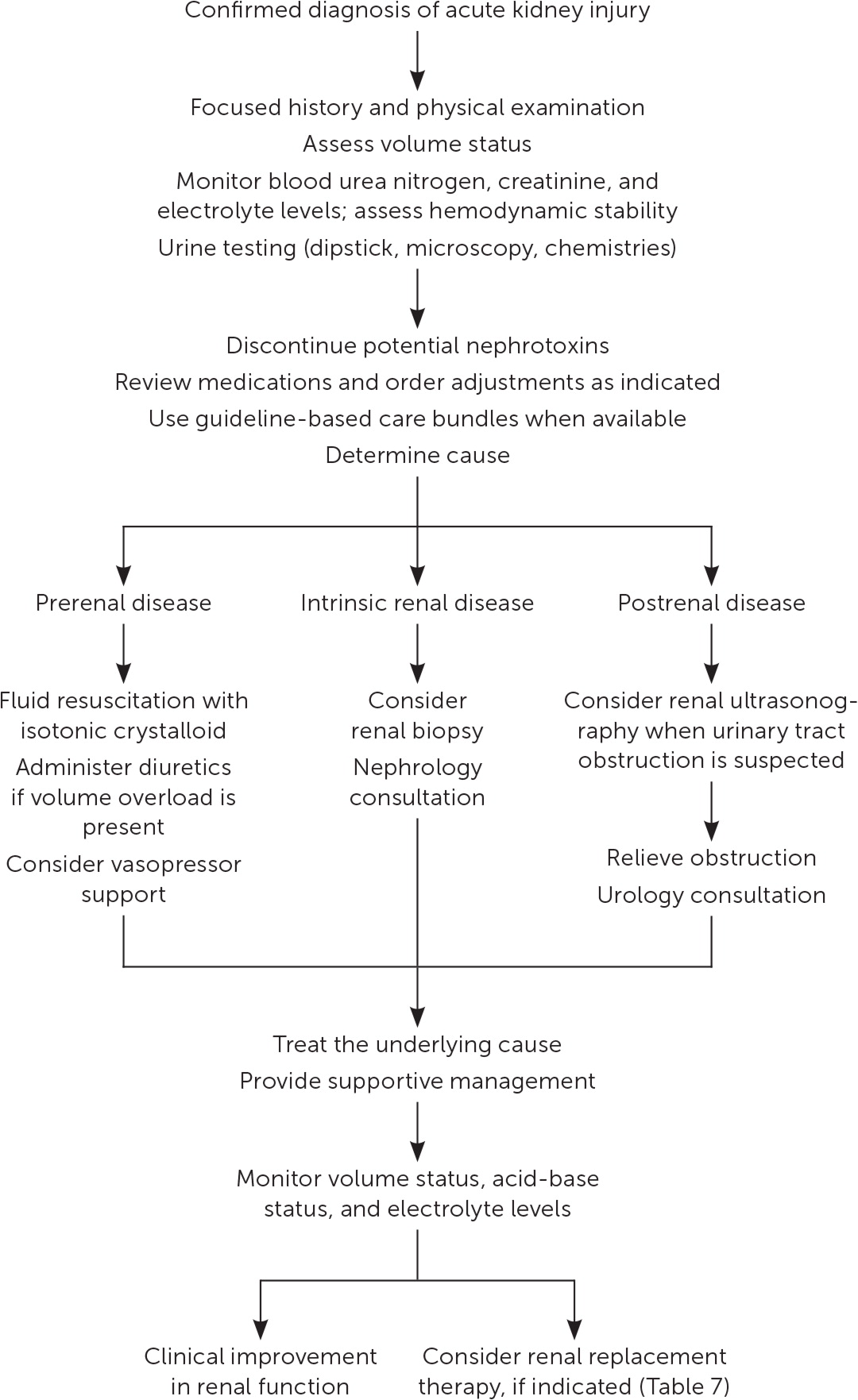
FLUID RESUSCITATION
An assessment of volume status and hemodynamic stability is a key component in the management of patients with acute kidney injury because fluid overload is associated with increased mortality. 25 Consequently, a delicate balance exists between optimizing renal perfusion and avoiding fluid overload. 26
If fluid resuscitation is indicated, isotonic crystalloids (e.g., 0.9% normal saline, lactated Ringer solution, Plasma-Lyte A) are recommended over colloids (e.g., albumin, dextran) as the initial therapy. 7 , 27 , 28 Excess chloride may be associated with worsening renal function and acid-base disturbances. 29 A prospective study of patients in the ICU found that a chloride-restrictive strategy for resuscitation was associated with a lower incidence of acute kidney injury and need for renal replacement therapy. 30 Subsequently, two trials comparing balanced crystalloids with 0.9% sodium chloride demonstrated improved composite renal outcomes (mortality, need for renal replacement therapy, and persistent renal dysfunction) in the balanced crystalloid group for both critically ill patients (absolute risk reduction [ARR] = 1.1%; number needed to treat [NNT] = 91) and non– critically ill patients (ARR = 0.9%; NNT = 111). 31 , 32
A mean arterial pressure goal of 65 mm Hg or greater is acceptable, and vasopressors may be required if this is not achieved through fluid resuscitation. An online calculator to determine mean arterial pressure is available at https://www.mdcalc.com/mean-arterial-pressure-map . Protocol-based strategies are recommended to prevent and improve acute kidney injury in high-risk patients (e.g., those who are postoperative or in septic shock). 7 A randomized controlled trial (RCT) of 776 patients with septic shock compared outcomes with a mean arterial pressure goal of 65 to 70 mm Hg vs. a goal of 80 to 85 mm Hg. No mortality difference was observed between the groups, but in a subset of patients with chronic hypertension, the higher goal group had lower rates of acute kidney injury (ARR = 13%; NNT = 8) and renal replacement therapy (ARR = 11%; NNT = 10). 33
AVOIDANCE OF NEPHROTOXICITY
A review of medications requiring discontinuation, dose adjustment, or monitoring is critical to the management of acute kidney injury ( Table 5 and Table 6 ) . 12 In addition, the implementation of pharmacist-led quality-improvement programs is associated with reductions in nephrotoxic exposures and rates of acute kidney injury in the hospital setting. 34
ADDITIONAL MANAGEMENT CONSIDERATIONS
