

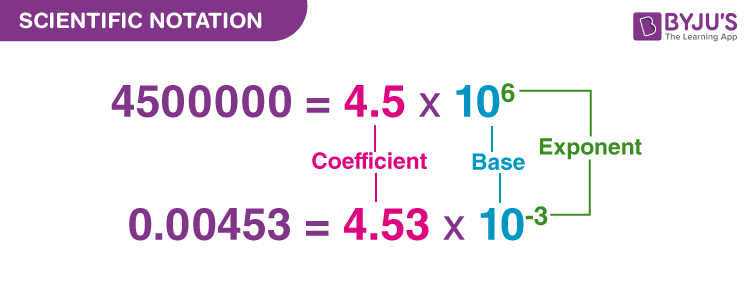
Scientific Notation – Examples and Practice Problems
Scientific notation allows us to write very large numbers or very small numbers in a more convenient way. Scientific notation is widely used by engineers and scientists. In this article, we will explore a summary of scientific notation. In addition, we will look at several examples with answers to improve our understanding of the concepts.
Also, we will look at some exercises to solve and practice what we have learned.
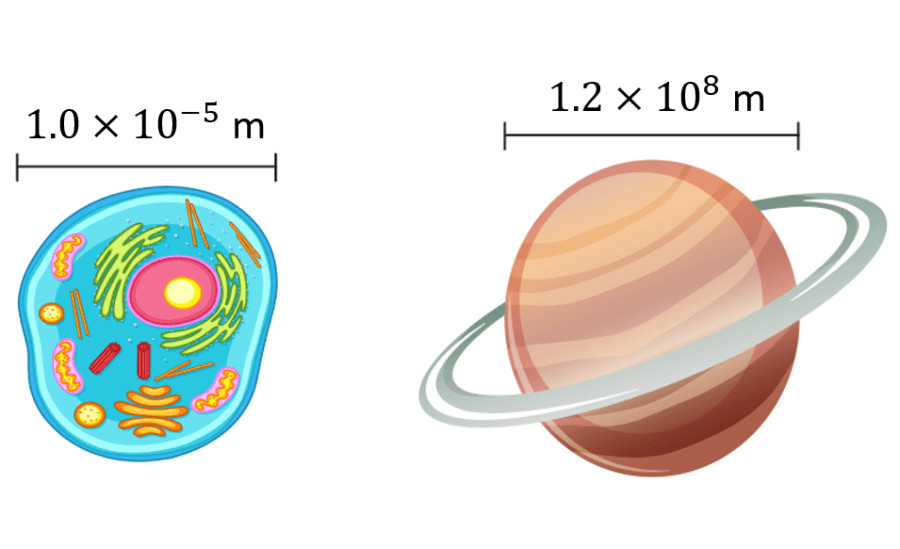
Relevant for …
Solving various exercises in scientific notation.
See examples
Summary of scientific notation
Scientific notation – examples with answers, scientific notation – practice problems.
Scientific notation is the way scientists and engineers handle numbers that are very large or numbers that are very small. For example, instead of writing 0.0000045, we write $latex 4.5\times{{10}^{- 6}}$.
We can think of the number $latex 4.5 \times{{10}^{- 6}}$ as the product of two numbers: 4.5 (the digit term) and $latex {{10}^{- 6}}$ (the exponential term). The following are some examples of scientific notation:
| $latex 1000=1\times {{10}^3}$ | $latex 4562=4.562\times {{10}^3}$ |
| $latex 100=1\times {{10}^2}$ | $latex 251=2.51\times {{10}^2}$ |
| $latex 10=1\times {{10}^1}$ | $latex 42=4.2\times {{10}^1}$ |
| $latex 1=1\times {{10}^0}$ | |
| $latex 0.1=1\times {{10}^{-1}}$ | $latex 0.41=4.1\times {{10}^{-1}}$ |
| $latex 0.01=1\times {{10}^{-2}}$ | $latex 0.024=2.4\times {{10}^{-2}}$ |
| $latex 0.001=1\times {{10}^{-3}}$ | $latex 0.0065=6.5\times {{10}^{-3}}$ |
The exponent of 10 is the number of places that the decimal point must be moved to get the number in long form. A positive exponent shows that the decimal point is shifted that number of places to the right. A negative exponent shows that the decimal point is shifted that number of places to the left.
The following examples with answers can be used to improve understanding of the concepts. The reasoning in the solution of each exercise is useful to be applied to other similar scientific notation problems.
Write the number 34100000 in scientific notation.
In scientific notation, the digit term indicates the number of significant figures in the number. The exponential term only places the decimal point.
In this case, the given number only has 3 significant figures. The zeros are not significant, the zeros only occupy one place. Therefore, we move the decimal point 7 places to the left and we have:
$latex 34100000=3.41\times {{10}^7}$
Write the number 0.00041 in scientific notation.
In this case, the given number only has 2 significant figures. Now, we move the decimal point 4 places to the right and we have:
$latex 0.00041=4.1\times {{10}^{-4}}$
Write the number 568200000000 in scientific notation.
Here, we have a number with 4 significant figures. In this case, we have to move the decimal point 11 places to the left, so we have the following:
$latex 568200000000=5.682\times {{10}^{11}}$
Write the number 0.00000345 in scientific notation.
The given number has 3 significant figures. Also, we have to move the decimal point 6 places to the right. By doing this, we get the following:
$latex 0.00000345=3.45\times {{10}^{-6}}$
Add the numbers $latex 5.321\times {{10}^{-2}}+4.5\times {{10}^{-4}}$.
To perform a sum of numbers written in scientific notation, we have to make sure that all the numbers are converted to the same power of 10. Once the numbers have the same power of 10, we simply add the digit terms:
$latex 5.321\times {{10}^{-2}}+4.5\times {{10}^{-4}}$
$latex =5.321\times {{10}^{-2}}+0.045\times {{10}^{-2}}$
$latex =5.366\times {{10}^{-2}}$
Subtract the numbers $latex 6.67\times {{10}^4}-3.61\times {{10}^{3}}$.
Similar to the previous exercise, we have to have the same power of 10 in both numbers to be able to subtract. After converting them to the same power, we simply add the digit part:
$latex 6.67\times {{10}^4}-3.61\times {{10}^{3}}$
$latex =6.67\times {{10}^4}-0.361\times {{10}^{4}}$
$latex =6.31\times {{10}^{4}}$
Multiply the numbers $latex (3.4\times {{10}^6})(4.2\times {{10}^{3}})$.
The digit part is multiplied in the normal way and the exponents are added. The final result is changed so that there is only one non-zero digit to the left of the decimal:
$latex (3.4\times {{10}^6})(4.2\times {{10}^{3}})$
$latex =(3.4)(4.2)\times {{10}^{6+3}}$
$latex =14.28\times {{10}^{9}}$
$latex =1.4\times {{10}^{10}}$
After having reviewed the examples with answers, try to solve the following scientific notation problems. Simply choose an answer and select the “Check” button to check your chosen answer. If you are having trouble with these problems, you can look back at the above examples carefully.
Write the number 325000000 in scientific notation.
Choose an answer
Write the number 0.0000000425 in scientific notation.
Simplify the expression $latex (4.215\times {{10}^{-2}})+(3.2\times {{10}^{-4}})$., simplify the expression $latex (8.97\times {{10}^4})-(2.62\times {{10}^{3}})$., find the product $latex (6.73\times {{10}^4})(2.91\times {{10}^3})$..
Interested in learning more about algebraic topics? Take a look at these pages:
- Exercises of Prime and Composite Numbers
- Examples of Square Roots
- Examples of Mixture Problems

Jefferson Huera Guzman
Jefferson is the lead author and administrator of Neurochispas.com. The interactive Mathematics and Physics content that I have created has helped many students.

Learn mathematics with our additional resources in different topics

Copyright © 2024 Neurochispas
All rights reserved.
INFORMATION
Terms and Conditions
Privacy Policy
About Neurochispas
About the Author
Scientific Notation with Negative Powers of 10
Related documents.

Study collections
- Maths higher
Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
SCIENTIFIC NOTATION WITH NEGATIVE POWERS OF 10 WORKSHEET
Problem 1 :
Write the given number in scientific notation.
Problem 2 :
Problem 3 :
Problem 4 :
The average size of an atom is about 0.00000003 centimeter across. Write the average size of an atom in scientific notation.

Detailed Answer Key
In the given number, number of digits from decimal point up to the first non zero digit is 5.
So, the decimal point has to be moved 5 digits to the right and exponent of 10 should be -5 (negative integer).
Therefore,
0.00006 = 6.0 x 10 -5
Solution :
In the given number, number of digits from decimal point up to the first non zero digit is 4.
So, the decimal point has to be moved 4 digits to the right and exponent of 10 should be -4 (negative integer).
0.000216 = 2.16 x 10 -4
In the given number, number of digits from decimal point up to the first non zero digit is 7.
So, the decimal point has to be moved 7 digits to the right and exponent of 10 should be -7 (negative integer).
0.0000009 = 9.0 x 10 -7
In 0.00000003, number of digits from decimal point up to the first non zero digit is 8.
So, the decimal point has to be moved 8 digits to the right and exponent of 10 should be -8 (negative integer)
0.00000003 = 3.0 x 10 -8
Therefore, the average size of an atom in scientific notation is about
3.0 x 10 -8 centimeter across
Kindly mail your feedback to [email protected]
We always appreciate your feedback.
© All rights reserved. onlinemath4all.com
- Sat Math Practice
- SAT Math Worksheets
- PEMDAS Rule
- BODMAS rule
- GEMDAS Order of Operations
- Math Calculators
- Transformations of Functions
- Order of rotational symmetry
- Lines of symmetry
- Compound Angles
- Quantitative Aptitude Tricks
- Trigonometric ratio table
- Word Problems
- Times Table Shortcuts
- 10th CBSE solution
- PSAT Math Preparation
- Privacy Policy
- Laws of Exponents
Recent Articles
Digital sat math problems and solutions (part - 15).
Sep 12, 24 08:16 AM
AP Calculus BC : Indeterminate Forms and L'Hopital's Rule
Sep 11, 24 07:32 PM
Derivative Problems and Solutions (Part - 3)
Sep 11, 24 06:33 PM
- Math Article
- Scientific Notation
Scientific notation

