An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Biological, Psychological, and Social Determinants of Depression: A Review of Recent Literature
Olivia remes.
1 Institute for Manufacturing, University of Cambridge, Cambridge CB3 0FS, UK
João Francisco Mendes
2 NOVA Medical School, Universidade NOVA de Lisboa, 1099-085 Lisbon, Portugal; ku.ca.mac@94cfj
Peter Templeton
3 IfM Engage Limited, Institute for Manufacturing, University of Cambridge, Cambridge CB3 0FS, UK; ku.ca.mac@32twp
4 The William Templeton Foundation for Young People’s Mental Health (YPMH), Cambridge CB2 0AH, UK
Associated Data
Depression is one of the leading causes of disability, and, if left unmanaged, it can increase the risk for suicide. The evidence base on the determinants of depression is fragmented, which makes the interpretation of the results across studies difficult. The objective of this study is to conduct a thorough synthesis of the literature assessing the biological, psychological, and social determinants of depression in order to piece together the puzzle of the key factors that are related to this condition. Titles and abstracts published between 2017 and 2020 were identified in PubMed, as well as Medline, Scopus, and PsycInfo. Key words relating to biological, social, and psychological determinants as well as depression were applied to the databases, and the screening and data charting of the documents took place. We included 470 documents in this literature review. The findings showed that there are a plethora of risk and protective factors (relating to biological, psychological, and social determinants) that are related to depression; these determinants are interlinked and influence depression outcomes through a web of causation. In this paper, we describe and present the vast, fragmented, and complex literature related to this topic. This review may be used to guide practice, public health efforts, policy, and research related to mental health and, specifically, depression.
1. Introduction
Depression is one of the most common mental health issues, with an estimated prevalence of 5% among adults [ 1 , 2 ]. Symptoms may include anhedonia, feelings of worthlessness, concentration and sleep difficulties, and suicidal ideation. According to the World Health Organization, depression is a leading cause of disability; research shows that it is a burdensome condition with a negative impact on educational trajectories, work performance, and other areas of life [ 1 , 3 ]. Depression can start early in the lifecourse and, if it remains unmanaged, may increase the risk for substance abuse, chronic conditions, such as cardiovascular disease, and premature mortality [ 4 , 5 , 6 , 7 , 8 ].
Treatment for depression exists, such as pharmacotherapy, cognitive behavioural therapy, and other modalities. A meta-analysis of randomized, placebo-controlled trials of patients shows that 56–60% of people respond well to active treatment with antidepressants (selective serotonin reuptake inhibitors, tricyclic antidepressants) [ 9 ]. However, pharmacotherapy may be associated with problems, such as side-effects, relapse issues, a potential duration of weeks until the medication starts working, and possible limited efficacy in mild cases [ 10 , 11 , 12 , 13 , 14 ]. Psychotherapy is also available, but access barriers can make it difficult for a number of people to get the necessary help.
Studies on depression have increased significantly over the past few decades. However, the literature remains fragmented and the interpretation of heterogeneous findings across studies and between fields is difficult. The cross-pollination of ideas between disciplines, such as genetics, neurology, immunology, and psychology, is limited. Reviews on the determinants of depression have been conducted, but they either focus exclusively on a particular set of determinants (ex. genetic risk factors [ 15 ]) or population sub-group (ex. children and adolescents [ 16 ]) or focus on characteristics measured predominantly at the individual level (ex. focus on social support, history of depression [ 17 ]) without taking the wider context (ex. area-level variables) into account. An integrated approach paying attention to key determinants from the biological, psychological, and social spheres, as well as key themes, such as the lifecourse perspective, enables clinicians and public health authorities to develop tailored, person-centred approaches.
The primary aim of this literature review: to address the aforementioned challenges, we have synthesized recent research on the biological, psychological, and social determinants of depression and we have reviewed research from fields including genetics, immunology, neurology, psychology, public health, and epidemiology, among others.
The subsidiary aim: we have paid special attention to important themes, including the lifecourse perspective and interactions between determinants, to guide further efforts by public health and medical professionals.
This literature review can be used as an evidence base by those in public health and the clinical setting and can be used to inform targeted interventions.
2. Materials and Methods
We conducted a review of the literature on the biological, psychological, and social determinants of depression in the last 4 years. We decided to focus on these determinants after discussions with academics (from the Manchester Metropolitan University, University of Cardiff, University of Colorado, Boulder, University of Cork, University of Leuven, University of Texas), charity representatives, and people with lived experience at workshops held by the University of Cambridge in 2020. In several aspects, we attempted to conduct this review according to PRISMA guidelines [ 18 ].
The inclusion and exclusion criteria are the following:
- - We included documents, such as primary studies, literature reviews, systematic reviews, meta-analyses, reports, and commentaries on the determinants of depression. The determinants refer to variables that appear to be linked to the development of depression, such as physiological factors (e.g., the nervous system, genetics), but also factors that are further away or more distal to the condition. Determinants may be risk or protective factors, and individual- or wider-area-level variables.
- - We focused on major depressive disorder, treatment-resistant depression, dysthymia, depressive symptoms, poststroke depression, perinatal depression, as well as depressive-like behaviour (common in animal studies), among others.
- - We included papers regardless of the measurement methods of depression.
- - We included papers that focused on human and/or rodent research.
- - This review focused on articles written in the English language.
- - Documents published between 2017–2020 were captured to provide an understanding of the latest research on this topic.
- - Studies that assessed depression as a comorbidity or secondary to another disorder.
- - Studies that did not focus on rodent and/or human research.
- - Studies that focused on the treatment of depression. We made this decision, because this is an in-depth topic that would warrant a separate stand-alone review.
- Next, we searched PubMed (2017–2020) using keywords related to depression and determinants. Appendix A contains the search strategy used. We also conducted focused searches in Medline, Scopus, and PsycInfo (2017–2020).
- Once the documents were identified through the databases, the inclusion and exclusion criteria were applied to the titles and abstracts. Screening of documents was conducted by O.R., and a subsample was screened by J.M.; any discrepancies were resolved through a communication process.
- The full texts of documents were retrieved, and the inclusion and exclusion criteria were again applied. A subsample of documents underwent double screening by two authors (O.R., J.M.); again, any discrepancies were resolved through communication.
- a. A data charting form was created to capture the data elements of interest, including the authors, titles, determinants (biological, psychological, social), and the type of depression assessed by the research (e.g., major depression, depressive symptoms, depressive behaviour).
- b. The data charting form was piloted on a subset of documents, and refinements to it were made. The data charting form was created with the data elements described above and tested in 20 studies to determine whether refinements in the wording or language were needed.
- c. Data charting was conducted on the documents.
- d. Narrative analysis was conducted on the data charting table to identify key themes. When a particular finding was noted more than once, it was logged as a potential theme, with a review of these notes yielding key themes that appeared on multiple occasions. When key themes were identified, one researcher (O.R.) reviewed each document pertaining to that theme and derived concepts (key determinants and related outcomes). This process (a subsample) was verified by a second author (J.M.), and the two authors resolved any discrepancies through communication. Key themes were also checked as to whether they were of major significance to public mental health and at the forefront of public health discourse according to consultations we held with stakeholders from the Manchester Metropolitan University, University of Cardiff, University of Colorado, Boulder, University of Cork, University of Leuven, University of Texas, charity representatives, and people with lived experience at workshops held by the University of Cambridge in 2020.
We condensed the extensive information gleaned through our review into short summaries (with key points boxes for ease of understanding and interpretation of the data).
Through the searches, 6335 documents, such as primary studies, literature reviews, systematic reviews, meta-analyses, reports, and commentaries, were identified. After applying the inclusion and exclusion criteria, 470 papers were included in this review ( Supplementary Table S1 ). We focused on aspects related to biological, psychological, and social determinants of depression (examples of determinants and related outcomes are provided under each of the following sections.
3.1. Biological Factors
The following aspects will be discussed in this section: physical health conditions; then specific biological factors, including genetics; the microbiome; inflammatory factors; stress and hypothalamic–pituitary–adrenal (HPA) axis dysfunction, and the kynurenine pathway. Finally, aspects related to cognition will also be discussed in the context of depression.
3.1.1. Physical Health Conditions
Studies on physical health conditions—key points:
- The presence of a physical health condition can increase the risk for depression
- Psychological evaluation in physically sick populations is needed
- There is large heterogeneity in study design and measurement; this makes the comparison of findings between and across studies difficult
A number of studies examined the links between the outcome of depression and physical health-related factors, such as bladder outlet obstruction, cerebral atrophy, cataract, stroke, epilepsy, body mass index and obesity, diabetes, urinary tract infection, forms of cancer, inflammatory bowel disorder, glaucoma, acne, urea accumulation, cerebral small vessel disease, traumatic brain injury, and disability in multiple sclerosis [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 ]. For example, bladder outlet obstruction has been linked to inflammation and depressive behaviour in rodent research [ 24 ]. The presence of head and neck cancer also seemed to be related to an increased risk for depressive disorder [ 45 ]. Gestational diabetes mellitus has been linked to depressive symptoms in the postpartum period (but no association has been found with depression in the third pregnancy trimester) [ 50 ], and a plethora of other such examples of relationships between depression and physical conditions exist. As such, the assessment of psychopathology and the provision of support are necessary in individuals of ill health [ 45 ]. Despite the large evidence base on physical health-related factors, differences in study methodology and design, the lack of standardization when it comes to the measurement of various physical health conditions and depression, and heterogeneity in the study populations makes it difficult to compare studies [ 50 ].
The next subsections discuss specific biological factors, including genetics; the microbiome; inflammatory factors; stress and hypothalamic–pituitary–adrenal (HPA) axis dysfunction, and the kynurenine pathway; and aspects related to cognition.
3.1.2. Genetics
Studies on genetics—key points:
There were associations between genetic factors and depression; for example:
- The brain-derived neurotrophic factor (BDNF) plays an important role in depression
- Links exist between major histocompatibility complex region genes, as well as various gene polymorphisms and depression
- Single nucleotide polymorphisms (SNPs) of genes involved in the tryptophan catabolites pathway are of interest in relation to depression
A number of genetic-related factors, genomic regions, polymorphisms, and other related aspects have been examined with respect to depression [ 61 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 ]. The influence of BDNF in relation to depression has been amply studied [ 117 , 118 , 141 , 142 , 143 ]. Research has shown associations between depression and BDNF (as well as candidate SNPs of the BDNF gene, polymorphisms of the BDNF gene, and the interaction of these polymorphisms with other determinants, such as stress) [ 129 , 144 , 145 ]. Specific findings have been reported: for example, a study reported a link between the BDNF rs6265 allele (A) and major depressive disorder [ 117 ].
Other research focused on major histocompatibility complex region genes, endocannabinoid receptor gene polymorphisms, as well as tissue-specific genes and gene co-expression networks and their links to depression [ 99 , 110 , 112 ]. The SNPs of genes involved in the tryptophan catabolites pathway have also been of interest when studying the pathogenesis of depression.
The results from genetics studies are compelling; however, the findings remain mixed. One study indicated no support for depression candidate gene findings [ 122 ]. Another study found no association between specific polymorphisms and major depressive disorder [ 132 ]. As such, further research using larger samples is needed to corroborate the statistically significant associations reported in the literature.
3.1.3. Microbiome
Studies on the microbiome—key points:
- The gut bacteria and the brain communicate via both direct and indirect pathways called the gut-microbiota-brain axis (the bidirectional communication networks between the central nervous system and the gastrointestinal tract; this axis plays an important role in maintaining homeostasis).
- A disordered microbiome can lead to inflammation, which can then lead to depression
- There are possible links between the gut microbiome, host liver metabolism, brain inflammation, and depression
The common themes of this review have focused on the microbiome/microbiota or gut metabolome [ 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 ], the microbiota-gut-brain axis, and related factors [ 152 , 162 , 163 , 164 , 165 , 166 , 167 ]. When there is an imbalance in the intestinal bacteria, this can interfere with emotional regulation and contribute to harmful inflammatory processes and mood disorders [ 148 , 151 , 153 , 155 , 157 ]. Rodent research has shown that there may be a bidirectional association between the gut microbiota and depression: a disordered gut microbiota can play a role in the onset of this mental health problem, but, at the same time, the existence of stress and depression may also lead to a lower level of richness and diversity in the microbiome [ 158 ].
Research has also attempted to disentangle the links between the gut microbiome, host liver metabolism, brain inflammation, and depression, as well as the role of the ratio of lactobacillus to clostridium [ 152 ]. The literature has also examined the links between medication, such as antibiotics, and mood and behaviour, with the findings showing that antibiotics may be related to depression [ 159 , 168 ]. The links between the microbiome and depression are complex, and further studies are needed to determine the underpinning causal mechanisms.
3.1.4. Inflammation
Studies on inflammation—key points:
- Pro-inflammatory cytokines are linked to depression
- Pro-inflammatory cytokines, such as the tumour necrosis factor (TNF)-alpha, may play an important role
- Different methods of measurement are used, making the comparison of findings across studies difficult
Inflammation has been a theme in this literature review [ 60 , 161 , 164 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 ]. The findings show that raised levels of inflammation (because of factors such as pro-inflammatory cytokines) have been associated with depression [ 60 , 161 , 174 , 175 , 178 ]. For example, pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-alpha, have been linked to depression [ 185 ]. Various determinants, such as early life stress, have also been linked to systemic inflammation, and this can increase the risk for depression [ 186 ].
Nevertheless, not everyone with elevated inflammation develops depression; therefore, this is just one route out of many linked to pathogenesis. Despite the compelling evidence reported with respect to inflammation, it is difficult to compare the findings across studies because of different methods used to assess depression and its risk factors.
3.1.5. Stress and HPA Axis Dysfunction
Studies on stress and HPA axis dysfunction—key points:
- Stress is linked to the release of proinflammatory factors
- The dysregulation of the HPA axis is linked to depression
- Determinants are interlinked in a complex web of causation
Stress was studied in various forms in rodent populations and humans [ 144 , 145 , 155 , 174 , 176 , 180 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 ].
Although this section has some overlap with others (as is to be expected because all of these determinants and body systems are interlinked), a number of studies have focused on the impact of stress on mental health. Stress has been mentioned in the literature as a risk factor of poor mental health and has emerged as an important determinant of depression. The effects of this variable are wide-ranging, and a short discussion is warranted.
Stress has been linked to the release of inflammatory factors, as well as the development of depression [ 204 ]. When the stress is high or lasts for a long period of time, this may negatively impact the brain. Chronic stress can impact the dendrites and synapses of various neurons, and may be implicated in the pathway leading to major depressive disorder [ 114 ]. As a review by Uchida et al. indicates, stress may be associated with the “dysregulation of neuronal and synaptic plasticity” [ 114 ]. Even in rodent studies, stress has a negative impact: chronic and unpredictable stress (and other forms of tension or stress) have been linked to unusual behaviour and depression symptoms [ 114 ].
The depression process and related brain changes, however, have also been linked to the hyperactivity or dysregulation of the HPA axis [ 127 , 130 , 131 , 182 , 212 ]. One review indicates that a potential underpinning mechanism of depression relates to “HPA axis abnormalities involved in chronic stress” [ 213 ]. There is a complex relationship between the HPA axis, glucocorticoid receptors, epigenetic mechanisms, and psychiatric sequelae [ 130 , 212 ].
In terms of the relationship between the HPA axis and stress and their influence on depression, the diathesis–stress model offers an explanation: it could be that early stress plays a role in the hyperactivation of the HPA axis, thus creating a predisposition “towards a maladaptive reaction to stress”. When this predisposition then meets an acute stressor, depression may ensue; thus, in line with the diathesis–stress model, a pre-existing vulnerability and stressor can create fertile ground for a mood disorder [ 213 ]. An integrated review by Dean and Keshavan [ 213 ] suggests that HPA axis hyperactivity is, in turn, related to other determinants, such as early deprivation and insecure early attachment; this again shows the complex web of causation between the different determinants.
3.1.6. Kynurenine Pathway
Studies on the kynurenine pathway—key points:
- The kynurenine pathway is linked to depression
- Indolamine 2,3-dioxegenase (IDO) polymorphisms are linked to postpartum depression
The kynurenine pathway was another theme that emerged in this review [ 120 , 178 , 181 , 184 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 ]. The kynurenine pathway has been implicated not only in general depressed mood (inflammation-induced depression) [ 184 , 214 , 219 ] but also postpartum depression [ 120 ]. When the kynurenine metabolism pathway is activated, this results in metabolites, which are neurotoxic.
A review by Jeon et al. notes a link between the impairment of the kynurenine pathway and inflammation-induced depression (triggered by treatment for various physical diseases, such as malignancy). The authors note that this could represent an important opportunity for immunopharmacology [ 214 ]. Another review by Danzer et al. suggests links between the inflammation-induced activation of indolamine 2,3-dioxegenase (the enzyme that converts tryptophan to kynurenine), the kynurenine metabolism pathway, and depression, and also remarks about the “opportunities for treatment of inflammation-induced depression” [ 184 ].
3.1.7. Cognition
Studies on cognition and the brain—key points:
- Cognitive decline and cognitive deficits are linked to increased depression risk
- Cognitive reserve is important in the disability/depression relationship
- Family history of cognitive impairment is linked to depression
A number of studies have focused on the theme of cognition and the brain. The results show that factors, such as low cognitive ability/function, cognitive vulnerability, cognitive impairment or deficits, subjective cognitive decline, regression of dendritic branching and hippocampal atrophy/death of hippocampal cells, impaired neuroplasticity, and neurogenesis-related aspects, have been linked to depression [ 131 , 212 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 ]. The cognitive reserve appears to act as a moderator and can magnify the impact of certain determinants on poor mental health. For example, in a study in which participants with multiple sclerosis also had low cognitive reserve, disability was shown to increase the risk for depression [ 63 ]. Cognitive deficits can be both causal and resultant in depression. A study on individuals attending outpatient stroke clinics showed that lower scores in cognition were related to depression; thus, cognitive impairment appears to be associated with depressive symptomatology [ 226 ]. Further, Halahakoon et al. [ 222 ] note a meta-analysis [ 240 ] that shows that a family history of cognitive impairment (in first degree relatives) is also linked to depression.
In addition to cognitive deficits, low-level cognitive ability [ 231 ] and cognitive vulnerability [ 232 ] have also been linked to depression. While cognitive impairment may be implicated in the pathogenesis of depressive symptoms [ 222 ], negative information processing biases are also important; according to the ‘cognitive neuropsychological’ model of depression, negative affective biases play a central part in the development of depression [ 222 , 241 ]. Nevertheless, the evidence on this topic is mixed and further work is needed to determine the underpinning mechanisms between these states.
3.2. Psychological Factors
Studies on psychological factors—key points:
- There are many affective risk factors linked to depression
- Determinants of depression include negative self-concept, sensitivity to rejection, neuroticism, rumination, negative emotionality, and others
A number of studies have been undertaken on the psychological factors linked to depression (including mastery, self-esteem, optimism, negative self-image, current or past mental health conditions, and various other aspects, including neuroticism, brooding, conflict, negative thinking, insight, cognitive fusion, emotional clarity, rumination, dysfunctional attitudes, interpretation bias, and attachment style) [ 66 , 128 , 140 , 205 , 210 , 228 , 235 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 ]. Determinants related to this condition include low self-esteem and shame, among other factors [ 269 , 270 , 275 , 278 ]. Several emotional states and traits, such as neuroticism [ 235 , 260 , 271 , 278 ], negative self-concept (with self-perceptions of worthlessness and uselessness), and negative interpretation or attention biases have been linked to depression [ 261 , 271 , 282 , 283 , 286 ]. Moreover, low emotional clarity has been associated with depression [ 267 ]. When it comes to the severity of the disorder, it appears that meta-emotions (“emotions that occur in response to other emotions (e.g., guilt about anger)” [ 268 ]) have a role to play in depression [ 268 ].
A determinant that has received much attention in mental health research concerns rumination. Rumination has been presented as a mediator but also as a risk factor for depression [ 57 , 210 , 259 ]. When studied as a risk factor, it appears that the relationship of rumination with depression is mediated by variables that include limited problem-solving ability and insufficient social support [ 259 ]. However, rumination also appears to act as a mediator: for example, this variable (particularly brooding rumination) lies on the causal pathway between poor attention control and depression [ 265 ]. This shows that determinants may present in several forms: as moderators or mediators, risk factors or outcomes, and this is why disentangling the relationships between the various factors linked to depression is a complex task.
The psychological determinants are commonly researched variables in the mental health literature. A wide range of factors have been linked to depression, such as the aforementioned determinants, but also: (low) optimism levels, maladaptive coping (such as avoidance), body image issues, and maladaptive perfectionism, among others [ 269 , 270 , 272 , 273 , 275 , 276 , 279 , 285 , 286 ]. Various mechanisms have been proposed to explain the way these determinants increase the risk for depression. One of the underpinning mechanisms linking the determinants and depression concerns coping. For example, positive fantasy engagement, cognitive biases, or personality dispositions may lead to emotion-focused coping, such as brooding, and subsequently increase the risk for depression [ 272 , 284 , 287 ]. Knowing the causal mechanisms linking the determinants to outcomes provides insight for the development of targeted interventions.
3.3. Social Determinants
Studies on social determinants—key points:
- Social determinants are the conditions in the environments where people are born, live, learn, work, play, etc.; these influence (mental) health [ 291 ]
- There are many social determinants linked to depression, such as sociodemographics, social support, adverse childhood experiences
- Determinants can be at the individual, social network, community, and societal levels
Studies also focused on the social determinants of (mental) health; these are the conditions in which people are born, live, learn, work, play, and age, and have a significant influence on wellbeing [ 291 ]. Factors such as age, social or socioeconomic status, social support, financial strain and deprivation, food insecurity, education, employment status, living arrangements, marital status, race, childhood conflict and bullying, violent crime exposure, abuse, discrimination, (self)-stigma, ethnicity and migrant status, working conditions, adverse or significant life events, illiteracy or health literacy, environmental events, job strain, and the built environment have been linked to depression, among others [ 52 , 133 , 235 , 236 , 239 , 252 , 269 , 280 , 292 , 293 , 294 , 295 , 296 , 297 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 , 310 , 311 , 312 , 313 , 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 , 322 , 323 , 324 , 325 , 326 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 , 336 , 337 , 338 , 339 , 340 , 341 , 342 , 343 , 344 , 345 , 346 , 347 , 348 , 349 , 350 , 351 , 352 , 353 , 354 , 355 , 356 , 357 , 358 , 359 , 360 , 361 , 362 , 363 , 364 , 365 , 366 , 367 , 368 , 369 , 370 , 371 ]. Social support and cohesion, as well as structural social capital, have also been identified as determinants [ 140 , 228 , 239 , 269 , 293 , 372 , 373 , 374 , 375 , 376 , 377 , 378 , 379 ]. In a study, part of the findings showed that low levels of education have been shown to be linked to post-stroke depression (but not severe or clinical depression outcomes) [ 299 ]. A study within a systematic review indicated that having only primary education was associated with a higher risk of depression compared to having secondary or higher education (although another study contrasted this finding) [ 296 ]. Various studies on socioeconomic status-related factors have been undertaken [ 239 , 297 ]; the research has shown that a low level of education is linked to depression [ 297 ]. Low income is also related to depressive disorders [ 312 ]. By contrast, high levels of education and income are protective [ 335 ].
A group of determinants touched upon by several studies included adverse childhood or early life experiences: ex. conflict with parents, early exposure to traumatic life events, bullying and childhood trauma were found to increase the risk of depression (ex. through pathways, such as inflammation, interaction effects, or cognitive biases) [ 161 , 182 , 258 , 358 , 362 , 380 ].
Gender-related factors were also found to play an important role with respect to mental health [ 235 , 381 , 382 , 383 , 384 , 385 ]. Gender inequalities can start early on in the lifecourse, and women were found to be twice as likely to have depression as men. Gender-related factors were linked to cognitive biases, resilience and vulnerabilities [ 362 , 384 ].
Determinants can impact mental health outcomes through underpinning mechanisms. For example, harmful determinants can influence the uptake of risk behaviours. Risk behaviours, such as sedentary behaviour, substance abuse and smoking/nicotine exposure, have been linked to depression [ 226 , 335 , 355 , 385 , 386 , 387 , 388 , 389 , 390 , 391 , 392 , 393 , 394 , 395 , 396 , 397 , 398 , 399 , 400 , 401 ]. Harmful determinants can also have an impact on diet. Indeed, dietary aspects and diet components (ex. vitamin D, folate, selenium intake, iron, vitamin B12, vitamin K, fiber intake, zinc) as well as diet-related inflammatory potential have been linked to depression outcomes [ 161 , 208 , 236 , 312 , 396 , 402 , 403 , 404 , 405 , 406 , 407 , 408 , 409 , 410 , 411 , 412 , 413 , 414 , 415 , 416 , 417 , 418 , 419 , 420 , 421 , 422 , 423 , 424 , 425 , 426 , 427 , 428 ]. A poor diet has been linked to depression through mechanisms such as inflammation [ 428 ].
Again, it is difficult to constrict diet to the ‘social determinants of health’ category as it also relates to inflammation (biological determinants) and could even stand alone as its own category. Nevertheless, all of these factors are interlinked and influence one another in a complex web of causation, as mentioned elsewhere in the paper.
Supplementary Figure S1 contains a representation of key determinants acting at various levels: the individual, social network, community, and societal levels. The determinants have an influence on risk behaviours, and this, in turn, can affect the mood (i.e., depression), body processes (ex. can increase inflammation), and may negatively influence brain structure and function.
3.4. Others
Studies on ‘other’ determinants—key points:
- A number of factors are related to depression
- These may not be as easily categorized as the other determinants in this paper
A number of factors arose in this review that were related to depression; it was difficult to place these under a specific heading above, so this ‘other’ category was created. A number of these could be sorted under the ‘social determinants of depression’ category. For example, being exposed to deprivation, hardship, or adversity may increase the risk for air pollution exposure and nighttime shift work, among others, and the latter determinants have been found to increase the risk for depression. Air pollution could also be regarded as an ecologic-level (environmental) determinant of mental health.
Nevertheless, we have decided to leave these factors in a separate category (because their categorization may not be as immediately clear-cut as others), and these factors include: low-level light [ 429 ], weight cycling [ 430 ], water contaminants [ 431 ], trade [ 432 ], air pollution [ 433 , 434 ], program-level variables (ex. feedback and learning experience) [ 435 ], TV viewing [ 436 ], falls [ 437 ], various other biological factors [ 116 , 136 , 141 , 151 , 164 , 182 , 363 , 364 , 438 , 439 , 440 , 441 , 442 , 443 , 444 , 445 , 446 , 447 , 448 , 449 , 450 , 451 , 452 , 453 , 454 , 455 , 456 , 457 , 458 , 459 , 460 , 461 , 462 , 463 , 464 , 465 , 466 , 467 , 468 , 469 ], mobile phone use [ 470 ], ultrasound chronic exposure [ 471 ], nighttime shift work [ 472 ], work accidents [ 473 ], therapy enrollment [ 226 ], and exposure to light at night [ 474 ].
4. Cross-Cutting Themes
4.1. lifecourse perspective.
Studies on the lifecourse perspective—key points:
- Early life has an importance on mental health
- Stress has been linked to depression
- In old age, the decline in social capital is important
Trajectories and life events are important when it comes to the lifecourse perspective. Research has touched on the influence of prenatal or early life stress on an individual’s mental health trajectory [ 164 , 199 , 475 ]. Severe stress that occurs in the form of early-life trauma has also been associated with depressive symptoms [ 362 , 380 ]. It may be that some individuals exposed to trauma develop thoughts of personal failure, which then serve as a catalyst of depression [ 380 ].
At the other end of the life trajectory—old age—specific determinants have been linked to an increased risk for depression. Older people are at a heightened risk of losing their social networks, and structural social capital has been identified as important in relation to depression in old age [ 293 ].
4.2. Gene–Environment Interactions
Studies on gene–environment interactions—key points:
- The environment and genetics interact to increase the risk of depression
- The etiology of depression is multifactorial
- Adolescence is a time of vulnerability
A number of studies have touched on gene–environment interactions [ 72 , 77 , 82 , 119 , 381 , 476 , 477 , 478 , 479 , 480 , 481 ]. The interactions between genetic factors and determinants, such as negative life events (ex. relationship and social difficulties, serious illness, unemployment and financial crises) and stressors (ex. death of spouse, minor violations of law, neighbourhood socioeconomic status) have been studied in relation to depression [ 82 , 135 , 298 , 449 , 481 ]. A study reported an interaction of significant life events with functional variation in the serotonin-transporter-linked polymorphic region (5-HTTLPR) allele type (in the context of multiple sclerosis) and linked this to depression [ 361 ], while another reported an interaction between stress and 5-HTTLPR in relation to depression [ 480 ]. Other research reported that the genetic variation of HPA-axis genes has moderating effects on the relationship between stressors and depression [ 198 ]. Another study showed that early-life stress interacts with gene variants to increase the risk for depression [ 77 ].
Adolescence is a time of vulnerability [ 111 , 480 ]. Perceived parental support has been found to interact with genes (GABRR1, GABRR2), and this appears to be associated with depressive symptoms in adolescence [ 480 ]. It is important to pay special attention to critical periods in the lifecourse so that adequate support is provided to those who are most vulnerable.
The etiology of depression is multifactorial, and it is worthwhile to examine the interaction between multiple factors, such as epigenetic, genetic, and environmental factors, in order to truly understand this mental health condition. Finally, taking into account critical periods of life when assessing gene–environment interactions is important for developing targeted interventions.
5. Discussion
Depression is one of the most common mental health conditions, and, if left untreated, it can increase the risk for substance abuse, anxiety disorders, and suicide. In the past 20 years, a large number of studies on the risk and protective factors of depression have been undertaken in various fields, such as genetics, neurology, immunology, and epidemiology. However, there are limitations associated with the extant evidence base. The previous syntheses on depression are limited in scope and focus exclusively on social or biological factors, population sub-groups, or examine depression as a comorbidity (rather than an independent disorder). The research on the determinants and causal pathways of depression is fragmentated and heterogeneous, and this has not helped to stimulate progress when it comes to the prevention and intervention of this condition—specifically unravelling the complexity of the determinants related to this condition and thus refining the prevention and intervention methods.
The scope of this paper was to bring together the heterogeneous, vast, and fragmented literature on depression and paint a picture of the key factors that contribute to this condition. The findings from this review show that there are important themes when it comes to the determinants of depression, such as: the microbiome, dysregulation of the HPA axis, inflammatory reactions, the kynurenine pathway, as well as psychological and social factors. It may be that physical factors are proximal determinants of depression, which, in turn, are acted on by more distal social factors, such as deprivation, environmental events, and social capital.
The Marmot Report [ 291 ], the World Health Organization [ 482 ], and Compton et al. [ 483 ] highlight that the most disadvantaged segments of society are suffering (the socioeconomic context is important), and this inequality in resources has translated to inequality in mental health outcomes [ 483 ]. To tackle the issue of egalitarianism and restore equality in the health between the groups, the social determinants need to be addressed [ 483 ]. A wide range of determinants of mental health have been identified in the literature: age, gender, ethnicity, family upbringing and early attachment patterns, social support, access to food, water and proper nutrition, and community factors. People spiral downwards because of individual- and societal-level circumstances; therefore, these circumstances along with the interactions between the determinants need to be considered.
Another important theme in the mental health literature is the lifecourse perspective. This shows that the timing of events has significance when it comes to mental health. Early life is a critical period during the lifespan at which cognitive processes develop. Exposure to harmful determinants, such as stress, during this period can place an individual on a trajectory of depression in adulthood or later life. When an individual is exposed to harmful determinants during critical periods and is also genetically predisposed to depression, the risk for the disorder can be compounded. This is why aspects such as the lifecourse perspective and gene–environment interactions need to be taken into account. Insight into this can also help to refine targeted interventions.
A number of interventions for depression have been developed or recommended, addressing, for example, the physical factors described here and lifestyle modifications. Interventions targeting various factors, such as education and socioeconomic status, are needed to help prevent and reduce the burden of depression. Further research on the efficacy of various interventions is needed. Additional studies are also needed on each of the themes described in this paper, for example: the biological factors related to postpartum depression [ 134 ], and further work is needed on depression outcomes, such as chronic, recurrent depression [ 452 ]. Previous literature has shown that chronic stress (associated with depression) is also linked to glucocorticoid receptor resistance, as well as problems with the regulation of the inflammatory response [ 484 ]. Further work is needed on this and the underpinning mechanisms between the determinants and outcomes. This review highlighted the myriad ways of measuring depression and its determinants [ 66 , 85 , 281 , 298 , 451 , 485 ]. Thus, the standardization of the measurements of the outcomes (ex. a gold standard for measuring depression) and determinants is essential; this can facilitate comparisons of findings across studies.
5.1. Strengths
This paper has important strengths. It brings together the wide literature on depression and helps to bridge disciplines in relation to one of the most common mental health problems. We identified, selected, and extracted data from studies, and provided concise summaries.
5.2. Limitations
The limitations of the review include missing potentially important studies; however, this is a weakness that cannot be avoided by literature reviews. Nevertheless, the aim of the review was not to identify each study that has been conducted on the risk and protective factors of depression (which a single review is unable to capture) but rather to gain insight into the breadth of literature on this topic, highlight key biological, psychological, and social determinants, and shed light on important themes, such as the lifecourse perspective and gene–environment interactions.
6. Conclusions
We have reviewed the determinants of depression and recognize that there are a multitude of risk and protective factors at the individual and wider ecologic levels. These determinants are interlinked and influence one another. We have attempted to describe the wide literature on this topic, and we have brought to light major factors that are of public mental health significance. This review may be used as an evidence base by those in public health, clinical practice, and research.
This paper discusses key areas in depression research; however, an exhaustive discussion of all the risk factors and determinants linked to depression and their mechanisms is not possible in one journal article—which, by its very nature, a single paper cannot do. We have brought to light overarching factors linked to depression and a workable conceptual framework that may guide clinical and public health practice; however, we encourage other researchers to continue to expand on this timely and relevant work—particularly as depression is a top priority on the policy agenda now.
Acknowledgments
Thank you to Isla Kuhn for the help with the Medline, Scopus, and PsycInfo database searches.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci11121633/s1 , Figure S1: Conceptual framework: Determinants of depression, Table S1: Data charting—A selection of determinants from the literature.
Appendix A.1. Search Strategy
Search: ((((((((((((((((“Gene-Environment Interaction”[Majr]) OR (“Genetics”[Mesh])) OR (“Genome-Wide Association Study”[Majr])) OR (“Microbiota”[Mesh] OR “Gastrointestinal Microbiome”[Mesh])) OR (“Neurogenic Inflammation”[Mesh])) OR (“genetic determinant”)) OR (“gut-brain-axis”)) OR (“Kynurenine”[Majr])) OR (“Cognition”[Mesh])) OR (“Neuronal Plasticity”[Majr])) OR (“Neurogenesis”[Mesh])) OR (“Genes”[Mesh])) OR (“Neurology”[Majr])) OR (“Social Determinants of Health”[Majr])) OR (“Glucocorticoids”[Mesh])) OR (“Tryptophan”[Mesh])) AND (“Depression”[Mesh] OR “Depressive Disorder”[Mesh]) Filters: from 2017—2020.
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations, Daily and Versions(R)
- exp *Depression/
- exp *Depressive Disorder/
- exp *”Social Determinants of Health”/
- exp *Tryptophan/
- exp *Glucocorticoids/
- exp *Neurology/
- exp *Genes/
- exp *Neurogenesis/
- exp *Neuronal Plasticity/
- exp *Kynurenine/
- exp *Genetics/
- exp *Neurogenic Inflammation/
- exp *Gastrointestinal Microbiome/
- exp *Genome-Wide Association Study/
- exp *Gene-Environment Interaction/
- exp *Depression/et [Etiology]
- exp *Depressive Disorder/et
- or/4-16 637368
- limit 22 to yr = “2017–Current”
- “cause* of depression”.mp.
- “cause* of depression”.ti.
- (cause adj3 (depression or depressive)).ti.
- (caus* adj3 (depression or depressive)).ti.
Appendix A.2. PsycInfo
(TITLE ( depression OR “ Depressive Disorder ”) AND TITLE (“ Social Determinants of Health ” OR tryptophan OR glucocorticoids OR neurology OR genes OR neurogenesis OR “ Neuronal Plasticity ” OR kynurenine OR genetics OR “ Neurogenic Inflammation ” OR “ Gastrointestinal Microbiome ” OR “ Genome-Wide Association Study ” OR “ Gene-Environment Interaction ” OR aetiology OR etiology )) OR TITLE ( cause* W/3 ( depression OR depressive )).
Author Contributions
O.R. was responsible for the design of the study and methodology undertaken. Despite P.T.’s involvement in YPMH, he had no role in the design of the study; P.T. was responsible for the conceptualization of the study. Validation was conducted by O.R. and J.F.M. Formal analysis (data charting) was undertaken by O.R. O.R. and P.T. were involved in the investigation, resource acquisition, and data presentation. The original draft preparation was undertaken by O.R. The writing was conducted by O.R., with review and editing by P.T. and J.F.M. Funding acquisition was undertaken by O.R. and P.T. All authors have read and agreed to the published version of the manuscript.
This research was funded by The William Templeton Foundation for Young People’s Mental Health, Cambridge Philosophical Society, and the Aviva Foundation.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
The Critical Relationship Between Anxiety and Depression
- Ned H. Kalin , M.D.
Search for more papers by this author
Anxiety and depressive disorders are among the most common psychiatric illnesses; they are highly comorbid with each other, and together they are considered to belong to the broader category of internalizing disorders. Based on statistics from the Substance Abuse and Mental Health Services Administration, the 12-month prevalence of major depressive disorder in 2017 was estimated to be 7.1% for adults and 13.3% for adolescents ( 1 ). Data for anxiety disorders are less current, but in 2001–2003, their 12-month prevalence was estimated to be 19.1% in adults, and 2001–2004 data estimated that the lifetime prevalence in adolescents was 31.9% ( 2 , 3 ). Both anxiety and depressive disorders are more prevalent in women, with an approximate 2:1 ratio in women compared with men during women’s reproductive years ( 1 , 2 ).
Across all psychiatric disorders, comorbidity is the rule ( 4 ), which is definitely the case for anxiety and depressive disorders, as well as their symptoms. With respect to major depression, a worldwide survey reported that 45.7% of individuals with lifetime major depressive disorder had a lifetime history of one or more anxiety disorder ( 5 ). These disorders also commonly coexist during the same time frame, as 41.6% of individuals with 12-month major depression also had one or more anxiety disorder over the same 12-month period. From the perspective of anxiety disorders, the lifetime comorbidity with depression is estimated to range from 20% to 70% for patients with social anxiety disorder ( 6 ), 50% for patients with panic disorder ( 6 ), 48% for patients with posttraumatic stress disorder (PTSD) ( 7 ), and 43% for patients with generalized anxiety disorder ( 8 ). Data from the well-known Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrate comorbidity at the symptom level, as 53% of the patients with major depression had significant anxiety and were considered to have an anxious depression ( 9 ).
Anxiety and depressive disorders are moderately heritable (approximately 40%), and evidence suggests shared genetic risk across the internalizing disorders ( 10 ). Among internalizing disorders, the highest level of shared genetic risk appears to be between major depressive disorder and generalized anxiety disorder. Neuroticism is a personality trait or temperamental characteristic that is associated with the development of both anxiety and depression, and the genetic risk for developing neuroticism also appears to be shared with that of the internalizing disorders ( 11 ). Common nongenetic risk factors associated with the development of anxiety and depression include earlier life adversity, such as trauma or neglect, as well as parenting style and current stress exposure. At the level of neural circuits, alterations in prefrontal-limbic pathways that mediate emotion regulatory processes are common to anxiety and depressive disorders ( 12 , 13 ). These findings are consistent with meta-analyses that reveal shared structural and functional brain alterations across various psychiatric illnesses, including anxiety and major depression, in circuits involving emotion regulation ( 13 ), executive function ( 14 ), and cognitive control ( 15 ).
Anxiety disorders and major depression occur during development, with anxiety disorders commonly beginning during preadolescence and early adolescence and major depression tending to emerge during adolescence and early to mid-adulthood ( 16 – 18 ). In relation to the evolution of their comorbidity, studies demonstrate that anxiety disorders generally precede the presentation of major depressive disorder ( 17 ). A European community-based study revealed, beginning at age 15, the developmental relation between comorbid anxiety and major depression by specifically focusing on social phobia (based on DSM-IV criteria) and then asking the question regarding concurrent major depressive disorder ( 18 ). The findings revealed a 19% concurrent comorbidity between these disorders, and in 65% of the cases, social phobia preceded major depressive disorder by at least 2 years. In addition, initial presentation with social phobia was associated with a 5.7-fold increased risk of developing major depressive disorder. These associations between anxiety and depression can be traced back even earlier in life. For example, childhood behavioral inhibition in response to novelty or strangers, or an extreme anxious temperament, is associated with a three- to fourfold increase in the likelihood of developing social anxiety disorder, which in turn is associated with an increased risk to develop major depressive disorder and substance abuse ( 19 ).
It is important to emphasize that the presence of comor‐bid anxiety symptoms and disorders matters in relation to treatment. Across psychiatric disorders, the presence of significant anxiety symptoms generally predicts worse outcomes, and this has been well demonstrated for depression. In the STAR*D study, patients with anxious major depressive disorder were more likely to be severely depressed and to have more suicidal ideation ( 9 ). This is consistent with the study by Kessler and colleagues ( 5 ), in which patients with anxious major depressive disorder, compared with patients with nonanxious major depressive disorder, were found to have more severe role impairment and more suicidal ideation. Data from level 1 of the STAR*D study (citalopram treatment) nicely illustrate the impact of comorbid anxiety symptoms on treatment. Compared with patients with nonanxious major depressive disorder, those 53% of patients with an anxious depression were less likely to remit and also had a greater side effect burden ( 20 ). Other data examining patients with major depressive disorder and comorbid anxiety disorders support the greater difficulty and challenge in treating patients with these comorbidities ( 21 ).
This issue of the Journal presents new findings relevant to the issues discussed above in relation to understanding and treating anxiety and depressive disorders. Drs. Conor Liston and Timothy Spellman, from Weill Cornell Medicine, provide an overview for this issue ( 22 ) that is focused on understanding mechanisms at the neural circuit level that underlie the pathophysiology of depression. Their piece nicely integrates human neuroimaging studies with complementary data from animal models that allow for the manipulation of selective circuits to test hypotheses generated from the human data. Also included in this issue is a review of the data addressing the reemergence of the use of psychedelic drugs in psychiatry, particularly for the treatment of depression, anxiety, and PTSD ( 23 ). This timely piece, authored by Dr. Collin Reiff along with a subgroup from the APA Council of Research, provides the current state of evidence supporting the further exploration of these interventions. Dr. Alan Schatzberg, from Stanford University, contributes an editorial in which he comments on where the field is in relation to clinical trials with psychedelics and to some of the difficulties, such as adequate blinding, in reliably studying the efficacy of these drugs ( 24 ).
In an article by McTeague et al. ( 25 ), the authors use meta-analytic strategies to understand the neural alterations that are related to aberrant emotion processing that are shared across psychiatric disorders. Findings support alterations in the salience, reward, and lateral orbital nonreward networks as common across disorders, including anxiety and depressive disorders. These findings add to the growing body of work that supports the concept that there are common underlying factors across all types of psychopathology that include internalizing, externalizing, and thought disorder dimensions ( 26 ). Dr. Deanna Barch, from Washington University in St. Louis, writes an editorial commenting on these findings and, importantly, discusses criteria that should be met when we consider whether the findings are actually transdiagnostic ( 27 ).
Another article, from Gray and colleagues ( 28 ), addresses whether there is a convergence of findings, specifically in major depression, when examining data from different structural and functional neuroimaging modalities. The authors report that, consistent with what we know about regions involved in emotion processing, the subgenual anterior cingulate cortex, hippocampus, and amygdala were among the regions that showed convergence across multimodal imaging modalities.
In relation to treatment and building on our understanding of neural circuit alterations, Siddiqi et al. ( 29 ) present data suggesting that transcranial magnetic stimulation (TMS) targeting can be linked to symptom-specific treatments. Their findings identify different TMS targets in the left dorsolateral prefrontal cortex that modulate different downstream networks. The modulation of these different networks appears to be associated with a reduction in different types of symptoms. In an editorial, Drs. Sean Nestor and Daniel Blumberger, from the University of Toronto ( 30 ), comment on the novel approach used in this study to link the TMS-related engagement of circuits with symptom improvement. They also provide a perspective on how we can view these and other circuit-based findings in relation to conceptualizing personalized treatment approaches.
Kendler et al. ( 31 ), in this issue, contribute an article that demonstrates the important role of the rearing environment in the risk to develop major depression. Using a unique design from a Swedish sample, the analytic strategy involves comparing outcomes from high-risk full sibships and high-risk half sibships where at least one of the siblings was home reared and one was adopted out of the home. The findings support the importance of the quality of the rearing environment as well as the presence of parental depression in mitigating or enhancing the likelihood of developing major depression. In an accompanying editorial ( 32 ), Dr. Myrna Weissman, from Columbia University, reviews the methods and findings of the Kendler et al. article and also emphasizes the critical significance of the early nurturing environment in relation to general health.
This issue concludes with an intriguing article on anxiety disorders, by Gold and colleagues ( 33 ), that demonstrates neural alterations during extinction recall that differ in children relative to adults. With increasing age, and in relation to fear and safety cues, nonanxious adults demonstrated greater connectivity between the amygdala and the ventromedial prefrontal cortex compared with anxious adults, as the cues were being perceived as safer. In contrast, neural differences between anxious and nonanxious youths were more robust when rating the memory of faces that were associated with threat. Specifically, these differences were observed in the activation of the inferior temporal cortex. In their editorial ( 34 ), Dr. Dylan Gee and Sahana Kribakaran, from Yale University, emphasize the importance of developmental work in relation to understanding anxiety disorders, place these findings into the context of other work, and suggest the possibility that these and other data point to neuroscientifically informed age-specific interventions.
Taken together, the papers in this issue of the Journal present new findings that shed light onto alterations in neural function that underlie major depressive disorder and anxiety disorders. It is important to remember that these disorders are highly comorbid and that their symptoms are frequently not separable. The papers in this issue also provide a developmental perspective emphasizing the importance of early rearing in the risk to develop depression and age-related findings important for understanding threat processing in patients with anxiety disorders. From a treatment perspective, the papers introduce data supporting more selective prefrontal cortical TMS targeting in relation to different symptoms, address the potential and drawbacks for considering the future use of psychedelics in our treatments, and present new ideas supporting age-specific interventions for youths and adults with anxiety disorders.
Disclosures of Editors’ financial relationships appear in the April 2020 issue of the Journal .
1 Substance Abuse and Mental Health Services Administration (SAMHSA): Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSDUH Series H-53). Rockville, Md, Center for Behavioral Health Statistics and Quality, SAMHSA, 2018. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm Google Scholar
2 Kessler RC, Chiu WT, Demler O, et al. : Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication . Arch Gen Psychiatry 2005 ; 62:617–627, correction, 62:709 Crossref , Medline , Google Scholar
3 Merikangas KR, He JP, Burstein M, et al. : Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) . J Am Acad Child Adolesc Psychiatry 2010 ; 49:980–989 Crossref , Medline , Google Scholar
4 Kessler RC, McGonagle KA, Zhao S, et al. : Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey . Arch Gen Psychiatry 1994 ; 51:8–19 Crossref , Medline , Google Scholar
5 Kessler RC, Sampson NA, Berglund P, et al. : Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys . Epidemiol Psychiatr Sci 2015 ; 24:210–226 Crossref , Medline , Google Scholar
6 Dunner DL : Management of anxiety disorders: the added challenge of comorbidity . Depress Anxiety 2001 ; 13:57–71 Crossref , Medline , Google Scholar
7 Kessler RC, Sonnega A, Bromet E, et al. : Posttraumatic stress disorder in the National Comorbidity Survey . Arch Gen Psychiatry 1995 ; 52:1048–1060 Crossref , Medline , Google Scholar
8 Brawman-Mintzer O, Lydiard RB, Emmanuel N, et al. : Psychiatric comorbidity in patients with generalized anxiety disorder . Am J Psychiatry 1993 ; 150:1216–1218 Link , Google Scholar
9 Fava M, Alpert JE, Carmin CN, et al. : Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D . Psychol Med 2004 ; 34:1299–1308 Crossref , Medline , Google Scholar
10 Hettema JM : What is the genetic relationship between anxiety and depression? Am J Med Genet C Semin Med Genet 2008 ; 148C:140–146 Crossref , Medline , Google Scholar
11 Hettema JM, Neale MC, Myers JM, et al. : A population-based twin study of the relationship between neuroticism and internalizing disorders . Am J Psychiatry 2006 ; 163:857–864 Link , Google Scholar
12 Kovner R, Oler JA, Kalin NH : Cortico-limbic interactions mediate adaptive and maladaptive responses relevant to psychopathology . Am J Psychiatry 2019 ; 176:987–999 Link , Google Scholar
13 Etkin A, Schatzberg AF : Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders . Am J Psychiatry 2011 ; 168:968–978 Link , Google Scholar
14 Goodkind M, Eickhoff SB, Oathes DJ, et al. : Identification of a common neurobiological substrate for mental illness . JAMA Psychiatry 2015 ; 72:305–315 Crossref , Medline , Google Scholar
15 McTeague LM, Huemer J, Carreon DM, et al. : Identification of common neural circuit disruptions in cognitive control across psychiatric disorders . Am J Psychiatry 2017 ; 174:676–685 Link , Google Scholar
16 Beesdo K, Knappe S, Pine DS : Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V . Psychiatr Clin North Am 2009 ; 32:483–524 Crossref , Medline , Google Scholar
17 Kessler RC, Wang PS : The descriptive epidemiology of commonly occurring mental disorders in the United States . Annu Rev Public Health 2008 ; 29:115–129 Crossref , Medline , Google Scholar
18 Ohayon MM, Schatzberg AF : Social phobia and depression: prevalence and comorbidity . J Psychosom Res 2010 ; 68:235–243 Crossref , Medline , Google Scholar
19 Clauss JA, Blackford JU : Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study . J Am Acad Child Adolesc Psychiatry 2012 ; 51:1066–1075 Crossref , Medline , Google Scholar
20 Fava M, Rush AJ, Alpert JE, et al. : Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report . Am J Psychiatry 2008 ; 165:342–351 Link , Google Scholar
21 Dold M, Bartova L, Souery D, et al. : Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders: results from a European multicenter study . J Psychiatr Res 2017 ; 91:1–13 Crossref , Medline , Google Scholar
22 Spellman T, Liston C : Toward circuit mechanisms of pathophysiology in depression . Am J Psychiatry 2020 ; 177:381–390 Link , Google Scholar
23 Reiff CM, Richman EE, Nemeroff CB, et al. : Psychedelics and psychedelic-assisted psychotherapy . Am J Psychiatry 2020 ; 177:391–410 Link , Google Scholar
24 Schatzberg AF : Some comments on psychedelic research (editorial). Am J Psychiatry 2020 ; 177:368–369 Link , Google Scholar
25 McTeague LM, Rosenberg BM, Lopez JW, et al. : Identification of common neural circuit disruptions in emotional processing across psychiatric disorders . Am J Psychiatry 2020 ; 177:411–421 Link , Google Scholar
26 Caspi A, Moffitt TE : All for one and one for all: mental disorders in one dimension . Am J Psychiatry 2018 ; 175:831–844 Link , Google Scholar
27 Barch DM : What does it mean to be transdiagnostic and how would we know? (editorial). Am J Psychiatry 2020 ; 177:370–372 Abstract , Google Scholar
28 Gray JP, Müller VI, Eickhoff SB, et al. : Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies . Am J Psychiatry 2020 ; 177:422–434 Link , Google Scholar
29 Siddiqi SH, Taylor SF, Cooke D, et al. : Distinct symptom-specific treatment targets for circuit-based neuromodulation . Am J Psychiatry 2020 ; 177:435–446 Link , Google Scholar
30 Nestor SM, Blumberger DM : Mapping symptom clusters to circuits: toward personalizing TMS targets to improve treatment outcomes in depression (editorial). Am J Psychiatry 2020 ; 177:373–375 Abstract , Google Scholar
31 Kendler KS, Ohlsson H, Sundquist J, et al. : The rearing environment and risk for major depression: a Swedish national high-risk home-reared and adopted-away co-sibling control study . Am J Psychiatry 2020 ; 177:447–453 Abstract , Google Scholar
32 Weissman MM : Is depression nature or nurture? Yes (editorial). Am J Psychiatry 2020 ; 177:376–377 Abstract , Google Scholar
33 Gold AL, Abend R, Britton JC, et al. : Age differences in the neural correlates of anxiety disorders: an fMRI study of response to learned threat . Am J Psychiatry 2020 ; 177:454–463 Link , Google Scholar
34 Gee DG, Kribakaran S : Developmental differences in neural responding to threat and safety: implications for treating youths with anxiety (editorial). Am J Psychiatry 2020 ; 177:378–380 Abstract , Google Scholar
- Cited by None

- Neuroanatomy
- Neurochemistry
- Neuroendocrinology
- Other Research Areas
Change Password
Your password must have 8 characters or more and contain 3 of the following:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
- Sign in / Register
Request Username
Can't sign in? Forgot your username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
An Exploratory Study of Students with Depression in Undergraduate Research Experiences
- Katelyn M. Cooper
- Logan E. Gin
- M. Elizabeth Barnes
- Sara E. Brownell
*Address correspondence to: Katelyn M. Cooper ( E-mail Address: [email protected] ).
