An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis
Matti uusitupa, tauseef a khan, effie viguiliouk, hana kahleova, angela a rivellese, kjeld hermansen, andreas pfeiffer, anastasia thanopoulou, jordi salas-salvadó, ursula schwab, john l sievenpiper.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] ; Tel.: +358-400-615661
Received 2019 Sep 3; Accepted 2019 Oct 18; Collection date 2019 Nov.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/ ).
Prevention of type 2 diabetes (T2D) is a great challenge worldwide. The aim of this evidence synthesis was to summarize the available evidence in order to update the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy. We conducted a systematic review and, where appropriate, meta-analyses of randomized controlled trials (RCTs) carried out in people with impaired glucose tolerance (IGT) (six studies) or dysmetabolism (one study) to answer the following questions: What is the evidence that T2D is preventable by lifestyle changes? What is the optimal diet (with a particular focus on diet quality) for prevention, and does the prevention of T2D result in a lower risk of late complications of T2D? The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was applied to assess the certainty of the trial evidence. Altogether seven RCTs (N = 4090) fulfilled the eligibility criteria and were included in the meta-analysis. The diagnosis of incident diabetes was based on an oral glucose tolerance test (OGTT). The overall risk reduction of T2D by the lifestyle interventions was 0.53 (95% CI 0.41; 0.67). Most of the trials aimed to reduce weight, increase physical activity, and apply a diet relatively low in saturated fat and high in fiber. The PREDIMED trial that did not meet eligibility criteria for inclusion in the meta-analysis was used in the final assessment of diet quality. We conclude that T2D is preventable by changing lifestyle and the risk reduction is sustained for many years after the active intervention (high certainty of evidence). Healthy dietary changes based on the current recommendations and the Mediterranean dietary pattern can be recommended for the long-term prevention of diabetes. There is limited or insufficient data to show that prevention of T2D by lifestyle changes results in a lower risk of cardiovascular and microvascular complications.
Keywords: prevention, type 2 diabetes, diet, lifestyles, complications
1. Introduction
Both the prevalence and incidence of type 2 diabetes (T2D) are increasing rapidly worldwide. Worldwide, in 2017, approximately 425 million people had diabetes. This figure may rise to 629 million by 2045. However, the figures for different European countries are not as dramatic as the figures in America and in many low- and middle-income countries. In Europe, the prevalence of T2D is also increasing in parallel to the obesity epidemic. In 2017, the number of patients with diabetes in Europe was 66 million (prevalence 9.1%) and it is estimated to be 81 million by 2045. [ 1 , 2 ]. T2D is a potent risk factor for cardiovascular diseases, but also for blindness, renal failure, and lower limb amputation, decreasing the quality of life of people affected. The burden of diabetes is not only a public health issue, but it also has marked economic consequences. More specifically, the expenses for the treatment of diabetes are increasing mostly due to its long-term complications but also modern drug treatment options [ 3 ]. Furthermore, bariatric surgery is becoming more popular for markedly obese patients with T2D due to its significant beneficial effects on metabolic control, long-term complications, and prognosis of T2D [ 4 , 5 ].
The interest in preventing diabetes through lifestyle changes was already present in the 1980s [ 6 ], and the opportunity to prevent T2D through lifestyle changes was re-emphasized in the 2004 recommendations of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) [ 7 ]. Since then, a number of randomized controlled trials (RCTs) have been published that show that T2D is preventable, or its onset can be markedly postponed, by increasing physical activity, reducing weight, and changing dietary habits.
To update the evidence for the EASD clinical practice guidelines for nutrition therapy, we conducted a systematic review and, where appropriate, meta-analyses of the available randomized controlled trials assessing lifestyle interventions in the prevention of T2D with the aim of answering the following questions:
(a) What is the evidence that T2D is preventable by lifestyle changes in adults with impaired glucose tolerance (IGT) and (b) what are the long-term results on the prevention of T2D?
What is the evidence that the lifestyle changes aimed to prevent T2D also modify the risk of cardiovascular disease and microvascular complications in people with IGT?
What is the optimal dietary composition for the prevention of T2D in people with IGT?
A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to assess the role of lifestyle changes on the prevention of T2D using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. In addition, we discuss the lifestyle including dietary changes that have been successfully used for the prevention of T2D and summarize the long-term follow-up results after the active intervention periods from the major T2D prevention trials on the incidence of T2D and micro- and macrovascular diseases, and finally make the conclusions regarding the three study questions.
We attempt to answer these three questions in turn, summarizing the evidence following by making conclusions at the end of the paper.
2. Evidence That T2D Is Preventable by Changing Lifestyles
A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to assess the role of lifestyle changes on the prevention of T2D using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
3.1. Search Strategy and Study Selection
We conducted our systematic review and meta-analysis according to the Cochrane Handbook for Systematic Reviews of Interventions [ 8 ], and reported the results according to the PRISMA guidelines ( www.prisma-statement.org ). We conducted standard literature searches of PubMed (MEDLINE), EMBASE, and Cochrane Library through 21 June 2019 to identify both original RCTs and recent systematic reviews [ 9 , 10 , 11 , 12 ] that have examined the association of lifestyle intervention with T2D. The following key words were used in selecting original RCTs for this search: type 2 diabetes, RCT, prevention, systematic reviews, impaired glucose tolerance (IGT), diet, dietary pattern, physical activity, and lifestyle. We supplemented the systematic search with a manual search of reference lists. We selected RCTs comparing the effect of lifestyle intervention (exercise-plus-diet or exercise-plus-diet-plus-weight loss) versus control (no lifestyle intervention) on incident T2D defined using study-specific criteria based on a 2 h oral glucose tolerance test (OGTT) in all populations in an outpatient setting with a minimum follow-up of 1 year. We included studies that were conducted in a high-risk population including those with IGT and metabolic syndrome. Studies that only assessed exercise intervention without diet or weight-loss, used a drug(s) as part of the lifestyle intervention, or only reported observational cohort studies were excluded. In case of the multiple publication of the same trial, we used the one with the end-trial data.
3.2. Data Extraction
Two investigators (EV and TAK) independently reviewed and extracted relevant data from each included report. A standardized form was used to extract data on sample size, participant characteristics, study setting and design, level of monitoring of eating habits, intervention and control arm, macronutrient composition of diets, energy balance, follow-up duration, funding source and outcome data. All discrepancies and disagreements were resolved through consensus.
3.3. Risk of Bias Assessment
Included trials were independently assessed by two investigators (EV and TAK) for the risk of bias using the Cochrane Risk of Bias Tool [ 8 ]. An assessment was performed across 5 domains of bias (sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting). The risk of bias was assessed as either low (proper methods taken to reduce bias), high (improper methods creating bias) or unclear (insufficient information provided to determine the bias level). All discrepancies and disagreements were resolved through consensus or, where necessary, by a third author (JLS). The methods applied are described in the individual publications [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ].
3.4. Data Syntheses
All analyses were conducted using Stata 16 ((StataCorp, College Station, TX, USA). Data were expressed as risk ratios (RRs) with 95% confidence intervals (CIs) and pooled using the restricted maximum likelihood (REML) random-effects models [ 29 ]. A random-effects model assumes that study estimates are estimating different, yet related, intervention effects and thus incorporates heterogeneity among studies. This is a more appropriate method to pool studies that may differ slightly in distribution of risk factors, population, size, and outcomes [ 30 ]. Heterogeneity was assessed using the Cochran Q statistic and quantified using the I 2 statistic. Significance for heterogeneity was set at p < 0.10, with an I 2 > 50% considered to be evidence of substantial heterogeneity [ 15 ]. Sources of heterogeneity were explored using sensitivity and subgroup analyses. Sensitivity analyses were performed in which each individual trial was removed from the meta-analysis and the effect size recalculated to determine whether a single trial exerted an undue influence. If ≥10 trials were available, then a priori subgroup analyses were conducted using meta-regression by baseline values, study design, follow-up, comparator arm, risk of bias and diabetes duration [ 16 ]. If ≥10 trials were available, then we also assessed publication bias by visual inspection of funnel plots and formal testing by the Egger and Begg tests [ 17 ].
3.5. Grading of the Evidence
The GRADE approach was used to assess the certainty of the evidence [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ]. The certainty of the evidence was graded as high, moderate, low, or very low. Randomized controlled trials receive an initial grade of high by default and are downgraded based on the following pre-specified criteria: risk of bias (weight of trials showing risk of bias by the Cochrane Risk of Bias Tool), inconsistency (substantial unexplained inter-study heterogeneity, I 2 > 50% and p < 0.10), indirectness (presence of factors that limit the generalizability of the results), imprecision (the 95% CI for effect estimates were wide or cross minimally important differences (MIDs) for benefit or harm), and publication bias (significant evidence of small-study effects). The MID for T2D was set at 5 percent based on increased cardiovascular disease risk [ 31 ].
4.1. Search Results
Figure 1 outlines our systematic search. We identified 5286 articles from PubMed (MEDLINE), EMBASE, and Cochrane Library.
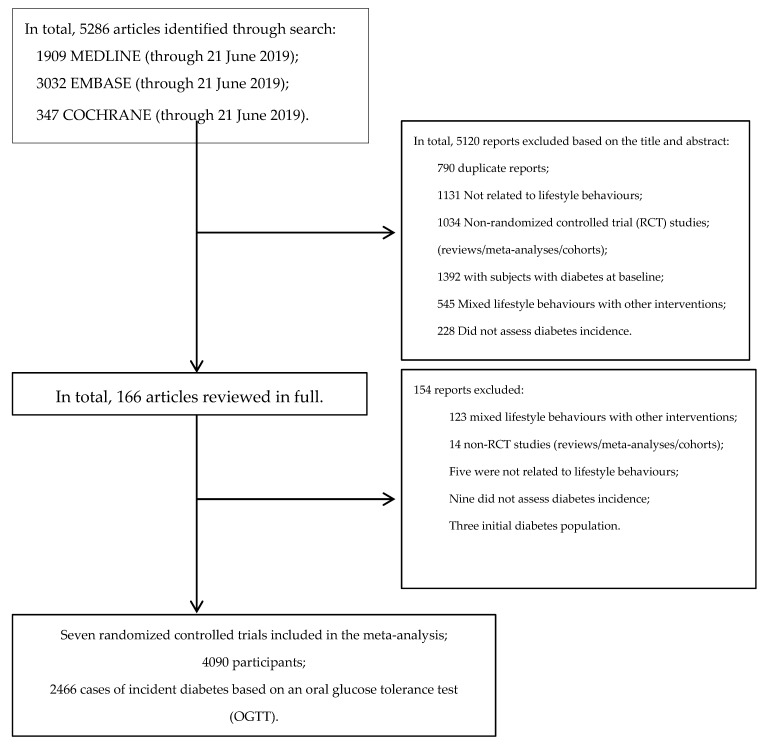
Flow diagram outlining the systematic search and article selection process.
4.2. Randomized Controlled Trials
We identified seven RCTs comprising 4090 study participants and 2466 incident type 2 diabetes cases [ 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 ] (see Table 1 and Figure 1 ). Except for the study by Bo et al. [ 38 , 39 ] (which was conducted in people with dysmetabolism), all studies were carried out in people with impaired glucose tolerance (IGT) based on an OGTT, and the diagnosis of incident diabetes was confirmed by OGTT applying contemporary WHO criteria for diabetes mellitus. Detailed data on the intervention measures and the follow-up of the control groups have been reported in individual publications and summarized in Table 1 .
Summary results on the randomized controlled trials aimed to prevent type 2 diabetes in people with impaired glucose tolerance or in people at high increased risk for diabetes.
IGT = impaired glucose tolerance based on OGTT, CHO = carbohydrates, prot = protein, SFA = saturated fatty acids, PUFA = polyunsaturated fatty acids, intervention = intervention group, control = control group, minus = reduction from baseline, NA = not available, and NS = not significant, LSM = lifestyle modification, Met = Metformin. Da Qing IGT: The Da Qing IGT and Diabetes Study; FDPS: Finnish Diabetes Prevention Study; DPP: The Diabetes Prevention Program; IDDP-1: The Indian Diabetes Prevention Programme; EDIPS: European Diabetes Prevention Study; LSM: lifestyle modification; Met: metformin; yrs: years; IGT: Impaired glucose tolerance.
4.3. Risk of Bias
Figure 2 shows the individual Cochrane Risk of Bias assessments of seven trials included in the current meta-analysis (see Figure 1 and Table 1 for details). The majority of trials were judged as having unclear or low risk of bias across domains. No evidence of a serious risk of bias was detected.
