

Site Search
- How to Search
- Advisory Group
- Editorial Board
- OEC Fellows
- History and Funding
- Using OEC Materials
- Collections
- Research Ethics Resources
- Ethics Projects
- Communities of Practice
- Get Involved
- Submit Content
- Open Access Membership
- Become a Partner
Introduction: What is Research Ethics?
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. This introduction covers what research ethics is, its ethical distinctions, approaches to teaching research ethics, and other resources on this topic.
What is Research Ethics
Why Teach Research Ethics
Animal Subjects
Biosecurity
Collaboration
Conflicts of Interest
Data Management
Human Subjects
Peer Review
Publication
Research Misconduct
Social Responsibility
Stem Cell Research
Whistleblowing
Descriptions of educational settings , including in the classroom, and in research contexts.
Case Studies
Other Discussion Tools
Information about the history and authors of the Resources for Research Ethics Collection
What is Research Ethics?
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include:
- Protections of human and animal subjects
However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research subjects. Other ethical challenges are rooted in many dimensions of research, including the:
- Collection, use, and interpretation of research data
- Methods for reporting and reviewing research plans or findings
- Relationships among researchers with one another
- Relationships between researchers and those that will be affected by their research
- Means for responding to misunderstandings, disputes, or misconduct
- Options for promoting ethical conduct in research
The domain of research ethics is intended to include nothing less than the fostering of research that protects the interests of the public, the subjects of research, and the researchers themselves.
Ethical Distinctions
In discussing or teaching research ethics, it is important to keep some basic distinctions in mind.
- It is important not to confuse moral claims about how people ought to behave with descriptive claims about how they in fact do behave. From the fact that gift authorship or signing off on un-reviewed data may be "common practice" in some contexts, it doesn't follow that they are morally or professionally justified. Nor is morality to be confused with the moral beliefs or ethical codes that a given group or society holds (how some group thinks people should live). A belief in segregation is not morally justified simply because it is widely held by a group of people or given society. Philosophers term this distinction between prescriptive and descriptive claims the 'is-ought distinction.'
- A second important distinction is that between morality and the law. The law may or may not conform to the demands of ethics (Kagan, 1998). To take a contemporary example: many believe that the law prohibiting federally funded stem cell research is objectionable on moral (as well as scientific) grounds, i.e., that such research can save lives and prevent much human misery. History is full of examples of bad laws, that is laws now regarded as morally unjustifiable, e.g., the laws of apartheid, laws prohibiting women from voting or inter-racial couples from marrying.
- It is also helpful to distinguish between two different levels of discussion (or two different kinds of ethical questions): first-order or "ground-level" questions and second-order questions.
- First-order moral questions concern what we should do. Such questions may be very general or quite specific. One might ask whether the tradition of 'senior' authorship should be defended and preserved or, more generally, what are the principles that should go into deciding the issue of 'senior' authorship. Such questions and the substantive proposals regarding how to answer them belong to the domain of what moral philosophers call 'normative ethics.'
- Second-order moral questions concern the nature and purpose of morality itself. When someone claims that falsifying data is wrong, what exactly is the standing of this claim? What exactly does the word 'wrong' mean in the conduct of scientific research? And what are we doing when we make claims about right and wrong, scientific integrity and research misconduct? These second-order questions are quite different from the ground-level questions about how to conduct one's private or professional life raised above. They concern the nature of morality rather than its content, i.e., what acts are required, permitted or prohibited. This is the domain of what moral philosophers call 'metaethics' (Kagan, 1998).
Ethical Approaches
Each of these approaches provides moral principles and ways of thinking about the responsibilities, duties and obligations of moral life. Individually and jointly, they can provide practical guidance in ethical decision-making.
- One of the most influential and familiar approaches to ethics is deontological ethics, associated with Immanuel Kant (1742-1804). Deontological ethics hold certain acts as right or wrong in themselves, e.g., promise breaking or lying. So, for example, in the context of research, fraud, plagiarism and misrepresentation are regarded as morally wrong in themselves, not simply because they (tend to) have bad consequences. The deontological approach is generally grounded in a single fundamental principle: Act as you would wish others to act towards you OR always treat persons as an end, never as a means to an end.