Department of Biology, University of Central Florida, Orlando, FL, 32816
Search for more papers by this author
Biology Education Research Lab, Research for Inclusive STEM Education Center, School of Life Sciences, Arizona State University, Tempe, AZ 85281
Depression is a top mental health concern among undergraduates and has been shown to disproportionately affect individuals who are underserved and underrepresented in science. As we aim to create a more inclusive scientific community, we argue that we need to examine the relationship between depression and scientific research. While studies have identified aspects of research that affect graduate student depression, we know of no studies that have explored the relationship between depression and undergraduate research. In this study, we sought to understand how undergraduates’ symptoms of depression affect their research experiences and how research affects undergraduates’ feelings of depression. We interviewed 35 undergraduate researchers majoring in the life sciences from 12 research-intensive public universities across the United States who identify with having depression. Using inductive and deductive coding, we identified that students’ depression affected their motivation and productivity, creativity and risk-taking, engagement and concentration, and self-perception and socializing in undergraduate research experiences. We found that students’ social connections, experiencing failure in research, getting help, receiving feedback, and the demands of research affected students’ depression. Based on this work, we articulate an initial set of evidence-based recommendations for research mentors to consider in promoting an inclusive research experience for students with depression.
INTRODUCTION
Depression is described as a common and serious mood disorder that results in persistent feelings of sadness and hopelessness, as well as a loss of interest in activities that one once enjoyed ( American Psychiatric Association [APA], 2013 ). Additional symptoms of depression include weight changes, difficulty sleeping, loss of energy, difficulty thinking or concentrating, feelings of worthlessness or excessive guilt, and suicidality ( APA, 2013 ). While depression results from a complex interaction of psychological, social, and biological factors ( World Health Organization, 2018 ), studies have shown that increased stress caused by college can be a significant contributor to student depression ( Dyson and Renk, 2006 ).
Depression is one of the top undergraduate mental health concerns, and the rate of depression among undergraduates continues to rise ( Center for Collegiate Mental Health, 2017 ). While we cannot discern whether these increasing rates of depression are due to increased awareness or increased incidence, it is clear that is a serious problem on college campuses. The percent of U.S. college students who self-reported a diagnosis with depression was recently estimated to be about 25% ( American College Health Association, 2019 ). However, higher rates have been reported, with one study estimating that up to 84% of undergraduates experience some level of depression ( Garlow et al. , 2008 ). Depression rates are typically higher among university students compared with the general population, despite being a more socially privileged group ( Ibrahim et al. , 2013 ). Prior studies have found that depression is negatively correlated with overall undergraduate academic performance ( Hysenbegasi et al. , 2005 ; Deroma et al. , 2009 ; American College Health Association, 2019 ). Specifically, diagnosed depression is associated with half a letter grade decrease in students’ grade point average ( Hysenbegasi et al. , 2005 ), and 21.6% of undergraduates reported that depression negatively affected their academic performance within the last year ( American College Health Association, 2019 ). Provided with a list of academic factors that may be affected by depression, students reported that depression contributed to lower exam grades, lower course grades, and not completing or dropping a course.
Students in the natural sciences may be particularly at risk for depression, given that such majors are noted to be particularly stressful due to their competitive nature and course work that is often perceived to “weed students out”( Everson et al. , 1993 ; Strenta et al. , 1994 ; American College Health Association, 2019 ; Seymour and Hunter, 2019 ). Science course instruction has also been described to be boring, repetitive, difficult, and math-intensive; these factors can create an environment that can trigger depression ( Seymour and Hewitt, 1997 ; Osborne and Collins, 2001 ; Armbruster et al ., 2009 ; Ceci and Williams, 2010 ). What also distinguishes science degree programs from other degree programs is that, increasingly, undergraduate research experiences are being proposed as an essential element of a science degree ( American Association for the Advancement of Science, 2011 ; President’s Council of Advisors on Science and Technology, 2012 ; National Academies of Sciences, Engineering, and Medicine [NASEM], 2017 ). However, there is some evidence that undergraduate research experiences can add to the stress of college for some students ( Cooper et al. , 2019c ). Students can garner multiple benefits from undergraduate research, including enhanced abilities to think critically ( Ishiyama, 2002 ; Bauer and Bennett, 2003 ; Brownell et al. , 2015 ), improved student learning ( Rauckhorst et al. , 2001 ; Brownell et al. , 2015 ), and increased student persistence in undergraduate science degree programs ( Jones et al. , 2010 ; Hernandez et al. , 2018 ). Notably, undergraduate research experiences are increasingly becoming a prerequisite for entry into medical and graduate programs in science, particularly elite programs ( Cooper et al. , 2019d ). Although some research experiences are embedded into formal lab courses as course-based undergraduate research experiences (CUREs; Auchincloss et al. , 2014 ; Brownell and Kloser, 2015 ), the majority likely entail working with faculty in their research labs. These undergraduate research experiences in faculty labs are often added on top of a student’s normal course work, so they essentially become an extracurricular activity that they have to juggle with course work, working, and/or personal obligations ( Cooper et al. , 2019c ). While the majority of the literature surrounding undergraduate research highlights undergraduate research as a positive experience ( NASEM, 2017 ), studies have demonstrated that undergraduate research experiences can be academically and emotionally challenging for students ( Mabrouk and Peters, 2000 ; Seymour et al. , 2004 ; Cooper et al. , 2019c ; Limeri et al. , 2019 ). In fact, 50% of students sampled nationally from public R1 institutions consider leaving their undergraduate research experience prematurely, and about half of those students, or 25% of all students, ultimately leave their undergraduate research experience ( Cooper et al. , 2019c ). Notably, 33.8% of these individuals cited a negative lab environment and 33.3% cited negative relationships with their mentors as factors that influenced their decision about whether to leave ( Cooper et al. , 2019c ). Therefore, students’ depression may be exacerbated in challenging undergraduate research experiences, because studies have shown that depression is positively correlated with student stress ( Hish et al. , 2019 ).
While depression has not been explored in the context of undergraduate research experiences, depression has become a prominent concern surrounding graduate students conducting scientific research. A recent study that examined the “graduate student mental health crisis” ( Flaherty, 2018 ) found that work–life balance and graduate students’ relationships with their research advisors may be contributing to their depression ( Evans et al. , 2018 ). Specifically, this survey of 2279 PhD and master’s students from diverse fields of study, including the biological/physical sciences, showed that 39% of graduate students have experienced moderate to severe depression. Fifty-five percent of the graduate students with depression who were surveyed disagreed with the statement “I have good work life balance,” compared to only 21% of students with depression who agreed. Additionally, the study highlighted that more students with depression disagreed than agreed with the following statements: their advisors provided “real” mentorship, their advisors provided ample support, their advisors positively impacted their emotional or mental well-being, their advisors were assets to their careers, and they felt valued by their mentors. Another recent study identified that depression severity in biomedical doctoral students was significantly associated with graduate program climate, a perceived lack of employment opportunities, and the quality of students’ research training environment ( Nagy et al. , 2019 ). Environmental stress, academic stress, and family and monetary stress have also been shown to be predictive of depression severity in biomedical doctoral students ( Hish et al. , 2019 ). Further, one study found that self-esteem is negatively correlated and stress is positively correlated with graduate student depression; presumably research environments that challenge students’ self-esteem and induce stress are likely contributing to depressive symptoms among graduate students ( Kreger, 1995 ). While these studies have focused on graduate students, and there are certainly notable distinctions between graduate and undergraduate research, the research-related factors that affect graduate student depression, including work–life balance, relationships with mentors, research environment, stress, and self-esteem, may also be relevant to depression among undergraduates conducting research. Importantly, undergraduates in the United States have reported identical levels of depression as graduate students but are often less likely to seek mental health care services ( Wyatt and Oswalt, 2013 ), which is concerning if undergraduate research experiences exacerbate depression.
Based on the literature on the stressors of undergraduate research experiences and the literature identifying some potential causes of graduate student depression, we identified three aspects of undergraduate research that may exacerbate undergraduates’ depression. Mentoring: Mentors can be an integral part of a students’ research experience, bolstering their connections with others in the science community, scholarly productivity, and science identity, as well as providing many other benefits ( Thiry and Laursen, 2011 ; Prunuske et al. , 2013 ; Byars-Winston et al. , 2015 ; Aikens et al. , 2016 , 2017 ; Thompson et al. , 2016 ; Estrada et al. , 2018 ). However, recent literature has highlighted that poor mentoring can negatively affect undergraduate researchers ( Cooper et al. , 2019c ; Limeri et al. , 2019 ). Specifically, one study of 33 undergraduate researchers who had conducted research at 10 institutions identified seven major ways that they experienced negative mentoring, which included absenteeism, abuse of power, interpersonal mismatch, lack of career support, lack of psychosocial support, misaligned expectations, and unequal treatment ( Limeri et al. , 2019 ). We hypothesize negative mentoring experiences may be particularly harmful for students with depression, because support, particularly social support, has been shown to be important for helping individuals with depression cope with difficult circumstances ( Aneshensel and Stone, 1982 ; Grav et al. , 2012 ). Failure: Experiencing failure has been hypothesized to be an important aspect of undergraduate research experiences that may help students develop some the most distinguishing abilities of outstanding scientists, such as coping with failure, navigating challenges, and persevering ( Laursen et al. , 2010 ; Gin et al. , 2018 ; Henry et al. , 2019 ). However, experiencing failure and the stress and fatigue that often accompany it may be particularly tough for students with depression ( Aldwin and Greenberger, 1987 ; Mongrain and Blackburn, 2005 ). Lab environment: Fairness, inclusion/exclusion, and social support within one’s organizational environment have been shown to be key factors that cause people to either want to remain in the work place and be productive or to want to leave ( Barak et al. , 2006 ; Cooper et al. , 2019c ). We hypothesize that dealing with exclusion or a lack of social support may exacerbate depression for some students; patients with clinical depression react to social exclusion with more pronounced negative emotions than do individuals without clinical depression ( Jobst et al. , 2015 ). While there are likely other aspects of undergraduate research that affect student depression, we hypothesize that these factors have the potential to exacerbate negative research experiences for students with depression.
Depression has been shown to disproportionately affect many populations that are underrepresented or underserved within the scientific community, including females ( American College Health Association, 2018 ; Evans et al. , 2018 ), first-generation college students ( Jenkins et al. , 2013 ), individuals from low socioeconomic backgrounds ( Eisenberg et al. , 2007 ), members of the LGBTQ+ community ( Eisenberg et al. , 2007 ; Evans et al. , 2018 ), and people with disabilities ( Turner and Noh, 1988 ). Therefore, as the science community strives to be more diverse and inclusive ( Intemann, 2009 ), it is important that we understand more about the relationship between depression and scientific research, because negative experiences with depression in scientific research may be contributing to the underrepresentation of these groups. Specifically, more information is needed about how the research process and environment of research experiences may affect depression.
Given the high rate of depression among undergraduates, the links between depression and graduate research, the potentially challenging environment of undergraduate research, and how depression could disproportionately impact students from underserved communities, it is imperative to begin to explore the relationship between scientific research and depression among undergraduates to create research experiences that could maximize student success. In this exploratory interview study, we aimed to 1) describe how undergraduates’ symptoms of depression affect their research experiences, 2) understand how undergraduate research affects students’ feelings of depression, and 3) identify recommendations based on the literature and undergraduates’ reported experiences to promote a positive research experience for students with depression.
This study was done with an approved Arizona State University Institutional Review Board protocol #7247.
In Fall 2018, we surveyed undergraduate researchers majoring in the life sciences across 25 research-intensive (R1) public institutions across the United States (specific details about the recruitment of the students who completed the survey can be found in Cooper et al. (2019c) ). The survey asked students for their opinions about their undergraduate research experiences and their demographic information and whether they would be interested in participating in a follow-up interview related to their research experiences. For the purpose of this study, we exclusively interviewed students about their undergraduate research experiences in faculty member labs; we did not consider students’ experiences in CUREs. Of the 768 undergraduate researchers who completed the survey, 65% ( n = 496) indicated that they would be interested in participating in a follow-up interview. In Spring 2019, we emailed the 496 students, explaining that we were interested in interviewing students with depression about their experiences in undergraduate research. Our specific prompt was: “If you identify as having depression, we would be interested in hearing about your experience in undergraduate research in a 30–60 minute online interview.” We did not define depression in our email recruitment because we conducted think-aloud interviews with four undergraduates who all correctly interpreted what we meant by depression ( APA, 2013 ). We had 35 students agree to participate in the interview study. The interview participants represented 12 of the 25 R1 public institutions that were represented in the initial survey.
Student Interviews
We developed an interview script to explore our research questions. Specifically, we were interested in how students’ symptoms of depression affect their research experiences, how undergraduate research negatively affects student depression, and how undergraduate research positively affects student depression.
We recognized that mental health, and specifically depression, can be a sensitive topic to discuss with undergraduates, and therefore we tried to minimize any discomfort that the interviewees might experience during the interview. Specifically, we conducted think-aloud interviews with three graduate students who self-identified with having depression at the time of the interview. We asked them to note whether any interview questions made them uncomfortable. We also sought their feedback on questions given their experiences as persons with depression who had once engaged in undergraduate research. We revised the interview protocol after each think-aloud interview. Next, we conducted four additional think-aloud interviews with undergraduates conducting basic science or biology education research who identified with having depression to establish cognitive validity of the questions and to elicit additional feedback about any questions that might make someone uncomfortable. The questions were revised after each think-aloud interview until no question was unclear or misinterpreted by the students and we were confident that the questions minimized students’ potential discomfort ( Trenor et al. , 2011 ). A copy of the final interview script can be found in the Supplemental Material.
All interviews were individually conducted by one of two researchers (K.M.C. and L.E.G.) who conducted the think-aloud interviews together to ensure that their interviewing practices were as similar as possible. The interviews were approximately an hour long, and students received a $15 gift card for their participation.
Personal, Research, and Depression Demographics
All student demographics and information about students’ research experiences were collected using the survey distributed to students in Fall 2018. We collected personal demographics, including the participants’ gender, race/ethnicity, college generation status, transfer status, financial stability, year in college, major, and age. We also collected information about the students’ research experiences, including the length of their first research experiences, the average number of hours they spend in research per week, how they were compensated for research, who their primary mentors were, and the focus areas of their research.
In the United States, mental healthcare is disproportionately unavailable to Black and Latinx individuals, as well as those who come from low socioeconomic backgrounds ( Kataoka et al. , 2002 ; Howell and McFeeters, 2008 ; Santiago et al. , 2013 ). Therefore, to minimize a biased sample, we invited anyone who identified with having depression to participate in our study; we did not require students to be diagnosed with depression or to be treated for depression in order to participate. However, we did collect information about whether students had been formally diagnosed with depression and whether they had been treated for depression. After the interview, all participants were sent a link to a short survey that asked them if they had ever been diagnosed with depression and how, if at all, they had ever been treated for depression. A copy of these survey questions can be found in the Supplemental Material. The combined demographic information of the participants is in Table 1 . The demographics for each individual student can be found in the Supplemental Material.
a Students reported the time they had spent in research 6 months before being interviewed and only reported on the length of time of their first research experiences.
b Students were invited to report multiple ways in which they were treated for their depression; other treatments included lifestyle changes and meditation.
c Students were invited to report multiple means of compensation for their research if they had been compensated for their time in different ways.
d Students were asked whether they felt financially stable, particularly during the undergraduate research experience.
e Students reported who they work/worked with most closely during their research experiences.
f Staff members included lab coordinators or lab managers.
g Other focus areas of research included sociology, linguistics, psychology, and public health.
Interview Analysis
The initial interview analysis aimed to explore each idea that a participant expressed ( Charmaz, 2006 ) and to identify reoccurring ideas throughout the interviews. First, three authors (K.M.C., L.E.G., and S.E.B.) individually reviewed a different set of 10 interviews and took detailed analytic notes ( Birks and Mills, 2015 ). Afterward, the authors compared their notes and identified reoccurring themes throughout the interviews using open coding methods ( Saldaña, 2015 ).
Once an initial set of themes was established, two researchers (K.M.C. and L.E.G.) individually reviewed the same set of 15 randomly selected interviews to validate the themes identified in the initial analysis and to screen for any additional themes that the initial analysis may have missed. Each researcher took detailed analytic notes throughout the review of an interview, which they discussed after reviewing each interview. The researchers compared what quotes from each interview they categorized into each theme. Using constant comparison methods, they assigned quotes to each theme and constantly compared the quotes to ensure that each quote fit within the description of the theme ( Glesne and Peshkin, 1992 ). In cases in which quotes were too different from other quotes, a new theme was created. This approach allowed for multiple revisions of the themes and allowed the authors to define a final set of codes; the researchers created a final codebook with refined definitions of emergent themes (the final coding rubric can be found in the Supplemental Material). Once the final codebook was established, the researchers (K.M.C. and L.E.G.) individually coded seven additional interviews (20% of all interviews) using the coding rubric. The researchers compared their codes, and their Cohen’s κ interrater score for these seven interviews was at an acceptable level (κ = 0.88; Landis and Koch, 1977 ). One researcher (L.E.G.) coded the remaining 28 out of 35 interviews. The researchers determined that data saturation had been reached with the current sample and no further recruitment was needed ( Guest et al. , 2006 ). We report on themes that were mentioned by at least 20% of students in the interview study. In the Supplemental Material, we provide the final coding rubric with the number of participants whose interview reflected each theme ( Hannah and Lautsch, 2011 ). Reporting the number of individuals who reported themes within qualitative data can lead to inaccurate conclusions about the generalizability of the results to a broader population. These qualitative data are meant to characterize a landscape of experiences that students with depression have in undergraduate research rather than to make claims about the prevalence of these experiences ( Glesne and Peshkin, 1992 ). Because inferences about the importance of these themes cannot be drawn from these counts, they are not included in the results of the paper ( Maxwell, 2010 ). Further, the limited number of interviewees made it not possible to examine whether there were trends based on students’ demographics or characteristics of their research experiences (e.g., their specific area of study). Quotes were lightly edited for clarity by inserting clarification brackets and using ellipses to indicate excluded text. Pseudonyms were given to all students to protect their privacy.
The Effect of Depressive Symptoms on Undergraduate Research
We asked students to describe the symptoms associated with their depression. Students described experiencing anxiety that is associated with their depression; this could be anxiety that precedes their depression or anxiety that results from a depressive episode or a period of time when an individual has depression symptoms. Further, students described difficulty getting out of bed or leaving the house, feeling tired, a lack of motivation, being overly self-critical, feeling apathetic, and having difficulty concentrating. We were particularly interested in how students’ symptoms of depression affected their experiences in undergraduate research. During the think-aloud interviews that were conducted before the interview study, graduate and undergraduate students consistently described that their depression affected their motivation in research, their creativity in research, and their productivity in research. Therefore, we explicitly asked undergraduate researchers how, if at all, their depression affected these three factors. We also asked students to describe any additional ways in which their depression affected their research experiences. Undergraduate researchers commonly described five additional ways in which their depression affected their research; for a detailed description of each way students’ research was affected and for example quotes, see Table 2 . Students described that their depression negatively affected their productivity in the lab. Commonly, students described that their productivity was directly affected by a lack of motivation or because they felt less creative, which hindered the research process. Additionally, students highlighted that they were sometimes less productive because their depression sometimes caused them to struggle to engage intellectually with their research or caused them to have difficulty remembering or concentrating; students described that they could do mundane or routine tasks when they felt depressed, but that they had difficulty with more complex and intellectually demanding tasks. However, students sometimes described that even mundane tasks could be difficult when they were required to remember specific steps; for example, some students struggled recalling a protocol from memory when their depression was particularly severe. Additionally, students noted that their depression made them more self-conscious, which sometimes held them back from sharing research ideas with their mentors or from taking risks such as applying to competitive programs. In addition to being self-conscious, students highlighted that their depression caused them to be overly self-critical, and some described experiencing imposter phenomenon ( Clance and Imes, 1978 ) or feeling like they were not talented enough to be in research and were accepted into a lab by a fluke or through luck. Finally, students described that depression often made them feel less social, and they struggled to socially engage with other members of the lab when they were feeling down.
The Effect of Undergraduate Research Experiences on Student Depression
We also wanted to explore how research impacted students’ feelings of depression. Undergraduates described how research both positively and negatively affected their depression. In the following sections, we present aspects of undergraduate research and examine how each positively and/or negatively affected students’ depression using embedded student quotes to highlight the relationships between related ideas.
Lab Environment: Relationships with Others in the Lab.
Some aspects of the lab environment, which we define as students’ physical, social, or psychological research space, could be particularly beneficial for students with depression.
Specifically, undergraduate researchers perceived that comfortable and positive social interactions with others in the lab helped their depression. Students acknowledged how beneficial their relationships with graduate students and postdocs could be.
Marta: “I think always checking in on undergrads is important. It’s really easy [for us] to go a whole day without talking to anybody in the lab. But our grad students are like ‘Hey, what’s up? How’s school? What’s going on?’ (…) What helps me the most is having that strong support system. Sometimes just talking makes you feel better, but also having people that believe in you can really help you get out of that negative spiral. I think that can really help with depression.”
Kelley: “I know that anytime I need to talk to [my postdoc mentors] about something they’re always there for me. Over time we’ve developed a relationship where I know that outside of work and outside of the lab if I did want to talk to them about something I could talk to them. Even just talking to someone about hobbies and having that relationship alone is really helpful [for depression].”
In addition to highlighting the importance of developing relationships with graduate students or postdocs in the lab, students described that forming relationships with other undergraduates in the lab also helped their depression. Particularly, students described that other undergraduate researchers often validated their feelings about research, which in turn helped them realize that what they are thinking or feeling is normal, which tended to alleviate their negative thoughts. Interestingly, other undergraduates experiencing the same issues could sometimes help buffer them from perceiving that a mentor did not like them or that they were uniquely bad at research. In this article, we use the term “mentor” to refer to anyone who students referred to in the interviews as being their mentors or managing their research experiences; this includes graduate students, postdoctoral scholars, lab managers, and primary investigators (PIs).
Abby: “One of my best friends is in the lab with me. A lot of that friendship just comes from complaining about our stress with the lab and our annoyance with people in the lab. Like when we both agree like, ‘Yeah, the grad students were really off today, it wasn’t us,’ that helps. ‘It wasn’t me, it wasn’t my fault that we were having a rough day in lab; it was the grad students.’ Just being able to realize, ‘Hey, this isn’t all caused by us,’ you know? (…) We understand the stresses in the lab. We understand the details of what each other are doing in the lab, so when something doesn’t work out, we understand that it took them like eight hours to do that and it didn’t work. We provide empathy on a different level.”
Meleana: “It’s great to have solidarity in being confused about something, and it’s just that is a form of validation for me too. When we leave a lab meeting and I look at [another undergrad] I’m like, ‘Did you understand anything that they were just saying?’ And they’re like, ‘Oh, no.’ (…) It’s just really validating to hear from the other undergrads that we all seem to be struggling with the same things.”
Developing positive relationships with faculty mentors or PIs also helped alleviate some students’ depressive feelings, particularly when PIs shared their own struggles with students. This also seemed to normalize students’ concerns about their own experiences.
Alexandra: “[Talking with my PI] is helpful because he would talk about his struggles, and what he faced. A lot of it was very similar to my struggles. For example, he would say, ‘Oh, yeah, I failed this exam that I studied so hard for. I failed the GRE and I paid so much money to prepare for it.’ It just makes [my depression] better, like okay, this is normal for students to go through this. It’s not an out of this world thing where if you fail, you’re a failure and you can’t move on from it.”
Students’ relationships with others in the lab did not always positively impact their depression. Students described instances when the negative moods of the graduate students and PIs would often set the tone of the lab, which in turn worsened the mood of the undergraduate researchers.
Abby: “Sometimes [the grad students] are not in a good mood. The entire vibe of the lab is just off, and if you make a joke and it hits somebody wrong, they get all mad. It really depends on the grad students and the leadership and the mood that they’re in.”
Interviewer: “How does it affect your depression when the grad students are in a bad mood?”
Abby: “It definitely makes me feel worse. It feels like, again, that I really shouldn’t go ask them for help because they’re just not in the mood to help out. It makes me have more pressure on myself, and I have deadlines I need to meet, but I have a question for them, but they’re in a bad mood so I can’t ask. That’s another day wasted for me and it just puts more stress, which just adds to the depression.”
Additionally, some students described even more concerning behavior from research mentors, which negatively affected their depression.
Julie: “I had a primary investigator who is notorious in the department for screaming at people, being emotionally abusive, unreasonable, et cetera. (…) [He was] kind of harassing people, demeaning them, lying to them, et cetera, et cetera. (…) Being yelled at and constantly demeaned and harassed at all hours of the day and night, that was probably pretty bad for me.”
While the relationships between undergraduates and graduate, postdoc, and faculty mentors seemed to either alleviate or worsen students’ depressive symptoms, depending on the quality of the relationship, students in this study exclusively described their relationships with other undergraduates as positive for their depression. However, students did note that undergraduate research puts some of the best and brightest undergraduates in the same environment, which can result in students comparing themselves with their peers. Students described that this comparison would often lead them to feel badly about themselves, even though they would describe their personal relationship with a person to be good.
Meleana: “In just the research field in general, just feeling like I don’t really measure up to the people around me [can affect my depression]. A lot of the times it’s the beginning of a little spiral, mental spiral. There are some past undergrads that are talked about as they’re on this pedestal of being the ideal undergrads and that they were just so smart and contributed so much to the lab. I can never stop myself from wondering like, ‘Oh, I wonder if I’m having a contribution to the lab that’s similar or if I’m just another one of the undergrads that does the bare minimum and passes through and is just there.’”
Natasha: “But, on the other hand, [having another undergrad in the lab] also reminded me constantly that some people are invested in this and meant to do this and it’s not me. And that some people know a lot more than I do and will go further in this than I will.”
While students primarily expressed that their relationships with others in the lab affected their depression, some students explained that they struggled most with depression when the lab was empty; they described that they did not like being alone in the lab, because a lack of stimulation allowed their minds to be filled with negative thoughts.
Mia: “Those late nights definitely didn’t help [my depression]. I am alone, in the entire building. I’m left alone to think about my thoughts more, so not distracted by talking to people or interacting with people. I think more about how I’m feeling and the lack of progress I’m making, and the hopelessness I’m feeling. That kind of dragged things on, and I guess deepened my depression.”
Freddy: “Often times when I go to my office in the evening, that is when I would [ sic ] be prone to be more depressed. It’s being alone. I think about myself or mistakes or trying to correct mistakes or whatever’s going on in my life at the time. I become very introspective. I think I’m way too self-evaluating, way too self-deprecating and it’s when I’m alone when those things are really, really triggered. When I’m talking with somebody else, I forget about those things.”
In sum, students with depression highlighted that a lab environment full of positive and encouraging individuals was helpful for their depression, whereas isolating or competitive environments and negative interactions with others often resulted in more depressive feelings.
Doing Science: Experiencing Failure in Research, Getting Help, Receiving Feedback, Time Demands, and Important Contributions.
In addition to the lab environment, students also described that the process of doing science could affect their depression. Specifically, students explained that a large contributor to their depression was experiencing failure in research.
Interviewer: “Considering your experience in undergraduate research, what tends to trigger your feelings of depression?”
Heather: “Probably just not getting things right. Having to do an experiment over and over again. You don’t get the results you want. (…) The work is pretty meticulous and it’s frustrating when I do all this work, I do a whole experiment, and then I don’t get any results that I can use. That can be really frustrating. It adds to the stress. (…) It’s hard because you did all this other stuff before so you can plan for the research, and then something happens and all the stuff you did was worthless basically.”
Julie: “I felt very negatively about myself [when a project failed] and pretty panicked whenever something didn’t work because I felt like it was a direct reflection on my effort and/or intelligence, and then it was a big glaring personal failure.”
Students explained that their depression related to failing in research was exacerbated if they felt as though they could not seek help from their research mentors. Perceived insufficient mentor guidance has been shown to be a factor influencing student intention to leave undergraduate research ( Cooper et al. , 2019c ). Sometimes students talked about their research mentors being unavailable or unapproachable.
Michelle: “It just feels like [the graduate students] are not approachable. I feel like I can’t approach them to ask for their understanding in a certain situation. It makes [my depression] worse because I feel like I’m stuck, and that I’m being limited, and like there’s nothing I can do. So then I kind of feel like it’s my fault that I can’t do anything.”
Other times, students described that they did not seek help in fear that they would be negatively evaluated in research, which is a fear of being judged by others ( Watson and Friend, 1969 ; Weeks et al. , 2005 ; Cooper et al. , 2018 ). That is, students fear that their mentor would think negatively about them or judge them if they were to ask questions that their mentor thought they should know the answer to.
Meleana: “I would say [my depression] tends to come out more in being more reserved in asking questions because I think that comes more like a fear-based thing where I’m like, ‘Oh, I don’t feel like I’m good enough and so I don’t want to ask these questions because then my mentors will, I don’t know, think that I’m dumb or something.’”
Conversely, students described that mentors who were willing to help them alleviated their depressive feelings.
Crystal: “Yeah [my grad student] is always like, ‘Hey, I can check in on things in the lab because you’re allowed to ask me for that, you’re not totally alone in this,’ because he knows that I tend to take on all this responsibility and I don’t always know how to ask for help. He’s like, ‘You know, this is my lab too and I am here to help you as well,’ and just reminds me that I’m not shouldering this burden by myself.”
Ashlyn: “The graduate student who I work with is very kind and has a lot of patience and he really understands a lot of things and provides simple explanations. He does remind me about things and he will keep on me about certain tasks that I need to do in an understanding way, and it’s just because he’s patient and he listens.”
In addition to experiencing failure in science, students described that making mistakes when doing science also negatively affected their depression.
Abby: “I guess not making mistakes on experiments [is important in avoiding my depression]. Not necessarily that your experiment didn’t turn out to produce the data that you wanted, but just adding the wrong enzyme or messing something up like that. It’s like, ‘Oh, man,’ you know? You can get really down on yourself about that because it can be embarrassing.”
Commonly, students described that the potential for making mistakes increased their stress and anxiety regarding research; however, they explained that how other people responded to a potential mistake was what ultimately affected their depression.
Briana: “Sometimes if I made a mistake in correctly identifying an eye color [of a fly], [my PI] would just ridicule me in front of the other students. He corrected me but his method of correcting was very discouraging because it was a ridicule. It made the others laugh and I didn’t like that.”
Julie: “[My PI] explicitly [asked] if I had the dedication for science. A lot of times he said I had terrible judgment. A lot of times he said I couldn’t be trusted. Once I went to a conference with him, and, unfortunately, in front of another professor, he called me a klutz several times and there was another comment about how I never learn from my mistakes.”
When students did do things correctly, they described how important it could be for them to receive praise from their mentors. They explained that hearing praise and validation can be particularly helpful for students with depression, because their thoughts are often very negative and/or because they have low self-esteem.
Crystal: “[Something that helps my depression is] I have text messages from [my graduate student mentor] thanking me [and another undergraduate researcher] for all of the work that we’ve put in, that he would not be able to be as on track to finish as he is if he didn’t have our help.”
Interviewer: “Why is hearing praise from your mentor helpful?”
Crystal: “Because a lot of my depression focuses on everybody secretly hates you, nobody likes you, you’re going to die alone. So having that validation [from my graduate mentor] is important, because it flies in the face of what my depression tells me.”
Brian: “It reminds you that you exist outside of this negative world that you’ve created for yourself, and people don’t see you how you see yourself sometimes.”
Students also highlighted how research could be overwhelming, which negatively affected their depression. Particularly, students described that research demanded a lot of their time and that their mentors did not always seem to be aware that they were juggling school and other commitments in addition to their research. This stress exacerbated their depression.
Rose: “I feel like sometimes [my grad mentors] are not very understanding because grad students don’t take as many classes as [undergrads] do. I think sometimes they don’t understand when I say I can’t come in at all this week because I have finals and they’re like, ‘Why though?’”
Abby: “I just think being more understanding of student life would be great. We have classes as well as the lab, and classes are the priority. They forget what it’s like to be a student. You feel like they don’t understand and they could never understand when you say like, ‘I have three exams this week,’ and they’re like, ‘I don’t care. You need to finish this.’”
Conversely, some students reported that their research labs were very understanding of students’ schedules. Interestingly, these students talked most about how helpful it was to be able to take a mental health day and not do research on days when they felt down or depressed.
Marta: “My lab tech is very open, so she’ll tell us, ‘I can’t come in today. I have to take a mental health day.’ So she’s a really big advocate for that. And I think I won’t personally tell her that I’m taking a mental health day, but I’ll say, ‘I can’t come in today, but I’ll come in Friday and do those extra hours.’ And she’s like, ‘OK great, I’ll see you then.’ And it makes me feel good, because it helps me take care of myself first and then I can take care of everything else I need to do, which is amazing.”
Meleana: “Knowing that [my mentors] would be flexible if I told them that I’m crazy busy and can’t come into work nearly as much this week [helps my depression]. There is flexibility in allowing me to then care for myself.”
Interviewer: “Why is the flexibility helpful given the depression?”
Meleana: “Because sometimes for me things just take a little bit longer when I’m feeling down. I’m just less efficient to be honest, and so it’s helpful if I feel like I can only go into work for 10 hours in a week. It declutters my brain a little bit to not have to worry about all the things I have to do in work in addition the things that I need to do for school or clubs, or family or whatever.”
Despite the demanding nature of research, a subset of students highlighted that their research and research lab provided a sense of stability or familiarity that distracted them from their depression.
Freddy: “I’ll [do research] to run away from those [depressive] feelings or whatever. (…) I find sadly, I hate to admit it, but I do kind of run to [my lab]. I throw myself into work to distract myself from the feelings of depression and sadness.”
Rose: “When you’re sad or when you’re stressed you want to go to things you’re familiar with. So because lab has always been in my life, it’s this thing where it’s going to be there for me I guess. It’s like a good book that you always go back to and it’s familiar and it makes you feel good. So that’s how lab is. It’s not like the greatest thing in the world but it’s something that I’m used to, which is what I feel like a lot of people need when they’re sad and life is not going well.”
Many students also explained that research positively affects their depression because they perceive their research contribution to be important.
Ashlyn: “I feel like I’m dedicating myself to something that’s worthy and something that I believe in. It’s really important because it contextualizes those times when I am feeling depressed. It’s like, no, I do have these better things that I’m working on. Even when I don’t like myself and I don’t like who I am, which is again, depression brain, I can at least say, ‘Well, I have all these other people relying on me in research and in this area and that’s super important.’”
Jessica: “I mean, it just felt like the work that I was doing had meaning and when I feel like what I’m doing is actually going to contribute to the world, that usually really helps with [depression] because it’s like not every day you can feel like you’re doing something impactful.”
In sum, students highlighted that experiencing failure in research and making mistakes negatively contributed to depression, especially when help was unavailable or research mentors had a negative reaction. Additionally, students acknowledged that the research could be time-consuming, but that research mentors who were flexible helped assuage depressive feelings that were associated with feeling overwhelmed. Finally, research helped some students’ depression, because it felt familiar, provided a distraction from depression, and reminded students that they were contributing to a greater cause.
We believe that creating more inclusive research environments for students with depression is an important step toward broadening participation in science, not only to ensure that we are not discouraging students with depression from persisting in science, but also because depression has been shown to disproportionately affect underserved and underrepresented groups in science ( Turner and Noh, 1988 ; Eisenberg et al. , 2007 ; Jenkins et al. , 2013 ; American College Health Association, 2018 ). We initially hypothesized that three features of undergraduate research—research mentors, the lab environment, and failure—may have the potential to exacerbate student depression. We found this to be true; students highlighted that their relationships with their mentors as well as the overall lab environment could negatively affect their depression, but could also positively affect their research experiences. Students also noted that they struggled with failure, which is likely true of most students, but is known to be particularly difficult for students with depression ( Elliott et al. , 1997 ). We expand upon our findings by integrating literature on depression with the information that students provided in the interviews about how research mentors can best support students. We provide a set of evidence-based recommendations focused on mentoring, the lab environment, and failure for research mentors wanting to create more inclusive research environments for students with depression. Notably, only the first recommendation is specific to students with depression; the others reflect recommendations that have previously been described as “best practices” for research mentors ( NASEM, 2017 , 2019 ; Sorkness et al. , 2017 ) and likely would benefit most students. However, we examine how these recommendations may be particularly important for students with depression. As we hypothesized, these recommendations directly address three aspects of research: mentors, lab environment, and failure. A caveat of these recommendations is that more research needs to be done to explore the experiences of students with depression and how these practices actually impact students with depression, but our national sample of undergraduate researchers with depression can provide an initial starting point for a discussion about how to improve research experiences for these students.
Recommendations to Make Undergraduate Research Experiences More Inclusive for Students with Depression
Recognize student depression as a valid illness..
Allow students with depression to take time off of research by simply saying that they are sick and provide appropriate time for students to recover from depressive episodes. Also, make an effort to destigmatize mental health issues.
Undergraduate researchers described both psychological and physical symptoms that manifested as a result of their depression and highlighted how such symptoms prevented them from performing to their full potential in undergraduate research. For example, students described how their depression would cause them to feel unmotivated, which would often negatively affect their research productivity. In cases in which students were motivated enough to come in and do their research, they described having difficulty concentrating or engaging in the work. Further, when doing research, students felt less creative and less willing to take risks, which may alter the quality of their work. Students also sometimes struggled to socialize in the lab. They described feeling less social and feeling overly self-critical. In sum, students described that, when they experienced a depressive episode, they were not able to perform to the best of their ability, and it sometimes took a toll on them to try to act like nothing was wrong, when they were internally struggling with depression. We recommend that research mentors treat depression like any other physical illness; allowing students the chance to recover when they are experiencing a depressive episode can be extremely important to students and can allow them to maximize their productivity upon returning to research ( Judd et al. , 2000 ). Students explained that if they are not able to take the time to focus on recovering during a depressive episode, then they typically continue to struggle with depression, which negatively affects their research. This sentiment is echoed by researchers in psychiatry who have found that patients who do not fully recover from a depressive episode are more likely to relapse and to experience chronic depression ( Judd et al. , 2000 ). Students described not doing tasks or not showing up to research because of their depression but struggling with how to share that information with their research mentors. Often, students would not say anything, which caused them anxiety because they were worried about what others in the lab would say to them when they returned. Admittedly, many students understood why this behavior would cause their research mentors to be angry or frustrated, but they weighed the consequences of their research mentors’ displeasure against the consequences of revealing their depression and decided it was not worth admitting to being depressed. This aligns with literature that suggests that when individuals have concealable stigmatized identities, or identities that can be hidden and that carry negative stereotypes, such as depression, they will often keep them concealed to avoid negative judgment or criticism ( Link and Phelan, 2001 ; Quinn and Earnshaw, 2011 ; Jones and King, 2014 ; Cooper and Brownell, 2016 ; Cooper et al. , 2019b ; Cooper et al ., unpublished data ). Therefore, it is important for research mentors to be explicit with students that 1) they recognize mental illness as a valid sickness and 2) that students with mental illness can simply explain that they are sick if they need to take time off. This may be useful to overtly state on a research website or in a research syllabus, contract, or agreement if mentors use such documents when mentoring undergraduates in their lab. Further, research mentors can purposefully work to destigmatize mental health issues by explicitly stating that struggling with mental health issues, such as depression and anxiety, is common. While we do not recommend that mentors ask students directly about depression, because this can force students to share when they are not comfortable sharing, we do recommend providing opportunities for students to reveal their depression ( Chaudoir and Fisher, 2010 ). Mentors can regularly check in with students about how they’re doing, and talk openly about the importance of mental health, which may increase the chance that students may feel comfortable revealing their depression ( Chaudoir and Quinn, 2010 ; Cooper et al ., unpublished data ).
Foster a Positive Lab Environment.
Encourage positivity in the research lab, promote working in shared spaces to enhance social support among lab members, and alleviate competition among undergraduates.
Students in this study highlighted that the “leadership” of the lab, meaning graduate students, postdocs, lab managers, and PIs, were often responsible for establishing the tone of the lab; that is, if they were in a bad mood it would trickle down and negatively affect the moods of the undergraduates. Explicitly reminding lab leadership that their moods can both positively and negatively affect undergraduates may be important in establishing a positive lab environment. Further, students highlighted how they were most likely to experience negative thoughts when they were alone in the lab. Therefore, it may be helpful to encourage all lab members to work in a shared space to enhance social interactions among students and to maximize the likelihood that undergraduates have access to help when needed. A review of 51 studies in psychiatry supported our undergraduate researchers’ perceptions that social relationships positively impacted their depression; the study found that perceived emotional support (e.g., someone available to listen or give advice), perceived instrumental support (e.g., someone available to help with tasks), and large diverse social networks (e.g., being socially connected to a large number of people) were significantly protective against depression ( Santini et al. , 2015 ). Additionally, despite forming positive relationships with other undergraduates in the lab, many undergraduate researchers admitted to constantly comparing themselves with other undergraduates, which led them to feel inferior, negatively affecting their depression. Some students talked about mentors favoring current undergraduates or talking positively about past undergraduates, which further exacerbated their feelings of inferiority. A recent study of students in undergraduate research experiences highlighted that inequitable distribution of praise to undergraduates can create negative perceptions of lab environments for students (Cooper et al. , 2019). Further, the psychology literature has demonstrated that when people feel insecure in their social environments, it can cause them to focus on a hierarchical view of themselves and others, which can foster feelings of inferiority and increase their vulnerability to depression ( Gilbert et al. , 2009 ). Thus, we recommend that mentors be conscious of their behaviors so that they do not unintentionally promote competition among undergraduates or express favoritism toward current or past undergraduates. Praise is likely best used without comparison with others and not done in a public way, although more research on the impact of praise on undergraduate researchers needs to be done. While significant research has been done on mentoring and mentoring relationships in the context of undergraduate research ( Byars-Winston et al. , 2015 ; Aikens et al. , 2017 ; Estrada et al. , 2018 ; Limeri et al. , 2019 ; NASEM, 2019 ), much less has been done on the influence of the lab environment broadly and how people in nonmentoring roles can influence one another. Yet, this study indicates the potential influence of many different members of the lab, not only their mentors, on students with depression.
Develop More Personal Relationships with Undergraduate Researchers and Provide Sufficient Guidance.
Make an effort to establish more personal relationships with undergraduates and ensure that they perceive that they have access to sufficient help and guidance with regard to their research.
When we asked students explicitly how research mentors could help create more inclusive environments for undergraduate researchers with depression, students overwhelmingly said that building mentor–student relationships would be extremely helpful. Students suggested that mentors could get to know students on a more personal level by asking about their career interests or interests outside of academia. Students also remarked that establishing a more personal relationship could help build the trust needed in order for undergraduates to confide in their research mentors about their depression, which they perceived would strengthen their relationships further because they could be honest about when they were not feeling well or their mentors might even “check in” with them in times where they were acting differently than normal. This aligns with studies showing that undergraduates are most likely to reveal a stigmatized identity, such as depression, when they form a close relationship with someone ( Chaudoir and Quinn, 2010 ). Many were intimidated to ask for research-related help from their mentors and expressed that they wished they had established a better relationship so that they would feel more comfortable. Therefore, we recommend that research mentors try to establish relationships with their undergraduates and explicitly invite them to ask questions or seek help when needed. These recommendations are supported by national recommendations for mentoring ( NASEM, 2019 ) and by literature that demonstrates that both social support (listening and talking with students) and instrumental support (providing students with help) have been shown to be protective against depression ( Santini et al. , 2015 ).
Treat Undergraduates with Respect and Remember to Praise Them.
Avoid providing harsh criticism and remember to praise undergraduates. Students with depression often have low self-esteem and are especially self-critical. Therefore, praise can help calibrate their overly negative self-perceptions.
Students in this study described that receiving criticism from others, especially harsh criticism, was particularly difficult for them given their depression. Multiple studies have demonstrated that people with depression can have an abnormal or maladaptive response to negative feedback; scientists hypothesize that perceived failure on a particular task can trigger failure-related thoughts that interfere with subsequent performance ( Eshel and Roiser, 2010 ). Thus, it is important for research mentors to remember to make sure to avoid unnecessarily harsh criticisms that make students feel like they have failed (more about failure is described in the next recommendation). Further, students with depression often have low self-esteem or low “personal judgment of the worthiness that is expressed in the attitudes the individual holds towards oneself” ( Heatherton et al. , 2003 , p. 220; Sowislo and Orth, 2013 ). Specifically, a meta-analysis of longitudinal studies found that low self-esteem is predictive of depression ( Sowislo and Orth, 2013 ), and depression has also been shown to be highly related to self-criticism ( Luyten et al. , 2007 ). Indeed, nearly all of the students in our study described thinking that they are “not good enough,” “worthless,” or “inadequate,” which is consistent with literature showing that people with depression are self-critical ( Blatt et al. , 1982 ; Gilbert et al. , 2006 ) and can be less optimistic of their performance on future tasks and rate their overall performance on tasks less favorably than their peers without depression ( Cane and Gotlib, 1985 ). When we asked students what aspects of undergraduate research helped their depression, students described that praise from their mentors was especially impactful, because they thought so poorly of themselves and they needed to hear something positive from someone else in order to believe it could be true. Praise has been highlighted as an important aspect of mentoring in research for many years ( Ashford, 1996 ; Gelso and Lent, 2000 ; Brown et al. , 2009 ) and may be particularly important for students with depression. In fact, praise has been shown to enhance individuals’ motivation and subsequent productivity ( Hancock, 2002 ; Henderlong and Lepper, 2002 ), factors highlighted by students as negatively affecting their depression. However, something to keep in mind is that a student with depression and a student without depression may process praise differently. For a student with depression, a small comment that praises the student’s work may not be sufficient for the student to process that comment as praise. People with depression are hyposensitive to reward or have reward-processing deficits ( Eshel and Roiser, 2010 ); therefore, praise may affect students without depression more positively than it would affect students with depression. Research mentors should be mindful that students with depression often have a negative view of themselves, and while students report that praise is extremely important, they may have trouble processing such positive feedback.
Normalize Failure and Be Explicit about the Importance of Research Contributions.
Explicitly remind students that experiencing failure is expected in research. Also explain to students how their individual work relates to the overall project so that they can understand how their contributions are important. It can also be helpful to explain to students why the research project as a whole is important in the context of the greater scientific community.
Experiencing failure has been thought to be a potentially important aspect of undergraduate research, because it may provide students with the potential to develop integral scientific skills such as the ability to navigate challenges and persevere ( Laursen et al. , 2010 ; Gin et al. , 2018 ; Henry et al. , 2019 ). However, in the interviews, students described that when their science experiments failed, it was particularly tough for their depression. Students’ negative reaction to experiencing failure in research is unsurprising, given recent literature that has predicted that students may be inadequately prepared to approach failure in science ( Henry et al. , 2019 ). However, the literature suggests that students with depression may find experiencing failure in research to be especially difficult ( Elliott et al. , 1997 ; Mongrain and Blackburn, 2005 ; Jones et al. , 2009 ). One potential hypothesis is that students with depression may be more likely to have fixed mindsets or more likely to believe that their intelligence and capacity for specific abilities are unchangeable traits ( Schleider and Weisz, 2018 ); students with a fixed mindset have been hypothesized to have particularly negative responses to experiencing failure in research, because they are prone to quitting easily in the face of challenges and becoming defensive when criticized ( Forsythe and Johnson, 2017 ; Dweck, 2008 ). A study of life sciences undergraduates enrolled in CUREs identified three strategies of students who adopted adaptive coping mechanisms, or mechanisms that help an individual maintain well-being and/or move beyond the stressor when faced with failure in undergraduate research: 1) problem solving or engaging in strategic planning and decision making, 2) support seeking or finding comfort and help with research, and 3) cognitive restructuring or reframing a problem from negative to positive and engaging in self encouragement ( Gin et al. , 2018 ). We recommend that, when undergraduates experience failure in science, their mentors be proactive in helping them problem solve, providing help and support, and encouraging them. Students also explained that mentors sharing their own struggles as undergraduate and graduate students was helpful, because it normalized failure. Sharing personal failures in research has been recommended as an important way to provide students with psychosocial support during research ( NASEM, 2019 ). We also suggest that research mentors take time to explain to students why their tasks in the lab, no matter how small, contribute to the greater research project ( Cooper et al. , 2019a ). Additionally, it is important to make sure that students can explain how the research project as a whole is contributing to the scientific community ( Gin et al. , 2018 ). Students highlighted that contributing to something important was really helpful for their depression, which is unsurprising, given that studies have shown that meaning in life or people’s comprehension of their life experiences along with a sense of overarching purpose one is working toward has been shown to be inversely related to depression ( Steger, 2013 ).
Limitations and Future Directions
This work was a qualitative interview study intended to document a previously unstudied phenomenon: depression in the context of undergraduate research experiences. We chose to conduct semistructured interviews rather than a survey because of the need for initial exploration of this area, given the paucity of prior research. A strength of this study is the sampling approach. We recruited a national sample of 35 undergraduates engaged in undergraduate research at 12 different public R1 institutions. Despite our representative sample from R1 institutions, these findings may not be generalizable to students at other types of institutions; lab environments, mentoring structures, and interactions between faculty and undergraduate researchers may be different at other institution types (e.g., private R1 institutions, R2 institutions, master’s-granting institutions, primarily undergraduate institutions, and community colleges), so we caution against making generalizations about this work to all undergraduate research experiences. Future work could assess whether students with depression at other types of institutions have similar experiences to students at research-intensive institutions. Additionally, we intentionally did not explore the experiences of students with specific identities owing to our sample size and the small number of students in any particular group (e.g., students of a particular race, students with a graduate mentor as the primary mentor). We intend to conduct future quantitative studies to further explore how students’ identities and aspects of their research affect their experiences with depression in undergraduate research.