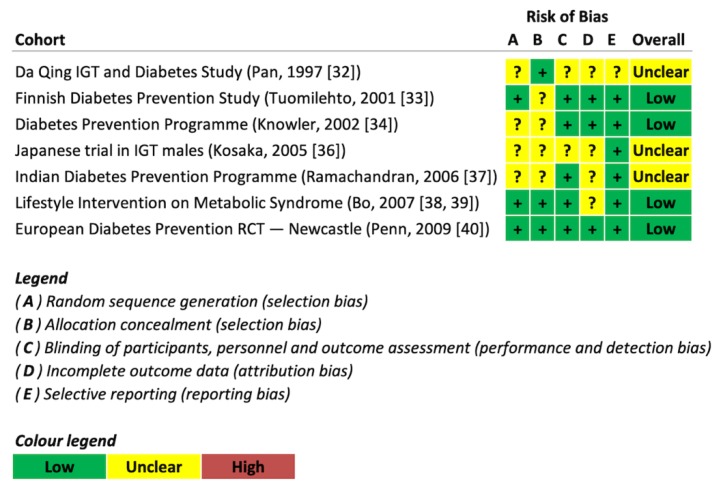
Risk of bias assessment.
4.4. Effect of Lifestyle Changes on Type 2 Diabetes Risk
Figure 3 shows the effect of lifestyle changes on T2D risk based on the meta-analysis. In seven trials involving 4090 participants [ 32 , 33 , 34 , 36 , 37 , 38 , 40 ], lifestyle intervention significantly decreased T2D risk compared to control groups (RR = 0.53 (95% CI: 0.41, 0.67), p < 0.001), with evidence of substantial inter-study heterogeneity (I 2 = 63%, p = 0.01).
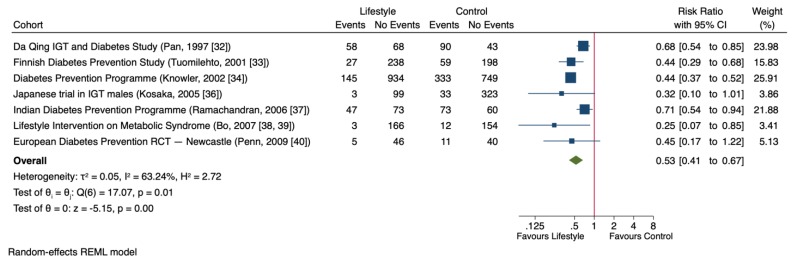
Forest plot of randomized controlled trials investigating the effect of lifestyle changes on type 2 diabetes risk (T2D). The pooled effect estimate for the overall effect is represented by the green diamond. Data are expressed as weighted risk ratios with 95% confidence intervals (CIs) using the restricted maximum likelihood (REML) random-effects model. Inter-study heterogeneity was tested by the Cochrane Q-statistic at a significance level of p < 0.10 and quantified by I 2 , where a level of ≥50% represented substantial heterogeneity.
4.5. Sensitivity and Subgroup Analyses
Table 2 shows selected sensitivity analyses in which the systematic removal of individual trials altered the results. The evidence of substantial heterogeneity was partially explained by the removal of Knowler et al. [ 34 ], which changed the evidence for heterogeneity from significant (I 2 = 65%, p = 0.009) to non-significant (I 2 = 43%, p = 0.16). However, this did not appreciably change the overall effect estimate (RR = 0.49 (95% CI: 0.37, 0.64), p < 0.001). Subgroup analyses were not conducted for any outcome as <10 trials were available.
Influence analysis assessment for the effect of lifestyle changes on T2D risk.
CI = confidence interval.
4.6. Publication Bias
Publication bias was not assessed for any outcome as <10 trials were available.
4.7. GRADE Assessment
Table 3 shows a summary of the GRADE assessments of the overall certainty of the effect of lifestyle changes on the risk of transition from IGT to T2D. The evidence was graded as high for the effect of lifestyle intervention on T2D risk reduction without any downgrading for risk of bias, inconsistency, indirectness, imprecision, or other considerations.
GRADE assessment for the effect of lifestyle changes on T2D risk.
CI = confidence interval; GRADE = grading of recommendations assessment, development, and evaluation; RR = risk ratio; T2D = type 2 diabetes. a Although there was significant heterogeneity (I 2 = 65%, p = 0.01), the removal of one study [ 34 ] explained some of the heterogeneity, which changed it from significant to non-significant (I 2 = 36%, p = 0.16). However, the estimate of effect did not change appreciably. Furthermore, this inconsistency was not considered serious as the magnitude of effect remained large and in the same direction across all the studies (RR < 0.72).
5. Discussion on the Systematic Review and Meta-Analysis
We conducted a systematic review and meta-analysis of seven randomized controlled trials involving 4090 predominantly middle-aged participants with glucose impairment (IGT or dysmetabolism), which showed that lifestyle modification including improved diet and physical activity reduced the risk of type 2 diabetes by 47 percent.
5.1. Results in the Context of Existing Literature
Recent systematic reviews published on the prevention of T2D in high-risk groups uniformly conclude that the onset of T2D can be delayed or prevented with lifestyle changes. Furthermore, these systematic reviews conclude that lifestyle changes may result in the sustained reduction of T2D [ 9 , 10 , 11 , 12 ]. On the other hand, a recent Cochrane review concluded that the evidence took into account only the combined effect of physical activity and dietary changes, and the evidence on the effect of diet or physical activity alone is insufficient [ 12 ].
A brief discussion of the included studies and other literature is helpful here as these will also be referred to in the subsequent sections of this paper. The Chinese Da Qing study [ 32 ] had altogether 577 IGT individuals in 33 study clinics that were randomized to control, exercise, healthy diet, and healthy diet plus exercise clinics, with a follow-up of 6 years. The risk of diabetes was reduced by 33% in the diet-only group, 47% in the exercise-only group and 38% in the diet-plus-exercise group as compared to the control group, without significant differences between the intervention groups. The study individuals were normal weight or overweight at baseline, and the reduction in total energy intake was 100–240 kcal depending on the intervention ( Table 1 ).
In the Finnish Diabetes Prevention Study (FDPS) [ 33 ], 522 individuals with IGT were randomized into a control or lifestyle intervention group (healthy diet and physical activity promotion). The diagnosis of T2D was based on repeated OGTT. After 3.2 years of follow-up, there was a significant decrease in the incidence of T2D, and the trial was prematurely stopped based on the decision of the independent advisory committee. The risk reduction was 58% in the intervention group compared to the control group. Weight loss was larger in the intervention group: the difference in weight reduction between the groups was 3.5 and 2.6 kg at 1 and 3 years, respectively. The intervention group also showed an increase in physical activity and the number of sedentary people was smaller in the intervention (17%) than in the control group (29%).
In the Diabetes Prevention Program (DPP) study conducted in the USA [ 34 ], altogether, 3234 individuals with IGT in 27 centers were randomized into the lifestyle intervention, metformin or control groups. The mean follow-up was 2.8 years. The risk of T2D was reduced by 58% in the lifestyle intervention group as compared to the control group. In the metformin group, the risk of diabetes was 31% lower than in the control group. At year 1, weight reduction in the intervention group was 5.6 kg and 0.1 kg in the control group. No detailed changes in physical activity were reported. It is of note that the initial BMI in the DPP was 34 kg/m 2 when in the FDPS it was 30–31 kg/m 2 .
In a Japanese study on 458 men with IGT [ 36 ], compared to the control group, a remarkable relative risk reduction of 67.4% was found in the intervention group that aimed for weight reduction, increased vegetable intake and physical activity during the 4 year follow-up. The BMI goal was 22 kg/m 2 and the majority of participants had either normal BMI or they were overweight with IGT. Still, the average weight loss was 2.2 kg in the intervention group.
In the Indian Diabetes Prevention Programme (IDPP-1) study [ 37 ], consisting of 531 subjects with IGT, there was a 28.5% reduction in the risk of T2D after 3 years of follow-up in the lifestyle modification group (LSM) compared to the control group, 28.2% reduction in the LSM-plus-metformin (Met) group and 26.4% reduction in the Met group. No significant group differences were found in the preventative effect with regard to LSM, Met and LSM-plus-Met groups. This study did not report significant changes in body weight.
Bo et al. in Italy carried out a lifestyle intervention aimed at the prevention of metabolic syndrome (MetS) in 335 subjects with dysmetabolism. This group included subjects with metabolic syndrome together with those having only two components of metabolic syndrome plus high hs-CRP values. In addition to an effect on metabolic syndrome, this study also reported 1 and 4 year results on the incidence of T2D [ 38 , 39 ]. After one year, there was a marked risk reduction in the incidence of T2D [OR 0.23; 95% CI 0.06–0.85]. The difference in weight reduction between the intervention and control groups was approximately 2.3 kg. After 4 years, the incidence of T2D was 5.4% in the intervention group and 10.2% in the control group.
In the Newcastle arm of the European Diabetes Prevention Study (EDIPS) study [ 40 ] consisting of 102 subjects with IGT, after 3 years of lifestyle intervention following mostly principles of the FDPS, the incidence of T2D was 5.0% and 11.1% in the intervention and the control groups, respectively. The average weight loss was 2.5 kg in the intervention group and sustained beneficial changes in lifestyles predicted better outcome in the T2D risk.
Before the above randomized trials that are included in the meta-analysis, Eriksson and Lindgarde reported in 1991 [ 41 ] that a 6 month sequential intervention of dietary change or increased physical activity may have prevented the development of T2D in 181 Swedish men who volunteered to take part in the lifestyle intervention compared to those who did not volunteer to participate.
In a smaller study of 88 subjects (the SLIM Study) [ 35 ], with 2 years of lifestyle intervention, not included in the current meta-analysis because it did not fulfill the inclusion criteria, there was a significant improvement in 2 h glucose values in the active intervention group. The beneficial changes could be ascribed to moderate weight loss and dietary changes (i.e., reduction in saturated fat intake) in combination with increased physical activity. Incidence data on T2D after 3 years were included in the European Diabetes Prevention Study RCT [ 42 ], where the preventative effect of ≥5% weight loss was particularly high, especially if maintained for 3 years.
Two post-hoc reports from the PREDIMED study also suggest that it is possible to prevent T2D even without significant weight loss in individuals at high risk for cardiovascular disease (CVD), using the Mediterranean diet including extra virgin olive oil or nuts. The risk reduction using the Mediterranean diet intervention, either supplemented with virgin olive oil or nuts, compared to the control group was 30% to 50% depending on the baseline population [ 43 , 44 ]. These studies are discussed in greater detail later in the manuscript with regard to the optimal diet for the prevention of T2D and cardiovascular disease.
5.2. Strengths and Limitations
Our systematic review and meta-analysis have several strengths. These include a rigorous search and selection strategy that identified all available randomized controlled trials examining the effect of lifestyle modification on T2D in individuals; the inclusion of predominantly high-quality randomized controlled trials, which give the greatest protection against bias; the use of the REML random-effects model, which is robust to non-normal distributions and has been recommended for use in meta-analyses over other random-effects estimators [ 29 ]; and the assessment of the overall certainty of the evidence using the GRADE approach.
There were no major limitations of our systematic review and meta-analysis. There was an issue of high heterogeneity, but we did not downgrade for the observed inconsistency. We did not consider the statistical heterogeneity to be a limitation as our meta-analysis included large studies with narrow confidence intervals and similar estimates in the same direction. Therefore, this apparent inconsistency was an artefact of non-overlapping narrow CIs rather than a limitation of the certainty of the overall estimate [ 23 , 45 ]. Balancing the strengths and limitations, the evidence as assessed using GRADE was of high certainty for the effect of lifestyle modification on the reduction of T2D.
6. Long-Term Results on the Prevention of Type 2 Diabetes
Three follow-up studies, the Da Qing Chinese study [ 46 ], FDPS [ 47 , 48 ] and DPP [ 49 ], showed that the beneficial lifestyle changes achieved in the prevention of T2D trials resulted in a sustained risk reduction of T2D over 10 years of follow-up ( Table 4 ).
Long-term post-intervention preventative effect on the incidence of type 2 diabetes in the former intervention groups compared to control groups in three randomized controlled lifestyle intervention studies.
Figure 4 shows the effect of lifestyle changes on the T2D risk based on the meta-analysis of the selected trials that had the long-term follow-up after the lifestyle intervention phase. In three trials consisting a total of 3855 participants with a median follow-up of 13 years [ 46 , 47 , 49 ], lifestyle intervention was associated with significantly lower T2D risk compared to control groups (RR = 0.63 [95% CI: 0.54, 0.74], p < 0.001) with no evidence of inter-study heterogeneity (I 2 = 0%, p = 0.76).
