- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History

... and beyond
- Socratic Meta
- Featured Answers

How can I balance synthesis reactions?

Related questions
- Question #dc389
- Question #a1081
- Question #19eb4
- Question #bc744
- How do you know when a decomposition reaction is complete?
- Question #c5c0c
- How do decomposition reactions differ from synthesis reactions?
- Question #b986f
- Why are synthesis reactions particularly important in the body?
- Why are dehydration synthesis reactions important?
Impact of this question

If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
High school chemistry
Course: high school chemistry > unit 3, balancing chemical equations.
- Balancing more complex chemical equations
- Visually understanding balancing chemical equations
- Balancing another combustion reaction
- Apply: balancing equations
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
4.1 Writing and Balancing Chemical Equations
Learning objectives.
By the end of this section, you will be able to:
- Derive chemical equations from narrative descriptions of chemical reactions.
- Write and balance chemical equations in molecular, total ionic, and net ionic formats.
An earlier chapter of this text introduced the use of element symbols to represent individual atoms. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation . Consider as an example the reaction between one methane molecule (CH 4 ) and two diatomic oxygen molecules (O 2 ) to produce one carbon dioxide molecule (CO 2 ) and two water molecules (H 2 O). The chemical equation representing this process is provided in the upper half of Figure 4.2 , with space-filling molecular models shown in the lower half of the figure.
This example illustrates the fundamental aspects of any chemical equation:
- The substances undergoing reaction are called reactants , and their formulas are placed on the left side of the equation.
- The substances generated by the reaction are called products , and their formulas are placed on the right side of the equation.
- Plus signs (+) separate individual reactant and product formulas, and an arrow (⟶) (⟶) separates the reactant and product (left and right) sides of the equation.
- The relative numbers of reactant and product species are represented by coefficients (numbers placed immediately to the left of each formula). A coefficient of 1 is typically omitted.
It is common practice to use the smallest possible whole-number coefficients in a chemical equation, as is done in this example. Realize, however, that these coefficients represent the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios. Methane and oxygen react to yield carbon dioxide and water in a 1:2:1:2 ratio. This ratio is satisfied if the numbers of these molecules are, respectively, 1-2-1-2, or 2-4-2-4, or 3-6-3-6, and so on ( Figure 4.3 ). Likewise, these coefficients may be interpreted with regard to any amount (number) unit, and so this equation may be correctly read in many ways, including:
- One methane molecule and two oxygen molecules react to yield one carbon dioxide molecule and two water molecules.
- One dozen methane molecules and two dozen oxygen molecules react to yield one dozen carbon dioxide molecules and two dozen water molecules.
- One mole of methane molecules and 2 moles of oxygen molecules react to yield 1 mole of carbon dioxide molecules and 2 moles of water molecules.
Balancing Equations
The chemical equation described in section 4.1 is balanced , meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. It may be confirmed by simply summing the numbers of atoms on either side of the arrow and comparing these sums to ensure they are equal. Note that the number of atoms for a given element is calculated by multiplying the coefficient of any formula containing that element by the element’s subscript in the formula. If an element appears in more than one formula on a given side of the equation, the number of atoms represented in each must be computed and then added together. For example, both product species in the example reaction, CO 2 and H 2 O, contain the element oxygen, and so the number of oxygen atoms on the product side of the equation is
The equation for the reaction between methane and oxygen to yield carbon dioxide and water is confirmed to be balanced per this approach, as shown here:
A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. This process is represented qualitatively by an unbalanced chemical equation:
Comparing the number of H and O atoms on either side of this equation confirms its imbalance:
The numbers of H atoms on the reactant and product sides of the equation are equal, but the numbers of O atoms are not. To achieve balance, the coefficients of the equation may be changed as needed. Keep in mind, of course, that the formula subscripts define, in part, the identity of the substance, and so these cannot be changed without altering the qualitative meaning of the equation. For example, changing the reactant formula from H 2 O to H 2 O 2 would yield balance in the number of atoms, but doing so also changes the reactant’s identity (it’s now hydrogen peroxide and not water). The O atom balance may be achieved by changing the coefficient for H 2 O to 2.
The H atom balance was upset by this change, but it is easily reestablished by changing the coefficient for the H 2 product to 2.
These coefficients yield equal numbers of both H and O atoms on the reactant and product sides, and the balanced equation is, therefore:
Example 4.1
Balancing chemical equations.
Next, count the number of each type of atom present in the unbalanced equation.
Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. To balance the number of oxygen atoms, a reasonable first attempt would be to change the coefficients for the O 2 and N 2 O 5 to integers that will yield 10 O atoms (the least common multiple for the O atom subscripts in these two formulas).
The N atom balance has been upset by this change; it is restored by changing the coefficient for the reactant N 2 to 2.
The numbers of N and O atoms on either side of the equation are now equal, and so the equation is balanced.
Check Your Learning
It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. When balance is achieved, all the equation’s coefficients may then be multiplied by a whole number to convert the fractional coefficients to integers without upsetting the atom balance. For example, consider the reaction of ethane (C 2 H 6 ) with oxygen to yield H 2 O and CO 2 , represented by the unbalanced equation:
Following the usual inspection approach, one might first balance C and H atoms by changing the coefficients for the two product species, as shown:
This results in seven O atoms on the product side of the equation, an odd number—no integer coefficient can be used with the O 2 reactant to yield an odd number, so a fractional coefficient, 7 2 , 7 2 , is used instead to yield a provisional balanced equation:
A conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by 2:
Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients . Although the equation for the reaction between molecular nitrogen and molecular hydrogen to produce ammonia is, indeed, balanced,
the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. Dividing each coefficient by the greatest common factor, 3, gives the preferred equation:
Link to Learning
Use this interactive tutorial for additional practice balancing equations.
Additional Information in Chemical Equations
The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water ( aqueous solutions , as introduced in the preceding chapter). These notations are illustrated in the example equation here:
This equation represents the reaction that takes place when sodium metal is placed in water. The solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid in pure form, but readily dissolved in water).
Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equation’s arrow. For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta (Δ) over the arrow.
Other examples of these special conditions will be encountered in more depth in later chapters.
Equations for Ionic Reactions
Given the abundance of water on earth, it stands to reason that a great many chemical reactions take place in aqueous media. When ions are involved in these reactions, the chemical equations may be written with various levels of detail appropriate to their intended use. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. When aqueous solutions of CaCl 2 and AgNO 3 are mixed, a reaction takes place producing aqueous Ca(NO 3 ) 2 and solid AgCl:
This balanced equation, derived in the usual fashion, is called a molecular equation because it doesn’t explicitly represent the ionic species that are present in solution. When ionic compounds dissolve in water, they may dissociate into their constituent ions, which are subsequently dispersed homogenously throughout the resulting solution (a thorough discussion of this important process is provided in the chapter on solutions). Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case:
Unlike these three ionic compounds, AgCl does not dissolve in water to a significant extent, as signified by its physical state notation, s .
Explicitly representing all dissolved ions results in a complete ionic equation . In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions:
Examining this equation shows that two chemical species are present in identical form on both sides of the arrow, Ca 2+ ( aq ) and NO 3 − ( a q ) . NO 3 − ( a q ) . These spectator ions —ions whose presence is required to maintain charge neutrality—are neither chemically nor physically changed by the process, and so they may be eliminated from the equation to yield a more succinct representation called a net ionic equation :
Following the convention of using the smallest possible integers as coefficients, this equation is then written:
This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(I) ions, regardless of the source of these ions. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of Cl − and Ag + .
Example 4.2
Ionic and molecular equations.
Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction:
The two dissolved ionic compounds, NaOH and Na 2 CO 3 , can be represented as dissociated ions to yield the complete ionic equation:
Finally, identify the spectator ion(s), in this case Na + ( aq ), and remove it from each side of the equation to generate the net ionic equation:
Write balanced molecular, complete ionic, and net ionic equations for this process.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/4-1-writing-and-balancing-chemical-equations
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
How to Balance Chemical Equations
Last Updated: October 13, 2023 Fact Checked
This article was co-authored by Bess Ruff, MA . Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 4,941,570 times.
Taking a dive into the world of chemical equations? These problems can seem tricky at a glance, but they’re easy to figure out once you learn the basic steps and rules to balancing them. Not to worry; we’ll walk you through exactly how to figure out just about any problem, no matter how many atoms and molecules you're working with. Dealing with especially complex equations? We’ve got you covered there, too—scroll to section 2 for a handy tutorial on solving trickier equations with an algebraic balance.
Doing a Traditional Balance

