- The Open University
- Guest user / Sign out
- Study with The Open University

My OpenLearn Profile
Personalise your OpenLearn profile, save your favourite content and get recognition for your learning

Addressing ethical issues in your research proposal
This article explores the ethical issues that may arise in your proposed study during your doctoral research degree.
What ethical principles apply when planning and conducting research?
Research ethics are the moral principles that govern how researchers conduct their studies (Wellcome Trust, 2014). As there are elements of uncertainty and risk involved in any study, every researcher has to consider how they can uphold these ethical principles and conduct the research in a way that protects the interests and welfare of participants and other stakeholders (such as organisations).
You will need to consider the ethical issues that might arise in your proposed study. Consideration of the fundamental ethical principles that underpin all research will help you to identify the key issues and how these could be addressed. As you are probably a practitioner who wants to undertake research within your workplace, consider how your role as an ‘insider’ influences how you will conduct your study. Think about the ethical issues that might arise when you become an insider researcher (for example, relating to trust, confidentiality and anonymity).
What key ethical principles do you think will be important when planning or conducting your research, particularly as an insider? Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination.
Key ethical issues that you will address as an insider researcher include:
- Gaining trust
- Avoiding coercion when recruiting colleagues or other participants (such as students or service users)
- Practical challenges relating to ensuring the confidentiality and anonymity of organisations and staff or other participants.
(Heslop et al, 2018)
A fuller discussion of ethical principles is available from the British Psychological Society’s Code of Human Research Ethics (BPS, 2021).
You can also refer to guidance from the British Educational Research Association and the British Association for Applied Linguistics .

Ethical principles are essential for protecting the interests of research participants, including maximising the benefits and minimising any risks associated with taking part in a study. These principles describe ethical conduct which reflects the integrity of the researcher, promotes the wellbeing of participants and ensures high-quality research is conducted (Health Research Authority, 2022).
Research ethics is therefore not simply about gaining ethical approval for your study to be conducted. Research ethics relates to your moral conduct as a doctoral researcher and will apply throughout your study from design to dissemination (British Psychological Society, 2021). When you apply to undertake a doctorate, you will need to clearly indicate in your proposal that you understand these ethical principles and are committed to upholding them.
Where can I find ethical guidance and resources?
Professional bodies, learned societies, health and social care authorities, academic publications, Research Ethics Committees and research organisations provide a range of ethical guidance and resources. International codes such as the Universal Declaration of Human Rights underpin ethical frameworks (United Nations, 1948).
You may be aware of key legislation in your own country or the country where you plan to undertake the research, including laws relating to consent, data protection and decision-making capacity, for example, the Data Protection Act, 2018 (UK). If you want to find out more about becoming an ethical researcher, check out this Open University short course: Becoming an ethical researcher: Introduction and guidance: What is a badged course? - OpenLearn - Open University
You should be able to justify the research decisions you make. Utilising these resources will guide your ethical judgements when writing your proposal and ultimately when designing and conducting your research study. The Ethical Guidelines for Educational Research (British Educational Research Association, 2018) identifies the key responsibilities you will have when you conduct your research, including the range of stakeholders that you will have responsibilities to, as follows:
- to your participants (e.g. to appropriately inform them, facilitate their participation and support them)
- clients, stakeholders and sponsors
- the community of educational or health and social care researchers
- for publication and dissemination
- your wellbeing and development
The National Institute for Health and Care Research (no date) has emphasised the need to promote equality, diversity and inclusion when undertaking research, particularly to address long-standing social and health inequalities. Research should be informed by the diversity of people’s experiences and insights, so that it will lead to the development of practice that addresses genuine need. A commitment to equality, diversity and inclusion aims to eradicate prejudice and discrimination on the basis of an individual or group of individuals' protected characteristics such as sex (gender), disability, race, sexual orientation, in line with the Equality Act 2010.
The NIHR has produced guidance for enhancing the inclusion of ‘under-served groups’ when designing a research study (2020). Although the guidance refers to clinical research it is relevant to research more broadly.
You should consider how you will promote equality and diversity in your planned study, including through aspects such as your research topic or question, the methodology you will use, the participants you plan to recruit and how you will analyse and interpret your data.
What ethical issues do I need to consider when writing my research proposal?

You might be planning to undertake research in a health, social care, educational or other setting, including observations and interviews. The following prompts should help you to identify key ethical issues that you need to bear in mind when undertaking research in such settings.
1. Imagine you are a potential participant. Think about the questions and concerns that you might have:
- How would you feel if a researcher sat in your space and took notes, completed a checklist, or made an audio or film recording?
- What harm might a researcher cause by observing or interviewing you and others?
- What would you want to know about the researcher and ask them about the study before giving consent?
- When imagining you are the participant, how could the researcher make you feel more comfortable to be observed or interviewed?
2. Having considered the perspective of your potential participant, how would you take account of concerns such as privacy, consent, wellbeing and power in your research proposal?
[Adapted from OpenLearn course: Becoming an ethical researcher, Week 2 Activity 3: Becoming an ethical researcher - OpenLearn - Open University ]
The ethical issues to be considered will vary depending on your organisational context/role, the types of participants you plan to recruit (for example, children, adults with mental health problems), the research methods you will use, and the types of data you will collect. You will need to decide how to recruit your participants so you do not inappropriately exclude anyone. Consider what methods may be necessary to facilitate their voice and how you can obtain their consent to taking part or ensure that consent is obtained from someone else as necessary, for example, a parent in the case of a child.
You should also think about how to avoid imposing an unnecessary burden or costs on your participants. For example, by minimising the length of time they will have to commit to the study and by providing travel or other expenses. Identify the measures that you will take to store your participants’ data safely and maintain their confidentiality and anonymity when you report your findings. You could do this by storing interview and video recordings in a secure server and anonymising their names and those of their organisations using pseudonyms.
Professional codes such as the Code of Human Research Ethics (BPS, 2021) provide guidance on undertaking research with children. Being an ‘insider’ researching within your own organisation has advantages. However, you should also consider how this might impact on your research, such as power dynamics, consent, potential bias and any conflict of interest between your professional and researcher roles (Sapiro and Matthews, 2020).
How have other researchers addressed any ethical challenges?
The literature provides researchers’ accounts explaining how they addressed ethical challenges when undertaking studies. For example, Turcotte-Tremblay and McSween-Cadieux (2018) discuss strategies for protecting participants’ confidentiality when disseminating findings locally, such as undertaking fieldwork in multiple sites and providing findings in a generalised form. In addition, professional guidance includes case studies illustrating how ethical issues can be addressed, including when researching online forums (British Sociological Association, no date).
Watch the videos below and consider what insights the postgraduate researcher and supervisor provide regarding issues such as being an ‘insider researcher’, power relations, avoiding intrusion, maintaining participant anonymity and complying with research ethics and professional standards. How might their experiences inform the design and conduct of your own study?
Postgraduate researcher and supervisor talk about ethical considerations
Your thoughtful consideration of the ethical issues that might arise and how you would address these should enable you to propose an ethically informed study and conduct it in a responsible, fair and sensitive manner.
British Educational Research Association (2018) Ethical Guidelines for Educational Research. Available at: https://www.bera.ac.uk/publication/ethical-guidelines-for-educational-research-2018 (Accessed: 9 June 2023).
British Psychological Society (2021) Code of Human Research Ethics . Available at: https://cms.bps.org.uk/sites/default/files/2022-06/BPS%20Code%20of%20Human%20Research%20Ethics%20%281%29.pdf (Accessed: 9 June 2023).
British Sociological Association (2016) Researching online forums . Available at: https://www.britsoc.co.uk/media/24834/j000208_researching_online_forums_-cs1-_v3.pdf (Accessed: 9 June 2023).
Health Research Authority (2022) UK Policy Framework for Health and Social Care Research . Available at: https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/uk-policy-framework-health-social-care-research/uk-policy-framework-health-and-social-care-research/#chiefinvestigators (Accessed: 9 June 2023).
Heslop, C., Burns, S., Lobo, R. (2018) ‘Managing qualitative research as insider-research in small rural communities’, Rural and Remote Health , 18: pp. 4576.
Equality Act 2010, c. 15. Available at: https://www.legislation.gov.uk/ukpga/2010/15/introduction (Accessed: 9 June 2023).
National Institute for Health and Care Research (no date) Equality, Diversity and Inclusion (EDI) . Available at: https://arc-kss.nihr.ac.uk/public-and-community-involvement/pcie-guide/how-to-do-pcie/equality-diversity-and-inclusion-edi (Accessed: 9 June 2023).
National Institute for Health and Care Research (2020) Improving inclusion of under-served groups in clinical research: Guidance from INCLUDE project. Available at: https://www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 (Accessed: 9 June 2023).
Sapiro, B. and Matthews, E. (2020) ‘Both Insider and Outsider. On Conducting Social Work Research in Mental Health Settings’, Advances in Social Work , 20(3). Available at: https://doi.org/10.18060/23926
Turcotte-Tremblay, A. and McSween-Cadieux, E. (2018) ‘A reflection on the challenge of protecting confidentiality of participants when disseminating research results locally’, BMC Medical Ethics, 19(supplement 1), no. 45. Available at: https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-018-0279-0
United Nations General Assembly (1948) The Universal Declaration of Human Rights . Resolution A/RES/217/A. Available at: https://www.un.org/en/about-us/universal-declaration-of-human-rights#:~:text=Drafted%20by%20representatives%20with%20different,all%20peoples%20and%20all%20nations . (Accessed: 9 June 2023).
Wellcome Trust (2014) Ensuring your research is ethical: A guide for Extended Project Qualification students . Available at: https://wellcome.org/sites/default/files/wtp057673_0.pdf (Accessed: 9 June 2023).
More articles from the research proposal collection

Writing your research proposal
A doctoral research degree is the highest academic qualification that a student can achieve. The guidance provided in these articles will help you apply for one of the two main types of research degree offered by The Open University.
Level: 1 Introductory
Defining your research methodology
Your research methodology is the approach you will take to guide your research process and explain why you use particular methods. This article explains more.

Writing your proposal and preparing for your interview
The final article looks at writing your research proposal - from the introduction through to citations and referencing - as well as preparing for your interview.
Free courses on postgraduate study

Are you ready for postgraduate study?
This free course, Are you ready for postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and ensure you are ready to develop the skills and confidence to pursue your learning further.

Succeeding in postgraduate study
This free course, Succeeding in postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and to develop the skills and confidence to pursue your learning further.

Applying to study for a PhD in psychology
This free OpenLearn course is for psychology students and graduates who are interested in PhD study at some future point. Even if you have met PhD students and heard about their projects, it is likely that you have only a vague idea of what PhD study entails. This course is intended to give you more information.
Become an OU student
Ratings & comments, share this free course, copyright information, publication details.
- Originally published: Tuesday, 27 June 2023
- Body text - Creative Commons BY-NC-SA 4.0 : The Open University
- Image 'Pebbles balance on a stone see-saw' - Copyright: Photo 51106733 / Balance © Anatoli Styf | Dreamstime.com
- Image 'Camera equipment set up filming a man talking' - Copyright: Photo 42631221 © Gabriel Robledo | Dreamstime.com
- Image 'Applying to study for a PhD in psychology' - Copyright free
- Image 'Succeeding in postgraduate study' - Copyright: © Everste/Getty Images
- Image 'Addressing ethical issues in your research proposal' - Copyright: Photo 50384175 / Children Playing © Lenutaidi | Dreamstime.com
- Image 'Writing your proposal and preparing for your interview' - Copyright: Photo 133038259 / Black Student © Fizkes | Dreamstime.com
- Image 'Defining your research methodology' - Copyright free
- Image 'Writing your research proposal' - Copyright free
- Image 'Are you ready for postgraduate study?' - Copyright free
Rate and Review
Rate this article, review this article.
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews
For further information, take a look at our frequently asked questions which may give you the support you need.
Research Design Review
A discussion of qualitative & quantitative research design, writing ethics into your qualitative proposal.
Every research proposal for studying human beings must carefully consider the ethical ramifications of engaging individuals for research purposes, and this is particularly true in the relatively intimate, in-depth nature of qualitative research. It is incumbent on qualitative researchers to honestly assure research participants their confidentiality and right to privacy, safety from harm, and right to terminate their voluntary participation at any time with no untoward repercussions from doing so. The proposal should describe the procedures that will be taken to implement these assurances, including gaining informed consent, gaining approval from the relevant Institutional Review Board, and anonymizing participants’ names, places mentioned, and other potentially identifying information.
Special consideration should be given in the proposal to ethical matters when the proposed research (a) pertains to vulnerable populations such as children or the elderly; (b) concerns a marginalized segment of the population such as people with disabilities, same-sex couples, or the economically disadvantaged; (c) involves covert observation that will be conducted in association with an ethnographic study; or (d) is a narrative study in which the researcher may withhold the full true intent of the research in order not to stifle or bias participants’ telling of their stories.
Furthermore, the researcher should pay particular attention to ethical considerations when writing a proposal for a focus group study. The focus group method (regardless of mode) brings together (typically) a number of strangers who are often asked to offer their candid thoughts on personal and sensitive topics. For this reason (and other reasons, e.g., the moderator may be sharing confidential information with the participants), it is important to gain a signed consent form from all participants; however, the reality is that there is no way the researcher can totally guarantee confidentiality. These and other associated ethical considerations should be discussed in the Design section of the focus group proposal.
Share this:
- Click to share on Reddit (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
- Pingback: The TQF Qualitative Research Proposal: Limitations | Research Design Review
- Pingback: The TQF Qualitative Research Proposal: The Research Team | Research Design Review
- Pingback: The Total Quality Framework Proposal: Design Section — Credibility | Research Design Review
Great article! Ethical considerations become even more significant as we incorporate more and more technology.
- Pingback: Writing Ethics Into Your Qualitative Proposal | Managementpublic
Leave a comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
The Research Whisperer
Just like the thesis whisperer – but with more money, how to write a successful ethics application.

She has a particular interest in tuberculosis, viral hepatitis, adolescent health, and the health of people in criminal justice settings.
Kat advises colleagues from diverse backgrounds on research ethics, study design, and data analysis.
She tweets from @epi_punk .

The word “ethics” strikes fear into the hearts of most early career researchers.
Some of the reasons are beyond our control, but there’s actually a lot we can do to make our own experiences of the ethics approval process less painful.
I’m writing this from two perspectives: as an early career researcher (I finished my PhD in 2019), and as a committee member (I’ve sat on an ethics advisory group since the start of my PhD in 2014).
The job of ethics committees is to identify the possible risks in a project, and then assess whether the research team:
- are aware of the risks.
- are taking appropriate steps to minimise them.
- have a plan to handle anything that does go wrong.
To do this, ethics committees need information. If you want your ethics application to get through the process as quickly as possible, you need to give the committee enough detail so that they understand your project and how you are managing any risks.
Getting your application as right as possible the first time makes the whole process go more quickly. If you don’t provide enough information, the committee will come back with questions. You may need to resubmit your application to the next meeting, which could be a month or two away.
Spending more time on your application for the first meeting can save you months later on!
Here are the main questions ethics committees will ask themselves when they assess your project:
- Are there any risks to the researchers? (e.g. Injuries in the lab, safety risks travelling to study sites, exposure to distressing topics during interviews or data analysis.)
- Are there any risks to the study participants? (From the study procedures themselves; risks to their privacy; risks of distress if they are asked about or exposed to upsetting content)
- Are there any risks to third parties? (i.e. people who aren’t directly participating)
- Could anybody’s privacy be invaded by the data collection process?
- Are there other staff in a lab who might be hurt if there were an accident?
- Are the research team aware of these risks, are they taking steps to minimise them, and do they have a plan if things go wrong?
The only way for the ethics committee to assess this is from the information you put into your application. Carefully think through your project and ask yourself those questions. And then put all of the answers into your application.
Here’s an example:
I am planning a project at the moment that involves interviewing health care providers about vulnerable people that they work with.
What are the risks to me? There aren’t any physical safety risks – I’ll be sitting in my office on the phone.
What about psychological risks? Could I be distressed by the content of the interviews? It’s possible. Some of the people I’ll interview are working with clients who have experienced child abuse, and some of their stories about their work might be upsetting.
What am I doing about these risks? I’m conducting interviews on the phone, rather than travelling to other people’s workplaces or homes. I won’t ask specifically about any distressing topics (minimising the risk), although they might come up anyway. If I get upset about the content of the interviews, I will probably be okay: I’ve worked in this area for many years, and I have strategies for dealing with it when my work upsets me (taking a break, talking to a colleague on the same project later on to help me process my feelings about it).
All of this goes into my application! I don’t write “I will conduct interviews with providers” and then say there are no risks, or that I have managed the risks. I give the committee all the details about each of the foreseeable risks I’ve identified, and exactly what I’m doing about them.
What about the risks to my participants? They could also find the content of the interviews upsetting. Again, my interview tool doesn’t ask directly about any distressing topics (minimising the risk), but it may come up. What’s my plan if my participants get upset? I’ll offer to change the topic, take a break, or stop the interview entirely. I mention this risk in the consent form, and the form will tell participants that they will have these options if they feel distressed. I will repeat this to them verbally at the start of the interview, and remind them that they don’t need to discuss anything with me that they don’t want to. Again, all these details go into my application.
What about risks to other people? Some health care providers might tell me private or sensitive information about their clients, by giving me specific examples instead of talking in general terms. To avoid this, I will ask them at the start of the interview not to talk about specific individuals, but to rather keep their answers general. If a participant does start to talk about an individual, I’ll remind them that this isn’t appropriate. I’ll also erase that part of the recording later on, so that those information isn’t transcribed. Again, all these details go into my application so that the ethics committee can see that I’m aware of the risk and I have a plan to manage it if it occurs.
As a committee member, I see applications get into trouble for a few common reasons.
The first is a lack of information , giving a very brief description of what will be done, without enough detail for the committee to understand the risks and what is being done about them.
The second is inconsistency , when a researcher says one thing on their application form, and something else in their consent form. Check carefully for consistency across all your documents before you submit.
A third is when a researcher proposes to do something that directly goes against the national ethical standards for research (e.g. collecting data without consent when they could get consent, or storing sensitive data in an insecure manner). Do not do this.
Some general tips:
- Find out the deadlines for your committee now, and start your application well in advance. It’s very hard to do a good job at the last minute, especially if you need details from your supervisor or other people in the project.
- Ask a colleague for a previous successful application for a similar project. Take note of the risks they identified, and how they managed them. Look at their consent forms and other documents, and see what you can adapt and reuse.
- Use grant applications for the project as a source of information on background, aims, methods, and outcomes. The format and level of detail required by the ethics committee is often similar.
- Read your country’s ethical guidance for research projects: this is what the ethics committee is working off. Think about which issues apply to your project, and how you can meet each of the standards. Spell this out for the committee.
- Find out whether your institution has specific requirements regarding wording in consent forms, storage of data, handling chemicals in the lab, etc. In your application, tell the committee that you are aware of these requirements and say how your project will meet them. Make sure that your consent forms and other documents are consistent with your institution’s standards. If your institution offers templates, use them!
- Ethics committees also assess the technical soundness of the research because poor quality research wastes time and resources, and exposes people to risks that aren’t justified by adequate benefits. Most committees include statistician and methods experts specifically for this reason (I’m one of them). Give a detailed explanation of your methods, and make sure they are appropriate to your research question. Get advice from a methods expert or a statistician to check that your project is sound – it’s much better to identify problems at the planning stage, rather than after you’ve gotten approval and collected your data.
- If you are doing an application for the first time, get help from your supervisor or thesis advisor. They shouldn’t make you do the application on your own. The more help you can get before you submit, the more quickly your project will get approved.
Share this:
Also I suggest doing the ethics training offered by your institution, or professional body. Recently I attended ANU’s Human Ethics training session. While I occasionally teach ethics, and have been a Chief Investigator on a project, I still found it useful. https://services.anu.edu.au/training/aries-human-ethics-training-sessions
Another useful resource is The Research Ethics Application Database (TREAD), an online database of successful research ethics applications from around the world, some of which include supporting documents such as consent forms and information sheets. (TREAD is also glad to have new submissions so if you have made a successful application, please consider sharing your paperwork – fully anonymised of course.) Info here https://tread.tghn.org/
Like Liked by 1 person
[…] Writing your ethics application? Here’s some tips! […]
Leave a comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar

Library Guides
Dissertations 4: methodology: ethics.
- Introduction & Philosophy
- Methodology
Research Ethics
In the research context, ethics can be defined as "the standards of behaviour that guide your conduct in relation to the rights of those who become the subject of your work, or are affected by it" (Saunders, Lewis and Thornhill 2015, p239).
The University itself is guided by the fundamental principle that research involving humans and /or animals and/or the environment should involve no more than minimal risk of harm to physical and psychological wellbeing.
Thus, ethics relates to many aspects of your research, including the conduct towards:
The participants of your primary research (experiments, interviews etc). You will need to explain that participation is voluntary, and they have the right to withdraw at any time. You will need the participants' informed consent. You will need to avoid harming the participants, physically as well as mentally. You will need to respect the participants’ privacy and offer the right to anonymity. You will need to manage their personal data confidentially, also according to legislation such as the Data Protection Act 2018. You will need to be truthful and accurate when using the information provided by the participants.
The authors you have used as secondary sources. You will need to acknowledge their work and avoid plagiarism by doing the proper citing and referencing.
The readers of your research. You will need to exercise the utmost integrity, honesty, accuracy and objectivity in the writing of your work.
The researcher . You will need to ensure that the research will be safe for you to undertake.
Your research may entail some risk, but risk has to be analysed and minimised through risk assessment. Depending on the type of your research, your research proposal may need to be approved by an Ethics Committee, which will assess your research proposal in light of the elements mentioned above. Again, you are advised to use a research methods book for further guidance.
Research Ethics Online Course
Introduction to Research Ethics: Working with People
Find out how to conduct ethical research when working with people by studying this online course for university students. Course developed by the University of Leeds.

