StudyMonkey
Your personal ai chemistry tutor.


Learn Smarter, Not Harder with Chemistry AI
Introducing StudyMonkey, your AI-powered Chemistry tutor .
StudyMonkey AI can tutor complex Chemistry homework questions, enhance your essay writing and assess your work—all in seconds.
No more long all-nighters
24/7 solutions to Chemistry questions you're stumped on and essays you procrastinated on.
No more stress and anxiety
Get all your Chemistry assignments done with helpful answers in 10 seconds or less.
No more asking friends for Chemistry help
StudyMonkey is your new smart bestie that will never ghost you.
No more staying after school
AI Chemistry tutoring is available 24/7, on-demand when you need it most.
Chemistry is the branch of science that studies the properties and behavior of matter, the changes it undergoes during chemical reactions, and the energy that accompanies those processes.
AI Tutor for any subject
American college testing (act), anthropology, advanced placement exams (ap exams), arabic language, archaeology, biochemistry, chartered financial analyst (cfa) exam, communications, computer science, certified public accountant (cpa) exam, cultural studies, cyber security, dental admission test (dat), discrete mathematics, earth science, elementary school, entrepreneurship, environmental science, essay writer, farsi (persian) language, fundamentals of engineering (fe) exam, gender studies, graduate management admission test (gmat), graduate record examination (gre), greek language, hebrew language, high school entrance exam, high school, human geography, human resources, international english language testing system (ielts), information technology, international relations, independent school entrance exam (isee), lesson planner, linear algebra, linguistics, law school admission test (lsat), machine learning, master's degree, medical college admission test (mcat), meteorology, microbiology, middle school, national council licensure examination (nclex), national merit scholarship qualifying test (nmsqt), number theory, organic chemistry, project management professional (pmp), political science, portuguese language, probability, project management, preliminary sat (psat), public policy, public relations, russian language, scholastic assessment test (sat), social sciences, secondary school admission test (ssat), sustainability, swahili language, test of english as a foreign language (toefl), trigonometry, turkish language, united states medical licensing examination (usmle), web development, step-by-step guidance 24/7.
Receive step-by-step guidance & homework help for any homework problem & any subject 24/7
Ask any Chemistry question
StudyMonkey supports every subject and every level of education from 1st grade to masters level.
Get an answer
StudyMonkey will give you an answer in seconds—multiple choice questions, short answers, and even an essays are supported!
Review your history
See your past questions and answers so you can review for tests and improve your grades.
Try StudyMonkey for free
It's not cheating....
Try the AI homework helper for free and upgrade at your convenience
You're just learning smarter than everyone else
How Can StudyMonkey Help You?
Hear From Our Happy Students
"The AI tutor is available 24/7, making it a convenient and accessible resource for students who need help with their homework at any time."
"Overall, StudyMonkey is an excellent tool for students looking to improve their understanding of homework topics and boost their academic success."

