- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
18k Accesses
36 Citations
14 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
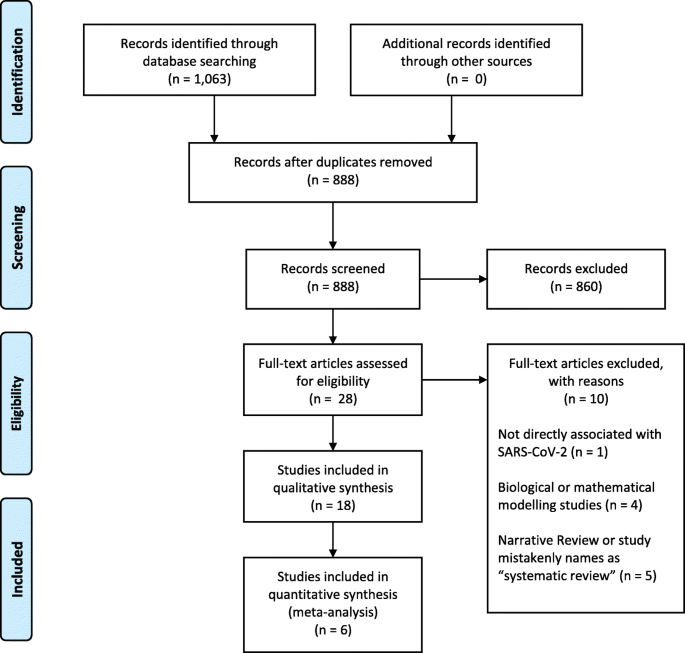
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
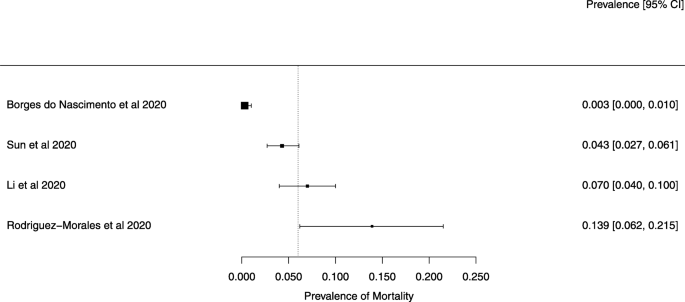
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
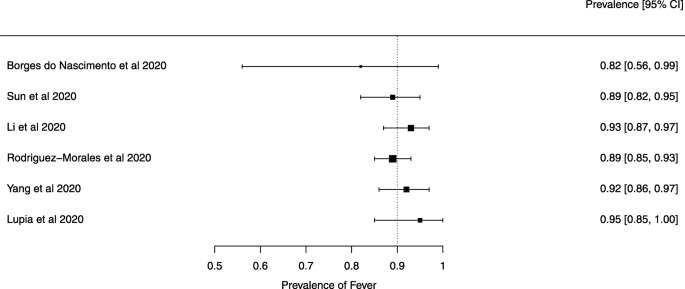
A meta-analysis of the prevalence of fever
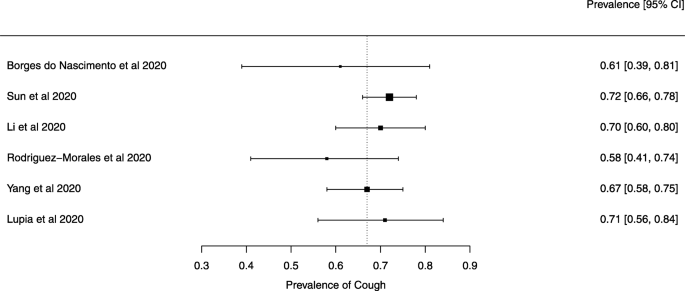
A meta-analysis of the prevalence of cough
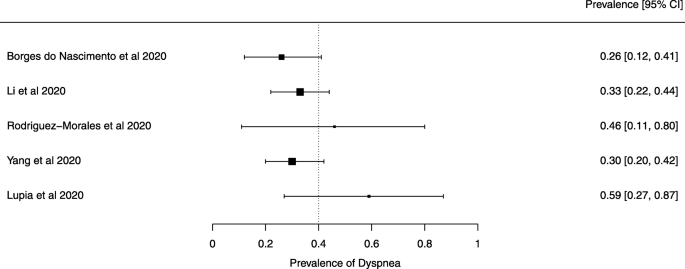
A meta-analysis of the prevalence of dyspnea
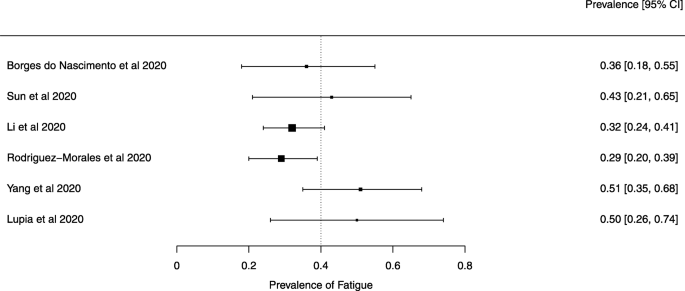
A meta-analysis of the prevalence of fatigue or myalgia
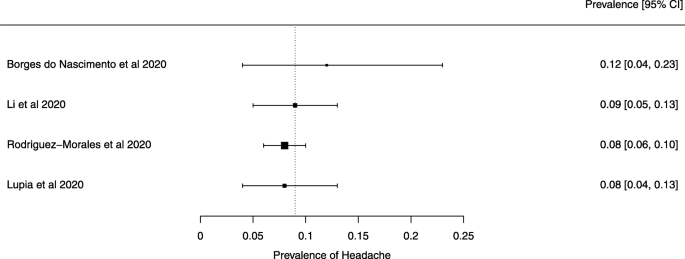
A meta-analysis of the prevalence of headache
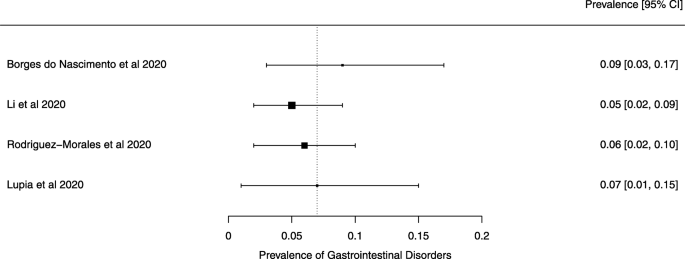
A meta-analysis of the prevalence of gastrointestinal disorders
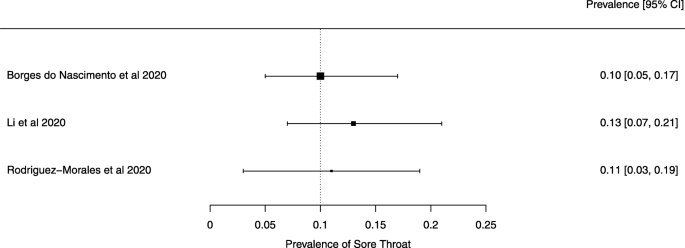
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
The most influential COVID-19 articles: A systematic review
Suhaib js ahmad, konstantinos degiannis, joseph borucki, sjaak pouwels, david laith rawaf, marion head, chun hei li, rami archid, ahmed r ahmed, katie mellor, doerte wichmann, aristomenis exadaktylos.
- Author information
- Article notes
- Copyright and License information
Corresponding author. Department of General Surgery, Betsi Cadwaladr University Health Board, Wales, UK. [email protected]
Suhaib JS. Ahmad and Konstantinos Degiannis contributed equally to this manuscript.
Received 2022 Aug 22; Revised 2023 Jan 27; Accepted 2023 Feb 2; Collection date 2023 Mar.
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),causative pathogen of coronavirus disease 2019 (COVID-19), has triggered a pandemic with challenges for health care systems around the world. Researchers have studied and published on the subject of SARS-CoV-2 and the disease extensively. What is the significance of articles published, shared and cited in the early stages of such a pandemic?
Materials and methods
A systematic literature search in a time frame of 12 months and analysis rating using Principle Component Analysis (PCA) and Multiple Factor Analysis (MFA) were performed.
The 100 most cited COVID-19 articles were identified. The majority of these articles were from China (n = 54), followed by United States of America (USA) (n = 21) and United Kingdom (UK) (n = 8). All articles were published in high-ranked, peer-reviewed journals, with research focusing onthe the diagnosis, transmission and therapy of COVID-19. The level of evidence of the 100 most cited COVID-19 articles on average was low.
In the early stages of a pandemic, new and innovative research can emerge and be highly cited, regardless of the level of evidence.
Keywords: COVID-19, Intensive care, ICU care, Citations, SARS-CoV-2
1. Introduction
The onset of the COVID-19 pandemic was not only a test of resilience for the human race, but it also put scientists through their paces. In being a novel virus there was initally a lack of literature to aid the medical workforce; it fast became a race for scientists to contribute to the evidence-base to guide management of unwell patients accordingly. Newly proposed treatments based on anecdotal evidence were being used across the world, however policy-makers and those treating patients on the ‘front-line’ were unable to rely on such data alone for assurance that these novel treatments would be best for patient care. Some countries such as the UK with NICE guidelines, heavily rely on validated and peer-reviewed evidence in order to formulate treatment guidelines and regimens.
One of the largest barriers to clinical confidence in hastily published ‘COVID-19’ articles is the distinct lack of high hierarchical levels of evidence. Whilst this could largely be due to the lack of time alongside intense pressure to publish research, there may also be a general lack of understanding that results from case-studies of small sample sizes cannot be extrapolated to be true of entire populations.