- << Previous: Methods
- Next: Methodology >>
- Last Updated: Sep 14, 2022 12:58 PM
- URL: https://libguides.westminster.ac.uk/methodology-for-dissertations
CONNECT WITH US

Ethics statement examples - ESRC
Introduction.
Proposals submitted to the ESRC must provide a full ethics statement that confirms that proper consideration has been given to any ethics issues raised. All ESRC-funded grants must be approved by at least a light-touch ethics review.
The ESRC does not require a favourable ethics opinion to be secured prior to submission of a research proposal. However, a proposal must state what the applicant considers to be the possible ethics implications throughout the research project lifecycle, what measures will be taken for ongoing consideration of ethics issues, what review will be required for their proposed research and how and when it will be obtained.
Risk and benefit to researchers, participants and others (for example, potentially stigmatised or marginalised groups) as a result of the research and the potential impact, knowledge exchange, dissemination activity and future re-use of the data should also be considered as part of the ethical statement.
If an ethics review is required at a later stage in the project, this should be discussed and funding arrangements agreed in advance with the ESRC. At a minimum we expect that ethics review will be undertaken prior to the stage in the project that the actual research is carried out.
During peer review, reviewers and assessors will be asked to consider the ethical statement in the proposal. If they disagree with the proposed approach to ethics issues, or the statement does not adequately address these issues, this could lead to the rejection of a proposal, or the award of a conditional grant to ensure the necessary ethical considerations and ethical review are undertaken.
Last updated: 28 January 2022
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
Research Ethics Step by Step
- Open Access
- First Online: 15 September 2020
Cite this chapter
You have full access to this open access chapter

- Jaap Bos 4
42k Accesses
After Reading This Chapter, You Will:
Have a general knowledge of Institutional Review Board (IRB) procedures
Have the capacity to anticipate the basic ethical pitfalls in research designs
Know how to counter common ethical objections
Be able to design an informed consent form
This chapter has been co-authored by Dorota Lepianka.
You have full access to this open access chapter, Download chapter PDF
- Added value
Anonymization
- Avoiding harm
- Conflicting loyalties
- Cost-benefit analysis
- Data management
- Data storage
- Equitability
- Ethics creep
- Expenditures
- False negatives
- False positives
- Gatekeepers
- Informed consent
- Intrusive questioning
- Invasion of integrity
Pseudonymization
- Reciprocity
- Responsibility
- Risk assessment
- Seeking justice
- Vulnerability
1 Introduction
1.1 research design and ethical approval.
In this chapter, we aim to guide you through some of the most important ethical issues you may encounter throughout the process of finalizing your research design and preparing it for the process of ethical approval. The issues discussed here range from broad topics about the relevancy of the research itself, to detailed questions regarding confidentiality, establishing informed consent, briefing and debriefing research participants, dealing with invasive techniques, deception, and safe storage of your data.
The majority of these ethical dilemmas coincide largely with the concerns voiced by independent Institutional Review Boards (IRBs, also referred to as Research Ethics Committees , or RECs). IRBs register, review, and oversee local research applications that involve human participants. They are established to protect the rights of research participants and to foster a sustainable research environment. The task of such boards is to evaluate whether or not a research design meets the institutional ethics standards and facilitates a necessary risk assessment .
The necessity of ethical reviewing is reflected in national laws as well as international declarations and has become a mandatory procedure in universities and research institutes worldwide (see Israel 2015 , for an overview of ethical reviewing practices). Failing to seek the approval of an IRB can have serious consequence for the researchers involved. For example, the retraction note attached to an article on bullying, published in 2017 in the International Journal of Pediatrics revealed ‘The study was conducted in agreement with the school principal and the authors received verbal approval, but they did not receive formal ethical approval from the designated committee of the Ministry of Education ’ (entry at ‘Retraction Watch’, March 13th 2019).
A number of scholars focusing on ethical review processes have critiqued the institutionalization of ethical reviewing, because, as one author observed, it seems to assume that unscrupulous researchers are restrained only by the leash and muzzle of the IRB system (Schneider 2015 , p. 6).
Indeed, by setting aside ethics as a separate issue and submitting it to an ‘administrative logic’ (procedural, formalistic approach), scholarly research has fallen prey to a form of ethics creep , a process whereby the regulatory system expands and intensifies at the expense of genuine ethical reflection (Haggerty 2004 ). Scott ( 2017 ) remembers how a simple study once was killed by such formalistic procedures. Understandably, researchers sometimes see the completion of an IRB application form to be a mere ‘formality, a hurdle to surmount to get on and do the research’ (Guillemin and Gillam 2004 , p. 263).
We agree that ethical considerations should inform our discussions about research, and that these discussions should not be obstructed by regulatory procedures. The aim of this chapter is therefore to assist you in your ethical deliberations. This chapter seeks to guide you through the process of making important ethical decisions at all stages of formulating a research design, and to help you identify the common pitfalls, objections, and critiques. To facilitate this process, we have designed a series of queries at the end of each paragraph, that could be taken into consideration whenever you plan to carry out a research project. Not all questions may be relevant to all research projects, but as a whole, they should facilitate a fairly thorough preparation.
In the sections to follow, we map out the various ethical dimensions of designing a research project step by step: addressing the fundamental question of why and for whom we do research (Sect. 10.2 ); an exploration of the ethical considerations of the research design itself, including the recruitment of study participants (Sects. 10.3 and 10.4 ); violation of integrity (Sect. 10.5 ); avoiding deception (Sect. 10.6 ); informed consent (Sect. 10.7 ); collecting data during field work (Sect. 10.8 ); what to do with incidental findings (Sect. 10.9 ); analyzing collected data (Sect. 10.10 ); reporting and dissemination of research findings (Sect. 10.11 ); and finally data management and storage (Sect. 10.12 ). This chapter closes with a summary (Sect. 10.13 ) and we include a brief ethics checklist and offer a model informed consent form that can be used in the future to help you cross all your ‘t’s and dot all your ‘i’s (Box 10.1 ).
We highlight our discussions with multiple case studies selected from a wide range of disciplines within the social sciences, including specializations within psychology, anthropology, educational sciences, interdisciplinary studies, and others. For the sake of brevity, we refrain from seeking examples from all disciplines for each individual dilemma, but instead focus on those that seem most poignant. We hope this overview will prepare you to face the rigors of research with confidence.
Box 10.1: Rules of Thumb for Ethical Assessment of Research Designs
Avoiding Harm Researchers have a responsibility to ensure that their study does no harm to any participants or communities involved. They also need to assess the risks that participants (and communities) may face.
How likely is your research project to cause harm to the individuals or communities you choose to research? How serious is the possible harm? What measures need be taken to offset the risks? Is there any way in which harm could be justified or excused? How do you ensure that your study does not endanger the values, cultural traditions, and practices of the community you study?
Doing Good Researchers have the complementary obligation to do research that contributes to the furthering of others’ well-being.
Who are the beneficiaries of your study? What specific benevolence might flow from it and for whom? What can participants reasonably expect in return from you and what should you offer them, if anything? What does your study offer to promote the well-being of others? How does the community or society at large benefit?
Seeking Justice Finally, researchers should ensure that participants are treated justly and that no one has been favored or discriminated against.
Do you treat your participants fairly and have you taken their needs into consideration? How do you ensure a fair distribution of the burdens and benefits in both the participant’s experience and research outcomes? How are the (perhaps contradictory) needs of the communities taken into account?
Whereas all three criteria seem ‘self-evident’ if not trivial, there remains the critical and difficult question of how to interpret them, and whether they apply in any given case (i.e. everybody will agree that one should not harm people and do good or seek justice but what does this mean in practice?). For further discussion, see Beauchamp and Childress ( 2001 ), Principles of Biomedical Ethics .
2 Relevancy: Choice of Research Area
2.1 what for.
There are few subjects or questions that researchers cannot study, but are they all worth researching? That is a different question. Contrary to what you may think, completely new research questions do not exist. Research builds upon the pre-existing research lexicon. In fact, researchers have an obligation to enhance or critique theories, improve established bodies of knowledge, and adapt or alter relevant methodologies.
Failing to acknowledge research traditions may come with the risk of wasting valuable resources, but also of self-disqualification. The relevancy of a research project is thus not so much measured in terms of how much knowledge it generates, but rather in how much knowledge it generates in relation to what is already known (see the imperative of originality, discussed in Chap. 2 ).
2.2 For Whom?
Some research is fundamental – for the sake of knowledge – but most is not. Often, results have certain practical uses for other parties, sciences’ stakeholders . They can be commissionaires who act as patrons of research projects, professionals working in a ‘field of practice’ who make use of scientific knowledge, or their clients. Research can have implications for policy makers, teachers, therapists, professionals working with minority groups, or indeed, minority groups themselves, to name but a few.
The question how research projects impact various (potential) stakeholders is not always explicitly addressed, but we feel that this is something that deserves careful attention. Who is addressed, who will be influenced, and who can make use of research in which ways? Consider the following two examples where the stakeholders are specifically targeted and even addressed.
Ran et al. ( 2003 ) describe a comparative research study into the effectiveness of psychoeducational intervention programs in the treating of schizophrenics in rural China. The program specifically targets patients’ relatives, who, the researchers conclude, need to improve their knowledge of the illness and change their attitude towards the patient.
A qualitative study on experiences with prejudice and discrimination among Afghan and Iranian immigrant youth in Canada singles out the media as a ‘major contributor to shaping prejudicial attitudes and behaviors,’ and schools as one of the first places youth may encounter discrimination (Khanlou et al. 2008 )
2.3 At What Cost?
Thirdly, there is the question of balancing costs and benefits of research. Costs comprise of salaries, investments, use of equipment, but also of sacrifices or (health) risks run by all those involved. Benefits can be expected revenue and earnings, but also gained knowledge and expertise, certain privileges allotted to participants, or even access to particular facilities.
The fact that the costs and benefits can be of a material and immaterial nature makes them both difficult to measure and predict (see Diener and Crandall 1978 ). How do you value and weigh costs and benefits? Who should profit and who should run which risks?
While there is no way to answer these questions in general, there are different models that you can use to assess risks and benefits, based on what you think counts as important.
In the first model, science is committed to the principle of impartiality . Researchers and research participants partake in research primarily because they value science, want to promote its cause, and feel that their contribution helps further scientific knowledge. In this model, costs consist just of the salaries of the researchers and the marginal compensation of the participants for their time. Knowledge acquisition is the most important gain, and risks are understood in the immediate context of research (health hazards).
In the second model, knowledge production is regarded as a commercial activity. Universities and their researchers are seen as entrepreneurs who collaborate with other parties (mainly industry and government) and are committed to the principle of profit . In this model, costs are seen as investments, gains as (potential) revenues. Compensation of participants is an expense item and any risks they run can be ‘bought off.’
The third model proposes knowledge production from the principle of equitability (fairness for all). It accepts that knowledge may be profitable, but rejects a one-sided distribution of gains, where all the profits (patents, publication, prestige, grants) go to the researchers only, and none to the participants. Participants should not merely be monetarily compensated, but profit in a much more direct way, for example by giving them access to health facilities, providing better knowledge of the topic in question (Anderson 2019 ) or even empowering whole communities (Benatar 2002 ).
These different models not only perceive parties or stakeholders differently, they also perceive of risks, costs and benefits differently. Consequently, researchers may come to weigh the costs, benefits, and risks differently depending on what they value most (Box 10.2 ).
Box 10.2: Fair Compensation?
In a research application for a study on coping with undesirable social behavior at the workplace in China, the researchers planned to ask participants to complete a questionnaire which was estimated to takes up to 15 min. Participants would receive ¥8 (roughly 1 Euro) in compensation for their effort, but only once they completed the questionnaire. When queried by their local IRB why every participant wouldn’t be compensated regardless, rather than only those who complete the questionnaire, the researchers presented four arguments:
Rewarding participation before finishing the research leads to high dropout rates
It is difficult to organize payment with non-completers
The questions are non-invasive
In comparable cases, applications are always approved by IRBs
Evaluate these responses by ranking the arguments. Which argument do you find most and which least convincing, and why?
(Case communicated to one of the authors of this chapter)
2.4 Trauma Research: A Case in Point
Consider the question of whether research on traumatic experiences itself should be regarded as harmful. It is argued, on the one hand, that asking about traumatic experience is risky, as survivors may be more vulnerable and easier to stigmatize. On the other hand, there is also evidence that suggests that talking or writing about traumatic experiences can in fact be beneficial, psychologically as well as physiologically (Marshall et al. 2001 ). How does one weigh the (potential) risks against the (possible) benefits (DePrince and Freyd 2006 )?
In a study among 517 undergraduate students, Marno Cromer et al. ( 2006 ) asked subjects to rate how distressing it was for them to discuss a range of traumatic experiences and found that a vast majority did not find it difficult at all. However, argued the authors sensibly, it’s not the average that counts here, but the exception . And indeed, 24 participants reported the trauma research to be ‘much more distressing’ than everyday life. Of these 24, all but one still believed the research to be important enough to be carried out. The one exception reported that the research seemed ‘a somewhat bad idea.’
These findings concur with Newman et al. ( 1999 ), who did research on childhood abuse and found that a minority of the respondents reported feeling upset after the research. Of these, a few indicated that they would have preferred to have not participated had they known what the experience would be like.
In weighing the (immaterial) benefits against the costs of talking about traumatic experiences (distress), the former were deemed to outweigh the latter, provided that interviewers are carefully selected and trained.
Of note, however, one consideration is left out of this comparison, namely the question of whether not doing the research should be considered a risk (Box 10.3 ). Indeed, Becker-Blease and Freyd ( 2006 , p. 225) reason that ‘silence is part of the problem’, and there is a real ‘possibility that the social forces that keep so many people silent about abuse play out in the institution, research labs, and IRBs.’
Will the cost-benefit balance shift if the risk of not doing research be taken into consideration?
Q1: What is the added value of my research project and for whom does it benefit?
Which research traditions and methodologies do I relate to and why?
Who is addressed by my research project (who are my possible stakeholders)?
Which costs and benefits can be expected, for whom, and how do I balance them?
3 Choice of Participants
3.1 ethical limitations in choice of participants.
Researchers must make many decisions regarding the choice of participants. Is the sample randomly selected and does it give a fair representation of the population? Will the N be large enough to test my hypothesis? Has non-response been taken into account? Et cetera. Some of these methodological questions have ethical consequences, as we will explore below.
3.2 Number of Participants
This is of ethical concern because research is considered (at least to a degree) a burden on participants and often times on society at large as well. The number of participants should therefore be no more than absolutely necessary.
In quantitative studies, a reasonable estimate can be given with a power analysis . ‘Statistical power’ in hypothesis testing signifies the probability that the test will detect an effect that actually exists. By calculating the power of a study, it becomes possible to determine the required sample size, given a particular statistical method, and a predetermined degree of confidence. For example, to detect a small interaction effect between two variables, using a linear mixed-effect method, a sample of N = 120 would suffice at a default alpha of.05. Remaining space in this book does not permit a detailed discussion of how to calculate the power of a study, but see Cohen 1988 , for an explanation of power in the behavioral sciences in general.
In qualitative studies, no such power analysis would be suitable. Instead, the principle of saturation is often used. Saturation implies approaching new informants until enough knowledge is gained to answer the research question, or until the categories used are fully accounted for. What exactly constitutes ‘saturation’ may differ from one field of expertise to the next and may need further problematization moving forward (see O’Reilly and Parker 2012 ).
3.3 Selection of Participants
Laboratory studies often use undergraduate students as research subjects (usually in exchange for ‘credits’). These are called subject pools . In some fields of research in the social sciences, subject pools make up the majority of research participants, as Diener and Crandall ( 1978 ) pointed out long ago.
Convenience sampling (using groups of people who are easy to contact or to reach) not only has methodological drawbacks, but also ethical implications. Heinrich, Heine, and Norenzayan ( 2010 ) called attention to the social science’s ‘usual subjects’ and named them WEIRDOs, an acronym for Western, Educated, Industrialized, Rich and coming from Democratic cultures.
They maintain that WEIRDOs aren’t representative of humans as a whole, and that psychologists shouldn’t routinely use them to make broad claims about the drivers of human behavior because WEIRDOs differ in fundamental aspects with non-WEIRDOs. Different cultural experiences result in differing styles of reasoning, conceptions of the self, notions of fairness, and even visual perception.
Box 10.3: Risks and Benefits
Risk : The probability of harm (physical, psychological, social, legal, or economic) occurring as a result of participation in a research study. The probability and the magnitude of possible harm may vary from minimal (or none) to significant.
Minimal Risk : A risk is considered to be minimal when the probability and magnitude of harm or discomfort anticipated in the proposed research are not greater, in and of themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychological examination or tests.
Benefit : A valued or desired outcome, of material or physical nature (i.e. money, goods), or immaterial nature (i.e. knowledge, skills, privileges). Individuals may not only benefit from the research, but also communities as a whole.
(Adapted from the Policy Manual of the University of Louisville.)
3.4 Online Communities
As a specific target group for research, online communities pose their own dilemmas. Legally, researchers must be aware that they may be bound by the ‘general terms and conditions’ of these online platforms, which can restrict the use of their data for research purposes. Morally, it is important to ask whether it is right to record the activities of an online public place without the participant’s consent, regardless of whether it is allowed (see Chap. 7 for a discussion of this question). There are two viewpoints we will explore on this matter.
Oliver ( 2010 , p. 133) argues that although communication in an online environment may be mediated in different ways, it is still communication between people. In essence, the same ethical principles should apply, including the receipt of active consent.
Burbules ( 2009 , p. 538) on the other hand, argues that in online or web-based research, notions regarding privacy, anonymity, and the right to ‘own’ information needs to be radically reconsidered.
What matters online, Burbules argues, is not so much anonymity, but rather access. In the digital universe, people want to share information. But they also want to control who can make use of it. A challenging dichotomy to navigate indeed.
This problem (the question of who can access which data) has become even more urgent today. This urgency can be traced to new information and communication technologies that enable researchers to build extremely complex models based on massive and diverse databases, allowing increasingly accurate predictions about an individual’s actions and choices.
3.5 Control Groups
Research on the effects of certain interventions that involve control groups (participants who receive either less effective or no treatment) leads to the question of whether it is fair for a participant to be placed in a disadvantageous position.
This is referred to as asymmetrical treatment . The question is grounded in considerations of egalitarian justice , which is in other words, the idea that individuals should have an equal share of the benefits, rather than just the baseline avoidance of harm.
It is suggested that participants in the control group be offered the more effective treatment once the study is completed (Mark and Lenz-Watson 2011 ). A problem with this being that it applies to certain research designs only (typically RCT, or ‘Randomized Controlled Trial’) and not to others (policy interventions, for example, or education; for further discussion, see Diener and Crandall 1978 ). With research on policy interventions, (as opposed to treatment research), the question is whether or not it is fair to offer certain policies to certain groups and not to others.
Q2: Who are the participants in my research project?
Which ethical consequences may be involved in selecting participants for my research project?
How do I ensure that my selection of participants does not result in unfair treatment?
4 Vulnerable Participants
4.1 vulnerable participants.
Vulnerable participants are properly conceived of as those who have ‘an identifiably increased likelihood of incurring additional or greater wrong’ (Hurst quoted in Bracken-Roche et al. 2017 ). Seeking the cooperation of vulnerable people may be problematic for various reasons, but that does not imply that they cannot or should not be involved in research. It does mean that these groups need special attention, however.
4.2 Minors and Children
Working with minors and children requires consideration from both a moral and a legal perspective. Often in place are somewhat arbitrary age limits that will differ from country to country, which require that researchers seek active consent from the parents or legal representatives of a child. This says little, however, about the minor’s moral capacity to participate in research.
IRBs generally acknowledge that children can be involved, but that different age-groups should be treated on par with their stages of psychological development, that will inform what a six-year-old or a twelve-year-old child can or cannot do, or what an eight-year-old is capable of comparatively. In general, the younger the child, the shorter and less intense the inquiry should be.
We concur with Schenk and Rama Rao ( 2016 , p. 451) who argue that young children should be excluded from providing detailed information on potentially traumatic topics that may cause strong emotional distress. As is usually the case, exceptions can be made under particular circumstances, but they remain outliers. We also agree with Vargas and Montaya ( 2009 ) that it is sensible to consider any contextual and cultural factors, as this may make a difference in a child’s understanding of the research environment.
Finally, we emphasize that researchers who work with minors (children) should have special training on how to interview or collect data from them.
4.3 Disadvantaged Participants
When cognitively impaired individuals are included in a research design, special attention must be paid to the potential level of invasiveness, the degree of risk, the potential for benefit, and the participant’s severity of cognitive impairment (Szala-Meneok 2009 ). Likewise, people who are in dependent circumstances (such as detainees, elderly people in nursing homes, or the unemployed), may not always have the capacity to refuse consent, or may fail to understand that they have the power to refuse cooperation. A reasonable assessment regarding the perceived ability to participate and to refuse participation must thus be made for every case in which these populations are involved (see Box 10.4 for an overview of vulnerable participants).
4.4 Mixed Vulnerability
At times, several forms of vulnerability coincide within one research proposal. Consider as a case in point a proposed study into health problems (suicidal ideation), sexual risk-taking behavior, and substance use of LGBT adolescents of between 16–17 years old, as reported by Brian Mustanski ( 2011 ). An Institutional Review Board (IRB) was hesitant to approve Mustanski’s application for a number of reasons. We will discuss those reasons below, together with Mustanski’s responses.
The first problem the IRB encountered was that the researcher was seeking a waiver for parental permission. Adolescents at this age are legally minors and any waiver requires the provision of an appropriate mechanism for protecting the minor. Mustanski argued, however, that the goal of waiving parental permission was not to circumvent the authority of parents. ‘Instead, it is to allow for scientists to conduct research that could improve the health of adolescents in cases where parental permission is not a reasonable requirement to protect the participating youth’ ( 2011 , p. 677).
The second concern of the IRB was the vulnerability of the LGBT community collectively, who have historically been more prone to stigmatization and discrimination. Mustanski replied that he knew of no evidence that demonstrated any decision-making impairment of members of the LGBT community, and that he believed many of them would be insulted to have it implied otherwise.
Finally, and perhaps most importantly, the IRB was worried that participants in this research might be exposed to sensitive information that could lead to psychological harm. Mustanski agreed that IRBs should have a role in protecting participants’ interests, but argued that IRBs tended to overestimate risks. This can lead to time-consuming procedures and the implementation of supposed protections that may mitigate the scientific validity of the research, or discourage future behavioral research involving certain populations. After a number of required adjustments (such as a more detailed risk assessment), the proposal was ultimately accepted.
Box 10.4: Vulnerable Populations by Category
Adapted with permission from the guidelines of the Institutional Review Board for Social and Behavioral Sciences at the University of Virginia.
Q3: How do you ensure appropriate and equitable selection of participants?
Who are your research subjects?
Are your research subjects part of a vulnerable population, and if so, what risks do you anticipate?
Where do you expect to find them and how do you intend to recruit them?
5 Use of Invasive Techniques
5.1 invasive techniques.
By invasive techniques , we mean any procedure or intervention that affects the body or mind of a research participant such that it results in psychological or physical harm. Some argue that our definition of invasiveness should not be limited to individual participants but should include entire communities as well (Box 10.5 ).
Invasive techniques, by definition, violate the principle of nonmaleficence (‘do no harm’), and are among the most urgent concerns of IRBs the world over. However, harm is broadly (and vaguely) defined, ranging from trauma to strong disagreeable feelings, and from short-term to long-lasting. The European Textbook on Ethics ( 2010 , p. 200) defines harm as such: ‘To be harmed is to have one’s interests set back or to be made worse off than one would otherwise have been. Harms can relate to any aspect of an individual’s welfare, for example physical or social. Institutions can also be harmed insofar as they can be thought of as having interests distinct from those of their members.’
Invasive techniques may include exposure to insensitive stimuli, intrusive interrogation, excessive measurements, or any procedures that can cause damage. We exclude from our discussion any medical practices or intervention, such as administering drugs or the use of clinical health trials and refer anyone who intends to use these techniques to specialized Medical Research Ethics Committees (MRECs).
5.2 Examples of Invasive Research
Some of psychology’s most famous experiments were hampered by the ethical quandaries of invasive research. For example, John Watson’s 1919 behavioral experiments with ‘Little Albert,’ an eleven-month-old child, who was exposed to loud, frightening sounds when presented with specific fearsome images. Although it is unclear what the net effects were on the child, by today’s standards, the design would be considered unethical for its gross lack on concern for the wellbeing of the child (see Harris 1979; Beck et al. 2009 ) (Fig. 10.1 ).