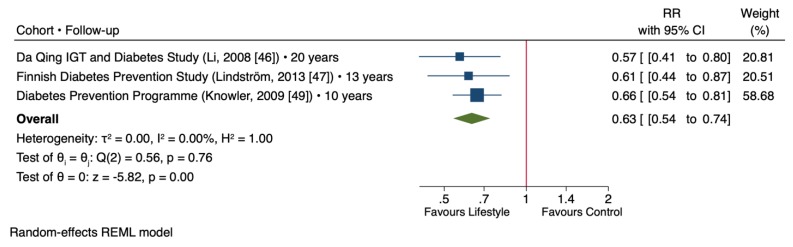
Forest plot of randomized controlled trials investigating the long-term post-intervention effect of lifestyle changes on type 2 diabetes risk. The pooled effect estimate for the overall effect is represented by the green diamond. Data are expressed as weighted risk ratios with 95% confidence intervals (CIs) using the REML random-effects model. Inter-study heterogeneity was tested by the Cochrane Q-statistic at a significance level of p < 0.10 and quantified by I 2 , where a level of ≥50% represented substantial heterogeneity.
Based on the results from FDPS [ 47 , 48 ], 22 subjects with IGT must be treated for one year or 5 subjects for five years to prevent one case of diabetes. Accordingly, in DPP [ 49 ], the respective figure was 6.9 subjects for a 3 year intervention.
7. Evidence That the Prevention of T2D in High-Risk Individuals Results in a Lower Risk of Cardiovascular Disease (CVD) and Microvascular Complications
Among the selected intervention trials, three follow-up post-intervention studies reported cardiovascular and/or microvascular complications ( Table 5 ). Furthermore, we considered the PREDIMED intervention trial results for this question as this study was carried out in high-risk individuals [ 43 , 44 ].
Long-term post-intervention data on mortality, cardiovascular (CVD) mortality and microvascular complications in the former intervention groups compared to the control groups in three randomized controlled lifestyle intervention studies.
NA: Not available.
This question is of particular importance, since the ultimate goal of the prevention and treatment of diabetes is the prevention of the long-term complications of diabetes associated with long-term hyperglycemia, dyslipidemias, hypertension, and other metabolic abnormalities, including low-grade inflammation [ 50 ]. Indeed, long-term intervention trials on the prevention of T2D have shown that besides improved glycemia, due to the correction of insulin resistance and possibly the preservation of beta-cell capacity [ 33 , 34 , 51 ], many of the well-known cardiovascular risk factors and characteristics of metabolic syndrome are corrected by changing to a healthier diet, increasing physical activity and losing weight [ 43 , 44 , 51 , 52 , 53 ]. However, there has been little evidence that the incidence of CVD or microvascular complications can be postponed or prevented by changing lifestyles. Recent data from the Da Qing Diabetes Prevention Outcome study reported results for both mortality and morbidity that suggest long-term benefits as a result of changing lifestyle habits. To summarize, there was a significant reduction in all cause deaths (26%), CVD deaths (33%) and total CVD events (26%) in the combined intervention groups as compared to the control group. Furthermore, composite microvascular diseases (35%) and the incidence of any retinopathy (40%) were significantly lower in the combined intervention groups in this cohort [ 54 ].
Furthermore, the PREDIMED study reported a significant reduction in combined stroke and all cardiovascular events in individuals randomized to the Mediterranean diet (MedDiet) plus extra-virgin olive oil or MedDiet plus nuts group [ 58 ]. Recently, the incidence of retinopathy was reported to be lower in the PREDIMED study in individuals randomized to MedDiet plus extra-virgin olive oil group (RR 0.56; 95%CI 0.32–0.97) or MedDiet plus nuts group (0.63; 95% CI 0.35–1.11). By contrast, no effect of the Mediterranean diet interventions on diabetic nephropathy was reported in the PREDIMED [ 59 ]. In the DPP follow-up study [ 55 ], retinopathic changes in women were lower in the former lifestyle intervention group than in the control group. Similarly, individuals who developed T2D had higher incidence of retinopathy than those who were non-diabetic after a long follow-up period ( Table 5 ). In FDPS, no difference was found in CVD morbidity or mortality between the intervention and control groups after 10 years, but incident cases remained low in both intervention and control groups [ 56 ]. In a sub-group analysis, the occurrence of retinopathy (microaneurysms) was significantly higher in the control (37/98, 38%) than in the intervention group (27/113, 24%; p = 0.026, see Table 4 for adjusted results) of the former FDPS participants [ 56 ].
An original report from the Look AHEAD trial showed no benefit of lifestyle intervention for the prevention of cardiovascular disease in patients with T2D, but a post-hoc analysis showed a 21% risk reduction in combined cardiovascular events in individuals who were able lose at least 10 kg of body weight as compared to patients with a stable body weight or long-term weight gain [ 60 ].
A recent systematic review and meta-analysis of prospective cohort studies and randomized clinical trials suggests that MedDiet has a beneficial role on the CVD prevention in populations inclusive of the individuals with T2D [ 61 ].
Discussion on Macro- and Microvascular Risk Reduction in the T2D Prevention Trials
Among the diabetes prevention trials which have examined follow-up data, only the Chinese Da Qing Diabetes Prevention Outcome Study has reported lower mortality and morbidity from any cause and cardiovascular disease in the people with IGT randomized into lifestyle intervention groups ( Table 5 ). Furthermore, the Chinese study found a clear decrease in composite microvascular diseases and retinopathy [ 54 ]. Indeed, these long-term results are of particular interest, since one long-term goal of the prevention of T2D is to prevent its complications as well. A longer follow-up of a relatively younger age cohort that is also less obese is a possible reason why significant risk reduction in CVD mortality and morbidity is only seen in the Chinese study and not in the American DPP Outcome Study [ 55 ] or in the FDPS [ 56 ]. After the active intervention phase, both the American and Finnish study participants, on average, remained relatively obese compared to the Chinese study. There may also be genetic or ethnic differences between the study populations, resulting in different distributions of the risk factors for T2D and of T2D rate itself [ 3 ]. For example, smoking was particularly common among the Chinese study participants [ 54 ]. Furthermore, the management of the main risk factors and health care resources available may offer other explanations for divergent results. In terms of microvascular complications, which are closely associated to hyperglycemia, the Chinese study results were encouraging with a 35% reduction in composite microvascular complications and 40% reduction in any retinopathy in the intervention groups. The results from both the DPP Outcome Study and the FDPS supported the long-term benefit achieved by changing lifestyles with regard to incident retinopathy [ 55 , 57 ]. Finally, it should be emphasized that the statistical power of the intervention studies on the prevention of T2D may not be sufficient to show significant differences in CVD outcomes between the intervention and the control groups [ 62 ].
8. Discussion on the Factors Explaining the Risk Reduction of T2D Including the Optimal Dietary Composition for the Prevention of T2D
8.1. what are the factors explaining the risk reduction of t2d in randomized controlled trials.
This question is of particular importance as it is related to strategies in preventing T2D. The Da Qing IGT study is the only study with both diet and physical activity arms randomized by clinic [ 32 ], and the PREDIMED trial is the only study testing the effect of a food pattern enriched with key foods (nuts or virgin olive oil) without physical activity or energy restriction [ 43 , 44 ]. All other lifestyle intervention studies combine dietary changes, weight reduction for overweight or obese people, and physical activity. It is of note that Chinese people with IGT in the Da Qing study [ 32 ], Japanese men with IGT [ 36 ], and individuals in the Indian IDD-1 study [ 37 ] had a much lower BMI than in study populations carried out in Europe or in the U.S.A.
8.2. Weight Reduction
Based on secondary analyses of randomized controlled trials, it can be concluded that a better adherence to lifestyle changes in general results in the better long-term prevention of T2D [ 33 , 48 , 49 ]. Furthermore, based on the evidence coming from observational studies on T2D risk factors [ 2 , 63 ] and the remarkable beneficial effects of weight reduction on glucose metabolism [ 51 , 64 , 65 , 66 ], weight reduction has been considered as a cornerstone in the prevention of T2D; with larger weight reductions associated with a lower risk of T2D. In the EDIPS study on 771 participants with IGT combining data from the FDPS, and SLIM and Newcastle studies, the risk of T2D was 89% lower in individuals who were able to sustain weight loss of at least 5% over 3 years than in individuals without significant weight changes [ 42 ]. Nevertheless, it is impossible to conclude that weight reduction is the only means to reduce the risk of T2D in overweight and obese people with impaired glucose metabolism, since weight loss is almost always associated with simultaneous changes in physical activity and/or diet. Indeed, the studies in people with Asian origin suggest that changing diet and increasing physical activity also seem to play a significant role in the prevention of T2D in individuals at risk for T2D with both normal body weight and over-weight people [ 32 , 36 , 37 ]. The importance of weight reduction in T2D can be gauged from a recent weight-management trial, in which 306 individuals with T2D in 39 primary care practices demonstrated a remission rate of 86% in individuals who lost 15 kg or more (24% of participants) [ 67 ]; an overall weight-loss difference of 9 kg resulted in a remission rate of 46% in the intervention group versus 4% in the control group in the full study.

8.3. Optimal Diet
8.3.1. individual nutrients and foods.
Several observational studies have been conducted to analyze the associations between food groups or nutrient consumption and T2D incidence. Ley et al. [ 68 ] conducted a series of meta-analyses of prospective cohort studies on food and beverage intake and T2D risk. Processed and unprocessed red meat, white rice, and sugar-sweetened beverages have shown a consistent positive relation with T2D, whereas green leafy vegetables, total dairy products, whole grains, alcohol in moderation in women, and coffee have been inversely associated with T2D. The consumption of berries and fruits rich in anthocyanins, such as bilberries, blueberries, grapes, apples, and pears, has also been associated with a lower risk of T2D [ 69 ]. Recent evidence also shows that yogurt intake [ 70 ] and nut intake (in women) is inversely associated with T2D. Legumes are another food group with cardiometabolic benefits [ 71 , 72 , 73 , 74 , 75 , 76 , 77 ] and legumes show an inverse association with the risk of diabetes and gestational diabetes [ 77 , 78 ]. In the same meta-analysis of prospective studies by Ley et al. [ 68 ], heme-iron, glycemic index and glycemic load of the diet were directly associated with T2D incidence, whereas total magnesium and vitamin D in the diet, as well as cereal fiber, were inversely related to T2D. A recent review based on meta-analyses and earlier reviews emphasize the preventive effect of whole grains and dietary fiber on the incidence of T2D [ 79 ].
8.3.2. Dietary Patterns
In addition to individual nutrients and foods, several studies have looked at dietary patterns and prevention of T2D. A Western dietary pattern, which is high in sugar-sweetened soft drinks, refined grains, diet soft drinks, and processed meat, was associated with an increased risk of diabetes in the Nurses Health Study (NHS) I and NHS II studies [ 80 ].
In contrast, some prospective cohort studies have demonstrated that adherence to plant-based dietary patterns, such as Mediterranean [ 81 , 82 ] DASH (Dietary Approaches to Stop Hypertension) or vegetarian dietary patterns [ 82 , 83 , 84 , 85 ], are associated with a lower risk of T2D incidence. In two prospective studies, a Mediterranean-type or healthy dietary pattern has also been inversely related to gestational diabetes [ 78 , 86 ].
Meal frequency and timing may also have a role in the T2D risk. Skipping breakfast and snacking have been associated with increased risk of T2D in both men and women [ 87 , 88 ]. Based on limited evidence, consuming breakfast regularly and not eating snacks between main meals may also be a strategy to reduce the risk of T2D [ 89 ].
8.3.3. Diet and Weight Loss
Current evidence from randomized intervention trials ( Table 1 ) suggests that weight loss by means of a healthy diet with lower saturated fat intake, but rich in vegetables, fruit, and whole grain products is beneficial in the prevention of T2D, especially when combined with physical activity. Indeed, all of the seven randomized lifestyle intervention studies in our systematic review and meta-analysis applied this kind of dietary approach. In FDPS, the best results in the prevention of T2D were achieved in IGT individuals with high fiber but moderate fat intake [ 47 , 90 ]. Similarly, in the American DPP study, 1 year weight loss success was associated with a high carbohydrate, high fiber, but a rather low total and saturated fat diet intake [ 91 ]. Regarding the quality of dietary fat, current evidence suggests that unsaturated fatty acids may have beneficial effects on insulin sensitivity and it is suggested to lower the risk of T2D [ 92 , 93 ].