The students who participated in the study volunteered to be interviewed about their depression; therefore, it is possible that depression is a more salient part of these students’ identities and/or that they are more comfortable talking about their depression than the average population of students with depression. It is also important to acknowledge the personal nature of the topic and that some students may not have fully shared their experiences ( Krumpal, 2013 ), particularly those experiences that may be emotional or traumatizing ( Kahn and Garrison, 2009 ). Additionally, our sample was skewed toward females (77%). While females do make up approximately 60% of students in biology programs on average ( Eddy et al. , 2014 ), they are also more likely to report experiencing depression ( American College Health Association, 2018 ; Evans et al. , 2018 ). However, this could be because women have higher rates of depression or because males are less likely to report having depression; clinical bias, or practitioners’ subconscious tendencies to overlook male distress, may underestimate depression rates in men ( Smith et al. , 2018 ). Further, females are also more likely to volunteer to participate in studies ( Porter and Whitcomb, 2005 ); therefore, many interview studies have disproportionately more females in the data set (e.g., Cooper et al. , 2017 ). If we had been able to interview more male students, we might have identified different findings. Additionally, we limited our sample to life sciences students engaged in undergraduate research at public R1 institutions. It is possible that students in other majors may have different challenges and opportunities for students with depression, as well as different disciplinary stigmas associated with mental health.
In this exploratory interview study, we identified a variety of ways in which depression in undergraduates negatively affected their undergraduate research experiences. Specifically, we found that depression interfered with students’ motivation and productivity, creativity and risk-taking, engagement and concentration, and self-perception and socializing. We also identified that research can negatively affect depression in undergraduates. Experiencing failure in research can exacerbate student depression, especially when students do not have access to adequate guidance. Additionally, being alone or having negative interactions with others in the lab worsened students’ depression. However, we also found that undergraduate research can positively affect students’ depression. Research can provide a familiar space where students can feel as though they are contributing to something meaningful. Additionally, students reported that having access to adequate guidance and a social support network within the research lab also positively affected their depression. We hope that this work can spark conversations about how to make undergraduate research experiences more inclusive of students with depression and that it can stimulate additional research that more broadly explores the experiences of undergraduate researchers with depression.
Important note
If you or a student experience symptoms of depression and want help, there are resources available to you. Many campuses provide counseling centers equipped to provide students, staff, and faculty with treatment for depression, as well as university-dedicated crisis hotlines. Additionally, there are free 24/7 services such as Crisis Text Line, which allows you to text a trained live crisis counselor (Text “CONNECT” to 741741; Text Depression Hotline , 2019 ), and phone hotlines such as the National Suicide Prevention Lifeline at 1-800-273-8255 (TALK). You can also learn more about depression and where to find help near you through the Anxiety and Depression Association of American website: https://adaa.org ( Anxiety and Depression Association of America, 2019 ) and the Depression and Biopolar Support Alliance: http://dbsalliance.org ( Depression and Biopolar Support Alliance, 2019 ).
ACKNOWLEDGMENTS
We are extremely grateful to the undergraduate researchers who shared their thoughts and experiences about depression with us. We acknowledge the ASU LEAP Scholars for helping us create the original survey and Rachel Scott for her helpful feedback on earlier drafts of this article. L.E.G. was supported by a National Science Foundation (NSF) Graduate Fellowship (DGE-1311230) and K.M.C. was partially supported by a Howard Hughes Medical Institute (HHMI) Inclusive Excellence grant (no. 11046) and an NSF grant (no. 1644236). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF or HHMI.
- Aikens, M. L., Robertson, M. M., Sadselia, S., Watkins, K., Evans, M., Runyon, C. R. , … & Dolan, E. L. ( 2017 ). Race and gender differences in undergraduate research mentoring structures and research outcomes . CBE—Life Sciences Education , 16 (2), ar34. Link , Google Scholar
- Aikens, M. L., Sadselia, S., Watkins, K., Evans, M., Eby, L. T., & Dolan, E. L. ( 2016 ). A social capital perspective on the mentoring of undergraduate life science researchers: An empirical study of undergraduate–postgraduate–faculty triads . CBE—Life Sciences Education , 15 (2), ar16. Link , Google Scholar
- Aldwin, C., & Greenberger, E. ( 1987 ). Cultural differences in the predictors of depression . American Journal of Community Psychology , 15 (6), 789–813. Medline , Google Scholar
- American Association for the Advancement of Science . ( 2011 ). Vision and change in undergraduate biology education: A call to action . Retrieved November 29, 2019, from http://visionandchange.org/files/2013/11/aaas-VISchange-web1113.pdf Google Scholar
- American College Health Association . ( 2018 ). Undergraduate reference group executive summary, Fall 2018 . Retrieved November 29, 2019, from www.acha.org/documents/ncha/NCHA-II_Fall_2018_Reference_Group_Executive_Summary.pdf Google Scholar
- American College Health Association . ( 2019 ). Retrieved November 29, 2019, from NCHA-II_SPRING_2019_UNDERGRADUATE_REFERENCE_GROUP_DATA_REPORT.pdf www.acha.org/documents/ncha/NCHA-II_SPRING_2019_UNDERGRADUATE_REFERENCE_GROUP_DATA_REPORT.pdf Google Scholar
- American Psychiatric Association . ( 2013 ). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Publishing. Google Scholar
- Aneshensel, C. S., & Stone, J. D. ( 1982 ). Stress and depression: A test of the buffering model of social support . Archives of General Psychiatry , 39 (12), 1392–1396. Medline , Google Scholar
- Anxiety and Depression Association of America . ( 2019 ). Home page . Retrieved November 29, 2019, from https://adaa.org Google Scholar
- Armbruster, P., Patel, M., Johnson, E., & Weiss, M. ( 2009 ). Active learning and student-centered pedagogy improve student attitudes and performance in introductory biology . CBE—Life Sciences Education , 8 (3), 203–213. Link , Google Scholar
- Ashford, S. J. ( 1996 ). Working with doctoral students: Rhythms of Academic Life: Personal Accounts of Careers in Academia . In Front, P. J.Taylor, M. S. (Eds.), Rhythms of Academic Life: Personal Accounts of Careers in Academia (pp. 153–158). Thousand Oaks, CA: Sage. Google Scholar
- Auchincloss, L. C., Laursen, S. L., Branchaw, J. L., Eagan, K., Graham, M., Hanauer, D. I. , … & Rowland, S. ( 2014 ). Assessment of course-based undergraduate research experiences: A meeting report . CBE—Life Sciences Education , 13 (1), 29–40. Link , Google Scholar
- Barak, M. E. M., Levin, A., Nissly, J. A., & Lane, C. J. ( 2006 ). Why do they leave? Modeling child welfare workers’ turnover intentions . Children and Youth Services Review , 28 (5), 548–577. Google Scholar
- Bauer, K. W., & Bennett, J. S. ( 2003 ). Alumni perceptions used to assess undergraduate research experience . Journal of Higher Education , 74 (2), 210–230. Google Scholar
- Birks, M., & Mills, J. ( 2015 ). Grounded theory: A practical guide . Thousand Oaks, CA: Sage. Google Scholar
- Blatt, S. J., Quinlan, D. M., Chevron, E. S., McDonald, C., & Zuroff, D. ( 1982 ). Dependency and self-criticism: Psychological dimensions of depression . Journal of Consulting and Clinical Psychology , 50 (1), 113. Medline , Google Scholar
- Brown, R. T., Daly, B. P., & Leong, F. T. ( 2009 ). Mentoring in research: A developmental approach . Professional Psychology: Research and Practice , 40 (3), 306. Google Scholar
- Brownell, S. E., Hekmat-Scafe, D. S., Singla, V., Seawell, P. C., Imam, J. F. C., Eddy, S. L. , … & Cyert, M. S. ( 2015 ). A high-enrollment course-based undergraduate research experience improves student conceptions of scientific thinking and ability to interpret data . CBE—Life Sciences Education , 14 (2), ar21. Link , Google Scholar
- Brownell, S. E., & Kloser, M. J. ( 2015 ). Toward a conceptual framework for measuring the effectiveness of course-based undergraduate research experiences in undergraduate biology . Studies in Higher Education , 40 (3), 525–544. Google Scholar
- Byars-Winston, A. M., Branchaw, J., Pfund, C., Leverett, P., & Newton, J. ( 2015 ). Culturally diverse undergraduate researchers’ academic outcomes and perceptions of their research mentoring relationships . International Journal of Science Education , 37 (15), 2533–2554. Medline , Google Scholar
- Cane, D. B., & Gotlib, I. H. ( 1985 ). Depression and the effects of positive and negative feedback on expectations, evaluations, and performance . Cognitive Therapy and Research , 9 (2), 145–160. Google Scholar
- Ceci, S. J., & Williams, W. M. ( 2010 ). Sex differences in math-intensive fields . Current Directions in Psychological Science , 19 (5), 275–279. Medline , Google Scholar
- Center for Collegiate Mental Health . ( 2017 ). Center for Collegiate Mental Health 2017 Annual Report . State College, PA: Penn State Universit. Google Scholar
- Charmaz, K. ( 2006 ). Constructing grounded theory: A practical guide through qualitative research . Thousand Oaks, CA: Sage. Google Scholar
- Chaudoir, S. R., & Fisher, J. D. ( 2010 ). The disclosure processes model: Understanding disclosure decision making and postdisclosure outcomes among people living with a concealable stigmatized identity . Psychological Bulletin , 136 (2), 236. Medline , Google Scholar
- Chaudoir, S. R., & Quinn, D. M. ( 2010 ). Revealing concealable stigmatized identities: The impact of disclosure motivations and positive first-disclosure experiences on fear of disclosure and well-being . Journal of Social Issues , 66 (3), 570–584. Medline , Google Scholar
- Clance, P. R., & Imes, S. A. ( 1978 ). The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention . Psychotherapy: Theory, Research & Practice , 15 (3), 241. Google Scholar
- Cooper, K. M., Ashley, M., & Brownell, S. E. ( 2017 ). A bridge to active learning: A summer bridge program helps students maximize their active-learning experiences and the active-learning experiences of others . CBE—Life Sciences Education , 16 (1), ar17. Link , Google Scholar
- Cooper, K. M., Blattman, J. N., Hendrix, T., & Brownell, S. E. ( 2019a ). The impact of broadly relevant novel discoveries on student project ownership in a traditional lab course turned CURE . CBE—Life Sciences Education , 18 (4), ar57. Link , Google Scholar
- Cooper, K. M., & Brownell, S. E. ( 2016 ). Coming out in class: Challenges and benefits of active learning in a biology classroom for LGBTQIA students . CBE—Life Sciences Education , 15 (3), ar37. https://doi.org/10.1187/cbe.16-01-0074 Link , Google Scholar
- Cooper, K. M., Brownell, S. E., & Gormally, C. C. ( 2019b ). Coming out to the class: Identifying factors that influence college biology instructor decisions about whether to reveal their LGBQ identity in class . Journal of Women and Minorities in Science and Engineering , 25 (3). Google Scholar
- Cooper, K. M., Downing, V. R., & Brownell, S. E. ( 2018 ). The influence of active learning practices on student anxiety in large-enrollment college science classrooms . International Journal of STEM Education , 5 (1), 23. Medline , Google Scholar
- Cooper, K. M., Gin, L. E., Akeeh, B., Clark, C. E., Hunter, J. S., Roderick, T. B. , … & Brownell, S. E. ( 2019c ). Factors that predict life sciences student persistence in undergraduate research experiences . PLoS ONE , 14 (8). https://doi.org/10.1371/journal.pone.0220186 Google Scholar
- Cooper, K. M., Gin, L. E., & Brownell, S. E. ( 2019d ). Diagnosing differences in what introductory biology students in a fully online and an in-person biology degree program know and do regarding medical school admission . Advances in Physiology Education , 43 (2), 221–232. Medline , Google Scholar
- Cooper, K. M., Gin, L. E., & Brownell, S. E. ( In press ). Depression as a concealable stigmatized identity: What influences whether students conceal or reveal their depression in undergraduate research experiences? International Journal of STEM Education , ( in press ). Google Scholar
- Depression and Biopolar Support Alliance . ( 2019 ). Home page . Retrieved November 28, 2019, from www.dbsalliance.org Google Scholar
- Deroma, V. M., Leach, J. B., & Leverett, J. P. ( 2009 ). The relationship between depression and college academic performance . College Student Journal , 43 (2), 325–335. Google Scholar
- Dweck, C. S. ( 2008 ). Mindset: The new psychology of success . New York, NY: Random House Digital. Google Scholar
- Dyson, R., & Renk, K. ( 2006 ). Freshmen adaptation to university life: Depressive symptoms, stress, and coping . Journal of Clinical Psychology , 62 (10), 1231–1244. Medline , Google Scholar
- Eddy, S. L., Brownell, S. E., & Wenderoth, M. P. ( 2014 ). Gender gaps in achievement and participation in multiple introductory biology classrooms . CBE—Life Sciences Education , 13 (3), 478–492. https://doi.org/10.1187/cbe.13-10-0204 Link , Google Scholar
- Eisenberg, D., Gollust, S. E., Golberstein, E., & Hefner, J. L. ( 2007 ). Prevalence and correlates of depression, anxiety, and suicidality among university students . American Journal of Orthopsychiatry , 77 (4), 534–542. Medline , Google Scholar
- Elliott, R., Sahakian, B. J., Herrod, J. J., Robbins, T. W., & Paykel, E. S. ( 1997 ). Abnormal response to negative feedback in unipolar depression: Evidence for a diagnosis specific impairment . Journal of Neurology, Neurosurgery & Psychiatry , 63 (1), 74–82. Medline , Google Scholar
- Eshel, N., & Roiser, J. P. ( 2010 ). Reward and punishment processing in depression . Biological Psychiatry , 68 (2), 118–124. Medline , Google Scholar
- Estrada, M., Hernandez, P. R., & Schultz, P. W. ( 2018 ). A longitudinal study of how quality mentorship and research experience integrate underrepresented minorities into STEM careers . CBE—Life Sciences Education , 17 (1), ar9. Link , Google Scholar
- Evans, T. M., Bira, L., Gastelum, J. B., Weiss, L. T., & Vanderford, N. L. ( 2018 ). Evidence for a mental health crisis in graduate education . Nature Biotechnology , 36 (3), 282. Medline , Google Scholar
- Everson, H. T., Tobias, S., Hartman, H., & Gourgey, A. ( 1993 ). Test anxiety and the curriculum: The subject matters . Anxiety, Stress, and Coping , 6 (1), 1–8. Google Scholar
- Flaherty, C. ( 2018 ). New study says graduate students’ mental health is a “crisis.” Retrieved November 29, 2019, from www.insidehighered.com/news/2018/03/06/new-study-says-graduate-students-mental-health-crisis Google Scholar
- Forsythe, A., & Johnson, S. ( 2017 ). Thanks, but no-thanks for the feedback . Assessment & Evaluation in Higher Education , 42 (6), 850–859. Google Scholar
- Garlow, S. J., Rosenberg, J., Moore, J. D., Haas, A. P., Koestner, B., Hendin, H., & Nemeroff, C. B. ( 2008 ). Depression, desperation, and suicidal ideation in college students: Results from the American Foundation for Suicide Prevention College Screening Project at Emory University . Depression and Anxiety , 25 (6), 482–488. Medline , Google Scholar
- Gelso, C. J., & Lent, R. W. ( 2000 ). Scientific training and scholarly productivity: The person, the training environment, and their interaction . In Brown, S. D.Lent, R. W. (Eds.), Handbook of counseling psychology (pp. 109–139). Hoboken, NJ: John Wiley & Sons Inc. Google Scholar
- Gilbert, P., Baldwin, M. W., Irons, C., Baccus, J. R., & Palmer, M. ( 2006 ). Self-criticism and self-warmth: An imagery study exploring their relation to depression . Journal of Cognitive Psychotherapy , 20 (2), 183. Google Scholar
- Gilbert, P., McEwan, K., Bellew, R., Mills, A., & Gale, C. ( 2009 ). The dark side of competition: How competitive behaviour and striving to avoid inferiority are linked to depression, anxiety, stress and self-harm . Psychology and Psychotherapy: Theory, Research and Practice , 82 (2), 123–136. Medline , Google Scholar
- Gin, L. E., Rowland, A. A., Steinwand, B., Bruno, J., & Corwin, L. A. ( 2018 ). Students who fail to achieve predefined research goals may still experience many positive outcomes as a result of CURE participation . CBE—Life Sciences Education , 17 (4), ar57. Link , Google Scholar
- Glesne, C., & Peshkin, A. ( 1992 ). Becoming qualitative researchers: An introduction . London, England, UK: Longman. Google Scholar
- Grav, S., Hellzèn, O., Romild, U., & Stordal, E. ( 2012 ). Association between social support and depression in the general population: The HUNT study, a cross-sectional survey . Journal of Clinical Nursing , 21 (1–2), 111–120. Medline , Google Scholar
- Guest, G., Bunce, A., & Johnson, L. ( 2006 ). How many interviews are enough? An experiment with data saturation and variability . Field Methods , 18 (1), 59–82. Google Scholar
- Hancock, D. R. ( 2002 ). Influencing graduate students’ classroom achievement, homework habits and motivation to learn with verbal praise . Educational Research , 44 (1), 83–95. Google Scholar
- Hannah, D. R., & Lautsch, B. A. ( 2011 ). Counting in qualitative research: Why to conduct it, when to avoid it, and when to closet it . Journal of Management Inquiry , 20 (1), 14–22. Google Scholar
- Heatherton, T. F., & Wyland, C. L. ( 2003 ). Assessing self-esteem . In Lopez, S. J.Snyder, C. R. (Eds.), Positive psychological assessment: A handbook of models and measures (pp. 219–233). Washington, DC: American Psychological Association. https://doi.org/10.1037/10612-014 . Google Scholar
- Henderlong, J., & Lepper, M. R. ( 2002 ). The effects of praise on children’s intrinsic motivation: A review and synthesis . Psychological Bulletin , 128 (5), 774. Medline , Google Scholar
- Henry, M. A., Shorter, S., Charkoudian, L., Heemstra, J. M., & Corwin, L. A. ( 2019 ). FAIL is not a four-letter word: A theoretical framework for exploring undergraduate students’ approaches to academic challenge and responses to failure in STEM learning environments . CBE—Life Sciences Education , 18 (1), ar11. Link , Google Scholar
- Hernandez, P. R., Woodcock, A., Estrada, M., & Schultz, P. W. ( 2018 ). Undergraduate research experiences broaden diversity in the scientific workforce . BioScience , 68 (3), 204–211. Google Scholar
- Hish, A. J., Nagy, G. A., Fang, C. M., Kelley, L., Nicchitta, C. V., Dzirasa, K., & Rosenthal, M. Z. ( 2019 ). Applying the stress process model to stress–burnout and stress–depression relationships in biomedical doctoral students: A cross-sectional pilot study . CBE—Life Sciences Education , 18 (4), ar51. Link , Google Scholar
- Howell, E., & McFeeters, J. ( 2008 ). Children’s mental health care: Differences by race/ethnicity in urban/rural areas . Journal of Health Care for the Poor and Underserved , 19 (1), 237–247. Medline , Google Scholar
- Hysenbegasi, A., Hass, S. L., & Rowland, C. R. ( 2005 ). The impact of depression on the academic productivity of university students . Journal of Mental Health Policy and Economics , 8 (3), 145. Medline , Google Scholar
- Ibrahim, A. K., Kelly, S. J., Adams, C. E., & Glazebrook, C. ( 2013 ). A systematic review of studies of depression prevalence in university students . Journal of Psychiatric Research , 47 (3), 391–400. Medline , Google Scholar
- Intemann, K. ( 2009 ). Why diversity matters: Understanding and applying the diversity component of the National Science Foundation’s broader impacts criterion . Social Epistemology , 23 (3–4), 249–266. Google Scholar
- Ishiyama, J. ( 2002 ). Does early participation in undergraduate research benefit social science and humanities students? College Student Journal , 36 (3), 381–387. Google Scholar
- Jenkins, S. R., Belanger, A., Connally, M. L., Boals, A., & Durón, K. M. ( 2013 ). First-generation undergraduate students’ social support, depression, and life satisfaction . Journal of College Counseling , 16 (2), 129–142. Google Scholar
- Jobst, A., Sabass, L., Palagyi, A., Bauriedl-Schmidt, C., Mauer, M. C., Sarubin, N. , … & Zill, P. ( 2015 ). Effects of social exclusion on emotions and oxytocin and cortisol levels in patients with chronic depression . Journal of Psychiatric Research , 60 , 170–177. Medline , Google Scholar
- Jones, K. P., & King, E. B. ( 2014 ). Managing concealable stigmas at work: A review and multilevel model . Journal of Management , 40 (5), 1466–1494. Google Scholar
- Jones, M. T., Barlow, A. E., & Villarejo, M. ( 2010 ). Importance of undergraduate research for minority persistence and achievement in biology . Journal of Higher Education , 81 (1), 82–115. Google Scholar
- Jones, N. P., Papadakis, A. A., Hogan, C. M., & Strauman, T. J. ( 2009 ). Over and over again: Rumination, reflection, and promotion goal failure and their interactive effects on depressive symptoms . Behaviour Research and Therapy , 47 (3), 254–259. Medline , Google Scholar
- Judd, L. L., Paulus, M. J., Schettler, P. J., Akiskal, H. S., Endicott, J., Leon, A. C. , … & Keller, M. B. ( 2000 ). Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? American Journal of Psychiatry , 157 (9), 1501–1504. Medline , Google Scholar
- Kahn, J. H., & Garrison, A. M. ( 2009 ). Emotional self-disclosure and emotional avoidance: Relations with symptoms of depression and anxiety . Journal of Counseling Psychology , 56 (4), 573. Google Scholar
- Kataoka, S. H., Zhang, L., & Wells, K. B. ( 2002 ). Unmet need for mental health care among US children: Variation by ethnicity and insurance status . American Journal of Psychiatry , 159 (9), 1548–1555. Medline , Google Scholar
- Kreger, D. W. ( 1995 ). Self-esteem, stress, and depression among graduate students . Psychological Reports , 76 (1), 345–346. Medline , Google Scholar
- Krumpal, I. ( 2013 ). Determinants of social desirability bias in sensitive surveys: A literature review . Quality & Quantity , 47 (4), 2025–2047. Google Scholar
- Landis, J. R., & Koch, G. G. ( 1977 ). An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers . Biometrics , 33 (2), 363–374. Medline , Google Scholar
- Laursen, S., Hunter, A.-B., Seymour, E., Thiry, H., & Melton, G. ( 2010 ). Undergraduate research in the sciences: Engaging students in real science . Hoboken, NJ: Wiley. Google Scholar
- Limeri, L. B., Asif, M. Z., Bridges, B. H., Esparza, D., Tuma, T. T., Sanders, D. , … & Maltese, A. V. ( 2019 ). “Where’s my mentor?” Characterizing negative mentoring experiences in undergraduate life science research . CBE—Life Sciences Education , 18 (4), ar61. Link , Google Scholar
- Link, B. G., & Phelan, J. C. ( 2001 ). Conceptualizing stigma . Annual Review of Sociology , 27 (1), 363–385. Google Scholar
- Luyten, P., Sabbe, B., Blatt, S. J., Meganck, S., Jansen, B., De Grave, C. , … & Corveleyn, J. ( 2007 ). Dependency and self-criticism: Relationship with major depressive disorder, severity of depression, and clinical presentation . Depression and Anxiety , 24 (8), 586–596. Medline , Google Scholar
- Mabrouk, P. A., & Peters, K. ( 2000 ). Student perspectives on undergraduate research (UR) experiences in chemistry and biology . CUR Quarterly , 21 (1), 25–33. Google Scholar
- Maxwell, J. A. ( 2010 ). Using numbers in qualitative research . Qualitative Inquiry , 16 (6), 475–482. Google Scholar
- Mongrain, M., & Blackburn, S. ( 2005 ). Cognitive vulnerability, lifetime risk, and the recurrence of major depression in graduate students . Cognitive Therapy and Research , 29 (6), 747–768. Google Scholar
- Nagy, G. A., Fang, C. M., Hish, A. J., Kelly, L., Nicchitta, C. V., Dzirasa, K., & Rosenthal, M. Z. ( 2019 ). Burnout and mental health problems in biomedical doctoral students . CBE—Life Sciences Education , 18 (2), ar27. Link , Google Scholar
- National Academies of Sciences, Engineering, and Medicine (NASEM) . ( 2017 ). Undergraduate research experiences for STEM students: Successes, challenges, and opportunities . Washington, DC: National Academies Press. https://doi.org/10.17226/24622 Google Scholar
- NASEM . ( 2019 ). The science of effective mentorship in STEMM . Washington, DC: National Academies Press. Retrieved November 29, 2019, from www.nap.edu/download/25568 Google Scholar
- Osborne, J., & Collins, S. ( 2001 ). Pupils’ views of the role and value of the science curriculum: A focus-group study . International Journal of Science Education , 23 (5), 441–467. https://doi.org/10.1080/09500690010006518 Google Scholar
- Porter, S. R., & Whitcomb, M. E. ( 2005 ). Non-response in student surveys: The role of demographics, engagement and personality . Research in Higher Education , 46 (2), 127–152. Google Scholar
- President’s Council of Advisors on Science and Technology . ( 2012 ). Engage to excel: Producing one million additional college graduates with degrees in science, Technology, Engineering, and mathematics . Washington, DC: U.S. Government Office of Science and Technology. Google Scholar
- Prunuske, A. J., Wilson, J., Walls, M., & Clarke, B. ( 2013 ). Experiences of mentors training underrepresented undergraduates in the research laboratory . CBE—Life Sciences Education , 12 (3), 403–409. Link , Google Scholar
- Quinn, D. M., & Earnshaw, V. A. ( 2011 ). Understanding concealable stigmatized identities: The role of identity in psychological, physical, and behavioral outcomes . Social Issues and Policy Review , 5 (1), 160–190. Google Scholar
- Rauckhorst, W. H., Czaja, J. A., & Baxter Magolda, M. ( 2001 ). Measuring the impact of the undergraduate research experience on student intellectual development . Snowbird, UT: Project Kaleidoscope Summer Institute. Google Scholar
- Saldaña, J. ( 2015 ). The coding manual for qualitative researchers . Thousand Oaks, CA: Sage. Google Scholar
- Santiago, C. D., Kaltman, S., & Miranda, J. ( 2013 ). Poverty and mental health: How do low-income adults and children fare in psychotherapy? Journal of Clinical Psychology , 69 (2), 115–126. Medline , Google Scholar
- Santini, Z. I., Koyanagi, A., Tyrovolas, S., Mason, C., & Haro, J. M. ( 2015 ). The association between social relationships and depression: A systematic review . Journal of Affective Disorders , 175 , 53–65. Medline , Google Scholar
- Schleider, J., & Weisz, J. ( 2018 ). A single-session growth mindset intervention for adolescent anxiety and depression: 9-month outcomes of a randomized trial . Journal of Child Psychology and Psychiatry , 59 (2), 160–170. Medline , Google Scholar
- Seymour, E., & Hewitt, N. M. ( 1997 ). Talking about leaving: Why undergraduates leave the sciences . Westview Press. Google Scholar
- Seymour, E., & Hunter, A.-B. ( 2019 ). Talking about leaving revisited . New York, NY: Springer. Google Scholar
- Seymour, E., Hunter, A.-B., Laursen, S. L., & DeAntoni, T. ( 2004 ). Establishing the benefits of research experiences for undergraduates in the sciences: First findings from a three-year study . Science Education , 88 (4), 493–534. Google Scholar
- Smith, D. T., Mouzon, D. M., & Elliott, M. ( 2018 ). Reviewing the assumptions about men’s mental health: An exploration of the gender binary . American Journal of Men’s Health , 12 (1), 78–89. Medline , Google Scholar
- Sorkness, C. A., Pfund, C., Ofili, E. O., Okuyemi, K. S., Vishwanatha, J. K., Zavala, M. E. , … & Deveci, A. ( 2017 ). A new approach to mentoring for research careers: The National Research Mentoring Network . BMC Proceedings , 11 , 22. Medline , Google Scholar
- Sowislo, J. F., & Orth, U. ( 2013 ). Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies . Psychological Bulletin , 139 (1), 213. Medline , Google Scholar
- Steger, M. F. ( 2013 ). Experiencing meaning in life: Optimal functioning at the nexus of well-being, psychopathology, and spirituality . In Wong, P. T. P. (Ed.), The human quest for meaning (pp. 211–230). England, UK: Routledge. Google Scholar
- Strenta, A. C., Elliott, R., Adair, R., Matier, M., & Scott, J. ( 1994 ). Choosing and leaving science in highly selective institutions . Research in Higher Education , 35 (5), 513–547. Google Scholar
- Text Depression Hotline . ( 2019 ). Crisis text line . Retrieved November 29, 2019, from www.crisistextline.org/depression Google Scholar
- Thiry, H., & Laursen, S. L. ( 2011 ). The role of student–advisor interactions in apprenticing undergraduate researchers into a scientific community of practice . Journal of Science Education and Technology , 20 (6), 771–784. Google Scholar
- Thompson, J. J., Conaway, E., & Dolan, E. L. ( 2016 ). Undergraduate students’ development of social, cultural, and human capital in a networked research experience . Cultural Studies of Science Education , 11 (4), 959–990. Google Scholar
- Trenor, J. M., Miller, M. K., & Gipson, K. G. ( 2011 ). Utilization of a think-aloud protocol to cognitively validate a survey instrument identifying social capital resources of engineering undergraduates . 118th American Society for Engineering Education Annual Conference and Exposition, Vancouver, BC, Canada . Google Scholar
- Turner, R. J., & Noh, S. ( 1988 ). Physical disability and depression: A longitudinal analysis . Journal of Health and Social Behavior , 29 (1), 23–37. Medline , Google Scholar
- Watson, D., & Friend, R. ( 1969 ). Measurement of social-evaluative anxiety . Journal of Consulting and Clinical Psychology , 33 (4), 448. Medline , Google Scholar
- Weeks, J. W., Heimberg, R. G., Fresco, D. M., Hart, T. A., Turk, C. L., Schneier, F. R., & Liebowitz, M. R. ( 2005 ). Empirical validation and psychometric evaluation of the Brief Fear of Negative Evaluation Scale in patients with social anxiety disorder . Psychological Assessment , 17 (2), 179. Medline , Google Scholar
- World Health Organization . ( 2018 ). Depression . Retrieved November 29, 2019, from www.who.int/news-room/fact-sheets/detail/depression Google Scholar
- Wyatt, T., & Oswalt, S. B. ( 2013 ). Comparing mental health issues among undergraduate and graduate students . American Journal of Health Education , 44 (2), 96–107. Google Scholar
- Carly A. Busch ,
- Tala Araghi ,
- Jingyi He ,
- Katelyn M. Cooper , and
- Colin Harrison, Monitoring Editor
- Emma C. Goodwin ,
- Danielle Pais ,
- Logan E. Gin , and
- Derek Braun, Monitoring Editor
- Baylee A. Edwards ,
- Chloe Bowen ,
- M. Elizabeth Barnes , and
- Tati Russo-Tait, Monitoring Editor
- Sara E. Grineski ,
- Danielle X. Morales , and
- Timothy W. Collins
- Carly A. Busch , and
- Tasneem F. Mohammed ,
- Erika M. Nadile ,
- Madison L. Witt ,
- Cindy Vargas ,
- Missy Tran ,
- Joseph Gazing Wolf ,
- Danielle Brister , and
- Sehoya Cotner, Monitoring Editor
- Katelyn M. Cooper ,
- Sarah L. Eddy , and
- Coping behavior versus coping style: characterizing a measure of coping in undergraduate STEM contexts 14 February 2022 | International Journal of STEM Education, Vol. 9, No. 1
- Lisa A. Corwin ,
- Michael E. Ramsey ,
- Eric A. Vance ,
- Elizabeth Woolner ,
- Stevie Maiden ,
- Nina Gustafson and
- Joseph A. Harsh
- Erin Shortlidge, Monitoring Editor
- K. Supriya ,
- Brian Sato, Monitoring Editor
- Logan E. Gin ,
- Clark Coffman, Monitoring Editor
- Nicholas J. Wiesenthal , and
- Maryrose Weatherton and
- Elisabeth E. Schussler
- Erika Offerdahl, Monitoring Editor
- Eight Recommendations to Promote Effective Study Habits for Biology Students Enrolled in Online Courses Journal of Microbiology & Biology Education, Vol. 23, No. 1
- Fostering professional development through undergraduate research: supporting faculty mentors and student researchers 30 March 2022 | Mentoring & Tutoring: Partnership in Learning, Vol. 30, No. 2
- Jeffrey Maloy ,
- Monika B. Kwapisz , and
- Bryce E. Hughes
- Terrell Morton, Monitoring Editor
- Anxiety and depression among US college students engaging in undergraduate research during the COVID-19 pandemic 14 December 2021 | Journal of American College Health, Vol. 9
- Danielle Brister ,
- Sara E. Brownell ,
- Chade T. Claiborne ,
- Curtis Lunt ,
- Kobe M. Walker ,
- Tamiru D. Warkina ,
- Yi Zheng , and
- Rebecca Price, Monitoring Editor
- Dominant Learning Styles of Interior Design Students in Generation Z 26 July 2021 | Journal of Interior Design, Vol. 46, No. 4
- Linking Emotional Intelligence, Physical Activity and Aggression among Undergraduates 26 November 2021 | International Journal of Environmental Research and Public Health, Vol. 18, No. 23
- Advancing undergraduate synthetic biology education: insights from a Canadian iGEM student perspective Canadian Journal of Microbiology, Vol. 67, No. 10
- Frank A. Guerrero ,
- Sara E. Brownell , and
- Jennifer Momsen, Monitoring Editor
- Nicholas J. Wiesenthal ,
- Isabella Ferreira , and
- Grant Ean Gardner, Monitoring Editor
- Carolyn E. Clark ,
- Deanna B. Elliott ,
- Travis B. Roderick ,
- Rachel A. Scott ,
- Denisse Arellano ,
- Diana Ramirez ,
- Kimberly Velarde ,
- Allyson Aeschliman ,
- Sarah T. Avalle ,
- Jessica Berkheimer ,
- Rachel Campos ,
- Michael Gerbasi ,
- Sophia Hughes ,
- Julie A. Roberts ,
- Quinn M. White ,
- Ehren Wittekind ,
- Rachelle Spell, Monitoring Editor
- Christine Pfund ,
- Janet L. Branchaw ,
- Melissa McDaniels ,
- Angela Byars-Winston ,
- Steven P. Lee ,, and
- Bruce Birren
- Vladimir Anokhin ,
- MacKenzie J. Gray ,
- Daniel E. Zajic ,
- Jason E. Podrabsky , and
- Erin E. Shortlidge
- Depression as a concealable stigmatized identity: what influences whether students conceal or reveal their depression in undergraduate research experiences? 4 June 2020 | International Journal of STEM Education, Vol. 7, No. 1

Submitted: 4 November 2019 Revised: 24 February 2020 Accepted: 6 March 2020
© 2020 K. M. Cooper, L. E. Gin, et al. CBE—Life Sciences Education © 2020 The American Society for Cell Biology. This article is distributed by The American Society for Cell Biology under license from the author(s). It is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2023 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
7 Depression Research Paper Topic Ideas
Nancy Schimelpfening, MS is the administrator for the non-profit depression support group Depression Sanctuary. Nancy has a lifetime of experience with depression, experiencing firsthand how devastating this illness can be.
Cara Lustik is a fact-checker and copywriter.
:max_bytes(150000):strip_icc():format(webp)/Cara-Lustik-1000-77abe13cf6c14a34a58c2a0ffb7297da.jpg)
In psychology classes, it's common for students to write a depression research paper. Researching depression may be beneficial if you have a personal interest in this topic and want to learn more, or if you're simply passionate about this mental health issue. However, since depression is a very complex subject, it offers many possible topics to focus on, which may leave you wondering where to begin.
If this is how you feel, here are a few research titles about depression to help inspire your topic choice. You can use these suggestions as actual research titles about depression, or you can use them to lead you to other more in-depth topics that you can look into further for your depression research paper.
What Is Depression?
Everyone experiences times when they feel a little bit blue or sad. This is a normal part of being human. Depression, however, is a medical condition that is quite different from everyday moodiness.
Your depression research paper may explore the basics, or it might delve deeper into the definition of clinical depression or the difference between clinical depression and sadness .
What Research Says About the Psychology of Depression
Studies suggest that there are biological, psychological, and social aspects to depression, giving you many different areas to consider for your research title about depression.
Types of Depression
There are several different types of depression that are dependent on how an individual's depression symptoms manifest themselves. Depression symptoms may vary in severity or in what is causing them. For instance, major depressive disorder (MDD) may have no identifiable cause, while postpartum depression is typically linked to pregnancy and childbirth.
Depressive symptoms may also be part of an illness called bipolar disorder. This includes fluctuations between depressive episodes and a state of extreme elation called mania. Bipolar disorder is a topic that offers many research opportunities, from its definition and its causes to associated risks, symptoms, and treatment.
Causes of Depression
The possible causes of depression are many and not yet well understood. However, it most likely results from an interplay of genetic vulnerability and environmental factors. Your depression research paper could explore one or more of these causes and reference the latest research on the topic.
For instance, how does an imbalance in brain chemistry or poor nutrition relate to depression? Is there a relationship between the stressful, busier lives of today's society and the rise of depression? How can grief or a major medical condition lead to overwhelming sadness and depression?
Who Is at Risk for Depression?
This is a good research question about depression as certain risk factors may make a person more prone to developing this mental health condition, such as a family history of depression, adverse childhood experiences, stress , illness, and gender . This is not a complete list of all risk factors, however, it's a good place to start.
The growing rate of depression in children, teenagers, and young adults is an interesting subtopic you can focus on as well. Whether you dive into the reasons behind the increase in rates of depression or discuss the treatment options that are safe for young people, there is a lot of research available in this area and many unanswered questions to consider.
Depression Signs and Symptoms
The signs of depression are those outward manifestations of the illness that a doctor can observe when they examine a patient. For example, a lack of emotional responsiveness is a visible sign. On the other hand, symptoms are subjective things about the illness that only the patient can observe, such as feelings of guilt or sadness.
An illness such as depression is often invisible to the outside observer. That is why it is very important for patients to make an accurate accounting of all of their symptoms so their doctor can diagnose them properly. In your depression research paper, you may explore these "invisible" symptoms of depression in adults or explore how depression symptoms can be different in children .
How Is Depression Diagnosed?
This is another good depression research topic because, in some ways, the diagnosis of depression is more of an art than a science. Doctors must generally rely upon the patient's set of symptoms and what they can observe about them during their examination to make a diagnosis.
While there are certain laboratory tests that can be performed to rule out other medical illnesses as a cause of depression, there is not yet a definitive test for depression itself.
If you'd like to pursue this topic, you may want to start with the Diagnostic and Statistical Manual of Mental Disorders (DSM). The fifth edition, known as DSM-5, offers a very detailed explanation that guides doctors to a diagnosis. You can also compare the current model of diagnosing depression to historical methods of diagnosis—how have these updates improved the way depression is treated?

Treatment Options for Depression
The first choice for depression treatment is generally an antidepressant medication. Selective serotonin reuptake inhibitors (SSRIs) are the most popular choice because they can be quite effective and tend to have fewer side effects than other types of antidepressants.
Psychotherapy, or talk therapy, is another effective and common choice. It is especially efficacious when combined with antidepressant therapy. Certain other treatments, such as electroconvulsive therapy (ECT) or vagus nerve stimulation (VNS), are most commonly used for patients who do not respond to more common forms of treatment.
Focusing on one of these treatments is an option for your depression research paper. Comparing and contrasting several different types of treatment can also make a good research title about depression.
A Word From Verywell
The topic of depression really can take you down many different roads. When making your final decision on which to pursue in your depression research paper, it's often helpful to start by listing a few areas that pique your interest.
From there, consider doing a little preliminary research. You may come across something that grabs your attention like a new study, a controversial topic you didn't know about, or something that hits a personal note. This will help you narrow your focus, giving you your final research title about depression.
Remes O, Mendes JF, Templeton P. Biological, psychological, and social determinants of depression: A review of recent literature . Brain Sci . 2021;11(12):1633. doi:10.3390/brainsci11121633
National Institute of Mental Health. Depression .
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition . American Psychiatric Association.
National Institute of Mental Health. Mental health medications .
Ferri, F. F. (2019). Ferri's Clinical Advisor 2020 E-Book: 5 Books in 1 . Netherlands: Elsevier Health Sciences.
By Nancy Schimelpfening Nancy Schimelpfening, MS is the administrator for the non-profit depression support group Depression Sanctuary. Nancy has a lifetime of experience with depression, experiencing firsthand how devastating this illness can be.
ORIGINAL RESEARCH article
Evolution and emerging trends in depression research from 2004 to 2019: a literature visualization analysis.

- 1 School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2 School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
Depression has become a major threat to human health, and researchers around the world are actively engaged in research on depression. In order to promote closer research, the study of the global depression knowledge map is significant. This study aims to map the knowledge map of depression research and show the current research distribution, hotspots, frontiers, and trends in the field of depression research, providing researchers with worthwhile information and ideas. Based on the Web of Science core collection of depression research from 2004 to 2019, this study systematically analyzed the country, journal, category, author, institution, cited article, and keyword aspects using bibliometric and data visualization methods. A relationship network of depression research was established, highlighting the highly influential countries, journals, categories, authors, institutions, cited articles, and keywords in this research field. The study identifies great research potential in the field of depression, provides scientific guidance for researchers to find potential collaborations through collaboration networks and coexistence networks, and systematically and accurately presents the hotspots, frontiers, and shortcomings of depression research through the knowledge map of global research on depression with the help of information analysis and fusion methods, which provides valuable information for researchers and institutions to determine meaningful research directions.
Introduction
Health issues are becoming more and more important to people due to the continuous development of health care. The social pressures on people are becoming more and more pronounced in a social environment that is developing at an increasing rate. Prolonged exposure to stress can have a negative impact on brain development ( 1 ), and depression is one of the more typical disorders that accompany it. Stress will increase the incidence of depression ( 2 ), depression has become a common disease ( 3 ), endangering people's physical health. Depression is a debilitating mental illness with mood disorders, also known as major depression, clinical depression, or melancholia. In human studies of the disease, it has been found that depression accounts for a large proportion of the affected population. According to the latest data from the World Health Organization (WHO) statistics in 2019, there are more than 350 million people with depression worldwide, with an increase of about 18% in the last decade and an estimated lifetime prevalence of 15% ( 4 ), it is a major cause of global disability and disease burden ( 5 ), and depression has quietly become a disease that threatens hundreds of millions of people worldwide.
Along with the rise of science communication research, the quantification of science is also flourishing. As a combination of “data science” and modern science, bibliometrics takes advantage of the explosive growth of research output in the era of big data, and uses topics, authors, publications, keywords, references, citations, etc. as research targets to reveal the current status and impact of the discipline more accurately and scientifically. Whereas, there is not a wealth of bibliometric studies related to depression. Fusar-Poli et al. ( 6 ) used bibliometrics to systematically evaluate cross-diagnostic psychiatry. Hammarström et al. ( 7 ) used bibliometrics to analyze the scientific quality of gender-related explanatory models of depression in the medical database PubMed. Tran et al. ( 8 ) used the bibliometric analysis of research progress and effective interventions for depression in AIDS patients. Wang et al. ( 9 ) used bibliometric methods to analyze scientific studies on the comorbidity of pain and depression. Shi et al. ( 10 ) performed a bibliometric analysis of the top 100 cited articles on biomarkers in the field of depression. Dongping et al. ( 11 ) used bibliometric analysis of studies on the association between depression and gut flora. An Chunping et al. ( 12 ) analyzed the literature on acupuncture for depression included in PubMed based on bibliometrics. Yi and Xiaoli ( 13 ) used a bibliometric method to analyze the characteristics of the literature on the treatment of depression by Chinese medicine in the last 10 years. Zhou and Yan ( 14 ) used bibliometric method to analyze the distribution of scientific and technological achievements on depression in Peoples R China. Guaijuan ( 15 ) performed a bibliometric analysis of the interrelationship between psoriasis and depression. Econometric analysis of the relationship between vitamin D deficiency and depression was performed by Yunzhi et al. ( 16 ) and Shauni et al. ( 17 ) performed a bibliometric analysis of domestic and international research papers on depression-related genes from 2003 to 2007. A previous review of depression-related bibliometric studies revealed that there is no bibliometric analysis of global studies in the field of depression, including country network analysis, journal network analysis, category network analysis, author network analysis, institutional network analysis, literature co-citation analysis, keyword co-presentation analysis, and cluster analysis.
The aim of this study was to conduct a comprehensive and systematic literature-based data mining and metrics analysis of depression-related research. More specifically, this analysis focuses on cooperative network and co-presentation analysis, based on the 36,477 papers included in the Web of Science Core Collection database from 2004 to 2019, and provides an in-depth analysis of cooperative network, co-presentation network, and co-citation through modern metrics and data visualization methods. Through the mining of key data, the data correlation is further explored, and the results obtained can be used to scientifically and reasonably predict the depression-related information. This study aims to show the spatial and temporal distribution of research countries, journals, authors, and institutions in the field of depression in a more concise manner through a relational network. A deeper understanding of the internal structure of the research community will help researchers and institutions to establish more accurate and effective global collaborations, in line with the trend of human destiny and globalization. In addition, the study will allow for the timely identification of gaps in current research. A more targeted research direction will be established, a more complete picture of the new developments in the field of depression today will be obtained, and the research protocol will be informed for further adjustments. The results of these analyses will help researchers understand the evolution of this field of study. Overall, this paper uses literature data analysis to find research hotspots in the field of depression, analyze the knowledge structure within different studies, and provide a basis for predicting research frontiers. This study analyzed the literature in the field of depression using CiteSpace 5.8.R2 (64-bit) to analyze collaborative networks, including country network analysis, journal network analysis, category network analysis, researcher network analysis, and institutional network analysis using CiteSpace 5.8.R2 (64-bit). In addition, literature co-citation, keyword co-presentation, and cluster analysis of depression research hotspots were also performed. Thus, exploring the knowledge dimensions of the field, quantifying the research patterns in the field, and uncovering emerging trends in the field will help to obtain more accurate and complete information. The large amount of current research results related to depression will be presented more intuitively and accurately with the medium of information technology, and the scientific evaluation of research themes and trend prediction will be provided from a new perspective.
Data Sources
The data in this paper comes from the Web of Science (WoS) core collection. The time years were selected as 2004–2019. First, the literature was retrieved after entering “depression” using the title search method. A total of 73,829 articles, excluding “depression” as “suppression,” “decline,” “sunken,” “pothole,” “slump,” “low pressure,” “frustration.” The total number of articles with other meanings such as “depression” was 5,606, and the total number of valid articles related to depression was 68,223. Next, the title search method was used to search for studies related to “major depressive disorder” not “depression,” and a total of 8,070 articles were retrieved. For the two search strategies, a total of 76,293 records were collected. The relevant literature retrieved under the two methods were combined and exported in “plain text” file format. The exported records included: “full records and references cited.” CiteSpace processed the data to obtain 41,408 valid records, covering all depression-related research articles for the period 2004–2019, and used this as the basis for analysis.
Processing Tools
CiteSpace ( 18 ), developed by Chao-Mei Chen, a professor in the School of Information Science and Technology at Drexel University, is a Java-based program with powerful data visualization capabilities and is one of the most widely used knowledge mapping tools. The software version used in this study is CiteSpace 5.8.R2 (64-bit).
Methods of Analysis
This study uses bibliometrics and data visualization as analytical methods. First, the application of bibliometrics to the field of depression helped to identify established and emerging research clusters, demonstrating the value of research in this area. Second, data visualization provides multiple perspectives on the data, presenting correlations in a clearer “knowledge graph” that can reveal underestimated and overlooked trends, patterns, and differences ( 19 ). CiteSpace is mainly based on the “co-occurrence clustering idea,” which extracts the information units (keywords, authors, institutions, countries, journals, etc.) in the data by classification, and then further reconstructs the data in the information units to form networks based on different types and strengths of connections (e.g., keyword co-occurrence, author collaboration, etc.). The resulting networks include nodes and links, where the nodes represent the information units of the literature and the links represent the existence of connections (co-occurrence) between the nodes. Finally, the network is measured, statistically analyzed, and presented in a visual way. The analysis needs to focus on: the overall structure of the network, key nodes and paths. The key evaluation indicators in this study are: betweenness centrality, year, keyword frequency, and burst strength. Betweenness centrality (BC) is the number of times a node acts as the shortest bridge between two other nodes. The higher the number of times a node acts as an “intermediary,” the greater its betweenness centrality. Betweenness centrality is a measure of the importance of articles found and measured by nodes in the network by labeling the category (or authors, journals, institutions, etc.) with purple circles. There may be many shortest paths between two nodes in the network, and by counting all the shortest paths of any two nodes in the network, if many of the shortest paths pass through a node, then the node is considered to have high betweenness centrality. In CiteSpace, nodes with betweenness centrality over 0.1 are called critical nodes. Year, which represents the publication time of the article. Frequency, which represents the number of occurrences. Burst strength, an indicator used to measure articles with sudden rise or sudden decline in citations. Nodes with high burst strength usually represent a shift in a certain research area and need to be focused on, and the burst article points are indicated in red. The nodes and their sizes and colors are first analyzed initially, and further analyzed by betweenness centrality indicators for evaluation. Each node represents an article, and the larger the node, the greater the frequency of the keyword word and the greater the relevance to the topic. Similarly, the color of the node represents time: the warmer the color, the more recent the time; the colder the color, the older the era; the node with a purple outer ring is a node with high betweenness centrality; the color of each annual ring can determine the time distribution: the color of the annual ring represents the corresponding time, and the thickness of one annual ring is proportional to the number of articles within the corresponding time division; the dominant color can reflect the relative concentration of the emergence time; the node The appearance of red annual rings in the annual rings means hot spots, and the frequency of citations has been or is still increasing rapidly.
Large-Scale Assessment
Country analysis.
During the period 2004–2019, a total of 157 countries/territories have conducted research on depression, which is about 67.38% of 233 countries/territories worldwide. This shows that depression is receiving attention from many countries/regions around the world. Figure 1 shows the geographical distribution of published articles for 157 countries. The top 15 countries are ranked according to the number of articles published. Table 1 lists the top 15 countries with the highest number of publications in the field of depression worldwide from 2004 to 2019. These 15 countries include 4 Asian countries (Peoples R China, Japan, South Korea, Turkey), 2 North American countries (USA, Canada), 1 South American country (Brazil), 7 European countries (UK, Germany, Netherlands, Italy, France, Spain, Sweden), and 1 Oceania country (Australia).