Scientific notation is a form of presenting very large numbers or very small numbers in a simpler form. As we know, the whole numbers can be extended till infinity, but we cannot write such huge numbers on a piece of paper. Also, the numbers which are present at the millions place after the decimal needed to be represented in a simpler form. Thus, it is difficult to represent a few numbers in their expanded form. Hence, we use scientific notations. Also learn, Numbers In General Form .
For example, 100000000 can be written as 10 8 , which is the scientific notation. Here the exponent is positive. Similarly, 0.0000001 is a very small number which can be represented as 10 -8 , where the exponent is negative.
Scientific Notation Definition
As discussed in the introduction, the scientific notation helps us to represent the numbers which are very huge or very tiny in a form of multiplication of single-digit numbers and 10 raised to the power of the respective exponent. The exponent is positive if the number is very large and it is negative if the number is very small. Learn power and exponents for better understanding.
The general representation of scientific notation is:
| ; 1 ≤ a < 10 |
Also, read:
- Scientific notation formula calculator
- Scientific Notation Calculator
Scientific Notation Rules
To determine the power or exponent of 10, we must follow the rule listed below:
- The base should be always 10
- The exponent must be a non-zero integer, that means it can be either positive or negative
- The absolute value of the coefficient is greater than or equal to 1 but it should be less than 10
- Coefficients can be positive or negative numbers including whole and decimal numbers
- The mantissa carries the rest of the significant digits of the number
Let us understand how many places we need to move the decimal point after the single-digit number with the help of the below representation.
- If the given number is multiples of 10 then the decimal point has to move to the left, and the power of 10 will be positive. Example: 6000 = 6 × 10 3 is in scientific notation.
- If the given number is smaller than 1, then the decimal point has to move to the right, so the power of 10 will be negative. Example: 0.006 = 6 × 0.001 = 6 × 10 -3 is in scientific notation.
Scientific Notation Examples
The examples of scientific notation are: 490000000 = 4.9×10 8 1230000000 = 1.23×10 9 50500000 = 5.05 x 10 7 0.000000097 = 9.7 x 10 -8 0.0000212 = 2.12 x 10 -5
Positive and Negative Exponent
When the scientific notation of any large numbers is expressed, then we use positive exponents for base 10. For example: 20000 = 2 x 10 4 , where 4 is the positive exponent.
When the scientific notation of any small numbers is expressed, then we use negative exponents for base 10. For example: 0.0002 = 2 x 10 -4 , where -4 is the negative exponent.
From the above, we can say that the number greater than 1 can be written as the expression with positive exponent, whereas the numbers less than 1 with negative exponent.
Problems and Solutions
Question 1: Convert 0.00000046 into scientific notation.
Solution: Move the decimal point to the right of 0.00000046 up to 7 places.
The decimal point was moved 7 places to the right to form the number 4.6
Since the numbers are less than 10 and the decimal is moved to the right. Hence, we use a negative exponent here.
⇒ 0.00000046 = 4.6 × 10 -7
This is the scientific notation.
Question 2: Convert 301000000 in scientific notation.
Solution: Move the decimal to the left 8 places so it is positioned to the right of the leftmost non zero digits 3.01000000. Remove all the zeroes and multiply the number by 10.
Now the number has become = 3.01.
Since the number is greater than 10 and the decimal is moved to left, therefore, we use here a positive exponent.
Hence, 3.01 × 10 8 is the scientific notation of the number.
Question 3:Convert 1.36 × 10 7 from scientific notation to standard notation.
Solution: Given, 1.36 × 10 7 in scientific notation.
Exponent = 7
Since the exponent is positive we need to move the decimal place 7 places to the right.
1.36 × 10 7 = 1.36 × 10000000 = 1,36,00,000.
Practice Questions
Problem 1: Convert the following numbers into scientific notation.
Problem 2: Convert the following into standard form.
- 2.89 × 10 -6
- 9.8 × 10 -2
Frequently Asked Questions on Scientific Notation – FAQs
How do you write 0.00001 in scientific notation, what are the 5 rules of scientific notation, what are the 3 parts of a scientific notation, how do you write 75 in scientific notation, how do you put scientific notation into standard form.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Maths related queries and study materials
Your result is as below
Request OTP on Voice Call
| MATHS Related Links | |
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Module 7: Exponents
Problem solving with scientific notation, learning outcome.
- Solve application problems involving scientific notation
A water molecule.
Solve Application Problems
Learning rules for exponents seems pointless without context, so let us explore some examples of using scientific notation that involve real problems. First, let us look at an example of how scientific notation can be used to describe real measurements.
Think About It
Match each length in the table with the appropriate number of meters described in scientific notation below. Write your ideas in the textboxes provided before you look at the solution.
| The height of a desk | Diameter of a water molecule | Diameter of the sun at its equator |
| Distance from Earth to Neptune | Diameter of Earth at the equator | Height of Mt. Everest (rounded) |
| Diameter of an average human cell | Diameter of a large grain of sand | Distance a bullet travels in one second |
| Power of 10, units in meters | Length from table above |
| [latex]10^{12}[/latex] | |
| [latex]10^{9}[/latex] | |
| [latex]10^{6}[/latex] | |
| [latex]10^{4}[/latex] | |
| [latex]10^{2}[/latex] | |
| [latex]10^{0}[/latex] | |
| [latex]10^{-3}[/latex] | |
| [latex]10^{-5}[/latex] | |
| [latex]10^{-10}[/latex] |
| Power of 10, units in meters | Length from table above |
| [latex]10^{12}[/latex] | Distance from Earth to Neptune |
| [latex]10^{9}[/latex] | Diameter of the sun at its equator |
| [latex]10^{6}[/latex] | Diameter of Earth at the equator |
| [latex]10^{4}[/latex] | Height of Mt. Everest (rounded) |
| [latex]10^{2}[/latex] | Distance a bullet travels in one second |
| [latex]10^{0}[/latex] | The height of a desk |
| [latex]10^{-3}[/latex] | Diameter of a large grain of sand |
| [latex]10^{-5}[/latex] | Diameter of an average human cell |
| [latex]10^{-10}[/latex] | Diameter of a water molecule |
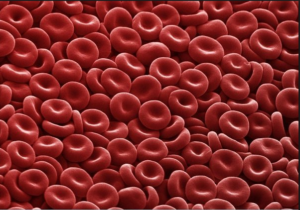
Several red blood cells.
One of the most important parts of solving a “real-world” problem is translating the words into appropriate mathematical terms and recognizing when a well known formula may help. Here is an example that requires you to find the density of a cell given its mass and volume. Cells are not visible to the naked eye, so their measurements, as described with scientific notation, involve negative exponents.
Human cells come in a wide variety of shapes and sizes. The mass of an average human cell is about [latex]2\times10^{-11}[/latex] grams. [1] Red blood cells are one of the smallest types of cells [2] , clocking in at a volume of approximately [latex]10^{-6}\text{ meters }^3[/latex]. [3] Biologists have recently discovered how to use the density of some types of cells to indicate the presence of disorders such as sickle cell anemia or leukemia. [4] Density is calculated as [latex]\frac{\text{ mass }}{\text{ volume }}[/latex]. Calculate the density of an average human cell.
Read and Understand: We are given an average cellular mass and volume as well as the formula for density. We are looking for the density of an average human cell.
Define and Translate: [latex]m=\text{mass}=2\times10^{-11}[/latex], [latex]v=\text{volume}=10^{-6}\text{ meters}^3[/latex], [latex]\text{density}=\frac{\text{ mass }}{\text{ volume }}[/latex]
Write and Solve: Use the quotient rule to simplify the ratio.
[latex]\begin{array}{c}\text{ density }=\frac{2\times10^{-11}\text{ grams }}{10^{-6}\text{ meter }^3}\\\text{ }\\\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,=2\times10^{-11-\left(-6\right)}\frac{\text{ grams }}{\text{ meter }^3}\\\text{ }\\\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,=2\times10^{-5}\frac{\text{ grams }}{\text{ meter }^3}\\\end{array}[/latex]
If scientists know the density of healthy cells, they can compare the density of a sick person’s cells to that to rule out or test for disorders or diseases that may affect cellular density.
The average density of a human cell is [latex]2\times10^{-5}\frac{\text{ grams }}{\text{ meter }^3}[/latex]
The following video provides an example of how to find the number of operations a computer can perform in a very short amount of time.
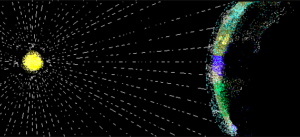
Light traveling from the sun to the earth.
In the next example, you will use another well known formula, [latex]d=r\cdot{t}[/latex], to find how long it takes light to travel from the sun to Earth. Unlike the previous example, the distance between the earth and the sun is massive, so the numbers you will work with have positive exponents.
The speed of light is [latex]3\times10^{8}\frac{\text{ meters }}{\text{ second }}[/latex]. If the sun is [latex]1.5\times10^{11}[/latex] meters from earth, how many seconds does it take for sunlight to reach the earth? Write your answer in scientific notation.
Read and Understand: We are looking for how long—an amount of time. We are given a rate which has units of meters per second and a distance in meters. This is a [latex]d=r\cdot{t}[/latex] problem.
Define and Translate:
[latex]\begin{array}{l}d=1.5\times10^{11}\\r=3\times10^{8}\frac{\text{ meters }}{\text{ second }}\\t=\text{ ? }\end{array}[/latex]
Write and Solve: Substitute the values we are given into the [latex]d=r\cdot{t}[/latex] equation. We will work without units to make it easier. Often, scientists will work with units to make sure they have made correct calculations.
[latex]\begin{array}{c}d=r\cdot{t}\\1.5\times10^{11}=3\times10^{8}\cdot{t}\end{array}[/latex]
Divide both sides of the equation by [latex]3\times10^{8}[/latex] to isolate t.
[latex]\begin{array}{c}\frac{1.5\times10^{11}}{3\times10^{8}}=\frac{3\times10^{8}}{3\times10^{8}}\cdot{t}\end{array}[/latex]
On the left side, you will need to use the quotient rule of exponents to simplify, and on the right, you are left with t.
[latex]\begin{array}{c}\text{ }\\\left(\frac{1.5}{3}\right)\times\left(\frac{10^{11}}{10^{8}}\right)=t\\\text{ }\\\left(0.5\right)\times\left(10^{11-8}\right)=t\\0.5\times10^3=t\end{array}[/latex]
This answer is not in scientific notation, so we will move the decimal to the right, which means we need to subtract one factor of [latex]10[/latex].
[latex]0.5\times10^3=5.0\times10^2=t[/latex]
The time it takes light to travel from the sun to Earth is [latex]5.0\times10^2[/latex] seconds, or in standard notation, [latex]500[/latex] seconds. That is not bad considering how far it has to travel!
Scientific notation was developed to assist mathematicians, scientists, and others when expressing and working with very large and very small numbers. Scientific notation follows a very specific format in which a number is expressed as the product of a number greater than or equal to one and less than ten times a power of [latex]10[/latex]. The format is written [latex]a\times10^{n}[/latex], where [latex]1\leq{a}<10[/latex] and n is an integer. To multiply or divide numbers in scientific notation, you can use the commutative and associative properties to group the exponential terms together and apply the rules of exponents.
- Orders of magnitude (mass). (n.d.). Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/Orders_of_magnitude_(mass) ↵
- How Big is a Human Cell? ↵
- How big is a human cell? - Weizmann Institute of Science. (n.d.). Retrieved May [latex]26, 2016[/latex], from http://www.weizmann.ac.il/plants/Milo/images/humanCellSize120116Clean.pdf ↵
- Grover, W. H., Bryan, A. K., Diez-Silva, M., Suresh, S., Higgins, J. M., & Manalis, S. R. (2011). Measuring single-cell density. Proceedings of the National Academy of Sciences, 108(27), 10992-10996. doi:10.1073/pnas.1104651108 ↵
- Revision and Adaptation. Provided by : Lumen Learning. License : CC BY: Attribution
- Application of Scientific Notation - Quotient 1 (Number of Times Around the Earth). Authored by : James Sousa (Mathispower4u.com) for Lumen Learning. Located at : https://youtu.be/san2avgwu6k . License : CC BY: Attribution
- Application of Scientific Notation - Quotient 2 (Time for Computer Operations). Authored by : James Sousa (Mathispower4u.com) for Lumen Learning. Located at : https://youtu.be/Cbm6ejEbu-o . License : CC BY: Attribution
- Unit 11: Exponents and Polynomials, from Developmental Math: An Open Program. Provided by : Monterey Institute of Technology and Education. Located at : http://nrocnetwork.org/dm-opentext . License : CC BY: Attribution


Learn maths at home

A Complete Guide to Scientific Notation (Standard Form)
Scientific notation: video lesson, what is scientific notation.
Scientific notation can be used to represent both decimals less than one whole and very large whole numbers.
Scientific notation is a concise way to represent both very large or very small numbers. Scientific notation involves a number between 1 and 10 being multiplied to a power of 10. The power of 10 is positive if the number being represented is large and it is negative if the number is small.
- A positive power of 10 tells us how many digits the number has between its first digit and the decimal point
- A negative power of 10 tells us how many zero digits are in front of the non-zero digits.
For example, 6×10 5 is a 6 followed by 5 digits. It represents 600 000.
For example, 6×10 -5 is a 6 with 5 zeros before it. It represents 0.00006.