This paper aims to highlight, understand and assess the top 100 most-cited articles published under the topic of COVID-19 through a systematic search using stringent inclusion and exclusion criteria. As shown in the results section, most papers originated from China (n = 54) and USA (n = 21). Difficulties with translations of Chinese papers were found to be an issue (although most were published in English), with their focus on diagnosis, mechanism, transmission and treatment, whilst Western papers focused only on transmission and treatment.
Using Principle Component Analysis (PCA) and Multiple Factor Analysis (MFA) of the filtered search results, this systematic review explores the possible correlations between objective metrics including: number of citations, density, article age, hierarchical evidence level and impact factors. Our findings suggest that pioneering evidence was published and subsequently heavily cited regardless of the level of evidence (mainly levels IV & V). We hope that this review will be of use to those contributing to the evidence base in future time-pressured scenarios such as subsequentnovel pathogen emergences.
2. Materials and methods
The Web of Science and the iSearch COVID-19 portfolio were utilised as effective tools for retrieval of citation information of published Covid-19 articles.
The Web of Science provides comprehensive citation data for articles published in Medline, Web of Science Core Collection, BIOSIS Citation Index, KCI-Korean Journal Database, Russian Science Citation Index, and SciELo Citation index [ [1] , [2] , [3] ]. Topic fields of articles (title, abstract, author's keywords and keywords within a record) were searched for the following keywords:
“Wuhan Coronavirus” OR “Wuhan Seafood Market Pneumonia Virus” OR “COVID19” OR “COVID-19” OR “COVID-2019” OR “Coronavirus Disease 2019” OR “SARS-CoV-2” OR SARS2 OR “2019-nCoV” OR “2019 Novel Coronavirus” OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “2019 Novel Coronavirus Infection” OR “Coronavirus Disease 2019” OR “Coronavirus Disease-19” OR “Novel Coronavirus” OR “Coronavirus” OR “SARS-CoV-2019” OR “SARS-CoV-19”.
The iSearch COVID-19 portfolio is the National Institute of Health's comprehensive source for publications related to COVID-19. It demonstrates cutting-edge analytical capabilities and is updated daily.
Only COVID-related articles submitted after 31/12/2019 (first reported COVID-19 case) were included in the study and the 100 most cited articles were identified and evaluated by two independent reviewers ( Fig. 1 ).
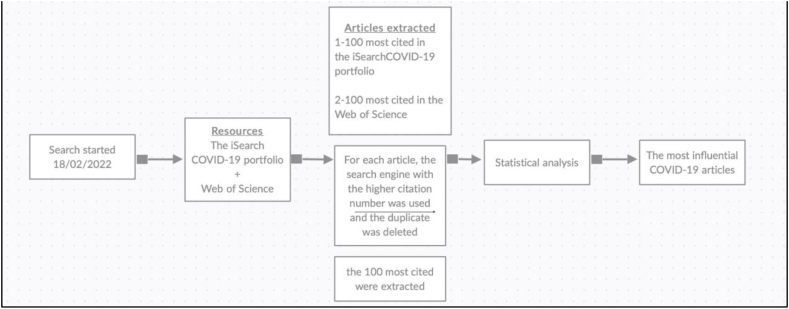
Flow diagram demonstrating the methodology and data extraction.
COVID-19 articles were classified and assigned a level of evidence.
The levels of evidence (I–V) were adapted from the National Health and Medical Research Council (NHMRC) and The Centre for Evidence-Based Medicine (CEBM) [ 4 ].
Articles were categorized, using LitCovid, by different research topics as following:
Clinical Features, Mechanism, Diagnosis, Treatment, Transmission, Prevention, Forecasting and General [ 5 ].
2.1. Statistical analysis
Statistical analyses were conducted using the R programming language. Normality of data was checked using the Shapiro-Wilk test. The distribution of a parameter was characterised by the median and interquartile range. The Kendall rank correlation coefficient was used to measure the ordinal association between two values. Multiple Factor Analysis (MFA) was used to analyse quantitative variables simultaneously. A P -value of <0.05 was considered statistically significant. Microsoft Excel software was used for descriptive statistical analysis.
∗Any disagreements between the reviewers were resolved via consensus.
Table 1 gives an overview of the top 100 most cited articles on COVID-19. All articles were published in 2020 (100%). The highest number of citations was 18958 and the lowest number was 1410. The median age of the articles was 21 months (range 13–24). In terms of levels of evidence - 14 articles were evidence level I, 7 were level II, 12 were III, 45 were IV and 22 were V.
Overview of the top 100 cited COVID-19 articles (∗ next to rank number indicates systematic review).
13 articles were basic science, 2 case control studies, 4 case reports, 32 case series, 3 clinical consensus articles, 12 cohort studies, 8 cross sectional studies, 5 expert opinions, 7 randomised controlled trials and 14 systematic reviews ( Fig. 2 ).
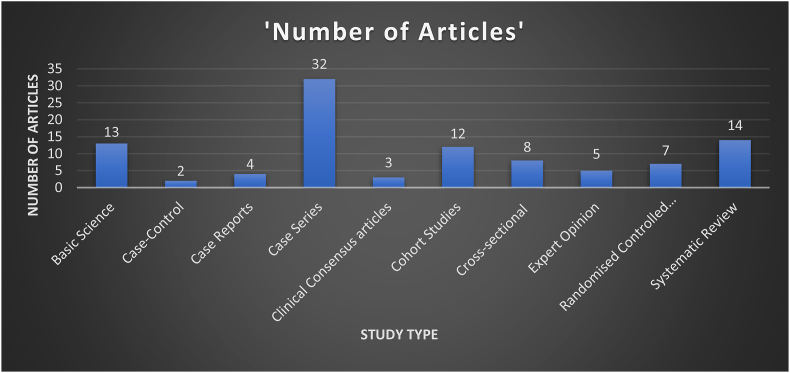
The hundred most cited Covid-19 articles study types.
The Top 100 cited COVID-19 originated from 13 countries ( Fig. 3 ), of which more than half were from China (n = 54), followed by United States of America (USA) (n = 21); United Kingdom (UK) (n = 8); Germany (n = 4); Italy (n = 4); Netherlands (n = 2); Brazil (n = 1); Canada (n = 1); Colombia (n = 1); France (n = 1); Singapore (n = 1); Switzerland (n = 1) and Taiwan (n = 1).
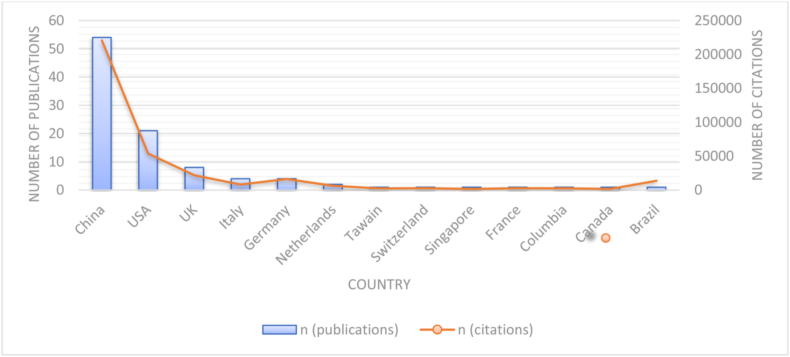
Number of publications and citations per country.
Table 2 shows the journals in which the top 100 cited COVID-19 articles were published with accompanying journal metrics. Of the 100 cited COVID-19 articles, 13 were published in the New England Journal of Medicine (NEJM), followed by 21 in the Lancet or associated journals (The Lancet (n = 13), Lancet Infectious Disease (n = 4), Lancet Respiratory medicine (n = 2), Lancet Psychiatry (n = 1), Lancet Oncology (n = 1)); 13 in Nature or associated journal (Nature (n = 7), Nature Medicine (n = 3), Nature Microbiology (n = 1), Nature Reviews Cardiology (n = 1), Nature Review Immunology (n = 1)); 13 in JAMA or associated journals (JAMA (n = 8), JAMA Neurology (n = 1), JAMA Network Open (n = 1), JAMA Cardiology (n = 2), JAMA Internal Medicine (n = 1)), 4 in Cell and 3 articles in Science.
Journals in which top 100 cited COVID-19 articles were published with accompanying journal metrics.
Articles published in China concentrated on the diagnosis, mechanism, transmission and treatment of COVID-19. On the other hand, articles published in Europe and the USA mainly focused on the transmission and the treatment of the virus ( Table 3 ).
Articles topic field.
Principle Component Analysis (PCA) revealed a strong correlation between the number of citations and the citation density of citations. Furthermore, there was also a strong correlation between the age of the article, the level of the evidence and the impact factor. There was a significant trend towards increased frequency of citations with age of the article (r = 0.26, P = 0.0004). The number of citations an article had was not significantly associated with the level of evidence (r = 0.152, p = 0.152) ( Fig. 4 ).
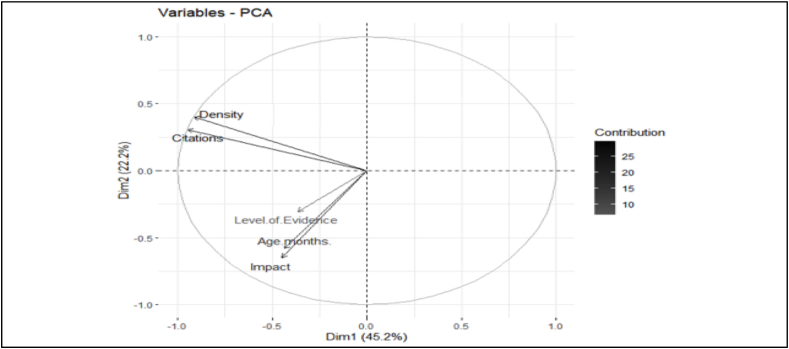
Principal component analysis of the relationship between citation number, citation density, level of evidence and Impact.