Because of a lack of benefit, diuretics are not recommended for the treatment or prevention of acute kidney injury, except to alleviate volume overload. 7 For ICU patients, a plasma glucose target of 110 to 149 mg per dL (6.1 to 8.3 mmol per L) is recommended, although this target has not been studied in RCTs. 7 Nutritional status should be evaluated, and dietary recommendations should be based on the underlying cause and severity of the acute kidney injury. 7 , 12
If metabolic derangements from acute kidney injury do not respond to conservative treatment, renal replacement therapy, in consultation with a nephrologist, may be required. Table 7 includes indications for initiating renal replacement therapy. 7 , 35 – 37 A multicenter RCT of 488 patients with acute kidney injury and septic shock compared early initiation of renal replacement therapy (within 12 hours) with delayed initiation (48 hours) and found no difference in 90-day mortality. 38
Early nephrology consultation (within 48 hours) appears to be beneficial for patients with acute kidney injury. 39 In addition to when initiating renal replacement therapy, nephrology consultation should be considered when there is inadequate response to supportive treatment and for acute kidney injury without a clear cause, stage 3 or higher acute kidney injury, stage 4 or higher chronic kidney disease, and other situations requiring specialist expertise (e.g., renal transplant, glomerulonephritis, multiple myeloma). 36
Inpatient data from a health care system found acute kidney injury care to be optimal only 50% of the time. 40 Multimodal educational programs delivered to clinicians have shown improvements in clinician self-assessment of acute kidney injury care. 41 Acute kidney injury care bundles, a specific set of guideline-based diagnostic and therapeutic interventions, are associated with improved in-hospital mortality rates and reduced risk of progression in observational studies. 42
The transition from the hospital to the outpatient setting presents an opportunity to improve the care of patients with acute kidney injury. Follow-up three months after hospitalization is reasonable if renal function is recovered (90% or greater from baseline), with earlier follow-up intervals (at three weeks and then again at three months) for patients with a slower recovery. 43 Blood pressure, weight, serum creatinine level, and GFR should be measured at each visit. Nephrology consultation is recommended if the estimated GFR remains less than 60 mL per minute per 1.73 m 2 . 43 The optimal duration of monitoring after acute kidney injury is unclear.