How Scientific Notation is Used in Real Life
Scientific notation is used whenever very large or very small numbers are involved. For example in physics, astronomy, chemistry and finance.
Some examples of scientific notation used in real-life include:
- The very large distances involved in astronomy. For example, the distance between the Earth and the Sun is said to be 93 million miles, which can be written as 9.3 × 10 7 miles.
- In Physics, very large or very small known constants are represented in scientific notation such as the speed of light (2.998 × 10 8 meters per second) or Planck’s constant (6.63 × 10 -34 joule-seconds).
- In chemistry, the mass of atoms, molecules and molar mass is often written in scientific notation. For example, Avogadro’s number is 6.02 × 10 23 particles per mole.
- In finance and economics, figures such as national debt, gross domestic product (GDP), or market capitalisation are often expressed in scientific notation. For instance, if a country’s GDP is $2.5 trillion, it would be expressed as 2.5 × 10 12 dollars.
- Engineers often work with values that span many orders of magnitude, such as voltage, current, or power.
- In medicine and biology, scientific notation is used to express values like cell counts, DNA base pairs, or concentrations of substances in body fluids.
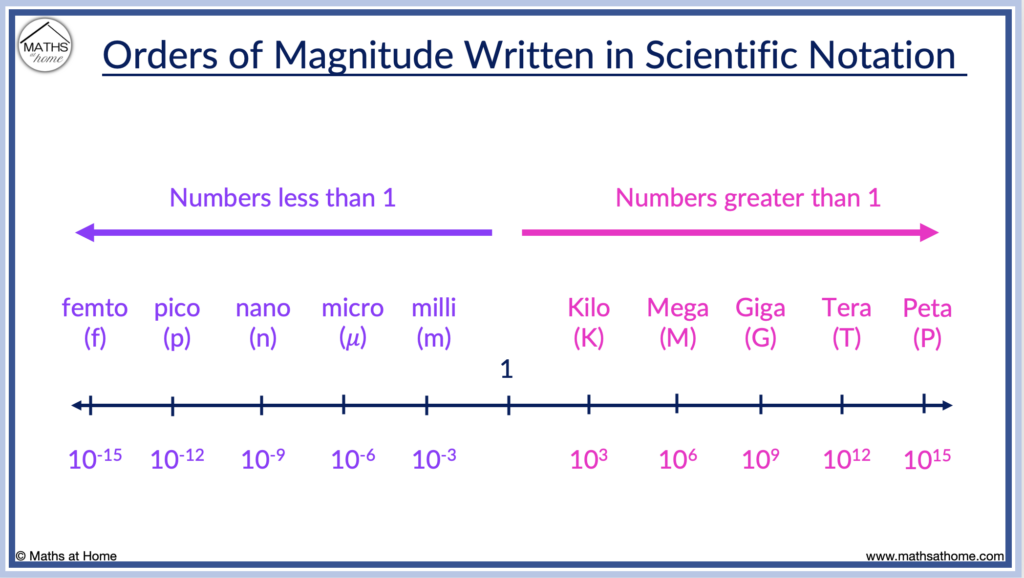
In science, abbreviations are commonly used to refer to given orders of magnitude such as:
| femto | ×10 |
| pico | ×10 |
| nano | ×10 |
| micro | ×10 |
| milli | ×10 |
| centi | ×10 |
| deci | ×10 |
| deca | ×10 |
| hecto | ×10 |
| kilo | ×10 |
| mega | ×10 |
| giga | ×10 |
| tera | ×10 |
| peta | ×10 |
Here are some common values of scientific notation listed on a number line from least to greatest.
Scientific Notation Explained
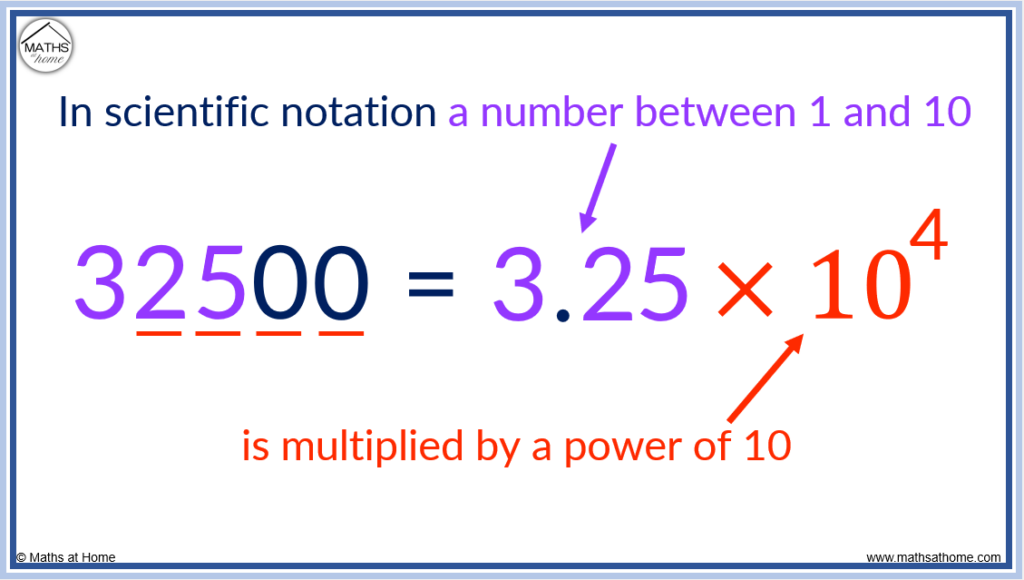
Scientific notation is used when a very large or very small number is too long to write in the normal manner. In scientific notation numbers are written as a number between 1 and 10 multiplied by a power of 10. For example, 32500 is written as 3.25×10 4 .
Scientific notation is a more concise way to write long numbers whilst indicating their order of magnitude.
Scientific notation is also commonly known as standard form. Both scientific notation and standard form refer to numbers written as a number between 1 and 10 multiplied by 10 to the power of some other positive or negative number.
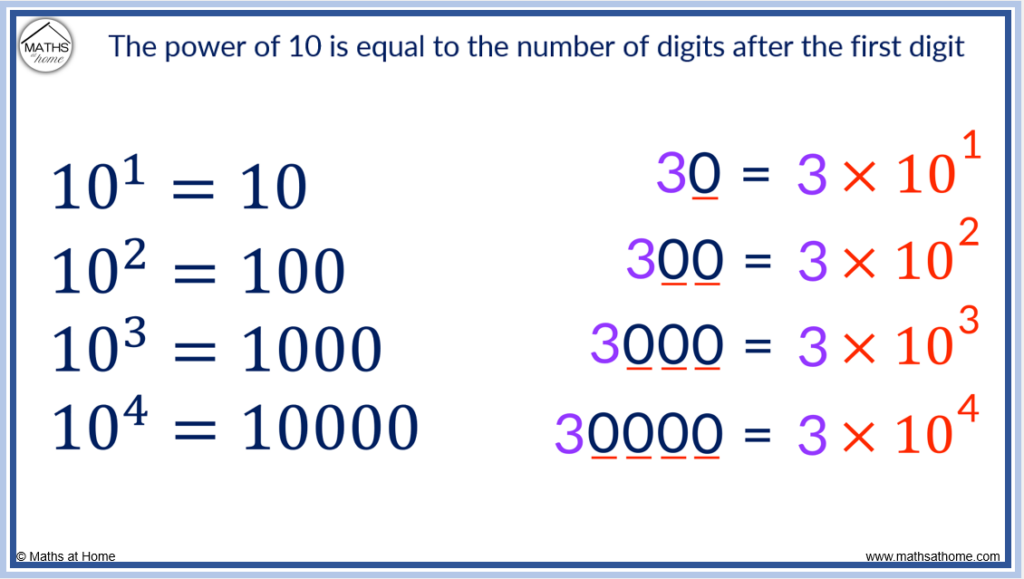
For example consider the following lists:
- 10 3 = 1000
- 10 4 = 10000
The power that 10 is raised to in each case is equal to the number of digits after the 1.
- 3 × 10 1 = 30
- 3 × 10 2 = 300
- 3 × 10 3 = 3000
- 3 × 10 4 = 30000
The power that 10 is raised to in each case is equal to the number of digits after the 3.
A number written in scientific notation always consists of the following parts:
- A number between the values of 1 and 10
- A multiplication by 10 raised to a power
The name for a number not written in scientific notation is simply ‘standard notation’ or ‘decimal notation’. When comparing a number written in standard notation to a number written in scientific notation, it may also be referred to as ‘expanded form’.
The first step is to write the first part of the number which must always be between 1 and 10.
We take 32500 and write it as 3.25 so that it is now a number that is bigger than 1 and less than 10.
The next step is to find the power of 10 that 3.25 is raised to to make it equal to 32500.
The power that ten is raised to is equal to the number of digits after the first digit.
In 32500, there are 4 digits after the first digit of 3. Therefore the power that 10 is raised to is 4.
32500 can be written as 3.25 × 10 4 .
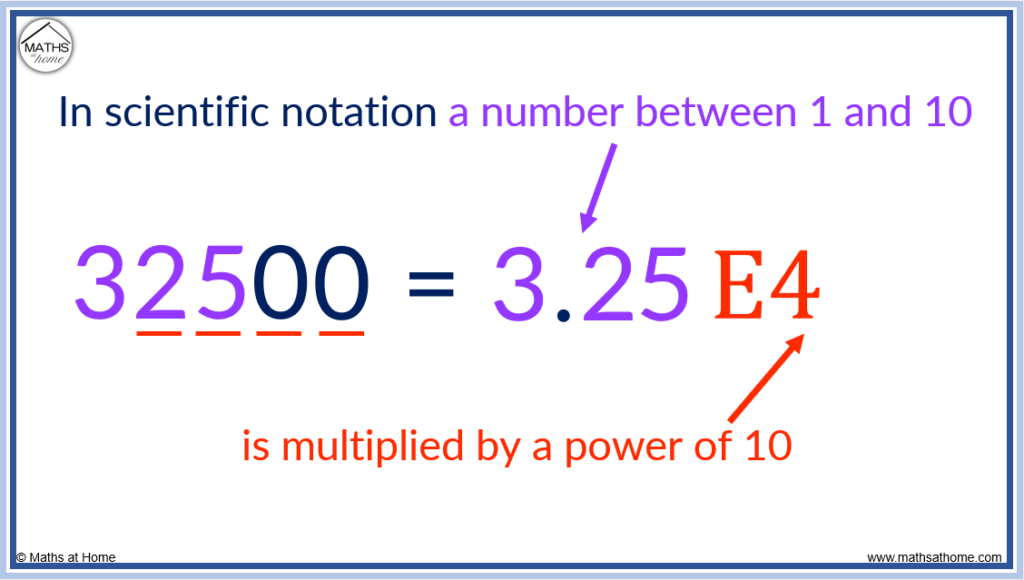
Scientific notation is often used on calculator displays (since the calculator screen cannot fit very long numbers). On a calculator, scientifi notation is written using ‘E’ to represent × 10^.
For example, 32500 can be written as 3.25 × 10 4 or alternatively as 3.25E4.
In scientific notation, E stands for × 10 raised to the power of the number that comes after the E.
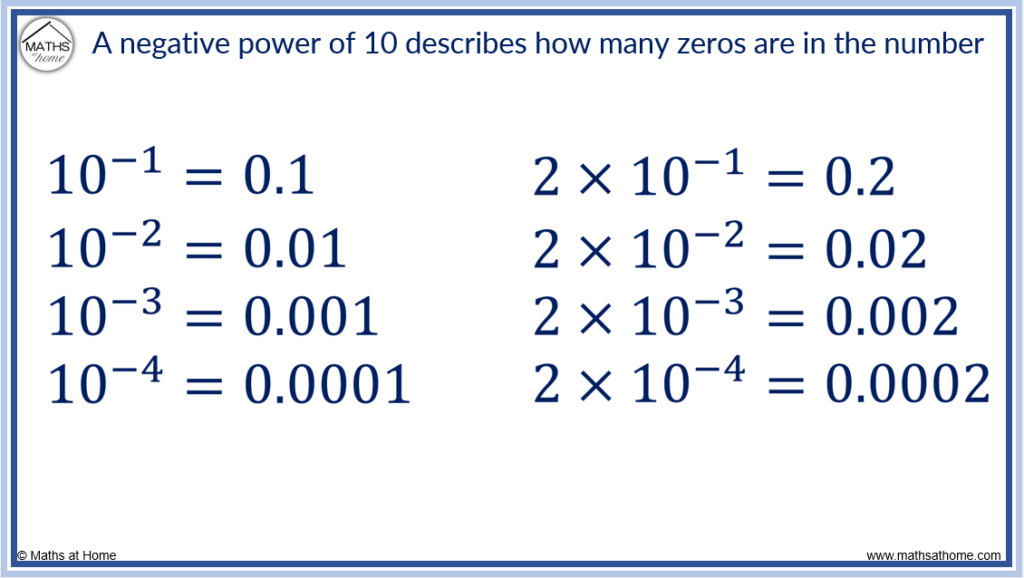
Scientific notation can also be used to write very small numbers by using negative powers of 10.
We can see that the negative power of 10 describes how many zeros are at the start of the decimal number.
For example, 10 -3 has 3 zeros when written as 0.001.
The number 0.000034 can be written as 3.4 × 10 -5 . There are 5 zeros before the digits of 3 and 4.
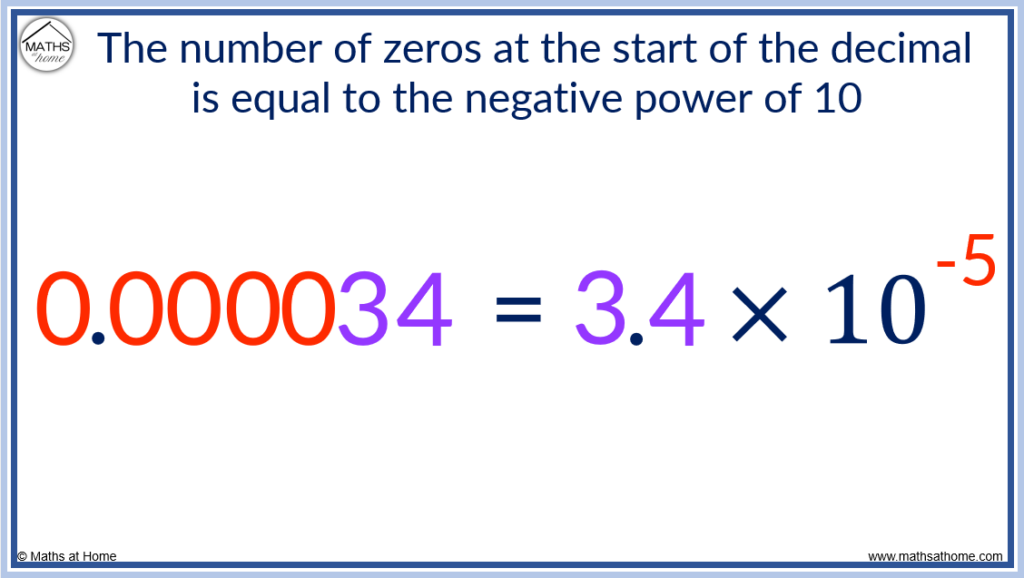
Rules of Scientific Notation
Numbers written in scientific notation must adhere to the following rules:
- The number before the multiplication sign must always be at least 1 and less than 10.
- There is always a multiplication by a power of 10 (as opposed to a multiplication by any other number).
- The power of 10 can be negative, zero or positive.
- If the number being represented is 10 or more, the power of 10 is positive.
- If the number being represented is 10 or more, the power of 10 is equal to the number of digits that come after the first digit of the number.
- If the number being represented is less than 1, the power of 10 is negative.
- If the number being represented is less than 1, the power of 10 is equal to (-1) multiplied by the number of zeros at the start of the number.
- If the number being represented is greater than 1 but less than 10, the power of 10 is zero.
How to Write Large Numbers in Scientific Notation
- Write a decimal point after the first digit of the number to form a number between 1 and 10.
- Multiply this by a power of 10, where the power is equal to the number of digits after the first digit of the large number.
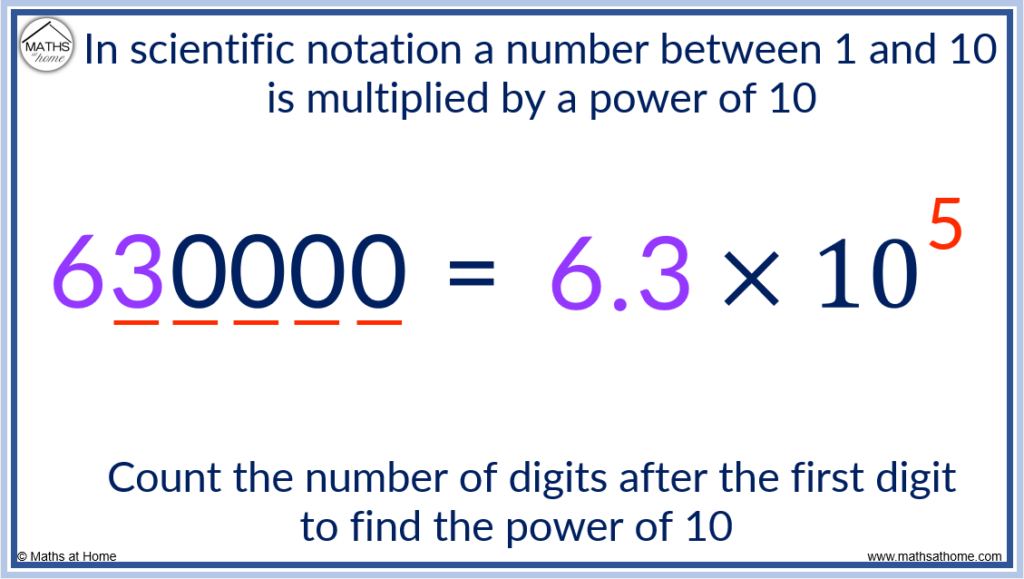
For example, write 630000 in scientific notation:
Step 1. Write a decimal point after the first digit of the number to form a number between 1 and 10
A decimal point is placed after the 6 to make 6.30000 which is just 6.3
6.3 is a number between 1 and 10.
Step 2. Multiply this by a power of 10, where the power is equal to the number of digits after the first digit of the large number
The first digit of 630000 is 6. We count the number of digits after this first digit of 6.
There are 5 digits after the first digit. That is, the 3, 0, 0, 0 and 0.
Therefore we multiply by 10 5 .
630000 is written in scientific notation as 6.3×10 5 .
How to Write Small Numbers in Scientific Notation
- Write the non-zero digits as a number between 1 and 10.
- Multiply this by a negative power of 10 equal to the number of zeros in front of the first non-zero digit.
For example, write 0.000008 in scientific notation.
Step 1. Write the non-zero digits as a number between 1 and 10
The zeros at the start of 0.000008 are ignored to obtain the non-zero digit of 8.
8 is written because it is a number between 1 and 10.
Step 2. Multiply this by a negative power of 10 equal to the number of zeros in front of the first non-zero digit
The first non-zero digit in 0.000008 is the 8.
The number of zeros in front of this non-zero digit is 6.
Since there are 6 zeros, we multiply the 8 by 10 to the power of -6.
0.000008 is written in scientific notation as 8×10 -6 .

