- Search Menu
- Advance articles
- Editor's Choice
- Graphical Abstracts and Tidbit
- Author Guidelines
- Submission Site
- Open Access
- About American Journal of Hypertension
- Editorial Board
- Board of Directors
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- AJH Summer School
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Mean arterial pressure and urinary protein excretion responses to chronic reductions in uterine perfusion pressure (rupp) in pregnant rats. all data are expressed as mean ± sem., vascular responses to acetylcholine are reduced in pregnant rats with chronic reductions in uterine perfusion pressure (rupp). all data are expressed as mean ± sem., glomerular filtration rate and renal plasma flow responses to chronic reductions in uterine perfusion pressure (rupp) in pregnant rats. all data are expressed as mean ± sem., does a reduction in renal nitric oxide synthesis mediate the abnormal pressure natriuresis and elevation in arterial pressure during pih, does enhanced endothelin synthesis contribute to the elevation in arterial pressure during pih, does enhanced thromboxane and/or reduced prostacyclin synthesis mediate the renal and cardiovascular abnormalities in pih.
- < Previous
Pathophysiology of pregnancy-induced hypertension
- Article contents
- Figures & tables
- Supplementary Data
Joey P. Granger, Barbara T. Alexander, William A. Bennett, Raouf A. Khalil, Pathophysiology of pregnancy-induced hypertension, American Journal of Hypertension , Volume 14, Issue S3, June 2001, Pages 178S–185S, https://doi.org/10.1016/S0895-7061(01)02086-6
- Permissions Icon Permissions
Pregnancy-induced hypertension (PIH) is estimated to affect 7% to 10% of all pregnancies in the United States. Despite being the leading cause of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PIH have not yet been fully elucidated. Studies during the past decade, however, have provided a better understanding of the potential mechanisms responsible for the pathogenesis of PIH. The initiating event in PIH appears to be reduced uteroplacental perfusion as a result of abnormal cytotrophoblast invasion of spiral arterioles. Placental ischemia is thought to lead to widespread activation/dysfunction of the maternal vascular endothelium that results in enhanced formation of endothelin and thromboxane, increased vascular sensitivity to angiotensin II, and decreased formation of vasodilators such as nitric oxide and prostacyclin. The quantitative importance of the various endothelial and humoral factors in mediating the reduction in renal hemodynamic and excretory function and elevation in arterial pressure during PIH is still unclear. Investigators are also attempting to elucidate the placental factors that are responsible for mediating activation/dysfunction of the maternal vascular endothelium. Microarray analysis of genes within the ischemic placenta should provide new insights into the link between placental ischemia and hypertension. More effective strategies for the prevention of preeclampsia should be forthcoming once the underlying pathophysiologic mechanisms that are involved in PIH are completely understood. Am J Hypertens 2001;14:178S–185S © 2001 American Journal of Hypertension, Ltd.
Pregnancy-induced hypertension (PIH) is estimated to affect 7% to 10% of all pregnancies in the United States. 1–4 Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PIH are unclear. Hypertension associated with preeclampsia develops during pregnancy and remits after delivery, implicating the placenta as a central culprit in the disease. An initiating event in PIH has been postulated to be reduced placental perfusion that leads to widespread dysfunction of the maternal vascular endothelium by mechanisms that remain to be defined. 1–4 The mechanisms leading to reduced placental perfusion in PIH may be multiple, but most studies in humans suggest abnormal cytotrophoblast invasion of spiral arterioles as an important factor. 1–5
Several lines of experimental evidence support this hypothesis. For example, studies in various animal models, including sheep, dog, rabbit, and rat have shown that reductions in uteroplacental blood flow leads to a hypertensive state that closely resembles PIH in women. 6 , 7 Additional support for this concept derives from studies in humans that indicate increased circulating fibronectin and factor VIII antigen, both markers of endothelial cell injury. 1–4 , 8–10 Decreases in the production of endothelial-derived relaxing factors, such as nitric oxide and prostacyclin, increase production of endothelin and thromboxane, and enhanced vascular reactivity to angiotensin II in women with PIH also suggest abnormal endothelial function. 1–4 , 11
During normal pregnancy, significant changes in cardiovascular and renal function occur to meet the metabolic needs of the mother and the fetus. 1–3 For example, maternal cardiac output and blood volume increase by approximately 40% to 50%, whereas total peripheral resistance and arterial blood pressure (BP) tend to decrease. 1–3 In addition, there are marked changes in renal function such as elevations in renal plasma flow and glomerular filtration rate of approximately 30% to 40%. 12 Renin concentration, renin activity, and angiotensin II levels are elevated; however, the vascular responsiveness to angiotensin II appears to be reduced. 13 The mechanisms that are involved in mediating these significant cardiovascular and renal changes during pregnancy have been studied extensively, and it appears that endothelial factors such as nitric oxide play an important role. 1–3 , 14 , 15
The marked hemodynamic and renal changes that normally occur during pregnancy do not manifest themselves in women who develop PIH. Pregnancy-induced hypertension is associated with significant elevations in total peripheral resistance, enhanced responsiveness to angiotensin II, and marked reductions in renal blood flow and glomerular filtration rate and proteinuria. 1–3 Although the physiologic mechanisms that mediate the alterations in cardiovascular and renal function have been extensively studied during normal pregnancy, information regarding the mediators of the reduction in renal and cardiovascular function during PIH has been limited because of the difficulty in performing mechanistic studies in pregnant women. Although several animal models have been developed to study PIH, information on the mechanisms involved in mediating the long-term reduction in kidney function and increase in arterial pressure is lacking. Experimental induction of chronic uteroplacental ischemia appears to be the most promising animal model to study potential mechanisms of PIH, as reductions in uteroplacental blood flow in a variety of animal models lead to a hypertensive state that closely resembles PIH in women. 1–3 , 6 , 7 , 16
Chronic reductions in uteroplacental perfusion pressure in gravid rats after day 14 of gestation, as reported by Eder and MacDonald 17 and Abitbol, 18 lead to significant increases in arterial pressure and proteinuria. We have recently begun to work with this model to examine potential pathophysiologic mechanisms that mediate the hypertension during chronic reductions in uteroplacental perfusion pressure. 19 We reduced uterine perfusion pressure in the gravid rat by approximately 40% by placing a silver clip around the aorta below the renal arteries. Because this procedure has been shown to cause an adaptive increase in uterine blood flow through the ovarian artery, we also placed a silver clip on both the right and left uterine arcade at the ovarian end just before the first segmental artery. 20 We found that reducing uteroplacental perfusion with this approach results in significant and consistent elevations in arterial pressure of 20 to 30 mm Hg as compared to control pregnant rats at day 19 of gestation (Fig. 1) . Our data also indicate that this hypertension is associated with proteinuria, reductions in renal plasma flow and glomerular filtration rate (Figs. 1 and 2 ), and a hypertensive shift in the pressure natriuresis relationship. 20 , 67 Moreover, our data indicate that endothelial function (Fig. 3) is significantly altered in response to chronic reductions in uteroplacental perfusion pressure in the pregnant rat. 21 , 22 Finally, we have found intrauterine growth restriction in response to chronic reductions in uteroplacental perfusion pressure in the pregnant rat, as the average pup size in this group is smaller than in normal pregnant rats. 20 Thus, a chronic reduction in uteroplacental perfusion pressure in the pregnant rat has many of the features of PIH in women. The role of various endothelial, autacoid, and hormonal factors in mediating the reduction in renal hemodynamic and excretory function and elevation in arterial pressure produced by chronic reductions in uteroplacental perfusion pressure will be the main focus of the remaining portion of this brief review.
02086-6/2/m_ajh.178S.f1.jpeg?Expires=1718760697&Signature=lJJCs78zXMs~JFNUOrANZCPGk~4DVv0KB9i~eV6sRm7XlAW0FsJt6NtEPohgqS-eImfFWVxtdtXHX7gxZNepSahdFCzedAudux8-j8jqBnyGVg70SnZ8cMDMxKQVOiJNYkYZyRf81uJaPKnRvdLljLDr5j1F0epmz0BFGm8REFY~1povVXjfeW4zbh0wec-kJJ1nP~8r1EpIweorqKVaAcrryYpZFxyoOu9rnZ83fWq4CrZNsvTHBccUlgMq3gqlnKRpzH1zYLngj5CzlQd9ncB5qUO4LEDdVnKp~ib5Dkq00ObHnn2yoBh-E5zSHVmu20sXDZIFP8KJasr5BDT-vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
One potential mechanism for the reduction in pressure natriuresis and elevation in arterial pressure in response to a chronic reduction in uteroplacental perfusion pressure in the pregnant rat is a reduction in renal nitric oxide (NO) synthesis. 23–26 Nitric oxide is synthesized from L-arginine by a family of enzymes known as NO synthases (NOS). Nitric oxide synthase is readily inhibited by L-arginine analogs such as N -methyl-arginine (L-NMMA), N -nitro-L-arginine (L-NNA), and N -nitro-L-arginine methyl ester (L-NAME). Studies from our laboratories and others have indicated that NO plays an important role in the regulation of renal function and arterial pressure under various physiologic and pathophysiologic conditions. 24 , 27–33 Of particular relevance to PIH is the finding that reducing NO synthesis results in a hypertensive shift in the pressure natriuresis relationship. 28 , 33 This impairment in pressure natriuresis is also associated with reductions in renal plasma flow and glomerular filtration rate and an inability to transmit renal perfusion pressure into the renal interstitium. 28 , 33
Substantial evidence indicates that NO production is elevated in normal pregnancy. 14 , 15 Plasma and urinary levels of cGMP, the second messenger of NO, increase during pregnancy in rats. 14 , 15 Marked increases in 24-h urinary nitrate/nitrite excretion have also been reported to be normal during pregnancy in the rat. 14 , 15 Studies have also shown that pregnancy increases activity of calcium-dependent NOS in uterine artery and heart in early and late pregnancy. 14 , 15 Increased expression of mRNA levels for both constitutive NOS isoforms have been observed in a variety of tissues in late pregnancy. 14 , 15 Plasma arginine levels are also reduced in pregnancy. These findings presumably reflect increased utilization of substrate in response to increased formation of NO.
Increases in NO production appear to play an important role in the renal vasodilatation of pregnancy. 14 , 15 Recent studies by Conrad 14 and other researchers 15 clearly demonstrated that the renal vasodilatation in the pregnant rat is due to an increased NO production. Because NO appears be an important physiologic vasodilator in normal pregnancy, NO deficiency during preeclampsia might be involved in the disease process. Studies from several laboratories have found that chronic NOS inhibition in pregnant rats produces a hypertension associated with peripheral and renal vasoconstriction, proteinuria, intrauterine growth retardation, and increased fetal morbidity, a pattern that closely resembles the symptoms of human pregnancy-induced hypertension. 22 , 34 , 35 However, whether there is a reduction in NO production during pregnancy-induced hypertension is unclear. Much of the uncertainty originates from the difficulty in directly assessing the activity of the NO system in a clinical setting. 1–3 Assessment of whole body NO production by measurement of 24-h nitrate/nitrite excretion has yielded variable results due to difficulties in controlling for factors such as nitrate intake. We have recently reported that normal pregnancy in the rat is associated with significant increases in whole body NO production and renal protein expression of neuronal and inducible NOS. 36 We also recently determined whether whole body and renal NO production is reduced in a rat model of PIH produced by chronically reducing uterine perfusion pressure. 20 Chronic reductions in uterine perfusion pressure resulted in increases in arterial pressure of 20 to 25 mm Hg, decreases in renal plasma flow and glomerular filtration rate, but no difference in urinary nitrite/nitrate excretion relative to control pregnant rats. In contrast, reductions in uterine perfusion pressure in virgin rats resulted in no significant effects on arterial pressure. Renal endothelial and inducible NOS protein expression did not decrease significantly in the chronically reduced uterine perfusion pressure rats relative to normal pregnant rats; however, significant reductions in neuronal NOS were observed. The results of this study indicate that the increase in arterial pressure observed in response to chronic decreases in uterine perfusion pressure in pregnant rats is associated with no change in whole body NO production and a decrease in renal protein expression of neuronal NOS. Whether the reduction in renal protein expression of neuronal NOS occurs as a result of the hypertension or the reduction in renal protein expression of neuronal NOS plays a role in mediating the reduction in renal hemodynamics and elevation in arterial pressure remains to be determined.
Another endothelial-derived factor that may play a role in PIH is the vasoconstrictor endothelin. In 1988, Yanagisawa and co-workers 37 characterized an endothelial-derived vasoconstrictor, a 21-amino-acid peptide subsequently called endothelin. Endothelin is derived from a 23-amino-acid peptide precursor preproendothelin that is cleaved after translation to form proendothelin. In the presence of a converting enzyme located within the endothelial cells, proendothelin or big endothelin is cleaved to produce the 21-amino-acid peptide endothelin. Endothelin receptor-binding sites have been identified throughout the body with the greatest number of receptors in the kidneys and lungs. 38 The vasoconstrictor effects of endothelin are mediated by endothelin A receptors on the vascular smooth muscle. In addition, evidence is accumulating that endothelin B receptors located on vascular smooth muscle also contribute to the vasoconstrictor effects of this peptide. 39 Endothelin B receptors located on endothelium are thought to release NO and prostacyclin. Endothelin reduces renal hemodynamic and sodium excretory function and plays an important role in mediating the altered pressure natriuresis and other hemodynamic changes in several models of hypertension including the deoxycorticosterone salt hypertensive rat and the Dahl salt-sensitive hypertensive rat. 39–41
Because endothelial damage is a known stimulus for endothelin synthesis, increases in the production of endothelin may participate in PIH. Plasma concentration of endothelin has been measured in a number of studies involving normal pregnant women and women with pregnancy-induced hypertension. 42–45 Most investigators have found higher plasma concentrations of endothelin of approximately two- to threefold in women with PIH. 42–45 Typically, plasma levels of endothelin are highest during the latter stage of the disease, suggesting that endothelin may not be involved in the initiation of PIH, but rather in the progression of disease into a malignant phase. 42–45 Although the elevation in plasma levels of endothelin are only two- or threefold above normal during PIH, we found that this level of plasma endothelin can have significant long-term effects on systemic hemodynamics and arterial pressure regulation. 46 , 47 We found that increasing the plasma levels of endothelin within the two- to threefold range for 2 to 3 h had no effect on arterial pressure, whereas increasing endothelin levels for 7 days resulted in significant reductions in renal hemodynamics, renal pressure natriuresis, and significant elevations in mean arterial pressure. 46 , 47 The increase in mean arterial pressure was also associated with significant reductions in cardiac output and renal plasma flow and elevations in total peripheral resistance. 46 , 47 Thus, long-term elevations in plasma levels of endothelin comparable to those measured in patients with PIH could play a role in mediating the reductions in renal function and elevations in arterial pressure observed in women with PIH.
Although some studies have reported no significant changes in circulating levels of endothelin during PIH, a role for endothelin as a paracrine or autocrine agent in PIH remains worthy of consideration. Many of the experimental and genetic rat models of hypertension are not associated with elevations in plasma endothelin. 39 Yet, elevations in endothelin synthesis have been reported in specific tissues including the kidney. 39 For example, investigators have reported enhanced expression of preproendothelin in vascular tissues from various organ systems, including the kidney. 38 , 39 Several studies have also reported an increase in local production of endothelin in women with PIH. 42–44 Whether increased synthesis of endothelin occurs within the kidney during PIH remains uncertain, as some investigators have found no differences between preeclamptic and normal pregnant women in urinary excretion of endothelin—a measure of local renal synthesis. 42–44
We recently examined the role of endothelin in mediating the hypertension in response to chronic reductions in uterine perfusion pressure in conscious, chronically instrumented pregnant rats. 48 Renal expression of preproendothelin was significantly elevated in both the medulla and in the cortex of the pregnant rats with chronic reductions in uterine perfusion pressure as compared to control pregnant rats. Chronic administration of the selective endothelin type A receptor antagonist (ABT-627, 5 mg/kg/day for 10 days) markedly attenuated the increase in mean arterial pressure observed in the pregnant rats with chronic reductions in uterine perfusion pressure (Fig. 4) . However, endothelin type A receptor blockade had no significant effect on BP in the normal pregnant animals. These findings suggest that endothelin plays a major role in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats.
Mean arterial pressure in response to chronic reductions in uterine perfusion pressure (RUPP) in control pregnant rats and pregnant rats pretreated with an ET A receptor antagonist (+ET A ). All data are expressed as mean ± SEM.
02086-6/2/m_ajh.178S.f4.jpeg?Expires=1718760697&Signature=zkDgcoB9g24kEWFTd4Zf3vsBYjvmxfqEMqS1a3rtyzG1~23sRzQFJ2quDuJxk0EnbcKQmeE8Kow6TY5x56jJtZ1Ix14~iLyw34iJ3J2BhWcfE8~K~Pyile--61eOvqBZHIuUo27WkjUZQCrsktcsWDA~WKmyZnY5zWG4F3m9b1tYppbxFdU9tOqFYutYsVYQ9ipnz4LBk6O0jE7SHRyfpkoKFTEW1-Y-fnyhbQffdfmeNf4LGdR2a11I3kSRl8kPBcGYModp0eHsipWQ1G7mZZUp8Kt2ZUeXTuzXHlDcY7cal4vFmAyLQcE8xkryTNxv6KcpfKr0HS751Gr3RBAuSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Several lines of evidence suggest that changes in the prostaglandin system may play a role in mediating the renal dysfunction and increase in arterial pressure during PIH. Significant alterations in prostacyclin and thromboxane production occur in women with PIH. 49–52 Plasma and urine levels of thromboxane are elevated in women with PIH, whereas syntheses of prostaglandins, such as prostacyclin, are reduced. 49–52 Additional evidence for a potential role of thromboxane in PIH derives from a study by Woods. 53 She demonstrated that short-term increases in systemic arterial pressure produced by acute reductions in uterine perfusion in pregnant dogs can be prevented by thromboxane receptor antagonism. Further evidence of a potential role for thromboxane is supported by studies in humans, indicating that low dose aspirin attenuates the development of PIH in women at risk for the disease. 1–3
Although some studies suggest a potential role for thromboxane in PIH, the quantitative importance of this substance in mediating the long-term reduction in renal hemodynamics and elevation in arterial pressure produced by chronic reductions in uterine perfusion pressure in pregnant rats is still uncertain. Thromboxane is not only produced by platelets and macrophages, but also by multiple renal cells. 54 , 55 Furthermore, the receptor for thromboxane appears to be abundant within the vasculature of the kidney. 54 , 55 Finally, there is considerable evidence that thromboxane-induced constriction contributes to the renal vasoconstriction in several experimental models of hypertension, 54 , 55 Whether thromboxane mediates the renal hemodynamic and arterial pressure changes observed in the rat model of PIH is unknown. In preliminary experiments, however, we found that urinary excretion of thromboxane B 2 was higher in the hypertensive pregnant rats with chronic reductions in uterine perfusion pressure than normal pregnant rats at day 19 of gestation. 56
Is the renin-angiotensin system important in mediating the reduction in renal function and increase in arterial pressure during PIH?