- From such central principles are derived rules or guidelines for what is permitted, required and prohibited. Objections to principle-based or deontological ethics include the difficulty of applying highly general principles to specific cases, e.g.: Does treating persons as ends rule out physician-assisted suicide, or require it? Deontological ethics is generally contrasted to consequentialist ethics (Honderich, 1995).
- According to consequentialist approaches, the rightness or wrongness of an action depends solely on its consequences. One should act in such a way as to bring about the best state of affairs, where the best state of affairs may be understood in various ways, e.g., as the greatest happiness for the greatest number of people, maximizing pleasure and minimizing pain or maximizing the satisfaction of preferences. A theory such as Utilitarianism (with its roots in the work of Jeremy Bentham and John Stuart Mill) is generally taken as the paradigm example of consequentialism. Objections to consequentialist ethics tend to focus on its willingness to regard individual rights and values as "negotiable." So, for example, most people would regard murder as wrong independently of the fact that killing one person might allow several others to be saved (the infamous sacrifice of an ailing patient to provide organs for several other needy patients). Similarly, widespread moral opinion holds certain values important (integrity, justice) not only because they generally lead to good outcomes, but in and of themselves.
- Virtue ethics focuses on moral character rather than action and behavior considered in isolation. Central to this approach is the question what ought we (as individuals, as scientists, as physicians) to be rather than simply what we ought to do. The emphasis here is on inner states, that is, moral dispositions and habits such as courage or a developed sense of personal integrity. Virtue ethics can be a useful approach in the context of RCR and professional ethics, emphasizing the importance of moral virtues such as compassion, honesty, and respect. This approach has also a great deal to offer in discussions of bioethical issues where a traditional emphasis on rights and abstract principles frequently results in polarized, stalled discussions (e.g., abortion debates contrasting the rights of the mother against the rights of the fetus).
- The term 'an ethics of care' grows out of the work of Carol Gilligan, whose empirical work in moral psychology claimed to discover a "different voice," a mode of moral thinking distinct from principle-based moral thinking (e.g., the theories of Kant and Mill). An ethics of care stresses compassion and empathetic understanding, virtues Gilligan associated with traditional care-giving roles, especially those of women.
- This approach differs from traditional moral theories in two important ways. First, it assumes that it is the connections between persons, e.g., lab teams, colleagues, parents and children, student and mentor, not merely the rights and obligations of discrete individuals that matter. The moral world, on this view, is best seen not as the interaction of discrete individuals, each with his or her own interests and rights, but as an interrelated web of obligations and commitment. We interact, much of the time, not as private individuals, but as members of families, couples, institutions, research groups, a given profession and so on. Second, these human relationships, including relationships of dependency, play a crucial role on this account in determining what our moral obligations and responsibilities are. So, for example, individuals have special responsibilities to care for their children, students, patients, and research subjects.
- An ethics of care is thus particularly useful in discussing human and animal subjects research, issues of informed consent, and the treatment of vulnerable populations such as children, the infirm or the ill.
- The case study approach begins from real or hypothetical cases. Its objective is to identify the intuitively plausible principles that should be taken into account in resolving the issues at hand. The case study approach then proceeds to critically evaluate those principles. In discussing whistle-blowing, for example, a good starting point is with recent cases of research misconduct, seeking to identify and evaluate principles such as a commitment to the integrity of science, protecting privacy, or avoiding false or unsubstantiated charges. In the context of RCR instruction, case studies provide one of the most interesting and effective approaches to developing sensitivity to ethical issues and to honing ethical decision-making skills.
- Strictly speaking, casuistry is more properly understood as a method for doing ethics rather than as itself an ethical theory. However, casuistry is not wholly unconnected to ethical theory. The need for a basis upon which to evaluate competing principles, e.g., the importance of the well-being of an individual patient vs. a concern for just allocation of scarce medical resources, makes ethical theory relevant even with case study approaches.