4. Discussion
This systematic review identified the 100 most cited articles on Covid-19 and sought to identify trends within them by applying citation analysis techniques. In late 2019, the COVID-19 pandemic presented one of the greatest challenges of the modern scientific era. With an estimated 503,862 deaths worldwide reported within the first 6 months of 2020 [ 6 ], the gravity and urgency of the problem required rapid advancement in knowledge to a degree not previously seen. It is unsurprising that with the amount of funding and resource invested, great volumes of scientific literature were produced in a relatively short period of time. What is surprising is the degree to which this occurred. Despite the first case of COVID-19 being only reported in December 2019, by July 2020 over 27,000 COVID-19 related articles had been published [ 7 ], with Covid related articles accounting for more scientific publication then all other topics combined. This unprecedented level of publication provokes many questions around the quality of research and the readiness of article acceptance [ [8] , [9] , [10] , [11] , [12] ].
All the articles were published in 2020 with a mean article age of 21 months (range 13–24 months). There was a weak but significant association between age and citation number; as citations take time to accumulate and consequently more recently published articles may not yet have achieved sufficient citations to have entered the review. The weakness of this relationship is likely a result of the short time-frame over which the articles have accrued the citations. The most highly cited article has been cited 18958 times and had a citation density of 790, the median citations was 2434.5 (IQR 1989.5–3749.0) and median citation density was 117.5 (IQR 89.5–185.2). This is particularly impressive as a variety of other citation classics have reported significantly lower median citations despite covering time periods of many years [ 2 , [13] , [14] , [15] , [16] ]. On average a journal article will peak in citation density approximately 3 years after publication [ 17 ] which presents a potential problem in applying citation analysis to a novel and rapidly evolving field. The strong correlation between density and citation number combined suggests that highly cited articles continue to be cited and may be establishing ‘authority’ status. Given the ongoing expansion in literature there is a risk that articles, considered powerful by traditional metrics, may already be scientifically out of date but not yet past their peak in terms of citation accrual.
54% of the articles originated from China which is unusual for citation classics reviews. Similar reviews on other topics tend to draw most of their articles from the USA [ 1 , 2 , [13] , [14] , [15] ].This is likely explained by early geographic distribution of cases which would have granted a significant advantage for Chinese-based labs, resulting in earlier publication and thus citation accrual. Interestingly the USA provided almost half of the remaining articles, which allowing for the above explanation is in keeping with what would be expected. The early geographical distribution of cases may also explain diagnosis playing a significant role in articles from China but not from the rest of the world.
Articles representing level IV and V levels of evidence account for 67% of those identified. Whilst citation classics often demonstrate the inclusion of the lowest levels of evidence, it is seldom to this degree. For example, a review into general medical articles found 38% of articles were drawn from the lowest two levels [ 1 ] and another review into GI surgery 44% [ 2 ]. Only 7 RCTs were identified which is significantly lower than what would have been expected. It must be considered that higher levels of evidence such as RCTs (and systematic reviews of these) can take many months to conduct. It is probable that the lack of high-level evidence, and the over-representation of lower levels of evidence, is partially a result of the literature not yet reaching maturity. Another interesting finding of this review is the degree to which high impact factor journals are publishing low levels of evidence. It has been previously shown that in the top three general medical journals (The Lancet, New England Journal of Medicine and Journal of the American Medical Association) the level of evidence represented by an article regarding COVID-19 was significantly lower when compared to both contemporary and historic controls [ 9 ].
The main limitation of this review is the time at which it was conducted; this makes comparisons to similar reviews of different topics difficult. Due to the short publication span of the papers the definition of citation density had to be modified, using a reference period of a month rather than a year. It is likely that as the literature around COVID-19 matures trends in publications will change. It is possible that in the early stages of an emerging topic traditional citation metrics may not be the most reliable way of identifying the most influential research in the longer term. Presence on social media may play an important role in identification of future influential articles; number of tweets within the first 7 days of a publication are shown to correlate with high levels of citation [ 18 ]. The simple and easily repeatable methods of this review, however, allow for later comparative review to examine how these trends have changed.
5. Conclusion
This review has collated the 100 most influential COVID-19 papers and assessed trends within them. We have established that in the early phases of a pandemic new and ground-breaking research surfaces regardless of the evidence level and can gain high levels of citation.
This study received funding from Insel Gruppe AG, Bern, Switzerland.
CRediT authorship contribution statement
Suhaib JS. Ahmad: Conceptualization, Methodology, Literature search, Data curation, Project administration, Writing – original draft, Writing – review & editing, Validation, Final approval. Konstantinos Degiannis: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Joseph Borucki: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Sjaak Pouwels: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. David Laith Rawaf: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Marion Head: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Chun Hei Li: Methodology, Data curation, Visualization, Writing – original draft, Writing – review & editing, Validation, Final approval. Rami Archid: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Ahmed R. Ahmed: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Anil Lala: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Wasif Raza: Writing – original draft, Writing – review & editing, Validation, Final approval. Katie Mellor: Methodology, Writing – original draft, Writing – review & editing, Validation, Final approval. Doerte Wichmann: Writing – original draft, Writing – review & editing, Validation, Final approval. Aristomenis Exadaktylos: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition, Writing – original draft, Writing – review & editing, Validation, Final approval.
Declaration of competing interest
The authors declared no conflict of interest.
- 1. Ahmad S.J., Ahmed A.R., Kowalewski K.F., Nickel F., Rostami K., Stocker C.J., Hakky S.M., Archid R., McWhinnie D., Mohajer-Bastami A., Seimenis D.S., Ahmad S., Mansour S., Ahmed M.H., Mital D., Exadaktylos A.K. Citation classics in general medical journals: assessing the quality of evidence; a systematic review. Gastroenterol Hepatol Bed Bench. 2020;13(2):101–114. Spring. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Ahmad S.J.S., Ahmed A.R., Exadaktylos A.K., McWhinnie D., Nickel F., Hakky S.M., Ramdin A., Müller P.C. Systematic review on citation classics in minimally invasive gastrointestinal surgery. J Minimal Access Surg. 2018 Oct-Dec;14(4):265–272. doi: 10.4103/jmas.JMAS_149_18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Ahmad S.S., Ahmad S.S., Kohl S., Ahmad S., Ahmed A.R. The hundred most cited articles in bariatric surgery. Obes Surg. 2015 May;25(5):900–909. doi: 10.1007/s11695-014-1542-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Straus S., Glasziou W., Richardson S., Haynes R.B. fourth ed. Churchill Livingstone; Edinburgh, UK: 2011. Evidence based medicine: how to practice and teach it. [ Google Scholar ]
- 5. Chen Q., Allot A., Lu Z. LitCovid: an open database of COVID-19 literature. Nucleic Acids Res. 2021;49(D1):D1534–D1540. doi: 10.1093/nar/gkaa952. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Organisation WH . 2020. (Coronavirus disease (COVID-19) situation report – 162 (30th june 2020)). [ Google Scholar ]
- 7. Wang J., Hong N. The COVID-19 research landscape: measuring topics and collaborations using scientific literature. Medicine. 2020;99(43) doi: 10.1097/MD.0000000000022849. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. London Alex J., Kimmelman J. Against pandemic research exceptionalism. Science. 2020;368(6490):476–477. doi: 10.1126/science.abc1731. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Zdravkovic M., Berger-Estilita J., Zdravkovic B., Berger D. Scientific quality of COVID-19 and SARS CoV-2 publications in the highest impact medical journals during the early phase of the pandemic: a case control study. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241826. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Jung R.G., Di Santo P., Clifford C., Prosperi-Porta G., Skanes S., Hung A., et al. Methodological quality of COVID-19 clinical research. Nat Commun. 2021;12(1):943. doi: 10.1038/s41467-021-21220-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Locher C., Moher D., Cristea I.A., Naudet F. Publication by association: how the COVID-19 pandemic has shown relationships between authors and editorial board members in the field of infectious diseases. BMJ Evid Based Med. 2022 Jun;27(3):133–136. doi: 10.1136/bmjebm-2021-111670. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Horbach S.P.J.M. Pandemic publishing: medical journals strongly speed up their publication process for COVID-19. Quantitative Sci Stud. 2020;1(3):1056–1067. [ Google Scholar ]
- 13. Brandt J.S., Hadaya O., Schuster M., Rosen T., Sauer M.V., Ananth C.V. A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw Open. 2019;2(12) doi: 10.1001/jamanetworkopen.2019.18007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Hennessey K., Afshar K., Macneily A.E. The top 100 cited articles in urology. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2009;3(4):293–302. doi: 10.5489/cuaj.1123. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Loomes D.E., van Zanten S.V. Bibliometrics of the top 100 clinical articles in digestive disease. Gastroenterology. 2013;144(4) doi: 10.1053/j.gastro.2013.02.013. 673-6.e5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Yoon D.Y., Yun E.J., Ku Y.J., Baek S., Lim K.J., Seo Y.L., et al. Citation classics in radiology journals: the 100 top-cited articles, 1945-2012. AJR Am J Roentgenol. 2013;201(3):471–481. doi: 10.2214/AJR.12.10489. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Margolis J. Citation Indexing and Evaluation of Scientific Papers: the spread of influence in populations of scientific papers may become a subject for quantitative analysis. Science. 1967;155(3767):1213–1219. doi: 10.1126/science.155.3767.1213. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Eysenbach G. Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123–e. doi: 10.2196/jmir.2012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (575.5 KB)
- Collections

Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
SARS-CoV-2 articles from across Nature Portfolio
SARS-CoV-2 is a positive-sense single-stranded RNA virus. It is contagious in humans and is the cause of the coronavirus disease 2019 (COVID-19).