The renin-angiotensin system plays an important role in the long-term regulation of renal function and arterial pressure during a variety of physiologic and pathophysiologic conditions. 57 During normal pregnancy, plasma renin concentration, renin activity, and angiotensin II (Ang II) levels are all elevated; however, the vascular responsiveness to Ang II appears to be reduced. 1–3 The importance of the renin-angiotensin in the regulation of renal function and arterial pressure during PIH is unclear. Although some studies have reported that reductions in uterine perfusion pressure enhances uteroplacental renin release, most animal studies have reported decreased or normal plasma renin activity and Ang II concentrations. 1–3 In addition, most investigators have observed that in established human preeclampsia, plasma renin activity and Ang II levels are usually low or normal. 1–3 Although circulating levels of Ang II may be normal during PIH, it is possible that reducing uteroplacental perfusion pressure could increase the renal sensitivity to Ang II through reductions in NO or prostacyclin synthesis or by enhanced formation of thromboxane. Consistent with this suggestion are studies indicating enhanced vascular responsiveness to Ang II in vessels from animals or humans with PIH. 1–3 Furthermore, previous studies from our laboratory and others have found that, unlike normal conditions, the preglomerular vessels of the renal circulation become extremely sensitive to the vasoconstrictor actions of Ang II when the renal synthesis of NO or prostacyclin is reduced or when thromboxane synthesis is elevated. 29 , 30 , 57 Increased vascular Ang II responsiveness during PIH, however, does not prove Ang II as an important endogenous mediator of the vasoconstriction or hypertension in experimental models of PIH, as increased responsiveness may only reflect low endogenous Ang II formation. Thus, the importance of increased Ang II to the control of renal function and BP during PIH is unclear. A previous study by Woods and Brooks, 58 however, indicates that Ang II may not be important in mediating the acute rise in arterial pressure during short-term reductions in uterine perfusion pressure in dogs. They demonstrated that the increase in arterial pressure in response to reduced uterine perfusion pressure was unaltered in animals whose renin-angiotensin system had been fixed by prior infusion of captopril plus Ang II infusion. Although the results from this acute study suggest that the renin-angiotensin system might not be involved in mediating increases in systemic arterial pressure during acute reductions in uteroplacental blood flow, the mechanisms causing hypertension under acute conditions may not necessarily be the same as those that contribute to the chronic hypertension induced by long-term reductions in uteroplacental perfusion pressure.
We recently determined the importance of Ang II in mediating the long-term reduction in renal hemodynamic and the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats. 59 Chronic oral administration of a converting enzyme inhibitor (enalapril, 250 mg/L for 6 days) decreased mean arterial pressure to a similar extent in pregnant rats with reduced uterine perfusion pressure (RUPP) and normal pregnant rats. Blockade of the renin-angiotensin system (RAS), however, had no significant effect on the BP response to chronic reductions in uterine perfusion pressure as the differences in BP between the normal pregnant and RUPP rats were similar in control and converting enzyme inhibitor-treated groups. These findings suggest that the RAS does not play a major role in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats. 59
Is maternal endothelial activation/dysfunction in preeclampsia due to enhanced cytokine production in response to placental ischemia?
Although reductions in blood flow to the uteroplacental unit are known to result in cardiovascular and renal abnormalities consistent with the pathophysiologic features of human PIH, the physiologic mechanisms linking placental ischemia with the abnormalities in the maternal circulation are unclear. 60 Several lines of evidence support the hypothesis that the ischemic placenta contributes to endothelial cell activation/dysfunction of the maternal circulation by enhancing the synthesis of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1). 60 TNF-α and IL-1 are inflammatory cytokines that have been shown to induced structural as well as functional alterations in endothelial cells. 61 These inflammatory cytokines also enhance the formation of a number of endothelial cell substances such as endothelin and reduce acetylcholine-induced vasodilatation. 60–62 Also supporting a potential role of TNF-α in preeclampsia are findings that plasma levels of TNF-α are significantly elevated in women with preeclampsia by approximately twofold. 60 , 63 Furthermore, IL-6, which is activated by TNF-α, has also been reported to be elevated in preeclamptic women. 60 Although high levels of TNF-α, as observed during septic shock or after lipopolysaccharide administration, activate gene expression of inducible nitric oxide synthase, modest levels of TNF-α have been shown to destabilize the mRNA of endothelial nitric oxide synthase. 64
Whether chronic and modest increases in plasma TNF-α can activate the endothelium during pregnancy and lead to reduced kidney function, high BP, and other features of PIH is unknown. Consistent with a potential role of cytokine activation in PIH is the recent study by Faas and colleagues. 65 They reported that an intravenous infusion of a high dose of lipopolysaccharide (LPS) decreased BP in pregnant rats, whereas a very low dose infusion of the endotoxin resulted in significant and long-term increases in BP and urinary albumin excretion and significant platelet aggregation in conscious pregnant rats. Although LPS is known to activate TNF-α, it is unclear whether the effects of low dose LPS on cardiovascular and kidney function were mediated through TNF-α or IL-1, as these cytokines were not measured in that study.
Although plasma levels of TNF-α are elevated by two- to threefold in women with PIH, the importance of TNF-α in mediating the systemic and renal hemodynamic changes associated with this disease is unclear. To determine the long-term effects of a two- to threefold elevation in plasma TNF-α on renal and systemic hemodynamics in pregnant rats we recently infused TNF-α for 5 days at a rate of 50 ng/day during days 14 to 19 of gestation in pregnant rats. 66 Plasma levels doubled in the TNF-α-treated pregnant rats. Arterial pressure was significantly higher in the TNF-α-treated pregnant rat as compared to pregnant controls at day 19 of gestation. A twofold elevation in plasma TNF-α in pregnant rats also caused a significant reduction in renal hemodynamics. These data suggest that elevated plasma levels of TNF-α observed in preeclamptic women may play an important role in the pathogenesis of PIH.
Although these preliminary findings with TNF-α support the cytokine hypothesis, finding the link between placental ischemia and maternal endothelial and vascular abnormalities remains an important area of investigation. Microarray analysis of genes within the ischemic placenta of women with preeclampsia and in animal models of chronic reductions in uterine perfusion pressure should provide new insights into the link between placental ischemia and hypertension. More effective strategies for the prevention of preeclampsia should be forthcoming once the underlying pathophysiologic mechanisms that are involved in PIH are completely understood.
Studies during the past decade have provided a better understanding of the potential mechanisms responsible for the pathogenesis of PIH. The initiating event in PIH has been postulated to be reduced uteroplacental perfusion as a result of abnormal cytotrophoblast invasion of spiral arterioles (Fig. 5) . Placental ischemia is thought to lead to widespread activation/dysfunction of the maternal vascular endothelium that results in enhanced formation of endothelin and thromboxane, increased vascular sensitivity to Ang II, and decreased formation of vasodilators such as NO and prostacyclin. These endothelial abnormalities, in turn, cause chronic hypertension by impairing renal pressure natriuresis and increasing total peripheral resistance. The quantitative importance of the various endothelial and humoral factors in mediating the reduction in renal hemodynamic and excretory function and elevation in arterial pressure during PIH is still unclear. Results from ongoing basic and clinical studies, however, should provide new and important information regarding the physiologic mechanisms responsible for the elevation in arterial pressure in women with preeclampsia. More effective strategies for the prevention of preeclampsia should be forthcoming once the underlying pathophysiologic mechanisms that are involved in PIH are completely understood.
Potential mechanism whereby chronic reductions in uteroplacental perfusion may lead to hypertension. ET = endothelin; TBX = thromboxane; PGI 2 = prostacyclin; NO = nitric oxide; ANG II = angiotensin II.
02086-6/2/m_ajh.178S.f5.jpeg?Expires=1718760697&Signature=ofDyJMp5gwY~irkN2DdPDaPZQS8zJaHEy1Jy9Ba7DJltNd7RusiKQchX8QoPOnW0idk~ezTaZLxh2Wbok1DBzCTkjdQHcxiWxbyrb9obZXultcj~t773YM0gYrz5ZrBoq9-tv-VbddIKuT6UJ6oJ9ZyhnRyH-KVYUwIms1DbW91W~DG~Tv8qKTcJZCqEudn0P6bTeY2vZHBfeoE9~A7GItIJiJ4FuAbXmWOOWvKhUuBfojfY6siKtEWn3U8vh2KgM0UXybAjTyAAzxTVosJiZ2WfgubQ~19DGz9ZJ-YhkBxleDBxjv-1m0HqbCysla-ABgjx6ghoiWZ79D5qe83P8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
1. August P , Lindheimer MD : Pathophysiology of preeclampsia . Hypertension 1995 ; 142 : 2407 – 2426 .
Google Scholar
2. Lindheimer MD , Katz AI : Renal physiology and disease in pregnancy , in Seldin D.W. and Giebisch G. (Eds). The Kidney: Physiology and Pathophysiology . 2nd ed. Raven Press : New York , 1992 . 3371 – 3431 .
Google Preview
3. Chesley LC Hypertensive disorders in pregnancy . Appleton-Century-Crofts : New York , 1978 .
4. Saftlas AF , Olson DR , Franks AL , Atrash HK , Pokras R : Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986 . Am J Obstet Gynecol 1990 ; 163 : 460 – 465 .
5. Gerretsen G , Huisjes HJ , Elema JD : Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation . Br J Obstet Gynecol 1981 ; 88 : 876 – 881 .
6. Conrad KP : Animal models of pre-eclampsia: do they exist? . Fetal Med Rev 1990 ; 2 : 67 – 88 .
7. Douglas BH : The rat as a model for preeclampsia , in Lindheimer M.D., Katz A.I. and Zuspan F.P. (Eds). Hypertension in Pregnancy . John Wiley : New York , 1976 . 411 – 419 .
8. Roberts JM , Taylor RN , Goldfien A : Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia . Am J Hypertens 1991 ; 4 : 700 – 708 .
9. Morris NH , Eaton BM , Dekker G : Nitric oxide, the endothelium, pregnancy and pre-eclampsia . Br J Obstet Gynecol 1996 ; 103 : 4 – 15 .
10. Rodgers GM , Taylor RN , Roberts JM : Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells . Am J Obstet Gynecol 1988 ; 159 : 908 – 914 .
11. Keith JC Jr , Thatcher CD , Schaub RG : Beneficial effects of U-63, 557A, a thromboxane synthetase inhibitor, in an ovine model of pregnancy-induced hypertension . Am J Obstet Gynecol 1987 ; 157 : 199 – 203 .
12. Davison JM , Hytten FE : Glomerular filtration during and after pregnancy . J Obstet Gynecol (Brit Common) 1974 ; 81 : 588 – 595 .
13. Gant NF , Daley GL , Chand S , Whalley PJ , McDonald PC : A study of angiotensin II pressor response throughout primigravid pregnancy . J Clin Invest 1973 ; 52 : 2682 – 2689 .
14. Conrad KP : Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models . Am J Kidney Dis 1987 ; 9 : 253 – 263 .
15. Baylis C , Suto T , Conrad K : Importance of nitric oxide in control of systemic and renal hemodynamics during normal pregnancy: studies in the rat and implications for preeclampsia . Hypertens Pregnancy 1996 ; 15 : 147 – 169 .
16. Losonczy G , Brown G , Venuto RC : Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits . Am J Med Sci 1992 ; 303 : 233 – 240 .
17. Eder DJ , McDonald MT : A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats . Clin Exp Hyper Hyper Preg 1987 ; B6 : 431 – 451 .
18. Abitbol MM : Simplified technique to produce toxemia in the rat: consideration on cause of toxemia . Clin Exp Hyper Hyper Preg 1982 ; B1 : 93 – 103 .
19. Alexander BT , Kassab SE , Miller MT , Abram SR , Reckelhoff JF , Bennett WA , Granger JP : Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide . Hypertension 2001 ; 37 : 1191 – 1195 .
20. Nienartowicz A , Link S , Moll W : Adaptation of the uterine arcade in rats during pregnancy . J Develop Physiol 1989 ; 21 : 101 – 108 .
21. Crews JK , Herrington JN , Granger JP , Khalil RA : Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rats . Hypertension 2000 ; 35 : 71 – 76 .
22. Khalil RA , Crews JK , Novak J , Kassab S , Granger JP : Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats . Hypertension 1998 ; 31 : 1065 – 1069 .
23. Granger JP , Alexander BT : Pathophysiology of pregnancy-induced hypertension . Curr Concepts Hypertens 1999 ; 3 : 5 – 6 .
24. Granger JP , Alexander BT : Abnormal pressure natriuresis in hypertension: role of nitric oxide . Acta Physiol Scand 2000 ; 168 : 161 – 168 .
25. Seligman SP , Buyon JP , Clancy RM , Young BK , Abramson SB : The role of nitric oxide in the pathogenesis of preeclampsia . Am J Obstet Gynecol 1994 ; 171 : 944 – 948 .
26. Baylis C , Engels K : Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of endothelial derived relaxing factor (EDRF) in the rat . Clin Exp Hypertens 1992 ; B11 : 117 – 129 .
27. Bachmann S , Mundel P : NO in the kidney: synthesis, localization and function . Am J Kidney Dis 1994 ; 24 : 112 – 129 .
28. Nakamura T , Alberola A , Granger JP : Role of renal interstitial pressure as a mediator of sodium retention during blockade of endothelium derived nitric oxide hypertension . Hypertension 1993 ; 21 : 956 – 960 .
29. Alberola A , Salazar FJ , Nakamura T , Granger JP : Renal hemodynamic effects of angiotensin II (AII): interactions with endothelium derived nitric oxide . Am J Physiol 1994 ; 267 : R1472 – R1478 .
30. Schnackenberg C , Wilkins C , Granger JP : Role of nitric oxide in modulating the vasoconstrictor actions of angiotensin II in preglomerular and postglomerular vessels in dogs . Hypertension 1995 ; 26 : 1024 – 1029 .
31. Novak J , Reckelhoff JF , Bumgarner L , Cockrell K , Kassab SE , Granger JP : Role of nitric oxide in mediating the reduced sensitivity of the renal circulation to angiotensin II in pregnant rats . Hypertension 1997 ; 30 : 580 – 584 .
32. Kassab S , Miller T , Novak J , Reckelhoff JF , Hester RL , Granger JP : Systemic hemodynamics and regional blood flows during chronic nitric oxide synthesis inhibition in pregnancy . Hypertension 1998 ; 30 : 315 – 320 .
33. Nakamura T , Salazar FJ , Alberola A , Granger JP : Effect of renal perfusion pressure on renal interstitial hydrostatic pressure and Na excretion: role of endothelium-derived nitric oxide . Nephron 1998 ; 78 : 104 – 111 .
34. Yallampalli C , Garfield RE : Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia . Am J Obstet Gynecol 1993 ; 169 : 1316 – 1320 .
35. Molnar M , Suto T , Toth T , Hertelendy F : Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation . Am J Obstet Gynecol 1994 ; 170 : 1458 – 1466 .
36. Alexander BT , Reckelhoff JF , Kassab S , Granger JP : Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats . Hypertension 1999 ; 33 : 435 – 439 .
37. Yanagisawa M , Kurihara H , Kimura S : A novel potent vasoconstrictor peptide produced by vascular endothelial cells . Nature 1988 ; 332 : 411 – 415 .
38. Kohan DE : Endothelins in the normal and diseased kidney . Am J Kidney Dis 1997 ; 29 : 2 – 26 .
39. Schiffrin EL : Endothelin: potential role in hypertension and vascular hypertrophy . Hypertension 1995 ; 25 : 1135 – 1143 .
40. Kato T , Kassab S , Wilkins FC , Kirchner K , Keiser J , Granger JP : Endothelin antagonist improve renal function in spontaneously hypertensive rats . Hypertension 1995 ; 25 : 883 – 887 .
41. Kassab S , Novak J , Miller T , Granger J : Cardiovascular and renal actions of endothelin receptor antagonism in Dahl salt-sensitive hypertension . Hypertension 1997 ; 30 : 682 – 686 .
42. Dekker GA , Kraayenbrink AA , Zeeman GG , van Kamp GJ : Increased plasma levels of the novel vasoconstrictor peptide endothelin in severe pre-eclampsia . Eur J Obstet Gynecol Reprod Biol 1991 ; 40 : 215 – 220 .
43. Clark BA , Halvorson L , Sachs B , Epstein FH : Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment . Am J Obstet Gynecol 1992 ; 166 : 962 – 968 .
44. Roberts JM , Taylor RN , Musci TJ , Rogers GM , Hubel CA , McLaughlin MK : Preeclampsia: an endothelial cell disorder . Am J Obstet Gynecol 1989 ; 161 : 1200 – 1204 .
45. Taylor RN , Varma M , Teng NNH , Roberts JM : Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies . J Clin Endocrinol Metab 1990 ; 71 : 1675 – 1677 .
46. Wilkins FC Jr , Alberola A , Mizelle HL , Opgenorth TJ , Granger JP : Chronic hypertension produced by long-term pathophysiological increases in circulating endothelin levels in conscious dogs . J Cardiovasc Res 1993 ; 22 : 325 – 328 .
47. Wilkins FC Jr , Alberola A , Mizelle HL , Opgenorth TJ , Granger JP : Systemic hemodynamics and renal function during long-term pathophysiological increases in circulating endothelin . Am J Physiol 1995 ; 268 : R375 – R381 .
48. Alexander BT , Rinewalt AN , Cockrell KL , Bennett WA , Granger JP : Endothelin-A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure . Hypertension 2001 ; 37 : 485 – 489 .
49. Wang Y , Walsh S , Kay H : Placenta lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia . Am J Obstet Gynecol 1992 ; 167 : 946 – 949 .
50. Friedman SA : Preeclampsia: a review of the role of prostaglandins . Obstet Gynecol 1988 ; 71 : 122 – 137 .
51. Conrad KP , Dunn MJ : Renal synthesis and urinary excretion of eicosanoids during pregnancy in rats . Am J Physiol 1987 ; 253 : F1197 .
52. Wang Y , Walsh SW , Guo J , Zhang J : The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood . Am J Obstet Gynecol 1991 ; 165 : 1695 – 1700 .
53. Woods LL : Importance of prostaglandins in hypertension during reduced uteroplacental perfusion pressure . Am J Physiol 1989 ; 257 : R1558 – R1561 .
54. Remuzzi G , Fitzgerald GA , Patrono C : Thromboxane synthesis and action within the kidney . Kidney Int 1992 ; 41 : 1483 – 1493 .
55. Ogletree ML : Overview of physiological and pathophysiological effects of thromboxane A2 . Fed Proc 1987 ; 46 : 133 – 138 .
56. Llinas MT , Alexander BT , Abram SR , Sedeek M , Granger JP : Enhanced production of thromboxane A2 in response to chronic reductions in uterine perfusion pressure in pregnant rats . FASEB J 2001 ; 15 : A288 . (Abstract)
57. Hall JE , Granger JP : Role of sodium and fluid excretion in hypertension , in Swales J.D. (Ed). Textbook of Hypertension . Blackwell Scientific Pubs : Oxford , 1994 . 360 – 387 .
58. Woods LL , Brooks VL : Role of the renin-angiotensin system in hypertension during reduced uteroplacental perfusion pressure . Am J Physiol 1989 ; 257 : R204 – R209 .
59. Alexander BT , Cockrell KL , Sedeek M , Granger JP : Role of the renin-angiotensin system in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in the pregnant rat . Hypertension 2001 ; 37 : 986 . (Abstract)
60. Conrad KP , Benyo DF : Placental cytokines and the pathogenesis of preeclampsia . Am J Reprod Immunol 1997 ; 37 : 240 – 249 .
61. Pober JS , Cotran RS : Cytokines and endothelial cell biology . Physiol Rev 1990 ; 70 : 427 – 451 .
62. Marsden PA , Brenner BM : Transcriptional regulation of the endothelin-1 gene by TNFα . Am J Physiol 1992 ; 262 : C854 – C861 .
63. Kupferminc MJ , Peaceman AM , Wigton TR , Rehnberg KA , Socol ML : Tumor necrosis factor-alpha I is elevated in plasma and amniotic fluid of patients with severe preeclampsia . Am J Obstet Gynecol 1994 ; 170 : 1752 – 1759 .
64. Yoshizumi M , Perrella MA , Burnett JC , Lee ME : Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life . Circ Res 1993 ; 73 : 205 – 209 .
65. Faas MM , Schulling GA , Baller JFW , Visscher CA , Bakker WW : A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats . Am J Obstet Gynecol 1994 ; 171 : 158 – 164 .
