- 20 Most Unethical Experiments in Psychology
Humanity often pays a high price for progress and understanding — at least, that seems to be the case in many famous psychological experiments. Human experimentation is a very interesting topic in the world of human psychology. While some famous experiments in psychology have left test subjects temporarily distressed, others have left their participants with life-long psychological issues . In either case, it’s easy to ask the question: “What’s ethical when it comes to science?” Then there are the experiments that involve children, animals, and test subjects who are unaware they’re being experimented on. How far is too far, if the result means a better understanding of the human mind and behavior ? We think we’ve found 20 answers to that question with our list of the most unethical experiments in psychology .

Emma Eckstein

Electroshock Therapy on Children

Operation Midnight Climax

The Monster Study

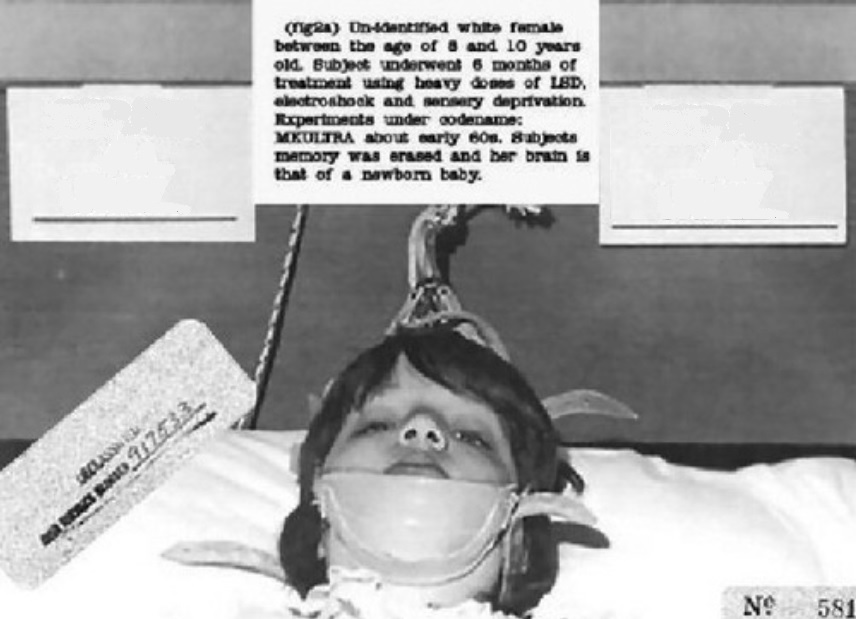
Project MKUltra
The Aversion Project

Unnecessary Sexual Reassignment

Stanford Prison Experiment

Milgram Experiment

The Monkey Drug Trials

Featured Programs
Facial expressions experiment.

Little Albert

Bobo Doll Experiment

The Pit of Despair

The Bystander Effect

Learned Helplessness Experiment

Racism Among Elementary School Students

UCLA Schizophrenia Experiments

The Good Samaritan Experiment

Robbers Cave Experiment

Related Resources:
- What Careers are in Experimental Psychology?
- What is Experimental Psychology?
- The 25 Most Influential Psychological Experiments in History
- 5 Best Online Ph.D. Marriage and Family Counseling Programs
- Top 5 Online Doctorate in Educational Psychology
- 5 Best Online Ph.D. in Industrial and Organizational Psychology Programs
- Top 10 Online Master’s in Forensic Psychology
- 10 Most Affordable Counseling Psychology Online Programs
- 10 Most Affordable Online Industrial Organizational Psychology Programs
- 10 Most Affordable Online Developmental Psychology Online Programs
- 15 Most Affordable Online Sport Psychology Programs
- 10 Most Affordable School Psychology Online Degree Programs
- Top 50 Online Psychology Master’s Degree Programs
- Top 25 Online Master’s in Educational Psychology
- Top 25 Online Master’s in Industrial/Organizational Psychology
- Top 10 Most Affordable Online Master’s in Clinical Psychology Degree Programs
- Top 6 Most Affordable Online PhD/PsyD Programs in Clinical Psychology
- 50 Great Small Colleges for a Bachelor’s in Psychology
- 50 Most Innovative University Psychology Departments
- The 30 Most Influential Cognitive Psychologists Alive Today
- Top 30 Affordable Online Psychology Degree Programs
- 30 Most Influential Neuroscientists
- Top 40 Websites for Psychology Students and Professionals
- Top 30 Psychology Blogs
- 25 Celebrities With Animal Phobias
- Your Phobias Illustrated (Infographic)
- 15 Inspiring TED Talks on Overcoming Challenges
- 10 Fascinating Facts About the Psychology of Color
- 15 Scariest Mental Disorders of All Time
- 15 Things to Know About Mental Disorders in Animals
- 13 Most Deranged Serial Killers of All Time

Site Information
- About Online Psychology Degree Guide
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Research ethics.
Jennifer M. Barrow ; Grace D. Brannan ; Paras B. Khandhar .
Affiliations
Last Update: September 18, 2022 .
- Introduction
Multiple examples of unethical research studies conducted in the past throughout the world have cast a significant historical shadow on research involving human subjects. Examples include the Tuskegee Syphilis Study from 1932 to 1972, Nazi medical experimentation in the 1930s and 1940s, and research conducted at the Willowbrook State School in the 1950s and 1960s. [1] As the aftermath of these practices, wherein uninformed and unaware patients were exposed to disease or subject to other unproven treatments, became known, the need for rules governing the design and implementation of human-subject research protocols became very evident.
The first such ethical code for research was the Nuremberg Code, arising in the aftermath of Nazi research atrocities brought to light in the post-World War II Nuremberg Trials. [1] This set of international research standards sought to prevent gross research misconduct and abuse of vulnerable and unwitting research subjects by establishing specific human subject protective factors. A direct descendant of this code was drafted in 1978 in the United States, known as the Belmont Report, and this legislation forms the backbone of regulation of clinical research in the USA since its adoption. [2] The Belmont Report contains 3 basic ethical principles:
- Respect for persons
- Beneficence
Additionally, the Belmont Report details research-based protective applications for informed consent, risk/benefit assessment, and participant selection. [3]
- Issues of Concern
The first protective principle stemming from the 1978 Belmont Report is the principle of Respect for Persons, also known as human dignity. [2] This dictates researchers must work to protect research participants' autonomy while also ensuring full disclosure of factors surrounding the study, including potential harms and benefits. According to the Belmont Report, "an autonomous person is an individual capable of deliberation about personal goals and acting under the direction of such deliberation." [1]
To ensure participants have the autonomous right to self-determination, researchers must ensure that potential participants understand that they have the right to decide whether or not to participate in research studies voluntarily and that declining to participate in any research does not affect in any way their access to current or subsequent care. Also, self-determined participants must be able to ask the researcher questions and comprehend the questions asked by the researcher. Researchers must also inform participants that they may stop participating in the study without fear of penalty. [4] As noted in the Belmont Report definition above, not all individuals can be autonomous concerning research participation. Whether because of the individual's developmental level or because of various illnesses or disabilities, some individuals require special research protections that may involve exclusion from research activities that can cause potential harm or appointing a third-party guardian to oversee the participation of such vulnerable persons. [5]
Researchers must also ensure they do not coerce potential participants into agreeing to participate in studies. Coercion refers to threats of penalty, whether implied or explicit, if participants decline to participate or opt out of a study. Additionally, giving potential participants extreme rewards for agreeing to participate can be a form of coercion. The rewards may provide an enticing enough incentive that the participant feels they need to participate. In contrast, they would otherwise have declined if such a reward were not offered. While researchers often use various rewards and incentives in studies, they must carefully review this possibility of coercion. Some incentives may pressure potential participants into joining a study, thereby stripping participants of complete self-determination. [3]
An additional aspect of respecting potential participants' self-determination is to ensure that researchers have fully disclosed information about the study and explained the voluntary nature of participation (including the right to refuse without repercussion) and possible benefits and risks related to study participation. A potential participant cannot make a truly informed decision without complete information. This aspect of the Belmont Report can be troublesome for some researchers based on their study designs and research questions. Noted biases related to reactivity may occur when study participants know the exact guiding research questions and purposes. Some researchers may avoid reactivity biases using covert data collection methods or masking critical study information. Masking frequently occurs in pharmaceutical trials with placebos because knowledge of placebo receipt can affect study outcomes. However, masking and concealed data collection methods may not fully respect participants' rights to autonomy and the associated informed consent process. Any researcher considering hidden data collection or masking of some research information from participants must present their plans to an Institutional Review Board (IRB) for oversight, as well as explain the potential masking to prospective patients in the consent process (ie, explaining to potential participants in a medication trial that they are randomly assigned either the medication or a placebo). The IRB determines if studies warrant concealed data collection or masking methods in light of the research design, methods, and study-specific protections. [6]
The second Belmont Report principle is the principle of beneficence. Beneficence refers to acting in such a way to benefit others while promoting their welfare and safety. [7] Although not explicitly mentioned by name, the biomedical ethical principle of nonmaleficence (not harm) also appears within the Belmont Report's section on beneficence. The beneficence principle includes 2 specific research aspects:
- Participants' right to freedom from harm and discomfort
- Participants' rights to protection from exploitation [8]
Before seeking IRB approval and conducting a study, researchers must analyze potential risks and benefits to research participants. Examples of possible participant risks include physical harm, loss of privacy, unforeseen side effects, emotional distress or embarrassment, monetary costs, physical discomfort, and loss of time. Possible benefits include access to a potentially valuable intervention, increased understanding of a medical condition, and satisfaction with helping others with similar issues. [8] These potential risks and benefits should explicitly appear in the written informed consent document used in the study. Researchers must implement specific protections to minimize discomfort and harm to align with the principle of beneficence. Under the principle of beneficence, researchers must also protect participants from exploitation. Any information provided by participants through their study involvement must be protected.
The final principle contained in the Belmont Report is the principle of justice, which pertains to participants' right to fair treatment and right to privacy. The selection of the types of participants desired for a research study should be guided by research questions and requirements not to exclude any group and to be as representative of the overall target population as possible. Researchers and IRBs must scrutinize the selection of research participants to determine whether researchers are systematically selecting some groups (eg, participants receiving public financial assistance, specific ethnic and racial minorities, or institutionalized) because of their vulnerability or ease of access. The right to fair treatment also relates to researchers treating those who refuse to participate in a study fairly without prejudice. [3]
The right to privacy also falls under the Belmont Report's principle of justice. Researchers must keep any shared information in their strictest confidence. Upholding the right to privacy often involves procedures for anonymity or confidentiality. For participants' data to be completely anonymous, the researcher cannot have the ability to connect the participants to their data. The study is no longer anonymous if researchers can make participant-data connections, even if they use codes or pseudonyms instead of personal identifiers. Instead, researchers are providing participant confidentiality. Various methods can help researchers assure confidentiality, including locking any participant identifying data and substituting code numbers instead of names, with a correlation key available only to a safety or oversight functionary in an emergency but not readily available to researchers. [3]
- Clinical Significance
One of the most common safeguards for the ethical conduct of research involves using external reviewers, such as an Institutional Review Board (IRB). Researchers seeking to begin a study must submit a full research proposal to the IRB, which includes specific data collection instruments, research advertisements, and informed consent documentation. The IRB may perform a complete or expedited review depending on the nature of the study and the risks involved. Researchers cannot contact potential participants or start collecting data until they obtain full IRB approval. Sometimes, multi-site studies require approvals from several IRBs, which may have different forms and review processes. [3]
A significant study aspect of interest to IRB members is using participants from vulnerable groups. Vulnerable groups may include individuals who cannot give fully informed consent or those individuals who may be at elevated risk of unplanned side effects. Examples of vulnerable participants include pregnant women, children younger than the age of consent, terminally ill individuals, institutionalized individuals, and those with mental or emotional disabilities. In the case of minors, assent is also an element that must be addressed per Subpart D of the Code of Federal Regulations, 45 CFR 46.402, which defines consent as "a child's affirmative agreement to participate in research; mere failure to object should not, absent affirmative agreement, be construed as assent." [9] There is a lack in the literature on when minors can understand research, although current research suggests that the age by which a minor could assent is around 14. [10] Anytime researchers include vulnerable groups in their studies, they must have extra safeguards to uphold the Belmont Report's ethical principles, especially beneficence. [3]
- Enhancing Healthcare Team Outcomes
Research ethics is a foundational principle of modern medical research across all disciplines. The overarching body, the IRB, is intentionally comprised of experts across various disciplines, including ethicists, social workers, physicians, nurses, other scientific researchers, counselors, mental health professionals, and advocates for vulnerable subjects. There is also often a legal expert on the panel or available to discuss any questions regarding the legality or ramifications of studies.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Jennifer Barrow declares no relevant financial relationships with ineligible companies.
Disclosure: Grace Brannan declares no relevant financial relationships with ineligible companies.
Disclosure: Paras Khandhar declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Barrow JM, Brannan GD, Khandhar PB. Research Ethics. [Updated 2022 Sep 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The historical, ethical, and legal background of human-subjects research. [Respir Care. 2008] The historical, ethical, and legal background of human-subjects research. Rice TW. Respir Care. 2008 Oct; 53(10):1325-9.
- The Belmont Report at 40: Reckoning With Time. [Am J Public Health. 2018] The Belmont Report at 40: Reckoning With Time. Adashi EY, Walters LB, Menikoff JA. Am J Public Health. 2018 Oct; 108(10):1345-1348. Epub 2018 Aug 23.
- Informed consent in human experimentation before the Nuremberg code. [BMJ. 1996] Informed consent in human experimentation before the Nuremberg code. Vollmann J, Winau R. BMJ. 1996 Dec 7; 313(7070):1445-9.
- Review The History of Human Subjects Research and Rationale for Institutional Review Board Oversight. [Nutr Clin Pract. 2021] Review The History of Human Subjects Research and Rationale for Institutional Review Board Oversight. Spellecy R, Busse K. Nutr Clin Pract. 2021 Jun; 36(3):560-567. Epub 2021 Jan 13.
- Review Ethical issues in research. [Best Pract Res Clin Obstet Gyn...] Review Ethical issues in research. Artal R, Rubenfeld S. Best Pract Res Clin Obstet Gynaecol. 2017 Aug; 43:107-114. Epub 2017 Jan 23.
Recent Activity
- Research Ethics - StatPearls Research Ethics - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- The Magazine
- Stay Curious
- The Sciences
- Environment
- Planet Earth
5 Unethical Medical Experiments Brought Out of the Shadows of History
Prisoners and other vulnerable populations often bore the brunt of unethical medical experimentation..
Most people are aware of some of the heinous medical experiments of the past that violated human rights. Participation in these studies was either forced or coerced under false pretenses. Some of the most notorious examples include the experiments by the Nazis, the Tuskegee syphilis study, the Stanford Prison Experiment, and the CIA’s LSD studies.
But there are many other lesser-known experiments on vulnerable populations that have flown under the radar. Study subjects often didn’t — or couldn’t — give consent. Sometimes they were lured into participating with a promise of improved health or a small amount of compensation. Other times, details about the experiment were disclosed but the extent of risks involved weren’t.
This perhaps isn’t surprising, as doctors who conducted these experiments were representative of prevailing attitudes at the time of their work. But unfortunately, even after informed consent was introduced in the 1950s , disregard for the rights of certain populations continued. Some of these researchers’ work did result in scientific advances — but they came at the expense of harmful and painful procedures on unknowing subjects.
Here are five medical experiments of the past that you probably haven’t heard about. They illustrate just how far the ethical and legal guidepost, which emphasizes respect for human dignity above all else, has moved.
The Prison Doctor Who Did Testicular Transplants
From 1913 to 1951, eugenicist Leo Stanley was the chief surgeon at San Quentin State Prison, California’s oldest correctional institution. After performing vasectomies on prisoners, whom he recruited through promises of improved health and vigor, Stanley turned his attention to the emerging field of endocrinology, which involves the study of certain glands and the hormones they regulate. He believed the effects of aging and decreased hormones contributed to criminality, weak morality, and poor physical attributes. Transplanting the testicles of younger men into those who were older would restore masculinity, he thought.
Stanley began by using the testicles of executed prisoners — but he ran into a supply shortage. He solved this by using the testicles of animals, including goats and deer. At first, he physically implanted the testicles directly into the inmates. But that had complications, so he switched to a new plan: He ground up the animal testicles into a paste, which he injected into prisoners’ abdomens. By the end of his time at San Quentin, Stanley did an estimated 10,000 testicular procedures .
The Oncologist Who Injected Cancer Cells Into Patients and Prisoners
During the 1950s and 1960s, Sloan-Kettering Institute oncologist Chester Southam conducted research to learn how people’s immune systems would react when exposed to cancer cells. In order to find out, he injected live HeLa cancer cells into patients, generally without their permission. When patient consent was given, details around the true nature of the experiment were often kept secret. Southam first experimented on terminally ill cancer patients, to whom he had easy access. The result of the injection was the growth of cancerous nodules , which led to metastasis in one person.
Next, Southam experimented on healthy subjects , which he felt would yield more accurate results. He recruited prisoners, and, perhaps not surprisingly, their healthier immune systems responded better than those of cancer patients. Eventually, Southam returned to infecting the sick and arranged to have patients at the Jewish Chronic Disease Hospital in Brooklyn, NY, injected with HeLa cells. But this time, there was resistance. Three doctors who were asked to participate in the experiment refused, resigned, and went public.
The scandalous newspaper headlines shocked the public, and legal proceedings were initiated against Southern. Some in the scientific and medical community condemned his experiments, while others supported him. Initially, Southam’s medical license was suspended for one year, but it was then reduced to a probation. His career continued to be illustrious, and he was subsequently elected president of the American Association for Cancer Research.
The Aptly Named ‘Monster Study’
Pioneering speech pathologist Wendell Johnson suffered from severe stuttering that began early in his childhood. His own experience motivated his focus on finding the cause, and hopefully a cure, for stuttering. He theorized that stuttering in children could be impacted by external factors, such as negative reinforcement. In 1939, under Johnson’s supervision, graduate student Mary Tudor conducted a stuttering experiment, using 22 children at an Iowa orphanage. Half received positive reinforcement. But the other half were ridiculed and criticized for their speech, whether or not they actually stuttered. This resulted in a worsening of speech issues for the children who were given negative feedback.
The study was never published due to the multitude of ethical violations. According to The Washington Post , Tudor was remorseful about the damage caused by the experiment and returned to the orphanage to help the children with their speech. Despite his ethical mistakes, the Wendell Johnson Speech and Hearing Clinic at the University of Iowa bears Johnson's name and is a nod to his contributions to the field.
The Dermatologist Who Used Prisoners As Guinea Pigs
One of the biggest breakthroughs in dermatology was the invention of Retin-A, a cream that can treat sun damage, wrinkles, and other skin conditions. Its success led to fortune and fame for co-inventor Albert Kligman, a dermatologist at the University of Pennsylvania . But Kligman is also known for his nefarious dermatology experiments on prisoners that began in 1951 and continued for around 20 years. He conducted his research on behalf of companies including DuPont and Johnson & Johnson.
Kligman’s work often left prisoners with pain and scars as he used them as study subjects in wound healing and exposed them to deodorants, foot powders, and more for chemical and cosmetic companies. Dow once enlisted Kligman to study the effects of dioxin, a chemical in Agent Orange, on 75 inmates at Pennsylvania's Holmesburg Prison. The prisoners were paid a small amount for their participation but were not told about the potential side effects.
In the University of Pennsylvania’s journal, Almanac , Kligman’s obituary focused on his medical advancements, awards, and philanthropy. There was no acknowledgement of his prison experiments. However, it did mention that as a “giant in the field,” he “also experienced his fair share of controversy.”
The Endocrinologist Who Irradiated Prisoners
When the Atomic Energy Commission wanted to know how radiation affected male reproductive function, they looked to endocrinologist Carl Heller . In a study involving Oregon State Penitentiary prisoners between 1963 and 1973, Heller designed a contraption that would radiate their testicles at varying amounts to see what effect it had, particularly on sperm production. The prisoners also were subjected to repeated biopsies and were required to undergo vasectomies once the experiments concluded.
Although study participants were paid, it raised ethical issues about the potential coercive nature of financial compensation to prison populations. The prisoners were informed about the risks of skin burns, but likely were not told about the possibility of significant pain, inflammation, and the small risk of testicular cancer.
- personal health
- behavior & society
Already a subscriber?
Register or Log In