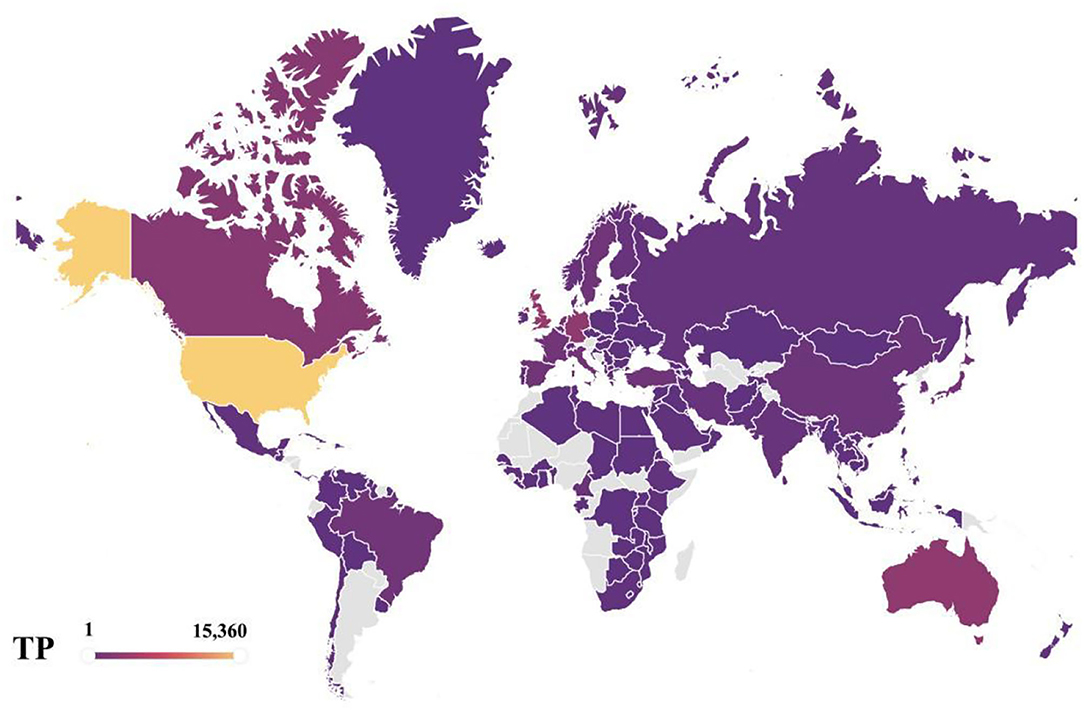
Figure 1 . Geographical distributions of publications, 2004–2019.
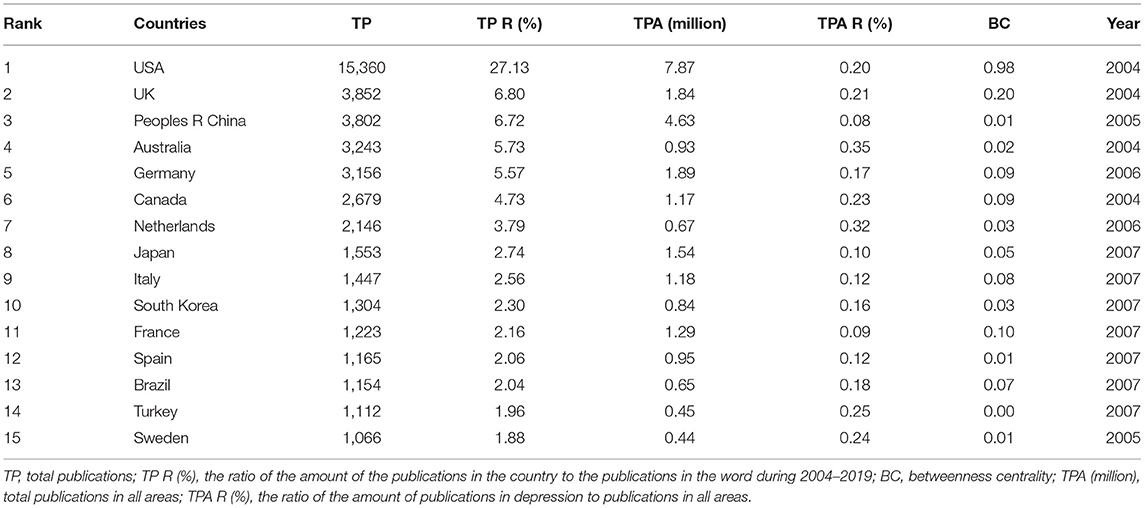
Table 1 . The top 15 productive countries.
Overall, the main distribution of these articles is in USA and some European countries, such as UK, Germany, Netherlands, Italy, France, Spain, and Sweden. This means that these countries are more interested and focused on research on depression compared to others. The total number of publications across all research areas in the Web of Science core collection is similar to the distribution of depression research areas, with the trend toward USA, UK, and Peoples R China as leading countries being unmistakable, and USA has been a leader in the field of depression, with far more articles published than any other country. It can also be seen that USA is the country with the highest betweenness centrality in the network of national collaborations analyzed in this paper. USA research in the field of depression is closely linked to global research, and is an important part of the global collaborative network for depression research. As of 2019, the total number of articles published in depression performance research in USA represents 27.13% of the total number of articles published in depression worldwide, which is ~4 times more than the second-place country, UK, which is far ahead of other countries. Peoples R China, as the third most published country, has a dominant number of articles, but its betweenness centrality is 0.01, reflecting the fact that Peoples R China has less collaborative research with other countries, so Peoples R China should strengthen its foreign collaborative research and actively establish global scientific research partnerships to seek development and generate breakthroughs in cooperation. The average percentage of scientific research on depression in each country is about 0.19%, also highlighting the urgent need to address depression as one of the global human health problems. The four Asian countries included in the top 15 countries are Peoples R China, Japan, South Korea, and Turkey, with Peoples R China ranking third with 6.72% of the total number of all articles counted. The distribution may be explained by the fact that Peoples R China is the largest developing country with a rapid development rate as the largest. Along with the steady rise in the country's economic power, people are creating economic benefits and their health is becoming a consumable commodity. The lifetime prevalence and duration of depression varies by country and region ( 2 ), but the high prevalence and persistence of depression worldwide confirms the increasing severity of the disease worldwide. The WHO estimates that more than 300 million people, or 4.4% of the world's population, suffer from depression ( 20 ), with the number of people suffering from depression increasing at a patient rate of 18.4% between 2005 and 2015. Depression, one of the most prevalent mental illnesses of our time, has caused both physical and psychological harm to many people, and it has become the leading cause of disability worldwide today, and in this context, there is increased interest and focus on research into depression. It is expected that a more comprehensive understanding of depression and finding ways to prevent and cope with the occurrence of this disease can help people get rid of the pain and shadow brought by depression, obtain a healthy and comfortable physical and mental environment and physical health, and make Chinese contributions to the cause of human health. Undoubtedly, the occurrence of depressive illnesses in the context of irreversible human social development has stimulated a vigorous scientific research environment on depression in Peoples R China and other developing countries and contributed to the improvement of research capacity in these countries. Moreover, from a different perspective, the geographical distribution of articles in this field also represents the fundamental position of the country in the overall scientific and academic research field.
Growth Trend Analysis
Figure 2 depicts the distribution of 38,433 articles from the top 10 countries in terms of the number of publications and the trend of growth during 2004–2019.
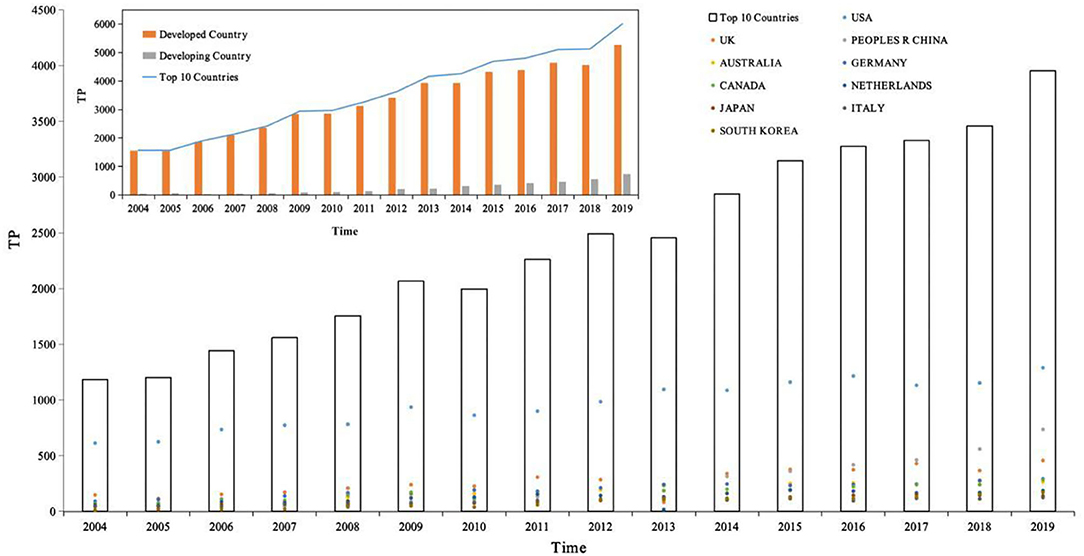
Figure 2 . The distribution of publications in top 10 productive countries, 2004–2019. Source: author's calculation. National development classification criteria refer to “Human Development Report 2020” ( 21 ).
First, the number of articles published per year for the top 10 countries in terms of productivity was counted and then the white bar chart in Figure 2 was plotted, with the year as the horizontal coordinate and total publications as the vertical coordinate, showing the distribution of the productivity of articles in the field of depression per year. The total number of publications for the period 2004–2019 is 38,433. Based on the white bars and line graphs in Figure 2 , we can divide this time period into three growth periods. The number of publications in each growth period is calculated based on the number of publications per year. As can be seen from the figure, the period 2004–2019 can be divided into three main growth periods, namely 2004–2009, 2010–2012, and 2013–2019, the first growth period being from 2004 to 2009, the number of publications totaled 6,749, accounting for 23.97% of all publications; from 2010 to 2012, the number of publications totaled 8,236, accounting for 17.56% of all publications; and from 2013 to 2019, the number of publications totaled 22,473, accounting for 58.47% of all publications. Of these, 2006 was the first year of sharp growth with an annual growth rate of 19.97%, 2009 was the second year of sharp growth with an annual growth rate of 17.64%, and 2008 was the third year of sharp growth with an annual growth rate of 16.09%. In the last 5 years, 2019 has also shown a sharp growth trend with a growth rate of 14.34%. Notably, in 2010 and 2013, there was negative growth with the growth rate of −3.39 and −1.45%. In the last 10 years, depression research has become one of the most valuable areas of human research. It can also be noted that the number of publications in the field of depression in these 10 countries has been increasing year after year.
Second, the analysis is conducted from the perspective of national development, divided into developed and developing countries, as shown in the orange bar chart in Figure 2 , where the horizontal coordinate is year and the vertical coordinate is total publications, comparing the article productivity variability between developed and developing countries. The top 10 most productive countries in the field of depression globally include nine developed countries and one developing country, respectively. During the period 2004–2019, 34,631 papers were published in developed countries and 3,802 papers were published in developing countries, with developed countries accounting for 90.11% of the 38,433 articles and developing countries accounting for 9.89%, and the total number of publications in developed countries was about 9 times higher than that in developing countries. During the period 2004–2019, the number of publications in developed countries showed negative growth in 2 years (2010 and 2013) with growth rates of −3.39 and −1.45%, respectively. The rest of the years showed positive growth with growth rates of 1.52% (2005), 19.97 (2006), 8.11 (2007), 12.70 (2008), 17.64 (2009), 13.22 (2011), 10.17 (2012), 16.09 (2014), 10.46 (2015), 4.10 (2016), 1.59 (2017), 3.91 (2018), and 14.34 (2019), showing three periods of positive growth: 2004–2009, 2011–2012, and 2014–2019, with the highest growth rate of 19.97% in 2006. Recent years have also shown a higher growth trend, with a growth rate of 14.34% in 2019. It is worth noting that developing countries have been showing positive growth in the number of articles in the period 2004–2019, with annual growth rates of 81.25 (2005), 17.24 (2006), 35.29 (2007), 19.57 (2008), 65.45 (2009), 13.19 (2010), 29.13 (2011), 54.89 (2012), 12.14 (2013), 36.36 (2014), 14.92 (2015), 16.02 (2016), 10.24 (2017), 21.17 (2018), and 31.37 (2019), with the highest growth rate of 81.25% in 2005. In the field of depression research, developed countries are still the main force and occupy an important position.
Further, 10 countries with the highest productivity in the field of depression are compared, total publications in the vertical coordinate, and the colored scatter plot contains 10 colored dots, representing 10 different countries. On the one hand, the variability of the contributions of different countries in the same time frame can be compared horizontally. On the other hand, it is possible to compare vertically the variability of the growth of different countries over time. Among them, USA, with about 40.29% of the world's publications in the field of depression, has always been a leader in the field of depression with its rich research results. Peoples R China, as the only developing country, ranks 3rd in the top 10 countries with high production of research papers in the field of depression, and Peoples R China's research in the field of depression has shown a rapid growth trend, and by 2016, it has jumped to become the 2nd largest country in the world, with the number of published papers increasing year by year, which has a broad prospect and great potential for development.
Distribution of Periodicals
Table 2 lists the top 15 journals in order of number of journal co-citations. In the field of depression, the top 15 cited journals accounted for 19.06% of the total number of co-citations, nearly one in five of the total number of journal co-citations. In particular, the top 3 journals were ARCH GEN PSYCHIAT (ARCHIVES OF GENERAL PSYCHIATRY), J AFFECT DISORDERS (JOURNAL OF AFFECTIVE DISORDERS), and AM J PSYCHIAT (AMERICAN JOURNAL OF PSYCHIATRY), with co-citation counts of 20,499, 20,302, and 20,143, with co-citation rates of 2.09, 2.07, and 2.06%, respectively. The main research area of ARCH GEN PSYCHIAT is Psychiatry; the main research area of the journal J AFFECT DISORDERS is Neurosciences and Neurology, Psychiatry; AM J PSYCHIAT is the main research area of Psychiatry, and the three journals have “psychiatry” in common, making them the most frequently co-cited journals in the field of depression.
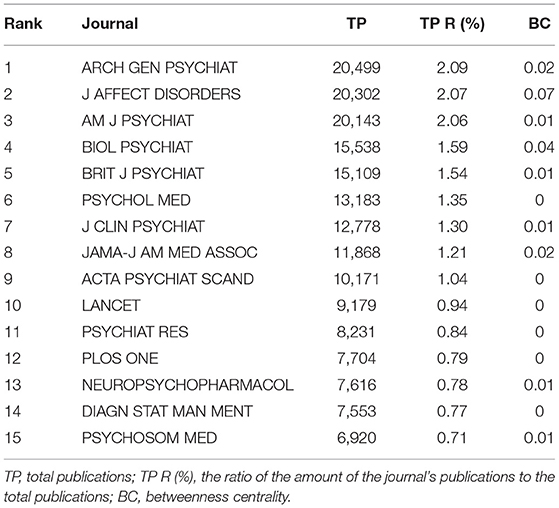
Table 2 . The top 15 co-cited journals.
Figure 3 shows the network relationship graph of the cited journals from 2004 to 2019. The figure takes g-index as the selection criteria, the scale factor k = 25 to include more nodes. Each node of the graph represents each journal, the node size represents the number of citation frequencies, the label size represents the size of the betweenness centrality of the journal in the network, and the links between journals represent the co-citation relationships. The journal co-citation map reflects the structure of the journals, indicating that there are links between journals and that the journals include similar research topics. These journals included research topics related to neuroscience, psychiatry, neurology, and psychology. The journal with betweenness centrality size in the top 1 was ARCH GEN PSYCHIAT, with betweenness centrality size of 0.07, and impact shadows of 14.48. ARCH GEN PSYCHIAT, has research themes of Psychiatry. In all, these journals in Figure 3 occupy an important position in the journal's co-citation network and have strong links with other journals.
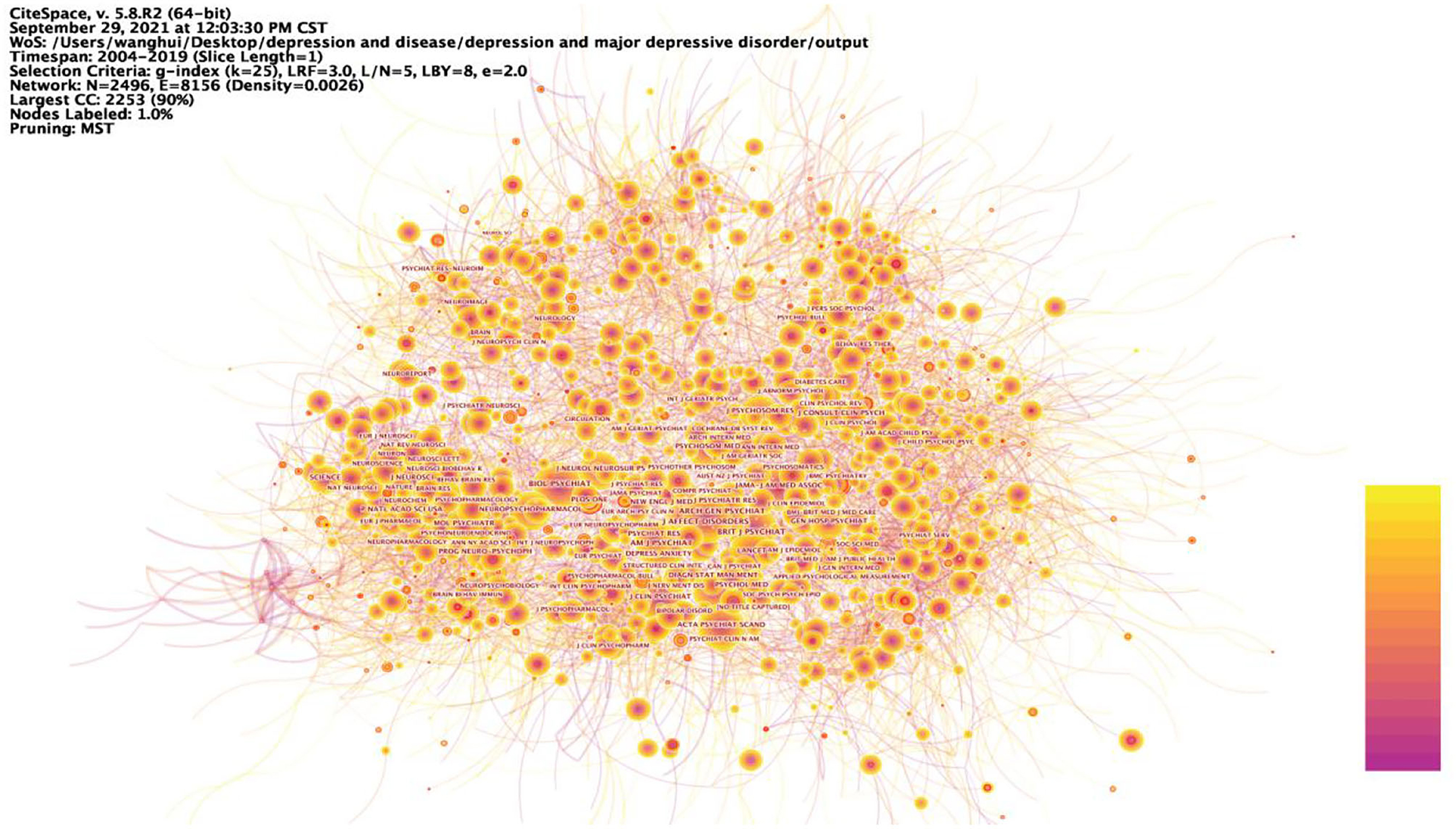
Figure 3 . Prominent journals involved in depression. The betweenness centrality of a node in the network measures the importance of the position of the node in the network. Two types of nodes may have high betweenness centrality scores: (1) Nodes that are highly connected to other nodes, (2) Nodes are positioned between different groups of nodes. The lines represent the link between two different nodes.
Distribution of Categories
Table 3 lists the 15 most popular categories in the field of depression research during the period 2004–2019. In general, the main disciplines involved are neuroscience, psychology, pharmacy, medicine, and health care, which are closely related to human life and health issues. Of these, psychiatry accounted for 20.78%, or about one-five, making it the most researched category. The study of depression focuses on neuroscience, reflecting the essential characteristics of depression as a category of mental illness and better reflecting the fact that depression is an important link in the human public health care. In addition, Table 3 shows that the category with the highest betweenness centrality is Neuroscience, followed by Public, Environment & Occupational Health, and then Pharmacology & Pharmacy, with betweenness centrality of 0.16, 0.13, and 0.11, respectively. It is found that the research categories of depression are also centered on disciplines such as neuroscience, public health and pharmacology, indicating that research on depression requires a high degree of integration of multidisciplinary knowledge and integration of information from various disciplines in order to have a more comprehensive and in-depth understanding of the depression.
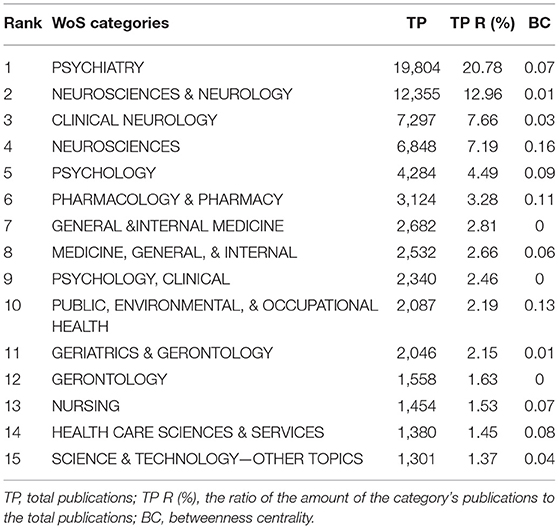
Table 3 . The top 15 productive categories, 2004–2019.
Figure 4 shows the nine categories with the betweenness centrality in the category research network, with Neuroscience being the node with the highest betweenness centrality in this network, meaning that Neuroscience is most strongly linked to all research categories in the field of depression research. Depression is a debilitating psychiatric disorder with mood disorders. It is worth noting that the development of depression not only has psychological effects on humans, but also triggers many somatic symptoms that have a bad impact on their daily work and life, giving rise to the second major mediating central point of research with public health as its theme. The somatization symptoms of depression often manifest as abnormalities in the cardiovascular system, and many studies have looked at the pathology of the cardiovascular system in the hope of finding factors that influence the onset of depression, mechanisms that trigger it or new ways to treat it. Thus, depression involves not only the nervous system, but also interacts with the human cardiovascular system, for example, and the complexity of depression dictates that the study of depression is an in-depth study based on complex systems.
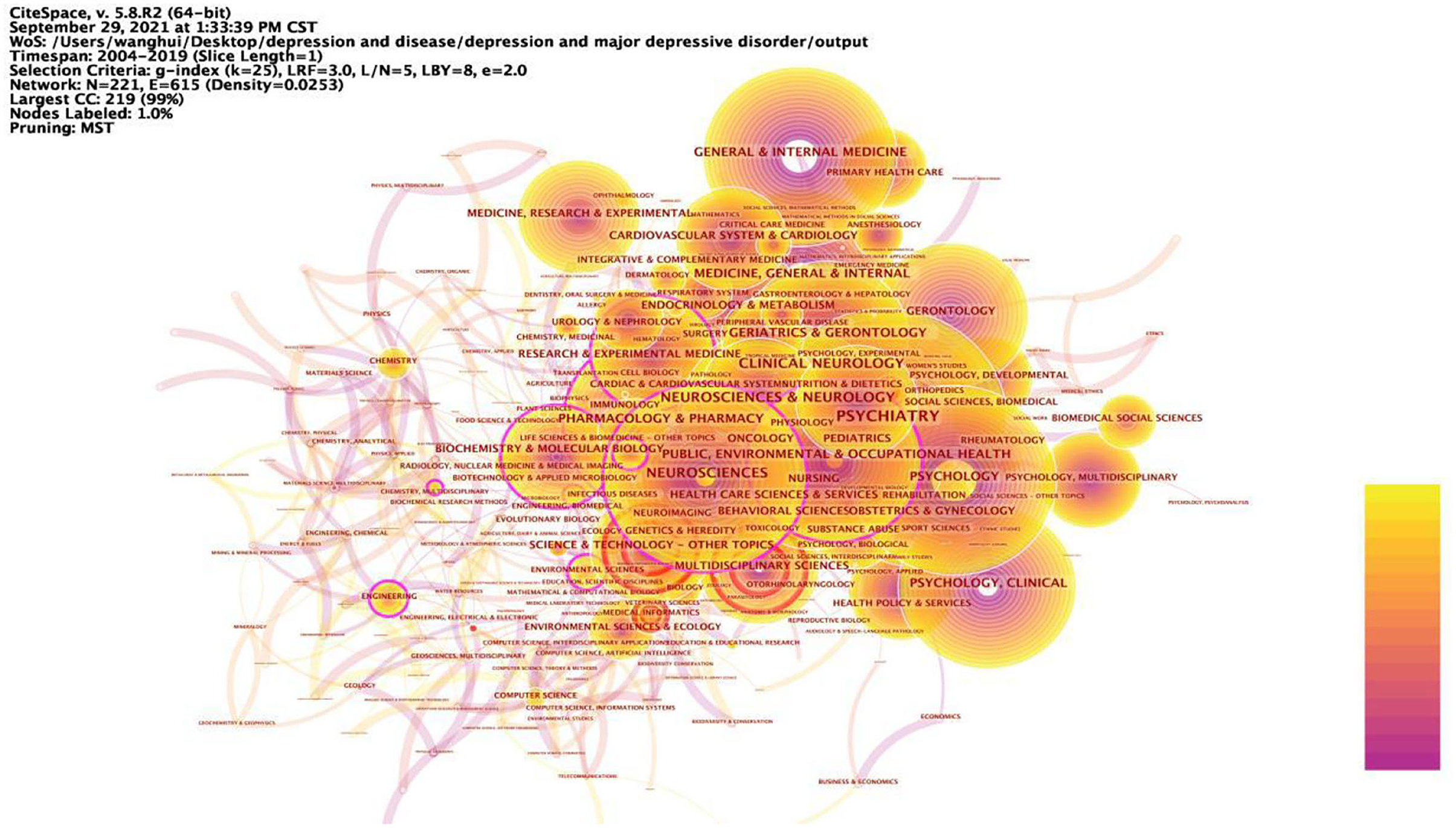
Figure 4 . Prominent categories involved in depression, 2004–2019. The betweenness centrality of a node in the network measures the importance of the position of the node in the network. Two types of nodes may have high betweenness centrality scores: (1) Nodes that are highly connected to other nodes, (2) Nodes are positioned between different groups of nodes. The lines represent the link between two different nodes.
Author Statistics
The results of the analysis showed that there were many researchers working in the field of depression over the past 16 years, and 63 of the authors published at least 30 articles related to depression. Table 4 lists the 15 authors with the highest number of articles published. It includes the rank of the number of articles published, author, country, number of articles published in depression-related studies, total number of articles included in Web of Science, total number of citations, average number of citations, and H-index. According to the statistics, seven of the top 15 authors are from USA, three from the Netherlands, one from Canada, one from Australia, one from New Zealand, one from Italy, and one from Germany. From this, it can be seen that these productive authors are from developed countries, thus it can be inferred that developed countries have a better research environment, more advanced research technology and more abundant research funding. The evaluation indicators in the author co-occurrence network are frequency, betweenness centrality and time of first appearance. The higher the frequency, i.e., the higher the number of collaborative publications, the more collaboration, the higher the information dissemination rate, the three authors with the highest frequency in this author co-occurrence network are MAURIZIO FAVA, BRENDA W. J. H. PENNINX, MADHUKAR H. TRIVEDI; the higher the betweenness centrality, i.e., the closer the relationship with other authors, the more collaboration, the higher the information dissemination rate, the three authors with the highest betweenness centrality are the three authors with the highest betweenness centrality are MICHAEL E. THASE, A. JOHN RUSH; the time of first appearance, i.e., the longer the influence generated by the author's research, the higher the information dissemination rate; in addition, the impact factor and citations can also reflect the information dissemination efficiency of the authors.
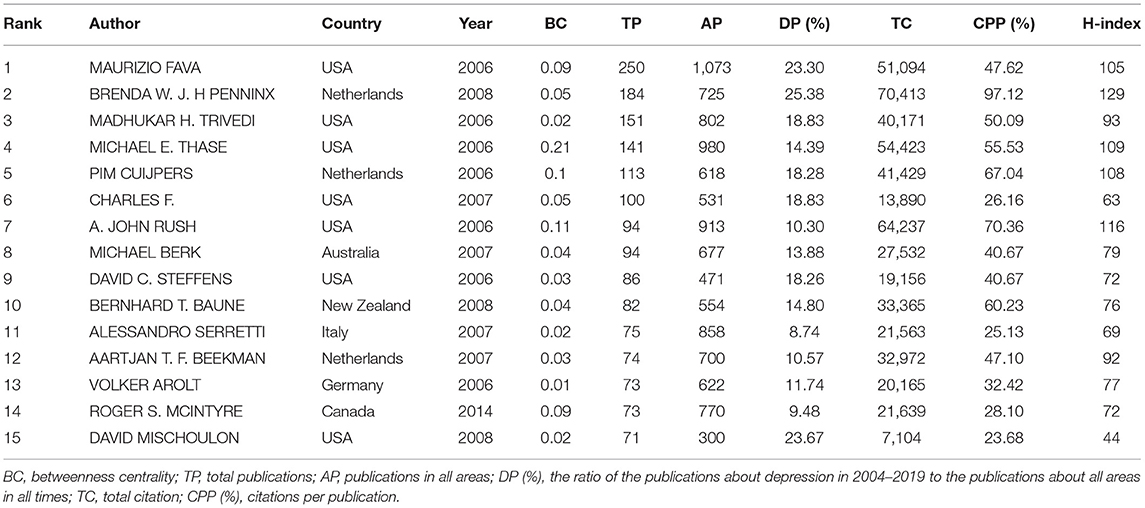
Table 4 . The top 15 authors in network of co-authorship, 2004–2019.
The timezone view ( Figure 5 ) in the author co-occurrence network clearly shows the updates and interactions of author collaborations, for example. All nodes are positioned in a two-dimensional coordinate with the horizontal axis of time, and according to the time of first posting, the nodes are set in different time zones, and their positions are sequentially upward with the time axis, showing a left-to-right, bottom-up knowledge evolution diagram. The time period 2004–2019 is divided into 16 time zones, one for each year, and each circle in the figure represents an author, and the time zone in which the circle appears is the year when the author first published an article in the data set of this study. The closer the color, the warmer the color, the closer the time, the colder the color, the older the era, the thickness of an annual circle, and the number of articles within the corresponding time division is proportional, the dominant color can reflect the relative concentration of the emergence time, the nodes appear in the annual circle of the red annual circle, that is, on behalf of the hot spot, the frequency of being cited was or is still increasing sharply. Nodes with purple outer circles are nodes with high betweenness centrality. The time zone view demonstrates the growth of author collaboration in the field, and it can be found from the graph that the number of author collaborations increases over time, and the frequency of publications in the author collaboration network is high; observe that the thickness of the warm annual rings in the graph is much greater than the thickness of the cold annual rings, which represents the increase of collaboration in time; there are many authors in all time zones, which indicates that there are many research collaborations and achievements in the field, and the field is in a period of collaborative prosperity. The linkage relationship between the sub-time-periods can be seen by the linkage relationship between the time periods, and it can be found from the figure that there are many linkages in the field in all time periods, which indicates that the author collaboration in the field of depression research is strong.
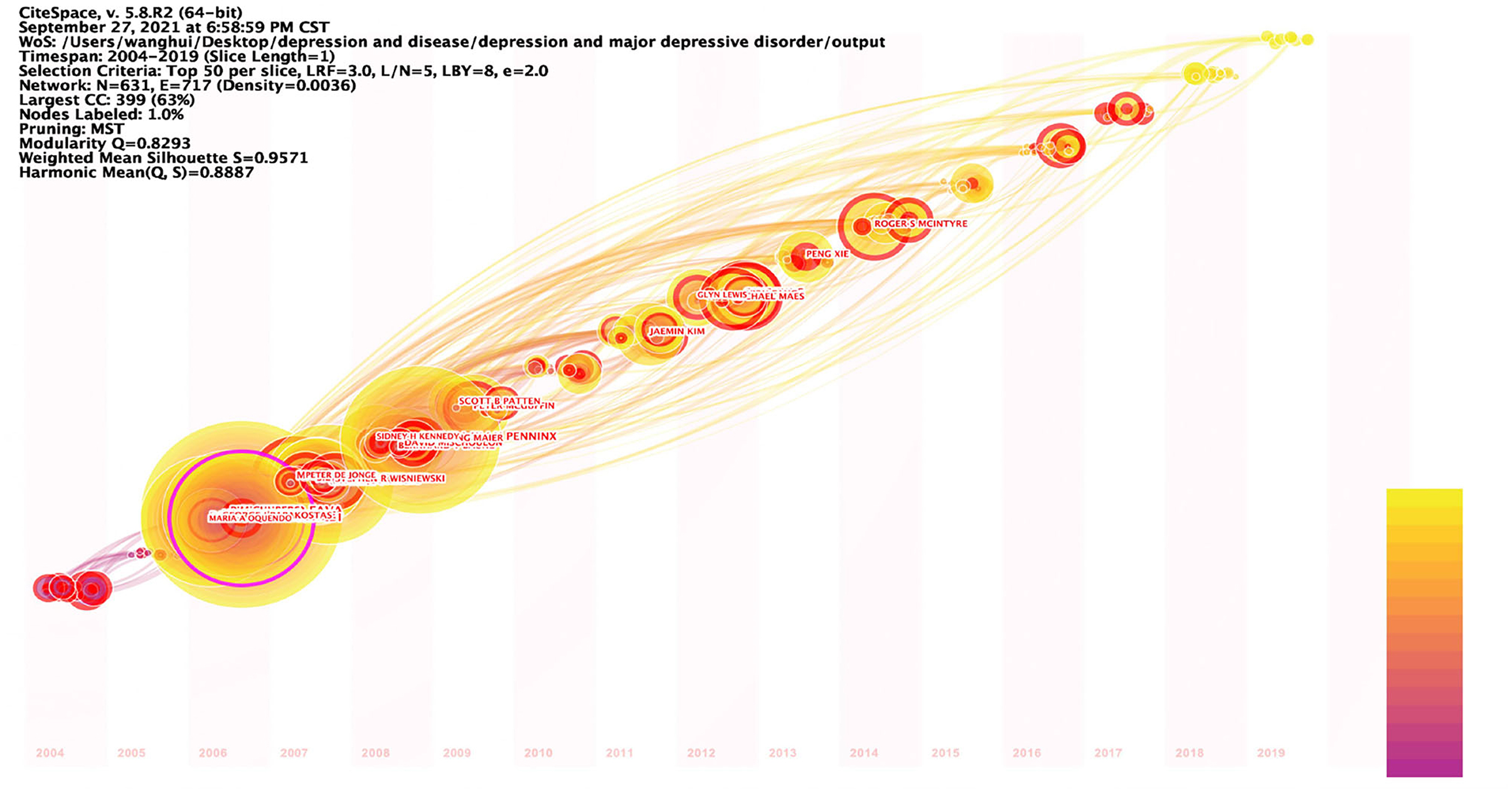
Figure 5 . Timezone view of the author's co-existing network in depression, 2004–2019. The circle represents the author, the time zone in which the circle appears is the year in which the author first published in this study dataset, the radius of the circle represents the frequency of appearance, the color represents the different posting times, the lines represent the connections between authors, and the time zone diagram shows the evolution of author collaboration.
Institutional Statistics
Table 5 lists the top 15 research institutions in network of co-authors' institutions. These include 10 American research institutions, two Netherlands research institutions, one UK research institution, one Canadian research institution and one Australian research institution, all of which, according to the statistics, are from developed countries. Of these influential research institutions, 66.7% are from USA. Figure 6 shows the collaborative network with these influential research institutions as nodes. Kings Coll London (0.2), Univ Michigan (0.17), Univ Toronto (0.15), Stanford Univ (0.14), Univ Penn (0.14), Univ Pittsburgh (0.14), Univ Melbourne (0.12), Virginia Commonwealth Univ (0.12), Columbia Univ (0.1), Duke Univ (0.1), Massachusetts Gen Hosp (0.1), Vrije Univ Amsterdam (0.1), with betweenness centrality >0.1. Kings Coll London has a central place in this collaborative network and is influential in the field of depression research. Table 6 lists the 15 institutions with the strong burst strength. The top 3 institutions are all from USA. Univ Copenhagen, Univ Illinois, Harvard Med Sch, Boston Univ, Univ Adelaide, Heidelberg Univ, Univ New South Wales, and Icahn Sch Med Mt Sinai have had strong burst strength in recent years. It suggests that these institutions may have made a greater contribution to the field of depression over the course of this year and more attention could be paid to their research.
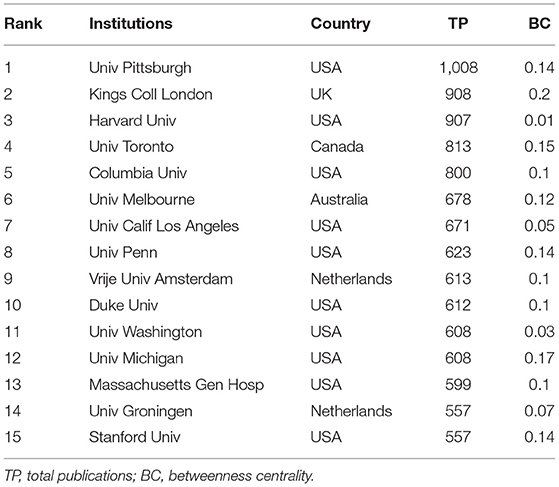
Table 5 . The top 15 institutions in network of co-authors' institutions, 2004–2019.
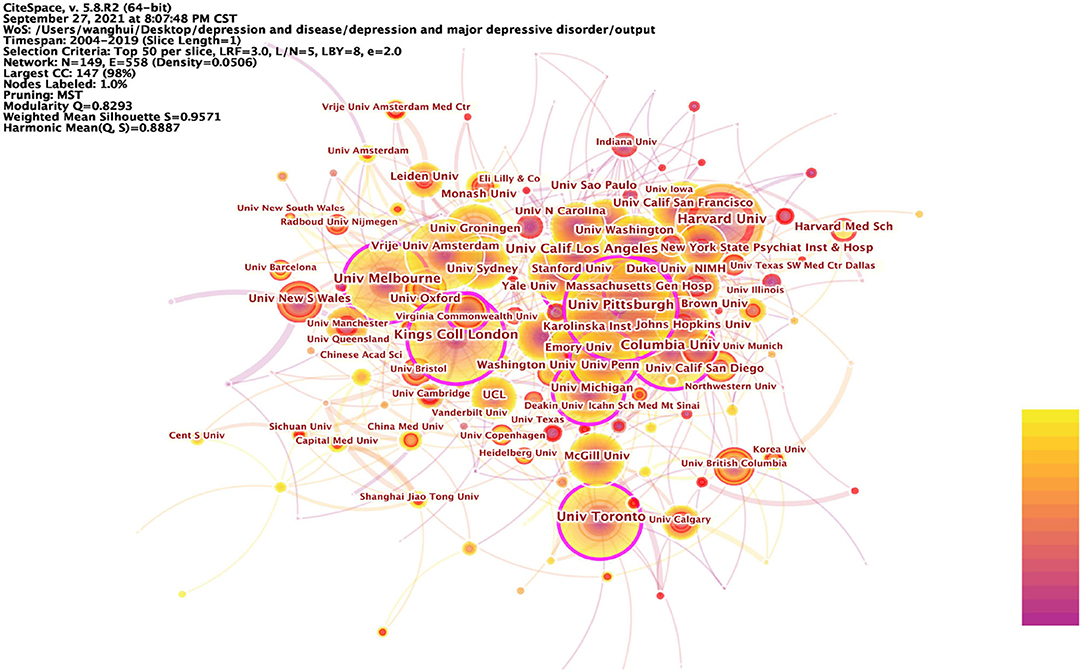
Figure 6 . Prominent institutions involved in depression, 2004–2019. The betweenness centrality of a node in the network measures the importance of the position of the node in the network. Two types of nodes may have high betweenness centrality scores: (1) Nodes that are highly connected to other nodes, (2) Nodes are positioned between different groups of nodes. The lines represent the link between two different nodes.
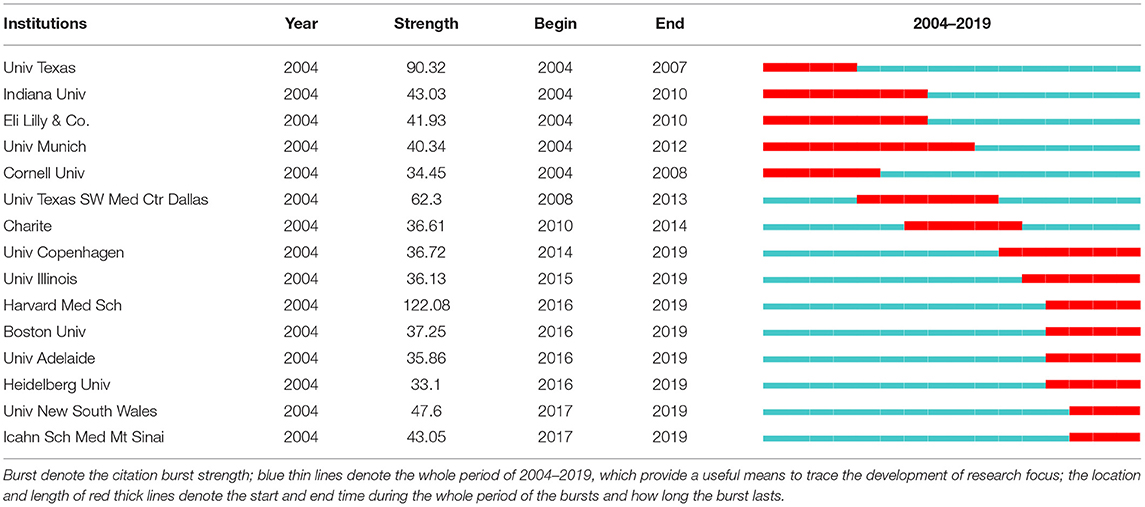
Table 6 . The top 15 institutions with the strongest citation bursts, 2004–2019.
Summing up the above analysis, it can be seen that the research institutions in USA are at the center of the depression research field, are at the top of the world in terms of quantity and quality of research, and are showing continuous growth in vitality. Research institutions in USA, as pioneers among all research institutions, lead and drive the development of depression research and play an important role in cutting-edge research in the field of depression.
Article Citations
Table 7 lists the 16 articles that have been cited more than 1,000 times within the statistical range of this paper from 2004 to 2019. As can be seen from the table, the most cited article was written by Dowlati et al. from Canada and published in BIOLOGICAL PSYCHIATRY 2010, which was cited 2,556 times. In addition, 11 of these 16 highly cited articles were from the USA. Notably, two articles by Kroenke, K as first author appear in this list, ranked 7th and 11th, respectively. In addition, there are three articles from Canada, one article from Switzerland, and one article from the UK. And interestingly, all of these countries are developed countries. It can be reflected that developed countries have ample research experience and high quality of research in the field of depression research. On the other hand, it also reflects that depression is a key concern in developed countries. These highly cited articles provide useful information to many researchers and are of high academic and exploratory value.
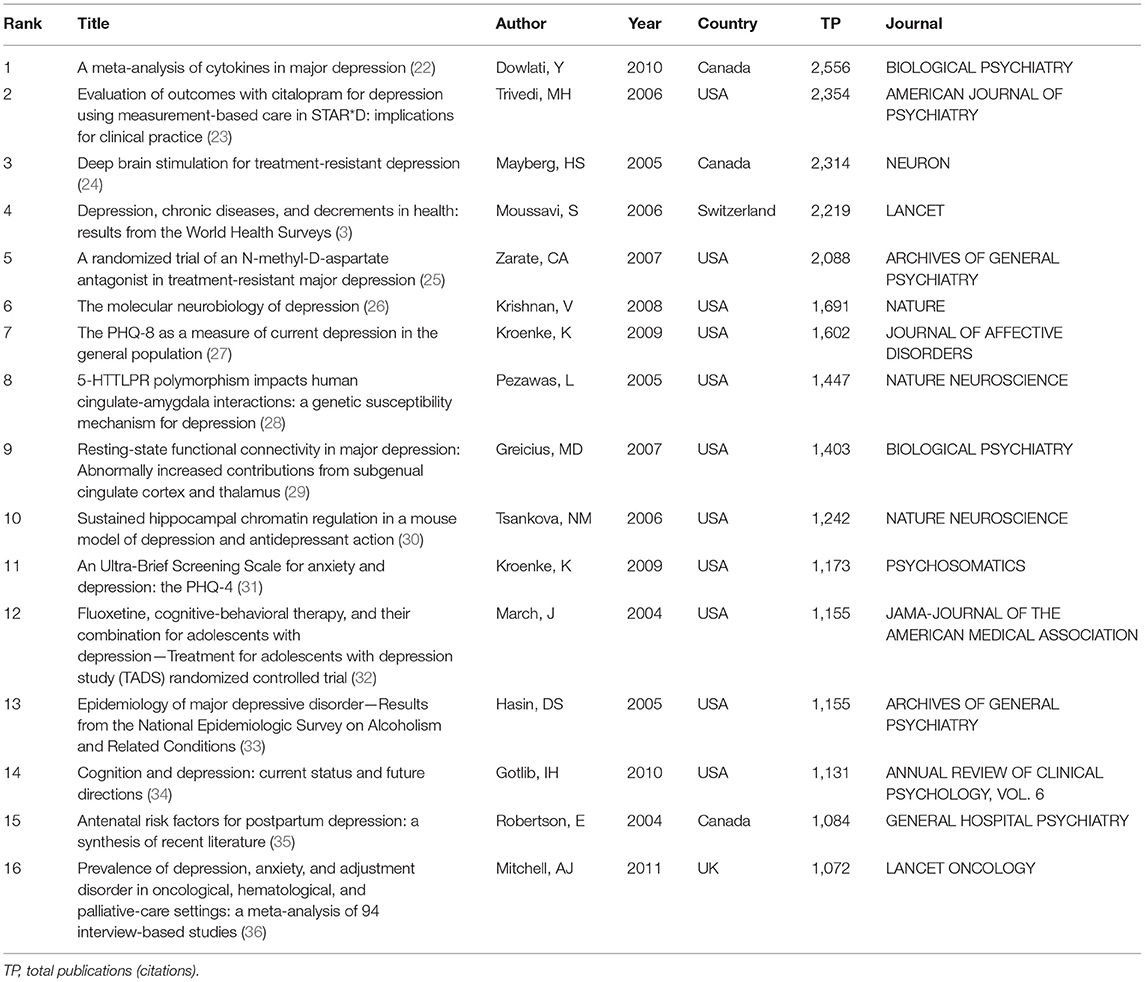
Table 7 . The top 15 frequency cited articles, 2004–2019.
Research Hotspots Ang Frontiers
Keyword analysis.
The keyword analysis of depression yielded the 25 most frequent keywords in Table 8 and the keyword co-occurrence network in Figure 7 . Also, the data from this study were detected by burst, the 25 keywords with the strongest burst strength were obtained in Table 9 . These results bring out the popular and cutting-edge research directions in the field clearly.
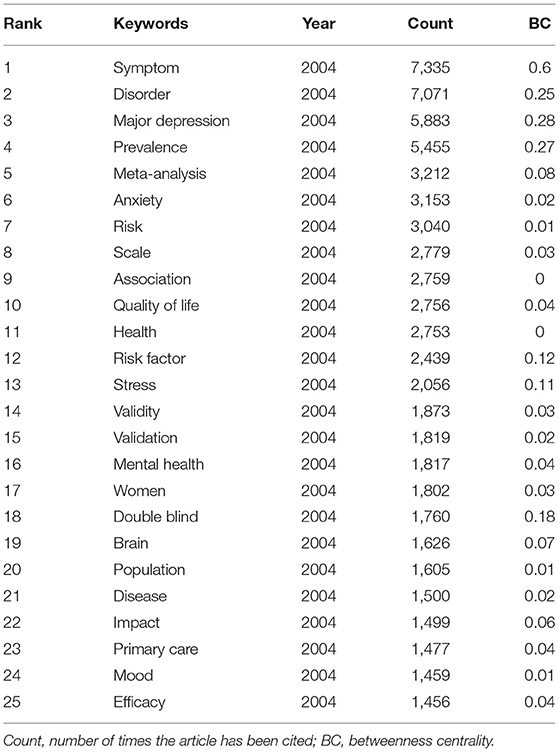
Table 8 . Top 25 frequent keywords in the period of 2004–2019.
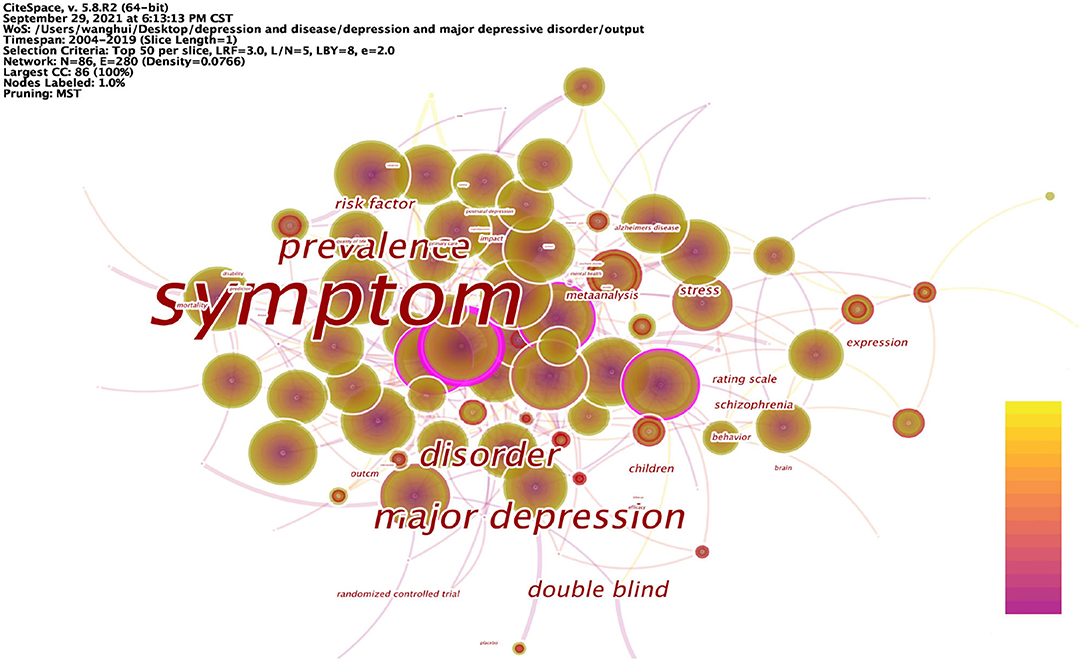
Figure 7 . Keyword co-occurrence network in depression, 2004–2019.