Pathogenic T cells in post-viral lung disease in mice
A hallmark of post-viral lung disease, including post-acute sequelae of COVID-19, is dysplastic lung repair. CD8 + T cells contribute to aberrant repair through induction by IFNγ and TNF of IL-1β in macrophages, halting differentiation of alveolar type 2 cells.
- Stanley Perlman
- Alan Sariol
Latest Research and Reviews
Improved efficacy of SARS-CoV-2 isolation from COVID-19 clinical specimens using VeroE6 cells overexpressing TMPRSS2 and human ACE2
- Hitomi Kinoshita
- Tsukasa Yamamoto
- Shuetsu Fukushi
Epitope-anchored contrastive transfer learning for paired CD8 + T cell receptor–antigen recognition
Accurate prediction of T cell receptor (TCR)–antigen recognition remains a challenge. Zhang et al. propose a contrastive transfer learning model to predict TCR–pMHC binding that enables interpretable analyses of epitope-specific T cells and can decipher residue-level interactions.
- Yumeng Zhang
- Zhikang Wang
- Jiangning Song
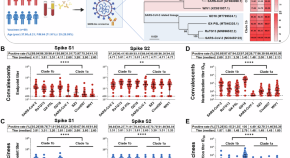
Broad cross neutralizing antibodies against sarbecoviruses generated by SARS-CoV-2 infection and vaccination in humans
- Xiaowang Qu
In vitro analysis suggests that SARS-CoV-2 infection differentially modulates cancer-like phenotypes and cytokine expression in colorectal and prostate cancer cells
- Alberta Serwaa
- Fatima Oyawoye
- Anastasia Rosebud Aikins
Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 omicron strains: a retrospective study in Beijing
- Mengzhao Wang
Lineage-specific pathogenicity, immune evasion, and virological features of SARS-CoV-2 BA.2.86/JN.1 and EG.5.1/HK.3
Here, the authors show that a single mutation in JN.1 spike, L455S, confers better spike cleavage and enhances virus infectivity in differentiated primary human nasal epithelial cells while increasing immune evasiveness, contributing to the efficient spread of JN.1.
- Yuanchen Liu
- Xiaoyu Zhao
News and Comment

The youngest among us fight COVID-19 in their own way
Children under the age of five infected with SARS-CoV-2 produce lower levels of some immune cells than older children and adults do.

COVID pandemic started in Wuhan market animals after all, suggests latest study
The finding comes from a reanalysis of genomic data.
- Smriti Mallapaty
Mpox: apply COVID lessons to control outbreak in Africa
As mpox becomes a global health emergency, global health leaders must better coordinate their plans and pharmaceutical companies must be prepared to make vaccines affordable.
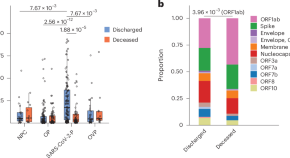
Phenotype and specificity of lung T cell responses correlate with outcome in SARS-CoV-2 pneumonia
Profiling of T cell responses in the lungs of patients with pneumonia revealed that early and persistent enrichment of T cells correlates with survival from SARS-CoV-2 pneumonia. Notably, lung T cells with an interferon-stimulated profile and specific for SARS-CoV-2 structural proteins support survival, whereas those that gain a nuclear factor-κB (NF-κB)-driven inflammatory profile and are directed against nonstructural proteins were associated with poor clinical outcomes.
Long COVID lung damage linked to immune system response
Inhibiting a protein associated with chronic inflammation improves lung health in mice with COVID-19.
- Helena Kudiabor
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Current treatment in COVID-19 disease: a rapid review
Miguel rodriguez-guerra, preeti jadhav, timothy j vittorio.
- Author information
- Article notes
- Copyright and License information
Correspondence: Timothy J Vittorio, BronxCare Hospital Center, Department of Medicine/Division of Cardiology, 1650 Grand Concourse, 12th Floor, Bronx, NY 10457, USA. [email protected]
Corresponding author.
Received 2020 Oct 12; Accepted 2020 Dec 11; Collection date 2021.
Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
The world has faced the most challenging pandemic of the modern era, that of severe acute respiratory syndrome coronavirus 2 infection, causing coronavirus disease and affecting over 35 million people globally. The wide range of clinical manifestations associated with this viral disease is thought to be related to the overexpression of inflammatory markers. Due to a dysregulated host response, the most severe form involves multi-organ failure and thromboembolic complications. Immunomodulatory therapies may help prevent its progression and anticoagulation has been shown to reduce the risk of thrombotic complications. As this is a new entity for the medical world, there are no known therapeutic options nor has the prevention of complications been established. Anti-inflammatory agents, antimicrobial therapy, and vitamin supplements are short of clear benefits, but there is limited data to review. Other agents, such as convalescent plasma, eculizumab, immunoglobulins, neutralizing IgG1 monoclonal antibodies, remdesivir, steroids, and tocilizumab, have shown a possible impact on inpatient length of stay and mortality rate. This review aims to assess the efficacy and safety of these available therapies in light of current evidence. We compare these treatment options based on their impact on symptom management, inpatient length of stay, and overall morbidity and mortality.
Keywords: convalescent plasma, COVID-19, eculizumab, immunoglobulins, neutralizing IgG1 monoclonal antibodies, remdesivir, SARS-CoV-2, steroids, tocilizumab
Introduction
The world is facing the most challenging pandemic of the modern era, that of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, causing coronavirus disease (COVID-19), which has affected over 35 million people worldwide. This condition has been shown to be related to the overexpression of inflammatory markers, including interleukins. The widespread inflammatory dysregulated host response can result in multi-organ failure, thromboembolism and death. 1 , 2 Immunomodulatory agents and systemic anticoagulation might be useful to prevent disease progression and thromboembolic complications if initiated in selected groups of severely ill patients. 3 Here, we review various options that are available for the management of this condition using multiple sources, including journals, electronic libraries, and university portals. Our goal is to assist healthcare workers engaged in this ongoing pandemic to guide their management with this rapid and concise review.
We performed a retrospective analysis of 41 prominent original studies along with a review of widely published literature in medical journals regarding the available treatment options for COVID-19 infection in the period March to November 2020. These studies were reviewed after a cautious selection from university portals, multiple journals, and libraries, including but not limited to Nebraska University Library, medRxiv , European Review for Medical and Pharmacological Sciences , Journal of Medical Virology , Science Translational Medicine , HCA Lung Biological Network, Journal of Thrombosis and Hemostasis , Journal of Thrombosis and Thrombolysis , Lancet Respiratory Medicine , JAMA , NEJM , Journal of the American College of Cardiology , Journal of the American Heart Association , Journal of Heart Failure , Chest , BMJ , Levy Library (Icahn School of Medicine), PubMed, Medscape, and EBSCO host. The search was performed using the following keywords: SARS, SARS-CoV-2, COVID-19, coronavirus, virus, virology, COVID-19 treatment, SARS-CoV-2 treatment, SARS treatment, remdesivir, plasma, convalescent plasma, antivirals, azithromycin, oseltamivir, enoxaparin, heparin, apixaban, NOAC, ACE-inhibitor, angiotensin receptor blocker, chloroquine, hydroxychloroquine, vitamin D, vitamin C, eculizumab, immunoglobulins, tocilizumab, steroids, dexamethasone and methylprednisolone.
Role of anti-inflammatory agents including steroids and tocilizumab
Several studies suggest that the cytokine release syndrome or ‘cytokine storm’, the release of IL-1, IL-6, IL-12, and IL-18 along with TNFα, as well as other inflammatory mediators play an important role in the clinical manifestations of COVID-19 infection. 4 – 7
SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) located on the target cells in the respiratory system leading to activation of the SARS-CoV-2 S protein. 8 , 9 This results in a further activation of the inflammatory cascade. In patients with SARS-CoV-2 infection, the postmortem pathology has revealed tissue necrosis as well as macrophage and monocyte infiltrations in the gastrointestinal mucosa, heart, and lung tissues. Severe lymphopaenia with hyperactivated pro-inflammatory T cells and decreased regulatory T cells are commonly seen in critically ill patients, suggesting the importance of using immunosuppressive therapy to control the overactivated cytokine release syndrome. 10 – 12 Like other viral entities, profound lymphopaenia may also be present when the virus infects and destroys T cells. This mechanism includes both the adaptive and innate immune responses (comprising cell-mediated and humoral immunity), impairing lymphopoiesis and increasing lymphocyte apoptosis. Later, the virus replicates faster, compromising the endothelial–epithelial barrier, increasing the inflammatory response, and triggering the influx of monocytes and neutrophils.