In the PREDIMED trial, the Mediterranean diet enriched in nuts or extra virgin olive oil, resulted in a significant reduction in the incidence of T2D independent of weight loss or physical activity changes. This suggests that the quality of the diet may play a role in the prevention of T2D independent of weight changes [ 43 , 44 ]. However, these results are based on post-hoc analyses of a population at high cardiovascular risk and may not be extrapolated to healthy populations. In the SLIM and Newcastle studies, better adherence to the diet also predicted lower T2D risk [ 42 ]. To conclude, a diet with low consumption of red and processed meat, sugar, and sugar-sweetened beverages, but rich in vegetables, fruit, legumes, and whole grain products seems to be beneficial in the prevention of T2D.
8.3.4. Physical Activity
The Chinese Da Qing study [ 32 ] is the only intervention study that has examined the effect of exercise without weight loss or dietary changes. In the physical activity clinics, the risk of T2D was reduced by 47% as compared to clinics serving as control clinics, but no significant differences were observed between different randomization groups ( Table 1 ). There are no other long-term controlled intervention trials in this field. In FDPS, the impact of physical activity was examined as a secondary analysis taking into account the effect of diet and weight reduction. Based on different criteria used to evaluate physical activity, it was concluded that being physically active may reduce T2D risk by approximately 50% [ 94 ]. The recommendations to increase physical activity are strongly grounded by short-term controlled interventions that show improved glucose metabolism after increasing physical activity. Furthermore, epidemiological and trial evidence support the view that physical inactivity/sedentary lifestyle, along with being overweight and/or obese, are important risk factors for T2D and contribute to the current epidemic of T2D [ 1 , 2 , 95 , 96 ]. A recent PREDIMED-Plus Trial on overweight/obese individuals with metabolic syndrome who combined an energy-reduced Mediterranean-type diet and exercise promotion showed significant weight reduction (3.2 vs. 0.7 kg) and improvements in glucose metabolism, serum concentrations of triglycerides, HDL-cholesterol, and some inflammatory factors, compared to controls. These results confirm that a multifactorial approach, including physical activity, is successful in the prevention and treatment of disturbances in glucose metabolism [ 52 ].
9. Conclusions
We have a high certainty of evidence that T2D is preventable by changing lifestyle, i.e., weight reduction by diet change according to the current recommendations in terms of quality of fat, fiber intake, increased use of whole grain products, fruit, and vegetables, and increasing physical activity. The risk reduction of T2D is strongly related to the degree of long-term weight loss and adherence to lifestyle changes, and this preventive effect has been demonstrated to sustain for many years after active intervention.
Additional well-controlled intervention studies are needed to identify the optimal diet to prevent T2D. Currently, a diet moderate in fat, low in saturated fat intake, rich in fiber, whole grains, and fruit and vegetables, as well as a Mediterranean-type diet, may be recommended for the prevention of T2D in prediabetes.
There is still limited/insufficient evidence that the prevention of T2D by changing lifestyle may also prevent CVD or microvascular diseases.
Author Contributions
Conceptualization, M.U., H.K., A.A.R., K.H., A.P., A.T., J.S.-S., U.S. and J.L.S.; Methodology, M.U., T.A.K., E.V. and J.L.S.; Software, T.A.K. and E.V.; Validation, M.U., E.V. and T.A.K.; Formal Analysis, E.V., T.A.K.; Investigation, M.U., E.V., T.A.K. and J.L.S.; Resources, M.U., E.V., T.A.K., and J.L.S.; Data Curation, M.U., E.V. and T.A.K.; Writing—Original Draft Preparation, M.U., E.V., T.A.K., and J.L.S.; Writing—Review & Editing, M.U., T.A.K., E.V., H.K., A.A.R., K.H., A.P., A.T., J.S.-S., U.S. and J.L.S.; Visualization, M.U. and T.A.K.; Supervision, M.U. and J.L.S.; Project Administration, M.U. and J.L.S.; Funding Acquisition, M.U. and J.L.S.
The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) commissioned this systematic review and meta-analysis and provided funding and logistical support for meetings as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. This work was also supported by the Canadian Institutes of Health Research [funding reference number, 129920] through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3-D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation’s Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. Effie Viguiliouk was supported by a Toronto 3D Knowledge Synthesis and Clinical Trials foundation Internship Award. John L Sievenpiper was funded by a Diabetes Canada Clinician Scientist award. With the exception of the Clinical Practice Guidelines Committee of the DNSG of the EASD, none of the sponsors had a role in any aspect of the present study, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of the manuscript or decision to publish.
Conflicts of Interest
JSS serves on the board of, and has received a grant through, his institution from the International Nut and Dried Fruit Council and the Eroski Foundation. He serves on the Executive Committee of the Instituto Danone Spain and on the Scientific Committee of the Danone International Institute. He has received research support from the Instituto de Salud Carlos III, Spain; the Ministerio de Educación y Ciencia, Spain; the Departament de Salut Pública de la Generalitat de Catalunya, Catalonia, Spain; and the European Commission. Further research support has come from the California Walnut Commission, Sacramento CA, USA; the Patrimonio Comunal Olivarero, Spain; the La Morella Nuts, Spain; and Borges S.A., Spain. He reports receiving consulting fees or travel expenses from Danone; California Walnut Commission, the Eroski Foundation, the Instituto Danone–Spain, Nuts for Life, Australian Nut Industry Council, Nestlé, Abbot Laboratories, and Font Vella Lanjarón. He is on the Clinical Practice Guidelines Expert Committee of the European Association for the study of Diabetes (EASD) and has served on the Scientific Committee of the Spanish Food and Safety Agency, and the Spanish Federation of the Scientific Societies of Food, Nutrition and Dietetics. He is an Executive Board Member of the Diabetes and Nutrition Study Group [DNSG] of the EASD. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, and WhiteWave Foods. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Mott’s LLP, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, and Wirtschaftliche Vereinigung Zucker e.V. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Sobeys Inc. TAK has received research support from the Canadian Institutes of Health Research (CIHR) and an unrestricted travel donation from Bee Maid Honey Ltd. He was an invited speaker at a Calorie Control Council annual general meeting for which he received an honorarium. No competing interests were declared by the other authors (MU, EV, HK, AAR, KH, AP, AT, US).
- 1. International Diabetes Federation . IDF Atlas. 8th ed. International Diabetes Federation; Brussels, Belgium: 2017. [ Google Scholar ]
- 2. World Health Organization . Global Report on Diabetes 2016. WHO; Geneva, Switzerland: 2017. [ Google Scholar ]
- 3. Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Gloy V.L., Briel M., Bhatt D.L., Kashyap S.R., Schauer P.R., Mingrone G., Bucher H.C., Nordmann A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:5934. doi: 10.1136/bmj.f5934. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Douglas I.J., Bhaskaran K., Batterham R.L., Smeeth L. Bariatric Surgery in the United Kingdom: A Cohort Study of Weight Loss and Clinical Outcomes in Routine Clinical Care. PLoS Med. 2015;12:e1001925. doi: 10.1371/journal.pmed.1001925. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. World Health Organization . Diabetes Mellitus, Report of a WHO Study Group. WHO; Geneva, Switzerland: 1985. WHO Technical Report. [ PubMed ] [ Google Scholar ]
- 7. Mann J.I., De Leeuw I., Hermansen K., Karamanos B., Karlstrom B., Katsilambros N., Riccardi G., Rivellese A.A., Rizkalla S., Slama G., et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004;14:373–394. doi: 10.1016/S0939-4753(04)80028-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration; Oxford, UK: 2011. [ Google Scholar ]
- 9. Schellenberg E.S., Dryden D.M., Vandermeer B., Ha C., Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013;159:543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Barry E., Roberts S., Oke J., Vijayaraghavan S., Normansell R., Greenhalgh T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: Systematic review and meta-analysis of screening tests and interventions. BMJ. 2017;356:6538. doi: 10.1136/bmj.i6538. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Haw J.S., Galaviz K.I., Straus A.N., Kowalski A.J., Magee M.J., Weber M.B., Wei J., Narayan K.M.V., Ali M.K. Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2017;177:1808–1817. doi: 10.1001/jamainternmed.2017.6040. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Hemmingsen B., Gimenez-Perez G., Mauricio D., Roque I.F.M., Metzendorf M.I., Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2017;12:003054. doi: 10.1002/14651858.CD003054.pub4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Brunetti M., Shemilt I., Pregno S., Vale L., Oxman A.D., Lord J., Sisk J., Ruiz F., Hill S., Guyatt G.H., et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J. Clin. Epidemiol. 2013;66:140–150. doi: 10.1016/j.jclinepi.2012.04.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; Hoboken, NJ, USA: 2008. p. 672. [ Google Scholar ]
- 16. Thompson S.G., Higgins J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Guyatt G., Oxman A.D., Sultan S., Brozek J., Glasziou P., Alonso-Coello P., Atkins D., Kunz R., Montori V., Jaeschke R., et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Guyatt G.H., Oxman A.D., Kunz R., Atkins D., Brozek J., Vist G., Alderson P., Glasziou P., Falck-Ytter Y., Schunemann H.J. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Guyatt G.H., Oxman A.D., Kunz R., Brozek J., Alonso-Coello P., Rind D., Devereaux P.J., Montori V.M., Freyschuss B., Vist G., et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M., Alonso-Coello P., Falck-Ytter Y., Jaeschke R., Vist G., et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M., Alonso-Coello P., Glasziou P., Jaeschke R., Akl E.A., et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Guyatt G.H., Oxman A.D., Montori V., Vist G., Kunz R., Brozek J., Alonso-Coello P., Djulbegovic B., Atkins D., Falck-Ytter Y., et al. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. J. Clin. Epidemiol. 2011;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Guyatt G.H., Oxman A.D., Santesso N., Helfand M., Vist G., Kunz R., Brozek J., Norris S., Meerpohl J., Djulbegovic B., et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J. Clin. Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Guyatt G.H., Oxman A.D., Sultan S., Glasziou P., Akl E.A., Alonso-Coello P., Atkins D., Kunz R., Brozek J., Montori V., et al. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Guyatt G.H., Oxman A.D., Vist G., Kunz R., Brozek J., Alonso-Coello P., Montori V., Akl E.A., Djulbegovic B., Falck-Ytter Y., et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias) J. Clin. Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Guyatt G.H., Thorlund K., Oxman A.D., Walter S.D., Patrick D., Furukawa T.A., Johnston B.C., Karanicolas P., Akl E.A., Vist G., et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J. Clin. Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Langan D., Higgins J.P.T., Jackson D., Bowden J., Veroniki A.A., Kontopantelis E., Viechtbauer W., Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res. Synth. Methods. 2019;10:83–98. doi: 10.1002/jrsm.1316. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:549. doi: 10.1136/bmj.d549. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Booth G.L., Kapral M.K., Fung K., Tu J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: A population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X., Hu Z.X., Lin J., Xiao J.Z., Cao H.B., et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Mensink M., Blaak E.E., Corpeleijn E., Saris W.H., de Bruin T.W., Feskens E.J. Lifestyle intervention according to general recommendations improves glucose tolerance. Obes. Res. 2003;11:1588–1596. doi: 10.1038/oby.2003.211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Kosaka K., Noda M., Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Ramachandran A., Snehalatha C., Mary S., Mukesh B., Bhaskar A.D., Vijay V. Indian Diabetes Prevention, P. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Bo S., Ciccone G., Baldi C., Benini L., Dusio F., Forastiere G., Lucia C., Nuti C., Durazzo M., Cassader M., et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J. Gen. Intern. Med. 2007;22:1695–1703. doi: 10.1007/s11606-007-0399-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Bo S., Gambino R., Ciccone G., Rosato R., Milanesio N., Villois P., Pagano G., Cassader M., Gentile L., Durazzo M., et al. Effects of TCF7L2 polymorphisms on glucose values after a lifestyle intervention. Am. J. Clin. Nutr. 2009;90:1502–1508. doi: 10.3945/ajcn.2009.28379. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Penn L., White M., Oldroyd J., Walker M., Alberti K.G., Mathers J.C. Prevention of type 2 diabetes in adults with impaired glucose tolerance: The European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9:342. doi: 10.1186/1471-2458-9-342. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Eriksson K.F., Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Penn L., White M., Lindstrom J., den Boer A.T., Blaak E., Eriksson J.G., Feskens E., Ilanne-Parikka P., Keinanen-Kiukaanniemi S.M., Walker M., et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: Analysis of European Diabetes Prevention Study RCT. PLoS ONE. 2013;8:e57143. doi: 10.1371/journal.pone.0057143. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Salas-Salvado J., Bullo M., Babio N., Martinez-Gonzalez M.A., Ibarrola-Jurado N., Basora J., Estruch R., Covas M.I., Corella D., Aros F., et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Salas-Salvado J., Bullo M., Estruch R., Ros E., Covas M.I., Ibarrola-Jurado N., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160:1–10. doi: 10.7326/M13-1725. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Rücker G., Schwarzer G., Carpenter J.R., Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Li G., Zhang P., Wang J., Gregg E.W., Yang W., Gong Q., Li H., Li H., Jiang Y., An Y., et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet. 2008;371:1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Lindstrom J., Peltonen M., Eriksson J.G., Ilanne-Parikka P., Aunola S., Keinanen-Kiukaanniemi S., Uusitupa M., Tuomilehto J. Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56:284–293. doi: 10.1007/s00125-012-2752-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Lindstrom J., Ilanne-Parikka P., Peltonen M., Aunola S., Eriksson J.G., Hemio K., Hamalainen H., Harkonen P., Keinanen-Kiukaanniemi S., Laakso M., et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Nathan D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. de Mello V.D., Lindström J., Eriksson J., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Sundvall J., Laakso M., Tuomilehto J., Uusitupa M. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: The Finnish Diabetes Prevention Study. Diabetes Care. 2012;35:211–217. doi: 10.2337/dc11-1272. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Salas-Salvado J., Diaz-Lopez A., Ruiz-Canela M., Basora J., Fito M., Corella D., Serra-Majem L., Warnberg J., Romaguera D., Estruch R., et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care. 2019;42:777–788. doi: 10.2337/dc18-0836. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Uusitupa M., Lindstrom J., Tuomilehto J. Prevention of type 2 diabetes-success story that is waiting for next steps. Eur. J. Clin. Nutr. 2018;72:1260–1266. doi: 10.1038/s41430-018-0223-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Gong Q., Gregg E.W., Wang J., An Y., Zhang P., Yang W., Li H., Li H., Jiang Y., Shuai Y., et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: The China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011;54:300–307. doi: 10.1007/s00125-010-1948-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Uusitupa M., Peltonen M., Lindstrom J., Aunola S., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Valle T.T., Eriksson J.G., Tuomilehto J. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—Secondary analysis of the randomized trial. PLoS ONE. 2009;4:e5656. doi: 10.1371/journal.pone.0005656. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Aro A., Kauppinen A., Kivinen N., Selander T., Kinnunen K., Tuomilehto J., Keinanen-Kiukaanniemi S., Lindstrom J., Uusitupa M., Kaarniranta K. Life Style Intervention Improves Retinopathy Status-The Finnish Diabetes Prevention Study. Nutrients. 2019;11:1691. doi: 10.3390/nu11071691. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., Fiol M., Lapetra J., et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Diaz-Lopez A., Babio N., Martinez-Gonzalez M.A., Corella D., Amor A.J., Fito M., Estruch R., Aros F., Gomez-Gracia E., Fiol M., et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care. 2015;38:2134–2141. doi: 10.2337/dc15-1117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Look Ahead Research Group. Gregg E.W., Jakicic J.M., Blackburn G., Bloomquist P., Bray G.A., Clark J.M., Coday M., Curtis J.M., Egan C., et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. doi: 10.1016/S2213-8587(16)30162-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Becerra-Tomas N., Blanco Mejia S., Viguiliouk E., Khan T., Kendall C.W.C., Kahleova H., Rahelic D., Sievenpiper J.L., Salas-Salvado J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019 doi: 10.1080/10408398.2019.1565281. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Nathan D.M., Bennett P.H., Crandall J.P., Edelstein S.L., Goldberg R.B., Kahn S.E., Knowler W.C., Mather K.J., Mudaliar S., Orchard T.J., et al. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia. 2019;62:1319–1328. doi: 10.1007/s00125-019-4928-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res. Clin. Pract. 2017;132:169–170. doi: 10.1016/j.diabres.2017.09.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Uusitupa M., Lindi V., Louheranta A., Salopuro T., Lindstrom J., Tuomilehto J. Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes. 2003;52:2532–2538. doi: 10.2337/diabetes.52.10.2532. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Kitabchi A.E., Temprosa M., Knowler W.C., Kahn S.E., Fowler S.E., Haffner S.M., Andres R., Saudek C., Edelstein S.L., Arakaki R., et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Hamman R.F., Horton E., Barrett-Connor E., Bray G.A., Christophi C.A., Crandall J., Florez J.C., Fowler S., Goldberg R., Kahn S.E., et al. Factors affecting the decline in incidence of diabetes in the Diabetes Prevention Program Outcomes Study (DPPOS) Diabetes. 2015;64:989–998. doi: 10.2337/db14-0333. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Muraki I., Imamura F., Manson J.E., Hu F.B., Willett W.C., van Dam R.M., Sun Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Salas-Salvado J., Guasch-Ferre M., Diaz-Lopez A., Babio N. Yogurt and Diabetes: Overview of Recent Observational Studies. J. Nutr. 2017;147:1452–1461. doi: 10.3945/jn.117.248229. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Meyer K.A., Kushi L.H., Jacobs D.R., Slavin J., Sellers T.A., Folsom A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Hodge A.M., English D.R., O’Dea K., Giles G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–2706. doi: 10.2337/diacare.27.11.2701. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Bazzano L.A., Li T.Y., Joshipura K.J., Hu F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes care. 2008;31:1311–1317. doi: 10.2337/dc08-0080. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Villegas R., Gao Y.T., Yang G., Li H.L., Elasy T.A., Zheng W., Shu X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008;87:162–167. doi: 10.1093/ajcn/87.1.162. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Ericson U., Sonestedt E., Gullberg B., Hellstrand S., Hindy G., Wirfalt E., Orho-Melander M. High intakes of protein and processed meat associate with increased incidence of type 2 diabetes. Br. J. Nutr. 2013;109:1143–1153. doi: 10.1017/S0007114512003017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. von Ruesten A., Feller S., Bergmann M.M., Boeing H. Diet and risk of chronic diseases: Results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur. J. Clin. Nutr. 2013;67:412–419. doi: 10.1038/ejcn.2013.7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Becerra-Tomas N., Diaz-Lopez A., Rosique-Esteban N., Ros E., Buil-Cosiales P., Corella D., Estruch R., Fito M., Serra-Majem L., Aros F., et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin. Nutr. 2018;37:906–913. doi: 10.1016/j.clnu.2017.03.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Karamanos B., Thanopoulou A., Anastasiou E., Assaad-Khalil S., Albache N., Bachaoui M., Slama C.B., El Ghomari H., Jotic A., Lalic N., et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014;68:8–13. doi: 10.1038/ejcn.2013.177. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Fung T.T., Schulze M., Manson J.E., Willett W.C., Hu F.B. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch. Intern. Med. 2004;164:2235–2240. doi: 10.1001/archinte.164.20.2235. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Salas-Salvado J., Guasch-Ferre M., Lee C.H., Estruch R., Clish C.B., Ros E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2016;146:920–927. doi: 10.3945/jn.115.218487. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Jannasch F., Kroger J., Schulze M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017;147:1174–1182. doi: 10.3945/jn.116.242552. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Tonstad S., Stewart K., Oda K., Batech M., Herring R.P., Fraser G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013;23:292–299. doi: 10.1016/j.numecd.2011.07.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Snowdon D.A., Phillips R.L. Does a vegetarian diet reduce the occurrence of diabetes? Am. J. Public Health. 1985;75:507–512. doi: 10.2105/AJPH.75.5.507. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Chiu T.H.T., Pan W.H., Lin M.N., Lin C.L. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr. Diabetes. 2018;8:12. doi: 10.1038/s41387-018-0022-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Zhang C., Tobias D.K., Chavarro J.E., Bao W., Wang D., Ley S.H., Hu F.B. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: Prospective cohort study. BMJ. 2014;349:g5450. doi: 10.1136/bmj.g5450. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Mekary R.A., Giovannucci E., Willett W.C., van Dam R.M., Hu F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012;95:1182–1189. doi: 10.3945/ajcn.111.028209. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Mekary R.A., Giovannucci E., Cahill L., Willett W.C., van Dam R.M., Hu F.B. Eating patterns and type 2 diabetes risk in older women: Breakfast consumption and eating frequency. Am. J. Clin. Nutr. 2013;98:436–443. doi: 10.3945/ajcn.112.057521. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Ballon A., Neuenschwander M., Schlesinger S. Breakfast Skipping Is Associated with Increased Risk of Type 2 Diabetes among Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Nutr. 2019;149:106–113. doi: 10.1093/jn/nxy194. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Lindstrom J., Peltonen M., Eriksson J.G., Louheranta A., Fogelholm M., Uusitupa M., Tuomilehto J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia. 2006;49:912–920. doi: 10.1007/s00125-006-0198-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Sylvetsky A.C., Edelstein S.L., Walford G., Boyko E.J., Horton E.S., Ibebuogu U.N., Knowler W.C., Montez M.G., Temprosa M., Hoskin M., et al. A High-Carbohydrate, High-Fiber, Low-Fat Diet Results in Weight Loss among Adults at High Risk of Type 2 Diabetes. J. Nutr. 2017;147:2060–2066. doi: 10.3945/jn.117.252395. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Wu J.H.Y., Marklund M., Imamura F., Tintle N., Ardisson Korat A.V., de Goede J., Zhou X., Yang W.S., de Oliveira Otto M.C., Kröger J., et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5:965–974. doi: 10.1016/S2213-8587(17)30307-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Schwab U., Lauritzen L., Tholstrup T., Haldorssoni T., Riserus U., Uusitupa M., Becker W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014;58:25145. doi: 10.3402/fnr.v58.25145. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Laaksonen D.E., Lindstrom J., Lakka T.A., Eriksson J.G., Niskanen L., Wikstrom K., Aunola S., Keinanen-Kiukaanniemi S., Laakso M., Valle T.T., et al. Physical activity in the prevention of type 2 diabetes: The Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Aune D., Norat T., Leitzmann M., Tonstad S., Vatten L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015;30:529–542. doi: 10.1007/s10654-015-0056-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Burr J.F., Rowan C.P., Jamnik V.K., Riddell M.C. The role of physical activity in type 2 diabetes prevention: Physiological and practical perspectives. Phys. Sportsmed. 2010;38:72–82. doi: 10.3810/psm.2010.04.1764. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (2.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Advances in the management of type 2 diabetes in adults
Author affiliations
Rodolfo J Galindo 1 2
Jennifer M Trujillo 3
Cecilia C Low Wang 4
Type 2 diabetes is a chronic and progressive cardiometabolic disorder that affects more than 10% of adults worldwide and is a major cause of morbidity, mortality, disability, and high costs. Over the past decade, the pattern of management of diabetes has shifted from a predominantly glucose centric approach, focused on lowering levels of haemoglobin A 1c (HbA 1c ), to a directed complications centric approach, aimed at preventing short term and long term complications of diabetes, and a pathogenesis centric approach, which looks at the underlying metabolic dysfunction of excess adiposity that both causes and complicates the management of diabetes. In this review, we discuss the latest advances in patient centred care for type 2 diabetes, focusing on drug and non-drug approaches to reducing the risks of complications of diabetes in adults. We also discuss the effects of social determinants of health on the management of diabetes, particularly as they affect the treatment of hyperglycaemia in type 2 diabetes.
- Introduction
Diabetes, a chronic and progressive cardiometabolic disorder, is a major cause of morbidity, disability, and mortality worldwide. Comprehensive person centred management of diabetes requires attention to glycaemic control and risk factors for cardiovascular disease (hyperlipidaemia, hypertension, and tobacco use), weight management, early detection and treatment of microvascular, macrovascular, and metabolic complications of diabetes and mental health concerns, mitigation of burden of treatment, addressing social determinants of health, and improving quality of life. 1 The past decade has seen multiple developments in each aspect of the management of diabetes. This review focuses specifically on recent advances in the management of hyperglycaemia in diabetes, including drug and non-drug treatments. People with diabetes, caregivers, clinicians, health systems, payers, and policy makers need to appreciate the complexity and cost associated with optimal care of diabetes to meaningfully improve the health and well being of people living with diabetes.