- C 3 H 8 + O 2 --> H 2 O + CO 2
- This reaction occurs when propane (C 3 H 8 ) is burned in the presence of oxygen to produce water and carbon dioxide.

- For example, you have 3 oxygen atoms on the right side, but that total results from addition.
- Left side: 3 carbon (C3), 8 hydrogen (H8) and 2 oxygen (O2).
- Right side: 1 carbon (C), 2 hydrogen (H2) and 3 oxygen (O + O2).

- You'll need to recount your atoms before balancing the hydrogen and oxygen, as you'll likely need to use coefficients to balance the other atoms in the equation.

- C 3 H 8 + O 2 --> H 2 O + 3 CO 2
- The coefficient 3 in front of carbon on the right side indicates 3 carbon atoms just as the subscript 3 on the left side indicates 3 carbon atoms.
- In a chemical equation, you can change coefficients, but you must never alter the subscripts.

- C 3 H 8 + O 2 --> 4 H 2 O + 3CO 2
- On the right side, you now added a 4 as the coefficient because the subscript showed that you already had 2 hydrogen atoms.
- When you multiply the coefficient 4 times by the subscript 2, you end up with 8.

- Add a coefficient of 5 to the oxygen molecule on the left side of the equation. You now have 10 oxygen atoms on each side.