- Applied ethics is a branch of normative ethics. It deals with practical questions particularly in relation to the professions. Perhaps the best known area of applied ethics is bioethics, which deals with ethical questions arising in medicine and the biological sciences, e.g., questions concerning the application of new areas of technology (stem cells, cloning, genetic screening, nanotechnology, etc.), end of life issues, organ transplants, and just distribution of healthcare. Training in responsible conduct of research or "research ethics" is merely one among various forms of professional ethics that have come to prominence since the 1960s. Worth noting, however, is that concern with professional ethics is not new, as ancient codes such as the Hippocratic Oath and guild standards attest (Singer, 1986).
- Adams D, Pimple KD (2005): Research Misconduct and Crime: Lessons from Criminal Science on Preventing Misconduct and Promoting Integrity. Accountability in Research 12(3):225-240.
- Anderson MS, Horn AS, Risbey KR, Ronning EA, De Vries R, Martinson BC (2007): What Do Mentoring and Training in the Responsible Conduct of Research Have To Do with Scientists' Misbehavior? Findings from a National Survey of NIH-Funded Scientists . Academic Medicine 82(9):853-860.
- Bulger RE, Heitman E (2007): Expanding Responsible Conduct of Research Instruction across the University. Academic Medicine. 82(9):876-878.
- Kalichman MW (2006): Ethics and Science: A 0.1% solution. Issues in Science and Technology 23:34-36.
- Kalichman MW (2007): Responding to Challenges in Educating for the Responsible Conduct of Research, Academic Medicine. 82(9):870-875.
- Kalichman MW, Plemmons DK (2007): Reported Goals for Responsible Conduct of Research Courses. Academic Medicine. 82(9):846-852.
- Kalichman MW (2009): Evidence-based research ethics. The American Journal of Bioethics 9(6&7): 85-87.
- Pimple KD (2002): Six Domains of Research Ethics: A Heuristic Framework for the Responsible Conduct of Research. Science and Engineering Ethics 8(2):191-205.
- Steneck NH (2006): Fostering Integrity in Research: Definitions, Current Knowledge, and Future Directions. Science and Engineering Ethics 12:53-74.
- Steneck NH, Bulger RE (2007): The History, Purpose, and Future of Instruction in the Responsible Conduct of Research. Academic Medicine. 82(9):829-834.
- Vasgird DR (2007): Prevention over Cure: The Administrative Rationale for Education in the Responsible Conduct of Research. Academic Medicine. 82(9):835-837.
- Aristotle. The Nichomachean Ethics.
- Beauchamp RL, Childress JF (2001): Principles of Biomedical Ethics, 5th edition, NY: Oxford University Press.
- Bentham, J (1781): An Introduction to the Principles of Morals and Legislation.
- Gilligan C (1993): In a Different Voice: Psychological Theory and Women's Development. Cambridge: Harvard University Press.
- Glover, Jonathan (1977): Penguin Books.
- Honderich T, ed. (1995): The Oxford Companion to Philosophy, Oxford and New York: Oxford University Press.
- Kagan S (1998): Normative Ethics. Westview Press.
- Kant I (1785): Groundwork of the Metaphysics of Morals.
- Kant I (1788): Critique of Practical Reason.
- Kant I (1797): The Metaphysics of Morals.
- Kant I (1797): On a Supposed right to Lie from Benevolent Motives.
- Kuhse H, Singer P (1999): Bioethics: An Anthology. Blackwell Publishers.
- Mill JS (1861): Utilitarianism.
- Rachels J (1999): The Elements of Moral Philosophy, 3rd edition, Boston: McGraw-Hill.
- Regan T (1993): Matters of Life and Death: New Introductory Essays in Moral Philosophy, 3rd edition. New York: McGraw-Hill. The history of ethics.
- Singer P (1993): Practical Ethics, 2nd ed. Cambridge University Press.
The Resources for Research Ethics Education site was originally developed and maintained by Dr. Michael Kalichman, Director of the Research Ethics Program at the University of California San Diego. The site was transferred to the Online Ethics Center in 2021 with the permission of the author.