Drugs such as tocilizumab work by binding to the cell-related and soluble IL-6 receptors, inhibiting classic signalling and trans-signalling, which results in improved outcomes of patients with significant pneumonia. 13 , 14 A single dose of tocilizumab 400 mg improved lung function in 91% of patients and decreased the length of hospitalization in a large single-centre trial. 15 Another small study demonstrated the usefulness of repeated doses in severely ill patients, although the sample size in the study was not significant. 16
During the initial phase of the pandemic, a vast majority of patients received systemic steroids due to the established evidence of the efficacy of steroid use in systemic inflammatory response syndrome and patients mechanically ventilated due to respiratory illness. Multiple studies showed some benefit of steroid use, including a decrease in mortality, as observed in a trial at the University of Oxford including 6000 patients receiving 6 mg of dexamethasone daily. 17 The study showed a lower risk of death amongst the ventilated patients (rate ratio (RR) 0.65) and other patients receiving oxygen therapy (RR 0.80). Furthermore, the RECOVERY trial found that dexamethasone reduced 28-day all-cause mortality (21.6% versus 24.6%; age-adjusted rate ratio 0.83, 95% CI 0.74–0.92; p <0.001). Based on emerging data, dexamethasone is recommended in hypoxic patients but shows no difference in the routine use for mild disease manifestations. 18 , 19
There are ongoing trials to evaluate the efficacy of using neutralizing antibodies (Abs) in patients with mild to moderate disease. The BLAZE-1 randomized, double-blind clinical trial by Eli Lilly and Company was designed to assess the efficacy and safety of LY-CoV555 (bamlanivimab) and LY-CoV016. The investigators showed that LY-CoV555 improved viral clearance at an earlier time point (3 days) when compared to placebo as well as the rate of hospitalizations and emergency room visits (1.7% (5/302) for LY-CoV555 versus 6% (9/150) for placebo, 72% risk reduction). The mechanism of LY-CoV555 is based on the neutralization of the IgG1 monoclonal Ab against the spike protein of SARS-CoV-2. It inhibits the viral attachment and entry into human cells, thus offering the possibility of treating and even preventing disease progression to a severe form. 20 REGN-COV2 is a combination of two monoclonal Abs, casirivimab (REGN10933) and imdevimab (REGN10987), being investigated by Regeneron Pharmaceuticals, Inc. in phases I–III randomized clinical trials in non-hospitalized patients with mild to moderate COVID-19. This investigational Ab cocktail was granted emergency use authorization due to a decrement in viraemia and the time to alleviation of symptoms. 21 Further follow-up on the final results of this treatment modality in ongoing randomized clinical trials is required.
Role of immunomodulatory agents and convalescent plasma transfusion
During the pandemics of the Spanish and Avian influenza in 1918 and 1996, respectively, significant benefits were noted with the use of convalescent plasma (CP) and immunoglobulin (Ig) infusions. 22 – 24 These therapies showed decreased morbidity, improved hospital length of stay, as well as a decrease in mechanical ventilation and mortality. Based on the usefulness during prior pandemics, these treatment modalities were evaluated for their efficacy in the management of COVID-19 infection. As noted in a recent publication by Rojas et al., 25 the neutralization of the Abs, including the procoagulative reaction, was noted in patients who received CP or Ig infusions. In a trial headed by the Mayo Clinic for COVID-19 disease, CP was used in approximately 100,000 hospitalized patients (52% of these patients were in the intensive care unit (ICU) and 27% required mechanical ventilation) with moderate to severe illness in more than 2780 institutions. 26 The trial demonstrated that the use of CP with higher Ab levels was associated with reduced 30-day mortality. The side-effects identified were allergy, haemolytic reactions, volume overload, and transfusion-related reactions, including transfusion-related acute lung injury. The authors noted a reduced morbidity and mortality in patients who received CP, but the effective dose still remains unknown. Furthermore, Zhang et al. discussed the positive effect of CP decreasing the autoimmune Abs (e.g. antiphospholipid, systemic lupus erythematosus, etc.) which elevation of and their decreased levels after the administration of CP. 27 In a recent study conducted at the Center for Infectious Disease Research and Policy, Van Beusekom showed no clear benefit of CP. 28 Additionally, Casadevall et al. 29 posed the question of monotherapy with CP and discussed a potential use in combination with antiviral drugs. In terms of Ig, numerous case reports, case series, and small studies have been published but do not offer an established conclusion in favour of Ig infusions. 30 – 33 However, most of the studies have shown a possible lead-time bias in terms of reliable outcomes.
Eculizumab is monoclonal Ab targeted against complement C5. Clinical improvement is related to the prevention of membrane attack complex formation, resulting in the avoidance of end-organ damage and possibly of microthrombi phenomenon. 34 In other studies, patients received eculizumab (off-label), enoxaparin 4000 IU daily, lopinavir 800 mg daily with ritonavir 200 mg daily, hydroxychloroquine 400 mg daily, ceftriaxone 2 g daily intravenously, and vitamin C 6 g daily for 4 days and were on non-invasive ventilation, showing a potential benefit in terms of recovery, duration of the disease, and decrease in inflammatory markers. 35 , 36
In summary, our review of the literature showed a lack of evidence for routine use of Ig, CP, or eculizumab, but the selected treatment modalities can be useful in a specific patient population based on the severity of disease presentation. The individualization of treatment options and close monitoring of outcomes are a must, particularly as the long-term effects of these treatment options are unknown.
Role of systemic anticoagulation
In severe forms of COVID-19 infection, activation of the coagulation cascade and consumption of clotting factors occurs. Reports from Wuhan, China, indicated that up to 71% of patients who died met the criteria for diffuse intravascular coagulation. 37 Inflammation of the lung tissue and dysfunction of its endothelium may lead to a microthrombic phenomenon causing deep venous thrombosis, pulmonary embolism, and thrombotic arterial complications (e.g. limb ischaemia, ischaemic stroke, myocardial infarction). Prolonged thromboprophylaxis must be individualized based on risk versus benefit. In a retrospective analysis of a nationwide survey published in BMJ , low-molecular-weight heparin (LMWH) was associated with a decrease in overall 28-day mortality compared to subcutaneous mini-heparin (40% versus 64.2%). 38 The anti-inflammatory benefits of LMWH are related to its positive outcomes. Warfarin and direct-oral anticoagulants are not the drugs of choice due to the hepatic dysfunction present in severe illness, which leads to unpredictable levels in serum. 39 – 41
Based on the review of the literature, we recommend prophylactic systemic anticoagulation to all patients who have no contraindication (no active bleeding). Subcutaneous LMWH is the preferred agent for prophylactic anticoagulation but, in patients with renal failure, apixaban can be considered. Patients with clinical or radiographic signs of clotting should receive a therapeutic form of anticoagulation (preferred agent subcutaneous LWMH). Whenever possible, mechanical thromboembolism prophylaxis should be considered if a clinical contraindication for system anticoagulation exists.
Role of antiviral agents including remdesivir
Antiviral drugs are thought to work in different phases of viraemia, including in the prevention of viral entry into the host cell and in the prevention of both viral activation and replication. Remdesivir works by inhibiting viral replication via an adenosine analogue that becomes incorporated into the viral RNA, resulting in the inhibition of further viral replication and in early termination of the viral cycle. Sheahan et al. showed a potential reduction in viral load and lung infection in mice after the use of remdesivir. 42 In a recent study performed in North America, Europe, and Japan, clinical improvement was seen in 68% of patients with the use of remdesivir 200 mg i.v. first, followed by 100 mg daily to complete 10 days while diagnosed with COVID-19 disease. However, some evidence from clinical trials in China showed no such clinical benefit of remdesivir treatment and were terminated earlier due to an increased side-effect profile. 43 The uncertainty with the clinical benefit of remdesivir warrants more research and analysis before considering the widespread use of this drug. 44 – 46
Another antiviral agent widely considered for COVID-19 treatment is oseltamivir. The results of most observational studies showed inconsistent data about possible benefits versus no benefit. The main possible benefit is directed to the decrease in the time of recovery (1 day), but this topic is still controversial due to the heterogenetic evidence that is available.
The latest evidence on the efficacy of these therapies is not very promising. The World Health Organization launched new interim results from the SOLIDARITY trial that did not show a difference in mortality with remdesivir, hydroxychloroquine, lopinavir/ritonavir, and interferon regimens. 47 It is important to mention that this trial included data from multiple countries.
Role of antibiotics, hydroxychloroquine, and RAS modulators
As discussed earlier, activation of the immune response to SARS-CoV-2 involves the binding of viral particles to ACE2 receptors. Based on this mechanism of viremia, it was hypothesized that medications such as ACE inhibitors (ACEi) and angiotensin receptor blockers might lead to increased susceptibility to COVID-19 infections due to receptor upregulation. Observation studies evaluated this relationship, and evidence-based data did not show any association between ACEi/angiotensin receptor blockers and infection risk or hospital mortality. 48 In a large population-based study from Denmark, 4480 infected patients were compared based on prehospitalization use of ACEi, and no difference in mortality was noted. 49 , 50
Chloroquine/hydroxychloroquine (CQ/HCQ) were used widely during the initial phase of the pandemic due to their ability to inhibit viral entry, prevent endocytosis, downregulate the immune system, and decrease the cytokine storm. However, early data from clinical trials in the inpatient setting have not demonstrated clear benefits. The meta-analysis from multiple randomized clinical trials showed that CQ/HCQ with or without azithromycin were ineffective and rather may have increased the risk of adverse events. 47 HCQ did not show benefit in terms of mortality (RR 1.0, 95% CI 1.0–1.2, I 2 0%, n =6 studies), ICU use or need for mechanical ventilation (RR 1.1, 95% CI 0.9–1.4, I 2 0%, n =3 studies), virological cure (RR 1.0, 95% CI 0.9–1.2, I 2 =55%, n =5 studies), or disease exacerbation (RR 1.2, 95% CI 0.3–5.0, I 2 =29%, n =3 studies). There is clear data showing that CQ/HCQ, with or without azithromycin, is indeed ineffective in treating COVID-19 disease or its exacerbation. 51 , 52
Role of vitamins C and D
Vitamin C has gained interest in the management of COVID-19 due to its inherent anti-oxidative properties. There are ongoing clinical trials in various centres to evaluate the outcomes of early vitamin C treatment in patients with various clinical manifestations, but, currently, insufficient data establishing the use of vitamin C is available. Prior studies have shown the potential benefits of vitamin C supplementation in the management of acute respiratory distress syndrome and septic shock due to other viral illnesses. 53 It is prudent to follow-up on the outcomes of current ongoing trials to guide therapy in particular for COVID-19 infection. 54
There is growing interest to suggest that vitamin D deficiency is related to the risk of infection. This vitamin acts as an immune system modulator by providing an effective physical barrier and strengthening both adaptive and innate immunity. A meta-analysis of 25 randomized clinical trials in the past suggests that regular supplementation with vitamin D2/D3 (up to 2000 IU/d) is protective against acute respiratory infections. 55 Therefore, the prophylactic use of vitamin D in patients with COVID-19 is proposed. There is a lack of clinical data to support this treatment modality, and further clinical studies are needed to establish vitamin D supplementation as part of therapy. 56
Tables 1 and 2 briefly summarize the current clinical trials on the treatment of COVID-19 infection.