- The carbon, hydrogen, and oxygen atoms are balanced. Your equation is complete.
- The other 6 atoms of oxygen come from 3CO 2 .(3x2=6 atoms of oxygen+ the other 4=10)
Completing an Algebraic Balance
This method, also known as Bottomley's method, is especially useful for more complex reactions, although it does take a bit longer.

- PCl 5 + H 2 O --> H 3 PO 4 + HCl

- a PCl 5 + b H 2 O --> c H 3 PO 4 + d HCl

- On the left side, there are 2 b atoms of hydrogen (2 for every molecule of H 2 O), while on the right side, there are 3 c + d atoms of hydrogen (3 for every molecule of H 3 PO 4 and 1 for every molecule of HCl). Since the number of atoms of hydrogen has to be equal on both sides, 2 b must be equal to 3 c + d .
- Cl: 5 a = d
- H: 2 b =3 c + d

- To quickly do this, take one variable and assign a value to it. Let's make a = 1. Then start solving the system of equations to get the following values:
- Since P: a = c, we know that c = 1.
- Since Cl: 5a = d, we know that d = 5
- 2b = 3(1) + 5
- If the value you assigned returns fractional values, just multiply all values by the least common multiple (LCM) of the denominators to get rid of the fractions. If there is only one fraction, multiply all values by that values denominator.
- If the value you assigned returns coefficients that have a greatest common factor (GCF), simplify the chemical equation by dividing each value by the GCF.
Community Q&A
- Remember to simplify! If all of your coefficients can be divided by the same number, do so to get the simplest result. Thanks Helpful 46 Not Helpful 11
- If you're stuck, you can type the equation into the online balancer to balance it. Just remember that you won't have access to an online balancer when you're taking an exam, so don't become dependent on it. Thanks Helpful 55 Not Helpful 24
- To get rid of fractions, multiply the entire equation (both the left and right sides) by the number in the denominator of your fraction. Thanks Helpful 28 Not Helpful 11

- During the balancing process, you may use fractions to assist you, but the equation is not balanced as long as there are still coefficients using fractions. You never make half of a molecule or half of an atom in a chemical reaction. Thanks Helpful 26 Not Helpful 12
You Might Also Like

- ↑ https://www.khanacademy.org/science/ap-biology/chemistry-of-life/elements-of-life/a/matter-elements-atoms-article
- ↑ https://www.mathsisfun.com/algebra/add-subtract-balance.html
- ↑ https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/balancing-chemical-equations/v/balancing-chemical-equations-introduction
- ↑ https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Balancing_Redox_reactions
- ↑ https://www.mathsisfun.com/algebra/completing-square.html
About This Article

To balance a chemical equation, first write out your given formula with the reactants on the left of the arrow and the products on the right. For example, your equation should look something like "H2 + O2 → H2O." Count the number of atoms in each element on each side of the equation and list them under that side. For the equation H2 + O2 → H2O, there are 2 hydrogen atoms being added to 2 oxygen atoms on the left, so you would write "H=2" and "O=2" under the left side. There are 2 hydrogen atoms and 1 oxygen atom on the right, so you would write "H=2" and "O=1" under the right side. Since the number of atoms in each element isn't identical on both sides, the equation is not balanced. To balance the equation, you'll need to add coefficients to change the number of atoms on one side to match the other. For the equation H2 + O2 → H2O, you would add the coefficient 2 before H2O on the right side so that there are 2 oxygen atoms on each side of the equation, like H2 + O2 → 2H2O. However, subscripts can't be changed and are always multiplied by the coefficient, which means there are now 4 hydrogen atoms on the right side of the equation and only 2 hydrogen atoms on the left side. To balance this, add the coefficient 2 before H2 on the left side of the equation so there are 4 hydrogen atoms on each side, like 2H2 + O2 → 2H2O. Now the number of atoms in each element is the same on both sides of the equation, so the equation is balanced. Remember that if there's no coefficient in front of an element, it's assumed that the coefficient is 1. To learn how to balance chemical equations algebraically, scroll down! Did this summary help you? Yes No
- Send fan mail to authors

Reader Success Stories
Mar 11, 2022
Did this article help you?