Keep reading for as low as $1.99!
Sign up for our weekly science updates.
Save up to 40% off the cover price when you subscribe to Discover magazine.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
The Stanford Prison Experiment
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Cara Lustik is a fact-checker and copywriter.
:max_bytes(150000):strip_icc():format(webp)/Cara-Lustik-1000-77abe13cf6c14a34a58c2a0ffb7297da.jpg)
- Participants
- Setting and Procedure
In August of 1971, psychologist Philip Zimbardo and his colleagues created an experiment to determine the impacts of being a prisoner or prison guard. The Stanford Prison Experiment, also known as the Zimbardo Prison Experiment, went on to become one of the best-known studies in psychology's history —and one of the most controversial.
This study has long been a staple in textbooks, articles, psychology classes, and even movies. Learn what it entailed, what was learned, and the criticisms that have called the experiment's scientific merits and value into question.
Purpose of the Stanford Prison Experiment
Zimbardo was a former classmate of the psychologist Stanley Milgram . Milgram is best known for his famous obedience experiment , and Zimbardo was interested in expanding upon Milgram's research. He wanted to further investigate the impact of situational variables on human behavior.
Specifically, the researchers wanted to know how participants would react when placed in a simulated prison environment. They wondered if physically and psychologically healthy people who knew they were participating in an experiment would change their behavior in a prison-like setting.
Participants in the Stanford Prison Experiment
To carry out the experiment, researchers set up a mock prison in the basement of Stanford University's psychology building. They then selected 24 undergraduate students to play the roles of both prisoners and guards.
Participants were chosen from a larger group of 70 volunteers based on having no criminal background, no psychological issues , and no significant medical conditions. Each volunteer agreed to participate in the Stanford Prison Experiment for one to two weeks in exchange for $15 a day.
Setting and Procedures
The simulated prison included three six-by-nine-foot prison cells. Each cell held three prisoners and included three cots. Other rooms across from the cells were utilized for the jail guards and warden. One tiny space was designated as the solitary confinement room, and yet another small room served as the prison yard.
The 24 volunteers were randomly assigned to either the prisoner or guard group. Prisoners were to remain in the mock prison 24 hours a day during the study. Guards were assigned to work in three-man teams for eight-hour shifts. After each shift, they were allowed to return to their homes until their next shift.
Researchers were able to observe the behavior of the prisoners and guards using hidden cameras and microphones.
Results of the Stanford Prison Experiment
So what happened in the Zimbardo experiment? While originally slated to last 14 days, it had to be stopped after just six due to what was happening to the student participants. The guards became abusive and the prisoners began to show signs of extreme stress and anxiety .
It was noted that:
- While the prisoners and guards were allowed to interact in any way they wanted, the interactions were hostile or even dehumanizing.
- The guards began to become aggressive and abusive toward the prisoners while the prisoners became passive and depressed.
- Five of the prisoners began to experience severe negative emotions , including crying and acute anxiety, and had to be released from the study early.
Even the researchers themselves began to lose sight of the reality of the situation. Zimbardo, who acted as the prison warden, overlooked the abusive behavior of the jail guards until graduate student Christina Maslach voiced objections to the conditions in the simulated prison and the morality of continuing the experiment.
One possible explanation for the results of this experiment is the idea of deindividuation , which states that being part of a large group can make us more likely to perform behaviors we would otherwise not do on our own.
Impact of the Zimbardo Prison Experiment
The experiment became famous and was widely cited in textbooks and other publications. According to Zimbardo and his colleagues, the Stanford Prison Experiment demonstrated the powerful role that the situation can play in human behavior.
Because the guards were placed in a position of power, they began to behave in ways they would not usually act in their everyday lives or other situations. The prisoners, placed in a situation where they had no real control , became submissive and depressed.
In 2011, the Stanford Alumni Magazine featured a retrospective of the Stanford Prison Experiment in honor of the experiment’s 40th anniversary. The article contained interviews with several people involved, including Zimbardo and other researchers as well as some of the participants.
In the interviews, Richard Yacco, one of the prisoners in the experiment, suggested that the experiment demonstrated the power that societal roles and expectations can play in a person's behavior.
In 2015, the experiment became the topic of a feature film titled The Stanford Prison Experiment that dramatized the events of the 1971 study.
Criticisms of the Stanford Prison Experiment
In the years since the experiment was conducted, there have been a number of critiques of the study. Some of these include:
Ethical Issues
The Stanford Prison Experiment is frequently cited as an example of unethical research. It could not be replicated by researchers today because it fails to meet the standards established by numerous ethical codes, including the Code of Ethics of the American Psychological Association .
Why was Zimbardo's experiment unethical?
Zimbardo's experiment was unethical due to a lack of fully informed consent, abuse of participants, and lack of appropriate debriefings. More recent findings suggest there were other significant ethical issues that compromise the experiment's scientific standing, including the fact that experimenters may have encouraged abusive behaviors.
Lack of Generalizability
Other critics suggest that the study lacks generalizability due to a variety of factors. The unrepresentative sample of participants (mostly white and middle-class males) makes it difficult to apply the results to a wider population.
Lack of Realism
The Zimbardo Prison Experiment is also criticized for its lack of ecological validity. Ecological validity refers to the degree of realism with which a simulated experimental setup matches the real-world situation it seeks to emulate.
While the researchers did their best to recreate a prison setting, it is simply not possible to perfectly mimic all the environmental and situational variables of prison life. Because there may have been factors related to the setting and situation that influenced how the participants behaved, it may not truly represent what might happen outside of the lab.
Recent Criticisms
More recent examination of the experiment's archives and interviews with participants have revealed major issues with the research method , design, and procedures used. Together, these call the study's validity, value, and even authenticity into question.
These reports, including examinations of the study's records and new interviews with participants, have also cast doubt on some of its key findings and assumptions.
Among the issues described:
- One participant suggested that he faked a breakdown so he could leave the experiment because he was worried about failing his classes.
- Other participants also reported altering their behavior in a way designed to "help" the experiment .
- Evidence suggests that the experimenters encouraged the guards' behavior and played a role in fostering the abusive actions of the guards.
In 2019, the journal American Psychologist published an article debunking the famed experiment. It detailed the study's lack of scientific merit and concluded that the Stanford Prison Experiment was "an incredibly flawed study that should have died an early death."
In a statement posted on the experiment's official website, Zimbardo maintains that these criticisms do not undermine the main conclusion of the study—that situational forces can alter individual actions both in positive and negative ways.
The Stanford Prison Experiment is well known both inside and outside the field of psychology . While the study has long been criticized for many reasons, more recent criticisms of the study's procedures shine a brighter light on the experiment's scientific shortcomings.
Stanford University. About the Stanford Prison Experiment .
Stanford Prison Experiment. 2. Setting up .
Sommers T. An interview with Philip Zimbardo . The Believer.
Ratnesar R. The menace within . Stanford Magazine.
Jabbar A, Muazzam A, Sadaqat S. An unveiling the ethical quandaries: A critical analysis of the Stanford Prison Experiment as a mirror of Pakistani society . J Bus Manage Res . 2024;3(1):629-638.
Horn S. Landmark Stanford Prison Experiment criticized as a sham . Prison Legal News .
Bartels JM. The Stanford Prison Experiment in introductory psychology textbooks: A content analysis . Psychol Learn Teach . 2015;14(1):36-50. doi:10.1177/1475725714568007
American Psychological Association. Ecological validity .
Blum B. The lifespan of a lie . Medium .
Le Texier T. Debunking the Stanford Prison Experiment . Am Psychol . 2019;74(7):823-839. doi:10.1037/amp0000401
Stanford Prison Experiment. Philip Zimbardo's response to recent criticisms of the Stanford Prison Experiment .
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
- Unethical Research Practices to Avoid: Examples & Detection

