Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 October 2022

Health effects associated with smoking: a Burden of Proof study
- Xiaochen Dai ORCID: orcid.org/0000-0002-0289-7814 1 , 2 ,
- Gabriela F. Gil 1 ,
- Marissa B. Reitsma 1 ,
- Noah S. Ahmad 1 ,
- Jason A. Anderson 1 ,
- Catherine Bisignano 1 ,
- Sinclair Carr 1 ,
- Rachel Feldman 1 ,
- Simon I. Hay ORCID: orcid.org/0000-0002-0611-7272 1 , 2 ,
- Jiawei He 1 , 2 ,
- Vincent Iannucci 1 ,
- Hilary R. Lawlor 1 ,
- Matthew J. Malloy 1 ,
- Laurie B. Marczak 1 ,
- Susan A. McLaughlin 1 ,
- Larissa Morikawa ORCID: orcid.org/0000-0001-9749-8033 1 ,
- Erin C. Mullany 1 ,
- Sneha I. Nicholson 1 ,
- Erin M. O’Connell 1 ,
- Chukwuma Okereke 1 ,
- Reed J. D. Sorensen 1 ,
- Joanna Whisnant 1 ,
- Aleksandr Y. Aravkin 1 , 3 ,
- Peng Zheng 1 , 2 ,
- Christopher J. L. Murray ORCID: orcid.org/0000-0002-4930-9450 1 , 2 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1 , 2
Nature Medicine volume 28 , pages 2045–2055 ( 2022 ) Cite this article
26k Accesses
43 Citations
168 Altmetric
Metrics details
- Risk factors
Matters Arising to this article was published on 14 April 2023
As a leading behavioral risk factor for numerous health outcomes, smoking is a major ongoing public health challenge. Although evidence on the health effects of smoking has been widely reported, few attempts have evaluated the dose–response relationship between smoking and a diverse range of health outcomes systematically and comprehensively. In the present study, we re-estimated the dose–response relationships between current smoking and 36 health outcomes by conducting systematic reviews up to 31 May 2022, employing a meta-analytic method that incorporates between-study heterogeneity into estimates of uncertainty. Among the 36 selected outcomes, 8 had strong-to-very-strong evidence of an association with smoking, 21 had weak-to-moderate evidence of association and 7 had no evidence of association. By overcoming many of the limitations of traditional meta-analyses, our approach provides comprehensive, up-to-date and easy-to-use estimates of the evidence on the health effects of smoking. These estimates provide important information for tobacco control advocates, policy makers, researchers, physicians, smokers and the public.
Similar content being viewed by others
The Burden of Proof studies: assessing the evidence of risk
Health effects associated with exposure to secondhand smoke: a Burden of Proof study