E. M. Brown
Dec 3, 2016
Mar 23, 2022
Theresa Hill
Nov 21, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Get all the best how-tos!
Sign up for wikiHow's weekly email newsletter
How to Balance Chemical Equations
Jeffrey Coolidge/Getty Images
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A chemical equation is a written description of what happens in a chemical reaction. The starting materials, called reactants , are listed on the lefthand side of the equation. Next comes an arrow that indicates the direction of the reaction. The righthand side of the reaction lists the substances that are made, called products .
A balanced chemical equation tells you the amounts of reactants and products needed to satisfy the Law of Conservation of Mass. Basically, this means there are the same numbers of each type of atoms on the left side of the equation as there are on the right side of the equation. It sounds like it should be simple to balance equations , but it's a skill that takes practice. So, while you might feel like a dummy, you're not! Here's the process you follow, step by step, to balance equations. You can apply these same steps to balance any unbalanced chemical equation...
Easy Steps for Balancing Chemical Equations
Follow four easy steps to balance a chemical equation:
- Write the unbalanced equation to show the reactants and products.
- Write down how many atoms of each element there are on each side of the reaction arrow.
- Add coefficients (the numbers in front of the formulas) so the number of atoms of each element is the same on both sides of the equation. It's easiest to balance the hydrogen and oxygen atoms last.
- Indicate the state of matter of the reactants and products and check your work.
Write the Unbalanced Chemical Equation
The first step is to write down the unbalanced chemical equation. If you're lucky, this will be given to you. If you're told to balance a chemical equation and only given the names of the products and reactants, you'll need to either look them up or apply rules of naming compounds to determine their formulas.
Let's practice using a reaction from real life, the rusting of iron in the air. To write the reaction, you need to identify the reactants (iron and oxygen) and the products (rust). Next, write the unbalanced chemical equation:
Fe + O 2 → Fe 2 O 3
Note the reactants always go on the left side of the arrow. A "plus" sign separates them. Next, there is an arrow indicating the direction of the reaction (reactants become products). The products are always on the right side of the arrow. The order in which you write the reactants and products is not important.
Write Down Number of Atoms
The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow:
To do this, keep in mind a subscript indicates the number of atoms. For example, O 2 has 2 atoms of oxygen. There are 2 atoms of iron and 3 atoms of oxygen in Fe 2 O 3 . There is 1 atom in Fe. When there is no subscript, it means there is 1 atom.
On the reactant side:
On the product side:
How do you know the equation isn't already balanced? Because the number of atoms on each side isn't the same! Conservation of Mass states mass isn't created or destroyed in a chemical reaction, so you need to add coefficients in front of the chemical formulas to adjust the number of atoms so they will be the same on both sides.
Add Coefficients To Balance Mass in a Chemical Equation
When balancing equations, you never change subscripts . You add coefficients . Coefficients are whole number multipliers. If, for example, you write 2 H 2 O, that means you have 2 times the number of atoms in each water molecule, which would be 4 hydrogen atoms and 2 oxygen atoms. As with subscripts, you don't write the coefficient of "1", so if you don't see a coefficient, it means there is one molecule.
There is a strategy that will help you balance equations more quickly. It is called balancing by inspection . Basically, you look at how many atoms you have on each side of the equation and add coefficients to the molecules to balance out the number of atoms.
- Balance atoms present in a single molecule of reactant and product first.
- Balance any oxygen or hydrogen atoms last.
In the example:
Iron is present in one reactant and one product, so balance its atoms first. There is one atom of iron on the left and two on the right, so you might think putting 2 Fe on the left would work. While that would balance iron, you already know you're going to have to adjust oxygen, too, because it isn't balanced. By inspection (i.e., looking at it), you know you have to discard a coefficient of 2 for some higher number.
3 Fe doesn't work on the left because you can't put a coefficient in from of Fe 2 O 3 that would balance it.
4 Fe works, if you then add a coefficient of 2 in front of the rust (iron oxide) molecule, making it 2 Fe 2 O 3 . This gives you:
4 Fe + O 2 → 2 Fe 2 O 3
Iron is balanced, with 4 atoms of iron on each side of the equation. Next you need to balance oxygen.
Balance Oxygen and Hydrogen Atoms Last
This is the equation balanced for iron:
When balancing chemical equations, the last step is to add coefficients to oxygen and hydrogen atoms. The reason is that they usually appear in multiple reactants and products, so if you tackle them first you're usually making extra work for yourself.
Now, look at the equation (use inspection) to see which coefficient will work to balance oxygen. If you put a 2 in from of O 2 , that will give you 4 atoms of oxygen, but you have 6 atoms of oxygen in the product (coefficient of 2 multiplied by the subscript of 3). So, 2 does not work.
If you try 3 O 2 , then you have 6 oxygen atoms on the reactant side and also 6 oxygen atoms on the product side. This works! The balanced chemical equation is:
4 Fe + 3 O 2 → 2 Fe 2 O 3
Note: You could have written a balanced equation using multiples of the coefficients. For example, if you double all of the coefficients, you still have a balanced equation:
8 Fe + 6 O 2 → 4 Fe 2 O 3
However, chemists always write the simplest equation, so check your work to make sure you can't reduce your coefficients.
This is how you balance a simple chemical equation for mass. You may also need to balance equations for both mass and charge. Also, you may need to indicate the state of matter (solid, liquid, aqueous, gas) of reactants and products.
Balanced Equations with States of Matter (plus examples)
Step By Step Instructions for Balancing Oxidation-Reduction Equations
- Balancing Chemical Equations
- Balanced Equation Definition and Examples
- Examples of 10 Balanced Chemical Equations
- 5 Steps for Balancing Chemical Equations
- What Is a Chemical Reaction?
- What Is a Chemical Equation?
- Mole Ratio: Definition and Examples
- Mole Relations in Balanced Equations
- What Is a Word Equation in Chemistry?
- Balancing Equations Test Questions
- How to Balance Equations - Printable Worksheets
- Reactant Definition and Examples
- Example Problem of Mass Relations in Balanced Equations
- Limiting Reactant Definition (Limiting Reagent)
- Redox Reactions: Balanced Equation Example Problem
- Stoichiometry Definition in Chemistry
Balancing Equations & Reaction Types
Tutorials and problem sets, other resources.
Balance Chemical Equation - Online Balancer

WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy .
© 2024 webqc.org All rights reserved

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.3: Types of Reactions
- Last updated
- Save as PDF
- Page ID 188838
Learning Objectives
- Categorize reactions by type: synthesis, decomposition, single replacement, double replacement, and combustion.
Given a redox reaction equation, identify the element being oxidized, the one being reduced, and any spectator ions.
Write balanced equations for single replacement, double replacement, and combustion reactions.
Humans interact with one another in various and complex ways, and we classify these interactions according to common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their fists or feet, we say they are fighting. Faced with a wide range of varied interactions between chemical substances, scientists have likewise found it convenient (or even necessary) to classify chemical interactions by identifying common patterns of reactivity.
Decomposition Reactions
A decomposition reaction is a type of chemical reaction in which one reactant yields two or more products. The general form for a decomposition reaction is:
AB → A + B
Consider the decomposition of calcium carbonate:
CaCO 3(s) → CaO (s) + CO 2(g)
calcium carbonate calcium oxide carbon dioxide
Synthesis Reactions
A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound. The general equation for a synthesis reaction is:
A + B → AB
For example, the synthesis of hydrochloric acid from hydrogen and chlorine:
2Cl + H 2 → 2HCl
Oxidation-Reduction Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O 2 , a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O 2 , but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions . A few examples of such reactions will be used to develop a clear picture of this classification.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:
It is helpful to view the process with regard to each individual reactant, that is, to represent the fate of each reactant in the form of an equation called a half-reaction :
\[ \begin{align*} \ce{2Na}(s) &\rightarrow \ce{2Na+}(s)+\ce{2e-} \\[4pt] \ce{Cl2}(g)+\ce{2e-} &\rightarrow \ce{2Cl-}(s) \end{align*}\]
These equations show that Na atoms lose electrons while Cl atoms (in the Cl 2 molecule) gain electrons , the “ s ” subscripts for the resulting ions signifying they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
In this reaction, then, sodium is oxidized and chlorine undergoes reduction . Viewed from a more active perspective, sodium functions as a reducing agent (reductant) , since it provides electrons to (or reduces) chlorine. Likewise, chlorine functions as an oxidizing agent (oxidant) , as it effectively removes electrons from (oxidizes) sodium.
Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding \(\ce{NaCl}\):
The product of this reaction is a covalent compound, so transfer of electrons in the explicit sense is not involved. To clarify the similarity of this reaction to the previous one and permit an unambiguous definition of redox reactions, a property called oxidation number has been defined. The oxidation number (or oxidation state ) of an element in a compound is the charge its atoms would possess if the compound was ionic . The following guidelines are used to assign oxidation numbers to each element in a molecule or ion.
- The oxidation number of an atom in an elemental substance is zero.
- The oxidation number of a monatomic ion is equal to the ion’s charge.
- Hydrogen: +1 when combined with nonmetals, −1 when combined with metals
- Oxygen: −2 in most compounds, sometimes −1 (so-called peroxides, \(\ce{O2^2-}\)), very rarely \(-\dfrac{1}{2}\) (so-called superoxides, \(\ce{O2-}\)), positive values when combined with F (values vary)
- Halogens: −1 for F always, −1 for other halogens except when combined with oxygen or other halogens (positive oxidation numbers in these cases, varying values)
- The sum of oxidation numbers for all atoms in a molecule or polyatomic ion equals the charge on the molecule or ion.
Note: The proper convention for reporting charge is to write the number first, followed by the sign (e.g., 2+), while oxidation number is written with the reversed sequence, sign followed by number (e.g., +2). This convention aims to emphasize the distinction between these two related properties.
Example \(\PageIndex{3}\): Assigning Oxidation Numbers
Follow the guidelines in this section of the text to assign oxidation numbers to all the elements in the following species:
- \(\ce{SO3^2-}\)
(a) According to guideline 1, the oxidation number for H is +1.
Using this oxidation number and the compound’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
\(\ce{charge\: on\: H2S}=0=(2\times +1)+(1\times x)\)
\(x=0-(2\times +1)=-2\)
(b) Guideline 3 suggests the oxidation number for oxygen is −2.
Using this oxidation number and the ion’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
\(\ce{charge\: on\: SO3^2-}=-2=(3\times -2)+(1\times x)\) \(x=-2-(3\times -2)=+4\)
(c) For ionic compounds, it’s convenient to assign oxidation numbers for the cation and anion separately.
According to guideline 2, the oxidation number for sodium is +1.
Assuming the usual oxidation number for oxygen (−2 per guideline 3), the oxidation number for sulfur is calculated as directed by guideline 4:
\(x=-2-(4\times -2)=+6\)
Exercise \(\PageIndex{3}\)
Assign oxidation states to the elements whose atoms are underlined in each of the following compounds or ions:
- \(\mathrm{\underline{N}H_4^+}\)
- \(\mathrm{\sideset{ }{_{\large{4}}^{-}}{H_2\underline{P}O}}\)
N, −3
Using the oxidation number concept, an all-inclusive definition of redox reaction has been established. Oxidation-reduction (redox) reactions are those in which one or more elements involved undergo a change in oxidation number. While the vast majority of redox reactions involve changes in oxidation number for two or more elements, a few interesting exceptions to this rule do exist as shown below\). Definitions for the complementary processes of this reaction class are correspondingly revised as shown here:
Returning to the reactions used to introduce this topic, they may now both be identified as redox processes. In the reaction between sodium and chlorine to yield sodium chloride, sodium is oxidized (its oxidation number increases from 0 in Na to +1 in NaCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl 2 to −1 in NaCl). In the reaction between molecular hydrogen and chlorine, hydrogen is oxidized (its oxidation number increases from 0 in H 2 to +1 in HCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl 2 to −1 in HCl). Single-replacement reactions are redox reactions in which an ion in solution is displaced (or replaced) via the oxidation of a metallic element. One common example of this type of reaction is the acid oxidation of certain metals:
Metallic elements may also be oxidized by solutions of other metal salts; for example:
This reaction may be observed by placing copper wire in a solution containing a dissolved silver salt. Silver ions in solution are reduced to elemental silver at the surface of the copper wire, and the resulting Cu 2+ ions dissolve in the solution to yield a characteristic blue color (Figure \(\PageIndex{4}\)).
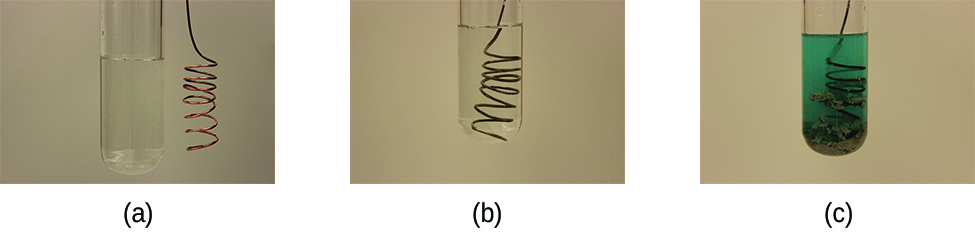
Example \(\PageIndex{4}\): Describing Redox Reactions
Identify which equations represent redox reactions, providing a name for the reaction if appropriate. For those reactions identified as redox, name the oxidant and reductant.
- \(\ce{ZnCO3}(s)\rightarrow \ce{ZnO}(s)+\ce{CO2}(g)\)
- \(\ce{2Ga}(l)+\ce{3Br2}(l)\rightarrow \ce{2GaBr3}(s)\)
- \(\ce{2H2O2}(aq)\rightarrow \ce{2H2O}(l)+\ce{O2}(g)\)
- \(\ce{BaCl2}(aq)+\ce{K2SO4}(aq)\rightarrow \ce{BaSO4}(s)+\ce{2KCl}(aq)\)
- \(\ce{C2H4}(g)+\ce{3O2}(g)\rightarrow \ce{2CO2}(g)+\ce{2H2O}(l)\)
Redox reactions are identified per definition if one or more elements undergo a change in oxidation number.
- This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
- This is a redox reaction. Gallium is oxidized, its oxidation number increasing from 0 in Ga( l ) to +3 in GaBr 3 ( s ). The reducing agent is Ga( l ). Bromine is reduced, its oxidation number decreasing from 0 in Br 2 ( l ) to −1 in GaBr 3 ( s ). The oxidizing agent is Br 2 ( l ).
- This is a redox reaction. It is a particularly interesting process, as it involves the same element, oxygen, undergoing both oxidation and reduction (a so-called disproportionation reaction) . Oxygen is oxidized, its oxidation number increasing from −1 in H 2 O 2 ( aq ) to 0 in O 2 ( g ). Oxygen is also reduced, its oxidation number decreasing from −1 in H 2 O 2 ( aq ) to −2 in H 2 O( l ). For disproportionation reactions, the same substance functions as an oxidant and a reductant.
- This is a redox reaction (combustion). Carbon is oxidized, its oxidation number increasing from −2 in C 2 H 4 ( g ) to +4 in CO 2 ( g ). The reducing agent (fuel) is C 2 H 4 ( g ). Oxygen is reduced, its oxidation number decreasing from 0 in O 2 ( g ) to −2 in H 2 O( l ). The oxidizing agent is O 2 ( g ).
Exercise \(\PageIndex{4}\)
This equation describes the production of tin(II) chloride:
Is this a redox reaction? If so, provide a more specific name for the reaction if appropriate, and identify the oxidant and reductant.
Yes, a single-replacement reaction. Sn( s ) is the reductant, HCl( g ) is the oxidant.
Combustion Reactions
Several subclasses of redox reactions are recognized, including combustion reactions in which the reductant (also called a fuel ) and oxidant (often, but not necessarily, molecular oxygen) react vigorously and produce significant amounts of heat, and often light, in the form of a flame. This is the basic structure of a combustion reaction in which oxygen from air is the oxidizing agent:
\[\ce{fuel}+\ce{O2} \rightarrow \ce{CO2}+\ce{H2O}\]
Note that the equation must be balanced based on the type of fuel being burnt.
Solid rocket-fuel reactions such as the one depicted below are combustion processes. A typical propellant reaction in which solid aluminum is oxidized by ammonium perchlorate is represented by this equation:
\[\ce{10Al}(s)+\ce{6NH4ClO4}(s)\rightarrow \ce{4Al2O3}(s)+\ce{2AlCl3}(s)+\ce{12H2O}(g)+\ce{3N2}(g)\]
Watch a brief video showing the test firing of a small-scale, prototype, hybrid rocket engine planned for use in the new Space Launch System being developed by NASA. The first engines firing at 3 s (green flame) use a liquid fuel/oxidant mixture, and the second, more powerful engines firing at 4 s (yellow flame) use a solid mixture.
Precipitation Reactions and Solubility Rules
A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement , double replacement , or metathesis reactions. These reactions are common in nature and are responsible for the formation of coral reefs in ocean waters and kidney stones in animals. They are used widely in industry for production of a number of commodity and specialty chemicals. Precipitation reactions also play a central role in many chemical analysis techniques, including spot tests used to identify metal ions and gravimetric methods for determining the composition of matter (see the last module of this chapter).
The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility , defined as the maximum concentration of a substance that can be achieved under specified conditions. Substances with relatively large solubilities are said to be soluble . A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Substances with relatively low solubilities are said to be insoluble , and these are the substances that readily precipitate from solution. More information on these important concepts is provided in the text chapter on solutions. For purposes of predicting the identities of solids formed by precipitation reactions, one may simply refer to patterns of solubility that have been observed for many ionic compounds (Table \(\PageIndex{1}\)).
A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead iodide:
\[\ce{2KI}(aq)+\ce{Pb(NO3)2}(aq)\rightarrow \ce{PbI2}(s)+\ce{2KNO3}(aq)\]
This observation is consistent with the solubility guidelines: The only insoluble compound among all those involved is lead iodide, one of the exceptions to the general solubility of iodide salts.
The net ionic equation representing this reaction is:
\[\ce{Pb^2+}(aq)+\ce{2I-}(aq)\rightarrow \ce{PbI2}(s)\]
Lead iodide is a bright yellow solid that was formerly used as an artist’s pigment known as iodine yellow (Figure \(\PageIndex{1}\)). The properties of pure PbI 2 crystals make them useful for fabrication of X-ray and gamma ray detectors.