66. Granger JP , Bennett WM , Alexander BT , Cockrell KL , Whitworth NS : Long-term elevation of plasma TNF-alpha increases arterial pressure and reduces kidney function in pregnant rats . Hypertension 1999 ; 34 : 337 . (abst)
67. Granger JP , Alexander BT , Abram SR , Reckelhoff JF , Wilson J , Rinewalt AN : Chronic reductions in uterine perfusion pressure in the pregnant rat produces hypertension and reduces pressure-natriuresis . Hypertension 2001 ; 37 : 682 . (Abstract)
- epoprostenol
- hypertension, pregnancy-induced
- nitric oxide
- pre-eclampsia
- hypertension
- hemodynamics
- excretory function
- angiotensin ii
- thromboxane
- vascular endothelium
- vasodilators
- endothelins
- endothelium
- maternal mortality
- arterial pressure
- perinatal period
- cytotrophoblast
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1941-7225
- Copyright © 2024 American Journal of Hypertension, Ltd.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Diagnosis and...
Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance
Visual summary: Resumen visual
Spanish version: Versión en español
- Related content
- Peer review
- Katie Webster , senior systematic reviewer 1 ,
- Sarah Fishburn , chair of guideline committee 1 ,
- Mike Maresh , clinical adviser 1 ,
- Sarah C Findlay , lay member ,
- Lucy C Chappell , topic advisor and NIHR research professor in obstetrics 2
- on behalf of the Guideline Committee
- 1 National Guideline Alliance, Royal College of Obstetricians and Gynaecologists, London
- 2 King’s College London
- Correspondence to: L C Chappell lucy.chappell{at}kcl.ac.uk
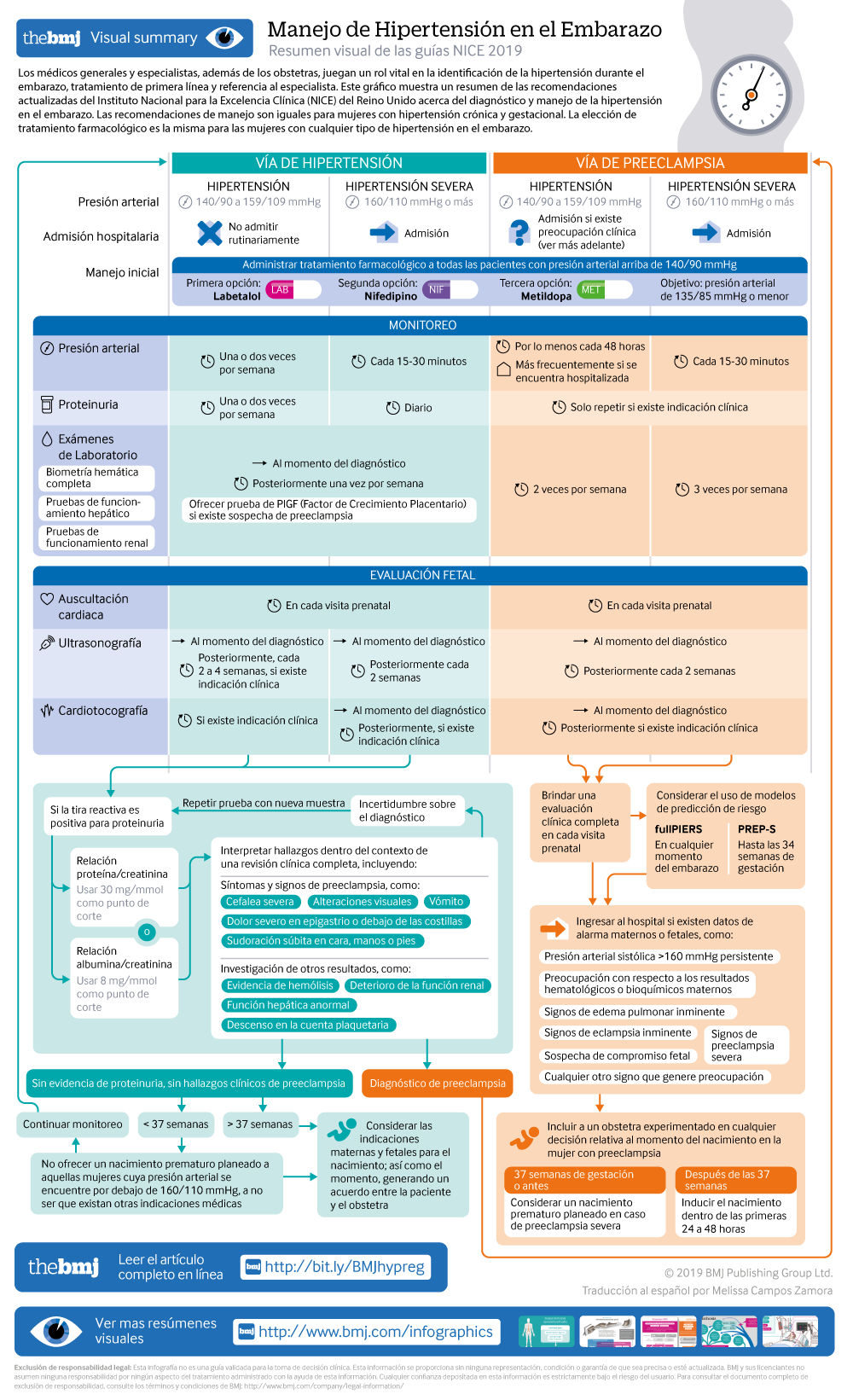
What you need to know
Hypertension affects about 10% of pregnant women, including those with pre-existing hypertension, chronic hypertension that is first diagnosed during pregnancy, and hypertension related to pregnancy (gestational hypertension and pre-eclampsia)
Target blood pressure during the antenatal period should be 135/85 mm Hg for women with hypertension during pregnancy
Hypertension during pregnancy is associated with an increased risk of hypertension and cardiovascular disorders in later life. Women should be offered appropriate lifestyle and dietary advice to minimise this risk
Hypertension in pregnancy is a common condition, affecting about 10% of pregnant women. This includes women with chronic hypertension—which may be diagnosed before pregnancy or in the early stages of pregnancy (<20 weeks’ gestation)—and women with hypertension related to pregnancy (gestational hypertension and pre-eclampsia) (see box 1 ). If not identified and treated, hypertension can lead to adverse events for both the woman and her baby, including increased risk of maternal stroke, lower birth weight, and increased risk of the baby requiring neonatal intensive care.
Definitions for hypertensive disorders of pregnancy
Chronic hypertension— Hypertension that is present at the booking visit or before 20 weeks’ gestation, or if the woman is already taking antihypertensive medication when starting maternity care. It can be primary or secondary in aetiology
Gestational hypertension— New hypertension presenting after 20 weeks of pregnancy without significant proteinuria
Pre-eclampsia— New onset hypertension (>140 mm Hg systolic or >90 mm Hg diastolic) after 20 weeks of pregnancy and the coexistence of one or both of the following new-onset conditions:
Proteinuria (urine protein:creatinine ratio ≥30 mg/mmol, or albumin:creatinine ratio ≥8 mg/mmol, or ≥1 g/L [2+] on dipstick testing)
Other maternal organ dysfunction, including features such as renal or liver involvement, neurological or haematological complications, or uteroplacental dysfunction (such as fetal growth restriction, abnormal umbilical artery Doppler waveform analysis, or stillbirth)
General practitioners and specialists other than obstetricians play a vital role in the identification of hypertension during pregnancy, first line management, and appropriate referral to specialist care. Women with pre-existing (chronic) hypertension may require pre-pregnancy counselling from their primary or secondary care team, modifications to their usual treatment, and referral to specialist care. Women are likely to have shared care between specialists and non-specialists throughout their pregnancy, meaning that GPs need to be aware of current blood pressure targets, suitable medication, and thresholds for urgent referral to specialist care. Furthermore, hypertensive disorders of pregnancy are known to predispose women to ongoing hypertension and associated cardiovascular morbidity in later life. The primary care team plays a crucial role in risk reduction and surveillance for these conditions. It is therefore vital that all healthcare professionals have an understanding of the optimal management of hypertension during pregnancy and the postpartum period.
This article summarises the updated recommendations from the National Institute for Health and Care Excellence (NICE) on the diagnosis and management of hypertension in pregnancy. 1
What's new in this guidance?
Initiation of antihypertensive medication is now recommended for women with a blood pressure measurement of 140/90 mm Hg
Target blood pressure for those taking antihypertensive medication is now 135/85 mm Hg
Categories of hypertension have now been simplified to “hypertension” and “severe hypertension” (rather than mild, moderate, and severe)
24 hour urine collection is no longer recommended for routine quantification of proteinuria during pregnancy
Hospital admission is no longer recommended for every woman with pre-eclampsia—risk assessment should be carried out on an individual basis to determine place of care
Pharmacological therapy for hypertension in the postnatal period now reflects stepped treatment recommended for adults, adapted for women who are breastfeeding
Estimates for the likelihood of recurrent hypertensive disorders in future pregnancies and of long term cardiovascular disease are provided
Recommendations
NICE recommendations are based on systematic reviews of best available evidence and explicit consideration of cost effectiveness. When minimal evidence is available, recommendations are based on the guideline committee’s experience and opinion of what constitutes good practice. Evidence levels for the recommendations are given in italic in square brackets.
Treatment of chronic hypertension
For women with chronic hypertension, recommended diet and lifestyle advice have been brought in line with that given to non-pregnant individuals. However, the choice of anti-hypertensive drugs is different during pregnancy, because of the need to consider the effects of the drug on the fetus. No specific medication was recommended in the previous version of the guideline, but labetalol, nifedipine, and methyldopa are now specified as suitable options to discuss with women for use in pregnancy. New evidence was identified to provide guidance on blood pressure targets during pregnancy, and the target has now been amended to 135/85 mm Hg (reduced from the previous guidance of 150/100 mm Hg), also reflecting evidence informing the management of hypertension in adults.
In addition to the new recommendations, NICE diagnostic guidance DG23 has been published since the previous guideline, and provides guidance on the use of placental growth factor (PlGF)-based testing. 2 This offers an additional diagnostic test to rule out pre-eclampsia in women with suspected pre-eclampsia (including those at increased risk of developing it, such as women with chronic hypertension or gestational hypertension), and so a link has been included in the updated guideline.
The recommendations are summarised below.
Offer pregnant women with chronic hypertension advice on:
Weight management
Healthy eating
Lowering the amount of salt in their diet.
Provide this advice in line with the NICE guideline on hypertension in adults: diagnosis and treatment 3
[ Based on the experience and opinion of the Guideline Committee (GC) ]
Continue with existing antihypertensive treatment if it is safe in pregnancy, or switch to an alternative treatment, unless:
Sustained systolic blood pressure is <110 mm Hg or
Sustained diastolic blood pressure is <70 mm Hg or
The woman has symptomatic hypotension.
[ Based on the experience and opinion of the GC ]
Offer antihypertensive treatment to pregnant women who have chronic hypertension and who are not already on treatment if they have:
Sustained systolic blood pressure ≥140 mm Hg or
Sustained diastolic blood pressure ≥90 mm Hg.
[ Based on very low to moderate quality evidence and the experience and opinion of the GC ]
When using antihypertensive treatment in pregnancy, aim for a target blood pressure of 135/85 mm Hg. [ Based on very low to moderate quality evidence and the experience and opinion of the GC ]
Consider labetalol to treat chronic hypertension in pregnant women. Consider nifedipine for women in whom labetalol is not suitable, or methyldopa if both labetalol and nifedipine are not suitable. Base the choice on any pre-existing treatment, side effect profiles, risks (including fetal effects), and the woman’s preference. [ Based on very low quality evidence and the experience and opinion of the GC ]
Offer pregnant women with chronic hypertension aspirin 75-150 mg once daily from 12 weeks. [ Based on very low to high quality evidence and the experience and opinion of the GC ]
Offer placental growth factor (PlGF)-based testing to help rule out pre-eclampsia between 20 weeks and up to 35 weeks of pregnancy, if women with chronic hypertension are suspected of developing pre-eclampsia.
Management of gestational hypertension
Management of gestational hypertension requires regular monitoring, to ensure that blood pressure control is maintained and that there is not progression to pre-eclampsia. The evidence for the type and frequency of monitoring was reviewed as part of this update, and the recommendations amended. The blood pressure target has been reduced to 135/85 mm Hg (in line with that for chronic hypertension), and the drug choices aligned to those used in chronic hypertension to simplify management for clinicians.
The recommendations are summarised in the infographic [ based on very low to moderate quality evidence and the experience and opinion of the GC ]

- Download figure
- Open in new tab
- Download powerpoint
Assessment of proteinuria in hypertensive disorders of pregnancy
Proteinuria is one of the key features of pre-eclampsia and should be assessed at each antenatal visit alongside blood pressure monitoring (see related NICE guidance on antenatal care for uncomplicated pregnancies 4 ). The updated recommendations stress that proteinuria measurements should always be interpreted alongside a full clinical review—to highlight that women may develop pre-eclampsia in the absence of proteinuria, and that there may be value in repeating a measurement if there is doubt over the diagnosis of pre-eclampsia.
Previous NICE guidelines recommended that proteinuria was assessed using a 24-hour urine collection or a spot urinary protein:creatinine ratio. The updated guideline assessed the evidence for the accuracy of protein:creatinine ratio and of the alternative test albumin:creatinine ratio and found both to have high specificity and sensitivity, meaning they can be used instead of 24-hour urine collection, which is no longer recommended.
Interpret proteinuria measurements for pregnant women in the context of a full clinical review of symptoms, signs, and other investigations for pre-eclampsia. [ Based on the experience and opinion of the GC ]
Use an automated reagent-strip reading device for dipstick screening for proteinuria in pregnant women in secondary care settings. [ Based on high quality evidence and the experience and opinion of the GC ]
If dipstick screening is positive (1+ or more) use albumin:creatinine ratio or protein:creatinine ratio to quantify proteinuria in pregnant women. [ Based on very low to low quality evidence and the experience and opinion of the GC ]
Do not use first morning urine void to quantify proteinuria in pregnant women. [ Based on very low quality evidence ]
Do not routinely use 24-hour urine collection to quantify proteinuria in pregnant women.
If using protein:creatinine ratio to quantify proteinuria in pregnant women:
Use 30 mg/mmol as a threshold for significant proteinuria
If the result is ≥30 mg/mmol and there is still uncertainty about the diagnosis of pre-eclampsia, consider re-testing on a new sample, alongside clinical review.
[ Based on very low quality evidence and the experience and opinion of the GC ]
If using albumin:creatinine ratio as an alternative to diagnose pre-eclampsia in pregnant women with hypertension:
Use 8 mg/mmol as a diagnostic threshold
If the result is ≥8 mg/mmol and there is still uncertainty about the diagnosis of pre-eclampsia, consider re-testing on a new sample alongside clinical review.
[ Based on low quality evidence and the experience and opinion of the GC ]
Pre-eclampsia
Pre-eclampsia can be associated with severe complications for a woman and her baby, so appropriate risk assessment and management is critical. The updated guidance uses the same blood pressure target and treatment choices as for chronic and gestational hypertension, simplifying management for the clinician, but no longer recommends that all women with pre-eclampsia be admitted to hospital as evidence for this approach was lacking. Instead, the guideline provides more information on the features which may indicate more severe disease requiring admission and provides information on new risk prediction models that may help identify women at risk of severe complications.
Carry out a full clinical assessment at each antenatal appointment for women with pre-eclampsia and offer admission to hospital for surveillance and any interventions needed if there are concerns for the wellbeing of the woman or baby. Concerns could include any of the following:
Sustained systolic blood pressure ≥160 mm Hg
Any maternal biochemical or haematological investigations that cause concern, such as a new and persistent
Rise in creatinine concentration (≥90 μmol/L, ≥1 mg/100 mL) or
Rise in alanine transaminase (>70 IU/L or twice upper limit of normal range) or
Fall in platelet count (<150 000/μL)
Signs of impending eclampsia
Signs of impending pulmonary oedema
Other signs of severe pre-eclampsia
Suspected fetal compromise
Any other clinical signs that cause concern.
Consider using either the fullPIERS or PREP-S validated risk prediction models to help guide decisions about the most appropriate place of care (such as the need for in utero transfer) and thresholds for intervention. [ Based on moderate to high quality evidence ]
When using a risk prediction model, take into account:
fullPIERS is intended for use at any time during pregnancy
PREP-S is intended for use only up to 34 weeks of pregnancy
fullPIERS and PREP-S models do not predict outcomes for babies.
[ Based on moderate to high quality evidence ]
Recommendations for management of pre-eclampsia are described in the infographic [ based on very low to moderate quality evidence and the experience and opinion of the GC ]
Planned early birth
Guidance on the indications and optimum timing ( box 2 ) for birth in women with pre-eclampsia has also been updated.
Timing of birth in women with pre-eclampsia
Before 34 weeks’ pregnancy— Continue surveillance unless there are indications for planned early birth (see recommendation). Offer intravenous magnesium sulfate and a course of antenatal corticosteroids in line with the NICE guideline on preterm labour and birth 5
From 34 to 36+6 weeks— Continue surveillance unless there are indications for planned early birth (see recommendation). When considering planned early birth, take into account the woman’s and baby’s condition, risk factors (such as maternal comorbidities, multi-fetal pregnancy), and availability of neonatal unit beds. Consider a course of antenatal corticosteroids in line with the NICE guideline on preterm labour and birth 5
From 37 weeks onwards —Initiate birth within 24-48 hours.
Record maternal and fetal thresholds for planned early birth before 37 weeks in women with pre-eclampsia. Thresholds for considering planned early birth could include (but are not limited to) any of the following known features of severe pre-eclampsia:
Inability to control maternal blood pressure despite using three or more classes of antihypertensives in appropriate doses
Maternal pulse oximetry <90%
Progressive deterioration in liver function, renal function, haemolysis, or platelet count
Ongoing neurological features, such as severe intractable headache, repeated visual scotomata, or eclampsia
Placental abruption
Reversed end-diastolic flow seen in umbilical artery Doppler velocimetry, a non-reassuring cardiotocograph, or stillbirth.
Other features not listed above may also be considered in the decision to plan early birth.
Involve a senior obstetrician in any decisions on timing of birth for women with pre-eclampsia. [ Based on the experience and opinion of the GC ]
Discuss with the anaesthetic team if birth is planned in a woman with pre-eclampsia. [ Based on the experience and opinion of the GC ]
Discuss with the neonatal team if birth is planned in a woman with pre-eclampsia, and neonatal complications are anticipated. [ Based on the experience and opinion of the GC ]
Offer intravenous magnesium sulfate and a course of antenatal corticosteroids if indicated, if early birth is planned for women with preterm pre-eclampsia, in line with the NICE guideline on preterm labour and birth. 5
Postnatal care for women with hypertension during pregnancy
Many women with hypertension during pregnancy will require antihypertensive treatment in the postnatal period, although the duration of treatment required will vary. Selection of an appropriate antihypertensive depends on the efficacy, safety, and tolerability of the different medications. To improve adherence, preparations with once daily use that are compatible with breast feeding are recommended. The recommendations were updated, based on the NICE guideline for the management of hypertension in adults, 3 adapted to support breastfeeding in women who may choose to breastfeed and to minimise the chance of women choosing not to breastfeed because of their medication.
Advise women with hypertension who wish to breastfeed that their treatment can be adapted to accommodate breastfeeding and that the need to take antihypertensive medication does not prevent them from breastfeeding. [ Based on the experience and opinion of the GC ]
Explain to women with hypertension who wish to breastfeed that:
Antihypertensive medicines can pass into breast milk
Most antihypertensive medicines taken while breastfeeding only lead to very low levels in breast milk, so the amounts taken in by babies are very small and would be unlikely to have any clinical effect
Most medicines are not tested in pregnant or breastfeeding women, so disclaimers in the manufacturer’s information are not because of any specific safety concerns or evidence of harm.