Small numbers less than one whole are written in scientific notation with a negative exponent representing the number of zeros at the start of the number.
For example, write the number 0.0257 in scientific notation.
The zeros at the start of 0.0257 are ignored and the decimal point is placed after the first non-zero digit of 2.
This results in 2.57 which is a number between 1 and 10.
The first non-zero digit is 2.
There are 2 zeros before the 2 and so, we multiply 2.57 by 10 to the power of -2.
0.0257 is written in scientific notation as 2.57×10 -2 .
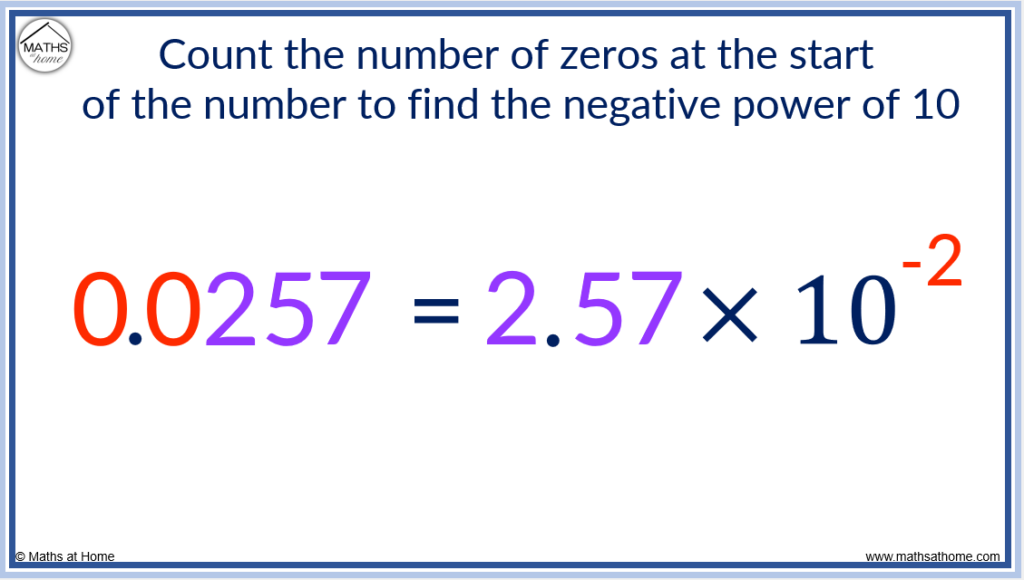
The power of the 10 is equal to the number of zeros at the start of the number multiplied by -1.
How to Read Scientific Notation
- If the power of 10 is positive, this is the number of digits after the first digit. So 2.11×10 6 has 6 digits after the 2. It is written as 2110000.
- If the power of 10 is negative, this is the number of zeros in front of the digits. So 5.3 × 10 -3 has 3 zeros at the start. It is written as 0.0053.
Scientific Notation for a Large Number
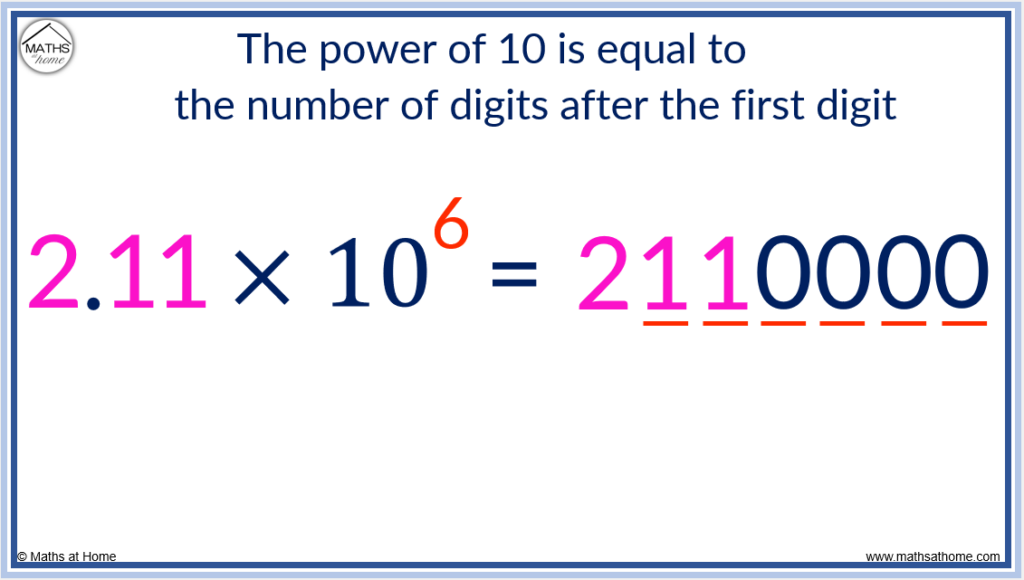
The power of 10 is 6 and so, there are 6 digits after the 2.
We already have two digits of 1 and so, four more 0 digits are needed.
Scientific Notation for a Small Number
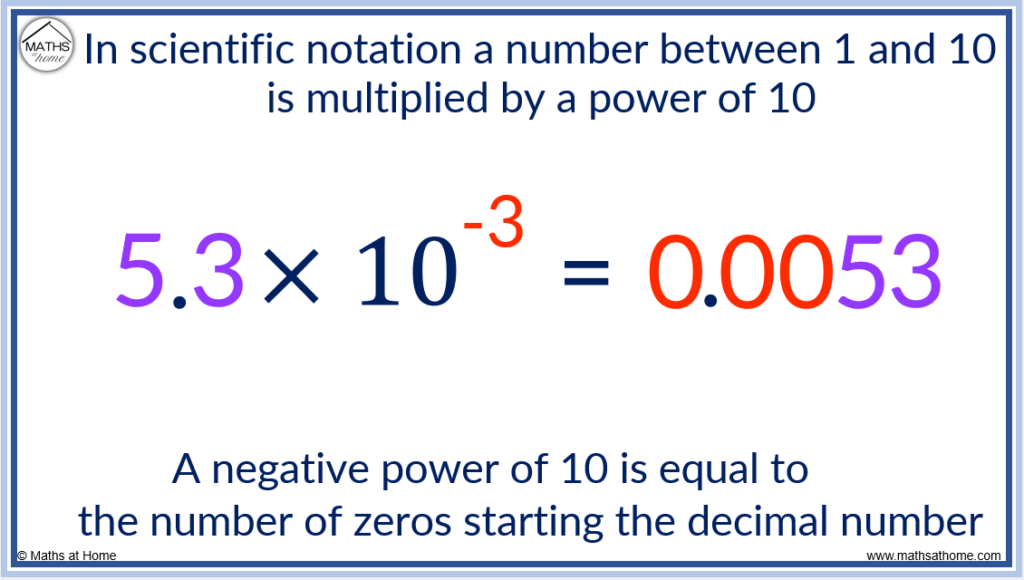
The power of 10 is negative 3.
Negative powers tell us how many 0 digits are at the start of the number.
We put three 0 digits and then the 5 and the 3.
The decimal point always comes after the first 0 digit.
For example, 2.93 × 10 4 has 4 digits after the first digit of 2.
Following the 2, there is a 9 then a 3. We need two more 0 digits to obtain 4 digits after the 2.
2.93 × 10 4. = 29300.