The solubility guidelines in Table \(\PageIndex{1}\) may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble compound. For example, mixing solutions of silver nitrate and sodium fluoride will yield a solution containing Ag + , \(\ce{NO3-}\), Na + , and F − ions. Aside from the two ionic compounds originally present in the solutions, AgNO 3 and NaF, two additional ionic compounds may be derived from this collection of ions: NaNO 3 and AgF. The solubility guidelines indicate all nitrate salts are soluble but that AgF is one of the exceptions to the general solubility of fluoride salts. A precipitation reaction, therefore, is predicted to occur, as described by the following equations:
\[\ce{NaF}(aq)+\ce{AgNO3}(aq)\rightarrow \ce{AgF}(s)+\ce{NaNO3}(aq)\hspace{20px}\ce{(molecular)}\]
Example \(\PageIndex{1}\): P redicting Precipitation Reactions
Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a balanced net ionic equation for the reaction.
- potassium sulfate and barium nitrate
- lithium chloride and silver acetate
- lead nitrate and ammonium carbonate
(a) The two possible products for this combination are KNO 3 and BaSO 4 . The solubility guidelines indicate BaSO 4 is insoluble, and so a precipitation reaction is expected. The net ionic equation for this reaction, derived in the manner detailed in the previous module, is
(b) The two possible products for this combination are LiC 2 H 3 O 2 and AgCl. The solubility guidelines indicate AgCl is insoluble, and so a precipitation reaction is expected. The net ionic equation for this reaction, derived in the manner detailed in the previous module, is
(c) The two possible products for this combination are PbCO 3 and NH 4 NO 3 . The solubility guidelines indicate PbCO 3 is insoluble, and so a precipitation reaction is expected. The net ionic equation for this reaction, derived in the manner detailed in the previous module, is
\[\ce{Pb^2+}(aq)+\ce{CO3^2-}(aq)\rightarrow \ce{PbCO3}(s) onumber\]
Steps for Writing Displacement Reactions
- The cations will switch places in the products for double replacement reactions.
- The element will replace the cation in the reacting compound and result in a new product for single replacement reactions.
- Make sure that all of the compound formulas are correctly written based on the oxidation state of the elements involved.
- Balance the equation.
Chemical reactions are classified according to similar patterns of behavior. Redox reactions involve a change in oxidation number for one or more reactant elements. Precipitation reactions involve the formation of one or more insoluble products. A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A decomposition reaction is a type of chemical reaction in which one reactant yields two or more products.