Health effects associated with chewing tobacco: a Burden of Proof study
Among both the public and the health experts, smoking is recognized as a major behavioral risk factor with a leading attributable health burden worldwide. The health risks of smoking were clearly outlined in a canonical study of disease rates (including lung cancer) and smoking habits in British doctors in 1950 and have been further elaborated in detail over the following seven decades 1 , 2 . In 2005, evidence of the health consequences of smoking galvanized the adoption of the first World Health Organization (WHO) treaty, the Framework Convention on Tobacco Control, in an attempt to drive reductions in global tobacco use and second-hand smoke exposure 3 . However, as of 2020, an estimated 1.18 billion individuals globally were current smokers and 7 million deaths and 177 million disability-adjusted life-years were attributed to smoking, reflecting a persistent public health challenge 4 . Quantifying the relationship between smoking and various important health outcomes—in particular, highlighting any significant dose–response relationships—is crucial to understanding the attributable health risk experienced by these individuals and informing responsive public policy.
Existing literature on the relationship between smoking and specific health outcomes is prolific, including meta-analyses, cohort studies and case–control studies analyzing the risk of outcomes such as lung cancer 5 , 6 , 7 , chronic obstructive pulmonary disease (COPD) 8 , 9 , 10 and ischemic heart disease 11 , 12 , 13 , 14 due to smoking. There are few if any attempts, however, to systematically and comprehensively evaluate the landscape of evidence on smoking risk across a diverse range of health outcomes, with most current research focusing on risk or attributable burden of smoking for a specific condition 7 , 15 , thereby missing the opportunity to provide a comprehensive picture of the health risk experienced by smokers. Furthermore, although evidence surrounding specific health outcomes, such as lung cancer, has generated widespread consensus, findings about the attributable risk of other outcomes are much more heterogeneous and inconclusive 16 , 17 , 18 . These studies also vary in their risk definitions, with many comparing dichotomous exposure measures of ever smokers versus nonsmokers 19 , 20 . Others examine the distinct risks of current smokers and former smokers compared with never smokers 21 , 22 , 23 . Among the studies that do analyze dose–response relationships, there is large variation in the units and dose categories used in reporting their findings (for example, the use of pack-years or cigarettes per day) 24 , 25 , which complicates the comparability and consolidation of evidence. This, in turn, can obscure data that could inform personal health choices, public health practices and policy measures. Guidance on the health risks of smoking, such as the Surgeon General’s Reports on smoking 26 , 27 , is often based on experts’ evaluation of heterogenous evidence, which, although extremely useful and well suited to carefully consider nuances in the evidence, is fundamentally subjective.
The present study, as part of the Global Burden of Diseases, Risk Factors, and Injuries Study (GBD) 2020, re-estimated the continuous dose–response relationships (the mean risk functions and associated uncertainty estimates) between current smoking and 36 health outcomes (Supplementary Table 1 ) by identifying input studies using a systematic review approach and employing a meta-analytic method 28 . The 36 health outcomes that were selected based on existing evidence of a relationship included 16 cancers (lung cancer, esophageal cancer, stomach cancer, leukemia, liver cancer, laryngeal cancer, breast cancer, cervical cancer, colorectal cancer, lip and oral cavity cancer, nasopharyngeal cancer, other pharynx cancer (excluding nasopharynx cancer), pancreatic cancer, bladder cancer, kidney cancer and prostate cancer), 5 cardiovascular diseases (CVDs: ischemic heart disease, stroke, atrial fibrillation and flutter, aortic aneurysm and peripheral artery disease) and 15 other diseases (COPD, lower respiratory tract infections, tuberculosis, asthma, type 2 diabetes, Alzheimer’s disease and related dementias, Parkinson’s disease, multiple sclerosis, cataracts, gallbladder diseases, low back pain, peptic ulcer disease, rheumatoid arthritis, macular degeneration and fractures). Definitions of the outcomes are described in Supplementary Table 1 . We conducted a separate systematic review for each risk–outcome pair with the exception of cancers, which were done together in a single systematic review. This approach allowed us to systematically identify all relevant studies indexed in PubMed up to 31 May 2022, and we extracted relevant data on risk of smoking, including study characteristics, following a pre-specified template (Supplementary Table 2 ). The meta-analytic tool overcomes many of the limitations of traditional meta-analyses by incorporating between-study heterogeneity into the uncertainty of risk estimates, accounting for small numbers of studies, relaxing the assumption of log(linearity) applied to the risk functions, handling differences in exposure ranges between comparison groups, and systematically testing and adjusting for bias due to study designs and characteristics. We then estimated the burden-of-proof risk function (BPRF) for each risk–outcome pair, as proposed by Zheng et al. 29 ; the BPRF is a conservative risk function defined as the 5th quantile curve (for harmful risks) that reflects the smallest harmful effect at each level of exposure consistent with the available evidence. Given all available data for each outcome, the risk of smoking is at least as harmful as the BPRF indicates.
We used the BPRF for each risk–outcome pair to calculate risk–outcome scores (ROSs) and categorize the strength of evidence for the association between smoking and each health outcome using a star rating from 1 to 5. The interpretation of the star ratings is as follows: 1 star (*) indicates no evidence of association; 2 stars (**) correspond to a 0–15% increase in risk across average range of exposures for harmful risks; 3 stars (***) represent a 15–50% increase in risk; 4 stars (****) refer to >50–85% increase in risk; and 5 stars (*****) equal >85% increase in risk. The thresholds for each star rating were developed in consultation with collaborators and other stakeholders.
The increasing disease burden attributable to current smoking, particularly in low- and middle-income countries 4 , demonstrates the relevance of the present study, which quantifies the strength of the evidence using an objective, quantitative, comprehensive and comparative framework. Findings from the present study can be used to support policy makers in making informed smoking recommendations and regulations focusing on the associations for which the evidence is strongest (that is, the 4- and 5-star associations). However, associations with a lower star rating cannot be ignored, especially when the outcome has high prevalence or severity. A summary of the main findings, limitations and policy implications of the study is presented in Table 1 .
We evaluated the mean risk functions and the BPRFs for 36 health outcomes that are associated with current smoking 30 (Table 2 ). Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 31 for each of our systematic reviews, we identified studies reporting relative risk (RR) of incidence or mortality from each of the 36 selected outcomes for smokers compared with nonsmokers. We reviewed 21,108 records, which were identified to have been published between 1 May 2018 and 31 May 2022; this represents the most recent time period since the last systematic review of the available evidence for the GBD at the time of publication. The meta-analyses reported in the present study for each of the 36 health outcomes are based on evidence from a total of 793 studies published between 1970 and 2022 (Extended Data Fig. 1 – 5 and Supplementary Information 1.5 show the PRISMA diagrams for each outcome). Only prospective cohort and case–control studies were included for estimating dose–response risk curves, but cross-sectional studies were also included for estimating the age pattern of smoking risk on cardiovascular and circulatory disease (CVD) outcomes. Details on each, including the study’s design, data sources, number of participants, length of follow-up, confounders adjusted for in the input data and bias covariates included in the dose–response risk model, can be found in Supplementary Information 2 and 3 . The theoretical minimum risk exposure level used for current smoking was never smoking or zero 30 .
Five-star associations
When the most conservative interpretation of the evidence, that is, the BPRF, suggests that the average exposure (15th–85th percentiles of exposure) of smoking increases the risk of a health outcome by >85% (that is, ROS > 0.62), smoking and that outcome are categorized as a 5-star pair. Among the 36 outcomes, there are 5 that have a 5-star association with current smoking: laryngeal cancer (375% increase in risk based on the BPRF, 1.56 ROS), aortic aneurysm (150%, 0.92), peripheral artery disease (137%, 0.86), lung cancer (107%, 0.73) and other pharynx cancer (excluding nasopharynx cancer) (92%, 0.65).
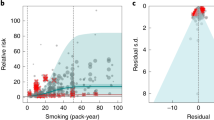
Results for all 5-star risk–outcome pairs are available in Table 2 and Supplementary Information 4.1 . In the present study, we provide detailed results for one example 5-star association: current smoking and lung cancer. We extracted 371 observations from 25 prospective cohort studies and 53 case–control studies across 25 locations (Supplementary Table 3 ) 5 , 6 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 . Exposure ranged from 1 pack-year to >112 pack-years, with the 85th percentile of exposure being 50.88 pack-years (Fig. 1a ).
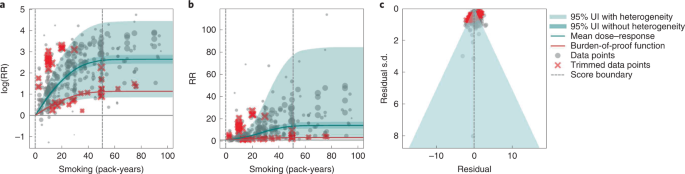
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on the x axis and the estimated s.d. that includes reported s.d. and between-study heterogeneity on the y axis.
We found a very strong and significant harmful relationship between pack-years of current smoking and the RR of lung cancer (Fig. 1b ). The mean RR of lung cancer at 20 pack-years of smoking was 5.11 (95% uncertainty interval (UI) inclusive of between-study heterogeneity = 1.84–14.99). At 50.88 pack-years (85th percentile of exposure), the mean RR of lung cancer was 13.42 (2.63–74.59). See Table 2 for mean RRs at other exposure levels. The BPRF, which represents the most conservative interpretation of the evidence (Fig. 1a ), suggests that smoking in the 15th–85th percentiles of exposure increases the risk of lung cancer by an average of 107%, yielding an ROS of 0.73.
The relationship between pack-years of current smoking and RR of lung cancer is nonlinear, with diminishing impact of further pack-years of smoking, particularly for middle-to-high exposure levels (Fig. 1b ). To reduce the effect of bias, we adjusted observations that did not account for more than five confounders, including age and sex, because they were the significant bias covariates identified by the bias covariate selection algorithm 29 (Supplementary Table 7 ). The reported RRs across studies were very heterogeneous. Our meta-analytic method, which accounts for the reported uncertainty in both the data and between-study heterogeneity, fit the data and covered the estimated residuals well (Fig. 1c ). After trimming 10% of outliers, we still detected publication bias in the results for lung cancer. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 5-star pairs.
Four-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of a health outcome by 50–85% (that is, ROS > 0.41–0.62), smoking is categorized as having a 4-star association with that outcome. We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer (52%, 0.42).
In the present study, we provide detailed results for one example 4-star association: current smoking and COPD. We extracted 51 observations from 11 prospective cohort studies and 4 case–control studies across 36 locations (Supplementary Table 3 ) 6 , 8 , 9 , 10 , 78 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 . Exposure ranged from 1 pack-year to 100 pack-years, with the 85th percentile of exposure in the exposed group being 49.75 pack-years.
We found a strong and significant harmful relationship between pack-years of current smoking and RR of COPD (Fig. 2b ). The mean RR of COPD at 20 pack-years was 3.17 (1.60–6.55; Table 2 reports RRs at other exposure levels). At the 85th percentile of exposure, the mean RR of COPD was 6.01 (2.08–18.58). The BPRF suggests that average smoking exposure raises the risk of COPD by an average of 72%, yielding an ROS of 0.54. The results for the other health outcomes that have an association with smoking rated as 4 stars are shown in Table 2 and Supplementary Information 4.2 .
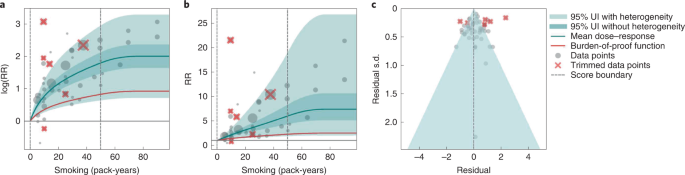
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on th e x axis and the estimated s.d. that includes the reported s.d. and between-study heterogeneity on the y axis.
The relationship between smoking and COPD is nonlinear, with diminishing impact of further pack-years of current smoking on risk of COPD, particularly for middle-to-high exposure levels (Fig. 2a ). To reduce the effect of bias, we adjusted observations that did not account for age and sex and/or were generated for individuals aged >65 years 116 , because they were the two significant bias covariates identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was large heterogeneity in the reported RRs across studies, and our meta-analytic method fit the data and covered the estimated residuals well (Fig. 2b ). Although we trimmed 10% of outliers, publication bias was still detected in the results for COPD. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for reported RR data and alternative exposures across studies for the remaining health outcomes that have a 4-star association with smoking.
Three-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of a health outcome by 15–50% (or, when protective, decreases the risk of an outcome by 13–34%; that is, ROS >0.14–0.41), the association between smoking and that outcome is categorized as having a 3-star rating. We identified 15 outcomes with a 3-star association: bladder cancer (40% increase in risk, 0.34 ROS); tuberculosis (31%, 0.27); esophageal cancer (29%, 0.26); cervical cancer, multiple sclerosis and rheumatoid arthritis (each 23–24%, 0.21); lower back pain (22%, 0.20); ischemic heart disease (20%, 0.19); peptic ulcer and macular degeneration (each 19–20%, 0.18); Parkinson's disease (protective risk, 15% decrease in risk, 0.16); and stomach cancer, stroke, type 2 diabetes and cataracts (each 15–17%, 0.14–0.16).
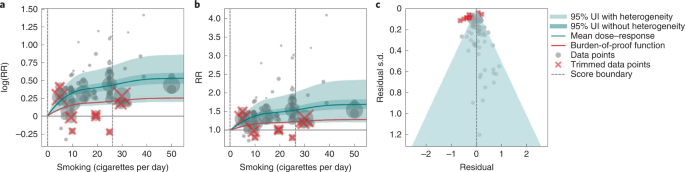
We present the findings on smoking and type 2 diabetes as an example of a 3-star risk association. We extracted 102 observations from 24 prospective cohort studies and 4 case–control studies across 15 locations (Supplementary Table 3 ) 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 . The exposure ranged from 1 cigarette to 60 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 26.25 cigarettes smoked per day.
We found a moderate and significant harmful relationship between cigarettes smoked per day and the RR of type 2 diabetes (Fig. 3b ). The mean RR of type 2 diabetes at 20 cigarettes smoked per day was 1.49 (1.18–1.90; see Table 2 for other exposure levels). At the 85th percentile of exposure, the mean RR of type 2 diabetes was 1.54 (1.20–2.01). The BPRF suggests that average smoking exposure raises the risk of type 2 diabetes by an average of 16%, yielding an ROS of 0.15. See Table 2 and Supplementary Information 4.3 for results for the additional health outcomes with an association with smoking rated as 3 stars.
a , The log(RR) function. b , RR function. c , A modified funnel plot showing the residuals (relative to 0) on the x axis and the estimated s.d. that includes the reported s.d. and between-study heterogeneity on the y axis.
The relationship between smoking and type 2 diabetes is nonlinear, particularly for high exposure levels where the mean risk curve becomes flat (Fig. 3a ). We adjusted observations that were generated in subpopulations, because it was the only significant bias covariate identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was moderate heterogeneity in the observed RR data across studies and our meta-analytic method fit the data and covered the estimated residuals extremely well (Fig. 3b,c ). After trimming 10% of outliers, we still detected publication bias in the results for type 2 diabetes. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 3-star pairs.
Two-star associations
When the BPRF suggests that the average exposure of smoking increases the risk of an outcome by 0–15% (that is, ROS 0.0–0.14), the association between smoking and that outcome is categorized as a 2-star rating. We identified six 2-star outcomes: nasopharyngeal cancer (14% increase in risk, 0.13 ROS); Alzheimer’s and other dementia (10%, 0.09); gallbladder diseases and atrial fibrillation and flutter (each 6%, 0.06); lip and oral cavity cancer (5%, 0.05); and breast cancer (4%, 0.04).
We present the findings on smoking and breast cancer as an example of a 2-star association. We extracted 93 observations from 14 prospective cohort studies and 9 case–control studies across 14 locations (Supplementary Table 3 ) 84 , 87 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 . The exposure ranged from 1 cigarette to >76 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 34.10 cigarettes smoked per day.
We found a weak but significant relationship between pack-years of current smoking and RR of breast cancer (Extended Data Fig. 6 ). The mean RR of breast cancer at 20 pack-years was 1.17 (1.04–1.31; Table 2 reports other exposure levels). The BPRF suggests that average smoking exposure raises the risk of breast cancer by an average of 4%, yielding an ROS of 0.04. See Table 2 and Supplementary Information 4.4 for results on the additional health outcomes for which the association with smoking has been categorized as 2 stars.
The relationship between smoking and breast cancer is nonlinear, particularly for high exposure levels where the mean risk curve becomes flat (Extended Data Fig. 6a ). To reduce the effect of bias, we adjusted observations that were generated in subpopulations, because it was the only significant bias covariate identified by the bias covariate selection algorithm (Supplementary Table 7 ). There was heterogeneity in the reported RRs across studies, but our meta-analytic method fit the data and covered the estimated residuals (Extended Data Fig. 6b ). After trimming 10% of outliers, we did not detect publication bias in the results for breast cancer. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 2-star pairs.
One-star associations
When average exposure to smoking does not significantly increase (or decrease) the risk of an outcome, once between-study heterogeneity and other sources of uncertainty are accounted for (that is, ROS < 0), the association between smoking and that outcome is categorized as 1 star, indicating that there is not sufficient evidence for the effect of smoking on the outcome to reject the null (that is, there may be no association). There were seven outcomes with an association with smoking that rated as 1 star: colorectal and kidney cancer (each –0.01 ROS); leukemia (−0.04); fractures (−0.05); prostate cancer (−0.06); liver cancer (−0.32); and asthma (−0.64).
We use smoking and prostate cancer as examples of a 1-star association. We extracted 78 observations from 21 prospective cohort studies and 1 nested case–control study across 15 locations (Supplementary Table 3 ) 157 , 160 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 . The exposure among the exposed group ranged from 1 cigarette to 90 cigarettes smoked per day, with the 85th percentile of exposure in the exposed group being 29.73 cigarettes smoked per day.
Based on our conservative interpretation of the data, we did not find a significant relationship between cigarettes smoked per day and the RR of prostate cancer (Fig. 4B ). The exposure-averaged BPRF for prostate cancer was 0.94, which was opposite null from the full range of mean RRs, such as 1.16 (0.89–1.53) at 20 cigarettes smoked per day. The corresponding ROS was −0.06, which is consistent with no evidence of an association between smoking and increased risk of prostate cancer. See Table 2 and Supplementary Information 4.5 for results for the additional outcomes that have a 1-star association with smoking.
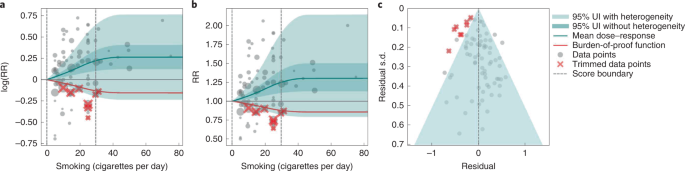
The relationship between smoking and prostate cancer is nonlinear, particularly for middle-to-high exposure levels where the mean risk curve becomes flat (Fig. 4a ). We did not adjust for any bias covariate because no significant bias covariates were selected by the algorithm (Supplementary Table 7 ). The RRs reported across studies were very heterogeneous, but our meta-analytic method fit the data and covered the estimated residuals well (Fig. 4b,c ). The ROS associated with the BPRF is −0.05, suggesting that the most conservative interpretation of all evidence, after accounting for between-study heterogeneity, indicates an inconclusive relationship between smoking exposure and the risk of prostate cancer. After trimming 10% of outliers, we still detected publication bias in the results for prostate cancer, which warrants further studies using sample populations. See Supplementary Tables 4 and 7 for study bias characteristics and selected bias covariates, Supplementary Fig. 5 for results without 10% trimming and Supplementary Table 8 for observed RR data and alternative exposures across studies for the remaining 1-star pairs.
Age-specific dose–response risk for CVD outcomes
We produced age-specific dose–response risk curves for the five selected CVD outcomes ( Methods ). The ROS associated with each smoking–CVD pair was calculated based on the reference risk curve estimated using all risk data regardless of age information. Estimation of the BPRF, calculation of the associated ROS and star rating of the smoking–CVD pairs follow the same rules as the other non-CVD smoking–outcome pairs (Table 1 and Supplementary Figs. 2 – 4 ). Once we had estimated the reference dose–response risk curve for each CVD outcome, we determined the age group of the reference risk curve. The reference age group is 55–59 years for all CVD outcomes, except for peripheral artery disease, the reference age group for which is 60–64 years. We then estimated the age pattern of smoking on all CVD outcomes (Supplementary Fig. 2 ) and calculated age attenuation factors of the risk for each age group by comparing the risk of each age group with that of the reference age group, using the estimated age pattern (Supplementary Fig. 3 ). Last, we applied the draws of age attenuation factors of each age group to the dose–response risk curve for the reference age group to produce the age group-specific dose–response risk curves for each CVD outcome (Supplementary Fig. 4 ).
Using our burden-of-proof meta-analytic methods, we re-estimated the dose–response risk of smoking on 36 health outcomes that had previously been demonstrated to be associated with smoking 30 , 186 . Using these methods, which account for both the reported uncertainty of the data and the between-study heterogeneity, we found that 29 of the 36 smoking–outcome pairs are supported by evidence that suggests a significant dose–response relationship between smoking and the given outcome (28 with a harmful association and 1 with a protective association). Conversely, after accounting for between-study heterogeneity, the available evidence of smoking risk on seven outcomes (that is, colon and rectum cancer, kidney cancer, leukemia, prostate cancer, fractures, liver cancer and asthma) was insufficient to reject the null or draw definitive conclusions on their relationship to smoking. Among the 29 outcomes that have evidence supporting a significant relationship to smoking, 8 had strong-to-very-strong evidence of a relationship, meaning that, given all the available data on smoking risk, we estimate that average exposure to smoking increases the risk of those outcomes by >50% (4- and 5-star outcomes). The currently available evidence for the remaining 21 outcomes with a significant association with current smoking was weak to moderate, indicating that smoking increases the risk of those outcomes by at least >0–50% (2- and 3-star associations).
Even under our conservative interpretation of the data, smoking is irrefutably harmful to human health, with the greatest increases in risk occurring for laryngeal cancer, aortic aneurysm, peripheral artery disease, lung cancer and other pharynx cancer (excluding nasopharynx cancer), which collectively represent large causes of death and ill-health. The magnitude of and evidence for the associations between smoking and its leading health outcomes are among the highest currently analyzed in the burden-of-proof framework 29 . The star ratings assigned to each smoking–outcome pair offer policy makers a way of categorizing and comparing the evidence for a relationship between smoking and its potential health outcomes ( https://vizhub.healthdata.org/burden-of-proof ). We found that, for seven outcomes in our analysis, there was insufficient or inconsistent evidence to demonstrate a significant association with smoking. This is a key finding because it demonstrates the need for more high-quality data for these particular outcomes; availability of more data should improve the strength of evidence for whether or not there is an association between smoking and these health outcomes.
Our systematic review approach and meta-analytic methods have numerous benefits over existing systematic reviews and meta-analyses on the same topic that use traditional random effects models. First, our approach relaxes the log(linear) assumption, using a spline ensemble to estimate the risk 29 . Second, our approach allows variable reference groups and exposure ranges, allowing for more accurate estimates regardless of whether or not the underlying relative risk is log(linear). Furthermore, it can detect outliers in the data automatically. Finally, it quantifies uncertainty due to between-study heterogeneity while accounting for small numbers of studies, minimizing the risk that conclusions will be drawn based on spurious findings.
We believe that the results for the association between smoking and each of the 36 health outcomes generated by the present study, including the mean risk function, BPRF, ROS, average excess risk and star rating, could be useful to a range of stakeholders. Policy makers can formulate their decisions on smoking control priorities and resource allocation based on the magnitude of the effect and the consistency of the evidence relating smoking to each of the 36 outcomes, as represented by the ROS and star rating for each smoking–outcome association 187 . Physicians and public health practitioners can use the estimates of average increased risk and the star rating to educate patients and the general public about the risk of smoking and to promote smoking cessation 188 . Researchers can use the estimated mean risk function or BPRF to obtain the risk of an outcome at a given smoking exposure level, as well as uncertainty surrounding that estimate of risk. The results can also be used in the estimation of risk-attributable burden, that is, the deaths and disability-adjusted life-years due to each outcome that are attributable to smoking 30 , 186 . For the general public, these results could help them to better understand the risk of smoking and manage their health 189 .
Although our meta-analysis was comprehensive and carefully conducted, there are limitations to acknowledge. First, the bias covariates used, although carefully extracted and evaluated, were based on observable study characteristics and thus may not fully capture unobserved characteristics such as study quality or context, which might be major sources of bias. Second, if multiple risk estimates with different adjustment levels were reported in a given study, we included only the fully adjusted risk estimate and modeled the adjustment level according to the number of covariates adjusted for (rather than which covariates were adjusted for) and whether a standard adjustment for age and sex had been applied. This approach limited our ability to make full use of all available risk estimates in the literature. Third, although we evaluated the potential for publication bias in the data, we did not test for other forms of bias such as when studies are more consistent with each other than expected by chance 29 . Fourth, our analysis assumes that the relationships between smoking and health outcomes are similar across geographical regions and over time. We do not have sufficient evidence to quantify how the relationships may have evolved over time because the composition of smoking products has also changed over time. Perhaps some of the heterogeneity of the effect sizes in published studies reflects this; however, this cannot be discerned with the currently available information.
In the future, we plan to include crude and partially adjusted risk estimates in our analyses to fully incorporate all available risk estimates, to model the adjusted covariates in a more comprehensive way by mapping the adjusted covariates across all studies comprehensively and systematically, and to develop methods to evaluate additional forms of potential bias. We plan to update our results on a regular basis to provide timely and up-to-date evidence to stakeholders.
To conclude, we have re-estimated the dose–response risk of smoking on 36 health outcomes while synthesizing all the available evidence up to 31 May 2022. We found that, even after factoring in the heterogeneity between studies and other sources of uncertainty, smoking has a strong-to-very-strong association with a range of health outcomes and confirmed that smoking is irrefutably highly harmful to human health. We found that, due to small numbers of studies, inconsistency in the data, small effect sizes or a combination of these reasons, seven outcomes for which some previous research had found an association with smoking did not—under our meta-analytic framework and conservative approach to interpreting the data—have evidence of an association. Our estimates of the evidence for risk of smoking on 36 selected health outcomes have the potential to inform the many stakeholders of smoking control, including policy makers, researchers, public health professionals, physicians, smokers and the general public.
For the present study, we used a meta-analytic tool, MR-BRT (metaregression—Bayesian, regularized, trimmed), to estimate the dose–response risk curves of the risk of a health outcome across the range of current smoking levels along with uncertainty estimates 28 . Compared with traditional meta-analysis using linear mixed effect models, MR-BRT relaxes the assumption of a log(linear) relationship between exposure and risk, incorporates between-study heterogeneity into the uncertainty of risk estimates, handles estimates reported across different exposure categories, automatically identifies and trims outliers, and systematically tests and adjusts for bias due to study designs and characteristics. The meta-analytic methods employed by the present study followed the six main steps proposed by Zheng et al. 28 , 29 , namely: (1) enacting a systematic review approach and data extraction following a pre-specified and standardized protocol; (2) estimating the shape of the relationship between exposure and RR; (3) evaluating and adjusting for systematic bias as a function of study characteristics and risk estimation; (4) quantifying between-study heterogeneity while adjusting for within-study correlation and the number of studies; (5) evaluating potential publication or reporting biases; and (6) estimating the mean risk function and the BPRF, calculating the ROS and categorizing smoking–outcome pairs using a star-rating scheme from 1 to 5.
The estimates for our primary indicators of this work—mean RRs across a range of exposures, BRPFs, ROSs and star ratings for each risk–outcome pair—are not specific to or disaggregated by specific populations. We did not estimate RRs separately for different locations, sexes (although the RR of prostate cancer was estimated only for males and of cervical and breast cancer only for females) or age groups (although this analysis was applied to disease endpoints in adults aged ≥30 years only and, as detailed below, age-specific estimates were produced for the five CVD outcomes).
The present study complies with the PRISMA guidelines 190 (Supplementary Tables 9 and 10 and Supplementary Information 1.5 ) and Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations 191 (Supplementary Table 11 ). The study was approved by the University of Washington Institutional Review Board (study no. 9060). The systematic review approach was not registered.
Selecting health outcomes
In the present study, current smoking is defined as the current use of any smoked tobacco product on a daily or occasional basis. Health outcomes were initially selected using the World Cancer Research Fund criteria for convincing or probable evidence as described in Murray et al. 186 . The 36 health outcomes that were selected based on existing evidence of a relationship included 16 cancers (lung cancer, esophageal cancer, stomach cancer, leukemia, liver cancer, laryngeal cancer, breast cancer, cervical cancer, colorectal cancer, lip and oral cavity cancer, nasopharyngeal cancer, other pharynx cancer (excluding nasopharynx cancer), pancreatic cancer, bladder cancer, kidney cancer and prostate cancer), 5 CVDs (ischemic heart disease, stroke, atrial fibrillation and flutter, aortic aneurysm and peripheral artery disease) and 15 other diseases (COPD, lower respiratory tract infections, tuberculosis, asthma, type 2 diabetes, Alzheimer’s disease and related dementias, Parkinson’s disease, multiple sclerosis, cataracts, gallbladder diseases, low back pain, peptic ulcer disease, rheumatoid arthritis, macular degeneration and fracture). Definitions of the outcomes are described in Supplementary Table 1 .
Step 1: systematic review approach to literature search and data extraction
Informed by the systematic review approach we took for the GBD 2019 (ref. 30 ), for the present study we identified input studies in the literature using a systematic review approach for all 36 smoking–outcome pairs using updated search strings to identify all relevant studies indexed in PubMed up to 31 May 2022 and extracted data on smoking risk estimates. Briefly, the studies that were extracted represented several types of study design (for example, cohort and case–control studies), measured exposure in several different ways and varied in their choice of reference categories (where some compared current smokers with never smokers, whereas others compared current smokers with nonsmokers or former smokers). All these study characteristics were catalogued systematically and taken into consideration during the modeling part of the analysis.
In addition, for CVD outcomes, we also estimated the age pattern of risk associated with smoking. We applied a systematic review of literature approach for smoking risk for the five CVD outcomes. We developed a search string to search for studies reporting any association between binary smoking status (that is, current, former and ever smokers) and the five CVD outcomes from 1 January 1970 to 31 May 2022, and included only studies reporting age-specific risk (RR, odds ratio (OR), hazard ratio (HR)) of smoking status. The inclusion criteria and results of the systematic review approach are reported in accordance with PRISMA guidelines 31 . Details for each outcome on the search string used in the systematic review approach, refined inclusion and exclusion criteria, data extraction template and PRISMA diagram are given in Supplementary Information 1 . Title and/or abstract screening, full text screening and data extraction were conducted by 14 members of the research team and extracted data underwent manual quality assurance by the research team to verify accuracy.
Selecting exposure categories
Cumulative exposure in pack-years was the measure of exposure used for COPD and all cancer outcomes except for prostate cancer, to reflect the risk of both duration and intensity of current smoking on these outcomes. For prostate cancer, CVDs and all the other outcomes except for fractures, we used cigarette-equivalents smoked per day as the exposure for current smoking, because smoking intensity is generally thought to be more important than duration for these outcomes. For fractures, we used binary exposure, because there were few studies examining intensity or duration of smoking on fractures. The smoking–outcome pairs and the corresponding exposures are summarized in Supplementary Table 4 and are congruent with the GBD 2019 (refs. 30 , 186 ).
Steps 2–5: modeling dose–response RR of smoking on the selected health outcomes
Of the six steps proposed by Zheng et al. 29 , steps 2–5 cover the process of modeling dose–response risk curves. In step 2, we estimated the shape (or the ‘signal’) of the dose–response risk curves, integrating over different exposure ranges. To relax the log(linear) assumption usually applied to continuous dose–response risk and make the estimates robust to the placement of spline knots, we used an ensemble spline approach to fit the functional form of the dose–response relationship. The final ensemble model was a weighted combination of 50 models with random knot placement, with the weight of each model proportional to measures of model fit and total variation. To avoid the influence of extreme data and reduce publication bias, we trimmed 10% of data for each outcome as outliers. We also applied a monotonicity constraint to ensure that the mean risk curves were nondecreasing (or nonincreasing in the case of Parkinson’s disease).
In step 3, following the GRADE approach 192 , 193 , we quantified risk of bias across six domains, namely, representativeness of the study population, exposure, outcome, reverse causation, control for confounding and selection bias. Details about the bias covariates are provided in Supplementary Table 4 . We systematically tested for the effect of bias covariates using metaregression, selected significant bias covariates using the Lasso approach 194 , 195 and adjusted for the selected bias covariates in the final risk curve.
In step 4, we quantified between-study heterogeneity accounting for within-study correlation, uncertainty of the heterogeneity, as well as small number of studies. Specifically, we used a random intercept in the mixed-effects model to account for the within-study correlation and used a study-specific random slope with respect to the ‘signal’ to capture between-study heterogeneity. As between-study heterogeneity can be underestimated or even zero when the number of studies is small 196 , 197 , we used Fisher’s information matrix to estimate the uncertainty of the heterogeneity 198 and incorporated that uncertainty into the final results.
In step 5, in addition to generating funnel plots and visually inspecting for asymmetry (Figs. 1c , 2c , 3c and 4c and Extended Data Fig. 6c ) to identify potential publication bias, we also statistically tested for potential publication or reporting bias using Egger’s regression 199 . We flagged potential publication bias in the data but did not correct for it, which is in line with the general literature 10 , 200 , 201 . Full details about the modeling process have been published elsewhere 29 and model specifications for each outcome are in Supplementary Table 6 .
Step 6: estimating the mean risk function and the BPRF
In the final step, step 6, the metaregression model inclusive of the selected bias covariates from step 3 (for example, the highest adjustment level) was used to predict the mean risk function and its 95% UI, which incorporated the uncertainty of the mean effect, between-study heterogeneity and the uncertainty in the heterogeneity estimate accounting for small numbers of studies. Specifically, 1,000 draws were created for each 0.1 level of doses from 0 pack-years to 100 pack-years or cigarette-equivalents smoked per day using the Bayesian metaregression model. The mean of the 1,000 draws was used to estimate the mean risk at each exposure level, and the 25th and 95th draws were used to estimate the 95% UIs for the mean risk at each exposure level.
The BPRF 29 is a conservative estimate of risk function consistent with the available evidence, correcting for both between-study heterogeneity and systemic biases related to study characteristics. The BPRF is defined as either the 5th (if harmful) or 95th (if protective) quantile curve closest to the line of log(RR) of 0, which defines the null (Figs. 1a , 2b , 3a and 4a ). The BPRF represents the smallest harmful (or protective) effect of smoking on the corresponding outcome at each level of exposure that is consistent with the available evidence. A BPRF opposite null from the mean risk function indicates that insufficient evidence is available to reject null, that is, that there may not be an association between risk and outcome. Likewise, the further the BPRF is from null on the same side of null as the mean risk function, the higher the magnitude and evidence for the relationship. The BPRF can be interpreted as indicating that, even accounting for between-study heterogeneity and its uncertainty, the log(RR) across the studied smoking range is at least as high as the BPRF (or at least as low as the BPRF for a protective risk).
To quantify the strength of the evidence, we calculated the ROS for each smoking–outcome association as the signed value of the log(BPRF) averaged between the 15th and 85th percentiles of observed exposure levels for each outcome. The ROS is a single summary of the effect of smoking on the outcome, with higher positive ROSs corresponding to stronger and more consistent evidence and a higher average effect size of smoking and a negative ROS, suggesting that, based on the available evidence, there is no significant effect of smoking on the outcome after accounting for between-study heterogeneity.
For ease of communication, we further classified each smoking–outcome association into a star rating from 1 to 5. Briefly, 1-star associations have an ROS <0, indicating that there is insufficient evidence to find a significant association between smoking and the selected outcome. We divided the positive ROSs into ranges 0.0–0.14 (2-star), >0.14–0.41 (3-star), >0.41–0.62 (4-star) and >0.62 (5-star). These categories correspond to excess risk ranges for harmful risks of 0–15%, >15–50%, >50–85% and >85%. For protective risks, the ranges of exposure-averaged decreases in risk by star rating are 0–13% (2 stars), >13–34% (3 stars), >34–46% (4 stars) and >46% (5 stars).
Among the 36 smoking–outcome pairs analyzed, smoking fracture was the only binary risk–outcome pair, which was due to limited data on the dose–response risk of smoking on fracture 202 . The estimation of binary risk was simplified because the RR was merely a comparison between current smokers and nonsmokers or never smokers. The concept of ROS for continuous risk can naturally extend to binary risk because the BPRF is still defined as the 5th percentile of the effect size accounting for data uncertainty and between-study heterogeneity. However, binary ROSs must be divided by 2 to make them comparable with continuous ROSs, which were calculated by averaging the risk over the range between the 15th and the 85th percentiles of observed exposure levels. Full details about estimating mean risk functions, BPRFs and ROSs for both continuous and binary risk–outcome pairs can be found elsewhere 29 .
Estimating the age-specific risk function for CVD outcomes
For non-CVD outcomes, we assumed that the risk function was the same for all ages and all sexes, except for breast, cervical and prostate cancer, which were assumed to apply only to females or males, respectively. As the risk of smoking on CVD outcomes is known to attenuate with increasing age 203 , 204 , 205 , 206 , we adopted a four-step approach for GBD 2020 to produce age-specific dose–response risk curves for CVD outcomes.
First, we estimated the reference dose–response risk of smoking for each CVD outcome using dose-specific RR data for each outcome regardless of the age group information. This step was identical to that implemented for the other non-CVD outcomes. Once we had generated the reference curve, we determined the age group associated with it by calculating the weighted mean age across all dose-specific RR data (weighted by the reciprocal of the s.e.m. of each datum). For example, if the weighted mean age of all dose-specific RR data was 56.5, we estimated the age group associated with the reference risk curve to be aged 55–59 years. For cohort studies, the age range associated with the RR estimate was calculated as a mean age at baseline plus the mean/median years of follow-up (if only the maximum years of follow-up were reported, we would halve this value and add it to the mean age at baseline). For case–control studies, the age range associated with the OR estimate was simply the reported mean age at baseline (if mean age was not reported, we used the midpoint of the age range instead).
In the third step, we extracted age group-specific RR data and relevant bias covariates from the studies identified in our systematic review approach of age-specific smoking risk on CVD outcomes, and used MR-BRT to model the age pattern of excess risk (that is, RR-1) of smoking on CVD outcomes with age group-specific excess RR data for all CVD outcomes. We modeled the age pattern of smoking risk on CVDs following the same steps we implemented for modeling dose–response risk curves. In the final model, we included a spline on age, random slope on age by study and the bias covariate encoding exposure definition (that is, current, former and ever smokers), which was picked by the variable selection algorithm 28 , 29 . When predicting the age pattern of the excess risk of smoking on CVD outcomes using the fitted model, we did not include between-study heterogeneity to reduce uncertainty in the prediction.
In the fourth step, we calculated the age attenuation factors of excess risk compared with the reference age group for each CVD outcome as the ratio of the estimated excess risk for each age group to the excess risk for the reference age group. We performed the calculation at the draw level to obtain 1,000 draws of the age attenuation factors for each age group. Once we had estimated the age attenuation factors, we carried out the last step, which consisted of adjusting the risk curve for the reference age group from step 1 using equation (1) to produce the age group-specific risk curves for each CVD outcome:
We implemented the age adjustment at the draw level so that the uncertainty of the age attenuation factors could be naturally incorporated into the final adjusted age-specific RR curves. A PRISMA diagram detailing the systematic review approach, a description of the studies included and the full details about the methods are in Supplementary Information 1.5 and 5.2 .
Estimating the theoretical minimum risk exposure level
The theoretical minimum risk exposure level for smoking was 0, that is, no individuals in the population are current or former smokers.
Model validation
The validity of the meta-analytic tool has been extensively evaluated by Zheng and colleagues using simulation experiments 28 , 29 . For the present study, we conducted two additional sensitivity analyses to examine how the shape of the risk curves was impacted by applying a monotonicity constraint and trimming 10% of data. We present the results of these sensitivity analyses in Supplementary Information 6 . In addition to the sensitivity analyses, the dose–response risk estimates were also validated by plotting the mean risk function along with its 95% UI against both the extracted dose-specific RR data from the studies included and our previous dose–response risk estimates from the GBD 2019 (ref. 30 ). The mean risk functions along with the 95% UIs were validated based on data fit and the level, shape and plausibility of the dose–response risk curves. All curves were validated by all authors and reviewed by an external expert panel, comprising professors with relevant experience from universities including Johns Hopkins University, Karolinska Institute and University of Barcelona; senior scientists working in relevant departments at the WHO and the Center for Disease Control and Prevention (CDC) and directors of nongovernmental organizations such as the Campaign for Tobacco-Free Kids.
Statistical analysis
Analyses were carried out using R v.3.6.3, Python v.3.8 and Stata v.16.
Statistics and reproducibility
The study was a secondary analysis of existing data involving systematic reviews and meta-analyses. No statistical method was used to predetermine sample size. As the study did not involve primary data collection, randomization and blinding, data exclusions were not relevant to the present study, and, as such, no data were excluded and we performed no randomization or blinding. We have made our data and code available to foster reproducibility.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The findings from the present study are supported by data available in the published literature. Data sources and citations for each risk–outcome pair can be downloaded using the ‘download’ button on each risk curve page currently available at https://vizhub.healthdata.org/burden-of-proof . Study characteristics and citations for all input data used in the analyses are also provided in Supplementary Table 3 , and Supplementary Table 2 provides a template of the data collection form.
Code availability
All code used for these analyses is publicly available online ( https://github.com/ihmeuw-msca/burden-of-proof ).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung. Br. Med. J. 2 , 739–748 (1950).
Article CAS PubMed PubMed Central Google Scholar
Di Cicco, M. E., Ragazzo, V. & Jacinto, T. Mortality in relation to smoking: the British Doctors Study. Breathe 12 , 275–276 (2016).
Article PubMed PubMed Central Google Scholar
World Health Organization. WHO Framework Convention on Tobacco Control 36 (WHO, 2003).
Dai, X., Gakidou, E. & Lopez, A. D. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob. Control 31 , 129–137 (2022).
Article PubMed Google Scholar
Dikshit, R. P. & Kanhere, S. Tobacco habits and risk of lung, oropharyngeal and oral cavity cancer: a population-based case-control study in Bhopal, India. Int. J. Epidemiol. 29 , 609–614 (2000).
Article CAS PubMed Google Scholar
Liaw, K. M. & Chen, C. J. Mortality attributable to cigarette smoking in Taiwan: a 12-year follow-up study. Tob. Control 7 , 141–148 (1998).
Gandini, S. et al. Tobacco smoking and cancer: a meta-analysis. Int. J. Cancer 122 , 155–164 (2008).
Deng, X., Yuan, C. & Chang, D. Interactions between single nucleotide polymorphism of SERPINA1 gene and smoking in association with COPD: a case–control study. Int. J. Chron. Obstruct. Pulmon. Dis. 12 , 259–265 (2017).
Leem, A. Y., Park, B., Kim, Y. S., Jung, J. Y. & Won, S. Incidence and risk of chronic obstructive pulmonary disease in a Korean community-based cohort. Int. J. Chron. Obstruct. Pulmon. Dis. 13 , 509–517 (2018).
Forey, B. A., Thornton, A. J. & Lee, P. N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulmon. Med. 11 , 36 (2011).
Article Google Scholar
Tan, J. et al. Smoking, blood pressure, and cardiovascular disease mortality in a large cohort of Chinese men with 15 years follow-up. Int. J. Environ. Res. Public Health 15 , E1026 (2018).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Br. Med. J. 328 , 1519 (2004).
Huxley, R. R. & Woodward, M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 378 , 1297–1305 (2011).
Hbejan, K. Smoking effect on ischemic heart disease in young patients. Heart Views 12 , 1–6 (2011).
Chao, H. et al. A meta-analysis of active smoking and risk of meningioma. Tob. Induc. Dis. 19 , 34 (2021).
Shi, H., Shao, X. & Hong, Y. Association between cigarette smoking and the susceptibility of acute myeloid leukemia: a systematic review and meta-analysis. Eur. Rev. Med Pharm. Sci. 23 , 10049–10057 (2019).
CAS Google Scholar
Macacu, A., Autier, P., Boniol, M. & Boyle, P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res. Treat. 154 , 213–224 (2015).
Pujades-Rodriguez, M. et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1 937 360 people in England: lifetime risks and implications for risk prediction. Int. J. Epidemiol. 44 , 129–141 (2015).
Kanazir, M. et al. Risk factors for hepatocellular carcinoma: a case-control study in Belgrade (Serbia). Tumori 96 , 911–917 (2010).
Pytynia, K. B. et al. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J. Clin. Oncol. 22 , 3981–3988 (2004).
Barengo, N. C., Antikainen, R., Harald, K. & Jousilahti, P. Smoking and cancer, cardiovascular and total mortality among older adults: the Finrisk Study. Prev. Med. Rep. 14 , 100875 (2019).
Guo, Y. et al. Modifiable risk factors for cognitive impairment in Parkinson’s disease: a systematic review and meta-analysis of prospective cohort studies. Mov. Disord. 34 , 876–883 (2019).
Aune, D., Vatten, L. J. & Boffetta, P. Tobacco smoking and the risk of gallbladder disease. Eur. J. Epidemiol. 31 , 643–653 (2016).
Qin, L., Deng, H.-Y., Chen, S.-J. & Wei, W. Relationship between cigarette smoking and risk of chronic myeloid leukaemia: a meta-analysis of epidemiological studies. Hematology 22 , 193–200 (2017).
Petrick, J. L. et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br. J. Cancer 118 , 1005–1012 (2018).
United States Department of Health, Education and Welfare. Smoking and Health. Report of the Advisory Committee on Smoking and Health to the Surgeon General of the United States Public Health Service https://www.cdc.gov/tobacco/data_statistics/sgr/index.htm (US DHEW, 1964).
United States Public Health Service Office of the Surgeon General & National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General . (US Department of Health and Human Services, 2020).
Zheng, P., Barber, R., Sorensen, R. J. D., Murray, C. J. L. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph Stat. 30 , 544–556 (2021).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. in press (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br. Med. J. 339 , b2535 (2009).
Liu, Z. Y., He, X. Z. & Chapman, R. S. Smoking and other risk factors for lung cancer in Xuanwei, China. Int. J. Epidemiol. 20 , 26–31 (1991).
Brownson, R. C., Reif, J. S., Keefe, T. J., Ferguson, S. W. & Pritzl, J. A. Risk factors for adenocarcinoma of the lung. Am. J. Epidemiol. 125 , 25–34 (1987).
Marugame, T. et al. Lung cancer death rates by smoking status: comparison of the Three-Prefecture Cohort study in Japan to the Cancer Prevention Study II in the USA. Cancer Sci. 96 , 120–126 (2005).
Dosemeci, M., Gokmen, I., Unsal, M., Hayes, R. B. & Blair, A. Tobacco, alcohol use, and risks of laryngeal and lung cancer by subsite and histologic type in Turkey. Cancer Causes Control 8 , 729–737 (1997).
Freedman, N. D. et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int. J. Epidemiol. 45 , 846–856 (2016).
Bae, J.-M. et al. Lung cancer incidence by smoking status in Korean men: 16 years of observations in the Seoul Male Cancer Cohort study. J. Korean Med. Sci. 28 , 636–637 (2013).
Everatt, R., Kuzmickienė, I., Virvičiūtė, D. & Tamošiūnas, A. Cigarette smoking, educational level and total and site-specific cancer: a cohort study in men in Lithuania. Eur. J. Cancer Prev. 23 , 579–586 (2014).
Nordlund, L. A., Carstensen, J. M. & Pershagen, G. Are male and female smokers at equal risk of smoking-related cancer: evidence from a Swedish prospective study. Scand. J. Public Health 27 , 56–62 (1999).
Siemiatycki, J., Krewski, D., Franco, E. & Kaiserman, M. Associations between cigarette smoking and each of 21 types of cancer: a multi-site case–control study. Int. J. Epidemiol. 24 , 504–514 (1995).
Chyou, P. H., Nomura, A. M. & Stemmermann, G. N. A prospective study of the attributable risk of cancer due to cigarette smoking. Am. J. Public Health 82 , 37–40 (1992).
Potter, J. D., Sellers, T. A., Folsom, A. R. & McGovern, P. G. Alcohol, beer, and lung cancer in postmenopausal women. The Iowa Women’s Health Study. Ann. Epidemiol. 2 , 587–595 (1992).
Chyou, P. H., Nomura, A. M., Stemmermann, G. N. & Kato, I. Lung cancer: a prospective study of smoking, occupation, and nutrient intake. Arch. Environ. Health 48 , 69–72 (1993).
Pesch, B. et al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int. J. Cancer 131 , 1210–1219 (2012).
Jöckel, K. H. et al. Occupational and environmental hazards associated with lung cancer. Int. J. Epidemiol. 21 , 202–213 (1992).
Jöckel, K. H., Ahrens, W., Jahn, I., Pohlabeln, H. & Bolm-Audorff, U. Occupational risk factors for lung cancer: a case-control study in West Germany. Int. J. Epidemiol. 27 , 549–560 (1998).
Lei, Y. X., Cai, W. C., Chen, Y. Z. & Du, Y. X. Some lifestyle factors in human lung cancer: a case-control study of 792 lung cancer cases. Lung Cancer 14 , S121–S136 (1996).
Pawlega, J., Rachtan, J. & Dyba, T. Evaluation of certain risk factors for lung cancer in Cracow (Poland)—a case–control study. Acta Oncol. 36 , 471–476 (1997).
Mao, Y. et al. Socioeconomic status and lung cancer risk in Canada. Int. J. Epidemiol. 30 , 809–817 (2001).
Barbone, F., Bovenzi, M., Cavallieri, F. & Stanta, G. Cigarette smoking and histologic type of lung cancer in men. Chest 112 , 1474–1479 (1997).
Matos, E., Vilensky, M., Boffetta, P. & Kogevinas, M. Lung cancer and smoking: a case–control study in Buenos Aires, Argentina. Lung Cancer 21 , 155–163 (1998).
Simonato, L. et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int. J. Cancer 91 , 876–887 (2001).
Risch, H. A. et al. Are female smokers at higher risk for lung cancer than male smokers? A case–control analysis by histologic type. Am. J. Epidemiol. 138 , 281–293 (1993).
Sankaranarayanan, R. et al. A case–control study of diet and lung cancer in Kerala, south India. Int. J. Cancer 58 , 644–649 (1994).
Band, P. R. et al. Identification of occupational cancer risks in British Columbia. Part I: methodology, descriptive results, and analysis of cancer risks, by cigarette smoking categories of 15,463 incident cancer cases. J. Occup. Environ. Med. 41 , 224–232 (1999).
Becher, H., Jöckel, K. H., Timm, J., Wichmann, H. E. & Drescher, K. Smoking cessation and nonsmoking intervals: effect of different smoking patterns on lung cancer risk. Cancer Causes Control 2 , 381–387 (1991).
Brockmöller, J., Kerb, R., Drakoulis, N., Nitz, M. & Roots, I. Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. Cancer Res. 53 , 1004–1011 (1993).
PubMed Google Scholar
Vena, J. E., Byers, T. E., Cookfair, D. & Swanson, M. Occupation and lung cancer risk. An analysis by histologic subtypes. Cancer 56 , 910–917 (1985).
Cascorbi, I. et al. Homozygous rapid arylamine N -acetyltransferase (NAT2) genotype as a susceptibility factor for lung cancer. Cancer Res. 56 , 3961–3966 (1996).
CAS PubMed Google Scholar
Chiazze, L., Watkins, D. K. & Fryar, C. A case–control study of malignant and non-malignant respiratory disease among employees of a fiberglass manufacturing facility. Br. J. Ind. Med 49 , 326–331 (1992).
CAS PubMed PubMed Central Google Scholar
Ando, M. et al. Attributable and absolute risk of lung cancer death by smoking status: findings from the Japan Collaborative Cohort Study. Int. J. Cancer 105 , 249–254 (2003).
De Matteis, S. et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am. J. Epidemiol. 177 , 601–612 (2013).
He, Y. et al. Changes in smoking behavior and subsequent mortality risk during a 35-year follow-up of a cohort in Xi’an, China. Am. J. Epidemiol. 179 , 1060–1070 (2014).
Nishino, Y. et al. Cancer incidence profiles in the Miyagi Cohort Study. J. Epidemiol. 14 , S7–S11 (2004).
Papadopoulos, A. et al. Cigarette smoking and lung cancer in women: results of the French ICARE case–control study. Lung Cancer 74 , 369–377 (2011).
Shimazu, T. et al. Alcohol and risk of lung cancer among Japanese men: data from a large-scale population-based cohort study, the JPHC study. Cancer Causes Control 19 , 1095–1102 (2008).
Tindle, H. A. et al. Lifetime smoking history and risk of lung cancer: results from the Framingham Heart Study. J. Natl Cancer Inst. 110 , 1201–1207 (2018).
PubMed PubMed Central Google Scholar
Yong, L. C. et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 146 , 231–243 (1997).
Hansen, M. S. et al. Sex differences in risk of smoking-associated lung cancer: results from a cohort of 600,000 Norwegians. Am. J. Epidemiol. 187 , 971–981 (2018).
Boffetta, P. et al. Tobacco smoking as a risk factor of bronchioloalveolar carcinoma of the lung: pooled analysis of seven case-control studies in the International Lung Cancer Consortium (ILCCO). Cancer Causes Control 22 , 73–79 (2011).
Yun, Y. D. et al. Hazard ratio of smoking on lung cancer in Korea according to histological type and gender. Lung 194 , 281–289 (2016).
Suzuki, I. et al. Risk factors for lung cancer in Rio de Janeiro, Brazil: a case–control study. Lung Cancer 11 , 179–190 (1994).
De Stefani, E., Deneo-Pellegrini, H., Carzoglio, J. C., Ronco, A. & Mendilaharsu, M. Dietary nitrosodimethylamine and the risk of lung cancer: a case–control study from Uruguay. Cancer Epidemiol. Biomark. Prev. 5 , 679–682 (1996).
Google Scholar
Kreuzer, M. et al. Risk factors for lung cancer in young adults. Am. J. Epidemiol. 147 , 1028–1037 (1998).
Armadans-Gil, L., Vaqué-Rafart, J., Rosselló, J., Olona, M. & Alseda, M. Cigarette smoking and male lung cancer risk with special regard to type of tobacco. Int. J. Epidemiol. 28 , 614–619 (1999).
Kubík, A. K., Zatloukal, P., Tomásek, L. & Petruzelka, L. Lung cancer risk among Czech women: a case–control study. Prev. Med. 34 , 436–444 (2002).
Rachtan, J. Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer 35 , 129–136 (2002).
Thun, M. J. et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 368 , 351–364 (2013).
Zatloukal, P., Kubík, A., Pauk, N., Tomásek, L. & Petruzelka, L. Adenocarcinoma of the lung among women: risk associated with smoking, prior lung disease, diet and menstrual and pregnancy history. Lung Cancer 41 , 283–293 (2003).
Hansen, M. S., Licaj, I., Braaten, T., Lund, E. & Gram, I. T. The fraction of lung cancer attributable to smoking in the Norwegian Women and Cancer (NOWAC) Study. Br. J. Cancer 124 , 658–662 (2021).
Zhang, P. et al. Association of smoking and polygenic risk with the incidence of lung cancer: a prospective cohort study. Br. J. Cancer 126 , 1637–1646 (2022).
Weber, M. F. et al. Cancer incidence and cancer death in relation to tobacco smoking in a population-based Australian cohort study. Int. J. Cancer 149 , 1076–1088 (2021).
Guo, L.-W. et al. A risk prediction model for selecting high-risk population for computed tomography lung cancer screening in China. Lung Cancer 163 , 27–34 (2022).
Mezzoiuso, A. G., Odone, A., Signorelli, C. & Russo, A. G. Association between smoking and cancers among women: results from the FRiCaM multisite cohort study. J. Cancer 12 , 3136–3144 (2021).
Hawrysz, I., Wadolowska, L., Slowinska, M. A., Czerwinska, A. & Golota, J. J. Adherence to prudent and mediterranean dietary patterns is inversely associated with lung cancer in moderate but not heavy male Polish smokers: a case–control study. Nutrients 12 , E3788 (2020).
Huang, C.-C., Lai, C.-Y., Tsai, C.-H., Wang, J.-Y. & Wong, R.-H. Combined effects of cigarette smoking, DNA methyltransferase 3B genetic polymorphism, and DNA damage on lung cancer. BMC Cancer 21 , 1066 (2021).
Viner, B., Barberio, A. M., Haig, T. R., Friedenreich, C. M. & Brenner, D. R. The individual and combined effects of alcohol consumption and cigarette smoking on site-specific cancer risk in a prospective cohort of 26,607 adults: results from Alberta’s Tomorrow Project. Cancer Causes Control 30 , 1313–1326 (2019).
Park, E. Y., Lim, M. K., Park, E., Oh, J.-K. & Lee, D.-H. Relationship between urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and lung cancer risk in the general population: a community-based prospective cohort study. Front. Oncol. 11 , 611674 (2021).
De Stefani, E., Deneo-Pellegrini, H., Mendilaharsu, M., Carzoglio, J. C. & Ronco, A. Dietary fat and lung cancer: a case–control study in Uruguay. Cancer Causes Control 8 , 913–921 (1997).
Wünsch-Filho, V., Moncau, J. E., Mirabelli, D. & Boffetta, P. Occupational risk factors of lung cancer in São Paulo, Brazil. Scand. J. Work Environ. Health 24 , 118–124 (1998).
Hu, J. et al. A case-control study of diet and lung cancer in northeast China. Int. J. Cancer 71 , 924–931 (1997).
Jia, G., Wen, W., Massion, P. P., Shu, X.-O. & Zheng, W. Incorporating both genetic and tobacco smoking data to identify high-risk smokers for lung cancer screening. Carcinogenesis 42 , 874–879 (2021).
Rusmaully, J. et al. Risk of lung cancer among women in relation to lifetime history of tobacco smoking: a population-based case–control study in France (the WELCA study). BMC Cancer 21 , 711 (2021).
Jin, K. et al. Tobacco smoking modifies the association between hormonal factors and lung cancer occurrence among post-menopausal Chinese women. Transl. Oncol. 12 , 819–827 (2019).
Tse, L. A., Wang, F., Wong, M. C.-S., Au, J. S.-K. & Yu, I. T.-S. Risk assessment and prediction for lung cancer among Hong Kong Chinese men. BMC Cancer 22 , 585 (2022).
Huang, C.-C. et al. Joint effects of cigarette smoking and green tea consumption with miR-29b and DNMT3b mRNA expression in the development of lung cancer. Genes 13 , 836 (2022).
Hosseini, M. et al. Environmental risk factors for lung cancer in Iran: a case–control study. Int. J. Epidemiol. 38 , 989–996 (2009).
Naghibzadeh-Tahami, A. et al. Is opium use associated with an increased risk of lung cancer? A case–control study. BMC Cancer 20 , 807 (2020).
Shimatani, K., Ito, H., Matsuo, K., Tajima, K. & Takezaki, T. Cumulative cigarette tar exposure and lung cancer risk among Japanese smokers. Jpn J. Clin. Oncol. 50 , 1009–1017 (2020).
Lai, C.-Y. et al. Genetic polymorphism of catechol- O -methyltransferase modulates the association of green tea consumption and lung cancer. Eur. J. Cancer Prev. 28 , 316–322 (2019).
Schwartz, A. G. et al. Hormone use, reproductive history, and risk of lung cancer: the Women’s Health Initiative studies. J. Thorac. Oncol. 10 , 1004–1013 (2015).
Kreuzer, M., Gerken, M., Heinrich, J., Kreienbrock, L. & Wichmann, H.-E. Hormonal factors and risk of lung cancer among women? Int. J. Epidemiol. 32 , 263–271 (2003).
Sreeja, L. et al. Possible risk modification by CYP1A1, GSTM1 and GSTT1 gene polymorphisms in lung cancer susceptibility in a South Indian population. J. Hum. Genet. 50 , 618–627 (2005).
Siemiatycki, J. et al. Are the apparent effects of cigarette smoking on lung and bladder cancers due to uncontrolled confounding by occupational exposures? Epidemiology 5 , 57–65 (1994).
Chan-Yeung, M. et al. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 40 , 131–140 (2003).
Lawania, S., Singh, N., Behera, D. & Sharma, S. Xeroderma pigmentosum complementation group D polymorphism toward lung cancer susceptibility survival and response in patients treated with platinum chemotherapy. Future Oncol. 13 , 2645–2665 (2017).
De Stefani, E. et al. Mate drinking and risk of lung cancer in males: a case-control study from Uruguay. Cancer Epidemiol. Biomark. Prev. 5 , 515–519 (1996).
Pérez-Padilla, R. et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am. J. Respir. Crit. Care Med. 154 , 701–706 (1996).
Zhang, X.-R. et al. Glucosamine use, smoking and risk of incident chronic obstructive pulmonary disease: a large prospective cohort study. Br. J. Nutr . https://doi.org/10.1017/S000711452100372X (2021).
Johannessen, A., Omenaas, E., Bakke, P. & Gulsvik, A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int. J. Tuberc. Lung Dis. 9 , 926–932 (2005).
Fox, J. Life-style and mortality: a large-scale census-based cohort study in Japan. J. Epidemiol. Community Health 45 , 173 (1991).
Article PubMed Central Google Scholar
Thomson, B. et al. Low-intensity daily smoking and cause-specific mortality in Mexico: prospective study of 150 000 adults. Int. J. Epidemiol. 50 , 955–964 (2021).
van Durme, Y. M. T. A. et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest 135 , 368–377 (2009).
Li, L. et al. SERPINE2 rs16865421 polymorphism is associated with a lower risk of chronic obstructive pulmonary disease in the Uygur population: a case–control study. J. Gene Med. 21 , e3106 (2019).
Ganbold, C. et al. The cumulative effect of gene-gene interactions between GSTM1 , CHRNA3 , CHRNA5 and SOD3 gene polymorphisms combined with smoking on COPD risk. Int. J. Chron. Obstruct. Pulmon. Dis. 16 , 2857–2868 (2021).
Omori, H. et al. Twelve-year cumulative incidence of airflow obstruction among Japanese males. Intern. Med. 50 , 1537–1544 (2011).
Manson, J. E., Ajani, U. A., Liu, S., Nathan, D. M. & Hennekens, C. H. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am. J. Med. 109 , 538–542 (2000).
Lv, J. et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int. J. Epidemiol. 46 , 1410–1420 (2017).
Waki, K. et al. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet. Med. 22 , 323–331 (2005).
Meisinger, C., Döring, A., Thorand, B. & Löwel, H. Association of cigarette smoking and tar and nicotine intake with development of type 2 diabetes mellitus in men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Diabetologia 49 , 1770–1776 (2006).
Huh, Y. et al. Association of smoking status with the risk of type 2 diabetes among young adults: a nationwide cohort study in South Korea. Nicotine Tob. Res. 24 , 1234–1240 (2022).
Sawada, S. S., Lee, I.-M., Muto, T., Matuszaki, K. & Blair, S. N. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care 26 , 2918–2922 (2003).
Will, J. C., Galuska, D. A., Ford, E. S., Mokdad, A. & Calle, E. E. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int. J. Epidemiol. 30 , 540–546 (2001).
Nakanishi, N., Nakamura, K., Matsuo, Y., Suzuki, K. & Tatara, K. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann. Intern. Med. 133 , 183–191 (2000).
Sairenchi, T. et al. Cigarette smoking and risk of type 2 diabetes mellitus among middle-aged and elderly Japanese men and women. Am. J. Epidemiol. 160 , 158–162 (2004).
Hou, X. et al. Cigarette smoking is associated with a lower prevalence of newly diagnosed diabetes screened by OGTT than non-smoking in Chinese men with normal weight. PLoS ONE 11 , e0149234 (2016).
Hu, F. B. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345 , 790–797 (2001).
Teratani, T. et al. Dose-response relationship between tobacco or alcohol consumption and the development of diabetes mellitus in Japanese male workers. Drug Alcohol Depend. 125 , 276–282 (2012).
Kawakami, N., Takatsuka, N., Shimizu, H. & Ishibashi, H. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus. Replication and extension in a Japanese cohort of male employees. Am. J. Epidemiol. 145 , 103–109 (1997).
Patja, K. et al. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J. Intern. Med. 258 , 356–362 (2005).
White, W. B. et al. High-intensity cigarette smoking is associated with incident diabetes mellitus in Black adults: the Jackson Heart Study. J. Am. Heart Assoc. 7 , e007413 (2018).
Uchimoto, S. et al. Impact of cigarette smoking on the incidence of Type 2 diabetes mellitus in middle-aged Japanese men: the Osaka Health Survey. Diabet. Med . 16 , 951–955 (1999).
Rimm, E. B., Chan, J., Stampfer, M. J., Colditz, G. A. & Willett, W. C. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. Br. Med. J. 310 , 555–559 (1995).
Article CAS Google Scholar
Hilawe, E. H. et al. Smoking and diabetes: is the association mediated by adiponectin, leptin, or C-reactive protein? J. Epidemiol. 25 , 99–109 (2015).
InterAct, Consortium et al. Smoking and long-term risk of type 2 diabetes: the EPIC-InterAct study in European populations. Diabetes Care 37 , 3164–3171 (2014).
Jee, S. H., Foong, A. W., Hur, N. W. & Samet, J. M. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care 33 , 2567–2572 (2010).
Rasouli, B. et al. Smoking and the risk of LADA: results from a Swedish population-based case-control study. Diabetes Care 39 , 794–800 (2016).
Wannamethee, S. G., Shaper, A. G. & Perry, I. J., British Regional Heart Study. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care 24 , 1590–1595 (2001).
Radzeviciene, L. & Ostrauskas, R. Smoking habits and type 2 diabetes mellitus in women. Women Health 58 , 884–897 (2018).
Carlsson, S., Midthjell, K. & Grill, V., Nord-Trøndelag Study. Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the Nord-Trøndelag study. Diabetologia 47 , 1953–1956 (2004).
Akter, S. et al. Smoking, smoking cessation, and the risk of type 2 diabetes among Japanese adults: Japan Epidemiology Collaboration on Occupational Health Study. PLoS ONE 10 , e0132166 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Park, C.-H. et al. [The effect of smoking status upon occurrence of impaired fasting glucose or type 2 diabetes in Korean men]. J. Prev. Med. Public Health 41 , 249–254 (2008).
Doi, Y. et al. Two risk score models for predicting incident Type 2 diabetes in Japan. Diabet. Med. 29 , 107–114 (2012).
van den Brandt, P. A. A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. Eur. J. Epidemiol. 32 , 683–690 (2017).
Dossus, L. et al. Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int. J. Cancer 134 , 1871–1888 (2014).
Kawai, M., Malone, K. E., Tang, M.-T. C. & Li, C. I. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer 120 , 1026–1034 (2014).
Reynolds, P. et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J. Natl Cancer Inst. 96 , 29–37 (2004).
Ellingjord-Dale, M. et al. Alcohol, physical activity, smoking, and breast cancer subtypes in a large, nested case-control study from the Norwegian Breast Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 26 , 1736–1744 (2017).
Arthur, R. et al. Association between lifestyle, menstrual/reproductive history, and histological factors and risk of breast cancer in women biopsied for benign breast disease. Breast Cancer Res. Treat. 165 , 623–631 (2017).
Luo, J. et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. Br. Med. J. 342 , d1016 (2011).
White, A. J., D’Aloisio, A. A., Nichols, H. B., DeRoo, L. A. & Sandler, D. P. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control 28 , 667–675 (2017).
Gram, I. T. et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol. Biomark. Prev. 14 , 61–66 (2005).
Gammon, M. D. et al. Cigarette smoking and breast cancer risk among young women (United States). Cancer Causes Control 9 , 583–590 (1998).
Magnusson, C., Wedrén, S. & Rosenberg, L. U. Cigarette smoking and breast cancer risk: a population-based study in Sweden. Br. J. Cancer 97 , 1287–1290 (2007).
Chu, S. Y. et al. Cigarette smoking and the risk of breast cancer. Am. J. Epidemiol. 131 , 244–253 (1990).
Lemogne, C. et al. Depression and the risk of cancer: a 15-year follow-up study of the GAZEL cohort. Am. J. Epidemiol. 178 , 1712–1720 (2013).
Morabia, A., Bernstein, M., Héritier, S. & Khatchatrian, N. Relation of breast cancer with passive and active exposure to tobacco smoke. Am. J. Epidemiol. 143 , 918–928 (1996).
Conlon, M. S. C., Johnson, K. C., Bewick, M. A., Lafrenie, R. M. & Donner, A. Smoking (active and passive), N -acetyltransferase 2, and risk of breast cancer. Cancer Epidemiol. 34 , 142–149 (2010).
Ozasa, K., Japan Collaborative Cohort Study for Evaluation of Cancer. Smoking and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac. J. Cancer Prev. 8 , 89–96 (2007).
Jones, M. E., Schoemaker, M. J., Wright, L. B., Ashworth, A. & Swerdlow, A. J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 19 , 118 (2017).
Bjerkaas, E. et al. Smoking duration before first childbirth: an emerging risk factor for breast cancer? Results from 302,865 Norwegian women. Cancer Causes Control 24 , 1347–1356 (2013).
Gram, I. T., Little, M. A., Lund, E. & Braaten, T. The fraction of breast cancer attributable to smoking: the Norwegian women and cancer study 1991–2012. Br. J. Cancer 115 , 616–623 (2016).
Li, C. I., Malone, K. E. & Daling, J. R. The relationship between various measures of cigarette smoking and risk of breast cancer among older women 65–79 years of age (United States). Cancer Causes Control 16 , 975–985 (2005).
Xue, F., Willett, W. C., Rosner, B. A., Hankinson, S. E. & Michels, K. B. Cigarette smoking and the incidence of breast cancer. Arch. Intern. Med. 171 , 125–133 (2011).
Parker, A. S., Cerhan, J. R., Putnam, S. D., Cantor, K. P. & Lynch, C. F. A cohort study of farming and risk of prostate cancer in Iowa. Epidemiology 10 , 452–455 (1999).
Sawada, N. et al. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int. J. Cancer 134 , 971–978 (2014).
Hiatt, R. A., Armstrong, M. A., Klatsky, A. L. & Sidney, S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control 5 , 66–72 (1994).
Cerhan, J. R. et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 8 , 229–238 (1997).
Watters, J. L., Park, Y., Hollenbeck, A., Schatzkin, A. & Albanes, D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol. Biomark. Prev. 18 , 2427–2435 (2009).
Butler, L. M., Wang, R., Wong, A. S., Koh, W.-P. & Yu, M. C. Cigarette smoking and risk of prostate cancer among Singapore Chinese. Cancer Causes Control 20 , 1967–1974 (2009).
Lotufo, P. A., Lee, I. M., Ajani, U. A., Hennekens, C. H. & Manson, J. E. Cigarette smoking and risk of prostate cancer in the physicians’ health study (United States). Int. J. Cancer 87 , 141–144 (2000).
Hsing, A. W. et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 50 , 6836–6840 (1990).
Veierød, M. B., Laake, P. & Thelle, D. S. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int. J. Cancer 73 , 634–638 (1997).
Meyer, J., Rohrmann, S., Bopp, M. & Faeh, D. & Swiss National Cohort Study Group. Impact of smoking and excess body weight on overall and site-specific cancer mortality risk. Cancer Epidemiol. Biomark. Prev . 24 , 1516–1522 (2015).
Putnam, S. D. et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann. Epidemiol. 10 , 361–369 (2000).
Taghizadeh, N., Vonk, J. M. & Boezen, H. M. Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS ONE 11 , e0153310 (2016).
Park, S.-Y. et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the Multiethnic Cohort Study. Cancer Causes Control 26 , 1507–1515 (2015).
Nomura, A. M., Lee, J., Stemmermann, G. N. & Combs, G. F. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 9 , 883–887 (2000).
Rodriguez, C., Tatham, L. M., Thun, M. J., Calle, E. E. & Heath, C. W. Smoking and fatal prostate cancer in a large cohort of adult men. Am. J. Epidemiol. 145 , 466–475 (1997).
Rohrmann, S. et al. Smoking and risk of fatal prostate cancer in a prospective U.S. study. Urology 69 , 721–725 (2007).
Giovannucci, E. et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol. Biomark. Prev. 8 , 277–282 (1999).
Rohrmann, S. et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 108 , 708–714 (2013).
Lund Nilsen, T. I., Johnsen, R. & Vatten, L. J. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br. J. Cancer 82 , 1358–1363 (2000).
Hsing, A. W., McLaughlin, J. K., Hrubec, Z., Blot, W. J. & Fraumeni, J. F. Tobacco use and prostate cancer: 26-year follow-up of US veterans. Am. J. Epidemiol. 133 , 437–441 (1991).
Murray, C. J. L. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1223–1249 (2020).
Bero, L. A. & Jadad, A. R. How consumers and policymakers can use systematic reviews for decision making. Ann. Intern. Med. 127 , 37–42 (1997).
Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb. Mortal. Wkly Rep. 58 , 1227–1232 (2009).
Prochaska, J. O. & Goldstein, M. G. Process of smoking cessation: implications for clinicians. Clin. Chest Med. 12 , 727–735 (1991).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br. Med. J. 372 , n71 (2021).
Stevens, G. A. et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet 388 , e19–e23 (2016).
BMJ Best Practice. What is GRADE? https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade (BMJ, 2021).
The GRADE Working Group. GRADE handbook . https://gdt.gradepro.org/app/handbook/handbook.html (The GRADE Working Group, 2013).
Efron, B., Hastie, T., Johnstone, I. & Tibshirani, R. Least angle regression. Ann. Stat. 32 , 407–499 (2004).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 58 , 267–288 (1996).
von Hippel, P. T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 15 , 35 (2015).
Kontopantelis, E., Springate, D. A. & Reeves, D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS ONE 8 , e69930 (2013).
Biggerstaff, B. J. & Tweedie, R. L. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat. Med. 16 , 753–768 (1997).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315 , 629–634 (1997).
Lee, P. N., Forey, B. A. & Coombs, K. J. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 12 , 385 (2012).
Rücker, G., Carpenter, J. R. & Schwarzer, G. Detecting and adjusting for small-study effects in meta-analysis. Biometr. J. 53 , 351–368 (2011).
Wu, Z.-J., Zhao, P., Liu, B. & Yuan, Z.-C. Effect of cigarette smoking on risk of hip fracture in men: a meta-analysis of 14 prospective cohort studies. PLoS ONE 11 , e0168990 (2016).
Thun, M. J. et al. in Cigarette Smoking Behaviour in the United States: changes in cigarette-related disease risks and their implication for prevention and control (eds Burns, D.M. et al.) Tobacco Control Monograph No. 8 Ch. 4 (National Cancer Institute, 1997).
Tolstrup, J. S. et al. Smoking and risk of coronary heart disease in younger, middle-aged, and older adults. Am. J. Public Health 104 , 96–102 (2014).
Jonas, M. A., Oates, J. A., Ockene, J. K. & Hennekens, C. H. Statement on smoking and cardiovascular disease for health care professionals. American Heart Association. Circulation 86 , 1664–1669 (1992).
Khan, S. S. et al. Cigarette smoking and competing risks for fatal and nonfatal cardiovascular disease subtypes across the life course. J. Am. Heart Assoc. 10 , e021751 (2021).
Download references
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation and Bloomberg Philanthropies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The study funders had no role in study design, data collection, data analysis, data interpretation, writing of the final report or the decision to publish.
We thank the Tobacco Metrics Team Advisory Group for their valuable input and review of the work. The members of the Advisory Group are: P. Allebeck, R. Chandora, J. Drope, M. Eriksen, E. Fernández, H. Gouda, R. Kennedy, D. McGoldrick, L. Pan, K. Schotte, E. Sebrie, J. Soriano, M. Tynan and K. Welding.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Xiaochen Dai, Gabriela F. Gil, Marissa B. Reitsma, Noah S. Ahmad, Jason A. Anderson, Catherine Bisignano, Sinclair Carr, Rachel Feldman, Simon I. Hay, Jiawei He, Vincent Iannucci, Hilary R. Lawlor, Matthew J. Malloy, Laurie B. Marczak, Susan A. McLaughlin, Larissa Morikawa, Erin C. Mullany, Sneha I. Nicholson, Erin M. O’Connell, Chukwuma Okereke, Reed J. D. Sorensen, Joanna Whisnant, Aleksandr Y. Aravkin, Peng Zheng, Christopher J. L. Murray & Emmanuela Gakidou
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Xiaochen Dai, Simon I. Hay, Jiawei He, Peng Zheng, Christopher J. L. Murray & Emmanuela Gakidou
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin
You can also search for this author in PubMed Google Scholar
Contributions
X.D., S.I.H., S.A.M., E.C.M., E.M.O., C.J.L.M. and E.G. managed the estimation or publications process. X.D. and G.F.G. wrote the first draft of the manuscript. X.D. and P.Z. had primary responsibility for applying analytical methods to produce estimates. X.D., G.F.G., N.S.A., J.A.A., S.C., R.F., V.I., M.J.M., L.M., S.I.N., C.O., M.B.R. and J.W. had primary responsibility for seeking, cataloguing, extracting or cleaning data, and for designing or coding figures and tables. X.D., G.F.G., M.B.R., N.S.A., H.R.L., C.O. and J.W. provided data or critical feedback on data sources. X.D., J.H., R.J.D.S., A.Y.A., P.Z., C.J.L.M. and E.G. developed methods or computational machinery. X.D., G.F.G., M.B.R., S.I.H., J.H., R.J.D.S., A.Y.A., P.Z., C.J.L.M. and E.G. provided critical feedback on methods or results. X.D., G.F.G., M.B.R., C.B., S.I.H., L.B.M., S.A.M., A.Y.A. and E.G. drafted the work or revised it critically for important intellectual content. X.D., S.I.H., L.B.M., E.C.M., E.M.O. and E.G. managed the overall research enterprise.
Corresponding author
Correspondence to Xiaochen Dai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Frederic Sitas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jennifer Sargent and Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 prisma 2020 flow diagram for an updated systematic review of the smoking and tracheal, bronchus, and lung cancer risk-outcome pair..
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and lung cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 2 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Chronic obstructive pulmonary disease risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and chronic obstructive pulmonary disease conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 3 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Diabetes mellitus type 2 risk- outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and type 2 diabetes conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 4 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Breast cancer risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and breast cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 5 PRISMA 2020 flow diagram for an updated systematic review of the Smoking and Prostate cancer risk-outcome pair.
The PRISMA flow diagram of an updated systematic review on the relationship between smoking and prostate cancer conducted on PubMed to update historical review from previous cycles of the Global Burden of Disease Study. Template is from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/ .
Extended Data Fig. 6 Smoking and Breast Cancer.
a , log-relative risk function. b , relative risk function. c , A modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard deviation (SD) that includes reported SD and between-study heterogeneity on the y-axis.
Supplementary information
Supplementary information.
Supplementary Information 1: Data source identification and assessment. Supplementary Information 2: Data inputs. Supplementary Information 3: Study quality and bias assessment. Supplementary Information 4: The dose–response RR curves and their 95% UIs for all smoking–outcome pairs. Supplementary Information 5: Supplementary methods. Supplementary Information 6: Sensitivity analysis. Supplementary Information 7: Binary smoking–outcome pair. Supplementary Information 8: Risk curve details. Supplementary Information 9: GATHER and PRISMA checklists.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dai, X., Gil, G.F., Reitsma, M.B. et al. Health effects associated with smoking: a Burden of Proof study. Nat Med 28 , 2045–2055 (2022). https://doi.org/10.1038/s41591-022-01978-x
Download citation
Received : 11 April 2022
Accepted : 28 July 2022
Published : 10 October 2022
Issue Date : October 2022
DOI : https://doi.org/10.1038/s41591-022-01978-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Estimating effects of whole grain consumption on type 2 diabetes, colorectal cancer and cardiovascular disease: a burden of proof study.
Nutrition Journal (2024)
- Gabriela F. Gil
- Jason A. Anderson
- Emmanuela Gakidou
Nature Communications (2024)
A multi-ancestry cerebral cortex transcriptome-wide association study identifies genes associated with smoking behaviors
- Xiaohang Xu
Molecular Psychiatry (2024)
- Luisa S. Flor
Nature Medicine (2024)
Metabolic profiling of smoking, associations with type 2 diabetes and interaction with genetic susceptibility
- Sofia Carlsson
European Journal of Epidemiology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Search Menu
- Sign in through your institution
- Supplements
- Cohort Profiles
- Education Corner
- Author Guidelines
- Submission Site
- Open Access
- About the International Journal of Epidemiology
- About the International Epidemiological Association
- Editorial Team
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the IEA
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
I. mortality and population data, ii. retrospective and prospective studies, iii. studies on pathogenesis, iv. other laboratory investigations, v. interpretation.
- < Previous
Smoking and lung cancer: recent evidence and a discussion of some questions *
- Article contents
- Figures & tables
- Supplementary Data
Jerome Cornfield, William Haenszel, E. Cuyler Hammond, Abraham M. Lilienfeld, Michael B. Shimkin, Ernst L. Wynder, Smoking and lung cancer: recent evidence and a discussion of some questions, International Journal of Epidemiology , Volume 38, Issue 5, October 2009, Pages 1175–1191, https://doi.org/10.1093/ije/dyp289
- Permissions Icon Permissions
Summary This report reviews some of the more recent epidemiologic and experimental findings on the relationship of tobacco smoking to lung cancer, and discusses some criticisms directed against the conclusion that tobacco smoking, especially cigarettes, has a causal role in the increase in broncho-genic carcinoma. The magnitude of the excess lung-cancer risk among cigarette smokers is so great that the results can not be interpreted as arising from an indirect association of cigarette smoking with some other agent or characteristic, since this hypothetical agent would have to be at least as strongly associated with lung cancer as cigarette use; no such agent has been found or suggested. The consistency of all the epidemiologic and experimental evidence also supports the conclusion of a causal relationship with cigarette smoking, while there are serious inconsistencies in reconciling the evidence with other hypotheses which have been advanced. Unquestionably there are areas where more research is necessary, and, of course, no single cause accounts for all lung cancer. The information already available, however, is sufficient for planning and activating public health measures. – J. Nat. Cancer Inst . 22: 173–203, 1959.
“The sum total of scientific evidence establishes beyond reasonable doubt that cigarette smoking is a causative factor in the rapidly increasing incidence of human epidermoid carcinoma of the lung.”