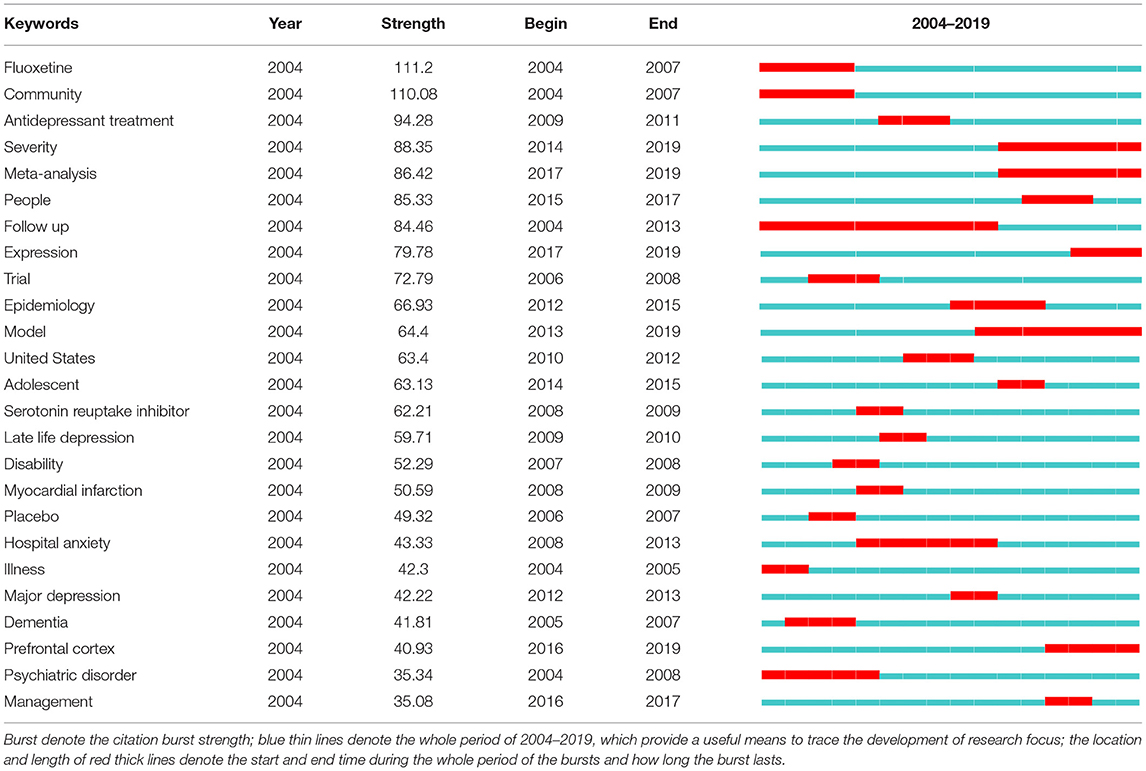
Table 9 . Top 25 keywords with strongest citation bursts in the period of 2004–2019.
The articles on depression during 2004–2019 were analyzed in 1-year time slices, and the top 25 keywords with the highest frequency of occurrence were selected from each slice to obtain the keyword network shown in Table 8 . The top 25 keywords with the highest frequencies were: symptom, disorder, major depression, prevalence, meta-analysis, anxiety, risk, scale, association, quality of life, health, risk factor, stress, validity, validation, mental health, women, double blind, brain, population, disease, impact, primary care, mood, and efficacy. High-frequency nodes respond to popular keywords and are an important basis for the field of depression research.
Figure 7 shows the co-occurrence network mapping of keywords regarding depression research. Each circle in the figure is a node representing a keyword, and the greater the betweenness centrality, the more critical the position of the node in the network. The top 10 keywords in terms of betweenness centrality are: symptom (0.6), major depression (0.28), prevalence (0.27), disorder (0.25), double blind (0.18), risk factor (0.12), stress (0.11), children (0.1), schizophrenia (0.1), and expression (0.1). Nodes with high betweenness centrality reflect that the keyword forms a co-occurrence relationship with multiple other keywords in the domain. A higher betweenness centrality indicates that it is more related to other keywords, and therefore, the node plays an important role in the study. Relatively speaking, these nodes represent the main research directions in the field of depression; they are also the key research directions in this period, and to a certain extent, represent the research hotspots in this period.
Burst detection was performed on the keywords, and the 25 keywords with the strongest strength were extracted, as shown in Table 9 . These keywords contain: fluoxetine, community, follow up, illness, psychiatric disorder, dementia, trial, placebo, disability, serotonin reuptake inhibitor, myocardial infarction, hospital anxiety, antidepressant treatment, late life depression, United States, epidemiology, major depression, model, severity, adolescent, people, prefrontal cortex, management, meta-analysis, and expression. The keywords that burst earlier include fluoxetine (2004), community (2004), follow up (2004), illness (2004), and psychiatric disorder (2004), are keywords that imply that researchers focused on themes early in the field of depression. As researchers continue to explore, the study of depression is changing day by day, and the keywords that have burst in recent years are people (2015), prefrontal cortex (2016), management (2016), meta-analysis (2017), and expression (2017). Reflecting the fact that depression research in recent years has mainly focused on human subjects, the focus has been on the characterization of populations with depression onset. The relationship between depression and the brain has aroused the curiosity of researchers, what exactly are the causes that trigger depression and what are the effects of depression for the manifestation of depression have caused a wide range of discussions in the research community, and the topics related to it have become the most popular studies and have been the focus of research in recent years. All of these research areas showed considerable growth, indicating that research into this area is gaining traction, suggesting that it is becoming a future research priority. The keywords with the strongest burst strength are fluoxetine (111.2), community (110.08), antidepressant treatment (94.28), severity (88.35), meta-analysis (86.42), people (85.33), and follow up (84.46). The rapid growth of research based on these keywords indicates that these topics are the most promising and interesting. The keywords that has been around the longest burst are follow up (2004–2013), model (2013–2019), hospital anxiety (2008–2013), severity (2014–2019), and psychiatric disorder (2004–2008), researchers have invested a lot of research time in these research directions, making many research results, and responding to the exploratory value and significance of research on these topics. At the same time, the longer duration of burst also proves that these research directions have research potential and important value.
Research Hotspots
Hotspots must mainly have the characteristics of high frequency, high betweenness centrality, strong burst, and time of emergence can be used as secondary evaluation indicators. The higher the number of occurrences, the higher the degree of popularity and attention. The higher betweenness centrality means the greater the influence and the higher the importance. Nodes with strong burst usually represent key shift nodes and need to be focused on. The time can be dynamically adjusted according to the target time horizon of the analysis. Thus, based on the results of statistical analysis, it is clear that the research hotspots in the field of depression can be divided into four main areas: etiology (external factors, internal factors), impact (quality of life, disease symptoms, co-morbid symptoms), treatment (interventions, drug development, care modalities), and assessment (population, size, symptoms, duration of disease, morbidity, mortality, effectiveness).
Risk factors for depression include a family history of depression, early life abuse and neglect, and female sexuality and recent life stressors. Physical illnesses also increase the risk of depression, particularly increasing the prevalence associated with metabolic (e.g., cardiovascular disease) and autoimmune disorders.
Research on the etiology of depression can be divided into internal and external factors. In recent years, researchers have increasingly focused on the impact of external factors on depression. Depression is influenced by environmental factors related to social issues, such as childhood experiences, social interactions, and lifestyles. Adverse childhood experiences are risk factors for depression and anxiety in adolescence ( 37 ) and are a common pathway to depression in adults ( 38 ). Poor interpersonal relationships with classmates, family, teachers, and friends increase the prevalence of depression in adolescents ( 39 ). Related studies assessed three important, specific indicators of the self-esteem domain: social confidence, academic ability, and appearance ( 40 ). The results suggest that these three dimensions of self-esteem are key risk factors for increased depressive symptoms in Chinese adolescents. The vulnerability model ( 41 ) suggests that low self-esteem is a causal risk factor for depression, and low self-esteem is thought to be one of the main causes of the onset and progression of depression, with individuals who exhibit low self-esteem being more likely to develop social anxiety and social withdrawal, and thus having a sense of isolation ( 42 ), which in turn leads to subsequent depression. Loneliness predicts depression in adolescents. Individuals with high levels of loneliness experience more stress and tension from psychological and physical sources in their daily lives, which, combined with insufficient care from society, can lead to depression ( 43 ). A mechanism of association exists between life events and mood disorders, with negative life events being directly associated with depressive symptoms ( 44 ). In a cross-sectional study conducted in Shanghai, the prevalence of depression was higher among people who worked longer hours, and daily lifestyle greatly influenced the prevalence of depression ( 45 ). A number of studies in recent years have presented a number of interesting ideas, and they suggest that depression is related to different environmental factors, such as temperature, sunlight hours, and air pollution. Environmental factors have been associated with suicidal behavior. Traffic noise is a variable that triggers depression and is associated with personality disorders such as depression ( 46 ). The harmful effects of air pollution on mental health, inhalation of air pollutants can trigger neuroinflammation and oxidative stress and induce dopaminergic neurotoxicity. A study showed that depression was associated with an increase in ambient fine particulate matter (PM2.5) ( 47 ).
Increased inflammation is a feature of many diseases and even systemic disorders, such as some autoimmune diseases [e.g., type 1 diabetes ( 48 ) or rheumatoid arthritis ( 49 )] and infectious diseases [e.g., hepatitis and sepsis ( 50 )], are associated with an inflammatory response and have been found to increase the risk of depression. A growing body of evidence supports a bidirectional association between depression and inflammatory processes, with stressors and pathogens leading to excessive or prolonged inflammatory responses when combined with predisposing factors (e.g., childhood adversity and modifying factors such as obesity). The resulting illnesses (e.g., pain, sleep disorders), depressive symptoms, and negative health (e.g., poor diet, sedentary lifestyle) may act as mediating pathways leading to inflammation and depression. In terms of mechanistic pathways, cytokines induce depression by affecting different mood-related processes. Elevated inflammatory signals can dysregulate the metabolism of neurotransmitters, damaging neurons, and thus altering neural activity in the brain. In addition cytokines can modulate depression by regulating hormone levels. Inflammation can have different effects on different populations depending on individual physiology, and even lower levels of inflammation may have a depressive effect on vulnerable individuals. This may be due to lower parasympathetic activity, poorer sensitivity to glucocorticoid inhibitory feedback, a greater response to social threat in the anterior oral cortex or amygdala and a smaller hippocampus. Indeed, these are all factors associated with major depression that can affect the sensitivity to the inhibitory consequences of inflammatory stimuli.
Depression triggers many somatization symptoms, which can manifest as insomnia, menopausal syndrome, cardiovascular problems, pain, and other somatic symptoms. There is a link between sleep deprivation and depression, with insomnia being a trigger and maintenance of depression, and more severe insomnia and chronic symptoms predicting more severe depression. Major depression is considered to be an independent risk factor for the development of coronary heart disease and a predictor of cardiovascular events ( 51 ). Patients with depression are extremely sensitive to pain and have increased pain perception ( 52 ) and is associated with an increased risk of suicide ( 53 , 54 ), and generally the symptoms of these pains are not relieved by medication.
Studies have shown that depression triggers an inflammatory response, promoting an increase in cytokines in response to stressors vs. pathogens. For example, mild depressive symptoms have been associated with an amplified and prolonged inflammatory response ( 55 , 56 ) following influenza vaccination in older adults and pregnant women. Among women who have recently given birth, those with a lifetime history of major depression have greater increases in both serum IL-6 and soluble IL-6 receptors after delivery than women without a history of depression ( 57 ). Pro-inflammatory agents, such as interferon-alpha (IFN-alpha), for specific somatization disorders [e.g., hepatitis C or malignant melanoma ( 58 , 59 )], although effective for somatic disorders, pro-inflammatory therapy often leads to psychiatric side effects. Up to 80% of patients treated with IFN-α have been reported to suffer from mild to moderate depressive symptoms.
Clinical trials have shown better antidepressant treatment with anti-inflammatory drugs compared to placebo, either as monotherapy ( 60 , 61 ) or as an add-on treatment ( 62 – 65 ) to antidepressants ( 66 , 67 ). However, findings like whether NSAIDs can be safely used in combination with antidepressants are controversial. Patients with depression often suffer from somatic co-morbidities, which must be included in the benefit/risk assessment. It is important to consider the type of medication, duration of treatment, and dose, and always balance the potential treatment effect with the risk of adverse events in individual patients. Depression, childhood adversity, stressors, and diet all affect the gut microbiota and promote gut permeability, another pathway that enhances the inflammatory response, and effective depression treatment may have profound effects on mood, inflammation, and health. Early in life gut flora colonization is associated with hypothalamic-pituitary-adrenal (HPA) axis activation and affects the enteric nervous system, which is associated with the risk of major depression, gut flora dysbiosis leads to the onset of TLR4-mediated inflammatory responses, and pro-inflammatory factors are closely associated with depression. Clinical studies have shown that in the gut flora of depressed patients, pro-inflammatory bacteria such as Enterobacteriaceae and Desulfovibrio are enriched, while short-chain fatty acid producing bacteria are reduced, and some of these bacterial taxa may transmit peripheral inflammation into the brain via the brain-gut axis ( 68 ). In addition, gut flora can affect the immune system by modulating neurotransmitters (5-hydroxytryptamine, gamma-aminobutyric acid, norepinephrine, etc.), which in turn can influence the development of depression ( 69 ). Therefore, antidepressant drugs targeting gut flora are a future research direction, and diet can have a significant impact on mood by regulating gut flora.
As the molecular basis of clinical depression remains unclear, and treatments and therapeutic effects are limited and associated with side effects, researchers have worked to discover new treatment modalities for depression. High-amplitude low-frequency musical impulse stimulation as an additional treatment modality seems to produce beneficial effects ( 70 ). Studies have found electroconvulsive therapy to be one of the most effective antidepressant treatment therapies ( 71 ). Physical exercise can promote molecular changes that lead to a shift from a chronic pro-inflammatory to an anti-inflammatory state in the peripheral and central nervous system ( 72 ). Aromatherapy is widely used in the treatment of central nervous system disorders ( 73 ). By activating the parasympathetic nervous system, qigong can be effective in reducing depression ( 74 ). The exploration of these new treatment modalities provides more reference options for the treatment of depression.
Large-scale assessments of depression have found that the probability of developing depression varies across populations. Depression affects some specific populations more significantly, for example: adolescents, mothers, and older adults. Depression is one of the disorders that predispose to adolescence, and depression is associated with an increased risk of suicide among college students ( 75 ). Many women develop depression after childbirth. Depression that develops after childbirth is one of the most common complications for women in the postpartum period ( 76 ). The health of children born to mothers who suffer from postpartum depression can also be adversely affected ( 77 ). Depression can cause many symptoms within the central nervous system, especially in the elderly population ( 78 ).
Furthermore, one of the most consistent findings of the association between inflammation and depression is the elevated levels of peripheral pro-inflammatory markers in depressed individuals, and peripheral pro-inflammatory marker levels can also be used as a basis for the assessment of depressed patients. Studies have shown that the following pro-inflammatory markers have been found to be at increased levels in depressed individuals: CRP ( 79 , 80 ), IL-6 ( 22 , 79 , 81 , 82 ), TNF–α, and interleukin-1 receptor antagonist (IL-1ra) ( 79 , 82 ), however, this association is not unidirectional and the subsequent development of depression also increases pro-inflammatory markers ( 82 , 83 ). These biomarkers are of great interest, and depressed patients with increased inflammatory markers may represent a relatively drug-resistant population.
Frontier Analysis
The exploration and analysis of frontier areas of depression were based on the results of the analysis of the previous section on keywords. According to the evaluation index and analysis idea of this study, the frontier research topics need to have the following four characteristics: low to medium frequency, strong burst, high betweenness centrality, and the research direction in recent years. Therefore, combining the results of keyword analysis and these characteristics, it can be found that the frontier research on depression also becomes clear.
Research on Depression Characterized by Psychosexual Disorders
Exploration of biological mechanisms based on depression-associated neurological disorders and analysis of depression from a neurological perspective have always been the focus of research. Activation of neuroinflammatory pathways may contribute to the development of depression ( 84 ). A research model based on the microbial-gut-brain axis facilitates the neurobiology of depression ( 85 ). Some probiotics positively affect the central nervous system due to modulation of neuroinflammation and thus may be able to modulate depression ( 86 ). The combination of environmental issues and the neurobiological study of depression opens new research directions ( 46 ).
Research on Relevant Models of Depression
How to develop a model that meets the purpose of the study determines the outcome of the study and has become the direction that researchers have been exploring in recent years. Martínez et al. ( 87 ) developed a predictive model to assess factors that modify the treatment pathway for postpartum depression. Nie et al. ( 88 ) extended the work on predictive modeling of treatment-resistant depression to establish a predictive model for treatment-resistant depression. Rational modeling methods and behavioral testing facilitate a more comprehensive exploration of depression, with richer studies and more scientifically valid findings.
Research and Characterization of the Depressed Patient Population
Current research on special groups and depression has received much attention. In a study of a group of children, 4% were found to suffer from depression ( 89 ). The diagnosis and treatment of mental health disorders is an important component of pediatric care. Second, some studies of populations with distinct characteristics have been based primarily on female populations. Maternal perinatal depression is also a common mental disorder with a prevalence of over 10% ( 90 ). In addition, geriatric depression is a chronic and specific disorder ( 91 ). Studies based on these populations highlight the characteristics of the disorder more directly than large-scale population explorations and are useful for conducting extended explorations from specific to generalized.
Somatic Comorbidities Associated With Depression
Depression often accompanies the onset and development of many other disorders, making the study of physical comorbidities associated with depression a new landing place for depression research. Depression is a complication of many neurological or psychopathological disorders. Depression is a common co-morbidity of glioblastoma multiforme ( 92 ). Depression is an important disorder associated with stroke ( 93 ). Chronic liver disease is associated with depression ( 94 ). The link between depressive and anxiety states and cancer has been well-documented ( 95 ). In conclusion, depression is associated with an increased risk of lung, oral, prostate, and skin cancers, an increased risk of cancer-specific death from lung, bladder, breast, colorectal, hematopoietic system, kidney, and prostate cancers, and an increased risk of all-cause mortality in lung cancer patients. The early detection and effective intervention of depression and its complications has public health and clinical implications.
Research on Mechanisms of Depression
Research based on the mechanisms of depression includes the study of disease pathogenesis, the study of drug action mechanisms, and the study of disease treatment mechanisms. Research on the pathogenesis of depression has focused more on the study of the hypothalamic-pituitary-adrenal axis. Social pressure can change the hypothalamic-pituitary-adrenal axis ( 96 ). Studies on the mechanism of action of drugs are mostly based on their effects on the central nervous system. The antidepressant effects of Tanshinone IIA are mediated by the ERK-CREB-BDNF pathway in the hippocampus of mice ( 97 ). Research on the mechanisms of depression treatment has also centered on the central nervous system. It has been shown that the vagus nerve can transmit signals to the brain that can lead to a reduction in depressive behavior ( 98 ).
In this study, based on the 2004–2019 time period, this wealth of data is effectively integrated through data analysis and processing to reproduce the research process in a particular field and to co-present global trends in homogenous fields while organizing past research.
Journals that have made outstanding contributions in this field include ARCH GEN PSYCHIAT, J AFFECT DISORDERS and AM J PSYCHIAT. PSYCHIATRY, NEUROSCIENCES & NEUROLOGY and CLINICAL NEUROLOGY are the three most popular categories. The three researchers with the highest number of articles were MAURIZIO FAVA (USA), BRENDA W. J. H. PENNINX (NETHERLANDS) and MADHUKAR H TRIVEDI (USA). Univ Pittsburgh (USA), Kings Coll London (UK) and Harvard Univ (USA) are three of the most productive and influential research institutions. A Meta-Analysis of Cytokines in Major Depression, Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice and Deep brain stimulation for treatment-resistant depression are key articles. Through keyword analysis, a distribution network centered on depression was formed. Although there are good trends in the research on depression, there are still many directions to be explored in depth. Some recommendations regarding depression are as follows.
(1) The prevention of depression can be considered by focusing on treating external factors and guiding the individual.
Faced with the rising incidence of depression worldwide and the difficulty of treating depression, researchers can think more about how to prevent the occurrence of depression. Depressed moods are often the result of stress, not only social pressures on the individual, but also environmental pressures in the developmental process, which in turn have an unhealthy relationship with the body and increase the likelihood of depression. The correlation between external factors and depression is less well-studied, but the control of external factors may be more effective in the short term than in the long term, and may be guided by self-adjustment to avoid major depressive disorder.
(2) The measurement and evaluation of the degree of depression should be developed in the direction of precision.
In the course of research, it has been found that the Depression Rating Scale is mostly used for the detection and evaluation of depression. This kind of assessment is more objective, but it still lacks accuracy, and the research on measurement techniques and methods is less, which is still at a low stage. Patients with depression usually have a variety of causes, conditions, and duration of illness that determine the degree of depression. Therefore, whether these scales can truly accurately measure depression in depressed patients needs further consideration. Accurate measurement is an important basis for evidence-based treatment of depression, and thus how to achieve accurate measurement of depression is a research direction that researchers can move toward.
Therefore, there is an urgent need for further research to address these issues.
A systematic analysis of research in the field of depression in this study concludes that the distribution of countries, journals, categories, authors, institutions, and citations may help researchers and research institutions to establish closer collaboration, develop appropriate publication plans, grasp research hotspots, identify valuable research ideas, understand current emerging research, and determine research directions. In addition, there are still some limitations that can be overcome in future work. First, due to the lack of author and address information in older published articles, it may not be possible to accurately calculate their collaboration; second, although the data scope of this paper is limited to the Web of Science, it can adequately meet our objectives.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
HW conceived and designed the analysis, collected the data, performed the analysis, and wrote the paper. XT, XW, and YW conceived and designed the analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China under Grant No. 81973495.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. (2009) 10:434–45. doi: 10.1038/nrn2639
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
3. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. (2007) 370:851–8. doi: 10.1016/S0140-6736(07)61415-9
4. Peter Heutink MR. The genetics of MDD – a review of challenges and opportunities. J Depress Anxiety. (2014) 3:150. doi: 10.4172/2167-1044.1000150
CrossRef Full Text | Google Scholar
5. Melchior M, Caspi A, Milne BJ, Danese A, Poulton R, Moffitt TE. Work stress precipitates depression and anxiety in young, working women and men. Psychol Med. (2007) 37:1119–29. doi: 10.1017/S0033291707000414
6. Fusar-Poli P, Solmi M, Brondino N, Davies C, Chae C, Politi P, et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry. (2019) 18:192–207. doi: 10.1002/wps.20631
7. Hammarström A, Lehti A, Danielsson U, Bengs C, Johansson EE. Gender-related explanatory models of depression: a critical evaluation of medical articles. Public Health. (2009) 123:689–93. doi: 10.1016/j.puhe.2009.09.010
8. Tran BX, Ho RCM, Ho CSH, Latkin CA, Phan HT, Ha GH, et al. Depression among patients with HIV/AIDS: research development and effective interventions (gapresearch). Int J Environ Res Public Health. (2019) 16:1772. doi: 10.3390/ijerph16101772
9. Wang XQ, Peng MS, Weng LM, Zheng YL, Zhang ZJ, Chen PJ. Bibliometric study of the comorbidity of pain and depression research. Neural Plast. (2019) 2019:1657498. doi: 10.1155/2019/1657498
10. Shi S, Gao Y, Sun Y, Liu M, Shao L, Zhang J, et al. The top-100 cited articles on biomarkers in the depression field: a bibliometric analysis. Psychol Heal Med. (2020) 26:533–42. doi: 10.1080/13548506.2020.1752924
11. Dongping G, Ami D, Ranran D, Yuan Y, Xiaobei S, Xiaoyao W, et al. A study of the relationship between depression and intestinal flora based on bibliometrics. China Med Her. (2017) 14:173–6.
12. Chunping A, Haiyan W, Nina H, Pinghui H. Bibiometrics analysis of acupuncture for treating depression based on GoPubMed. J Clin Acupunct Moxibustion. (2017) 33:54–7.
13. Yi Y, Xiaoli W. Bibiometrics analysis of acupuncture for treating depression based on GoPubMed. Mod Chinese Med. (2012) 14:35–8. doi: 10.13313/j.issn.1673-4890.2012.09.013
CrossRef Full Text
14. Zhou X, Yan F. Analysis on scientific and technological achievements in the field of depression during 1999-2017. J Prev Med Inf. (2019) 35:777–82.
15. Guaijuan W. Bibliometric analysis of literatures of psoriasis and depression based on GoPubmed. J Dermatol Venereol. (2017) 39:391–4.
16. Yunzhi C, Jie G, Yihui C, Chen G, Chen W, Wang H, et al. A Bibliometric analysis on research of vitamin D deficiency and depression caused. J Guiyang Coll Tradit Chinese Med. (2016) 38:32–5.
17. Shaoni L, Shumin R, Chunhui Z, Yongxiao T, Qizheng Z. Bibliometric analysis of depression-related genes. Chinese J Med Libr Inf Sci. (2009) 18:75–8.
18. Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2014) 57:359–77. doi: 10.1002/asi.20317
19. Elgendi M. Scientists need data visualization training. Nat Biotechnol. (2017) 35:990–1. doi: 10.1038/nbt.3986
20. World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates (2017).
Google Scholar
21. World Health Organization. Human Development Report 2020 (2020).
22. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
23. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR * D: implications for clinical practice. Am J Psychiatry. (2006) 163:28–40. doi: 10.1176/appi.ajp.163.1.28
24. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. (2005) 45:651–60. doi: 10.1016/j.neuron.2005.02.014
25. Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry . (2006) 67:793–802. doi: 10.1001/archgenpsychiatry.2010.90
26. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. (2008) 455:894–902. doi: 10.1038/nature07455
27. Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. (2009) 114:163–73. doi: 10.1016/j.jad.2008.06.026
28. Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. (2005) 8:828–34. doi: 10.1038/nn1463
29. Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. (2007) 62:429–37. doi: 10.1016/j.biopsych.2006.09.020
30. Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. (2006) 9:519–25. doi: 10.1038/nn1659
31. Kroenke K, Spitzer RL, Williams JBW, Löwe B. An Ultra-Brief Screening Scale for anxiety and depression: the PHQ−4. Psychosomatics. (2009) 50:613–21. doi: 10.1016/s0033-3182(09)70864-3
32. March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. J Pediatr. (2005) 146:145. doi: 10.1016/j.jpeds.2004.10.032
33. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder. Arch Gen Psychiatry. (2005) 62:1097. doi: 10.1001/archpsyc.62.10.1097
34. Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. (2010) 6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305
35. Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. (2004) 26:289–95. doi: 10.1016/j.genhosppsych.2004.02.006
36. Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. (2011) 12:160–74. doi: 10.1016/S1470-2045(11)70002-X
37. Wang D, Jiang Q, Yang Z, Choi JK. The longitudinal influences of adverse childhood experiences and positive childhood experiences at family, school, and neighborhood on adolescent depression and anxiety. J Affect Disord. (2021) 292:542–51. doi: 10.1016/j.jad.2021.05.108
38. LeMasters K, Bates LM, Chung EO, Gallis JA, Hagaman A, Scherer E, et al. Adverse childhood experiences and depression among women in rural Pakistan. BMC Public Health. (2021) 21:400. doi: 10.1186/s12889-021-10409-4
39. Zhao Y, Zhao Y, Lee YT, Chen L. Cumulative interpersonal relationship risk and resilience models for bullying victimization and depression in adolescents. Pers Individ Dif. (2020) 155:109706. doi: 10.1016/j.paid.2019.109706
40. Zhou J, Li X, Tian L, Huebner ES. Longitudinal association between low self-esteem and depression in early adolescents: the role of rejection sensitivity and loneliness. Psychol Psychother Theor Res Pract. (2020) 93:54–71. doi: 10.1111/papt.12207
41. Butler AC, Hokanson JE, Flynn HA. A comparison of self-esteem lability and low trait self-esteem as vulnerability factors for depression. J Pers Soc Psychol. (1994) 66:166–77. doi: 10.1037/0022-3514.66.1.166
42. Watson J, Nesdale D. Rejection sensitivity, social withdrawal, and loneliness in young adults. J Appl Soc Psychol. (2012) 42:1984–2005. doi: 10.1111/j.1559-1816.2012.00927.x
43. Teo AR, Marsh HE, Forsberg CW, Nicolaidis C, Chen JI, Newsom J, et al. Loneliness is closely associated with depression outcomes and suicidal ideation among military veterans in primary care. J Affect Disord. (2018) 230:42–9. doi: 10.1016/j.jad.2018.01.003
44. Sarubin N, Goerigk S, Padberg F, Übleis A, Jobst A, Erfurt L, et al. Self-esteem fully mediates positive life events and depressive symptoms in a sample of 173 patients with affective disorders. Psychol Psychother Theor Res Pract. (2020) 93:21–35. doi: 10.1111/papt.12205
45. Li Z, Dai J, Wu N, Jia Y, Gao J, Fu H. Effect of long working hours on depression and mental well-being among employees in Shanghai: the role of having leisure hobbies. Int J Environ Res Public Health. (2019) 16:4980. doi: 10.3390/ijerph16244980
46. Díaz J, López-Bueno JA, López-Ossorio JJ, Gónzález JL, Sánchez F, Linares C. Short-term effects of traffic noise on suicides and emergency hospital admissions due to anxiety and depression in Madrid (Spain). Sci Total Environ. (2020) 710:136315. doi: 10.1016/j.scitotenv.2019.136315
47. Fan SJ, Heinrich J, Bloom MS, Zhao TY, Shi TX, Feng WR, et al. Ambient air pollution and depression: a systematic review with meta-analysis up to 2019. Sci Total Environ . (2020) 701:134721. doi: 10.1016/j.scitotenv.2019.134721
48. Korczak DJ, Pereira S, Koulajian K, Matejcek A, Giacca A. Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia. (2011) 54:2483–93. doi: 10.1007/s00125-011-2240-3
49. Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. (2002) 64:52–60. doi: 10.1097/00006842-200201000-00008
50. Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, et al. Autoimmune diseases and severe infections as risk factors for mood disorders a nationwide study. JAMA Psychiatry. (2013) 70:812–20. doi: 10.1001/jamapsychiatry.2013.1111
51. Charlson FJ, Stapelberg NJC, Baxter AJ, Whiteford HA. Should global burden of disease estimates include depression as a risk factor for coronary heart disease? BMC Med. (2011) 9:47. doi: 10.1186/1741-7015-9-47
52. Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuro Psychopharmacol Biol Psychiatry. (2018) 87:159–67. doi: 10.1016/j.pnpbp.2017.05.012
53. Razali SM, Khalib AQ. Pain symptoms in Malay patients with major depression. Asian J Psychiatr. (2012) 5:297–302. doi: 10.1016/j.ajp.2012.02.015
54. Bahk WM, Park S, Jon DI, Yoon BH, Min KJ, Hong JP. Relationship between painful physical symptoms and severity of depressive symptomatology and suicidality. Psychiatry Res. (2011) 189:357–61. doi: 10.1016/j.psychres.2011.01.009
55. Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. (2003) 60:1009–14. doi: 10.1001/archpsyc.60.10.1009
56. Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun. (2010) 24:49–53. doi: 10.1016/j.bbi.2009.05.055
57. Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord. (2001) 63:85–92. doi: 10.1016/S0165-0327(00)00156-7
58. Friebe A, Horn M, Schmidt F, Janssen G, Schmid-Wendtner MH, Volkenandt M, et al. Dose-dependent development of depressive symptoms during adjuvant interferon-α treatment of patients with malignant melanoma. Psychosomatics . (2010) 51:466–73. doi: 10.1016/s0033-3182(10)70738-6
59. Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. (2008) 372:117–26. doi: 10.1016/S0140-6736(08)61033-8
60. Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. (2006) 367:29–35. doi: 10.1016/S0140-6736(05)67763-X
61. Iyengar RL, Gandhi S, Aneja A, Thorpe K, Razzouk L, Greenberg J, et al. NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med . (2013) 126:1017.e11–8. doi: 10.1016/j.amjmed.2013.02.037
62. Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. (2006) 11:680–4. doi: 10.1038/sj.mp.4001805
63. Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. (2009) 26:607–11. doi: 10.1002/da.20589
64. Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: Randomized double-blind placebo-controlled study. J Affect Disord. (2012) 141:308–14. doi: 10.1016/j.jad.2012.03.033
65. Majd M, Hashemian F, Hosseinib SM, Shariatpanahi MV, Sharifid A. A randomized, double-blind, placebo-controlled trial of celecoxib augmentation of sertraline in treatment of drug-naive depressed women: a pilot study. Iran J Pharm Res. (2015) 14:891–9. doi: 10.22037/ijpr.2015.1637
66. Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci USA. (2011) 108:9262–7. doi: 10.1073/pnas.1104836108
67. Uher R, E TK, Tracy D, Wolfgang M, Ole M, Joanna H, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. (2014) 171:1278–86. doi: 10.1176/appi.ajp.2014.14010094
68. Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression – a systematic review. Clin Psychol Rev. (2021) 83:101943. doi: 10.1016/j.cpr.2020.101943
69. Yang Z, Li J, Gui X, Shi X, Bao Z, Han H, et al. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol Psychiatry. (2020) 25:2759–72. doi: 10.1038/s41380-020-0729-1
70. Sigurdardóttir GA, Nielsen PM, Rønager J, Wang AG. A pilot study on high amplitude low frequency–music impulse stimulation as an add-on treatment for depression. Brain Behav. (2019) 9:e01399. doi: 10.1002/brb3.1399
71. Maffioletti E, Gennarelli M, Gainelli G, Bocchio-Chiavetto L, Bortolomasi M, Minelli A. BDNF genotype and baseline serum levels in relation to electroconvulsive therapy effectiveness in treatment-resistant depressed patients. J ECT. (2019) 35:189–94. doi: 10.1097/YCT.0000000000000583
72. Ignácio ZM, da Silva RS, Plissari ME, Quevedo J, Réus GZ. Physical exercise and neuroinflammation in major depressive disorder. Mol Neurobiol. (2019) 56:8323–35. doi: 10.1007/s12035-019-01670-1
73. Lu Z, Zhang T, Wang X, Wang J, Shen J, Xiao Z, et al. Zwitterionic polymer-based nanoparticles encapsulated with linalool for regulating central nervous system. ACS Biomater Sci Eng. (2020) 6:442–9. doi: 10.1021/acsbiomaterials.9b01451
74. Koo JW, Chaudhury D, Han MH, Nestler EJ. Role of mesolimbic brain-derived neurotrophic factor in depression. Biol Psychiatry. (2019) 86:738–48. doi: 10.1016/j.biopsych.2019.05.020
75. Seppälä EM, Bradley C, Moeller J, Harouni L, Nandamudi D, Brackett MA. Promoting mental health and psychological thriving in university students: a randomized controlled trial of three well-being interventions. Front Psychiatry. (2020) 11:590. doi: 10.3389/fpsyt.2020.00590
76. Weissman M, Olfson M. Depression in women: implications for health care research. Science. (1995) 269:799–801. doi: 10.2105/AJPH.2020.305798
77. Savory K, Garay SM, Sumption LA, Kelleher JS, Daughters K, Janssen AB, et al. Prenatal symptoms of anxiety and depression associated with sex differences in both maternal perceptions of one year old infant temperament and researcher observed infant characteristics. J Affect Disord. (2020) 264:383–92. doi: 10.1016/j.jad.2019.11.057
78. Eraydin IE, Mueller C, Corbett A, Ballard C, Brooker H, Wesnes K, et al. Investigating the relationship between age of onset of depressive disorder and cognitive function. Int J Geriatr Psychiatry. (2019) 34:38–46. doi: 10.1002/gps.4979
79. Howren MB, Lamkin DM, Suls J. Associations of depression with c-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. (2009) 71:171–86. doi: 10.1097/PSY.0b013e3181907c1b
80. Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73131 individuals. JAMA Psychiatry. (2013) 70:176–84. doi: 10.1001/2013.jamapsychiatry.102
81. Liu Y, Ho RCM, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. (2012) 139:230–9. doi: 10.1016/j.jad.2011.08.003
82. Dahl J, Ormstad H, Aass HCD, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. (2014) 45:77–86. doi: 10.1016/j.psyneuen.2014.03.019
83. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life a population-based longitudinal study. JAMA Psychiatry. (2014) 71:1121–8. doi: 10.1001/jamapsychiatry.2014.1332
84. Zhang Z, xuan, Li E, Yan J, ping, Fu W, Shen P, Tian SW, et al. Apelin attenuates depressive-like behavior and neuroinflammation in rats co-treated with chronic stress and lipopolysaccharide. Neuropeptides. (2019) 77:101959. doi: 10.1016/j.npep.2019.101959
85. Tian P, O'Riordan KJ, Lee Y, kun, Wang G, Zhao J, Zhang H, et al. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress. (2020) 12:100216. doi: 10.1016/j.ynstr.2020.100216
86. Paiva IHR, Duarte-Silva E, Peixoto CA. The role of prebiotics in cognition, anxiety, and depression. Eur Neuropsychopharmacol. (2020) 34:1–18. doi: 10.1016/j.euroneuro.2020.03.006
87. Martínez P, Vöhringer PA, Rojas G. Barreiras de acesso a tratamento para mães com depressão pós-parto em centros de atenção primária: Um modelo preditivo. Rev Lat Am Enfermagem. (2016) 24:e2675. doi: 10.1590/1518-8345.0982.2675
88. Nie Z, Vairavan S, Narayan VA, Ye J, Li QS. Predictive modeling of treatment resistant depression using data from STARD and an independent clinical study. PLoS ONE. (2018) 13:e0197268. doi: 10.1371/journal.pone.0197268
89. Elmore AL, Crouch E. The Association of adverse childhood experiences with anxiety and depression for children and youth, 8 to 17 years of age. Acad Pediatr. (2020) 20:600–8. doi: 10.1016/j.acap.2020.02.012
90. Cheng B, Wang X, Zhou Y, Li J, Zhao Y, Xia S, et al. Regional cerebral activity abnormality in pregnant women with antenatal depression. J Affect Disord. (2020) 274:381–8. doi: 10.1016/j.jad.2020.05.107
91. Senel B, Özel-Kizil ET, Sorgun MH, Tezcan-Aydemir S, Kirici S. Transcranial sonography imaging of brainstem raphe, substantia nigra and cerebral ventricles in patients with geriatric depression. Int J Geriatr Psychiatry. (2020) 35:702–11. doi: 10.1002/gps.5287
PubMed Abstract | CrossRef Full Text
92. Mugge L, Mansour TR, Crippen M, Alam Y, Schroeder J. Depression and glioblastoma, complicated concomitant diseases: a systemic review of published literature. Neurosurg Rev. (2020) 43:497–511. doi: 10.1007/s10143-018-1017-2
93. Allida S, Kl C, Cf H, Lang H, House A, Ml H. Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke. Cochrane Database Syst Rev. (2020) 1:CD003437. doi: 10.1002/14651858.CD003437.pub4
94. Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: a population-based study. Psychosomatics. (2013) 54:52–9. doi: 10.1016/j.psym.2012.09.005
95. Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. (2020) 25:1487–99. doi: 10.1038/s41380-019-0595-x
96. Colaianna M, Schiavone S, Zotti M, Tucci P, Morgese MG, Bäckdahl L, et al. Neuroendocrine profile in a rat model of psychosocial stress: relation to oxidative stress. Antioxida Redox Signal. (2013) 18:1385–99. doi: 10.1089/ars.2012.4569
97. Lu J, Zhou H, Meng D, Zhang J, Pan K, Wan B, et al. Tanshinone IIA Improves depression-like behavior in mice by activating the ERK-CREB-BDNF signaling pathway. Neuroscience. (2020) 430:1–11. doi: 10.1016/j.neuroscience.2020.01.026
98. McVey Neufeld KA, Bienenstock J, Bharwani A, Champagne-Jorgensen K, Mao YK, West C, et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci Rep. (2019) 9:14290. doi: 10.1038/s41598-019-50807-8
Keywords: depression, major depressive disorder, bibliometrics, visual analysis, knowledge graphs, CiteSpace
Citation: Wang H, Tian X, Wang X and Wang Y (2021) Evolution and Emerging Trends in Depression Research From 2004 to 2019: A Literature Visualization Analysis. Front. Psychiatry 12:705749. doi: 10.3389/fpsyt.2021.705749
Received: 06 May 2021; Accepted: 05 October 2021; Published: 29 October 2021.
Reviewed by:
Copyright © 2021 Wang, Tian, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Wang, wangyun@bucm.edu.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 08 April 2024
Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease
- Felicity Liew 1 na1 ,
- Claudia Efstathiou ORCID: orcid.org/0000-0001-6125-8126 1 na1 ,
- Sara Fontanella 1 ,
- Matthew Richardson 2 ,
- Ruth Saunders 2 ,
- Dawid Swieboda 1 ,
- Jasmin K. Sidhu 1 ,
- Stephanie Ascough 1 ,
- Shona C. Moore ORCID: orcid.org/0000-0001-8610-2806 3 ,
- Noura Mohamed 4 ,
- Jose Nunag ORCID: orcid.org/0000-0002-4218-0500 5 ,
- Clara King 5 ,
- Olivia C. Leavy 2 , 6 ,
- Omer Elneima 2 ,
- Hamish J. C. McAuley 2 ,
- Aarti Shikotra 7 ,
- Amisha Singapuri ORCID: orcid.org/0009-0002-4711-7516 2 ,
- Marco Sereno ORCID: orcid.org/0000-0003-4573-9303 2 ,
- Victoria C. Harris 2 ,
- Linzy Houchen-Wolloff ORCID: orcid.org/0000-0003-4940-8835 8 ,
- Neil J. Greening ORCID: orcid.org/0000-0003-0453-7529 2 ,
- Nazir I. Lone ORCID: orcid.org/0000-0003-2707-2779 9 ,
- Matthew Thorpe 10 ,
- A. A. Roger Thompson ORCID: orcid.org/0000-0002-0717-4551 11 ,
- Sarah L. Rowland-Jones 11 ,
- Annemarie B. Docherty ORCID: orcid.org/0000-0001-8277-420X 10 ,
- James D. Chalmers 12 ,
- Ling-Pei Ho ORCID: orcid.org/0000-0001-8319-301X 13 ,
- Alexander Horsley ORCID: orcid.org/0000-0003-1828-0058 14 ,
- Betty Raman 15 ,
- Krisnah Poinasamy 16 ,
- Michael Marks 17 , 18 , 19 ,
- Onn Min Kon 1 ,
- Luke S. Howard ORCID: orcid.org/0000-0003-2822-210X 1 ,
- Daniel G. Wootton 3 ,
- Jennifer K. Quint 1 ,
- Thushan I. de Silva ORCID: orcid.org/0000-0002-6498-9212 11 ,
- Antonia Ho 20 ,
- Christopher Chiu ORCID: orcid.org/0000-0003-0914-920X 1 ,
- Ewen M. Harrison ORCID: orcid.org/0000-0002-5018-3066 10 ,
- William Greenhalf 21 ,
- J. Kenneth Baillie ORCID: orcid.org/0000-0001-5258-793X 10 , 22 , 23 ,
- Malcolm G. Semple ORCID: orcid.org/0000-0001-9700-0418 3 , 24 ,
- Lance Turtle 3 , 24 ,
- Rachael A. Evans ORCID: orcid.org/0000-0002-1667-868X 2 ,
- Louise V. Wain 2 , 6 ,
- Christopher Brightling 2 ,
- Ryan S. Thwaites ORCID: orcid.org/0000-0003-3052-2793 1 na1 ,
- Peter J. M. Openshaw ORCID: orcid.org/0000-0002-7220-2555 1 na1 ,
- PHOSP-COVID collaborative group &
ISARIC investigators
Nature Immunology volume 25 , pages 607–621 ( 2024 ) Cite this article
26k Accesses
1 Citations
2147 Altmetric
Metrics details
- Inflammasome
- Inflammation
- Innate immunity
One in ten severe acute respiratory syndrome coronavirus 2 infections result in prolonged symptoms termed long coronavirus disease (COVID), yet disease phenotypes and mechanisms are poorly understood 1 . Here we profiled 368 plasma proteins in 657 participants ≥3 months following hospitalization. Of these, 426 had at least one long COVID symptom and 233 had fully recovered. Elevated markers of myeloid inflammation and complement activation were associated with long COVID. IL-1R2, MATN2 and COLEC12 were associated with cardiorespiratory symptoms, fatigue and anxiety/depression; MATN2, CSF3 and C1QA were elevated in gastrointestinal symptoms and C1QA was elevated in cognitive impairment. Additional markers of alterations in nerve tissue repair (SPON-1 and NFASC) were elevated in those with cognitive impairment and SCG3, suggestive of brain–gut axis disturbance, was elevated in gastrointestinal symptoms. Severe acute respiratory syndrome coronavirus 2-specific immunoglobulin G (IgG) was persistently elevated in some individuals with long COVID, but virus was not detected in sputum. Analysis of inflammatory markers in nasal fluids showed no association with symptoms. Our study aimed to understand inflammatory processes that underlie long COVID and was not designed for biomarker discovery. Our findings suggest that specific inflammatory pathways related to tissue damage are implicated in subtypes of long COVID, which might be targeted in future therapeutic trials.
Similar content being viewed by others
Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19
Keith Sacco, Riccardo Castagnoli, … Luigi D. Notarangelo
In COVID-19, NLRP3 inflammasome genetic variants are associated with critical disease and these effects are partly mediated by the sickness symptom complex: a nomothetic network approach
Michael Maes, Walton Luiz Del Tedesco Junior, … Andréa Name Colado Simão
Epidemiology, clinical presentation, pathophysiology, and management of long COVID: an update
Sizhen Su, Yimiao Zhao, … Lin Lu
One in ten severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections results in post-acute sequelae of coronavirus disease 2019 (PASC) or long coronavirus disease (COVID), which affects 65 million people worldwide 1 . Long COVID (LC) remains common, even after mild acute infection with recent variants 2 , and it is likely LC will continue to cause substantial long-term ill health, requiring targeted management based on an understanding of how disease phenotypes relate to underlying mechanisms. Persistent inflammation has been reported in adults with LC 1 , 3 , but studies have been limited in size, timing of samples or breadth of immune mediators measured, leading to inconsistent or absent associations with symptoms. Markers of oxidative stress, metabolic disturbance, vasculoproliferative processes and IFN-, NF-κB- or monocyte-related inflammation have been suggested 3 , 4 , 5 , 6 .
The PHOSP-COVID study, a multicenter United Kingdom study of patients previously hospitalized with COVID-19, has reported inflammatory profiles in 626 adults with health impairment after COVID-19, identified through clustering. Elevated IL-6 and markers of mucosal inflammation were observed in those with severe impairment compared with individuals with milder impairment 7 . However, LC is a heterogeneous condition that may be a distinct form of health impairment after COVID-19, and it remains unclear whether there are inflammatory changes specific to LC symptom subtypes. Determining whether activated inflammatory pathways underlie all cases of LC or if mechanisms differ according to clinical presentation is essential for developing effective therapies and has been highlighted as a top research priority by patients and clinicians 8 .
In this Letter, in a prospective multicenter study, we measured 368 plasma proteins in 657 adults previously hospitalized for COVID-19 (Fig. 1a and Table 1 ). Individuals in our cohort experienced a range of acute COVID-19 severities based on World Health Organization (WHO) progression scores 9 ; WHO 3–4 (no oxygen support, n = 133 and median age of 55 years), WHO 5–6 (oxygen support, n = 353 and median age of 59 years) and WHO 7–9 (critical care, n = 171 and median age of 57 years). Participants were hospitalized for COVID-19 ≥3 months before sample collection (median 6.1 months, interquartile range (IQR) 5.1–6.8 months and range 3.0–8.3 months) and confirmed clinically ( n = 36/657) or by PCR ( n = 621/657). Symptom data indicated 233/657 (35%) felt fully recovered at 6 months (hereafter ‘recovered’) and the remaining 424 (65%) reported symptoms consistent with the WHO definition for LC (symptoms ≥3 months post infection 10 ). Given the diversity of LC presentations, patients were grouped according to symptom type (Fig. 1b ). Groups were defined using symptoms and health deficits that have been commonly reported in the literature 1 ( Methods ). A multivariate penalized logistic regression model (PLR) was used to explore associations of clinical covariates and immune mediators at 6 months between recovered patients ( n = 233) and each LC group (cardiorespiratory symptoms, cardioresp, n = 398, Fig. 1c ; fatigue, n = 384, Fig. 1d ; affective symptoms, anxiety/depression, n = 202, Fig. 1e ; gastrointestinal symptoms, GI, n = 132, Fig. 1f ; and cognitive impairment, cognitive, n = 61, Fig. 1g ). Women ( n = 239) were more likely to experience CardioResp (odds ratio (OR 1.14), Fatigue (OR 1.22), GI (OR 1.13) and Cognitive (OR 1.03) outcomes (Fig. 1c,d,f,g ). Repeated cross-validation was used to optimize and assess model performance ( Methods and Extended Data Fig. 1 ). Pre-existing conditions, such as chronic lung disease, neurological disease and cardiovascular disease (Supplementary Table 1 ), were associated with all LC groups (Fig. 1c–g ). Age, C-reactive protein (CRP) and acute disease severity were not associated with any LC group (Table 1 ).
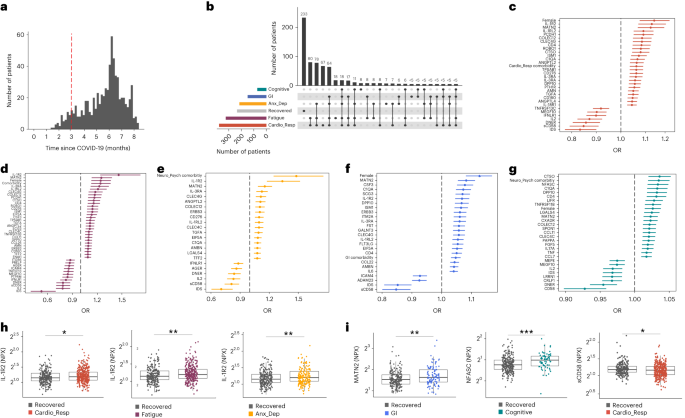
a , Distribution of time from COVID-19 hospitalization at sample collection. All samples were cross-sectional. The vertical red line indicates the 3 month cutoff used to define our final cohort and samples collected before 3 months were excluded. b , An UpSet plot describing pooled LC groups. The horizontal colored bars represent the number of patients in each symptom group: cardiorespiratory (Cardio_Resp), fatigue, cognitive, GI and anxiety/depression (Anx_Dep). Vertical black bars represent the number of patients in each symptom combination group. To prevent patient identification, where less than five patients belong to a combination group, this has been represented as ‘<5’. The recovered group ( n = 233) were used as controls. c – g , Forest plots of Olink protein concentrations (NPX) associated with Cardio_Resp ( n = 365) ( c ), fatigue (n = 314) ( d ), Anx_Dep ( n = 202) ( e ), GI ( n = 124) ( f ) and cognitive ( n = 60) ( g ). Neuro_Psych, neuropsychiatric. The error bars represent the median accuracy of the model. h , i , Distribution of Olink values (NPX) for IL-1R2 ( h ) and MATN2, neurofascin and sCD58 ( i ) measured between symptomatic and recovered individuals in recovered ( n = 233), Cardio_Resp ( n = 365), fatigue ( n = 314) and Anx_Dep ( n = 202) groups ( h ) and MATN2 in GI ( n = 124), neurofascin in cognitive ( n = 60) and sCD58 in Cardio_Resp and recovered groups ( i ). The box plot center line represents the median, the boundaries represent IQR and the whisker length represents 1.5× IQR. The median values were compared between groups using two-sided Wilcoxon signed-rank test, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001.