Stage 3 acute kidney injury requiring renal replacement therapy is associated with mortality rates between 44% and 52%. 44 , 45 Observational studies have shown an increased risk of developing chronic kidney disease following acute kidney injury. 3 In a cohort study that followed hospitalized Medicare beneficiaries for two years after discharge, acute kidney injury was associated with a 13-fold increased risk of end-stage renal disease in patients without preexisting chronic kidney disease and a 40-fold increase in patients with both acute kidney injury and chronic kidney disease. 5 Acute kidney injury is also associated with an increased risk of cardiovascular mortality, acute myocardial infarction, and heart failure. 46 , 47 A retrospective cohort study of 2,451 hospitalized patients with acute kidney injury found that they had a 22% increased risk of developing hypertension within six months. 48
An individualized approach to implementing preventive strategies is based on the presence of clinical situations that increase the risk of acute kidney injury, such as exposure to intravenous contrast media and being in the perioperative period. Use of periprocedural normal saline and minimizing the volume of contrast media reduce the risk of contrast media–induced acute kidney injury. 49 Sodium bicarbonate–based intravenous fluids are not superior to normal saline in preventing acute kidney injury. 50
A meta-analysis of 15 RCTs (n = 6,532) showed that in patients undergoing coronary angiography or percutaneous coronary intervention, high-dose statins (e.g., atorvastatin [Lipitor], rosuvastatin [Crestor], simvastatin [Zocor]) reduced the incidence of contrast media–induced acute kidney injury when compared with low-dose statins or placebo (ARR = 2.8%; NNT = 36). 51 A Cochrane review of 72 studies (n = 4,378) found no convincing evidence that any pharmacologic intervention reduces the risk of acute kidney injury during the perioperative period. 52
This article updates previous articles on this topic by Rahman, et al. 13 ; Needham 53 ; and Agrawal and Swartz . 54
Data Sources: This manuscript was based on literature identified in Essential Evidence Plus, PubMed Clinical Queries, the Agency for Healthcare Research and Quality, the Cochrane Database of Systematic Reviews, and Google Scholar using the search terms acute kidney injury and acute renal failure. References from those sources were also searched. Search dates: October 2018, January 2019, April 2019, and August 2019.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Navy, U.S. Air Force, Department of Defense, or the U.S. government.
Moore BJ, Torio CM. Healthcare Cost and Utilization Project. Statistical brief #231. Acute renal failure hospitalizations, 2005–2014. Accessed January 22, 2019. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb231-Acute-Renal-Failure-Hospitalizations.pdf
Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361-1369.
Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58-66.
Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223-228.
Macedo E, Mehta RL. Clinical approach to the diagnosis of acute kidney injury. In: Gilbert SJ, Weiner DE, eds. Primer on Kidney Diseases . 7th ed. National Kidney Foundation; 2018:300–310.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(suppl 1):1-138.
Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212.
Mehta RL, Kellum JA, Shah SV, et al. Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204.
Sharfuddin AA, Weisbord SD, Palevsky P, et al. Acute kidney injury. In: Skorecki K, Chertow GM, Marsden PA, et al., eds. Brenner and Rector's The Kidney . 10th ed. Elsevier; 2016:958–1011.
Moore PK, Hsu RK, Liu KD. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis. 2018;72(1):136-148.
Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639. Accessed September 5, 2019. https://www.aafp.org/afp/2012/1001/p631.html
Badr KF, Ichikawa I. Prerenal failure: a deleterious shift from renal compensation to decompensation. N Engl J Med. 1988;319(10):623-629.
Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816-828.
Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956-961.
Muriithi AK, Leung N, Valeri AM, et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int. 2015;87(2):458-464.
Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26(9):2231-2238.
Pickering JW, Frampton CM, Walker RJ, et al. Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit Care. 2012;16(3):R107.