The consideration of the accumulated scientific evidence has led to the acceptance of a similar viewpoint by responsible public health officials in Great Britain, the Netherlands, Norway, and the United States. This consensus of scientific and public health opinion does not mean that all problems, regarding smoking and lung cancer have now been solved or that valid questions and reservations about some aspects of the subject do not remain. An excellent collection of primary references and opinions expressing both “sides” of the question was issued by a committee of the House of Representatives 3 which sought to examine the claims of filter-tip cigarette advertisements.
The general acceptance of the cigarette-lung-cancer relationship has not decreased research interest but has accelerated research in this and in such related fields as respiratory physiology and environmental carcinogens, and on the effect of tobacco smoke in a wide range of physiological and pathological reactions.
The result is that considerably more information has been published or has become available through other media. Included in the recent scientific evidence are the following:
Additional retrospective studies 4 , 5 , 6 on men with lung cancer and on matched controls have appeared. All show an association between cigarette smoking and epidermoid-undifferentiated lung cancer.
Additional retrospective studies on women 7 , 6 also show the association.
The first results of a third large prospective study 8 , which included 200,000 United States veterans who were observed for 30 months, duplicate closely the reported findings of the Hammond-Horn 9 and the Doll-Hill 10 studies.
Analyses by Kreyberg and others 11 , 12 substantiate that, epidemiologically, primary lung cancer must be divided into epidermoid-undifferentiated and adenocarcinoma. The latter is much less related to smoking and, so far as is know at present, to other carcinogenic inhalants.
Additional findings have become available on the impingement of tobacco-smoke particles in the bronchi of animals, ciliary paralysis, and penetration of unidentified fluorescent materials into the bronchial cells. 13 , 14 , 15
Additional data have been published 16 , 17 on the more frequent occurrence of hyperplastic and metaplastic changes in the lungs of smokers as compared with the lungs of nonsmokers. Hyperplastic and metaplastic changes have been produced in bronchi of dogs exposed to direct contact with tobacco “tars” 18 and in bronchi of mice exposed to tobacco smoke. 19
Additional confirmations have been obtained on the induction of cancer of the skin in mice painted with tobacco-smoke condensates. 20 , 21 , 22 , 23 , 24
Progress continues on the isolation and identification of chemical constituents in tobacco smoke, including compounds of the carcinogenic polycyclic type. 23 , 25 , 26 , 27 , 28
The growing and consistent body of evidence has had no noticeable effect upon the viewpoint of a small but important group of individuals who would deny a causal role of cigarette smoking in cancer of the lung. Among these critics are Little 29 and Hartnett 30 , spokesmen for the American tobacco industry. Berkson 31 , 32 has been critical of many aspects of the statistical studies, and his reservations are, in part, also evident in papers by Neyman 33 and Arkin 34 . More general objections by Fisher 35 , 36 , Greene 37 , Hueper 38 , Macdonald 39 . Rigdon 40 , and Rosenblatt 41 have been published.
We have reviewed the criticism that have been made regarding the cigarette-lung cancer relationship in the light of new evidence. In this review we have several objectives: a) to point out recorded facts that directly answer some of the criticisms; b) to define more precisely some inadequacies of information, with the hope that this will lead to further research. The particular references we have used were selected because in our opinion the criticism was well stated; it is not our intention to reply to any specific publication or to any specific critic. Our view is that all valid questions should be answered. However, some questions may not be relevant, or there may be no information presently available for an answer. In the latter case, we believe that a distinction should be made between data that are unavailable and data that have been found to be contradictory.
For convenience, we have divided the criticisms and answers into five major topics, as follows: (I) Mortality and population data; (II) Retrospective and prospective studies; (III) Studies on pathogenesis; (IV) Other laboratory investigations; and (V) Interpretation.
The rising death rate from lung cancer in all countries that have sufficiently detailed mortality statistics is the most striking neoplastic phenomenon of this century. That this increase is a fact and not a spurious result of statistical classification is now commonly accepted An entirely contrary view is held by only a few persons 40 , though there are dissenting opinions 42 , 38 regarding the extent and time relationship of this recorded increase.
Obviously, the case for the etiologic role of cigarette smoking would be seriously compromised if it could be demonstrated that the lung-cancer rate over the past half century had been stationary, particularly after 1920 when much of the rise in cigarette consumption, instead of other forms of tobacco, occurred 43 .
In a recent review, Rigdon and Kirchoff 44 document that primary lung cancer was first recognized as an entity during the early part of the 19 th century, and that its occurrence has increased steadily since then, as manifested by the recorded relative frequency with which it was recognized in the clinic and at necropsy. This is undoubtedly correct but does not constitute evidence against a true increase in the incidence of the disease during the whole, or a more recent part, of the last 100 years.
Hueper 38 , accepting a true increase in the incidence of lung cancer, regards an increase dating back to 1900, or before the widespread use of cigarettes, as evidence against the cigarette-lung-cancer relationship. His contention would have crucial import only if it were maintained that cigarette smoking is the sole cause of lung cancer.
The vital statistics and the necropsy data that support the presumption of a real increase in lung-cancer risk certainly apply to the years after 1920. Because of the uncertainties associated with changes in diagnostic accuracy, no firm conclusions can be reached on whether the rate of increase in lung-cancer mortality has, in truth, accelerated since 1920.
Effect of Aging
Rosenblatt 41 has raised the question about the effect of the aging population on the lung-cancer rate. This particular point has been investigated by the use of age-adjusted rates. Dunn 45 has noted that only one sixth of the over-all increase in lung-cancer mortality among males in the United States (from 4 to 24 deaths per 100,000 males between 1930 and 1951) could be attributed to an aging population. Similar findings 46 have been presented for England and Wales where observations on lung-cancer mortality date back to 1900; the 1953 mortality rate for both sexes, 34 per 100,000 population, was 43 times the corresponding 1900 rate, 0.8 per 100,000 population. Allowance for increased average age of the population could account for only half this rise in lung-cancer mortality, with a 24-fold difference between 1900 and 1953.
Also, an aging population, does not affect the age-specific death rates and cannot account for the phenomenon of increasingly higher lung-cancer mortality at all ages throughout the lifespan, which has occurred among successively younger groups of males born in the United States and England and Wales since 1850. A similar but less pronounced “cohort displacement” has been shown for females.
Diagnostic Factors
Little 29 and others 40 have raised the important question on whether better diagnostic measures and more complete reporting have resulted in a spurious increase in the recorded attack rate. Several special features of the increase in lung-cancer mortality would be difficult to account for on diagnostic grounds. These include the continuous rising ratio of male to female deaths, the increasing lung-cancer mortality rate among successively younger cohorts, and the magnitude of the current, continuing, increase in lung-cancer mortality 46 . By 1955, among white males, 50 to 64 years of age, in the United States, more deaths were attributed to lung cancer than to all other respiratory diseases combined.
Gilliam 42 has made a careful study of the potential effect of improved diagnosis on the course of the lung-cancer death rate. Even assuming that 2 percent of the deaths certified in past years as tuberculosis or other respiratory disease were really due to lung cancer, he concluded that “… all of the increase in mortality attributed to cancer of the lung since 1914 in United States white males and females cannot be accounted for by erroneous death certification to other respiratory diseases without unreasonable assumptions of age and sex differences in diagnostic error.” His computations reduced the respective 26-fold and sevenfold increase in lung-cancer mortality among males and females, between 1914 and 1950, to the more modestly estimated dimensions of fourfold and 30 percent, respectively. These estimates are certainly the lower bound on the magnitude of the true rate of increase during this period.
The Copenhagen Tuberculosis Station data, examined by Clemmesen et al . 47 , provide the greatest measure of control on the diagnostic improvement factor. In a tuberculosis referral service, used extensively by local physicians, where diagnostic standards and procedures including systematic bronchoscopy remained virtually unchanged between 1941 and 1950, the lung-cancer prevalence rate among male examinees increased at a rate comparable to that recorded by the Danish cancer registry for the total male population. This can be regarded as evidence that the reported increase in Danish incidence is not due to diagnostic changes.
Necropsy Data
Most necropsy data agree with mortality data on the increase in lung-cancer risk. To establish this point we referred to a necropsy series summarized by Steiner 48 , and returned to the original sources for evidence on the nature of changes over time. Since an existing compilation was chosen, the results do not represent a culling of autopsy series for data favorable to this thesis. The findings from 13 series are summarized in text- figure 1 as the proportion of lung cancers in relation to all necropsies. The relative frequency in terms of total tumors or total carcinomas yielded results which would lead to substantially the same inferences.
Mortality and necropsy data have their own virtues and weaknesses. Death certificates provide a complete report of deaths, but do not emphasize a high quality of diagnostic evidence, while the reverse holds true for necropsies. However, since both approaches lead to the same inferences, neither great variation in the quality of diagnostic evidence nor the unrepresentative nature of some of the necropsy observations can be viewed as plausible interpretations of the results. The alternative conclusion of a real increase in lung-cancer risk remains.
Urban-Rural Differences
Emphasis has been placed on the alleged incompatibility of the excess lung-cancer mortality, among urban residents, with the cigarette-smoking hypothesis 38 , 49 . Mortality data from several countries indicate strongly that lung-cancer rates are much higher in cities than in rural areas, and the observation that urban males in general have higher lung-cancer mortality than rural males is undoubtedly correct.
The assertion of Macdonald 39 that “ … country people smoke as much, if not more, than do city people …” is not borne out by the facts 50 . Nevertheless, the evidence indicates that adjustment for smoking history could account for only a fraction of this urban-rural difference 51 .
However, this does not establish the converse proposition that control of residence history in the analysis of collected data would account for the excess lung-cancer risk among cigarette smokers. Evidence now in hand weighs strongly against this last assertion. Stocks and Campbell 67 , in their report on lung-cancer mortality among persons in Liverpool, the suburban environs, and rural towns in North Wales, showed that heavy smokers have higher lung-cancer rates when urban and rural males were studied separately. Mills and Porter 52 reported similar findings in Ohio. These results agree with the experience of the Hammond-Horn 9 study, which revealed markedly higher death rates for bronchogenic carcinoma among smokers regardless of whether they lived in cities or in rural areas. No contradictory observations are known to us.
Sex Differences
The sex disparity in lung-cancer mortality has also been cited 35 , 7 as grounds for discarding the cigarette-smoking hypothesis. In this connection it should be noted that persons advocating this line of argument have minimized sex differences in smoking habits to a degree not supported by available facts. A survey of smoking habits in a cross section of the United States population 50 demonstrated that men, on the average, have been smoking for longer periods than women. The sex differences in tobacco use were especially pronounced at ages over 55, when most lung-cancer deaths occur; 0.6 percent of United States females in this age group have been reported as current users of more than 1 pack of cigarettes daily compared to 6.9 percent of United States males. British data 53 also revealed much lower tobacco consumption among females, particularly for the years before World War II.
The present data contrasting the experience by sex would appear to support the cigarette-smoking hypothesis rather than discredit it. When differences in smoking habits are considered, it is possible to reduce the observed fivefold excess lung-cancer mortality among males to the 40 percent excess mortality which prevails for many other causes of death 51 . One intriguing finding from these studies is that the estimated death rates for female nonsmokers agree closely with the death rates derived from retrospective studies on male nonsmokers 7 .
Evidence for Other Etiological Factors
Etiologic factors of industrial origin, such as exposure to chromates and coal gas, are well established 46 . Excess lung-cancer risks among such groups as asbestos workers who develop asbestosis, appear likely 46 . One epidemiologic study 54 of British, World War I, veterans exposed to mustard gas and/or with a wartime history of influenza revealed virtually no excess lung-cancer risk among these groups.
The existence of other important lung-cancer effects associated with such characteristics as socioeconomic class cannot be questioned. Cohart 55 found that the poorest economic class had a 40 percent higher lung-cancer incidence than the remaining population of New Haven, Connecticut. Results from the 10-city morbidity survey 56 have revealed a sharp gradient in lung-cancer incidence, by income class, for white males, which is consistent with Cohart's findings. Since cigarette smoking is not inversely related to socioeconomic status, we can agree with Cohart “… that important environmental factors other than cigarette smoking exist that contribute to the causation of lung cancer.” These and other findings are convincing evidence for multiple causes of lung cancer. It is obviously untenable to regard smoking of tobacco as the sole cause of lung cancer.
Two points should be made: The population exposed to established industrial carcinogens is small, and these agents cannot account for the increasing lung-cancer risk in the remainder of the population. Also, the effects associated with socioeconomic class and related characteristics are smaller than those noted for smoking history, and the smoking-class differences cannot be accounted for in terms of these other effects.
Special population Groups
Haag and Hanmer 57 reported that employees in 9 processing plants of the American Tobacco Company, with an above-average proportion of smokers, had a lower mortality than the general population of Virginia and North Carolina for all causes and for cancer and cardiovascular diseases, but no higher mortality for respiratory cancer and coronary disease. They concluded: “The existence of such a population makes it evident that cigarette smoking per se is not necessarily or invariably associated with a higher risk of lung cancer or cardiovascular diseases or with diminished longevity.”
The group studied by Haag and Hanmer was too small to yield significant results on respiratory cancer. Moreover, a major flaw in the conclusion has been pointed out by Case 58 . It is well known that mortality comparisons cannot be drawn directly between employee groups and the general population, since the death rates for many groups of employed persons are lower than death rates for the general population with age, sex, and race taken into consideration. This is true because there is a strong tendency to exclude from employment those persons who have acute or chronic diseases or who are seriously disabled from any cause and those employees who develop permanent disabilities from disease or other causes are usually discharged, retired, or dropped from the list of regular employees. Reasons of this nature undoubtedly account for the deficit in deaths from all causes noted in the group of employees under consideration.
A different picture is provided by the Society of Actuaries 59 who made a study for 1946 through 1954. The death claims for employees of the tobacco industry were reported to be slightly higher than, and the permanent disability claims were reported to be over three times as high as, those for employees in nonrated industries as a whole. This latter comparison indicates that the basic assumption of the Haag and Hanmer study is incorrect. Also, interpretation of group comparisons in this field should account separately for the experience of smokers and nonsmokers. We hope that Haag and Hanmer will supplement the report to provide data for smokers and nonsmokers in the study population.
The association between smoking and lung cancer has now been investigated and reported by at least 21 independent groups of investigators in 8 different countries, who employed what is known as the retrospective method 1 , 4 , 5 , 6 , 7 , 46 . In these studies, patients with lung cancer, or their relatives, were questioned about their smoking history and other past events, and the answers compared with those of individuals without lung cancer who were selected as controls. Although these 21 studies have certain features in common, they varied greatly in the methods of selecting the groups, the methods of interview, and other important aspects.
The association between smoking and lung cancer was further investigated in two countries by three independent groups 8 , 9 , 10 , using the prospective method. In these studies, large groups were questioned on smoking habits and other characteristics, and the groups were observed for several years for data on mortality and causes of death. The three prospective studies also varied in several important details including the type of subjects, the selection of subjects, and the method of obtaining information on smoking habits.
In each of these studies, an association was found between smoking and lung cancer. In every investigation where the type of smoking and lung cancer. In every investigation where the type of smoking was considered, a higher degree of association was found between lung cancer and cigarette smoking than between lung cancer and pipe or cigar smoking. In every instance where amount of smoking was considered, it was found that the degree of association with lung cancer increased as the amount of smoking increased. When ex-cigarette smokers were compared with current cigarette smokers, it was found that lung-cancer death rates were higher among current cigarette smokers than among ex-cigarette smokers.
A number of investigators 60 have criticized the retrospective method but, for the most part, the specific points of criticism apply only to some of the studies and not to others. Some features of the three prospective studies on smoking also have been criticized. Again, certain of the points of criticism apply to one or another of the three prospective studies but not to all three. Specifically, doubts raised as to the validity of the early findings of the prospective studies have been eliminated by the persistence of the findings in the later phases of the same studies.
The validity of the findings on these extensive investigations has been questioned in regard to two major aspects: 1) the methods of selection of the study groups, and 2) the accuracy of information regarding smoking habits and the diagnosis of lung cancer.
Selection of Study Groups
Neyman 33 pointed out that a study based on a survey of a population at some given instant of time may yield misleading results. Suppose that a study is made on a day when all patients with lung cancer and a group of people without lung cancer are questioned about their smoking habits. If smokers with lung cancer live longer than nonsmokers with lung cancer, there would be a higher proportion of smokers in the lung-cancer group than in the control group – this would follow without questioning the proposition on which the model is based. However, only two of the retrospective studies were conducted in a way approximating an “instantaneous survey” procedure, so that this criticism does not apply to most of the studies. Furthermore, this difficulty is completely avoided in prospective studies.
Berkson 31 indicated that people with two specific complaints are more likely to be hospitalized than people with only one of these complaints. If a retrospective study were conducted exclusively on hospital patients an association would be found between these two specific complaints, even if there were no association between the same two complaints in the general population. This would influence the results if smokers with lung cancer are more likely to be hospitalized than nonsmokers with lung cancer. However, Berkson showed that this difficulty is trivial if a high percentage of people with either one of these two conditions is hospitalized, which is the situation with lung-cancer patients. Furthermore, one retrospective study 67 included all lung cancer patients who were in the study area, including those not hospitalized; another retrospective study 61 was based on individuals who died of lung cancer and other diseases regardless of whether they had been hospitalized or not. This difficulty does not arise in prospective studies.
In all but one of the 21 retrospective studies, the procedure was to compare the smoking habits of lung-cancer patients with the smoking habits of a control group who did not have lung cancer. Hammond 60 , Berkson 31 , and others have pointed out the grave danger of bias if the control group is not selected in such a way as to represent (in respect to smoking habits) the general population which includes the lung-cancer patients. Subsequent events have proved that this criticism is well founded, though the direction of the bias in most studies turned out to yield an underestimate of the degree of association between cigarette smoking and lung cancer. The reason was that in most of the retrospective studies the control group consisted of patients with diseases other than lung cancer. The choice of such a control group is tantamount to assuming that there is no association between smoking and diseases which resulted in hospitalization of the control subjects. This was an incorrect assumption since other studies have indicated an association between smoking and a number of diseases, such as coronary artery disease, thromboangiitis obliterans, and cancer of the buccal cavity.
Doll and Hill 62 , recognizing the possibility of bias in a control group selected from hospital patients, obtained an additional control group by ascertaining the smoking habits of the general population in a random sample of the area in which their hospital was located. The largest percentage of smokers (particularly heavy smokers) was found in the lung-cancer group, the smallest percentage of smokers was found in the general population sample, and an intermediate percentage of smokers was found in the hospital-control group. Similar results have been reported in a recent study of women 7 .
Berkson 31 pointed out that the criticisms in regard to selection bias in the retrospective studies are also applicable to the earlier findings in a prospective study. Suppose that, in selecting subjects for a prospective study, sick smokers are overrepresented in relation to well smokers and/or well non-smokers are overrepresented in relation to sick nonsmokers. In this event, during the earlier period after selection, the death rate of the smokers in the study would be higher than the death rate of the nonsmokers in the study, even if death rates were unrelated to smoking habits of the general population. If smoking is unrelated to death from lung cancer (or other causes), the death rate of the smokers would tend to equalize with that of the nonsmokers as the study progressed. Thus, the bias would diminish with time, and a relationship due to such bias would disappear. This general principle is well known to actuaries and is one of the cornerstones of the life insurance business.
Hammond and Horn 9 , recognizing this possible difficulty, excluded from the study all persons who were obviously ill at the time of selection. As expected, the total death rate of the study population was low and very few deaths from lung cancer occurred during the first 8 months after selection. The total death rate, and particularly the death rate from lung cancer, rose considerably in the subsequent 3 years. What is more important, the observed association between cigarette smoking and lung cancer was considerably higher in the latter part than in the early part of the study, and the association between cigarette smoking and total death rates was also somewhat greater in the latter part of the study. This showed that the original bias in the selection of the subjects was slight and that it yielded an underestimate of the degree of association between smoking and death rates.
This particular problem was not encountered in the prospective studies of Doll and Hill 10 who could observe the death rates of all physicians in Great Britain (nonresponders as well as responders to the smoking questionnaire). The prospective study of Dorn 8 also had a defined population of veterans holding insurance policies, and nonresponders were observed as well as responders. Moreover, these two studies also showed that higher mortality from lung cancer among smokers was more evident during the later period than in the earlier period of observation. Thus, in the course of time, there was no disappearance of any selection bias factors that may have been introduced into the original study groups.
The subjects for the Hammond and Horn prospective study 9 were selected by volunteer workers with specific instructions on how it should be done. Mainland and Herrera 63 have suggested that the volunteer workers may have introduced a bias in the way they selected the subjects. The foregoing evidence of persistence and accentuation of the differences between smokers and nonsmokers, in time, effectively counters purposeful, as well as unknown, sources of such selection.
Accuracy of Information
Berkson 31 , 32 has remarked that the two major variables considered in all these studies – the ascertainment of smoking habits and the diagnosis of disease – are both subject to considerable error. The accuracy of diagnosis is not a major problem in retrospective studies because the investigator can restrict his study to those patients whose diagnosis of lung cancer has been thoroughly confirmed. This feature has been taken into consideration in several retrospective studies. It is more of a problem in prospective studies since all deaths that occur must be included, and certainly some of the diagnoses will be uncertain. However, in all three prospective studies, the total death rate was found to be higher in cigarette smokers than in nonsmokers and found to increase with the amount of cigarette smoking. If some of the excess deaths associated with cigarette smoking and ascribed to lung cancer were actually due to some other disease, then it means that: a) the association between cigarette smoking and lung cancer was somewhat overestimated, but b) the association between smoking and some other disease was somewhat underestimated. The reverse would be true if some of the excess deaths associated with cigarette smoking and ascribed to diseases other than lung cancer were actually due to lung cancer. Hammond and Horn 9 found that the association with cigarette smoking was greater for patients with a well-established diagnosis of lung cancer than for patients with less convincing evidence for a diagnosis of lung cancer. This suggests that inaccuracies in diagnosis resulted somewhat in an underestimate of the degree of association between smoking and lung cancer.
The study on physicians, by Doll and Hill 10 , in which presumably the clinical and pathologic evidence of the cause of death would be somewhat more than in the general population considered by Hammond and Horn and by Dorn, yields almost identical risks to lung cancer by smoking class.
In regard to information about smoking, Finkner et al . 64 have made a thorough study of the accuracy of replies to questionnaires on smoking habits. Their results indicate that replies are not completely accurate but that most of the errors are relatively minor – very few heavy smokers are classified as light smokers. Random and independent errors simply tend to diminish the apparent degree of association between two variables. A national survey of smoking habits in the United States 50 yielded results on tobacco consumption that were consistent with figures on tobacco production and taxation.
On two occasions several years apart, Hammond and Horn 9 and Dorn 8 questioned a proportion of their subjects. The results indicated close reproducibility in the answers.
Hammond 60 and others 39 have questioned the reliability of the retrospective method on the grounds that the illness may bias the responses given by the patient or his family when they are questioned about smoking habits, and that knowledge of the diagnosis may bias the interviewer. This possible difficulty was minimized in several of the 21 retrospective studies on smoking in relation to lung cancer. For example, in the study conducted by Levin 65 , all patients admitted to a hospital during the course of several years were questioned about their smoking habits before a diagnosis was made. Only a small proportion later turned out to have lung cancer, though many had lung disease symptoms or lung diseases other than lung cancer. Doll and Hill 10 also showed that patients whose diagnosis of lung cancer was subsequently established to be erroneous had smoking histories characteristic of the control rather than of the lung-cancer group. Furthermore, a larger percentage of cigarette smokers have been found among patients with epidermoid carcinoma of the lungs than among patients with adenocarcinoma of the lungs 66 . This could hardly have resulted from bias either on the part of the patient or on the part of the interviewer.
Multiple Variables
Arkin 34 , Little 29 , Macdonald 39 , and others have criticized the studies of cigarette-lung cancer relationship on the grounds that only smoking habits were really investigated, and that numerous other possible variables were not considered.
This criticism may seem especially appropriate in view of the accepted fact that no single etiologic factor has been proposed for any neoplastic disease. The criticism may also be valid in relation to any one of the retrospective and prospective studies. However, in the aggregate, quite a number of other variables have been specifically investigated or can be inferentially derived. Of course, all studies considered the basic factors of age and sex; some dealt with geographic distribution 67 , occupation 68 , urban or rural residence 67 , marital and parous status 7 , and some other habits such as coffee consumption 7 .
The Doll and Hill 10 prospective study was confined to a single professional group, physicians. Thus there could be no great variation attributable to occupation or socioeconomic status. Stocks and Campbell 67 put particular emphasis on the study of air pollution and occupational exposure and included a number of other factors in addition to smoking. It is evident, in the Hammond-Horn 9 study and other investigations, that there is a consistent relationship between urban residence and a higher mortality due to lung cancer. The important fact is that in all studies, when other variables are held constant, cigarette smoking retains its high association with lung cancer.
The only factors that may show a higher correlation with lung cancer than heavy cigarette smoking are such occupations as those of the Schneeberg miners and manufacturers of chromate 46 . We are not acquainted with actual studies of these and related occupation groups in which cigarette and other tobacco consumption is also considered. Such studies, we suggest, would be useful additions to our knowledge of other etiologic agents of the interplay between multiple causes in human pulmonary cancer.
Inhalation of Smoke
If cigarette smoking produces cancer of the lungs as a result of direct contact between tobacco smoke and the bronchial mucosa, smokers who inhale cigarette smoke should be exposed to higher concentrations of the carcinogens than noninhalers and therefore have a higher risk to the development of lung cancer. The retrospective study of Doll and Hill 62 , however, elicited no difference between patients with lung cancer and the controls in the proportion of smokers who stated that they inhaled. Fisher 35 , Hueper 38 , and Macdonald 39 have emphasized this point as contradictory to the smoking-lung-cancer relationship, and, of course, it is. Unfortunately, this particular finding was not reinvestigated in the prospective study of Doll and Hill 10 .
Three authors, Lickint 69 , Breslow et al . 68 , and Schwartz and Denoix 4 , however, did find the relative risk of lung cancer to be greater among inhalers than among noninhalers when age, type, and amount of smoking were held constant. It must be admitted that there is no clear explanation of the contradiction posed by the Doll-Hill 62 findings, though a number of plausible hypotheses could be advanced. More experimental work is required, including some objective definition and measurement of the depth and length of inhalation.
Hammond 70 has recently queried male smokers about their inhalation practices. He found that very few pipe and cigar smokers inhale; that most men inhale who smoke only cigarettes; and that there are proportionally fewer inhalers among men who smoke both cigars and cigarettes than among men who smoke only cigarettes. These findings are compatible with the view that differences in inhaling account for the fact that the lung-cancer death rate of cigar and pipe smokers is less than the lung-cancer death rate of cigarette smokers; and that the lung-cancer death rate of men who smoke both cigars and cigarettes is somewhat lower than the lung-cancer death rate of men who smoke only cigarettes.
Upper-Respiratory Cancer
Rosenblatt 41 has drawn attention to the fact that increased consumption of cigarettes has not been accompanied by an increase in upper-respiratory cancer similar to that noted in cancer of the lung and bronchus. Hueper 38 also has expressed doubts about the causative role of cigarette smoking on the basis that cigarette smoking is not associated with cancer of the oral cavity or of the fingers, which are often stained with tobacco tar.
The premise that a carcinogen should act equally on different tissues is not supported by experimental or clinical evidence 71 . Carcinogens, which produce liver tumors in animals, may be noncarcinogenic when applied to the skin. Coal soot, accepted as etiologically related to carcinoma of the scrotum in chimney sweeps, does not increase the risk to cancer of the penis. There is no a priori reason why a carcinogen that produces bronchogenic cancer in man should also produce neoplastic changes in the nasopharynx or in other sites. It is an intriguing fact, deserving further research, that carcinoma of the trachea is a rarity, whereas carcinoma of the bronchus is common among individuals exposed to chromates, as well as among chronic cigarette smokers.
Several studies have established the association of all types of tobacco smoking, including cigarettes, with cancer of the oral cavity 72 . However, the relative risk of developing cancer of the mouth is greater for cigar and pipe smokers than for cigarette smokers. The risk of laryngeal cancer is increased by smoking and an equal risk exists among cigarette, cigar, and pipe smokers 73 . The per capita consumption of cigars and pipe tobacco has decreased since 1920, while cigarette smoking has increased 43 .
These associations contrast sharply with the findings on lung cancer, which have consistently shown that cigarette smokers have much higher risks than either cigar or pipe smokers. Since 1920 the increase in tobacco consumption has been primarily due to the rise in cigarette consumption 43 , and the stabler rates for intra-oral and laryngeal cancer, while the lung-cancer rates have increased steeply, can be considered compatible with the causal role of cigarette smoking in lung cancer.
Effect of Tobacco Smoke on Bronchial Mucosa
Statements by Hartnett 30 , Macdonald 39 , and others 31 , 29 imply that the relationship of cigarette smoking and lung cancer is based exclusively on “statistics” and lacks “experimental” evidence. The differentiation between various methods of scientific inquiry escapes us as being a valid basis for the acceptance or the rejection of facts. Nevertheless it is true that historically the retrospective studies on lung cancer preceded the intensive interest in laboratory investigations stimulated by the statistical findings.
Hilding 13 has shown experimentally that exposure to cigarette smoke inhibited ciliary action in the isolated bronchial epithelium of cows. Kotin and Falk 15 obtained essentially the same results in experiments on rats and rabbits. Hilding 14 further showed that inhibition of ciliary action interfered with the mechanism whereby foreign material is ordinarily removed from the surface of bronchial epithelium. In addition, he found that foreign material deposited on the surface tended to accumulate in any area where the cilia have been destroyed. Auerbach et al . 16 found that the small areas of the bronchial epithelium where ciliated columnar cells were absent appeared more frequently in smokers than in nonsmokers. Chang 17 found that cilia were shorter, on an average, in the bronchial epithelium of smokers than in that of nonsmokers.
These studies have demonstrated the existence of a mechanism whereby foreign material from any source (e.g. tobacco smoke, industrial dusts, fumes from automobile exhausts, general air pollutants, and, perhaps, pathogenic organisms) is likely to remain in contact with the bronchial epithelium for a longer period in smokers than in nonsmokers.
Auerbach and his associates 16 studied the microscopic appearance of the bronchial epithelium of patients who died of lung cancer and patients who died of other diseases. Each of these two groups of patients was classified according to whether they were nonsmokers, light smokers, or heavy cigarette smokers. Among the cancer patients there were no nonsmokers. Approximately 208 sections from all parts of the tracheobronchial tree from each patient were examined. Many areas of basal cell hyperplasia, squamous metaplasia, and marked atypism, with loss of columnar epithelium were found in the tracheo-bronchial tree of men who had died of lung cancer. Almost as many such lesions were found in heavy cigarette smokers who had died of other diseases; somewhat less were found in light cigarette smokers; and much less in nonsmokers. Chang 17 has reported similar findings in the bronchial epithelium of smokers compared with nonsmokers.
The chief criticism of Auerbach's study has concerned terminology. Following the definition previously set forth by Black and Ackerman 74 , Auerbach et al . used the term “carcinoma- in-situ ” to describe certain lesions with marked atypical changes and loss of columnar epithelium. Whether this is an appropriate term may be questioned, but it is not relevant to the validity of the findings. Certainly there are no data to indicate what proportion of these morphologically abnormal areas would progress to invasive carcinoma.
The recent findings of Auerbach et al . and Chang have been reproduced experimentally in animals. Rockey and his associates 75 applied tobacco ”tar” directly to the bronchial mucosa of dogs. Within 3 to 6 weeks, the tar-treated surface became granular and later developed wart like elevations. Upon microscopic examination, hyperplasia, transitional metaplasia, and squamous metaplasia were found in these areas. Leuchtenberger et al . 19 exposed mice to cigarette smoke for periods up to 200 days. The bronchial epithelium was then examined microscopically. Bronchitis, basal-cell hyperplasia, and atypical basal-cell hyperplasia were found in the majority of the animals and squamous metaplasia in a few. Further work and longer periods of observation are necessary to establish whether some of these lesions would progress to frank neoplasia.
Skin Cancer in Rodents
One of the links in the total evidence for the causal relationship of cigarette smoking and lung cancer is the demonstration that tobacco smoke condensates (usually referred to as “tars”) have the biologic property of evoking carcinoma in certain laboratory animals, particularly mice. The production of skin cancer in mice, following repeated, long-term applications of tobacco tar, has now been reported from at least six different laboratories 20 , 21 , 22 , 23 , 24 , 76 . It is undeniable that some investigators did not obtain positive results, perhaps because the dose and other experimental conditions were different, or because the complex tobacco tars probably varied widely in their composition. The negative results of Passey et al . 18 have been quoted by Hueper 38 and others, but a more recent experiment by Passey 24 with Swiss strain mice did lead to the appearance of at least two carcinomas after repeated applications of tobacco-smoke condensate.
Little 29 indicated that “… the extrapolation to the human lung of results obtained by painting of or injection into the skin of mice is decidedly questionable”. Direct extrapolation from one species to another is, of course, not justified. Nevertheless, results in animals are fully consistent with the epidemiologic findings in man. A quotation from Kotin 49 is appropriate: “The chemical demonstration of carcinogenic agents in the environment and their successful use for the production of tumours in experimental animals do not prove or even especially strongly suggest a like relationship in the instance of man. When, however, a demonstrable parallelism exists between epidemiologic data and laboratory findings, greater significance accrues to both. Medical history is replete with examples in which laboratory findings have been proved ultimately to have their counterpart in the human experience. Exceptions have been very few.”
Greene 37 , while discounting the significance of the induction of skin carcinoma in Swiss mice because of the constitutionally “high differential susceptibility” of the strain, believes that the failure to induce neoplasms in embryonic transplants exposed to tobacco tar is more important evidence. Greene's interesting technique does produce positive results when pure chemicals such as benzo[α]pyrene are used, and this chemical has been recovered from some samples of tobacco-smoke condensate. We are not acquainted with reports of neoplasms arising in embryonic tissue that has been exposed in vitro to coal tar, another crude mixture that contains carcinogens.
The high frequency of carcinoma induction reported by Wynder et al . 76 has not been achieved by other investigators, who reported that no more than 20 percent of animals, and usually considerably less, developed carcinoma of the skin. The presence of cocarcinogenic materials in tobacco-smoke condensates has been demonstrated by Gellhorn 22 and by Bock and Moore 20 . To the mouse data are now added the data on the induction of skin cancer in some rabbits painted with tobacco-smoke condensate 77 ; this condensate, when combined with a killed suspension of tubercle bacilli, and introduced into a bronchus, produced a carcinoma of the bronchus in one rat 78 .
Since malignant neoplasms have been obtained in several strains of mice, and a few neoplasms have been produced in rabbits and rats, the issue of strain or species limitation to the reaction is more difficult to maintain. It is, of course, a fact that many agents shown to be carcinogenic to the skin of mice have not been proved carcinogenic to man. In most instances there is simply no experience with such agents in man, so that lack of proof really represents lack of data, pro and con.
The Problem of Dosage
Little 29 has further questioned the applicability of animal data to man, as follows: “Tobacco smoke or smoke condensate has failed to produce cancer even on the skin of susceptible strains of mice when applied in the quantity and at an exposure rate that would simulate conditions of human smoking.”
The differences in species, tissues, and conditions between the induction of neoplasms on the skin of mice and in the bronchi of man, preclude fine comparisons of dose and time relationships.
Bronchogenic Cancer in Animals
The pulmonary adenomatous tumor in mice, rats, and guinea pigs cannot be compared with the bronchogenic carcinoma in man 71 . Until a few years ago, the experimental induction of epidermoid carcinoma had been achieved only in a few mice by passing strings impregnated with carcinogenic hydrocarbons through the lung. Epidermoid carcinoma of the lung was consistently produced in rats by beryllium 79 , by carcinogenic hydrocarbons introduced as fixed pellets into bronchi of rats 80 , and by inhalation of radioactive particles 81 .
Little 29 has noted that “… prolonged exposure of the lungs of rodents to massive doses of cigarette smoke has failed to produce bronchogenic cancer.” This remains true at the time of this report, although it can be questioned whether any animal receives as large a dose of cigarette smoke through indirect exposure as a human being does by voluntary deep inhalation. Therefore the failure may be a technical one, which may be solved by further experimentation. The early results of Leuchtenberger et al . 19 suggested that this may be achieved.
Carcinogens in Tobacco Smoke
The isolation and identification of specific chemical constituents in tobacco smoke, which are carcinogenic for the pulmonary tissue of man, is an important area for research.
It has been clear for some time that combustion or pyrolysis of most organic material, including tobacco, will form higher aromatic polycyclics of established carcinogenic activity 28 . A number of higher aromatic polycyclics have been identified and isolated ( 23 , 25 , 26 , 27 ). These materials include benzo[ e ]pyrene, benzo[ a ]pyrene, dibenz[ a,h ]anthracene, chrysene, and, most recently, a newly established carcinogen, 3,4-benz-fluoranthene. Whether these compounds are equally involved in human pulmonary carcinogenesis is, of course, conjectural.
Little 29 has implied that a specific constituent must be found to account for the biologic activity of tobacco smoke. This is not necessary. The situation is similar to the establishment of the carcinogenic activity of tar, which was accepted before the isolation of benzo[ a ]pyrene by Kennaway and his coworkers. In this instance, also, benzo[ a ]pyrene is most probably not the only carcinogen in the complex mixture called tar, and there are strong indications that some noncarcinogenic components in tar may have cocarcinogenic effects.
Three interpretations of the observed association of lung cancer and cigarette smoking are possible: 1) that cigarette smoking “causes” lung cancer, either (a) through the direct carcinogenic action of smoke on human bronchial epithelium or (b) by a more indirect mode of action such as making the individual susceptible to some other specific carcinogenic agent in the environment; 2) that lung cancer “causes” cigarette smoking, perhaps because a precancerous condition sets up a process which leads to a craving for tobacco; 3) that cigarette smoking and lung cancer both have a common cause, usually specified as a special constitutional make-up, perhaps genetic in origin, which predisposes certain individuals to lung cancer and also makes them cigarette smokers.
The second hypothesis was advanced by Fisher 36 , apparently for the sake of logical completeness, and it is not clear whether it is intended to be regarded as a serious possibility. Since we know of no evidence to support the view that the bronchogenic carcinoma diagnosed after age 50 began before age 18, the median age at which cigarette smokers begin smoking, we shall not discuss it further.
The Constitutional Hypothesis
The first hypothesis may be referred to as the causal hypothesis and the third as the constitutional hypothesis. Nothing short of a series of independently conducted, controlled, experiments on human subjects, continued for 30 to 60 years, could provide a clear-cut and unequivocal choice between them. We nevertheless argue that evidence, in addition to that associating an increased mortality from lung cancer with cigarette smoking, is entirely consistent with the causal hypothesis but inconsistent, in many respects, with the constitutional hypothesis, so that even in the absence of controlled experimentation on human beings the weight of the evidence is for the one and against the other.
The difficulties with the constitutional hypothesis include the following considerations: (a) changes in lung-cancer mortality over the last half century; (b) the carcinogenicity of tobacco tars for experimental animals; (c) the existence of a large effect from pipe and cigar tobacco on cancer of the buccal cavity and larynx but not on cancer of the lung; (d) the reduced lung-cancer mortality among discontinued cigarette smokers. No one of these considerations is perhaps sufficient by itself to counter the constitutional hypothesis ad hoc modification of which can accommodate each additional piece of evidence. A point is reached, however, when a continuously modified hypothesis becomes difficult to entertain seriously.
Changes in Mortality
Mortality from lung cancer has increased continuously in the last 50 years, and considerably more for males than females. Such an increase can be explained either as the result of an environmental change (to which males are more exposed or more sensitive than females, if both are equally exposed) or as the result of a sex-linked mutation. The constitutional hypothesis must be modified in the light of this increase, since an unchanging constitutional make-up cannot by itself explain an increase in mortality. Proponents of the constitutional hypothesis have not indicated the type of modification they would consider. Three suggest themselves to us: 1) differences in constitutional make-up are genetic in origin, but rather than predisposing one to lung cancer, they make one sensitive to some new environmental agent (other than tobacco), which does induce lung cancer; 2) differences in constitutional make-up are not genetic but are the result of differential exposure to some new environmental agent, which both predisposes to lung cancer and creates a craving for cigarette smoke; 3) the mutation has led to a greater susceptibility to lung cancer and a preference for cigarette smoke.
In the first two situations the effect of the postulated constitutional make-up would be mediated through an environmental agent. The modified hypothesis thus requires the existence of an environmental agent other than tobacco, exposure to which would be at least as highly correlated with lung-cancer mortality as exposure to cigarettes, and which also would be highly correlated with cigarette consumption. No such agent has yet been found or even suggested. In view of the magnitude of the increase in mortality from lung cancer, the third situation would require a mutation rate exceeding anything previously observed.
Experimental Carcinogenesis With Tobacco Tar
Condensed tobacco smoke contains substances that are carcinogenic for mouse and rabbit skin. It does not necessarily follow that these substances are also carcinogenic for human lungs nor does it follow that they are not. However, the constitutional hypothesis asserts they are not; and that it is simply a coincidence that these materials which are carcinogenic for experimental animals are also associated with a higher lung-cancer mortality in man.
Types of Tobacco and Cancer Site
A greatly increased lung-cancer risk is associated with increased cigarette consumption but not with increased consumption of pipe and cigar tobacco. Studies on cancer of the buccal cavity and larynx, however, have demonstrated a considerably higher risk among smokers, irrespective of the form or tobacco used. Only two ways of modifying the constitutional hypothesis to take account of this evidence occur to us: 1) There are two different constitutional make-ups, one of which predisposes to cigarettes but not to pipe and cigar consumption and to cancer of the lung, and the other predisposes to cancer of the buccal cavity and larynx but not of the lung and to tobacco consumption in any form. 2) Constitutional make-up predisposes to cigarette consumption and lung cancer only, but tobacco smoke, whether from cigarettes, cigars, or pipes, is carcinogenic for the mucosa of the buccal cavity and the larynx but not for the bronchial epithelium.
Mortality Among Discontinued Smokers
Mortality from lung cancer among discontinued cigarette smokers is less than that among those continuing to smoke 9 , 10 ; the magnitude of the reduction depending on amount previously smoked and the length of the discontinuance. The hypothetical constitutional factor which predisposes to lung cancer and cigarette smoking cannot therefore be a constant characteristic of an individual over his lifetime but must decrease in force at some time in life, thus resulting in the cessation of cigarette smoking and a concomitant, but not causally related, reduction in the lung-cancer risk. Furthermore, since cigarette smoking is rarely begun after age 35 50 , it must be inferred that the constitutional factor cannot increase in force with the passage of time, even though it may decrease.
In summary, the constitutional hypothesis does not provide a satisfactory explanation of all the evidence. It is natural, therefore, to inquire about the positive findings which support it. Even those who regard this hypothesis with favor would agree, we believe, that supporting evidence is quite scanty.
There are a number of characteristics in which cigarette smokers are known to differ from nonsmokers and presumably more will be discovered. Thus, cigarette smokers consume more alcohol, more black coffee, change jobs more often, engage more in athletics, and are more likely to have had at least one parent with hypertension or coronary artery disease 82 . Discontinued cigarette smokers are weaned at a later age than those continuing to smoke 83 . Recently, Fisher 83 reported that 51 monozygotic twins resembled each other more in their smoking habits than 33 dizygotic twins, thus suggesting a genetic determinant.
Two somewhat obvious, but necessary, comments on results of this type are in order: 1) The demonstration that a characteristic is related to smoking status does not by itself create a presumption that it is a common cause. It must also be shown to be related to the development of lung cancer among subgroups of individuals with the same smoking status. Alcohol and coffee fail to meet this test, while none of the other characteristics related to smoking status have been investigated from this point of view. 2) There is a quantitative question. Cigarette smokers have a ninefold greater risk of developing lung cancer than nonsmokers, while over-two-pack-a-day smokers have at least a 60-fold greater risk. Any characteristic proposed as a measure of the postulated cause common to both smoking status and lung-cancer risk must therefore be at least nine-fold more prevalent among cigarette smokers than among nonsmokers and at least 60-fold more prevalent among two-pack-a-day smokers. No such characteristic has yet been produced despite diligent search.
These comments on the quantitative aspects of association apply also to the relationship of certain characteristics with lung cancer. Thus, a possible genetic basis to lung cancer has been suggested to some by the association between gastric cancer and blood group. The difference, in risk of developing gastric cancer, between blood groups A and O, however, is 20 percent, while the only study of lung cancer and blood groups 84 with which we are familiar shows a difference of 27 percent (and is not quite significant at the P = 0.01 level. 1 Such differences are suggestive for further work, but cannot be considered as casting much light on differences of magnitude, ninefold to 60-fold.
Measures of Differences
The comments in the last two paragraphs have utilized a relative measure of differences in lung-cancer risk. Since Berkson 32 has argued that a relative measure is inappropriate in the investigation of smoking and mortality, we now discuss the use of relative and absolute measures of differences in risk. When an agent has an apparent effect on several diseases, the ranking of the diseases by the magnitude of the effect will depend on whether an absolute or a relative measure is used. Thus in Dorn's study 8 of American veterans there were 187 lung-cancer deaths among cigarette smokers compared with an expectation of 20 deaths, based on the rates for nonsmokers. This yields a mortality ratio of 9.35 as a relative measure and an excess of 167 deaths as an absolute measure. For cardiovascular diseases there were 1,780 deaths among cigarette smokers compared to an expectation of 1,165. This gives a relative measure of 1.53 and an absolute measure of 615 deaths. Relatively, cigarettes have much larger effect on lung cancer than on cardiovascular disease, while the reverse is true if an absolute measure is used.
Both the absolute and the relative measures serve a purpose. The relative measure is helpful in 1) appraising the possible noncausal nature of an agent having an apparent effect; 2) appraising the importance of an agent with respect to other possible agents inducing the same effect; and 3) properly reflecting the effects of disease misclassification or further refinement of classification. The absolute measure would be important in appraising the public health significance of an effect known to be causal.
If an agent, A, with no causal effect upon the risk of a disease, nevertheless, because of a positive correlation with some other causal agent, B, shows an apparent risk, r, for those exposed to A, relative to those not so exposed, then the prevalence of B, among those exposed to A, relative to the prevalence among those not so exposed, must be greater than r.
If two uncorrelated agents, A and B, each increase the risk of a disease, and if the risk of the disease in the absence of either agent is small (in a sense to be defined), then the apparent relative risk for A, r, is less than the risk for A in the absence of B.
If a causal agent A increases the risk for disease I and has no effect on the risk for disease II, then the relative risk of developing disease I, alone, is greater than the relative risk of developing disease I and II combined, while the absolute measure is unaffected.
The Causal Hypothesis
When the sexes are compared it is found that lung cancer has been increasing more rapidly in men relatively to women … But it is notorious, and conspicuous in the memory of the most of us, that over the last 50 years the increase of smoking among women has been great, and that among men (even if positive) certainly small. The theory that increasing smoking is ‘the cause’ of the change in apparent incidence of lung cancer is not even tenable in the face of this contrast.
It would thus appear that cigarette smoking is one of the causes of all ills and contributes to the over-all death rate, remembering that this rate includes such causes as accident, homicide, etc. It seems quite clear that cigarette smoking is a symptom, not a cause. It is possible – even though this is a conjecture – that they type of person who is careful of his health is less likely to be a cigarette smoker and that the cigarette smoker is likely to be the person who generally takes greater health risks.
Berkson 32 also has pointed to the multiple findings in both the Hammond-Horn and the Doll-Hill results and concluded that the observed associations may have some other explanation than a causal one. He suggests three: 1) “The observed associations are ‘spurious’ …. 2) The observed associations have a constitutional basis. Persons who are nonsmokers, or relatively light smokers, are the kind of people who are biologically self-protective, and biologically this is correlated with robustness in meeting mortal stress from disease generally. 3) Smoking increases the ‘rate of living’ (Pearl), and smokers at a given age are, biologically, at an age older than their chronologic age.”
One might ask why the finding of an association with a number of diseases, rather than just one, is necessarily contradictory and must be regarded as supporting the constitutional hypothesis. Arkin 34 supplied no answer, while the relevant statements of Berkson 32 on this point were:
When an investigation set up to test the theory, suggested by evidence previously obtained, that smoking causes lung cancer, turns out to indicate that smoking causes or provokes a whole gamut of diseases, inevitably it raises the suspicion that something is amiss. It is not logical to take such a set of results [e.g., an association of smoking with a ‘wide variety of diseases’] as confirming the theory that tobacco smoke contains carcinogenic substances which, by contact with the pulmonary tissues, initiate cancerous changes at the site of contact.
The apparent multiple effects of tobacco do raise a question with respect to the mode of action, however, and since this question is related to another alleged contradiction – the apparent lack of an inhalation effect – we shall discuss them together. What mode of action, it has been asked, can one postulate to explain these diverse effects? Two remarks are in order: 1) The evidence that tobacco is a causal agent in the development of other diseases seems weaker than the evidence for lung cancer simply because the effects are smaller. While we could not exclude the possibility that cigarettes play a causal role in, for instance, the development of arteriosclerotic-coronary heart disease, the possibility that a common third factor will be discovered, which explains a 70 percent elevation in risk from coronary heart disease among cigarette smokers, is less remote than the possibility that the ninefold risk for lung cancer will be so explained. 2) Accepting, for the sake of discussion, the causal role of cigarettes for any disease showing an elevated mortality ratio, no mater how small, the presence of other causes will be manifested in a lowered mortality ratio. Thus, even if cigarette consumption causes an elevation of 70 percent in mortality from coronary heart disease, other causes of great importance must also be present, as is manifested by the high mortality from this disease among nonsmokers. The existence of a small number of nonsmokers who develop lung cancer is a definite indication, by the same token, that cigarettes are not an absolutely necessary condition and that there are other causes of lung cancer.
If tobacco smoke does have multiple effects, each of these effects must be studied separately because of the complex nature of the agent. To postulate in advance that a single mode of action will be found to characterize them all is an unwarranted oversimplification. It is generally accepted, for example, that tobacco smoke causes thromboangiitis obliterans in susceptible humans by interfering with the peripheral circulation, and that it causes tumors when painted on the backs of susceptible mice because of the presence of carcinogenics in the tars. The a priori postulation of a single mode of action for these two effects is no substitute for detailed study of each.
As to the possible mode of action of tobacco smoke in inducing lung cancer, the evidence at this writing suggests direct action of substances in tobacco smoke on susceptible tissues with which they are in contact. Aside from background knowledge derived from experimental carcinogensis which suggests this explanation, the following evidence favors it: 1) Cigarette smoke, which is usually drawn into the lungs is associated with mortality from lung cancer, while smoke from pipes and cigars, which is usually not inhaled, if not. 2) For sites with which smoke is in direct contact, whether or not inhaled, particularly buccal cavity and larynx, the type of tobacco used makes less difference in incidence. 3) In experimental carcinogenesis, which uses tobacco tars, tumors have appeared at the site of application, and their incidence has not yet seriously dependent on the type of tobacco used. 4) The relative risk of lung cancer is higher among cigarette smokers who inhale than among those smoking the same number of cigarettes per day, but who do not inhale.
Several critics 36 , 38 , 39 have stressed the failure of Doll and Hill 62 , in their preliminary report, to find a difference in risk between inhalers and noninhalers, but this finding was contradicted in three other studies 4 , 68 , 69 . Further work on this point is desirable, but would be more convincing if a more objective measure were found of the amount of smoke to which human bronchial epithelium is exposed in the course of smoking a cigarette.
Why, it is sometimes asked, do most heavy cigarette smokers fail to develop lung cancer if cigarettes are in fact a causal agent? We have no answer to this question. But neither can we say why most of the Lübeck babies who were exposed to massive doses of virulent tubercle bacilli failed to develop tuberculosis. This is not a reason, however, for doubting the causal role of the bacilli in the development of the disease.
One cannot discuss the mode of action of tobacco without becoming aware of the necessity of vastly expanded research in the field. The idea that the subject of tobacco and mortality is a closed one requiring no further study is not one we share. As in other fields of science, new findings lead to new questions, and new experimental techniques will continue to cast further light on old ones. This does not imply that judgment must be suspended until all the evidence is in, or that there are hierarchies of evidence, only some types of which are acceptable. The doctrine that one must never assess what has already been learned until the last possible piece of evidence would be a novel one for science.
It would be desirable to have a set of findings on the subject of smoking and lung cancer so clear-cut and unequivocal that they were self-interpreting. The findings now available on tobacco, as in most other fields of science, particularly biologic science, do not meet this ideal. Nevertheless, if the findings had been made on a new agent, to which hundreds of millions of adults were not already addicted, and on one which did not support a large industry, skilled in the arts of mass persuasion, the evidence for the hazardous nature of the agent would be generally regarded as beyond dispute. In the light of all the evidence on tobacco, and after careful consideration of all the criticisms of this evidence that have been made, we find ourselves unable to agree with the proposition that cigarette smoking is a harmless habit with no important effects on health or longevity. The concern shown by medical and public health authorities with the increasing diffusion to ever younger groups of an agent that is a health hazard seems to us to be well founded.
* Cornfield J et al. Smoking and lung cancer: recent evidence and a discussion of some questions. JNCI 1959;22:173–203. Reprinted with permission.
1 Our attention has been called to a summary of three additional studies, which report no association between ABO blood groups and lung cancer, by Roberts JAF. Blood groups and susceptibility to disease. Brit. J. Prev. & Social Med. 11: 107–125, 1957.
Google Scholar
Google Preview
We feel obliged to give proof of the rather obvious statement on the magnitudes of relative risk because it has been suggested that the use of a relative measurement is merely "instinctive" and lacking in rational justification. Let the disease rate for those exposed to the causal agent, B, be r 1 and for those not exposed, r 2 , each rate being unaffected by exposure or nonexposure to the noncausal agent, A. Let r 1 > r 2 . Of those exposed to A, let the proportion exposed to B be p 1 , and of those not exposed to A, let the proportion exposed to B be p 2 . Because of the assumed positive correlation between A and B, p 1 > p 2 . Then
R 1 = rate for those exposed to A = p 1 r 1 + (1 – p 1 ) r 2
The proof again is simple. Let r 11 denote the risk of the disease in the presence of both A and B, r 12 , the risk in the presence of A and absence of B, r 12 , the risk in the absence of A and presence of B, and r 22 the risk in the absence of both A and B. It is reasonable to assume r 22 = 0, but the less restrictive specification r 22 < r 12 r 21 / r 11 is sufficient for what follows. The proportion of the population exposed to B is denoted by p , and this, by hypothesis, is the same whether A is present or absent. Then
R 1 = rate for those exposed to A = pr 11 + (1 – p ) r 12
- lung cancer
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 63 |
| February 2017 | 228 |
| March 2017 | 248 |
| April 2017 | 115 |
| May 2017 | 88 |
| June 2017 | 48 |
| July 2017 | 36 |
| August 2017 | 188 |
| September 2017 | 170 |
| October 2017 | 245 |
| November 2017 | 369 |
| December 2017 | 1,272 |
| January 2018 | 1,287 |
| February 2018 | 1,474 |
| March 2018 | 1,444 |
| April 2018 | 1,304 |
| May 2018 | 1,270 |
| June 2018 | 1,004 |
| July 2018 | 621 |
| August 2018 | 721 |
| September 2018 | 762 |
| October 2018 | 826 |
| November 2018 | 890 |
| December 2018 | 537 |
| January 2019 | 453 |
| February 2019 | 642 |
| March 2019 | 883 |
| April 2019 | 660 |
| May 2019 | 717 |
| June 2019 | 584 |
| July 2019 | 664 |
| August 2019 | 483 |
| September 2019 | 520 |
| October 2019 | 477 |
| November 2019 | 401 |
| December 2019 | 707 |
| January 2020 | 477 |
| February 2020 | 456 |
| March 2020 | 363 |
| April 2020 | 628 |
| May 2020 | 369 |
| June 2020 | 504 |
| July 2020 | 345 |
| August 2020 | 261 |
| September 2020 | 473 |
| October 2020 | 441 |
| November 2020 | 398 |
| December 2020 | 386 |
| January 2021 | 415 |
| February 2021 | 519 |
| March 2021 | 640 |
| April 2021 | 563 |
| May 2021 | 448 |
| June 2021 | 348 |
| July 2021 | 321 |
| August 2021 | 419 |
| September 2021 | 495 |
| October 2021 | 563 |
| November 2021 | 519 |
| December 2021 | 370 |
| January 2022 | 506 |
| February 2022 | 506 |
| March 2022 | 854 |
| April 2022 | 745 |
| May 2022 | 559 |
| June 2022 | 327 |
| July 2022 | 302 |
| August 2022 | 339 |
| September 2022 | 457 |
| October 2022 | 469 |
| November 2022 | 598 |
| December 2022 | 439 |
| January 2023 | 489 |
| February 2023 | 435 |
| March 2023 | 471 |
| April 2023 | 402 |
| May 2023 | 413 |
| June 2023 | 267 |
| July 2023 | 290 |
| August 2023 | 268 |
| September 2023 | 404 |
| October 2023 | 369 |
| November 2023 | 428 |
| December 2023 | 467 |
| January 2024 | 525 |
| February 2024 | 466 |
| March 2024 | 549 |
| April 2024 | 519 |
| May 2024 | 379 |
| June 2024 | 295 |
Email alerts
Citing articles via, looking for your next opportunity.
- About International Journal of Epidemiology
- Recommend to your Library
Affiliations
- Online ISSN 1464-3685
- Copyright © 2024 International Epidemiological Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
Lung cancer
- Lung cancer is the leading cause of cancer-related deaths worldwide, accounting for the highest mortality rates among both men and women.
- Smoking is the leading cause of lung cancer, responsible for approximately 85% of all cases.
- Lung cancer is often diagnosed at advanced stages when treatment options are limited.
- Screening high risk individuals has the potential to allow early detection and to dramatically improve survival rates.
- Primary prevention (such as tobacco control measures and reducing exposure to environmental risk factors) can reduce the incidence of lung cancer and save lives.
Lung cancer is a type of cancer that starts when abnormal cells grow in an uncontrolled way in the lungs. It is a serious health issue that can cause severe harm and death.
Symptoms of lung cancer include a cough that does not go away, chest pain and shortness of breath.
It is important to seek medical care early to avoid serious health effects. Treatments depend on the person’s medical history and the stage of the disease.
The most common types of lung cancer are non-small cell carcinoma (NSCLC) and small cell carcinoma (SCLC). NSCLC is more common and grows slowly, while SCLC is less common but often grows quickly.
Lung cancer is a significant public health concern, causing a considerable number of deaths globally. GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer (IARC) show as lung cancer remains the leading cause of cancer death, with an estimated 1.8 million deaths (18%) in 2020.
Smoking tobacco (including cigarettes, cigars, and pipes) is the primary risk factor for lung cancer but it can also affect non-smokers. Other risk factors include exposure to secondhand smoke, occupational hazards (such as asbestos, radon and certain chemicals), air pollution, hereditary cancer syndromes, and previous chronic lung diseases.
Lung cancer can cause several symptoms that may indicate a problem in the lungs.
The most common symptoms include:
- cough that does not go away
- shortness of breath
- coughing up blood (haemoptysis)
- weight loss with no known cause
- lung infections that keep coming back.
Early symptoms may be mild or dismissed as common respiratory issues, leading to delayed diagnosis.
Not smoking tobacco is the best way to prevent lung cancer.
Other risk factors to avoid include:
- secondhand smoke
- air pollution
- workplace hazards like chemicals and asbestos.
Early treatment can prevent lung cancer from becoming worse and spreading to other parts of the body.
Prevention of lung cancer include primary and secondary prevention measures. Primary prevention aims to prevent the initial occurrence of a disease through risk reduction and promoting healthy behaviour. In public health, these preventive measures include smoking cessation, promoting smoke-free environments, implementing tobacco control policies, addressing occupational hazards, and reducing air pollution levels.
Secondary prevention for lung cancer involves screening methods that aim to detect the disease in its early stages, before symptoms become apparent and can be indicated for high-risk individuals. In this population, early detection can significantly increase the chances of successful treatment and improve outcomes. The primary screening method for lung cancer is low-dose computed tomography (LDCT).
Diagnostic methods for lung cancer include physical examination, imaging (such as chest X-rays, computed tomography scans, and magnetic resonance imaging), examination of the inside of the lung using a bronchoscopy, taking a sample of tissue (biopsy) for histopathology examination and definition of the specific subtype (NSCLC versus SCLC), and molecular testing to identify specific genetic mutations or biomarkers to guide the best treatment option.
Treatment and care
Treatments for lung cancer are based on the type of cancer, how much it has spread, and the person’s medical history. Early detection of lung cancer can lead to better treatments and outcomes.
Treatments include:
- radiotherapy (radiation)
- chemotherapy
- targeted therapy
- immunotherapy.
Surgery is often used in the early stages of lung cancer if the tumour has not spread to other areas of the body. Chemotherapy and radiation therapy can help shrink the tumour.
Doctors from several disciplines often work together to provide treatment and care of people with lung cancer.
Supportive care is important for people with lung cancer. It aims to manage symptoms, provide pain relief, and give emotional support. It can help to increase quality of life for people with lung cancer and their families.
Stages of care
a) Early stage disease : The primary treatment for early stage lung cancer (i.e. tumour limited to the lung, with no metastatic dissemination to distant organs or lymph nodes) is surgical removal of the tumour through procedures such as lobectomy, segmentectomy, or wedge resection. Neoadjuvant therapy (chemotherapy and/or radiation therapy before surgery) can help reduce tumour size, making it more manageable for surgical removal. Adjuvant treatment (chemotherapy and/or radiation therapy) is very often recommended after surgery to reduce the risk of cancer recurrence. In cases where surgery is not feasible, radiation therapy or stereotactic body radiation therapy (SBRT) may be used as the primary treatment. Targeted therapy and immunotherapy may also be considered based on specific tumour characteristics. Individualized treatment plans should be discussed with healthcare professionals.
b) Advanced disease: The treatment for metastatic stage lung cancer, where the cancer has spread to distant organs or lymph nodes, is based on various factors, including the patient's overall health, the extent and location of metastases, histology, genetic profile, and individual preferences. The primary goal is to prolong survival, alleviate symptoms, and improve quality of life. Systemic therapies, such as chemotherapy, targeted therapy, and immunotherapy, play a crucial role in the treatment of metastatic lung cancer.
Chemotherapy is often the first-line treatment for the majority of patients around the world and involves the use of drugs that circulate throughout the body to kill cancer cells. Combination chemotherapy regimens are commonly used, and the choice of drugs depends on factors such as the histological type of the cancer and the patient's general health conditions. Targeted therapy, designed to block the signalling pathways that drive the growth of cancer cells, is an important option for patients with specific genetic mutations or biomarkers identified in their tumour. Immunotherapy, specifically immune checkpoint inhibitors, has revolutionized the treatment of metastatic lung cancer. These drugs help to stimulate the immune system to recognize and attack cancer cells. Local treatments, such as radiation therapy and surgery, may be used to manage specific metastatic sites or alleviate symptoms caused by tumour growth.
Clinical Trials
Clinical trials offer opportunities to access novel treatments or experimental therapies for patients. Participation in clinical trials helps advance medical knowledge and potentially offers new treatment options.
WHO response
WHO recognizes the significant impact of lung cancer on global health and has implemented several initiatives to address the disease comprehensively. The WHO's response focuses on tobacco control, cancer prevention, early detection, and improving access to quality treatment and care. WHO supports countries in implementing evidence-based tobacco control policies, including increasing tobacco taxes, enforcing comprehensive bans on tobacco advertising, promotion, and sponsorship, and implementing strong graphic health warnings on tobacco products.
The Organization also promotes cancer prevention strategies by advocating for healthy lifestyles, including regular physical activity, a healthy diet, and minimizing exposure to environmental risk factors. Additionally, WHO supports early detection programs and encourages countries to implement screening measures for high-risk populations to detect lung cancer at earlier stages when treatment options are more effective. Last, WHO works towards ensuring access to quality treatment and care for lung cancer patients by providing technical guidance to member states, promoting equitable access to essential cancer medicines, and fostering international collaboration to share best practices and improve cancer care outcomes.
International Agency for Research on Cancer: Lung cancer
WHO's work on tobacco cessation
WHO's work on cancer
ESMO Clinical Practice Guidelines: Lung and Chest Tumours
- Patient Care & Health Information
- Diseases & Conditions
- Lung cancer
Lung cancer begins in the cells of the lungs.
Lung cancer is a kind of cancer that starts as a growth of cells in the lungs. The lungs are two spongy organs in the chest that control breathing.
Lung cancer is the leading cause of cancer deaths worldwide.
People who smoke have the greatest risk of lung cancer. The risk of lung cancer increases with the length of time and number of cigarettes smoked. Quitting smoking, even after smoking for many years, significantly lowers the chances of developing lung cancer. Lung cancer also can happen in people who have never smoked.
Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Lung cancer typically doesn't cause symptoms early on. Symptoms of lung cancer usually happen when the disease is advanced.
Signs and symptoms of lung cancer that happen in and around the lungs may include:
- A new cough that doesn't go away.
- Chest pain.
- Coughing up blood, even a small amount.
- Hoarseness.
- Shortness of breath.
Signs and symptoms that happen when lung cancer spreads to other parts of the body may include:
- Losing weight without trying.
- Loss of appetite.
- Swelling in the face or neck.
When to see a doctor
Make an appointment with your doctor or other healthcare professional if you have any symptoms that worry you.
If you smoke and haven't been able to quit, make an appointment. Your healthcare professional can recommend strategies for quitting smoking. These may include counseling, medicines and nicotine replacement products.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Lung cancer happens when cells in the lungs develop changes in their DNA. A cell's DNA holds the instructions that tell a cell what to do. In healthy cells, the DNA gives instructions to grow and multiply at a set rate. The instructions tell the cells to die at a set time. In cancer cells, the DNA changes give different instructions. The changes tell the cancer cells to make many more cells quickly. Cancer cells can keep living when healthy cells would die. This causes too many cells.
The cancer cells might form a mass called a tumor. The tumor can grow to invade and destroy healthy body tissue. In time, cancer cells can break away and spread to other parts of the body. When cancer spreads, it's called metastatic cancer.
Smoking causes most lung cancers. It can cause lung cancer in both people who smoke and in people exposed to secondhand smoke. But lung cancer also happens in people who never smoked or been exposed to secondhand smoke. In these people, there may be no clear cause of lung cancer.
How smoking causes lung cancer
Researchers believe smoking causes lung cancer by damaging the cells that line the lungs. Cigarette smoke is full of cancer-causing substances, called carcinogens. When you inhale cigarette smoke, the carcinogens cause changes in the lung tissue almost immediately.
At first your body may be able to repair this damage. But with each repeated exposure, healthy cells that line your lungs become more damaged. Over time, the damage causes cells to change and eventually cancer may develop.
Types of lung cancer
Lung cancer is divided into two major types based on the appearance of the cells under a microscope. Your healthcare professional makes treatment decisions based on which major type of lung cancer you have.
The two general types of lung cancer include:
- Small cell lung cancer. Small cell lung cancer usually only happens in people who have smoked heavily for years. Small cell lung cancer is less common than non-small cell lung cancer.
- Non-small cell lung cancer. Non-small cell lung cancer is a category that includes several types of lung cancers. Non-small cell lung cancers include squamous cell carcinoma, adenocarcinoma and large cell carcinoma.
Risk factors
A number of factors may increase the risk of lung cancer. Some risk factors can be controlled, for instance, by quitting smoking. Other factors can't be controlled, such as your family history.
Risk factors for lung cancer include:
Your risk of lung cancer increases with the number of cigarettes you smoke each day. Your risk also increases with the number of years you have smoked. Quitting at any age can significantly lower your risk of developing lung cancer.
Exposure to secondhand smoke
Even if you don't smoke, your risk of lung cancer increases if you're around people who are smoking. Breathing the smoke in the air from other people who are smoking is called secondhand smoke.
Previous radiation therapy
If you've had radiation therapy to the chest for another type of cancer, you may have an increased risk of developing lung cancer.
Exposure to radon gas
Radon is produced by the natural breakdown of uranium in soil, rock and water. Radon eventually becomes part of the air you breathe. Unsafe levels of radon can build up in any building, including homes.
Exposure to cancer-causing substances
Workplace exposure to cancer-causing substances, called carcinogens, can increase your risk of developing lung cancer. The risk may be higher if you smoke. Carcinogens linked to lung cancer risk include asbestos, arsenic, chromium and nickel.
Family history of lung cancer
People with a parent, sibling or child with lung cancer have an increased risk of the disease.
Complications
Lung cancer can cause complications, such as:
Shortness of breath
People with lung cancer can experience shortness of breath if cancer grows to block the major airways. Lung cancer also can cause fluid to collect around the lungs and heart. The fluid makes it harder for the affected lung to expand fully when you inhale.
Coughing up blood
Lung cancer can cause bleeding in the airway. This can cause you to cough up blood. Sometimes bleeding can become severe. Treatments are available to control bleeding.
Advanced lung cancer that spreads can cause pain. It may spread to the lining of a lung or to another area of the body, such as a bone. Tell your healthcare professional if you experience pain. Many treatments are available to control pain.
Fluid in the chest
Lung cancer can cause fluid to accumulate in the chest, called pleural effusion. The fluid collects in the space that surrounds the affected lung in the chest cavity, called the pleural space.
Pleural effusion can cause shortness of breath. Treatments are available to drain the fluid from your chest. Treatments can reduce the risk that pleural effusion will happen again.
Cancer that spreads to other parts of the body
Lung cancer often spreads to other parts of the body. Lung cancer may spread to the brain and the bones.
Cancer that spreads can cause pain, nausea, headaches or other symptoms depending on what organ is affected. Once lung cancer has spread beyond the lungs, it's generally not curable. Treatments are available to decrease symptoms and to help you live longer.
There's no sure way to prevent lung cancer, but you can reduce your risk if you:
Don't smoke
If you've never smoked, don't start. Talk to your children about not smoking so that they can understand how to avoid this major risk factor for lung cancer. Begin conversations about the dangers of smoking with your children early so that they know how to react to peer pressure.
Stop smoking
Stop smoking now. Quitting reduces your risk of lung cancer, even if you've smoked for years. Talk to your healthcare team about strategies and aids that can help you quit. Options include nicotine replacement products, medicines and support groups.
Avoid secondhand smoke
If you live or work with a person who smokes, urge them to quit. At the very least, ask them to smoke outside. Avoid areas where people smoke, such as bars. Seek out smoke-free options.
Test your home for radon
Have the radon levels in your home checked, especially if you live in an area where radon is known to be a problem. High radon levels can be fixed to make your home safer. Radon test kits are often sold at hardware stores and can be purchased online. For more information on radon testing, contact your local department of public health.
Avoid carcinogens at work
Take precautions to protect yourself from exposure to toxic chemicals at work. Follow your employer's precautions. For instance, if you're given a face mask for protection, always wear it. Ask your healthcare professional what more you can do to protect yourself at work. Your risk of lung damage from workplace carcinogens increases if you smoke.
Eat a diet full of fruits and vegetables
Choose a healthy diet with a variety of fruits and vegetables. Food sources of vitamins and nutrients are best. Avoid taking large doses of vitamins in pill form, as they may be harmful. For instance, researchers hoping to reduce the risk of lung cancer in people who smoked heavily gave them beta carotene supplements. Results showed the supplements increased the risk of cancer in people who smoke.
Exercise most days of the week
If you don't exercise regularly, start out slowly. Try to exercise most days of the week.
Lung cancer care at Mayo Clinic
Living with lung cancer?
Connect with others like you for support and answers to your questions in the Lung Cancer support group on Mayo Clinic Connect, a patient community.
Lung Cancer Discussions