IMAGES
VIDEO
COMMENTS
webpage-http://www.kentchemistry.com/links/Math/balancing.htmThis short video shows you how to balance a few synthesis chemical reactions.___Mg+ ___ O2==___ ...
A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. We can relate a balanced chemical equation to the structural formulas of its reactants and products. ... Synthesis, decomposition, single displacement, double displacement, and combustion are the main few. The type of reaction (and thus, the ...
A +B → AB. We will look at two examples of synthesis, first the formation of Ammonia N H 3 and the oxidation of iron to form rust. To produce ammonia the basic equation is. N + H → N H 3. We must remember that Nitrogen and Hydrogen are both diatomic molecules in their standard gas form. This adjusts the equation to. N 2 + H 2 → N H 3.
In a balanced chemical equation, both the numbers of each type of atom and the total charge are the same on both sides. Equations \(\ref{3.1.1}\) and \(\ref{3.1.2}\) are balanced chemical equations. What is different on each side of the equation is how the atoms are arranged to make molecules or ions. A chemical reaction represents a change in ...
N2 + H2 -> NH3. On the left there is 2 N and 2 H. On the right there is 1 N and 3 H. If we tried to balance starting with H you'd need to use a fraction or decimal and would get messy, so let's start with N. There's 2 on the left and 1 on the right, so we need to change the coefficient of NH3 to 2. Now we have.
Balancing Equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter.
The chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that occurred, its word equation, the thermodynamics of the reaction, and provide the steps to balance it using both the inspection and algebraic methods covered earlier. You can also use it to double-check your work and perform ...
Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, more complex compound. These pieces can be elements or simpler compounds. Complex simply means that the product compound has more atoms than the reactant molecules.
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/science/chemistry/chemical-reaction...
Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products. Practice with different levels of difficulty and get immediate feedback. Compare your results with real-life examples and simulations of chemical reactions.
Chemical Equations and the Law of Conservation of Matter. In the previous section, the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation:. H 2 (g) + O 2 (g) → H 2 O (g) . At the molecular level, the reaction would look something like this: Notice that there are two oxygen atoms on the left hand side of the equation and only ...
This means that you will need to balance the carbon atoms first. 5. Use a coefficient to balance the single carbon atom. Add a coefficient to the single carbon atom on the right of the equation to balance it with the 3 carbon atoms on the left of the equation. C 3 H 8 + O 2 --> H 2 O + 3 CO 2.
Example of Balancing a Chemical Equation. Let's illustrate this with an example by balancing this chemical equation: P 4 O 10 + H 2 O → H 3 PO 4. First, let's look at the element that appears least often. Notice that oxygen occurs twice on the left-hand side, so that is not a good element to start out with.
Learn how to balance chemical equations by adjusting the coefficients of reactants and products. Explore different types of reactions and check your answers with the built-in hints. Try the game mode to challenge yourself and earn rewards.
Balancing a synthesis chemical equation involves making sure that the number of atoms of each element is the same on both sides of the equation. Here are the steps: 1. Write the unbalanced equation: Reactants → Products 2. Count the number of atoms of each element on both sides of the equation. 3. Use coefficients (the numbers in front of the chemical formulas) to balance the atoms.
Write Down Number of Atoms. The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow: Fe + O 2 → Fe 2 O 3. To do this, keep in mind a subscript indicates the number of atoms. For example, O 2 has 2 atoms of oxygen.
Sixteen balance redox equations by sight. Single Replacement. Double Replacement. Decomposition. Synthesis. Combustion. Writing molecular and net ionic equations (10) (15) (20) A list of the seven diatomic elements (plus two friends) Reaction types and product prediction in a PDF file.
There are twice as many chloride ions in the reactants as there are in the products. Place a 2 in front of the NaCl in order to balance the chloride ions. Pb(NO 3) 2(aq) + 2 _ NaCl(aq) → NaNO 3(aq) + PbCl 2(s) 1 Pb atom on both reactant and product sides. 2 Na atoms on reactant side, 1 Na atom on product side.
Describes the basics of synthesis reactions, how to identify them, predict the product and balance the chemical equation. Two examples are also shown, synth...
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below; Always use the upper case for the first character in the element name and the lower case for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Compare: Co - cobalt and CO - carbon monoxide
Synthesis Reactions. A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound. The general equation for a synthesis reaction is: A + B → AB.