Related Resources
Submit Content to the OEC Donate

This material is based upon work supported by the National Science Foundation under Award No. 2055332. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
- Essay Guides
- Other Essays
- How to Write an Ethics Paper: Guide & Ethical Essay Examples
- Speech Topics
- Basics of Essay Writing
- Essay Topics
- Main Academic Essays
- Research Paper Topics
- Basics of Research Paper Writing
- Miscellaneous
- Chicago/ Turabian
- Data & Statistics
- Methodology
- Admission Writing Tips
- Admission Advice
- Other Guides
- Student Life
- Studying Tips
- Understanding Plagiarism
- Academic Writing Tips
- Basics of Dissertation & Thesis Writing
- Research Paper Guides
- Formatting Guides
- Basics of Research Process
- Admission Guides
- Dissertation & Thesis Guides
How to Write an Ethics Paper: Guide & Ethical Essay Examples

Table of contents
Use our free Readability checker
An ethics essay is a type of academic writing that explores ethical issues and dilemmas. Students should evaluates them in terms of moral principles and values. The purpose of an ethics essay is to examine the moral implications of a particular issue, and provide a reasoned argument in support of an ethical perspective.
Writing an essay about ethics is a tough task for most students. The process involves creating an outline to guide your arguments about a topic and planning your ideas to convince the reader of your feelings about a difficult issue. If you still need assistance putting together your thoughts in composing a good paper, you have come to the right place. We have provided a series of steps and tips to show how you can achieve success in writing. This guide will tell you how to write an ethics paper using ethical essay examples to understand every step it takes to be proficient. In case you don’t have time for writing, get in touch with our professional essay writers for hire . Our experts work hard to supply students with excellent essays.
What Is an Ethics Essay?
An ethics essay uses moral theories to build arguments on an issue. You describe a controversial problem and examine it to determine how it affects individuals or society. Ethics papers analyze arguments on both sides of a possible dilemma, focusing on right and wrong. The analysis gained can be used to solve real-life cases. Before embarking on writing an ethical essay, keep in mind that most individuals follow moral principles. From a social context perspective, these rules define how a human behaves or acts towards another. Therefore, your theme essay on ethics needs to demonstrate how a person feels about these moral principles. More specifically, your task is to show how significant that issue is and discuss if you value or discredit it.
Purpose of an Essay on Ethics
The primary purpose of an ethics essay is to initiate an argument on a moral issue using reasoning and critical evidence. Instead of providing general information about a problem, you present solid arguments about how you view the moral concern and how it affects you or society. When writing an ethical paper, you demonstrate philosophical competence, using appropriate moral perspectives and principles.
Things to Write an Essay About Ethics On
Before you start to write ethics essays, consider a topic you can easily address. In most cases, an ethical issues essay analyzes right and wrong. This includes discussing ethics and morals and how they contribute to the right behaviors. You can also talk about work ethic, code of conduct, and how employees promote or disregard the need for change. However, you can explore other areas by asking yourself what ethics mean to you. Think about how a recent game you watched with friends started a controversial argument. Or maybe a newspaper that highlighted a story you felt was misunderstood or blown out of proportion. This way, you can come up with an excellent topic that resonates with your personal ethics and beliefs.
Ethics Paper Outline
Sometimes, you will be asked to submit an outline before writing an ethics paper. Creating an outline for an ethics paper is an essential step in creating a good essay. You can use it to arrange your points and supporting evidence before writing. It also helps organize your thoughts, enabling you to fill any gaps in your ideas. The outline for an essay should contain short and numbered sentences to cover the format and outline. Each section is structured to enable you to plan your work and include all sources in writing an ethics paper. An ethics essay outline is as follows:
- Background information
- Thesis statement
- Restate thesis statement
- Summarize key points
- Final thoughts on the topic
Using this outline will improve clarity and focus throughout your writing process.
Ethical Essay Structure
Ethics essays are similar to other essays based on their format, outline, and structure. An ethical essay should have a well-defined introduction, body, and conclusion section as its structure. When planning your ideas, make sure that the introduction and conclusion are around 20 percent of the paper, leaving the rest to the body. We will take a detailed look at what each part entails and give examples that are going to help you understand them better. Refer to our essay structure examples to find a fitting way of organizing your writing.