435 Replies Fri, Jun 28, 2024

194 Replies Thu, Jun 27, 2024

52 Replies Tue, Jun 25, 2024
- Non-small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450. Accessed Dec. 4, 2023.
- Small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1462. Accessed Dec. 4, 2023.
- Niederhuber JE, et al., eds. Cancer of the lung: Non-small cell lung cancer and small cell lung cancer. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 4, 2023.
- Non-small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Lung cancer – non-small cell. Cancer.Net. https://www.cancer.net/cancer-types/lung-cancer/view-all. Accessed Dec. 4, 2023.
- Lung cancer – small cell. Cancer.Net. https://www.cancer.net/cancer-types/33776/view-all. Accessed Dec. 4, 2023.
- Detterbeck FC, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; doi:10.1378/chest.12-2377.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed Dec. 4, 2023.
- Lung cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/lung-cancer. Accessed Dec. 4, 2023.
- Cairns LM. Managing breathlessness in patients with lung cancer. Nursing Standard. 2012; doi:10.7748/ns2012.11.27.13.44.c9450.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Jan. 13, 2020.
- Brown AY. Allscripts EPSi. Mayo Clinic. July 30, 2019.
- Searching for cancer centers. American College of Surgeons. https://www.facs.org/search/cancer-programs. Accessed Dec. 4, 2023.
- Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine. 2010; doi:10.1056/NEJMoa1000678.
- Dunning J, et al. Microlobectomy: A novel form of endoscopic lobectomy. Innovations. 2017; doi:10.1097/IMI.0000000000000394.
- Leventakos K, et al. Advances in the treatment of non-small cell lung cancer: Focus on nivolumab, pembrolizumab and atezolizumab. BioDrugs. 2016; doi:10.1007/s40259-016-0187-0.
- Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Medicine. 1999;5:1365.
- Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine. 2011; doi:10.1056/NEJMoa1102873.
- Infographic: Lung Cancer
- Lung cancer surgery
- Lung nodules: Can they be cancerous?
- Super Survivor Conquers Cancer
Associated Procedures
- Ablation therapy
- Brachytherapy
- Bronchoscopy
- Chemotherapy
- Lung cancer screening
- Positron emission tomography scan
- Proton therapy
- Radiation therapy
- Stop-smoking services
News from Mayo Clinic
- Science Saturday: Study finds senescent immune cells promote lung tumor growth June 17, 2023, 11:00 a.m. CDT
- Era of hope for patients with lung cancer Nov. 16, 2022, 03:00 p.m. CDT
- Mayo Clinic Q&A podcast: Survivorship after surgery for lung cancer Nov. 15, 2022, 01:30 p.m. CDT
- Mayo Clinic Minute: Understanding lung cancer Nov. 02, 2022, 04:00 p.m. CDT
- Lung cancer diagnosis innovation leads to higher survival rates Nov. 02, 2022, 02:30 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been recognized among the top Pulmonology hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be part of the cutting-edge research and care that's changing medicine.
- Undergraduate
- High School
- Architecture
- American History
- Asian History
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- Linguistics
- Criminal Justice
- Legal Issues
- Anthropology
- Archaeology
- Political Science
- World Affairs
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Pharmacology
- Earth science
- Agriculture
- Agricultural Studies
- Computer Science
- IT Management
- Mathematics
- Investments
- Engineering and Technology
- Engineering
- Aeronautics
- Medicine and Health
- Alternative Medicine
- Communications and Media
- Advertising
- Communication Strategies
- Public Relations
- Educational Theories
- Teacher's Career
- Chicago/Turabian
- Company Analysis
- Education Theories
- Shakespeare
- Canadian Studies
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
- Movie Review
- Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Marketing Plan
- Multiple Choice Quiz
- Personal Statement
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Reaction Paper
- Research Paper
- Research Proposal
- SWOT analysis
- Thesis Paper
- Online Quiz
- Literature Review
- Movie Analysis
Statistics problem
- Math Problem
- All papers examples
- How It Works
- Money Back Policy
- Terms of Use
- Privacy Policy
- We Are Hiring
Relationship Between Smoking and Lung Cancer, Essay Example
Pages: 6
Words: 1722
Hire a Writer for Custom Essay
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.
For many years, there has been a direct causal relationship between lung cancer and smoking.( Chest-xRay.com ). There have been many different carcinogens contained in cigarette smoke, so that there is a high chance of developing lung cancer from either directly smoking or from secondhand smoke. In addition, despite the fact that many people have quit smoking, the risk of lung cancer is not instantly gone once someone stop smoking. A large percentage of newly diagnosed lung cancer, with the median abstinence duration nine years, has occurred in former smokers. (Ibid.)
This discussion examines a group of smokers and non-smokers, and their diagnosis of lung cancer. The purpose is to enhance general knowledge about how devastating tobacco is on the health status of smokers and non-smokers.
Chapter I: Introduction
Problem statement
The problem is the increasing number of patients suffering from lung cancer as reported by the World Health Organization..
The purpose of the study is to discuss the association between smoking and lung cancer with the goal of highlighting this correlation and contributing to the body of knowledge reporting it, so as to shed a spotlight on this global problem.
My project will be useful in contributing to the knowledge base regarding lung cancer and its correlation to smoking.
Literature review
According to www.lungcancer.org , lung cancer is the” uncontrolled growth of abnormal cells in one or both lungs.” Because they are abnormal, the cells do not carry out the typical purposes of normal cells and do not develop into healthy lung tissue. When they increase in size, these abnormal cells may form tumors and be an obstacle towards the lungs’ functioning, specifically in the area of providing oxygen to the body through the blood.
DNA is contained in all the cells of the body, including those of the lungs. Every time a mature cell divides into two daughter cells, it produces the same exact DNA. These cells are clones of the original cells, exact replicas of the starting cell. This is the way that the cells of our skin continually replenish themselves so that once old cells die, new ones take their place.
Cancer begins with a mutation in a cell’s DNA which can be caused by the typical aging process or through environmental factors such as exposure to asbestos or cigarette smoking.
A lung cancer cell is caused by a series of genetic changes. When the cells have only some irregularities but still function as lung cells, they would be considered precancerous. Such changes can signify progression towards cancer. Eventually, if the cell becomes more irregular it will not be effective in performing functions as lung tissue. In addition, as the disease progresses, some cells might travel away from the main tumor and start growing elsewhere in the body.
There are two different forms of lung cancer: primary and secondary. Primary lung cancers are those which originate in the lungs. Secondary lung cancers are those which have a distant tissue of origin but invade the lung tissue.
The main causes of lung cancer include the following: carcinogens such as tobacco smoke, viral infections and ionizing radiation. These factors cause disruption in the DNA structure of the tissues of the lungs. As this damaged DNA continues to accumulate, cancer of the lungs develops. Tobacco is the main contributor to lung cancers in the world. In a 2007 study (Jean & James, 2007) there was an indication that out of every 100 lung cancer deaths in the United States, 90 were secondary to smoking. Jean and James established that all 90 victims were chronic smokers of tobacco while about six of them had close relatives who smoked.
In another study from London, Marry established that 80% of her experimental population that were suffering from lung cancer were smokers (2008). The remainder had a close family relative who smoked or were working or living near a smoker. These two studies show that there is a significant relationship between the smoking of tobacco and lung cancer.
Study design
This is a retrospective study. Questionnaires were administered to 1000 respondents who were all suffering from lung cancer. In order to find respondents, a random sampling technique was used in which out of 2020 people suffering from lung cancer, 1000 were given papers on which the word “yes” was printed, and 1000 were given papers on which the word “no” was printed. Those who had a paper written “yes” were considered as the sample and were given questionnaires to fill out.. All 1000 questionnaires were collected and all of them were filled in. The questionnaires were then analyzed and the data entered into Excel workbook and the results presented in Microsoft Office Word.
Justification for this method
This method was deemed the most appropriate since it easily collected data from the respondents anonymously. That way, it was hoped that respondents would feel comfortable being open with their smoking habits. It was retrospective in that the study would analyze the past behavioral characteristics of the individual.
Sequence of events
The first issue to determine was the identification of the study population. The population criteria was patients with lung cancer. In order to find a sample population, a simple random sampling technique was used. This was achieved by giving the patients one of two randomly assigned papers with the word yes or no written on them The collection of the data was done by administration of questionnaires to the respondents. The questionnaires were then collected and analyzed.
The only tool that was used to collect data was the questionnaire because it was simple and inexpensive to use with a large population. The patients’ files were analyzed to confirm that they were indeed suffering from lung cancer.
In the study, three variables were considered: the independent, the dependent, and the control. The control value was constant: the study sample suffering from lung cancer. The dependent variables were the whether the patient was a smoker or not.
The hypothesis for this study was that there is relationship between smoking and lung cancer. Most people who were suffering from lung cancer were almost all current or past smokers. As a result, a relationship between the two was supported.
Data collection
The data was collected by use of questionnaires. A sample of the questionnaires is indicated in the appendix. The respondents gave age in years, their place of residence and what disease they were suffering from. They also indicated whether they smoke or not.
The results were collected and entered into table as follows.
Table: number of people who smoke and they are suffering lung cancer
| Those who smoke | 930 |
| Those who don’t smoke | 70 |
| No response | 0 |
According to the above table, the total number of patients who were not smokers was 70. Those who had lung cancer and were smokers were 930. Also studied was the number of those who were not smokers and gave the following results:
Non-smokers with lung cancer
| Have a close relative who smokes | 45 |
| Have no close relative who is a smoker | 25 |
Forty five respondents who were not smokers said that they were living or working close to a person who is a smoker. Only 25 were not close to a person who smokes tobacco.
Smokers and non-smokers suffering from lung cancer
Non-smokers
The results above show that there is a close relationship between lung cancer and smoking. Ninety three percent of the total respondents indicated that they smoke. Of the seven percent remaining who do not smoke, four and half percent of the total respondents had a close relative or lived closely to a person who smokes. Only two and half of the total were not smokers and had no relative or a person living near them who smoke.
In establishing the relationship between lung cancer and smoking, the research indicated the above results. From the data collected, 93% of the respondents who had lung cancer were smokers. Again, out of those who were not smokers, 64% of them were living or working close to people who smoke. Only two and half percent of the total respondents had no history of smoking or living near a smoker. This shows that a causal relationship between lung cancer and smoking given the high number of the people who smoke started smoking before they developed lung cancer.
Obviously, if the number of people who smoke is reduced, then the rates of lung cancer will decline as well. This will result in a decrease of the huge costs involved in treating such patients. It is also evident that the living near a person who smokes is a risk factor for lung cancer.
In conclusion, it is not a coincidence that 93 of the patients examined suffering from lung cancer were smokers. As Edwards puts it, smoking tobacco leads to production of carcinogens which break up the DNA in the nucleus of a cell (1999). This breakage leads to accumulation of defective DNA in the nucleus. The defective nucleus constantly multiplies but the cell does not undergo a process called apoptosis. Apoptosis is the process of programmed cell death and it ensures that defective cells die before they become cancerous (Joy & Ben 2006). These findings points towards a problem in the current society. There is an increase in the number of people smoking. Therefore, if the current tread does not change there is the probability that in the future we will see more lung cancers and related diseases.
This study supported all the other studies that preceded it in showing that smoking is a direct causative agent of lung cancer. Ideally, the antismoking campaigns that are so prevalent these days will be effective ways to minimize the risks of lung cancer. Reducing the rate of tobacco use will lower the rate of lung cancer and as a result, the huge costs involved in treating patients with this illness could substantially be decreased.
Edwards, P. M. (1999). Pathogenesis of Cancer . London: Free Press.
http://www.chestx-ray.com/Smoke/Smoke.html Retrieved 2010, May.
http://www.lungcancer.org/reading/about.php Retrieved 2010, May.
Jean, W. J. & Jane, B. J. (2007). Research advances in Cancer. Journal Of Association of Medics , 675 (8), 76-89.
Joy, C.,& Ben, D. (2006). Textbook of medicine . New York: education puplishers Marry, F. (2006). Smoking and cancer. A Journal on Cancer , 77(2) 67-89.
Stuck with your Essay?
Get in touch with one of our experts for instant help!
System of Linear Equations, Statistics Problem Example
Research Methods in Criminal Justice & Security, Essay Example
Time is precious
don’t waste it!
Plagiarism-free guarantee
Privacy guarantee
Secure checkout
Money back guarantee