Upgrade to StudyMonkey Premium!
Why not upgrade to StudyMonkey Premium and get access to all features?
Chemistry AI Homework Solver
Master the elements with Smodin’s AI Chemistry problem solver – your ultimate assistant for tackling tough assignments!
See Our Chemistry AI Homework Solver in Action
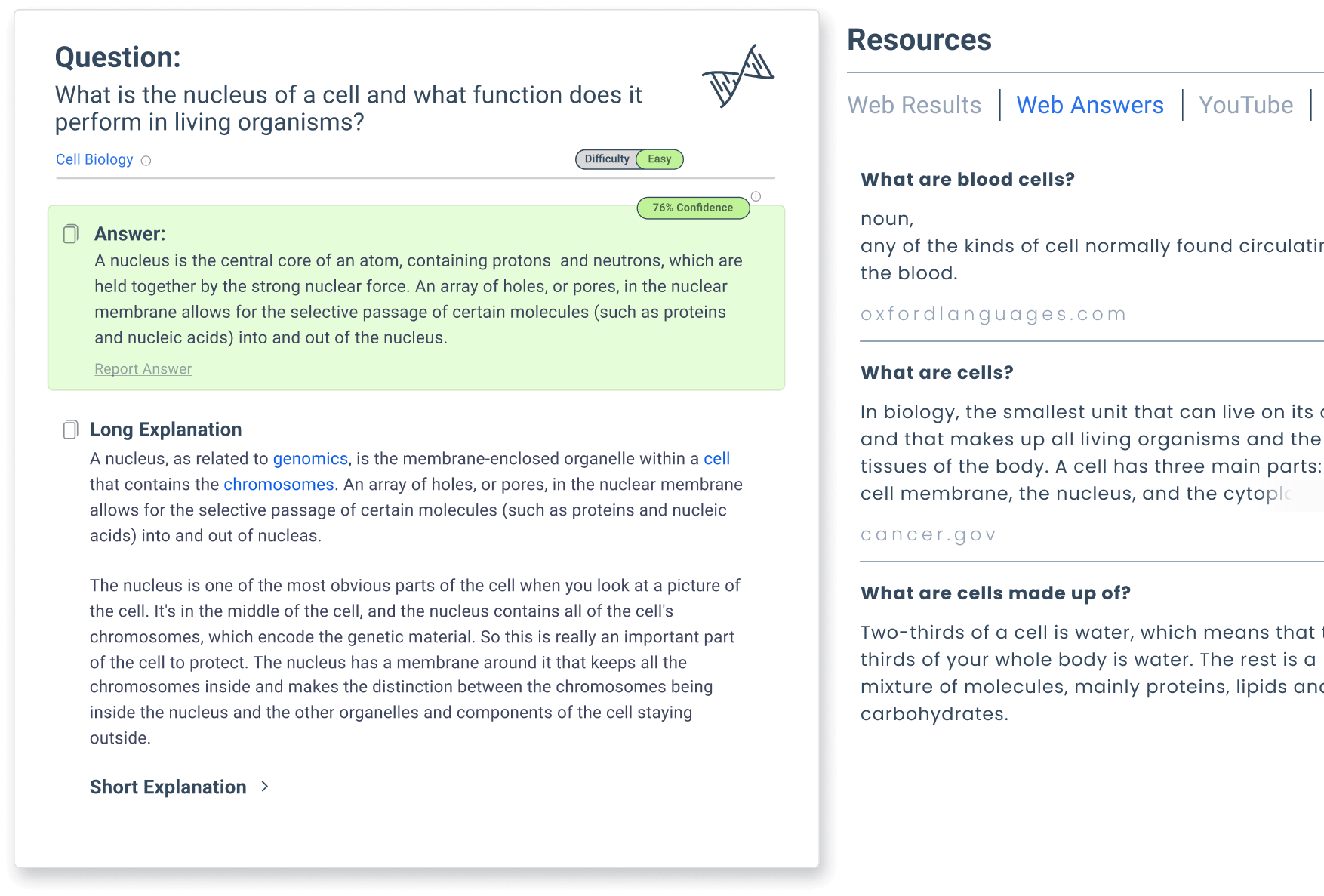
Smodin Is the Ultimate Solution to Your Chemistry Assignments
How does it work.
Our tool uses advanced technology, such as machine learning and algorithms, to accurately answer all your chemistry-related questions. All you need to do is share your question or assignment, and you’ll find the best answer in no time.
Why Choose an AI Homework Solver?
- Saves time : With our chemistry homework solver, you no longer have to spend hours completing your assignments. Ask your questions here, get all your answers, and finish your homework in a fraction of the time. It’s like paying someone to do your homework , but you also get to learn simultaneously.
- Accurate answers : You no longer have to worry about placing letters and numbers in the wrong places while solving equations. Our tool provides accurate solutions to all your queries, ensuring you don’t make unnecessary mistakes. In other words, it’s the ultimate website for all your homework answers .
- Improve your grades : Complete your chemistry homework with accurate answers and submit them on time, ensuring your grades go up.
- 24/7 Availability : Do you have a chemistry-related question you need to ask, but everyone’s asleep? Our tool never sleeps and is always available to answer all your queries immediately.
Experience the Best Chemistry AI Homework Solver
Get instant chemistry help with smodin’s ai-powered homework solver.
Do you have a question you wish you had asked your teacher during class hours? Now, it’s too late, and you must turn in your assignment the next day. With Smodin’s AI Chemistry Problem Solver, you can ask all your questions, even during the middle of the night.
Ace Your Exams with the Help of Smodin’s Chemistry Homework Assistant
The benefits of using a chemistry ai solver for your homework.
- You save time as you get accurate answers in a couple of seconds.
- Understanding complex topics is easier, as you can break them down into smaller, bite-sized subjects.
- You get step-by-step solutions, which makes it easier to grasp the subject. The knowledge you learn this way will help you in your future assignments.
Say Goodbye to Late-Night Study Sessions with Smodin’s Chemistry Question Solver
Unlock your potential with smodin’s chemistry ai homework solver, frequently asked questions, how can smodin help with my chemistry homework, how does the ai generate solutions for chemistry problems, can i use this tool to prepare for chemistry exams, what other subjects does smodin support besides chemistry, what are the restrictions or limitations associated with using this service, how do i get started.
© 2024 Smodin LLC