Almost every aspect of human life is guided by guidelines, rules, and regulations. That is why, from time immemorial, there have been set ethics and rules that serve as a guide and moderator in human activities.
Without these recognized ethics, everyone will approach issues in ways they deem appropriate. This applies to the research and research community.
Research guidelines provide information about accepted research ethics to the research community and the researchers. These guidelines provide ethics, advice, and guidance. They help researchers develop ethical discretion and also to prevent the researchers from committing scientific misconduct, and promote good scientific practice.
We are going to discuss research ethics, what they mean, how they are adopted, how important they are, and how they affect research and research institutions.
What are Research Ethics?
Ethics are a set of rules, which can be broadly written and unwritten. They govern a human’s behavioral expectations and that of others.
While society broadly agrees on some ethical values such as murder is terrible and disallowed, a wide variation also exists on how we can interpret these set values in practice.
Now research ethics refers to the values and the norms an institution has put in place to help regulate scientific activities. It is a collection of scientific morals in the line of duty. This guideline specifies the traits or behaviors that are recognized by the research community based on the general ethics of science and society at large.
Research guidelines are binding on both the researcher and the institution. This is because there are responsibilities to be carried out by both the researcher and the institution to ensure that their research is reliable. However, it is important that the institutions are clear on the research ethics roles and responsibilities at every point. Part of the duties of the institution is to have good administrational management and funding that would allow researchers to comply with designed ethical guidelines and norms.
Read: Research Bias: Definition, Types + Examples
The guidelines primarily cover research and other research-related activities, which include teaching, dissemination of research information, and also the management of institutions. Research ethical guidelines are also used as tools in the assessment of an individual case in the planning of research and even when reporting or publishing the outcomes and findings of that study.
The research ethics guideline covers the projects of students at all levels, and that of the doctoral research fellows. It is also the responsibility of the institution to provide relevant training regarding research ethics to the students and doctoral research Fellows. This is because the research norms and guidelines apply to all researches regardless of whether they are commissioned research, applied research, or basic research.
Research conducted by the public, or private institutions is also subjected to these guidelines and ethics. Consulting firms that perform research-related tasks, such as systematic acquisition and information processing about individuals, groups, and organizations, are also not excluded.
Read: Consent Letter: Writing Guide, Types, [+12 Consent Samples]
There are guidelines regulating research at different levels based on recognized norms for research ethics. We are going to look at these research norms below.
- There are norms for good scientific practice. They relate to finding accuracy and relevant knowledge in research. These norms are Originality, trustworthiness, academic freedom, and openness.
- There are norms for the research communities. They guide the relationship between the people that partake together in research. These norms are respect, accountability, confidentiality, integrity, impartiality, constructive criticism, informed and free consent, and human dignity.
- There are norms that guide the researchers’ relationship with the rest of society. These norms are social responsibility, dissemination of research, and independence.
The first two groups listed above are internal ethical norms. They relate to regulating the research communities, while the other relates to the relationship that exists between the research and the outside world or the society. Many times, the lines between these norms get blurred.
Research ethics are the standard ethics set by the supervising institution or bodies to govern how scientific research and other types of research are conducted in various research institutions such as the universities, and also moderate how they are interpreted.
Read: Sampling Bias: Definition, Types + [Examples]
Why is Research Ethics Important?
The aim of research ethics is to guide researchers to conduct their studies and report their findings without deception or intent to directly or indirectly cause harm to their subjects or any member of society, as the case may be.
Also, research ethics establish the validity of a researcher’s study or research. It establishes that the research is authentic and error/bias-free. This will give the researcher credibility within the institution and in the public.
Research ethics also ensure the safety of research or study subjects and the researcher. This is because it is a must that all researchers follow these guidelines.
Another importance of research ethics is that it shows that your research publications are not plagiarized, and your readers are not reading unverified data. This is achieved through the research manuscript. Your research findings must adhere to the set guidelines.
The last point to consider is that research ethics provide the researcher with a sense of responsibility. This makes it easy to find appropriate solutions in the case of any misconduct.
Read: Systematic Errors in Research: Definition, Examples
Examples of Unethical Research Practices
Here’s a list of unethical practices every researcher must avoid
1. Duplicate publication
It is unethical for a researcher to submit a research paper or publication that has two or more seminal journals which could be with or without acknowledgment of these other journals. This practice is known as duplicate submission or duplicate publication.
Some authors practice duplication of publication so as to increase their numbers of submissions; however, it is unethical and it also amounts to the wasting of time of the publication resources and the journal reviewers why it also serves no benefits today to the scientific community and humanity at large.
You can only submit your research paper to just one journal.
2. Research data falsification
the falsification or fabrication of research data occurs when a researcher tries to manipulate the procedures used in conducting research or the important findings just to have the researcher’s desired result.
Recording non-existent data or falsifying a data recording is known as research fabrication.
Research data fabrication is common in the pharmaceutical industry. They do this fabrication 2 market a specific drug today to the general public without considering the drug’s side effects. This act is unethical and it is also a wastage of the limited resources available for research.
This can result in revoking the physician’s clinical license, the prosecution of the physician, and also create huge mistrust in the mind of the public.
3. Plagiarism
Plagiarism is a huge offense in the research community. It is the practice of taking another person’s research or work or even idea and inculcating it in your own writing without giving them the dual credit. In some cases, just for recognition, the researcher can even use another person’s research as their own publication journal.
In other cases, the researcher may change the letters of someone else’s publication to their own words without referencing the original author. This is known as self-plagiarism.
With technology, there are more tools to detect plagiarism. This means it is now very easy for journal editors to detect plagiarism. Plagiarism may not be intentional sometimes, it may just happen accidentally. However, you can avoid it by referencing all the sources you used in writing your own scientific journal.
Ensure that all the authors whose work you have used are properly cited in your paper, regardless of if they’re from previous publications.
4. Authorship Conflict
ICMJE (The International Committee of Medical Journal Editors) guidelines provided that anyone who has contributed to the conception, the designing of research data, contributed to the data analysis, helped to draft or revise the journal and seek approval before the journal is published has an authorship claim to the journal.
Now an authorship conflict can arise if the name of a person who has contributed to the journal in any form is not included in the publication.
If one of the persons whose name was cited in the journal does not give consent or agree to its publication. That is an authorship conflict and it is unethical.
If the name of one additional author is cited while the name of an already cited author is removed, whether before publication or after publication, it is an authorship conflict.
Another cause of authorship conflict is citing a person’s name based on “senior in practice” or family affiliation when the said person has contributed nothing to the research and the documentation of the research findings.
Authorship conflict can be avoided before conducting the research by selecting the authors in the beginning and also by the journals asking the authors to submit a checklist that contains the criteria for authorship.
5. Conflict of interest
Conflict of interest arises in research when the author or the researcher gets influenced by financial reasons or personal issues that ultimately affect the quality of the outcome of the study.
When these conflicts of interest arise, which could be personal conditions and financial consideration or other types of conflicts, the researcher should truthfully disclose the current situation to the editorial team, and do so completely without leaving out a detail.
Read: Undercoverage Bias: Definition, Examples in Survey Research
Research ethical guidelines are designed to guide researchers in research conduct and publications.
This is why all researchers should develop habits of self-consciousness, self-restraint, and self of responsibility. This will enable them to take importance to the welfare of the members of the research community, the public, and their own reputation. Bearing all this in mind what wood prevents them from partaking in any misconduct in their research and publication.
Implications and Consequences of Unethical Research Practices
A researcher’s ethical obligations are to be taken seriously because they are truly no laughing matter. Here are the things at stake if violated.
- A researcher risks an unapproved study or publication if the research proposal submitted to the supervising institution does not meet the research ethical requirements. This implies that the researcher will not be allowed to proceed with the research until the ethical conditions have been met according to the standard by the supervising institution.
- If you have gotten a go-ahead for your study or research, failure to comply with the guidelines can result in your research being declared void and retracted. This means that you have to follow every step throughout the research else you can face disciplinary actions.
- If your research publication is connected to your doctorate degree, your doctorate title might be revoked. If the nature of your ethics breach is criminal, then the supervising institution can take legal action against the researcher. This may lead to prison sentences for the researcher.
- Also, an unethical omission can cause the characteristics of the researcher to be questioned in terms of reliability, and also, the validity of the test will be questioned.
- Unethical conduct in research can put in bad media coverage and damage the reputation of you and your research institution.
Researchers should remember the consequence of unethical conduct is humiliation. Aside from the loss of reputation, there could be legal consequences. That is why researchers should not take shortcuts when conducting their research.
Retraction watch is a website where retracted researchers are publicized and no researcher would want to be featured on this website for unethical conduct.
Read: Type I vs Type II Errors: Causes, Examples & Prevention
How to Detect Unethical Research Practices
Here are some tools and mechanisms you can use to prevent and detect unethical practices.
1. Management responsibility
In its day-to-day dealings, the management must maintain the highest standards of integrity. This is because if senior management is dishonest and corrupt, they will spread dishonest and fraudulent acts to all levels. It is their responsibility to be the highest standards when it comes to integrity.
The management is to serve as an example for all in the research organization to follow by pointing out correct and acceptable behavior to the staff. To ensure maximum security, they must make sure that the organization has all the procedures and control measures in place to ensure maximum security.
2. Code of ethics
There must be a setup code of ethics for all employees to follow in every establishment. This should be a formal statement containing ethical codes of conduct for the organization’s employees to follow. The Code of Ethics should unambiguously state the type of behavior expected from the employees, and what is unacceptable.
3. Personnel policies and procedures
If policies and procedures are open and fair, and also efficient personnel is in an organization, the organization’s exposure to fraud will be minimal. Organizations should consider putting effective policies in place.
Ethical Principles in Research
Respect for individuals.
- Researchers must base their study on the fundamental respect for human dignity.
- The research must respect their privacy. The autonomy and integrity of the individual must be protected.
- It must perform its duty to inform participants of what they’re being given. They must be provided with adequate information about the research and the purpose of the research.
- It must derive consent to notify. The participants must willingly grant consent before the research can proceed.
- It must also practice confidentiality. All personal data must be handled with utmost care and confidentiality.
- There must also be a responsibility to protect children and not cause others harm.
Respect for Institutions
- The research must be in accordance with the rules of the public administration.
- There must be adequate respect given to private establishments that do not want to give out their data or information.
- The interest of the vulnerable groups must be protected at all times.
- Research should be carried out on other cultures appropriately. And these cultures must be respected.
- Cultural monuments such as archives, artifacts, and texts must be treated with maximum care and preserved.
Explore: 21 Chrome Extensions for Academic Researchers in 2021
Formplus Features for Ethical Research
If you want to conduct a survey or make use of questionnaires in your research, the best website to use is Formplus.
Formplus has all you need to develop your form, administer your form, gather the data from your survey or questionnaire, and interpret it.
Formplus also has in place all the requirements a researcher needs to follow ethical guidelines. Your forms are secured, private, and protected.
- GDPR-Compliant
The GDPR-compliant consent form helps you to maintain the European Union’s data privacy laws. You can collect personal information such as names, emails, phone numbers, and addresses using the GDPR compliant form builder on Formplus.
- Privacy Policy and Security
The privacy protection policy of Formplus is so transparent that it will tell you what the data collected from you will be used for, and who it is shared with. Also, your data is so secure that not even Formplus can access it without your permission.
Security is also 100% guaranteed. Formplus takes extra steps to ensure the privacy of its users is protected. Formplus website complies with all international laws and requirements you can think of. All your sensitive information is protected online and offline.
Rules and guidelines are important. Not only because they give you instructions on how to carry out a procedure in research, but also because they make you responsible and respectable humans in society.
There are set ethical guidelines that protect the researcher, the research community, and the general public. It is in a researcher’s best interest to follow these laid down rules because the consequences are a long-lasting dent to whoever is involved and that might even be the end of their career.

Connect to Formplus, Get Started Now - It's Free!
- code of ethics
- data falsification
- duplicate publication
- principles of research
- research ethics
- unethical research
- unethical research practices
- busayo.longe

You may also like:
Exploratory Research: What are its Method & Examples?
Overview on exploratory research, examples and methodology. Shows guides on how to conduct exploratory research with online surveys

Recall Bias: Definition, Types, Examples & Mitigation
This article will discuss the impact of recall bias in studies and the best ways to avoid them during research.
What is Pure or Basic Research? + [Examples & Method]
Simple guide on pure or basic research, its methods, characteristics, advantages, and examples in science, medicine, education and psychology
Market Research: Types, Methods & Survey Examples
A complete guide on market research; definitions, survey examples, templates, importance and tips.

Formplus - For Seamless Data Collection
Collect data the right way with a versatile data collection tool. try formplus and transform your work productivity today..
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Common Ethical Issues In Research And Publication
Ng chirk jenn.
- Author information
- Article notes
- Copyright and License information
Dr Ng Chirk Jenn, Senior lecturer, Department of Primary Care Medicine, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Tel: 03-79492306, Email: [email protected]
Corresponding author.
Collection date 2006.
INTRODUCTION
Research is the pillar of knowledge, and it constitutes an integral part of progress. In the fast-expanding field of biomedical research, this has improved the quality and quantity of life. Historically, medical doctors have been in the privileged position to carry out research, especially in clinical research which involves people. They are able to control “life and death” of patients and have free access to their confidential information. Moreover, medical researchers have also enjoyed immunity from accountability due to high public regard for science and medicine. This has resulted in some researchers conducting unethical researches. For instance, in World War II, medical doctors had conducted unethical experiments on human in the name of science, resulting in harm and even death in some cases. 1 More recently, the involvement of pharmaceutical industry in clinical trials have raised issues about how to safeguard patient’s care and to ensure the published research findings are objective. 2
In the light of these ethical controversies, the Declaration of Helsinki was established to inform biomedical researchers the principles of clinical research. 3 This declaration highlighted a tripartite guidelines for good clinical practice which include respect for the dignity of the person; research should not override the health, well-being and care of subjects; principles of justice. Committee on Publication Ethics (COPE) was also founded in 1997 to address the breaches of research and publication ethics. 4
How do we apply all these principles in our daily conduct of research? This paper will discuss different ethical issues in research, including study design and ethical approval, data analysis, authorship, conflict of interest and redundant publication and plagiarism. I have also included two case scenarios in this paper to illustrate common ethical issues in research and publication.
ETHICAL ISSUES IN RESEARCH
1. study design and ethics approval.
According to COPE, “good research should be well adjusted, well-planned, appropriately designed, and ethically approved. To conduct research to a lower standard may constitute misconduct.” 3 This may appear to be a stringent criterion, but it highlights the basic requirement of a researcher is to conduct a research responsibly. To achieve this, a research protocol should be developed and adhered to. It must be carefully agreed to by all contributors and collaborators, and the precise roles of each team member should be spelled out early, including matters of authorship and publications. Research should seek to answer specific questions, rather than just collect data.
It is essential to obtain approval from the Institutional Review Board, or Ethics Committee, of the respective organisations for studies involving people, medical records, and anonymised human tissues. The research proposal should discuss potential ethical issues pertaining to the research. The researchers should pay special attention to vulnerable subjects to avoid breech of ethical codes (e.g. children, prisoners, pregnant women, mentally challenged, educationally and economically disadvantaged). Patient information sheet should be given to the subjects during recruitment, detailing the objectives, procedures, potential benefits and harms, as well as rights to refuse participation in the research. Consent should be explained and obtained from the subjects or guardians, and steps should be taken to ensure confidentiality of information provided by the subjects.
2. Data analysis
It is the responsibility of the researcher to analyse the data appropriately. Although inappropriate analysis does not necessarily amount to misconduct, intentional omission of result may cause misinterpretation and mislead the readers. Fabrication and falsification of data do constitute misconduct. For example, in a clinical trial, if a drug is found to be ineffective, this study should be reported. There is a tendency for the researchers to under-report negative research findings, 5 and this is partly contributed by pressure from the pharmaceutical industry which funds the clinical trial.
To ensure appropriate data analysis, all sources and methods used to obtain and analyse data should be fully disclosed. Failure to do so may lead the readers to misinterpret the results without considering possibility of the study being underpowered. The discussion section of a paper should mention any issues of bias, and explain how they have been dealt with in the design and interpretation of the study.
3. Authorship
There is no universally agreed definition of authorship. 6 It is generally agreed that an author should have made substantial contribution to the intellectual content, including conceptualising and designing the study; acquiring, analysing and interpreting the data. The author should also take responsibility to certify that the manuscript represents valid work and take public responsibility for the work. Finally, an author is usually involved in drafting or revising the manuscript, as well as approving the submitted manuscript. Data collection, editing of grammar and language, and other routine works by itself, do not deserve an authorship.
It is crucial to decide early on in the planning of a research who will be credited as authors, as contributors, and who will be acknowledged. It is also advisable to read carefully the “Advice to Authors” of the target journal which may serve as a guide to the issue of authorship.
4. Conflicts of interest
This happens when researchers have interests that are not fully apparent and that may influence their judgments on what is published. These conflicts include personal, commercial, political, academic or financial interest. Financial interests may include employment, research funding, stock or share ownership, payment for lecture or travel, consultancies and company support for staff. This issue is especially pertinent in biomedical research where a substantial number of clinical trials are funded by pharmaceutical company.
Such interests, where relevant, should be discussed in the early stage of research. The researchers need to take extra effort to ensure that their conflicts of interest do not influence the methodology and outcome of the research. It would be useful to consult an independent researcher, or Ethics Committee, on this issue if in doubt. When publishing, these conflicts of interest should be declared to editors, and readers will judge for themselves whether the research findings are trustworthy.
5. Redundant publication and plagiarism
Redundant publication occurs when two or more papers, without full cross reference, share the same hypothesis, data, discussion points, or conclusions. However, previous publication of an abstract during the proceedings of meetings does not preclude subsequent submission for publication, but full disclosure should be made at the time of submission. This is also known as self-plagiarism. In the increasing competitive environment where appointments, promotions and grant applications are strongly influenced by publication record, researchers are under intense pressure to publish, and a growing minority is seeking to bump up their CV through dishonest means. 7
On the other hand, plagiarism ranges from unreferenced use of others’ published and unpublished ideas, including research grant applications to submission under “new” authorship of a complete paper, sometimes in different language.
Therefore, it is important to disclose all sources of information, and if large amount of other people’s written or illustrative materials is to be used, permission must be sought.
It is the duty of the researcher to ensure that research is conducted in an ethical and responsible manner from planning to publication. Researchers and authors should familiarise themselves with these principles and follows them strictly. Any potential ethical issues in research and publication should be discussed openly within the research team. If in doubt, it is advisable to consult the respective institutional review board (IRB) for their expert opinions.
Case Scenario 1:
“A community survey on prevalence of domestic violence among secondary school students.”
Who should we obtain the consent? Students, parents, teachers or Ministry of Education?
To conduct this study, we need to seek approval from the Ministry of Education and permission from the school principal. However, consent should be taken from parents, who are the legal guardians of the students.
If the results show that 50% of the students have ever been abused, should I report them to the police?
These ethical issues should be discussed at the proposal stage, and the participants/guardians should be informed about the decision to report to the police while taking the consent. This will potentially affect the response rate; but this is also the responsibility of the researcher to protect the participants and their families.
I have decided to publish it. Can I send an abstract for presentation as part of the conference proceedings, and later submit similar abstract with the full text for publication. Is that redundant publication?
Yes, you can. However, you need to declare to the publisher that you have presented the paper in the conference. Redundant publication happens when an author has submitted two papers with similar objective, methodology and results, without cross referencing.
Can I submit the same paper in a different language?
Yes, you can. However, you have to declare to the publisher that you have published an identical paper in a different language.
Case Scenario 2:
“Does HRT improve vasomotor symptoms among menopausal women in a Malaysian primary care clinic?”
Some people say it is “unethical” to do this study because it has been proven in many studies. But no such research has ever been done locally!
HRT has been proven to be effective in relieving vasomotor symptoms in many well-designed studies. It is inappropriate for the researcher to repeat an established therapy which may potentially cause harm to them (e.g. deep vein thrombosis and breast cancer). However, it is appropriate to repeat research if the researchers feel that it may yield a different outcome in the local setting based on a firm theoretical basis.
Do we still need to obtain ethics approval if it is part of daily clinical practice?
Yes, even though it is part of our normal practice, all research involving human subjects, especially when it involves drugs, should be subjected to ethics approval. (E.g. “How did the researchers ensure that they explain to the patients fully about the potential harm of HRT?”)
I’m worried that if I start explaining to the participants about the possibility of Ca breast, they won’t want to participate. How can I “play down” this possible side effect?
As mentioned earlier, it is the duty of the researcher to ensure that the participant understands the benefits and risks of the treatment. The information should be conveyed in an objectively manner in the patient information sheet. Any queries from the patient should be answered truthfully, and it is the patient’s rights to refuse to participate in the research.
While writing the introduction and discussion of my paper/thesis, I copied sentences from some papers. But I referred to them in my reference. Is that acceptable?
It is acceptable to quote sentences from a paper as long as they are duly referenced.
- 1. Human D, Fluss SS. World Medical Association; 2001. The World Medical Association Declaration of Helsinki: historical and contemporary perspectives. [ Google Scholar ]
- 2. Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality. BMJ. 2003;326:1167–70. doi: 10.1136/bmj.326.7400.1167. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. World Medical association. 2004. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. [ PubMed ] [ Google Scholar ]
- 4. Committee on Publication Ethics (COPE) 2005. Guidelines on good publication and the Code of Conduct. [ Google Scholar ]
- 5. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2:100–2. doi: 10.1371/journal.pmed.0020138. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Medical Research Council. London: MRC; 1998. MRC Guidelines for good clinical practice in clinical trials. [ Google Scholar ]
- 7. Giles J. Taking on the cheats. Nature. 2005;435:258–9. doi: 10.1038/435258a. [ DOI ] [ PubMed ] [ Google Scholar ]
- PDF (104.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Educational resources and simple solutions for your research journey