To study the association of peripheral inflammation with symptoms, we analyzed cross-sectional data collected approximately 6 months after hospitalizations. We measured 368 immune mediators from plasma collected contemporaneously with symptom data. Mediators suggestive of myeloid inflammation were associated with all symptoms (Fig. 1c–h ). Elevated IL-1R2, an IL-1 receptor expressed by monocytes and macrophages modulating inflammation 11 and MATN2, an extracellular matrix protein that modulates tissue inflammation through recruitment of innate immune cells 12 , were associated with cardioresp (IL-1R2 OR 1.14, Fig. 1c,h ), fatigue (IL-1R2 OR 1.45, Fig. 1d,h ), anxiety/depression (IL-1R2 OR 1.34. Fig. 1e,h ) and GI (MATN2 OR 1.08, Fig. 1f ). IL-3RA, an IL-3 receptor, was associated with cardioresp (OR 1.07, Fig. 1c ), fatigue (OR 1.21, Fig. 1d ), anxiety/depression (OR 1.12, Fig. 1e ) and GI (OR 1.06, Fig. 1f ) groups, while CSF3, a cytokine promoting neutrophilic inflammation 13 , was elevated in cardioresp (OR 1.06, Fig. 1c ), fatigue (OR 1.12, Fig. 1d ) and GI (OR 1.08, Fig. 1f ).
Elevated COLEC12, which initiates inflammation in tissues by activating the alternative complement pathway 14 , associated with cardioresp (OR 1.09, Fig. 1c ), fatigue (OR 1.19, Fig. 1d ) and anxiety/depression (OR 1.11, Fig. 1e ), but not with GI (Fig. 1f ) and only weakly with cognitive (OR 1.02, Fig. 1g ). C1QA, a degradation product released by complement activation 15 was associated with GI (OR 1.08, Fig. 1f ) and cognitive (OR 1.03, Fig. 1g ). C1QA, which is known to mediate dementia-related neuroinflammation 16 , had the third strongest association with cognitive (Fig. 1g ). These observations indicated that myeloid inflammation and complement activation were associated with LC.
Increased expression of DPP10 and SCG3 was observed in the GI group compared with recovered (DPP10 OR 1.07 and SCG3 OR 1.08, Fig. 1f ). DPP10 is a membrane protein that modulates tissue inflammation, and increased DPP10 expression is associated with inflammatory bowel disease 17 , 18 , suggesting that GI symptoms may result from enteric inflammation. Elevated SCG3, a multifunctional protein that has been associated with irritable bowel syndrome 19 , suggested that noninflammatory disturbance of the brain–gut axis or dysbiosis, may occur in the GI group. The cognitive group was associated with elevated CTSO (OR 1.04), NFASC (OR 1.03) and SPON-1 (OR 1.02, Fig. 1g,i ). NFASC and SPON-1 regulate neural growth 20 , 21 , while CTSO is a cysteine proteinase supporting tissue turnover 22 . The increased expression of these three proteins as well as C1QA and DPP10 in the cognitive group (Fig. 1g ) suggested neuroinflammation and alterations in nerve tissue repair, possibly resulting in neurodegeneration. Together, our findings indicated that complement activation and myeloid inflammation were common to all LC groups, but subtle differences were observed in the GI and cognitive groups, which may have mechanistic importance. Acutely elevated fibrinogen during hospitalization has been reported to be predictive of LC cognitive deficits 23 . We found elevated fibrinogen in LC relative to recovered (Extended Data Fig. 2a ; P = 0.0077), although this was not significant when restricted to the cognitive group ( P = 0.074), supporting our observation of complement pathway activation in LC and in keeping with reports that complement dysregulation and thrombosis drive severe COVID-19 (ref. 24 ).
Elevated sCD58 was associated with lower odds of all LC symptoms and was most pronounced in cardioresp (OR 0.85, Fig. 1c,i ), fatigue (OR 0.80, Fig. 1d ) and anxiety/depression (OR 0.83, Fig. 1e ). IL-2 was negatively associated with the cardioresp (Fig. 1c , OR 0.87), fatigue (Fig. 1d , OR 0.80), anxiety/depression (Fig. 1e , OR 0.84) and cognitive (Fig. 1g , OR 0.96) groups. Both IL-2 and sCD58 have immunoregulatory functions 25 , 26 . Specifically, sCD58 suppresses IL-1- or IL-6-dependent interactions between CD2 + monocytes and CD58 + T or natural killer cells 26 . The association of sCD58 with recovered suggests a central role of dysregulated myeloid inflammation in LC. Elevated markers of tissue repair, IDS and DNER 27 , 28 , were also associated with recovered relative to all LC groups (Fig. 1c–g ). Taken together, our data suggest that suppression of myeloid inflammation and enhanced tissue repair were associated with recovered, supporting the use of immunomodulatory agents in therapeutic trials 29 (Supplementary Table 2 ).
We next sought to validate the experimental and analytical approaches used. Although Olink has been validated against other immunoassay platforms, showing superior sensitivity and specificity 30 , 31 , we confirmed the performance of Olink against chemiluminescent immunoassays within our cohort. We performed chemiluminescent immunoassays on plasma from a subgroup of 58 participants (recovered n = 13 and LC n = 45). There were good correlations between results from Olink (normalized protein expression (NPX)) and chemiluminescent immunoassays (pg ml −1 ) for CSF3, IL-1R2, IL-3RA, TNF and TFF2 (Extended Data Fig. 3 ). Most samples did not have concentrations of IL-2 detectable using a mesoscale discovery chemiluminescent assay, limiting this analysis to 14 samples (recovered n = 4, LC n = 10, R = 0.55 and P = 0.053, Extended Data Fig. 3 ). We next repeated our analysis using alternative definitions of LC. The Centers for Disease Control and Prevention and National Institute for Health and Care Excellence definitions for LC include symptoms occurring 1 month post infection 32 , 33 . Using the 1 month post-infection definition included 62 additional participants to our analysis (recovered n = 21, 3 females and median age 61 years and LC n = 41, 15 females and median age 60 years, Extended Data Fig. 2c ) and found that inflammatory associations with each LC group were consistent with our analysis based on the WHO definition (Extended Data Fig. 2d–h ). Finally, to validate the analytical approach (PLR) we examined the distribution of data, prioritizing proteins that were most strongly associated with each LC/recovered group (IL-1R2, MATN2, NFASC and sCD58). Each protein was significantly elevated in the LC group compared with recovered (Fig. 1h,i and Extended Data Fig. 4 ), consistent with the PLR. Alternative regression approaches (unadjusted regression models and partial least squares, PLS) reported results consistent with the original analysis of protein associations and LC outcome in the WHO-defined cohort (Fig. 1c–g , Supplementary Table 3 and Extended Data Figs. 5 and 6 ). The standard errors of PLS estimates were wide (Extended Data Fig. 6 ), consistent with previous demonstrations that PLR is the optimal method to analyze high-dimensional data where variables may have combined effects 34 . As inflammatory proteins are often colinear, working in-tandem to mediate effects, we prioritized PLR results to draw conclusions.
To explore the relationship between inflammatory mediators associated with different LC symptoms, we performed a network analysis of Olink mediators highlighted by PLR within each LC group. COLEC12 and markers of endothelial and mucosal inflammation (MATN2, PCDH1, ROBO1, ISM1, ANGPTL2, TGF-α and TFF2) were highly correlated within the cardioresp, fatigue and anxiety/depression groups (Fig. 2 and Extended Data Fig. 7 ). Elevated PCDH1, an adhesion protein modulating airway inflammation 35 , was highly correlated with other inflammatory proteins associated with the cardioresp group (Fig. 2 ), suggesting that systemic inflammation may arise from the lung in these individuals. This was supported by increased expression of IL-3RA, which regulates innate immune responses in the lung through interactions with circulating IL-3 (ref. 36 ), in fatigue (Figs. 1d and 2 ), which correlated with markers of tissue inflammation, including PCDH1 (Fig. 2 ). MATN2 and ISM1, mucosal proteins that enhance inflammation 37 , 38 , were highly correlated in the GI group (Fig. 2 ), highlighting the role of tissue-specific inflammation in different LC groups. SCG3 correlated less closely with mediators in the GI group (Fig. 2 ), suggesting that the brain–gut axis may contribute separately to some GI symptoms. SPON-1, which regulates neural growth 21 , was the most highly correlated mediator in the cognitive group (Fig. 2 and Extended Data Fig. 7 ), highlighting that processes within nerve tissue may underlie this group. These observations suggested that inflammation might arise from mucosal tissues and that additional mechanisms may contribute to pathophysiology underlying the GI and cognitive groups.
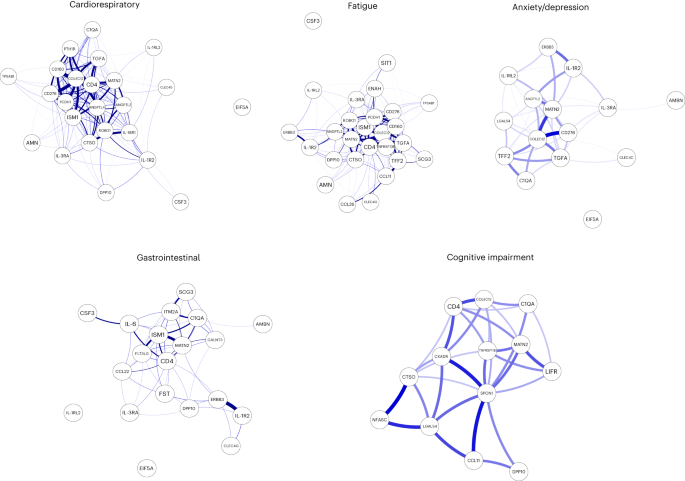
Network analysis of Olink mediators associated with cardioresp ( n = 365), fatigue ( n = 314), anxiety/depression ( n = 202), GI ( n = 124) and cognitive groups ( n = 60). Each node corresponds to a protein mediator identified by PLR. The edges (blue lines) were weighted according to the size of Spearman’s rank correlation coefficient between proteins. All edges represent positive and significant correlations ( P < 0.05) after FDR adjustment.
Women were more likely to experience LC (Table 1 ), as found in previous studies 1 . As estrogen can influence immunological responses 39 , we investigated whether hormonal differences between men and women with LC in our cohort explained this trend. We grouped men and women with LC symptoms into two age groups (those younger than 50 years and those 50 years and older, using age as a proxy for menopause status in women) and compared mediator levels between men and women in each age group, prioritizing those identified by PLR to be higher in LC compared with recovered. As we aimed to understand whether women with LC had stronger inflammatory responses than men with LC, we did not assess differences in men and women in the recovered group. IL-1R2 and MATN2 were significantly higher in women ≥50 years than men ≥50 years in the cardioresp group (Fig. 3a , IL-1R2 and MATN2) and the fatigue group (Fig. 3b ). In the GI group, CSF3 was higher in women ≥50 years compared with men ≥50 years (Fig. 3c ), indicating that the inflammatory markers observed in women were not likely to be estrogen-dependent. Women have been reported to have stronger innate immune responses to infection and to be at greater risk of autoimmunity 39 , possibly explaining why some women in the ≥50 years group had higher inflammatory proteins than men the same group. Proteins associated with the anxiety/depression (IL-1R2 P = 0.11 and MATN2 P = 0.61, Extended Data Fig. 8a ) and cognitive groups (CTSO P = 0.64 and NFASC P = 0.41, Extended Data Fig. 8b ) were not different between men and women in either age group, consistent with the absent/weak association between sex and these outcomes identified by PLR (Fig. 1e,g ). Though our findings suggested that nonhormonal differences in inflammatory responses may explain why some women are more likely to have LC, they require confirmation in adequately powered studies.
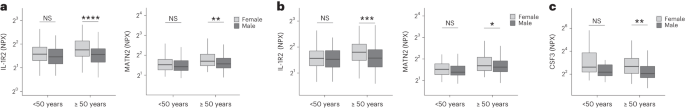
a – c , Olink-measured plasma protein levels (NPX) of IL-1R2 and MATN2 ( a and b ) and CSF3 ( c ) between LC men and LC women divided by age (<50 or ≥50 years) in the cardiorespiratory group (<50 years n = 8 and ≥50 years n = 270) ( a ), fatigue group (<50 years n = 81 and ≥50 years n = 227) ( b ) and GI group (<50 years n = 34 and ≥50 years n = 82) ( c ). the median values were compared between men and women using two-sided Wilcoxon signed-rank test, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001. The box plot center line represents the median, the boundaries represent IQR and the whisker length represents 1.5× IQR.
To test whether local respiratory tract inflammation persisted after COVID-19, we compared nasosorption samples from 89 participants (recovered, n = 31; LC, n = 33; and healthy SARS-CoV-2 naive controls, n = 25, Supplementary Tables 4 and 5 ). Several inflammatory markers were elevated in the upper respiratory tract post COVID (including IL-1α, CXCL10, CXCL11, TNF, VEGF and TFF2) when compared with naive controls, but similar between recovered and LC (Fig. 4a ). In the cardioresp group ( n = 29), inflammatory mediators elevated in plasma (for example, IL-6, APO-2, TGF-α and TFF2) were not elevated in the upper respiratory tract (Extended Data Fig. 9a ) and there was no correlation between plasma and nasal mediator levels (Extended Data Fig. 9b ). This exploratory analysis suggested upper respiratory tract inflammation post COVID was not specifically associated with cardiorespiratory symptoms.
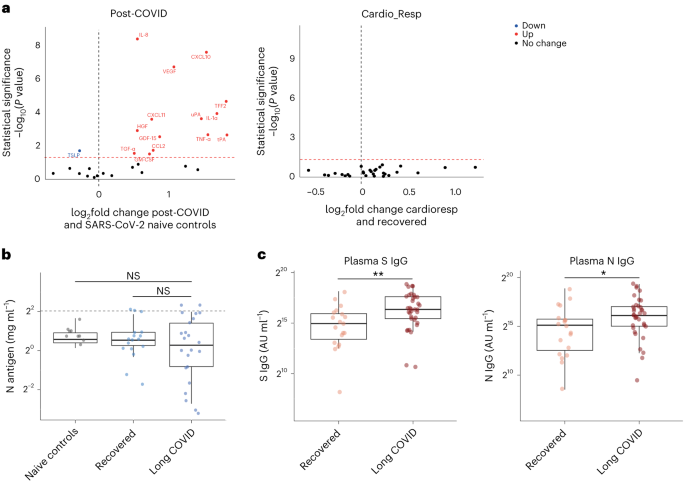
a , Nasal cytokines measured by immunoassay in post-COVID participants ( n = 64) compared with healthy SARS-CoV-2 naive controls ( n = 25), and between the the cardioresp group ( n = 29) and the recovered group ( n = 31). The red values indicate significantly increased cytokine levels after FDR adjustment ( P < 0.05) using two-tailed Wilcoxon signed-rank test. b , SARS-CoV-2 N antigen measured in sputum by electrochemiluminescence from recovered ( n = 17) and pooled LC ( n = 23) groups, compared with BALF from SARS-CoV-2 naive controls ( n = 9). The horizontal dashed line indicates the lower limit of detection of the assay. c , Plasma S- and N-specific IgG responses measured by electrochemiluminescence in the LC ( n = 35) and recovered ( n = 19) groups. The median values were compared using two-sided Wilcoxon signed-rank tests, NS P > 0.05, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001. The box plot center lines represent the median, the boundaries represent IQR and the whisker length represents 1.5× IQR.
To explore whether SARS-CoV-2 persistence might explain the inflammatory profiles observed in the cardioresp group, we measured SARS-CoV-2 nucleocapsid (N) antigen in sputum from 40 participants (recovered n = 17 and LC n = 23) collected approximately 6 months post hospitalization (Supplementary Table 6 ). All samples were compared with prepandemic bronchoalveolar lavage fluid ( n = 9, Supplementary Table 4 ). Only four samples (recovered n = 2 and LC n = 2) had N antigen above the assay’s lower limit of detection, and there was no difference in N antigen concentrations between LC and recovered (Fig. 4b , P = 0.78). These observations did not exclude viral persistence, which might require tissues samples for detection 40 , 41 . On the basis of the hypothesis that persistent viral antigen might prevent a decline in antibody levels over time, we examined the titers of SARS-CoV-2-specific antibodies in unvaccinated individuals (recovered n = 19 and LC n = 35). SARS-CoV-2 N-specific ( P = 0.023) and spike (S)-specific ( P = 0.0040) immunoglobulin G (IgG) levels were elevated in LC compared with recovered (Fig. 4c ).
Overall, we identified myeloid inflammation and complement activation in the cardioresp, fatigue, anxiety/depression, cognitive and GI groups 6 months after hospitalization (Extended Data Fig. 10 ). Our findings build on results of smaller studies 5 , 6 , 42 and are consistent with a genome-wide association study that identified an independent association between LC and FOXP4 , which modulates neutrophilic inflammation and immune cell function 43 , 44 . In addition, we identified tissue-specific inflammatory elements, indicating that myeloid disturbance in different tissues may result in distinct symptoms. Multiple mechanisms for LC have been suggested, including autoimmunity, thrombosis, vascular dysfunction, SARS-CoV-2 persistence and latent virus reactivation 1 . All these processes involve myeloid inflammation and complement activation 45 . Complement activation in LC has been suggested in a proteomic study in 97 mostly nonhospitalized COVID-19 cases 42 and a study of 48 LC patients, of which one-third experienced severe acute disease 46 . As components of the complement system are known to have a short half-life 47 , ongoing complement activation suggests active inflammation rather than past tissue damage from acute infection.
Despite the heterogeneity of LC and the likelihood of coexisting or multiple etiologies, our work suggests some common pathways that might be targeted therapeutically and supports the rationale for several drugs currently under trial. Our finding of increased sCD58 levels (associated with suppression of monocyte–lymphocyte interactions 26 ) in the recovered group, strengthens our conclusion that myeloid inflammation is central to the biology of LC and that trials of steroids, IL-1 antagonists, JAK inhibitors, naltrexone and colchicine are justified. Although anticoagulants such as apixaban might prevent thrombosis downstream of complement dysregulation, they can also increase the risk of serious bleeding when given after COVID-19 hospitalization 48 . Thus, clinical trials, already underway, need to carefully assess the risks and benefits of anticoagulants (Supplementary Table 2 ).
Our finding of elevated S- and N-specific IgG in LC could suggest viral persistence, as found in other studies 6 , 42 , 49 . Our network analysis indicated that inflammatory proteins in the cardioresp group interacted strongly with ISM1 and ROBO1, which are expressed during respiratory tract infection and regulate lung inflammation 50 , 51 . Although we were unable to find SARS-CoV-2 antigen in sputum from our LC cases, we did not test for viral persistence in GI tract and lung tissue 40 , 41 or in plasma 52 . Evidence of SARS-CoV-2 persistence would justify trials of antiviral drugs (singly or in combination) in LC. It is also possible that autoimmune processes could result in an innate inflammatory profile in LC. Autoreactive B cells have been identified in LC patients with higher SARS-CoV-2-specific antibody titers in a study of mostly mild acute COVID cases (59% WHO 2–3) 42 , a different population from our study of hospitalized cases.
Our observations of distinct protein profiles in GI and cognitive groups support previous reports on distinct associations between Epstein–Barr virus reactivation and neurological symptoms, or autoantibodies and GI symptoms relative to other forms of LC 49 , 53 . We did not assess autoantibody induction but found evidence of brain–gut axis disturbance (SCG3) in the GI group, which occurs in many autoimmune diseases 54 . We found signatures suggestive of neuroinflammation (C1QA) in the cognitive group, consistent with findings of brain abnormalities on magnetic resonance imaging after COVID-19 hospitalization 55 , as well as findings of microglial activation in mice after COVID-19 (ref. 56 ). Proinflammatory signatures dominated in the cardioresp, fatigue and anxiety/depression groups and were consistent with those seen in non-COVID depression, suggesting shared mechanisms 57 . The association between markers of myeloid inflammation, including IL-3RA, and symptoms was greatest for fatigue. Whilst membrane-bound IL-3RA facilitates IL-3 signaling upstream of myelopoesis 36 its soluble form (measured in plasma) can bind IL-3 and can act as a decoy receptor, preventing monocyte maturation and enhancing immunopathology 58 . Monocytes from individuals with post-COVID fatigue are reported to have abnormal expression profiles (including reduced CXCR2), suggestive of altered maturation and migration 5 , 59 . Lung-specific inflammation was suggested by the association between PCDH1 (an airway epithelial adhesion molecule 35 ) and cardioresp symptoms.
Our observations do not align with all published observations on LC. One proteomic study of 55 LC cases after generally mild (WHO 2–3) acute disease found that TNF and IFN signatures were elevated in LC 3 . Vasculoproliferative processes and metabolic disturbance have been reported in LC 4 , 60 , but these studies used uninfected healthy individuals for comparison and cannot distinguish between LC-specific phenomena and residual post-COVID inflammation. A study of 63 adults (LC, n = 50 and recovered, n = 13) reported no association between immune cell activation and LC 3 months after infection 61 , though myeloid inflammation was not directly measured, and 3 months post infection may be too early to detect subtle differences between LC and recovered cases due to residual acute inflammation.
Our study has limitations. We designed the study to identify inflammatory markers identifying pathways underlying LC subgroups rather than diagnostic biomarkers. The ORs we report are small, but associations were consistent across alternative methods of analysis and when using different LC definitions. Small effect sizes can be expected when using PLR, which shrinks correlated mediator coefficients to reflect combined effects and prevent colinear inflation 62 , and could also result from measurement of plasma mediators that may underestimate tissue inflammation. Although our LC cohort is large compared with most other published studies, some of our subgroups are small (only 60 cases were designated cognitive). Though the performance of the cognitive PLR model was adequate, our findings should be validated in larger studies. It should be noted that our cohort of hospitalized cases may not represent all types of LC, especially those occurring after mild infection. We looked for an effect of acute disease severity within our study and did not find it, and are reassured that the inflammatory profiles we observed were consistent with those seen in smaller studies including nonhospitalized cases 42 , 46 . Studies of posthospital LC may be confounded by ‘posthospital syndrome’, which encompasses general and nonspecific effects of hospitalization (particularly intensive care) 63 .
In conclusion, we found markers of myeloid inflammation and complement activation in our large prospective posthospital cohort of patients with LC, in addition to distinct inflammatory patterns in patients with cognitive impairment or gastrointestinal symptoms. These findings show the need to consider subphenotypes in managing patients with LC and support the use of antiviral or immunomodulatory agents in controlled therapeutic trials.
Study design and ethics
After hospitalization for COVID-19, adults who had no comorbidity resulting in a prognosis of less than 6 months were recruited to the PHOSP-COVID study ( n = 719). Patients hospitalized between February 2020 and January 2021 were recruited. Both sexes were recruited and gender was self-reported (female, n = 257 and male, n = 462). Written informed consent was obtained from all patients. Ethical approvals for the PHOSP-COVID study were given by Leeds West Research Ethics Committee (20/YH/0225).
Symptom data and samples were prospectively collected from individuals approximately 6 months (IQR 5.1–6.8 months and range 3.0–8.3 months) post hospitalization (Fig. 1a ), via the PHOSP-COVID multicenter United Kingdom study 64 . Data relating to patient demographics and acute admission were collected via the International Severe Acute Respiratory and Emerging Infection Consortium World Health Organization Clinical Characterisation Protocol United Kingdom (ISARIC4C study; IRAS260007/IRAS126600) (ref. 65 ). Adults hospitalized during the SARS-CoV-2 pandemic were systematically recruited into ISARIC4C. Written informed consent was obtained from all patients. Ethical approval was given by the South Central–Oxford C Research Ethics Committee in England (reference 13:/SC/0149), Scotland A Research Ethics Committee (20/SS/0028) and WHO Ethics Review Committee (RPC571 and RPC572l, 25 April 2013).
Data were collected to account for variables affecting symptom outcome, via hospital records and self-reporting. Acute disease severity was classified according to the WHO clinical progression score: WHO class 3–4: no oxygen therapy; class 5: oxygen therapy; class 6: noninvasive ventilation or high-flow nasal oxygen; and class 7–9: managed in critical care 9 . Clinical data were used to place patients into six categories: ‘recovered’, ‘GI’, ‘cardiorespiratory’, ‘fatigue’, ‘cognitive impairment’ and ‘anxiety/depression’ (Supplementary Table 7 ). Patient-reported symptoms and validated clinical scores were used when feasible, including Medical Research Council (MRC) breathlessness score, dyspnea-12 score, Functional Assessment of Chronic Illness Therapy (FACIT) score, Patient Health Questionnaire (PHQ)-9 and Generalized Anxiety Disorder (GAD)-7. Cognitive impairment was defined as a Montreal Cognitive Assessment score <26. GI symptoms were defined as answering ‘Yes’ to the presence of at least two of the listed symptoms. ‘Recovered’ was defined by self-reporting. Patients were placed in multiple groups if they experienced a combination of symptoms.
Matched nasal fluid and sputum samples were prospectively collected from a subgroup of convalescent patients approximately 6 months after hospitalization via the PHOSP-COVID study. Nasal and bronchoalveolar lavage fluid (BALF) collected from healthy volunteers before the COVID-19 pandemic were used as controls (Supplementary Table 4 ). Written consent was obtained for all individuals and ethical approvals were given by London–Harrow Research Ethics Committee (13/LO/1899) for the collection of nasal samples and the Health Research Authority London–Fulham Research Ethics Committee (IRAS project ID 154109; references 14/LO/1023, 10/H0711/94 and 11/LO/1826) for BALF samples.
Ethylenediaminetetraacetic acid plasma was collected from whole blood taken by venepuncture and frozen at −80 °C as previously described 7 , 66 . Nasal fluid was collected using a NasosorptionTM FX·I device (Hunt Developments), which uses a synthetic absorptive matrix to collect concentrated nasal fluid. Samples were eluted and stored as previously described 67 . Sputum samples were collected via passive expectoration and frozen at −80 °C without the addition of buffers. Sputum samples from convalescent individuals were compared with BALF from healthy SARS-CoV-2-naive controls, collected before the pandemic. BALF samples were used to act as a comparison for lower respiratory tract samples since passively expectorated sputum from healthy SARS-CoV-2-naive individuals was not available. BALF samples were obtained by instillation and recovery of up to 240 ml of normal saline via a fiberoptic bronchoscope. BALF was filtered through 100 µM strainers into sterile 50 ml Falcon tubes, then centrifuged for 10 min at 400 g at 4 °C. The resulting supernatant was transferred into sterile 50 ml Falcon tubes and frozen at −80 °C until use. The full methods for BALF collection and processing have been described previously 68 , 69 .
Immunoassays
To determine inflammatory signatures that associated with symptom outcomes, plasma samples were analyzed on an Olink Explore 384 Inflammation panel 70 . Supplementary Table 8 (Appendix 1 ) lists all the analytes measured. To ensure the validity of results, samples were run in a single batch with the use of negative controls, plate controls in triplicate and repeated measurement of patient samples between plates in duplicate. Samples were randomized between plates according to site and sample collection date. Randomization between plates was blind to LC/recovered outcome. Data were first normalized to an internal extension control that was included in each sample well. Plates were standardized by normalizing to interplate controls, run in triplicate on each plate. Each plate contained a minimum of four patient samples, which were duplicates on another plate; these duplicate pairs allowed any plate to be linked to any other through the duplicates. Data were then intensity normalized across all cohort samples. Finally, Olink results underwent quality control processing and samples or analytes that did not reach quality control standards were excluded. Final normalized relative protein quantities were reported as log 2 NPX values.
To further validate our findings, we performed conventional electrochemiluminescence (ECL) assays and enzyme-linked immunosorbent assay for Olink mediators that were associated with symptom outcome ( Supplementary Methods ). Contemporaneously collected plasma samples were available from 58 individuals. Like most omics platforms, Olink measures relative quantities, so perfect agreement with conventional assays that measure absolute concentrations is not expected.
Sputum samples were thawed before analysis and sputum plugs were extracted with the addition of 0.1% dithiothreitol creating a one in two sample dilution, as previously described 71 . SARS-CoV-2 S and N proteins were measured by ECL S-plex assay at a fixed dilution of one in two (Mesoscale Diagnostics), as per the manufacturers protocol 72 . Control BALF samples were thawed and measured on the same plate, neat. The S-plex assay is highly sensitive in detecting viral antigen in respiratory tract samples 73 .
Nasal cytokines were measured by ECL (mesoscale discovery) and Luminex bead multiplex assays (Biotechne). The full methods and list of analytes are detailed in Supplementary Methods .
Statistics and reproducibility
Clinical data was collected via the PHOSP REDCap database, to which access is available under reasonable request as per the data sharing statement in the manuscript. All analyses were performed within the Outbreak Data Analysis Platform (ODAP). All data and code can be accessed using information in the ‘Data sharing’ and ‘Code sharing’ statements at the end of the manuscript. No statistical method was used to predetermine sample size. Data distribution was assumed to be normal but this was not formally tested. Olink assays and immunoassays were randomized and investigators were blinded to outcomes.
To determine protein signatures that associated with each symptom outcome, a ridge PLR was used. PLR shrinks coefficients to account for combined effects within high-dimensional data, preventing false discovery while managing multicollinearity 34 . Thus, PLR was chosen a priori as the most appropriate model to assess associations between a large number of explanatory variables (that may work together to mediate effects) and symptom outcome 34 , 62 , 70 , 74 . In keeping with our aim to perform an unbiased exploration of inflammatory process, the model alpha was set to zero, facilitating regularization without complete penalization of any mediator. This enabled review of all possible mediators that might associate with LC 62 .
A 50 repeats tenfold nested cross-validation was used to select the optimal lambda for each model and assess its accuracy (Extended Data Fig. 1 ). The performance of the cognitive impairment model was influenced by the imbalance in size of the symptom group ( n = 60) relative to recovered ( n = 250). The model was weighted to account for this imbalance resulting in a sensitivity of 0.98, indicating its validity. We have expanded on the model performance and validation approaches in Supplementary Information .
Age, sex, acute disease severity and preexisting comorbidities were included as covariates in the PLR analysis (Supplementary Tables 1 and 3 ). Covariates were selected a priori using features reported to influence the risk of LC and inflammatory responses 1 , 39 , 64 , 75 . Ethnicity was not included since it has been shown not to predict symptom outcome in this cohort 64 . Individuals with missing data were excluded from the regression analysis. Each symptom group was compared with the ‘recovered’ group. The model coefficients of each covariate were converted into ORs for each outcome and visualized in a forest plot, after removing variables associated with regularized OR between 0.98 and 1.02 or in cases where most variables fell outside of this range, using mediators associated with the highest decile of coefficients either side of this range. This enabled exclusion of mediators with effect sizes that were unlikely to have clinical or mechanistic importance since the ridge PLR shrinks and orders coefficients according to their relative importance rather than making estimates with standard error. Thus, confidence intervals cannot be appropriately derived from PLR, and forest plot error bars were calculated using the median accuracy of the model generated by the nested cross-validation. To verify observations made through PLR analysis, we also performed an unadjusted PLR, an unadjusted logistic regression and a PLS analysis. Univariate analyses using Wilcoxon signed-rank test was also performed (Supplementary Table 8 , Appendix 1 ). Analyses were performed in R version 4.2.0 using ‘data.table v1.14.2’, ‘EnvStats v2.7.0’ ‘tidyverse v1.3.2’, ‘lme4 v1.1-32’, ‘caret v6.0-93’, ‘glmnet v4.1-6’, ‘mdatools v0.14.0’, ‘ggpubbr v0.4.0’ and ‘ggplot2 v3.3.6’ packages.
To further investigate the relationship between proteins elevated in each symptom group, we performed a correlation network analysis using Spearman’s rank correlation coefficient and false discovery rate (FDR) thresholding. The mediators visualized in the PLR forest plots, which were associated with cardiorespiratory symptoms, fatigue, anxiety/depression GI symptoms and cognitive impairment were used, respectively. Analyses were performed in R version 4.2.0 using ‘bootnet v1.5.6 ’ and ‘qgraph v1.9.8 ’ packages.
To determine whether differences in protein levels between men and women related to hormonal differences, we divided each symptom group into premenopausal and postmenopausal groups using an age cutoff of 50 years old. Differences between sexes in each group were determined using the Wilcoxon signed-rank test. To understand whether antigen persistence contributed to inflammation in adults with LC, the median viral antigen concentration from sputum/BALF samples and cytokine concentrations from nasal samples were compared using the Wilcoxon signed-rank test. All tests were two-tailed and statistical significance was defined as a P value < 0.05 after adjustment for FDR ( q -value of 0.05). Analyses were performed in R version 4.2.0 using ‘bootnet v1.5.6’ and ‘qgraph v1.9.8’ packages.
Extended Data Fig. 10 was made using Biorender, accessed at www.biorender.com .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This is an open access article under the CC BY 4.0 license.
The PHOSP-COVID protocol, consent form, definition and derivation of clinical characteristics and outcomes, training materials, regulatory documents, information about requests for data access, and other relevant study materials are available online at ref. 76 . Access to these materials can be granted by contacting [email protected] and [email protected].
The ISARIC4C protocol, data sharing and publication policy are available at https://isaric4c.net . ISARIC4C’s Independent Data and Material Access Committee welcomes applications for access to data and materials ( https://isaric4c.net ).
The datasets used in the study contain extensive clinical information at an individual level that prevent them from being deposited in an public depository due to data protection policies of the study. Study data can only be accessed via the ODAP, a protected research environment. All data used in this study are available within ODAP and accessible under reasonable request. Data access criteria and information about how to request access is available online at ref. 76 . If criteria are met and a request is made, access can be gained by signing the eDRIS user agreement.
Code availability
Code was written within the ODAP, using R v4.2.0 and publicly available packages (‘data.table v1.14.2’, ‘EnvStats v2.7.0’, ‘tidyverse v1.3.2’, ‘lme4 v1.1-32’, ‘caret v6.0-93’, ‘glmnet v4.1-6’, ‘mdatools v0.14.0’, ‘ggpubbr v0.4.0’, ‘ggplot2 v3.3.6’, ‘bootnet v1.5.6’ and ‘qgraph v1.9.8’ packages). No new algorithms or functions were created and code used in-built functions in listed packages available on CRAN. The code used to generate data and to analyze data is publicly available at https://github.com/isaric4c/wiki/wiki/ISARIC ; https://github.com/SurgicalInformatics/cocin_cc and https://github.com/ClaudiaEfstath/PHOSP_Olink_NatImm .
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21 , 133–146 (2023).
Article CAS PubMed PubMed Central Google Scholar
Antonelli, M., Pujol, J. C., Spector, T. D., Ourselin, S. & Steves, C. J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 399 , 2263–2264 (2022).
Talla, A. et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat. Commun. 14 , 3417 (2023).
Captur, G. et al. Plasma proteomic signature predicts who will get persistent symptoms following SARS-CoV-2 infection. EBioMedicine 85 , 104293 (2022).
Scott, N. A. et al. Monocyte migration profiles define disease severity in acute COVID-19 and unique features of long COVID. Eur. Respir. J. https://doi.org/10.1183/13993003.02226-2022 (2023).
Klein, J. et al. Distinguishing features of Long COVID identified through immune profiling. Nature https://doi.org/10.1038/s41586-023-06651-y (2023).
Article PubMed PubMed Central Google Scholar
Evans, R. A. et al. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir. Med . 10 , 761–775 (2022).
Article CAS Google Scholar
Houchen-Wolloff, L. et al. Joint patient and clinician priority setting to identify 10 key research questions regarding the long-term sequelae of COVID-19. Thorax 77 , 717–720 (2022).
Article PubMed Google Scholar
Marshall, J. C. et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20 , e192–e197 (2020).
Post COVID-19 condition (long COVID). World Health Organization https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:~:text=Definition,months%20with%20no%20other%20explanation (2022).
Peters, V. A., Joesting, J. J. & Freund, G. G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32 , 1–8 (2013).
Article CAS PubMed Google Scholar
Luo, Z. et al. Monocytes augment inflammatory responses in human aortic valve interstitial cells via β2-integrin/ICAM-1-mediated signaling. Inflamm. Res. 71 , 681–694 (2022).
Bendall, L. J. & Bradstock, K. F. G-CSF: from granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 25 , 355–367 (2014).
Ma, Y. J. et al. Soluble collectin-12 (CL-12) is a pattern recognition molecule initiating complement activation via the alternative pathway. J. Immunol. 195 , 3365–3373 (2015).
Laursen, N. S. et al. Functional and structural characterization of a potent C1q inhibitor targeting the classical pathway of the complement system. Front. Immunol. 11 , 1504 (2020).
Dejanovic, B. et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2 , 837–850 (2022).
Xue, G., Hua, L., Zhou, N. & Li, J. Characteristics of immune cell infiltration and associated diagnostic biomarkers in ulcerative colitis: results from bioinformatics analysis. Bioengineered 12 , 252–265 (2021).
He, T. et al. Integrative computational approach identifies immune‐relevant biomarkers in ulcerative colitis. FEBS Open Bio. 12 , 500–515 (2022).
Sundin, J. et al. Fecal chromogranins and secretogranins are linked to the fecal and mucosal intestinal bacterial composition of IBS patients and healthy subjects. Sci. Rep. 8 , 16821 (2018).
Kriebel, M., Wuchter, J., Trinks, S. & Volkmer, H. Neurofascin: a switch between neuronal plasticity and stability. Int. J. Biochem. Cell Biol. 44 , 694–697 (2012).
Woo, W.-M. et al. The C. elegans F-spondin family protein SPON-1 maintains cell adhesion in neural and non-neural tissues. Development 135 , 2747–2756 (2008).
Yadati, T., Houben, T., Bitorina, A. & Shiri-Sverdlov, R. The ins and outs of cathepsins: physiological function and role in disease management. Cells 9 , 1679 (2020).
Taquet, M. et al. Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat. Med. https://doi.org/10.1038/s41591-023-02525-y (2023).
Siggins, M. K. et al. Alternative pathway dysregulation in tissues drives sustained complement activation and predicts outcome across the disease course in COVID‐19. Immunology 168 , 473–492 (2023).
Pol, J. G., Caudana, P., Paillet, J., Piaggio, E. & Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 217 , e20191247 (2020).
Zhang, Y., Liu, Q., Yang, S. & Liao, Q. CD58 immunobiology at a glance. Front. Immunol. 12 , 705260 (2021).
Demydchuk, M. et al. Insights into Hunter syndrome from the structure of iduronate-2-sulfatase. Nat. Commun. 8 , 15786 (2017).
Wang, Z. et al. DNER promotes epithelial–mesenchymal transition and prevents chemosensitivity through the Wnt/β-catenin pathway in breast cancer. Cell Death Dis. 11 , 642 (2020).
Bonilla, H. et al. Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 14 , 1129459 (2023).
Wik, L. et al. Proximity extension assay in combination with next-generation sequencing for high-throughput proteome-wide analysis. Mol. Cell. Proteomics 20 , 100168 (2021).
Measuring protein biomarkers with Olink—technical comparisons and orthogonal validation. Olink Proteomics https://www.olink.com/content/uploads/2021/09/olink-technical-comparisons-and-orthogonal-validation-1118-v2.0.pdf (2021).
COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN) and Royal College of General Practitioners (RCGP) https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (2022).
Long COVID or post-COVID conditions. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html#:~:text=Long%20COVID%20is%20broadly%20defined,after%20acute%20COVID%2D19%20infection (2023).
Firinguetti, L., Kibria, G. & Araya, R. Study of partial least squares and ridge regression methods. Commun. Stat. Simul. Comput 46 , 6631–6644 (2017).
Article Google Scholar
Mortensen, L. J., Kreiner-Moller, E., Hakonarson, H., Bonnelykke, K. & Bisgaard, H. The PCDH1 gene and asthma in early childhood. Eur. Respir. J. 43 , 792–800 (2014).
Tong, Y. et al. The RNFT2/IL-3Rα axis regulates IL-3 signaling and innate immunity. JCI Insight 5 , e133652 (2020).
Wu, Y. et al. Effect of ISM1 on the immune microenvironment and epithelial-mesenchymal transition in colorectal cancer. Front. Cell Dev. Biol. 9 , 681240 (2021).
Luo, G. G. & Ou, J. J. Oncogenic viruses and cancer. Virol. Sin. 30 , 83–84 (2015).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16 , 626–638 (2016).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591 , 639–644 (2021).
Bussani, R. et al. Persistent SARS‐CoV‐2 infection in patients seemingly recovered from COVID‐19. J. Pathol. 259 , 254–263 (2023).
Woodruff, M. C. et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat. Commun. 14 , 4201 (2023).
Lammi, V. et al. Genome-wide association study of long COVID. Preprint at medRxiv https://doi.org/10.1101/2023.06.29.23292056 (2023).
Ismailova, A. et al. Identification of a forkhead box protein transcriptional network induced in human neutrophils in response to inflammatory stimuli. Front. Immunol. 14 , 1123344 (2023).
Beurskens, F. J., van Schaarenburg, R. A. & Trouw, L. A. C1q, antibodies and anti-C1q autoantibodies. Mol. Immunol. 68 , 6–13 (2015).
Cervia-Hasler, C. et al. Persistent complement dysregulation with signs of thromboinflammation in active long Covid. Science 383 , eadg7942 (2024).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14 , 857–877 (2015).
Toshner, M. R. et al. Apixaban following discharge in hospitalised adults with COVID-19: preliminary results from a multicentre, open-label, randomised controlled platform clinical trial. Preprint at medRxiv , https://doi.org/10.1101/2022.12.07.22283175 (2022).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185 , 881–895.e20 (2022).
Branchfield, K. et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351 , 707–710 (2016).
Rivera-Torruco, G. et al. Isthmin 1 identifies a subset of lung hematopoietic stem cells and it is associated with systemic inflammation. J. Immunol. 202 , 118.18 (2019).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76 , e487–e490 (2023).
Peluso, M. J. et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J. Clin. Invest. 133 , e163669 (2023).
Bellocchi, C. et al. The interplay between autonomic nervous system and inflammation across systemic autoimmune diseases. Int. J. Mol. Sci. 23 , 2449 (2022).
Raman, B. et al. Multiorgan MRI findings after hospitalisation with COVID-19 in the UK (C-MORE): a prospective, multicentre, observational cohort study. Lancet Respir. Med 11 , 1003–1019 (2023).
Fernández-Castañeda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185 , 2452–2468.e16 (2022).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9 , 46–56 (2008).
Broughton, S. E. et al. Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody. Cell Rep. 8 , 410–419 (2014).
Ley, K., Miller, Y. I. & Hedrick, C. C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31 , 1506–1516 (2011).
Iosef, C. et al. Plasma proteome of long-COVID patients indicates HIF-mediated vasculo-proliferative disease with impact on brain and heart function. J. Transl. Med. 21 , 377 (2023).
Santopaolo, M. et al. Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months. eLife 12 , e85009 (2023).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33 , 1–22 (2010).
Voiriot, G. et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann. Intensive Care 12 , 58 (2022).
Evans, R. A. et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir. Med 9 , 1275–1287 (2021).
Docherty, A. B. et al. Features of 20,133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ https://doi.org/10.1136/bmj.m1985 (2020).
Elneima, O. et al. Cohort profile: post-hospitalisation COVID-19 study (PHOSP-COVID). Preprint at medRxiv https://doi.org/10.1101/2023.05.08.23289442 (2023).
Liew, F. et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine 87 , 104402 (2023).
Ascough, S. et al. Divergent age-related humoral correlates of protection against respiratory syncytial virus infection in older and young adults: a pilot, controlled, human infection challenge model. Lancet Healthy Longev. 3 , e405–e416 (2022).
Guvenel, A. et al. Epitope-specific airway-resident CD4+ T cell dynamics during experimental human RSV infection. J. Clin. Invest. 130 , 523–538 (2019).
Article PubMed Central Google Scholar
Greenwood, C. J. et al. A comparison of penalised regression methods for informing the selection of predictive markers. PLoS ONE 15 , e0242730 (2020).
Higham, A. et al. Leukotriene B4 levels in sputum from asthma patients. ERJ Open Res. 2 , 00088–02015 (2016).
SARS-CoV-2 spike kit. MSD https://www.mesoscale.com/~/media/files/product%20inserts/s-plex%20sars-cov-2%20spike%20kit%20product%20insert.pdf (2023).
Ren, A. et al. Ultrasensitive assay for saliva-based SARS-CoV-2 antigen detection. Clin. Chem. Lab. Med. 60 , 771–777 (2022).
Breheny, P. & Huang, J. Penalized methods for bi-level variable selection. Stat. Interface 2 , 369–380 (2009).
Thwaites, R. S. et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci. Immunol. 6 , eabg9873 (2021).
Resources. PHOSP-COVID https://phosp.org/resource/ (2022).
Download references
Acknowledgements
This research used data assets made available by ODAP as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant ref. MC_PC_20058). This work is supported by the following grants: the PHOSP-COVD study is jointly funded by UK Research and Innovation and National Institute of Health and Care Research (NIHR; grant references MR/V027859/1 and COV0319). ISARIC4C is supported by grants from the National Institute for Health and Care Research (award CO-CIN-01) and the MRC (grant MC_PC_19059) Liverpool Experimental Cancer Medicine Centre provided infrastructure support for this research (grant reference C18616/A25153). Other grants that have supported this work include the UK Coronavirus Immunology Consortium (funder reference 1257927), the Imperial Biomedical Research Centre (NIHR Imperial BRC, grant IS-BRC-1215-20013), the Health Protection Research Unit in Respiratory Infections at Imperial College London and NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at University of Liverpool, both in partnership with Public Health England, (NIHR award 200907), Wellcome Trust and Department for International Development (215091/Z/18/Z), Health Data Research UK (grant code 2021.0155), MRC (grant code MC_UU_12014/12) and NIHR Clinical Research Network for providing infrastructure support for this research. We also acknowledge the support of the MRC EMINENT Network (MR/R502121/1), which is cofunded by GSK, the Comprehensive Local Research Networks, the MRC HIC-Vac network (MR/R005982/1) and the RSV Consortium in Europe Horizon 2020 Framework Grant 116019. F.L. is supported by an MRC clinical training fellowship (award MR/W000970/1). C.E. is funded by NIHR (grant P91258-4). L.-P.H. is supported by Oxford NIHR Biomedical Research Centre. A.A.R.T. is supported by a British Heart Foundation (BHF) Intermediate Clinical Fellowship (FS/18/13/33281). S.L.R.-J. receives support from UK Research and Innovation (UKRI), Global Challenges Research Fund (GCRF), Rosetrees Trust, British HIV association (BHIVA), European & Developing Countries Clinical Trials Partnership (EDCTP) and Globvac. J.D.C. has grants from AstraZeneca, Boehringer Ingelheim, GSK, Gilead Sciences, Grifols, Novartis and Insmed. R.A.E. holds a NIHR Clinician Scientist Fellowship (CS-2016-16-020). A. Horsley is currently supported by UK Research and Innovation, NIHR and NIHR Manchester BRC. B.R. receives support from BHF Oxford Centre of Research Excellence, NIHR Oxford BRC and MRC. D.G.W. is supported by an NIHR Advanced Fellowship. A. Ho has received support from MRC and for the Coronavirus Immunology Consortium (MR/V028448/1). L.T. is supported by the US Food and Drug Administration Medical Countermeasures Initiative contract 75F40120C00085 and the National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections (NIHR200907) at the University of Liverpool in partnership with UK Health Security Agency (UK-HSA), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford. L.V.W. has received support from UKRI, GSK/Asthma and Lung UK and NIHR for this study. M.G.S. has received support from NIHR UK, MRC UK and Health Protection Research Unit in Emerging and Zoonotic Infections, University of Liverpool. J.K.B. is supported by the Wellcome Trust (223164/Z/21/Z) and UKRI (MC_PC_20004, MC_PC_19025, MC_PC_1905, MRNO2995X/1 and MC_PC_20029). The funders were not involved in the study design, interpretation of data or writing of this manuscript. The views expressed are those of the authors and not necessarily those of the Department of Health and Social Care (DHSC), the Department for International Development (DID), NIHR, MRC, the Wellcome Trust, UK-HSA, the National Health Service or the Department of Health. P.J.M.O. is supported by a NIHR Senior Investigator Award (award 201385). We thank all the participants and their families. We thank the many research administrators, health-care and social-care professionals who contributed to setting up and delivering the PHOSP-COVID study at all of the 65 NHS trusts/health boards and 25 research institutions across the United Kingdom, as well as those who contributed to setting up and delivering the ISARIC4C study at 305 NHS trusts/health boards. We also thank all the supporting staff at the NIHR Clinical Research Network, Health Research Authority, Research Ethics Committee, Department of Health and Social Care, Public Health Scotland and Public Health England. We thank K. Holmes at the NIHR Office for Clinical Research Infrastructure for her support in coordinating the charities group. The PHOSP-COVID industry framework was formed to provide advice and support in commercial discussions, and we thank the Association of the British Pharmaceutical Industry as well the NIHR Office for Clinical Research Infrastructure for coordinating this. We are very grateful to all the charities that have provided insight to the study: Action Pulmonary Fibrosis, Alzheimer’s Research UK, Asthma and Lung UK, British Heart Foundation, Diabetes UK, Cystic Fibrosis Trust, Kidney Research UK, MQ Mental Health, Muscular Dystrophy UK, Stroke Association Blood Cancer UK, McPin Foundations and Versus Arthritis. We thank the NIHR Leicester Biomedical Research Centre patient and public involvement group and Long Covid Support. We also thank G. Khandaker and D. C. Newcomb who provided valuable feedback on this work. Extended Data Fig. 10 was created using Biorender.
Author information
These authors contributed equally: Felicity Liew, Claudia Efstathiou, Ryan S. Thwaites, Peter J. M. Openshaw.