Chemistry Assistant
Ai-powered chemistry problem solver.
- Homework Help: Students can use the Chemistry Assistant to help understand and work through chemistry problems in their homework.
- Teaching Aid: Teachers can use this tool to generate solutions to chemistry problems, aiding in lesson planning and student instruction.
- Exam Preparation: Use the Chemistry Assistant to prepare for chemistry exams by solving practice problems and getting explanations of chemistry terms and principles.
- Research Assistance: Researchers can use this tool to help work through chemistry problems in their work.
Yes, the Chemistry Assistant is designed to handle a wide range of chemistry problems, from basic to advanced. However, it's always important to cross-verify the solutions provided by the AI with trusted resources or professionals in the field to ensure accuracy and understanding, especially with more complex problems and principles.
While the Chemistry Assistant is specifically designed for chemistry problems, HyperWrite offers other AI tools for different subjects and needs. You can explore more tools at app.hyperwriteai.com/tools .
New & Trending Tools
Wedding toast generator, ai answer science questions, ai text analyzer and highlights.
- How it works
- Homework answers

Chemistry Answers
Questions: 44 357
Answers by our Experts: 42 759
Ask Your question
Need a fast expert's response?
and get a quick answer at the best price
for any assignment or question with DETAILED EXPLANATIONS !

IMAGES
VIDEO
COMMENTS
1. Initiate Your Chemistry Inquiry. Enter or upload your chemistry assignment questions into HIX Tutor's Chemistry AI homework solver to get started. 2. Experience AI-Driven Problem Solving. Once you input your query, our chemistry equation solver will use AI algorithms to dissect and process your question. 3. Obtain Comprehensive Solutions.
A 24/7 free Chemistry homework AI tutor that instantly provides personalized step-by-step guidance, explanations, and examples for any Chemistry homework problem. Improve your grades with our AI homework helper!
Our tool uses advanced technology, such as machine learning and algorithms, to accurately answer all your chemistry-related questions. All you need to do is share your question or assignment, and you’ll find the best answer in no time.
HyperWrite's Chemistry Assistant is an AI-powered tool designed to answer chemistry questions and think through solving chemistry problems. By leveraging advanced AI models, this tool simplifies complex chemistry problems and provides detailed, understandable solutions.
Get homework answers from experts in Chemistry. Submit your question, choose a relevant category and get a detailed answer for free.
Get your Pearson Chemistry homework done with Quizlet! Browse through thousands of step-by-step solutions to end-of-chapter questions from the most popular Pearson Chemistry textbooks. It's never been a better time to #LearnOn.