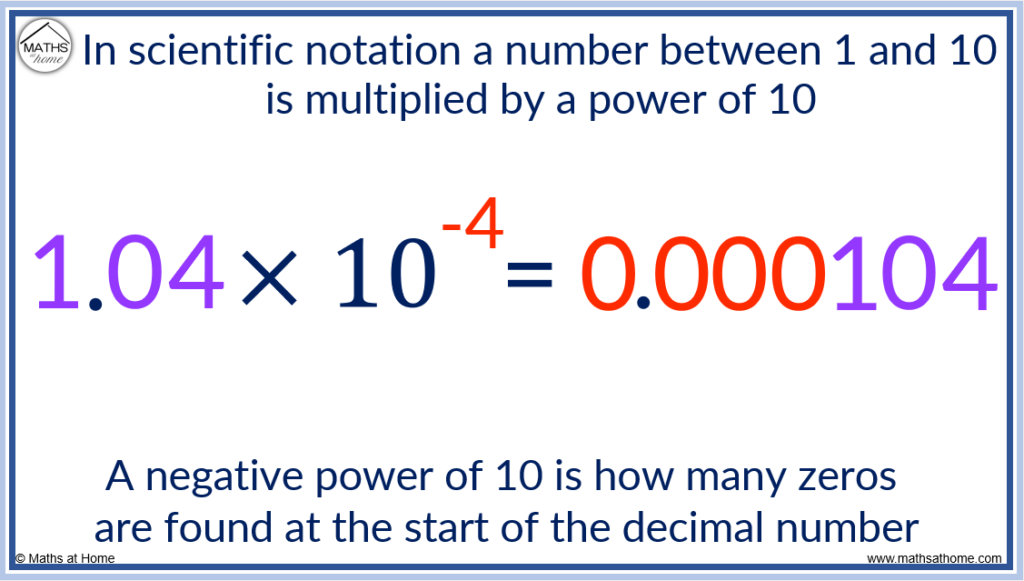
For example, 1.04 × 10 -4 has a negative power of 10.
Therefore this power is equivalent to the number of zeros at the start of the number. There will be 4 zeros followed by the digits of 1, 0 and 4.
1.04 × 10 -4 = 0.000104.
Notice that the power of 10 is only equal to the number of zeros at the start of the number, not the total number of zeros in the answer.
Examples of Scientific Notation
Notice that as an extra zero digit is added to the value, the exponent in scientific notation increases by 1.
Notice that as we move from one thousand to one million to one billion to one trillion, three zero digits are added and so, the exponent in scientific notation increases by 3.
Here are some common values listed in scientific notation:
| One Thousandth | 0.001 | 1×10 |
| One Hundredth | 0.01 | 1×10 |
| One Tenth | 0.1 | 1×10 |
| One | 1 | 1×10 |
| Ten | 10 | 1×10 |
| One Hundred | 100 | 1×10 |
| One Thousand | 1 000 | 1×10 |
| Ten Thousand | 10 000 | 1×10 |
| One Hundred Thousand | 100 000 | 1×10 |
| One Million | 1 000 000 | 1×10 |
| One Billion | 1 000 000 000 | 1×10 |
| One Trillion | 1 000 000 000 000 | 1×10 |
The power of zero in 1×10 0 means that zero tens have been multiplied by.
That is, 10 0 = 1 and so 1×10 0 simply means 1×1 which is just equal to 1.
Here are some examples of numbers written in scientific notation:
| 0.0006 | 6×10 |
| 0.005 | 5×10 |
| 0.028 | 2.8×10 |
| 0.349 | 3.49×10 |
| 5.4 | 5.4×10 |
| 12.7 | 1.27×10 |
| 400 | 4×10 |
| 500 | 5×10 |
| 1230 | 1.23×10 |
| 54400000 | 5.44×10 |
How to Round Numbers in Scientific Notation
It is common for numbers to be written to a particular number of significant figures and then written in scientific notation. This is because numbers in scientific notation are very large or very small and so, the final digits do not really impact on the overall size of the number.
Typically, numbers in scientific notation are given to 2 or 3 decimal places although further accuracy may be required.
It is common to round numbers in scientific notation to 3 significant figures.
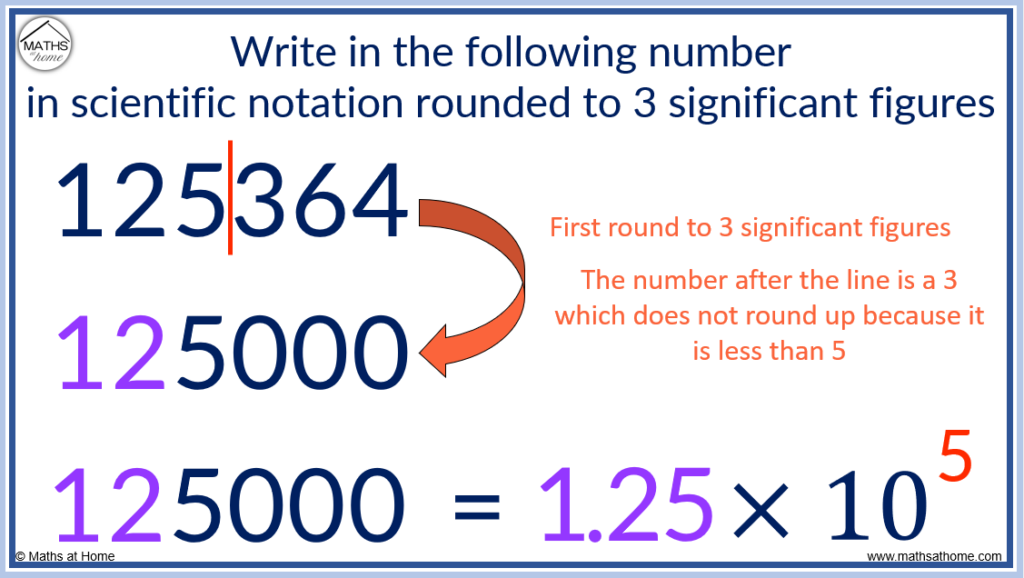
For example, write 125364 in scientific notation, rounded to 3 significant figures.
The first step is to round the number to 3 significant figures.
That is, we look at the first 3 non-zero digits of the number, which are 1, 2 and 5. We then look at the next digit after this to decide if the 3rd significant figure of 5 remains as a 5 or rounds up to a 6.
Only round up the 3rd significant figure if the 4th significant figure is equal to 5 or more.
Since the digit after the 5 is a 3, we do not round up.
We write 125 and then replace the other digits with zeros, so that 125364 written to 3 significant figures is 125000.
We now write this in scientific notation as 1.25 × 10 5 , since there are 5 digits after the first digit of 1 in the number 125000.
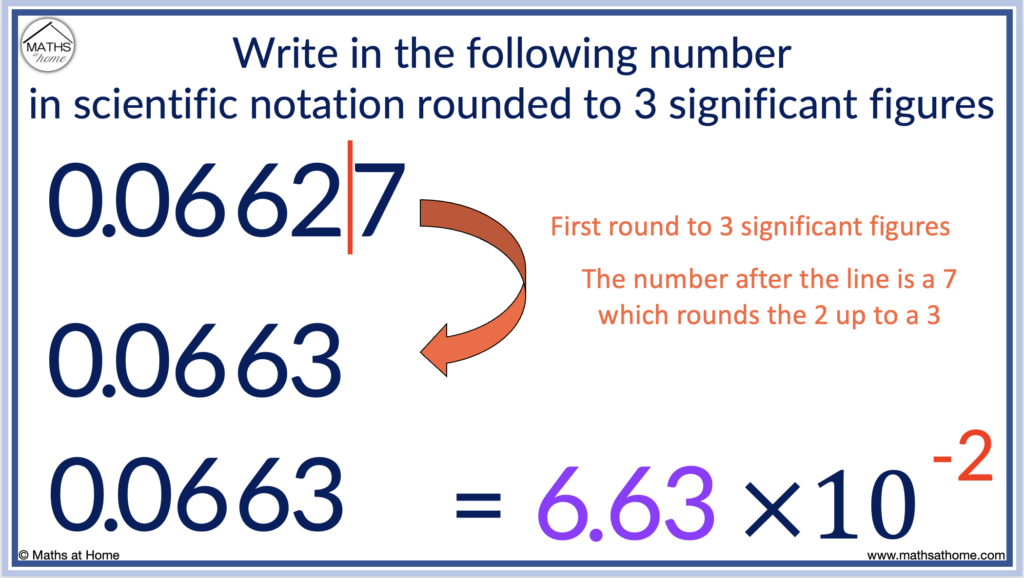
For example, write the number 0.06627 in scientific notation to 3 significant figures.
Significant figures are counted after the zeros at the start of the number.
We look at the first 3 significant figures of the number which are 6, 6 and 2.
We only round up the 3rd significant figure if the 4th significant figure is equal to 5 or more.
The 4th significant figure is a 7 and so, we round the 2 up to a 3.
0.06627 rounded to 3 significant figures is 0.0663.
This number starts with 2 zeros, so writing it in scientific notation we have 6.33 × 10 -2 .
How to Add and Subtract Numbers in Scientific Notation
The ease of adding and subtracting numbers in scientific notation depends on whether the size of the exponents are the same in each number.
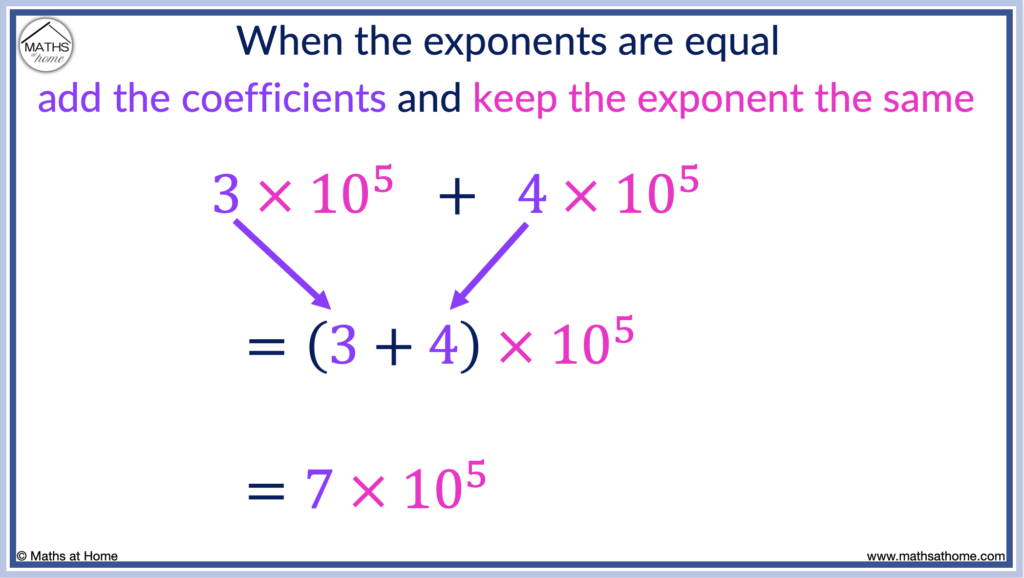
To add or subtract numbers in scientific notation that have the same exponent, simply add or subtract the coefficients and keep the exponent the same. For example, 3×10 5 + 4 × 10 5 is (3+4) × 10 5 which equals 7 × 10 5 .
In this example, the exponents on both 3×10 5 and 4×10 5 are both 5.
Therefore, we can simply add the 3 and the 4 together to obtain 7, whilst the exponent in the answer remains as 5.
3×10 5 + 4×10 5 = 7×10 5 .
This addition is essentially the same as 300 000 + 400 000 = 700 000.
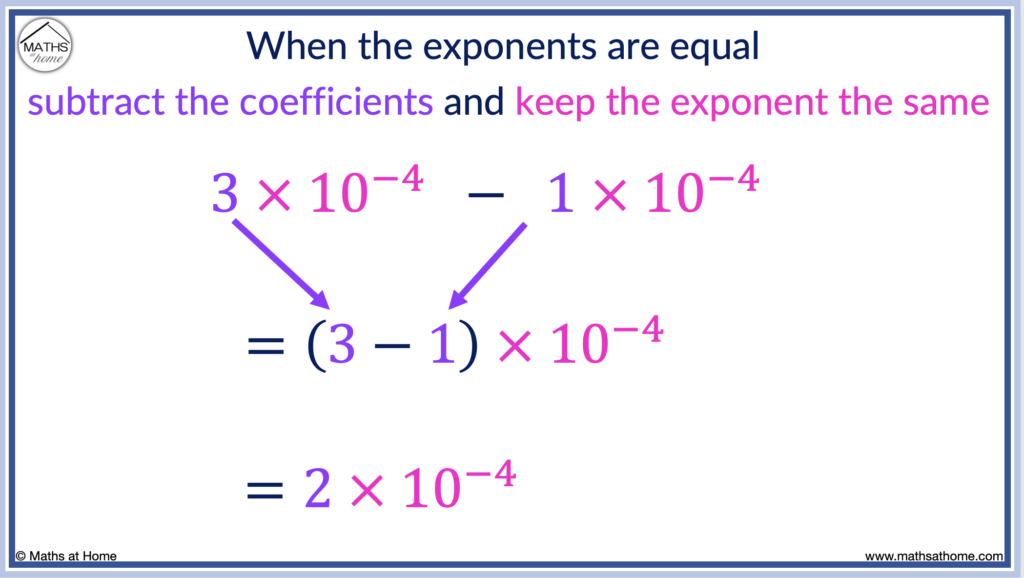
For example, 3×10 -4 – 1×10 -4 = (3-1)×10 -4 which is just 2×10 -4 .
In this example, we simply subtract the coefficients and keep the exponent as -4.