Related Essay Samples & Examples
Voting as a civic responsibility, essay example.
Pages: 1
Words: 287
Utilitarianism and Its Applications, Essay Example
Words: 356
The Age-Related Changes of the Older Person, Essay Example
Pages: 2
Words: 448
The Problems ESOL Teachers Face, Essay Example
Pages: 8
Words: 2293
Should English Be the Primary Language? Essay Example
Pages: 4
Words: 999
The Term “Social Construction of Reality”, Essay Example
Words: 371
Lung Cancer Research Results and Study Updates
See Advances in Lung Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
The immunotherapy drug durvalumab (Imfinzi) can help people with early-stage small cell lung cancer live longer, results from a large clinical trial show. Three years after starting treatment, nearly 60% of people who received the drug were still alive.
FDA has approved alectinib (Alecensa) as adjuvant therapy for people with lung cancer who have ALK-positive tumors. In a clinical trial, alectinib helped people live longer after surgery without their cancer returning than chemotherapy.
The results of the clinical trial that led to FDA’s 2023 approval of repotrectinib (Augtyro) for lung cancers with ROS1 fusions have been published. The drug shrank tumors in 80% of people receiving the drug as an initial treatment.
A collection of material about the ALCHEMIST lung cancer trials that will examine tumor tissue from patients with certain types of early-stage, completely resected non-small cell lung cancer for gene mutations in the EGFR and ALK genes, and assign patients with these gene mutations to treatment trials testing post-surgical use of drugs targeted against these mutations.
Tarlatamab, a new type of targeted immunotherapy, shrank small cell lung cancer (SCLC) tumors in more than 30% of participants in an early-stage clinical trial. Participants had SCLC that had progressed after previous treatments with other drugs.
For people with lung cancer and medullary thyroid cancer whose tumors have changes in the RET gene, selpercatinib improved progression-free survival compared with other common treatments, according to new clinical trial results.
In the ADAURA clinical trial, people with early-stage lung cancer treated with osimertinib (Tagrisso) after surgery lived longer than people treated with a placebo after surgery. Despite some criticisms about its design, the trial is expected to change patient care.
For certain people with early-stage non-small cell lung cancer, sublobar surgery to remove only a piece of the affected lung lobe is as effective as surgery to remove the whole lobe, new research shows.
Pragmatica-Lung is a clinical trial for people with non-small cell lung cancer that has spread beyond the lungs (stage 4 cancer). The trial will help confirm if the combination of pembrolizumab and ramucirumab helps people with advanced lung cancer live longer.
On August 11, the Food and Drug Administration (FDA) gave accelerated approval to trastuzumab deruxtecan (Enhertu) for adults with non-small cell lung cancer (NSCLC) that has a specific mutation in the HER2 gene. Around 3% of people with NSCLC have this kind of HER2 mutation.
Giving people with early-stage lung cancer the immunotherapy drug nivolumab (Opdivo) and chemotherapy before surgery can substantially delay the progression or return of their cancer, a large clinical trial found.
Atezolizumab (Tecentriq) is now the first immunotherapy approved by FDA for use as an additional, or adjuvant, treatment for some patients with non-small cell lung cancer. The approval was based on results of a clinical trial called IMpower010.
Quitting smoking after a diagnosis of early-stage lung cancer may help people live longer, a new study finds. The study, which included more than 500 patients, also found that quitting smoking delayed the cancer from returning or getting worse.
NCI scientists and their international collaborators have found that the majority of lung cancers in never smokers arise when mutations caused by natural processes in the body accumulate. They also identified three subtypes of lung cancer these individuals.
FDA has approved the first KRAS-blocking drug, sotorasib (Lumakras). The approval, which covers the use of sotorasib to treat some patients with advanced lung cancer, sets the stage for other KRAS inhibitors already in development, researchers said.
Combining the chemotherapy drug topotecan and the investigational drug berzosertib shrank tumors in some patients with small cell lung cancer, results from an NCI-supported phase 1 clinical trial show. Two phase 2 trials of the combination are planned.
Mortality rates from the most common lung cancer, non-small cell lung cancer (NSCLC), have fallen sharply in the United States in recent years, due primarily to recent advances in treatment, an NCI study shows.
In a study of more than 50,000 veterans with lung cancer, those with mental illness who received mental health treatment—including for substance use—lived substantially longer than those who didn’t participate in such programs.
FDA has granted accelerated approval for selpercatinib (Retevmo) to treat certain patients with thyroid cancer or non-small cell lung cancer whose tumors have RET gene alterations. The drug, which works by blocking the activity of RET proteins, was approved based on the results of the LIBRETTO-001 trial.
Osimertinib (Tagrisso) improves survival in people with non-small cell lung cancer with EGFR mutations, updated clinical trial results show. People treated with osimertinib lived longer than those treated with earlier-generation EGFR-targeted drugs.
A large clinical trial showed that adding the immunotherapy drug durvalumab (Imfinzi) to standard chemotherapy can prolong survival in some people with previously untreated advanced small cell lung cancer.
The investigational drug selpercatinib may benefit patients with lung cancer whose tumors have alterations in the RET gene, including fusions with other genes, according to results from a small clinical trial.
FDA has approved entrectinib (Rozlytrek) for the treatment of children and adults with tumors bearing an NTRK gene fusion. The approval also covers adults with non-small cell lung cancer harboring a ROS1 gene fusion.
Clinical recommendations on who should be screened for lung cancer may need to be reviewed when it comes to African Americans who smoke, findings from a new study suggest.
Use of a multipronged approach within hospitals, including community centers, not only eliminated treatment disparities among black and white patients with early-stage lung cancer, it also improved treatment rates for all patients, results from a new study show.
In everyday medical care, there may be more complications from invasive diagnostic procedures performed after lung cancer screening than has been reported in large studies.
The Lung Cancer Master Protocol, or Lung-MAP, is a precision medicine research study for people with advanced non-small cell lung cancer that has continued to grow after treatment. Patients are assigned to different study drug combinations based on the results of genomic profiling of their tumors.
On December 6, 2018, the Food and Drug Administration (FDA) approved atezolizumab (Tecentriq) in combination with a standard three-drug regimen as an initial treatment for advanced lung cancer that does not have EGFR or ALK mutations.
A new study has identified a potential biomarker of early-stage non–small cell lung cancer (NSCLC). The biomarker, the study’s leaders said, could help diagnose precancerous lung growths and early-stage lung cancers noninvasively and distinguish them from noncancerous growths.
Results from two large clinical trials should cement the value of the drugs brigatinib (Alunbrig) and durvalumab (Imfinzi) in treating non-small cell lung cancer (NSCLC). The trial results, several experts said, confirm that the drugs can improve the outcomes of patients with advanced NSCLC.
Cancer researchers have trained a computer program to scan images of tissue samples to differentiate normal lung tissue from the two most common forms of lung cancer. The program also learned to detect cancer-related genetic mutations in the samples.
- Introduction
- Conclusions
- Article Information
eTable 1A. Age- and Sex-Specific Number of Cancer Survivors (Patients Diagnosed With Any Invasive Cancer) at the End of Each Year From 2010 to 2019, Based on the Linkage of TCR, TCOD, and NHIRD
eTable 1B. Age- and Sex-Specific Number of Cancer Survivors at the End of Each Year From 2010 to 2019 in Taiwan, by Year of Diagnosis for Each Year From 1979 to 1988
eTable 1C. Age- and Sex-Specific Cancer Prevalence at Midyear of Each of the 9 Years From 2011 to 2019
eTable 2. Age- and Sex-Specific Midyear Population Size for 2011 to 2019 in Taiwan According to the Monthly Bulletin of Interior Statistics
eTable 3. Age- and Sex-Specific Cancer-Free Midyear Population Size in Taiwan From 2011 to 2019
eTable 4. Age- and Sex-Specific Smoking Rate for Each Year From 2011 to 2019
eTable 5. Midyear Age-Specific Number of Cancer-Free Individuals by Sex and Smoking Status for Each Year From 2011 to 2019
eTable 6. Age- and Sex-Specific Number of Patients With Invasive Lung ADC Diagnosed for Each Year From 2011 to 2019 According to the TCR
eTable 7A. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Late-Stage (Stage 2-4) ADC Among Patients With All Stages (1-4), Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 7B. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Late-Stage (2-4) ADC in Taiwan
eTable 7C. Age-, Sex-, and Year-Specific Percentages of Ever-Smokers Among Corresponding Patients With Early-Stage (1) ADC in Taiwan
eTable 7D. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Stage 3-4 ADC Among Patients With All Stages (1-4) of Invasive ADC, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 7E. Age-, Sex-, and Year-Specific Percentages of Ever-Smokers Among Corresponding Patients With Stage 3-4 ADC in Taiwan
eTable 8A. Estimated Age- and Sex-Specific Number of Patients With Late-Stage Lung ADC in the TCR, by Smoking Status
eTable 8B. Estimated Age- and Sex-Specific Number of Patients With Early-Stage Lung ADC in the TCR, by Smoking Status
eTable 8C. Estimated Age- and Sex-Specific Number of Patients With Stage 3-4 Lung ADC in the TCR, by Smoking Status
eTable 9. Number of Patients With Invasive Lung ADC in the TCR and TCRLF Having Known Smoking Status (Ever-Smoker or Never-Smoker), a Known Number of Pack-Years Smoked, and a Known Number of Years Since Quitting Smoking, 2011-2019
eTable 10A. Late-Stage ADC Age-Specific Incidence Rates for Each Single Year and for the Period 2011-2019, by Sex and Smoking Status
eTable 10B. Early-Stage ADC Age-Specific Incidence Rates For Each Single Year and for the Period 2011-2019, by Sex and Smoking Status
eTable 10C. Stage 3-4 ADC Age-Specific Incidence Rates for Each Single Year and for the Period 2011-2019, by Sex and Smoking Status
eTable 11A. Age-Specific Late-Stage Lung ADC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 11B. Age-Specific Early-Stage Lung ADC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 11C. Age-Specific Late-Stage 3-4 Lung ADC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 12A. Age-Specific Numbers of Never- and Ever-Smoking Males in the Taiwan Biobank
eTable 12B. Age-Specific Numbers of Never- and Ever-Smoking Females in the Taiwan Biobank
eTable 13. Histology Codes for Lung ADC That Appeared in the TCR for Each Year From 2011 to 2019
eTable 14. Age- and Sex-Specific Number of Patients With Invasive Lung SCC Diagnosed for Each Year From 2011 to 2019 According to the TCR
eTable 15A. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Late-Stage (2-4) SCC Among Patients With All Stages (1-4) of Invasive SCC, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 15B. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Late-Stage (2-4) SCC in Taiwan
eTable 15C. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Early-Stage (1) SCC in Taiwan
eTable 15D. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Stage 3-4 SCC Among Patients With All Stages (1-4) of Invasive SCC, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 15E. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Stage 3-4 SCC in Taiwan
eTable 16A. Estimated Age- and Sex-Specific Number of Patients With Late-Stage Lung SCC in the TCR, by Smoking Status
eTable 16B. Estimated Age- and Sex-Specific Number of Patients With Early-Stage Lung SCC in the TCR, by Smoking Status
eTable 16C. Estimated Age- and Sex-Specific Number of Patients With Stage 3-4 Lung SCC in the TCR, by Smoking Status
eTable 17. Number of Patients With Invasive Lung SCC in the TCR and TCRLF Having Known Smoking Status (Ever-Smoker or Never-Smoker), a Known Number of Pack-Years Smoked, and a Known Number of Years Since Quitting Smoking, 2011-2019
eTable 18A. Age-Specific Incidence Rates of Late-Stage SCC for the Period 2011-2019, by Sex and Smoking Status
eTable 18B. Age-Specific Incidence Rates of Early-Stage SCC for the Period 2011-2019, by Sex and Smoking Status
eTable 18C. Age-Specific Incidence Rates of Stage 3-4 SCC for the Period 2011-2019, by Sex and Smoking Status
eTable 19A. Age-Specific Late-Stage Lung SCC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 19B. Age-Specific Early-Stage Lung SCC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 19C. Age-Specific Late-Stage 3-4 Lung SCC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 20. Histology Codes for Lung SCC That Appeared in the TCR for Each Year From 2011 to 2019
eTable 21. Age- and Sex-Specific Number of Patients With Invasive SCLC Diagnosed From 2011 to 2019 According to the TCR
eTable 22A. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Late-Stage (2-4) SCLC Among Patients With All Stages (1-4) of Invasive SCLC, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 22B. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Late-Stage (2-4) SCLC in Taiwan
eTable 22C. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Early-Stage (1) SCLC in Taiwan
eTable 22D. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Stage 3-4 SCLC Among Patients With All Stages (1-4) of Invasive SCLC, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 22E. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Stage 3-4 SCLC in Taiwan
eTable 23A. Estimated Age- and Sex-Specific Number of Patients With Late-Stage Lung SCLC in the TCR, by Smoking Status
eTable 23B. Estimated Age- and Sex-Specific Number of Patients With Early-Stage Lung SCLC in the TCR, by Smoking Status
eTable 23C. Estimated Age- and Sex-Specific Number of Patients With Stage 3-4 Lung SCLC in the TCR, by Smoking Status
eTable 24. Number of Patients With Invasive Lung SCLC in the TCR and TCRLF Having Known Smoking Status (Ever-Smoker or Never-Smoker), a Known Number of Pack-Years Smoked, and a Known Number of Years Since Quitting Smoking, 2011-2019
eTable 25A. Late-Stage SCLC Age-Specific Incidence Rates for the Period 2011-2019, by Sex and Smoking Status
eTable 25B. Early-Stage SCLC Age-Specific Incidence Rates for the Period 2011-2019, by Sex and Smoking Status
eTable 25C. Stage 3-4 SCLC Age-Specific Incidence Rates for the Period 2011-2019, by Sex and Smoking Status
eTable 26A. Age-Specific Late-Stage Lung SCLC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 26B. Age-Specific Early-Stage Lung SCLC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 26C. Age-Specific Late-Stage 3-4 Lung SCLC Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 27. Histology Codes for Lung SCLC That Appeared in the TCR for Each Year From 2011 to 2019
eTable 28. Age- and Sex-Specific Number of Patients With Invasive Lung Cancer Diagnosed for Each Year From 2011 to 2019 According to the TCR
eTable 29A. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Late-Stage (2-4) Lung Cancer Among All Stages (1-4) of Invasive Cancer, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 29B. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Late-Stage (2-4) Lung Cancer in Taiwan
eTable 29C. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Early-Stage Lung Cancer in Taiwan
eTable 29D. Age-, Sex-, and Calendar Year–Specific Percentages of Patients With Stage 3-4 Lung Cancer Among Patients With All Stages (1-4) of Invasive Lung Cancer, Estimated Using the TCRLF for Each Year From 2011 to 2019
eTable 29E. Age-, Sex-, and Calendar Year–Specific Percentages of Ever-Smokers Among Corresponding Patients With Stage 3-4 Lung Cancer in Taiwan
eTable 30A. Estimated Age- and Sex-Specific Number of Patients With Late-Stage Lung Cancer in the TCR, by Smoking Status
eTable 30B. Estimated Age- and Sex-Specific Number of Patients With Early-Stage Lung Cancer in the TCR, by Smoking Status
eTable 30C. Estimated Age- and Sex-Specific Number of Patients With Stage 3-4 Lung Cancer in the TCR, by Smoking Status
eTable 31. Number of Patients With Invasive Lung Cancer in the TCR and TCRLF Having Known Smoking Status (Ever-Smoker or Never-Smoker), a Known Number of Pack-Years Smoked, and a Known Number of Years Since Quitting Smoking, 2011-2019
eTable 32A. Age-Specific Incidence Rates of Late-Stage Lung Cancer for Each Single Year and for the Period 2011-2019, by Sex and Smoking Status
eTable 32B. Age-Specific Incidence Rates of Early-Stage Lung Cancer for Each Single Year and for the Period 2011-2019, by Sex and Smoking Status
eTable 32C. Age-Specific Incidence Rates of Stage 3-4 Lung Cancer for Each Single Year and for the Period 2011-2019, by Sex and Smoking Status
eTable 33A. Age-Specific Late-Stage Lung Cancer Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 33B. Age-Specific Early-Stage Lung Cancer Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eTable 33C. Age-Specific Late-Stage 3-4 Lung Cancer Incidence Rate Ratios and Their 95% CIs Comparing Sex, Smoking Status, and Periods
eReferences
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Chien L , Jiang H , Tsai F, et al. Incidence of Lung Adenocarcinoma by Age, Sex, and Smoking Status in Taiwan. JAMA Netw Open. 2023;6(11):e2340704. doi:10.1001/jamanetworkopen.2023.40704
Manage citations:
© 2024
- Permissions
Incidence of Lung Adenocarcinoma by Age, Sex, and Smoking Status in Taiwan
- 1 Institute of Population Health Sciences, National Health Research Institutes, Zhunan, Taiwan
- 2 Department of Applied Mathematics, Chung-Yuan Christian University, Chung-Li, Taiwan
- 3 National Institute of Cancer Research, National Health Research Institutes, Zhunan, Taiwan
- 4 Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland
Question Do women have higher lung adenocarcinoma (ADC) rates than men in Taiwan regardless of smoking status?
Findings In this cohort study of 61 285 patients with lung ADC, incidence of lung ADC was significantly higher among females than among males irrespective of age, tumor stage, and smoking status.
Meaning The findings suggest a possible difference in lung ADC incidence by sex; there is a need to determine whether this is replicated in other populations and to evaluate the etiologic and translational implications.
Importance Knowing whether the effects of smoking and other risk factors with lung adenocarcinoma (ADC) incidence varies by sex would provide information on lung cancer prevention strategies.
Objective To evaluate whether women in Taiwan have higher age- and tumor stage–specific lung ADC incidence rates than men irrespective of smoking status (ie, ever smoker or never smoker).
Design, Setting, and Participants This population-based cohort study used data sets synthesized from the Taiwan Cancer Registry (TCR) from 1979 to 2019; the TCR Long Form (TCRLF) from 2011 to 2019, which provides individual-level smoking and tumor stage information; the Taiwan Cause of Death Database (TCOD) from 1985 to 2019; the National Health Insurance Research Database (NHIRD) from 2000 to 2020; the Monthly Bulletin of Interior Statistics (MBIS) from 2011 to 2019; the National Health Interview Survey from 2001, 2005, 2009, 2013, and 2017; and Taiwan Biobank data from 2008 to 2021. Included patients were aged 40 to 84 years and had any invasive lung cancer from January 1, 2011, to December 31, 2019.
Exposure Smoking status.
Main Outcomes and Measures The main outcomes were age-specific female-to-male incidence rate ratios (IRRs) of lung ADC by smoking status and tumor stage. Linked data from the TCR, TCOD, NHIRD, Taiwan National Health Interview Survey, and MBIS were used to estimate the age- and sex-specific numbers of cancer-free individuals at midyears from 2011 to 2019 by smoking status. Using the TCR and TCRLF, age-, sex-, tumor stage–, and diagnosis year–specific numbers of patients with lung ADC from 2011 to 2019 by smoking status were estimated.
Results A total of 61 285 patients (32 599 women [53.2%]) aged 40 to 84 years (mean [SD] age, 64.66 [10.79] years) in the Taiwanese population of approximately 23 million were diagnosed with invasive lung ADC as their first lifetime cancer between 2011 and 2019. Among smokers, men had higher tobacco use by almost all examined metrics, including nearly twice the mean (SD) number of pack-years smoked (eg, 7.87 [8.30] for men aged 30-34 years vs 4.38 [5.27] for women aged 30-34 years). For 5-year age bands between 40 and 84 years, incidence of lung ADC was significantly higher among females than males for nearly all age groups irrespective of tumor stage and smoking status (eg, for the age group 70-74 years, the female-to-male IRR for late-stage lung ADC among never smokers was 1.38 [95% CI, 1.30-1.50]).
Conclusions and Relevance In this cohort study, women had higher age- and stage-specific lung ADC incidence rates than men in Taiwan for both never and ever smokers, suggesting the possibility of differential exposures between sexes to risk factors other than smoking and the potential modification of ADC risk factors by sex. Further work is needed to determine whether this pattern replicates in other populations, discover the causes of lung ADC, and put preventive measures in place.
Lung cancer is the most common incident cancer among men and the second most common cancer among women worldwide. 1 Historically, lung cancer incidence rates have been higher among men than among women, reflecting higher smoking rates among men. 2 Among ever smokers, men smoke more cigarettes and initiate smoking at an earlier age. 3 , 4 However, emerging studies have observed a convergence of lung cancer incidence rates between sexes. 5 , 6 In some populations, rates have even become higher among young women than among young men. 5 , 6 In Western countries, where the age-specific smoking prevalence in women approached but did not exceed that of men, increasing lung adenocarcinoma (ADC) incidence rates have been observed, especially in women. 2 , 7
Having reviewed risk factors for lung cancer, Fidler-Benaoudia and colleagues 6 mentioned the possibility that women may be at an increased risk of lung cancer compared with men. Indeed, lifelong female never smokers have had slightly higher age-standardized rates of lung cancer than their male counterparts in population cohorts 8 - 11 ; however, the case numbers in these studies were not large enough for age-specific studies. When smokers were compared with never smokers, some studies reported a higher relative risk for smoking females than for smoking males, while others found no significant difference. 11 - 14 All these reports prompt the study of age- and sex-specific lung cancer incidence rates by smoking status.
In fact, such study of incidence rates is especially desirable and timely in East Asia in view of the recent observations that in East Asia, there exists a nearly simultaneous decrease in smoking rates, extensive introduction of low-dose computed tomography (LDCT) lung cancer screening, and a shift in lung cancer stage at diagnosis. 15 - 20 These studies pointed out overdiagnosis due to LDCT lung cancer screening, 15 , 18 an increase in incidence rates in females, 18 and the relevance of the subtype ADC in this context. 18 These findings reinforce the importance of the study of sex differences in lung ADC incidence and overdiagnosis by smoking status. To address some of these problems, we examined age-, sex-, and stage-specific lung ADC incidence rates by smoking status (ever or never smoker) in Taiwan.
This population-based ecological cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline and was approved by the institutional review board of Taiwan’s National Health Research Institutes, which waived informed consent because all the data sets used were provided to us after deidentification. The study conformed to the Declaration of Helsinki provisions. 21 All the analyses were performed in a secured area of the Data Science Center, Ministry of Health and Welfare, Taiwan.
We used data from 2011 to 2019 from the Taiwan Cancer Registry (TCR), including the long form (TCRLF), which collected individual-level smoking information for each patient with lung cancer starting in 2011; the Taiwan National Health Interview Survey (NHIS), which estimated age- and sex-specific ever smoking rates for 2001, 2005, 2009, 2013, and 2017; and the Taiwan Monthly Bulletin of Interior Statistics (MBIS), which reports age- and sex-specific population size. Because of case numbers, we report results for lung ADC more completely than for lung squamous cell carcinoma (SCC) and small cell lung cancer (SCLC) and excluded other histologic subtypes. Based on the NHIS, we estimated age- and sex-specific smoking rates in the Taiwanese population for each year between January 1, 2011, and December 31, 2019.
Linking the TCR from 1979 to 2019, the Taiwan Cause of Death Database (TCOD) from 1985 to 2019, and the National Health Insurance Research Database (NHIRD) from 2000 to 2020, we estimated the age- and sex-specific numbers of cancer survivors (patients diagnosed with any invasive cancer) in Taiwan at each midyear from January 1, 2011, to December 31, 2019; in general, an individual in the NHIRD at a certain time point, in the TCR before that time point, and not in the TCOD before that time point was considered a cancer survivor at that time point. Subtracting these individuals from the corresponding age- and sex-specific midyear population size reported in the MBIS and then multiplying by the corresponding smoking rates derived from the NHIS resulted in the age- and sex-specific numbers of cancer-free individuals for each midyear from 2011 to 2019 by smoking status. These data provided the person-years for estimating age-, sex-, and stage-specific lung ADC incidence rates by smoking status. The data sets, procedures, and estimates are detailed in the eMethods and eTables 1A to 5 in Supplement 1 .
The quality of the TCR and TCRLF is high in terms of completeness, timeliness, and accuracy. 22 , 23 The quality of the TCOD has been described elsewhere. 24 The NHIRD is a valuable research resource in population health studies. 25 Some of us have used the linkage of these 3 data sets in epidemiologic studies of cancer 26 , 27 and in the prediction of lung cancer risk among ever smokers. 28 The study design and quality of the NHIS was reported previously. 29 , 30
Given a combination of age, sex, and diagnosis year, we identified the set of patients whose first invasive cancer was lung ADC and who were included in the TCR specified by the combination. Restricting this set to the subset of patients included in the TCRLF, we estimated the percentage of patients with late-stage (stages 2-4) lung ADC among all patients with stages 1 to 4 invasive lung ADC for the combination. We also used the TCRLF to estimate the percentage of ever smokers among the subset of patients with invasive lung ADC specified by each age, sex, stage, and diagnosis year combination. Combining all these data resulted in estimated age-, sex-, stage-, and diagnosis year–specific numbers of patients with lung ADC in the TCR by smoking status (detailed in the eMethods and eTables 6 to 8C in Supplement 1 ). For comparison, we also studied patients diagnosed with stage 3 or 4 lung ADC (eTable 8C in Supplement 1 ). eTable 9 in Supplement 1 reports the number of patients with invasive lung ADC in the TCR with individual-level smoking information from 2011 to 2019 using the TCRLF.
We used the estimated age-, sex-, and diagnosis year–specific numbers of patients with late-stage or early-stage lung ADC by smoking status (reported in eTables 8A and 8B in Supplement 1 ) and the corresponding person-years (reported in eTable 5 in Supplement 1 ) to obtain the age-, sex-, and stage-specific lung ADC incidence rates by smoking status and period (2011-2015, 2016-2019, and 2011-2019), shown in eTables 10A to 10C in Supplement 1 for late-stage, early-stage, and stages 3 and 4, respectively. We also obtained the incidence rate ratios (IRRs) comparing incidence rates between sexes by smoking status and period, between ever and never smokers by sex and period, and between periods by sex and smoking status, shown in eTables 11A to 11C in Supplement 1 for late-stage, early-stage, and stages 3 and 4, respectively. All 95% CIs were the intervals between the 2.5th percentiles and the 97.5th percentiles estimated using bootstrapping methods. 31 The estimation procedures are detailed in the eMethods in Supplement 1 and were implemented using the boot package in R, version 4.3.0 (R Project for Statistical Computing). We sometimes used the binomial distribution to report a 95% CI or did not report a 95% CI when the estimand was too small; situations such as these are noted in the relevant tables. The data analyses were performed between March 1 and September 9, 2023.
We used the Taiwan Biobank from 2008 to 2021, including a total of 132 720 participants, to compare smoking exposures between sexes, including number of pack-years smoked, number of cigarettes smoked per day, number of years smoked, and years since cessation (eMethods and eTables 12A and 12B in Supplement 1 ). eTable 13 in Supplement 1 reports codes for lung ADC. For privacy protection consideration, the Data Science Center did not allow us to bring the number of patients specified by an age, sex, stage, histologic subtype, and smoking status combination out of the center for subsequent analyses if that number was between 1 and 4. This was the main reason that we limited our study to ADC, SCC, and SCLC and considered only age groups 40 to 64 years and 65 to 84 years for some results about early-stage SCC and SCLC.
With this in mind, we prepared corresponding tables for SCC and SCLC. Specifically, eTables 14 to 20 in Supplement 1 are the SCC counterparts of eTables 6 to 11 and 13 for ADC; eTables 21 to 27 in Supplement 1 are the SCLC counterparts. We also prepared corresponding eTables 28 to 33C in Supplement 1 for lung cancer including all the subtypes.
From 2011 to 2019, a total of 61 285 patients (28 686 men [46.8%]; 32 599 women [53.2%]) aged 40 to 84 years (mean [SD] age, 64.66 [10.79] years) were diagnosed with invasive lung ADC as their first cancer in the Taiwanese population of approximately 23 million. Table 1 reports the number of person-years and the number of patients with invasive lung ADC in this population from 2011 to 2019 by age, sex, stage (late or early), and smoking status, derived from eTables 5, 8A, and 8B in Supplement 1 . Table 1 also includes similar information for SCC, SCLC, and lung cancer overall, derived from eTables 16A, 16B, 23A, and 23B in Supplement 1 . Table 1 indicates that over 96.5 million person-years and 61 285 patients with invasive lung ADC were used for rate computation. Among them, an estimated 30 570 patients were female never smokers, 9893 were male never smokers, 2029 were female ever smokers, and 18 793 were male ever smokers. Among smokers, men had higher tobacco use by almost all examined metrics, including nearly twice the mean (SD) number of pack-years smoked (eg, 7.87 [8.30] for men aged 30-34 years vs 4.38 [5.27] for women aged 30-34 years) (eTables 12A and 12B in Supplement 1 ).
Based on eTables 10A, 10B, 18A, 18B, 25A, and 25B in Supplement 1 , we report age-specific incidence rates and their 95% CIs by age, histologic subtype, sex, stage, smoking status, and period (2011-2015 or 2016-2019) in Table 2 . Single-year incidence rates and other related information for lung ADC are presented in eTables 10A to 10C in Supplement 1 . Table 2 shows that females had higher lung ADC rates than males by age, stage, and smoking status except for groups older than 75 years and compares incidence rates across age, sex, stage, smoking status, and periods. For example, for the age group 70 to 74 years in the period 2016-2019, the incidence of late-stage lung ADC for female never smokers was 99.53 (95% CI, 94.33-104.91) and for male never smokers was 65.73 (95% CI, 60.01-72.13).
Table 3 reports age-specific female-to-male IRRs from 2011 to 2019 by smoking status and stage. For any given smoking status and stage combination, age-specific female-to-male IRRs for ADC were higher than 1, with nearly all 95% CIs higher than 1 (eg, for the age group 70-74 years, the female-to-male IRR for late-stage lung ADC was 1.38 [95% CI, 1.30-1.50] for never smokers and 1.26 [95% CI, 1.07-1.50] for ever smokers).
These results imply that never-smoking females had a higher rate of invasive lung ADC than their male counterparts for most combinations. Findings were similar for ever smokers because men had higher age-specific smoking exposure by nearly all examined metrics, including number of pack-years smoked, number of cigarettes smoked per day, number of years smoked, age at smoking initiation, and number of years since cessation. Only for the age groups 55 to 59 and 60 to 64 years had men experienced slightly longer times since cessation; details are provided in eTables 12A and 12B and the eMethods in Supplement 1 .
Table 3 shows that almost all never- and ever-smoking females had lower rates of late-stage SCC and SCLC than their male counterparts. For early-stage SCC, never-smoking females older than 60 years also had lower rates than their male counterparts. No such statements can be made for early-stage SCLC because of small case numbers.
Table 4 reports age-specific IRRs comparing ever smokers with never smokers from 2011 to 2019 by histologic subtype, sex, and stage. The IRRs were higher than 1 for late-stage lung ADC for any given sex and age group combination, but they were not always higher than 1 for early-stage lung ADC among people younger than 60 years. For late-stage SCC and SCLC, these IRRs were higher than 1. For early-stage SCC, they were also higher than 1 among people aged 60 years or older. No similar statements can be made for early-stage SCLC because of small case numbers.
Table 5 reports age-specific IRRs for 2016 to 2019 vs 2011 to 2015 by histologic subtype, stage, smoking status, and sex. For late-stage lung ADC among never smokers, age- and sex-specific incidence rates were slightly lower from 2016 to 2019 than from 2011 to 2015; for late-stage lung ADC among ever smokers, the IRRs were slightly higher than 1 for most of the age groups. For early-stage lung ADC, both the lower and upper bounds of the 95% CIs for nearly all of the IRRs were higher than 1, indicating significantly higher incidence rates from 2016 to 2019 than from 2011 to 2015. For late-stage SCC and SCLC, there were few differences between the rates in these 2 periods. For early-stage SCC and SCLC, there seemed to be no support that the later period had higher incidence rates, mainly due to their small case numbers. The results for stages 3 and 4 ADC (eTables 10C and 11C in Supplement 1 ), SCC (eTables 18C and 19C in Supplement 1 ), SCLC (eTables 25C and 26C in Supplement 1 ), and lung cancer (eTables 32C and 33C in Supplement 1 ) were similar to the respective results for late-stage disease.
Based on a large Taiwanese population study with individual-level smoking information, we studied age-, sex-, stage-, and diagnosis year–specific incidence rates of lung ADC by smoking status from 2011 to 2019, a period in which LDCT lung cancer screening was not reimbursed by the National Health Insurance Program but was available commercially. 15 , 32 , 33 We found that although smoking was generally more common among males, higher incidence rates of stage-specific lung ADC in females were observed among both never and ever smokers, and IRRs comparing ever smokers with never smokers were higher than 1 for late-stage lung ADC but not always for early-stage lung ADC among younger people.
According to Gao and colleagues, 15 we may assume that LDCT lung cancer screening in Taiwan increased from the 2011 to 2015 period to the 2016 to 2019 period. This assumption is in line with the higher age- and sex-specific incidence rates that we found from 2016 to 2019 than from 2011 to 2015 for early-stage lung ADC. These rates may indicate overdiagnosis if we assume that the distributions of risk factors for ADC in Taiwan varied little with calendar year in this period. The observation that lung cancer detected by LDCT is mostly in an early stage 34 , 35 and the finding that the rates of late-stage lung ADC from 2016 to 2019 were at most slightly larger than those from 2011 to 2015 jointly suggest that most late-stage lung ADCs were not detected by screening or incidentally.
Thus, the finding that females had higher incidence rates of late-stage lung ADC than males calls for other explanations, which might be attributed to a combination of genetic, hormonal, and societal factors. Because Taiwan’s National Health Insurance Program reduces the impact of accessibility, we first suggest the possibility of differential exposure to risk factors other than tobacco smoking (eg, home cooking fumes, environmental tobacco smoke 36 ) and the potential effect modification of ADC risk factors by sex. 37 We may also consider the sex differences in comorbidity before ADC diagnosis. 27
In view of a US report that women had approximately twice as large an autopsy reservoir of indolent lesions of the lung as men 38 (which nonetheless meet the criteria for lung cancer) and a Japanese report that never-smoking women had approximately twice as large an LDCT lung cancer detection rate as men, 39 the current study provides support that women may be more susceptible to late-stage lung ADC. That some of the IRRs when comparing periods were higher than 1 for late-stage lung ADC among ever smokers deserves further study to see whether these findings were also due to the wide availability of LDCT lung cancer screening.
This study’s finding that some of the IRRs for ever vs never smokers for early-stage lung ADC were less than 1 among people younger than 60 years deserves attention. These findings might be an artifact of low accumulated tobacco exposure among younger people, the existence of risk factors other than tobacco smoking, and low incidence rates among younger people, among other factors.
Our finding that age-specific IRRs for late-stage lung ADC comparing ever smokers with never smokers were similar between sexes seems to be in line with findings of Freedman and colleagues 13 and suggests that the relative risk of lung ADC among current smokers vs never smokers is similar in women and men. However, the IRRs for late-stage SCLC were larger among older females, which is an indication for more in-depth study. 13
A recent report on the overdiagnosis of lung cancer associated with LDCT lung cancer screening in East Asia emphasizes that LDCT screening should be restricted to those at highest risk while rigorously maintaining adherence to growth assessment protocols. 19 These suggestions are valid in general, but the overdiagnosis reported in the current study concerns particularly early-stage invasive lung ADC and prompts a comparison of the prognosis of early-stage ADC detected by screening with that detected by symptoms. 17
The focus of this study was on lung ADC because of its large burden worldwide and its large number of cases in this study. For late-stage SCC and SCLC, this study found that males had higher rates than females irrespective of smoking status; because men had higher smoking exposure, the results regarding never smokers are more compelling. For early-stage SCC and SCLC, the case numbers were not large enough to draw definitive conclusions.
The main strength of this study is that it was a large population study with the availability of individual-level smoking information for 92% of all patients diagnosed with lung ADC from 2011 to 2019 in Taiwan. The number of never-smoking patients with invasive lung ADC was larger than in other studies in the literature. 8 - 11
A limitation of this study is its short study period. Although the excess early-stage incidence rates for lung ADC suggest some overdiagnosis, we may need a longer period to see if late-stage incidence rates decrease and if there is a sex difference in overdiagnosis. Another limitation resulted from the large error involved in the estimates of age-specific female smoking rates due to the low incidence of smoking among females, especially in older age groups.
This cohort study found higher age- and stage-specific lung ADC incidence rates among women than men in Taiwan for both never and ever smokers, suggesting the possibility of differential exposures between sexes to risk factors other than smoking and potential modification of ADC risk factors by sex. Further work is needed to determine whether the sex differences in incidence of lung ADC replicate in other populations and other geographic regions. 40 , 41 Given the large number of patients with invasive lung ADC among never smokers in this population and worldwide, there is urgency to discover the causes of this tumor and put preventive measures in place.
Accepted for Publication: September 19, 2023.
Published: November 1, 2023. doi:10.1001/jamanetworkopen.2023.40704
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Chien LH et al. JAMA Network Open .
Corresponding Author: I-Shou Chang, PhD, National Institute of Cancer Research, National Health Research Institutes, 35 Keyan Rd, Zhunan, Miaoli, Taiwan ( [email protected] ).
Author Contributions: Prof Hsiung and Dr I.-S. Chang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Lan, Prof Hsiung, and Dr I.-S. Chang contributed equally to this work.
Concept and design: Chien, Freedman, Lan, Hsiung, I.-S. Chang.
Acquisition, analysis, or interpretation of data: Chien, Jiang, Tsai, H.-Y. Chang, Rothman, Lan, Hsiung, I.-S. Chang.
Drafting of the manuscript: Chien, Rothman, Lan, I.-S. Chang.
Critical review of the manuscript for important intellectual content: Chien, Jiang, Tsai, H.-Y. Chang, Freedman, Lan, Hsiung, I.-S. Chang.
Statistical analysis: Chien, Jiang, Tsai, Rothman, Lan, I.-S. Chang.
Obtained funding: Lan, Hsiung, I.-S. Chang.
Administrative, technical, or material support: H.-Y. Chang, Hsiung, I.-S. Chang.
Supervision: Lan, Hsiung, I.-S. Chang.
Conflict of Interest Disclosures: Prof Hsiung reported receiving grants from Taiwan’s National Health Research Institutes during the conduct of the study. Dr I.-S. Chang reported receiving grants from the Ministry of Health and Welfare (MOHW), Taiwan, during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was supported by project grants MOHW103-TDU-212-114001, MOHW104-TDU-B-212-124-008, MOHW105-TDU-B-212-134013, MOHW106-TDU-B-212-144013, MOHW107-TDU-B-212-114026A, MOHW108-TDU-B-212-124026, MOHW109-TDU-B-212-134026, MOHW110-TDU-B-212-144026, and NHRI-PH-110-GP-01 from the MOHW, Taiwan (Prof Hsiung and Dr I.-S. Chang), and by the intramural research program of the National Cancer Institute, US National Institutes of Health (Drs Rothman and Lan).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: Chia-Yu Chen, MS, and Hsiao-Han Hung, MS, National Health Research Institutes, Taiwan, prepared eTables 1A to 1C and 12A and 12B, respectively, in Supplement 1 . They did not receive compensation.
Additional Information: The data sets of the Taiwan Cancer Registry, Taiwan Cause of Death Database, and National Health Insurance Research Database analyzed in this study were provided by and analyzed in the Data Science Center, MOHW, Taiwan.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Smoking and the Risk of Developing Lung Cancer
This paper delves into the evidence-based practice problem encapsulated in the pivotal question: “Among adult smokers, do ongoing smoking cessation strategies and interventions reduce the prevalence of lung cancer within five years of continuous follow-up?” This is a significant problem, given its damaging influence on the nation and economy through high healthcare costs impact besides low output. With many options for smoking cessation, including cognitive-behavioural therapy to pharmacological intervention, offering some hope, this work investigates the ins and outs of solving the problem.
Evidence-based Practice Problem Discussion
The chosen problem is that cigarette smoking leads to a high prevalence of lung cancer. Statistics show that the risk associated with active smoking is stupendous. The latter implies that individuals who smoke cigarettes and tobacco are at least 15-30 times more likely to contract lung cancer or even die from it compared to nonsmokers (Al Achkar et al., 2020). This risk is further underscored by estimates demonstrating that smoking causes approximately 90% of lung cancer cases in men and about 80 %of those in women (Al Achkar et al.,2020).
Significance of the Selected Evidence-Based Practice Problem
The impact of the evidence-based practice problem is highlighted by disturbing statistics and potential positive results emanating from appropriate intervention. Globally, over one million people die of lung cancer each year, demonstrating the life-threatening consequences (Gourlay et al., 2021). This issue is a private health problem and a social and economic burden. Without the appropriate interventions, individuals and their families suffer from the increased cost of medication. Costs associated with these diseases may precipitate poverty and poor productivity. Further, the social implication is far-reaching, affecting families and communities.
Addressing this problem can be seen in the positive results that follow interventions. Various smoking cessation interventions such as cognitive behavioural therapy, motivational interviewing, support groups and pharmacological aids have proved to be effective in minimizing the number of relapses as well as reducing emerging cases of lung cancer. With efficient interventions, the pressure off healthcare systems can be reduced while individuals’ quality of life increases and provide a complete elimination from lung cancer-related mortality rates. With thorough management of the smoking epidemic, we can envision tomorrow filled with lower cases of lung cancer, healthy populations and a lighter burden on healthcare facilities.

PICOT Question Discussion
The PICOT question is formulated as follows:
“Among adult smokers (P), do ongoing smoking cessation strategies and interventions (I) compared to no intervention (C) reduce the prevalence of lung cancer (O) within five years of continuous follow-up (T)?”
The PICOT question developed for the evidence-based practice problem of the association between cigarette smoking and the increased risk of lung cancer is as follows:
Population (P)
This selection is composed of adult smokers who are more prone to suffer from lung cancer.
Intervention (I)
The different treatment options studied represent various interventions; these include cognitive-behavioural therapy, motivational interviewing support groups, face-to-face therapists and health education campaigns. Other pharmacological aids that include nicotine replacement therapies (nasal sprays, oral inhalers, gum) and drugs incl, including bupropion SR varenicline, are also considered.
Comparison (C)
The comparison is based on assessing current smoking cessation strategies and interventions versus no intervention. This makes it possible to evaluate the performance of such approaches in lowering lung cancer prevalence.
Outcome (O)
The targeted outcome is the reduction in lung cancer prevalence among the adult smoking population after five years of continuous follow-up.
Timeframe (T)
The course of the interventions, especially cognitive therapy sessions, is critical for evaluating their long-term efficiencies in lowering relapses and avoiding the progression of smoking behaviour.
The three Articles Selected
Kotz et al., 2020: smoking cessation attempts and methods in germany.
Smoking cessation trials are sparsely mentioned in this study, as only 19.0% of smokers undertake annual attempts to stop smoking. Most importantly, only 13.0% of these attempts used evidence-based methods. The findings highlight a significant gap in the use of effective smoking cessation techniques among smokers (Kotz et al., 2020b). This directly connects to the PICOT question, indicating that one needs to compare ongoing smoking cessation interventions with no intervention for decreasing lung cancer prevalence in a specified time.
Moldovanu et al., 2021: Lung Cancer Screening and Smoking Cessation Efforts
The paper identifies the connection between lung cancer screening and smoking cessation. It shows different termination rates in screening trials, indicating the intricacy of getting positive results (Moldovanu et al., 2021b). The finding that not all screening results conclusively affected smoking behaviour also highlights the importance of evaluating such interventions further in contingent with lung cancer screening.
de Ruiter et al., 2022: Smoking Cessation Training and Treatment in Cancer Centers
The paper discusses the importance of smoking cessation training and treatment in cancer centres. It shows that cancer survivors, especially smokers, do not know the effect of smoking on treatment outcomes (de Ruiter et al., 2022b). The 5 A’s model in the study stresses patient readiness to quit smoking and provides evidence-based treatment.
Search Strategy
To find the three articles, I used a rigorous search method on PubMed with pertinent keywords and criteria. The main terms were “smoking cessation,” “lung cancer screening,” and“cancer centre smoking cessation.” These. The search was initially designed to be pretty broad so as not to miss articles detailing the connection between smoking cessation and lung cancer. To make the search more precise, I used special filters in PubMed that limited publication dates of articles to those from five years ago. This temporal limitation was to ensure that the chosen articles were current and provided insight into recent trends in research. Besides, I chose English articles to satisfy the language requirements.
The search strategy included critically evaluating the relevance of each article title, abstract and keywords. Studies that specifically addressed the effectiveness of current smoking cessation programs and their relation to lung cancer incidence or adaptation in cancer centres were given priority.
In conclusion, the study of smoking cessation approaches and their relationship with lung cancer rates reveals a complex picture. Considering the general theme addressed by each of these articles, it is evident that smoking cessation among adults calls for immediate interventions to prevent lung cancer. The statistics elaborate on smoking prevalence, utilization of evidence-based methods and socioeconomic features controlling cessation efforts. The importance of the problem in evidence-based practice is that it could be used to relieve pressure on health systems and increase overall wellness by slowing down smoking-related lung cancer rates. The interventions discussed, including cognitive behavioural therapy and pharmacological aid, highlight the various strategies associated with smoking cessation. However, without such interventions, repercussions affect individuals, societal health and economic factors.
Al Achkar, M., Marchand, L., Thompson, M., Chow, L. Q., Revere, D., & Baldwin, L. (2020). Unmet needs and opportunities for improving care for patients with advanced lung cancer on targeted therapies: A qualitative study. BMJ Open , 10 (3), e032639. https://doi.org/10.1136/bmjopen-2019-032639
De Ruiter, W. K., Barker, M., Rahimi, A., Ivanova, A., Zawertailo, L., Melamed, O. C., & Selby, P. (2022b). Smoking cessation training and treatment: options for cancer centres. Current Oncology, 29(4), 2252–2262. https://doi.org/10.3390/curroncol29040183
Gourlay, E., Atikins, A., & Grundy, S. (2021). Should patients with an unknown smoking status be routinely invited for targeted lung health checks? Lung Cancer , 156 , S24. https://doi.org/10.1016/s0169-5002(21)00257-9
Kotz, D., Batra, A., & Kastaun, S. (2020b). Smoking cessation attempts and common strategies employed. Deutsches Arzteblatt International . https://doi.org/10.3238/arztebl.2020.0007
Moldovanu, D., De Koning, H. J., & Van Der Aalst, C. M. (2021b). Lung cancer screening and smoking cessation efforts. Translational Lung Cancer Research , 10 (2), 1099–1109. https://doi.org/10.21037/tlcr-20-899
Cite This Work
To export a reference to this article please select a referencing style below:
Related Essays
Gender soccer equal pay, nursing assignment: evaluating health information, the river city youth basketball tournament, cannabis for pain management, advanced levels of clinical inquiry and systematic reviews, which wound care interventions are effective in improving wound healing among clients with diabetic foot ulcers, popular essay topics.
- American Dream
- Artificial Intelligence
- Black Lives Matter
- Bullying Essay
- Career Goals Essay
- Causes of the Civil War
- Child Abusing
- Civil Rights Movement
- Community Service
- Cultural Identity
- Cyber Bullying
- Death Penalty
- Depression Essay
- Domestic Violence
- Freedom of Speech
- Global Warming
- Gun Control
- Human Trafficking
- I Believe Essay
- Immigration
- Importance of Education
- Israel and Palestine Conflict
- Leadership Essay
- Legalizing Marijuanas
- Mental Health
- National Honor Society
- Police Brutality
- Pollution Essay
- Racism Essay
- Romeo and Juliet
- Same Sex Marriages
- Social Media
- The Great Gatsby
- The Yellow Wallpaper
- Time Management
- To Kill a Mockingbird
- Violent Video Games
- What Makes You Unique
- Why I Want to Be a Nurse
- Send us an e-mail
Essay Service Examples Health Lung Cancer
Lung Cancer and Smoking: Analytical Essay
Introduction
- Proper editing and formatting
- Free revision, title page, and bibliography
- Flexible prices and money-back guarantee
Lung cancer risk factors in never-smoking women
Genetic profiling in lung cancer, the association between human papilloma virus and lung cancer, areas of research.
Our writers will provide you with an essay sample written from scratch: any topic, any deadline, any instructions.
Cite this paper
Related essay topics.
Get your paper done in as fast as 3 hours, 24/7.
Related articles

Most popular essays
- Artificial Intelligence
- Lung Cancer
In recent years, AI has gradually entered the medical field. Artificial intelligence continues to...
Natural resources are simply the naturally occurring of resources where. People use these natural...
As a point of departure, to understand how a tumour appears in our lungs we have to know how is...
Image processing techniques are widely used in several medical problems for image enhancement in...
- Effects of Technology
The mortality rate due to lung cancer is increasing rapidly day by day. The major reason behind...
Lung cancer is one of the most increasing diseases in the rapidly changing world. This disease can...
Cancer is the disease that results when cellular changes cause the uncontrolled growth of cells....
Lung cancer has become the most prevalent and threatening cancer worldwide. Parallel to most...
Lung cancer is one of the most serious cancers in the world, with the smallest survival rate after...
Join our 150k of happy users
- Get original paper written according to your instructions
- Save time for what matters most
Fair Use Policy
EduBirdie considers academic integrity to be the essential part of the learning process and does not support any violation of the academic standards. Should you have any questions regarding our Fair Use Policy or become aware of any violations, please do not hesitate to contact us via [email protected].
We are here 24/7 to write your paper in as fast as 3 hours.
Provide your email, and we'll send you this sample!
By providing your email, you agree to our Terms & Conditions and Privacy Policy .
Say goodbye to copy-pasting!
Get custom-crafted papers for you.
Enter your email, and we'll promptly send you the full essay. No need to copy piece by piece. It's in your inbox!
- News and Features
- Conferences
- Clinical Tools
- Special Collections
Lung Cancer Statistics: Understanding the Numbers for Informed Care