Make decisions on treatment together with the woman, based on her preferences. [ Based on the experience and opinion of the GC ]
As antihypertensive agents have the potential to transfer into breast milk:
Consider monitoring the blood pressure of babies, especially those born preterm, who have symptoms of low blood pressure for the first few weeks
When women are discharged home, advise them to monitor their babies for drowsiness, lethargy, pallor, cold peripheries, or poor feeding.
Offer enalapril to treat hypertension in women during the postnatal period, with appropriate monitoring of maternal renal function and maternal serum potassium. [ Based on the experience and opinion of the GC ]
For women of black African or Caribbean family origin with hypertension during the postnatal period, consider antihypertensive treatment with:
Nifedipine or
Amlodipine if the woman has previously used this successfully to control her blood pressure.
[ Based on very low to low quality evidence and the experience and opinion of the GC ]
For women with hypertension in the postnatal period, if blood pressure is not controlled with a single medicine consider a combination of nifedipine (or amlodipine) and enalapril. If this combination is not tolerated or is ineffective, consider:
Adding atenolol or labetalol to the combination treatment or
Swapping one of the medicines already being used for atenolol or labetalol.
When treating women with antihypertensive medication during the postnatal period, use medicines that are taken once daily when possible. [ Based on the experience and opinion of the GC ]
When possible, avoid using diuretics or angiotensin receptor blockers to treat hypertension in women in the postnatal period who are breastfeeding or expressing milk. [ Based on the experience and opinion of the GC ]
Treat women with hypertension in the postnatal period who are not breastfeeding and who are not planning to breastfeed in line with the NICE guideline on hypertension in adults. 3 [ Based on the experience and opinion of the GC ]
Long term consequences of hypertension during pregnancy
The occurrence of hypertension during one pregnancy is known to predispose women to hypertension in the future—with an increased likelihood of recurrence of hypertensive disorders of pregnancy in future pregnancies and of long term hypertension in later life.
Precisely quantifying the likelihood of recurrence during pregnancy is challenging, but the updated guidance provides some estimates of how likely hypertensive disorders are to recur ( table 1 ). Advise women with hypertensive disorders of pregnancy that the overall risk of recurrence in future pregnancies is approximately 1 in 5.
Prevalence of hypertensive disorder in a future pregnancy in women with hypertension in previous or current pregnancy [ Based on very low to high quality evidence and the experience and opinion of the GC ]
- View inline
In addition, hypertensive disorders during pregnancy are known to be associated with an increased likelihood of hypertension, and associated cardiovascular morbidity, in later life. The updated guideline provides estimates of this increase in likelihood for women with hypertensive disorders during pregnancy, to enable them to modify their lifestyle accordingly.
Advise women who have had a hypertensive disorder of pregnancy that this is associated with an increased risk of hypertension and cardiovascular disease in later life (see table 2 ). [ Based on moderate to high quality evidence and the experience and opinion of the GC ]
Advise women who have had a hypertensive disorder of pregnancy to discuss how to reduce their risk of cardiovascular disease, including hypertensive disorders, with their GP or specialist. This may include:
Avoiding smoking, as recommended in the NICE guideline on stopping smoking 6
Maintaining a healthy lifestyle, as recommended in the NICE guideline on cardiovascular disease 7
Maintaining a healthy weight, as recommended in the NICE guideline on obesity. 8
In women who have had pre-eclampsia or hypertension with early birth before 34 weeks consider pre-pregnancy counselling to discuss possible risks of recurrent hypertensive disorders of pregnancy and how to lower them for any future pregnancies. [ Based on the experience and opinion of the GC ]
Relative risk* of future cardiovascular morbidity in women with hypertension in previous or current pregnancy
Implementation
A patient decision aid has been developed to support implementation of this guideline and is available at https://action-on-pre-eclampsia.org.uk/public-area/high-blood-pressure-in-pregnancy/#resources .
PREP and fullPIERS clinical prediction tools are freely available online
Future research
Further research is needed on the efficacy and safety of antihypertensive agents during pregnancy and the postnatal period—including the comparative efficacy of different antihypertensives to treat chronic hypertension, the neonatal effects of β blockers and mixed α and β blockers, and the efficacy of different antihypertensives in the postnatal period.
Two areas of antenatal care were prioritised for future research—to assess whether inpatient care is associated with better outcomes for women with pre-eclampsia, and to establish the optimal fetal monitoring strategy to identify infants that are small for gestational age.
Future research should concentrate on the efficacy of interventions to reduce the risk of recurrence of hypertension in future pregnancies, and the risk of long term cardiovascular complications.
Guidelines into practice
Do you refer women with chronic hypertension to a specialist in hypertensive disorders of pregnancy for pre-pregnancy advice?
Do you stop ACE inhibitors or angiotensin II receptor blockers within two days of notification of pregnancy?
Do you provide information for postnatal women after pregnancy hypertension on long term cardiovascular risk and interventions to reduce that risk?
How women with lived experience were involved in the creation of this article
Committee members involved in this guideline update included lay members who contributed to the formulation of the recommendations summarised here.
Further information on the guidance
The guideline update was developed using the methods described in Developing NICE guidelines: the manual , 2014 ( https://www.nice.org.uk/media/default/about/what-we-do/our-programmes/developing-nice-guidelines-the-manual.pdf ). Systematic literature searches were undertaken to identify all published clinical evidence and health economic evidence relevant to the review questions. The guideline committee comprised healthcare professionals and lay members, who considered the evidence identified and drafted recommendations on the basis of the evidence and the expertise and opinion of the committee. Draft recommendations were subject to stakeholder consultation and revision before publication of the final guideline.
Other details
This guideline has been published by NICE and is available at https://www.nice.org.uk/guidance/ng133 .
Quick reference guides are being developed by NICE and will be available at https://www.nice.org.uk/guidance/ng133 .
Acknowledgments
The members of the guideline committee were (in alphabetical order) Philip Barclay, Sarah Beswick, Lucy Chappell, Alena Chong, Maria Clark, Sarah Findlay, Sarah Fishburn (chair), Christine Harding, Pramod Mainie, Maryam Parisaei, Lisa Smith, Mark Tighe, Ashifa Trivedi, and Pensee Wu.
The members of the National Guideline Alliance team were (in alphabetical order) Offiong Ani, Hilary Eadon, Louise Geneen, Eva Gonzalez-Viana, Matthew Prettyjohns, Tim Reeves, and Katie Webster.
Contributors: All authors contributed to the initial draft of this article, helped revise the manuscript, and approved the final version for publication.
Funding: The National Guideline Alliance was commissioned and funded by the National Institute for Health and Care Excellence to develop this guideline and write this BMJ summary.
Disclaimer: The guideline referred to in this article was produced by the National Guideline Alliance for the National Institute for Health and Care Excellence (NICE). The views expressed in this article are those of the authors and not necessarily those of NICE.
Competing interests: We declare the following interests based on NICE's policy on conflicts of interests ( https://www.nice.org.uk/Media/Default/About/Who-we-are/Policies-and-procedures/declaration-of-interests-policy.pdf ): SF has received funding from NICE, National Institute for Health Research, Royal College of Obstetricians and Gynaecology and Mott MacDonald. SCF has received funding from the BMJ. The authors’ full statements can be viewed at https://www.nice.org.uk/guidance/ng133/documents/register-of-interests-2 .
- ↵ National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management (NICE guideline NG133). 2019. https://www.nice.org.uk/guidance/ng133 .
- ↵ National Institute for Health and Care Excellence. PlGF-based testing to help diagnose suspected pre-eclampsia (Triage PlGF test, Elecsys immunoassay sFlt-1/PlGF ratio, DELFIA Xpress PlGF 1-2-3 test, and BRAHMS sFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio) (diagnostics guidance DG23). 2016. https://www.nice.org.uk/guidance/dg23 .
- ↵ National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management (clinical guideline CG127). Updated 2016. https://www.nice.org.uk/guidance/cg127 .
- ↵ National Institute for Health and Care Excellence. Antenatal care for uncomplicated pregnancies (clinical guideline CG62). Updated 2019. https://www.nice.org.uk/guidance/cg62 .
- ↵ National Institute for Health and Care Excellence. Preterm labour and birth (NICE guideline NG25). Updated 2019. https://www.nice.org.uk/guidance/ng25 .
- ↵ National Institute for Health and Care Excellence. Stop smoking interventions and services (NICE guideline NG92). 2018. https://www.nice.org.uk/guidance/ng92 .
- ↵ National Institute for Health and Care Excellence. Cardiovascular disease prevention (Public health guideline PH25). 2010. https://www.nice.org.uk/guidance/ph25 .
- ↵ National Institute for Health and Care Excellence. Obesity prevention (Clinical guideline CG43). Updated 2015. https://www.nice.org.uk/guidance/cg43 .
Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia Essay
Pregnancy induced hypertension.
A woman with pregnancy induced hypertension (PIH) experiences high blood pressure and protein deposits are found in her urine. In most cases, this condition occurs after twenty weeks of pregnancy and is common among first time mothers, teenagers and old mothers above forty years and who have had multiple pregnancies.
- Rushes or spots on the face.
- Protein in the urine.
- Sudden weight gain
- Sharp pain in the stomach especially on upper right side and around the ribs
The condition can be detected through urine tests that check protein levels in the urine and using a Doppler scan that monitors flow of blood in the placenta. If the PIH is mild, the doctor recommends less salt consumption and resting by lying on left side so as to suppress the weight of the baby acting on the blood vessels. For severe cases, the doctor may use medications such as a magnesium sulfate injection to lower the blood pressure. Other medications include methyldopa, labetalol, and calcium channel blockers. Among these medications, research has shown methyldopa is the best of all because it has very few side effects to both the mother and fetus (Seneviratne, 1998, p. 167).
Pre-Eclampsia
Pre-eclampsia is a form of pregnancy induced hypertension that is associated with presence of proteins in the urine commonly known as proteinuria. It occurs at about twenty weeks of pregnancy but it is mostly common beyond twenty four weeks. It affects women who are having their first pregnancy as well as those who get pregnant in the course of pre-existing hypertension conditions.
The condition is characterized by a rise in blood pressure that can go above 140/90mmHg. It is usually diagnosed during a routine antenatal checkup and in some cases the condition may warrant admission of the patient for close monitoring. Medical researchers have not fully discovered the pathophysiology of pre-eclampsia. However, it is believed to be a placental disorder that could result from poor perfusion in the placenta. It could also result from poor nutrition and high body fat. The underlying effect is poor development of the fetus, which is normally smaller than usual, mainly due inadequate flow of blood in the placenta. Severe pre-eclampsia may be experienced by a pregnant woman who previously had a mild type of this disease. The most dangerous thing about this condition is that it often appears with little or no warning. The blood pressure rises to about 160/110mmHg and there is a high quantity of protein deposits in the urine. The patient may have one or a combination of the following symptoms: severe headache, blurred vision, epigastric sharp pain similar to a heartburn, nausea and vomiting, muscle twitching and swelling of limbs (Wickham, 2008, p. 212).
Treatment for pre-eclampsia in particular focuses on the high blood pressure. Doctors usually advise bed rest and antihypertensive medication may be administered to lower the blood pressure if the patient is in critical condition. In cases where the patient has convulsions, drugs to counter convulsions may be given. Doctors believe the best treatment for pre-eclampsia is induced premature birth, which is usually done through caesarean section. The following medications are used for reducing blood pressure:
- Magnesium sulfate, which prevents the risk of developing eclampsia
- Calcium channel blockers
Methyldopa, which is administered orally, is considered the best medication among these since it has fewer side effects.
One of the complications that could results from pre-eclampsia is where the pregnant woman develops seizures and eventually goes into coma. The symptoms for eclampsia are similar to those of pre-eclampsia. However, the most common symptom for eclampsia is seizures. The tests for both pre-eclampsia and eclampsia include, blood platelet count, protein check in the urine and kidney function analysis.
An intravenous injection of magnesium sulfate helps to reduce the chances of seizures recurring. Other medications may also be given to manage the level of blood pressure. These medications include hydralazine and labetalol. Premature birth may also be induced by use of oxytocin or prostaglandins, which can induce labor pains and hence prepare the cervix for delivery.
Side Effects of the Various Medications
Magnesium sulfate has adverse effects on muscles, as it makes them to grow weaker. It may also cause dizziness and slow breathing. Hydralazine may cause loss of appetite, mild diarrhea and vomiting. He patient could experience severe side effects such as yellowing of eyes, irregular heartbeat, joint pains and swelling of the mouth. Labetalol could induce side effects such as nose stuffiness, fatigue, indigestion, wheezing, persistent cough, chest pains and yellowing of the eyes. Oxytocin’s side effects include abrupt uterus contraction, vomiting, heavy bleeding during childbirth and blood clotting problems. Calcium channel blockers could cause side effects such as reduced heart rate and constipation. The use of Nifedipine has been known to have side effects such as blurred vision, heartburn, swelling of gums and limbs and constipation (Lyall & Belfort, 2007, p. 250).
Lyall, F., & Belfort, M. A. (2007). Pre-eclampsia: Etiology and clinical practice . New York, NY: Cambridge University Press.
Seneviratne, H. (1998). Pregnancy induced hypertension. Himayatnagar, Hyderabad: Orient Longman.
Wickham, S. (2008). Midwifery: Best practice, volume 5. New York, NY: New York Press.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, March 28). Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/
"Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." IvyPanda , 28 Mar. 2022, ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
IvyPanda . (2022) 'Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia'. 28 March.
IvyPanda . 2022. "Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." March 28, 2022. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
1. IvyPanda . "Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." March 28, 2022. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
Bibliography
IvyPanda . "Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." March 28, 2022. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
- The Major Medical Causes of Maternal Deaths and Ways to Reduce It
- Supporting the Health Needs of Patients With Parkinson’s, Preeclampsia, and Postpartum Depression
- Placenta Previa: A Literature Review
- Male Sexual Performance: Complementary and Alternative Medicine
- Column Agglutination Technology (CAT) in Blood Bank
- Bardet-Bield Syndrome (BBS): Overview
- Raising Awareness on Food Poisoning Among Children Riyadh
- Spinal Anatomy: A Discussion of Cases of Spinal Defects
Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)
Teaching plan.
Pregnant women are prone to complications, which threaten their lives and that of the infants. Pregnancy-induced hypertension is one of the complications that pregnant women experience. To prevent or manage complications, parents usually attend childbirth classes.
The childbirth class, which was held at Sibley Memorial Hospital taught parents how to handle experiences that they undergo during the first, second, and third trimesters. Despite the fact that the instructor covered extensive topics, she did not cover the topic of pregnancy-induced hypertension. Hence, the teaching plan focused on signs and symptoms, the nature of the complication, risk factors, and applicable interventions.
On February 7, 2015, I attended a boot camp for childbirth class that was hosted at Sibley Memorial Hospital. The session of the childbirth class started at 9am and ended at 5pm. The instructor’s name was Rosemarie Antunes, a registered nurse with the experience of 30 years in obstetric, labor, and delivery nursing acquired from different hospitals in different states.
She obtained her licenses as a registered nurse (RN) from the Virginia Department of Health Professions in 2004 and State of Connecticut, Department of Public Health in 1980. She has also received professional certification from Prepared Childbirth Educators in 2006 and Certified Labor Doula in 2009. Rosemarie took her diploma education for RN at Saint Francis Hospital School in 1977-1980.
Currently, she works for Fauquier Health System, a family birthing center, since 2004. She also works for Labor and Delivery, Postpartum, and Normal Newborn as a certified childbirth educator with experience of 10 years in preparing expectant mothers and their partners for what is ahead of them. Rosemarie is a mother of six children with 10 grandchildren (R. Antunes, personal communication, February 7, 2015).
The instructor employed constructivism as a teaching philosophy because she aided the participants to understand experiences that they expect during pregnancy and delivery by using questions and demonstrations. To construct the foundation of teaching, the instructor told the participants to ask questions that they might have before she commences each session.
Moreover, the instructor informed the participants that she would stop at any time during presentation to allow them to ask questions. To enhance understanding among the participants, the instructor demonstrated her teachings using various methods. The teaching methods that the instructor employed in demonstrations are videos, PowerPoint presentation, handouts, dolls, and birth balls.
The childbirth class took place on February 7, 2015 at Sibley Memorial Hospital in one of their lecture halls from 9am to 5pm. The childbirth class comprised of Caucasian couples, African couples, and Asians couples. Interestingly, during their introduction, all of them indicated that they were expecting their babies in March 2015.
From the introduction, it became apparent that half of the couples were married while the remaining couples were just partners. The participants were young adults between the ages of 20 to 30 years, who were expecting their babies for the first time. The couples were ready for the childbirth lessons as they brought with them pillows, birth balls, and blankets.
The topics covered in childbirth class aimed at enhancing the understanding of pregnancy (3 rd trimester), labor, Christina Birth story, comfort techniques, medical procedures, cesarean birth, newborn procedures, postpartum, and labor rehabilitation. The instructor covered anatomy and physiology of pregnancy from first trimester to the third trimester, and hormonal proliferations that happen during pregnancy.
To improve their health, the instructor encouraged the pregnant women to eat food high in fiber, drink water at all times, and call HCP whenever they experience pain during urination. The instructor also covered signs of labor and expected medical emergencies such as induction and cesarean births, which are essential in saving babies and mothers.
She taught the participants how to employ exercise, relaxation, massage, and music in improving the birth of the newborn in a natural way. The instructor also mentioned postpartum and gave healthcare instructions for newborn, such as safety and breastfeeding techniques.
Pregnancy-Induced Hypertension (PIH)
Although the instructor extensively covered diverse areas of childbirth, I noted that she did not delve deep into complications of pregnancy, and thus, she should have examined the topic of pregnancy-induced hypertension (PIH).
Pregnant women are susceptible to PIH or gestational hypertension owing to changes in their bodies. Essentially, PIH has medical importance because it threatens the lives of the baby and the mother. Therefore, I will explore the topic of PIH with a view of equipping the participants with the appropriate knowledge that is critical in prevention, treatment, and management of the complication.
Summary of Teaching
The first objective of teaching pregnant women is to enable them to identify signs and symptoms of PIH. As PIH requires early detection for treatment and management interventions to be effective, pregnant women need to understand how to identify the signs and symptoms of PIH very early.
Excessive swelling of hands and feet, dizziness, excessive nausea, rapid heartbeat, severe headaches, drowsiness, fever, blurred vision, and pain in the abdomen are some of the signs and symptoms of PIH, which pregnant women need to watch so that they can seek early medical attention.
According to Jwa et al. (2013), early detection of PIH is critical for fetal and maternal health because it enhances the effectiveness of treatment and management interventions. As teaching methods, I will employ PowerPoint presentation, brochures, handouts, and discussion.
In teaching about PIH, the second objective is to enable the participating couples to understand the nature of PIH. Given that pregnant women experience diverse forms of hypertension, PIH is a unique form of hypertension because it only happens after the 20th week of pregnancy and can be either transient or chronic (Sajith et al., 2014).
When blood pressure of a pregnant woman is higher than 140/90 in two different occasions, and her urine contains no proteins, the differential diagnosis indicates PIH. As a teaching method, I will demonstrate diagnosis of PIH by measuring blood pressure of the pregnant women and undertaking urinalysis to determine the presence of proteins in urine.
The third objective is to enable the participating couples to understand risks of PIH. The common risk factors for PIH are women with the first-time pregnancy, increased maternal age, family history, multiple gestations, proteinuria, hypertension, and diabetes mellitus (Jwa et al., 2013).
Moreover, nutrition also has other risk factors for PIH because an increased consumption of vitamin E and mono- and poly-unsaturated fatty acids increases the risk for PIH, while an increased consumption of magnesium, potassium, and vitamin C reduces the risk for PIH (Kazemian et al., 2012).
Sleep disturbance is also a possible risk factor for PHI because it correlates with hypertension (Haney, Buysse, & Okun, 2011). To expose these findings, I will employ PowerPoint presentation, brochures, handouts, and discussion.