To add or subtract numbers in scientific notation that have different exponents, it is easiest to convert the numbers to standard notation first. Then perform the addition or subtraction and write the result back in scientific notation if needed.
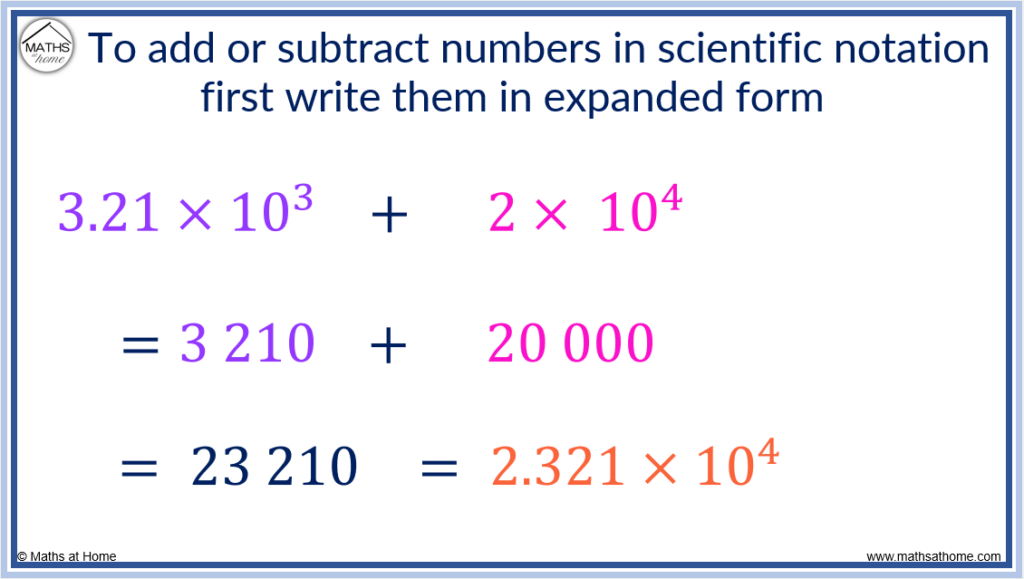
In the example of 3.21×10 3 + 2×10 4 , the exponents are different sizes.
- Converting 3.21×10 3 to standard notation, we have 3 210. The exponent of 3 means there are 3 digits after the first digit.
After the 3, we have the 2 then the 1 and so, another digit of 0 is needed to make 3 digits after the first digit in total.
Converting 2×10 4 to standard notation, we have 20 000. That is, we have 4 digits after the first digit of 2.
Now, these numbers can be added following the standard addition process.
3 210 + 20 000 = 23 210.
We can write this in scientific notation if needed as 2.321×10 4 since there are 4 digits after the first digit in 23 210.
Here is an alternate method for adding numbers in scientific notation when they have different exponents.
The same numbers as above are used but here we are demonstrating a different method to solve the problem.
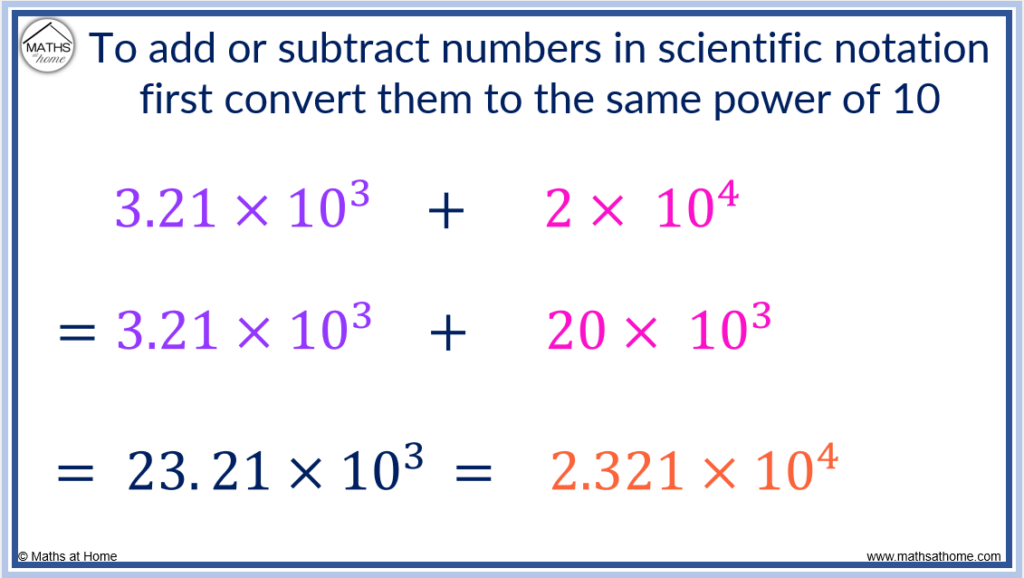
To add or subtract numbers in scientific notation, first convert the numbers to have the same exponent. To do this, multiply the coefficient of the number with the largest exponent by 10 each time the exponent is reduced by 1.
For example, in 3.21×10 3 + 2×10 4 , we wish to reduce the exponent in 2×10 4 from a 4 to a 3 so that it is the same exponent as in 3.21×10 3 .
Since we need to reduce the exponent by 1, we multiply the 2 by 10 to make 20.
2×10 4 is the same as 20×10 3 .
Therefore 3.21×10 3 + 2×10 4 is rewritten as 3.21×10 3 + 20×10 3 .
Now the coefficients can be added so that 3.21×10 3 + 20×10 3 = (3.21+20)×10 3 .
This equals 23.21×10 3 which can be readjusted to 2.321×10 4 as we divide the coefficient by 10 as we increase the exponent of 10 by 1.
Here is an example of subtracting numbers in scientific notation by first converting them to have the same exponents.
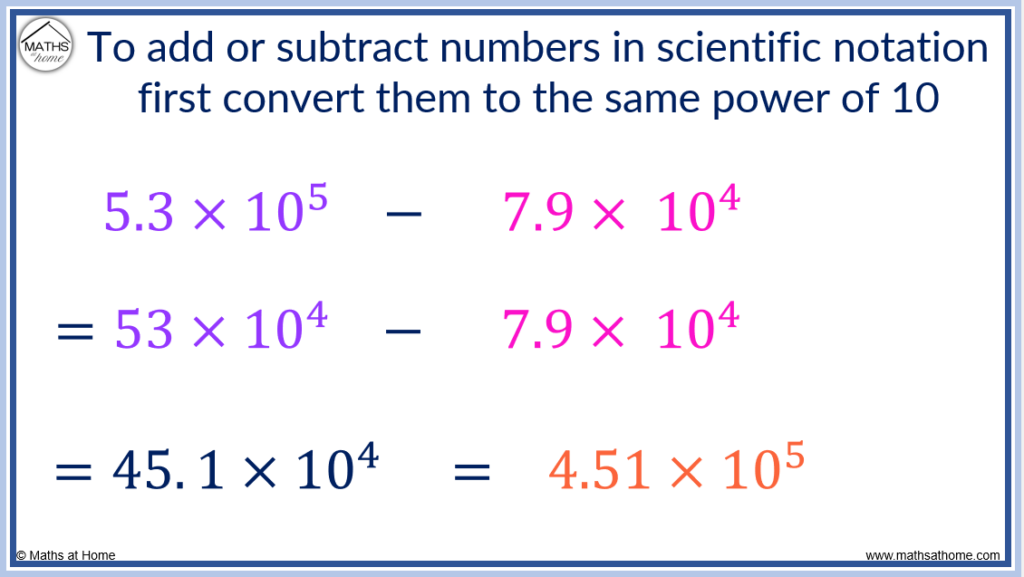
In the example of 5.3×10 5 – 7.9×10 4 , we need to change the exponent of 5.3×10 5 to a 4.
To do this, we multiply the 5.3 by 10 to make 53.
5.3×10 5 is the same as 53×10 4 .
Therefore 5.3×10 5 – 7.9×10 4 can be written as 53×10 4 – 7.9×10 4 .
Now the numbers have the same exponent, subtract the coefficients like so:
(53 – 7.9)×10 4 = 45.1×10 4 .
Finally, this is rewritten so that the coefficient is a number between 1 and 10. We divide 45.1 by 10 and increase the exponent by 1 to compensate.
45.1×10 4 = 4.51×10 5 .
How to Multiply and Divide Numbers in Scientific Notation
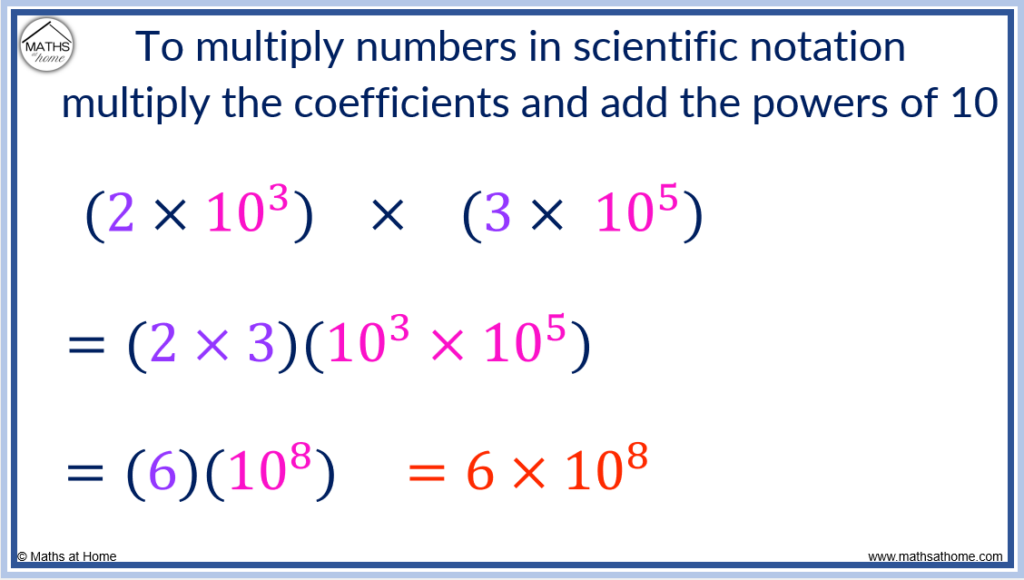
To multiply numbers in scientific notation, multiply the coefficients and add the exponents. For example, (2×10 3 ) × (3×10 5 ) = 6×10 8 . The coefficients of 2 and 3 were multiplied to make 6 and the exponents of 3 and 5 were added to make 8.
This occurs since exponents are always added when they are multiplied together.
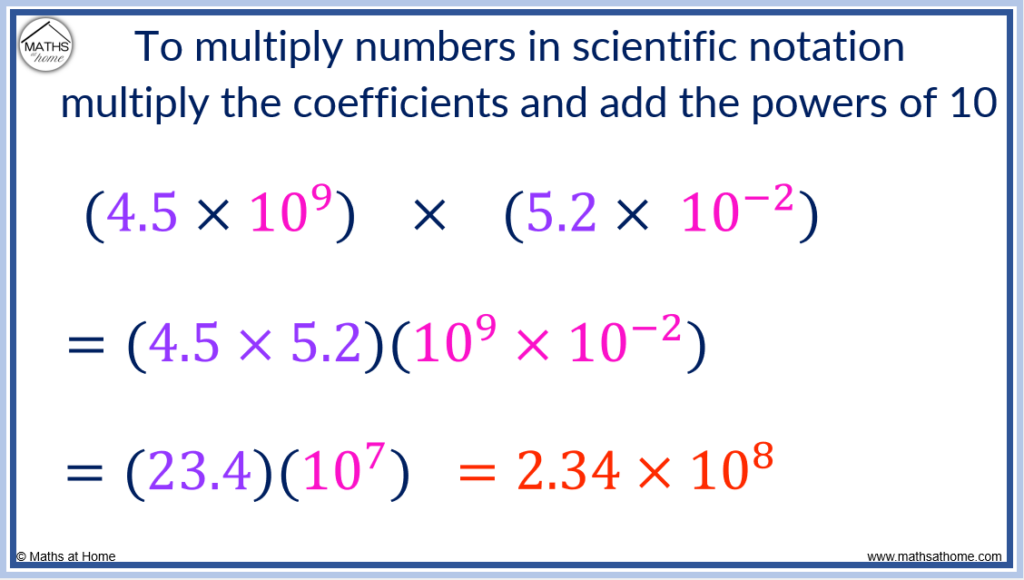
Here is an example of multiplying numbers in scientific notation: (4.5×10 9 ) × (5.2×10 -2 ).
Firstly, the coefficients of 4.5 and 5.2 are multiplied to obtain 23.4.
Secondly, the exponents are added so that 9 + -2 = 7.
Therefore, (4.5×10 9 ) × (5.2×10 -2 ) = 23.4×10 7 .
However, 23.4 must be written as a number between 1 and 10.
We divide 23.4 by 10 to obtain 2.34 and we increase the exponent from 7 to 8 to compensate.
(4.5×10 9 ) × (5.2×10 -2 ) = 2.34×10 8 .
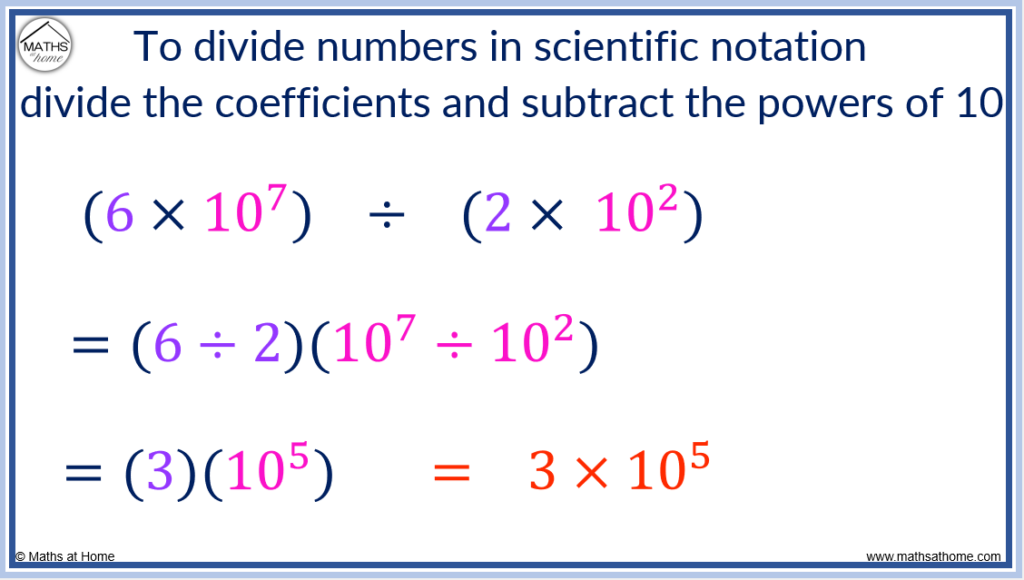
To divide numbers in scientific notation, divide the coefficients and subtract the exponents. For example, (6×10 7 ) ÷ (2×10 2 ) = 3×10 5 . The coefficients of 6 and 2 were divided to obtain 3 and the exponent of 2 was subtracted from the exponent of 7 to obtain 5.
This occurs since exponents are subtracted when they a division takes place.
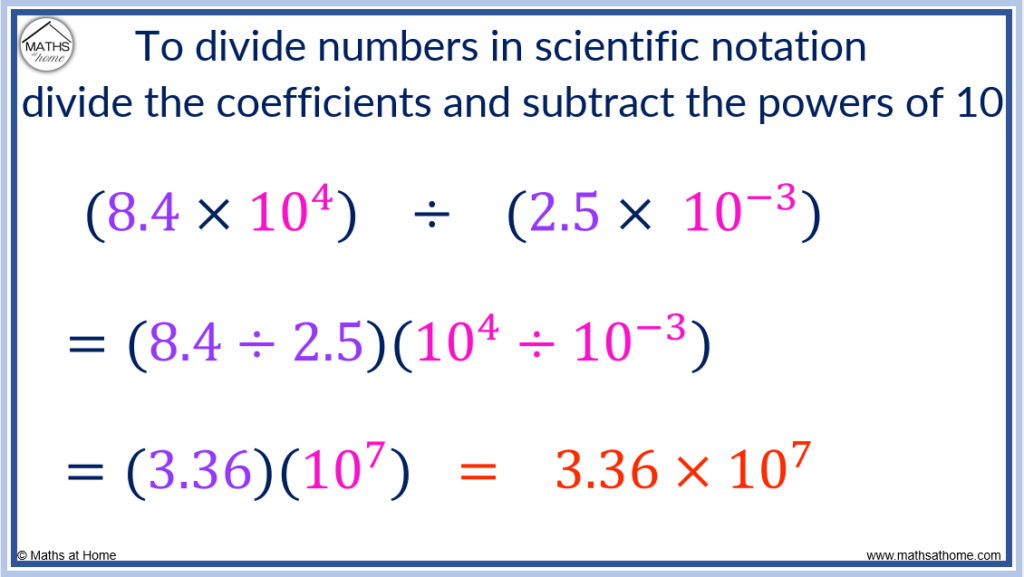
Here is another example of dividing numbers in scientific notation: (8.4×10 4 ) ÷ (2.5×10 -3 ).
Firstly, the coefficients are divided so 8.4 ÷ 2.5 = 3.36.
Secondly, the exponents are subtracted. 4 – -3 is the same as 4 + 3 which equals 7.
(8.4×10 4 ) ÷ (2.5×10 -3 ) = 3.36×10 7 .
Scientific Notation on Calculator
Scientific notation is commonly used in calculator displays.
To write scientific notation on a calculator, use the button labelled as ‘×10 𝑥 ‘ or just 10 𝑥 ‘.
- Enter the coefficient (the number between 1 and 10)
- Then press the ‘×10 𝑥 ‘ button
- Then enter the power of 10