Little Albert . Still from the film made by Watson. (Source: Wikipedia)
Harry Harlow’s ‘Pit of Despair Studies’ from the 1950s involved infant primates who were raised in social isolation, without their protective mothers or with surrogate mothers (dolls). They consequently developed signs of what humans call ‘panic disorder.’ This complete lack of concern for animal welfare would certainly be considered unethical by today’s standards.
Psychologist Stanley Milgram’s well-known 1961 experiments, that involved participants who were led to believe that they were administering electric shocks to fellow participants are deemed invasive, despite the researcher attempting to minimize harm by debriefing his participants (see Tolich 2014 ).
Diana Baumrind ( 1964 ) was quick to recognize the ethical perils of the Milgram studies: ‘From the subject’s point of view procedures which involve loss of dignity, self-esteem, and trust in rational authority are probably most harmful in the long run and require the most thoughtfully planned reparations, if engaged in at all’ (p. 423).
5.3 Avoiding Invasive Routines?
Can (or should) invasiveness be avoided at all times? The answer seems obvious: no techniques that cause harm should be put to use. In practice, however, the answer is more ambiguous.
Some research topics are inherently ‘sensitive’ (i.e. psychological trauma, loss, bereavement, discrimination, sexism, or suicide). Merely discussing these subjects can be perceived as painful. Similarly, some techniques necessitate a physical response from participants and can result in some harm. Does that imply these subjects cannot be researched, and that these stimuli cannot be used? Not necessarily.
In an experiment that provides a telling example, researchers tried to establish the causal relationship between workload and stress response. To do so, they had to induce a potentially harmful stimulus, namely some form of stress. The results showed that such stimuli do indeed have an influence on a participant’s perceived well-being and impacted their physical health, as indicated by an increased cardiovascular response (see Hjortskov et al. 2004 ).
Is it justifiable to expose respondents to harmful stimuli, even when the effect is likely short-term? Hjortskov et al. answered the question in the affirmative and took refuge in what is considered by many as a safe baseline in research ethics. If harm does not exceed the equivalence of what can be expected to occur in everyday life, they argued, then the procedure should be safe.
It has been maintained that invasive techniques using stressors, unpleasant noises, rude or unkind remarks, among other forms of aggravators, are acceptable when (a) there are no other non-invasive techniques at hand, (b) the effects are equivalent to what people can expect to encounter in everyday situations, (c) have no long-lasting impact, and (d) everything is done to minimize harm.
Some retort that this will not (always) be sufficient. People who face systematic stigmatization in everyday life, or social exclusion, would be harmed in a way that is not acceptable should they be exposed to such stimuli, even though that is exactly what they expect to occur in everyday life.
Q4: Will the research design procedures result in any (unacceptable form of) harm or risk?
Which possible risks of harm are feasible in this research?
How do you plan to minimize harm (if any)?
Box 10.5: Invasive or Intrusive?
The term invasive originates from the medical sciences, where it means : entering the body, by cutting or inserting instruments . In the social sciences, it describes techniques that enter one’s privacy. Questions about one’s sexual orientation, political preferences, and other privately sensitive subjects are considered ‘invasive’, as is exposure to strong aversive stimuli or traumatizing experiences.
Intrusive was originally a legal term, described as entering without invitation or welcome . In the social sciences, it describes techniques that invoke ‘unwelcome feelings.’ Research may be regarded as ‘intrusive’ when it concerns topics that respondents dislike talking about or find difficult to discuss (Elam and Fenton 2003 , p. 16). Intrusive techniques can also involve prolonged procedures and processes that involve substantial physical contact. Intrusive questions can make a participant feel uneasy, uncomfortable, even shameful: ‘Are you anorexic?’ ‘Do you masturbate?’
Some examples of invasive and intrusive technique include:
EEG, PAT scans, CAT scans, (f)MRI, or measuring heart rate, are all non-invasive in the medical and psychological sense, but can be intrusive.
Questions about race, ethnicity, and sexual health can be both invasive and intrusive.
Queries about personal information, including name, date and place of birth, biometric records, education, financial, and employment history, are often thought to be neither invasive nor intrusive. However, to some people some of these questions can be intrusive. Regardless, use of this information is strictly limited under data protection regulation in most countries.
6 Deception
6.1 deceptive techniques.
Any research procedure in which a participant is deliberately provided with misinformation is labeled as a deceptive technique . Deception involves (a) giving false information, or (b) generating false assumptions, or (c) withholding any information that participants may request, or (d) withholding information that is relevant to appropriate informed consent (Lawson 2001 , p. 120). Just because early research on the harmfulness of deception does not indicate that deceived participants feel harmed (Christensen 1988 ) or that they become resentful (Kimmel 1998 ), does not mean it is without moral consequence.
By default, deception excludes consent (see below). Participants are therefore not at liberty to decide to participate (or to continue participating) on the conditions known to them, regardless of whether consent was given afterwards, or even whether participants agreed to be deceived beforehand (when they agree to be fooled in some way).
Deception thus suggests a possible breach of two important ethical principles: the protection of people’s autonomy and dignity, and the fair and equitable treatment of participants. Some have called for the abandonment of deception in research altogether, while others maintain that certain research areas, particularly in psychology, cannot do without it (see Christensen 1988 ). At any rate, IRBs have become more cautious in the last decade and generally insist on a full debriefing at minimum (see Mertens and Ginsberg 2009 , p. 331). But even a full debriefing may not always be possible.
To summarize: forms of deception include providing false or misleading information about:
Research goals or aims
Research setup
The researcher’s identity
The nature of a participants’ tasks or role
Any possible risks or consequences of participation.
The distinction between false information and defective information is noteworthy. False information means presenting an (oftentimes completely) wrong picture of the true research goals, while defective or misleading information might only mean withholding some (key) aspects thereof. Some argue that not telling participants certain things is not a form of deception (Hey 1998 , p. 397), but we concur with Lawson ( 2001 ) that it certainly can be, especially on a relational level (pertaining to the relationship between researcher and participant) (Fig. 10.2 ).
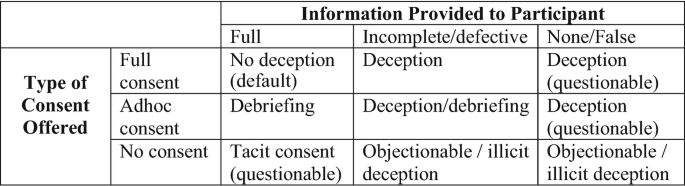
Degrees of deception as a function of consent/debriefing and provision of information
6.2 Four Cases
Consider the following four cases in which (some form) of deception was deployed. How does the form and level of deception differ in these cases?
In the first, that came to the attention of one of the authors of this chapter, a group of researchers proposed to approach a number of intermediaries with mock job application letters and matching CV’s that differed only with respect to the ethnicity of the ‘applicant’. The researchers intended to measure the response rate of the intermediaries as an indication of hidden discrimination. The ‘participants’ (the intermediaries) were neither informed of nor debriefed about the research project, and thus would not be able to not participate or retort to its findings. Deception was deemed necessary to elicit true behavioral response.
The second pertained to an unpublished ethnographic study into social exclusion of the poor in Poland, carried out by one of the authors of this chapter. The researcher asked participants if they could be interviewed about their ‘lifestyles,’ deliberately not mentioning the goal of the study (social exclusion) because the researcher reasoned it might instill them (against their own conviction) with an idea that they are marginalized and excluded. The researcher feared that this idea would impose on them an identity that they could perceive as harmful. The research participants who were asked for consent were not informed about the true nature of the research project, nor were they debriefed afterwards. In this case, deception was considered both necessary as well as in the interest of the participants.
The third case concerns a covert participant observation project in an online anorexia support community performed by Brotsky and Giles ( 2007 ). The researchers created a mock identity of an anorexic young woman who said she wanted to continue losing weight. The researchers wanted to study the psychological support offered to her by the community, who was not informed about the research project. Throughout the course of the project, the invented character of the researchers developed close (online) relationships with some of its members. They justified the use of a manufactured identity on the grounds that if the purpose of the study was disclosed, access to the site would probably not be granted. Deception was deemed acceptable because of the ‘potential benefit of our findings to the eating disorders clinical field’ ( 2007 , p. 96). The research participants were never asked for their consent, nor informed about the nature of the research.
The fourth case concerns social psychology research into the bystander effect (the inclination not to intervene in a situation when other people are present). Experiments on the bystander effect rely heavily on giving false information about the roles of other participants involved in the study, because they are in reality in cahoots with the researcher.
In a recent study into the bystander effect, Van Bommel et al. ( 2014 ) wanted to know whether the presence of security cameras would have any influence on said effect. The researchers designed a realistic face-to-face situation featuring a security camera (not featured in the control group). They exposed participants to a mock ‘criminal act’ to see whether they would respond or not. Immediately afterwards, participants were informed of the true nature of the setup.
In all cases, some form of deception was considered necessary, though for different reasons. Deception contributes to inequity between the research and the participant. By debriefing the participant (i.e. informing them of the true nature or purpose of the research), some of this can be countered under certain circumstances. In the first case discussed above, debriefing was not considered, in the second it was ruled out. In the fourth case it was part of the design by default and not questioned as such. In the third case, it could (and some would argue should) have been used.
6.3 Deception and Misinformation
Arguably what matters most in considering the use of deception are found within two parameters: the degree of misinformation and the degree to which participants may give consent or can be debriefed (Ortmann and Hertwig 2002 ) (see Fig. 10.3 ). The questions any researcher must answer regardless are (1) whether or not it is really necessary to use deception, and (2) how to repair inequity if it were to be used.