The fourth objective of teaching is to enhance understanding of available treatment and management interventions of PIH. When pregnant women know the nature of available interventions, they can discuss with their doctors and choose the best intervention that fits them, hence, promote therapeutic adherence.
Sajith et al. (2014) state that both mono- and combined therapies of antihypertensive drugs are used in the treatment and management of PIH because they are safe for mothers and infants. Kazemian et al., (2012) recommends the application of nutrition in the prevention, treatment, and management of PIH.
Moreover, Haney, Buysse, and Okun (2011) recommend that alleviation of sleep disturbance reduces blood pressure, and hence, prevents the occurrence of PIH. The methods of teaching will comprise the use of the PowerPoint presentation, brochures, handouts, and discussion.
Pregnancy-Induced Hypertension
What is pregnancy-induced hypertension.
Pregnancy-induced hypertension refers to the high blood pressure, which women experience when they are pregnant.
Why is it important for pregnant women?
Pregnancy-induced hypertension affects pregnant women because their body changes during pregnancy. If doctors do not detect and treat pregnancy-induced hypertension, the mother and the baby will die. Therefore, pregnant women need to understand this disease so that they can seek medical attention whenever they experience signs and symptoms and save themselves and the unborn babies.
Signs and Symptoms
The common signs and symptoms of pregnancy-induced hypertension are excessive swelling of hands and feet, severe morning sickness, dizziness, fast heartbeat, severe headaches, drowsiness, high temperature, poor vision, and pain in the abdomen.
Nature of Pregnancy-Induced Hypertension
Pregnancy-induced hypertension is different from other types of hypertensions because it affects women only, occurs after 20 weeks of pregnancy, and there are no proteins in the urine. However, when not treated, it progresses into a disease called preeclampsia, which causes urine to appear in urine.
Risk Factors
The risk factors for pregnancy-induced hypertension are first-time pregnancy, age of the mother, bloodline with this disease, proteins in urine, many pregnancies, diabetes, nutrition, high blood pressure, and sleep disturbance.
Treatment and Management Interventions
- Use medications that reduce high blood pressure (antihypertensive drugs).
- Control food intake by reducing the amount of oils while increasing the amount of potassium, magnesium, and vitamin C.
- Avoid disturbance during sleep and have peace of mind.
Haney, A., Buysse, D., & Okun, M. (2011). Sleep and pregnancy-induced hypertension: A possible target for intervention? Journal of Clinical Sleep Medicine, 9 (12), 1349-1356.
Jwa, S., Arata, N., Sakamoto, N., Watanabe, N., Aoki, H., Kurauchi-Mito, A., Dongmei, Q., Ohya, Y., Ichihara, A., & Kitagawa. (2011). Prediction of pregnancy-induced hypertension by shift of blood pressure class according to the JSH 2009 guidelines. Hypertension Research, 34 (1), 1203-1208.
Kazemian, E., Dorosti-Motiagh, A., Sotoudeh, G., Eshraghian, M., & Ansary, S. (2012). The nutritional status of women with gestational hypertension compared to normal pregnant women. Women’s Health Care, 1 (10), 1-6.
Sajith, M., Nimbargi, V., Modi, A., Sumariya, R., & Pawar, A. (2014). Incidence of pregnancy induced hypertension and prescription pattern of antihypertensive drugs in pregnancy. International Journal of Pharma Sciences and Research, 5 (4), 163-170.
Cite this paper
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2020, January 15). Complication of Pregnancy: Pregnancy Induced Hypertension (PIH). https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/
"Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." StudyCorgi , 15 Jan. 2020, studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
StudyCorgi . (2020) 'Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)'. 15 January.
1. StudyCorgi . "Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." January 15, 2020. https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
Bibliography
StudyCorgi . "Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." January 15, 2020. https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
StudyCorgi . 2020. "Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." January 15, 2020. https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
This paper, “Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)”, was written and voluntary submitted to our free essay database by a straight-A student. Please ensure you properly reference the paper if you're using it to write your assignment.
Before publication, the StudyCorgi editorial team proofread and checked the paper to make sure it meets the highest standards in terms of grammar, punctuation, style, fact accuracy, copyright issues, and inclusive language. Last updated: April 30, 2024 .
If you are the author of this paper and no longer wish to have it published on StudyCorgi, request the removal . Please use the “ Donate your paper ” form to submit an essay.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health


Preeclampsia: Recent Advances in Predicting, Preventing, and Managing the Maternal and Fetal Life-Threatening Condition
Kai-jung chang.
1 Department of Obstetrics and Gynecology, Taipei Tzu-Chi Hospital, The Buddhist Tzu-Chi Medical Foundation, Taipei 231, Taiwan
Kok-Min Seow
2 Department of Obstetrics and Gynecology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei 111, Taiwan
3 Department of Obstetrics and Gynecology, National Yang-Ming Chiao-Tung University, Taipei 112, Taiwan
Kuo-Hu Chen
4 School of Medicine, Tzu-Chi University, Hualien 970, Taiwan
Associated Data
Not applicable.
Preeclampsia accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies. Defined as gestational hypertension after 20 weeks of pregnancy and coexisting proteinuria or generalized edema, and certain forms of organ damage, it is life-threatening for both the mother and the fetus, in terms of increasing the rate of mortality and morbidity. Preeclamptic pregnancies are strongly associated with significantly higher medical costs. The maternal costs are related to the extra utility of the healthcare system, more resources used during hospitalization, and likely more surgical spending due to an elevated rate of cesarean deliveries. The infant costs also contribute to a large percentage of the expenses as the babies are prone to preterm deliveries and relevant or causative adverse events. Preeclampsia imposes a considerable financial burden on our societies. It is important for healthcare providers and policy-makers to recognize this phenomenon and allocate enough economic budgets and medical and social resources accordingly. The true cellular and molecular mechanisms underlying preeclampsia remain largely unexplained, which is assumed to be a two-stage process of impaired uteroplacental perfusion with or without prior defective trophoblast invasion (stage 1), followed by general endothelial dysfunction and vascular inflammation that lead to systemic organ damages (stage 2). Risk factors for preeclampsia including race, advanced maternal age, obesity, nulliparity, multi-fetal pregnancy, and co-existing medical disorders, can serve as warnings or markers that call for enhanced surveillance of maternal and fetal well-being. Doppler ultrasonography and biomarkers including the mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and serum pregnancy-associated plasma protein A (PAPP-A) can be used for the prediction of preeclampsia. For women perceived as high-risk individuals for developing preeclampsia, the administration of low-dose aspirin on a daily basis since early pregnancy has proven to be the most effective way to prevent preeclampsia. For preeclamptic females, relevant information, counseling, and suggestions should be provided to facilitate timely intervention or specialty referral. In pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance including the Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test can be arranged. If the results are unfavorable, early intervention and aggressive therapy should be considered. Affected females should have access to higher levels of obstetric units and neonatal institutes. Before, during, and after delivery, monitoring and preparation should be intensified for affected gravidas to avoid serious complications of preeclampsia. In severe cases, delivery of the fetus and the placenta is the ultimate solution to treat preeclampsia. The current review is a summary of recent advances regarding the knowledge of preeclampsia. However, the detailed etiology, pathophysiology, and effect of preeclampsia seem complicated, and further research to address the primary etiology and pathophysiology underlying the clinical manifestations and outcomes is warranted.
1. Introduction
Hypertensive disorder during pregnancy poses a substantial threat to both maternal and fetal health conditions [ 1 ]. Preeclampsia is one of the most well-known medical conditions that belong to this disease spectrum, which also accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies [ 2 , 3 ]. It is depicted as a gestational condition with a hypertensive disorder diagnosed after 20 weeks of gestation and coexisting proteinuria or generalized edema, and certain forms of hematologic disorders such as thrombocytopenia or signs of end organ damage including renal impairment, abnormal liver function, pulmonary edema, and cerebral and visual disturbance [ 4 , 5 ]. The definitions of gestational hypertension (pregnancy-induced hypertension) and preeclampsia are shown in Figure 1 . Serious or long-term complications may result when preeclampsia turns into a severe type or is left without being sufficiently treated. Multiorgan involvement may be seen in such cases, and the impairment of uteroplacental perfusion could potentially lead to gestational complications and poor fetal outcomes including intrauterine fetal growth restriction and preterm delivery. As the situation worsens, it may become life-threatening for both the mother and the fetus, in terms of increasing the rate of mortality and morbidity [ 5 ].

The definitions of gestational hypertension (pregnancy-induced hypertension) and preeclampsia.
One straightforward way to categorize preeclampsia is to subdivide it into early-onset and late-onset groups in accordance with the gestational age (GA). The cutoff point is usually set as GA 34 weeks or GA 37 weeks, and we can subcategorize preeclampsia into the early-onset (GA < 34 weeks), late-onset (GA ≥ 34 weeks), preterm (GA < 37 weeks), and term (GA ≥ 37weeks) subgroups ( Table 1 ). The diagnoses made at different timings during the pregnancy course may suggest different pathophysiologic and etiologic pathways [ 6 ].
Classification of preeclampsia according to gestational age.
Preeclampsia should be viewed as a disease spectrum in which different subtypes may vary greatly in disease mechanisms and clinical presentations. The status of a previously normotensive pregnant woman developing new onset of hypertension after GA 20 weeks is termed “gestational hypertension” or “pregnancy induced hypertension”. If aside from gestational hypertension, a patient is also noted with proteinuria, thrombocytopenia, impairment in renal or liver function, cerebral symptoms, visual symptoms, or pulmonary edema, then she meets the diagnostic criteria of preeclampsia ( Figure 1 ). In terms of severity, preeclampsia could be classified as “nonsevere” or “severe” types ( Table 2 ), with the latter group exhibiting clinical features including blood pressure exceeding 160/100 mmHg, headache, visual disturbances, upper abdominal pain, oliguria, elevated serum creatinine, thrombocytopenia (<100,000/µL), elevated level of liver enzymes, fetal growth restriction, pulmonary edema, onset at an early gestational age, and the presence of convulsion (eclampsia) [ 7 ].
Classification of preeclampsia according to severity.
Although the definite cause of preeclampsia remains unknown to date, several hypotheses have been made to explain its pathophysiology. One of the most commonly accepted theories is the two-stage model, which proposes that inadequate trophoblast invasion would lead to shallow placentation and subsequent poor uteroplacental perfusion (stage I), thus causing widespread endothelial dysfunction and systemic clinical manifestations (stage II) [ 8 ]. The window between the first and second stages provides an optimal opportunity for prediction during the subclinical phase [ 5 ]. Known as a safe and effective drug in the prevention of pregnancy-related vascular disorders including but not limited to preeclampsia, aspirin has been applied for preeclampsia prevention with a low dosage starting as early as before GA 16 weeks and until approximately GA 36 weeks [ 9 ]. Nevertheless, non-pregnant women throughout the world enjoy the privilege of early prevention and intervention. Even if they do, sometimes preeclampsia may still develop. The only definite solution for preeclampsia is the delivery or termination of pregnancy. When a diagnosis is made, antihypertensive medication is, however, one of the most important treatments before delivery. Fluid control, prevention, and treatment for end organ damage should be applied as well [ 10 ].
Due to the notable prevalence and influence of preeclampsia in pregnancy, an understanding of preeclampsia, as thorough as possible, is crucial. The review aimed to summarize existing studies in the literature to explore the epidemiology, etiology (risk factors), socioeconomic burdens, pathophysiologic mechanisms, prediction, prevention, and treatment of preeclampsia. The cutting-edge studies will be analyzed and integrated into this review to provide state-of-the-art knowledge.
2. Materials and Methods
Searching terms and strategies in the literature.
The literature was searched to identify basic and clinical studies, which investigated the epidemiology, etiology (risk factors), socioeconomic burdens, and underlying pathophysiological mechanisms of preeclampsia, along with its prediction, prevention, and treatment. Figure 2 illustrates the flowchart of database searching, screening, and inclusion of the references that we selected from the literature. In this review, all of the articles were retrieved from the databases Medline and PubMed using the search terms “preeclampsia”, “gestational hypertension”, and “pregnancy induced hypertension” for the research topic. For screening and selection in the next stage, only full-text articles were considered for inclusion in further analysis. In the second stage, the articles published before 1983 were excluded to ensure the novelty of the current review. Duplicated articles were also excluded. From a total of 152 articles identified in the screening process, 126 potential articles (1983–2022) met the criteria for inclusion.

Flowchart of database searching, screening, and inclusion of the references selected from the literature.
Hereafter, two experts in the field independently inspected the contents of articles including demographics, research designs, and outcomes, and identified eligible basic and clinical studies for inclusion. The solicited articles with poor research designs, questionable sampling methods, or mismatched outcomes would be excluded at this stage. The discrepancies between the experts were discussed via mutual communication to reach a consensus. All eligible studies were included in the review using the search terms and strategies (identification from the database, screening of the studies, selection of potential articles, and final inclusion). Finally, a total of 103 articles were collected for review from 152 articles identified in the initial search.
3. Epidemiology, Etiology (Risk Factors), and Economic Burden of Preeclampsia
3.1. epidemiology and risk factors.
Preeclampsia is a gestational disorder affecting women worldwide from different nations, ethnicities, age groups, etc. Overall, a prevalence rate of approximately 2 to 15% of pregnant women is documented, with an average prevalence rate of approximately 4.6% [ 2 , 3 , 11 ]. The pathophysiology of preeclampsia is complex and remains incompletely unveiled, which makes it sensible that its prevalence and traits would vary under different circumstances. In other words, different populations with preeclampsia may display different prevalence, patterns, or distribution of risk factors and pregnancy outcomes. The study of its epidemiology and risk factors could thereby demonstrate its complexity and heterogeneity. Table 3 presents a list of risk factors for preeclampsia.
A brief summary of risk factors for preeclampsia.
3.2. Race and Ethnicity
In a cross-sectional study conducted by Yang et al., a thorough comparison between the characteristics of preeclampsia among the Chinese and Swedish populations was made. The study included a total of 634,689 pregnancies, among which the Chinese and the Swedish exhibited similar prevalence rates of approximately 2 to 3%. However, there were marked variabilities in the other descriptive results. The maternal age, mean body mass index (BMI), and obesity rates were higher in Sweden, while more nulliparous women and cesarean deliveries were identified in their Chinese counterparts. The disease extent and pregnancy outcomes also differed. Mild preeclampsia was more common in the Swedish population, while there were more severe cases in China. The Chinese also had overall higher rates of stillbirth, preterm birth, and low birth weight. Ethnicity, lifestyle, metabolic perturbations, genetic factors, and seeking medical help may all contribute to these variabilities [ 11 ]. While race is a potential contributory factor, it may not be completely persuasive in this scenario since the Swedish comprised relatively richer ethnicities whereas the Chinese were primarily Hans.
The role of race in preeclampsia has been investigated in various studies. A review article written by Zhang et al. pointed out that African Americans had a higher rate and severity of preeclampsia, which was likely related to, if not directly resulting from, multifactorial causes including previous history of preeclampsia, system lupus erythematosus, sickle cell anemia, gestational diabetes mellitus, and a history of chronic hypertension [ 12 ]. Another study conducted by Ghosh et al. suggested that non-Hispanic women had higher odds of developing preeclampsia and had greater severity of disease, compared with Hispanic women and Asian/Pacific Islanders. An expert review written by Johnson also mentioned a higher risk of preeclampsia among Black, Native American, and Native Alaskan races. Nevertheless, it is worth noting that research focusing on the role of race or ethnicity in the disparities of preeclampsia shares some common limitations. Firstly, race or ethnicity is not a scientifically biological or genetic trait; rather, it is more often self-reported and thus may become subjective. Secondly, a person could belong to more than one racial or ethnic group instead of being assigned to one single category. Thirdly, a standardized method of classification used in medical research may fail to reflect the cultural, lingual, or historical origins and distributions in reality. Instead of serving as a direct or independent factor, the role that race or ethnicity plays in preeclampsia may correspond to the reflection or marker for the influence of cultural, socioeconomic, or healthcare resources, etc. [ 13 ].
Many real-world statistics suggest that advanced or extremely young maternal age is an important risk factor for preeclampsia. Furthermore, these mothers at risk may also face more adverse maternal and neonatal outcomes and hence should raise special concerns throughout their pregnancy courses.
A cohort study that included preeclamptic individuals from 1998–2014 in the U.S. suggested that women at extreme ages (<25 years or >45 years) tended to develop severe morbidities. Women younger than 25 years of age had a significantly higher rate of developing eclampsia. On the other hand, women more than 45 years old were more likely to suffer from acute heart failure and acute kidney injury (acute renal failure). The results suggested that both extremely young and elderly mothers were exposed to a greater threat during gestation but possibly from different perspectives [ 14 ].
Some studies focused mainly on the advanced maternal age (AMA) groups. A registry-based study in Finland suggested that women older than 35 years exhibited more preeclampsia, early and late preterm deliveries, cesarean deliveries, and poorer neonatal outcomes [ 15 ]. In a retrospective cross-sectional study in Indonesia conducted in 2016-2017, preeclamptic women over 35 years of age developed more severe complications in general, with postpartum hemorrhage in particular, while no significant increase in the developments of HELLP syndrome, visual disturbances, pulmonary edema, or eclampsia was identified. Regarding neonatal outcomes, there were more preterm deliveries (GA < 37 weeks), intrauterine growth restrictions, neonatal asphyxia, and neonatal infections in the group of advanced-age women [ 16 ].
3.4. Parity
Nulliparity has long been classified as a risk factor, as it may triple the risk for preeclampsia [ 17 ]. Some studies have concluded that nulliparous women were found with a higher percentage of preeclampsia compared to other cohorts [ 18 ]. Many hypotheses attribute this to immunological reasons. A feasible explanation is that a suboptimal maternal adaptation to fetal or paternal alloantigens may indirectly result in impaired uteroplacental perfusion, which accounts for the pathogenesis of preeclampsia [ 19 ]. Nulliparous women were also proposed to endure an “angiogenic imbalance”, manifested as a higher circulating sFlt1 level and sFlt1/PIGF ratio, which may also contribute to their tendency of developing preeclampsia [ 20 ]. From a fundamental viewpoint, meanwhile, there may be little difference between nulliparous and multiparous women regarding other risk factors including AMA, diabetes mellitus, multifetal gestations, etc. [ 21 ].
3.5. Obesity
Obesity is an alarming issue in the modern world and has proven to confer many hazards to human health. With time, it has raised concerns in both developed and developing countries. As one of the leading attributable risk factors, the mechanism of how obesity could potentially lead to preeclampsia has been studied. Obesity is known to be associated with systemic inflammatory reactions, insulin resistance, and oxidative stress. The pathways through which obesity could result in hypertensive disorder include increased oxidative stress, increased sympathetic tone, and increased expression of angiotensinogen [ 22 ]. Insulin resistance, on the other hand, is linked to reduced cytotrophoblast migration and consequent placental ischemia [ 23 ].
For pregnant women, maternal obesity, maternal overweight, and even a BMI increase within the normal range may indicate an increased risk of maternal and fetal morbidities, including preeclampsia. Accordingly, obese or overweight pregnant women should be advised to lose weight through diet control, a moderate amount of physical activity, and lifestyle modification.
3.6. Other Maternal Conditions
One of the most important risk factors for preeclampsia is a history of preeclampsia in a previous pregnancy. Women with preeclampsia in their first pregnancies have a notably higher risk of developing it again in their second pregnancies. Many cohort studies have demonstrated this phenomenon. [ref. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies]. Aside from her own medical history, a woman’s family history of preeclampsia should warrant special concerns as well, as a positive family history is also a powerful indicator of preeclampsia at all stages of the pregnancy course [ 24 , 25 ].