Current treatment in COVID-19 infection.
Clinical trials of current treatment in COVID-19 infection.
Post-COVID-19 disease
Important signs or symptoms that follow acute infection are pain, physical competence, renal function, hypercoagulability, impaired renal function, myopathy or polyneuropathy, residual pulmonary infections, psychiatric or psychological disorders (anxiety, cognitive disorder, depression, insomnia, post-traumatic stress disorder), and cardiac manifestations, including arrhythmias and myocardial injury. Patients will therefore need to be closely monitored for chronic conditions, especially cardiovascular, metabolic, and neurologic disorders. 57 , 58
The SARS-CoV-2 infection/COVID-19 is a pro-inflammatory process with multiple consequences, including an increased mortality rate, especially in patients with a medical history of heart failure, hypertension, or renal disease. Multiple data have shown the potential sequelae that patients might experience, including chronic fatigue, thrombotic events post infection, non-reversible lung disease, and altered mental and physical disability; however, these cannot yet be fully determined. Future studies following up on the cardiovascular, neurovascular, renal, and other potential complications that could result from this viral illness are warranted.
Acknowledgements
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/01/dic.2020-10-3-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2021 Rodriguez-Guerra M, Jadhav P, Vittorio TJ. https://doi.org/10.7573/dic.2020-10-3 . Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/current-treatment-in-covid-19-disease:-a-rapid-review
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office [email protected]
For all permissions, rights and reprints, contact David Hughes [email protected]
- 1. Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Van de Veerdonk FL, Netea MG, van Deuren M, et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. [Published online April 27, 2020] Elife. doi: 10.7554/eLife.57555. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Hall MW, Joshi I, Leal L, Ooi EE. Immune immunomodulation in coronavirus disease 2019 (COVID-19): strategic considerations for personalized therapeutic intervention. Clin Infect Dis. 2020. p. ciaa904. [ DOI ] [ PMC free article ] [ PubMed ]
- 4. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Cohort Multiple Randomized Controlled Trials Open-label of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients - Tocilizumab Trial - CORIMUNO-19 - TOCI (CORIMUNO-TOCI) https://clinicaltrials.gov/ct2/show/NCT04331808 .
- 14. National Health Commission and State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia. [Accessed April 25, 2020]. https://www.chinalawtranslate.com/wp-content/uploads/2020/03/Who-translation.pdf .
- 15. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Cennimo DJ, Bergman SJ. Coronavirus disease 2019 (COVID-19) treatment & management. Updated 2020. [Medscape]. https://emedicine.medscape.com/article/2500114-clinical .
- 17. Young K. COVID-19 steroid lowered mortality/rural America/potential drug-drug interaction. https://www.jwatch.org/fw116743/2020/06/16/covid-19-steroid-lowered-mortality-rural-america .
- 18. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report [Published online July 17, 2020] N Engl J Med. doi: 10.1056/NEJMoa2021436. [ DOI ] [ Google Scholar ]
- 19. Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34(6):1503–1511. doi: 10.1038/s41375-020-0848-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Lilly announces proof of concept data for neutralizing antibody LY-CoV555 in the COVID-19 outpatient setting. https://investor.lilly.com/news-releases/news-release-details/lilly-announces-proof-concept-data-neutralizing-antibody-ly .
- 21. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of casirivimab and imdevimab. 2020. [Accessed November 13, 2020]. https://www.fda.gov/media/143892/download .
- 22. Francis FD, Hall MW, Gaines AR. Early use of convalescent serum in influenza. Mil Surg. 1920;47:177–179. [ Google Scholar ]
- 23. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Davey RT, Jr, et al. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. Lancet Respir Med. 2019;7:951–963. doi: 10.1016/S2213-2600(19)30253-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Rojas M, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020;19(7):102554. doi: 10.1016/j.autrev.2020.102554. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Coronavirus Web Archive. https://www.uscovidplasma.org/
- 27. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Van Beusekom M. Adults, too, develop rare but severe COVID-related syndrome. CIDRAP News. https://www.cidrap.umn.edu/news-perspective/2020/10/adults-too-develop-rare-severe-covid-related-syndrome . [ Google Scholar ]
- 29. Casadevall A, Joyner MJ, Pirofski L. A randomized trial of convalescent plasma for COVID-19—potentially hopeful signals. JAMA. 2020;324(5):455–457. doi: 10.1001/jama.2020.10218. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Song Y, Zhang M, Yin L, et al. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2) Int J Antimicrob Agents. 2020;56(2):106080. doi: 10.1016/j.ijantimicag.2020.106080. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:E9–E13. doi: 10.1016/j.chest.2020.03.039. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Pitts T. Hudson Medical: A Preliminary update to the Soliris to stop immune mediated death in COVID-19 (SOLID-C19) compassionate use study. https://hudsonmedical.com/articles/soliris-stop-death-covid-19/ [ DOI ] [ PMC free article ] [ PubMed ]
- 35. Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046. doi: 10.1136/bmjopen-2017-017046. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: Interim clinical guidance from the Anticoagulation Forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Moores LK, Tritschler T, Brosnahan S. Prevention, diagnosis and treatment of venous thromboembolism in patients with COVID-19: CHEST Guideline and Expert Panel Report. Chest. 2020 doi: 10.1016/j.chest.2020.05.559. [ DOI ] [ Google Scholar ]
- 42. Norrie JD. Remdesivir for COVID-19: challenges of underpowered studies. Lancet. 2020;395(10236):1525–1527. doi: 10.1016/S0140-6736(20)31023-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Pruijssers AJ, George AS, Schäfer A, et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 2020;32:107940. doi: 10.1016/j.celrep.2020.107940. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Yoo JH. Uncertainty about the efficacy of Remdesivir on COVID-19. J Korean Med Sci. 2020;35(23):e221. doi: 10.3346/jkms.2020.35.e221. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168–177. doi: 10.1001/jama.2020.11301. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. World Health Organization. Solidarity Therapeutics Trial produces conclusive evidence on the effectiveness of repurposed drugs for COVID-19 in record time. https://www.who.int/news/item/15-10-2020-solidarity-therapeutics-trial-produces-conclusive-evidence-on-the-effectiveness-of-repurposed-drugs-for-covid-19-in-record-time .
- 49. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Borba MGS, et al. CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial JAMA Netw Open 202034e208857. 10.1001/jamanetworkopen.2020.8857 [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Hoang BX, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Chivese T, Musa OAH, Hindy G, et al. A meta-review of systematic reviews and an updated meta-analysis on the efficacy of chloroquine and hydroxychloroquine in treating COVID19 infection. medRxiv. 2020 doi: 10.1101/2020.07.28.20164012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24(1):133. doi: 10.1186/s13054-020-02851-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Kochav SM, et al. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020 doi: 10.1161/CIRCEP.120.008719. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370:m3001. doi: 10.1136/bmj.m3001. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (298.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Virology, transmission...
Virology, transmission, and pathogenesis of SARS-CoV-2
Read our latest coverage of the coronavirus outbreak.
- Related content
- Peer review
- Muge Cevik , clinical lecturer 1 2 ,
- Krutika Kuppalli , assistant professor 3 ,
- Jason Kindrachuk , assistant professor of virology 4 ,
- Malik Peiris , professor of virology 5
- 1 Division of Infection and Global Health Research, School of Medicine, University of St Andrews, St Andrews, UK
- 2 Specialist Virology Laboratory, Royal Infirmary of Edinburgh, Edinburgh, UK and Regional Infectious Diseases Unit, Western General Hospital, Edinburgh, UK
- 3 Division of Infectious Diseases, Medical University of South Carolina, Charleston, SC, USA
- 4 Laboratory of Emerging and Re-Emerging Viruses, Department of Medical Microbiology, University of Manitoba, Winnipeg, MB, Canada
- 5 School of Public Health, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China
- Correspondence to M Cevik mc349{at}st-andrews.ac.uk
What you need to know
SARS-CoV-2 is genetically similar to SARS-CoV-1, but characteristics of SARS-CoV-2—eg, structural differences in its surface proteins and viral load kinetics—may help explain its enhanced rate of transmission
In the respiratory tract, peak SARS-CoV-2 load is observed at the time of symptom onset or in the first week of illness, with subsequent decline thereafter, indicating the highest infectiousness potential just before or within the first five days of symptom onset
Reverse transcription polymerase chain reaction (RT-PCR) tests can detect viral SARS-CoV-2 RNA in the upper respiratory tract for a mean of 17 days; however, detection of viral RNA does not necessarily equate to infectiousness, and viral culture from PCR positive upper respiratory tract samples has been rarely positive beyond nine days of illness
Symptomatic and pre-symptomatic transmission (1-2 days before symptom onset), is likely to play a greater role in the spread of SARS-CoV-2 than asymptomatic transmission
A wide range of virus-neutralising antibodies have been reported, and emerging evidence suggests that these may correlate with severity of illness but wane over time
Since the emergence of SARS-CoV-2 in December 2019, there has been an unparalleled global effort to characterise the virus and the clinical course of disease. Coronavirus disease 2019 (covid-19), caused by SARS-CoV-2, follows a biphasic pattern of illness that likely results from the combination of an early viral response phase and an inflammatory second phase. Most clinical presentations are mild, and the typical pattern of covid-19 more resembles an influenza-like illness—which includes fever, cough, malaise, myalgia, headache, and taste and smell disturbance—rather than severe pneumonia (although emerging evidence about long term consequences is yet to be understood in detail). 1 In this review, we provide a broad update on the emerging understanding of SARS-CoV-2 pathophysiology, including virology, transmission dynamics, and the immune response to the virus. Any of the mechanisms and assumptions discussed in the article and in our understanding of covid-19 may be revised as further evidence emerges.