Research Misconduct: Reasons and Types of Research Misconduct

Science and is built on the foundation of integrity and trust, and questionable research practices or research misconduct is counter-productive to the production and use of scientific knowledge. Not only does it dilute the research findings and lead to misinterpretations, it also leads to an overall loss of trust in the work done by the scientific community. Hence any kind of research misconduct or malpractice is considered a grave issue that needs to be strictly avoided. This makes it critical for scientists and researchers to gain an understanding of the best practices and ethical research to avoid unknowingly venturing into such grey areas. This article delves into what is scientific misconduct, different types of research misconduct, and 5 reasons for committing research misconduct.
Table of Contents
What is research misconduct?
Research misconduct or scientific misconduct refers to actions and behaviors by researchers that fail to honor the integrity of research. The Office of Research Integrity defines research misconduct as the falsification, fabrication or plagiarism in conducting, planning, reporting or reviewing research [1] . Simply put, research misconduct is any intentional deviation from ethical research practices. A scientific misconduct example is deliberately creating names and details of survey participants for the purpose of generating data, which is an unethical research practice. Other common scientific misconduct examples include modifying or omitting data to influence study findings or withholding critical information from human participants in clinical trials or experiments.
Types of research misconduct
There are different types of research misconduct or scientific misconduct and unethical practices in research. The most serious ethical infractions are fabrication, falsification, and plagiarism in research. Some of the most common types of research misconduct have been detailed below.
- Fabrication: This refers to the practice of making up data without having done the required research. Research misconduct covers not only the act of fabrication, but also the sharing, discussing, or publishing of this fabricated data or results.
- Falsification: This type of scientific misconduct involves the wilful manipulation of data, materials, processes, or equipment to arrive at a predefined conclusion. One such example would be selectively omitting or changing data, which results in the erroneous representation of research results.
- Plagiarism: This is one of the most common types of scientific misconduct, and involves using another person’s ideas, content, writing, processes, or results without giving due credit. This also includes self-plagiarism , which occurs when you replicate your own writings or ideas from previously published research without providing proper credit.
- Authorship: This type of scientific misconduct in research includes attempts to assign false authorships without adequate contribution to research, mentioning authors without their consent, or failing to include authors who are original contributors. Naming authors in the wrong order or incorrectly is also considered unethical.
- Conflicts of interest: This can be classified under general scientific misconduct and involves lapses by researchers in declaring any conflict of interests in their research work. These conflicts of interest may be financial, personal, and professional and need to be reported appropriately to avoid any ethical issues.
- Approvals: One of the most important aspect of research that involves human or animal subjects is adhering to all the ethical approvals and legal guidelines. Non-compliance with this ethical mandate is considered a serious type of research misconduct.

5 Reasons for committing research misconduct
Over time there have been varied reasons for researchers to succumb to scientific misconduct. Let us look at 5 reasons for committing research misconduct.
- Career pressures : An important factor often associated with research misconduct is the undue pressure researchers face. They need to conduct original research in a fast-paced environment, publish frequently in peer reviewed journals, and procure funding for research projects to advance their research career. This along with the need to juggle multiple responsibilities against tight deadlines create undue stress to succeed at any cost, leading to a lack of care or even deliberate research misconduct.
- Researcher’s personal psychology: Some researchers may be overly driven by a desire to quickly attain a strong professional reputation or even financial gains, which could push them to research misconduct.
- Lack of appropriate training and skills: The lack of training on the best practices and ethical guidelines to be followed as researchers is another reason for research misconduct. Poor awareness and understanding on these issues often lead to unethical conduct in research.
- Insufficient supervision or mentoring: Related to the point above, this relates to situations where researchers, especially early career researchers, fail to receive sufficient and appropriate support from immediate supervisors or from their affiliated institution. A lack of oversight and guidance may knowingly or unknowingly lead to research misconduct.
- Inadequate knowledge: Scientific misconduct can occur if the researcher does not have sufficient knowledge of the topic/subject or on research best practices. Carelessness when conducting research and reporting it are also considered research misconduct.
Researchers and institutions should adopt various measures to prevent the occurrence of scientific misconduct. The most significant aspect is the provision of adequate training that builds researcher knowledge as to what constitutes research misconduct and how best to avoid this. It is vital for institutions to have guidelines and procedures related to good research practices and ethical conduct and ensure it is disseminated effectively among their research community.
It is also important for supervisors to mentor budding researchers on the correct procedures and practices, including what constitutes ethical misconduct in research. Without sufficient awareness, it will be difficult to effectively address this important issue. Finally, researchers must take it upon themselves to check the global standards and guidelines for ethical research to ensure they are not engaging in any scientific misconduct.
Q: What are the consequences of research misconduct?
Research misconduct, which includes fabrication, falsification, and plagiarism, can lead to severe repercussions. These may involve damage to a researcher’s reputation, loss of funding, retraction of published papers, and academic sanctions. Additionally, it undermines scientific integrity, erodes public trust, and hampers the advancement of knowledge. Institutions may conduct investigations, resulting in job loss and legal actions. Overall, research misconduct has far-reaching negative impacts on individuals, institutions, and the scientific community as a whole.
Q: What are the major causes of research misconduct?
Research misconduct arises from various factors such as pressure to publish, competition for grants, and career advancement. Lack of supervision, inadequate training in ethical research practices, and poor research culture can contribute. High publication demands may drive researchers to cut corners, leading to fabrication of data, falsification of results, and even plagiarism. Ethical lapses might also stem from personal ambition, greed, or the desire to bolster one’s professional standing, ultimately undermining the credibility of scientific work.
Q: What is meant by falsification in research?
Falsification refers to the deliberate manipulation or alteration of research data, methods, or results to present inaccurate or misleading information. This unethical practice involves misrepresenting findings by selectively omitting data, changing results, or altering graphs and images. Falsification distorts the truth, compromises research integrity, and can have profound implications for scientific progress and public trust in research outcomes.
Q: What are some examples of scientific misconduct?
Scientific misconduct encompasses various behaviors such as plagiarism, where one presents others’ work as their own. Fabrication involves inventing data or results that do not exist. Falsification includes altering or manipulating data to fit a desired outcome. Misleading authorship and inadequate citation of sources are also forms of misconduct. Additionally, improper handling of human or animal subjects, failure to disclose conflicts of interest, and misrepresentation of credentials can occur. These actions breach ethical standards, erode scientific credibility, and can lead to severe consequences for individuals and the research community.
- Definition of Research Misconduct. The Office of Research Integrity. Available online at https://ori.hhs.gov/definition-research-misconduct
R Discovery is a literature search and research reading platform that accelerates your research discovery journey by keeping you updated on the latest, most relevant scholarly content. With 250M+ research articles sourced from trusted aggregators like CrossRef, Unpaywall, PubMed, PubMed Central, Open Alex and top publishing houses like Springer Nature, JAMA, IOP, Taylor & Francis, NEJM, BMJ, Karger, SAGE, Emerald Publishing and more, R Discovery puts a world of research at your fingertips.
Try R Discovery Prime FREE for 1 week or upgrade at just US$72 a year to access premium features that let you listen to research on the go, read in your language, collaborate with peers, auto sync with reference managers, and much more. Choose a simpler, smarter way to find and read research – Download the app and start your free 7-day trial today !
Related Posts

How does R Discovery Translate Complex Academic Papers Accurately

What Constitutes Good Research in Academia?
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, guiding principles for ethical research.
Pursuing Potential Research Participants Protections

“When people are invited to participate in research, there is a strong belief that it should be their choice based on their understanding of what the study is about, and what the risks and benefits of the study are,” said Dr. Christine Grady, chief of the NIH Clinical Center Department of Bioethics, to Clinical Center Radio in a podcast.
Clinical research advances the understanding of science and promotes human health. However, it is important to remember the individuals who volunteer to participate in research. There are precautions researchers can take – in the planning, implementation and follow-up of studies – to protect these participants in research. Ethical guidelines are established for clinical research to protect patient volunteers and to preserve the integrity of the science.
NIH Clinical Center researchers published seven main principles to guide the conduct of ethical research:
Social and clinical value
Scientific validity, fair subject selection, favorable risk-benefit ratio, independent review, informed consent.
- Respect for potential and enrolled subjects
Every research study is designed to answer a specific question. The answer should be important enough to justify asking people to accept some risk or inconvenience for others. In other words, answers to the research question should contribute to scientific understanding of health or improve our ways of preventing, treating, or caring for people with a given disease to justify exposing participants to the risk and burden of research.
A study should be designed in a way that will get an understandable answer to the important research question. This includes considering whether the question asked is answerable, whether the research methods are valid and feasible, and whether the study is designed with accepted principles, clear methods, and reliable practices. Invalid research is unethical because it is a waste of resources and exposes people to risk for no purpose
The primary basis for recruiting participants should be the scientific goals of the study — not vulnerability, privilege, or other unrelated factors. Participants who accept the risks of research should be in a position to enjoy its benefits. Specific groups of participants (for example, women or children) should not be excluded from the research opportunities without a good scientific reason or a particular susceptibility to risk.
Uncertainty about the degree of risks and benefits associated with a clinical research study is inherent. Research risks may be trivial or serious, transient or long-term. Risks can be physical, psychological, economic, or social. Everything should be done to minimize the risks and inconvenience to research participants to maximize the potential benefits, and to determine that the potential benefits are proportionate to, or outweigh, the risks.
To minimize potential conflicts of interest and make sure a study is ethically acceptable before it starts, an independent review panel should review the proposal and ask important questions, including: Are those conducting the trial sufficiently free of bias? Is the study doing all it can to protect research participants? Has the trial been ethically designed and is the risk–benefit ratio favorable? The panel also monitors a study while it is ongoing.
Potential participants should make their own decision about whether they want to participate or continue participating in research. This is done through a process of informed consent in which individuals (1) are accurately informed of the purpose, methods, risks, benefits, and alternatives to the research, (2) understand this information and how it relates to their own clinical situation or interests, and (3) make a voluntary decision about whether to participate.
Respect for potential and enrolled participants
Individuals should be treated with respect from the time they are approached for possible participation — even if they refuse enrollment in a study — throughout their participation and after their participation ends. This includes:
- respecting their privacy and keeping their private information confidential
- respecting their right to change their mind, to decide that the research does not match their interests, and to withdraw without a penalty
- informing them of new information that might emerge in the course of research, which might change their assessment of the risks and benefits of participating
- monitoring their welfare and, if they experience adverse reactions, unexpected effects, or changes in clinical status, ensuring appropriate treatment and, when necessary, removal from the study
- informing them about what was learned from the research
More information on these seven guiding principles and on bioethics in general
This page last reviewed on March 16, 2016
Connect with Us
- More Social Media from NIH

What Are The Top 10 Unethical Psychology Experiments?
- By Cliff Stamp, BS Psychology, MS Rehabilitation Counseling
- Published September 9, 2019
- Last Updated November 13, 2023
- Read Time 8 mins