Multifetal gestation is associated with a three- to four-fold increased risk for preeclampsia. This may be more related to the gestation itself because multifetal gestation imposes a greater burden on the cardiovascular system. Individuals with multifetal gestations are frequently excluded from general studies, as they are often viewed as a special population. For these women who are diagnosed with preeclampsia, it may be confusing whether some of the adverse outcomes such as preterm deliveries are related to preeclampsia or multifetal gestation per se. Nevertheless, the fact that women with multifetal gestations are more prone to preeclampsia and serious complications should raise more attention, and allow an opportunity for screening, prevention, or early intervention [ 26 ].
Preeclamptic patients with pre-pregnancy chronic hypertension are classified to have “superimposed preeclampsia”. Chronic hypertension accounts for approximately 4% of pregnancies and is often associated with adverse gestational outcomes such as preeclampsia, preterm delivery, intrauterine fetal growth restriction, and placenta abruption. Approximately 20% of these patients eventually develop preeclampsia and tend to do so even earlier than the normotensive population. Poorer pregnancy outcomes are observed concurrently [ 27 ].
Both pre-existing type I and type II diabetes mellitus have been shown to possess a higher risk of preeclampsia. Statistically, 10–20% of diabetic women develop preeclampsia during pregnancy, which is a significantly higher percentage compared to their non-diabetic counterparts. On the other hand, gestational diabetes mellitus (GDM) is also considered to be an independent risk factor for preeclampsia by some researchers, while more investigations are required to determine whether GDM and preeclampsia share a common etiologic pathway [ 28 ].
While preeclamptic patients have an increased long-term risk of developing an end-stage renal disease, the renal disease itself may also serve as a risk factor for preeclampsia. To be more specific, microalbuminuria, diabetic nephropathy, and chronic kidney disease may predispose to preeclampsia [ 29 ]. This may be related to impaired glycocalyx integrity and alterations in the complement and renin-angiotensin-aldosterone systems [ 30 ].
Other than the aforementioned risk factors, mostly involving but not limited to pre-pregnancy health conditions, there are numerous other risk factors that could be mentioned. Aside from pre-existing hypertension, diabetes mellitus, and renal disease, particular medical conditions including autoimmune disease, periodontal disease, and antidepressant exposure have all been proven to play a part in this “disease of theories”. [ 31 , 32 , 33 ].
3.7. Socioeconomic Burden
Preeclampsia is one of the top causes of adverse maternal and fetal outcomes globally, and hence a short-term special medical care program is often required to take care of preeclamptic patients. This makes it not only a health-related issue but also a socioeconomic one, as greater manpower or resource consumption and extra spending in the healthcare system would be inevitable. Yet, few studies have aimed to make estimates of the potentially enormous socioeconomic burden related to preeclampsia. Relevant studies have made limited conclusions to date.
Jing Hao et al. conducted a retrospective study to investigate the economic burden of preeclampsia using data from the United States. Three cohorts were defined in this study: Women who had uncomplicated pregnancies until term, women with hypertension but not preeclampsia, and women diagnosed with preeclampsia. The maternal and infant costs were estimated from GA 20 weeks until 6 weeks postpartum for the former and 12 months post-delivery for the latter. The mean care cost of the preeclamptic group was $41,790 USD, which was significantly higher than the uncomplicated group ($13,187 USD) and the group with hypertension but without preeclampsia ($24,182 USD). The cost difference was largely dependent on the infant costs [ 34 ].
Another retrospective study focused on a similar issue in the United States was conducted by Warren et al. The maternal costs were estimated from 6 months before birth and 12 months afterward, while the infant costs were calculated until 12 months of age. The results suggested an estimated increased cost of $6583 USD per birth in the maternal model. On the other hand, the increased cost of the infant model was substantially influenced by the gestational age at birth. Costs devoted to the infant accounted for 26% of total healthcare costs at term delivery, and a tremendously increased percentage of 91% with deliveries at GA < 28 weeks. As a result of preeclampsia and preterm deliveries, these high costs were more closely related to adverse fetal or infant outcomes, including intraventricular hemorrhage, bronchopulmonary dysplasia, periventricular leukomalacia, and infant death [ 35 ].
An Irish study using data from the SCOPE (Screening for Pregnancy End Points) study disclosed a doubling average cost (5243 EUR) in preeclampsia-complicated pregnancies. The study included data from the initial antepartum visit until 12 months postpartum and drew the conclusion that these costs were primarily related to postpartum care, followed by antepartum and peripartum care, respectively. The increased medical costs were related to more and higher-level health services including antepartum examinations, more maternal hospitalization spending, longer infantile NICU stays, etc. [ 36 ].
Despite the lack of abundant research, present economic studies on preeclampsia in different parts of the world seem to have reached the consensus that preeclamptic pregnancies are strongly associated with significantly higher medical costs for both the mother and her baby. The maternal costs are related to the extra utility of the healthcare system, more resources used during hospitalization, and likely more surgical spending due to an elevated rate of cesarean deliveries. The infant costs also contribute to a large percentage of the expenses as the babies are prone to preterm deliveries and relevant or causative adverse events. Although studies cannot reflect the accurate amount of preeclampsia-related healthcare costs in reality, there is no doubt that preeclampsia imposes a considerable financial burden on our societies. It is important for healthcare providers and policy-makers to recognize this phenomenon and allocate enough economic budgets, medical, and social resources accordingly.
4. Pathophysiology of Preeclampsia
4.1. brief summary.
Preeclampsia has been termed a “disease of theories” by some as numerous studies have aimed to propose different concepts to explore its complex etiology and pathophysiology. Previous findings have suggested that the triggers of preeclampsia include placental factors and other predisposing maternal factors. The mechanisms of early- and late-onset preeclampsia may not be completely the same. Based on the current understanding of preeclampsia, the revised “two-stage model” has become one of the most widely accepted theories regarding its formation.
The classical two-stage model was first described in 1991, innovatively introducing the idea that preeclampsia should be viewed as a trophoblastic disease rather than merely a hypertensive disorder. In this model, the first stage of preeclampsia is described as the “placental stage”, in which deficient remodeling of spiral arteries results in impaired placental perfusion and placental ischemia. As the disease progresses with time and clinical maternal syndrome develops, it reaches the second stage [ 37 ]. The clinical manifestations of preeclampsia will be further discussed in the next section.
Ever since the initial proposal of the two-stage theory, ongoing research has expanded and refined our knowledge of the development of preeclampsia. Stage 1 is focused on the revised idea of impaired uteroplacental perfusion with or without poor placentation and subsequent spiral artery insufficiency. Stage 2 surrounds the concept that general endothelial dysfunction and vascular inflammation would lead to a systemic clinical response. Figure 3 displays the contributing factors and the two-stage models of preeclampsia.

Contributing factors and the two-stage models of preeclampsia.
4.2. Stage 1
In the current revised model, stage 1 is initiated when reduced placental perfusion develops. Poor placentation and the resultant deficient spiral artery remodeling in the intervillous space is one of the causes for this phase but may not be the sole mechanism. In addition to the impairment of placental perfusion, maternal factors are essential so as to result in the development of systemic maternal pathophysiological changes [ 37 ]. Stage 1 usually occurs in the first trimester, at the period of time when the deep invasion of the extravillous trophoblast (EVT) takes place. The migration of EVT cells into the decidua leads to the remodeling of maternal spiral arteries, which is a key element to uteroplacental perfusion and fetal blood supply. The process may initiate as early as before GA 8 weeks, while the establishment of uteroplacental circulation is completed at approximately GA 12 weeks. Hence, it is believed that stage 1 takes place before GA 12 to 20 weeks [ 38 ].
The differentiation and invasion of EVTs are regulated by various factors including cytokines, growth factors, chemokines, cell adhesion molecules, placental oxygen tension, extracellular matrix (ECM)-degrading enzymes, and membrane-bound cell surface peptidases. These factors are either directly or indirectly related to the differentiation and decidual invasion of EVT cells and may serve as markers for the first stage of preeclampsia formation [ 38 ]. When defective trophoblast invasion and insufficient transformation of the maternal uterine vasculature emerge, decreased maternal uterine blood flow follows, which may be detected and quantified by uterine artery Doppler studies. Persistent high vessel resistance in early pregnancy may suggest that the aforementioned phenomenon has occurred. Existing studies have demonstrated that the placental endothelial cells in women with high-resistance uterine arteries are more sensitive to TNFα and thus are more susceptible to cell injury and apoptosis.
In the normal process of trophoblast invasion, the resistance of uterine artery blood flow decreases, and the uterine artery blood flow increases in the term. The placenta is typically developed in the first trimester. If a relatively hypoxic environment is noted, the latter placental tissues may exhibit an altered balance of antioxidant enzyme activity. However, the histopathology findings of the placenta are nonspecific and not limited to preeclamptic pregnancies. These changes to the placenta can also be induced by other microscopic insults or toxins [ 39 ].
4.3. Stage 2
Stage 2 features the scenario where impaired uteroplacental perfusion interacts with other various maternal constitutional factors. Pathophysiological changes in the liver, kidney, and cardiovascular system are compatible with the concept of insufficient blood supply. Systemic endothelial dysfunction and injury are possible explanations for the maternal clinical manifestations and have been proven to be present in preeclamptic women.
One important issue that attracts interest is how the first stage links to or leads to the second stage. The clinical value of discovering the answer lies in the fact that it may shed light on a way to “prevent” the formation of preeclampsia, which will be further discussed in the article. One proposal suggests that microparticle particles produced during syncytiotrophoblast apoptosis may directly or indirectly result in endothelial dysfunction. An increased amount and concentration of inflammatory cells and substances have been found in women with preeclampsia, and they could potentially alter the systemic endothelial function. The renin-angiotensin system may also play a role in the process. In addition, some recent findings have suggested that vascular endothelial growth factor (VEGF) and placental growth factor (sFlt-1) could be involved in the linkage as well. Moreover, oxidative stress accumulated during the process may provide another possible explanation [ 40 ]. Table 4 lists the possible pathways and explanations of mechanisms underlying preeclampsia.
Possible pathways and explanations of mechanisms underlying preeclampsia.
Various factors contribute to the regulation of artery compliance during pregnancy. A failure of maternal vascular adaptation can cause hypertensive disorders such as preeclampsia. Some circulating cytokines and growth factors at abnormal levels may inhibit normal calcium signaling events, thereby damaging cell-to-cell contacts of the endothelium and leading to endothelial dysfunction. Important markers include endothelin-1 (ET-1), interleukin-8 (IL-8), ELAM, and the endothelial leukocyte adhesion molecule-1 [ 41 ]. There is also sound evidence of decreased production or bioavailability of nitric oxide (NO)—a stimulant of smooth muscle relaxation—in preeclamptic pregnancies [ 42 ]. Other potential influential vasodilators include prostacyclin (PGI2) and the endothelium-derived hyperpolarizing factor (EDHF) [ 41 ].
As mentioned above, the dysregulation of the renin-angiotensin system (RAS) may participate in the pathogenesis of preeclampsia. In 2007, Florian Herse et al. published a study that included preeclamptic and non-preeclamptic women who had undergone cesarean deliveries. Genetic characteristics and histopathological results of the maternal and placental tissues of the participants were investigated. A 4-fold increase in the angiotensin II type 1 (AT1) receptor in the decidua was found in preeclamptic pregnancies. Increases in corresponding gene and protein expression were also confirmed by RT-PCR and immunohistochemistry studies. Circulating agonistic autoantibodies (AAs) targeting the AT1 receptor have been described previously, with the ability to cross the placenta and enter fetal circulation. AT1-AAs could induce calcium signaling and initiate events that would later lead to preeclampsia [ 43 ]. Roxanna A. Irani et al. published a study with similar findings in 2010. Animal experiments showed that pregnant mice with AT1-AA injections developed preeclamptic features and also had increased levels of antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1) and endoglin. Additionally, AT1-AA might be associated with increased TNF-α, indirectly causing damage to the endothelium and end organs [ 44 ].
Oxidative stress describes the imbalance between the formation of oxidative reactive species (ROS) and the antioxidant capacity of the body [ 45 ]. A causal role of oxidative stress in hypertension has been demonstrated in previous research, with multiple possible pathogenic pathways including the alteration of NO bioavailability or signaling. A reduction of oxidative stress has been observed in hypertensive cases who received antihypertensive treatment [ 46 ]. Oxidative stress in the healthy placenta may be important for its organogenesis, but excess levels in the impaired placenta would lead to increased circulating placenta debris, damaging the maternal endothelial cells in the term. As a major source of ROS production, the mitochondria have been found to be swollen in the trophoblasts of preeclamptic animal models, which plays a crucial role in cell apoptosis. Altogether, any errors in the maintenance of the oxygen pressure may bring about placental diseases and maternal complications, such as preeclampsia [ 46 ].
4.4. Limitations of the Placenta Model
Even though the two-stage theory is the mainstream explanation of the origin of preeclampsia, some argue that further evaluation is needed to determine the causative relation between trophoblast development and spiral artery transformation. For instance, previous case reports have pointed out similar findings of the uterine artery Doppler waveforms in extra-uterine pregnancies, suggesting that the resistance of uterine artery blood flow may not accurately reflect the consequences of trophoblast invasion [ 47 ].
Some have suggested that the result of Doppler studies may be a reflection of systemic vascular resistance changes but not on the uterine artery itself. The argument is based on the paradox that a “de-transformation” of spiral arteries does not occur when the vascular resistance of the uterine artery is noted in the third trimester [ 48 ]. In the meantime, while it is fairly certain that impaired uteroplacental perfusion is associated with subsequent endothelial dysfunction, almost all the supporting evidence of different hypotheses of its linkage raises some challenges. To date, it is believed that many potential mechanisms underlie preeclampsia, and the disease is caused by complicated interactions between maternal and environmental factors, and potentially more than that. The incomplete understanding of its pathogenesis continues to provoke further research.
5. Systemic Manifestations of Preeclampsia
Preeclampsia is a systemic disorder that may present with various symptoms and signs. The manifestation of preeclampsia is widely perceived to be centered around hypertension and proteinuria, but clinical presentations could be variable in essence. Different organs and systems could all be influenced by preeclampsia. Systemic manifestations of preeclampsia are shown in Figure 4 .

Systemic manifestations of preeclampsia.
According to the two-stage theory, preeclampsia proceeds into the clinical stage once the systemic vascular response and inflammation have taken place as a result of endothelial dysfunction. This aptly explains why preeclampsia is a global syndrome as the endothelium is distributed all over the body. The most famous affected organs and systems include the central nervous system, cardiovascular system, liver, and kidney.
5.1. Central Nervous System (CNS)
The brain is a vital organ that requires approximately 20% of available oxygen to maintain normal function. In most physiological conditions, cerebral blood flow (CBF) has sufficient capability to autoregulate and remains rather stable to cope with its high metabolic demand. However, once brain injury to a certain extent occurs, sequelae including acute severe hypertension, the loss of myogenic tone of the vascular smooth muscle, and uncontrolled vasoconstriction may lead to the failure of autoregulation. As they key to the delicate homeostasis of the brain environment, both hypoperfusion and hyperperfusion may break the balance and bring great harm. Insufficient CBF could lead to ischemic brain injury and ischemic stroke. Hyperperfusion, on the other hand, may disrupt the blood–brain barrier (BBB) and cause edema formation, which is one of the classic findings in preeclamptic and eclamptic patients [ 49 , 50 ].
CBF could be assessed in patients with preeclampsia via transcranial Doppler imaging. The middle cerebral artery is often chosen to be the target of examinations. Perhaps somewhat surprisingly, the cerebral flow index (CFI) appears to be normal in most women with preeclampsia. However, cerebral perfusion pressure (CPP) exhibits greater elevation in preeclamptic women and may serve as the key to brain injury among these women [ 51 ]. Brain damage has been demonstrated in autopsies and image studies [ 52 ]. It has been proven in some studies that elevated CPP corresponds to hypertension, and antihypertensive treatment that decreases CPP lowers the rate of cerebral complications in these patients [ 53 ].
CNS manifestations that are suggestive of severe disease status are headaches, visual disturbances, changes in consciousness, and seizures. The spectrum is coined “preeclamptic encephalopathy” [ 53 ]. Once a seizure takes place, the impression of eclampsia is almost certain after the exclusion of other previously known neurological conditions that may also lead to convulsion events. Eclampsia is one of the most serious forms of preeclampsia and is highly related to obstetric morbidities and mortalities. Management for eclampsia is similar to those for any form of severe preeclampsia, and most patients recover well without neurological sequelae [ 54 ].
Another frequent neurological finding is posterior reversible encephalopathy syndrome (PRES). PRES is a result of hypoxia and vasogenic edema of the brain that is often related to acute uncontrolled hypertension or systemic endothelial dysfunction. The syndrome progresses in a rather rapid manner but also resolves rapidly with a good prognosis once the trigger is withdrawn [ 55 ]. PRES is high among pregnant women with severe preeclampsia or eclampsia, and usually indicates a better prognosis than PRES in non-pregnant women or is associated with other causes [ 56 ].
Stroke—or a cerebrovascular accident—refers to a brain attack when impairment of part of the CBF or a burst in a brain blood vessel occurs. Strongly related to hypertensive disorders, cerebrovascular events are yet another complication that is significantly linked to preeclampsia. Although uncommon in pregnancy, it shares similar disease pathways and risk factors with strokes that take place in non-pregnant patients, and thus is indicative of an increased long-term risk for stroke events [ 57 ].
5.2. Cardiovascular Systems
Preeclampsia has been classified as one independent gender-specific risk factor for cardiovascular events by the American Heart Association (AHA). Studies have proven that women with gestational hypertensive disorders carry a 2- to 4-fold risk for cardiovascular diseases [ 58 ]. In fact, preeclampsia and cardiovascular diseases share many predisposing factors such as elevated blood pressure and increased BMI. The disease spectrum includes coronary heart disease, heart failure, and cardiovascular disease death, and the influences may be life-long [ 59 ].
The long-term cardiovascular sequelae not only affect the mother but have also proven to bring hazards to her children at the same time. Although the establishment of a dependency relationship is difficult, many studies have shown an increased rate of congenital heart disease and future cardiovascular morbidities for offspring. Some scholars believe, however, that the influences of cardiovascular risks on offspring are limited to term infants or cases with early preeclampsia [ 60 ].
The complex multifactorial nature of preeclampsia and cardiovascular diseases makes it hard to make a straightforward ascription of the latter to the former. There is also a lack of a standardized protocol for cardiovascular prevention. Nevertheless, medical staff and preeclamptic patients should keep in mind the importance of continuous screening and early intervention of cardiovascular diseases. Monitoring of the body weight, blood pressure, lipid level, and lifestyle should be performed every five years until the age of 50 when women would qualify for most other international regular cardiovascular risk assessment guidelines [ 61 ].
Preeclampsia-related liver disease is frequently seen in the third trimester. Liver involvement is rare but indicative of severe disease extent. The most notorious example is H(Hemolysis)EL(Elevated Liver Enzymes)LP(Low Platelet Count) syndrome, which is a variant of severe preeclampsia. According to the diagnostic criteria of Tennessee Classification and Mississippi Classification, an elevated liver enzyme is usually defined by an elevated AST or ALT ≥ 70 U/L, although blood tests often reveal a level ≥ 500 U/L. Thrombotic microangiopathy serves as one of the possible explanations, while periportal hemorrhage and necrosis have been observed in histopathology studies. As rare as it may be, the condition could lead to hepatic rupture [ 62 ]. Women treated with corticosteroids exhibit overall improved laboratory results including liver function tests. Administration after delivery helps to avoid a rebound and further complications. Nevertheless, the natural course of HELLP could not be altered by corticosteroids [ 63 ].