Perazella MA. The urine sediment as a biomarker of kidney disease. Am J Kidney Dis. 2015;66(5):748-755.
Gotfried J, Wiesen J, Raina R, et al. Finding the cause of acute kidney injury. Cleve Clin J Med. 2012;79(2):121-126.
Podoll A, Walther C, Finkel K. Clinical utility of gray scale renal ultrasound in acute kidney injury. BMC Nephrol. 2013;14:188.
Kellum JA, Bellomo R, Ronco C. Progress in prevention and treatment of acute kidney injury: moving beyond kidney attack. JAMA. 2018;320(5):437-438.
Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422-427.
Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10(1):37-47.
Finfer S, Bellomo R, Boyce N, et al.; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247-2256.
Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis [published correction appears in JAMA . 2013;309(12):1220]. JAMA. 2013;309(7):678-688.
Vanmassenhove J, Kielstein J, Jörres A, et al. Management of patients at risk of acute kidney injury. Lancet. 2017;389(10084):2139-2151.
Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566-1572.
Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839.
Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828.
Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583-1593.
Goldstein SL, Mottes T, Simpson K, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90(1):212-221.
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756-766.
National Institute for Health and Care Excellence. Clinical guideline 169. Acute kidney injury: prevention, detection, and management. Accessed December 1, 2018. https://www.nice.org.uk/guidance/cg169
Mehta RL. Indications for dialysis in the ICU: renal replacement vs. renal support. Blood Purif. 2001;19(2):227-232.
Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431-1442.
Mehta RL, McDonald B, Gabbai F, et al. Nephrology consultation in acute renal failure: does timing matter?. Am J Med. 2002;113(6):456-461.
Stewart J, Findlay G, Smith N, et al. Adding insult to injury: a review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). Accessed April 3, 2019. https://www.ncepod.org.uk/2009report1/Downloads/AKI_report.pdf
Xu G, Baines R, Westacott R, et al. An educational approach to improve outcomes in acute kidney injury (AKI). BMJ Open. 2014;4(3):e004388.
Kolhe NV, Reilly T, Leung J, et al. A simple care bundle for use in acute kidney injury. Nephrol Dial Transplant. 2016;31(11):1846-1854.
Vanmassenhove J, Vanholder R, Lameire N. Points of concern in post acute kidney injury management. Nephron. 2018;138(2):92-103.
Palevsky PM, Zhang JH, O'Connor TZ, et al.; VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury [published correction appears in N Engl J Med . 2009;361(24):2391]. N Engl J Med. 2008;359(1):7-20.
Bellomo R, Cass A, Cole L, et al.; RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627-1638.
Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961-973.
Odutayo A, Wong CX, Farkouh M, et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377-387.
Hsu CY, Hsu RK, Yang J, et al. Elevated BP after AKI. J Am Soc Nephrol. 2016;27(3):914-923.
Ahmed K, McVeigh T, Cerneviciute R, et al. Effectiveness of contrast-associated acute kidney injury prevention methods: a systematic review and network meta-analysis. BMC Nephrol. 2018;19(1):323.
Solomon R, Gordon P, Manoukian SV, et al. Randomized trial of bicarbonate or saline study for the prevention of contrast-induced nephropathy in patients with CKD. Clin J Am Soc Nephrol. 2015;10(9):1519-1524.
Gandhi S, Mosleh W, Abdel-Qadir H, et al. Statins and contrast-induced acute kidney injury with coronary angiography. Am J Med. 2014;127(10):987-1000.
Zacharias M, Mugawar M, Herbison GP, et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2013(9):CD003590.
Needham E. Management of acute renal failure. Am Fam Physician. 2005;72(9):1739-1746. Accessed September 5, 2019. https://www.aafp.org/afp/2005/1101/p1739.html
Agrawal M, Swartz R. Acute renal failure [published correction appears in Am Fam Physician . 2001;63(3):445]. Am Fam Physician. 2000;61(7):2077-2088. Accessed September 5, 2019. https://www.aafp.org/afp/2000/0401/p2077.html
Continue Reading
More in AFP
More in pubmed.
Copyright © 2019 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

IMAGES
VIDEO