Posted September 2019 by Clifton Stamp, B.S. Psychology; M.A. Rehabilitation Counseling, M.A. English; 10 updates since. Reading time: 8 min. Reading level: Grade 9+. Questions on unethical psychology experiments? Email Toni at: [email protected] .
Like all sciences, psychology relies on experimentation to validate its hypotheses. This puts researchers in a bit of a bind, in that experimentation requires manipulation of one set of variables. Manipulating human beings can be unethical and has the potential to lead to outright harm. Nowadays experiments that involve human beings must meet a high standard for safety and security for all research participants. Although ethical and safety standards in the 21st century keep people safe from any potential ill effects of experiments and studies, conditions just a few decades ago were far from ideal and were in many cases out and out harmful.
The Top 10 Unethical Psychology Experiments
10. The Stanford Prison Experiment (1971). This example of unethical research studies occurred in August of 1971, Dr. Philip Zimbardo of Stanford University began a Navy-funded experiment examining the effects of power dynamics between prison officers and prisoners. It only took six days before the experiment collapsed. Participants so completely absorbed their roles that the “officers” began psychologically torturing the prisoners and the prisoners became aggressive toward the officers. The prisoners, in turn, fought the guards and refused to comply with requests. By the second day, prisoners refused to obey guards and the guards started threatening prisoners with violence, far over their instructions. By the 6 th day, guards were harassing the prisoners physically and mentally and some guards had harmed prisoners. Zimbardo stopped the experiment at that point.
9. The Monster Study (1939). The Monster Study is a prime example of an unethical psychology experiment on humans that changed the world. Wendell Johnson, a psychologist at the University of Iowa, conducted an experiment about stuttering on 22 orphans. His graduate student, Mary Tudor, experimented while Johnson supervised her work. She divided a group of 22 children into two groups. Each group was a mixture of children with and without speech problems. One group received encouragement and positive feedback, but the other was ridiculed for any speech problems, including non-existent problems. Children who received ridicule naturally made no progress, and some of the orphans with no speech problems developed those very problems.
The study continued for six months and caused lasting, chronic psychological issues for some of the children. The study caused so much harm that some of the former subjects secured a monetary award from the University of Iowa in 2007 due to the harm they’d suffered.
8. The Milgram Conformity Experiment (1961 ). After the horrors of the Second World War, psychological researchers like Stanley Milgram wondered what made average citizens act like those in Germany who had committed atrocities. Milgram wanted to determine how far people would go carrying out actions that might be detrimental to others if they were ordered or encouraged to do so by an authority figure. The Milgram experiment showed the tension between that obedience to the orders of the authority figure and personal conscience.
In each experiment, Milgram designated three people as either a teacher, learner or experimenter. The “learner” was an actor planted by Milgram and stayed in a room separate from the experimenter and teacher. The teacher attempted to teach the learner how to perform small sets of word associations. When the learner got a pair wrong, the teacher delivered an electric shock to the learner. In reality, there was no shock given. The learner pretended to be in increasingly greater amounts of distress. When some teachers expressed hesitation about increasing the level of shocks, the experimenter encouraged them to do so.
Many of the subjects (the teachers) experienced severe and lasting psychological distress. The Milgram Conformity Experiment has become the byword for well-intentioned psychological experiments gone wrong.
8. David Reimer (1967–1977) . When David Reimer was eighth months old, his penis was seriously damaged during a failed circumcision. His parents contacted John Money, a professor of psychology and pediatrics at Johns Hopkins, who was a researcher in the development of gender. As David had an identical twin brother, Money viewed the situation as a rare opportunity to conduct his own experiment into the nature of gender, by advising Reimer’s parents to have the David sexually reassigned as a female. Money’s theory was that gender was a completely sociological construct and primarily influenced by nurture, as opposed to nature. Money was catastrophically wrong.
Reimer never identified as female and developed strong psychological attachment to being a male. At age 14, Reimer’s parents told him the truth about his condition and he elected to switch to a male identity. Although he later had surgery to correct the initial sex re-assignment, he suffered from profound depression related to his sex and gender issues and committed suicide in 2004. Money’s desire to test his controversial psychology experiment on humans without their consent cost someone his life.
7. Landis’ Facial Expressions Experiment (1924). In 1924, at the University of Minnesota, Carney Landis created an experiment on humans to investigate the similarity of different people’s’ facial expressions. He wanted to determine if people displayed similar or different facial expressions while experiencing common emotions.
Carney chose students as participants, who were exposed to different situations to prompt emotional reactions. However, to elicit revulsion, he ordered the participants to behead a live rat. One-third of participants refused to do it, while another two-thirds complied, with a great deal of trauma done to them–and the rats. This unethical experiment is one of many reasons review boards were created and have made drastic changes in policy over experiments done on humans.
6. The Aversion Project (1970s and 80s). During the apartheid years in South Africa, doctors in the South African military tried to “cure” homosexuality in conscripts by forcing them to undergo electroshock therapy and chemical castration. The military also forced gay conscripts to undergo sex-change operations. This happened as one segment of a secret military program headed by Dr. Aubrey Levin that sought to study and eliminate homosexuality in the military as recently as 1989. Exception for several cases of lesbian soldiers who were abused, most of the 900 soldiers to be abused were very young, from 16 to 24-year-old male conscripts. Astoundingly, Dr. Levin relocated to Canada where he worked until he was sent to prison for assaulting a patient.
5. Monkey Drug Trials (1969). The Monkey Drug Trials experiment was ostensibly meant to test the effects of illicit drugs on monkeys. Given that monkeys and humans have similar reactions to drugs, and that animals have long been part of medical experiments, the face of this experiment might not look too bad. It’s actually horrific. Monkeys and rats learned to self-administer a range of drugs, from cocaine, amphetamines, codeine, morphine and alcohol. The animals suffered horribly, tearing their fingers off, clawing fur away, and in some cases breaking bones attempting to break out of their cages. This psychology experiment study had no purpose other than to re-validate studies that had been validated many times before.
4. Little Albert (1920). John Watson, the founder of the psychological school of behaviorism, believed that behaviors were primarily learned. Anxious to test his hypothesis, he used an orphan, “Little Albert,” as an experimental subject. He exposed the child to a laboratory rat, which caused no fear response from the boy, for several months. Next, at the same time, the child was exposed to the rat, he struck a steel bar with a hammer, scaring the little boy and causing a fear response. By associating the appearance of the rat with the loud noise, Little Albert became afraid of the rat. Naturally, the fear was a condition that needed to be fixed, but the boy left the facility before Watson could remedy things.
3. Harlow’s Pit of Despair (1970s). Harry Harlow, a psychologist at the University of Wisconsin-Madison, was a researcher in the field of maternal bonding. To investigate the effects of attachment on development, he took young monkeys and isolated them after they’d bonded with their mothers. He kept them completely isolated, in a vertical chamber that prevented all contact with other monkeys. They developed no social skills and became completely unable to function as normal rhesus monkeys. These controversial psychology experiments, were not only staggeringly cruel but revealed no data that wasn’t already known.
2. Learned Helplessness (1965). In 1965, Doctors Martin Seligman and Steve Maier investigated the concept of learned helplessness. Three sets of dogs were placed in harnesses. One group of dogs were control subjects. Nothing happened to them. Dogs from group two received electric shocks; however, they were able to stop the shocks when they pressed a lever. In group three, the subjects were shocked, but the levers didn’t abort the shocks. Next, the psychologists placed the dogs in an open box the dogs could easily jump out of, but even though they received shocks, they didn’t leap out of the box. The dogs developed a sense of helplessness, or an inability to take successful action to change a bad situation.
1. The Robbers Cave Experiment (1954). Although the Robbers Cave Experiment is much less disturbing than some of the others in the list, it’s still a good example of the need for informed consent. In 1954, Muzafer Sherif, a psychologist interested in group dynamics and conflict, brought a group of preteen boys to a summer camp. He divided them into groups and engaged the boys in competitions. However, Sherif manipulated the outcomes of the contests, keeping the results close for each group. Then he gave the boys tasks to complete as a unified group, with everyone working together. The conflicts that had arisen when the boys were competing vanished when they worked as one large group.
More Psychology Rankings of Interest:
- 10 Key Moments in the History of Psychology
- 5 Key Moments in Social Psychology
- 5 Learning Theories in Psychology
- Top 10 Movies About Psychology
Trending now
Unethical human research in the field of neuroscience: a historical review
- Review Article
- Published: 19 February 2018
- Volume 39 , pages 829–834, ( 2018 )
Cite this article

- Hussein Algahtani ORCID: orcid.org/0000-0001-9484-9838 1 ,
- Mohammed Bajunaid 2 &
- Bader Shirah 3
8722 Accesses
13 Citations
32 Altmetric
Explore all metrics
Understanding the historical foundations of ethics in human research are key to illuminating future human research and clinical trials. This paper gives an overview of the most remarkable unethical human research and how past misconducts helped develop ethical guidelines on human experimentation such as The Nuremberg Code 1947 following WWII. Unethical research in the field of neuroscience also proved to be incredibly distressing. Participants were often left with life-long cognitive disabilities. This emphasizes the importance of implicating strict rules and ethical guidelines in neuroscience research that protect participants and respects their dignity. The experiments conducted by German Nazi in the concentration camps during WWII are probably the most inhumane and brutal ever conducted. The Nuremberg Code of 1947, one of the few positive outcomes of the Nazi experiments, is often considered the first document to set out ethical regulations of human research. It consists of numerous necessary criteria, to highlight a few, the subject must give informed consent, there must be a concrete scientific basis for the experiment, and the experiment should yield positive results that cannot be obtained in any other way. In the end, we must remember, the interest of the patient must always prevail over the interest of science or society.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Research Ethics and Scientific Integrity in Neuroscience