Other liver diseases associated with preeclampsia include acute fatty liver of pregnancy (AFLP), hepatic infarction, and rupture. In cases of AFLP, laboratory abnormalities include elevated liver enzymes, prolonged prothrombin time and partial thromboplastin time, and increased bilirubin levels. Other typical clinical symptoms comprise central nervous system involvements such as headache and consciousness disturbances, jaundice, and gastrointestinal symptoms including anorexia, abdominal discomfort, nausea, and vomiting. If the expression of long-chain 3-hydroxyacyl-CoA dehydrogenase is not evident, the prognosis is usually good [ 64 ].
Hepatic complications in pregnancy are rare but could be fatal. They are more likely to be found in preeclamptic or eclamptic cases and indicate severe disease status. Hence, prompt termination of pregnancy or delivery is often indicated. Liver transplantation may be considered in patients with a grave prognosis [ 63 ].
5.4. Kidney
The imbalance of the renin-angiotensin aldosterone system (RAAS) along with the imbalance between proangiogenic and anti-angiogenic factors may explain the relationship between preeclampsia and renal impairment. Similar to cardiovascular risks, preeclampsia shares common predisposing factors with renal risks and confers a higher risk of chronic kidney diseases later in life [ 65 ].
The activation of RAAS is normal during pregnancy, which results in a volume increase. However, excessive activation possibly related to sFlt-1 and AT1-AAs—as may be seen in preeclamptic subjects—could lead to preeclampsia or preeclampsia-like syndrome. Once the delicate balance is disrupted, hypertension and renal involvements may be seen [ 66 ]. Thrombotic microangiopathies in renal cells have been observed in histopathology studies, suggesting glomerular injury in preeclamptic patients [ 67 ].
When acute kidney injury occurs, an abrupt increase in serum creatinine and a decrease in urine output could be detected. However, both the glomerular filtration rate and serum creatinine level are not perfectly reliable markers during pregnancy, as physiological changes allow an increase in the former and a reduction in the latter. The diagnosis may rely on other clinical manifestations such as oliguria, proteinuria, and edema, and is thereby delayed in some cases [ 68 ].
The condition may be life threatening, but also tends to regress rapidly in the postpartum period. Nonetheless, it still warrants concern for screening for later renal diseases. There is a significant association between preeclampsia—the early-onset subgroup in particular—and future chronic kidney diseases, hypertensive diseases, and glomerular or proteinuric diseases. For preeclamptic women, a 10- to 12-fold increase in end-stage renal disease has been proposed in existing statistical analyses. Hence, further screening for kidney diseases years after pregnancy should be implemented [ 69 ].
5.5. Other Targets
Preeclampsia is a global disorder that may present with symptoms and pathologic findings all over the body. Aside from the vital organs, for example, the hematologic system is another commonly affected target. In a study conducted by Neelam Jhajharia et al., lower hemoglobin and platelets were found in these patients, while higher WBC and hematocrit were observed [ 70 ]. Similar findings could also be found in other studies [ 71 , 72 ]. Some parameters may vary from study to study, but a trend of decreased hemoglobin and platelet levels is almost always observed in data analyses. Marked thrombocytopenia signifies a severe disease form, as manifested in HELLP syndrome.
Gastrointestinal involvements are common in preeclamptic patients. Symptoms of nausea and vomiting are frequently experienced by them, and some women complain of indigestion. The more devastating complications include hepatic involvement as described earlier, and pancreatic involvement, namely referring to the increased risk of pancreatitis and necrosis of the pancreas [ 73 ].
Another classic clinical manifestation of preeclampsia is edema. It is worth noting that edema is not essential to the diagnosis of preeclampsia and is often observed in normal pregnancies as a result of the increase in body fluids. General swelling due to water and salt retention is especially prominent in preeclampsia due to elevated blood pressure and endothelial injury causing extravasation from the vessels. As rare as it may be, one of the most severe presentations of fluid overload is pulmonary edema, which has been reported in cases of severe preeclampsia [ 74 ].
6. Prediction and Prevention of Preeclampsia
The potential consequences of preeclampsia pose great threat and harm to mothers and their children, the medical system, and society worldwide. To prevent adverse outcomes, various strategies have been invented and studied, including diet control, exercise, and medication. Among them, the administration of low-dose aspirin has been proven to be one of the most effective ways to prevent the development and progression of preeclampsia.
In order to apply preventive methods in a cost-effective manner, a precise prediction model and timing would be required. According to the two-stage theory of its pathophysiology, the first stage of preeclampsia typically takes place in the first trimester, when inadequate trophoblast invasion leads to abnormal placentation and subsequent uteroplacental insufficiency. During this process, the patient is usually in her subclinical phase, which allows a window for screening and prevention.
6.1. Prediction Models
Apparently, a good prediction tool would provide many benefits to the early prevention and intervention of preeclampsia. Different professional organizations have thus far proposed their own prediction models based on the currently acknowledged risk factors.
An expert review written by Piya Chaemsaithong et al. made a detailed comparison between some of the most widely accepted prediction models ( Table 5 ). Risk factors such as a history of preeclampsia in previous pregnancies, chronic hypertension, autoimmune diseases, renal diseases, diabetes mellitus, and multifetal gestation are included in almost all the prediction models and are primarily considered to be “high” risk factors if the models made a segmentation between “high” risk and “moderate” risk factors. Other risk factors that are taken into consideration include nulliparity, advanced maternal age, maternal obesity, and family history, among others. Some are classified as “moderate” risk factors. A previous medical record and chronic hypertension are considered to be the two most important contributory risk factors [ 5 ].
A comparison between different prediction models of preeclampsia.
Most prediction models have either low detection rates or high false-positive rates, however, and are insufficient for precise prediction. An alternative is to use the Bayes theorem and take the individual maternal history and characteristics into consideration. This competing model allows a more patient-specific and dynamic approach and is used by the Fetal Medicine Foundation (FMF) and is the only one that has undergone extensive internal and external validations. In addition to the checklist for risk factors, other maternal factors including the mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and serum pregnancy-associated plasma protein A (PAPP-A) are also taken into account [ 5 , 75 ]. The best timing of preeclampsia risk screening is around GA 11 to 13 weeks. As soon as the result is revealed, early prevention could be initiated if a high risk for preeclampsia is suspected [ 76 ].
A systematic review examined the performance of soluble fms-like tyrosine kinase-1 (sFlt-1), the placental growth factor (PlGF), and the sFlt-1/PlGF ratio in predicting adverse outcomes in women with preeclampsia. The literature search identified 33 eligible studies (n = 9426). Due to significant heterogeneity between studies, few studies (n = 4–8) were included in the final meta-analysis component. Nonetheless, both PlGF and the sFlt-1/PlGF ratio demonstrated areas ROC values between 0.68 and 0.87 for the prediction of composite adverse maternal and perinatal outcomes, preterm birth, and fetal growth restriction. Conclusively, PlGF and the sFlt-1/PlGF ratio show prognostic promise for adverse outcomes in preeclampsia, but study heterogeneity limits their clinical utility [ 77 ].
Currently, prediction models for gestational hypertension and preeclampsia have been developed with data and assumptions from developed countries. A review aimed to identify and assess the methodological quality of prediction models for gestational hypertension and pre-eclampsia with reference to their application in low-resource settings. The review retrieved 40 eligible articles and revealed 77% of all the prediction models’ combined biomarkers with maternal clinical characteristics. The biomarkers used as predictors in most models were PAPP-A and PlGF. Only five studies were conducted in low- and middle-income countries. Therefore, the review concluded that prediction models using maternal characteristics, with good discrimination and calibration, should be externally validated for use in low- and middle-income countries where biomarker assays are not routinely available [ 78 ].
6.2. Possible Preventative Measures
An article written by Sammya Bezerra Maia and Holanda Moura et al. offered a perspective on the potential preventative measures by classifying them as primary, secondary, or tertiary preventions. Primary prevention is unlikely to be successful since the concept is centered around the avoidance of pregnancy in high-risk populations and lifestyle modification in the whole population in order to decrease the incidence of preeclampsia. Secondary prevention focuses on the interruption of the pathogenic process before its development and is the main target of investigations. Tertiary prevention does not aim to prevent preeclampsia itself, but rather prevent its further complications. Aside from the aforementioned section on aspirin prevention, lifestyle management, nutritional supplementation, and antenatal surveillance may aid primary and secondary prevention. Accordingly, rest, exercise, diet modification such as a low-salt diet, and antioxidant use have all been suggested. Unfortunately, none of them have been proven to be effective [ 79 ]. In contrast, another systematic review solicited 28 RCTs studying the effects of various factors such as anticoagulants (heparin, enoxaparin, dalteparin, and nadroparin), aspirin, paravastatin, nitric oxide, yoga, micronutrients such as L-arginine, folic acid, vitamin E and C, phytonutrients, lycopene, and vitamin D alone or in combination with calcium. The results of this review showed that low-molecular-weight heparin, enoxaparin, yoga, L-arginine, folic acid, and vitamin D prevented preeclampsia alone or combined with calcium [ 80 ].
6.3. Low-Dose Aspirin
In fact, for women perceived as high-risk individuals for developing preeclampsia, the administration of low-dose aspirin on a daily basis since early pregnancy has proven to be the most effective way to prevent preeclampsia.
Aspirin is one of the oldest medications still in use to date. It is widely applied as an antithrombotic drug due to its effect on platelet inactivation. The mechanism of platelet inactivation relies on COX-1 inhibition, which blocks TXA2 synthesis. It is usually administered in a low dose (75–81 mg/day), which is sufficient for TXA2 but not PGI2 inhibition. The application of aspirin is primarily related to secondary prevention for cardiovascular diseases, while its use in primary prevention remains somewhat controversial. Another common use of aspirin is anticoagulant treatment in neurological diseases such as transient ischemic attack (TIA) or stroke. A recent history or increased risk for gastrointestinal bleeding, intracerebral bleeding, and other adverse events is worth noting, and aspirin should only be administered when the benefits outweigh the risks—primarily related to the increased bleeding tendency [ 76 ].
Ever since the first publication of a case report suggesting the role of aspirin in preeclampsia prevention in 1978, numerous studies have aimed to quantify the effects of aspirin on preventing preeclampsia but without a consensus owing to the heterogeneity of the study groups. Meanwhile, meta-analyses have suggested prophylactic aspirin use, which is best started before GA 16 weeks and ahead of the completion of placentation. Later, the ASPRE trial confirmed the effect of aspirin on early-onset preeclampsia. With good compliance, aspirin prophylaxis could reach 76–90% effect size [ 79 ].
A meta-analysis including a total of 18,907 participants in eight trials reported that the administration of aspirin was associated with a reduction in the risk of preterm preeclampsia (relative risk: 0.62; 95% confidence interval: 0.45–0.87), but there was no significant effect on term preeclampsia (relative risk: 0.92; 95% confidence interval: 0.70–1.21). The reduction in preterm preeclampsia was confined to the subgroup in which aspirin was initiated at ≤16 weeks of gestation and at a daily dose of ≥100 mg (relative risk: 0.33; 95% confidence interval: 0.19–0.57). Thus, aspirin can reduce the risk of preterm preeclampsia rather than term preeclampsia, and only when it is initiated at ≤16 weeks of gestation and at a daily dose of ≥100 mg [ 81 ].
Many organizations have proposed their own guidelines regarding the dosage and timing of aspirin use. Table 6 presents the current recommendations for the administration of low-dose aspirin in women at risk of future preeclampsia. While some differences exist, most agree on a daily dosage between 60 and 150 mg per day, and some have a precise dosage of 81 mg per day for low-dose aspirin tablets in the U.S. Administration is recommended late in the first trimester and could be initiated as early as GA 12 weeks and before GA 16 weeks. Low-dose aspirin is usually prescribed until the late preterm period. Bleeding disorders are uncommon, while some women might experience a certain degree of gastrointestinal discomfort [ 79 , 82 ].
Recommendations for administration of low-dose aspirin in women at risk of future preeclampsia.
Low-dose aspirin use for preeclampsia prevention has been proven to be cost-effective and safe in pregnancy. Therefore, when the prediction model suggests a high risk of preeclampsia or when a mother carries some risk factors, low-dose aspirin should be initiated if no contraindications are identified.
7. Management of Preeclampsia
Table 7 is a summary of the current management practices of preeclampsia.
A summary of current management practices of preeclampsia.
7.1. Antihypertensive Treatment
Elevated blood pressure is essential to the diagnosis of preeclampsia and is associated with increased cardiac, vascular, and neurological risks. Therefore, antihypertensive medication should be administered for the control of blood pressure.
Some studies divide gestational hypertension into “severe” and “non-severe” groups, typically setting 160 mmHg as the cutoff point for systemic blood pressure (SBP). While the choice of antihypertensive regimen may be designed in an individualized manner, most clinicians agree with the initiation of antihypertensive therapy when the SBP exceeds 140 or 160 mmHg or when the diastolic blood pressure (DBP) reaches above 100 mmHg. The treatment target also differs between different guidelines; some set no specific treatment target, while some target an SBP below 110 mmHg or a DBP less than 80 to 90 mmHg [ 83 ].
The majority of common antihypertensive medications are contraindicated during pregnancy, so the choice of drugs is rather limited. Currently, almost all the approved medications belong to class C, including labetalol, hydralazine, nifedipine, methyldopa (class B), diazoxide, and the relatively contraindicated nitroprusside. The former three are used more frequently even though the FDA has not approved the usage of nifedipine in hypertension management [ 83 ].
7.1.1. Labetalol
Labetalol could be administered in the intravenous or oral form, while the intravenous form is more often used in hypertensive emergencies or grave conditions. It is a combined alpha- and beta-adrenoceptor-blocking agent with more potency on the beta receptor. It is widely used as an antihypertensive agent and has the advantage of exerting a minimal effect on heart rate and cardiac output. Side effects and adverse events are primarily related to the influence on the RAAS and respiratory system. Therefore, other alternatives should be contemplated for patients with asthma [ 84 ].
When given in preeclamptic pregnancies, labetalol also decreases proteinuria and perinatal deaths. No other antihypertensive medications have proven to produce similar effects [ 85 ]. In non-severe cases, the oral dosage of 200 to 1200 mg per day can be divided into two to three doses, depending on the individual condition [ 83 ]. On the other hand, an intravenous bolus of 20 mg labetalol is indicated in severe cases and could be followed by a double dose in ten minutes [ 86 ].
7.1.2. Hydralazine
Hydralazine is another popular medication of choice, which also comes in intravenous and oral forms similar to labetalol. It lowers blood pressure by acting as a direct arteriole vasodilator. Headache, flushing, chest discomfort, and gastrointestinal upset have been reported with hydralazine use. It is also known to be associated with drug-induced lupus syndrome [ 87 ]. However, drug toxicity is uncommon. The drug is fairly safe except for contraindication in women with coronary artery disease since the increased cardiac output and oxygen demand may be hazardous [ 87 ]. Its effects have been proven, but it may be less efficacious than other antihypertensive drugs such as labetalol and nifedipine and is perceived by some as a second-line choice instead of a first-line option [ 85 ]. In regular oral use for blood pressure control, the recommended regimen is to start with 10 mg four times per day with gradual adjustment. The maintenance dosage could be as much as 50 mg four times per day and still has room for titration as long as it does not exceed the daily maximum dosage of 300 mg [ 87 ].
7.1.3. Nifedipine
Nifedipine is a safe and effective oral drug for lowering blood pressure in preeclamptic patients. The advantages include its relatively low cost and wider accessibility [ 88 ]. This medication functions as a calcium channel blocker with a rapid vasodilating effect but is associated with few adverse events and a low risk of hypotension [ 89 ]. Headache, flushing, and palpitations are the most frequent complaints encountered [ 90 ]. Its simultaneous relaxing effect on the myometrium also makes it a tocolytic drug commonly used to avoid preterm delivery [ 90 ]. It is usually initiated with a 10 mg dose and could be repeated later [ 86 ].
7.2. Magnesium Sulfate
Magnesium sulfate is used extensively for the prevention of seizures in preeclampsia and recurrent seizures in eclampsia for a lengthy period of time. Compared to placebo and other anticonvulsants such as phenytoin and diazepam, magnesium sulfate has proven to be more effective with fewer side effects. Although the mechanism is not fully understood, several possible explanations have been proposed [ 91 ].
Since the twentieth century, the action of magnesium sulfate used in preeclampsia has been studied. It is less likely related to antihypertensive effects as eclampsia does not necessarily take place in a hypertensive condition. Instead, magnesium sulfate may function as a calcium antagonist and inhibit acetylcholine-calcium-dependent release. While calcium may induce vasospasm and activate smooth muscle constriction, magnesium works in the opposite fashion. Since cerebral vasospasm is a common finding in preeclamptic seizures, this may serve as a plausible explanation to justify the use of magnesium sulfate. Another hypothesis suggests magnesium functions as a blocker of NMDA receptors, and thus prevents calcium influx [ 92 , 93 ].
In women with severe preeclampsia or eclampsia, magnesium could be given with an initial loading dose via the intravenous or intramuscular route, followed by a maintenance infusion. To reach the therapeutic serum concentration of 3.5 to 7 mEq/L (4.2 to 8.4 mg/dL), the recommended loading dose is 6 g intramuscularly, 2 to 4 g intravenously with a rate of 1 g/min, or a combination of both. Different guidelines and studies may suggest a slight modification. Some minor side effects such as a warm sensation, flushing, nausea, and vomiting may be encountered within the therapeutic window. However, serious adverse events may occur if marked hypermagnesemia is noted. The loss of the normal patellar reflex may be seen when the serum concentration of magnesium reaches 8 to 10 mEq/L, and more devastating respiratory depression could result when the serum concentration of magnesium reaches or exceeds 13 mEq/L. Therefore, persistent monitoring of the neurological performance such as the presence of the patellar reflex, respiratory pattern, and urine output should be implemented to avoid magnesium toxicity and severe adverse events. If a dosing error or toxicity is noted, calcium gluconate could be administered as an antidote [ 94 ].
Overall, magnesium sulfate use in pregnant women is still considered to be safe as long as close surveillance is performed. Its effects on seizure prevention have been assuring, and associated morbidity and mortality rates are low.
7.3. Delivery and Termination of Pregnancy
Preeclampsia is a pregnancy-specific condition, which would require delivery or termination of pregnancy under certain circumstances. A dilemma between expectant management and delivery is sometimes faced, especially when the patient has not reached term pregnancy. Hence, the timing of delivery in preeclamptic patients has raised keen discussions.
Lucy C Chappell et al. conducted a randomized clinical trial on late-preterm preeclamptic patients to survey this issue. A total of 901 gravidas with preeclampsia from GA 34 weeks to less than GA 37 weeks were included and randomly allocated to expectant management or planned delivery evenly. Planned delivery was initiated within 48 h of randomization to allow corticosteroid use if needed, and labor induction was prioritized unless an indication for cesarean delivery existed. The maternal outcomes included severe hypertension, deficits, impairments of different organs, placenta abruption, and maternal death. The perinatal outcomes were a composite of NICU stays, neonatal deaths, and further neurological developments. The statistical findings suggested a significantly lower rate of maternal morbidities and mortalities but more adverse perinatal outcomes in the planned delivery group. The higher rate of adverse perinatal outcomes, however, was primarily related to NICU admissions due to preterm birth, and other neonatal outcomes were similar to those in the expectant group otherwise. The total maternal and neonatal medical costs were lower in the planned delivery group. Collectively, the results suggested planned delivery in women with late-preterm preeclampsia, but the risk of increased NICU admission, although not associated with further morbidities, should be informed and discussed with the patient [ 95 ].
A systematic review in 2017 solicited six articles regarding preterm preeclampsia and the timing of delivery. The subjects of discussion included both early-preterm and late-preterm women. The statistics suggested postponing delivery until GA 37 weeks for better fetal outcomes, and no severe maternal complications or fetal distress existed. However, delivery should be considered even with early preterm patients once severe maternal complications or impaired fetal well-being was noted. It is important to note that suggested delivery is not equivalent to an emergency delivery and should still allow a 24-h interval for preparation (e.g., corticosteroid use to promote fetal lung maturity) since an immediate delivery is often associated with greater risks. On the other hand, women who chose expectant management should receive close monitoring and medication if needed. For example, magnesium sulfate could be used to reduce the risk of eclampsia, and more recently, has been suggested as a neuroprotective medication for the fetus before GA 32 weeks in particular [ 96 ].