Authors and Affiliations
National Heart and Lung Institute, Imperial College London, London, UK
Felicity Liew, Claudia Efstathiou, Sara Fontanella, Dawid Swieboda, Jasmin K. Sidhu, Stephanie Ascough, Onn Min Kon, Luke S. Howard, Jennifer K. Quint, Christopher Chiu, Ryan S. Thwaites, Peter J. M. Openshaw, Jake Dunning & Peter J. M. Openshaw
Institute for Lung Health, Leicester NIHR Biomedical Research Centre, University of Leicester, Leicester, UK
Matthew Richardson, Ruth Saunders, Olivia C. Leavy, Omer Elneima, Hamish J. C. McAuley, Amisha Singapuri, Marco Sereno, Victoria C. Harris, Neil J. Greening, Rachael A. Evans, Louise V. Wain, Christopher Brightling & Ananga Singapuri
NIHR Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK
Shona C. Moore, Daniel G. Wootton, Malcolm G. Semple, Lance Turtle, William A. Paxton & Georgios Pollakis
The Imperial Clinical Respiratory Research Unit, Imperial College NHS Trust, London, UK
Noura Mohamed
Cardiovascular Research Team, Imperial College Healthcare NHS Trust, London, UK
Jose Nunag & Clara King
Department of Population Health Sciences, University of Leicester, Leicester, UK
Olivia C. Leavy, Louise V. Wain & Beatriz Guillen-Guio
NIHR Leicester Biomedical Research Centre, University of Leicester, Leicester, UK
Aarti Shikotra
Centre for Exercise and Rehabilitation Science, NIHR Leicester Biomedical Research Centre-Respiratory, University of Leicester, Leicester, UK
Linzy Houchen-Wolloff
Usher Institute, University of Edinburgh, Edinburgh, UK
Nazir I. Lone, Luke Daines, Annemarie B. Docherty, Nazir I. Lone, Matthew Thorpe, Annemarie B. Docherty, Thomas M. Drake, Cameron J. Fairfield, Ewen M. Harrison, Stephen R. Knight, Kenneth A. Mclean, Derek Murphy, Lisa Norman, Riinu Pius & Catherine A. Shaw
Centre for Medical Informatics, The Usher Institute, University of Edinburgh, Edinburgh, UK
Matthew Thorpe, Annemarie B. Docherty, Ewen M. Harrison, J. Kenneth Baillie, Sarah L. Rowland-Jones, A. A. Roger Thompson & Thushan de Silva
Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK
A. A. Roger Thompson, Sarah L. Rowland-Jones, Thushan I. de Silva & James D. Chalmers
University of Dundee, Ninewells Hospital and Medical School, Dundee, UK
James D. Chalmers & Ling-Pei Ho
MRC Human Immunology Unit, University of Oxford, Oxford, UK
Ling-Pei Ho & Alexander Horsley
Division of Infection, Immunity and Respiratory Medicine, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK
Alexander Horsley & Betty Raman
Radcliffe Department of Medicine, University of Oxford, Oxford, UK
Betty Raman & Krisnah Poinasamy
Asthma + Lung UK, London, UK
Krisnah Poinasamy & Michael Marks
Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, UK
Michael Marks
Hospital for Tropical Diseases, University College London Hospital, London, UK
Division of Infection and Immunity, University College London, London, UK
Michael Marks & Mahdad Noursadeghi
MRC Centre for Virus Research, School of Infection and Immunity, University of Glasgow, Glasgow, UK
Antonia Ho & William Greenhalf
Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK
William Greenhalf & J. Kenneth Baillie
The Roslin Institute, University of Edinburgh, Edinburgh, UK
J. Kenneth Baillie, J. Kenneth Baillie, Sara Clohisey, Fiona Griffiths, Ross Hendry, Andrew Law & Wilna Oosthuyzen
Pandemic Science Hub, University of Edinburgh, Edinburgh, UK
J. Kenneth Baillie
The Pandemic Institute, University of Liverpool, Liverpool, UK
Malcolm G. Semple & Lance Turtle
University of Manchester, Manchester, UK
Kathryn Abel, Perdita Barran, H. Chinoy, Bill Deakin, M. Harvie, C. A. Miller, Stefan Stanel & Drupad Trivedi
Intensive Care Unit, Royal Infirmary of Edinburgh, Edinburgh, UK
Kathryn Abel & J. Kenneth Baillie
North Bristol NHS Trust and University of Bristol, Bristol, UK
H. Adamali, David Arnold, Shaney Barratt, A. Dipper, Sarah Dunn, Nick Maskell, Anna Morley, Leigh Morrison, Louise Stadon, Samuel Waterson & H. Welch
University of Edinburgh, Manchester, UK
Davies Adeloye, D. E. Newby, Riinu Pius, Igor Rudan, Manu Shankar-Hari, Catherine Sudlow, Sarah Walmsley & Bang Zheng
King’s College Hospital NHS Foundation Trust and King’s College London, London, UK
Oluwaseun Adeyemi, Rita Adrego, Hosanna Assefa-Kebede, Jonathon Breeze, S. Byrne, Pearl Dulawan, Amy Hoare, Caroline Jolley, Abigail Knighton, M. Malim, Sheetal Patale, Ida Peralta, Natassia Powell, Albert Ramos, K. Shevket, Fabio Speranza & Amelie Te
Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Laura Aguilar Jimenez, Gill Arbane, Sarah Betts, Karen Bisnauthsing, A. Dewar, Nicholas Hart, G. Kaltsakas, Helen Kerslake, Murphy Magtoto, Philip Marino, L. M. Martinez, Marlies Ostermann, Jennifer Rossdale & Teresa Solano
Royal Free London NHS Foundation Trust, London, UK
Shanaz Ahmad, Simon Brill, John Hurst, Hannah Jarvis, C. Laing, Lai Lim, S. Mandal, Darwin Matila, Olaoluwa Olaosebikan & Claire Singh
University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK
N. Ahmad Haider, Catherine Atkin, Rhiannon Baggott, Michelle Bates, A. Botkai, Anna Casey, B. Cooper, Joanne Dasgin, Camilla Dawson, Katharine Draxlbauer, N. Gautam, J. Hazeldine, T. Hiwot, Sophie Holden, Karen Isaacs, T. Jackson, Vicky Kamwa, D. Lewis, Janet Lord, S. Madathil, C. McGee, K. Mcgee, Aoife Neal, Alex Newton-Cox, Joseph Nyaboko, Dhruv Parekh, Z. Peterkin, H. Qureshi, Liz Ratcliffe, Elizabeth Sapey, J. Short, Tracy Soulsby, J. Stockley, Zehra Suleiman, Tamika Thompson, Maximina Ventura, Sinead Walder, Carly Welch, Daisy Wilson, S. Yasmin & Kay Por Yip
Stroke Association, London, UK
Rubina Ahmed & Richard Francis
University College London Hospital and University College London, London, UK
Nyarko Ahwireng, Dongchun Bang, Donna Basire, Jeremy Brown, Rachel Chambers, A. Checkley, R. Evans, M. Heightman, T. Hillman, Joseph Jacob, Roman Jastrub, M. Lipman, S. Logan, D. Lomas, Marta Merida Morillas, Hannah Plant, Joanna Porter, K. Roy & E. Wall
Oxford University Hospitals NHS Foundation Trust and University of Oxford, Oxford, UK
Mark Ainsworth, Asma Alamoudi, Angela Bloss, Penny Carter, M. Cassar, Jin Chen, Florence Conneh, T. Dong, Ranuromanana Evans, V. Ferreira, Emily Fraser, John Geddes, F. Gleeson, Paul Harrison, May Havinden-Williams, P. Jezzard, Ivan Koychev, Prathiba Kurupati, H. McShane, Clare Megson, Stefan Neubauer, Debby Nicoll, C. Nikolaidou, G. Ogg, Edmund Pacpaco, M. Pavlides, Yanchun Peng, Nayia Petousi, John Pimm, Najib Rahman, M. J. Rowland, Kathryn Saunders, Michael Sharpe, Nick Talbot, E. M. Tunnicliffe & C. Xie
St George’s University Hospitals NHS Foundation Trust, London, UK
Mariam Ali, Raminder Aul, A. Dunleavy, D. Forton, Mark Mencias, N. Msimanga, T. Samakomva, Sulman Siddique, Vera Tavoukjian & J. Teixeira
University Hospitals of Leicester NHS Trust and University of Leicester, Leicester, UK
M. Aljaroof, Natalie Armstrong, H. Arnold, Hnin Aung, Majda Bakali, M. Bakau, E. Baldry, Molly Baldwin, Charlotte Bourne, Michelle Bourne, Nigel Brunskill, P. Cairns, Liesel Carr, Amanda Charalambou, C. Christie, Melanie Davies, Enya Daynes, Sarah Diver, Rachael Dowling, Sarah Edwards, C. Edwardson, H. Evans, J. Finch, Sarah Glover, Nicola Goodman, Bibek Gooptu, Kate Hadley, Pranab Haldar, Beverley Hargadon, W. Ibrahim, L. Ingram, Kamlesh Khunti, A. Lea, D. Lee, Gerry McCann, P. McCourt, Teresa Mcnally, George Mills, Will Monteiro, Manish Pareek, S. Parker, Anne Prickett, I. N. Qureshi, A. Rowland, Richard Russell, Salman Siddiqui, Sally Singh, J. Skeemer, M. Soares, E. Stringer, T. Thornton, Martin Tobin, T. J. C. Ward, F. Woodhead, Tom Yates & A. J. Yousuf
University of Exeter, Exeter, UK
Louise Allan, Clive Ballard & Andrew McGovern
University of Leicester, Leicester, UK
Richard Allen, Michelle Bingham, Terry Brugha, Selina Finney, Rob Free, Don Jones, Claire Lawson, Daniel Lozano-Rojas, Gardiner Lucy, Alistair Moss, Elizabeta Mukaetova-Ladinska, Petr Novotny, Kimon Ntotsis, Charlotte Overton, John Pearl, Tatiana Plekhanova, M. Richardson, Nilesh Samani, Jack Sargant, Ruth Saunders, M. Sharma, Mike Steiner, Chris Taylor, Sarah Terry, C. Tong, E. Turner, J. Wormleighton & Bang Zhao
Liverpool University Hospitals NHS Foundation Trust and University of Liverpool, Liverpool, UK
Lisa Allerton, Ann Marie Allt, M. Beadsworth, Anthony Berridge, Jo Brown, Shirley Cooper, Andy Cross, Sylviane Defres, S. L. Dobson, Joanne Earley, N. French, Kera Hainey, Hayley Hardwick, Jenny Hawkes, Victoria Highett, Sabina Kaprowska, Angela Key, Lara Lavelle-Langham, N. Lewis-Burke, Gladys Madzamba, Flora Malein, Sophie Marsh, Chloe Mears, Lucy Melling, Matthew Noonan, L. Poll, James Pratt, Emma Richardson, Anna Rowe, Victoria Shaw, K. A. Tripp, Lilian Wajero, S. A. Williams-Howard, Dan Wootton & J. Wyles
Sherwood Forest Hospitals NHS Foundation Trust, Nottingham, UK
Lynne Allsop, Kaytie Bennett, Phil Buckley, Margaret Flynn, Mandy Gill, Camelia Goodwin, M. Greatorex, Heidi Gregory, Cheryl Heeley, Leah Holloway, Megan Holmes, John Hutchinson, Jill Kirk, Wayne Lovegrove, Terri Ann Sewell, Sarah Shelton, D. Sissons, Katie Slack, Susan Smith, D. Sowter, Sarah Turner, V. Whitworth & Inez Wynter
Nottingham University Hospitals NHS Trust and University of Nottingham, London, UK
Paula Almeida, Akram Hosseini, Robert Needham & Karen Shaw
Manchester University NHS Foundation Trust and University of Manchester, London, UK
Bashar Al-Sheklly, Cristina Avram, John Blaikely, M. Buch, N. Choudhury, David Faluyi, T. Felton, T. Gorsuch, Neil Hanley, Tracy Hussell, Zunaira Kausar, Natasha Odell, Rebecca Osbourne, Karen Piper Hanley, K. Radhakrishnan & Sue Stockdale
Imperial College London, London, UK
Danny Altmann, Anew Frankel, Luke S. Howard, Desmond Johnston, Liz Lightstone, Anne Lingford-Hughes, William Man, Steve McAdoo, Jane Mitchell, Philip Molyneaux, Christos Nicolaou, D. P. O’Regan, L. Price, Jennifer K. Quint, David Smith, Jonathon Valabhji, Simon Walsh, Martin Wilkins & Michelle Willicombe
Hampshire Hospitals NHS Foundation Trust, Basingstoke, UK
Maria Alvarez Corral, Ava Maria Arias, Emily Bevan, Denise Griffin, Jane Martin, J. Owen, Sheila Payne, A. Prabhu, Annabel Reed, Will Storrar, Nick Williams & Caroline Wrey Brown
British Heart Foundation, Birmingham, UK
Shannon Amoils
NHS Greater Glasgow and Clyde Health Board and University of Glasgow, Glasgow, UK
David Anderson, Neil Basu, Hannah Bayes, Colin Berry, Ammani Brown, Andrew Dougherty, K. Fallon, L. Gilmour, D. Grieve, K. Mangion, I. B. McInnes, A. Morrow, Kathryn Scott & R. Sykes
University of Oxford, Oxford, UK
Charalambos Antoniades, A. Bates, M. Beggs, Kamaldeep Bhui, Katie Breeze, K. M. Channon, David Clark, X. Fu, Masud Husain, Lucy Kingham, Paul Klenerman, Hanan Lamlum, X. Li, E. Lukaschuk, Celeste McCracken, K. McGlynn, R. Menke, K. Motohashi, T. E. Nichols, Godwin Ogbole, S. Piechnik, I. Propescu, J. Propescu, A. A. Samat, Z. B. Sanders, Louise Sigfrid & M. Webster
Belfast Health and Social Care Trust and Queen’s University Belfast, Belfast, UK
Cherie Armour, Vanessa Brown, John Busby, Bronwen Connolly, Thelma Craig, Stephen Drain, Liam Heaney, Bernie King, Nick Magee, E. Major, Danny McAulay, Lorcan McGarvey, Jade McGinness, Tunde Peto & Roisin Stone
Airedale NHS Foundation Trust, Keighley, UK
Lisa Armstrong, Brigid Hairsine, Helen Henson, Claire Kurasz, Alison Shaw & Liz Shenton
Wrightington Wigan and Leigh NHS Trust, Wigan, UK
A. Ashish, Josh Cooper & Emma Robinson
Leeds Teaching Hospitals and University of Leeds, Leeds, UK
Andrew Ashworth, Paul Beirne, Jude Clarke, C. Coupland, Matthhew Dalton, Clair Favager, Jodie Glossop, John Greenwood, Lucy Hall, Tim Hardy, Amy Humphries, Jennifer Murira, Dan Peckham, S. Plein, Jade Rangeley, Gwen Saalmink, Ai Lyn Tan, Elaine Wade, Beverley Whittam, Nicola Window & Janet Woods
University of Liverpool, Liverpool, UK
M. Ashworth, D. Cuthbertson, G. Kemp, Anne McArdle, Benedict Michael, Will Reynolds, Lisa Spencer, Ben Vinson, Katie A. Ahmed, Jane A. Armstrong, Milton Ashworth, Innocent G. Asiimwe, Siddharth Bakshi, Samantha L. Barlow, Laura Booth, Benjamin Brennan, Katie Bullock, Nicola Carlucci, Emily Cass, Benjamin W. A. Catterall, Jordan J. Clark, Emily A. Clarke, Sarah Cole, Louise Cooper, Helen Cox, Christopher Davis, Oslem Dincarslan, Alejandra Doce Carracedo, Chris Dunn, Philip Dyer, Angela Elliott, Anthony Evans, Lorna Finch, Lewis W. S. Fisher, Lisa Flaherty, Terry Foster, Isabel Garcia-Dorival, Philip Gunning, Catherine Hartley, Karl Holden, Anthony Holmes, Rebecca L. Jensen, Christopher B. Jones, Trevor R. Jones, Shadia Khandaker, Katharine King, Robyn T. Kiy, Chrysa Koukorava, Annette Lake, Suzannah Lant, Diane Latawiec, Lara Lavelle-Langham, Daniella Lefteri, Lauren Lett, Lucia A. Livoti, Maria Mancini, Hannah Massey, Nicole Maziere, Sarah McDonald, Laurence McEvoy, John McLauchlan, Soeren Metelmann, Nahida S. Miah, Joanna Middleton, Joyce Mitchell, Ellen G. Murphy, Rebekah Penrice-Randal, Jack Pilgrim, Tessa Prince, P. Matthew Ridley, Debby Sales, Rebecca K. Shears, Benjamin Small, Krishanthi S. Subramaniam, Agnieska Szemiel, Aislynn Taggart, Jolanta Tanianis-Hughes, Jordan Thomas, Erwan Trochu, Libby van Tonder, Eve Wilcock & J. Eunice Zhang
University College London, London, UK
Shahab Aslani, Amita Banerjee, R. Batterham, Gabrielle Baxter, Robert Bell, Anthony David, Emma Denneny, Alun Hughes, W. Lilaonitkul, P. Mehta, Ashkan Pakzad, Bojidar Rangelov, B. Williams, James Willoughby & Moucheng Xu
Hull University Teaching Hospitals NHS Trust and University of Hull, Hull, UK
Paul Atkin, K. Brindle, Michael Crooks, Katie Drury, Nicholas Easom, Rachel Flockton, L. Holdsworth, A. Richards, D. L. Sykes, Susannah Thackray-Nocera & C. Wright
East Kent Hospitals University NHS Foundation Trust, Canterbury, UK
Liam Austin, Eva Beranova, Tracey Cosier, Joanne Deery, Tracy Hazelton, Carly Price, Hazel Ramos, Reanne Solly, Sharon Turney & Heather Weston
Baillie Gifford Pandemic Science Hub, Centre for Inflammation Research, The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Nikos Avramidis, J. Kenneth Baillie, Erola Pairo-Castineira & Konrad Rawlik
Roslin Institute, University of Edinburgh, Edinburgh, UK
Nikos Avramidis, J. Kenneth Baillie & Erola Pairo-Castineira
Newcastle upon Tyne Hospitals NHS Foundation Trust and University of Newcastle, Newcastle upon Tyne, UK
A. Ayoub, J. Brown, G. Burns, Gareth Davies, Anthony De Soyza, Carlos Echevarria, Helen Fisher, C. Francis, Alan Greenhalgh, Philip Hogarth, Joan Hughes, Kasim Jiwa, G. Jones, G. MacGowan, D. Price, Avan Sayer, John Simpson, H. Tedd, S. Thomas, Sophie West, M. Witham, S. Wright & A. Young
East Cheshire NHS Trust, Macclesfield, UK
Marta Babores, Maureen Holland, Natalie Keenan, Sharlene Shashaa & Helen Wassall
Sheffield Teaching NHS Foundation Trust and University of Sheffield, Sheffield, UK
J. Bagshaw, M. Begum, K. Birchall, Robyn Butcher, H. Carborn, Flora Chan, Kerry Chapman, Yutung Cheng, Luke Chetham, Cameron Clark, Zach Coburn, Joby Cole, Myles Dixon, Alexandra Fairman, J. Finnigan, H. Foot, David Foote, Amber Ford, Rebecca Gregory, Kate Harrington, L. Haslam, L. Hesselden, J. Hockridge, Ailsa Holbourn, B. Holroyd-Hind, L. Holt, Alice Howell, E. Hurditch, F. Ilyas, Claire Jarman, Allan Lawrie, Ju Hee Lee, Elvina Lee, Rebecca Lenagh, Alison Lye, Irene Macharia, M. Marshall, Angeline Mbuyisa, J. McNeill, Sharon Megson, J. Meiring, L. Milner, S. Misra, Helen Newell, Tom Newman, C. Norman, Lorenza Nwafor, Dibya Pattenadk, Megan Plowright, Julie Porter, Phillip Ravencroft, C. Roddis, J. Rodger, Peter Saunders, J. Sidebottom, Jacqui Smith, Laurie Smith, N. Steele, G. Stephens, R. Stimpson, B. Thamu, N. Tinker, Kim Turner, Helena Turton, Phillip Wade, S. Walker, James Watson, Imogen Wilson & Amira Zawia
University of Nottingham, Nottingham, UK
David Baguley, Chris Coleman, E. Cox, Laura Fabbri, Susan Francis, Ian Hall, E. Hufton, Simon Johnson, Fasih Khan, Paaig Kitterick, Richard Morriss, Nick Selby, Iain Stewart & Louise Wright
Wirral University Teaching Hospital, Wirral, UK
Elisabeth Bailey, Anne Reddington & Andrew Wight
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Western General Hospital, Edinburgh, UK
University of Swansea, Swansea, UK
University of Southampton, London, UK
David Baldwin, P. C. Calder, Nathan Huneke & Gemma Simons
Royal Brompton and Harefield Clinical Group, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
R. E. Barker, Daniele Cristiano, N. Dormand, P. George, Mahitha Gummadi, S. Kon, Kamal Liyanage, C. M. Nolan, B. Patel, Suhani Patel, Oliver Polgar, L. Price, P. Shah, Suver Singh & J. A. Walsh
York and Scarborough NHS Foundation Trust, York, UK
Laura Barman, Claire Brookes, K. Elliott, L. Griffiths, Zoe Guy, Kate Howard, Diana Ionita, Heidi Redfearn, Carol Sarginson & Alison Turnbull
NHS Highland, Inverness, UK
Fiona Barrett, A. Donaldson & Beth Sage
Royal Papworth Hospital NHS Foundation Trust, Cambridge, UK
Helen Baxendale, Lucie Garner, C. Johnson, J. Mackie, Alice Michael, J. Newman, Jamie Pack, K. Paques, H. Parfrey, J. Parmar & A. Reddy
University Hospitals of Derby and Burton, Derby, UK
Paul Beckett, Caroline Dickens & Uttam Nanda
NHS Lanarkshire, Hamilton, UK
Murdina Bell, Angela Brown, M. Brown, R. Hamil, Karen Leitch, L. Macliver, Manish Patel, Jackie Quigley, Andrew Smith & B. Welsh
Cambridge University Hospitals NHS Foundation Trust, NIHR Cambridge Clinical Research Facility and University of Cambridge, Cambridge, UK
Areti Bermperi, Isabel Cruz, K. Dempsey, Anne Elmer, Jonathon Fuld, H. Jones, Sherly Jose, Stefan Marciniak, M. Parkes, Carla Ribeiro, Jessica Taylor, Mark Toshner, L. Watson & J. Worsley
Loughborough University, Loughborough, UK
Lettie Bishop & David Stensel
Betsi Cadwallader University Health Board, Bangor, UK
Annette Bolger, Ffyon Davies, Ahmed Haggar, Joanne Lewis, Arwel Lloyd, R. Manley, Emma McIvor, Daniel Menzies, K. Roberts, W. Saxon, David Southern, Christian Subbe & Victoria Whitehead
Nottingham University Hospitals NHS Trust and University of Nottingham, Nottingham, UK
Charlotte Bolton, J. Bonnington, Melanie Chrystal, Catherine Dupont, Paul Greenhaff, Ayushman Gupta, W. Jang, S. Linford, Laura Matthews, Athanasios Nikolaidis, Sabrina Prosper & Andrew Thomas
King’s College London, London, UK
Kate Bramham, M. Brown, Khalida Ismail, Tim Nicholson, Carmen Pariante, Claire Sharpe, Simon Wessely & J. Whitney
Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK
Lucy Brear, Karen Regan, Dinesh Saralaya & Kim Storton
South London and Maudsley NHS Foundation Trust and King’s College London, London, UK
G. Breen & M. Hotopf
London School of Hygiene and Tropical Medicine, London, UK
Andrew Briggs
Whittington Health NHS Trust, London, UK
E. Bright, P. Crisp, Ruvini Dharmagunawardena & M. Stern
Cardiff and Vale University Health Board, Cardiff, UK
Lauren Broad, Teriann Evans, Matthew Haynes, L. Jones, Lucy Knibbs, Alison McQueen, Catherine Oliver, Kerry Paradowski, Ramsey Sabit & Jenny Williams
Yeovil District Hospital NHS Foundation Trust, Yeovil, UK
Andrew Broadley
University of Birmingham, Birmingham, UK
Mattew Broome, Paul McArdle, Paul Moss, David Thickett, Rachel Upthegrove, Dan Wilkinson, David Wraith & Erin L. Aldera
BHF Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, UK
Anda Bularga
University of Cambridge, Cambridge, UK
Ed Bullmore, Jonathon Heeney, Claudia Langenberg, William Schwaeble, Charlotte Summers & J. Weir McCall
NIHR Leicester Biomedical Research Centre–Respiratory Patient and Public Involvement Group, Leicester, UK
Jenny Bunker, Rhyan Gill & Rashmita Nathu
Imperial College Healthcare NHS Trust and Imperial College London, London, UK
L. Burden, Ellen Calvelo, Bethany Card, Caitlin Carr, Edwin Chilvers, Donna Copeland, P. Cullinan, Patrick Daly, Lynsey Evison, Tamanah Fayzan, Hussain Gordon, Sulaimaan Haq, Gisli Jenkins, Clara King, Onn Min Kon, Katherine March, Myril Mariveles, Laura McLeavey, Silvia Moriera, Unber Munawar, Uchechi Nwanguma, Lorna Orriss-Dib, Alexandra Ross, Maura Roy, Emily Russell, Katherine Samuel, J. Schronce, Neil Simpson, Lawrence Tarusan, David Thomas, Chloe Wood & Najira Yasmin
Harrogate and District NHD Foundation Trust, Harrogate, UK
Tracy Burdett, James Featherstone, Cathy Lawson, Alison Layton, Clare Mills & Lorraine Stephenson
Newcastle University/Chair of NIHR Dementia TRC, Newcastle, UK
Oxford University Hospitals NHS Foundation Trust, Oxford, UK
A. Burns & N. Kanellakis
Tameside and Glossop Integrated Care NHS Foundation Trust, Ashton-under-Lyne, UK
Al-Tahoor Butt, Martina Coulding, Heather Jones, Susan Kilroy, Jacqueline McCormick, Jerome McIntosh, Heather Savill, Victoria Turner & Joanne Vere
University of Oxford, Nuffield Department of Medicine, Oxford, UK
University of Glasgow, Glasgow, UK
Jonathon Cavanagh, S. MacDonald, Kate O’Donnell, John Petrie, Naveed Sattar & Mark Spears
United Lincolnshire Hospitals NHS Trust, Grantham, UK
Manish Chablani & Lynn Osborne
Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
Trudie Chalder
University Hospital of South Manchester NHS Foundation Trust, Manchester, UK
N. Chaudhuri
University Hospital Southampton NHS Foundation Trust and University of Southampton, Southampton, UK
Caroline Childs, R. Djukanovic, S. Fletcher, Matt Harvey, Mark Jones, Elizabeth Marouzet, B. Marshall, Reena Samuel, T. Sass, Tim Wallis & Helen Wheeler
King’s College Hospital/Guy’s and St Thomas’ NHS FT, London, UK
A. Chiribiri & C. O’Brien
Barts Health NHS Trust, London, UK
K. Chong-James, C. David, W. Y. James, Paul Pfeffer & O. Zongo
NHS Lothian and University of Edinburgh, Edinburgh, UK
Gaunab Choudhury, S. Clohisey, Andrew Deans, J. Furniss, Ewen Harrison, S. Kelly & Aziz Sheikh
School of Cardiovascular Medicine and Sciences. King’s College London, London, UK
Phillip Chowienczyk
Lewisham and Greenwich NHS Trust, London, UK
Hywel Dda University Health Board, Haverfordwest, UK
S. Coetzee, Kim Davies, Rachel Ann Hughes, Ronda Loosley, Heather McGuinness, Abdelrahman Mohamed, Linda O’Brien, Zohra Omar, Emma Perkins, Janet Phipps, Gavin Ross, Abigail Taylor, Helen Tench & Rebecca Wolf-Roberts
NHS Tayside and University of Dundee, Dundee, UK
David Connell, C. Deas, Anne Elliott, J. George, S. Mohammed, J. Rowland, A. R. Solstice, Debbie Sutherland & Caroline Tee
Swansea Bay University Health Board, Port Talbot, UK
Lynda Connor, Amanda Cook, Gwyneth Davies, Tabitha Rees, Favas Thaivalappil & Caradog Thomas
Faculty of Medicine, Nursing and Health Sciences, School of Biomedical Sciences, Monash University, Melbourne, Victoria, Australia
Eamon Coughlan
Rotherham NHS Foundation Trust, Rotherham, UK
Alison Daniels, Anil Hormis, Julie Ingham & Lisa Zeidan
Salford Royal NHS Foundation Trust, Salford, UK
P. Dark, Nawar Diar-Bakerly, D. Evans, E. Hardy, Alice Harvey, D. Holgate, Sean Knight, N. Mairs, N. Majeed, L. McMorrow, J. Oxton, Jessica Pendlebury, C. Summersgill, R. Ugwuoke & S. Whittaker
Cwm Taf Morgannwg University Health Board, Mountain Ash, UK
Ellie Davies, Cerys Evenden, Alyson Hancock, Kia Hancock, Ceri Lynch, Meryl Rees, Lisa Roche, Natalie Stroud & T. Thomas-Woods
Borders General Hospital, NHS Borders, Melrose, UK
Joy Dawson, Hosni El-Taweel & Leanne Robinson
Aneurin Bevan University Health Board, Caerleon, UK
Amanda Dell, Sara Fairbairn, Nancy Hawkings, Jill Haworth, Michaela Hoare, Victoria Lewis, Alice Lucey, Georgia Mallison, Heeah Nassa, Chris Pennington, Andrea Price, Claire Price, Andrew Storrie, Gemma Willis & Susan Young
University of Exeter Medical School, Exeter, UK
London North West University Healthcare NHS Trust, London, UK
Shalin Diwanji, Sambasivarao Gurram, Padmasayee Papineni, Sheena Quaid, Gerlynn Tiongson & Ekaterina Watson
Alzheimer’s Research UK, Cambridge, UK
Hannah Dobson
Health and Care Research Wales, Cardiff, UK
Yvette Ellis
University of Bristol, Bristol, UK
Jonathon Evans
University of Sheffield, Sheffield, UK
L. Finnigan, Laura Saunders & James Wild
Great Western Hospital Foundation Trust, Swindon, UK
Eva Fraile & Jacinta Ugoji
Royal Devon and Exeter NHS Trust, Barnstaple, UK
Michael Gibbons
Kettering General Hospital NHS Trust, Kettering, UK
Anne-Marie Guerdette, Melanie Hewitt, R. Reddy, Katie Warwick & Sonia White
NIHR Leicester Biomedical Research Centre, Leicester, UK
Beatriz Guillen-Guio
University of Leeds, Leeds, UK
Elspeth Guthrie & Max Henderson
Royal Surrey NHS Foundation Trust, Cranleigh, UK
Mark Halling-Brown & Katherine McCullough
Chesterfield Royal Hospital NHS Trust, Calow, UK
Edward Harris & Claire Sampson
Long Covid Support, London, UK
Claire Hastie, Natalie Rogers & Nikki Smith
King’s College Hospital, NHS Foundation Trust and King’s College London, London, UK
Department of Oncology and Metabolism, University of Sheffield, Sheffield, UK
Simon Heller
NIHR Office for Clinical Research Infrastructure, London, UK
Katie Holmes
Asthma UK and British Lung Foundation Partnership, London, UK
Ian Jarrold & Samantha Walker
North Middlesex University Hospital NHS Trust, London, UK
Bhagy Jayaraman & Tessa Light
Action for Pulmonary Fibrosis, Peterborough, UK
Cardiff University, National Centre for Mental Health, Cardiff, UK
McPin Foundation, London, UK
Thomas Kabir
Roslin Institute, The University of Edinburgh, Edinburgh, UK
Steven Kerr
The Hillingdon Hospitals NHS Foundation Trust, London, UK
Samantha Kon, G. Landers, Harpreet Lota, Mariam Nasseri & Sofiya Portukhay
Queen Mary University of London, London, UK
Ania Korszun
Swansea University, Swansea Welsh Network, Hywel Dda University Health Board, Swansea, UK
Royal Infirmary of Edinburgh, NHS Lothian, Edinburgh, UK
Nazir I. Lone
Barts Heart Centre, London, UK
Barts Health NHS Trust and Queen Mary University of London, London, UK
Adrian Martineau
Salisbury NHS Foundation Trust, Salisbury, UK
Wadzanai Matimba-Mupaya & Sophia Strong-Sheldrake
University of Newcastle, Newcastle, UK
Hamish McAllister-Williams, Stella-Maria Paddick, Anthony Rostron & John Paul Taylor
Gateshead NHS Trust, Gateshead, UK
W. McCormick, Lorraine Pearce, S. Pugmire, Wendy Stoker & Ann Wilson
Manchester Centre for Clinical Neurosciences, Salford Royal NHS Foundation Trust, Manchester, UK
Katherine McIvor
Kidney Research UK, Peterborough, UK
Aisling McMahon
NHS Dumfries and Galloway, Dumfries, UK
Michael McMahon & Paula Neill
Swansea University, Swansea, UK
MQ Mental Health Research, London, UK
Lea Milligan
BHF Centre for Cardiovascular Science, Usher Institute of Population Health Sciences and Informatics, University of Edinburgh, Edinburgh, UK
Nicholas Mills
Shropshire Community Health NHS Trust, Shropshire, UK
Sharon Painter, Johanne Tomlinson & Louise Warburton
Somerset NHS Foundation Trust, Taunton, UK
Sue Palmer, Dawn Redwood, Jo Tilley, Carinna Vickers & Tania Wainwright
Francis Crick Institute, London, UK
Markus Ralser
Manchester University NHD Foundation Trust, Manchester, UK
Pilar Rivera-Ortega
Diabetes UK, University of Glasgow, Glasgow, UK
Elizabeth Robertson
Barnsley Hospital NHS Foundation Trust, Barnsley, UK
Amy Sanderson
MRC–University of Glasgow Centre for Virus Research, Glasgow, UK
Janet Scott
Diabetes UK, London, UK
Kamini Shah
British Heart Foundation Centre, King’s College London, London, UK
King’s College Hospital NHS Foundation Trust, London, UK
University Hospitals Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK
Institute of Cardiovascular and Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, UK
University College London NHS Foundation Trust, London and Barts Health NHS Trust, London, UK
Northumbria University, Newcastle upon Tyne, UK
Ioannis Vogiatzis
Swansea University and Swansea Welsh Network, Swansea, UK
N. Williams
DUK | NHS Digital, Salford Royal Foundation Trust, Salford, UK
Queen Alexandra Hospital, Portsmouth, UK
- Kayode Adeniji
Princess Royal Hospital, Haywards Heath, UK
Daniel Agranoff & Chi Eziefula
Bassetlaw Hospital, Bassetlaw, UK
Darent Valley Hospital, Dartford, UK
Queen Elizabeth the Queen Mother Hospital, Margate, UK
Ana Alegria
School of Informatics, University of Edinburgh, Edinburgh, UK
Beatrice Alex, Benjamin Bach & James Scott-Brown
North East and North Cumbria Ingerated, Newcastle upon Tyne, UK
Section of Biomolecular Medicine, Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Petros Andrikopoulos, Kanta Chechi, Marc-Emmanuel Dumas, Julian Griffin, Sonia Liggi & Zoltan Takats
Section of Genomic and Environmental Medicine, Respiratory Division, National Heart and Lung Institute, Imperial College London, London, UK
Petros Andrikopoulos, Marc-Emmanuel Dumas, Michael Olanipekun & Anthonia Osagie
John Radcliffe Hospital, Oxford, UK
Brian Angus
Royal Albert Edward Infirmary, Wigan, UK
Abdul Ashish
Manchester Royal Infirmary, Manchester, UK
Dougal Atkinson
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh, UK
Section of Molecular Virology, Imperial College London, London, UK
Wendy S. Barclay
Furness General Hospital, Barrow-in-Furness, UK
Shahedal Bari
Hull University Teaching Hospital Trust, Kingston upon Hull, UK
Gavin Barlow
Hillingdon Hospital, Hillingdon, UK
Stella Barnass
St Thomas’ Hospital, London, UK
Nicholas Barrett
Coventry and Warwickshire, Coventry, UK
Christopher Bassford
St Michael’s Hospital, Bristol, UK
Sneha Basude
Stepping Hill Hospital, Stockport, UK
David Baxter
Royal Liverpool University Hospital, Liverpool, UK
Michael Beadsworth
Bristol Royal Hospital Children’s, Bristol, UK
Jolanta Bernatoniene
Scarborough Hospital, Scarborough, UK
John Berridge
Golden Jubilee National Hospital, Clydebank, UK
Colin Berry
Liverpool Heart and Chest Hospital, Liverpool, UK
Nicola Best
Centre for Inflammation Research, The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Debby Bogaert & Clark D. Russell
James Paget University Hospital, Great Yarmouth, UK
Pieter Bothma & Darell Tupper-Carey
Aberdeen Royal Infirmary, Aberdeen, UK
Robin Brittain-Long
Adamson Hospital, Cupar, UK
Naomi Bulteel
Royal Devon and Exeter Hospital, Exeter, UK
Worcestershire Royal Hospital, Worcester, UK
Andrew Burtenshaw
ISARIC Global Support Centre, Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Gail Carson, Laura Merson & Louise Sigfrid
Conquest Hospital, Hastings, UK
Vikki Caruth
The James Cook University Hospital, Middlesbrough, UK
David Chadwick
Dorset County Hospital, Dorchester, UK
Duncan Chambler
Antimicrobial Resistance and Hospital Acquired Infection Department, Public Health England, London, UK
Meera Chand
Department of Epidemiology and Biostatistics, School of Public Health, Faculty of Medicine, Imperial College London, London, UK
Kanta Chechi
Royal Bournemouth General Hospital, Bournemouth, UK
Harrogate Hospital, Harrogate, UK
Jenny Child
Royal Blackburn Teaching Hospital, Blackburn, UK
Srikanth Chukkambotla
Edinburgh Clinical Research Facility, University of Edinburgh, Edinburgh, UK
Richard Clark, Audrey Coutts, Lorna Donelly, Angie Fawkes, Tammy Gilchrist, Katarzyna Hafezi, Louise MacGillivray, Alan Maclean, Sarah McCafferty, Kirstie Morrice, Lee Murphy & Nicola Wrobel
Torbay Hospital, Torquay, UK
Northern General Hospital, Sheffield, UK
Paul Collini, Cariad Evans & Gary Mills
Liverpool Clinical Trials Centre, University of Liverpool, Liverpool, UK
Marie Connor, Jo Dalton, Chloe Donohue, Carrol Gamble, Michelle Girvan, Sophie Halpin, Janet Harrison, Clare Jackson, Laura Marsh, Stephanie Roberts & Egle Saviciute
Department of Infectious Disease, Imperial College London, London, UK
Graham S. Cooke & Shiranee Sriskandan
St Georges Hospital (Tooting), London, UK
Catherine Cosgrove
Blackpool Victoria Hospital, Blackpool, UK
Jason Cupitt & Joanne Howard
The Royal London Hospital, London, UK
Maria-Teresa Cutino-Moguel
MRC-University of Glasgow Centre for Virus Research, Glasgow, UK
Ana da Silva Filipe, Antonia Y. W. Ho, Sarah E. McDonald, Massimo Palmarini, David L. Robertson, Janet T. Scott & Emma C. Thomson
Salford Royal Hospital, Salford, UK
University Hospital of North Durham, Durham, UK
Chris Dawson
Norfolk and Norwich University Hospital, Norwich, UK
Samir Dervisevic
Intensive Care Unit, Royal Infirmary Edinburgh, Edinburgh, UK
Annemarie B. Docherty & Seán Keating
Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK
Cara Donegan & Rebecca G. Spencer
Salisbury District Hospital, Salisbury, UK
Phil Donnison
National Phenome Centre, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Gonçalo dos Santos Correia, Matthew Lewis, Lynn Maslen, Caroline Sands, Zoltan Takats & Panteleimon Takis
Section of Bioanalytical Chemistry, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Gonçalo dos Santos Correia, Matthew Lewis, Lynn Maslen, Caroline Sands & Panteleimon Takis
Guy’s and St Thomas’, NHS Foundation Trust, London, UK
Sam Douthwaite, Michael MacMahon, Marlies Ostermann & Manu Shankar-Hari
The Royal Oldham Hospital, Oldham, UK
Andrew Drummond
European Genomic Institute for Diabetes, Institut Pasteur de Lille, Lille University Hospital, University of Lille, Lille, France
Marc-Emmanuel Dumas
McGill University and Genome Quebec Innovation Centre, Montreal, Qeubec, Canada
National Infection Service, Public Health England, London, UK
Jake Dunning & Maria Zambon
Hereford Count Hospital, Hereford, UK
Ingrid DuRand
Southampton General Hospital, Southampton, UK
Ahilanadan Dushianthan
Northampton General Hospital, Northampton, UK
Tristan Dyer
University Hospital of Wales, Cardiff, UK
Chrisopher Fegan
University Hospitals Bristol NHS Foundation Trust, Bristol, UK
Liverpool School of Tropical Medicine, Liverpool, UK
Tom Fletcher
Leighton Hospital, Crewe, UK
Duncan Fullerton & Elijah Matovu
Manor Hospital, Walsall, UK
Scunthorpe Hospital, Scunthorpe, UK
Sanjeev Garg
Cambridge University Hospital, Cambridge, UK
Effrossyni Gkrania-Klotsas
West Suffolk NHS Foundation Trust, Bury St Edmunds, UK
Basingstoke and North Hampshire Hospital, Basingstoke, UK
Arthur Goldsmith
North Cumberland Infirmary, Carlisle, UK
Clive Graham
Paediatric Liver, GI and Nutrition Centre and MowatLabs, King’s College Hospital, London, UK
Tassos Grammatikopoulos
Institute of Liver Studies, King’s College London, London, UK
Institute of Microbiology and Infection, University of Birmingham, Birmingham, UK
Christopher A. Green
Department of Molecular and Clinical Cancer Medicine, University of Liverpool, Liverpool, UK
William Greenhalf
Institute for Global Health, University College London, London, UK
Rishi K. Gupta
NIHR Health Protection Research Unit, Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK
Hayley Hardwick, Malcolm G. Semple, Tom Solomon & Lance C. W. Turtle
Warwick Hospital, Warwick, UK
Elaine Hardy
Birmingham Children’s Hospital, Birmingham, UK
Stuart Hartshorn
Nottingham City Hospital, Nottingham, UK
Daniel Harvey
Glangwili Hospital Child Health Section, Carmarthen, UK
Peter Havalda
Alder Hey Children’s Hospital, Liverpool, UK
Daniel B. Hawcutt
Department of Infectious Diseases, Queen Elizabeth University Hospital, Glasgow, UK
Antonia Y. W. Ho
Bronglais General Hospital, Aberystwyth, UK
Maria Hobrok
Worthing Hospital, Worthing, UK
Luke Hodgson
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Peter W. Horby
Rotheram District General Hospital, Rotheram, UK
Anil Hormis
Virology Reference Department, National Infection Service, Public Health England, Colindale Avenue, London, UK
Samreen Ijaz
Royal Free Hospital, London, UK
Michael Jacobs & Padmasayee Papineni
Homerton Hospital, London, UK
Airedale Hospital, Airedale, UK
Paul Jennings
Basildon Hospital, Basildon, UK
Agilan Kaliappan
The Christie NHS Foundation Trust, Manchester, UK
Vidya Kasipandian
University Hospital Lewisham, London, UK
Stephen Kegg
The Whittington Hospital, London, UK
Michael Kelsey
Southmead Hospital, Bristol, UK
Jason Kendall
Sheffield Childrens Hospital, Sheffield, UK
Caroline Kerrison
Royal United Hospital, Bath, UK
Ian Kerslake
Department of Pharmacology, University of Liverpool, Liverpool, UK
Nuffield Department of Medicine, Peter Medawar Building for Pathogen Research, University of Oxford, Oxford, UK
Paul Klenerman
Translational Gastroenterology Unit, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Public Health Scotland, Edinburgh, UK
Susan Knight, Eva Lahnsteiner & Sarah Tait
Western General Hospital, Edinburgh, UK
Oliver Koch
Southend University Hospital NHS Foundation Trust, Southend-on-Sea, UK
Gouri Koduri
Hinchingbrooke Hospital, Huntingdon, UK
George Koshy & Tamas Leiner
Royal Preston Hospital, Fulwood, UK
Shondipon Laha
University Hospital (Coventry), Coventry, UK
Steven Laird
The Walton Centre, Liverpool, UK
Susan Larkin
ISARIC, Global Support Centre, COVID-19 Clinical Research Resources, Epidemic diseases Research Group, Oxford (ERGO), University of Oxford, Oxford, UK
James Lee & Daniel Plotkin
Centre for Health Informatics, Division of Informatics, Imaging and Data Science, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
Gary Leeming
Hull Royal Infirmary, Hull, UK
Patrick Lillie
Nottingham University Hospitals NHS Trust:, Nottingham, UK
Wei Shen Lim
Darlington Memorial Hospital, Darlington, UK
Queen Elizabeth Hospital (Gateshead), Gateshead, UK
Vanessa Linnett
Warrington Hospital, Warrington, UK
Jeff Little
Bristol Royal Hospital for Children, Bristol, UK
Mark Lyttle
St Mary’s Hospital (Isle of Wight), Isle of Wight, UK
Emily MacNaughton
The Tunbridge Wells Hospital, Royal Tunbridge Wells, UK
Ravish Mankregod
Huddersfield Royal, Huddersfield, UK
Countess of Chester Hospital, Liverpool, UK
Ruth McEwen & Lawrence Wilson
Frimley Park Hospital, Frimley, UK
Manjula Meda
Nuffield Department of Medicine, John Radcliffe Hospital, Oxford, UK
Alexander J. Mentzer
Department of Microbiology/Infectious Diseases, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, UK
MRC Human Genetics Unit, MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK
Alison M. Meynert & Murray Wham
St James University Hospital, Leeds, UK
Jane Minton
Arrowe Park Hospital, Birkenhead, UK
Kavya Mohandas
Great Ormond Street Hospital, London, UK
Royal Shrewsbury Hospital, Shrewsbury, UK
Addenbrookes Hospital, Cambridge, UK
Elinoor Moore
Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK
Shona C. Moore, William A. Paxton & Georgios Pollakis
East Surrey Hospital (Redhill), Redhill, UK
Patrick Morgan
Burton Hospital, Burton, UK
Craig Morris & Tim Reynolds
Peterborough City Hospital, Peterborough, UK
Katherine Mortimore
Kent and Canterbury Hospital, Canterbury, UK
Samuel Moses
Weston Area General Trust, Bristol, UK
Mbiye Mpenge
Bedfordshire Hospital, Bedfordshire, UK
Rohinton Mulla
Glasgow Royal Infirmary, Glasgow, UK
Michael Murphy
Macclesfield General Hospital, Macclesfield, UK
Thapas Nagarajan
Derbyshire Healthcare, Derbyshire, UK
Megan Nagel
Chelsea and Westminster Hospital, London, UK
Mark Nelson & Matthew K. O’Shea
Watford General Hospital, Watford, UK
Lillian Norris & Tom Stambach
EPCC, University of Edinburgh, Edinburgh, UK
Lucy Norris
Section of Biomolecular Medicine, Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, London, UK
Michael Olanipekun
Imperial College Healthcare NHS Trust: London, London, UK
Peter J. M. Openshaw
Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Anthonia Osagie
Prince Philip Hospital, Llanelli, UK
Igor Otahal & Andrew Workman
George Eliot Hospital – Acute Services, Nuneaton, UK
Molecular and Clinical Cancer Medicine, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK
Carlo Palmieri
Clatterbridge Cancer Centre NHS Foundation Trust, Liverpool, UK
Kettering General Hospital, Kettering, UK
Selva Panchatsharam
University Hospitals of North Midlands NHS Trust, North Midlands, UK
Danai Papakonstantinou
Russells Hall Hospital, Dudley, UK
Hassan Paraiso
Harefield Hospital, Harefield, UK
Lister Hospital, Lister, UK
Natalie Pattison
Musgrove Park Hospital, Taunton, UK
Justin Pepperell
Kingston Hospital, Kingston, UK
Mark Peters
Queen’s Hospital, Romford, UK
Mandeep Phull
Southport and Formby District General Hospital, Southport, UK
Stefania Pintus
St George’s University of London, London, UK
Tim Planche
King’s College Hospital (Denmark Hill), London, UK
Centre for Clinical Infection and Diagnostics Research, Department of Infectious Diseases, School of Immunology and Microbial Sciences, King’s College London, London, UK
Nicholas Price
Department of Infectious Diseases, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
The Clatterbridge Cancer Centre NHS Foundation, Bebington, UK
David Price
The Great Western Hospital, Swindon, UK
Rachel Prout
Ninewells Hospital, Dundee, UK
Nikolas Rae
Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, UK
Andrew Rambaut
Poole Hospital NHS Trust, Poole, UK
Henrik Reschreiter
William Harvey Hospital, Ashford, UK
Neil Richardson
King’s Mill Hospital, Sutton-in-Ashfield, UK
Mark Roberts
Liverpool Women’s Hospital, Liverpool, UK
Devender Roberts
Pinderfields Hospital, Wakefield, UK
Alistair Rose
North Devon District Hospital, Barnstaple, UK
Guy Rousseau
Queen Elizabeth Hospital, Birmingham, UK
Tameside General Hospital, Ashton-under-Lyne, UK
Brendan Ryan
City Hospital (Birmingham), Birmingham, UK
Taranprit Saluja
Department of Pediatrics and Virology, St Mary’s Medical School Bldg, Imperial College London, London, UK
Vanessa Sancho-Shimizu
The Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, UK
Matthias Schmid
NHS Greater Glasgow and Clyde, Glasgow, UK
Janet T. Scott
Respiratory Medicine, Institute in The Park, University of Liverpool, Alder Hey Children’s Hospital, Liverpool, UK
Malcolm G. Semple
Broomfield Hospital, Broomfield, UK
Stoke Mandeville, UK
Prad Shanmuga
University Hospital of North Tees, Stockton-on-Tees, UK
Anil Sharma
Institute of Translational Medicine, University of, Liverpool, Merseyside, UK
Victoria E. Shaw
Royal Manchester Children’s Hospital, Manchester, UK
Anna Shawcross
New Cross Hospital, Wolverhampton, UK
Jagtur Singh Pooni
Bedford Hospital, Bedford, UK
Jeremy Sizer
Colchester General Hospital, Colchester, UK
Richard Smith
University Hospital Birmingham NHS Foundation Trust, Birmingham, UK
Catherine Snelson & Tony Whitehouse
Walton Centre NHS Foundation Trust, Liverpool, UK
Tom Solomon
Chesterfield Royal Hospital, Calow, UK
Nick Spittle
MRC Centre for Molecular Bacteriology and Infection, Imperial College London, London, UK
Shiranee Sriskandan
Princess Alexandra Hospital, Harlow, UK
Nikki Staines & Shico Visuvanathan
Milton Keynes Hospital, Eaglestone, UK
Richard Stewart
Division of Structural Biology, The Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK
David Stuart
Royal Bolton Hopital, Farnworth, UK
Pradeep Subudhi
Department of Medicine, University of Cambridge, Cambridge, UK
Charlotte Summers
Department of Child Life and Health, University of Edinburgh, Edinburgh, UK
Olivia V. Swann
Royal Gwent (Newport), Newport, UK
Tamas Szakmany
The Royal Marsden Hospital (London), London, UK
Kate Tatham
Blood Borne Virus Unit, Virus Reference Department, National Infection Service, Public Health England, London, UK
Richard S. Tedder
Transfusion Microbiology, National Health Service Blood and Transplant, London, UK
Department of Medicine, Imperial College London, London, UK
Queen Victoria Hospital (East Grinstead), East Grinstead, UK
Leeds Teaching Hospitals NHS Trust, Leeds, UK
Robert Thompson
Royal Stoke University Hospital, Stoke-on-Trent, UK
Chris Thompson
Whiston Hospital, Rainhill, UK
Ascanio Tridente
Tropical and Infectious Disease Unit, Royal Liverpool University Hospital, Liverpool, UK
Lance C. W. Turtle
Croydon University Hospital, Thornton Heath, UK
Mary Twagira
Gloucester Royal, Gloucester, UK
Nick Vallotton