- Epidemiology
The current prevalence of diabetes among adults is 10.5% worldwide (536.6 million adults), with marked variation across regions and countries, and is estimated to reach 12.2% (783.2 million adults) by 2045. 2 Diabetes is more prevalent in high income (11.1%) and middle income (10.8%) countries than in low income countries (5.5%). The prevalence of diabetes is rising everywhere, most rapidly in middle income countries where the prevalence is expected to reach 13.1% by 2045, 2 probably because of changing diet and lifestyle factors, rising rates of obesity, inadequate resources for early diagnosis and prevention, and potentially greater genetic or epigenetic susceptibility arising from inadequate fetal and childhood nutrition. Data for low and middle income countries are likely to be underestimated because of barriers to screening and timely diagnosis.
More than 90% of people with diabetes have type 2 diabetes, 3 characterised by insulin resistance and progressive beta cell failure, and commonly associated with other cardiometabolic disorders, including obesity, hypertension, cardiovascular disease, and hepatic steatosis. Diabetes contributed to 6.7 million deaths in 2021 alone, 4 highlighting the urgency of preventing diabetes and optimising its management to improve health outcomes and quality of life for all people at risk of or with the disease. 1
- Sources and selection criteria
We searched PubMed for articles published in English. We prioritised randomised controlled trials, clinical guidelines, consensus statements, and systematic reviews. Search terms were: ((type 2 diabetes mellitus AND management (medical subject headings (MeSH) terms)) AND (type 2 diabetes mellitus (MeSH terms))) AND (care management, patient (MeSH terms)). Filters applied were: clinical trial, guideline, meta-analysis, practice guideline, randomised controlled trial, and systematic review, from 1 January 2013 to 1 January 2023. The reference lists of these articles were screened for relevant publications.
- Goals and targets of management of type 2 diabetes
The primary objectives of the management of diabetes are to reduce the incidence and burden of complications and to improve quality of life ( figure 1 ). Historically, these objectives were pursued through control of hyperglycaemia. In this glucose centric approach, clinical practice guidelines recommend targeting haemoglobin A 1c (HbA 1c ) concentrations at <7% (53 mmol/mol) or <6.5% (47.5 mmol/mol) and, more recently, continuous glucose monitoring time in range >70% for most non-pregnant adults with type 2 diabetes, with lower or higher glycaemic thresholds individualised for each person. 5–7 These recommendations for levels of HbA 1c come from data from randomised controlled trials showing a reduction in microvascular complications with more intensive glycaemic control, 8–12 although data for the association between time in range and risk of complications of chronic diabetes are limited but emerging. 13 Implementation of glycaemic targets based on continuous glucose monitoring has also been limited by gaps in insurance coverage and accessibility, although continuous glucose monitoring is increasingly recommended for and used by people with type 2 diabetes. 14 15
Randomised controlled trials of older antihyperglycaemic treatments, such as sulfonylureas and insulins, however, have not shown a consistent association between intensive glycaemic control and reduction in macrovascular complications or mortality. 16 Nevertheless, longer term follow-up of intensively treated adults provides some evidence of a lower risk of macrovascular events and cardiovascular death. 8 17 Conversely, intensive glycaemic control in individuals with frailty, advanced age, and multimorbidity was associated with an increased risk of severe hypoglycaemia and death. 18–20 Therefore, future research is needed to examine the effect of intensive glycaemic control when achieved with newer glucose lowering drugs, which have a lower risk of hypoglycaemia and additional cardio-reno-metabolic benefits. Taken together, these data highlight the importance of individualised glycaemic management and the need to shift the emphasis away from the imperfect surrogate of levels of HbA 1c towards reducing hard outcomes of the adverse health effects of diabetes, while lessening the burden of treatment. 21 22
Shifting pattern of management of type 2 diabetes. HbA 1c =haemoglobin A 1c ; ASCVD=atherosclerotic cardiovascular disease; CKD=chronic kidney disease; HF=heart failure; GIP=glucose dependent insulinotropic polypeptide; GLP1RA=glucagon-like peptide 1 receptor agonist; SGLT2i=sodium glucose cotransporter 2 inhibitor; SU=sulfonylurea; DPP4i=dipeptidyl peptidase 4 inhibitor. *Insulin is preferred for acute management of severe hyperglycaemia; †Thiazolidinediones improve insulin resistance
Over the past decade, multiple randomised controlled trials have shown a reduction in cardiovascular disease, kidney disease, heart failure, and mortality with the use of glucagon-like peptide 1 receptor agonists (GLP1RAs) and sodium glucose cotransporter 2 inhibitors (SGLT2is), independent of a reduction in levels of HbA 1c . 23 These findings signalled a new complications centric era of the management of diabetes, focused directly on preventing or reducing macrovascular, microvascular, and other emerging complications of diabetes, such as heart failure. Many, 6 24 25 although not all, 26 clinical practice guidelines recommend treatment with GLP1RAs or SGLT2is, or both, for patients with cardiovascular or kidney disease, or both, or with risk factors for atherosclerotic cardiovascular disease, independent of glycaemic control, although all continue to stress the concurrent importance of achieving HbA 1c targets.
More recently, the pattern of management of diabetes has begun to shift further, with a renewed focus on looking at the causes of type 2 diabetes and its metabolic comorbidities and long term complications. This pathogenesis centric approach places the management of obesity at the centre of the prevention and treatment of the disease. 27 Even a relatively small amount (5-7%) of weight loss reduced the risk of incident diabetes and improved glycaemic control in people with type 2 diabetes. 28–31 Greater amounts of weight loss have been reported to have greater beneficial effects on glycaemic control (including remission of diabetes), metabolic dysfunction, and quality of life. 29 30 32–37 Weight loss achieved with metabolic surgery reduced the risks of microvascular and macrovascular complications of diabetes and reduced mortality. 38–41
By contrast, intensive lifestyle treatments in the Look AHEAD (Action for Health in Diabetes) randomised controlled trial of 5145 adults with type 2 diabetes and overweight/obesity did not reduce the risk of cardiovascular events compared to usual care. 30 The likelihood of detecting differences between the intensive lifestyle and conventional treatment groups might have been reduced because the cardiovascular event rate in the Look AHEAD population was much lower than anticipated (0.7% per year v estimated 3.1% per year). 42 A post hoc analysis suggested that those who lost at least 10% of their body weight in the first year had a significantly lower risk of the primary outcome, which was a composite of the first occurrence of death from cardiovascular causes, non-fatal acute myocardial infarction, stroke, and hospital admission for angina (adjusted hazard ratio 0.79, 95% confidence interval 0.64 to 0.98, P=0.034). 35 How weight loss achieved with drug treatment, particularly agents such as semaglutide and tirzepatide, compares with metabolic surgery for glycaemic control, microvascular and macrovascular complications, and mortality, should be examined.
- Lifestyle treatments: medical nutrition treatment, physical activity, and sleep
Successful management of type 2 diabetes must include consistent attention to behaviours that sustain a healthy lifestyle and are foundational for achieving glycaemic control, preventing complications, supporting quality of life, and preserving optimal health. Medical nutrition treatment for diabetes emphasises a balanced selection of nutrient dense foods while minimising or eliminating added sugar, refined grains, and highly processed foods. 6 7 43 Recommendations for optimal carbohydrate intake and composition vary, with the strongest evidence supporting an overall reduction in intake of carbohydrates. This principle can be applied to multiple dietary patterns, including a Mediterranean diet high in monounsaturated and polyunsaturated fats, low carbohydrate, vegetarian, or a plant based diets, and the Dietary Approaches to Stop Hypertension diet, with a focus on non-starchy vegetables, fruits, and legumes, and some dairy in those who are lactose tolerant. 6 43 Only the Mediterranean diet has been shown to reduce cardiovascular disease and mortality. 44 Also, evidence indicates the beneficial effects of involvement of community health workers to support education in self-management of diabetes and overall care, especially in rural or underserved communities, or both. 45 Because hypertension and cardiovascular disease are major causes of mortality in individuals with diabetes, more attention needs to be paid to overall sodium intake and limiting the content of saturated fat and trans fat in the diet. 6
Stopping smoking and abstinence from tobacco products is also imperative for cardiovascular health in adults with diabetes, and robust evidence supports the benefit of stopping smoking despite the potential for weight gain. 6 46 Although nicotine replacement products and electronic cigarettes might facilitate stopping smoking, nicotine itself can impair glucose tolerance and adversely affect the cardiovascular system through increased sympathetic activation. 47
Baseline levels of physical activity should be assessed to set reasonable and realistic behaviour oriented goals. Increasing the duration of physical activity and reducing sedentary time have been reported to improve cardiorespiratory fitness and HbA 1c levels. 48 Recommendations can be made to increase leisure time physical activity (walking, taking the stairs, and household chores), decrease sedentary time, and introduce physical activity on most days. 6 49 Physical activities include both aerobic and resistance training, as well as flexibility and balance training. 50
The length and quality of sleep are increasingly recognised as essential components of the management of diabetes and individuals should be screened for sleep related disorders. 6 43 Referral for diagnosis and treatment of obstructive sleep apnoea and other sleep disorders should be considered if indicated. Screening for psychosocial factors and social determinants of health that might affect an individual's diabetes care and quality of life should also be performed, with engagement of or referral to relevant clinical team members for further evaluation and care, as appropriate. 43
Lifestyle interventions in individuals with obesity or who are overweight are most successful when efforts are intensive and frequent follow-up is available, either in person or virtually. 6 49 Weight loss can be achieved in various ways, and is most effective when strategies are combined: caloric restriction, increased caloric expenditure, elimination or substitution of drugs that promote weight gain, use of weight reducing drugs and, in select individuals, metabolic or bariatric surgery. One dietary strategy that has received considerable attention in recent years is time restricted eating, 51 although data in adults with type 2 diabetes are limited to one randomised controlled trial 52 53 and a larger trial is ongoing (n=344; Using Early Time Restricted Feeding and Timed Light Therapy to Improve Glycemic Control in Adults With Type 2 Diabetes, NCT04155619 ). Weight management is discussed in more detail below.
- Drug treatment of type 2 diabetes
Initial management of type 2 diabetes has traditionally included metformin in most adults because of its glucose lowering effect, neutral effects on weight, minimal risk of hypoglycaemia, safety profile, low cost, and ease of administration. Now, in the light of evidence from trials of cardiovascular and kidney outcomes, decisions on treatment of diabetes with drugs should be made based on cardiac comorbidities (established atherosclerotic cardiovascular disease and heart failure), risk factors for atherosclerotic cardiovascular disease and kidney disease, engaging adults in shared decision making, and prioritising the use of drugs shown to reduce the risk of cardiovascular or kidney adverse outcomes, or both, in adults with specific comorbidities. 7 24–26
Adults with atherosclerotic cardiovascular disease or indicators of high risk
In people with established atherosclerotic cardiovascular disease or risk factors for atherosclerotic cardiovascular disease, a GLP1RA or SGLT2i with known cardiovascular benefit should be started, regardless of levels of HbA 1c or background glucose lowering treatments. 24 Drugs that have been shown to cause significant reductions in major adverse cardiovascular events in cardiovascular outcomes trials compared with placebo include the GLP1RAs dulaglutide (hazard ratio 0.88, 95% confidence interval 0.79 to 0.99), liraglutide (0.87, 0.78 to 0.97), and subcutaneous semaglutide (0.74, 0.58 to 0.95), and the SGLT2is canagliflozin (0.86, 0.75 to 0.97) and empagliflozin (0.85, 0.75 to 0.97). 54–58 None of the trials of cardiovascular outcomes involved head-to-head comparisons of GLP1RAs versus SGLT2is. 59
Individual components of the composite major adverse cardiovascular events outcome as well as secondary outcomes in the cardiovascular outcomes trials vary between GLP1RAs and SGLT2is. A reduction in stroke was seen in meta-analyses of randomised controlled trials of GLP1RAs compared with placebo (hazard ratio 0.83, 95% confidence interval 0.76 to 0.92) but not with SGLT2is compared with placebo (0.95, 0.85 to 1.05). 59 The mechanisms and benefits of GLP1RAs and SGLT2is seem to be complementary, and evidence is emerging to support combination treatment, which might provide more benefit than each used alone. 60–62 Currently, guidelines from the American Diabetes Association/European Association for the Study of Diabetes recommend the addition of the alternative class when more glucose lowering is needed. 24 25
Adults with heart failure
GLP1RAs have not shown benefit for heart failure outcomes in individual randomised controlled trials of cardiovascular outcomes, 55 56 58 although meta-analyses of these studies suggested a potential benefit. 59 63 64 SGLT2is, by contrast, have consistently shown significant benefit for heart failure outcomes. 54 57 65 Also, dapagliflozin and empagliflozin were beneficial in people with reduced or preserved ejection fraction without type 2 diabetes, and have an indication for improving heart failure outcomes. 66–69 Accordingly, in people with heart failure, an SGLT2i with known benefit should be started to reduce the risk of major adverse cardiovascular events and worsening heart failure. 24 26
Adults with chronic kidney disease
GLP1RAs have shown benefit for secondary kidney related outcomes in large individual randomised controlled trials 55 56 70 and meta-analyses 59 63 64 of cardiovascular outcomes, but dedicated kidney outcome trials are ongoing. 71 Several SGLT2is, including canagliflozin, dapagliflozin, and empagliflozin, have shown benefit in adults with chronic kidney disease with or without type 2 diabetes and in dedicated kidney outcome trials, and have an indication for improving chronic kidney disease outcomes. 24 72 Therefore, SGLT2is with primary evidence are preferred for individuals with an estimated glomerular filtration rate <60 mL/min/1.73 m 2 or albuminuria, or both, to reduce the progression of chronic kidney disease. If SGLT2is are not tolerated or cannot be used, GLP1RAs with demonstrated renal benefit are a reasonable alternative. 24 26 73 Current prescribing information allows SGLT2is to be started in adults with an estimated glomerular filtration rate of ≥20 mL/min/1.73 m 2 for kidney benefit, although the glucose lowering effects are substantially reduced at an estimated glomerular filtration rate <45 mL/min/1.73 m 2 . 74 A small reduction in the estimated glomerular filtration rate can be seen after starting treatment with SGLT2is because of reversal or correction of the previous hyperfiltration state in adults with diabetes, but it does not predict further reductions in estimated glomerular filtration rate or require discontinuation of treatment.