Human Brain Research and Ethics
Nardini C (2014) The ethics of clinical trials. Ecancermedicalscience 8:387
PubMed PubMed Central Google Scholar
Glenn Forister J, Dennis Blessing J (2015) Introduction to research and medical literature for health professionals. Jones & Bartlett Learning, Burlington
Google Scholar
NIH Clinical Center: Ethics in Clinical Research. Retrieved 24 August 2017. Available at https://clinicalcenter.nih.gov/recruit/ethics.html
Illes J, Bird SJ (2006) Neuroethics: a modern context for ethics in neuroscience. Trends Neurosci 29(9):511–517. https://doi.org/10.1016/j.tins.2006.07.002
Article CAS PubMed PubMed Central Google Scholar
Silverman F (1988) The “monster” study. J Fluen Disord 13(3):225–231. https://doi.org/10.1016/0094-730X(88)90049-6
Article Google Scholar
Stanford Prison Experiment. Retrieved 24 August 2017. Available at https://www.simplypsychology.org/zimbardo.html
Thomas SB, Quinn SC (1991) The Tuskegee Syphilis Study, 1932 to 1972: implications for HIV education and AIDS risk education programs in the black community. Am J Public Health 81(11):1498–1505. https://doi.org/10.2105/AJPH.81.11.1498
Brandt AM (1978) Racism and research: the case of the Tuskegee Syphilis Study. Hast Cent Rep 8(6):21–29. https://doi.org/10.2307/3561468
Article CAS Google Scholar
Korda A The Nazi medical experiments. ADF Health [online]. Retrieved 24 August 2017. Available at: http://www.defence.gov.au/health/infocentre/journals/adfhj_apr06/adfhealth_7_1_33-37.html
The 30 most disturbing human experiments in history. Retrieved 24 August 2017. Available at http://www.bestpsychologydegrees.com/30-most-disturbing-human-experiments-in-history/
Strous RD, Edelman MC (2007) Eponyms and the Nazi era: time to remember and time for change. Isr Med Assoc J 9(3):207–214
PubMed Google Scholar
Laud Humphreys and the Tearoom Sex Study. Retrieved 24 August 2017. Available at http://www.drjkoch.org/Intro/Readings/Humphreys.htm
Harris B (1979) Whatever happened to little Albert? Am Psychol 34(2):151–160. https://doi.org/10.1037/0003-066X.34.2.151
Slater M, Antley A, Davison A, Swapp D, Guger C, Barker C, Pistrang N, Sanchez-Vives MV (2006) A virtual reprise of the Stanley Milgram obedience experiments. PLoS One 1(1):e39. https://doi.org/10.1371/journal.pone.0000039
Article PubMed PubMed Central Google Scholar
Schell BH (1994) The ominous shadow of the CIA has imprinted itself on the brain research community. J Calif Alliance Ment Ill 5(1):38–40
Wilson ST, Stanley B (2006) Ethical concerns in schizophrenia research: looking back and moving forward. Schizophr Bull 32(1):30–36. https://doi.org/10.1093/schbul/sbj023
Article PubMed Google Scholar
Download references
Author information
Authors and affiliations.
King Abdulaziz Medical City, King Saud bin Abdulaziz University for Health Sciences, P.O. Box: 12723, Jeddah, 21483, Saudi Arabia
Hussein Algahtani
King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
Mohammed Bajunaid
King Abdullah International Medical Research Center, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
Bader Shirah
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hussein Algahtani .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Rights and permissions
Reprints and permissions
About this article
Algahtani, H., Bajunaid, M. & Shirah, B. Unethical human research in the field of neuroscience: a historical review. Neurol Sci 39 , 829–834 (2018). https://doi.org/10.1007/s10072-018-3245-1
Download citation
Received : 24 October 2017
Accepted : 03 January 2018
Published : 19 February 2018
Issue Date : May 2018
DOI : https://doi.org/10.1007/s10072-018-3245-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Unethical research
- Neuroscience
- Find a journal
- Publish with us
- Track your research
- Original article
- Open access
- Published: 01 April 2021
Unethical practices within medical research and publication – An exploratory study
- S. D. Sivasubramaniam 1 ,
- M. Cosentino 2 ,
- L. Ribeiro 3 &
- F. Marino 2
International Journal for Educational Integrity volume 17 , Article number: 7 ( 2021 ) Cite this article
29k Accesses
2 Citations
4 Altmetric
Metrics details
The data produced by the scientific community impacts on academia, clinicians, and the general public; therefore, the scientific community and other regulatory bodies have been focussing on ethical codes of conduct. Despite the measures taken by several research councils, unethical research, publishing and/or reviewing behaviours still take place. This exploratory study considers some of the current unethical practices and the reasons behind them and explores the ways to discourage these within research and other professional disciplinary bodies. These interviews/discussions with PhD students, technicians, and academics/principal investigators (PIs) (N=110) were conducted mostly in European higher education institutions including UK, Italy, Ireland, Portugal, Czech Republic and Netherlands.
Through collegiate discussions, sharing experiences and by examining previously published/reported information, authors have identified several less reported behaviours. Some of these practices are mainly influenced either by the undue institutional expectations of research esteem or by changes in the journal review process. These malpractices can be divided in two categories relating to (a) methodological malpractices including data management, and (b) those that contravene publishing ethics. The former is mostly related to “committed bias”, by which the author selectively uses the data to suit their own hypothesis, methodological malpractice relates to selection of out-dated protocols that are not suited to the intended work. Although these are usually unintentional, incidences of intentional manipulations have been reported to authors of this study. For example, carrying out investigations without positive (or negative) controls; but including these from a previous study. Other methodological malpractices include unfair repetitions to gain statistical significance, or retrospective ethical approvals. In contrast, the publication related malpractices such as authorship malpractices, ethical clearance irregularities have also been reported. The findings also suggest a globalised approach with clear punitive measures for offenders is needed to tackle this problem.
Introduction
Scientific research depends on effectively planned, innovative investigation coupled with truthful, critically analysed reporting. The research findings impact on academia, clinicians, and the general public, but the scientific community is usually expected to “self-regulate”, focussing on ethical codes of conduct (or behaviour). The concept of self-regulation is built-in from the early stages of research grant application until the submission of the manuscripts for gaining impact. However, increasing demands on research esteem, coupled with the way this is captured/assessed, has created a relentless pressure to publish at all costs; this has resulted in several scientific misconduct (Rawat and Meena 2014 ). Since the beginning of this century, cases of blatant scientific misconduct have received significant attention. For example, questionable research practices (QRP) have been exposed by whistle blowers within the scientific community and publicised by the media (Altman 2006 ; John et al. 2012 ). Moreover, organisations such as the Centre for Scientific Integrity (CSI) concentrate on the transparency, integrity and reproducibility of published data, and promote best practices (www1 n.d. ). These measures focus on “scholarly conduct” and promote ethical behaviour in research and the way it is reported/disseminated, yet the number of misconduct and/or QRP’s are on the rise. In 2008, a survey amongst researchers funded by the National Institutes of Health (NIH) suggested there might be as many as 1,000 cases of potential scientific misconduct going unreported each year (Titus et al. 2008 ). Another report on bioRxiv (an open access pre-print repository) showed 6% of the papers (59 out of 960) published in one journal (Molecular and Cellular Biology - MCB), between 2009 and 2016, contained inappropriately duplicated images (Bik et al. 2018 ). Brainard ( 2018 ) recently reported that the number of articles retracted by scientific journals had increased 10-fold in the past 10 years. If the reported incidence of scientific misconduct is this high, then one can predict the prevalence of other, unreported forms of misconduct. The World Association of Medical Editors (WAME) has identified the following as the most reported misconduct: fabrication, falsification, plagiarism/ghost writing, image/data manipulation, improprieties of authorship, misappropriation of the ideas of others, violation of local and international regulations (including animal/human rights and ethics), inappropriate/false reporting (i.e. wrongful whistle-blowing) (www2 n.d. ).
However, WAME failed to identify other forms of scientific misconduct, such as; reviewer bias (including reviewers’ own scientific, religious or political beliefs) (Adler and Stayer 2017 ), conflicts of interests (Bero 2017 ), and peer-review fixing, which is widespread, especially after the introduction of author appointed peer reviewers (Ferguson et al., 2014 ; Thomas 2018 ). The most recent Retraction Watch report has shown that more than 500 published manuscripts have been retracted due to peer-review fixing; many of these are from a small group of authors (cited in Meadows 2017 ). Other reasons for retraction include intentional/unintentional misconduct, fraud and to a lesser extent honest errors. According to Fang et al. ( 2012 ), in a detailed study using 2,047 retracted articles within biomedical and life-sciences, 67.4% of retractions were due to some form of misconduct (including fraud/suspected fraud, duplicate publication, and plagiarism). Only 21.3% of retractions were due to genuine error. As can be seen, most of the information regarding academic misconduct is reported, detected or meta-analysed from databases. As for reporting (or whistle blowing), many scientists have shown been reticent to raise concerns, mainly because of the fear of aftermath or implications of doing so (Bouter and Hendrix 2017 ). An anonymous information-gathering activity amongst scientists, junior scientists, technicians and PhD students may highlight the misconduct issues that are being encountered in their day-to-day laboratory, and scholarly, activities. Therefore, this exploratory study of an interview-based study reports potentially un-divulged misconduct and tries to form a link with previously reported misconduct that are either being enforced, practiced or discussed within scientific communities.
Methodology
This qualitative exploratory study was based on informal mini-interviews conducted through collegiate discussions with technicians, PhD scholars, and fellow academics (N=110) within medical and biomedical sciences mainly in European higher education institutions including UK, Italy, Ireland, Portugal, Czech Republic and Netherlands (only 5 PhD students). PhD students (n=75), technicians (mostly in the UK; n=25) and academics/principal investigators (PIs; n=10), around Europe, have taken part in this qualitative narrative exploration study. These mini-interviews were carried out in accordance with local ethical guidance and processes. The discussions or conversations were not voice recorded; nor the details of interviewees taken to maintain anonymity. The data was captured (in long-hand) by summarising their views on following three questions (see below).
These answers/notes were then grouped according to their similarities and summarised (see Tables 1 and 2 ). The mini-interviews were semi-structured, based around three questions.
Have you encountered any individual or institutional malpractices in your research area/laboratory?
If so, could you give a short description of this misconduct?
What are the measures, in your opinion, needed to minimise or remove these misconduct?
we also examined recently published and/or reported (in media) unethical practice or misconduct to compare our findings (see Table 2 ). Fig. 1 summarises the methodology and its meta-cognitive reflection (similar to Eaton et al. 2019 ).
Interactive enquiry-based explorative methodology used in this study
Results and discussion
As stated above, this manuscript is an exploratory study of unethical practice amongst medical researchers that are not well known or previously reported. Hence, the methodology applied was more exploratory with minimal focus on standardisation, using details of qualitative approach and paradigm, or the impact of researcher characteristics and reflexivity (British Medical Journal (BMJ) – www3 n.d. ). Most importantly, our initial informal meetings prior to this study clearly indicated that the participants were reluctant to provide information that would assist for an analysis linked to researcher characteristics and/or reflexivity. Thus, the level of data presented herein would not be suitable for a full thematic analysis. We do accept this as a research limitation.
This study has identified some less reported (not well-known) unethical behaviours or misconduct. These findings from technician/PhD scholars and academics/PIs are summarised in Tables 1 and 2 . The study initially aimed to identify any previously unreported unethical research conducts, however, the data shows that many previously identified misconduct are still common amongst researchers. Since the interviews were not audio recorded (to reassure anonymity), the participants were openly reported the unethical practices within their laboratories (or elsewhere). This may cast doubts on the accuracy of data interpretation. To minimise this, we have captured the summary of the conversation in long-hand.
We were able to generalise two emerging themes linked to the periods of a typical research cycle (as described by Hevner 2007 ); (a) methodological malpractices (including data management), and (b) those that contravene publishing ethics. Researcher-linked behaviours happen during laboratory investigation stage, where researchers employ questionable research practices, these include self-imposed as well as acquired (or taught) habits. As can be seen from Tables 1 and 2 , these misconduct are mainly carried out by either PhD scholars, post-doctoral scientists or early career researchers. These reported “practices” may be common amongst laboratory staff, especially given the fact that some of these practices have been nicknamed (e.g. ghost repeats, data mining etc. – see Table 1 ). Individual or researcher-linked unethical behaviours mostly related to “committed bias”, by which the researcher selectively uses the data to suit their own hypothesis or what they perceive as ground-breaking. This often results in conducts where research (and in some cases data/results) is statistically manipulated to suit the perceived conclusion.
Although this is a small-scale pilot study, we feel this reflects the common trend in laboratory-based research. As mentioned earlier, although this study was set out to detect unreported research misconduct/malpractices, study participant reported some of the behaviours that were already reported in previous studies.
In contrast, established academics, professors and PIs tend to commit publication-related misconduct. These can be divided into author-related or reviewer-related misconduct. The former includes QRPs during manuscript preparation (such as selective usage of data, omitting outliers, improper ethical clearance, authorship demands etc). The latter is carried out by the academics when they review others manuscripts and includes delaying review decisions, reciprocal reviewing etc.
From tables above, it seems that most of the reported misconduct can be easily prevented if specific and accurate guidelines or code of conduct are present in each research laboratory (see below). This aspect, for example is of minor impact in the clinical research, where the study protocol is rigorously detailed in advance, the specific analysis that will be included in the final report specified in advance with clear primary or secondary endpoints, and all the analysis/reports need to be stored for the final study revision/conclusion. All these different steps are regulated by Good Clinical Practice guidelines (GCP; National Institute for Health Research Clinical Research Network (NIHR CRN- www4 n.d. ).
This by no means indicates that in clinical research fraud does not exist, but that it is easier to discover it than in laboratory based-investigations. The paper of Verhagen et al. ( 2003 ) clearly refers to a specific situation that commonly happens in a research laboratory. The majority of experiments within biomedical research are conducted on tissues or cells. Therefore, the experimental set-ups, including negative and positive controls can easily (and frequently) be manipulated. This can only be prevented by using Standard Operating Procedures (SOP) and well written and clear regulation such as Good Laboratory Practice (GLP; Directive 2004 /9/EC), and written protocols. However, at present, no such regulations exist apart from in industry-based research, where GLP is mandatory. In a survey-based systematic review, Fanelli ( 2009 ) reported that approximately 2% of scientists claimed they had fabricated their data, at some point in their research career. It is worth noting, Fanelli’s study (as well as ours) only reported data from those who were willing admit engaging in these activities. This cast questions on actual number of occurrences, as many of them would not have reported misconduct. Other authors have highlighted the same issue and cast doubt on the reproducibility of scientific data (Resnik and Shamoo, 2017 ; Brall et al, 2017 ; Shamoo 2016 ; Collins and Tabak 2014 ; Kornfeld and Titus 2016 ).
The interview responses
We also wanted to understand the causes of these QRPs to obtain a clear picture of these misconduct. Based on interview responses, we have tried to give a narrative but critical description of individual perceptions, and their rationalisation in relation to previously published information.
Methodological malpractices
The data reported herein show that PhD scholars/post-doctoral fellows are mostly involved in laboratory-linked methodological misconduct. Many of them (especially the post-doctoral scientists) blamed supervisory/institutional pressures on not only enhancing publishing record, but also maintaining high impact. One post-doctoral scientist claimed “ there is always a constant pressure on publication; my supervisor said the reason you are not producing any meaningful data is because you are a perfectionist ”. He further recalled his supervisor once saying “ if the data is 80% correct, you should accept it as valid and stop repeating until you are satisfied ”.
Likewise, another researcher who recently returned from the US said “ I was an excellent researcher here (home country), but when I went to America, they demanded at least one paper every six months ”. “ When I was unable to deliver this (and missed a year without publishing any papers), my supervisor stopped meeting me, I was not invited for any laboratory meetings, presentations, and proposal discussions; in fact, they made me quit the job ”. A PhD student recalled his supervisor jokingly hinting “ if you want a perfect negative control, use water it will not produce any results ”. Comments and demands like these must have played a big role in encouraging laboratory based misconduct. In particular, the pressure to publish more papers in a limited period led to misconduct such as data manipulation (removing outliers, duplicate replications, etc.) or changing the aim of the study, and as a consequence including data set that were not previously considered, because the results are not in line with the original aim of the study. All these aspects force the young researchers to adopt an attitude that leads them to obtain publishable results by any means (ethical or not) – A “ Machiavellian personality trait ” as put by Tijdink et al. ( 2016 ). Indeed, an immoral message is being delivered to these young researchers (future scientists), enhancing cheating behaviours. In fact, Buljan et al. ( 2018 ) have recently highlighted the research environment, in which a scientist is working, as one of the potential causes of misconduct.
Behaviours that contravene publishing ethics
Academics (and PIs) have mostly identified misconduct linked to contravening publishing ethics. This finding itself shows that most of the academics who took part in this study has less “presence” within their laboratories. When confronted with the data obtained from PhD scholars and technicians, some of them vehemently denied these claims. Others came up with a variety of excuses. One lecturer/researcher said, “ I have got far too much teaching to be in my laboratory ”. Another professor said, “ I have post-docs within my laboratory, they will look after the rest; to be honest, my research skills are too old to refresh!” One PI replied, “ why should I check them? No one checked me when I was doing research ”. All these replies show a lack of care for research malpractices. It is true that academics are under pressure to deliver high impact research, carry out consultancy work, get involved with internationalisation within academia and teach (Edwards and Roy 2017 ). However, these pressures should not undermine research ethics.
One researcher claimed to have noticed at least two different versions of “ convenient ethical clearance ”. According to him, some researchers, especially those using human tissues, avoid specifying their research aims; and instead write an application in such a way that they can use these samples for a variety of different projects (bearing in mind of possible future developments). For example, if they aim to use the tissue to study a particular protein, the ethical application would mention all the related proteins and linked pathways. They justify this by claiming the tissues are precious, therefore they are “ maximising the effective use of available material ”. Whilst understanding the rationale within their argument, the academic who witnessed this practice asked a question “ how ethical it is to supply misleading information in an ethical application ?” He also highlighted issues with backdating ethical approval in one institution. That is, the ethical approval was obtained (or in his words “ staged ”) after the study has been completed. Although this is one incident reported by one whistle-blower, it highlights the institutional malpractices.