In 2024, approximately 235,000 people in the United States will be diagnosed with lung cancer, and 125,000 will die from the disease. 1,2 In light of the tremendous impact lung cancer has on patients, families, and the health care system, this article provides health care professionals with the most recent lung cancer statistics to inform their practice. It covers the 10 most important lung cancer statistics for clinicians to know, as well as historical trends in lung cancer statistics, factors that affect the incidence and mortality of lung cancer, and resources for patient education.
Lung Cancer in the United States: Top 10 Statistics
- 1. Of the estimated 234,580 new cases of lung cancer that will be diagnosed in 2024, 116,310 will occur in men and 118,270 in women. 3
- 2. Of the estimated 125,070 lung cancer deaths that will occur in 2024, 65,790 will be men and 59,280 will be women. 3
- 3. Each day, an estimated 340 people die from lung cancer. This is almost 2.5 times as many people who die from colorectal cancer, which is the second most common cause of cancer death. 3
- 4. Approximately 101,300 of the estimated 125,070 lung cancer deaths (81%) in 2024 will be directly caused by smoking. 3
- 5. Lung cancer is the leading cause of cancer death in men and women age 50 years and older. 3
- 6. The overall 5-year relative survival rate for lung cancer is 25%, but it is lower for men (21%) than for women (30%). 1
- 7. In 2021, an estimated 610,816 people in the United States were living with lung cancer. 2
- 8. As a result of earlier detection and advances in treatment, lung cancer mortality rates have been decreasing. The declines in lung cancer mortality rates accelerated from 2% per year during 2005 to 2013 to 4% per year during 2013 to 2021. 3
- 9. The 3-year relative survival rate for patients with non-small cell lung cancer (NSCLC) increased from 26% in 2004 to 40% in 2017, compared with an increase of 9% to 13% in individuals small cell lung cancer. 3
- 10. Although screening has reduced lung cancer mortality in high-risk individuals by 16% to 24%, in 2020, only approximately 6% of the 14.2 million individuals who met the criteria received screening. 3
Historical Trends in Lung Cancer Statistics
The National Institute of Health’s Surveillance, Epidemiology, and End Results Program (SEER) tracks the rates of cancer incidence and survival in the United States; it groups lung and bronchus cancer as a single category. According to SEER data, the rate of new cases of lung and bronchus cancer was 49.0 per 100,000 people per year, based on 2017-2021 cases, age-adjusted. 2 The death rate was 32.4 per 100,000 people per year, based on 2018-2022 deaths, age-adjusted. 2 Based on 2017-2019 data, approximately 6.1% of people will be diagnosed with lung and bronchus cancer at some point in their life. 2 Based on 2014-2020 data, the 5-year relative survival rate for lung and bronchus cancer was 26.7%. 2 From 2012 to 2021, the age-adjusted rates for new lung and bronchus cancer cases fell 2.0% each year on average. From 2013 to 2022, age-adjusted death rates fell an average of 4.1% each year. 2
Lung cancer is more common in men than in women, particularly among individuals who are non-Hispanic Black. 2 The incidence of lung cancer has been on the decline in men since the mid-1980s but in women only since the mid-2000s. 1 This is the result of differences between sexes in historical patterns of smoking and smoking cessation. The rate of decline in lung cancer incidence has been twice as high significant in men (2.5% per year) as in women (1% per year). 1
Mostly due to reductions in smoking, mortality rates for lung cancer have declined by 59% since 1990 in men and by 36% since 2002 in women. 1 These declines have also been accelerated by advances in treatment for NSCLC and earlier detection. From 2017 through 2021, the death rate declined by approximately 4% per year. 1
Ethnic and Racial Disparities in Lung Cancer
Ethnic and racial disparities in lung cancer reflect differences in the prevalence of certain risk factors due to cultural or other reasons. 1 Smoking is the leading cause of lung cancer. 2 Compared to individuals in other groups, those who are Hispanic or Asian American have lower rates of lung cancer because overall, they are less likely to smoke. 1 The incidence of lung cancer in people who are Asian American or Pacific Islander is approximately one-half that of those who are White. However, due to the high prevalence of smoking in people who are Native Hawaiian, the incidence of lung cancer in these individuals is similar to that of people who are White. 1,2
Socioeconomic Factors and Lung Cancer
Lower socioeconomic status — an approximation based on income, education, and/or health insurance — is associated with higher lung cancer incidence and mortality. 1 In 2015 to 2019, lung cancer mortality rates were 4.6 times higher among men with 12 or fewer years of education than among men with a 4-year college degree. This is because the prevalence of smoking is much higher in individuals with less education: 24% of men without a high school education smoke vs 6% of college graduates. 1
Geographical Variations in Lung Cancer Incidence
The incidence of lung cancer is at least 3 times higher in Kentucky, West Virginia, and Arkansas (75 to 84 per 100,000 persons) than in Utah (25 per 100,000 persons). 3 This is a reflection of the high rates of smoking in those states. In 2021, the prevalence of smoking was highest in West Virginia (24%), Arkansas (22%), and Kentucky, Mississippi, Tennessee, and Louisiana (20%) compared with 7% in Utah and 9% in California and Washington, DC. 3
Lung Cancer Risk Factors
Smoking .
Cigarette smoking is the leading cause of preventable disease and mortality worldwide. 4 There are more 7,000 chemicals in cigarette smoke, at least 69 of which have been linked to cancer. 4 Smoking is the direct cause of an estimated 90% of lung cancer deaths. 4 The risk of developing and dying from lung cancer increases with the quantity and duration of cigarette smoking. Cigar and pipe smoking also increases the risk of lung cancer. 1
Environmental Factors and Occupational Exposures
After smoking, the second most common cause of lung cancer in the United States is exposure to radon gas, which is released from soil and can accumulate in indoor air. 1 Secondhand smoke is the cause of 2.7% of lung cancers, which equates to 6,300 new cases in 2024. 1 Other risk factors include exposure to the following 1 :
- Asbestos (particularly among individuals who smoke);
- Metals such as chromium, cadmium, and arsenic;
- Some organic compounds;
- Radiation;
- Air pollution; and
- Diesel exhaust.
Occupational exposures that increase the risk of developing lung cancer include rubber manufacturing, paving, roofing, painting, and chimney sweeping. 1
Lung Cancer Stages
How lung cancer is staged depends on the type of cancer. Non-small cell lung cancer is most commonly staged using the American Joint Committee on Cancer TNM system. 5 The TNM system using a combination of numbers (0 and I to IV) and letters to evaluate the size and extent of the main tumor (T), spread to nearby lymph nodes (N), and spread (metastasis) to distant sites (M). 5 Lower numbers and earlier letters generally indicate less advanced disease.
The American Cancer Society offers a detailed description of the TNM lung cancer staging system . Small cell lung cancer is typically staged using a 2-stage system as either Limited (cancer is only on 1 side of the chest and can be treated with a single radiation field) or Extensive (cancer has spread widely throughout the lung, to the other lung, or to other parts of the body). 6 The TNM system is also used to stage small cell lung cancer, but TNM staging generally is not as important for this type of cancer. 6
Patient Education on Lung Cancer
Part of the reason lung cancer is a leading cause of cancer death is a failure to detect the disease in its early stages, which may be due to barriers such as patients’ lack of awareness of lung cancer symptoms, misappraisal of symptoms, poor relationship with clinicians, and/or lack of access to health care services. 7 In a review that included 16 studies, Saab et al found that large campaigns increased lung cancer awareness, promoted help-seeking, and resulted in early detection of lung cancer. 7
These researchers also noted that multimodal public health interventions, including educational campaigns, are best suited to detect lung cancer early and reduce barriers to help-seeking. 7 According to guidelines from the American Cancer Society, annual lung cancer screening is recommended for healthy individuals age 50 to 80 years who have a smoking history equal to or greater than 20 pack-years, regardless of how long it has been since they quit smoking. 3
The American Lung Association offers several resources to help people to quit smoking 4 :
- Freedom From Smoking is a smoking cessation program that is available in various formats and includes comprehensive, evidence-based techniques.
- The Lung Helpline and Tobacco Quitline offers free one-on-one telephone counseling from certified tobacco treatment specialists. It is available at 1-800-LUNGUSA (1-800-586-4872), Monday-Friday 7 am to 9 pm and weekends 9 am to 5 pm Central Time.
In 2024, lung cancer will be diagnosed in nearly a quarter of a million people in the United States. Understanding lung cancer statistics and trends is an important tool clinicians can leverage to more effectively diagnose and treat these patients.
Want to read more?
Please login or register first to view this content.
Login Register
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Anniversary
- BMJ Journals
You are here
- Online First
- Smoking history and all-cause, ischaemic heart disease and lung cancer mortality: follow-up study of 358 551 men and women aged 40–43 years
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-2909-5678 Aage Tverdal 1 ,
- http://orcid.org/0000-0002-8331-6363 Randi Selmer 2 ,
- Dag S Thelle 3 , 4
- 1 Centre for Fertility and Health , Norwegian Institute of Public Health , Oslo , Norway
- 2 Department of Mental and Physical Health , Norwegian Institute of Public Health , Oslo , Norway
- 3 Department of Biostatistics , University of Oslo , Oslo , Norway
- 4 Institute of Medicine,School of Public Health and Community Medicien , University of Gothenburg , Goteborg , Sweden
- Correspondence to Dr Aage Tverdal, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo 0213, Norway; aage.tverdal{at}fhi.no
Aims We studied the health consequences of quitting smoking before age 43 by time since quitting, number of years smoked and cigarettes smoked per day. The outcomes were all-cause, ischaemic heart disease and lung cancer mortality.
Design Prospective study.
Setting Norwegian counties.
Participants Men and women aged 40–43 years who participated in a national cardiovascular screening programme and who were followed from 1985 to 2018.
Measurements Self-reports from questionnaire on time since quitting smoking, years smoked and number of cigarettes per day, and measurements of height, weight and blood pressure, and a blood sample where serum was analysed for total serum cholesterol and triglycerides.
Findings The all-cause mortality rate was 30% higher among quitters less than 1 year ago compared with never smokers (adjusted HR=1.30, 95% CI 1.18–1.43 in men and HR=1.31, 95% CI 1.16 to 1.50 in women). Quitters who had smoked longer than 20 years had 23% higher mortality in men (HR=1.23, 95% CI 1.14 to 1.34) and 32% higher mortality in women (HR=1.32, 95% CI 1.18 to 1.49). Past smoking of more than 20 cigarettes/day was associated with HR=1.14 (1.05–1.23) in men and HR=1.16 (1.01–1.32) in women. The HR for lung cancer was 6.77 (95% CI 4.86 to 9.45) for quitting men who had smoked for more than 20 years compared with never smokers. The corresponding figure for women was 5.75 (95% CI 4.08 to 8.09).
Conclusions The mortality among quitters was close to that of never smokers, except for a higher mortality for lung cancer, which on the other hand was much lower than the lung cancer mortality in current smokers.
- harm reduction
- smoking caused disease
Data availability statement
No data are available.
https://doi.org/10.1136/tc-2023-057977
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Twitter @aatverdal
Contributors AT is responsible for the overall content and is the guarantor of the paper. AT, RS and DST conceptualised and designed the study and revised the manuscript. AT drafted the initial manuscript. AT, RS and DST critically reviewed the manuscript for intellectual content. AT, RS and DST approved the final revised manuscript and agree to be accountable for all aspects of the work.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Research article
- Open access
- Published: 24 June 2024
Lung cancer metabolomics: a pooled analysis in the Cancer Prevention Studies
- Ziyin Tang 1 ,
- Donghai Liang 1 ,
- Emily L. Deubler 2 ,
- Jeremy A. Sarnat 1 ,
- Sabrina S. Chow 1 ,
- W. Ryan Diver 2 , 3 , 4 &
- Ying Wang ORCID: orcid.org/0000-0002-1241-6252 2
BMC Medicine volume 22 , Article number: 262 ( 2024 ) Cite this article
197 Accesses
6 Altmetric
Metrics details
A better understanding of lung cancer etiology and the development of screening biomarkers have important implications for lung cancer prevention.
We included 623 matched case–control pairs from the Cancer Prevention Study (CPS) cohorts. Pre-diagnosis blood samples were collected between 1998 and 2001 in the CPS-II Nutrition cohort and 2006 and 2013 in the CPS-3 cohort and were sent for metabolomics profiling simultaneously. Cancer-free controls at the time of case diagnosis were 1:1 matched to cases on date of birth, blood draw date, sex, and race/ethnicity. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using conditional logistic regression, controlling for confounders. The Benjamini–Hochberg method was used to correct for multiple comparisons.
Sphingomyelin (d18:0/22:0) (OR: 1.32; 95% CI: 1.15, 1.53, FDR = 0.15) and taurodeoxycholic acid 3-sulfate (OR: 1.33; 95% CI: 1.14, 1.55, FDR = 0.15) were positively associated with lung cancer risk. Participants diagnosed within 3 years of blood draw had a 55% and 48% higher risk of lung cancer per standard deviation increase in natural log-transformed sphingomyelin (d18:0/22:0) and taurodeoxycholic acid 3-sulfate level, while 26% and 28% higher risk for those diagnosed beyond 3 years, compared to matched controls. Lipid and amino acid metabolism accounted for 47% to 80% of lung cancer-associated metabolites at P < 0.05 across all participants and subgroups. Notably, ever-smokers exhibited a higher proportion of lung cancer-associated metabolites ( P < 0.05) in xenobiotic- and lipid-associated pathways, whereas never-smokers showed a more pronounced involvement of amino acid- and lipid-associated metabolic pathways.
Conclusions
This is the largest prospective study examining untargeted metabolic profiles regarding lung cancer risk. Sphingomyelin (d18:0/22:0), a sphingolipid, and taurodeoxycholic acid 3-sulfate, a bile salt, may be risk factors and potential screening biomarkers for lung cancer. Lipid and amino acid metabolism may contribute significantly to lung cancer etiology which varied by smoking status.
Peer Review reports
According to Global Cancer Statistics 2020, lung cancer accounts for 11.4% of the 19.3 million newly diagnosed cancer cases and remains the leading cause of cancer mortality [ 1 ]. Lung cancer is a heterogeneous tumor with several differentiation types. It is often diagnosed at an advanced stage and the 5-year survival rate is 24.6% [ 2 , 3 , 4 ]. The pathogenesis of lung cancer is believed to be influenced by gene-environment interaction [ 5 , 6 ]. Variability in cellular, molecular, and genetic characteristics in lung cancer histological types has been well-documented [ 2 ]. Along with the change in the environmental and behavioral risk factors, the distribution of lung cancer displays great demographic, temporal, and geographical variability [ 7 ]. Surprisingly, epidemiological findings have shown that approximately 25% of lung cancer cases are not attributable to tobacco smoking, and the rate of lung cancer in never-smokers is increasing [ 8 , 9 ]. Numerous studies have shown disparities in epidemiological, clinical, and molecular characteristics arising in smokers and never-smokers, indicating the possibility of distinct etiologies for the development of lung cancer in each group [ 8 , 10 ]. A better understanding of the heterogeneity in lung cancer etiology has important implications in prevention, early detection and diagnosis, tumor classification, prognosis, and personalized therapeutic decision.
Over the past decades, metabolomics has emerged as a promising technique of studying the comprehensive metabolic profile in biospecimen, providing valuable information for the practice of precision medicine [ 11 ]. As substantially altered metabolism has been proven to be a hallmark in cancer cells [ 12 , 13 ], the application of metabolomics in lung cancer provides an outstanding opportunity to elucidate the etiology and identify potential screening and early detection biomarkers. A growing number of metabolomics studies have examined lung cancer-driven metabolic changes in different biosamples [ 14 ]. Most studies have focused on characterizing the metabolic signatures differentiated by histological types in blood-based samples [ 15 , 16 , 17 ], while few have focused on stage-differentiated metabolic signatures [ 17 , 18 , 19 , 20 ]. Notably, these previous studies were mostly targeted metabolomics analyses, which focused on a limited number of metabolic endpoints. Overall, the existing findings display considerable heterogeneity among the studies. No metabolites were replicated and validated across studies, thereby limiting broad inference and the potential for their development as clinically applicable biomarkers [ 14 ]. To our knowledge, only one untargeted metabolomics application has been conducted in lung cancer research [ 21 ] and none have been performed in the USA.
To address these critical knowledge gaps, we conducted a comprehensive and exploratory metabolomics study on lung cancer within the Cancer Prevention Studies (CPS) [ 22 , 23 ]. These well-constructed large prospective cohorts have pre-diagnosis samples with comprehensive information on lifestyle factors, and long-term follow-up provides a unique opportunity to better understand potential metabolic signatures in pre-diagnosis stage associated with lung cancer etiology.
Study design and population
Lung cancer cases and matched controls included in this analysis are participants from the CPS-II Nutrition cohort and ongoing CPS-3 cohort. At enrollment of the CPS-II Nutrition cohort in 1992–1993, participants completed a self-administered questionnaire that included anthropometric, demographic, dietary, lifestyle, and medical information. Follow-up questionnaires were sent to the cohort participants in 1997 and every other year thereafter to update exposures and to ascertain newly diagnosed cancers. A subset of 39,371 CPS-II Nutrition cohort participants provided a non-fasting blood samples between 1998 and 2001, and the information on demographic characteristics and other covariates in the analysis was assessed from the survey collected at blood draw or the 1999 survey. At enrollment of the CPS-3 cohort between 2006 and 2013, participants provided informed consent, a non-fasting blood sample and completed a brief enrollment survey on demographic characteristics and other covariates. Follow-up questionnaires were sent to active participants in 2015 and every 3 years to update exposures and ascertain newly diagnosed cancer cases. Detailed descriptions of the two cohorts can be found elsewhere [ 22 , 23 ]. All aspects of the CPS-II Nutrition cohort (IRB00045780) and CPS-3 cohort (IRB00059007) were reviewed and approved by the Emory University Institutional Review Board.
A total of 1913 lung cancer cases were identified in the CPS-II Nutrition cohort through June 2015 and 176 lung cancer cases were identified in the CPS-3 Cohort through December 2015. Cases in the CPS-II Nutrition cohort were first identified through self-report and then were verified with medical records, state cancer registry linkage, or linkage with the National Death Index (defined by ICD-10 codes C33 and C34, excluding histology codes ≥ 9590). Cases in the CPS-3 cohort were identified primarily through linkage with state cancer registries, and a small proportion were identified by self-report that were verified by medical records during tumor collection. We applied a series of exclusion criteria to include participants (Additional file 1: Fig. S1). As a result, 500 and 123 lung cancer cases from the CPS-II Nutrition cohort and CPS-3 cohort were included in the analysis, respectively. Controls who were cancer-free at the time of case diagnosis were matched 1:1 to cases on age at blood draw (± 6 months), sex, race/ethnicity, and blood draw date (± 30 days).
Metabolomics profiling
The pre-diagnosis blood samples collected from both cohorts were sent to Metabolon, Inc. (Durham, NC, USA) for untargeted metabolomics profiling simultaneously, using ultrahigh-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis techniques. A detailed process was described elsewhere [ 24 , 25 ] and in supplemental materials.
A total of 1,401 metabolites were detected. After filtering metabolites that were unknown ( n = 238), were missing technical intraclass correlation coefficient (ICC) ( n = 34), with ICC < 50% ( n = 201), and were undetectable in > 90% of samples ( n = 41), 887 known metabolites were included in the statistical analysis with an average ICC of 84% (interquartile range (IQR): 77–94%) and the coefficient of variation (CV)% of 24% (IQR: 12–30%).
Statistical analysis
As metabolomics assessments were conducted simultaneously for cases and controls in both cohorts, we performed a pooled analysis. Metabolites were naturally log-transformed and auto-scaled to approximate normal distribution before formal analysis.
Covariate data obtained in each cohort were harmonized. The characteristics between lung cancer cases and matched controls were compared using Student’s t -test for continuous variables and Pearson’s chi-squared test for categorical variables. For the primary pooled analysis, we applied conditional logistic regression to estimate the odds ratio (OR) and 95% confidence interval (CI) per one standard deviation increase in the naturally log-transformed level of each known metabolite with lung cancer risk. The statistical models were conditioned on the matching variables and controlled for the body mass index (BMI) group (underweight: < 18.5 kg/m 2 , normal weight: 18.5–25 kg/m 2 , overweight: 25–30 kg/m 2 , obese: ≥ 30 kg/m 2 ), hours since last meal (continuous; to account for length of fasting), physical activity (continuous; hours/week), fruits and vegetables consumption (continuous; servings/week), smoking status (categorical: never, former, current, and unknown), and hormone use (categorical: not a current user, current user, not applicable, unknown). Physical activity estimates the average total hours per week of walking or exercise in the CPS-II Nutrition cohort, while it estimates the average hours per day during the past 2 years in the CPS-3 cohort. We harmonized the variables and converted them into hours per week. The covariates were selected based on the literature review and a Directed Acyclic Graph. We removed the observations with any missing data for continuous covariates. We assigned an unknown category for missing data for categorical covariates. If a case was removed, its matching control was removed simultaneously, and vice versa. For the primary pooled analysis, 116 case–control pairs were removed due to missing values in hours since the last meal, physical activity, and fruit and vegetable consumption for either case or its matching control (Additional file 1: Fig. S1). Benjamini–Hochberg approach was used to calculate false discovery rates (FDRs) to correct for multiple comparisons. Metabolites associated with lung cancer risk at FDR < 0.2 were deemed statistically significant. To gain more biological responses of lung cancer, we focused on metabolites associated with lung cancer risk at P < 0.05 ( P -value from statistical models before multiple comparison corrections) and further described and summarized the pathways in which these metabolites were involved.
We conducted an agglomerative hierarchical clustering analysis to group the lung cancer-associated metabolites ( P < 0.05) based on their similarities. Pearson correlation was calculated between each pair of metabolites and then used for distance measure. Euclidean distance was computed between each pair of metabolites and returned the distance matrix. We then used the Ward clustering method to compute the similarity of the two clusters for merging [ 26 ]. The R package “ pheatmap ” was used for this analysis and result visualization.
We further examined the associations stratified by sex and by years between blood draw and lung cancer diagnosis (< 3 years, ≥ 3 years) using conditional logistic regression with the same set of covariates but excluding hormone use for males. The goal of the stratified analysis by years since the blood draw was to identify metabolites that may potentially serve as early detection biomarkers of lung cancer. Additionally, we stratified the analysis by smoking status (never, ever), by stage (localized, regional, distant), and by histological subtypes (squamous cell carcinoma, adenocarcinoma) using unconditional logistic regression, adjusting for matching variables as well as BMI group, hours since last meal, physical activity, fruits and vegetables consumption, hormone use, and smoking status (only for stage- and subtype-stratified analyses). For stratified analyses by smoking status, stage, and subtype where the unconditional logistic regression was applied, matching factors were adjusted as covariates in the model. If a case was removed due to missing covariates, its matching control would not be removed if the control has completed the covariates information, and vice versa. The lung cancer stage was examined according to the Surveillance, Epidemiology and End Results (SEER) stage at diagnosis: localized (invasive tumors confined to the lung); regional (tumors that extend to adjacent tissue or regional lymph nodes); distant (tumors are metastasized). The lung cancer histological subtype was categorized using ICD-O-3 morphology codes [ 27 ]. The morphology codes for each subtype can be found in supplementary materials. P for interaction was calculated using the likelihood ratio test. To test heterogeneity by stage and by subtype, we used the “ eh_test_subtype ” function in the R package “ riskclustr ” [ 28 ]. This function is designed for the test of etiologic heterogeneity across disease subtypes in the context of the case–control study. P -heterogeneity < 0.05 was considered statistically significant.
To examine the robustness of the results, we conducted a series of sensitivity analyses: (1) we recategorized the smoking variables into 7 categories (never, current smoker for < 50 years, current smoker for ≥ 50 years, former smoker quit < 10 years ago, former smoker quit 10–20 years ago, former smoker quit ≥ 20 years ago, unknown) based on smoking status and duration of time and adjusted it in the main analysis; (2) we further adjusted for cohort (CPS-II Nutrition, CPS-3) in the analysis to evaluate if any differences between cohorts (e.g., age of blood samples) would affect the main analysis results; (3) we also examined the associations stratified by years between blood draw and lung cancer diagnosis (< 5 years, ≥ 5 years).
All analyses were conducted using R (version 4.1.0.).
Population characteristics
A total of 623 case–control pairs with an average age of 66.9 (± 8.6) years at blood draw were included in the analysis. Among the 1246 participants, 52.5% were female and the majority (96.1% in cases and 96.6% in controls) were white. Compared with controls, the average hours since the last meal for lung cancer cases was smaller. Additionally, cases were more likely to be current and former smokers (Table 1 ). Lung cancer cases were on average diagnosed at an age of 72.9 (± 10.3) years and the median time between blood draw and lung cancer diagnosis was 5.0 years (IQR: 7.0 years). Among cases, 46.5% were at a distant stage and 50.1% were adenocarcinoma.
Sixty-two metabolites were associated with lung cancer risk, mainly in lipid and amino acid metabolism
In the main analysis, two metabolites were significantly associated with lung cancer risk: sphingomyelin (SM) (d18:0/22:0) (OR: 1.32, 95% CI: 1.13, 1.53; FDR = 0.15) and taurodeoxycholic acid 3-sulfate (OR: 1.33, 95% CI: 1.14, 1.55; FDR = 0.15) (Figs. 1 and 2 , Additional file 2: Table S1). A total of 62 metabolites were associated with lung cancer risk at P < 0.05 (Fig. 2 ). Among the 62 metabolites, 37 metabolites showed positive associations (OR range: 1.15–1.33) and 25 had negative associations (OR range: 0.78–0.87) with lung cancer risk. Agglomerative hierarchical clustering analysis among the 62 metabolites revealed that an additional 2 SMs and 1 dihydroceramide are moderately to highly correlated with SM (d18:0/22:0) (Fig. 3 ). These metabolites were characterized mainly as lipids (39%), amino acids (24%), and xenobiotics (11%). (Additional file 1: Fig. S2). The lipid metabolism can include seven categories including sphingolipids, bile acids, phospholipids, fatty acids, glycerolipids, steroids, and eicosanoids. Specifically, higher levels of the metabolites identified in sphingolipid metabolism (OR range: 1.15–1.32), bile acid metabolism (OR range: 1.17–1.33), and fatty acid metabolism (OR range: 1.18–1.28) were associated with a higher risk of developing lung cancer (Additional file 2: Table S2). For the metabolites in sphingolipid metabolism, three were dihydrophingomyelins, two were dihydroceramides, and one was sphingomyelin. For metabolites in bile acid metabolism, one belonged to primary bile acid metabolism, while the other five belonged to secondary bile acid metabolism. The amino acids metabolism mainly contains arginine and proline metabolism, branched-chain amino acid metabolism, and aromatic amino acid metabolism. Likewise, higher levels of the metabolites identified in branched-chain amino acid metabolism (OR range: 1.16–1.19) were associated with a higher risk of developing lung cancer (Additional file 2: Table S3).
A volcano plot of associations between metabolites and lung cancer risk in the entire population. The X -axis denotes the odds ratio of lung cancer-metabolite associations. Odds ratios (95% confidence intervals) per one standard deviation increase in natural log-transformed level of each known metabolite with lung cancer risk were estimated from conditional logistic regression models, matched on age at blood draw, sex, race, and date of blood draw. Models were adjusted for body mass index group (underweight, healthy weight, overweight, obesity), hours since last meal (continuous), physical activity (continuous, hours/week), fruits and vegetables consumption (continuous, servings/week), smoking status (never, former, current, unknown), hormone use (not a current user, current user, not applicable, unknown). The Y -axis denotes the negative log 10 of the P -value in the lung cancer-metabolite association. Different colors were used to represent different pathways where the metabolites are involved. The dark red dashed line represents P -value = 0.05. SM (d18:0/22:0) and taurodeoxycholic acid 3-sulfate were associated with lung cancer risk (FDR < 0.2). SM (d18:0/22:0), behenoyl dihydrosphingomyelin (d18:0/22:0)
A forest plot of associations between metabolites and lung cancer risk ( P < 0.05) in the entire population. Odds ratios (95% confidence intervals) per one standard deviation increase in natural log-transformed level of each known metabolite with lung cancer risk were estimated from conditional logistic regression models, matched on age at blood draw, sex, race, and date of blood draw. Models were adjusted for body mass index group (underweight, healthy weight, overweight, obesity), hours since last meal (continuous), physical activity (continuous, hours/week), fruits and vegetables consumption (continuous, servings/week), smoking status (never, former, current, unknown), hormone use (not a current user, current user, not applicable, unknown). Each dot represents the odds ratio of the association, with the whiskers representing the 95% confidence interval. The dots are arranged in ascending order based on the P -values of the associations, starting from the smallest P to the largest. Blue dots represent the metabolites associated with lung cancer risk at FDR < 0.2. The dashed vertical line represents the odds ratio of one. SM (d18:0/22:0), behenoyl dihydrosphingomyelin (d18:0/22:0); SM (d18:0/20:0, d16:0/22:0), sphingomyelin (d18:0/20:0, d16:0/22:0); SM (d18:0/18:0, d19:0/17:0), sphingomyelin (d18:0/18:0, d19:0/17:0); SM (d18:1/16:0 (OH)), hydroxypalmitoyl sphingomyelin (d18:1/16:0(OH)). * Putative identifications that are not confirmed with a purified standard (not tier 1). ** Putative identifications for which a standard is not available (not tier 1). Metabolites that are structurally similar but have a side group that could not be placed definitively in the molecule were given the same chemical name followed by a number in parentheses to differentiate them from each other
Agglomerative hierarchical clustering heatmap of the Pearson’s correlation coefficients among the sixty-two metabolites associated with lung cancer risk ( P- value < 0.05). * Putative identifications that are not confirmed with a purified standard (not tier 1). ** Putative identifications for which a standard is not available (not tier 1). Metabolites that are structurally similar but have a side group that could not be placed definitively in the molecule were given the same chemical name followed by a number in parentheses to differentiate them from each other
SM (d18:0/22:0) and taurodeoxycholic acid 3-sulfate were consistently positively associated with lung cancer risk across strata
SM (d18:0/22:0) was consistently positively associated with lung cancer risk across strata, though the associations in some strata were not statistically significant ( P < 0.05). When stratified by sex, SM (d18:0/22:0) was associated with ( P < 0.05) higher lung cancer risk in both men and women ( P -heterogeneity = 0.50) (Table 2 ). Notably, among cases diagnosed within 3 years of blood draw ( n = 177), one standard deviation increase in natural log-transformed SM (d18:0/22:0) levels was associated with 55% higher risk of lung cancer (OR: 1.55, 95% CI: 1.12, 2.13), while the same amount of increase was associated with 26% higher risk among cases diagnosed beyond 3 years after blood draw (OR: 1.26, 95% CI: 1.06, 1.50) ( n = 446), compared to matched controls. However, the association of SM (d18:0/22:0) with lung cancer risk did not differ by follow-up time ( P -heterogeneity = 0.33). When stratified by smoking status, SM (d18:0/22:0) was associated with higher lung cancer risk among ever-smokers ( P = 0.02). The association was also positive, albeit non-significant, among never-smokers ( P = 0.61). There was no interaction between SM (d18:0/22:0) and smoking status ( P -heterogeneity = 0.49). No heterogeneity was observed when stratified SM (d18:0/22:0) associations by lung cancer stage ( P -heterogeneity = 0.77) and subtype ( P -heterogeneity = 0.12).
Taurodeoxycholic acid 3-sulfate was associated with higher lung cancer risk in male, female, cases diagnosed within and beyond 3 years of blood draw, and those at localized stage ( P < 0.05) (Table 2 ). Likewise, among cases diagnosed within 3 years of blood draw ( n = 177), one standard deviation increase in natural log-transformed taurodeoxycholic acid 3-sulfate levels was associated with 48% higher risk of lung cancer (OR: 1.48, 95% CI: 1.08, 2.03), while the same amount of increase was associated with 28% higher risk among cases diagnosed beyond 3 years after blood draw (OR: 1.28, 95% CI: 1.07, 1.53) ( n = 446), compared to matched controls. No heterogeneity was observed when stratified taurodeoxycholic acid 3-sulfate associations by sex ( P -heterogeneity = 0.91), follow-up time ( P -heterogeneity = 0.50), smoking status ( P -heterogeneity = 0.62), lung cancer stage ( P -heterogeneity = 0.08), and subtype ( P -heterogeneity = 0.29).
Lung cancer -associated metabolic profiles varied between ever- and never-smokers
We observed that the distribution of metabolic pathways containing lung cancer-associated metabolites ( P < 0.05) varied by smoking status, sex, tumor stage, and histological subtypes (Additional file 1: Fig. S3). Results for stratified analyses can be found in supplementary materials (Additional file 2: Table S4–S14). We identified 65 metabolites associated with lung cancer risk (FDR < 0.2) in ever-smokers (Additional file 1: Fig. S4–S5, Additional file 2: Table S9), while none in never-smokers (Additional file 2: Table S8). Interestingly, the four most significant metabolites in ever-smokers were tobacco metabolites, which were cotinine, hydroxycotinine, cotinine N-oxide, and 3-hydroxycotinine glucuronide. Looking closely at the pathways where metabolites associated with lung cancer risk ( P < 0.05) were involved, there were greater proportion of metabolites in xenobiotic- and lipid-associated metabolic pathways in ever-smokers compared to never-smokers (Fig. 4 ). However, the amino acid- and lipid-associated metabolic pathways were more pronounced in never-smokers. As for stratified analysis by follow-up time, the proportion of lipid- and amino acid-associated metabolic pathways were similar (Fig. 4 ), but lung cancer-associated metabolites ( P < 0.05) were largely different (Additional file 2: Table S6–S7). A more distinct perturbation of metabolites in lipid pathways was observed in female cases than in male cases, those at regional and distant stages than those at a localized stage, adenocarcinoma cases than squamous cell carcinoma cases (Additional file 1: Fig. S3). A more distinct perturbation of metabolites in amino acids pathways was observed in cases at localized stages than those at other stages. For subtype-stratified analysis, we identified 12 metabolites significantly associated with lung cancer risk (FDR < 0.2) in squamous cell carcinoma (Additional file 2: Table S13), while one in adenocarcinoma (Additional file 2: Table S14). Notably, lipid and amino acid metabolism are major metabolic pathways involved in lung cancer development, accounting for 47% to 80% of all lung cancer-associated metabolites at P < 0.05, either among all participants or in subgroup analyses (Fig. 4 , Additional file 1: Fig. S2–S3).
Descriptive distribution of metabolic pathways that contain the lung cancer-associated metabolites at P- value < 0.05 by smoking status and follow-up time
Sensitivity analyses
Sensitivity analyses revealed that 63% of lung cancer-associated metabolites ( P < 0.05) remained when replacing four-category smoking variables with seven-category smoking variables that further included smoking duration in the model. The associations of SM (d18:0/22:0), taurodeoxycholic acid 3-sulfate, and lung cancer risk in cases diagnosed within 5 years of blood draw remained significant (OR: 1.47, 95% CI: 1.15, 1.89, P = 0.003 and OR: 1.60, 95% CI: 1.25, 2.07, P < 0.001, respectively). The number and identities of metabolites ( P < 0.05) and their corresponding ORs from models were nearly the same before and after including the cohort variable in the model, which indicates that the effects of any differences between cohorts were too small to detect (results not shown).
In this large pooled analysis of prospective cohort studies on examining metabolic profiles in association with lung cancer risk using untargeted metabolomics, SM (d18:0/22:0), a sphingolipid, and taurodeoxycholic acid 3-sulfate, a bile salt, were positively associated with lung cancer risk regardless of smoking status, follow-up time, sex, stage, and subtype, though the associations in some strata did not survive P < 0.05. Lipid (sphingolipid, bile acid, phospholipids, and fatty acids pathways) and amino acid metabolism (arginine and proline metabolism, branched-chain amino acids, and aromatic amino acids) may play an important role in lung cancer etiology. Distinct metabolic profiles between never and ever-smokers suggest heterogeneity in lung cancer etiology by smoking status.
Lipid metabolism has been associated with the initiation and progression of lung cancer [ 29 ]. Consistently, we observed an extensive perturbation of metabolites in lipid pathways in our study. Sphingolipids are ubiquitous bioactive components of cell membranes and also play an important role in cell signaling in various physiological processes [ 30 , 31 , 32 ]. Previous studies have ranked sphingolipid metabolism as one of the top dysregulated pathways in lung cancer development in human studies [ 33 , 34 ]. In particular, several key sphingolipids (e.g., sphingosine-1-phosphate (S1P), ceremide) and related enzymes (e.g., sphingosine kinases (SphK1/2), ceramide kinases (Cerk)) were found to play crucial roles in lung cancer etiology by disrupting universe cellular processes, regulating downstream signaling pathways, and affecting tumor microenvironment [ 32 , 35 , 36 , 37 , 38 , 39 ]. In our study, higher levels of several sphingolipids were associated with a higher risk of lung cancer, suggesting the aberrantly active activity of sphingolipids in lung cancer development. Upregulation of these metabolites, as precursors of ceramide, may be an indicator of increased synthesis of ceramide/S1P or abnormal ceramide-to-S1P ratio. Specifically, the imbalance of ceramide/S1P has been suggested to be associated with unrelenting airway inflammation which could ultimately cause increased oxidative stress and aberrant signaling [ 40 , 41 ], increased apoptosis and senescence [ 42 , 43 , 44 ], impaired immunity [ 45 , 46 ], lung remodeling [ 47 , 48 ], increased lung permeability, and altered surfactant [ 49 ].
Perturbation of bile acid metabolism in lung cancer cases also warrants attention. Bile acids are known for the promotion of the absorption of lipids, and they also play an important role in cell signaling and maintaining human body homeostasis. Recent studies have characterized the role of bile acids in cancer development and progression, albeit the research is in its infancy [ 50 , 51 , 52 ]. In our study, we identified one conjugated primary bile acid and five conjugated secondary bile acids and their derivatives, which were all positively associated with lung cancer risk. Consistently, another study reported much higher serum-free secondary bile acids (deoxycholic acid and ursodeoxycholic acid) and primary bile acid (chenodeoxycholic acid) in non-small cell lung cancer (NSCLC) patients than the healthy controls [ 52 ]. Due to the close link between bile acids and microbes in the gut [ 50 , 53 , 54 ], higher expression of secondary bile acids identified in the current study may be an indicator of the abnormal structure of microbial communities. However, details remain unclear on how bile acid metabolism is regulated in lung cancer. Further investigations on bile acid metabolism and the interaction between secondary bile acids and gut microbiota in lung cancer etiology are needed.
Particularly, we observed higher levels of SM (d18:0/22:0), a sphingolipid, was consistently associated with lung cancer risk among all participants (FDR < 0.2) and across different strata ( P < 0.05). SM (d18:0/22:0) is involved in the dihydrosphingomyelins pathway. Additionally, we observed higher levels of taurodeoxycholic acid 3-sulfate, a bile salt, was associated with higher lung cancer risk in the entire population (FDR < 0.2) and several subgroups (male, female, cases diagnosed within and beyond 3 years of blood draw, and those at localized stage) ( P < 0.05). Taurodeoxycholic acid 3-sulfate is involved in secondary bile acid metabolism. Notably, the association of SM (d18:0/22:0) and taurodeoxycholic acid 3-sulfate with lung cancer was the strongest among cases diagnosed within 3 years of follow-up, but the association was still significant though weaker among cases diagnosed beyond 3 years of follow-up, which shows their great potential as an early detection and possibly a screening biomarker for lung cancer. In addition, we identified three additional SMs positively associated with lung cancer risk before correcting for multiple comparisons, including SM (d18:1/16:0 (OH)), SM (d18:0/18:0, d19:0/17:0), and SM (d18:0/20:0, d16:0/22:0). Previous studies have shown the changes of SMs alone or in combination with other molecules can predict the recurrence of specific types of lung cancer [ 55 , 56 ] and can differentiate early-stage lung cancer from controls [ 57 ]. Additionally, our study replicated several metabolites previously found to be associated with lung cancer risk, including cotinine, lactate, and glutamate [ 14 ]. Increased plasma cotinine levels were associated with a 33% higher risk of lung cancer in the present study, which is consistent with previous findings [ 14 , 58 , 59 ].
In addition, we observed a certain degree of perturbation of amino acids metabolism in lung cancer cases compared to matched controls, including arginine and proline metabolism (arginine and proline metabolism, creatine metabolism), branched-chain amino acids metabolism (leucine, isoleucine, and valine metabolism), and aromatic amino acids metabolism (tryptophan metabolism). Amino acid metabolism plays a crucial role in various cellular processes including protein synthesis and energy production, which was found involved in tumor development and progression. More specifically, arginine and proline metabolism plays an important role in metabolic reprogramming in cancer [ 60 , 61 ]. An increase in branched-chain amino acids (BCAAs) metabolism was thought to provide energy sources and contribute to tumor growth [ 62 ]. Tryptophan and its metabolites have been reported to be significantly involved in the immune escape of lung cancer, such as promoting immune suppression [ 63 ].
We observed distinct metabolic profiles associated with lung cancer risk by smoking status, suggesting the heterogeneity in lung cancer etiology between never-smokers and ever-smokers to a certain degree, though the detailed mechanisms were not clear. Specifically, we identified 65 metabolites associated with lung cancer risk (FDR < 0.2) in ever-smokers, while none in never-smokers. When considering lung cancer-associated metabolites at P < 0.05, we observed a more prominent perturbation of metabolites in xenobiotic-associated and lipid-associated pathways in ever-smokers compared to never-smokers. The SM(d18:0/22:0) association was stronger in ever-smokers than in never-smokers, suggesting that this pathway may be particularly relevant to lung cancers that develop as a result of cigarette smoking. Our findings provide extra evidence that lung cancer mechanisms may differ by smoking status. Consistent with previous findings, lung cancer in never-smokers and ever-smokers was suggested as two distinct disease processes, with different epidemiologic, clinical, and genetic characteristics [ 8 , 10 , 64 , 65 , 66 , 67 ]. Lung cancer-associated metabolites ( P < 0.05) varied greatly between cases diagnosed within and beyond 3 years of blood draw, among different stages, as well as between squamous cell carcinoma and adenocarcinoma cases. These findings may suggest potential differences in metabolome associated with different rates of progression, stages, and subtypes. Limited studies have reported several metabolites in serum were differentially expressed in early stage versus advanced stage of lung cancer [ 68 ]. It is noteworthy that the number of cases is not very large in some strata in our analysis, which may lead to insufficient statistical power. Our findings should be validated by future studies. Overall, the perturbation of lipid levels was found to be a dominant characteristic across the entire study population, as well as in other subgroups, with the exception of lung cancer cases who were never-smokers, males, and at localized stage. A caveat is that the pathway differentiation by smoking status or by other strata was simply descriptive and did not involve statistical testing to determine the significance across the subgroups. We observed certain degrees of metabolites in xenobiotic-related pathways across the entire study population and subgroups, with the highest proportions in ever-smoking lung cancer cases (30%) followed by squamous cell carcinoma cases (27%). This may imply residual confounding arising from dietary factors as well as concurrent exposure to drugs and other chemical agents. Specifically, among ever-smoking lung cancer cases and squamous cell carcinoma cases, we observed ten and eight metabolites of caffeinated and decaffeinated coffee (e.g., caffeine, 1-methylurate, and 1,3-dimethylurate) [ 69 ], which were positively associated with the lung cancer risk. Previously, smoking has been associated with higher caffeine consumption [ 70 , 71 ].
Previously, Seow et al. conducted a prospective nested case–control study with a focus on lung cancer-associated metabolic perturbation in urine samples collected from never-smoking Chinese women [ 21 ]. They found extensive urinal metabolic perturbation among lung cancer cases compared to controls, which suggests systematic changes in 1-carbon metabolism, oxidative stress and inflammation pathways, and nucleotide metabolism. Among never-smoking cases in our study, we did observe pathways related to 1-carbon metabolism, oxidative stress, and inflammation, including methionine, cysteine, and taurine metabolism, tocopherol metabolism, glutamate metabolism, and histidine metabolism. It is not reasonable to directly compare the results between our and Seow’s studies given differences in biosamples and metabolic profiling procedures, and heterogeneities in populations including races, ages, sex proportion, and dietary patterns.
To our knowledge, this is the largest prospective study of untargeted metabolomics on lung cancer risk. The current study has a large sample size, based upon the established cohort with well-characterized risk factors (e.g., detailed smoking histories, hormone use). We can perform stratified and in-depth analyses. Besides, pre-diagnosis blood samples provide valuable information on metabolic perturbations associated with lung cancer initiation and development, which is beneficial for early-detection biomarkers identification. In this study, we only included 887 known metabolites, with high levels of confidence in the annotation (Levels 1 and 2) [ 72 ] and high data quality, which makes our results more reliable compared to prior studies that reported all detected signals in the biosamples and claimed all the signals are unique compounds. Our study also has limitations. This analysis is based on one-time metabolic measurement, neglecting within-person variations over time. Thus, the dynamics of metabolites during lung cancer development were unknown. Additionally, Metabolic profiling using non-fasting blood samples, potentially introduced measurement errors in diet-related metabolites. Yet, the impact of fasting status was minimized by controlling for hours since the last meal in analyses. From the perspective of hypothesis generation, a loose threshold, P < 0.05, was used for gaining more information on biological pathways associated with lung cancer by smoking status. Simultaneously, the possibility of a false discovery rate increased [ 73 ]. It is noteworthy that the metabolome is sensitive and susceptible to influences from both endogenous and exogenous factors along with the computational nature of this study, caution is warranted in interpreting the results as the causality was not able to be established. Potential selection bias may exist, as population characteristics including sex, race, BMI, and smoking differed between the lung cancer cases included in the present analysis and those excluded due to unavailable blood samples. Also, our findings may lack generalizability to races other than white or younger populations.
In this large pooled analysis of nested case–control studies of lung cancer metabolomics, we identified that pre-diagnosis changes in lipid metabolism and amino acid metabolism may play important roles in lung cancer etiology. Notably, SM (d18:0/22:0) and taurodeoxycholic acid 3-sulfate may be risk factors and potential screening biomarkers for lung cancer. Distinctive metabolic profiles by smoking status suggest heterogeneity in lung cancer etiology. Future studies are needed to validate our findings.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Abbreviations
Branched-chain amino acids
Body mass index
Ceramide kinases
Confidence interval
Cancer Prevention Study
False discovery rates
Intraclass correlation coefficient
Interquartile range
Non-small cell lung cancer
Sphingosine-1-phosphate
Surveillance, Epidemiology and End Results
- Sphingomyelin
Sphingosine kinases
Ultrahigh-performance liquid chromatography-tandem mass spectrometry
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J Clin. 2021;71(3):209–49.
de Sousa VML, Carvalho L. Heterogeneity in Lung Cancer. Pathobiology. 2018;85(1–2):96–107.
PubMed Google Scholar
American Lung Association. Lung Cancer Fact Sheet https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet2020 [updated May 27, 2020; cited 2022 Apr 7, 2022]. Available from: https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet .
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2018. Bethesda: National Cancer Institute; 2021.
Haugen A, Ryberg D, Mollerup S, Zienolddiny S, Skaug V, Svendsrud DH. Gene–environment interactions in human lung cancer. Toxicol Lett. 2000;112–113:233–7.
Article PubMed Google Scholar
Wk LAM. Lung cancer in Asian women—the environment and genes*. Respirology. 2005;10(4):408–17.
Article Google Scholar
Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. 2019;85(1):8.
Article PubMed PubMed Central Google Scholar
Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers — a different disease. Nat Rev Cancer. 2007;7(10):778–90.
Article CAS PubMed Google Scholar
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Gazdar AF, Zhou C. 4 - Lung Cancer in Never-Smokers: A Different Disease. In: Pass HI, Ball D, Scagliotti GV, editors. IASLC Thoracic Oncology. 2nd ed. Philadelphia: Elsevier; 2018. p. 23- 9.e3.
Chapter Google Scholar
Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud. 2015;1(1):a000588-a.
Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2(10):881–98.
Article CAS PubMed PubMed Central Google Scholar
Sciacovelli M, Gaude E, Hilvo M, Frezza C. The metabolic alterations of cancer cells. Methods Enzymol. 2014;542:1–23.
Lee KB, Ang L, Yau WP, Seow WJ. Association between Metabolites and the Risk of Lung Cancer: A Systematic Literature Review and Meta-Analysis of Observational Studies. Metabolites. 2020;10(9):362.
Chuang S-C, Fanidi A, Ueland PM, Relton C, Midttun Ø, Vollset SE, et al. Circulating Biomarkers of Tryptophan and the Kynurenine Pathway and Lung Cancer Risk. Cancer Epidemiol Biomark Prev. 2014;23(3):461–8.
Article CAS Google Scholar
Fanidi A, Muller DC, Yuan J-M, Stevens VL, Weinstein SJ, Albanes D, et al. Circulating Folate, Vitamin B6, and Methionine in Relation to Lung Cancer Risk in the Lung Cancer Cohort Consortium (LC3). J Natl Cancer Inst. 2018;110(1):57–67.
Esme H, Cemek M, Sezer M, Saglam H, Demir A, Melek H, et al. High levels of oxidative stress in patients with advanced lung cancer. Respirology. 2008;13(1):112–6.
Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, et al. Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLoS ONE. 2011;6(9): e24143.
Kim HJ, Jang SH, Ryu J-S, Lee JE, Kim YC, Lee MK, et al. The performance of a novel amino acid multivariate index for detecting lung cancer: A case control study in Korea. Lung Cancer. 2015;90(3):522–7.
Zhang L, Zheng J, Ahmed R, Huang G, Reid J, Mandal R, et al. A High-Performing Plasma Metabolite Panel for Early-Stage Lung Cancer Detection. Cancers. 2020;12(3):622.
Seow WJ, Shu XO, Nicholson JK, Holmes E, Walker DI, Hu W, et al. Association of Untargeted Urinary Metabolomics and Lung Cancer Risk Among Never-Smoking Women in China. JAMA Netw Open. 2019;2(9): e1911970.
Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(2):500–11.
Patel AV, Jacobs EJ, Dudas DM, Briggs PJ, Lichtman CJ, Bain EB, et al. The American Cancer Society’s Cancer Prevention Study 3 (CPS-3): Recruitment, study design, and baseline characteristics. Cancer. 2017;123(11):2014–24.
Evans AM, Bridgewater B, Liu Q, Mitchell M, Robinson R, Dai H, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4(2):1.
Google Scholar
Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal Chem. 2009;81(16):6656–67.
Murtagh F, Legendre P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J Classif. 2014;31(3):274–95.
Organization WH. International classification of diseases for oncology (ICD-O): World Health Organization; 2013.
Begg CB, Zabor EC, Bernstein JL, Bernstein L, Press MF, Seshan VE. A conceptual and methodological framework for investigating etiologic heterogeneity. Stat Med. 2013;32(29):5039–52.
Merino Salvador M, Gómez de Cedrón M, Moreno Rubio J, Falagán Martínez S, Sánchez Martínez R, Casado E, et al. Lipid metabolism and lung cancer. Critical Reviews in Oncology/Hematology. 2017;112:31–40.
Hannun YA, Bell RM. Lysosphingolipids Inhibit Protein Kinase C: Implications for the Sphingolipidoses. Science. 1987;235(4789):670–4.
Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-α activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255(5052):1715–8.
Lin M, Li Y, Wang S, Cao B, Li C, Li G. Sphingolipid Metabolism and Signaling in Lung Cancer: A Potential Therapeutic Target. J Oncol. 2022;2022:9099612.
Chen Y, Ma Z, Min L, Li H, Wang B, Zhong J, et al. Biomarker Identification and Pathway Analysis by Serum Metabolomics of Lung Cancer. Biomed Res Int. 2015;2015: 183624.
PubMed PubMed Central Google Scholar
Meng Q, Hu X, Zhao X, Kong X, Meng Y-M, Chen Y, et al. A circular network of coregulated sphingolipids dictates lung cancer growth and progression. EBioMedicine. 2021;66: 103301.
Liu L, Zhou XY, Zhang JQ, Wang GG, He J, Chen YY, et al. LncRNA HULC promotes non-small cell lung cancer cell proliferation and inhibits the apoptosis by up-regulating sphingosine kinase 1 (SPHK1) and its downstream PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2018;22(24):8722–30.
CAS PubMed Google Scholar
Ma Y, Xing X, Kong R, Cheng C, Li S, Yang X, et al. SphK1 promotes development of non-small cell lung cancer through activation of STAT3. Int J Mol Med. 2021;47(1):374–86.
Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60.
Pastukhov O, Schwalm S, Zangemeister-Wittke U, Fabbro D, Bornancin F, Japtok L, et al. The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br J Pharmacol. 2014;171(24):5829–44.
Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22(37):5897–906.
Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50.
Petrache I, Berdyshev EV. Ceramide Signaling and Metabolism in Pathophysiological States of the Lung. Annu Rev Physiol. 2016;78(1):463–80.
Justice MJ, Petrusca DN, Rogozea AL, Williams JA, Schweitzer KS, Petrache I, et al. Effects of lipid interactions on model vesicle engulfment by alveolar macrophages. Biophys J. 2014;106(3):598–609.
Petrusca DN, Gu Y, Adamowicz JJ, Rush NI, Hubbard WC, Smith PA, et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem. 2010;285(51):40322–32.
Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11(5):491–8.
Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–70.
Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11(6):403–15.
Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. 2010;181(4):344–52.
Tabeling C, Yu H, Wang L, Ranke H, Goldenberg NM, Zabini D, et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci. 2015;112(13):E1614–23.
Ryan AJ, McCoy DM, McGowan SE, Salome RG, Mallampalli RK. Alveolar sphingolipids generated in response to TNF-α modifies surfactant biophysical activity. J Appl Physiol. 2003;94(1):253–8.
Yang R, Qian L. Research on Gut Microbiota-Derived Secondary Bile Acids in Cancer Progression. Integr Cancer Ther. 2022;21:15347354221114100.
Fu J, Yu M, Xu W, Yu S. Research Progress of Bile Acids in Cancer. Frontiers in Oncology. 2022;11:778258.
Liu X, Chen B, You W, Xue S, Qin H, Jiang H. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett. 2018;412:194–207.
Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2018;16(1):21–6.
Zhan K, Zheng H, Li J, Wu H, Qin S, Luo L, et al. Gut microbiota-bile acid crosstalk in diarrhea-irritable bowel syndrome. BioMed Res Int. 2020;2020(1):3828249.
Takanashi Y, Funai K, Sato S, Kawase A, Tao H, Takahashi Y, et al. Sphingomyelin(d35:1) as a novel predictor for lung adenocarcinoma recurrence after a radical surgery: a case-control study. BMC Cancer. 2020;20(1):800.
Takanashi Y, Funai K, Eto F, Mizuno K, Kawase A, Tao H, et al. reased sphingomyelin (t34:1) is a candidate predictor for lung squamous cell carcinoma recurrence after radical surgery: a case-control study. BMC Cancer. 2021;21(1):1232.
Guo Y, Ren J, Li X, Liu X, Liu N, Wang Y, et al. Simultaneous Quantification of Serum Multi-Phospholipids as Potential Biomarkers for Differentiating Different Pathophysiological states of lung, stomach, intestine, and pancreas. J Cancer. 2017;8(12):2191–204.
Larose TL, Guida F, Fanidi A, Langhammer A, Kveem K, Stevens VL, et al. Circulating cotinine concentrations and lung cancer risk in the Lung Cancer Cohort Consortium (LC3). Int J Epidemiol. 2018;47(6):1760–71.
Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1184–8.
Proline Metabolism in Cell Regulation and Cancer Biology. Recent Advances and Hypotheses. Antioxid Redox Signal. 2019;30(4):635–49.
Chen CL, Hsu SC, Ann DK, Yen Y, Kung HJ. Arginine Signaling and Cancer Metabolism. Cancers. 2021;13(14):3541.
Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, Bauer MR, et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science. 2016;353(6304):1161–5.
Li C, Zhao H. Tryptophan and Its Metabolites in Lung Cancer: Basic Functions and Clinical Significance. Front Oncol. 2021;11: 707277.
Chapman AM, Sun KY, Ruestow P, Cowan DM, Madl AK. Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–34.
Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Annals of oncology. 2002;13(7):1087–93.
Santoro IL, Ramos RP, Franceschini J, Jamnik S, Fernandes ALG. Non-small cell lung cancer in never smokers: a clinical entity to be identified. Clinics. 2011;66:1873–7.
Toh C-K, Gao F, Lim W-T, Leong S-S, Fong K-W, Yap S-P, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol. 2006;24(15):2245–51.
Bamji-Stocke S, van Berkel V, Miller DM, Frieboes HB. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics. 2018;14(6):81.
Wang Y, Gapstur SM, Carter BD, Hartman TJ, Stevens VL, Gaudet MM, et al. Untargeted Metabolomics Identifies Novel Potential Biomarkers of Habitual Food Intake in a Cross-Sectional Study of Postmenopausal Women. J Nutr. 2018;148(6):932–43.
Treur JL, Taylor AE, Ware JJ, McMahon G, Hottenga JJ, Baselmans BM, et al. Associations between smoking and caffeine consumption in two European cohorts. Addiction. 2016;111(6):1059–68.
Swanson JA, Lee JW, Hopp JW. Caffeine and nicotine: a review of their joint use and possible interactive effects in tobacco withdrawal. Addict Behav. 1994;19(3):229–56.
Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics : Official journal of the Metabolomic Society. 2007;3(3):211–21.
Liang D, Li Z, Vlaanderen J, Tang Z, Jones DP, Vermeulen R, et al. A State-of-the-Science Review on High-Resolution Metabolomics Application in Air Pollution Health Research: Current Progress, Analytical Challenges, and Recommendations for Future Direction. Environ Health Perspect. 2023;131(5):56002.
Download references
Acknowledgements
The authors express sincere appreciation to all Cancer Prevention Study-II and Cancer Prevention Study-3 participants, and to each member of the study and biospecimen management group. The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention's National Program of Cancer Registries and cancer registries supported by the National Cancer Institute's Surveillance Epidemiology and End Results Program. Besides, we would like to acknowledge National Institute of Health (NIH) research grant [R21ES032117] and NIH Center Grants [P30ES019776] in supporting DL, JAS, and ZT’s efforts in this.
The views expressed here are those of the authors and do not necessarily represent the American Cancer Society or the American Cancer Society – Cancer Action Network.
The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study-II cohort and Cancer Prevention Study-3. Support of this project is from Michel & Claire Gudefin Family Foundation Inc. We also acknowledge the support from the National Institute of Health (NIH) research grant [R21ES032117] and the HERCULES Exposome Research Center, supported by the National Institute of Environmental Health Sciences of the NIH (P30ES019776).
Author information
Authors and affiliations.
Gangarosa Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA
Ziyin Tang, Donghai Liang, Jeremy A. Sarnat & Sabrina S. Chow
Department of Population Science, American Cancer Society, Atlanta, GA, USA
Emily L. Deubler, W. Ryan Diver & Ying Wang
Barcelona Institute for Global Health (ISGlobal), Barcelona, Spain
W. Ryan Diver
Universitat Pompeu Fabra (UPF), Barcelona, Spain
You can also search for this author in PubMed Google Scholar
Contributions
YW, DL, and ZT designed the study. YW, ELD, and WRD retrieved and compiled the dataset. YW and DL directed the analytical strategy’s implementation. ZT conducted the statistical analyses. ZT drafted the manuscript with input from YW, DL, JAS, and SSC. All authors contributed to interpreting the findings and revising the manuscript. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @ziyin_tang (Ziyin Tang),
@YingWang934550 (Ying Wang),
@donghai_liang (Donghai Liang).
Corresponding authors
Correspondence to Donghai Liang or Ying Wang .
Ethics declarations
Ethics approval and consent to participate.
All aspects of the CPS-II Nutrition cohort (IRB00045780) and CPS-3 cohort (IRB00059007) were reviewed and approved by the Emory University Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3473_moesm1_esm.docx.
Additional file 1: Figure S1. Flowchart of exclusion criteria for study participants in the primary pooled analysis. Figure S2. Distribution of super and sub pathways containing the sixty-two metabolites associated with lung cancer risk ( P -value < 0.05). Figure S3. Descriptive distribution of metabolic pathways that contain the lung cancer-associated metabolites at P -value < 0.05 by sex, lung cancer stage, and subtype. Figure S4. Agglomerative hierarchical clustering heatmap of the Pearson’s correlation coefficients among the sixty-five metabolites associated with lung cancer risk in ever smokers (FDR < 0.2). Figure S5. A volcano plot of associations between metabolites and lung cancer risk in ever smokers.
12916_2024_3473_MOESM2_ESM.xlsx
Additional file 2: Table S1. Metabolites associated with lung cancer risk at P -value < 0.05 in the entire population. Table S2. Lipid-associated metabolic pathways components identified in lung cancer cases compared to controls. Table S3. Amino acids-associated metabolic pathways components identified in lung cancer cases compared to controls. Table S4. Metabolites associated with lung cancer risk at P -value < 0.05 in female stratum. Table S5. Metabolites associated with lung cancer risk at P -value < 0.05 in male stratum. Table S6. Metabolites associated with lung cancer risk at P -value < 0.05 in follow-up time ≤ 3 years stratum. Table S7. Metabolites associated with lung cancer risk at P -value < 0.05 in follow-up time > 3 years stratum. Table S8. Metabolites associated with lung cancer risk at P -value < 0.05 in never smokers stratum. Table S9. Metabolites associated with lung cancer risk at P -value < 0.05 in ever smokers stratum. Table S10. Metabolites associated with lung cancer risk at P -value < 0.05 in localized lung cancer stage stratum. Table S11. Metabolites associated with lung cancer risk at P -value < 0.05 in reginal lung cancer stage stratum. Table S12. Metabolites associated with lung cancer risk at P -value < 0.05 in distant lung cancer stage stratum. Table S13. Metabolites associated with lung cancer risk at P -value < 0.05 in squamous cell carcinoma stratum. Table S14. Metabolites associated with lung cancer risk at P -value < 0.05 in adenocarcinoma stratum.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tang, Z., Liang, D., Deubler, E.L. et al. Lung cancer metabolomics: a pooled analysis in the Cancer Prevention Studies. BMC Med 22 , 262 (2024). https://doi.org/10.1186/s12916-024-03473-1
Download citation
Received : 06 December 2023
Accepted : 10 June 2024
Published : 24 June 2024
DOI : https://doi.org/10.1186/s12916-024-03473-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
- Metabolomics
- Lipid metabolism
- Amino acid metabolism
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]