Role of metformin
Although metformin was a commonly used background drug in most large trials of cardiovascular and kidney outcomes, 75 several post hoc analyses have demonstrated benefit with GLP1RAs or SGLT2is regardless of background use of metformin. 76–82 Current guidelines from the American Diabetes Association/European Association for the Study of Diabetes and the American Association of Clinical Endocrinology no longer recommend metformin as the preferred first line agent for all individuals with type 2 diabetes, and instead suggest consideration of cardiac and kidney comorbidities when selecting first line treatment. 6 24 25 Cost is a major consideration in selecting the most appropriate treatment, however, probably contributing to differences in these recommendations from guidelines used in other countries. In the US, insurers have not caught up with the guidelines, and require that metformin is used before other agents. Guidance from the National Institute for Health and Care Excellence (NICE) still recommends metformin as the first line treatment for people with cardiac or kidney comorbidities, or both, with introduction of an SGLT2i in people who cannot tolerate metformin or need intensification of treatment. 26 Despite robust outcome data, GLP1RAs are not recommended ny NICE until failure of triple oral drug treatment and only in people with a high body mass index or in whom insulin treatment cannot be used. 26 Insurance formulary restrictions on prescribing GLP1RAs and SGLT2is, including the requirement of step treatment starting with metformin, still persist but should be reconsidered to better align with scientific evidence.
Other situations when a drug other than metformin can be considered as first line treatment include severe or symptomatic hyperglycaemia (HbA 1c >10%, ketosis, or weight loss), creatinine clearance or estimated glomerular filtration rate <30 mL/min/1.73 m 2 , or when the person cannot tolerate metformin despite slow up titration of the dose or a trial of the extended release formulation, or both. Sulfonylureas and thiazolidinediones are now less commonly recommended because of their adverse effect profiles. Sulfonylureas can lead to weight gain and are associated with a high risk of hypoglycaemia, and thiazolidinediones can also cause weight gain, as well as fluid retention and osteoporosis. People treated with thiazolidinediones must be monitored for the development of heart failure; thiazolidinediones are not recommended for those with symptoms of heart failure and are contraindicated in class 3 or 4 heart failure. Because generic forms of sulfonylureas and thiazolidinediones are available, however, these drug classes are options when cost is a barrier to accessing other agents or the individual's clinical situation requires these drugs. Pioglitazone, a thiazolidinedione, has beneficial effects in hepatic steatosis and stroke, and can be considered in these contexts. 6 83
Effect on weight and weight related comorbidities
Clinicians should also consider the effect of the glucose lowering regimen on weight and weight related comorbidities, including overweight or obesity and non-alcoholic fatty liver disease or non-alcoholic steatohepatitis. Weight loss is greatest with the dual glucose dependent insulinotropic polypeptide (GIP)-GLP1RA, tirzepatide, and subcutaneous semaglutide, followed by dulaglutide and liraglutide. 27 Moderate weight loss is seen with the other GLP1RAs and SGLT2is. Drugs with neutral effects on weight include the dipeptidyl peptidase 4 inhibitors (DPP4is) and metformin, whereas the sulfonylureas, thiazolidinediones, and insulin all increase the risk of weight gain ( table 1 ). 24 27 84 Recent single centre and population based cross sectional studies in the US estimated that >70% of people with type 2 diabetes have non-alcoholic fatty liver disease and more than half of those with type 2 diabetes and non-alcoholic fatty liver disease have steatohepatitis. 85–88 Insulin resistance, impaired lipid and glucose metabolism, and altered insulin secretion play a part in non-alcoholic fatty liver disease and progression of type 2 diabetes, and might indicate why the two diseases are so closely linked. 89 Although limited evidence exists so far, current guidelines recommend the use of a GLP1RA or pioglitazone for the treatment of diabetes in people with non-alcoholic steatohepatitis. 90 91 Weight management, which is essential for the treatment of hepatic steatosis, is discussed below.
Glucose lowering efficacy
In addition to choosing a drug that targets cardiovascular, kidney, and metabolic outcomes, clinicians should also develop a treatment approach that has sufficient efficacy to achieve glycaemic targets. 24 Although some guidelines (most notably, the Australian Diabetes Society) cite a lack of evidence to support substantial differences in glucose lowering between antihyperglycaemic drug classes when used as monotherapy, 92 prior meta-analyses, including a meta-analysis of 453 trials assessing nine drug classes, and the recently completed Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness (GRADE) pragmatic randomised clinical trial comparing insulin glargine U-100, the sulfonylurea glimepiride, the GLP1RA liraglutide, and the DPP4i sitagliptin in 5047 individuals with moderately uncontrolled type 2 diabetes found insulin and GLP1RA to be significantly more effective at lowering HbA1c than the other examined drugs. 93 , 94 The American Diabetes Association Standards of Care there categorise drug classes as having very high, high, or intermediate glucose lowering efficacy ( table 1 ). 24 The greatest reductions in levels of HbA 1c are seen with the dual GIP-GLP1RAs, GLP1RAs, and insulin. In the GLP1RA class, subcutaneous semaglutide and dulaglutide had the highest efficacy for glucose lowering. The recently approved dual GIP-GLP1RA, tirzepatide, seems to have the greatest efficacy for reducing levels of glucose. SGLT2is and DPP4is have less robust HbA 1c lowering effects and are classified as intermediate to high (SGLT2is) and intermediate (DPP4is). 24 25 93
GRADE, a large scale, comparative effectiveness study of four drugs in combination with metformin, found that insulin glargine and liraglutide achieved and maintained HbA 1c targets more effectively than glimepiride and sitagliptin. The study did not, however, include newer agents, such as the SGLT2is, or once weekly GLP1RAs. 95 The GRADE study also highlighted the challenges of maintaining glucose targets over time, with 71% of study participants progressing to HbA 1c ≥7% within four years, regardless of the treatment option. 94 A meta-analysis of 229 randomised controlled trials comprising 121 914 participants suggested that glucose lowering efficacy was highest with GLP1RA and weakest with DPP4i, with other agents in between. 96 By contrast, a meta-analysis of 140 randomised trials and 26 observational studies showed that each new class of non-insulin drugs added to metformin monotherapy lowers levels of HbA 1c by about 0.7-1%. 95 A shift towards earlier use of combination treatment, in contrast with a stepwise approach, to reach glucose targets and provide better glycaemic durability has been reported. 24 97 For people with marked hyperglycaemia (eg, HbA 1c >10% or with symptoms), clinicians should start insulin, or a combination of insulin with GLP1RAs. 98 When improved glycaemic control is achieved, many people with type 2 diabetes can be safely transitioned to non-insulin treatments with close monitoring to prevent hypoglycemia and hyperglycemia.
- Safety considerations
Other considerations in the selection of treatment for diabetes are the risks of hypoglycaemia, other adverse effects and safety considerations, as well as cost and administration requirements that often result in barriers to adherence. Therefore, individuals with diabetes, care partners, and clinicians need to engage in shared decision making to identify treatment strategies that are aligned with the individual's goals of care, treatment preferences, the clinical and psychosocial context, and risks and benefits associated with each treatment option. Tables 1 and 2 summarise this information. Some key and controversial safety considerations are discussed below.
Acute pancreatitis
Acute pancreatitis has been reported in individuals who received GLP1RAs, DPP4is, and the GIP-GLP1RA, tirzepatide. After early post-marketing reports, the US Food and Drug Administration warned of a potential link between acute pancreatitis and GLP1RAs and DPP4is. 99 Multiple preclinical, observational, and randomised controlled studies were inconsistent, with some showing positive associations and others showing no association. 100 Ultimately, the FDA concluded that a causal relation could not be established and insufficient evidence existed to modify treatment. Systematic reviews and meta-analyses of randomised controlled trials (eg, long term cardiovascular outcomes trials) concluded that treatment with GLP1RAs or DPP4is was not associated with an increased risk of pancreatitis or pancreatic cancer. 101–103
Nonetheless, current prescribing information, FDA guidance, and treatment guidelines recommend cautious use of these drug classes in people with a history of pancreatitis, in part because these people were excluded from most trials. 24 If these drug classes are used, individuals should be monitored for signs and symptoms of pancreatitis and, if pancreatitis develops, treatment should be discontinued and not restarted. 24 99 We also suggest caution in starting these drugs in people with a previous history of pancreatitis, particularly when the cause of pancreatitis is unknown or persists. Monitoring of lipase levels in randomised controlled trials showed asymptomatic fluctuations in both groups (intervention and placebo). Hence no evidence exists to suggest ongoing monitoring during treatment.
Gallbladder or biliary disease
GLP1RAs, DPP4is, and GIP-GLP1RAs are also associated with an increased risk of gallbladder and biliary disease, including cholelithiasis and cholecystitis. 104–107 Although the absolute risk of biliary or gllbladder disease with GLP1RA therapy seems to be small, with a recent meta-analysis of 76 randomised controlled trials involving 103 371 103 371 participants reporting an additional 27 incidences per 10 000 patients per year, 104 this finding might under-represent the true risk, because many studies did not report biliary related events. The risk seems to be higher with higher doses of drugs, longer duration of use, and when used for weight loss rather than glycaemic control. We therefore advise caution with the use of GLP1RAs, DPP4is, and GIP-GLP1RAs in people at high risk of biliary complications.
Diabetic retinopathy
A significant increase in retinopathy complications (3% v 1.8%, P=0.02), including vitreous haemorrhage, blindness, or need for photocoagulation treatment or an intravitreal agent, was seen in people receiving semaglutide during the SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) randomised controlled trial with 3297 participants with type 2 diabetes. 56 Of those with retinopathy complications, 83.5% had a history of retinopathy at baseline. In a meta-analysis of four cardiovascular outcomes trials of dulaglutide, liraglutide, oral semaglutide, and subcutaneous semaglutide, use of GLP1RAs was associated with an increased risk of rapidly worsening retinopathy (odds ratio 1.23, 95% confidence interval 1.05 to 1.44). 108 In another meta-analysis, GLP1RAs were not independently associated with an increased risk of retinopathy, but an association between retinopathy and the magnitude of the reduction in levels of HbA 1c was found. 109 Rapid glucose lowering has previously been associated with worsening diabetic retinopathy, 110 and the GLP1RA cardiovascular outcomes trials were not powered to detect differences in retinopathy complications. Thus whether worsening retinopathy is caused by the drug itself, a change or rate of change in glucose levels, or a combination of both is unclear. We advise caution when GLP1RAs are used, particularly semaglutide, in people with diabetic retinopathy, and individuals should be monitored closely for progression of retinopathy. 111
Whether other GLP1RAs similarly increase the risk of progressive diabetic retinopathy is not known. Consultation with an ophthalmologist should be considered before starting GLP1RAs in people with pre-existing retinopathy. 111 A large randomised controlled trial (A Research Study to Look at How Semaglutide Compared to Placebo Affects Diabetic Eye Disease in People With Type 2 Diabetes (FOCUS), NCT03811561 ) evaluating the long term effects of subcutaneous semaglutide on eye disease in 1500 people with type 2 diabetes is ongoing and should provide more evidence.