What we know about the virus
SARS-CoV-2 is an enveloped β-coronavirus, with a genetic sequence very similar to SARS-CoV-1 (80%) and bat coronavirus RaTG13 (96.2%). 2 The viral envelope is coated by spike (S) glycoprotein, envelope (E), and membrane (M) proteins ( fig 1 ). Host cell binding and entry are mediated by the S protein. The first step in infection is virus binding to a host cell through its target receptor. The S1 sub-unit of the S protein contains the receptor binding domain that binds to the peptidase domain of angiotensin-converting enzyme 2 (ACE 2). In SARS-CoV-2 the S2 sub-unit is highly preserved and is considered a potential antiviral target. The virus structure and replication cycle are described in figure 1 .

(1) The virus binds to ACE 2 as the host target cell receptor in synergy with the host’s transmembrane serine protease 2 (cell surface protein), which is principally expressed in the airway epithelial cells and vascular endothelial cells. This leads to membrane fusion and releases the viral genome into the host cytoplasm (2). Stages (3-7) show the remaining steps of viral replication, leading to viral assembly, maturation, and virus release
- Download figure
- Open in new tab
- Download powerpoint
Coronaviruses have the capacity for proofreading during replication, and therefore mutation rates are lower than in other RNA viruses. As SARS-CoV-2 has spread globally it has, like other viruses, accumulated some mutations in the viral genome, which contains geographic signatures. Researchers have examined these mutations to study virus characterisation and understand epidemiology and transmission patterns. In general, the mutations have not been attributed to phenotypic changes affecting viral transmissibility or pathogenicity. The G614 variant in the S protein has been postulated to increase infectivity and transmissibility of the virus. 3 Higher viral loads were reported in clinical samples with virus containing G614 than previously circulating variant D614, although no association was made with severity of illness as measured by hospitalisation outcomes. 3 These findings have yet to be confirmed with regards to natural infection.
Why is SARS-CoV-2 more infectious than SARS-CoV-1?
SARS-CoV-2 has a higher reproductive number (R 0 ) than SARS-CoV-1, indicating much more efficient spread. 1 Several characteristics of SARS-CoV-2 may help explain this enhanced transmission. While both SARS-CoV-1 and SARS-CoV-2 preferentially interact with the angiotensin-converting enzyme 2 (ACE 2) receptor, SARS-CoV-2 has structural differences in its surface proteins that enable stronger binding to the ACE 2 receptor 4 and greater efficiency at invading host cells. 1 SARS-CoV-2 also has greater affinity (or bonding) for the upper respiratory tract and conjunctiva, 5 thus can infect the upper respiratory tract and can conduct airways more easily. 6
Viral load dynamics and duration of infectiousness
Viral load kinetics could also explain some of the differences between SARS-CoV-2 and SARS-CoV-1. In the respiratory tract, peak SARS-CoV-2 load is observed at the time of symptom onset or in the first week of illness, with subsequent decline thereafter, which indicates the highest infectiousness potential just before or within the first five days of symptom onset ( fig 2 ). 7 In contrast, in SARS-CoV-1 the highest viral loads were detected in the upper respiratory tract in the second week of illness, which explains its minimal contagiousness in the first week after symptom onset, enabling early case detection in the community. 7

After the initial exposure, patients typically develop symptoms within 5-6 days (incubation period). SARS-CoV-2 generates a diverse range of clinical manifestations, ranging from mild infection to severe disease accompanied by high mortality. In patients with mild infection, initial host immune response is capable of controlling the infection. In severe disease, excessive immune response leads to organ damage, intensive care admission, or death. The viral load peaks in the first week of infection, declines thereafter gradually, while the antibody response gradually increases and is often detectable by day 14 (figure adapted with permission from https://www.sciencedirect.com/science/article/pii/S009286742030475X ; https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30230-7/fulltext )
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) technology can detect viral SARS-CoV-2 RNA in the upper respiratory tract for a mean of 17 days (maximum 83 days) after symptom onset. 7 However, detection of viral RNA by qRT-PCR does not necessarily equate to infectiousness, and viral culture from PCR positive upper respiratory tract samples has been rarely positive beyond nine days of illness. 5 This corresponds to what is known about transmission based on contact tracing studies, which is that transmission capacity is maximal in the first week of illness, and that transmission after this period has not been documented. 8 Severely ill or immune-compromised patients may have relatively prolonged virus shedding, and some patients may have intermittent RNA shedding; however, low level results close to the detection limit may not constitute infectious viral particles. While asymptomatic individuals (those with no symptoms throughout the infection) can transmit the infection, their relative degree of infectiousness seems to be limited. 9 10 11 People with mild symptoms (paucisymptomatic) and those whose symptom have not yet appeared still carry large amounts of virus in the upper respiratory tract, which might contribute to the easy and rapid spread of SARS-CoV-2. 7 Symptomatic and pre-symptomatic transmission (one to two days before symptom onset) is likely to play a greater role in the spread of SARS-CoV-2. 10 12 A combination of preventive measures, such as physical distancing and testing, tracing, and self-isolation, continue to be needed.
Route of transmission and transmission dynamics
Like other coronaviruses, the primary mechanism of transmission of SARS-CoV-2 is via infected respiratory droplets, with viral infection occurring by direct or indirect contact with nasal, conjunctival, or oral mucosa, when respiratory particles are inhaled or deposited on these mucous membranes. 6 Target host receptors are found mainly in the human respiratory tract epithelium, including the oropharynx and upper airway. The conjunctiva and gastrointestinal tracts are also susceptible to infection and may serve as transmission portals. 6
Transmission risk depends on factors such as contact pattern, environment, infectiousness of the host, and socioeconomic factors, as described elsewhere. 12 Most transmission occurs through close range contact (such as 15 minutes face to face and within 2 m), 13 and spread is especially efficient within households and through gatherings of family and friends. 12 Household secondary attack rates (the proportion of susceptible individuals who become infected within a group of susceptible contacts with a primary case) ranges from 4% to 35%. 12 Sleeping in the same room as, or being a spouse of an infected individual increases the risk of infection, but isolation of the infected person away from the family is related to lower risk of infection. 12 Other activities identified as high risk include dining in close proximity with the infected person, sharing food, and taking part in group activities 12 The risk of infection substantially increases in enclosed environments compared with outdoor settings. 12 For example, a systematic review of transmission clusters found that most superspreading events occurred indoors. 11 Aerosol transmission can still factor during prolonged stay in crowded, poorly ventilated indoor settings (meaning transmission could occur at a distance >2 m). 12 14 15 16 17
The role of faecal shedding in SARS-CoV-2 transmission and the extent of fomite (through inanimate surfaces) transmission also remain to be fully understood. Both SARS-CoV-2 and SARS-CoV-1 remain viable for many days on smooth surfaces (stainless steel, plastic, glass) and at lower temperature and humidity (eg, air conditioned environments). 18 19 Thus, transferring infection from contaminated surfaces to the mucosa of eyes, nose, and mouth via unwashed hands is a possible route of transmission. This route of transmission may contribute especially in facilities with communal areas, with increased likelihood of environmental contamination. However, both SARS-CoV-1 and SARS-CoV-2 are readily inactivated by commonly used disinfectants, emphasising the potential value of surface cleaning and handwashing. SARS-CoV-2 RNA has been found in stool samples and RNA shedding often persists for longer than in respiratory samples 7 ; however, virus isolation has rarely been successful from the stool. 5 7 No published reports describe faecal-oral transmission. In SARS-CoV-1, faecal-oral transmission was not considered to occur in most circumstances; but, one explosive outbreak was attributed to aerosolisation and spread of the virus across an apartment block via a faulty sewage system. 20 It remains to be seen if similar transmission may occur with SARS-CoV-2.
Pathogenesis
Viral entry and interaction with target cells.
SARS-CoV-2 binds to ACE 2, the host target cell receptor. 1 Active replication and release of the virus in the lung cells lead to non-specific symptoms such as fever, myalgia, headache, and respiratory symptoms. 1 In an experimental hamster model, the virus causes transient damage to the cells in the olfactory epithelium, leading to olfactory dysfunction, which may explain temporary loss of taste and smell commonly seen in covid-19. 21 The distribution of ACE 2 receptors in different tissues may explain the sites of infection and patient symptoms. For example, the ACE 2 receptor is found on the epithelium of other organs such as the intestine and endothelial cells in the kidney and blood vessels, which may explain gastrointestinal symptoms and cardiovascular complications. 22 Lymphocytic endotheliitis has been observed in postmortem pathology examination of the lung, heart, kidney, and liver as well as liver cell necrosis and myocardial infarction in patients who died of covid-19. 1 23 These findings indicate that the virus directly affects many organs, as was seen in SARS-CoV-1 and influenzae.
Much remains unknown. Are the pathological changes in the respiratory tract or endothelial dysfunction the result of direct viral infection, cytokine dysregulation, coagulopathy, or are they multifactorial? And does direct viral invasion or coagulopathy directly contribute to some of the ischaemic complications such as ischaemic infarcts? These and more, will require further work to elucidate.