Selective use of data is another category reported here and elsewhere (Priya Satalkar & David Shaw, 2019 ; Blatt 2013 ; Bornmann 2013 ). One academic reported incidences of researchers purposely avoiding data to maximise the statistical significance. If this is the case, then the validity of reported work, its statistical significance, and in some cases its clinical usage are in question. What is interesting is that, as elegantly reported by Fanelli ( 2010 ), in the highest percentage of published papers, the findings always report the data that are in line with the original hypothesis. In fact, the number of papers published reporting negative results are very limited.
Misconduct relating to authorships have been highlighted in many previous studies (Ploug 2018 ; Vera-Badillo et al. 2016 ; Ng Chirk Jenn 2006 ). The British Medical Journal (BMJ – www5 n.d. ) has classified two main types of misconduct relating to authorships; (a) omission of a collaborator who has contributed significantly to the project and (b) inclusion of an author who has not (or minimally) contributed. Interestingly in this study, one academic claimed that he was under pressure to include the research co-ordinator of his department as an author in every publication.
He recalled the first instance when he was pressurised to include the co-ordinator, “ It was my first paper as a PI but due to my institutional policy, all potential publications needed to be scrutinised by the co-ordinator for their worthiness of publication ”, “ so when I submitted for internal scrutiny, I was called by the co-ordinator who simply said there is nothing wrong with this study, but one important name is missing in authors’ list ” (indirectly requesting her name to be included). Likewise, another PI said, “ it is an unwritten institutional policy to include at least one professor on every publication ”. Yet another PI claimed, “ this is common in my laboratory – all post-doctoral scientists would have a chance to be an author ” “ by this way we would build their research esteem ”. His justification for this was “ many post-doctoral scientists spend a substantial amount of time mentoring other scientists and PhD students, therefore they deserve honorary authorships ”. Similar malpractices have also been highlighted by other authors (Vera-Badillo et al. 2016 ; Gøtzsche et al. 2007 ) but the worrying finding is that in many cases, the practice is institutionalised. With regards to authorships, according to the International Committee of Medical Journal Editors (ICMJE – www6 n.d. ), authorships can only be given to those with (a) a substantial contribution (at least to a significant part of the investigation), (b) involvement in manuscript preparation including contribution to critical review. However, our discussions have revealed complementary authorships, authorship denial, etc.
Malpractices in peer-review process
The final QRP highlighted by our interviewees relates to the vreviewing process. One academic openly admitted, “ I and Dr X usually use each other as reviewers because we both understand our research”, he further added, “the blind reviewing is the thing of the past, every author has his own writing style, and if you are in one particular research field, with time, you would be able to predict the origin of the manuscript you are reviewing (whether it is your friend or a person with a conflicting research interest!”. Another academic said that “ the era of blind reviewing is long gone, authors are intentionally or unintentionally identifying themselves within the manuscripts with sentences such as ‘previously we have shown’. “This allows the reviewer to identify the authors from the reference list ”. He further claimed he also experienced reviewers intentionally delaying acceptance or asking for further experiments to be carried out, simply because they wanted their manuscript (on a related topic) to be published first! Incidences like this, though minimal, cast questions on the reviewing process itself.
Recent reports by Thomas ( 2018 ) and Preston ( 2017 ) (see also Adler and Stayer 2017 ) have highlighted issues (or scams) such as an author reviewing his own manuscripts! Of course, many journals do not use the suggested reviewers; instead, they build a database of reviewers and randomly select appropriate reviewers. Still, it is not clear how robust this approach is in curtailing reviewer-based misconduct. Organisations such as Retraction Watch constantly pick up and report these malpractices, yet there are no definite sanctions or punishment for the culprits (Zimmerman 2017 )
One of the academic interviewees recalled an incidence in which an author has been dismissed due to a serious image manipulation scam, yet obtained a research tenure in another institution within 3 months of dismissal. Galbraith ( 2017 ) reviewed summaries of 284 integrity-related cases published by the Office of Research Integrity (ORI), and found that in around 47% of cases the researchers received moderate punishment and were often permitted to continue their research. This highlights the need for a globalised approach with clear sanction measures to tackle research misconduct. Although this is a small-scale study, it has highlighted that despite measures taken by research regulatory bodies, the problem of misconduct is still there. The main problem behind this is “the lack of care” underpinned by pressures for esteem.
Limitations
This is an exploratory study with minimal focus on standardisation, using details of qualitative approach and paradigm, or the impact of researcher characteristics and reflexivity. Therefore, the level of data presented herein is not suited for a full thematic analysis. Also, this is a small-scale study with a sample size of of 110 participants who are further divided into sub-groups (such as PhD students, technicians and PIs). This limits the scope of analysing variability in the responses of individual sub-groups, and therefore might have resulted in voluntary response bias (i.e. responses are influenced by individual perceptions against research misconduct). Yet, the study has highlighted the issue of research misconduct is worth pursuing using a large sample. It also highlighted the common QRPs (both laboratory and publication related) that need to be focussed further, enabling us to establish a right research design for future studies.
The way forward
This exploratory study (and previously reported large scale studies) showed QRP is still a problem in science and medical research. So what are the way forward to stop these types of misconduct? Whilst it is important to set up confirmed criteria for individual research conduct, it is also important to set up institutional policies. These policies should aim at promoting academic/research integrity, with paramount attention on the training of young researchers about research integrity. The focus should be on young researchers attaining rigorous learning/application of the best methodological and professional standards in their research. In fact, the Singapore statement on research integrity (www7 n.d. ), not only highlights the importance of individual researchers maintaining integrity in their research, but also insists the roles of institutions creating/sustaining research integrity via educational programmes with continuous monitoring (Cosentino and Picozzi 2013 ). Considering the findings from this study, it would also be appropriate to suggest an international regulatory body to regularly monitor these practices involving all stakeholders including governments.
In fact, this (and other studies) have highlighted the importance of re-validating the “voluntary commitment” to follow the research integrity. With respect to individual researchers, we propose using a unified approach for early career researchers (ECRs). They should be educated about the importance of ethics/ethical behaviours (see Table 3 ) for our suggestion for ECRs). We feel it is vital to provide compulsory ethical training throughout their career (not just at the beginning). It is also advisable to regularly carry out “peer review” visits/processes between laboratories for ethical and health/safety aspects. Most importantly, it is time for the research community to move away from the expectation of “self-governance” establish and international research governance guidelines that can monitored by individual countries.
We, do agree this is a small-scale pilot study and due to the way it was conducted, we are unable to carry out a full thematic analysis. This was mainly because the participants were extremely reluctant to offer information to formulate researcher characteristics. Also, the study data in many cases conforms to the previously reported fact, that QRP and research misconduct is still a problem within science and medicine. Yet, this study has attempted to narrate the previously unreported justifications given by the interviewees. In addition, we were able to highlight that these activities are becoming regular occurrence (those nick-named behaviours). We also provided some directives on how academic pressures are inflicted upon early career researchers. We also provided some recommendations in regard to the training ECRs.
Significance
The study has highlighted the negative influence on supervisory/peer pressures and/or inappropriate training may be main causes for these misconducts, highlighting the importance on devising and implementing a universal research code of conduct. Although this was an exploratory investigation, the data presented herein have pointed out that unethical practices can still be widespread within biomedical field. It highlighted the fact that despite the proactive/reflective measures taken by the research governance organisations, these practices are still going on in different countries within Europe. As the study being explorative, we had the flexibility to adapt and evolve our questions in reflection to the responses. This would help us to carry out a detailed systematic research in this topic involving international audience/researchers.
Concluding remarks
To summarise, this small-scale interview-based narrative study has highlighted that QRP and research misconduct is still a problem within science and medicine. Although they may be influenced by institutional and career-related pressures, these practices seriously undermine ethical standards, and question the validity of data that are being reported. The findings also suggest that both methodological and publication-related malpractices continue, despite being widely reported. The measures taken by journal editors and other regulatory bodies such as WAME and ICMJE may not be efficient to curtail these practices. Therefore, it would be important to take steps in providing a universal research code of conduct. Without a globalised approach with clear punitive measures for offenders, research misconduct and QRP not only affect reliability, reproducibility, and integrity of research, but also hinder the public trustworthiness for medical research. This study has also highlighted the importance of carrying out large-scale studies to obtain a clear picture about misconduct undermining research ethics culture.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article
Adler AC, Stayer SA (2017) Bias Among Peer Reviewers. JAMA. 318(8):755. https://doi.org/10.1001/jama.2017.9186
Article Google Scholar
Altman, LK (2006). For science gatekeepers, a credibility gap. The New York Times. Retrieved from http://www.nytimes.com/2006/05/02/health/02docs.html?pagewanted=all . Accessed 26 July 2019
Bero L (2017) Addressing Bias and Conflict of Interest Among Biomedical Researchers. JAMA 317(17):1723–1724. https://doi.org/10.1001/jama.2017.3854
Bik EM, Fang FC, Kullas AL, Davis RJ, Casadevall A (2018) Analysis and Correction of Inappropriate Image Duplication: the. Mol Cell Biol Exp. https://doi.org/10.1128/MCB.00309-18
Blatt M (2013) Manipulation and Misconduct in the Handling of Image Data. Plant Physiol 163(1):3–4. https://doi.org/10.1104/pp.113.900471
Bornmann L (2013) Research Misconduct—Definitions, Manifestations and Extent. Publications. 1:87–98. https://doi.org/10.3390/publications1030087
Bouter LM, Hendrix S (2017) Both whistle-blowers and the scientists they accuse are vulnerable and deserve protection. Account Res 24(6):359–366. https://doi.org/10.1080/08989621.2017.1327814
Brainard J (2018) Rethinking retractions. Science. 362(6413):390–393. https://doi.org/10.1126/science.362.6413.390
Brall C, Maeckelberghe E, Porz R, Makhoul J, Schröder-Bäck P (2017) Research Ethics 2.0: New Perspectives on norms, values, and integrity in genomic research in times of even ccarcer resources. Public Health Genomics 20:27–35. https://doi.org/10.1159/000462960
Buljan I, Barać L, Marušić A (2018) How researchers perceive research misconduct in biomedicine and how they would prevent it: A qualitative study in a small scientific community. Account Res 25(4):220–238. https://doi.org/10.1080/08989621.2018.1463162
Collins FS and Tabak LA (2014) Policy: NIH plans to enhance reproducibility. NATURE (Comment) - https://www.nature.com/news/policy-nih-plans-to-enhance-reproducibility-1.14586
Cosentino M , and Picozzi M (2013) Transparency for each research article: Institutions must also be accountable for research integrity. BMJ 2013;347:f5477 doi: https://doi.org/10.1136/bmj.f5477 .
Directive 2004/9/EC of the European Parliament and of the Council of 11 February 2004 on the inspection and verification of good laboratory practice (GLP). https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:050:0028:0043:EN:PDF . Accessed 07 Sep 2019
Eaton SE, Chibry N, Toye MA, Toye MA, Rossi S (2019) Interinstitutional perspectives on contract cheating: a qualitative narrative exploration from Canada. Int J Educ Integr 15:9. https://doi.org/10.1007/s40979-019-0046-0
Edwards M, Roy (2017) Academic Research in the 21st Century: Maintaining Scientific Integrity in a Climate of Perverse Incentives and Hypercompetition. Environ Eng Sci 34(1):51–61. https://doi.org/10.1089/ees.2016.0223
Fanelli D (2009) How Many Scientists Fabricate and Falsify Research? A Systematic Review and Meta-Analysis of Survey Data. Plos One 4(5):e5738
Fanelli D (2010) Do Pressures to Publish Increase Scientists' Bias? An Empirical Support from US States Data. PLoS One 5(4):e10271. https://doi.org/10.1371/journal.pone.0010271
Fang FC, Steen RG, Casadevall A (2012) Misconduct accounts for the majority of retracted scientific publications. PNAS 109(42):17028–11703. https://doi.org/10.1073/pnas.1212247109
Ferguson C, Marcus A, Oransky I (2014) Publishing: Publishing: The peer-review scam. Nature (News review). 515(7528):480-2. http://www.nature.com/news/publishing-the-peer-review-scam-1.16400. Accessed 21 Nov 2019
Galbraith KL (2017) Life after research misconduct: Punishments and the pursuit of second chances. J Empir Res Hum Res Ethics 12(1):26–32. https://doi.org/10.1177/1556264616682568
Gøtzsche PC, Hróbjartsson A, Johansen HK, HaahrMT ADG, Chan A-W (2007) Ghost Authorship in Industry-Initiated Randomised Trial. Plos-Med. https://doi.org/10.1371/journal.pmed.0040019
Hevner AR (2007) A Three Cycle View of Design Science Research. Scand J Inf Syst 19(2):4 https://aisel.aisnet.org/sjis/vol19/iss2/4
Google Scholar
Jenn NC (2006) Common Ethical Issues In Research And Publication. Malays Fam Physician 1(2-3):74–76
John LK, Loewenstein G, Prelec D (2012) Measuring the prevalence of questionable research practices with incentives for truth telling. Psychol Sci. 23(5):524–532
Kornfeld DS, Titus SL (2016) (2016) Stop ignoring misconduct. Nature. 537(7618):29–30. https://doi.org/10.1038/537029a
Meadows, A. (2017). What does transparent peer review mean and why is it important? The Scholarly Kitchen, [blog of the Society for Scholarly Publishing.] [Google Scholar]
Ploug TJ (2018) Should all medical research be published? The moral responsibility of medical journal. Med Ethics 44:690–694
Preston A (2017) The future of peer review. Scie Am. Retrieved from https://blogs.scientificamerican.com/observations/the-future-of-peer-review/
Rawat S, Meena S (2014) Publish or perish: Where are we heading? J Res Med Sci. 19(2):87–89
Resnik DB, Shamoo AE (2017) Reproducibility and Research Integrity. Account Res. 24(2):116–123. https://doi.org/10.1080/08989621.2016.1257387
Satalka P, Shaw D (2019) How do researchers acquire and develop notions of research integrity? A qualitative study among biomedical researchers in Switzerland. BMC Med Ethics 20:72. https://doi.org/10.1186/s12910-019-0410-x
Shamoo AE (2016) Audit of research data. Account Res. 23(1):1–3. https://doi.org/10.1080/08989621.2015.1096727
Thomas SP (2018) Current controversies regarding peer review in scholarly journals. Issues Ment Health Nurs 39(2):99–101. https://doi.org/10.1080/01612840.2018.1431443.
Tijdink JK, Bouter LM, Veldkamp CL, van de Ven PM, Wicherts JM, Smulders YM (2016) Personality traits are associated with research misbehavior in Dutch scientists: A cross-sectional study. Plos One. https://doi.org/10.1371/journal.pone.0163251
Titus SL, Wells JA, Rhoades LJ (2008) Repairing research integrity. Nature 453:980–982
Vera-Badillo, Marc Napoleonea FE, Krzyzanowskaa MK, Alibhaib SMH, Chanc A-W, Ocanad A, Templetone AJ, Serugaf B, Amira E, Tannocka IF, (2016) Honorary and ghost authorship in reports of randomised clinical trials in oncology. Eur J Cancer (66)1 doi: https://doi.org/10.1016/j.ejca.2016.06.023
Verhagen H, Aruoma OI, van Delft JH, Dragsted LO, Ferguson LR, Knasmüller S, Pool-Zobel BL, Poulsen HE, Williamson G, Yannai S (2003) The 10 basic requirements for a scientific paper reporting antioxidant, antimutagenic or anticarcinogenic potential of test substances in in vitro experiments and animal studies in vivo. Food Chem Toxicol. 41(5):603–610
www1 n.d.: https://retractionwatch.com/the-center-for-scientific-integrity/ . Accessed 13 Nov 2019
www2 n.d.: http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html . Accessed 07 July 2019
www3 n.d.: https://bmjopen.bmj.com/content/bmjopen/8/12/e024499/DC1/embed/inline-supplementary-material-1.pdf?download=true . Accessed 26 July 2019
www4n.d.: http://www.crn.nihr.ac.uk/learning-development/ - National Institute for Health Research Clinical Research Network (NIHR CRN) - Accessed 13 Nov 2019
www5 n.d.: https://www.bmj.com/about-bmj/resources-authors/forms-policies-and-checklists/scientific-misconduct . Accessed 07 July 2019
www6 n.d.: http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html - Accessed 0 July 2019
www7 n.d.: http://www.singaporestatement.org . Accessed 10 Aug 2019
Zimmerman SV (2017), "The Canadian Experience: A Response to ‘Developing Standards for Research Practice: Some Issues for Consideration’ by James Parry", Finding Common Ground: Consensus in Research Ethics Across the Social Sciences (Advances in Research Ethics and Integrity, Vol. 1) Emerald Publishing Limited, pp. 103-109. https://doi.org/10.1108/S2398-601820170000001009
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference. We also sincerely thank Dr Carol Stalker, school of Psychology, University of Derby, for her critical advice on the statistical analysis.
Not applicable – the study was carried out as a collaborative effort amongst the authors.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, Derby, DE22 1GB, UK
S. D. Sivasubramaniam
Center of Research in Medical Pharmacology, University of Insubria, Via Ravasi, 2, 21100, Varese, VA, Italy
M. Cosentino & F. Marino
Faculty of Medicine, University of Porto, Porto, Portugal
You can also search for this author in PubMed Google Scholar
Contributions
Dr Sivasubramaniam has produced the questionnaire with interview format with the contribution of all other authors. He also has read the manuscript with the help of Prof Consentino. The latter also contributed for the initial literature survey and discussion. Drs Marino and Ribario have helped in the data collection and analysis. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to S. D. Sivasubramaniam .
Ethics declarations
Competing interests.
The authors can certify that they have NO affiliations with or involvement in any organization or entity with any financial or non-financial interests (including personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S.D., Cosentino, M., Ribeiro, L. et al. Unethical practices within medical research and publication – An exploratory study. Int J Educ Integr 17 , 7 (2021). https://doi.org/10.1007/s40979-021-00072-y
Download citation
Received : 17 July 2020
Accepted : 24 January 2021
Published : 01 April 2021
DOI : https://doi.org/10.1007/s40979-021-00072-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical research
- Research misconduct
- Committed bias
- Unethical practices
International Journal for Educational Integrity
ISSN: 1833-2595
- General enquiries: [email protected]
Unethical human research in the field of neuroscience: a historical review
Affiliations.
- 1 King Abdulaziz Medical City, King Saud bin Abdulaziz University for Health Sciences, P.O. Box: 12723, Jeddah, 21483, Saudi Arabia. [email protected].
- 2 King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
- 3 King Abdullah International Medical Research Center, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
- PMID: 29460160
- DOI: 10.1007/s10072-018-3245-1
Understanding the historical foundations of ethics in human research are key to illuminating future human research and clinical trials. This paper gives an overview of the most remarkable unethical human research and how past misconducts helped develop ethical guidelines on human experimentation such as The Nuremberg Code 1947 following WWII. Unethical research in the field of neuroscience also proved to be incredibly distressing. Participants were often left with life-long cognitive disabilities. This emphasizes the importance of implicating strict rules and ethical guidelines in neuroscience research that protect participants and respects their dignity. The experiments conducted by German Nazi in the concentration camps during WWII are probably the most inhumane and brutal ever conducted. The Nuremberg Code of 1947, one of the few positive outcomes of the Nazi experiments, is often considered the first document to set out ethical regulations of human research. It consists of numerous necessary criteria, to highlight a few, the subject must give informed consent, there must be a concrete scientific basis for the experiment, and the experiment should yield positive results that cannot be obtained in any other way. In the end, we must remember, the interest of the patient must always prevail over the interest of science or society.
Keywords: Ethics; History; Neuroscience; Unethical research.
Publication types
- Historical Article
- History, 20th Century
- Human Experimentation / ethics
- Human Experimentation / history*
- Neurosciences / ethics
- Neurosciences / history*