Here are the instructions for entering scientific notation on some common calculators:
Casio Fx-300
The ‘×10 𝑥 ‘ button is found in the middle of the bottom row.
Casio Fx-82
Casio fx-cg50.
The ’10 𝑥 ‘ option is found above the ‘log’ button. Press ‘2nd’ then ‘log’ to write numbers in scientific notation.
- Press the ‘2nd’ button
- Then press the ‘log’ button to select the ’10 𝑥 ‘ option
The Math You Need, When You Need It
math tutorials for students majoring in the earth sciences
- First Publication: August 11, 2023
- Revision: August 23, 2024 -- Revised based on classroom testing feedback.
Scientific Notation - Practice Problems
Solving earth science problems with scientific notation, × div[id^='image-'] {position:static}div[id^='image-'] div.hover{position:static} introductory problems.
These problems cover the fundamentals of writing scientific notation and using it to understand relative size of values and scientific prefixes.
Problem 1: The distance to the moon is 238,900 miles. Write this value in scientific notation.
Problem 3: The atomic radius of a magnesium atom is approximately 1.6 x 10 -10 meters (m). How do you write this length in standard form?
0.00000000016 m
Fissure A = 400,000 m Fissure B = 50,000 m
This shows fissure A is larger (by almost 10 times!). The shortcut to answer a question like this is to look at the exponent. If both coefficients are between 1-10, then the value with the larger exponent is the larger number.
Problem 5: The amount of carbon in the atmosphere is 750 petagrams (pg). One petagram equals 1 x 10 15 grams (g). Write out the amount of carbon in the atmosphere in (i) scientific notation and (ii) standard decimal format.
`750 xx 10^15 g = 7.5 xx 10^17 g`
The exponent is a positive number, so the decimal will move to the right in the next step.
750,000,000,000,000,000 g
Advanced Problems
Scientific notation is used in solving these earth and space science problems and they are provided to you as an example. Be forewarned that these problems move beyond this module and require some facility with unit conversions, rearranging equations, and algebraic rules for multiplying and dividing exponents. If you can solve these, you've mastered scientific notation!
Problem 7: Calculate the volume of water (in cubic meters and in liters) falling on a 10,000 km 2 watershed from 5 cm of rainfall.
`10,000 km^2 = 1 xx 10^4 km^2`
5 cm of rainfall = `5 xx 10^0 cm`
Let's start with meters as the common unit and convert to liters later. There are 1 x 10 3 m in a km and area is km x km (km 2 ), therefore you need to convert from km to m twice:
`1 xx 10^3 m/(km) * 1 xx 10^3 m/(km) = 1 xx 10^6 m^2/(km)^2` `1 xx 10 m^2/(km)^2 * 1 xx 10^4 km^2 = 1 xx 10^10 m^2` for the area of the watershed.
For the amount of rainfall, you should convert from centimeters to meters:
`5 cm * (1 m)/(100 cm)= 5 xx 10^-2 m`
`V = A * d`
When multiplying terms with exponents, you can multiply the coefficients and add the exponents:
`V = 1 xx 10^10 m^2 * 5.08 xx 10^(-2) m = 5.08 xx 10^8 m^3`
Given that there are 1 x 10 3 liters in a cubic meter we can make the following conversion:
`1 xx 10^3 L * 5.08 xx 10^8 m^3 = 5.08 xx 10^11 L`
Step 5. Check your units and your answer - do they make sense?
`V = 4/3 pi r^3`
Using this equation, plug in the radius (r) of the dust grains.
`V = 4/3 pi (2 xx10^(-6))^3m^3`
Notice the (-6) exponent is cubed. When you take an exponent to an exponent, you need to multiply the two terms
`V = 4/3 pi (8 xx10^(-18)m^3)`
Then, multiple the cubed radius times pi and 4/3
`V = 3.35 xx 10^(-17) m^3`
`m = 3.35 xx 10^(-17) m^3 * 3300 (kg)/m^3`
Notice in the equation above that the m 3 terms cancel each other out and you are left with kg
`m = 1.1 xx 10^(-13) kg`