What causes lung cancer other than cigarettes?
- By Health IQ digital
- Jun 21, 2024

Lung cancer is often associated with cigarette smoking, but various other factors can contribute to the development of this disease. Understanding these factors is crucial for prevention and early detection. This article explores several causes of lung cancer beyond cigarette smoking , providing insights into how each factor plays a role in increasing the risk.
Exposure to radon gas
Radon is a naturally occurring radioactive gas that can accumulate in homes and buildings, particularly in areas with poor ventilation. It is produced by the breakdown of uranium in soil, rock, and water. When radon gas is inhaled, it can damage the cells lining the lungs, leading to lung cancer over time. Testing for radon and mitigating high levels can significantly reduce the risk.
Secondhand smoke
While not as directly harmful as smoking itself, secondhand smoke is a significant risk factor for lung cancer. It involves inhaling smoke from burning tobacco products or the smoke exhaled by smokers. Even brief exposure to secondhand smoke can damage lung tissue, increasing the risk of lung cancer, particularly among non-smokers living or working with smokers.
Occupational hazards
Certain occupations expose workers to carcinogens that can increase the risk of lung cancer. For example, asbestos, a mineral fiber used in construction and manufacturing, has been linked to lung cancer and mesothelioma. Workers in mining, construction, and shipbuilding industries may be at higher risk due to exposure to asbestos and other harmful substances like silica, diesel exhaust, and certain chemicals.
Air pollution
Outdoor air pollution, particularly in urban areas with heavy traffic and industrial activities, has increased lung cancer risk. Pollutants such as particulate matter, nitrogen dioxide, and sulfur dioxide can damage lung tissue and lead to cancer over time. Additionally, indoor air pollution from burning wood or coal for cooking and heating can contribute to lung cancer risk.
Genetic factors
Genetics can play a role in an individual’s susceptibility to lung cancer. Some people inherit genetic mutations that increase their risk, regardless of smoking status. A family history of lung cancer can be an indicator of a genetic predisposition, making it essential for individuals with a family history to be vigilant about other risk factors and undergo regular screenings.
Radiation therapy
Individuals who have undergone radiation therapy to the chest for other cancers, such as breast or Hodgkin’s lymphoma, may have an increased risk of developing lung cancer later in life. The radiation used to treat these cancers can damage the lung tissue, leading to mutations and cancer development over time.
Diet and nutrition
While the relationship between diet and lung cancer is still being studied, some evidence suggests that poor diet and nutrition can contribute to the risk. Diets high in processed foods and low in fruits and vegetables may be linked to an increased risk of lung cancer. Antioxidants and other nutrients found in fruits and vegetables are believed to help protect cells from damage that can lead to cancer.
Certain infections have been associated with an increased risk of lung cancer. For instance, the human papillomavirus (HPV) has been linked to various cancers, including lung cancer. Additionally, tuberculosis and other chronic lung infections can cause scarring and inflammation in the lungs, potentially leading to cancer.
Previous lung disease
Chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis can increase the risk of lung cancer. These conditions cause long-term inflammation and damage to lung tissue, leading to cancerous cell development.
Age and gender
Age is a significant risk factor for lung cancer, with most cases occurring in individuals over 65. While men historically have higher rates of lung cancer, the gap between men and women is narrowing, possibly due to changes in smoking patterns and exposure to other risk factors.
Preventive measures
Preventing lung cancer involves addressing the various risk factors beyond smoking. Here are some preventive measures:
- Radon test: Ensure your home is tested for radon, and take mitigation steps if high levels are found.
- Avoid secondhand smoke: Avoid environments where you may be exposed to secondhand smoke and encourage smoking cessation among family members and colleagues.
- Occupational safety: Follow safety guidelines and use protective equipment if you work in an industry that exposes you to carcinogens.
- Reduce air pollution exposure: Limit outdoor activities on days with high air pollution and ensure good ventilation when using indoor stoves or fireplaces.
- Healthy diet: Maintain a diet rich in fruits and vegetables to give your body essential nutrients and antioxidants.
- Regular screenings: If you have a family history of lung cancer or other risk factors, undergo regular screenings to detect any early signs of lung cancer.
Reducing lung cancer risk
Lung cancer is a complex disease with multiple causes beyond cigarette smoking. Understanding these risk factors and taking preventive measures can significantly reduce the risk of developing lung cancer. By being aware of environmental exposures, occupational hazards, genetic predispositions, and lifestyle choices, individuals can take proactive steps to protect their lung health. Early detection through regular screenings and maintaining a healthy lifestyle are critical components in the fight against lung cancer.
By addressing these factors, we can work towards reducing the incidence of lung cancer and improving outcomes for those affected by this disease .
This story was created using AI technology.
Sign up for Rolling Out news straight to your inbox.

- air pollution , diet and nutrition , genetic factors , Infections , lung cancer , occupational hazards , preventive measures , radiation therapy , radon gas , secondhand smoke

7 common reasons people have panic attacks

What causes seizures in children

How commuting with co-workers can lead to an affair

Why arguing and talking loudly raise blood pressure

Is it a bad cold or a COVID symptom?

Why some men don’t wipe themselves properly
- More in Health IQ

How hair implants can help grow your hair

7 common symptoms of a heart attack families should know
- Community News
- Justice For All
- All Entertainment
- Reality Check
- All Culture
- Relationships
- Cocktail & Beer
- Creative Lens
- All Business
- Black Intellectuals
- Diversity Equity & Focus
- Sisters with Superpowers
- Home Ownership & Real Estate
- Entrepreneurs & Business Leaders
- Executive Suite
- Finance & Wealth
- Marketing & Branding
- Be the Match Atlanta
- Food & Nutrition
- Browse Places & Events
- Visit Hōtel
- HBCU Culture
- Privacy Policy
Change Location
Find awesome listings near you.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Office on Smoking and Health (US). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2006.