Ethics Paper Introduction
An ethics essay introduction gives a synopsis of your main argument. One step on how to write an introduction for an ethics paper is telling about the topic and describing its background information. This paragraph should be brief and straight to the point. It informs readers what your position is on that issue. Start with an essay hook to generate interest from your audience. It can be a question you will address or a misunderstanding that leads up to your main argument. You can also add more perspectives to be discussed; this will inform readers on what to expect in the paper.
Ethics Essay Introduction Example
You can find many ethics essay introduction examples on the internet. In this guide, we have written an excellent extract to demonstrate how it should be structured. As you read, examine how it begins with a hook and then provides background information on an issue.
Imagine living in a world where people only lie, and honesty is becoming a scarce commodity. Indeed, modern society is facing this reality as truth and deception can no longer be separated. Technology has facilitated a quick transmission of voluminous information, whereas it's hard separating facts from opinions.
In this example, the first sentence of the introduction makes a claim or uses a question to hook the reader.
Ethics Essay Thesis Statement
An ethics paper must contain a thesis statement in the first paragraph. Learning how to write a thesis statement for an ethics paper is necessary as readers often look at it to gauge whether the essay is worth their time.
When you deviate away from the thesis, your whole paper loses meaning. In ethics essays, your thesis statement is a roadmap in writing, stressing your position on the problem and giving reasons for taking that stance. It should focus on a specific element of the issue being discussed. When writing a thesis statement, ensure that you can easily make arguments for or against its stance.
Ethical Paper Thesis Example
Look at this example of an ethics paper thesis statement and examine how well it has been written to state a position and provide reasons for doing so:
The moral implications of dishonesty are far-reaching as they undermine trust, integrity, and other foundations of society, damaging personal and professional relationships.
The above thesis statement example is clear and concise, indicating that this paper will highlight the effects of dishonesty in society. Moreover, it focuses on aspects of personal and professional relationships.
Ethics Essay Body
The body section is the heart of an ethics paper as it presents the author's main points. In an ethical essay, each body paragraph has several elements that should explain your main idea. These include:
- A topic sentence that is precise and reiterates your stance on the issue.
- Evidence supporting it.
- Examples that illustrate your argument.
- A thorough analysis showing how the evidence and examples relate to that issue.
- A transition sentence that connects one paragraph to another with the help of essay transitions .
When you write an ethics essay, adding relevant examples strengthens your main point and makes it easy for others to understand and comprehend your argument.
Body Paragraph for Ethics Paper Example
A good body paragraph must have a well-defined topic sentence that makes a claim and includes evidence and examples to support it. Look at part of an example of ethics essay body paragraph below and see how its idea has been developed:
Honesty is an essential component of professional integrity. In many fields, trust and credibility are crucial for professionals to build relationships and success. For example, a doctor who is dishonest about a potential side effect of a medication is not only acting unethically but also putting the health and well-being of their patients at risk. Similarly, a dishonest businessman could achieve short-term benefits but will lose their client’s trust.
Ethics Essay Conclusion
A concluding paragraph shares the summary and overview of the author's main arguments. Many students need clarification on what should be included in the essay conclusion and how best to get a reader's attention. When writing an ethics paper conclusion, consider the following:
- Restate the thesis statement to emphasize your position.
- Summarize its main points and evidence.
- Final thoughts on the issue and any other considerations.
You can also reflect on the topic or acknowledge any possible challenges or questions that have not been answered. A closing statement should present a call to action on the problem based on your position.
Sample Ethics Paper Conclusion
The conclusion paragraph restates the thesis statement and summarizes the arguments presented in that paper. The sample conclusion for an ethical essay example below demonstrates how you should write a concluding statement.
In conclusion, the implications of dishonesty and the importance of honesty in our lives cannot be overstated. Honesty builds solid relationships, effective communication, and better decision-making. This essay has explored how dishonesty impacts people and that we should value honesty. We hope this essay will help readers assess their behavior and work towards being more honest in their lives.
In the above extract, the writer gives final thoughts on the topic, urging readers to adopt honest behavior.
How to Write an Ethics Paper?