`0.25 "light years" * 9.3xx10^15 m = 2.325xx10^15 m = r`
`V = 4/3 pi (2.325 xx10^(15) m)^3`
`V = 5.26 xx10^(46) m^3`
Number of dust grains = `5.26 xx10^(46) m^3 xx 0.001` grains/m 3
Number of dust grains = `5.26xx10^43 "grains"`
Total mass = `1.1xx10^(-13) (kg)/("grains") * 5.26xx10^43 "grains"`
Notice in the equation above the 'grains' terms cancel each other out and you are left with kg
Total mass = `5.79xx10^30 kg`
TAKE THE QUIZ!!
Or you can go back to the Scientific Notation explanation page .
« Previous Page Next Page »

- Parallelogram
- Quadrilateral
- Parallelepiped
- Tetrahedron
- Dodecahedron
- Fraction Calculator
- Mixed Fraction Calculator
- Greatest Common Factor Calulator
- Decimal to Fraction Calculator
- Whole Numbers
- Rational Numbers
- Place Value
- Irrational Numbers
- Natural Numbers
- Binary Operation
- Numerator and Denominator
- Order of Operations (PEMDAS)
Scientific Notation
Table of Contents
Last modified on June 8th, 2024
#ezw_tco-2 .ez-toc-title{ font-size: 120%; ; ; } #ezw_tco-2 .ez-toc-widget-container ul.ez-toc-list li.active{ background-color: #ededed; } chapter outline
Scientific notation is a special way of representing numbers which are too large or small in a unique way that makes it easier to remember and compare them. They are expressed in the form (a × 10n). Here ‘a’ is the coefficient, and ‘n’ is the power or exponent of the base 10.
The diagram below shows the standard form of writing numbers in scientific notation:
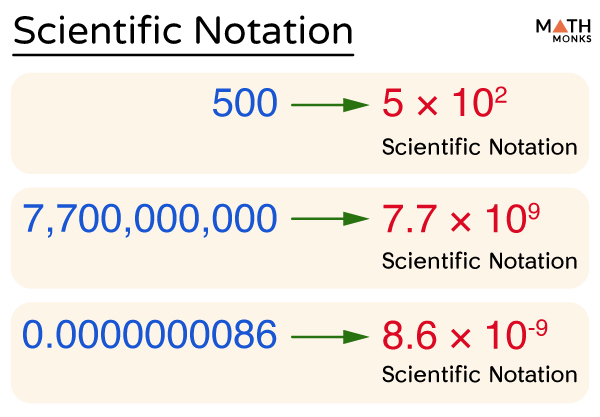
Thus, scientific notation is a floating-point system where numbers are expressed as products consisting of numbers between 1 and 10 multiplied with appropriate power of 10. It helps to represent big and small numbers in a much easier way.
The speed of light(c) measured in a vacuum is approximately 300,000,000 meters per second, which is written as 3 × 10 8 m/s in scientific notation. Again the mass of the sun is written as 1.988 × 10 30 kg. All these values, if written in scientific form, will reduce a lot of space and decrease the chances of errors.
Scientific Notation Rules
We must follow the five rules when writing numbers in scientific notation:
- The base should always be 10
- The exponent (n) must be a non-zero integer, positive or negative
- The absolute value of the coefficient (a) is greater than or equal to 1, but it should be less than 10 (1 ≤ a < 10)
- The coefficient (a) can be positive or negative numbers, including whole numbers and decimal numbers
- The mantissa contains the remaining significant digits of the number
How to Do Scientific Notation with Examples
As we know, in scientific notation, there are two parts:
- Part 1: Consisting of just the digits with the decimal point placed after the first digit
- Part 2: This part follows the first part by × 10 to a power that puts the decimal point where it should be
While writing numbers in scientific notation, we need to figure out how many places we should move the decimal point. The exponent of 10 determines the number of places the decimal point gets shifted to represent the number in long form.
There are two possibilities:
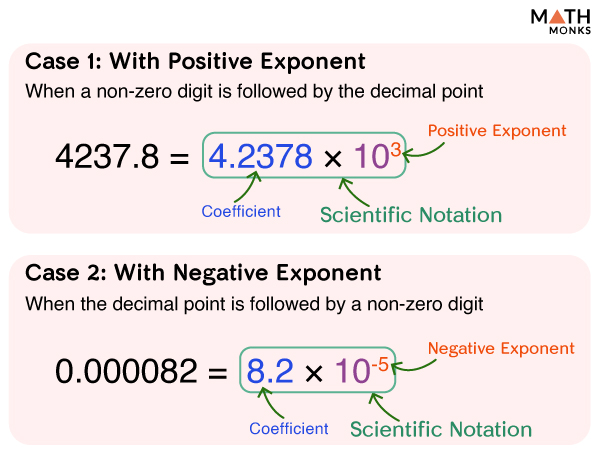
Case 1: With Positive Exponent
When the non-zero digit is followed by a decimal point
For example, if we want to represent 4237.8 in scientific notation, it will be:
- The first part will be 4.2378 (only the digit and the decimal point placed after the first digit)
- The second part following the first part will be × 10 3 (multiplied by 10 having a power of 3)
Case 2: With Negative Exponent
When the decimal point comes first, and the non-zero digit comes next
For example, if we want to represent 0.000082 in scientific notation, it will be:
- The first part will be 8.2 (only the coefficient in decimal form and the decimal point placed after the first digit)
- The second part following the first part will be × 10 -5 (multiplied by 10 having a power of -5)
Here is a table showing some more examples of numbers written in scientific notation:
| 1,000,000,000 (1 billion) | 1 x 10 |
| 24327 | 2.4327 x 10 |
| 0.053 | 5.3 x 10 |
| 0.00049386 | 4.9386 × 10 |
| 10,000,000 (10 million) | 1 x 10 |
| 31,000,000,000 (31 billion) | 31 x 109 |
| 7,63,000 | 7.63 × 10 |
| 1 nanometer (nm) | 10 m |
| 1 micrometer (μm) | 10 m |
Let us solve some more word problems involving writing numbers in scientific notation.
Write the number 0.0065 in scientific notation.
0.0065 is written in scientific notation as: 6.5 × 10 -3
Convert 4.5 in scientific notation.
4.5 is written in scientific notation as: 4.5 × 10 0
Write 53010000 in scientific notation.
53010000 is written in scientific notation as: 5.301 ×10 7
Light travels with a speed of 1.86 x 10 5 miles/second. It takes sunlight 4.8 x 10 3 seconds to reach Saturn. Find the approximate distance between Sun and Saturn. Express your answer in scientific notation.
As we know, Distance (d) = Speed (s) × Time (t), here s = 1.86 x 10 5 miles/second, t = 4.8 x 10 3 seconds = 8.928 x 10 8 miles
Other Ways of Writing in Scientific Notation
We sometimes use the ^ symbol instead of power while writing numbers in scientific notation. In such cases, the above number 4237.8, written in scientific notation as 4.2378 × 10 3 , can also be written as 4.2378 × 10^3 Similarly, calculators use the notation 4.2378E; here, E signifies 10 × 10 × 10
- Converting Scientific Notation to Standard Form
- Multiplying Numbers in Scientific Notation
- Dividing Numbers in Scientific Notation
- Adding and Subtracting Numbers in Scientific Notation
Leave a comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Related Materials
- Privacy Policy
- Trigonometry
Join Our Newsletter
© 2024 Mathmonks.com . All rights reserved. Reproduction in whole or in part without permission is prohibited.

IMAGES
VIDEO
COMMENTS
The reasoning in the solution of each exercise is useful to be applied to other similar scientific notation problems. EXAMPLE 1. Write the number 34100000 in scientific notation. ... we have to make sure that all the numbers are converted to the same power of 10. Once the numbers have the same power of 10, we simply add the digit terms ...
Name _____ Date _____ Class _____ LESSON 2-3 Scientific Notation with Negative Powers of 10 Practice and Problem Solving: A/B Write each number as a negative power of ten. ... 0.000473 20. 0.0024 Write each number in scientific notation. 17. ... square meters. 20. 2.4 × 10−3 Practice and Problem Solving: D 16. 0.0000001 17. 2.5 × ...
0.00006 = 6.0 x 10 -5. Problem 2 : Write the given number in scientific notation. 0.000216. Solution : In the given number, number of digits from decimal point up to the first non zero digit is 4. So, the decimal point has to be moved 4 digits to the right and exponent of 10 should be -4 (negative integer). Therefore, 0.000216 = 2.16 x 10 -4.
Regular Notation. How to Change. Scientific Notation. 420,000. Move the decimal after the 4 and before the 2 That is 5 places to the left Multiply 4.2 by 10 to the 5th power. 4.2 10. 735,000,000. Move the decimal after the 7 and before the 3 That is 8 places to the left Multiply 7.35 by 10 to the 8th power. 7.35 10.
Scientific notation, also called standard exponential notation, is a subset of exponential notation. Scientific notation represents numeric values using a significand that is 1 or greater, but less than 10, multiplied by the base 10 to a whole-number power. This means that to write a number in scientific notation, the decimal point in the ...
Example Problem 1: Using Scientific Notation with Negative Exponents: Write the number 5 × 10 − 2 in standard form. Step 1: The number multiplied by a power of ten is 5 whose decimal point is ...
Scientific Notation with Negative Powers of 10 Practice and Problem Solving: C Write each pair of numbers in standard notation. ... Write each pair of numbers in scientific notation. Write the numbers in scientific notation on the correct side of the comparison symbol. 5. 0.0012; 0.45 6. 0.0000023; 0.00032 ... 3.5 × 10−1 cm = 3.5 × 10−3 m ...
To write a number in scientific notation, move the decimal point to the right of the first digit in the number. Write the digits as a decimal number between and . Count the number of places that you moved the decimal point. Multiply the decimal number by raised to a power of .
Scientific Notation with Negative Powers of 10 Practice and Problem Solving: A/B Write each number as a negative power of ten. 1. 2 1 10 = _____ 2. 4 1 10 = _____ 3. 5 1 10 = _____ 4. 7 1 ... Write this measurement in scientific notation. _____ LESSON 15-3 . Author: prashant.shukla Created Date: 9/6/2017 4:34:31 PM ...
Scientific Notation with Powers of 10 Practice and Problem Solving: A/B Write each number as a negative power of ten. 1. 2 1 10 ... Practice and Problem Solving: D 1. 1 2. 1 000 3. 1 10,000 4. 1 1,000 5. 1
Problems and Solutions. Question 1: Convert 0.00000046 into scientific notation. Solution: Move the decimal point to the right of 0.00000046 up to 7 places. The decimal point was moved 7 places to the right to form the number 4.6. Since the numbers are less than 10 and the decimal is moved to the right. Hence, we use a negative exponent here.
The format is written [Math Processing Error] a × 10 n, where [Math Processing Error] 1 ≤ a <10 and n is an integer. To multiply or divide numbers in scientific notation, you can use the commutative and associative properties to group the exponential terms together and apply the rules of exponents. Orders of magnitude (mass). (n.d.).
Convert from decimal notation to scientific notation. To write a large number in scientific notation, move the decimal point to the left to obtain a number between 1 and 10. Since moving the decimal point changes the value, you have to multiply the decimal by a power of 10 so that the expression has the same value.
Express this number using scientific notation. 22. Violet light has the shortest wavelength of all visible light, measuring 0.0000004 metres. Express this wavelength in scientific notation. 23. Express each of the following values in standard notation. a) 8 10× 5 b) − ×2.9 10 8 c) 6.54 10× −3 d) 9 10× −11 e) 7.562 10× −9 24.
Multiply this by a negative power of 10 equal to the number of zeros in front of the first non-zero digit. For example, write 0.000008 in scientific notation. Step 1. Write the non-zero digits as a number between 1 and 10. The zeros at the start of 0.000008 are ignored to obtain the non-zero digit of 8.
In this example scientific notation calculation we're solving 1.225 × 10 5 + 3.655 × 10 3: 1.225 × 10 5 + 3.655 × 10 3 = 1.26155 x 10 5. E Notation. E notation is also known as exponential notation. E notation is the same as scientific notation where a decimal number between 1 and 10 is multiplied by 10 raised to some power. In E notation ...
Problem 1: The distance to the moon is 238,900 miles. Write this value in scientific notation. Show me how. Problem 2: The age of the Earth is roughly 4,560,000,000 years. Write this in scientific notation. Show me how. Problem 3: The atomic radius of a magnesium atom is approximately 1.6 x 10 -10 meters (m).
We must follow the five rules when writing numbers in scientific notation: The base should always be 10. The exponent (n) must be a non-zero integer, positive or negative. The absolute value of the coefficient (a) is greater than or equal to 1, but it should be less than 10 (1 ≤ a < 10) The coefficient (a) can be positive or negative numbers ...
Enter a number or a decimal number or scientific notation and the calculator converts to scientific notation, e notation, engineering notation, standard form and word form formats. To enter a number in scientific notation use a carat ^ to indicate the powers of 10. You can also enter numbers in e notation. Examples: 3.45 x 10^5 or 3.45e5.
Scientific Notation with Positive Powers of 10 Practice and Problem Solving: A/B Write each number as a power of 10. ... Write this number in scientific notation. _____ 34. The radius of Earth is about 6.38 × 103 kilometers. Write this distance in standard notation. ...