West Hertfordshire Teaching Hospitals NHS Trust, Hertfordshire, UK
Rama Vancheeswaran
North Middlesex Hospital, London, UK
Rachel Vincent
Medway Maritime Hospital, Gillingham, UK
Lisa Vincent-Smith
Royal Papworth Hospital Everard, Cambridge, UK
Alan Vuylsteke
Derriford (Plymouth), Plymouth, UK
St Helier Hospital, Sutton, UK
Rachel Wake
Royal Berkshire Hospital, Reading, UK
Andrew Walden
Royal Liverpool Hospital, Liverpool, UK
Ingeborg Welters
Bradford Royal infirmary, Bradford, UK
Paul Whittaker
Central Middlesex, London, UK
Ashley Whittington
Royal Cornwall Hospital (Tresliske), Truro, UK
Meme Wijesinghe
North Bristol NHS Trust, Bristol, UK
Martin Williams
St. Peter’s Hospital, Runnymede, UK
Stephen Winchester
Leicester Royal Infirmary, Leicester, UK
Martin Wiselka
Grantham and District Hospital, Grantham, UK
Adam Wolverson
Aintree University Hospital, Liverpool, UK
Daniel G. Wootton
North Tyneside General Hospital, North Shields, UK
Bryan Yates
Queen Elizabeth Hospital, King’s Lynn, UK
Peter Young
You can also search for this author in PubMed Google Scholar
PHOSP-COVID collaborative group
- Kathryn Abel
- , H. Adamali
- , Davies Adeloye
- , Oluwaseun Adeyemi
- , Rita Adrego
- , Laura Aguilar Jimenez
- , Shanaz Ahmad
- , N. Ahmad Haider
- , Rubina Ahmed
- , Nyarko Ahwireng
- , Mark Ainsworth
- , Asma Alamoudi
- , Mariam Ali
- , M. Aljaroof
- , Louise Allan
- , Richard Allen
- , Lisa Allerton
- , Lynne Allsop
- , Ann Marie Allt
- , Paula Almeida
- , Bashar Al-Sheklly
- , Danny Altmann
- , Maria Alvarez Corral
- , Shannon Amoils
- , David Anderson
- , Charalambos Antoniades
- , Gill Arbane
- , Ava Maria Arias
- , Cherie Armour
- , Lisa Armstrong
- , Natalie Armstrong
- , David Arnold
- , H. Arnold
- , A. Ashish
- , Andrew Ashworth
- , M. Ashworth
- , Shahab Aslani
- , Hosanna Assefa-Kebede
- , Paul Atkin
- , Catherine Atkin
- , Raminder Aul
- , Hnin Aung
- , Liam Austin
- , Cristina Avram
- , Nikos Avramidis
- , Marta Babores
- , Rhiannon Baggott
- , J. Bagshaw
- , David Baguley
- , Elisabeth Bailey
- , J. Kenneth Baillie
- , Steve Bain
- , Majda Bakali
- , E. Baldry
- , Molly Baldwin
- , David Baldwin
- , Clive Ballard
- , Amita Banerjee
- , Dongchun Bang
- , R. E. Barker
- , Laura Barman
- , Perdita Barran
- , Shaney Barratt
- , Fiona Barrett
- , Donna Basire
- , Neil Basu
- , Michelle Bates
- , R. Batterham
- , Helen Baxendale
- , Gabrielle Baxter
- , Hannah Bayes
- , M. Beadsworth
- , Paul Beckett
- , Paul Beirne
- , Murdina Bell
- , Robert Bell
- , Kaytie Bennett
- , Eva Beranova
- , Areti Bermperi
- , Anthony Berridge
- , Colin Berry
- , Sarah Betts
- , Emily Bevan
- , Kamaldeep Bhui
- , Michelle Bingham
- , K. Birchall
- , Lettie Bishop
- , Karen Bisnauthsing
- , John Blaikely
- , Angela Bloss
- , Annette Bolger
- , Charlotte Bolton
- , J. Bonnington
- , A. Botkai
- , Charlotte Bourne
- , Michelle Bourne
- , Kate Bramham
- , Lucy Brear
- , Jonathon Breeze
- , Katie Breeze
- , Andrew Briggs
- , E. Bright
- , Christopher Brightling
- , Simon Brill
- , K. Brindle
- , Lauren Broad
- , Andrew Broadley
- , Claire Brookes
- , Mattew Broome
- , Vanessa Brown
- , Ammani Brown
- , Angela Brown
- , Jeremy Brown
- , Terry Brugha
- , Nigel Brunskill
- , Phil Buckley
- , Anda Bularga
- , Ed Bullmore
- , Jenny Bunker
- , L. Burden
- , Tracy Burdett
- , David Burn
- , John Busby
- , Robyn Butcher
- , Al-Tahoor Butt
- , P. Cairns
- , P. C. Calder
- , Ellen Calvelo
- , H. Carborn
- , Bethany Card
- , Caitlin Carr
- , Liesel Carr
- , G. Carson
- , Penny Carter
- , Anna Casey
- , M. Cassar
- , Jonathon Cavanagh
- , Manish Chablani
- , Trudie Chalder
- , James D. Chalmers
- , Rachel Chambers
- , Flora Chan
- , K. M. Channon
- , Kerry Chapman
- , Amanda Charalambou
- , N. Chaudhuri
- , A. Checkley
- , Yutung Cheng
- , Luke Chetham
- , Caroline Childs
- , Edwin Chilvers
- , H. Chinoy
- , A. Chiribiri
- , K. Chong-James
- , N. Choudhury
- , Gaunab Choudhury
- , Phillip Chowienczyk
- , C. Christie
- , Melanie Chrystal
- , Cameron Clark
- , David Clark
- , Jude Clarke
- , S. Clohisey
- , G. Coakley
- , Zach Coburn
- , S. Coetzee
- , Joby Cole
- , Chris Coleman
- , Florence Conneh
- , David Connell
- , Bronwen Connolly
- , Lynda Connor
- , Amanda Cook
- , Shirley Cooper
- , B. Cooper
- , Josh Cooper
- , Donna Copeland
- , Tracey Cosier
- , Eamon Coughlan
- , Martina Coulding
- , C. Coupland
- , Thelma Craig
- , Daniele Cristiano
- , Michael Crooks
- , Andy Cross
- , Isabel Cruz
- , P. Cullinan
- , D. Cuthbertson
- , Luke Daines
- , Matthhew Dalton
- , Patrick Daly
- , Alison Daniels
- , Joanne Dasgin
- , Anthony David
- , Ffyon Davies
- , Ellie Davies
- , Kim Davies
- , Gareth Davies
- , Gwyneth Davies
- , Melanie Davies
- , Joy Dawson
- , Camilla Dawson
- , Enya Daynes
- , Anthony De Soyza
- , Bill Deakin
- , Andrew Deans
- , Joanne Deery
- , Sylviane Defres
- , Amanda Dell
- , K. Dempsey
- , Emma Denneny
- , J. Dennis
- , Ruvini Dharmagunawardena
- , Nawar Diar-Bakerly
- , Caroline Dickens
- , A. Dipper
- , Sarah Diver
- , Shalin Diwanji
- , Myles Dixon
- , R. Djukanovic
- , Hannah Dobson
- , S. L. Dobson
- , Annemarie B. Docherty
- , A. Donaldson
- , N. Dormand
- , Andrew Dougherty
- , Rachael Dowling
- , Stephen Drain
- , Katharine Draxlbauer
- , Katie Drury
- , Pearl Dulawan
- , A. Dunleavy
- , Sarah Dunn
- , Catherine Dupont
- , Joanne Earley
- , Nicholas Easom
- , Carlos Echevarria
- , Sarah Edwards
- , C. Edwardson
- , Claudia Efstathiou
- , Anne Elliott
- , K. Elliott
- , Yvette Ellis
- , Anne Elmer
- , Omer Elneima
- , Hosni El-Taweel
- , Teriann Evans
- , Ranuromanana Evans
- , Rachael A. Evans
- , Jonathon Evans
- , Cerys Evenden
- , Lynsey Evison
- , Laura Fabbri
- , Sara Fairbairn
- , Alexandra Fairman
- , K. Fallon
- , David Faluyi
- , Clair Favager
- , Tamanah Fayzan
- , James Featherstone
- , T. Felton
- , V. Ferreira
- , Selina Finney
- , J. Finnigan
- , L. Finnigan
- , Helen Fisher
- , S. Fletcher
- , Rachel Flockton
- , Margaret Flynn
- , David Foote
- , Amber Ford
- , D. Forton
- , Eva Fraile
- , C. Francis
- , Richard Francis
- , Susan Francis
- , Anew Frankel
- , Emily Fraser
- , N. French
- , Jonathon Fuld
- , J. Furniss
- , Lucie Garner
- , N. Gautam
- , John Geddes
- , J. George
- , P. George
- , Michael Gibbons
- , Rhyan Gill
- , Mandy Gill
- , L. Gilmour
- , F. Gleeson
- , Jodie Glossop
- , Sarah Glover
- , Nicola Goodman
- , Camelia Goodwin
- , Bibek Gooptu
- , Hussain Gordon
- , T. Gorsuch
- , M. Greatorex
- , Paul Greenhaff
- , William Greenhalf
- , Alan Greenhalgh
- , Neil J. Greening
- , John Greenwood
- , Rebecca Gregory
- , Heidi Gregory
- , D. Grieve
- , Denise Griffin
- , L. Griffiths
- , Anne-Marie Guerdette
- , Beatriz Guillen-Guio
- , Mahitha Gummadi
- , Ayushman Gupta
- , Sambasivarao Gurram
- , Elspeth Guthrie
- , Kate Hadley
- , Ahmed Haggar
- , Kera Hainey
- , Brigid Hairsine
- , Pranab Haldar
- , Lucy Hall
- , Mark Halling-Brown
- , Alyson Hancock
- , Kia Hancock
- , Neil Hanley
- , Sulaimaan Haq
- , Hayley Hardwick
- , Tim Hardy
- , Beverley Hargadon
- , Kate Harrington
- , Edward Harris
- , Victoria C. Harris
- , Ewen Harrison
- , Paul Harrison
- , Nicholas Hart
- , Alice Harvey
- , Matt Harvey
- , M. Harvie
- , L. Haslam
- , Claire Hastie
- , May Havinden-Williams
- , Jenny Hawkes
- , Nancy Hawkings
- , Jill Haworth
- , A. Hayday
- , Matthew Haynes
- , J. Hazeldine
- , Tracy Hazelton
- , Liam Heaney
- , Cheryl Heeley
- , Jonathon Heeney
- , M. Heightman
- , Simon Heller
- , Max Henderson
- , Helen Henson
- , L. Hesselden
- , Melanie Hewitt
- , Victoria Highett
- , T. Hillman
- , Ling-Pei Ho
- , Michaela Hoare
- , Amy Hoare
- , J. Hockridge
- , Philip Hogarth
- , Ailsa Holbourn
- , Sophie Holden
- , L. Holdsworth
- , D. Holgate
- , Maureen Holland
- , Leah Holloway
- , Katie Holmes
- , Megan Holmes
- , B. Holroyd-Hind
- , Anil Hormis
- , Alexander Horsley
- , Akram Hosseini
- , M. Hotopf
- , Linzy Houchen-Wolloff
- , Luke S. Howard
- , Kate Howard
- , Alice Howell
- , E. Hufton
- , Rachel Ann Hughes
- , Joan Hughes
- , Alun Hughes
- , Amy Humphries
- , Nathan Huneke
- , E. Hurditch
- , John Hurst
- , Masud Husain
- , Tracy Hussell
- , John Hutchinson
- , W. Ibrahim
- , Julie Ingham
- , L. Ingram
- , Diana Ionita
- , Karen Isaacs
- , Khalida Ismail
- , T. Jackson
- , Joseph Jacob
- , W. Y. James
- , Claire Jarman
- , Ian Jarrold
- , Hannah Jarvis
- , Roman Jastrub
- , Bhagy Jayaraman
- , Gisli Jenkins
- , P. Jezzard
- , Kasim Jiwa
- , C. Johnson
- , Simon Johnson
- , Desmond Johnston
- , Caroline Jolley
- , Ian Jones
- , Heather Jones
- , Mark Jones
- , Don Jones
- , Sherly Jose
- , Thomas Kabir
- , G. Kaltsakas
- , Vicky Kamwa
- , N. Kanellakis
- , Sabina Kaprowska
- , Zunaira Kausar
- , Natalie Keenan
- , Steven Kerr
- , Helen Kerslake
- , Angela Key
- , Fasih Khan
- , Kamlesh Khunti
- , Susan Kilroy
- , Bernie King
- , Clara King
- , Lucy Kingham
- , Jill Kirk
- , Paaig Kitterick
- , Paul Klenerman
- , Lucy Knibbs
- , Sean Knight
- , Abigail Knighton
- , Onn Min Kon
- , Samantha Kon
- , Ania Korszun
- , Ivan Koychev
- , Claire Kurasz
- , Prathiba Kurupati
- , Hanan Lamlum
- , G. Landers
- , Claudia Langenberg
- , Lara Lavelle-Langham
- , Allan Lawrie
- , Cathy Lawson
- , Claire Lawson
- , Alison Layton
- , Olivia C. Leavy
- , Ju Hee Lee
- , Elvina Lee
- , Karen Leitch
- , Rebecca Lenagh
- , Victoria Lewis
- , Joanne Lewis
- , Keir Lewis
- , N. Lewis-Burke
- , Felicity Liew
- , Tessa Light
- , Liz Lightstone
- , W. Lilaonitkul
- , S. Linford
- , Anne Lingford-Hughes
- , M. Lipman
- , Kamal Liyanage
- , Arwel Lloyd
- , Nazir I. Lone
- , Ronda Loosley
- , Janet Lord
- , Harpreet Lota
- , Wayne Lovegrove
- , Daniel Lozano-Rojas
- , Alice Lucey
- , Gardiner Lucy
- , E. Lukaschuk
- , Alison Lye
- , Ceri Lynch
- , S. MacDonald
- , G. MacGowan
- , Irene Macharia
- , J. Mackie
- , L. Macliver
- , S. Madathil
- , Gladys Madzamba
- , Nick Magee
- , Murphy Magtoto
- , N. Majeed
- , Flora Malein
- , Georgia Mallison
- , William Man
- , S. Mandal
- , K. Mangion
- , C. Manisty
- , R. Manley
- , Katherine March
- , Stefan Marciniak
- , Philip Marino
- , Myril Mariveles
- , Michael Marks
- , Elizabeth Marouzet
- , Sophie Marsh
- , M. Marshall
- , B. Marshall
- , Jane Martin
- , Adrian Martineau
- , L. M. Martinez
- , Nick Maskell
- , Darwin Matila
- , Wadzanai Matimba-Mupaya
- , Laura Matthews
- , Angeline Mbuyisa
- , Steve McAdoo
- , Hamish McAllister-Williams
- , Paul McArdle
- , Anne McArdle
- , Danny McAulay
- , Hamish J. C. McAuley
- , Gerry McCann
- , W. McCormick
- , Jacqueline McCormick
- , P. McCourt
- , Celeste McCracken
- , Lorcan McGarvey
- , Jade McGinness
- , K. McGlynn
- , Andrew McGovern
- , Heather McGuinness
- , I. B. McInnes
- , Jerome McIntosh
- , Emma McIvor
- , Katherine McIvor
- , Laura McLeavey
- , Aisling McMahon
- , Michael McMahon
- , L. McMorrow
- , Teresa Mcnally
- , M. McNarry
- , J. McNeill
- , Alison McQueen
- , H. McShane
- , Chloe Mears
- , Clare Megson
- , Sharon Megson
- , J. Meiring
- , Lucy Melling
- , Mark Mencias
- , Daniel Menzies
- , Marta Merida Morillas
- , Alice Michael
- , Benedict Michael
- , C. A. Miller
- , Lea Milligan
- , Nicholas Mills
- , Clare Mills
- , George Mills
- , L. Milner
- , Jane Mitchell
- , Abdelrahman Mohamed
- , Noura Mohamed
- , S. Mohammed
- , Philip Molyneaux
- , Will Monteiro
- , Silvia Moriera
- , Anna Morley
- , Leigh Morrison
- , Richard Morriss
- , A. Morrow
- , Paul Moss
- , Alistair Moss
- , K. Motohashi
- , N. Msimanga
- , Elizabeta Mukaetova-Ladinska
- , Unber Munawar
- , Jennifer Murira
- , Uttam Nanda
- , Heeah Nassa
- , Mariam Nasseri
- , Rashmita Nathu
- , Aoife Neal
- , Robert Needham
- , Paula Neill
- , Stefan Neubauer
- , D. E. Newby
- , Helen Newell
- , J. Newman
- , Tom Newman
- , Alex Newton-Cox
- , T. E. Nichols
- , Tim Nicholson
- , Christos Nicolaou
- , Debby Nicoll
- , Athanasios Nikolaidis
- , C. Nikolaidou
- , C. M. Nolan
- , Matthew Noonan
- , C. Norman
- , Petr Novotny
- , Kimon Ntotsis
- , Jose Nunag
- , Lorenza Nwafor
- , Uchechi Nwanguma
- , Joseph Nyaboko
- , Linda O’Brien
- , C. O’Brien
- , Natasha Odell
- , Kate O’Donnell
- , Godwin Ogbole
- , Olaoluwa Olaosebikan
- , Catherine Oliver
- , Zohra Omar
- , Peter J. M. Openshaw
- , D. P. O’Regan
- , Lorna Orriss-Dib
- , Lynn Osborne
- , Rebecca Osbourne
- , Marlies Ostermann
- , Charlotte Overton
- , Jamie Pack
- , Edmund Pacpaco
- , Stella-Maria Paddick
- , Sharon Painter
- , Erola Pairo-Castineira
- , Ashkan Pakzad
- , Sue Palmer
- , Padmasayee Papineni
- , K. Paques
- , Kerry Paradowski
- , Manish Pareek
- , Dhruv Parekh
- , H. Parfrey
- , Carmen Pariante
- , S. Parker
- , M. Parkes
- , J. Parmar
- , Sheetal Patale
- , Manish Patel
- , Suhani Patel
- , Dibya Pattenadk
- , M. Pavlides
- , Sheila Payne
- , Lorraine Pearce
- , John Pearl
- , Dan Peckham
- , Jessica Pendlebury
- , Yanchun Peng
- , Chris Pennington
- , Ida Peralta
- , Emma Perkins
- , Z. Peterkin
- , Tunde Peto
- , Nayia Petousi
- , John Petrie
- , Paul Pfeffer
- , Janet Phipps
- , S. Piechnik
- , John Pimm
- , Karen Piper Hanley
- , Riinu Pius
- , Hannah Plant
- , Tatiana Plekhanova
- , Megan Plowright
- , Krisnah Poinasamy
- , Oliver Polgar
- , Julie Porter
- , Joanna Porter
- , Sofiya Portukhay
- , Natassia Powell
- , A. Prabhu
- , James Pratt
- , Andrea Price
- , Claire Price
- , Carly Price
- , Anne Prickett
- , I. Propescu
- , J. Propescu
- , Sabrina Prosper
- , S. Pugmire
- , Sheena Quaid
- , Jackie Quigley
- , Jennifer K. Quint
- , H. Qureshi
- , I. N. Qureshi
- , K. Radhakrishnan
- , Najib Rahman
- , Markus Ralser
- , Betty Raman
- , Hazel Ramos
- , Albert Ramos
- , Jade Rangeley
- , Bojidar Rangelov
- , Liz Ratcliffe
- , Phillip Ravencroft
- , Konrad Rawlik
- , Anne Reddington
- , Heidi Redfearn
- , Dawn Redwood
- , Annabel Reed
- , Meryl Rees
- , Tabitha Rees
- , Karen Regan
- , Will Reynolds
- , Carla Ribeiro
- , A. Richards
- , Emma Richardson
- , M. Richardson
- , Pilar Rivera-Ortega
- , K. Roberts
- , Elizabeth Robertson
- , Leanne Robinson
- , Emma Robinson
- , Lisa Roche
- , C. Roddis
- , J. Rodger
- , Natalie Rogers
- , Gavin Ross
- , Alexandra Ross
- , Jennifer Rossdale
- , Anthony Rostron
- , Anna Rowe
- , J. Rowland
- , M. J. Rowland
- , A. Rowland
- , Sarah L. Rowland-Jones
- , Maura Roy
- , Igor Rudan
- , Richard Russell
- , Emily Russell
- , Gwen Saalmink
- , Ramsey Sabit
- , Beth Sage
- , T. Samakomva
- , Nilesh Samani
- , A. A. Samat
- , Claire Sampson
- , Katherine Samuel
- , Reena Samuel
- , Z. B. Sanders
- , Amy Sanderson
- , Elizabeth Sapey
- , Dinesh Saralaya
- , Jack Sargant
- , Carol Sarginson
- , Naveed Sattar
- , Kathryn Saunders
- , Peter Saunders
- , Ruth Saunders
- , Laura Saunders
- , Heather Savill
- , Avan Sayer
- , J. Schronce
- , William Schwaeble
- , Janet Scott
- , Kathryn Scott
- , Nick Selby
- , Malcolm G. Semple
- , Marco Sereno
- , Terri Ann Sewell
- , Kamini Shah
- , Ajay Shah
- , Manu Shankar-Hari
- , M. Sharma
- , Claire Sharpe
- , Michael Sharpe
- , Sharlene Shashaa
- , Alison Shaw
- , Victoria Shaw
- , Karen Shaw
- , Aziz Sheikh
- , Sarah Shelton
- , Liz Shenton
- , K. Shevket
- , Aarti Shikotra
- , Sulman Siddique
- , Salman Siddiqui
- , J. Sidebottom
- , Louise Sigfrid
- , Gemma Simons
- , Neil Simpson
- , John Simpson
- , Ananga Singapuri
- , Suver Singh
- , Claire Singh
- , Sally Singh
- , D. Sissons
- , J. Skeemer
- , Katie Slack
- , David Smith
- , Nikki Smith
- , Andrew Smith
- , Jacqui Smith
- , Laurie Smith
- , Susan Smith
- , M. Soares
- , Teresa Solano
- , Reanne Solly
- , A. R. Solstice
- , Tracy Soulsby
- , David Southern
- , D. Sowter
- , Mark Spears
- , Lisa Spencer
- , Fabio Speranza
- , Louise Stadon
- , Stefan Stanel
- , R. Steeds
- , N. Steele
- , Mike Steiner
- , David Stensel
- , G. Stephens
- , Lorraine Stephenson
- , Iain Stewart
- , R. Stimpson
- , Sue Stockdale
- , J. Stockley
- , Wendy Stoker
- , Roisin Stone
- , Will Storrar
- , Andrew Storrie
- , Kim Storton
- , E. Stringer
- , Sophia Strong-Sheldrake
- , Natalie Stroud
- , Christian Subbe
- , Catherine Sudlow
- , Zehra Suleiman
- , Charlotte Summers
- , C. Summersgill
- , Debbie Sutherland
- , D. L. Sykes
- , Nick Talbot
- , Ai Lyn Tan
- , Lawrence Tarusan
- , Vera Tavoukjian
- , Jessica Taylor
- , Abigail Taylor
- , Chris Taylor
- , John Paul Taylor
- , Amelie Te
- , Caroline Tee
- , J. Teixeira
- , Helen Tench
- , Sarah Terry
- , Susannah Thackray-Nocera
- , Favas Thaivalappil
- , David Thickett
- , David Thomas
- , S. Thomas
- , Caradog Thomas
- , Andrew Thomas
- , T. Thomas-Woods
- , A. A. Roger Thompson
- , Tamika Thompson
- , T. Thornton
- , Matthew Thorpe
- , Ryan S. Thwaites
- , Jo Tilley
- , N. Tinker
- , Gerlynn Tiongson
- , Martin Tobin
- , Johanne Tomlinson
- , Mark Toshner
- , T. Treibel
- , K. A. Tripp
- , Drupad Trivedi
- , E. M. Tunnicliffe
- , Alison Turnbull
- , Kim Turner
- , Sarah Turner
- , Victoria Turner
- , E. Turner
- , Sharon Turney
- , Lance Turtle
- , Helena Turton
- , Jacinta Ugoji
- , R. Ugwuoke
- , Rachel Upthegrove
- , Jonathon Valabhji
- , Maximina Ventura
- , Joanne Vere
- , Carinna Vickers
- , Ben Vinson
- , Ioannis Vogiatzis
- , Elaine Wade
- , Phillip Wade
- , Louise V. Wain
- , Tania Wainwright
- , Lilian Wajero
- , Sinead Walder
- , Samantha Walker
- , S. Walker
- , Tim Wallis
- , Sarah Walmsley
- , Simon Walsh
- , J. A. Walsh
- , Louise Warburton
- , T. J. C. Ward
- , Katie Warwick
- , Helen Wassall
- , Samuel Waterson
- , L. Watson
- , Ekaterina Watson
- , James Watson
- , M. Webster
- , J. Weir McCall
- , Carly Welch
- , Simon Wessely
- , Sophie West
- , Heather Weston
- , Helen Wheeler
- , Sonia White
- , Victoria Whitehead
- , J. Whitney
- , S. Whittaker
- , Beverley Whittam
- , V. Whitworth
- , Andrew Wight
- , James Wild
- , Martin Wilkins
- , Dan Wilkinson
- , Nick Williams
- , N. Williams
- , B. Williams
- , Jenny Williams
- , S. A. Williams-Howard
- , Michelle Willicombe
- , Gemma Willis
- , James Willoughby
- , Ann Wilson
- , Imogen Wilson
- , Daisy Wilson
- , Nicola Window
- , M. Witham
- , Rebecca Wolf-Roberts
- , Chloe Wood
- , F. Woodhead
- , Janet Woods
- , Dan Wootton
- , J. Wormleighton
- , J. Worsley
- , David Wraith
- , Caroline Wrey Brown
- , C. Wright
- , S. Wright
- , Louise Wright
- , Inez Wynter
- , Moucheng Xu
- , Najira Yasmin
- , S. Yasmin
- , Tom Yates
- , Kay Por Yip
- , Susan Young
- , Bob Young
- , A. J. Yousuf
- , Amira Zawia
- , Lisa Zeidan
- , Bang Zhao
- , Bang Zheng
- & O. Zongo
- , Daniel Agranoff
- , Ken Agwuh
- , Katie A. Ahmed
- , Dhiraj Ail
- , Erin L. Aldera
- , Ana Alegria
- , Beatrice Alex
- , Sam Allen
- , Petros Andrikopoulos
- , Brian Angus
- , Jane A. Armstrong
- , Abdul Ashish
- , Milton Ashworth
- , Innocent G. Asiimwe
- , Dougal Atkinson
- , Benjamin Bach
- , Siddharth Bakshi
- , Wendy S. Barclay
- , Shahedal Bari
- , Gavin Barlow
- , Samantha L. Barlow
- , Stella Barnass
- , Nicholas Barrett
- , Christopher Bassford
- , Sneha Basude
- , David Baxter
- , Michael Beadsworth
- , Jolanta Bernatoniene
- , John Berridge
- , Nicola Best
- , Debby Bogaert
- , Laura Booth
- , Pieter Bothma
- , Benjamin Brennan
- , Robin Brittain-Long
- , Katie Bullock
- , Naomi Bulteel
- , Tom Burden
- , Andrew Burtenshaw
- , Nicola Carlucci
- , Gail Carson
- , Vikki Caruth
- , Emily Cass
- , Benjamin W. A. Catterall
- , David Chadwick
- , Duncan Chambler
- , Meera Chand
- , Kanta Chechi
- , Nigel Chee
- , Jenny Child
- , Srikanth Chukkambotla
- , Richard Clark
- , Tom Clark
- , Jordan J. Clark
- , Emily A. Clarke
- , Sara Clohisey
- , Sarah Cole
- , Paul Collini
- , Marie Connor
- , Graham S. Cooke
- , Louise Cooper
- , Catherine Cosgrove
- , Audrey Coutts
- , Helen Cox
- , Jason Cupitt
- , Maria-Teresa Cutino-Moguel
- , Ana da Silva Filipe
- , Jo Dalton
- , Paul Dark
- , Christopher Davis
- , Chris Dawson
- , Thushan de Silva
- , Samir Dervisevic
- , Oslem Dincarslan
- , Alejandra Doce Carracedo
- , Cara Donegan
- , Lorna Donelly
- , Phil Donnison
- , Chloe Donohue
- , Gonçalo dos Santos Correia
- , Sam Douthwaite
- , Thomas M. Drake
- , Andrew Drummond
- , Marc-Emmanuel Dumas
- , Chris Dunn
- , Jake Dunning
- , Ingrid DuRand
- , Ahilanadan Dushianthan
- , Tristan Dyer
- , Philip Dyer
- , Angela Elliott
- , Cariad Evans
- , Anthony Evans
- , Chi Eziefula
- , Cameron J. Fairfield
- , Angie Fawkes
- , Chrisopher Fegan
- , Lorna Finch
- , Adam Finn
- , Lewis W. S. Fisher
- , Lisa Flaherty
- , Tom Fletcher
- , Terry Foster
- , Duncan Fullerton
- , Carrol Gamble
- , Isabel Garcia-Dorival
- , Atul Garg
- , Sanjeev Garg
- , Tammy Gilchrist
- , Michelle Girvan
- , Effrossyni Gkrania-Klotsas
- , Jo Godden
- , Arthur Goldsmith
- , Clive Graham
- , Tassos Grammatikopoulos
- , Christopher A. Green
- , Julian Griffin
- , Fiona Griffiths
- , Philip Gunning
- , Rishi K. Gupta
- , Katarzyna Hafezi
- , Sophie Halpin
- , Elaine Hardy
- , Ewen M. Harrison
- , Janet Harrison
- , Catherine Hartley
- , Stuart Hartshorn
- , Daniel Harvey
- , Peter Havalda
- , Daniel B. Hawcutt
- , Ross Hendry
- , Antonia Y. W. Ho
- , Maria Hobrok
- , Luke Hodgson
- , Karl Holden
- , Anthony Holmes
- , Peter W. Horby
- , Joanne Howard
- , Samreen Ijaz
- , Clare Jackson
- , Michael Jacobs
- , Susan Jain
- , Paul Jennings
- , Rebecca L. Jensen
- , Christopher B. Jones
- , Trevor R. Jones
- , Agilan Kaliappan
- , Vidya Kasipandian
- , Seán Keating
- , Stephen Kegg
- , Michael Kelsey
- , Jason Kendall
- , Caroline Kerrison
- , Ian Kerslake
- , Shadia Khandaker
- , Katharine King
- , Robyn T. Kiy
- , Stephen R. Knight
- , Susan Knight
- , Oliver Koch
- , Gouri Koduri
- , George Koshy
- , Chrysa Koukorava
- , Shondipon Laha
- , Eva Lahnsteiner
- , Steven Laird
- , Annette Lake
- , Suzannah Lant
- , Susan Larkin
- , Diane Latawiec
- , Andrew Law
- , James Lee
- , Gary Leeming
- , Daniella Lefteri
- , Tamas Leiner
- , Lauren Lett
- , Matthew Lewis
- , Sonia Liggi
- , Patrick Lillie
- , Wei Shen Lim
- , James Limb
- , Vanessa Linnett
- , Jeff Little
- , Lucia A. Livoti
- , Mark Lyttle
- , Louise MacGillivray
- , Alan Maclean
- , Michael MacMahon
- , Emily MacNaughton
- , Maria Mancini
- , Ravish Mankregod
- , Laura Marsh
- , Lynn Maslen
- , Hannah Massey
- , Huw Masson
- , Elijah Matovu
- , Nicole Maziere
- , Sarah McCafferty
- , Katherine McCullough
- , Sarah E. McDonald
- , Sarah McDonald
- , Laurence McEvoy
- , Ruth McEwen
- , John McLauchlan
- , Kenneth A. Mclean
- , Manjula Meda
- , Alexander J. Mentzer
- , Laura Merson
- , Soeren Metelmann
- , Alison M. Meynert
- , Nahida S. Miah
- , Joanna Middleton
- , Gary Mills
- , Jane Minton
- , Joyce Mitchell
- , Kavya Mohandas
- , James Moon
- , Elinoor Moore
- , Shona C. Moore
- , Patrick Morgan
- , Kirstie Morrice
- , Craig Morris
- , Katherine Mortimore
- , Samuel Moses
- , Mbiye Mpenge
- , Rohinton Mulla
- , Derek Murphy
- , Lee Murphy
- , Michael Murphy
- , Ellen G. Murphy
- , Thapas Nagarajan
- , Megan Nagel
- , Mark Nelson
- , Lisa Norman
- , Lillian Norris
- , Lucy Norris
- , Mahdad Noursadeghi
- , Michael Olanipekun
- , Wilna Oosthuyzen
- , Anthonia Osagie
- , Matthew K. O’Shea
- , Igor Otahal
- , Mark Pais
- , Massimo Palmarini
- , Carlo Palmieri
- , Selva Panchatsharam
- , Danai Papakonstantinou
- , Hassan Paraiso
- , Brij Patel
- , Natalie Pattison
- , William A. Paxton
- , Rebekah Penrice-Randal
- , Justin Pepperell
- , Mark Peters
- , Mandeep Phull
- , Jack Pilgrim
- , Stefania Pintus
- , Tim Planche
- , Daniel Plotkin
- , Georgios Pollakis
- , Frank Post
- , Nicholas Price
- , David Price
- , Tessa Prince
- , Rachel Prout
- , Nikolas Rae
- , Andrew Rambaut
- , Henrik Reschreiter
- , Tim Reynolds
- , Neil Richardson
- , P. Matthew Ridley
- , Mark Roberts
- , Stephanie Roberts
- , Devender Roberts
- , David L. Robertson
- , Alistair Rose
- , Guy Rousseau
- , Bobby Ruge
- , Clark D. Russell
- , Brendan Ryan
- , Debby Sales
- , Taranprit Saluja
- , Vanessa Sancho-Shimizu
- , Caroline Sands
- , Egle Saviciute
- , Matthias Schmid
- , Janet T. Scott
- , James Scott-Brown
- , Aarti Shah
- , Prad Shanmuga
- , Anil Sharma
- , Catherine A. Shaw
- , Victoria E. Shaw
- , Anna Shawcross
- , Rebecca K. Shears
- , Jagtur Singh Pooni
- , Jeremy Sizer
- , Benjamin Small
- , Richard Smith
- , Catherine Snelson
- , Tom Solomon
- , Rebecca G. Spencer
- , Nick Spittle
- , Shiranee Sriskandan
- , Nikki Staines
- , Tom Stambach
- , Richard Stewart
- , David Stuart
- , Krishanthi S. Subramaniam
- , Pradeep Subudhi
- , Olivia V. Swann
- , Tamas Szakmany
- , Agnieska Szemiel
- , Aislynn Taggart
- , Sarah Tait
- , Zoltan Takats
- , Panteleimon Takis
- , Jolanta Tanianis-Hughes
- , Kate Tatham
- , Richard S. Tedder
- , Jo Thomas
- , Jordan Thomas
- , Robert Thompson
- , Chris Thompson
- , Emma C. Thomson
- , Ascanio Tridente
- , Erwan Trochu
- , Darell Tupper-Carey
- , Lance C. W. Turtle
- , Mary Twagira
- , Nick Vallotton
- , Libby van Tonder
- , Rama Vancheeswaran
- , Rachel Vincent
- , Lisa Vincent-Smith
- , Shico Visuvanathan
- , Alan Vuylsteke
- , Sam Waddy
- , Rachel Wake
- , Andrew Walden
- , Ingeborg Welters
- , Murray Wham
- , Tony Whitehouse
- , Paul Whittaker
- , Ashley Whittington
- , Meme Wijesinghe
- , Eve Wilcock
- , Martin Williams
- , Lawrence Wilson
- , Stephen Winchester
- , Martin Wiselka
- , Adam Wolverson
- , Daniel G. Wootton
- , Andrew Workman
- , Nicola Wrobel
- , Bryan Yates
- , Peter Young
- , Maria Zambon
- & J. Eunice Zhang
Contributions
F.L. recruited participants, acquired clinical samples, analyzed and interpreted data and cowrote the manuscript, including all drafting and revisions. C.E. analyzed and interpreted data and cowrote this manuscript, including all drafting and revisions. S.F. and M.R. supported the analysis and interpretation of data as well as drafting and revisions. D.S., J.K.S., S.C.M., S.A., N.M., J.N., C.K., O.C.L., O.E., H.J.C.M., A. Shikotra, A. Singapuri, M.S., V.C.H., M.T., N.J.G., N.I.L. and C.C. contributed to acquisition of data underlying this study. L.H.-W., A.A.R.T., S.L.R.-J., L.S.H., O.M.K., D.G.W., T.I.d.S. and A. Ho made substantial contributions to conception/design and implementation of this work and/or acquisition of clinical samples for this work. They have supported drafting and revisions of the manuscript. E.M.H., J.K.Q. and A.B.D. made substantial contributions to the study design as well as data access, linkage and analysis. They have supported drafting and revisions of this work. J.D.C., L.-P.H., A. Horsley, B.R., K.P., M.M. and W.G. made substantial contributions to the conception and design of this work and have supported drafting and revisions of this work. J.K.B. obtained funding for ISARIC4C, is ISARIC4C consortium co-lead, has made substantial contributions to conception and design of this work and has supported drafting and revisions of this work. M.G.S. obtained funding for ISARIC4C, is ISARIC4C consortium co-lead, sponsor/protocol chief investigator, has made substantial contributions to conception and design of this work and has supported drafting and revisions of this work. R.A.E. and L.V.W. are co-leads of PHOSP-COVID, made substantial contributions to conception and design of this work, the acquisition and analysis of data, and have supported drafting and revisions of this work. C.B. is the chief investigator of PHOSP-COVID and has made substantial contributions to conception and design of this work. R.S.T. and L.T. made substantial contributions to the acquisition, analysis and interpretation of the data underlying this study and have contributed to drafting and revisions of this work. P.J.M.O. obtained funding for ISARIC4C, is ISARIC4C consortium co-lead, sponsor/protocol chief investigator and has made substantial contributions to conception and design of this work. R.S.T. and P.J.M.O. have also made key contributions to interpretation of data and have co-written this manuscript. All authors have read and approve the final version to be published. All authors agree to accountability for all aspects of this work. All investigators within ISARIC4C and the PHOSP-COVID consortia have made substantial contributions to the conception or design of this study and/or acquisition of data for this study. The full list of authors within these groups is available in Supplementary Information .
Corresponding authors
Correspondence to Ryan S. Thwaites or Peter J. M. Openshaw .
Ethics declarations
Competing interests.
F.L., C.E., D.S., J.K.S., S.C.M., C.D., C.K., N.M., L.N., E.M.H., A.B.D., J.K.Q., L.-P.H., K.P., L.S.H., O.M.K., S.F., T.I.d.S., D.G.W., R.S.T. and J.K.B. have no conflicts of interest. A.A.R.T. receives speaker fees and support to attend meetings from Janssen Pharmaceuticals. S.L.R.-J. is on the data safety monitoring board for Bexero trial in HIV+ adults in Kenya. J.D.C. is the deputy chief editor of the European Respiratory Journal and receives consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Insmed, Janssen, Novartis, Pfizer and Zambon. A. Horsley is deputy chair of NIHR Translational Research Collaboration (unpaid role). B.R. receives honoraria from Axcella therapeutics. R.A.E. is co-lead of PHOSP-COVID and receives fees from AstraZenaca/Evidera for consultancy on LC and from AstraZenaca for consultancy on digital health. R.A.E. has received speaker fees from Boehringer in June 2021 and has held a role as European Respiratory Society Assembly 01.02 Pulmonary Rehabilitation secretary. R.A.E. is on the American Thoracic Society Pulmonary Rehabilitation Assembly program committee. L.V.W. also receives funding from Orion pharma and GSK and holds contracts with Genentech and AstraZenaca. L.V.W. has received consulting fees from Galapagos and Boehringer, is on the data advisory board for Galapagos and is Associate Editor for the European Respiratory Journal . A. Ho is a member of NIHR Urgent Public Health Group (June 2020–March 2021). M.M. is an applicant on the PHOSP study funded by NIHR/DHSC. M.G.S. acts as an independent external and nonremunerated member of Pfizer’s External Data Monitoring Committee for their mRNA vaccine program(s), is Chair of Infectious Disease Scientific Advisory Board of Integrum Scientific LLC, and is director of MedEx Solutions Ltd. and majority owner of MedEx Solutions Ltd. and minority owner of Integrum Scientific LLC. M.G.S.’s institution has been in receipt of gifts from Chiesi Farmaceutici S.p.A. of Clinical Trial Investigational Medicinal Product without encumbrance and distribution of same to trial sites. M.G.S. is a nonrenumerated member of HMG UK New Emerging Respiratory Virus Threats Advisory Group and has previously been a nonrenumerated member of the Scientific Advisory Group for Emergencies (SAGE). C.B. has received consulting fees and/or grants from GSK, AstraZeneca, Genentech, Roche, Novartis, Sanofi, Regeneron, Chiesi, Mologic and 4DPharma. L.T. has received consulting fees from MHRA, AstraZeneca and Synairgen and speakers’ fees from Eisai Ltd., and support for conference attendance from AstraZeneca. L.T. has a patent pending with ZikaVac. P.J.M.O. reports grants from the EU Innovative Medicines Initiative 2 Joint Undertaking during the submitted work; grants from UK Medical Research Council, GSK, Wellcome Trust, EU Innovative Medicines Initiative, UK National Institute for Health Research and UK Research and Innovation–Department for Business, Energy and Industrial Strategy; and personal fees from Pfizer, Janssen and Seqirus, outside the submitted work.
Peer review
Peer review information.
Nature Immunology thanks Ziyad Al-Aly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Ioana Staicu was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 penalized logistic regression performance..
Graphs show classification error and Area under curve (AUC) from the 50 repeats tenfold nested cross-validation used to optimise and assess the performance of PLR testing associations with each LC outcome relative to Recovered (n = 233): Cardio_Resp (n = 398), Fatigue (n = 384), Anxiety/Depression (n = 202), GI (n = 132), ( e ) Cognitive (n = 6). The distributions of classification error and area under curve (AUC) from the nested cross-validation are shown. Box plot centre line represents the Median and boundaries of the box represent interquartile range (IQR), the whisker length represent 1.5xIQR.
Extended Data Fig. 2 Associations with long COVID symptoms in full study cohort.
( a ) Fibrinogen levels at 6 months were compared between pooled LC cases (n = 295) and Recovered (n = 233) and between the Cognitive group (n = 41) and Recovered (n = 233). Box plot centre line represent the Median and boundaries of the box represent interquartile range (IQR), the whisker length represents 1.5xIQR, any outliers beyond the whisker range are shown as individual dots. Median differences were compared using two-sided Wilcoxon signed-rank test *= p < 0·05, **= p < 0·01, ***= p < 0·001, ****= p < 0·0001. Unadjusted p-values are reported. b ) Distribution of time from COVID-19 hospitalisation at sample collection applying CDC and NICE definitions of LC (n = 719) ( c ) Upset plot of symptom groups. Horizontal coloured bars represent the number of patients in each symptom group: Cardiorespiratory (Cardio_Resp), Fatigue, Cognitive, Gastrointestinal (GI) and Anxiety/Depression (Anx_Dep). Vertical black bars represent the number of patients in each symptom combination group. To prevent patient identification, where less than 5 patients belong to a combination group, this has been represented as ‘<5’. The Recovered group (n = 250) were used as controls. Forest plots show Olink protein concentrations (NPX) associated with ( d ) Cardio_Resp (n = 398), ( e ) Fatigue (n = 342), ( f ) Anx_Dep (n = 219), ( g ) GI (n = 134), and ( h ) Cognitive (n = 65). Error bars represent the median accuracy of the model.
Extended Data Fig. 3 Validation of olink measurements using conventional assays in plasma.
Olink measured protein (NPX) were compared to chemiluminescence assays (ECL or ELISA, log2[pg/mL]) to validate our findings, where contemporaneously collected plasma samples were available (n = 58). Results from key mediators associated with LC groups were validated: CSF3, IL1R2, IL2, IL3RA, TNFa, TFF2. R = spearman rank correlation coefficient and shaded areas indicated the 95% confidence interval. Samples that fell below the lower limit of detection for a given assay were excluded and the ‘n’ value on each panel indicates the number of samples above this limit.
Extended Data Fig. 4 Univariate analysis of proteins associated with each symptom.
Olink measured plasma protein levels (NPX) compared between LC groups (Cardio_Resp, n = 398, Fatigue n = 384, Anxiety/Depression, n = 202, GI, n = 132 and Cognitive, n = 60) and Recovered (n = 233). Proteins identified by PLR were compared between groups. Median differences were compared using two-sided Wilcoxon signed-rank test. * = p < 0·05, ** = p < 0·01, *** = p < 0·001, ****= p < 0·0001 after FDR adjustment. Box plot centre line represent the Median and boundaries of the box represent interquartile range (IQR), the whisker length represents 1.5xIQR, any outliers beyond the whisker range are shown as individual dots.
Extended Data Fig. 5 Unadjusted Penalised Logistic Regression.
Olink measured proteins (NPX) and their association with Cardio_Resp (n = 398), Fatigue (n = 342), Anx_Dep (n = 219), GI (n = 134), and Cognitive (n = 65). Forest plots show odds of each LC outcome vs Recovered (n = 233), using PLR without adjusting for clinical co-variates. Error bars represent the median accuracy of the model.
Extended Data Fig. 6 Partial Least Squares analysis.
Olink measured proteins (NPX) and their association with Cardio_Resp (n = 398), Fatigue (n = 342), Anx_Dep (n = 219), GI (n = 134), and Cognitive (n = 65) groups. Forest plots show odds of LC outcome vs Recovered (n = 233), using PLS analysis. Error bars represent the standard error of the coefficient estimate.
Extended Data Fig. 7 Network analysis centrality.
Each graph shows the centrality score for each Olink measured protein (NPX) found to have significant associations with other proteins that were elevated in the Cardio_Resp (n = 398), Fatigue (n = 342), Anx_Dep (n = 219), GI (n = 134), and Cognitive (n = 65) groups relative to Recovered (n = 233).
Extended Data Fig. 8 Inflammation in men and women with long COVID.
Olink measured plasma protein levels (NPX) between men and women with symptoms, divided by age (<50 or >=50years): (a) shows IL1R2 and MATN2 in the Anxiety/Depression group (<50 n = 55, >=50 n = 133), (b) shows CTSO and NFASC in the Cognitive group (<50 n = 11, >=50 n = 50). Median values were compared between men and women using two-sided Wilcoxon signed-rank test. Box plot centre line represent the Median and boundaries represent interquartile range (IQR), the whisker length represents 1.5xIQR.
Extended Data Fig. 9 Inflammation in the upper respiratory tract.
Nasal cytokines measured by immunoassay in the CardioResp Group (n = 29) and Recovered (n = 31): ( a ) shows IL1a, IL1b, IL-6, APO-2, TGFa, TFF2. Median differences were compared using two-sided Wilcoxon signed-rank test. Box plot centre line represents the Median and boundaries of the box represent interquartile range (IQR), the whisker length represent 1.5xIQR. ( b ) Shows cytokines measured by immunoassay in paired plasma and nasal (n = 70). Correlations between IL1a, IL1b, IL-6, APO-2, TGFa and TFF2 in nasal and plasma samples were compared using Spearman’s rank correlation coefficient ( R ). Shaded areas indicated the 95% confidence interval of R.
Extended Data Fig. 10 Graphical abstract.
Summary of interpretation of key findings from Olink measured proteins and their association with CardioResp (n = 398), Fatigue (n = 342), Anx/Dep (n = 219), GI (n = 134), and Cognitive (n = 65) groups relative to Recovered (n = 233).
Supplementary information
Supplementary information.
Supplementary Methods, Statistics and reproducibility statement, Supplementary Results, Supplementary Tables 1–7, Extended data figure legends, Appendix 1 (Supplementary Table 8), Appendix 2 (PHOSP-COVID author list) and Appendix 3 (ISARIC4C author list).
Reporting Summary
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Liew, F., Efstathiou, C., Fontanella, S. et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat Immunol 25 , 607–621 (2024). https://doi.org/10.1038/s41590-024-01778-0
Download citation
Received : 11 August 2023
Accepted : 06 February 2024
Published : 08 April 2024
Issue Date : April 2024
DOI : https://doi.org/10.1038/s41590-024-01778-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Immune dysregulation in long covid.
- Laura Ceglarek
- Onur Boyman
Nature Immunology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
In this paper, we describe and present the vast, fragmented, and complex literature related to this topic. This review may be used to guide practice, public health efforts, policy, and research related to mental health and, specifically, depression. ... This paper discusses key areas in depression research; however, an exhaustive discussion of ...
In line with the Research Domain Criteria Project launched by the National Institute of Mental Health (Insel, 2014; Insel et al., 2010), a distinguished aim in developing an integrated neuroscientific model of depression therefore has to be the separation of distinct aetiological and pathophysiological trajectories which, although eventually ...
Our lack of knowledge cannot be put down to a scarcity of research in existing treatments. In the past decades, more than 500 randomised trials have examined the effects of antidepressant medications, and more than 600 trials have examined the effects of psychotherapies for depression (although comparatively few are conducted for early-onset depression).
The paper gives a complete overview of what is known about therapies for depression. ... we have developed a 'Meta-analytic Research Domain' (MARD) of all randomized trials of psychological treatments of depression. ... Depression in most studies was moderate to severe. Response (50 % improvement between baseline and endpoint) was the main ...
Authors of papers were contacted for clarification when data was missing or unclear. ... with most research on depression focusing on the 5-HT 1A receptor [11, 34].
Research Open Access 10 Apr 2024 Molecular Psychiatry. P: 1-8. Olfactory genes affect major depression in highly educated, emotionally stable, lean women: a bridge between animal models and ...
The current speed of progress in depression research is simply remarkable. We have therefore been able to create a second special issue of Molecular Psychiatry, 2020, focused on depression, with ...
Abstract. One of the most consistent findings in the depression literature is that stressful life events predict the onset and course of depressive episodes. Cognitive and biological responses to life stressors have both been identified, albeit largely independently, as central to understanding the association between stress and depression.
R. McShaneN Engl J Med 2023;389:1333-1334. Depression can become self-perpetuating as occupational, relationship, and social losses accrue. Sustained remission is achieved in less than half of ...
Abstract. Major depression is a mood disorder characterized by a sense of inadequacy, despondency, decreased activity, pessimism, anhedonia and sadness where these symptoms severely disrupt and ...
In addition, initial presentation with social phobia was associated with a 5.7-fold increased risk of developing major depressive disorder. These associations between anxiety and depression can be traced back even earlier in life. For example, childhood behavioral inhibition in response to novelty or strangers, or an extreme anxious temperament ...
Depression is a top mental health concern among undergraduates and has been shown to disproportionately affect individuals who are underserved and underrepresented in science. As we aim to create a more inclusive scientific community, we argue that we need to examine the relationship between depression and scientific research. While studies have identified aspects of research that affect ...
To improve our understanding, some research has been undertaken in which YP themselves are asked about their experience of depression. In a questionnaire study involving adolescents with depression in New Zealand, the researchers identified the aforementioned irritability as the most common characteristic alongside interpersonal problems and thought-processing symptoms (Crowe, Ward, Dunnachie ...
Background: Depression is a common mental health disease, especially in mid to late adolescence that, due to its. particularities, is a challenge and requires an effective diagnosis. Primary care ...
The possible causes of depression are many and not yet well understood. However, it most likely results from an interplay of genetic vulnerability and environmental factors. Your depression research paper could explore one or more of these causes and reference the latest research on the topic. For instance, how does an imbalance in brain ...
Econometric analysis of the relationship between vitamin D deficiency and depression was performed by Yunzhi et al. and Shauni et al. performed a bibliometric analysis of domestic and international research papers on depression-related genes from 2003 to 2007. A previous review of depression-related bibliometric studies revealed that there is ...
This research used data assets made available by ODAP as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National ...