Immune response and disease spectrum ( figure 2 )
After viral entry, the initial inflammatory response attracts virus-specific T cells to the site of infection, where the infected cells are eliminated before the virus spreads, leading to recovery in most people. 24 In patients who develop severe disease, SARS-CoV-2 elicits an aberrant host immune response. 24 25 For example, postmortem histology of lung tissues of patients who died of covid-19 have confirmed the inflammatory nature of the injury, with features of bilateral diffuse alveolar damage, hyaline-membrane formation, interstitial mononuclear inflammatory infiltrates, and desquamation consistent with acute respiratory distress syndrome (ARDS), and is similar to the lung pathology seen in severe Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). 26 27 A distinctive feature of covid-19 is the presence of mucus plugs with fibrinous exudate in the respiratory tract, which may explain the severity of covid-19 even in young adults. 28 This is potentially caused by the overproduction of pro-inflammatory cytokines that accumulate in the lungs, eventually damaging the lung parenchyma. 24
Some patients also experience septic shock and multi-organ dysfunction. 24 For example, the cardiovascular system is often involved early in covid-19 disease and is reflected in the release of highly sensitive troponin and natriuretic peptides. 29 Consistent with the clinical context of coagulopathy, focal intra-alveolar haemorrhage and presence of platelet-fibrin thrombi in small arterial vessels is also seen. 27 Cytokines normally mediate and regulate immunity, inflammation, and haematopoiesis; however, further exacerbation of immune reaction and accumulation of cytokines in other organs in some patients may cause extensive tissue damage, or a cytokine release syndrome (cytokine storm), resulting in capillary leak, thrombus formation, and organ dysfunction. 24 30
Mechanisms underlying the diverse clinical outcomes
Clinical outcomes are influenced by host factors such as older age, male sex, and underlying medical conditions, 1 as well as factors related to the virus (such as viral load kinetics), host-immune response, and potential cross-reactive immune memory from previous exposure to seasonal coronaviruses ( box 1 ).
Risk factors associated with the development of severe disease, admission to intensive care unit, and mortality
Underlying condition.
Hypertension
Cardiovascular disease
Chronic obstructive pulmonary disease
Presentation
Higher fever (≥39°C on admission)
Dyspnoea on admission
Higher qSOFA score
Laboratory markers
Neutrophilia/lymphopenia
Raised lactate and lactate dehydrogenase
Raised C reactive protein
Raised ferritin
Raised IL-6
Raised ACE2
D-dimer >1 μg/mL
Sex-related differences in immune response have been reported, revealing that men had higher plasma innate immune cytokines and chemokines at baseline than women. 31 In contrast, women had notably more robust T cell activation than men, and among male participants T cell activation declined with age, which was sustained among female patients. These findings suggest that adaptive immune response may be important in defining the clinical outcome as older age and male sex is associated with increased risk of severe disease and mortality.
Increased levels of pro-inflammatory cytokines correlate with severe pneumonia and increased ground glass opacities within the lungs. 30 32 In people with severe illness, increased plasma concentrations of inflammatory cytokines and biomarkers were observed compared with people with non-severe illness. 30 33 34
Emerging evidence suggests a correlation between viral dynamics, the severity of illness, and disease outcome. 7 Longitudinal characteristics of immune response show a correlation between the severity of illness, viral load, and IFN- α, IFN-γ, and TNF-α response. 34 In the same study many interferons, cytokines, and chemokines were elevated early in disease for patients who had severe disease and higher viral loads. This emphasises that viral load may drive these cytokines and the possible pathological roles associated with the host defence factors. This is in keeping with the pathogenesis of influenza, SARS, and MERS whereby prolonged viral shedding was also associated with severity of illness. 7 35
Given the substantial role of the immune response in determining clinical outcomes, several immunosuppressive therapies aimed at limiting immune-mediated damage are currently in various phases of development ( table 1 ).
Therapeutics currently under investigation
- View inline
Immune response to the virus and its role in protection
Covid-19 leads to an antibody response to a range of viral proteins, but the spike (S) protein and nucleocapsid are those most often used in serological diagnosis. Few antibodies are detectable in the first four days of illness, but patients progressively develop them, with most achieving a detectable response after four weeks. 36 A wide range of virus-neutralising antibodies have been reported, and emerging evidence suggests that these may correlate with severity but wane over time. 37 The duration and protectivity of antibody and T cell responses remain to be defined through studies with longer follow-up. CD-4 T cell responses to endemic human coronaviruses appear to manifest cross-reactivity with SARS-CoV-2, but their role in protection remains unclear. 38
Unanswered questions
Further understanding of the pathogenesis for SARS-CoV-2 will be vital in developing therapeutics, vaccines, and supportive care modalities in the treatment of covid-19. More data are needed to understand the determinants of healthy versus dysfunctional response and immune markers for protection and the severity of disease. Neutralising antibodies are potential correlates of protection, but other protective antibody mechanisms may exist. Similarly, the protective role of T cell immunity and duration of both antibody and T cell responses and the correlates of protection need to be defined. In addition, we need optimal testing systems and technologies to support and inform early detection and clinical management of infection. Greater understanding is needed regarding the long term consequences following acute illness and multisystem inflammatory disease, especially in children.
Education into practice
How would you describe SARS-CoV-2 transmission routes and ways to prevent infection?
How would you describe to a patient why cough, anosmia, and fever occur in covid-19?
Questions for future research
What is the role of the cytokine storm and how could it inform the development of therapeutics, vaccines, and supportive care modalities?
What is the window period when patients are most infectious?
Why do some patients develop severe disease while others, especially children, remain mildly symptomatic or do not develop symptoms?
What are the determinants of healthy versus dysfunctional response, and the biomarkers to define immune correlates of protection and disease severity for the effective triage of patients?
What is the protective role of T cell immunity and duration of both antibody and T cell responses, and how would you define the correlates of protection?
How patients were involved in the creation of this article
No patients were directly involved in the creation of this article.
How this article was created
We searched PubMed from 2000 to 18 September 2020, limited to publications in English. Our search strategy used a combination of key words including “COVID-19,” “SARS-CoV-2,” “SARS”, “MERS,” “Coronavirus,” “Novel Coronavirus,” “Pathogenesis,” “Transmission,” “Cytokine Release,” “immune response,” “antibody response.” These sources were supplemented with systematic reviews. We also reviewed technical documents produced by the Centers for Disease Control and Prevention and World Health Organization technical documents.
Author contributions: MC, KK, JK, MP drafted the first and subsequent versions of the manuscript and all authors provided critical feedback and contributed to the manuscript.
Competing interests The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: none.
Further details of The BMJ policy on financial interests are here: https://www.bmj.com/about-bmj/resources-authors/forms-policies-and-checklists/declaration-competing-interests
Provenance and peer review: commissioned; externally peer reviewed.
This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
- Bamford CGG ,
- Fischer WM ,
- Gnanakaran S ,
- Sheffield COVID-19 Genomics Group
- Corbett KS ,
- Corman VM ,
- Guggemos W ,
- Cheung MC ,
- Perera RAPM ,
- Cheng H-Y ,
- Taiwan COVID-19 Outbreak Investigation Team
- Meyerowitz E ,
- Richterman A ,
- Nergiz AI ,
- Maraolo AE ,
- Buitrago-Garcia D ,
- Egli-Gany D ,
- Counotte MJ ,
- ↵ European Centre for Disease Prevention and Control. Surveillance definitions for COVID-19. 2020. https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions .
- Klompas M ,
- ↵ Centers for Disease Control and Prevention. How COVID-19 spreads. 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- ↵ Centers for Disease Control and Prevention. Scientific brief: SARS-CoV-2 and potential airborne transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html
- Perera MRA ,
- van Doremalen N ,
- Bushmaker T ,
- Morris DH ,
- Monteil V ,
- Flammer AJ ,
- Steiger P ,
- Mangalmurti N ,
- Blanco-Melo D ,
- Nilsson-Payant BE ,
- Carsana L ,
- Sonzogni A ,
- ↵ Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020. https://pubmed.ncbi.nlm.nih.gov/32846427/
- Yale IMPACT Team
- CAP-China Network
- Perera RA ,
- Merrick B ,
- Grifoni A ,
- Weiskopf D ,
- Ramirez SI ,

IMAGES
VIDEO
COMMENTS
We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic. Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to ...
These papers highlight valuable research into the biology of coronavirus infection, its detection, treatment as well as into vaccine development and the epidemiology of the disease.
5. Conclusion. This review has collated the 100 most influential COVID-19 papers and assessed trends within them. We have established that in the early phases of a pandemic new and ground-breaking research surfaces regardless of the evidence level and can gain high levels of citation.
In this Review, we summarize the current understanding of the nature of SARS-CoV-2 and COVID-19. On the basis of recently published findings, this comprehensive Review covers the basic biology of...
Coronavirus disease 2019 (COVID-19) is a highly contagious infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide.
Since the outbreak of the COVID-19 pandemic, there has been a rapid expansion in vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
SARS-CoV-2 articles from across Nature Portfolio. SARS-CoV-2 is a positive-sense single-stranded RNA virus. It is contagious in humans and is the cause of the coronavirus disease 2019...
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature.
We performed a retrospective analysis of 41 prominent original studies along with a review of widely published literature in medical journals regarding the available treatment options for COVID-19 infection in the period March to November 2020.
Coronavirus disease 2019 (covid-19), caused by SARS-CoV-2, follows a biphasic pattern of illness that likely results from the combination of an early viral response phase and an inflammatory second phase.