Amputations
An increased risk of lower limb amputations was first reported in the cardiovascular outcomes trial for canagliflozin that included 10 142 participants with type 2 diabetes and high cardiovascular risk (6.3 v 3.4 participants per 1000 patient years; hazard ratio 1.97, 95% confidence interval 1.41 to 2.75) 57 and led to a warning added to the prescribing information for canagliflozin in 2017. 112 The FDA removed the warning in 2020 based on more clinical trial data that found that the risk was less than previously described. 113 Subsequent real world cohort studies, randomised controlled studies, and meta-analyses have reported conflicting results, with some suggesting an increased risk with all SGLT2is and others finding no increased risk. 114–121 Therefore, reasonable steps to take are to consider factors that increase the risk of amputations before starting an SGLT2i, closely monitor people for lower limb ulcers or infections, and discontinue the SGLT2i if these occur. Subgroup and exploratory analyses of the SGLT2i cardiovascular outcomes trials, however, suggest cardiovascular benefit in patients with peripheral arterial disease, 122–124 so clinicians should use shared decision making when assessing the benefits and risks of SGLT2is in those at high risk.
Diabetic ketoacidosis
SGLT2is are associated with an increased risk of diabetic ketoacidosis, particularly in people with type 1 diabetes and in the perioperative population. 125 126 Rates in adults with type 2 diabetes are low and range from 0.16 to 0.76 events per 1000 patient years. 127 In type 2 diabetes, the risk is increased in people who are insulin deficient, in older people, with prolonged use of SGLT2is, or in those with a combination of these factors. 128 Guidance on risk management of diabetic ketoacidosis is mainly from individuals with type 1 diabetes, with little guidance specific to type 2 diabetes, and recommendations are mostly extrapolated from the type 1 diabetes context. 126 129 People with diabetes should be informed of the importance of adherence to insulin treatment, avoiding very low carbohydrate diets (such as ketotic diets), and excessive intake of alcohol. Education on management of sick days should also be given, and insulin doses should be monitored carefully; basal insulin should not be discontinued completely during illness or planned activity, particularly in those receiving intensive insulin treatment.
Clinicians and people with diabetes should be aware of predisposing factors and the clinical presentation of diabetic ketoacidosis, which often occurs with lower serum glucose levels (so-called euglycaemic diabetic ketoacidosis), sometimes at glucose concentrations of ≤200 mg/dL (11.1 mmol/L). The SGLT2i should be discontinued and treatment started promptly if diabetic ketoacidosis is suspected. SGLT2is should also be discontinued 3-4 days before scheduled surgery, during prolonged fasting or low carbohydrate intake, or during critical illness to lessen the risk of diabetic ketoacidosis. 24 Some have suggested that absence of ketosis (<0.6 mmol/L blood ketones, negative urine ketones) should be confirmed in people with type 1 diabetes before the start of treatment if SGLT2is are being used off label in this population, 126 but no evidence exists in support of this practice for people with type 2 diabetes.
- Starting and titrating insulin treatment
Many people with type 2 diabetes will eventually require insulin because of the progressive nature of the disease. For most people, a GLP1RA should be considered as the first injectable agent before basal insulin, based on the strong evidence of similar efficacy, beneficial effect on weight, and less hypoglycaemia. 130 131 If more treatment is needed after a GLP1RA, basal insulin should be started first and titrated to a maximum effective dose in a safe and timely way. 7 98 Several steps are necessary to support optimisation of insulin treatment, including clear communication of expectations, adequacy of glucose monitoring (including continuous glucose monitoring for people with basal insulin or intensive insulin treatment, and remote telemonitoring), a feasible dose titration plan, clearly defined glycaemic targets, and education on proper administration of insulin and storage. 131–134 Whether the individual can self-titrate the dose or if more support is needed should be assessed. People who can self-titrate can be instructed to continue uptitrating the dose until fasting glucose levels are consistently between 80 and 130 mg/dL (4.4 to 7.2 mmol/L; or an individualised glycaemic target), an anticipated maximum basal dose is reached (eg, 0.5 units/kg/day), or have unexplained hypoglycaemia. Providing these endpoints is key to reducing the risk of being treated with an inappropriately high dose of basal insulin in an attempt to compensate for inadequate post-prandial glycaemic control (ie, overbasalisation) while facilitating continued titration to an effective dose. 135 If the individual cannot self-titrate, consider providing weekly follow-up healthcare remotely (ie, telehealth) for timely dose titrations.
If the basal insulin dose has been sufficiently titrated but levels of HbA 1c remain above the person's individualised target or concern for overbasalisation exists, targeting postprandial glucose excursions is warranted. Initially, consider adding a GLP1RA or GIP-GLPRA if not already being used. The next step is to add prandial insulin as a separate injection or by switching to a fixed ratio combination. Basal bolus insulin treatment requires more injections, more glucose testing, more education, and carries a higher risk of hypoglycaemia and weight gain. 98 Metformin or complication centric drugs (GLP1RAs and SGLT2is), or both, should be continued. Sulfonylureas should be discontinued because of the risk of hypoglycaemia with concurrent insulin treatment.
- Weight management in type 2 diabetes
Among adults with diabetes in the US, almost 28% are overweight (body mass index 25.0-29.9), 46% have obesity (body mass index 30.0-39.9), and 16% have severe obesity (body mass index ≥40.0). 136 Increasingly recognised as a chronic disease, obesity (termed adiposity based chronic disease) 137 138 is characterised by excessive, maldistributed, and dysfunctional adipose tissue, and is associated with increased risks of hyperglycaemia (ie, prediabetes and type 2 diabetes), cardiovascular disease, hyperlipidaemia, hypertension, chronic kidney disease, cancer, urinary incontinence, non-alcoholic fatty liver disease, osteoarthritis, infertility, obstructive sleep apnoea, and gastro-oesophageal reflux disease. 137
Obesity is closely related to the pathogenesis and pathophysiology of type 2 diabetes and it also affects the management and outcomes of diabetes. 137 139 Strong evidence indicates that weight loss, particularly if >10% of body weight, can prevent, improve, and even reverse type 2 diabetes. 140 The Diabetes Prevention Programme showed that people with prediabetes who were randomised to receive an intensive lifestyle intervention had a 16% reduction in the risk of progressing from prediabetes to diabetes for every kilogram of weight loss. 37 In the Look AHEAD study of people with type 2 diabetes and overweight or obesity, improvement in fasting glucose and HbA 1c levels was found with weight loss as little as ≥2 kg, and improvements were directly proportional to the amount of weight lost. 31 After initial weight loss from lifestyle interventions or pharmacotherapy, compensatory physiological responses often make efforts at further weight loss more difficult, less successful, or difficult to maintain, a biological phenomenon referred to as obesity protecting obesity. 141 Hence clinicians should provide a supportive approach, recognising personal biases, and avoiding stigma and judgment to facilitate weight management efforts. 141
Despite years of commercial availability, obesity drugs are rarely used, with fewer than 5-10% of people with diabetes and obesity receiving obesity drugs in the US. 142 This finding could be driven by the relatively low efficacy of historically available drugs for weight loss, with most drugs causing <7% body weight loss. 141 Recent developments with incretin treatments have closed this gap, however, with up to 20% weight loss reported with tirzepatide. 107 Several studies in people with obesity, with or without type 2 diabetes, treated with semaglutide or tirzepatide have reported reductions in body weight of at least 5-10% in up to 80-90% of people, and reductions of 15-20% in up to 40-50% of people. 106 107 143 144 Efforts to lose weight in people with type 2 diabetes and obesity should be supported through preferential use of glucose lowering drugs that are associated with weight loss, avoiding glucose lowering and non-diabetes drugs associated with weight gain, and aiming for weight loss of 12-15% as appropriate, to achieve maximum benefits. 7 140
- Equity and affordability of diabetes care
Affordability, accessibility, and feasibility of implementing the diabetes care plan are major considerations in shared decision making. In the US, the high and rising costs of insulin and non-insulin drugs 145 have contributed to diabetes distress, 146 cost related non-adherence 147 148 with a detrimental effect on diabetes health outcomes 149 and rationing of other vital expenses. 150 Therefore, healthcare providers must discuss concerns about affordability with all people with diabetes, ensure that prescribed drugs are available and accessible, and leverage care team and community support systems to reduce the financial burden of the management of diabetes. 151 152
To deal with the growing concerns about affordability of insulin in the US, out-of-pocket costs have been capped in 2023 by the Centers for Medicare and Medicaid Services (which oversee publicly funded insurance for seniors, low income individuals, and people with disabilities or end-stage kidney disease), several private insurance plans, and insulin manufacturers, and the effect of these changes on cost related non-adherence and rationing will need to be assessed. The cost of drugs is generally much lower outside of the US because of highly regulated policies on drug pricing and cost effectiveness in other high income countries, 153 154 but 80% of people with diabetes live in low and middle income countries 155 and half do not have access to recommended diabetes treatments. 156 These findings call for multifaceted policy solutions to lower costs, increase supply, and improve accessibility of evidence based diabetes treatments and technologies in all settings and populations. 157
Socioeconomic barriers to optimal management of diabetes are multifaceted and include not only the high costs of diabetes drugs, technology, and equipment, but also foundational social determinants of health, such as the home environment with access to healthy food choices and space for physical activity, environmental pollution and endocrine disrupting chemicals, stable housing with access to electricity and refrigeration, employment type and stability, and educational attainment. 152 Geographical differences in the quality of care and prevalence of type 2 diabetes and its complications exist across levels of rurality, 158 159 neighbourhood disadvantage, 158 160 and geopolitical environment. 161–163 Several interventions have been shown to be successful in improving the management of diabetes, including community health worker programmes, diabetes prevention and self-management programmes adapted specifically to the needs of underserved and disadvantaged populations, expansion of health insurance as part of the Affordable Care Act, food and housing support programmes, and others. 152
We must also be cognisant of pervasive racial and ethnic inequalities in the quality of diabetes care and health outcomes. In the US, racial and ethnic minority populations are disproportionately affected by diabetes 164 and its complications. 152 165 166 Multiple studies have shown worse glycaemic control 165 167 168 and higher rates of acute complications (hypoglycaemia, 160 165 169–172 diabetic ketoacidosis, and hyperglycaemic hyperosmolar state), 160 165 170 173 chronic complications (kidney disease, 165 174–178 amputation, 165 175 cardiovascular disease, 165 175 and retinopathy), 176 179 and mortality 180 181 among black people with type 2 diabetes relative to other racial and ethnic groups. People with type 2 diabetes from racial and ethnic minority groups are also substantially less likely to be treated with GLP1RAs and SGLT2is than non-Hispanic white people. 182 183 Similar inequalities in the prevalence, management, and health outcomes of diabetes have been described in Europe 184 185 and around the world. 186 187 These inequalities highlight the need for structural solutions and multisector collaborations that deal with the barriers to optimal diabetes management and health at all levels to ensure that all people, regardless of race, ethnic group, socioeconomic status, or place of residence, receive high quality care.
- Conclusions
The paradigm of diabetes management has shifted over the past decade from a predominantly glucose-centric approach to approaches that prioritise prevention of diabetes complications and addressing the underlying causes of diabetes and metabolic dysfunction, such as obesity ( figure 2 ). High quality, evidence based management of diabetes therefore requires reducing glucose levels to a safe, patient centred range; using glucose lowering drugs with a strong evidence base for reduction of diabetes complications and excess adiposity, not just lowering levels of HbA 1c ; minimising burden of treatment and improving quality of life; and implementing care delivery models that support high quality (effective, efficient, safe, equitable, timely, and person centred) care. 188 Access and affordability remain major barriers, as is the sustainable implementation of effective lifestyle interventions.
Person centred goals of treatment of type 2 diabetes
Questions for further research
What are the short term and long term health outcomes associated with combined GLP1RA and SGLT2i treatment?
What is the optimal weight loss target (>10% or 15%) in the management of type 2 diabetes?
What is the comparative effectiveness and safety of drug treatments for obesity compared with metabolic surgery for long term metabolic, microvascular, and macrovascular complications?
How can effective lifestyle treatments for long term weight loss be implemented effectively, sustainably, and equitably?
What are effective and sustainable ways to engage people with diabetes, care partners, and communities in the prevention and management of diabetes to ensure equitable access to care?
How can structural barriers to optimal metabolic health be removed?
Patient involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
- Publication history
- Rapid Responses

IMAGES
VIDEO