Alice Goffman, On the run
Q5: Will the research design provide a full disclosure of all information relevant to the participant? If not, why not?
How do you ensure your participants are adequately informed?
What do you do to prevent deception?
Box 10.6: Checkbox for Ethical Concerns in Social Sciences Research Design
Which research techniques do you use in your design, and to what extent is your design vulnerable to the ethical concerns above? Provide a detailed description.
7 Informed Consent
7.1 informed consent protocols.
Following established informed consent protocols are indispensable in any scientific research and serve to ensure that research is carried out in a manner that conforms to international regulations (such as the 1966 United Nations International Covenant on Civil and Political Rights, that explicitly prohibits that anyone be subjected to scientific experimentation without their permission).
Consent is based on four prerequisites: (1) it is given voluntarily (free from coercion), (2) the participant is a legally competent actor, (3) is well informed, and (4) comprehends what is asked of them.
To inform a participant means they must be notified about the objectives of the study, be informed about what is expected from them, and be told how their data will be used. Consent requires that a participant not only has a substantial understanding of the situation in which they will partake, but is also at liberty to refuse. Consent gives the researcher the right to involve the participant in the research project and at the same time assures that the respondent’s rights are protected.
Informed consent protocols assume different shapes and forms. Traditionally they were hard copy forms, physically signed by the participant. Lately, they are often digital (i.e. in an online questionnaire, the respondent is informed about the objective of the questionnaire and needs to agree by ticking a box before proceeding). In ethnographic studies, speech recordings are sometimes utilized.
Consent protocols require furthermore that participants are provided with information of whom to turn to in case of disagreement complaints, or in case of unexpected or accidental findings that may affect the participant. This can be an independent board or a professional not involved in the study.
7.2 Who Can and Cannot Consent?
From our definition, it follows that any adult capable of understanding what is communicated to them and is at liberty to say no, can give consent. This leaves out a list of people who cannot be expected to give consent for reasons of incompetence, incomprehension, or lack of freedom. These include:
Minors or children, who cannot legally give consent
Adults with cognitive impairment or diminished decision-making abilities, who may not comprehend properly
Adults in a dependent situation, such as refugees or undocumented immigrants, who may not be at liberty to refuse cooperation.
In some cases, others may consent for them (with children, this may be their parents or legal caregivers; with patients it can be their legal representatives). See Box 10.4 for an overview of vulnerability categories.
7.3 Active and Passive Consent
Consent is by default active , which means that the participant is knowledgeable about the purpose of the research and actively agrees to participate in it, under the conditions spelled out to them. Passive consent follows a different path. The participant is informed about the research, but it is assumed that they do not object, thus passively agreeing to participate. The researcher proceeds unless the participant actively refuses to participate.
Passive consent results in higher response rates and was more commonly sought after until the first decade of the twenty-first century. Today, a stricter view on subject autonomy is held, and consequently many IRBs no longer condone its practice, allowing it only in exceptional situations (Rangle et al. 2001 ).
7.4 Whose Responsibility?
It is the responsibility of the researcher that the participant fully understands what the research amounts to. The information provided must be comprehensive, to the point, and non-technical. Participants should be aware that they may refuse to (further) participate, withdraw their consent, and have their data removed from the study at any time (Box 10.7 ).
In some cases, it can be challenging to obtain informed consent, especially when participants are not accustomed with formal, written discourse, or come from a cultural background where such a formal permission would raise suspicion (see Israel 2015 , for further discussion on the pitfalls that informed consent may carry, especially in qualitative research) (Box 10.10 ).
In other cases, consent may need to be re-negotiated. This can occur in longitudinal studies where parents earlier agreed that their children could participate in the study, but the child meanwhile grows up and becomes an adult capable of making their own decisions.
7.5 Disclosure of Sensitive Information in Consent
Researchers are obliged to conceal information that might be damaging to the respondent’s reputation or affect their position within their community, organization, professional field, or could have an impact on their employability. For this reason, some institutions request that their researchers report only on data larger than a certain n-value, to prevent others from finding out who the participants may be (i.e. the Karolinksa Medical Institute at Stockholm set the norm at n > 6).
Such considerations are of relevance even when the study participants had, prior to their involvement in research, expressed their consent or even a wish to stay non- anonymous. The latter might happen with participants who are politicians or activists, who might treat their participation in research as a means to get publicity.
Q6: Have you obtained informed consent?
What information have you communicated to your participants?
In what ways have you ensured they are aware of what is expected from them?
Check with your local IRB for samples of an informed consent and/or guidelines (see end of this chapter for a sample).
Box 10.7: Informed Consent as a Universal Principle?
Although informed consent is accepted world-wide as a necessary requirement for research, the question can nevertheless be posed whether or not it is biased in favor of a Western notion of liberal individualism. Would the moral conception and ideal of informed consent be applicable in China, whose cultural and ethical traditions are often conspicuously different from those of the West, and are more specifically communally oriented (Nie 2001 )?
Liang and Lu ( 2006 ) did research on legal reforms in China, for which they conducted interviews. When seeking informed consent, they ran into what they called a conflict between the rigidity and inflexibility of informed consent and the relativity and informality of Chinese culture. For Chinese participants, consent would be regarded not so much as a legal formality but rather the foundation for continued friendship and trust. Consequently, they’d be hesitant to sign a consent form beforehand.
Furthermore, Chinese participants have a different view on the legal system. While Americans trust the confidentiality agreement because laws protect privileged information, in China no such laws or legal practices exist. As a result, Liang and Lu wrote, ‘a mere promise of confidentiality from the researcher to the participant would indeed raise red flags about the legitimacy of the research, therefore hurting rather than helping one’s research’ ( 2006 , p. 166).
Tangwa ( 2014 ) exemplifies the situation of West African women, who because of bride-price practices, are in unequal and therefore vulnerable relationships. These women are required by their communities to get approval from their husbands if they volunteer to enlist in medical research; by insisting only they themselves can give consent, their cause will not be furthered.
Castellano ( 2014 , p. 278) argues that the interests of Aboriginal peoples are not served with individualized consent procedures. The implementation of ethical standards for Aboriginal research should be in the hand of Aboriginal peoples. National committees should be formed, consisting of Aboriginal experts, who could develop such standards, and help prevent misrepresentation and stereotyping, and ensure that environmental research is included.
In these and similar cases, individualized informed consent procedures are all but appropriate. Instead, consent extends to communities, experts, or special committees, who oversee that interests of certain groups are served.
8 Fieldwork and Data Collection
8.1 entry strategy and conflicting loyalties.
When planning data collection, some considerations must be taken into account regarding strategies to access the ‘field’ (be that a school, a municipality, an internet community, or any another institute that houses participants) (Eysenbach and Till 2001 ).
Sometimes formal approval is required, other times approaching participants necessitates little more than the go-ahead from the head of an institute. Particularly when studying relatively small, tightly-knit communities or groups, caution is required. In those cases, researchers make use of gatekeepers , such as institutions or persons who have (direct) access to potential study participants. While gaining access to a field via gatekeepers has its obvious advantages, it may also involve some moral dilemmas.
Gatekeepers , just like research sponsors and research participants, usually have their own stake in research. When offering access to their networks, gatekeepers show trust and expect loyalty. Researchers thus engage in what is called relational ethics , which builds on mutual respect, dignity, and connectedness between the researcher and researched (Ellis 2014 , p. 4), although the researcher often cannot avoid politically embedded issues of power that require a ‘delicate balancing act’ (McAreavey and Das 2013 ).
8.2 Cooperation and Non-Cooperation
Once people agree to participate in a study, the researcher may count on their cooperation and benevolence. At a minimum, participants should not feel deceived, intimidated, or otherwise uncomfortable with the research, but there can be many other valid reasons why participants decide to leave a study. In some cases, such as in evaluation studies that involve a researcher’s prolonged presence in the field (perhaps even against the wish of some of the actual study participants), participants may become reluctant, mistrustful, or even non-cooperative. In other cases, participants my leave studies for no apparent reason at all (as they are free to do).
Research using large databases of raw, unstructured public data (‘big data’) poses its own ethical considerations, in particular with regard to privacy. In consumer behavior research, for example, Numan and Di Domenico ( 2012 ) observe that the volume and speed with which data must be analyzed often requires data collecting and analysis without an individual providing specific consent. This raises ethical concerns ‘relating to the extent to which organizations can control the collection and analysis of data when there is limited human involvement’ ( 2012 , p. 51).
Finally, there is the question of non-cooperation (or counter-cooperation), which can occur in a variety of ways. Researchers may find that participants avoid answering certain questions, are purposefully manipulative, or even lie about particular issues (because of shame or to protect their dignity).
All these forms of non-cooperation will pose researchers with a challenge, and it is therefore advisable to think ahead to strategies for what to do in case there are not sufficient data points to work with.
8.3 Interpersonal Dynamics
Ethical dilemmas may also arise as a consequence of interpersonal dynamics, both between the researcher and the study participants and (or) among study participants. In any case study that involves participant observation or repeated interviews with participants, continued interaction is likely to result in emotional and social engagement on the part of the researcher. This may lead to the formation of alliances and conflicting loyalties. As a result, the role of the researcher as an ‘objective observer’ of social life might be challenged.
In the course of any study, the researcher’s relationship with the gatekeepers or sponsors may also need to be renegotiated, for example, when gatekeepers try to influence the direction of the study. Commitments related to confidentiality and anonymity may need to be re-affirmed or redefined. Finally, in cases of intensive ethnographic observations, the prolonged presence of the researcher is also likely to re-define the community or group or organization studied, and may raise (moral) questions related to the role of the researcher and their relationship with the object of investigation (Mikesell et al. 2013 ) (Box 10.8 ).
Q7: How do you enter the ‘field’?
Which formal or informal agreements have you made and with whom?
Which expectations have been created when an agreement on cooperation has been made?
How do you deal with non-cooperation on the part of study participants?
How do you deal with competing loyalties?
Box 10.8: Alice Goffman – What are the Limits of a Researchers’ Involvement?
Sociologist Alice Goffman’s 2014 ethnographic study On the Run: Fugitive Life in an American City details the careers of poor black men in West Philadelphia. She paints a bleak picture of these men who follow ‘the other path into adulthood,’ leading them invariably to crime and eventually incarceration.
In the wake of the ‘Black Lives Matter’ movement, the book was received with ample praise. Goffman’s central claim, that it is the legal system itself creating crime and dysfunction in poor black communities, was supported by her critics, although some reviewers would argue that Goffman’s views are rather one-sided. For example, Heather Douglas ( 2014 ) wrote that Goffman refused to acknowledge that her participants create their own predicament through deliberate involvement in crime.
From a research ethics viewpoint, On the Run raises an alarming question about the limits of a researchers’ involvement. Some accounts in the book suggest that Goffman was so thoroughly involved with her participants that she became complicit in criminal activity herself, including even conspiring to commit murder (as one participant confided in his plans to kill someone). She thus violated perhaps the most basic precept of scholarly (and personal) responsibility – not to endanger somebody else’s life, and to do no harm (Lubet 2015 ). Whether she committed any crimes cannot be established, as she had carefully concealed the true identity of the participants involved and destroyed her field notes, which from an ethical viewpoint is also questionable.
9 Incidental Findings
9.1 incidental findings in clinical research.
Any research, including the most non-invasive varieties, can unearth Incidental Findings (IFs). For example, brain imaging research may bring to light undetected, clinically relevant abnormalities that are unexpectedly discovered and although unrelated to the purpose of the study, they may require urgent or immediate referral (Vernooij et al. 2007 ).
This raises the question of what to do with these findings. Is it in the interest of the participant that the researcher notifies them? The intuitive answer may be yes, but some argue that this is not evidently the case, as participants have a right not to know .
On the one hand, Miller et al. ( 2008 ) argue that clinical investigators do have an obligation to respond to incidental findings. They argue this point because the researcher entered into a professional relationship with the research participants, and thus they are granted privileged access to private information with potential relevance to the participants’ health. Appelbaum et al. ( 1987 ) on the other hand, warn against the false hopes that a confusion of roles might create, when participants feel that research protocols are designed to benefit them directly rather than to test or compare treatment methods.
Incidental findings call for the weighing of false positives (potential harm due to findings that have no clinical significance) against false negatives (failure to report a finding linked to a serious health problem). In an attempt to solve at least part of this quandary, IRBs often suggest that participants be offered an ‘opt-in’ / ‘opt-out’ choice in the informed consent. ‘Opt-in’ necessitates the researcher to communicate any accidental findings relevant to the participant, ‘opt-out’ prohibits the same.
However, even with such clear-cut arrangements, the researcher may still find it ethically problematic to remain silent when the participant chooses to ‘opt-out’ and a clearly identified, life-threatening, treatable condition is discovered (for further discussion see Illes et al. 2006 ).
9.2 Incidental Findings in Non-Clinical Research
DNA analysis is increasingly utilized in forensic anthropology, for example to identify damaged or fragmented human remains, for which DNA of family members is required for proper identification. Parker et al. ( 2012 ) argue that the increased prevalence of incidental findings (IFs) in non-clinical research (such as misattributed paternity or false beliefs about sibling relationships), calls for new policies to focused on minimizing the discovery of IFs.
Q8: How should you deal with incidental findings?
Which agreements have you made with your participants regarding any potential incidental findings?
Do you offer an opt-in/opt-out option in your informed consent?
How do you check for any unintended consequences of discovering incidental findings?
10 Analysis and Interpretation
10.1 analyzing results.
While fraud among academics is rare, questionable research practices do occur, leading to multiple forms of bias. These include (among others): publication bias (non-publication of null results), confirmation bias (tendency to look for confirmative results, disregarding of contradictory information), and funding bias (tendency to support the study’s financial supporter) (Box 10.9 ).
We believe that it is vitally important that researchers are aware of the forms of bias that lie in wait and operate as transparently as possible (see Chaps. 5 and 6 of this book for an extensive discussion). Below, we discuss three issues related to bias, namely significance , plausible objection , and limits of interpretation .
Box 10.9: Transparency: Steps Researchers Can Take to Reduce the Risk of Bias
Dr. Daniele Fanelli: ‘The way out of [bias], generally speaking, is to be transparent about what you did. I’m not naive enough to think that this is going to be the whole story, because publication space in journals is limited, and you will never be allowed to tell precisely everything that you have done. So in part, the system does need other ways also to allow researchers to make fully public their data, you know, all the results they obtained, etc.
Again the ideal to follow, I think, in any kind of research, is as much as possible, be transparent of the whole procedure. What were your original research questions, how you collected the data, what eventually was the data that went into this particular study, and so on.
(From online course site Epigeum, Research integrity: arts and humanities,
10.2 Significance
After having collected data, the main concern of a researcher is whether or not the data has a story to tell. Are the results indeed significant? Is the effect a sizeable proportion? Is there a convincing narrative pattern discernable in the interviews?
Given the study produced at least some results worthy of discussion, then the ethical question is: are the results significant and unique enough to warrant publication? This is not self-evident nor easily established. What is ‘significant’ in this context does not depend on a statistical or discursive measure, but on an overall evaluation of the results. This evaluation would also include the question of whether the phenomena observed was properly accounted for by available theories. Finding (statistically) significant results is one thing, finding something that is substantial is something else. It is the task of the researcher to carefully assess the weight of their findings.
10.3 Plausible Objection
Complementary to the question of significance is the question of plausible objection . One must ask themselves, if a study produces data containing significant results, how do those results line up with rivalling theories or other plausible explanations and objections? How do we know that novel findings were truly revealed, and not merely an exception to the rule, chance findings, or even false positives?
Again, the answer to these questions cannot be established on the basis of the data alone, but they need full consideration, nonetheless. The place to address these considerations is often in the discussion section of a scientific paper or as an addendum to the findings, although in reality they should precede any discussion of the findings.
10.4 Limits of Interpretation
Findings worthy of publication need to be framed such that their significance can be understood and eventually be communicated to others. But how far can our interpretation go?
In 2002, the then United States Secretary of Defense, Donald Rumsfeld, conceived of a way to interpret uncertain knowledge. He invented a concept that since found its way into scientific parlor: the ‘unknown unknowns.’ It plays a role in a distinction between ‘known knowns’ (the results of a study; certain evidence) and ‘known unknowns’ (certain variables not researched; certain contexts not taken into account). ‘Unknown unknowns’ are possible risks, future outcomes, or consequences one is not aware of.
While ‘known unknowns’ may point to the direction of future research, ‘unknown unknowns’ point to the fundamental uncertainty in scientific research. By imagining possible events and occurrences, certain ‘unknown unknowns’ may become ‘known unknowns.’ Similarly, by pointing out certain ‘blind spots’ in a frame of reference, ‘unknown unknowns’ may become ‘known unknowns,’ or even ‘known knowns’ once they are researched (Box 10.10 ).
Q9: What is the significance of your data?
What do your findings say in respect to alternative explanations and plausible objections?
What are the limits of interpretation of your study?
Box 10.10: Qualitative Research
In qualitative research, many of the steps described in this chapter cannot be separated clearly, but rather merge under certain conditions. The following flowchart, borrowed from Damianakis and Woodford ( 2012 , p. 715) details various considerations when planning a research project in small connected communities, recruiting participants, collecting data, and disseminating results (Fig. 10.4 ).
Considerations when planning a research project (Damianakis and Woodford 2012 , p. 715)
11 Reporting and Dissemination of Research Findings
11.1 dissemination and responsibility.
Research findings are commonly reported in scientific outlets, such as peer reviewed journals and scholarly books, or at international congresses and academic conferences. Alternative ways to reach various other audiences may include: articles in popular journals and newspapers; brochures and leaflets tageting certain lay audiences; appearances in seminars for professionals; participation in think tank research; radio and television performances; hosting of podcasts; involvement in internet forums; providing training sessions; and individual or group counseling (for further discussion see Oliver 2010 ).
We shall not discuss all of these individual forms of communicating research findings, but instead we will flesh out the ethical implications of three different role responsibilities (Mitcham 2003 ) inherent in the task of a researcher engaging with their audiences.
11.2 Responsibility to Participant
Researchers carry a responsibility to inform their participants about the results of their work. How do they fulfill this requirement? Often they suffice to just offer the possibility to learn about the findings of their research projects, and this usually entails no more than notifying the participants when the report is published.
In many cases, this may be sufficient, especially when participants have simply filled out a questionnaire or took part in an experiment, and were not otherwise involved in the research project. Considering that oftentimes research participants are not typical readers of academic journals, no further action may be required on the part of the researcher.
However, if, as in qualitative research, or in action research, respondents have dedicated time and energy into research projects, or are involved in it to some degree, the responsibility of the researcher to inform them of their research findings would not end there.
In either case it may be worthwhile to consider how respondents are affected by the research, and whether or not some ‘aftercare’ is needed in the form of ad hoc reevaluation or debriefing (Box 10.11 ).
Box 10.11: Whose Perspective Prevails?
Reporting research findings can be precarious, as the following example of an unpublished qualitative study reveals. The research project, financially supported by several municipalities, aimed to analyze the perspectives of policy makers, healthcare providers, and their clients on homeless shelters.
In several interviews, some of the clients (homeless individuals) complained about the poor living conditions in one of the shelters, and the inadequate support offered by one of the healthcare providing parties therein. One interviewee said: ‘It’s a mess. At least that’s how I see it. If you want to help people, you should do it completely different.’ Several of these complaints were included in the first report released, which was subsequently sent to the parties involved in the research project, including the healthcare providers.
Shortly thereafter, an argument arose between the researcher and the healthcare provider that had been criticized. The healthcare provider objected to the ‘uncritical publication’ of these complaints, which they believed were baseless and even harmful.
The researcher offered the healthcare provider an opportunity to contradict the complaints in a separate section, which would be inserted as an addendum in the next report sent to the relevant parties. The healthcare provider declined, insisting that it should be the researcher themselves that rectified what they considered to be a ‘grave mistake.’ The researcher’s supposed portrayal of a ‘crooked image’ of the organization would cause them serious damage, the healthcare provider argued.
The researcher objected, contending that it was their academic duty to report all research findings and not to favor one party. The healthcare provider thereupon threatened to file an official complaint against the researcher, with whom they would no longer collaborate if the researcher would not concede.
How would you advise this researcher? Should they:
Back down, revise the text, and omit the complaints to rescue the working relationship with the healthcare provider?
Persevere as a scientist who has the duty to report findings as objectively as possible, even at the cost of a working relationship?
Which option would you choose, and why?
(Case was communicated to one of the authors of this chapter.)
11.3 Responsibility to the Research Community
There is also an obligation to communicate research findings to the scientific community, and this obligation goes hand in hand with the requirement to be critical of one another’s work in service of furthering scientific knowledge. There are a few issues that can be raised here too.
Academic engagement with private industry is rapidly growing, and this is impacting academic research, as a literature review by Perkman et al. ( 2013 ) reveals. Commercialization may enhance productivity (on the short run), but it also impacts the agendas of researchers and promotes an environment of secrecy.
Although research commissioned by third parties is becoming more prevalent, it should be made perfectly clear whose interests are at stake. It has therefore become common practice for researchers to be required to disclose any affiliations with outside institutions; reveal specific financial arrangements, including arrangements concerning intellectual property; as well as divulge any other ties of a social, political, or personal nature that might indicate a conflict of interest. In short, researchers must be hyper-transparent (for further discussion, see Chap. 8 ).
11.4 Responsibility to Civic and Professional Communities
Disseminating knowledge to civic and professional communities entails a different focus than those of academic communities. Inasmuch as sharing knowledge is geared towards the application of theoretical insights, the information provided needs to be of practical value, which can be used for the purpose of training, evaluation, risk assessment, or other needs (Fig. 10.5 ).
‘Vaxxed’, the 2016 documentary defending Wakefield and his followers
This means a shift away from asking whether research conclusions are true (the focus of academic knowledge dissemination), toward asking under what conditions and assumptions the findings are valid (the focus of civic and professional knowledge dissemination), and this task has a number of ethical implications. This is especially true when vulnerable communities or developing countries are involved and questions of social responsibility emerges. What are the researchers’ obligations towards these communities? Due to their economic, political, and intellectual power, what are the duties of the scientist in relation to society and public interests (Payne, 2000 ) (Box 10.12 )?
Q10: Which information are you obliged to share with your research participants?
Which ties, commitments, and affiliations are of importance to the understanding of your research?
Which moral responsibilities do you have towards others, specifically to those with a stake in your research?
Box 10.12: The Anti-Vax Movement. Who is Responsible for What?
A 1998 paper published in The Lancet (a high-ranking peer reviewed medical journal) connecting MMR (Measles, Mumps and Rubella) vaccination and the onset of autism sparked a worldwide anti-vaccination movement, raging still today.
The paper describes twelve children, most of whom were diagnosed with autism, who had bowel and behavioral problems. Eight of those children were purported to have developed autistic symptoms within a few days after they had been given the MMR vaccine. The story was picked up by several large UK newspapers and became the center of a nationwide debate in the subsequent years. This fervor eventually spread to other countries, which brought the paper’s principal author, Andrew Wakefield, considerable fame but also substantial criticism.
The scope of the paper was very limited (it was merely a series of ‘case reports’ and the sample size was only twelve). Based upon scientific norms, it would not have led to any reputable conclusion about a relationship between the MMR vaccination and autism. In fact, subsequent studies failed to find any such connection. These later studies, that did systematically probe the relationship between MMR vaccines and autism, received far less media coverage than the original Wakefield paper (see Ben Goldacre’s 2009 Bad Science for details about the study and its media reception).
The Lancet study was retracted when it was found to be seriously flawed on several accounts. Data had been falsified and the research was deemed unethical because of Wakefield’s ‘callous disregard for any distress or pain the children might suffer.’ Additionally, it was argued that the author himself was compromised because of undisclosed financial conflicts of interest. Wakefield’s medical license was revoked in 2010.
All of this did nothing to deter the anti-vax movement (of which Wakefield was, and still is, the poster child) or halt its momentum. There was even a 2016 documentary with a pro-Wakefield spin, called Vaxxed, which in the tradition of conspiracy theories, was advertised: ‘from cover-up to catastrophe.’
The number of people who refuse to vaccine their children has now risen to dangerous levels. These individuals believe, misinformed as they may be, that measles is harmless, vaccines are dangerous, and that the government has no business interfering in their lives. Even worse, some believe that the government conceals ‘the truth’ for the sake of a ‘powerful medical-industrial complex.’ The result of this flood of misinformation? Rates of this deadly disease have begun increasing yet again.
The anti-vax case raises the serious question of who is to blame and for what are they to be blamed. Framed differently, where does a researcher’s responsibility begin and where does it end? And when do outside parties begin to share in this responsibility?
How would you define the responsibilities of the following actors in this anti-vax case, with regard to their obligation to communicate scientific findings?
Start with Wakefield, as the Principal Investigator (PI) of the study, who has an obligation to report not only truthfully but also responsibly about his research findings. Given that he honestly believes that MMR vaccines relate to (or even cause the onset of) autism, what responsibilities do you think he has as a scientist to communicate his findings? Is he to be blamed for what some consider a dangerous hoax? And how about the other parties involved in this case? Flesh out the responsibilities of all the parties involved as best as you can.
Actor Responsibility
Wakefield (as PI of the study)
Editor of The Lancet
Editors and journalists of newspapers
Documentary filmmakers
Medical researchers
Governments / authorities
Medical doctors
Anti-vaxxers
12 Data Management and Storage
12.1 secure storage.
Secure storage of research data is at the core of research ethics, especially today, in the age of hacking and data breaches, and is subject to expanding regulation worldwide. It serves two basic purposes: verification and reuse (for secondary analysis).
At first sight, it may appear desirable to simply preserve all data collected during research and to make it available to any and all researchers, in order to prevent fraud and render science more efficient. However, the issue in preserving research data touches on its confidential nature (see Chap. 7 ). Additionally, the competing interests of researchers, research participants, and other stakeholders presents a number of challenges. To deepen the challenge, conflicting database legislation in different countries makes the preservation of all research data near impossible.
Decisions have to be made as to whether or not data will be made available, to whom will have access, how it will be accessed, where it will be stored, and how long it will be stored (for an overview of these considerations, see Johnson and Bullock 2009 ). We will briefly discuss the main aspects of these questions below.
12.2 Sensitive Data
Before decisions are made about whether data should be archived and shared, the data’s sensitivity should be assessed first. What is deemed ‘sensitive’ in a legal sense may differ from country to country, though many would agree that any data containing personal information would classify as such. Sensitive data would thus include a participant’s identity, information about their ethnicity, gender, political opinions, medical history, sexual orientation, religious background, and philosophical beliefs.
How should researcher’s deal with sensitive data? Several strategies have been developed to confront this important issue.
Data is stripped of its identifying properties by assigning a code to specific pieces of information. For example, the name of the participant is replaced by a number. If a key is preserved that enables re-identification (linking names to number, for example), privacy policy requires that the key be stored separately, and shall not be shared.
Other policies insist, however, that no key be kept at all, and that data collection be anonymized right from the beginning, such that all data effectively becomes anonymous the moment it is collected, and can never be linked to individual participants. This strategy is most fitting for quantitative research practices.
The true identity of the research participant or interviewee is concealed by giving them a pseudonym (an alias) and by changing other identifying details that might make identification possible. This strategy is more commonly practiced in qualitative studies, such as ethnography and case histories.
Some regard pseudonymization as an insufficient guarantee of privacy, as clever detective work may enable the identification of participants. For example, almost all of Freud’s patients have since been identified, even though he went to great lengths to hide their identities. Destroying field notes to protect a respondents’ privacy might seem to be a solution to this issue, though in practice, it raises questions of its own.
Co-ownership
In some research practices, respondents define the goals of the research project in close collaboration with the researchers and remain actively involved in other stages of the project, including the interpretation and dissemination of the results. Typically, this is the case in ‘action research’ or ‘community engaged research’ (see Friedman Ross et al. 2009 ).
By becoming closely involved, research participants become co-owners of the research project, but this often means that the researcher cannot offer the same ethical guarantees concerning confidentiality and anonymity, informed consent, and protection from harm as in other research methodologies (Williamson and Prosser 2002 ). For example, when school professionals conduct action research, confidentiality will be much more difficult to secure (Nolen and van der Putten 2007 ).
12.3 Making Data available to Whom?
There appears to be a near consensus that data should be archived (if only for reasons of verification). There is dissent, however, over whether secondary researchers or other parties should be allowed access to said archived research data, even after anonymization or pseudonymization.
Large longitudinal research projects almost by definition require the sharing of data, if only for reasons of efficiency. However, legislation in many countries has become much more stringent about protecting the rights of research participants, and this can become an obstacle in these projects.
Legislation safeguards the rights of participants to:
Have access to their own data
Have their data corrected or removed at their request
Refuse any other use of their data than agreed upon
Should the foundational principles of privacy be followed strictly, as some argue, no other researchers should be allowed access to data unless participants consent to secondary analysis. Others, however, maintain a more liberal perspective, arguing that if data is entirely anonymized, then these restricting conditions need not apply. But even if that is the case, collaboration between teams of researchers from different countries can become quite difficult given that each country may possess different privacy rules.
12.4 Storing Data Where?
Securing data implies storage in a safe place. This could be an encrypted university hard drive, or the implementation of encryption software. Agreements must be made in advance as to who has access, and to which parts of the hard drive. Additionally, the question is who will maintain the data once it is stored.
For security reasons, data should never be kept on personal computers, laptops, or other information carriers. Similarly, hardcopy receptacles of sensitive information should be kept in safe places, such as a vault or a locker that can opened by designated people only.
Finally, some considerations must be given to possible data breaches, data leakages, and the accidental loss of sensitive data. What are the procedures that must be followed in the event that sensitive information is lost, or even made public by accident? Who needs to be informed, and who has which responsibilities?
12.5 Archiving Data for How Long?
Lastly, decisions must be made as to how long data should be stored. Some conflicts of interest may arise here. Some universities and research institutions insist on the extended storage of data (at least 10 years) for verification purposes, to prevent fraud and/or uncover forms of misconduct. This requirement, however, conflicts with certain privacy laws, which may demand the destruction of unnecessary data as soon as possible. It may also conflict with contractual obligations made with study participants (for example, when consent to participate in a study is given under the condition that collected data is destroyed immediately after the study report has been published).
Q11: How should you ensure that any sensitive data is rendered in a form that is fitting for the research purpose and stored in a safe manner?
With whom can your data safely be shared?
What are the data security and safe storage procedures at your institute or university? How do they differ from the agreements you’ve made with you research participants?
What is a safe amount of time to archive sensitive data?
13 Conclusions
13.1 summary.
In this chapter, we followed a step by step approach to the ethical questions you need to answer when planning a research project. The objective was to learn what questions to ask, and to reflect on the answers as you plan and design a research project (see Box 10.6 ).
First, we discussed what research questions must be asked, to consider how important they are, and to think about what your research can contribute to. This was followed by a cost-benefit analysis of the risks inherent to research in the social sciences.
Second, we examined the various implications in using a variety of research techniques, including the invasion of integrity and the risks of deception . We followed this with a brief outline of informed consent protocols and how you can avoid harm and do good.
Third, we considered the ethical issues involved in collecting , analyzing , and interpreting data. What (un)intended consequences does your presence in the field have on the participants and research outcomes? What promises do you have to live up to?
Finally, we reviewed the responsibilities that come with being a researcher, specifically when sharing your findings with others. We concluded with a discussion of the various issues involved in safely storing data.
Followed from start to finish, this chapter aimed to ensure social science researchers were made aware of any potential ethical pitfalls that may be encountered. Are you ready now to submit your research proposal? (Fig. 10.6 )