New Details Exposed In Unethical Liver Study At San Diego VA

For the second time, a federal watchdog agency found that the Department of Veterans Affairs’ investigation into unethical liver research performed on San Diego veterans was “not reasonable.”
The U.S. Office of Special Counsel published new reports on Tuesday revealing more details about the mistakes and violations that occurred during the research and its dissatisfaction with the VA’s investigation into what happened. inewsource broke the story about the unethical study in 2018 as the first article in its Risky Research series .
The study at the San Diego VA was part of a $6 million international project to find new therapies for people with alcoholic hepatitis. Researchers around the world were supposed to collect these patients’ leftover liver tissue after they received biopsies and look for patterns.
But that’s not what happened in San Diego.
In 2016, two whistleblowers filed complaints with the special counsel’s office, claiming researchers at the La Jolla hospital had persuaded veterans to undergo medically unnecessary biopsies and then used the samples for research purposes without telling the patients. The whistleblowers, who were both San Diego VA employees at the time, said it put seriously ill subjects at an unnecessary risk for bleeding and other complications.
VA investigators from Washington, D.C., flew to San Diego in 2017 to look into the concerns, and found violations of policies and procedures. But the special counsel’s office declared the VA’s work “unreasonable” for failing to address most of the whistleblowers' claims.
New documents reveal what the VA team found when it revisited the La Jolla hospital in 2019 and re-interviewed employees involved in the research. This time, much more serious wrongdoing was uncovered.
Reconstructing the research
The second investigation focused on the role of Dr. Samuel Ho, the San Diego VA’s former chief of gastroenterology who led the research when it began in 2013.
Investigators found that Ho instructed technicians to remove extra pieces of tissue from sick veterans during their liver biopsies for his research — without getting the patients’ consent — which increased the chance of complications. Investigators called this “noncompliance with federal regulation and VA policy.”
The technicians performing the biopsies never received a copy of the research plan, so they had no way of verifying that Ho was following protocol, according to the reports. They followed Ho’s direction because they were “susceptible to the influence from a senior member” of the San Diego VA staff.
The reports showcase a long list of other problems that occurred during the study, too.
Researchers used the wrong assessment forms to determine if someone was too cognitively impaired to agree to participate. And one patient had a liver sample taken the day before he gave consent to be in the study.
Plus, the study staff kept such poor records that inspectors were forced to reconstruct a timeline and a list of participants through interviews and partial documents. They found at least 22 subjects with alcoholic hepatitis had enrolled. At least 13 of them received biopsies and at least eight had their tissue samples sent to a repository on the East Coast to be used by other researchers.
RELATED: inewsource Risky Research series
The San Diego VA decided that the patients who received biopsies — and were severely ill during the research — should be tracked down and told about the ethics violations. By the time the letters were mailed two months later, only four of them were still alive.
There were at least 37 other subjects who did not have hepatitis and whose samples were supposed to serve as a comparison, but the details of what occurred in many of these cases were missing from research records.
When the special counsel’s office asked the VA why it didn’t discover these issues during its initial investigation, they were told that Ho had “intentionally provided false information and was untruthful” about his work.
“They could have easily discovered this in the first investigation if they had wanted to,” Martina Buck, one of the whistleblowers, told inewsource this week. “If they had wanted to really question the validity of a lot of these people as they questioned the whistleblowers' validity.”
Buck used to be on the research review board at the San Diego VA and first raised concerns about the study before it was approved in 2013. She said she was fired after she later alleged that misconduct occurred.
In response to questions from inewsource , VA spokesperson Randy Noller said Thursday the department requires employees to honestly answer questions from investigators and failing to do so could lead to disciplinary action.
An ‘unreasonable’ finding
Even though the VA’s new investigation corroborated more of the whistleblowers’ claims than it had before, it left major gaps, questions and inconsistencies.
For one, the reports show the San Diego VA never terminated the study after finding major ethics and policy violations. It ended when the funding ran out in November 2018.
Also, the investigation didn’t address claims about patient harm. Medical records from the whistleblowers show at least one patient left the procedure “oozing with blood,” with “stool scattered” on their body and in need of an emergency transfusion. Another had their medication delayed so they could fit the enrollment criteria, according to the special counsel’s report.
What is the Office of Special Counsel?
This federal office “ is an independent investigative and prosecutorial federal agency that protects the merit system for over 2.1 million federal employees.” It reports directly to the president about wrongdoing in federal agencies.
Its responsibilities include:
- Providing federal workers a safe channel to disclose violations of law, rule, or regulation; gross mismanagement; a gross waste of funds; an abuse of authority; or a substantial and specific danger to public health or safety. The office does not have investigative authority in disclosure cases but plays a critical oversight role in agency investigations of alleged misconduct.
- Investigating allegations of whistleblower retaliation to determine whether an employee has been fired, demoted, suspended or subjected to another personnel action for blowing the whistle. If the office can demonstrate that a personnel action was retaliatory, it works with the agency to provide relief to the employee.
After reviewing the VA’s reports, the special counsel’s office this week wrote letters to President Joe Biden and Congress, stating that the department’s investigation didn’t meet its standards.
“I continue to be concerned about the quality of care provided to veterans at (the San Diego VA), especially those who participated in the research protocol,” Special Counsel Henry J. Kerner wrote.
“I encourage the VA to consider additional critical review of the actions of the (researchers) during the lifecycle of the study,” Kerner added.
The special counsel’s office has a long history of concerns about the VA. An inewsource analysis in 2019 found the watchdog agency has declared about 16% of investigations by the VA medical inspector’s office “unreasonable.”
In the case of the liver research, the special counsel’s major concern was the reason patients were asked to undergo biopsies at all.

The whistleblowers contend that patients were persuaded to get biopsies as a part of their medical care even when they didn’t need them so their samples could be collected for research.
To support their claim, the whistleblowers pointed out that the kind of biopsies performed in the study — which involve inserting a catheter in the neck — had never been conducted at the San Diego VA on patients with alcoholic liver disease before this study began.
They also documented seven cases where the lead researcher ordered these biopsies over the objections of his colleagues.
But after conducting an external review of all seven cases, the VA concluded the procedures were appropriate given the patients’ medical conditions.
In a statement Thursday , the VA stood by its conclusion that the biopsies were appropriate.
Noller, the VA spokesperson, said the department’s external reviews were thorough and performed by physicians outside the VA with expertise in liver disease. He provided six written comments the experts submitted during the review and said the American College of Gastroenterology Guidelines don’t support the whistleblowers' opinions.
VA’s response
Read the VA’s full statement to inewsource here .
However, the special counsel’s report to the president said the VA’s reasoning “remains unconvincing.”
“The whistleblowers continue to provide consistent, clear support for their contention that transjugular biopsies were unnecessary for many of the patients in this study,” Kerner said in a statement announcing the newest findings.
‘No science here’
Despite the myriad of problems that occurred, there are no records indicating anyone has been disciplined for their role in the liver research, no publications using the tissue samples have been retracted and the VA has not acknowledged to the whistleblowers that many of their allegations were ultimately proven true.
Ho left the VA and took a job at a university in Dubai in mid-2018, while the investigations were ongoing. The VA said it couldn’t take any action against him since he’s no longer a federal employee, and it couldn’t determine that any other VA employees were responsible for what occurred.
Ho didn’t respond to questions inewsource emailed him for this story.
In 2019, Ho told investigators he didn’t follow the study protocol because the bureaucratic steps involved would have interfered with the research, which “goes against the purpose of what the patient had signed up for and what the (researchers) and ultimately what I believe society is interested in.”
Ho was replaced by Dr. Bernd Schnabl , a staff physician and attending at the San Diego VA and the UCSD Medical Center. Schnabl was previously working alongside Ho as one of the study’s researchers.

Schnabl, along with UCSD Vice Chancellor for Health Sciences Dr. David Brenner , also served on the steering committee for the international liver project. Their research proposal sent to the NIH stated they would use stool samples collected from the hepatitis patients to examine gut bacteria.
The whistleblowers filed complaints with UCSD about the roles that Brenner and Schnabl played in the research. A UCSD spokesperson wouldn’t comment on the complaints, saying the university doesn’t discuss personnel matters or ongoing investigations.
In 2019, Schnabl was the lead author on an article in Digestive Diseases and Sciences that used samples collected from the San Diego VA during the liver research . Ho was also listed as an author on the paper and his contribution line says he was responsible for enrolling participants.
Ethics experts have told inewsource that publishing academic papers based on unethical research is dangerous and encourages researchers to perform more problematic studies in the future.
Buck, the whistleblower, said the number of problems that occurred during the study should render the samples invalid.
“There was no science here,” Buck said. “There was no research achieved that you can glean anything from. No one should be basing any research studies using this as a foundation.”

inewsource contacted the journal’s publisher, Springer Nature, in 2019 and was told its staff would look into the issue.
On Thursday, Springer Nature spokesperson Alice Henchley said the study relied on serum and stool samples that the hepatitis patients provided, not their liver samples. She added that the publisher takes ethics complaints seriously and would reopen its investigation in light of the special counsel’s latest reports.
“We will be looking at the new reports you have provided to us in detail, so are unable to comment further at this time,” Henchley said. “We are grateful to you for bringing them to our attention.”
The whistleblowers have exhausted almost all avenues for reporting concerns about the liver research. Buck’s fellow whistleblower, Dr. Mario Chojkier, is a gastroenterologist at UCSD and the VA. They are married.

The two of them have filed complaints with at least three federal agencies, were interviewed twice by VA investigators and sent their allegations to other research institutions involved in the study.
They watched a congressional committee hearing in 2019 where Rep. Scott Peters, a San Diego Democrat, asked VA leaders to address how they handled the whistleblowers’ claims. But the committee didn’t take any official actions.
As for the special counsel’s newest reports, spokespeople for the House Committee on Veterans Affairs didn’t comment this week on whether they were reviewing the latest findings.
Rep. Mike Levin, a Democrat whose district stretches from Del Mar to Dana Point, was announced as the vice chair of the veterans committee Thursday. A spokesperson said Levin is reviewing the special counsel’s work.
Buck said the special counsel didn’t do enough to address the harms that occurred or prevent similar incidents from happening in the future.
“Something needs to be done,” she said.


IMAGES
VIDEO
COMMENTS
The study found that when a group of subjects witnessed the seizure, few people stepped forward to help. Subjects who were in the room alone with the actor, however, were willing to step up much more quickly. Learned Helplessness Experiment. In 1965, Martin Seligman conducted an unethical experiment using dogs.
Multiple examples of unethical research studies conducted in the past throughout the world have cast a significant historical shadow on research involving human subjects. Examples include the Tuskegee Syphilis Study from 1932 to 1972, Nazi medical experimentation in the 1930s and 1940s, and research conducted at the Willowbrook State School in the 1950s and 1960s.[1]
Most people are aware of some of the heinous medical experiments of the past that violated human rights. Participation in these studies was either forced or coerced under false pretenses. Some of the most notorious examples include the experiments by the Nazis, the Tuskegee syphilis study, the Stanford Prison Experiment, and the CIA's LSD ...
At a Glance. Some of the most controversial and unethical experiments in psychology include Harlow's monkey experiments, Milgram's obedience experiments, Zimbardo's prison experiment, Watson's Little Albert experiment, and Seligman's learned helplessness experiment. These and other controversial experiments led to the formation of rules and ...
The Stanford Prison Experiment is frequently cited as an example of unethical research. It could not be replicated by researchers today because it fails to meet the standards established by numerous ethical codes, including the Code of Ethics of the American Psychological Association.
Examples of Unethical Research Practices. Here's a list of unethical practices every researcher must avoid. 1. Duplicate publication. It is unethical for a researcher to submit a research paper or publication that has two or more seminal journals which could be with or without acknowledgment of these other journals.
Revised on October 1, 2024. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective ...
What makes some human experiments unethical, and what should we do with the ones that have contributed to current medicine? ... Research on smallpox vaccine is one example. ... The study observed ...
ETHICAL ISSUES IN RESEARCH 1. Study design and ethics approval. According to COPE, "good research should be well adjusted, well-planned, appropriately designed, and ethically approved. ... For example, in a clinical trial, if a drug is found to be ineffective, this study should be reported. ... Some people say it is "unethical" to do this ...
A scientific misconduct example is deliberately creating names and details of survey participants for the purpose of generating data, which is an unethical research practice. Other common scientific misconduct examples include modifying or omitting data to influence study findings or withholding critical information from human participants in ...
This includes considering whether the question asked is answerable, whether the research methods are valid and feasible, and whether the study is designed with accepted principles, clear methods, and reliable practices. Invalid research is unethical because it is a waste of resources and exposes people to risk for no purpose. Fair subject selection
Zimbardo stopped the experiment at that point. Featured Programs. 9. The Monster Study (1939). The Monster Study is a prime example of an unethical psychology experiment on humans that changed the world. Wendell Johnson, a psychologist at the University of Iowa, conducted an experiment about stuttering on 22 orphans.
In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making.
Practical Ethics for Psychologists. $74.99. Handbook of Research Ethics in Psychological Science. $51.99. Essentials of Consensual Qualitative Research. $24.99. Research Ethics in Psychological Science. $24.95. Ethical Conflicts in Psychology, 5th Ed.
Understanding the historical foundations of ethics in human research are key to illuminating future human research and clinical trials. This paper gives an overview of the most remarkable unethical human research and how past misconducts helped develop ethical guidelines on human experimentation such as The Nuremberg Code 1947 following WWII. Unethical research in the field of neuroscience ...
The data produced by the scientific community impacts on academia, clinicians, and the general public; therefore, the scientific community and other regulatory bodies have been focussing on ethical codes of conduct. Despite the measures taken by several research councils, unethical research, publishing and/or reviewing behaviours still take place. This exploratory study considers some of the ...
This paper gives an overview of the most remarkable unethical human research and how past misconducts helped develop ethical guidelines on human experimentation such as The Nuremberg Code 1947 following WWII. Unethical research in the field of neuroscience also proved to be incredibly distressing. Participants were often left with life-long ...
Similarly, the global pursuit of positive research findings by multiple investigators on a single subject also decreases the likelihood of true research findings due to a phenomenon called multiple hypothesis testing. From this framework, Ioannidis draws 6 conclusions: The smaller the sample size of a study, the lower its PPV.
A study carried out with former participants of a research, about their perspectives on the ethics in that very research, suggests that a good ethical practice is one that considers the impact of the research upon the participants, in a reflexive approach, rather than just following bureaucratic or prescriptive procedures.
The San Diego VA Medical Center in La Jolla is shown on Sept. 26, 2019. For the second time, a federal watchdog agency found that the Department of Veterans Affairs' investigation into unethical ...
Secondly, several studies are what we might call implication studies, i.e. using research scandals to study their effects or implications for other groups, individuals or for the research community. These studies address issues like how research misconduct spills over to uninvolved prior collaborators (Hussinger and Pellens Citation 2019), or ...