As you learn how to write an ethics essay, it is not advised to immediately choose a topic and begin writing. When you follow this method, you will get stuck or fail to present concrete ideas. A good writer understands the importance of planning. As a fact, you should organize your work and ensure it captures key elements that shed more light on your arguments. Hence, following the essay structure and creating an outline to guide your writing process is the best approach. In the following segment, we have highlighted step-by-step techniques on how to write a good ethics paper.
1. Pick a Topic
Before writing ethical papers, brainstorm to find ideal topics that can be easily debated. For starters, make a list, then select a title that presents a moral issue that may be explained and addressed from opposing sides. Make sure you choose one that interests you. Here are a few ideas to help you search for topics:
- Review current trends affecting people.
- Think about your personal experiences.
- Study different moral theories and principles.
- Examine classical moral dilemmas.
Once you find a suitable topic and are ready, start to write your ethics essay, conduct preliminary research, and ascertain that there are enough sources to support it.
2. Conduct In-Depth Research
Once you choose a topic for your essay, the next step is gathering sufficient information about it. Conducting in-depth research entails looking through scholarly journals to find credible material. Ensure you note down all sources you found helpful to assist you on how to write your ethics paper. Use the following steps to help you conduct your research:
- Clearly state and define a problem you want to discuss.
- This will guide your research process.
- Develop keywords that match the topic.
- Begin searching from a wide perspective. This will allow you to collect more information, then narrow it down by using the identified words above.
3. Develop an Ethics Essay Outline
An outline will ease up your writing process when developing an ethic essay. As you develop a paper on ethics, jot down factual ideas that will build your paragraphs for each section. Include the following steps in your process:
- Review the topic and information gathered to write a thesis statement.
- Identify the main arguments you want to discuss and include their evidence.
- Group them into sections, each presenting a new idea that supports the thesis.
- Write an outline.
- Review and refine it.
Examples can also be included to support your main arguments. The structure should be sequential, coherent, and with a good flow from beginning to end. When you follow all steps, you can create an engaging and organized outline that will help you write a good essay.
4. Write an Ethics Essay
Once you have selected a topic, conducted research, and outlined your main points, you can begin writing an essay . Ensure you adhere to the ethics paper format you have chosen. Start an ethics paper with an overview of your topic to capture the readers' attention. Build upon your paper by avoiding ambiguous arguments and using the outline to help you write your essay on ethics. Finish the introduction paragraph with a thesis statement that explains your main position. Expand on your thesis statement in all essay paragraphs. Each paragraph should start with a topic sentence and provide evidence plus an example to solidify your argument, strengthen the main point, and let readers see the reasoning behind your stance. Finally, conclude the essay by restating your thesis statement and summarizing all key ideas. Your conclusion should engage the reader, posing questions or urging them to reflect on the issue and how it will impact them.
5. Proofread Your Ethics Essay
Proofreading your essay is the last step as you countercheck any grammatical or structural errors in your essay. When writing your ethic paper, typical mistakes you could encounter include the following:
- Spelling errors: e.g., there, they’re, their.
- Homophone words: such as new vs. knew.
- Inconsistencies: like mixing British and American words, e.g., color vs. color.
- Formatting issues: e.g., double spacing, different font types.
While proofreading your ethical issue essay, read it aloud to detect lexical errors or ambiguous phrases that distort its meaning. Verify your information and ensure it is relevant and up-to-date. You can ask your fellow student to read the essay and give feedback on its structure and quality.
Ethics Essay Examples
Writing an essay is challenging without the right steps. There are so many ethics paper examples on the internet, however, we have provided a list of free ethics essay examples below that are well-structured and have a solid argument to help you write your paper. Click on them and see how each writing step has been integrated. Ethics essay example 1
Ethics essay example 2
Ethics essay example 3
Ethics essay example 4
College ethics essay example 5
Ethics Essay Writing Tips
When writing papers on ethics, here are several tips to help you complete an excellent essay:
- Choose a narrow topic and avoid broad subjects, as it is easy to cover the topic in detail.
- Ensure you have background information. A good understanding of a topic can make it easy to apply all necessary moral theories and principles in writing your paper.
- State your position clearly. It is important to be sure about your stance as it will allow you to draft your arguments accordingly.