Ready to submit your research proposal?
Suggested Reading
We highly recommend Research Ethics and Integrity for Social Scientists (Israel 2015 ) and The Student’s Guide of Research Ethics (Oliver 2010 ) as excellent reference books for students who want to learn more about the principles and philosophies of research ethics. Israel in particular gives an overview of the varying ethical policies found throughout the world. The Handbook of Social Research Ethics (edited by Mertens and Ginsberg 2009 ) offers an excellent selection of essays on a wide variety of topics in the history, theory, philosophy, and implementation of applied social research ethics. Especially worth mentioning is Chap. 8 , on IRBs, written by Spiegelman and Spears. Principles of Biomedical Ethics (Beauchamp and Childress 2001 ) is a classic in the field of research ethics. Diener and Crandall’s Ethics in Social and Behavioral Research ( 1978 ) offers an older yet still relevant insight into the field of research ethics as it first emerged.

14 Ethics Checklist
The following checklist may be useful when designing a research project. It is designed for students who do research under the supervision of a qualified researcher and can be adjusted at will and according to their own needs. It is emphatically not meant to replace local IRB’s protocols.
Appelbaum, P. S., Roth, L. H., Lidz, C. W., Benson, P., & Winslade, W. (1987). False hopes and best data: Consent to research and the therapeutic misconception. Hastings Center Report, 17 (2), 20–24. https://doi.org/10.2307/3562038 .
Article Google Scholar
Anderson, E. E. (2019). A proposal for fair compensation for research participants. The American Journal of Bioethics, 19 (9), 62–64. https://doi.org/10.1080/15265161.2019.1630501 .
Baumrind, D. (1964). Some thoughts of ethics of research: After reading Milgram’s study of obedience. American Psychologist, 19 (6), 421–423. https://psycnet.apa.org/doi/10.1037/h0040128 .
Beauchamp, T. L., & Childress, J. F. (2001). Principles of biomedical ethics . Oxford: Oxford University Press.
Google Scholar
Beck, H. P., Levinson, S., & Irons, G. (2009). Finding little Albert: A journey to John B. Watson’s infant laboratory. American Psychologist, 64 (7), 605–614. https://psycnet.apa.org/doi/10.1037/a0017234 .
Becker-Blease, K. A., & Freyd, J. J. (2006). Research participants telling the truth about their lives: The ethics of asking and not asking about abuse. American Psychologist, 61 (3), 218–226. https://doi.org/10.1037/0003-066X.61.3.218
Benatar, S. R. (2002). Reflections and recommendations on research ethics in developing countries. Social Science & Medicine, 54 (7), 1131–1141. https://doi.org/10.1016/S0277-9536(01)00327-6 .
Bracken-Roche, D., Bell, E., Macdonald, M. E., & Racine, E. (2017). The concept of ‘vulnerability’ in research ethics: An in-depth analysis of policies and guidelines. Health Research Policy and Systems, 15 (1), 8. https://doi.org/10.1186/s12961-016-0164-6 .
Brotsky, S. R., & Giles, D. (2007). Inside the “pro-ana” community: A covert online participant observation. Eating Disorders: The Journal of Treatment & Prevention, 15 (2), 93–109. https://doi.org/10.1080/10640260701190600 .
Burbules, N. (2009). Privacy and new technologies. In D. M. Mertens & P. E. Ginsberg (Eds.), The handbook of social research ethics (pp. 537–549). London: Sage.
Chapter Google Scholar
Castellano, M. B. (2014). Ethics of aboriginal research. In W. Teary, J. S. Gordon, & A. D. Renteln (Eds.), Global bioethics and human rights. Contemporary issues (pp. 273–288). Lanham: Rowman & Littlefield.
Christensen, L. (1988). Deception in psychological research: When is its use justified? Personality and Social Psychology Bulletin, 14 (4), 664–675.
Cohen, J. J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Erlbaum.
Cromer, L. D., Freyd, J. J., & Binder, A. K. (2006). What’s the risk in asking? Participant reaction to trauma history questions compared with reaction to other personal questions. Ethics and Behavior, 16 (4), 347–362. https://doi.org/10.1207/s15327019eb1604_5 .
Damianakis, T., & Woodford, M. R. (2012). Qualitative research with small connected communities: Generating new knowledge while upholding research ethics. Qualitative Health Research . https://doi.org/10.1016/j.fsigen.2012.10.002 .
DePrince, A. P., & Freyd, J. J. (2006). Costs and benefits of being asked about trauma history. Journal of Trauma Practice, 3 (4), 23–35. https://doi.org/10.1300/J189v03n04_02 .
Diener, E., & Crandall, R. (1978). Ethics in social and behavioral research . Oxford: University of Chicago Press.
Douglas, H. (2014). Values in Social Science. In N. Cartwright & E. Montuschi (Eds.), Philosophy of the social sciences. A new introduction (pp. 162–184). Oxford: Oxford University Press.
Elam, G., & Fenton, K. A. (2003). Researching sensitive issues and ethnicity: Lessons from sexual health. Ethnicity and Health, 8 (1), 15–27. https://doi.org/10.1080/13557850303557 .
Ellis, C. (2014). Telling secrets, revealing lives. Relational ethics in research with intimate others. Qualitative Inquiry, 19 (1), 3–29. https://doi.org/10.1177/2F1077800406294947 .
Eysenbach, G., & Till, J. E. (2001). Ethical issues in qualitative research on internet communities. British Medical Journal, 323 (7321), 1103–1105. https://doi.org/10.1136/bmj.323.7321.1103 .
Friedman Ross, L., Loup, A., Nelson, R. M., Botkin, J. R., Smith, G. R., & Gehler, S. (2009). The challenges of collaboration for academic and community partners in a research partnership: Points to consider. Journal of Empirical Research on Human Research Ethics, 5 (1), 19–31. https://doi.org/10.1525/2Fjer.2010.5.1.19 .
Goffman, A. (2014). On the run: Fugitive life in an American city . Chicago: University of Chicago Press.
Book Google Scholar
Goldacre, B. (2009). Bad science . London: Fourth Estate.
Guillemin, M., & Gillam, L. (2004). Ethics, reflexivity, and “ethically important moments” in research. Qualitative Inquiry, 10 (2), 261–280. https://doi.org/10.1177/2F1077800403262360 .
Haggerty, D. C. (2004). Ethics creep: Governing social science research in the name of ethics. Qualitative Sociology, 27 (4), 391–414.
Heinrich, J., Heine, S. J., & Norenzayan, A. (2010). The weirdest people in the world? Behavioral and Brain Sciences, 33 (2–3), 61–83. https://doi.org/10.1017/S0140525X0999152X .
Hey, J. D. (1998). Experimental economics and deception: A comment. Journal of Economic Psychology, 19 , 397–401.
Hjortskov, N., Rissén, D., Blangsted, A. K., et al. (2004). The effect of mental stress on heart rate variability and blood pressure during computer work. European Journal of Applied Physiology, 92 , 84–89. https://doi.org/10.1007/s00421-004-1055-z .
Hughes, J. (ed.) (2010). European Textbook on Ethics in Research. Brussels, European Union Directorate-General for Research.
Illes, J., Kirschen, M. P., Edwards, E., Stanford, L. R., Bandettini, P., Cho, M. K., Ford, P. J., Glover, G. H., Kulynych, J., Macklin, R., Michael, D. B., Wolf, S. M., et al. (2006). Incidental findings in brain imaging research. Science, 311 , 783–784. https://doi.org/10.1126/science.1124665 .
Israel, M. (2015). Research ethics and integrity for social scientists (2nd ed.). London: Sage.
Johnson, D., & Bullock, M. (2009). The ethics of data archiving: Issues from four perspectives. In D. Mertens & P. Ginsberg (Eds.), The handbook of social research ethics (pp. 214–228). London: Sage.
Khanlou, N., Koh, J. G., & Mill, C. (2008). Cultural identity and experiences of prejudice and discrimination of Afghan and Iranian immigrant youth. International Journal of Mental Health and Addiction, 6 (4), 494–513. https://doi.org/10.1007/s11469-008-9151-7 .
Kimmel, A. J. (1998). In defense of deception. American Psychologist, 53 , 803–805. https://psycnet.apa.org/doi/10.1037/0003-066X.53.7.803 .
Lawson, E. (2001). Informational and relational meanings of deception: Implications for deception methods in research. Ethics & Behavior, 11 (2), 115–130. https://doi.org/10.1207/S15327019EB1102_1 .
Liang, B., & Lu, H. (2006). Conducting fieldwork in China: Observations on collecting primary data regarding crime, law, and the criminal justice system. Journal of Contemporary Criminal Justice, 22 (2), 157–172. https://doi.org/10.1177/2F1043986206286918 .
Lubet, S. (2015). Ethics on the run. The New Rambler Review , May, 15–34. https://ssrn.com/abstract=2611742
Mark, M. M., & Lanz-Watson, A. L. (2011). Experiments and quasi-experiments in field settings. In A. T. Panter & S. K. Sterba (Eds.), Handbook of ethics in quantitative evaluation (pp. 185–209). New York: Routledge.
Marshall, R. D., Spitzer, R. L., Vaughan, R., Mellman, L. A., MacKinon, R. A., & Roose, S. P. (2001). Assessing the subjective experience of being a participant in psychiatric research. American Journal of Psychiatry, 158 , 319–321.
McAreavey, R., & Das, C. (2013). A delicate balancing act: Negotiating with gatekeepers for ethical research when researching minority communities. International Journal of Qualitative Methods, 12 (1), 113–131. https://doi.org/10.1177/2F160940691301200102 .
Mertens, D., & Ginsberg, P. E. (Eds.). (2009). The handbook of social research ethics . London: Sage.
Mikesell, L., Bromley, E., & Khodyakov, D. (2013). Ethical community-engaged research: A literature review. American Journal of Public Health, 103 (12), e7–e14. https://doi.org/10.2105/AJPH.2013.301605 .
Miller, F. G., Mello, M. M., & Joffe, S. (2008). Incidental findings in human subjects research: What do investigators owe research participants? Journal of Law, Medicine & Ethics, 36 (2), 271–279. https://doi.org/10.1111/j.1748-720X.2008.00269.x .
Mitcham, C. (2003). Co-responsibility for research integrity. Science and Engineering Ethics, 9 (2), 273–290. https://doi.org/10.1007/s11948-003-0014-0 .
Mustanski, B. (2011). Ethical and regulatory issues with conducting sexuality research with LGBT adolescents: A call to action for a scientifically informed approach. Archives of Sexual Behavior, 40 (4), 673–686. https://doi.org/10.1007/s10508-011-9745-1 .
Newman, E., Walker, E. A., & Gefland, A. (1999). Assessing the ethical costs and benefits of trauma-focused research. Law, Ethics, and Psychiatry in the General Hospital, 21 (3), 187–196.
Nie, J. B. (2001). Is informed consent not applicable in China? Intellectual flaws of the “cultural difference argument”. Formosan Journal of Medical Humanities, 2 (1–2), 67–74.
Nolen, A. L., & van der Putten, J. (2007). Action research in education: Addressing gaps in ethical principles and practices. Educational Researcher, 36 (7), 401–407. https://doi.org/10.3102/2F0013189X07309629 .
Numan, D., & Di Domenico, M. L. (2012). Market research and the ethics of big data. International Journal of Market Research, 55 (4), 505–520. https://doi.org/10.2501/2FIJMR-2013-015 .
O’Reilly, M., & Parker, N. (2012). ‘Unsatisfactory saturation’: A critical exploration of the notion of saturated sample sizes in qualitative research. Qualitative Research, 13 (2), 190–197. https://doi.org/10.1177/2F1468794112446106 .
Oliver, P. (2010). The student’s guide to research ethics (2nd ed.). Berkshire: Open University Press.
Ortmann, A., & Hertwig, R. (2002). The costs of deception: Evidence from psychology. Experimental Economics, 5 , 111–131. https://doi.org/10.1023/A:1020365204768 .
Parker, L., London, A. J., & Aronson, J. D. (2012). Incidental findings in the use of DNA to identify human remains: An ethical assessment. Forensic Science International Genetics, 7 (2), 221–229. https://doi.org/10.1016/j.fsigen.2012.10.002 .
Payne, S. L. (2000). Challenges for research ethics and moral knowledge construction in the applied social sciences. Journal of Business Ethics, 26 (4), 307–318. https://doi.org/10.1023/A:1006173106143 .
Perkman, M., Tartari, V., McKelvey, M., Autio, E., Broström, A., D’Esti, P., Fini, R., Geuna, A., Grimaldi, R., Hughes, A., Krabel, S., Kitson, M., Lierena, P., Lissoni, F., Salder, A., & Sobrero, M. (2013). Academic engagement and commercialization: A review of the literature on university-industry relations. Research Policy, 42 (2), 423–442. https://doi.org/10.1016/j.respol.2012.09.007 .
Ran, M. S., Ziang, M. Z., Chan, C. L. W., Leff, J., Simpson, P., Huang, M. S., Shan, Y. H., & Li, S. G. (2003). Effectiveness of psychoeducational intervention for rural Chinese families experiencing schizophrenia. Social Psychiatry and Psychiatric Epidemiology, 38 (2), 69–75. https://doi.org/10.1007/s00127-003-0601-z .
Rangle, L., Embry, T., & MacLeon, T. (2001). Active and passive consent: A comparison of actual research with children. Ethical Human Sciences and Services, 3 (1), 23–31. https://doi.org/10.1891/1523-150X.3.1.23 .
Schenk, K. D., & RamaRao, S. (2016). Ethical considerations of conducting research among children and young people affected by HIV: A view from an ethics review board. In P. Liamputtong (Ed.), Children and young people living with HIV/AIDS: A cross-cultural perspective (pp. 445–457). Cham: Springer.
Schneider, C. (2015). The censor’s hand: The misregulation of human-subject research . Cambridge: The MIT Press.
Scott, A. (2017). My IRB nightmare. Slate Star Codex . Retrieved March 17, 2020, from http://slatestarcodex.com/2017/08/29/my-irb-nightmare/#comments
Szala-Meneok, K. (2009). Ethical research with older adults. In D. M. Mertens & P. E. Ginsberg (Eds.), The handbook of social research ethics (pp. 507–517). London: Sage.
Tangwa, G. B. (2014). Ethics, human rights, and sexual/reproductive health in Africa: Exploratory sociocultural considerations. In W. Teary, J. S. Gordon, & A. D. Renteln (Eds.), Global bioethics and human rights: Contemporary issues (pp. 160–173). Lanham: Rowman & Littlefield.
Tolich, M. (2014). What can Milgram and Zimbardo teach ethics committees and qualitative researchers about minimizing harm? Research Ethics, 10 (2), 86–96. https://doi.org/10.1177/2F1747016114523771 .
Van Bommel, M., van Prooijen, J.-W., Elffers, H., & van Lange, P. A. M. (2014). Intervene to be seen: The power of a camera in attenuating the bystander effect. Social Psychological and Personality Science, 5 (4), 459–466. https://doi.org/10.1177/1948550613507958 .
Vargas, L. A., & Montaya, M. E. (2009). Involving minors in research. In D. M. Mertens & P. E. Ginsberg (Eds.), The handbook of social research ethics (pp. 489–509). London: Sage.
Vernooij, M. W., Arfan Ikram, M., Tanghe, H. L., Vincent, A. J. P. E., Hofman, A., Krestin, G. P., Niessen, W. J., Breteler, M. M. B., & van der Lugt, A. (2007). Incidental findings on brain MRI in the general population. The New England Journal of Medicine, 358 (18), 1821–1828. https://doi.org/10.1056/NEJMoa070972 .
Williamson, G. R., & Prosser, S. (2002). Action research: Politics, ethics and participation. Journal of Advanced Nursing, 40 (5), 587–593. https://doi.org/10.1046/j.1365-2648.2002.02416.x .
Download references
Author information
Authors and affiliations.
Department of Interdisciplinary Social Science, Utrecht University, Utrecht, The Netherlands
You can also search for this author in PubMed Google Scholar
Section Editor information
1 electronic supplementary materials.
AF_Ethical clip 10 (MP4 268358 kb)
Sample Informed Consent Form
[Adapt this form to your proposed research project].
Information about Participation in a Research Study at [your university or research institute]
[Title of the study:]
INTRODUCTION: Thank you for taking part in this study about [give brief explanation of the study]. Below is a description of the research procedures and an explanation of your rights as a research participant. In accordance with the ethics code of the [local institution], you are asked to read this information carefully. You are entitled to receive a copy of this form should you agree to proceed under the terms stated.
GENERAL INFORMATION: The purpose of this research is [give brief description of study purpose here]. This research is funded by [insert here, if applcable]. The potential conflicts of interest are [describe any that are known]. [OR] There are no known conflicts of interest in the conducting of this research study.
Your participation will last for approximately [duration estimate] and will take place at [location] at the following times [dates/times]. You will receive [insert reimbursement, i.e.. number of PPU, amount of money, a chance to win a voucher or, ‘no reimbursement’] for your participation in this study.
PROCEDURE: During this study, you will be asked to [insert brief description of what the participant will do]. You are aware that [describe any risks that are known]. [OR] There are no known or anticipated risks associated with participation in this study.
You have the right to end your participation at any moment, without citing a reason. If you choose to end your participation before the study terminates, you [will / will not] be reimbursed.
Regarding the use of your data, the following conditions apply:
Your data will be used for scientific purposes, including publication. Only the researchers have access to the data [OR] The data will be made available for other researchers on condition of confidentiality.
Your data will be handled and stored confidentially. This means that your data cannot be traced back to you. Specifically, the researcher will use a code number instead of your name to save your data.
[If the data from the study will be personally identifiable] The code number and other personally identifiable information, such as names, will be saved separately from each other in a secure location.
After publication, only the data that is necessary for the verification of the study results will be kept and stored safely for a minimum of 10 years and deleted once it is no longer needed. [OR] Personally identifiable data will be shared only if it is scientifically required to verify reported results.
You have the right to withhold any responses you have provided from subsequent analysis. This means we will not use your data for this or any follow-up research, nor will we share it anonymously for open science purposes. You can decide to withdraw your data until the study results are accepted for publication, or until the data is cleared of any and all identifying information, such that no-one will be able to trace you.
OFFER TO ANSWER QUESTIONS: You are now given the opportunity to ask questions. If you have any further questions or complaints about this study, you may contact the researcher, [name(s) of researcher(s) and email(s)—phone number(s) can be added if researchers prefer to use that method], of [your university or research institute].
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2020 The Author(s)
About this chapter
Bos, J. (2020). Research Ethics Step by Step. In: Research Ethics for Students in the Social Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-48415-6_10
Download citation
DOI : https://doi.org/10.1007/978-3-030-48415-6_10
Published : 15 September 2020
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-48414-9
Online ISBN : 978-3-030-48415-6
eBook Packages : Religion and Philosophy Philosophy and Religion (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Initial Review
- Post-Approval Submission
- Submission Document Format
- How to Write Research Proposal
- How to Write Informed Consent Form
- Clinical Trial vs Non-Clinical Trial
- Protecting Research Subject Information
- Importance of De-identifying Research Data
- Template & Guideline
- External link
iium rESEARCH eTHICS cOMMITTEE
How to write research proposals.