The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General.
1 introduction, summary, and conclusions.
- Introduction
The topic of passive or involuntary smoking was first addressed in the 1972 U.S. Surgeon General’s report ( The Health Consequences of Smoking , U.S. Department of Health, Education, and Welfare [USDHEW] 1972 ), only eight years after the first Surgeon General’s report on the health consequences of active smoking ( USDHEW 1964 ). Surgeon General Dr. Jesse Steinfeld had raised concerns about this topic, leading to its inclusion in that report. According to the 1972 report, nonsmokers inhale the mixture of sidestream smoke given off by a smoldering cigarette and mainstream smoke exhaled by a smoker, a mixture now referred to as “secondhand smoke” or “environmental tobacco smoke.” Cited experimental studies showed that smoking in enclosed spaces could lead to high levels of cigarette smoke components in the air. For carbon monoxide ( CO ) specifically, levels in enclosed spaces could exceed levels then permitted in outdoor air. The studies supported a conclusion that “an atmosphere contaminated with tobacco smoke can contribute to the discomfort of many individuals” ( USDHEW 1972 , p. 7). The possibility that CO emitted from cigarettes could harm persons with chronic heart or lung disease was also mentioned.
Secondhand tobacco smoke was then addressed in greater depth in Chapter 4 (Involuntary Smoking) of the 1975 Surgeon General’s report, The Health Consequences of Smoking ( USDHEW 1975 ). The chapter noted that involuntary smoking takes place when nonsmokers inhale both sidestream and exhaled mainstream smoke and that this “smoking” is “involuntary” when “the exposure occurs as an unavoidable consequence of breathing in a smoke-filled environment” (p. 87). The report covered exposures and potential health consequences of involuntary smoking, and the researchers concluded that smoking on buses and airplanes was annoying to nonsmokers and that involuntary smoking had potentially adverse consequences for persons with heart and lung diseases. Two studies on nicotine concentrations in nonsmokers raised concerns about nicotine as a contributing factor to atherosclerotic cardiovascular disease in nonsmokers.
The 1979 Surgeon General’s report, Smoking and Health: A Report of the Surgeon General ( USDHEW 1979 ), also contained a chapter entitled “Involuntary Smoking.” The chapter stressed that “attention to involuntary smoking is of recent vintage, and only limited information regarding the health effects of such exposure upon the nonsmoker is available” (p. 11–35). The chapter concluded with recommendations for research including epidemiologic and clinical studies. The 1982 Surgeon General’s report specifically addressed smoking and cancer ( U.S. Department of Health and Human Services [USDHHS] 1982 ). By 1982, there were three published epidemiologic studies on involuntary smoking and lung cancer, and the 1982 Surgeon General’s report included a brief chapter on this topic. That chapter commented on the methodologic difficulties inherent in such studies, including exposure assessment, the lengthy interval during which exposures are likely to be relevant, and accounting for exposures to other carcinogens. Nonetheless, the report concluded that “Although the currently available evidence is not sufficient to conclude that passive or involuntary smoking causes lung cancer in nonsmokers, the evidence does raise concern about a possible serious public health problem” (p. 251).
Involuntary smoking was also reviewed in the 1984 report, which focused on chronic obstructive pulmonary disease and smoking ( USDHHS 1984 ). Chapter 7 (Passive Smoking) of that report included a comprehensive review of the mounting information on smoking by parents and the effects on respiratory health of their children, data on irritation of the eye, and the more limited evidence on pulmonary effects of involuntary smoking on adults. The chapter began with a compilation of measurements of tobacco smoke components in various indoor environments. The extent of the data had increased substantially since 1972. By 1984, the data included measurements of more specific indicators such as acrolein and nicotine, and less specific indicators such as particulate matter ( PM ), nitrogen oxides, and CO . The report reviewed new evidence on exposures of nonsmokers using bio-markers, with substantial information on levels of cotinine, a major nicotine metabolite. The report anticipated future conclusions with regard to respiratory effects of parental smoking on child respiratory health ( Table 1.1 ).
Conclusions from previous Surgeon General’s reports on the health effects of secondhand smoke exposure
Involuntary smoking was the topic for the entire 1986 Surgeon General’s report, The Health Consequences of Involuntary Smoking ( USDHHS 1986 ). In its 359 pages, the report covered the full breadth of the topic, addressing toxicology and dosimetry of tobacco smoke; the relevant evidence on active smoking; patterns of exposure of nonsmokers to tobacco smoke; the epidemiologic evidence on involuntary smoking and disease risks for infants, children, and adults; and policies to control involuntary exposure to tobacco smoke. That report concluded that involuntary smoking caused lung cancer in lifetime nonsmoking adults and was associated with adverse effects on respiratory health in children. The report also stated that simply separating smokers and nonsmokers within the same airspace reduced but did not eliminate exposure to secondhand smoke. All of these findings are relevant to public health and public policy ( Table 1.1 ). The lung cancer conclusion was based on extensive information already available on the carcinogenicity of active smoking, the qualitative similarities between secondhand and mainstream smoke, the uptake of tobacco smoke components by nonsmokers, and the epidemiologic data on involuntary smoking. The three major conclusions of the report ( Table 1.2 ), led Dr. C. Everett Koop, Surgeon General at the time, to comment in his preface that “the right of smokers to smoke ends where their behavior affects the health and well-being of others; furthermore, it is the smokers’ responsibility to ensure that they do not expose nonsmokers to the potential [ sic ] harmful effects of tobacco smoke” ( USDHHS 1986 , p. xii).
Major conclusions of the 1986 Surgeon General’s report, The Health Consequences of Involuntary Smoking
Two other reports published in 1986 also reached the conclusion that involuntary smoking increased the risk for lung cancer. The International Agency for Research on Cancer ( IARC ) of the World Health Organization concluded that “passive smoking gives rise to some risk of cancer” ( IARC 1986 , p. 314). In its monograph on tobacco smoking, the agency supported this conclusion on the basis of the characteristics of sidestream and mainstream smoke, the absorption of tobacco smoke materials during an involuntary exposure, and the nature of dose-response relationships for carcinogenesis. In the same year, the National Research Council ( NRC ) also concluded that involuntary smoking increases the incidence of lung cancer in nonsmokers ( NRC 1986 ). In reaching this conclusion, the NRC report cited the biologic plausibility of the association between exposure to secondhand smoke and lung cancer and the supporting epidemiologic evidence. On the basis of a pooled analysis of the epidemiologic data adjusted for bias, the report concluded that the best estimate for the excess risk of lung cancer in nonsmokers married to smokers was 25 percent, compared with nonsmokers married to nonsmokers. With regard to the effects of involuntary smoking on children, the NRC report commented on the literature linking secondhand smoke exposures from parental smoking to increased risks for respiratory symptoms and infections and to a slightly diminished rate of lung growth.
Since 1986, the conclusions with regard to both the carcinogenicity of secondhand smoke and the adverse effects of parental smoking on the health of children have been echoed and expanded ( Table 1.3 ). In 1992, the U.S. Environmental Protection Agency ( EPA ) published its risk assessment of secondhand smoke as a carcinogen ( USEPA 1992 ). The agency’s evaluation drew on toxicologic information on secondhand smoke and the extensive literature on active smoking. A comprehensive meta-analysis of the 31 epidemiologic studies of secondhand smoke and lung cancer published up to that time was central to the decision to classify secondhand smoke as a group A carcinogen—namely, a known human carcinogen. Estimates of approximately 3,000 U.S. lung cancer deaths per year in non-smokers were attributed to secondhand smoke. The report also covered other respiratory health effects in children and adults and concluded that involuntary smoking is causally associated with several adverse respiratory effects in children. There was also a quantitative risk assessment for the impact of involuntary smoking on childhood asthma and lower respiratory tract infections in young children.
Selected major reports, other than those of the U.S. Surgeon General, addressing adverse effects from exposure to tobacco smoke
In the decade since the 1992 EPA report, scientific panels continued to evaluate the mounting evidence linking involuntary smoking to adverse health effects ( Table 1.3 ). The most recent was the 2005 report of the California EPA ( Cal/EPA 2005 ). Over time, research has repeatedly affirmed the conclusions of the 1986 Surgeon General’s reports and studies have further identified causal associations of involuntary smoking with diseases and other health disorders. The epidemiologic evidence on involuntary smoking has markedly expanded since 1986, as have the data on exposure to tobacco smoke in the many environments where people spend time. An understanding of the mechanisms by which involuntary smoking causes disease has also deepened.
As part of the environmental health hazard assessment, Cal/EPA identified specific health effects causally associated with exposure to secondhand smoke. The agency estimated the annual excess deaths in the United States that are attributable to secondhand smoke exposure for specific disorders: sudden infant death syndrome ( SIDS ), cardiac-related illnesses (ischemic heart disease), and lung cancer ( Cal/EPA 2005 ). For the excess incidence of other health outcomes, either new estimates were provided or estimates from the 1997 health hazard assessment were used without any revisions ( Cal/EPA 1997 ). Overall, Cal/EPA estimated that about 50,000 excess deaths result annually from exposure to secondhand smoke ( Cal/EPA 2005 ). Estimated annual excess deaths for the total U.S. population are about 3,400 (a range of 3,423 to 8,866) from lung cancer, 46,000 (a range of 22,700 to 69,600) from cardiac-related illnesses, and 430 from SIDS. The agency also estimated that between 24,300 and 71,900 low birth weight or pre-term deliveries, about 202,300 episodes of childhood asthma (new cases and exacerbations), between 150,000 and 300,000 cases of lower respiratory illness in children, and about 789,700 cases of middle ear infections in children occur each year in the United States as a result of exposure to secondhand smoke.
This new 2006 Surgeon General’s report returns to the topic of involuntary smoking. The health effects of involuntary smoking have not received comprehensive coverage in this series of reports since 1986. Reports since then have touched on selected aspects of the topic: the 1994 report on tobacco use among young people ( USDHHS 1994 ), the 1998 report on tobacco use among U.S. racial and ethnic minorities ( USDHHS 1998 ), and the 2001 report on women and smoking ( USDHHS 2001 ). As involuntary smoking remains widespread in the United States and elsewhere, the preparation of this report was motivated by the persistence of involuntary smoking as a public health problem and the need to evaluate the substantial new evidence reported since 1986. This report substantially expands the list of topics that were included in the 1986 report. Additional topics include SIDS , developmental effects, and other reproductive effects; heart disease in adults; and cancer sites beyond the lung. For some associations of involuntary smoking with adverse health effects, only a few studies were reviewed in 1986 (e. g ., ear disease in children); now, the relevant literature is substantial. Consequently, this report uses meta-analysis to quantitatively summarize evidence as appropriate. Following the approach used in the 2004 report ( The Health Consequences of Smoking , USDHHS 2004 ), this 2006 report also systematically evaluates the evidence for causality, judging the extent of the evidence available and then making an inference as to the nature of the association.
Organization of the Report
This twenty-ninth report of the Surgeon General examines the topics of toxicology of secondhand smoke, assessment and prevalence of exposure to secondhand smoke, reproductive and developmental health effects, respiratory effects of exposure to secondhand smoke in children and adults, cancer among adults, cardiovascular diseases, and the control of secondhand smoke exposure.
This introductory chapter (Chapter 1) includes a discussion of the concept of causation and introduces concepts of causality that are used throughout this report; this chapter also summarizes the major conclusions of the report. Chapter 2 (Toxicology of Secondhand Smoke) sets out a foundation for interpreting the observational evidence that is the focus of most of the following chapters. The discussion details the mechanisms that enable tobacco smoke components to injure the respiratory tract and cause nonmalignant and malignant diseases and other adverse effects. Chapter 3 (Assessment of Exposure to Secondhand Smoke) provides a perspective on key factors that determine exposures of people to secondhand smoke in indoor environments, including building designs and operations, atmospheric markers of secondhand smoke, exposure models, and biomarkers of exposure to secondhand smoke. Chapter 4 (Prevalence of Exposure to Secondhand Smoke) summarizes findings that focus on nicotine measurements in the air and cotinine measurements in biologic materials. The chapter includes exposures in the home, workplace, public places, and special populations. Chapter 5 (Reproductive and Developmental Effects from Exposure to Secondhand Smoke) reviews the health effects on reproduction, on infants, and on child development. Chapter 6 (Respiratory Effects in Children from Exposure to Secondhand Smoke) examines the effects of parental smoking on the respiratory health of children. Chapter 7 (Cancer Among Adults from Exposure to Secondhand Smoke) summarizes the evidence on cancer of the lung, breast, nasal sinuses, and the cervix. Chapter 8 (Cardiovascular Diseases from Exposure to Secondhand Smoke) discusses coronary heart disease ( CHD ), stroke, and subclinical vascular disease. Chapter 9 (Respiratory Effects in Adults from Exposure to Secondhand Smoke) examines odor and irritation, respiratory symptoms, lung function, and respiratory diseases such as asthma and chronic obstructive pulmonary disease. Chapter 10 (Control of Secondhand Smoke Exposure) considers measures used to control exposure to secondhand smoke in public places, including legislation, education, and approaches based on building designs and operations. The report concludes with “A Vision for the Future.” Major conclusions of the report were distilled from the chapter conclusions and appear later in this chapter.
Preparation of the Report
This report of the Surgeon General was prepared by the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Coordinating Center for Health Promotion, Centers for Disease Control and Prevention ( CDC ), and U.S. DHHS. Initial chapters were written by 22 experts who were selected because of their knowledge of a particular topic. The contributions of the initial experts were consolidated into 10 major chapters that were then reviewed by more than 40 peer reviewers. The entire manuscript was then sent to more than 30 scientists and experts who reviewed it for its scientific integrity. After each review cycle, the drafts were revised by the scientific editors on the basis of the experts’ comments. Subsequently, the report was reviewed by various institutes and agencies within U.S. DHHS. Publication lags, even short ones, prevent an up-to-the-minute inclusion of all recently published articles and data. Therefore, by the time the public reads this report, there may be additional published studies or data. To provide published information as current as possible, this report includes an Appendix of more recent studies that represent major additions to the literature.
This report is also accompanied by a companion database of key evidence that is accessible through the Internet ( http://www.cdc.gov/tobacco ). The database includes a uniform description of the studies and results on the health effects of exposure to secondhand smoke that were presented in a format compatible with abstraction into standardized tables. Readers of the report may access these data for additional analyses, tables, or figures.
- Definitions and Terminology
The inhalation of tobacco smoke by nonsmokers has been variably referred to as “passive smoking” or “involuntary smoking.” Smokers, of course, also inhale secondhand smoke. Cigarette smoke contains both particles and gases generated by the combustion at high temperatures of tobacco, paper, and additives. The smoke inhaled by nonsmokers that contaminates indoor spaces and outdoor environments has often been referred to as “secondhand smoke” or “environmental tobacco smoke.” This inhaled smoke is the mixture of sidestream smoke released by the smoldering cigarette and the mainstream smoke that is exhaled by a smoker. Sidestream smoke, generated at lower temperatures and under somewhat different combustion conditions than mainstream smoke, tends to have higher concentrations of many of the toxins found in cigarette smoke ( USDHHS 1986 ). However, it is rapidly diluted as it travels away from the burning cigarette.
Secondhand smoke is an inherently dynamic mixture that changes in characteristics and concentration with the time since it was formed and the distance it has traveled. The smoke particles change in size and composition as gaseous components are volatilized and moisture content changes; gaseous elements of secondhand smoke may be adsorbed onto materials, and particle concentrations drop with both dilution in the air or environment and impaction on surfaces, including the lungs or on the body. Because of its dynamic nature, a specific quantitative definition of secondhand smoke cannot be offered.
This report uses the term secondhand smoke in preference to environmental tobacco smoke, even though the latter may have been used more frequently in previous reports. The descriptor “secondhand” captures the involuntary nature of the exposure, while “environmental” does not. This report also refers to the inhalation of secondhand smoke as involuntary smoking, acknowledging that most nonsmokers do not want to inhale tobacco smoke. The exposure of the fetus to tobacco smoke, whether from active smoking by the mother or from her exposure to secondhand smoke, also constitutes involuntary smoking.
- Evidence Evaluation
Following the model of the 1964 report, the Surgeon General’s reports on smoking have included comprehensive compilations of the evidence on the health effects of smoking. The evidence is analyzed to identify causal associations between smoking and disease according to enunciated principles, sometimes referred to as the “Surgeon General’s criteria” or the “Hill” criteria (after Sir Austin Bradford Hill) for causality ( USDHEW 1964 ; USDHHS 2004 ). Application of these criteria involves covering all relevant observational and experimental evidence. The criteria, offered in a brief chapter of the 1964 report entitled “Criteria for Judgment,” included (1) the consistency of the association, (2) the strength of the association, (3) the specificity of the association, (4) the temporal relationship of the association, and (5) the coherence of the association. Although these criteria have been criticized (e. g ., Rothman and Greenland 1998 ), they have proved useful as a framework for interpreting evidence on smoking and other postulated causes of disease, and for judging whether causality can be inferred.
In the 2004 report of the Surgeon General, The Health Consequences of Smoking , the framework for interpreting evidence on smoking and health was revisited in depth for the first time since the 1964 report ( USDHHS 2004 ). The 2004 report provided a four-level hierarchy for interpreting evidence ( Table 1.4 ). The categories acknowledge that evidence can be “suggestive” but not adequate to infer a causal relationship, and also allows for evidence that is “suggestive of no causal relationship.” Since the 2004 report, the individual chapter conclusions have consistently used this four-level hierarchy ( Table 1.4 ), but evidence syntheses and other summary statements may use either the term “increased risk” or “cause” to describe instances in which there is sufficient evidence to conclude that active or involuntary smoking causes a disease or condition. This four-level framework also sharply and completely separates conclusions regarding causality from the implications of such conclusions.
Four-level hierarchy for classifying the strength of causal inferences based on available evidence
That same framework was used in this report on involuntary smoking and health. The criteria dating back to the 1964 Surgeon General’s report remain useful as guidelines for evaluating evidence ( USDHEW 1964 ), but they were not intended to be applied strictly or as a “checklist” that needed to be met before the designation of “causal” could be applied to an association. In fact, for involuntary smoking and health, several of the criteria will not be met for some associations. Specificity, referring to a unique exposure-disease relationship (e. g ., the association between thalidomide use during pregnancy and unusual birth defects), can be set aside as not relevant, as all of the health effects considered in this report have causes other than involuntary smoking. Associations are considered more likely to be causal as the strength of an association increases because competing explanations become less plausible alternatives. However, based on knowledge of dosimetry and mechanisms of injury and disease causation, the risk is anticipated to be only slightly or modestly increased for some associations of involuntary smoking with disease, such as lung cancer, particularly when the very strong relative risks found for active smokers are compared with those for lifetime nonsmokers. The finding of only a small elevation in risk, as in the example of spousal smoking and lung cancer risk in lifetime nonsmokers, does not weigh against a causal association; however, alternative explanations for a risk of a small magnitude need full exploration and cannot be so easily set aside as alternative explanations for a stronger association. Consistency, coherence, and the temporal relationship of involuntary smoking with disease are central to the interpretations in this report. To address coherence, the report draws not only on the evidence for involuntary smoking, but on the even more extensive literature on active smoking and disease.
Although the evidence reviewed in this report comes largely from investigations of secondhand smoke specifically, the larger body of evidence on active smoking is also relevant to many of the associations that were evaluated. The 1986 report found secondhand smoke to be qualitatively similar to mainstream smoke inhaled by the smoker and concluded that secondhand smoke would be expected to have “a toxic and carcinogenic potential that would not be expected to be qualitatively different from that of MS [mainstream smoke]” ( USDHHS 1986 , p. 23). The 2004 report of the Surgeon General revisited the health consequences of active smoking ( USDHHS 2004 ), and the conclusions substantially expanded the list of diseases and conditions caused by smoking. Chapters in the present report consider the evidence on active smoking that is relevant to biologic plausibility for causal associations between involuntary smoking and disease. The reviews included in this report cover evidence identified through search strategies set out in each chapter. Of necessity, the evidence on mechanisms was selectively reviewed. However, an attempt was made to cover all health studies through specified target dates. Because of the substantial amount of time involved in preparing this report, lists of new key references published after these cut-off dates are included in an Appendix . Literature reviews were extended when new evidence was sufficient to possibly change the level of a causal conclusion.
- Major Conclusions
This report returns to involuntary smoking, the topic of the 1986 Surgeon General’s report. Since then, there have been many advances in the research on secondhand smoke, and substantial evidence has been reported over the ensuing 20 years. This report uses the revised language for causal conclusions that was implemented in the 2004 Surgeon General’s report ( USDHHS 2004 ). Each chapter provides a comprehensive review of the evidence, a quantitative synthesis of the evidence if appropriate, and a rigorous assessment of sources of bias that may affect interpretations of the findings. The reviews in this report reaffirm and strengthen the findings of the 1986 report. With regard to the involuntary exposure of nonsmokers to tobacco smoke, the scientific evidence now supports the following major conclusions:
- Secondhand smoke causes premature death and disease in children and in adults who do not smoke.
- Children exposed to secondhand smoke are at an increased risk for sudden infant death syndrome ( SIDS ), acute respiratory infections, ear problems, and more severe asthma. Smoking by parents causes respiratory symptoms and slows lung growth in their children.
- Exposure of adults to secondhand smoke has immediate adverse effects on the cardiovascular system and causes coronary heart disease and lung cancer.
- The scientific evidence indicates that there is no risk-free level of exposure to secondhand smoke.
- Many millions of Americans, both children and adults, are still exposed to secondhand smoke in their homes and workplaces despite substantial progress in tobacco control.
- Eliminating smoking in indoor spaces fully protects nonsmokers from exposure to secondhand smoke. Separating smokers from nonsmokers, cleaning the air, and ventilating buildings cannot eliminate exposures of nonsmokers to secondhand smoke.
- Chapter Conclusions
Chapter 2 Toxicology of Secondhand Smoke
Evidence of carcinogenic effects from secondhand smoke exposure.
- 1. More than 50 carcinogens have been identified in sidestream and secondhand smoke.
- 2. The evidence is sufficient to infer a causal relationship between exposure to secondhand smoke and its condensates and tumors in laboratory animals.
- 3. The evidence is sufficient to infer that exposure of nonsmokers to secondhand smoke causes a significant increase in urinary levels of metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone ( NNK ). The presence of these metabolites links exposure to secondhand smoke with an increased risk for lung cancer.
- 4. The mechanisms by which secondhand smoke causes lung cancer are probably similar to those observed in smokers. The overall risk of secondhand smoke exposure, compared with active smoking, is diminished by a substantially lower carcinogenic dose.
Mechanisms of Respiratory Tract Injury and Disease Caused by Secondhand Smoke Exposure
- 5. The evidence indicates multiple mechanisms by which secondhand smoke exposure causes injury to the respiratory tract.
- 6. The evidence indicates mechanisms by which secondhand smoke exposure could increase the risk for sudden infant death syndrome.
Mechanisms of Secondhand Smoke Exposure and Heart Disease
- 7. The evidence is sufficient to infer that exposure to secondhand smoke has a prothrombotic effect.
- 8. The evidence is sufficient to infer that exposure to secondhand smoke causes endothelial cell dysfunctions.
- 9. The evidence is sufficient to infer that exposure to secondhand smoke causes atherosclerosis in animal models.
Chapter 3. Assessment of Exposure to Secondhand Smoke
Building designs and operations.
- 1. Current heating, ventilating, and air conditioning systems alone cannot control exposure to secondhand smoke.
- 2. The operation of a heating, ventilating, and air conditioning system can distribute secondhand smoke throughout a building.
Exposure Models
- 3. Atmospheric concentration of nicotine is a sensitive and specific indicator for secondhand smoke.
- 4. Smoking increases indoor particle concentrations.
- 5. Models can be used to estimate concentrations of secondhand smoke.
Biomarkers of Exposure to Secondhand Smoke
- 6. Biomarkers suitable for assessing recent exposures to secondhand smoke are available.
- 7. At this time, cotinine, the primary proximate metabolite of nicotine, remains the biomarker of choice for assessing secondhand smoke exposure.
- 8. Individual biomarkers of exposure to secondhand smoke represent only one component of a complex mixture, and measurements of one marker may not wholly reflect an exposure to other components of concern as a result of involuntary smoking.
Chapter 4. Prevalence of Exposure to Secondhand Smoke
- The evidence is sufficient to infer that large numbers of nonsmokers are still exposed to secondhand smoke.
- Exposure of nonsmokers to secondhand smoke has declined in the United States since the 1986 Surgeon General’s report, The Health Consequences of Involuntary Smoking .
- The evidence indicates that the extent of secondhand smoke exposure varies across the country.
- Homes and workplaces are the predominant locations for exposure to secondhand smoke.
- Exposure to secondhand smoke tends to be greater for persons with lower incomes.
- Exposure to secondhand smoke continues in restaurants, bars, casinos, gaming halls, and vehicles.
Chapter 5. Reproductive and Developmental Effects from Exposure to Secondhand Smoke
- 1. The evidence is inadequate to infer the presence or absence of a causal relationship between maternal exposure to secondhand smoke and female fertility or fecundability. No data were found on paternal exposure to secondhand smoke and male fertility or fecundability.
Pregnancy (Spontaneous Abortion and Perinatal Death)
- 2. The evidence is inadequate to infer the presence or absence of a causal relationship between maternal exposure to secondhand smoke during pregnancy and spontaneous abortion.
Infant Deaths
- 3. The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and neonatal mortality.
Sudden Infant Death Syndrome
- 4. The evidence is sufficient to infer a causal relationship between exposure to secondhand smoke and sudden infant death syndrome.
Preterm Delivery
- 5. The evidence is suggestive but not sufficient to infer a causal relationship between maternal exposure to secondhand smoke during pregnancy and preterm delivery.
Low Birth Weight
- 6. The evidence is sufficient to infer a causal relationship between maternal exposure to secondhand smoke during pregnancy and a small reduction in birth weight.
Congenital Malformations
- 7. The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and congenital malformations.
Cognitive Development
- 8. The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and cognitive functioning among children.
Behavioral Development
- 9. The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and behavioral problems among children.
Height/Growth
- 10. The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and children’s height/growth.
Childhood Cancer
- 11. The evidence is suggestive but not sufficient to infer a causal relationship between prenatal and postnatal exposure to secondhand smoke and childhood cancer.
- 12. The evidence is inadequate to infer the presence or absence of a causal relationship between maternal exposure to secondhand smoke during pregnancy and childhood cancer.
- 13. The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke during infancy and childhood cancer.
- 14. The evidence is suggestive but not sufficient to infer a causal relationship between prenatal and postnatal exposure to secondhand smoke and childhood leukemias.
- 15. The evidence is suggestive but not sufficient to infer a causal relationship between prenatal and postnatal exposure to secondhand smoke and childhood lymphomas.
- 16. The evidence is suggestive but not sufficient to infer a causal relationship between prenatal and postnatal exposure to secondhand smoke and childhood brain tumors.
- 17. The evidence is inadequate to infer the presence or absence of a causal relationship between prenatal and postnatal exposure to secondhand smoke and other childhood cancer types.
Chapter 6. Respiratory Effects in Children from Exposure to Secondhand Smoke
Lower respiratory illnesses in infancy and early childhood.
- 1. The evidence is sufficient to infer a causal relationship between secondhand smoke exposure from parental smoking and lower respiratory illnesses in infants and children.
- 2. The increased risk for lower respiratory illnesses is greatest from smoking by the mother.
Middle Ear Disease and Adenotonsillectomy
- 3. The evidence is sufficient to infer a causal relationship between parental smoking and middle ear disease in children, including acute and recurrent otitis media and chronic middle ear effusion.
- 4. The evidence is suggestive but not sufficient to infer a causal relationship between parental smoking and the natural history of middle ear effusion.
- 5. The evidence is inadequate to infer the presence or absence of a causal relationship between parental smoking and an increase in the risk of adenoidectomy or tonsillectomy among children.
Respiratory Symptoms and Prevalent Asthma in School-Age Children
- 6. The evidence is sufficient to infer a causal relationship between parental smoking and cough, phlegm, wheeze, and breathlessness among children of school age.
- 7. The evidence is sufficient to infer a causal relationship between parental smoking and ever having asthma among children of school age.
Childhood Asthma Onset
- 8. The evidence is sufficient to infer a causal relationship between secondhand smoke exposure from parental smoking and the onset of wheeze illnesses in early childhood.
- 9. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure from parental smoking and the onset of childhood asthma.
- 10. The evidence is inadequate to infer the presence or absence of a causal relationship between parental smoking and the risk of immunoglobulin E-mediated allergy in their children.
Lung Growth and Pulmonary Function
- 11. The evidence is sufficient to infer a causal relationship between maternal smoking during pregnancy and persistent adverse effects on lung function across childhood.
- 12. The evidence is sufficient to infer a causal relationship between exposure to secondhand smoke after birth and a lower level of lung function during childhood.
Chapter 7. Cancer Among Adults from Exposure to Secondhand Smoke
Lung cancer.
- 1. The evidence is sufficient to infer a causal relationship between secondhand smoke exposure and lung cancer among lifetime nonsmokers. This conclusion extends to all secondhand smoke exposure, regardless of location.
- 2. The pooled evidence indicates a 20 to 30 percent increase in the risk of lung cancer from secondhand smoke exposure associated with living with a smoker.
Breast Cancer
- 3. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke and breast cancer.
Nasal Sinus Cavity and Nasopharyngeal Carcinoma
- 4. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and a risk of nasal sinus cancer among nonsmokers.
- 5. The evidence is inadequate to infer the presence or absence of a causal relationship between secondhand smoke exposure and a risk of nasopharyngeal carcinoma among nonsmokers.
Cervical Cancer
- 6. The evidence is inadequate to infer the presence or absence of a causal relationship between secondhand smoke exposure and the risk of cervical cancer among lifetime nonsmokers.
Chapter 8. Cardiovascular Diseases from Exposure to Secondhand Smoke
- The evidence is sufficient to infer a causal relationship between exposure to secondhand smoke and increased risks of coronary heart disease morbidity and mortality among both men and women.
- Pooled relative risks from meta-analyses indicate a 25 to 30 percent increase in the risk of coronary heart disease from exposure to secondhand smoke.
- The evidence is suggestive but not sufficient to infer a causal relationship between exposure to secondhand smoke and an increased risk of stroke.
- Studies of secondhand smoke and subclinical vascular disease, particularly carotid arterial wall thickening, are suggestive but not sufficient to infer a causal relationship between exposure to secondhand smoke and atherosclerosis.
Chapter 9. Respiratory Effects in Adults from Exposure to Secondhand Smoke
Odor and irritation.
- 1. The evidence is sufficient to infer a causal relationship between secondhand smoke exposure and odor annoyance.
- 2. The evidence is sufficient to infer a causal relationship between secondhand smoke exposure and nasal irritation.
- 3. The evidence is suggestive but not sufficient to conclude that persons with nasal allergies or a history of respiratory illnesses are more susceptible to developing nasal irritation from secondhand smoke exposure.
Respiratory Symptoms
- 4. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and acute respiratory symptoms including cough, wheeze, chest tightness, and difficulty breathing among persons with asthma.
- 5. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and acute respiratory symptoms including cough, wheeze, chest tightness, and difficulty breathing among healthy persons.
- 6. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and chronic respiratory symptoms.
Lung Function
- 7. The evidence is suggestive but not sufficient to infer a causal relationship between short-term secondhand smoke exposure and an acute decline in lung function in persons with asthma.
- 8. The evidence is inadequate to infer the presence or absence of a causal relationship between short-term secondhand smoke exposure and an acute decline in lung function in healthy persons.
- 9. The evidence is suggestive but not sufficient to infer a causal relationship between chronic secondhand smoke exposure and a small decrement in lung function in the general population.
- 10. The evidence is inadequate to infer the presence or absence of a causal relationship between chronic secondhand smoke exposure and an accelerated decline in lung function.
- 11. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and adult-onset asthma.
- 12. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and a worsening of asthma control.
Chronic Obstructive Pulmonary Disease
- 13. The evidence is suggestive but not sufficient to infer a causal relationship between secondhand smoke exposure and risk for chronic obstructive pulmonary disease.
- 14. The evidence is inadequate to infer the presence or absence of a causal relationship between secondhand smoke exposure and morbidity in persons with chronic obstructive pulmonary disease.
Chapter 10. Control of Secondhand Smoke Exposure
- Workplace smoking restrictions are effective in reducing secondhand smoke exposure.
- Workplace smoking restrictions lead to less smoking among covered workers.
- Establishing smoke-free workplaces is the only effective way to ensure that secondhand smoke exposure does not occur in the workplace.
- The majority of workers in the United States are now covered by smoke-free policies.
- The extent to which workplaces are covered by smoke-free policies varies among worker groups, across states, and by sociodemographic factors. Workplaces related to the entertainment and hospitality industries have notably high potential for secondhand smoke exposure.
- Evidence from peer-reviewed studies shows that smoke-free policies and regulations do not have an adverse economic impact on the hospitality industry.
- Evidence suggests that exposure to secondhand smoke varies by ethnicity and gender.
- In the United States, the home is now becoming the predominant location for exposure of children and adults to secondhand smoke.
- Total bans on indoor smoking in hospitals, restaurants, bars, and offices substantially reduce secondhand smoke exposure, up to several orders of magnitude with incomplete compliance, and with full compliance, exposures are eliminated.
- Exposures of nonsmokers to secondhand smoke cannot be controlled by air cleaning or mechanical air exchange.
- Methodologic Issues
Much of the evidence on the health effects of involuntary smoking comes from observational epidemiologic studies that were carried out to test hypotheses related to secondhand smoke and risk for diseases and other adverse health effects. The challenges faced in carrying out these studies reflect those of observational research generally: assessment of the relevant exposures and outcomes with sufficient validity and precision, selection of an appropriate study design, identification of an appropriate and sufficiently large study population, and collection of information on other relevant factors that may confound or modify the association being studied. The challenge of accurately classifying secondhand smoke exposures confronts all studies of such exposures, and consequently the literature on approaches to and limitations of exposure classification is substantial. Sources of bias that can affect the findings of epidemiologic studies have been widely discussed ( Rothman and Greenland 1998 ), both in general and in relation to studies of involuntary smoking. Concerns about bias apply to any study of an environmental agent and disease risk: misclassification of exposures or outcomes, confounding effect modification, and proper selection of study participants. In addition, the generalizability of findings from one population to another (external validity) further determines the value of evidence from a study. Another methodologic concern affecting secondhand smoke literature comes from the use of meta-analysis to combine the findings of epidemiologic studies; general concerns related to the use of meta-analysis for observational data and more specific concerns related to involuntary smoking have also been raised. This chapter considers these methodologic issues in anticipation of more specific treatment in the following chapters.
Classification of Secondhand Smoke Exposure
For secondhand smoke, as for any environmental factor that may be a cause of disease, the exposure assessment might encompass the time and place of the exposure, cumulative exposures, exposure during a particular time, or a recent exposure ( Jaakkola and Jaakkola 1997 ; Jaakkola and Samet 1999 ). For example, exposures to secondhand smoke across the full life span may be of interest for lung cancer, while only more recent exposures may be relevant to the exacerbation of asthma. For CHD , both temporally remote and current exposures may affect risk. Assessments of exposures are further complicated by the multiplicity of environments where exposures take place and the difficulty of characterizing the exposure in some locations, such as public places or workplaces. Additionally, exposures probably vary qualitatively and quantitatively over time and across locations because of temporal changes and geographic differences in smoking patterns.
Nonetheless, researchers have used a variety of approaches for exposure assessments in epidemiologic studies of adverse health effects from involuntary smoking. Several core concepts that are fundamental to these approaches are illustrated in Figure 1.1 ( Samet and Jaakkola 1999 ). Cigarette smoking is, of course, the source of most secondhand smoke in the United States, followed by pipes, cigars, and other products. Epidemiologic studies generally focus on assessing the exposure, which is the contact with secondhand smoke. The concentrations of secondhand smoke components in a space depend on the number of smokers and the rate at which they are smoking, the volume into which the smoke is distributed, the rate at which the air in the space exchanges with uncontaminated air, and the rate at which the secondhand smoke is removed from the air. Concentration, exposure, and dose differ in their definitions, although the terms are sometimes used without sharp distinctions. However, surrogate indicators that generally describe a source of exposure may also be used to assess the exposure, such as marriage to a smoker or the number of cigarettes smoked in the home. Biomarkers can provide an indication of an exposure or possibly the dose, but for secondhand smoke they are used for recent exposure only.
The determinants of exposure, dose, and biologically effective dose that underlie the development of health effects from smoking. Source: Samet and Jaakkola (more...)
People are exposed to secondhand smoke in a number of different places, often referred to as “microenvironments” ( NRC 1991 ). A microenvironment is a definable location that has a constant concentration of the contaminant of interest, such as secondhand smoke, during the time that a person is there. Some key microenvironments for secondhand smoke include the home, the workplace, public places, and transportation environments ( Klepeis 1999 ). Based on the microenvironmental model, total exposure can be estimated as the weighted average of the concentrations of secondhand smoke or indicator compounds, such as nicotine, in the microenvironments where time is spent; the weights are the time spent in each microenvironment. Klepeis (1999) illustrates the application of the microenvironmental model with national data from the National Human Activity Pattern Survey conducted by the EPA . His calculations yield an overall estimate of exposure to airborne particles from smoking and of the contributions to this exposure from various microenvironments.
Much of the epidemiologic evidence addresses the consequences of an exposure in a particular microenvironment, such as the home (spousal smoking and lung cancer risk or maternal smoking and risk for asthma exacerbation), or the workplace (exacerbation of asthma by the presence of smokers). Some studies have attempted to cover multiple microenvironments and to characterize exposures over time. For example, in the multicenter study of secondhand smoke exposure and lung cancer carried out in the United States, Fontham and colleagues (1994) assessed exposures during childhood, in workplaces, and at home during adulthood. Questionnaires that assess exposures have been the primary tool used in epidemiologic studies of secondhand smoke and disease. Measurement of biomarkers has been added in some studies, either as an additional and complementary exposure assessment approach or for validating questionnaire responses. Some studies have also measured components of secondhand smoke in the air.
Questionnaires generally address sources of exposure in microenvironments and can be tailored to address the time period of interest. Questionnaires represent the only approach that can be used to assess exposures retrospectively over a life span, because available biomarkers only reflect exposures over recent days or, at most, weeks. Questionnaires on secondhand smoke exposure have been assessed for their reliability and validity, generally based on comparisons with either biomarker or air monitoring data as the “gold” standard ( Jaakkola and Jaakkola 1997 ). Two studies evaluated the reliability of questionnaires on lifetime exposures ( Pron et al. 1988 ; Coultas et al. 1989 ). Both showed a high degree of repeatability for questions concerning whether a spouse had smoked, but a lower reliability for responses concerning the quantitative aspects of an exposure. Emerson and colleagues (1995) evaluated the repeatability of information from parents of children with asthma. They found a high reliability for parent-reported tobacco use and for the number of cigarettes to which the child was exposed in the home during the past week.
To assess validity, questionnaire reports of current or recent exposures have been compared with levels of cotinine and other biomarkers. These studies tend to show a moderate correlation between levels of cotinine and questionnaire indicators of exposures ( Kawachi and Colditz 1996 ; Cal/EPA 1997 ; Jaakkola and Jaakkola 1997 ). However, cotinine levels reflect not only exposure but metabolism and excretion ( Benowitz 1999 ). Consequently, exposure is only one determinant of variation in cotinine levels among persons; there also are individual variations in metabolism and excretion rates. In spite of these sources of variability, mean levels of cotinine vary as anticipated across categories of self-reported exposures ( Cal/EPA 1997 ; Jaakkola and Jaakkola 1997 ), and self-reported exposures are moderately associated with measured levels of markers ( Cal/EPA 1997 ; Jaakkola and Jaakkola 1997 ).
Biomarkers are also used for assessing exposures to secondhand smoke. A number of biomarkers are available, but they vary in their specificity and in the dynamics of the temporal relationship between the exposure and the marker level ( Cal/EPA 1997 ; Benowitz 1999 ). These markers include specific tobacco smoke components (nicotine) or metabolites (cotinine and tobacco-specific nitrosamines), nonspecific biomarkers (thiocyanate and CO ), adducts with tobacco smoke components or metabolites (4-amino-biphenyl hemoglobin adducts, benzo[ a ]pyrene DNA adducts, and polycyclic aromatic hydrocarbon albumin adducts), and nonspecific assays (urinary mutagenicity). Cotinine has been the most widely used biomarker, primarily because of its specificity, half-life, and ease of measurement in body fluids (e. g ., urine, blood, and saliva). Biomarkers are discussed in detail in Chapter 3 (Assessment of Exposure to Secondhand Smoke).
Some epidemiologic studies have also incorporated air monitoring, either direct personal sampling or the indirect approach based on the microenvironmental model. Nicotine, present in the gas phase of secondhand smoke, can be monitored passively with a special filter or actively using a pump and a sorbent. Hammond and Leaderer (1987) first described a diffusion monitor for the passive sampling of nicotine in 1987; this device has now been widely used to assess concentrations in different environments and to study health effects. Airborne particles have also been measured using active monitoring devices.
Each of these approaches for assessing exposures has strengths and limitations, and preference for one over another will depend on the research question and its context ( Jaakkola and Jaakkola 1997 ; Jaakkola and Samet 1999 ). Questionnaires can be used to characterize sources of exposures, such as smoking by parents. With air concentrations of markers and time-activity information, estimates of secondhand smoke exposures can be made with the microenvironmental model. Biomarkers provide exposure measures that reflect the patterns of exposure and the kinetics of the marker; the cotinine level in body fluids, for example, reflects an exposure during several days. Air monitoring may be useful for validating measurements of exposure. Exposure assessment strategies are matched to the research question and often employ a mixture of approaches determined by feasibility and cost constraints.
Misclassification of Secondhand Smoke Exposure
Misclassification may occur when classifying exposures, outcomes, confounding factors, or modifying factors. Misclassification may be differential on either exposure or outcome, or it may be random ( Armstrong et al. 1992 ). Differential or nonrandom misclassification may either increase or decrease estimates of effect, while random misclassification tends to reduce the apparent effect and weaken the relationship of exposure with disease risk. In studies of secondhand smoke and disease risk, exposure misclassification has been a major consideration in the interpretation of the evidence, although misclassification of health outcome measures has not been a substantial issue in this research. The consequences for epidemiologic studies of misclassification in general are well established ( Rothman and Greenland 1998 ).
An extensive body of literature on the classification of exposures to secondhand smoke is reviewed in this and other chapters, as well as in some publications on the consequences of misclassification ( Wu 1999 ). Two general patterns of exposure misclassification are of concern to secondhand smoke: (1) random misclassification that is not differential by the presence or absence of the health outcome and (2) systematic misclassification that is differential by the health outcome. In studying the health effects of secondhand smoke in adults, there is a further concern as to the classification of the active smoking status (never, current, or former smoking); in studies of children, the accuracy of secondhand smoke exposure classification is the primary methodologic issue around exposure assessment, but unreported active smoking by adolescents is also a concern.
With regard to random misclassification of secondhand smoke exposures, there is an inherent degree of unavoidable measurement error in the exposure measures used in epidemiologic studies. Questionnaires generally assess contact with sources of an exposure (e. g ., smoking in the home or work-place) and cannot capture all exposures nor the intensity of exposures; biomarkers provide an exposure index for a particular time window and have intrinsic variability. Some building-related factors that determine an exposure cannot be assessed accurately by a questionnaire, such as the rate of air exchange and the size of the microenvironment where time is spent, nor can concentrations be assessed accurately by subjective reports of the perceived level of tobacco smoke. In general, random misclassification of exposures tends to reduce the likelihood that studies of secondhand smoke exposure will find an effect. This type of misclassification lessens the contrast between exposure groups, because some truly exposed persons are placed in the unexposed group and some truly unexposed persons are placed in the exposed group. Differential misclassification, also a concern, may increase or decrease associations, depending on the pattern of misreporting.
One particular form of misclassification has been raised with regard to secondhand smoke exposure and lung cancer: the classification of some current or former smokers as lifetime nonsmokers ( USEPA 1992 ; Lee and Forey 1995 ; Hackshaw et al. 1997 ; Wu 1999 ). The resulting bias would tend to increase the apparent association of secondhand smoke with lung cancer, if the misclassified active smokers are also more likely to be classified as involuntary smokers. Most studies of lung cancer and secondhand smoke have used spousal smoking as a main exposure variable. As smoking tends to aggregate between spouses (smokers are more likely to marry smokers), misclassification of active smoking would tend to be differential on the basis of spousal smoking (the exposure under investigation). Because active smoking is strongly associated with increased disease risk, greater misclassification of an actively smoking spouse as a non-smoker among spouses of smokers compared with spouses of nonsmokers would lead to risk estimates for spousal smoking that are biased upward by the effect of active smoking. This type of misclassification is also relevant to studies of spousal exposure and CHD risk or other diseases also caused by active smoking, although the potential for bias is less because the association of active smoking with CHD is not as strong as with lung cancer.
There have been a number of publications on this form of misclassification. Wu (1999) provides a review, and Lee and colleagues (2001) offer an assessment of potential consequences. A number of models have been developed to assess the extent of bias resulting from the misclassification of active smokers as lifetime nonsmokers ( USEPA 1992 ; Hackshaw et al. 1997 ). These models incorporate estimates of the rate of misclassification, the degree of aggregation of smokers by marriage, the prevalence of smoking in the population, and the risk of lung cancer in misclassified smokers ( Wu 1999 ). Although debate about this issue continues, analyses show that estimates of upward bias from misclassifying active smokers as lifetime nonsmokers cannot fully explain the observed increase in risk for lung cancer among lifetime non-smokers married to smokers ( Hackshaw et al. 1997 ; Wu 1999 ).
There is one additional issue related to exposure misclassification. During the time the epidemiologic studies of secondhand smoke have been carried out, exposure has been widespread and almost unavoidable. Therefore, the risk estimates may be biased downward because there are no truly unexposed persons. The 1986 Surgeon General’s report recognized this methodologic issue and noted the need for further data on population exposures to secondhand smoke ( USDHHS 1986 ). This bias was also recognized in the 1986 report of the NRC , and an adjustment for this misclassification was made to the lung cancer estimate ( NRC 1986 ). Similarly, the 1992 report of the EPA commented on background exposure and made an adjustment ( USEPA 1992 ). Some later studies have attempted to address this issue; for example, in a case-control study of active and involuntary smoking and breast cancer in Switzerland, Morabia and colleagues (2000) used a questionnaire to assess exposure and identified a small group of lifetime nonsmokers who also reported no exposure to secondhand smoke. With this subgroup of controls as the reference population, the risks of secondhand smoke exposure were substantially greater for active smoking than when the full control population was used.
This Surgeon General’s report further addresses specific issues of exposure misclassification when they are relevant to the health outcome under consideration.
Use of Meta-Analysis
Meta-analysis refers to the process of evaluating and combining a body of research literature that addresses a common question. Meta-analysis is composed of qualitative and quantitative components. The qualitative component involves the systematic identification of all relevant investigations, a systematic assessment of their characteristics and quality, and the decision to include or exclude studies based on predetermined criteria. Consideration can be directed toward sources of bias that might affect the findings. The quantitative component involves the calculation and display of study results on common scales and, if appropriate, the statistical combination of these results across studies and an exploration of the reasons for any heterogeneity of findings. Viewing the findings of all studies as a single plot provides insights into the consistency of results and the precision of the studies considered. Most meta-analyses are based on published summary results, although they are most powerful when applied to data at the level of individual participants. Meta-analysis is most widely used to synthesize evidence from randomized clinical trials, sometimes yielding findings that were not evident from the results of individual studies. Meta-analysis also has been used extensively to examine bodies of observational evidence.
Beginning with the 1986 NRC report, meta-analysis has been used to summarize the evidence on involuntary smoking and health. Meta-analysis was central to the 1992 EPA risk assessment of secondhand smoke, and a series of meta-analyses supported the conclusions of the 1998 report of the Scientific Committee on Tobacco and Health in the United Kingdom. The central role of meta-analysis in interpreting and applying the evidence related to involuntary smoking and disease has led to focused criticisms of the use of meta-analysis in this context. Several papers that acknowledged support from the tobacco industry have addressed the epidemiologic findings for lung cancer, including the selection and quality of the studies, the methods for meta-analysis, and dose-response associations ( Fleiss and Gross 1991 ; Tweedie and Mengersen 1995 ; Lee 1998 , 1999 ). In a lawsuit brought by the tobacco industry against the EPA, the 1998 decision handed down by Judge William L . Osteen, Sr., in the North Carolina Federal District Court criticized the approach EPA had used to select studies for its meta-analysis and criticized the use of 90 percent rather than 95 percent confidence intervals for the summary estimates ( Flue-Cured Tobacco Cooperative Stabilization Corp. v. United States Environmental Protection Agency , 857 F. Supp. 1137 [M.D.N.C. 1993]). In December 2002, the 4th U.S. Circuit Court of Appeals threw out the lawsuit on the basis that tobacco companies cannot sue the EPA over its secondhand smoke report because the report was not a final agency action and therefore not subject to court review ( Flue-Cured Tobacco Cooperative Stabilization Corp. v. The United States Environmental Protection Agency , No. 98–2407 [4th Cir., December 11, 2002], cited in 17.7 TPLR 2.472 [2003]).
Recognizing that there is still an active discussion around the use of meta-analysis to pool data from observational studies (versus clinical trials), the authors of this Surgeon General’s report used this methodology to summarize the available data when deemed appropriate and useful, even while recognizing that the uncertainty around the meta-analytic estimates may exceed the uncertainty indicated by conventional statistical indices, because of biases either within the observational studies or produced by the manner of their selection. However, a decision to not combine estimates might have produced conclusions that are far more uncertain than the data warrant because the review would have focused on individual study results without considering their overall pattern, and without allowing for a full accounting of different sample sizes and effect estimates.
The possibility of publication bias has been raised as a potential limitation to the interpretation of evidence on involuntary smoking and disease in general, and on lung cancer and secondhand smoke exposure specifically. A 1988 paper by Vandenbroucke used a descriptive approach, called a “funnel plot,” to assess the possibility that publication bias affected the 13 studies considered in a review by Wald and colleagues (1986) . This type of plot characterizes the relationship between the magnitude of estimates and their precision. Vandenbroucke suggested the possibility of publication bias only in reference to the studies of men. Bero and colleagues (1994) concluded that there had not been a publication bias against studies with statistically significant findings, nor against the publication of studies with nonsignificant or mixed findings in the research literature. The researchers were able to identify only five unpublished “negative” studies, of which two were dissertations that tend to be delayed in publication. A subsequent study by Misakian and Bero (1998) did find a delay in the publication of studies with nonsignificant results in comparison with studies having significant results; whether this pattern has varied over the several decades of research on secondhand smoke was not addressed. More recently, Copas and Shi (2000) assessed the 37 studies considered in the meta-analysis by Hackshaw and colleagues (1997) for publication bias. Copas and Shi (2000) found a significant correlation between the estimated risk of exposure and sample size, such that smaller studies tended to have higher values. This pattern suggests the possibility of publication bias. However, using a funnel plot of the same studies, Lubin (1999) found little evidence for publication bias.
On this issue of publication bias, it is critical to distinguish between indirect statistical arguments and arguments based on actual identification of previously unidentified research. The strongest case against substantive publication bias has been made by researchers who mounted intensive efforts to find the possibly missing studies; these efforts have yielded little nothing that would alter published conclusions ( Bero et al. 1994 ; Glantz 2000 ). Presumably because this exposure is a great public health concern, the findings of studies that do not have statistically significant outcomes continue to be published ( Kawachi and Colditz 1996 ).
The quantitative results of the meta-analyses, however, were not determinate in making causal inferences in this Surgeon General’s report. In particular, the level of statistical significance of estimates from the meta-analyses was not a predominant factor in making a causal conclusion. For that purpose, this report relied on the approach and criteria set out in the 1964 and 2004 reports of the Surgeon General, which involved judgments based on an array of quantitative and qualitative considerations that included the degree of heterogeneity in the designs of the studies that were examined. Sometimes this heterogeneity limits the inference from meta-analysis by weakening the rationale for pooling the study results. However, the availability of consistent evidence from heterogenous designs can strengthen the meta-analytic findings by making it unlikely that a common bias could persist across different study designs and populations.
Confounding
Confounding, which refers in this context to the mixing of the effect of another factor with that of secondhand smoke, has been proposed as an explanation for associations of secondhand smoke with adverse health consequences. Confounding occurs when the factor of interest (secondhand smoke) is associated in the data under consideration with another factor (the confounder) that, by itself, increases the risk for the disease ( Rothman and Greenland 1998 ). Correlates of secondhand smoke exposures are not confounding factors unless an exposure to them increases the risk of disease. A factor proposed as a potential confounder is not necessarily an actual confounder unless it fulfills the two elements of the definition. Although lengthy lists of potential confounding factors have been offered as alternatives to direct associations of secondhand smoke exposures with the risk for disease, the factors on these lists generally have not been shown to be confounding in the particular data of interest.
The term confounding also conveys an implicit conceptualization as to the causal pathways that link secondhand smoke and the confounding factor to disease risk. Confounding implies that the confounding factor has an effect on risk that is independent of secondhand smoke exposure. Some factors considered as potential confounders may, however, be in the same causal pathway as a secondhand smoke exposure. Although socioeconomic status ( SES ) is often cited as a potential confounding factor, it may not have an independent effect but can affect disease risk through its association with secondhand smoke exposure ( Figure 1.2 ). This figure shows general alternative relationships among SES, secondhand smoke exposure, and risk for an adverse effect. SES may have a direct effect, or it may indirectly exert its effect through an association with secondhand smoke exposure, or it may confound the relationship between secondhand smoke exposure and disease risk. To control for SES as a potential confounding factor without considering underlying relationships may lead to incorrect risk estimates. For example, controlling for SES would not be appropriate if it is a determinant of secondhand smoke exposure but has no direct effect.
Model for socioeconomic status (SES) and secondhand smoke (SHS) exposure. Arrows indicate directionality of association.
Nonetheless, because the health effects of involuntary smoking have other causes, the possibility of confounding needs careful exploration when assessing associations of secondhand smoke exposure with adverse health effects. In addition, survey data from the last several decades show that secondhand smoke exposure is associated with correlates of lifestyle that may influence the risk for some health effects, thus increasing concerns for the possibility of confounding ( Kawachi and Colditz 1996 ). Survey data from the United States ( Matanoski et al. 1995 ) and the United Kingdom ( Thornton et al. 1994 ) show that adults with secondhand smoke exposures generally tend to have less healthful lifestyles. However, the extent to which these patterns of association can be generalized, either to other countries or to the past, is uncertain.
The potential bias from confounding varies with the association of the confounder to secondhand smoke exposures in a particular study and to the strength of the confounder as a risk factor. The importance of confounding to the interpretation of evidence depends further on the magnitude of the effect of secondhand smoke on disease. As the strength of an association lessens, confounding as an alternative explanation for an association becomes an increasing concern. In prior reviews, confounding has been addressed either quantitatively ( Hackshaw et al. 1997 ) or qualitatively ( Cal/EPA 1997 ; Thun et al. 1999 ). In the chapters in this report that focus on specific diseases, confounding is specifically addressed in the context of potential confounding factors for the particular diseases.
- Tobacco Industry Activities
The evidence on secondhand smoke and disease risk, given the public health and public policy implications, has been reviewed extensively in the published peer-reviewed literature and in evaluations by a number of expert panels. In addition, the evidence has been criticized repeatedly by the tobacco industry and its consultants in venues that have included the peer-reviewed literature, public meetings and hearings, and scientific symposia that included symposia sponsored by the industry. Open criticism in the peer-reviewed literature can strengthen the credibility of scientific evidence by challenging researchers to consider the arguments proposed by critics and to rebut them.
Industry documents indicate that the tobacco industry has engaged in widespread activities, however, that have gone beyond the bounds of accepted scientific practice ( Glantz 1996 ; Ong and Glantz 2000 , 2001 ; Rampton and Stauber 2000 ; Yach and Bialous 2001 ; Hong and Bero 2002 ; Diethelm et al. 2004 ). Through a variety of organized tactics, the industry has attempted to undermine the credibility of the scientific evidence on secondhand smoke. The industry has funded or carried out research that has been judged to be biased, supported scientists to generate letters to editors that criticized research publications, attempted to undermine the findings of key studies, assisted in establishing a scientific society with a journal, and attempted to sustain controversy even as the scientific community reached consensus ( Garne et al. 2005 ). These tactics are not a topic of this report, but to the extent that the scientific literature has been distorted, they are addressed as the evidence is reviewed. This report does not specifically identify tobacco industry sponsorship of publications unless that information is relevant to the interpretation of the findings and conclusions.
- Armstrong BK, White E, Saracci R, editors. Monographs in Epidemiology and Biostatistics. Vol. 21. New York: Oxford University Press; 1992. Principles of Exposure Measurement in Epidemiology.
- Benowitz NL. Biomarkers of environmental tobacco smoke. Environmental Health Perspectives. 1999; 107 (Suppl 2):349–55. [ PMC free article : PMC1566286 ] [ PubMed : 10350520 ]
- Bero LA, Glantz SA, Rennie D. Publication bias and public health policy on environmental tobacco smoke. Journal of the American Medical Association. 1994; 272 (2):133–6. [ PubMed : 8015124 ]
- California Environmental Protection Agency. Health Effects of Exposure to Environmental Tobacco Smoke. Sacramento (CA): California Environmental Protection Agency, Office of Environmental Health Hazard Assessment, Reproductive and Cancer Hazard Assessment Section and Air Toxicology and Epidemiology Section; 1997.
- California Environmental Protection Agency. Part B: Health Effects. Sacramento (CA): California Environmental Protection Agency, Office of Environmental Health Hazard Assessment; 2005. Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant.
- Copas JB, Shi JQ. Reanalysis of epidemiological evidence on lung cancer and passive smoking. British Medical Journal. 2000; 320 (7232):417–8. [ PMC free article : PMC27286 ] [ PubMed : 10669446 ]
- Coultas DB, Peake GT, Samet JM. Questionnaire assessment of lifetime and recent exposure to environmental tobacco smoke. American Journal of Epidemiology. 1989; 130 (2):338–47. [ PubMed : 2750729 ]
- Diethelm PA, Rielle JC, McKee M.The whole truth and nothing but the truth? The research that Phillip Morris did not want you to see. Nov 11, 2004. [accessed: January 6, 2005]. http://image .thelancet .com/extras/03art7306web.pdf [ PubMed : 15993237 ]
- Emerson JA, Hovell MF, Meltzer SB, Zakarian JM, Hofstetter CR, Wahlgren DR, Leaderer BP, Meltzer EO. The accuracy of environmental tobacco smoke exposure measures among asthmatic children. Journal of Clinical Epidemiology. 1995; 48 (10):1251–9. [ PubMed : 7561987 ]
- Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. Journal of Clinical Epidemiology. 1991; 44 (2):127–39. [ PubMed : 1995774 ]
- Flue-Cured Tobacco Cooperative Stabilization Corp. v. United States Environmental Protection Agency (M.D.N.C. June 22, 1993), cited in 8.2 TPLR 3.97 (1993).
- Flue-Cured Tobacco Cooperative Stabilization Corp. v. The United States Environmental Protection Agency, No. 98–2407 (4th Cir., December 11, 2002), cited in 17.7 TPLR 2.472 (2003) (Overturning lower court’s decision invalidating EPA’s findings that secondhand smoke is a “known human carcinogen”).
- Fontham ET, Correa P, Reynolds P, Wu-Williams A, Buffler PA, Greenberg RS, Chen VW, Alterman T, Boyd P, Austin DF, Liff J. Environmental tobacco smoke and lung cancer in nonsmoking women: a multicenter study. Journal of the American Medical Association. 1994; 271 (22):1752–9. [ PubMed : 8196118 ]
- Garne D, Watson M, Chapman S, Byrne F. Environmental tobacco smoke research published in the journal Indoor and Built Environment and associations with the tobacco industry. Lancet. 2005; 365 (9461):804–9. [ PubMed : 15733724 ]
- Glantz SA. The ledger of tobacco control. Journal of the American Medical Association. 1996; 276 (11):871–2. [ PubMed : 8782631 ]
- Glantz SA. Lung cancer and passive smoking: nothing new was said. British Medical Journal. 2000; 321 (7270):1222–3. [ PubMed : 11073523 ]
- Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. British Medical Journal. 1997; 315 (7114):980–8. [ PMC free article : PMC2127653 ] [ PubMed : 9365295 ]
- Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environmental Science & Technology. 1987; 21 (5):494–7. [ PubMed : 22296139 ]
- Hong MK, Bero LA. How the tobacco industry responded to an influential study of the health effects of secondhand smoke. British Medical Journal. 2002; 325 (7377):1413–6. [ PMC free article : PMC1124865 ] [ PubMed : 12480862 ]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Tobacco Smoking. Vol. 38. Lyon (France): International Agency for Research on Cancer; 1986.
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking. Vol. 83. Lyon (France): International Agency for Research on Cancer; 2004. [ PMC free article : PMC4781536 ] [ PubMed : 15285078 ]
- Jaakkola MS, Jaakkola JJ. Assessment of exposure to environmental tobacco smoke. European Respiratory Journal. 1997; 10 (10):2384–97. [ PubMed : 9387970 ]
- Jaakkola MS, Samet JM. Environmental tobacco smoke: risk assessment. Environmental Health Perspectives. 1999; 107 (Suppl 6):823–904. [ PMC free article : PMC1566195 ] [ PubMed : 10592138 ]
- Kawachi I, Colditz GA. Invited commentary: confounding, measurement error, and publication bias in studies of passive smoking. American Journal of Epidemiology. 1996; 144 (10):909–15. [ PubMed : 8916501 ]
- Klepeis NE. An introduction to the indirect exposure assessment approach: modeling human exposure using microenvironmental measurements and the recent National Human Activity Pattern Survey. Environmental Health Perspectives. 1999; 107 (Suppl 2):365–74. [ PMC free article : PMC1566279 ] [ PubMed : 10350522 ]
- Lee PN. Difficulties in assessing the relationship between passive smoking and lung cancer. Statistical Methods in Medical Research. 1998; 7 (2):137–63. [ PubMed : 9654639 ]
- Lee PN. Simple methods for checking for possible errors in reported odds ratios, relative risks and confidence intervals. Statistics in Medicine. 1999; 18 (15):1973–81. [ PubMed : 10440880 ]
- Lee PN, Forey BA. Misclassification of smoking habits as determined by cotinine or by repeated self-report—summary of evidence from 42 studies. Journal of Smoking-Related Diseases. 1995; 6 :109–29.
- Lee PN, Forey B, Fry JS. Revisiting the association between environmental tobacco smoke exposure and lung cancer risk. III: Adjusting for the biasing effect of misclassification of smoking habits. Indoor and Built Environment. 2001; 10 (6):384–98.
- Lubin JH. Estimating lung cancer risk with exposure to environmental tobacco smoke. Environmental Health Perspectives. 1999; 107 (Suppl 6):879–83. [ PMC free article : PMC1566203 ] [ PubMed : 10592146 ]
- Matanoski G, Kanchanaraksa S, Lantry D, Chang Y. Characteristics of nonsmoking women in NHANES I and NHANES I Epidemiologic Follow-up Study with exposure to spouses who smoke. American Journal of Epidemiology. 1995; 142 (2):149–57. [ PubMed : 7598114 ]
- Misakian AL, Bero LA. Publication bias and research on passive smoking: comparison of published and unpublished studies. Journal of the American Medical Association. 1998; 280 (3):250–3. [ PubMed : 9676672 ]
- Morabia A, Bernstein MS, Bouchardy I, Kurtz J, Morris MA. Breast cancer and active and passive smoking: the role of the N -acetyltransferase 2 genotype. American Journal of Epidemiology. 2000; 152 (3):226–32. [ PubMed : 10933269 ]
- National Health and Medical Research Council. A scientific information paper. Canberra (Commonwealth of Australia): Canberra ACT; 1997. The Health Effects of Passive Smoking.
- National Research Council. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington: National Academy Press; 1986. [ PubMed : 25032469 ]
- National Research Council. Human Exposure Assessment for Airborne Pollutants: Advances and Opportunities. Washington: National Academy Press; 1991.
- Ong EK, Glantz SA. Tobacco industry efforts subverting International Agency for Research on Cancer’s second-hand smoke study. Lancet. 2000; 355 (9211):1253–9. [ PubMed : 10770318 ]
- Ong EK, Glantz SA. Constructing “sound science” and “good epidemiology”: tobacco, lawyers, and public relations rms. American Journal of Public Health. 2001; 91 (11):1749–57. [ PMC free article : PMC1446868 ] [ PubMed : 11684593 ]
- Pron GE, Burch JD, Howe GR, Miller AB. The reliability of passive smoking histories reported in a case-control study of lung cancer. American Journal of Epidemiology. 1988; 127 (2):267–73. [ PubMed : 3337082 ]
- Rampton S, Stauber J. Trust Us, We’re Experts: How Industry Manipulates Science and Gambles with Your Future. Los Angeles: J.P. Tarcher; 2000.
- Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott-Raven; 1998.
- Samet JM, Jaakkola JJK. The epidemiologic approach to investigating outdoor air pollution. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. Air Pollution and Health. San Diego: Academic Press; 1999. pp. 431–60.
- Scientific Committee on Tobacco and Health . Report of the Scientific Committee on Tobacco and Health. London: The Stationery Office; 1998.
- Thornton A, Lee P, Fry J. Differences between smokers, ex-smokers, passive smokers and non-smokers. Journal of Clinical Epidemiology. 1994; 47 (10):1143–62. [ PubMed : 7722548 ]
- Thun M, Henley J, Apicella L. Epidemiologic studies of fatal and nonfatal cardiovascular disease and ETS exposure from spousal smoking. Environmental Health Perspectives. 1999; 107 (Suppl 6):841–6. [ PMC free article : PMC1566204 ] [ PubMed : 10592140 ]
- Tweedie RL, Mengersen KL. Meta-analytic approaches to dose-response relationships, with application in studies of lung cancer and exposure to environmental tobacco smoke. Statistics in Medicine. 1995; 14 (5–7):545–69. [ PubMed : 7792447 ]
- US Department of Health and Human Services . The Health Consequences of Smoking: Cancer A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office on Smoking and Health; 1982. DHHS Publication No. (PHS) 82–50179.
- US Department of Health and Human Services. A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office on Smoking and Health; 1984. The Health Consequences of Smoking: Chronic Obstructive Lung Disease. DHHS Publication No. (PHS) 84–50205.
- US Department of Health and Human Services. A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health; 1986. The Health Consequences of Involuntary Smoking. DHHS Publication No. (CDC) 87–8398.
- US Department of Health and Human Services. A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1994. Preventing Tobacco Use Among Young People.
- US Department of Health and Human Services. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998. Tobacco Use Among US Racial/Ethnic Minority Groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics.
- US Department of Health and Human Services. A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. Women and Smoking.
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- US Department of Health, Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1964. PHS Publication No. 1103.
- US Department of Health, Education, and Welfare. A Report of the Surgeon General: 1972. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Health Services and Mental Health Administration; 1972. The Health Consequences of Smoking. DHEW Publication No. (HSM) 72–7516.
- US Department of Health, Education, and Welfare. A Report of the Surgeon General, 1975. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1975. The Health Consequences of Smoking. DHEW Publication No. (CDC) 77–8704.
- US Department of Health, Education, and Welfare. A Report of the Surgeon General. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Office of the Assistant Secretary for Health, Office of Smoking and Health; 1979. Smoking and Health. DHEW Publication No. (PHS) 79–50066.
- U.S. Environmental Protection Agency. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. Washington: U.S. Environmental Protection Agency, Office of Research and Development, Office of Air Radiation; 1992. Report No. EPA/600/6-90/0006F.
- Vandenbroucke JP. Passive smoking and lung cancer: a publication bias? British Medical Journal (Clinical Research Edition). 1988; 296 (6619):391–2. [ PMC free article : PMC2544973 ] [ PubMed : 3125912 ]
- Wald NJ, Nanchahal K, Thompson SG, Cuckle HS. Does breathing other people’s tobacco smoke cause lung cancer? British Medical Journal (Clinical Research Edition). 1986; 293 (6556):1217–22. [ PMC free article : PMC1341990 ] [ PubMed : 3096439 ]
- World Health Organization. International Consultation on Environmental Tobacco Smoke (ETS) and Child Health: Consultation Report. Geneva: World Health Organization; 1999.
- Wu AH. Exposure misclassification bias in studies of environmental tobacco smoke and lung cancer. Environmental Health Perspectives. 1999; 107 (Suppl 6):873–7. [ PMC free article : PMC1566193 ] [ PubMed : 10592145 ]
- Yach D, Bialous SA. Junking science to promote tobacco. American Journal of Public Health. 2001; 91 (11):1745–8. [ PMC free article : PMC1446867 ] [ PubMed : 11684592 ]
- Cite this Page Office on Smoking and Health (US). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2006. 1, Introduction, Summary, and Conclusions.
- PDF version of this title (20M)
- Disable Glossary Links
In this Page
Other titles in these collections.
- Publications and Reports of the Surgeon General
- Health Services/Technology Assessment Text (HSTAT)
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Introduction, Summary, and Conclusions - The Health Consequences of Involuntary ... Introduction, Summary, and Conclusions - The Health Consequences of Involuntary Exposure to Tobacco Smoke
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Introduction. Lung cancer is the leading cause of cancer death worldwide with 1.7 million global deaths attributed to cigarette smoking in 2015. 1 Tobacco use is the leading cause of lung cancer; 55% of lung cancer deaths in women and over 70% of lung cancer deaths in men are due to smoking. 1 These global estimates, however, mask major differences in smoking prevalence in men and women across ...
Worldwide, smoking causes 1.69 million deaths per year from lung cancer (1,2), the leading cause of cancer death for men and women.In the United States, 87% of lung cancer deaths are attributable to cigarette smoking ().Lung cancer screening can reduce the relative risk of dying from lung cancer by 20% (), but when combined with successful smoking cessation, this benefit has been estimated to ...
In the USA, tobacco use is responsible for nearly 1 in 5 deaths. 7 In 2012, the estimated percentage of new lung cancers in males (116,470 cases) and females (109,690 cases) was 14% each. Among these lung cancers, 29% of male and 26% of female cases were estimated to be fatal. 3 Smoking accounts for at least 30% of all cancer deaths and 87% of ...
Each year in the U.S., 10-20% of lung cancers happen in people who have either never smoked or smoked fewer than 100 cigarettes in their life. However, the CDC points out that quitting smoking ...
We found a very strong and significant harmful relationship between pack-years of current smoking and the RR of lung cancer (Fig. 1b).The mean RR of lung cancer at 20 pack-years of smoking was 5. ...
Abstract. Summary This report reviews some of the more recent epidemiologic and experimental findings on the relationship of tobacco smoking to lung cancer, and discusses some criticisms directed against the conclusion that tobacco smoking, especially cigarettes, has a causal role in the increase in broncho-genic carcinoma. The magnitude of the excess lung-cancer risk among cigarette smokers ...
Smoking tobacco (including cigarettes, cigars, and pipes) is the primary risk factor for lung cancer but it can also affect non-smokers. Other risk factors include exposure to secondhand smoke, occupational hazards (such as asbestos, radon and certain chemicals), air pollution, hereditary cancer syndromes, and previous chronic lung diseases.
1 INTRODUCTION. Lung cancer is the leading cause of cancer death in the United States and worldwide. 1, 2 Globally, it is the leading cause of cancer death for men and second only to breast cancer for women, while in the United States, it is the leading cause for both sexes. Each year in the United States, over 230,000 people are diagnosed with lung cancer, and more than 130,000 will die from ...
But lung cancer also happens in people who never smoked or been exposed to secondhand smoke. In these people, there may be no clear cause of lung cancer. How smoking causes lung cancer. Researchers believe smoking causes lung cancer by damaging the cells that line the lungs. Cigarette smoke is full of cancer-causing substances, called carcinogens.
Factors with uncertain or unproven effects on lung cancer risk Smoking marijuana. There are reasons to think smoking marijuana might increase lung cancer risk. Marijuana smoke contains tar and many of the same cancer-causing substances that are in tobacco smoke. (Tar is the sticky, solid material that remains after burning, which is thought to ...
In conclusion, it is not a coincidence that 93 of the patients examined suffering from lung cancer were smokers. As Edwards puts it, smoking tobacco leads to production of carcinogens which break up the DNA in the nucleus of a cell (1999). This breakage leads to accumulation of defective DNA in the nucleus.
Michael J Thun. Although tobacco smoking causes many diseases and affects most of the organ systems in the body, lung. cancer is typically the first condition that comes to mind when one considers the health impact of smoking. Lung cancer is also one of the most important and devastating illnesses caused by smoking, given its high.
Smoking causes more than 7 in 10 lung cancer cases in the UK. Both active smoking and passive smoking cause lung cancer. Passive smoking is when a person breathes in someone else's tobacco smoke. You can find out more about passive smoking on our webpage. There are some other things that increase your risk of lung cancer, which you can learn ...
Find research articles on lung cancer, which may include news stories, clinical trials, blog posts, and descriptions of active studies. ... Quitting smoking after a diagnosis of early-stage lung cancer may help people live longer, a new study finds. The study, which included more than 500 patients, also found that quitting smoking delayed the ...
Lung cancer is the most common incident cancer among men and the second most common cancer among women worldwide. 1 Historically, lung cancer incidence rates have been higher among men than among women, reflecting higher smoking rates among men. 2 Among ever smokers, men smoke more cigarettes and initiate smoking at an earlier age. 3,4 However ...
Preventable risk factors include smoking marijuana, inhaling certain chemicals like gasoline fuel or diesel exhaust, undergoing radiation therapy, and having a poor diet. But, the American Cancer Society affirms that about 87% of lung cancer cases are a result of smoking tobacco, and that some of the other 13% are caused by secondhand smoke (11).
Worldwide over 1 million people die due to lung cancer each year. It is estimated that cigarette smoking explains almost 90% of lung cancer risk in men and 70 to 80% in women. Clinically evident lung cancers have multiple genetic and epigenetic abnormalities. These abnormalities may result in activation of oncogenes and inactivation of tumor ...
Smoking and Lung Cancer Essay. Lung Cancer accounts for fifteen percent of all cancer cases, and an estimated 170,000 people in the United States get lung cancer a year. (5)About 155,000 of those people die from the cancer. Recently, the rate of women affected by lung cancer has increased, while the rate of men affected has decreased.
Moldovanu et al., 2021: Lung Cancer Screening and Smoking Cessation Efforts. The paper identifies the connection between lung cancer screening and smoking cessation. It shows different termination rates in screening trials, indicating the intricacy of getting positive results (Moldovanu et al., 2021b). ... Use our essay writing service and save ...
Cigarette smoking is a danger to our life and health. It is the leading known cause of lung cancer. Each year more than 30,000 people will die of lung cancer and 4 out of 5 of them will get it because of cigarette smoke. Studies have proven that there is no safe way to smoke. Tobacco contains many dangerous cancer causing chemicals that affects ...
In the U.S. and Europe, the incidence rates of people who have never smoked is higher in women than in men- 20% approximately of women with lung cancer have never smoked unlike to 2-6% of nonsmoking men. (Helland and Brustugun 2009). Lung cancer has become a concern for both genders due to a recent drastic increase in cigarette smoking.
Mostly due to reductions in smoking, mortality rates for lung cancer have declined by 59% since 1990 in men and by 36% since 2002 in women. 1 These declines have also been accelerated by advances ...
Lung cancer, encompassing both small cell and non-small cell types and affecting men and women almost equally, stands as the second most common cancer in the United States.According to the ...
Aims We studied the health consequences of quitting smoking before age 43 by time since quitting, number of years smoked and cigarettes smoked per day. The outcomes were all-cause, ischaemic heart disease and lung cancer mortality. Design Prospective study. Setting Norwegian counties. Participants Men and women aged 40-43 years who participated in a national cardiovascular screening ...
Introduction. Cigarette smoking is the single largest cause of cancer worldwide yet tobacco use is decreasing less rapidly in women than men, and lung cancer remains the leading cause of cancer death in women. 1 2 Data from mostly cross-sectional studies suggest that cancer screening services are underused in women, but other studies reported no association between smoking status and cancer ...
Lung cancer is a major global health problem, affecting both smokers and non-smokers. Smoking is the biggest risk factor, but environmental variables also play a significant role. In India, lung ...
Other than lung cancer, smoking causes several other types of cancers and complications, so smoking is not encouraged, therefore, if are are a user, you should consider quitting immediately.
A better understanding of lung cancer etiology and the development of screening biomarkers have important implications for lung cancer prevention. We included 623 matched case-control pairs from the Cancer Prevention Study (CPS) cohorts. Pre-diagnosis blood samples were collected between 1998 and 2001 in the CPS-II Nutrition cohort and 2006 and 2013 in the CPS-3 cohort and were sent for ...
Lung cancer is often associated with cigarette smoking, but various other factors can contribute to the development of this disease. Understanding these factors is crucial for prevention and early ...
By 1982, there were three published epidemiologic studies on involuntary smoking and lung cancer, and the 1982 Surgeon General's report included a brief chapter on this topic. ... Several papers that acknowledged support from the tobacco industry have addressed the epidemiologic findings for lung cancer, including the selection and ...