- When writing ethics essays, be mindful of your audience. Provide arguments that they can understand.
- Integrate solid examples into your essay. Morality can be hard to understand; therefore, using them will help a reader grasp these concepts.
Bottom Line on Writing an Ethics Paper
Creating this essay is a common exercise in academics that allows students to build critical skills. When you begin writing, state your stance on an issue and provide arguments to support your position. This guide gives information on how to write an ethics essay as well as examples of ethics papers. Remember to follow these points in your writing:
- Create an outline highlighting your main points.
- Write an effective introduction and provide background information on an issue.
- Include a thesis statement.
- Develop concrete arguments and their counterarguments, and use examples.
- Sum up all your key points in your conclusion and restate your thesis statement.
Contact our academic writing platform and have your challenge solved. Here, you can order essays and papers on any topic and enjoy top quality.

Daniel Howard is an Essay Writing guru. He helps students create essays that will strike a chord with the readers.
You may also like

When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
Understanding Scientific and Research Ethics

How to pass journal ethics checks to ensure a smooth submission and publication process
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it’s often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
The underlying principles of scientific and research ethics
Scientific and research ethics exist to safeguard human rights, ensure that we treat animals respectfully and humanely, and protect the natural environment.
The specific details may vary widely depending on the type of research you’re conducting, but there are clear themes running through all research and reporting ethical requirements:
Documented 3rd party oversight
- Consent and anonymity
- Full transparency
If you fulfill each of these broad requirements, your manuscript should sail through any journal’s ethics check.

If your research is 100% theoretical, you might be able to skip this one. But if you work with living organisms in any capacity—whether you’re administering a survey, collecting data from medical records, culturing cells, working with zebrafish, or counting plant species in a ring—oversight and approval by an ethics committee is a prerequisite for publication. This oversight can take many different forms:
For human studies and studies using human tissue or cells, obtain approval from your institutional review board (IRB). Register clinical trials with the World Health Organization (WHO) or International Committee of Medical Journal Editors (ICMJE). For animal research consult with your institutional animal care and use committee (IACUC). Note that there may be special requirements for non-human primates, cephalopods, and other specific species, as well as for wild animals. For field studies , anthropology and paleontology , the type of permission required will depend on many factors, like the location of the study, whether the site is publicly or privately owned, possible impacts on endangered or protected species, and local permit requirements.
TIP: You’re not exempt until your committee tells you so
Even if you think your study probably doesn’t require approval, submit it to the review board anyway. Many journals won’t consider retrospective approvals. Obtaining formal approval or an exemption up front is worth it to ensure your research is eligible for publication in the future.
TIP: Keep your committee records close
Clearly label your IRB/IACUC paperwork, permit numbers, and any participant permission forms (including blank copies), and keep them in a safe place. You will need them when you submit to a journal. Providing these details proactively as part of your initial submission can minimize delays and get your manuscript through journal checks and into the hands of reviewers sooner.
Consent & anonymity
Obtaining consent from human subjects.
You may not conduct research on human beings unless the subjects understand what you are doing and agree to be a part of your study. If you work with human subjects, you must obtain informed written consent from the participants or their legal guardians.
There are many circumstances where extra care may be required in order to obtain consent. The more vulnerable the population you are working with the stricter these guidelines will be. For example, your IRB may have special requirements for working with minors, the elderly, or developmentally delayed participants. Remember that these rules may vary from country to country. Providing a link to the relevant legal reference in your area can help speed the screening and approval process.
TIP: What if you are working with a population where reading and writing aren’t common?
Alternatives to written consent (such as verbal consent or a thumbprint) are acceptable in some cases, but consent still has to be clearly documented. To ensure eligibility for publication, be sure to:
- Get IRB approval for obtaining verbal rather than written consent
- Be prepared to explain why written consent could not be obtained
- Keep a copy of the script you used to obtain this consent, and record when consent was obtained for your own records
Consent and reporting for human tissue and cell lines
Consent from the participant or their next-of-kin is also required for the use of human tissue and cell lines. This includes discarded tissue, for example the by-products of surgery.