Writing a research proposal is an essential step in the process of initiating and conducting a research project. A well-crafted research proposal serves as a roadmap for your research and helps convince others (such as funding agencies or academic institutions) of the importance and feasibility of your study. Here’s a general guide on how to write a research proposal:
Start with a clear and concise title that reflects the main theme of your research.
2. Introduction
Provide a brief introduction to the research problem, background information, and the context of your study. Clearly state the research question or hypothesis.
3. Literature Review
Review existing literature related to your research topic. Discuss the key theories, findings, and gaps in the current knowledge. This section helps you position your research within the broader academic context.
4. Objectives and Research Questions
Specify the objectives of your study and the specific research questions you aim to address. Ensure that these are clear, focused, and directly related to your research problem.
5. Methodology
Detail the research design, methods, and techniques you plan to use. Include information about your sample size, data collection methods, and data analysis procedures. Discuss why these methods are appropriate for your study.
6. Significance of the Study
Explain the potential impact and significance of your research. Describe how your study contributes to existing knowledge and why it is relevant to the academic or practical field.
7. Theoretical Framework (if applicable)
If your research is grounded in a particular theoretical framework, provide a brief explanation of the framework and how it informs your study.
8. Research Timeline
Outline a timeline for your research, including key milestones and deadlines. This demonstrates that you have a realistic plan for completing the project.
9. Budget (if applicable)
If your research requires funding, provide a detailed budget. Include costs for materials, equipment, travel, and any other relevant expenses.
10. Ethical Considerations
Discuss any ethical considerations associated with your research, including how you plan to address them.
11. Conclusion
Summarize the main points of your proposal and restate the importance of your research. Conclude by emphasizing why your study is worth pursuing.
12. References
Include a list of references cited in your proposal. Ensure proper citation in a consistent format (e.g., APA, MLA).
13. Appendices (if necessary)
Attach any additional materials, such as survey instruments, consent forms, or supplementary information, in the appendices.
General Tips:
- Follow the guidelines provided by the funding agency or academic institution.
- Write in a clear, concise, and formal style.
- Proofread and edit your proposal for clarity, coherence, and correctness.
Remember that a well-organized and persuasive research proposal is crucial for gaining support and approval for your research project. Adjust the structure and content based on the specific requirements of your target audience and the nature of your research.
How To Write A Research Proposal
A Straightforward How-To Guide (With Examples)
By: Derek Jansen (MBA) | Reviewed By: Dr. Eunice Rautenbach | August 2019 (Updated April 2023)
Writing up a strong research proposal for a dissertation or thesis is much like a marriage proposal. It’s a task that calls on you to win somebody over and persuade them that what you’re planning is a great idea. An idea they’re happy to say ‘yes’ to. This means that your dissertation proposal needs to be persuasive , attractive and well-planned. In this post, I’ll show you how to write a winning dissertation proposal, from scratch.
Before you start:
– Understand exactly what a research proposal is – Ask yourself these 4 questions
The 5 essential ingredients:
- The title/topic
- The introduction chapter
- The scope/delimitations
- Preliminary literature review
- Design/ methodology
- Practical considerations and risks
What Is A Research Proposal?
The research proposal is literally that: a written document that communicates what you propose to research, in a concise format. It’s where you put all that stuff that’s spinning around in your head down on to paper, in a logical, convincing fashion.
Convincing is the keyword here, as your research proposal needs to convince the assessor that your research is clearly articulated (i.e., a clear research question) , worth doing (i.e., is unique and valuable enough to justify the effort), and doable within the restrictions you’ll face (time limits, budget, skill limits, etc.). If your proposal does not address these three criteria, your research won’t be approved, no matter how “exciting” the research idea might be.
PS – if you’re completely new to proposal writing, we’ve got a detailed walkthrough video covering two successful research proposals here .

How do I know I’m ready?
Before starting the writing process, you need to ask yourself 4 important questions . If you can’t answer them succinctly and confidently, you’re not ready – you need to go back and think more deeply about your dissertation topic .
You should be able to answer the following 4 questions before starting your dissertation or thesis research proposal:
- WHAT is my main research question? (the topic)
- WHO cares and why is this important? (the justification)
- WHAT data would I need to answer this question, and how will I analyse it? (the research design)
- HOW will I manage the completion of this research, within the given timelines? (project and risk management)
If you can’t answer these questions clearly and concisely, you’re not yet ready to write your research proposal – revisit our post on choosing a topic .
If you can, that’s great – it’s time to start writing up your dissertation proposal. Next, I’ll discuss what needs to go into your research proposal, and how to structure it all into an intuitive, convincing document with a linear narrative.
The 5 Essential Ingredients
Research proposals can vary in style between institutions and disciplines, but here I’ll share with you a handy 5-section structure you can use. These 5 sections directly address the core questions we spoke about earlier, ensuring that you present a convincing proposal. If your institution already provides a proposal template, there will likely be substantial overlap with this, so you’ll still get value from reading on.
For each section discussed below, make sure you use headers and sub-headers (ideally, numbered headers) to help the reader navigate through your document, and to support them when they need to revisit a previous section. Don’t just present an endless wall of text, paragraph after paragraph after paragraph…
Top Tip: Use MS Word Styles to format headings. This will allow you to be clear about whether a sub-heading is level 2, 3, or 4. Additionally, you can view your document in ‘outline view’ which will show you only your headings. This makes it much easier to check your structure, shift things around and make decisions about where a section needs to sit. You can also generate a 100% accurate table of contents using Word’s automatic functionality.

Ingredient #1 – Topic/Title Header
Your research proposal’s title should be your main research question in its simplest form, possibly with a sub-heading providing basic details on the specifics of the study. For example:
“Compliance with equality legislation in the charity sector: a study of the ‘reasonable adjustments’ made in three London care homes”
As you can see, this title provides a clear indication of what the research is about, in broad terms. It paints a high-level picture for the first-time reader, which gives them a taste of what to expect. Always aim for a clear, concise title . Don’t feel the need to capture every detail of your research in your title – your proposal will fill in the gaps.
Need a helping hand?
Ingredient #2 – Introduction
In this section of your research proposal, you’ll expand on what you’ve communicated in the title, by providing a few paragraphs which offer more detail about your research topic. Importantly, the focus here is the topic – what will you research and why is that worth researching? This is not the place to discuss methodology, practicalities, etc. – you’ll do that later.
You should cover the following:
- An overview of the broad area you’ll be researching – introduce the reader to key concepts and language
- An explanation of the specific (narrower) area you’ll be focusing, and why you’ll be focusing there
- Your research aims and objectives
- Your research question (s) and sub-questions (if applicable)
Importantly, you should aim to use short sentences and plain language – don’t babble on with extensive jargon, acronyms and complex language. Assume that the reader is an intelligent layman – not a subject area specialist (even if they are). Remember that the best writing is writing that can be easily understood and digested. Keep it simple.

Note that some universities may want some extra bits and pieces in your introduction section. For example, personal development objectives, a structural outline, etc. Check your brief to see if there are any other details they expect in your proposal, and make sure you find a place for these.
Ingredient #3 – Scope
Next, you’ll need to specify what the scope of your research will be – this is also known as the delimitations . In other words, you need to make it clear what you will be covering and, more importantly, what you won’t be covering in your research. Simply put, this is about ring fencing your research topic so that you have a laser-sharp focus.
All too often, students feel the need to go broad and try to address as many issues as possible, in the interest of producing comprehensive research. Whilst this is admirable, it’s a mistake. By tightly refining your scope, you’ll enable yourself to go deep with your research, which is what you need to earn good marks. If your scope is too broad, you’re likely going to land up with superficial research (which won’t earn marks), so don’t be afraid to narrow things down.
Ingredient #4 – Literature Review
In this section of your research proposal, you need to provide a (relatively) brief discussion of the existing literature. Naturally, this will not be as comprehensive as the literature review in your actual dissertation, but it will lay the foundation for that. In fact, if you put in the effort at this stage, you’ll make your life a lot easier when it’s time to write your actual literature review chapter.
There are a few things you need to achieve in this section:
- Demonstrate that you’ve done your reading and are familiar with the current state of the research in your topic area.
- Show that there’s a clear gap for your specific research – i.e., show that your topic is sufficiently unique and will add value to the existing research.
- Show how the existing research has shaped your thinking regarding research design . For example, you might use scales or questionnaires from previous studies.
When you write up your literature review, keep these three objectives front of mind, especially number two (revealing the gap in the literature), so that your literature review has a clear purpose and direction . Everything you write should be contributing towards one (or more) of these objectives in some way. If it doesn’t, you need to ask yourself whether it’s truly needed.
Top Tip: Don’t fall into the trap of just describing the main pieces of literature, for example, “A says this, B says that, C also says that…” and so on. Merely describing the literature provides no value. Instead, you need to synthesise it, and use it to address the three objectives above.

Ingredient #5 – Research Methodology
Now that you’ve clearly explained both your intended research topic (in the introduction) and the existing research it will draw on (in the literature review section), it’s time to get practical and explain exactly how you’ll be carrying out your own research. In other words, your research methodology.
In this section, you’ll need to answer two critical questions :
- How will you design your research? I.e., what research methodology will you adopt, what will your sample be, how will you collect data, etc.
- Why have you chosen this design? I.e., why does this approach suit your specific research aims, objectives and questions?
In other words, this is not just about explaining WHAT you’ll be doing, it’s also about explaining WHY. In fact, the justification is the most important part , because that justification is how you demonstrate a good understanding of research design (which is what assessors want to see).
Some essential design choices you need to cover in your research proposal include:
- Your intended research philosophy (e.g., positivism, interpretivism or pragmatism )
- What methodological approach you’ll be taking (e.g., qualitative , quantitative or mixed )
- The details of your sample (e.g., sample size, who they are, who they represent, etc.)
- What data you plan to collect (i.e. data about what, in what form?)
- How you plan to collect it (e.g., surveys , interviews , focus groups, etc.)
- How you plan to analyse it (e.g., regression analysis, thematic analysis , etc.)
- Ethical adherence (i.e., does this research satisfy all ethical requirements of your institution, or does it need further approval?)
This list is not exhaustive – these are just some core attributes of research design. Check with your institution what level of detail they expect. The “ research onion ” by Saunders et al (2009) provides a good summary of the various design choices you ultimately need to make – you can read more about that here .
Don’t forget the practicalities…
In addition to the technical aspects, you will need to address the practical side of the project. In other words, you need to explain what resources you’ll need (e.g., time, money, access to equipment or software, etc.) and how you intend to secure these resources. You need to show that your project is feasible, so any “make or break” type resources need to already be secured. The success or failure of your project cannot depend on some resource which you’re not yet sure you have access to.
Another part of the practicalities discussion is project and risk management . In other words, you need to show that you have a clear project plan to tackle your research with. Some key questions to address:
- What are the timelines for each phase of your project?
- Are the time allocations reasonable?
- What happens if something takes longer than anticipated (risk management)?
- What happens if you don’t get the response rate you expect?
A good way to demonstrate that you’ve thought this through is to include a Gantt chart and a risk register (in the appendix if word count is a problem). With these two tools, you can show that you’ve got a clear, feasible plan, and you’ve thought about and accounted for the potential risks.
Tip – Be honest about the potential difficulties – but show that you are anticipating solutions and workarounds. This is much more impressive to an assessor than an unrealistically optimistic proposal which does not anticipate any challenges whatsoever.
Final Touches: Read And Simplify
The final step is to edit and proofread your proposal – very carefully. It sounds obvious, but all too often poor editing and proofreading ruin a good proposal. Nothing is more off-putting for an assessor than a poorly edited, typo-strewn document. It sends the message that you either do not pay attention to detail, or just don’t care. Neither of these are good messages. Put the effort into editing and proofreading your proposal (or pay someone to do it for you) – it will pay dividends.
When you’re editing, watch out for ‘academese’. Many students can speak simply, passionately and clearly about their dissertation topic – but become incomprehensible the moment they turn the laptop on. You are not required to write in any kind of special, formal, complex language when you write academic work. Sure, there may be technical terms, jargon specific to your discipline, shorthand terms and so on. But, apart from those, keep your written language very close to natural spoken language – just as you would speak in the classroom. Imagine that you are explaining your project plans to your classmates or a family member. Remember, write for the intelligent layman, not the subject matter experts. Plain-language, concise writing is what wins hearts and minds – and marks!
Let’s Recap: Research Proposal 101
And there you have it – how to write your dissertation or thesis research proposal, from the title page to the final proof. Here’s a quick recap of the key takeaways:
- The purpose of the research proposal is to convince – therefore, you need to make a clear, concise argument of why your research is both worth doing and doable.
- Make sure you can ask the critical what, who, and how questions of your research before you put pen to paper.
- Title – provides the first taste of your research, in broad terms
- Introduction – explains what you’ll be researching in more detail
- Scope – explains the boundaries of your research
- Literature review – explains how your research fits into the existing research and why it’s unique and valuable
- Research methodology – explains and justifies how you will carry out your own research
Hopefully, this post has helped you better understand how to write up a winning research proposal. If you enjoyed it, be sure to check out the rest of the Grad Coach Blog . If your university doesn’t provide any template for your proposal, you might want to try out our free research proposal template .

Psst… there’s more!
This post is an extract from our bestselling short course, Research Proposal Bootcamp . If you want to work smart, you don't want to miss this .
You Might Also Like:

30 Comments
Thank you so much for the valuable insight that you have given, especially on the research proposal. That is what I have managed to cover. I still need to go back to the other parts as I got disturbed while still listening to Derek’s audio on you-tube. I am inspired. I will definitely continue with Grad-coach guidance on You-tube.
Thanks for the kind words :). All the best with your proposal.
First of all, thanks a lot for making such a wonderful presentation. The video was really useful and gave me a very clear insight of how a research proposal has to be written. I shall try implementing these ideas in my RP.
Once again, I thank you for this content.
I found reading your outline on writing research proposal very beneficial. I wish there was a way of submitting my draft proposal to you guys for critiquing before I submit to the institution.
Hi Bonginkosi
Thank you for the kind words. Yes, we do provide a review service. The best starting point is to have a chat with one of our coaches here: https://gradcoach.com/book/new/ .
Hello team GRADCOACH, may God bless you so much. I was totally green in research. Am so happy for your free superb tutorials and resources. Once again thank you so much Derek and his team.
You’re welcome, Erick. Good luck with your research proposal 🙂
thank you for the information. its precise and on point.
Really a remarkable piece of writing and great source of guidance for the researchers. GOD BLESS YOU for your guidance. Regards
Thanks so much for your guidance. It is easy and comprehensive the way you explain the steps for a winning research proposal.
Thank you guys so much for the rich post. I enjoyed and learn from every word in it. My problem now is how to get into your platform wherein I can always seek help on things related to my research work ? Secondly, I wish to find out if there is a way I can send my tentative proposal to you guys for examination before I take to my supervisor Once again thanks very much for the insights
Thanks for your kind words, Desire.
If you are based in a country where Grad Coach’s paid services are available, you can book a consultation by clicking the “Book” button in the top right.
Best of luck with your studies.
May God bless you team for the wonderful work you are doing,
If I have a topic, Can I submit it to you so that you can draft a proposal for me?? As I am expecting to go for masters degree in the near future.
Thanks for your comment. We definitely cannot draft a proposal for you, as that would constitute academic misconduct. The proposal needs to be your own work. We can coach you through the process, but it needs to be your own work and your own writing.
Best of luck with your research!
I found a lot of many essential concepts from your material. it is real a road map to write a research proposal. so thanks a lot. If there is any update material on your hand on MBA please forward to me.
GradCoach is a professional website that presents support and helps for MBA student like me through the useful online information on the page and with my 1-on-1 online coaching with the amazing and professional PhD Kerryen.
Thank you Kerryen so much for the support and help 🙂
I really recommend dealing with such a reliable services provider like Gradcoah and a coach like Kerryen.
Hi, Am happy for your service and effort to help students and researchers, Please, i have been given an assignment on research for strategic development, the task one is to formulate a research proposal to support the strategic development of a business area, my issue here is how to go about it, especially the topic or title and introduction. Please, i would like to know if you could help me and how much is the charge.
This content is practical, valuable, and just great!
Thank you very much!
Hi Derek, Thank you for the valuable presentation. It is very helpful especially for beginners like me. I am just starting my PhD.
This is quite instructive and research proposal made simple. Can I have a research proposal template?
Great! Thanks for rescuing me, because I had no former knowledge in this topic. But with this piece of information, I am now secured. Thank you once more.
I enjoyed listening to your video on how to write a proposal. I think I will be able to write a winning proposal with your advice. I wish you were to be my supervisor.
Dear Derek Jansen,
Thank you for your great content. I couldn’t learn these topics in MBA, but now I learned from GradCoach. Really appreciate your efforts….
From Afghanistan!
I have got very essential inputs for startup of my dissertation proposal. Well organized properly communicated with video presentation. Thank you for the presentation.
Wow, this is absolutely amazing guys. Thank you so much for the fruitful presentation, you’ve made my research much easier.
this helps me a lot. thank you all so much for impacting in us. may god richly bless you all
How I wish I’d learn about Grad Coach earlier. I’ve been stumbling around writing and rewriting! Now I have concise clear directions on how to put this thing together. Thank you!
Fantastic!! Thank You for this very concise yet comprehensive guidance.
Even if I am poor in English I would like to thank you very much.
Thank you very much, this is very insightful.
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Sex Transm Dis AIDS
- v.32(2); Jul-Dec 2011
Ethical considerations in scientific writing
Jane d. carver.
Department of Pediatrics, University of South Florida, and Clinical and Translational Science Institute (USF-CTSI), Tampa, Florida, USA
Patricia J. Emmanuel
Ritu parchure.
1 Prayas, Pune, India
INTRODUCTION
Fostering scientific advancement requires strict adherence to ethical guidelines for research and scientific writing. Several professional organizations have policies to address the ethics associated with scientific writing and publishing, including the Committee on Publication Ethics and the International Council of Medical Journal Editors (ICMJE); the majority of medical journals follow the ICMJE's Uniform Guidelines. We discuss two issues related to ethics in scientific writing: Plagiarism and authorship. Plagiarism, the most common form of scientific misconduct, is defined as the appropriation of another person's ideas, processes, results or words without giving appropriate credit. While plagiarism is often intentional, it may be unintentional due to confusion regarding the definition of plagiarism and how to avoid it. Other forms of plagiarism include self-plagiarism, whereby authors copy large parts of one of their previous manuscripts word-for-word. Duplicate publication is a form of plagiarism that occurs when an author submits a previously-published work as if it were original. An increasing number of manuscripts are retracted each year due to duplicate publication. The incidence of plagiarism is of particular concern among international trainees in the U.S. and in countries where English is not the primary language, and is often due to issues related to language barriers. The major issues related to authorship include determination of author responsibilities and author order. Awarding authorship to people who have not made sufficient contributions conveys benefit to them inappropriately and it reduces benefit to those who actually contributed to the work, while denying authorship to deserving contributors is a widespread violation of scientific integrity.
The benefits of research can only be realized if results of investigations are published in the literature for others to replicate and expand upon. Fostering scientific advancement requires strict adherence to ethical guidelines for research and scientific writing. Here, we discuss two issues related to ethics in scientific writing: plagiarism and authorship. Violations of the ethical principles associated with these issues are considered as scientific misconduct. However, authors and academic institutions often have difficulty in defining and addressing these complex issues. Fortunately, several professional organizations have developed policies to address these and other issues associated with the ethics of scientific writing. These policies can be readily adopted – and adapted – by academic institutions, but the process still requires that the policies be consistently adhered to. The Committee on Publication Ethics (COPE)-[ 1 ] defines best practice in the ethics of scholarly publication. The COPE's Code of Conduct and Best Practice Guidelines for Journal Editors, ascribed to by many major journals, defines ethical violations that involve publication issues, and provides guidelines for editors and publishers in dealing with these violations. The International Council of Medical Journal Editors (ICMJE)[ 2 ] developed the uniform requirements for manuscripts submitted to biomedical journals. The majority of medical journals follow the uniform guidelines, which provide guidance on many issues including plagiarism and authorship standards. The U.S. Office of Research Integrity (ORI)[ 3 ] oversees and directs public health research in the U.S. The ORI develops policies and procedures related to detecting, investigating and preventing research misconduct, and it implements programs to promote research integrity.
While preparing his dissertation, a graduate student used a colleague's previously-submitted paper to compose much of the introduction and background sections. The professor recognized the duplication and questioned the student. The student argued that the methods, results and discussion section are all original, and the background is mostly common knowledge. He admitted to using the colleague's paper but felt that he had changed enough words, and that citation wasn′t necessary because the information was common knowledge.
The U.S. Office of Science and Technology defines plagiarism as “the appropriation of another person's ideas, processes, results or words without giving appropriate credit, including those obtained through confidential review of others’ research proposal and manuscripts.”[ 4 ] Although plagiarism is considered as a form of scientific misconduct, it is often unintentional. Inexperienced writers and trainees may not be aware of the importance of strict adherence to plagiarism guidelines, they may be confused by vague and conflicting definitions of plagiarism, faculty may assume that trainees understand what plagiarism is and how to avoid it, and authors often have difficulty in paraphrasing complex ideas or methods.[ 5 , 6 ] Further complicating the issue is that institutions in some countries may not require strict adherence to plagiarism guidelines.
Plagiarism, the most common form of scientific misconduct, occurs quite often among students and faculty. Studies have documented persistent plagiarism among medical students, and have found that explicit warnings may not be enough to deter students from engaging in plagiarism.[ 7 ] Faculty at research institutions may succumb to plagiarism due to the tremendous pressure to publish their work, which is essential to effectively compete for grant money and to advance their careers.
It has been noted that the incidence of plagiarism is higher among international versus domestic trainees in the U.S. This difference is mainly attributed to differing perspectives of international students toward plagiarism, the lack of formal policies on research misconduct at their home institutions, and language barriers causing difficulties in writing English.[ 6 ] Plagiarism in countries where English is not the primary language is also a significant concern.[ 7 ] English is often the preferred language to communicate scientific ideas and results, and there is increasing pressure to publish papers in reputable English-language journals. However, many faculty and trainees are not skilled in expressing complex ideas in English. This language barrier, along with the ease of internet searches and the ability to “cut and paste” verbiage from Web pages, contribute to the increasing incidence of plagiarism. In all academic settings, the increasing pressure to publish as an important step in advancing careers further contributes to the increasing incidence of plagiarism. The Indian government, in particular, has expressed concern about the country's low research output, and its revised rules for academic promotion link the number of published papers to promotions.[ 8 ] If institutions and faculty are to be competitive in the global research arena, better policies to address research misconduct need to be developed, and training in the skill of scientific writing needs to be recognized as a critically important priority.
Several different forms of plagiarism encountered in scientific writing
Intentional plagiarism, in which one knowingly lifts text directly from other authors without giving appropriate credit, is the most common form of plagiarism. Fisher and Zigmond[ 5 ] believe the common factors that underlie intentional plagiarism are an individual's strong desire to succeed, coupled with a lack of time and lack of interest in learning how to write properly. As in the case study above, some authors may view “common knowledge” in their field quite broadly. However, even basic background information needs to be properly cited, both to give credit to the original author(s) and to aid readers in finding the information provided. When compiling background and introduction sections, it can be easy to lift phrases directly from notes taken from primary sources. However, it is important to remember that taking text directly from a source requires proper citation and the use of quotation marks when word-for-word text is cited.
Self-plagiarism
Also known as text recycling, is another common form of plagiarism. In self-plagiarism, the author copies large parts of one of his or her previous papers word-for-word. This form of plagiarism can be difficult to define, since there is no consensus on how many words of copied text constitute self-plagiarism. Although the ethical breach associated with self-plagiarism is generally less severe than with intentional plagiarism, it is still considered as scientific misconduct. Copying sections of previously published text, for example the methods section of a research paper, is occasionally legitimate. However, copying large parts of an original paper is considered as self-plagiarism, and submitting it for publication is considered as duplicate publication, as discussed below.[ 9 ]
Duplicate publication is a form of plagiarism that occurs when an author submits for publication a previously-published work as if it were original. Submitting previously published work is considered as plagiarism and a form of scientific misconduct, unless the author makes a clear statement that the article is being intentionally republished in part or in whole. Duplicate submission of manuscripts wastes the time of the editor and reviewers. Worse yet, duplicate publication of research distorts the scientific record, since it implies that more than one study has independently achieved the reported results. Readers of published manuscripts have a right to expect that what they read is original content, and they should not be misled into believing a report is original when it is a duplication of the author's own work or that of others.[ 10 ] At the time of submission, most journals require that authors make a statement about any previous submissions that were similar or that were based on the reported results. Some forms of duplicate publication are acceptable, such as clinical trial updates and conference proceedings. According to the ICME guidelines,[ 2 ] submitted manuscripts that are duplicates should be promptly rejected. If the editor is not made aware of the violation prior to publication, a notice of duplicate publication may be published with or without the author's explanation or approval.
The number of published manuscripts that are retracted each year is increasing, and plagiarism is making a significant contribution to this increase. Steen[ 11 ] investigated the reasons for retraction of 742 English language research papers from PubMed between 2000 and 2010. Sixteen percent of papers were retracted due to duplicate publication and 14% were retracted due to plagiarism. Errami and Garner[ 10 ] also searched the published biomedical literature and reported tens of thousands of highly similar articles, and that the number is growing. In their commentary in Nature, the authors state that the “three major sins of modern publishing” are duplication, co-submission and plagiarism.
Academic institutions are increasingly using plagiarism detection software to detect plagiarism in documents submitted by students. Likewise, journals use software tools to detect plagiarism and duplicate publications among submitted manuscripts. Plagiarism detection software compares the text of manuscripts with a database of the existing scholarly literature. The Lancet, which recently adopted the use of plagiarism detection software,[ 12 ] screens all submitted papers before sending them for peer review. If there is substantial overlap with previously published material, the editors may ask authors to put text in quotation marks, rewrite passages, or they may reject the manuscript and contact the head of the author's institution.
Table 1 lists the U.S. Department of Health and Human Services Office of Research Integrity's “Guidelines for Avoiding Plagiarism”. A good rule-of-thumb to follow is to always provide a citation if there is any question about the appropriateness of doing so. Our institution provides an on-line tutorial to assist faculty and students in differentiating plagiarism from paraphrasing,[ 13 ] and the student catalog provides specific definitions for plagiarism, along with punishment guidelines.[ 14 ] Most published guidelines for avoiding, detecting and dealing with plagiarism emphasize that a multi-faceted approach should be used to ensure that all persons understand the meaning of and consequences of plagiarism.[ 6 , 7 , 15 ]
The U.S. Department of Health and Human Services Office of Research Integrity<s “Guidelines for Avoiding Plagiarism”.[ 3 ]