According to the most updated ACOG guidelines in 2018, the choice between expectant management and prompt delivery should be made based on gestational age, maternal condition, and fetal well-being. Those with reassuring antenatal testing, no signs of preterm labor, and no severe disease features make good candidates for expectant management until GA 37 weeks. There are no benefits to delaying delivery afterward. Conversely, those with potentially severe features and a worrisome fetal condition should be advised for immediate delivery. For patients with severe preeclampsia, early delivery indicates a trade-off between fetal benefits and maternal risks, and a thorough discussion between the medical team and the patient should be performed. Delivery should be considered at any time when the maternal or fetal condition deteriorates or becomes unstable regardless of the gestational age. A complete course of corticosteroid administration is not always necessary, especially when the woman has reached late preterm (GA ≥ 34 weeks) [ 97 ].
7.4. Fluid Management
Fluid management is important in women with preeclampsia because they are more prone to fluid overload, which could lead to pulmonary edema. However, research on an ideal fluid strategy has been limited to date. Present data fail to suggest an optimal regimen, in turn provoking future research [ 98 ]. Nevertheless, intravenous medication with fluids is almost inevitable for hospitalized patients and should therefore be administered with caution. For instance, some clinicians have recently suggested avoiding intravenous fluid preloads before epidural or spinal anesthesia [ 98 ].
7.5. Diet Management
Fl Diet and nutrient intake may impact the risks of preeclampsia, and some studies have aimed to seek the best policy for diet management. In 2022, BMJ published a review based on data from 2000 to 2021 regarding the effects of dietary factors, nutritional supplements, and maternal weight on preeclampsia. Some of the findings may be contradictory to public belief; for instance, a low-salt diet to prevent hypertension and antioxidant (e.g., Vit. C and E) supplements to relieve oxidative stress seem to be plausible ways to prevent preeclampsia, but the review fails to show enough evidence in reality. However, it is important to note that a low amount or lack of evidence does not imply that they are not helpful and should not be recommended. In fact, the dietary factors that have proven to reduce the risk of preeclampsia include maternal weight control, high fiber intake, probiotics use, calcium and vitamin D supplements, multivitamin and multimineral supplements, and the avoidance of a high-salt diet and raw food [ 99 ].
A cohort study conducted by Anum S. Minhas et al. suggested that a self-reported Mediterranean-style diet is associated with lower preeclampsia risks. A Mediterranean-style diet is rich in vegetables, fruits, and healthy fats, of which the coherence could be assessed with a food-frequency questionnaire, in which individuals answer the questions by recalling their eating habits regarding meat, seafood, vegetables, beans, fruits, oil, wine, sweetened beverages, and commercially baked foods. It has been previously proven to lower cardiovascular risks in the non-pregnant population, and this study further suggested that greater adherence is associated with >20% lower odds of developing preeclampsia in the pregnant population compared to women with less adherence [ 100 ].
7.6. Exercise
Many clinical and animal studies have identified significant or non-significant effects of exercise during pregnancy on the reduction of gestational hypertensive disorders including preeclampsia.
There are a few possible explanations in so for the results observed. To begin with, maternal exercise creates a transient hypoxic environment, which in turn promotes the compensatory proliferation of the trophoblastic, endothelial, and stromal cells of the placenta, and consequently leads to improved placentation. Secondly, exercise stimulates antioxidant pathways and increases the number of mitochondria, relieving much of the oxidative stress that is linked to preeclampsia. Thirdly, exercise has an anti-inflammatory effect, which allows the body to maintain a healthy immune reaction, thus reducing the abnormal immune response to the fetus that is witnessed in preeclamptic patients [ 101 ].
A review study in 2017 suggested that aerobic exercise is beneficial in pregnancy and should be encouraged. Whether or not aerobic exercise could reduce preeclampsia remained controversial in some studies, but overall, the review concluded that 30 to 60 min aerobic exercise two to seven times per week during pregnancy reduced the incidence of gestational hypertensive disorders and the rate of cesarean deliveries [ 102 ]. However, this may not be the perfect regimen for every pregnant woman. The pre-pregnancy physical activity levels and maternal condition should always be taken into consideration for physicians to offer the best advice on the frequency, intensity, type, and time of exercise [ 103 ].
7.7. Long-Term Follow Up
Last but not least, preeclampsia is a syndrome that develops before delivery, yet also demands extra healthcare in the long run. Long-term follow-up for potential complications is indicated since sequelae of the cardiovascular system, liver, and kidney could take place. Close surveillance for years is suggested, which requires alertness from a good medical team and good medical compliance from the patient herself.
8. Discussion
The true cellular and molecular mechanisms underlying preeclampsia remain largely unexplained, which are assumed to be a two-stage process of impaired uteroplacental perfusion with or without prior defective trophoblast invasion (stage 1), followed by general endothelial dysfunction and vascular inflammation that lead to systemic organ damages (stage 2). Although the causes of preeclampsia are multi-factorial and cannot be described in a simple way, the aforementioned theories may provide a reasonable explanation for the results observed in past studies. Nevertheless, the detailed etiology, pathophysiology, and effect of preeclampsia seem complicated and remain to be clarified.
As mentioned above, overweight including pre-pregnancy obesity and excessive weight gain during pregnancy predisposes women to the progression of preeclampsia. As a state of chronic inflammation, overweight will increase the risk of preeclampsia by means of activating macrophages, NK cells, and peripheral helper T cells within the placenta to produce inflammatory cytokines such as IL-6, IL-7, and TNF-α. Established on these findings and reasons, avoiding excessive weight gain before and during pregnancy, rather than merely using overweight as a predictor, may be the best strategy to prevent the occurrence of preeclampsia. Therefore, proper weight control for pregnant females can not only decrease the physical burden on the body but also reduce the risk of preeclampsia.
Well-recognized risk factors for preeclampsia include race, advanced maternal age, obesity, nulliparity, multi-fetal pregnancy, and co-existing medical disorders. These factors can serve as warnings or markers to label pregnant women who need enhanced surveillance of maternal and fetal well-being. For at-risk females, appropriate information, counseling, and suggestions should be provided to facilitate a timely intervention or specialty referral. For pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance including a Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test can be arranged. If the results are unfavorable, early intervention and aggressive therapy should be considered. Moreover, affected females should have access to higher levels of obstetric units and neonatal institutes. Before, during, and after delivery, monitoring and preparation should be intensified for affected gravidas to avoid serious complications of preeclampsia. In response to the potential physiological effect and psychological impact, consultation and discussion are usually beneficial for females diagnosed with preeclampsia. For severe cases, delivery of the fetus and placenta is the ultimate solution to treat preeclampsia. However, the determination of the appropriate timing for delivery depends on the severity of maternal preeclampsia and the maturity of the fetus.
It remains not fully understood with regard to the molecular level and pathologic mechanism of preeclampsia and its associated treatment. More studies are still required to investigate the role of anticoagulant therapy (such as aspirin, as described above) in preeclampsia. To minimize the heterogeneity of research in the future, the standardization of several critical factors in preeclampsia and the related treatment should be carefully considered. Two of the important factors are the timing and intensity of screening and intervention, which have a remarkable impact on the therapeutic effects. Furthermore, the severity and outcome in the individuals diagnosed with preeclampsia need standardization. Moreover, a larger sample size is also required to draw a reliable conclusion and to improve the reproducibility of the study result.
9. Conclusions
Preeclampsia accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies. It is life-threatening for both the mother and the fetus, in turn, increasing the rate of mortality and morbidity.
Preeclamptic pregnancies are strongly associated with significantly higher medical costs. The maternal costs are related to the extra utility of the healthcare system, more resources used during hospitalization, and likely more surgical spending due to an elevated rate of cesarean deliveries. The infant costs also contribute to a large percentage of the expenses as the babies are prone to preterm deliveries and relevant or causative adverse events. Preeclampsia imposes a considerable financial burden on our societies. It is important for healthcare providers and policy-makers to recognize this phenomenon and allocate enough economic budgets and medical and social resources accordingly.
The true cellular and molecular mechanisms underlying preeclampsia remain largely unexplained, which are assumed to be a two-stage process of impaired uteroplacental perfusion with or without prior defective trophoblast invasion (stage 1), followed by general endothelial dysfunction and vascular inflammation that lead to systemic organ damages (stage 2).
Risk factors for preeclampsia, including race, advanced maternal age, obesity, nulliparity, multi-fetal pregnancy, and co-existing medical disorders, can serve as warnings or markers that call for enhanced surveillance of maternal and fetal well-being. Doppler ultrasonography and biomarkers including the mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and serum pregnancy-associated plasma protein A (PAPP-A) can be used for the prediction of preeclampsia. For women perceived as high-risk individuals for developing preeclampsia, the administration of low-dose aspirin on a daily basis since early pregnancy has proven to be the most effective way to prevent preeclampsia. For preeclamptic females, relevant information, counseling, and suggestions should be provided to facilitate timely intervention or specialty referral. In pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance including a Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test can be arranged. If the results are unfavorable, early intervention and aggressive therapy should be considered. Affected females should have access to higher levels of obstetric units and neonatal institutes. Before, during, and after delivery, monitoring and preparation should be intensified for affected gravidas to avoid serious complications of preeclampsia. In severe cases, delivery of the fetus and placenta is the ultimate solution to treat preeclampsia.
Although the aforementioned theories may provide a reasonable explanation for the results observed in the past studies, the detailed etiology, pathophysiology, and effect of preeclampsia seem complicated, and further research to address the primary etiology and pathophysiology underlying the clinical manifestations and outcomes is warranted.
Funding Statement
This review and APC were funded by a grant from Taipei Tzu-Chi Hospital, Taiwan (TCRD-TPE-111-10) for K.-H.C. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in writing the manuscript, or in deciding to publish the results.
Author Contributions
K.-J.C., K.-M.S. and K.-H.C. conceived the review and designed the search methods for the literature; K.-J.C. and K.-H.C. collected the data in the literature; K.-J.C., K.-M.S. and K.-H.C. performed data analyses; K.-J.C. and K.-H.C. wrote the review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Pregnancy-Induced hypertension
Affiliations.
- 1 Unit of Reproductive Endocrinology and Unit of Human Reproduction, First Department of Obstetrics and Gynecology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
- 2 Third Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
- PMID: 26158653
- DOI: 10.14310/horm.2002.1582
Pregnancy-induced hypertension (PIH) complicates 6-10% of pregnancies. It is defined as systolic blood pressure (SBP) >140 mmHg and diastolic blood pressure (DBP) >90 mmHg. It is classified as mild (SBP 140-149 and DBP 90-99 mmHg), moderate (SBP 150-159 and DBP 100-109 mmHg) and severe (SBP ≥ 160 and DBP ≥ 110 mmHg). PIH refers to one of four conditions: a) pre-existing hypertension, b) gestational hypertension and preeclampsia (PE), c) pre-existing hypertension plus superimposed gestational hypertension with proteinuria and d) unclassifiable hypertension. PIH is a major cause of maternal, fetal and newborn morbidity and mortality. Women with PIH are at a greater risk of abruptio placentae, cerebrovascular events, organ failure and disseminated intravascular coagulation. Fetuses of these mothers are at greater risk of intrauterine growth retardation, prematurity and intrauterine death. Ambulatory blood pressure monitoring over a period of 24 h seems to have a role in predicting deterioration from gestational hypertension to PE. Antiplatelet drugs have moderate benefits when used for prevention of PE. Treatment of PIH depends on blood pressure levels, gestational age, presence of symptoms and associated risk factors. Non-drug management is recommended when SBP ranges between 140-149 mmHg or DBP between 90-99 mmHg. Blood pressure thresholds for drug management in pregnancy vary between different health organizations. According to 2013 ESH/ESC guidelines, antihypertensive treatment is recommended in pregnancy when blood pressure levels are ≥ 150/95 mmHg. Initiation of antihypertensive treatment at values ≥ 140/90 mmHg is recommended in women with a) gestational hypertension, with or without proteinuria, b) pre-existing hypertension with the superimposition of gestational hypertension or c) hypertension with asymptomatic organ damage or symptoms at any time during pregnancy. Methyldopa is the drug of choice in pregnancy. Atenolol and metoprolol appear to be safe and effective in late pregnancy, while labetalol has an efficacy comparable to methyldopa. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II antagonists are contraindicated in pregnancy due to their association with increased risk of fetopathy.
Publication types
- Antihypertensive Agents / therapeutic use*
- Blood Pressure / physiology
- Blood Pressure Monitoring, Ambulatory
- Gestational Age
- Hypertension, Pregnancy-Induced / diagnosis*
- Hypertension, Pregnancy-Induced / drug therapy
- Hypertension, Pregnancy-Induced / therapy*
- Risk Factors
- Antihypertensive Agents

IMAGES
VIDEO
COMMENTS
The prevalence of hypertension in reproductive-aged women is estimated to be 7.7%. 1 Hypertensive disorders of pregnancy, an umbrella term that includes preexisting and gestational hypertension, preeclampsia, and eclampsia, complicate up to 10% of pregnancies and represent a significant cause of maternal and perinatal morbidity and mortality. 2 ...
The recent American College of Cardiology/AHA task force guidelines lowered the threshold for the diagnosis of hypertension in nonpregnant patients to 130/80 mm Hg for stage 1 hypertension and to 140/90 mm Hg for stage 2 hypertension, resulting in larger numbers of individuals being diagnosed and treated. 6 There is robust evidence in the ...
The Pregnancy-Induced Hypertension Essay. Pregnancy-induced hypertension (PIH) is among the major causes of maternal mortality and a significant contributor to maternal and perinatal morbidity. Preeclampsia is characterized by hypertension that develops throughout pregnancy and disappears after birth, suggesting that the placenta is a critical ...
Gestational hypertension is blood pressure greater than or equal to 140/90 that begins during the latter half of pregnancy (typically after 20 weeks). During pregnancy, high blood pressure can affect your body in different ways than it normally would. If high blood pressure goes unmanaged, both you and the fetus are at risk for complications.
Abstract. Pregnancy-induced hypertension (PIH), which includes both gestational hypertension and preeclampsia, is a common and morbid pregnancy complication for which the pathogenesis remains unclear. Emerging evidence suggests that insulin resistance, which has been linked to essential hypertension, may play a role in PIH.
Transient gestational hypertension is not a benign disorder; it is associated with ≈20% chance of developing preeclampsia and a further 20% chance of developing gestational hypertension. Therefore, such women should receive extra monitoring throughout their pregnancy, ideally including home BP measurements.
Pregnancy-induced hypertension (PIH) is estimated to affect 7% to 10% of all pregnancies in the United States. 1-4 Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PIH are unclear. Hypertension associated with preeclampsia develops during pregnancy and remits after ...
1 INTRODUCTION. Pregnancy-induced hypertension (PIH) is regarded as a multi-system disorder that is defined as persistent hypertension of more than 140/90 mmHg during the gestational period. 1, 2 Based on the presence of edema, proteinuria, seizures, and other related symptoms, PIH can be classified into four distinct categories: preeclampsia (PE)/eclampsia, gestational hypertension (GH ...
Hypertension in pregnancy is a common condition, affecting about 10% of pregnant women. This includes women with chronic hypertension—which may be diagnosed before pregnancy or in the early stages of pregnancy (<20 weeks' gestation)—and women with hypertension related to pregnancy (gestational hypertension and pre-eclampsia) (see box 1).If not identified and treated, hypertension can ...
8. Consequences of Hypertension in Pregnancy. Hypertension in pregnancy is a major cause of maternal morbidity and mortality in the United States. There is approximately one maternal death due to preeclampsia-eclampsia per 100,000 live births, with a case-fatality rate of 6.4 deaths per 10,000 cases [79, 80]. The outcome of hypertension in ...
Pregnancy Induced Hypertension. A woman with pregnancy induced hypertension (PIH) experiences high blood pressure and protein deposits are found in her urine. In most cases, this condition occurs after twenty weeks of pregnancy and is common among first time mothers, teenagers and old mothers above forty years and who have had multiple pregnancies.
INTRODUCTION. Pregnancy Induced Hypertension (PIH) is one of the commonest causes of both maternal 1 as well as neonatal mortality 2 and morbidity that develops as a result of pregnancy and generally regresses after delivery, affecting about 5-8 % of pregnant women, moreover 5-22% of pregnancies especially in developing countries. 3. The mortality is closely associated with the severity of ...
e22 February 2022 Hypertension. 2022;79:e21-e41. DOI: 10.1161/HYP.0000000000000208 Garovic et al Hypertension in Pregnancy: Diagnosis, BP Goals, and Pharmacotherapy Association (AHA) Hypertension Clinical Practice Guide-lines, the threshold for the diagnosis of stage 1 hyper-tension was further lowered to 130/80 from 140/90
Pregnancy-induced hypertension is different from other types of hypertensions because it affects women only, occurs after 20 weeks of pregnancy, and there are no proteins in the urine. However, when not treated, it progresses into a disease called preeclampsia, which causes urine to appear in urine. ... Need an essay on Complication of ...
During the gestation period, various complications in pregnant women are reported including hypertension, gestational diabetes mellitus (GDM), obesity, dyslipidemia, infections, and stillbirth [11, 12].Among them hypertensive disorders during pregnancy remain the major cause of maternal-fetal morbidity and mortality both in developing as well as in developed countries, affecting up to 10 % of ...
Pregnancy-Induced Hypertension - Science topic. A condition in pregnant women with elevated systolic (>140 mm Hg) and diastolic (>90 mm Hg) blood pressure on at least two occasions 6 h apart ...
Pregnancy-induced hypertension is a severe pregnancy complication, increasing risk of long-term cardiovascular disease in mothers and offspring. We hypothesized that maternal blood pressure in pregna ... Search for more papers by this author , Ralf Dechend. Ralf Dechend. From the experimental and Clinical Research Center, a joint cooperation ...
The definitions of gestational hypertension (pregnancy-induced hypertension) and preeclampsia are shown in Figure 1. Serious or long-term complications may result when preeclampsia turns into a severe type or is left without being sufficiently treated. Multiorgan involvement may be seen in such cases, and the impairment of uteroplacental ...
Pregnancy-induced hypertension (PIH) complicates 6-10% of pregnancies. It is defined as systolic blood pressure (SBP) >140 mmHg and diastolic blood pressure (DBP) >90 mmHg. It is classified as mild (SBP 140-149 and DBP 90-99 mmHg), moderate (SBP 150-159 and DBP 100-109 mmHg) and severe (SBP ≥ …
High blood pressure, also called hypertension, is very common. In the United States, high blood pressure happens in 1 in every 12 to 17 pregnancies among women ages 20 to 44. 3. High blood pressure in pregnancy has become more common. However, with good blood pressure control, you and your baby are more likely to stay healthy.
Pregnancy Induced Hypertension (PIH) is a multi-organ disease process that develops as a result of pregnancy and regresses in the postpartum period. It usually develops after 20 weeks of gestation in a woman who had normal blood pressure. It is defined as an elevation of systolic and diastolic pressures equal to or above 140/90 mm Hg.
901-909. The population-based study by Birukov et al in this issue of Hypertension 1 described maternal blood pressure patterns during pregnancy in >2400 women, 10% of whom developed a hypertensive disorder of pregnancy (HDP; gestational hypertension or preeclampsia). The authors then examined associations between these patterns and offspring ...
Introduction. New-onset hypertension is common during pregnancy, with gestational hypertension affecting an estimated 3% and preeclampsia 2% to 3% of pregnancies in the United States. 1 Although both conditions are characterized by new-onset high blood pressure late in gestation, the development of systemic organ dysfunction during preeclampsia 2 is associated with significant maternal and ...