A junior investigator prepared a case series and review article based on a group of interesting patients he has cared for. He worked with one student and a colleague to review the cases and prepare the manuscript, and they were both listed as authors on the paper. When the manuscript was close to completion, the investigator asked his senior mentor to review the manuscript. The mentor returned the paper with several edits and comments, and added his name as the senior author on the paper.
Authorship issues are often contentious and can affect personal and professional relationships. The major issues related to authorship include determination of author responsibilities and author order. There is tremendous pressure among academicians to be listed on as many publications as possible, and students in many graduate programs are required to publish one or more first-authored papers. However, awarding authorship to people who have not made sufficient contributions conveys benefit to them inappropriately, and it reduces the benefit to those who actually contributed to the work.[ 16 ]
Several forms of authorship abuse described by Kevin Strange[ 16 ]
- Coercion authorship, where intimidation is used to gain authorship. This type of authorship can occur when a senior person pressures a more junior person or a student to include their name on a paper to which they have not contributed enough to qualify for authorship;
- Honorary, guest or gift authorship that is awarded to acknowledge friendship, to gain favor, and/or to give the paper a greater sense of legitimacy. It is still quite common for authors to add well-known senior investigators as authors to their papers, even though the senior person may not have made significant contributions to the paper;
- Mutual support authorship, whereby two or more investigators place their names on each other's papers to enhance their perceived productivity;
- Ghost authorship, where papers are written by people who are not included as authors or are not acknowledged. Ghost authorship is quite common in the pharmaceutical industry, which often hires professional writers.
- Denial of authorship, where a work is published without providing authorship or acknowledgement to people who made substantial contributions to the work.
In the case described above, the senior mentor may expect to be added to the junior faculty member's paper because he feels that his position of authority qualifies him for authorship, and/or because he feels that he substantially contributed to the content through his edits and comments. However, even if he did make substantive changes and suggestions, the junior faculty member should not be made to feel coerced into adding the senior mentor as an author. The junior investigator should be able to confidently refer to published guidelines of authorship to determine if the senior mentor qualifies for authorship – and he should have the support of his institution in making this determination.
An often overlooked aspect of authorship is that the agreement implies support for the findings of the study, and a willingness to take public responsibility for the paper. Dr. Strange[ 16 ] describes several high-profile cases in which investigators inappropriately accepted authorship on papers. When serious charges of scientific misconduct were filed against the authors, the inappropriate authors tried to distance themselves from the study – after implicitly supporting the findings by accepting authorship. These cases illustrate the importance of not accepting authorship inappropriately, and of accepting the responsibility that accompanies authorship.
As with plagiarism, many institutions and professional organizations have established formal authorship guidelines. The U.S. Department of Health and Human Services Office of Research Integrity recommends that all research institutions, journals and scientific societies establish and make public their authorship policies.[ 3 ] The ICMJE's standards for authorship have been revised several times, have been adopted by hundreds of journals, and are the most widely accepted[ 2 ] [ Table 2 ]. In general, the ICMJE recommends that authorship be reserved for those who made substantive intellectual contributions to a published study.
Abbreviated version of the International Medical Journal Editors’ “Guidelines for Authorship”.[ 2 ]

Another authorship issue that can be problematic is authorship order. Generally speaking, the first and last author positions are considered as the most desirable. The first author, or “primary author”, is the person who conducted most of the work described in the paper, and is usually the person who drafted the manuscript. The “senior author” is usually the last person named, and is generally the person who directed or oversaw the project. Senior authors are often expected to take responsibility for the project as a whole. The names of “contributing authors’” appear between the primary and senior authors, and the order should reflect their relative contribution to the work.[ 16 ] The importance of these designations to medical school promotion committees, and clarification of these designations in published manuscripts have been described.[ 17 ] Increasingly, journals require that the role(s) of each listed author be specified at the time of submission, and many journals publish this information with the article.
CONCLUSIONS
Ethical lapses in writing and publishing are all too common. The cases presented illustrate a very small sample of the complex issues authors may face. We encourage institutions to adopt formal policies related to scientific misconduct – including plagiarism and authorship. Numerous established policies are available that can be adopted – or adapted – to meet the needs of individual institutions. Institutions should make their policies related to plagiarism readily available to both students and faculty, and they should provide clear guidelines to help students and faculty recognize and avoid plagiarism. Defining roles on projects and establishing authorship order on manuscripts before the writing begins – or even before the project begins – can often circumvent misunderstandings related to authorship. Authors should also clarify authorship expectations when they ask colleagues to review a working manuscript, and when they invite a colleague to participate on a project. Team science can help to foster ethical publishing if the team establishes guiding principles of authorship and publishing, and holds each member accountable to these principles.
Source of Support: Nil.
Conflict of Interest: None declared.
- Cookies & Privacy
- GETTING STARTED
- Introduction
- FUNDAMENTALS
- Acknowledgements
- Research questions & hypotheses
- Concepts, constructs & variables
- Research limitations
- Getting started
- Sampling Strategy
- Research Quality
- Research Ethics
- Data Analysis
Research ethics
When completing an undergraduate or master's level dissertation, there are a number of ethical requirements that must be taken into account. Some of these are formal requirements, such as the submission of an Ethics Proposal and/or the use of an Ethics Consent Form . However, at the undergraduate and master?s level, it is more likely that these ethical requirements simply have to be built into the way that you design and conduct your dissertation research. It is also important to understand what these ethical requirements are in order to write the Research Ethics section of your Research Strategy chapter (usually Chapter Three: Research Strategy ), as well as ensure that issues of research ethics are properly taken into account and do not slow you down.
When considering the research ethics in your dissertation, you need to think about: (a) the five basic ethical principles you need to take into account; and (b) how research ethics are influenced by your chosen research strategy . In addition, we set out some of the components that you will need to consider when writing an Ethics Consent Form .
- Principles of research ethics
- Research strategy and research ethics
- Ethics consent form

Ethical Considerations
Ethical Considerations can be specified as one of the most important parts of the research. Dissertations may even be doomed to failure if this part is missing.
According to Bryman and Bell (2007) [1] the following ten points represent the most important principles related to ethical considerations in dissertations:
- Research participants should not be subjected to harm in any ways whatsoever.
- Respect for the dignity of research participants should be prioritised.
- Full consent should be obtained from the participants prior to the study.
- The protection of the privacy of research participants has to be ensured.
- Adequate level of confidentiality of the research data should be ensured.
- Anonymity of individuals and organisations participating in the research has to be ensured.
- Any deception or exaggeration about the aims and objectives of the research must be avoided.
- Affiliations in any forms, sources of funding, as well as any possible conflicts of interests have to be declared.
- Any type of communication in relation to the research should be done with honesty and transparency.
- Any type of misleading information, as well as representation of primary data findings in a biased way must be avoided.
In order to address ethical considerations aspect of your dissertation in an effective manner, you will need to expand discussions of each of the following points to at least one paragraph:
1. Voluntary participation of respondents in the research is important. Moreover, participants have rights to withdraw from the study at any stage if they wish to do so.
2. Respondents should participate on the basis of informed consent. The principle of informed consent involves researchers providing sufficient information and assurances about taking part to allow individuals to understand the implications of participation and to reach a fully informed, considered and freely given decision about whether or not to do so, without the exercise of any pressure or coercion. [2]
3. The use of offensive, discriminatory, or other unacceptable language needs to be avoided in the formulation of Questionnaire/Interview/Focus group questions.
4. Privacy and anonymity or respondents is of a paramount importance.
5. Acknowledgement of works of other authors used in any part of the dissertation with the use of Harvard/APA/Vancouver referencing system according to the Dissertation Handbook
6. Maintenance of the highest level of objectivity in discussions and analyses throughout the research
7. Adherence to Data Protection Act (1998) if you are studying in the UK
In studies that do not involve primary data collection, on the other hand, ethical issues are going to be limited to the points d) and e) above.
Most universities have their own Code of Ethical Practice. It is critically important for you to thoroughly adhere to this code in every aspect of your research and declare your adherence in ethical considerations part of your dissertation.
My e-book, The Ultimate Guide to Writing a Dissertation in Business Studies: a step by step assistance offers practical assistance to complete a dissertation with minimum or no stress. The e-book covers all stages of writing a dissertation starting from the selection to the research area to submitting the completed version of the work within the deadline. John Dudovskiy

[1] Bryman, A. & Bell, E. (2007) “Business Research Methods”, 2nd edition. Oxford University Press.
[2] Saunders, M., Lewis, P. & Thornhill, A. (2012) “Research Methods for Business Students” 6th edition, Pearson Education Limited.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Ethical Considerations in Research | Types & Examples
Ethical Considerations in Research | Types & Examples
Published on 7 May 2022 by Pritha Bhandari .
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviours, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to:
- Protect the rights of research participants
- Enhance research validity
- Maintain scientific integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research aims with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Prevent plagiarism, run a free check.
Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process, so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
- What the study is about
- The risks and benefits of taking part
- How long the study will take
- Your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymise data collection. For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymisation is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants, but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study, as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources, counselling, or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine scientific integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Bhandari, P. (2022, May 07). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved 14 May 2024, from https://www.scribbr.co.uk/research-methods/ethical-considerations/
Is this article helpful?

Pritha Bhandari
Other students also liked, a quick guide to experimental design | 5 steps & examples, data collection methods | step-by-step guide & examples, how to avoid plagiarism | tips on citing sources.
You are using an outdated browser. Please upgrade your browser to improve your experience.
Example Documents
Each project is different and so the documentation required for different projects is different too. Below you will find some examples of study documentation, which you may use as a guide when producing your own.
General Tips
- Use simple words and sentences.
- Ensure the information is easy to follow - consider how you format the text and whether to use flowcharts/diagrams.
- Ask rather than demand.
- Avoid using jargon.
- Use the active (not passive) voice, e.g. 'We invite you...' instead of 'You are invited to...'
- Tailor your material to the audience, e.g. consent forms for preschool children will be different to those for young adults.
- For guidance on writing a good lay summary, see VoiceNorth's short video: Bitesize Training - How to Write a Good Lay Summar y.
Ethics Application Forms
At Newcastle University, researchers must complete an ethics application form, before any research commences, either by:
- completing the University Online Ethics Form or
- by completing the HRA IRAS form (if NHS/HSC Research Ethics Committee approval required)*
*Note, if you are unsure whether your study requires NHS/HSC REC approval, you should complete the University Online Ethics Form first, which will notify you accordingly if NHS/HSC REC approval is needed.
Ethics application forms will ask the researcher for key information about the research project, including:
- Principal Investigator contact details
- Project description
- Proposed project start and end dates
- Details of the risks associated with the research
- Proposed measures to prevent/minimise the risks
- Additional details, as applicable
The information provided should be written for a lay audience, and supporting documentation should be attached with the application form (e.g. information sheets, consent forms, data management plans and other relevant research materials, including for example research questionnaires, recruitment materials).
Below are examples of ethics application forms:
1. Example Ethics Form - Cyber Bullying [PDF: 122KB]
2. Example Ethics Form - Student Project [WORD: 50KB]
3. Example Ethics Form - Food & Nutrition [PDF: 496KB]
4. Example Ethics Form - Sexual Health [PDF: 201KB]
Participant Information Sheets (PIS)
The Participant Information Sheet (PIS) provides participants with sufficient information about the research study to allow them to make an appropriate (fully informed) decision about taking part. For further information, please see the Human Participation - Informing Participants section.
Example Information Sheet
Consent Forms
On receiving the information about the research study (typically through a Participant Information Sheet), the participant should be allowed time to consider whether or not to take part. If they wish to take part, typically participants will sign a Consent Form. For further information, please refer to the section on Human Participation - Acquiring Voluntary Consent and the University's Informed Consent Guidelines .
The University has also developed an Example Consent Form that can be downloaded and adapted to the research project.
Data Management Plans
A research data management plan outlines how a researcher will collect, use and store data, during and after the research study. For further information, please see the Data - Governance considerations for research data .
DMPOnline provides access to example Data Management Plans. The online tool can also be used to develop Data Management Plans that meet different funder requirements.
Further guidance is available through the University's Research Data Service (RDS) .
Privacy Notice
A Privacy Notice sets out how personal information will be processed in accordance with the UK General Data Protection Regulation (GDPR). Participants in a research project should be provided with a Privacy Notice alongside a Participant Information Sheet (PIS), and have the opportunity to ask questions before they sign a Consent Form .
To support researchers, the University has created a template form that can be downloaded and adapted to the project:
Template Privacy Notice for research
If you wish to recommend any changes to the information above, or have any example documents that may help other researchers, please contact [email protected] .
- Privacy Policy

Home » How To Write A Research Proposal – Step-by-Step [Template]
How To Write A Research Proposal – Step-by-Step [Template]
Table of Contents

How To Write a Research Proposal
Writing a Research proposal involves several steps to ensure a well-structured and comprehensive document. Here is an explanation of each step:
1. Title and Abstract
- Choose a concise and descriptive title that reflects the essence of your research.
- Write an abstract summarizing your research question, objectives, methodology, and expected outcomes. It should provide a brief overview of your proposal.
2. Introduction:
- Provide an introduction to your research topic, highlighting its significance and relevance.
- Clearly state the research problem or question you aim to address.
- Discuss the background and context of the study, including previous research in the field.
3. Research Objectives
- Outline the specific objectives or aims of your research. These objectives should be clear, achievable, and aligned with the research problem.
4. Literature Review:
- Conduct a comprehensive review of relevant literature and studies related to your research topic.
- Summarize key findings, identify gaps, and highlight how your research will contribute to the existing knowledge.
5. Methodology:
- Describe the research design and methodology you plan to employ to address your research objectives.
- Explain the data collection methods, instruments, and analysis techniques you will use.
- Justify why the chosen methods are appropriate and suitable for your research.
6. Timeline:
- Create a timeline or schedule that outlines the major milestones and activities of your research project.
- Break down the research process into smaller tasks and estimate the time required for each task.
7. Resources:
- Identify the resources needed for your research, such as access to specific databases, equipment, or funding.
- Explain how you will acquire or utilize these resources to carry out your research effectively.
8. Ethical Considerations:
- Discuss any ethical issues that may arise during your research and explain how you plan to address them.
- If your research involves human subjects, explain how you will ensure their informed consent and privacy.
9. Expected Outcomes and Significance:
- Clearly state the expected outcomes or results of your research.
- Highlight the potential impact and significance of your research in advancing knowledge or addressing practical issues.
10. References:
- Provide a list of all the references cited in your proposal, following a consistent citation style (e.g., APA, MLA).
11. Appendices:
- Include any additional supporting materials, such as survey questionnaires, interview guides, or data analysis plans.
Research Proposal Format
The format of a research proposal may vary depending on the specific requirements of the institution or funding agency. However, the following is a commonly used format for a research proposal:
1. Title Page:
- Include the title of your research proposal, your name, your affiliation or institution, and the date.
2. Abstract:
- Provide a brief summary of your research proposal, highlighting the research problem, objectives, methodology, and expected outcomes.
3. Introduction:
- Introduce the research topic and provide background information.
- State the research problem or question you aim to address.
- Explain the significance and relevance of the research.
- Review relevant literature and studies related to your research topic.
- Summarize key findings and identify gaps in the existing knowledge.
- Explain how your research will contribute to filling those gaps.
5. Research Objectives:
- Clearly state the specific objectives or aims of your research.
- Ensure that the objectives are clear, focused, and aligned with the research problem.
6. Methodology:
- Describe the research design and methodology you plan to use.
- Explain the data collection methods, instruments, and analysis techniques.
- Justify why the chosen methods are appropriate for your research.
7. Timeline:
8. Resources:
- Explain how you will acquire or utilize these resources effectively.
9. Ethical Considerations:
- If applicable, explain how you will ensure informed consent and protect the privacy of research participants.
10. Expected Outcomes and Significance:
11. References:
12. Appendices:
Research Proposal Template
Here’s a template for a research proposal:
1. Introduction:
2. Literature Review:
3. Research Objectives:
4. Methodology:
5. Timeline:
6. Resources:
7. Ethical Considerations:
8. Expected Outcomes and Significance:
9. References:
10. Appendices:
Research Proposal Sample
Title: The Impact of Online Education on Student Learning Outcomes: A Comparative Study
1. Introduction
Online education has gained significant prominence in recent years, especially due to the COVID-19 pandemic. This research proposal aims to investigate the impact of online education on student learning outcomes by comparing them with traditional face-to-face instruction. The study will explore various aspects of online education, such as instructional methods, student engagement, and academic performance, to provide insights into the effectiveness of online learning.
2. Objectives
The main objectives of this research are as follows:
- To compare student learning outcomes between online and traditional face-to-face education.
- To examine the factors influencing student engagement in online learning environments.
- To assess the effectiveness of different instructional methods employed in online education.
- To identify challenges and opportunities associated with online education and suggest recommendations for improvement.
3. Methodology
3.1 Study Design
This research will utilize a mixed-methods approach to gather both quantitative and qualitative data. The study will include the following components:
3.2 Participants
The research will involve undergraduate students from two universities, one offering online education and the other providing face-to-face instruction. A total of 500 students (250 from each university) will be selected randomly to participate in the study.
3.3 Data Collection
The research will employ the following data collection methods:
- Quantitative: Pre- and post-assessments will be conducted to measure students’ learning outcomes. Data on student demographics and academic performance will also be collected from university records.
- Qualitative: Focus group discussions and individual interviews will be conducted with students to gather their perceptions and experiences regarding online education.
3.4 Data Analysis
Quantitative data will be analyzed using statistical software, employing descriptive statistics, t-tests, and regression analysis. Qualitative data will be transcribed, coded, and analyzed thematically to identify recurring patterns and themes.
4. Ethical Considerations
The study will adhere to ethical guidelines, ensuring the privacy and confidentiality of participants. Informed consent will be obtained, and participants will have the right to withdraw from the study at any time.
5. Significance and Expected Outcomes
This research will contribute to the existing literature by providing empirical evidence on the impact of online education on student learning outcomes. The findings will help educational institutions and policymakers make informed decisions about incorporating online learning methods and improving the quality of online education. Moreover, the study will identify potential challenges and opportunities related to online education and offer recommendations for enhancing student engagement and overall learning outcomes.
6. Timeline
The proposed research will be conducted over a period of 12 months, including data collection, analysis, and report writing.
The estimated budget for this research includes expenses related to data collection, software licenses, participant compensation, and research assistance. A detailed budget breakdown will be provided in the final research plan.
8. Conclusion
This research proposal aims to investigate the impact of online education on student learning outcomes through a comparative study with traditional face-to-face instruction. By exploring various dimensions of online education, this research will provide valuable insights into the effectiveness and challenges associated with online learning. The findings will contribute to the ongoing discourse on educational practices and help shape future strategies for maximizing student learning outcomes in online education settings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How To Write A Proposal – Step By Step Guide...

Grant Proposal – Example, Template and Guide

How To Write A Business Proposal – Step-by-Step...

Business Proposal – Templates, Examples and Guide

Proposal – Types, Examples, and Writing Guide

How to choose an Appropriate Method for Research?
Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS 2024 Datasets and Benchmarks Track
If you'd like to become a reviewer for the track, or recommend someone, please use this form .
The Datasets and Benchmarks track serves as a venue for high-quality publications, talks, and posters on highly valuable machine learning datasets and benchmarks, as well as a forum for discussions on how to improve dataset development. Datasets and benchmarks are crucial for the development of machine learning methods, but also require their own publishing and reviewing guidelines. For instance, datasets can often not be reviewed in a double-blind fashion, and hence full anonymization will not be required. On the other hand, they do require additional specific checks, such as a proper description of how the data was collected, whether they show intrinsic bias, and whether they will remain accessible. The Datasets and Benchmarks track is proud to support the open source movement by encouraging submissions of open-source libraries and tools that enable or accelerate ML research.
The previous editions of the Datasets and Benchmarks track were highly successful; you can view the accepted papers from 2021 , 2002 , and 2023 , and the winners of the best paper awards 2021 , 2022 and 2023
CRITERIA. W e are aiming for an equally stringent review as the main conference, yet better suited to datasets and benchmarks. Submissions to this track will be reviewed according to a set of criteria and best practices specifically designed for datasets and benchmarks , as described below. A key criterion is accessibility: datasets should be available and accessible , i.e. the data can be found and obtained without a personal request to the PI, and any required code should be open source. We encourage the authors to use Croissant format ( https://mlcommons.org/working-groups/data/croissant/ ) to document their datasets in machine readable way. Next to a scientific paper, authors should also submit supplementary materials such as detail on how the data was collected and organised, what kind of information it contains, how it should be used ethically and responsibly, as well as how it will be made available and maintained.
RELATIONSHIP TO NeurIPS. Submissions to the track will be part of the main NeurIPS conference , presented alongside the main conference papers. Accepted papers will be officially published in the NeurIPS proceedings .
SUBMISSIONS. There will be one deadline this year. It is also still possible to submit datasets and benchmarks to the main conference (under the usual review process), but dual submission to both is not allowed (unless you retracted your paper from the main conference). We also cannot transfer papers from the main track to the D&B track. Authors can choose to submit either single-blind or double-blind . If it is possible to properly review the submission double-blind, i.e., reviewers do not need access to non-anonymous repositories to review the work, then authors can also choose to submit the work anonymously. Papers will not be publicly visible during the review process. Only accepted papers will become visible afterward. The reviews themselves are not visible during the review phase but will be published after decisions have been made. The datasets themselves should be accessible to reviewers but can be publicly released at a later date (see below). New authors cannot be added after the abstract deadline and they should have an OpenReview profile by the paper deadline. NeurIPS does not tolerate any collusion whereby authors secretly cooperate with reviewers, ACs or SACs to obtain favourable reviews.
SCOPE. This track welcomes all work on data-centric machine learning research (DMLR) and open-source libraries and tools that enable or accelerate ML research, covering ML datasets and benchmarks as well as algorithms, tools, methods, and analyses for working with ML data. This includes but is not limited to:
- New datasets, or carefully and thoughtfully designed (collections of) datasets based on previously available data.
- Data generators and reinforcement learning environments.
- Data-centric AI methods and tools, e.g. to measure and improve data quality or utility, or studies in data-centric AI that bring important new insight.
- Advanced practices in data collection and curation that are of general interest even if the data itself cannot be shared.
- Frameworks for responsible dataset development, audits of existing datasets, identifying significant problems with existing datasets and their use
- Benchmarks on new or existing datasets, as well as benchmarking tools.
- In-depth analyses of machine learning challenges and competitions (by organisers and/or participants) that yield important new insight.
- Systematic analyses of existing systems on novel datasets yielding important new insight.
Read our original blog post for more about why we started this track.
Important dates
- Abstract submission deadline: May 29, 2024
- Full paper submission and co-author registration deadline: Jun 5, 2024
- Supplementary materials submission deadline: Jun 12, 2024
- Review deadline - Jul 24, 2024
- Release of reviews and start of Author discussions on OpenReview: Aug 07, 2024
- End of author/reviewer discussions on OpenReview: Aug 31, 2024
- Author notification: Sep 26, 2024
- Camera-ready deadline: Oct 30, 2024 AOE
Note: The site will start accepting submissions on April 1 5 , 2024.
FREQUENTLY ASKED QUESTIONS
Q: My work is in scope for this track but possibly also for the main conference. Where should I submit it?
A: This is ultimately your choice. Consider the main contribution of the submission and how it should be reviewed. If the main contribution is a new dataset, benchmark, or other work that falls into the scope of the track (see above), then it is ideally reviewed accordingly. As discussed in our blog post, the reviewing procedures of the main conference are focused on algorithmic advances, analysis, and applications, while the reviewing in this track is equally stringent but designed to properly assess datasets and benchmarks. Other, more practical considerations are that this track allows single-blind reviewing (since anonymization is often impossible for hosted datasets) and intended audience, i.e., make your work more visible for people looking for datasets and benchmarks.
Q: How will paper accepted to this track be cited?
A: Accepted papers will appear as part of the official NeurIPS proceedings.
Q: Do I need to submit an abstract beforehand?
A: Yes, please check the important dates section for more information.
Q: My dataset requires open credentialized access. Can I submit to this track?
A: This will be possible on the condition that a credentialization is necessary for the public good (e.g. because of ethically sensitive medical data), and that an established credentialization procedure is in place that is 1) open to a large section of the public, 2) provides rapid response and access to the data, and 3) is guaranteed to be maintained for many years. A good example here is PhysioNet Credentialing, where users must first understand how to handle data with human subjects, yet is open to anyone who has learned and agrees with the rules. This should be seen as an exceptional measure, and NOT as a way to limit access to data for other reasons (e.g. to shield data behind a Data Transfer Agreement). Misuse would be grounds for desk rejection. During submission, you can indicate that your dataset involves open credentialized access, in which case the necessity, openness, and efficiency of the credentialization process itself will also be checked.
SUBMISSION INSTRUCTIONS
A submission consists of:
- Please carefully follow the Latex template for this track when preparing proposals. We follow the NeurIPS format, but with the appropriate headings, and without hiding the names of the authors. Download the template as a bundle here .
- Papers should be submitted via OpenReview
- Reviewing is in principle single-blind, hence the paper should not be anonymized. In cases where the work can be reviewed equally well anonymously, anonymous submission is also allowed.
- During submission, you can add a public link to the dataset or benchmark data. If the dataset can only be released later, you must include instructions for reviewers on how to access the dataset. This can only be done after the first submission by sending an official note to the reviewers in OpenReview. We highly recommend making the dataset publicly available immediately or before the start of the NeurIPS conference. In select cases, requiring solid motivation, the release date can be stretched up to a year after the submission deadline.
- Dataset documentation and intended uses. Recommended documentation frameworks include datasheets for datasets , dataset nutrition labels , data statements for NLP , data cards , and accountability frameworks .
- URL to website/platform where the dataset/benchmark can be viewed and downloaded by the reviewers.
- URL to Croissant metadata record documenting the dataset/benchmark available for viewing and downloading by the reviewers. You can create your Croissant metadata using e.g. the Python library available here: https://github.com/mlcommons/croissant
- Author statement that they bear all responsibility in case of violation of rights, etc., and confirmation of the data license.
- Hosting, licensing, and maintenance plan. The choice of hosting platform is yours, as long as you ensure access to the data (possibly through a curated interface) and will provide the necessary maintenance.
- Links to access the dataset and its metadata. This can be hidden upon submission if the dataset is not yet publicly available but must be added in the camera-ready version. In select cases, e.g when the data can only be released at a later date, this can be added afterward (up to a year after the submission deadline). Simulation environments should link to open source code repositories
- The dataset itself should ideally use an open and widely used data format. Provide a detailed explanation on how the dataset can be read. For simulation environments, use existing frameworks or explain how they can be used.
- Long-term preservation: It must be clear that the dataset will be available for a long time, either by uploading to a data repository or by explaining how the authors themselves will ensure this
- Explicit license: Authors must choose a license, ideally a CC license for datasets, or an open source license for code (e.g. RL environments). An overview of licenses can be found here: https://paperswithcode.com/datasets/license
- Add structured metadata to a dataset's meta-data page using Web standards (like schema.org and DCAT ): This allows it to be discovered and organized by anyone. A guide can be found here: https://developers.google.com/search/docs/data-types/dataset . If you use an existing data repository, this is often done automatically.
- Highly recommended: a persistent dereferenceable identifier (e.g. a DOI minted by a data repository or a prefix on identifiers.org ) for datasets, or a code repository (e.g. GitHub, GitLab,...) for code. If this is not possible or useful, please explain why.
- For benchmarks, the supplementary materials must ensure that all results are easily reproducible. Where possible, use a reproducibility framework such as the ML reproducibility checklist , or otherwise guarantee that all results can be easily reproduced, i.e. all necessary datasets, code, and evaluation procedures must be accessible and documented.
- For papers introducing best practices in creating or curating datasets and benchmarks, the above supplementary materials are not required.
- For papers resubmitted after being retracted from another venue: a brief discussion on the main concerns raised by previous reviewers and how you addressed them. You do not need to share the original reviews.
- For the dual submission and archiving, the policy follows the NeurIPS main track paper guideline .
Use of Large Language Models (LLMs): We welcome authors to use any tool that is suitable for preparing high-quality papers and research. However, we ask authors to keep in mind two important criteria. First, we expect papers to fully describe their methodology, and any tool that is important to that methodology, including the use of LLMs, should be described also. For example, authors should mention tools (including LLMs) that were used for data processing or filtering, visualization, facilitating or running experiments, and proving theorems. It may also be advisable to describe the use of LLMs in implementing the method (if this corresponds to an important, original, or non-standard component of the approach). Second, authors are responsible for the entire content of the paper, including all text and figures, so while authors are welcome to use any tool they wish for writing the paper, they must ensure that all text is correct and original.
REVIEWING AND SELECTION PROCESS
Reviewing will be single-blind, although authors can also submit anonymously if the submission allows that. A datasets and benchmarks program committee will be formed, consisting of experts on machine learning, dataset curation, and ethics. We will ensure diversity in the program committee, both in terms of background as well as technical expertise (e.g., data, ML, data ethics, social science expertise). Each paper will be reviewed by the members of the committee. In select cases where ethical concerns are flagged by reviewers, an ethics review may be performed as well.
Papers will not be publicly visible during the review process. Only accepted papers will become visible afterward. The reviews themselves are also not visible during the review phase but will be published after decisions have been made. Authors can choose to keep the datasets themselves hidden until a later release date, as long as reviewers have access.
The factors that will be considered when evaluating papers include:
- Utility and quality of the submission: Impact, originality, novelty, relevance to the NeurIPS community will all be considered.
- Reproducibility: All submissions should be accompanied by sufficient information to reproduce the results described i.e. all necessary datasets, code, and evaluation procedures must be accessible and documented. We encourage the use of a reproducibility framework such as the ML reproducibility checklist to guarantee that all results can be easily reproduced. Benchmark submissions in particular should take care to ensure sufficient details are provided to ensure reproducibility. If submissions include code, please refer to the NeurIPS code submission guidelines .
- Was code provided (e.g. in the supplementary material)? If provided, did you look at the code? Did you consider it useful in guiding your review? If not provided, did you wish code had been available?
- Ethics: Any ethical implications of the work should be addressed. Authors should rely on NeurIPS ethics guidelines as guidance for understanding ethical concerns.
- Completeness of the relevant documentation: Per NeurIPS ethics guidelines , datasets must be accompanied by documentation communicating the details of the dataset as part of their submissions via structured templates (e.g. TODO). Sufficient detail must be provided on how the data was collected and organized, what kind of information it contains, ethically and responsibly, and how it will be made available and maintained.
- Licensing and access: Per NeurIPS ethics guidelines , authors should provide licenses for any datasets released. These should consider the intended use and limitations of the dataset, and develop licenses and terms of use to prevent misuse or inappropriate use.
- Consent and privacy: Per NeurIPS ethics guidelines , datasets should minimize the exposure of any personally identifiable information, unless informed consent from those individuals is provided to do so. Any paper that chooses to create a dataset with real data of real people should ask for the explicit consent of participants, or explain why they were unable to do so.
- Ethics and responsible use: Any ethical implications of new datasets should be addressed and guidelines for responsible use should be provided where appropriate. Note that, if your submission includes publicly available datasets (e.g. as part of a larger benchmark), you should also check these datasets for ethical issues. You remain responsible for the ethical implications of including existing datasets or other data sources in your work.
- Legal compliance: For datasets, authors should ensure awareness and compliance with regional legal requirements.
ADVISORY COMMITTEE
The following committee will provide advice on the organization of the track over the coming years: Sergio Escalera, Isabelle Guyon, Neil Lawrence, Dina Machuve, Olga Russakovsky, Joaquin Vanschoren, Serena Yeung.
DATASETS AND BENCHMARKS CHAIRS
Lora Aroyo, Google Francesco Locatello, Institute of Science and Technology Austria Lingjuan Lyu, Sony AI
Contact: [email protected]

IMAGES
VIDEO
COMMENTS
Revised on May 9, 2024. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments ...
Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination. Key ethical issues that you will address as an insider researcher include: Gaining trust.
Ethical Considerations. Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
Writing Ethics Into Your Qualitative Proposal. A qualitative research proposal is comprised of many pieces and parts that are necessary to convey the researcher's justification for conducting the research, how the research will be conducted (including the strengths and limitations of the pr oposed approach), as well as what the sponsor of the ...
Writing the Research Proposal Arguments Presented in a Proposal. It is helpful to consider early in planning the study the major points that need to be addressed in a proposal. These points—or topics—all need to be interconnected to provide a cohesive picture of the entire project.
The job of ethics committees is to identify the possible risks in a project, and then assess whether the research team: are aware of the risks. are taking appropriate steps to minimise them. have a plan to handle anything that does go wrong. To do this, ethics committees need information. If you want your ethics application to get through the ...
INTRODUCTION. A clean, well-thought-out proposal forms the backbone for the research itself and hence becomes the most important step in the process of conduct of research.[] The objective of preparing a research proposal would be to obtain approvals from various committees including ethics committee [details under 'Research methodology II' section [Table 1] in this issue of IJA) and to ...
Research Ethics. In the research context, ethics can be defined as "the standards of behaviour that guide your conduct in relation to the rights of those who become the subject of your work, or are affected by it" (Saunders, Lewis and Thornhill 2015, p239). The University itself is guided by the fundamental principle that research involving ...
Introduction. Proposals submitted to the ESRC must provide a full ethics statement that confirms that proper consideration has been given to any ethics issues raised. All ESRC-funded grants must be approved by at least a light-touch ethics review. The ESRC does not require a favourable ethics opinion to be secured prior to submission of a ...
In the sections to follow, we map out the various ethical dimensions of designing a research project step by step: addressing the fundamental question of why and for whom we do research (Sect. 10.2); an exploration of the ethical considerations of the research design itself, including the recruitment of study participants (Sects. 10.3 and 10.4 ...
Here's a general guide on how to write a research proposal: 1. Title. Start with a clear and concise title that reflects the main theme of your research. 2. Introduction. Provide a brief introduction to the research problem, background information, and the context of your study.
Make sure you can ask the critical what, who, and how questions of your research before you put pen to paper. Your research proposal should include (at least) 5 essential components : Title - provides the first taste of your research, in broad terms. Introduction - explains what you'll be researching in more detail.
The Rationale specifies the reasons for conducting the research in light of current knowledge. It should include a well documented statement of the need/problem that is the basis of the project, the cause of this problem and its possible solutions. It is the equivalent to the introduction in a research paper and it puts the proposal in context.
We discuss two issues related to ethics in scientific writing: Plagiarism and authorship. Plagiarism, the most common form of scientific misconduct, is defined as the appropriation of another person's ideas, processes, results or words without giving appropriate credit. While plagiarism is often intentional, it may be unintentional due to ...
When considering the research ethics in your dissertation, you need to think about: (a) the five basic ethical principles you need to take into account; and (b) how research ethics are influenced by your chosen research strategy. In addition, we set out some of the components that you will need to consider when writing an Ethics Consent Form ...
In order to address ethical considerations aspect of your dissertation in an effective manner, you will need to expand discussions of each of the following points to at least one paragraph: 1. Voluntary participation of respondents in the research is important. Moreover, participants have rights to withdraw from the study at any stage if they ...
4 actce ct 020 Fact Sheet How to write a research proposal Context This resource is one of a suite prepared by BACP to enable members to engage with BACP's current Ethical Framework for the Counselling Professions in respect of research. Using Fact Sheet resources BAC P member s hav e a contractua l commitmen t to wor k in accordanc e with
In the upper right corner, the author of the research proposal should point the number of the page. Don't forget to number every page of your proposal. The full title of the document should be centered 1/3 of the way down your page. Don't forget to double space the text and write your name right below your title.
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating ...
At Newcastle University, researchers must complete an ethics application form, before any research commences, either by: completing the University Online Ethics Form or; by completing the HRA IRAS form (if NHS/HSC Research Ethics Committee approval required)* *Note, if you are unsure whether your study requires NHS/HSC REC approval, you should complete the University Online Ethics Form first ...
Identify the ethical principles and standards. 2. Describe your research design and methods. Be the first to add your personal experience. 3. Discuss the ethical implications and challenges. Be ...
Here is an explanation of each step: 1. Title and Abstract. Choose a concise and descriptive title that reflects the essence of your research. Write an abstract summarizing your research question, objectives, methodology, and expected outcomes. It should provide a brief overview of your proposal. 2.
The Datasets and Benchmarks track is proud to support the open source movement by encouraging submissions of open-source libraries and tools that enable or accelerate ML research. The previous editions of the Datasets and Benchmarks track were highly successful; you can view the accepted papers from 2021, 2002, and 2023, and the winners of the ...