When working with cell lines transparency and good record keeping are essential. Here are some basic guidelines to bear in mind:
- When working with established cell lines , cite the published article where the cell line was first described.
- If you’re using repository or commercial cell lines , explain exactly which ones, and provide the catalog or repository number.
- If you received a cell line from a colleague , rather than directly from a repository or company, be sure to mention it. Explain who gifted the cells and when.
- For a new cell line obtained from a colleague there may not be a published article to cite yet, but the work to generate the cell line must meet the usual requirements of consent—even if it was carried out by another research group. You’ll need to provide a copy of your colleagues’ IRB approval and details about the consent procedures in order to publish the work.
Finally, you’re obliged to keep your human subjects anonymous and to protect any identifying information in photos and raw data. Remove all names, birth dates, detailed addresses, or job information from files you plan to share. Blur faces and tattoos in any images. Details such as geography (city/country), gender, age, or profession may be shared at a generalized level and in aggregate. Read more about standards for de-identifying datasets in The BMJ .
TIP: Anonymity can be important in field work too
Be careful about revealing geographic data in fieldwork. You don’t want to tip poachers off to the location of the endangered elephant population you studied, or expose petroglyphs to vandalism.
Full Transparency
No matter the discipline, transparent reporting of methods, results, data, software and code is essential to ethical research practice. Transparency is also key to the future reproducibility of your work.
When you submit your study to a journal, you’ll be asked to provide a variety of statements certifying that you’ve obtained the appropriate permissions and clearances, and explaining how you conducted the work. You may also be asked to provide supporting documentation, including field records and raw data. Provide as much detail as you can at this stage. Clear and complete disclosure statements will minimize back-and-forth with the journal, helping your submission to clear ethics checks and move on to the assessment stage sooner.
TIP: Save that data
As you work, be sure to clearly label and organize your data files in a way that will make sense to you later. As close as you are to the work as you conduct your study, remember that two years could easily pass between capturing your data and publishing an article reporting the results. You don’t want to be stuck piecing together confusing records in order to create figures and data files for repositories.
Read our full guide to preparing data for submission .
Keep in mind that scientific and research ethics are always evolving. As laws change and as we learn more about influence, implicit bias and animal sentience, the scientific community continues to strive to elevate our research practice.
A checklist to ensure you’re ethics-check ready
Before you begin your research
Obtain approval from your IRB, IACUC or other approving body
Obtain written informed consent from human participants, guardians or next-of-kin
Obtain permits or permission from property owners, or confirm that permits are not required
Label and save all of records
As you work
Adhere strictly to the protocols approved by your committee
Clearly label your data, and store it in a way that will make sense to your future self
As you write, submit and deposit your results
Be ready to cite specific approval organizations, permit numbers, cell lines, and other details in your ethics statement and in the methods section of your manuscript
Anonymize all participant data (including human and in some cases animal or geographic data)
If a figure does include identifying information (e.g. a participant’s face) obtain special consent
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
- Original article
- Open access
- Published: 13 July 2021
Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures
- Shivadas Sivasubramaniam 1 ,
- Dita Henek Dlabolová 2 ,
- Veronika Kralikova 3 &
- Zeenath Reza Khan 3
International Journal for Educational Integrity volume 17 , Article number: 14 ( 2021 ) Cite this article
17k Accesses
12 Citations
4 Altmetric
Metrics details
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own committees to focus on or approve activities that have ethical impact. In contrast, lesser-developed countries (worldwide) are trying to set up these committees to govern their academia and research. As the first European consortium established to assist academic integrity, European Network for Academic Integrity (ENAI), we felt the importance of guiding those institutions and communities that are trying to conduct research with ethical principles. We have established an ethical advisory working group within ENAI with the aim to promote ethics within curriculum, research and institutional policies. We are constantly researching available data on this subject and committed to help the academia to convey and conduct ethical behaviour. Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications of research projects among peers. Therefore, this short paper preliminarily aims to critically review the available information on ethics, the history behind establishing ethical principles and its international guidelines to govern research.
The paper is based on the workshop conducted in the 5th International conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019. During the workshop, we have detailed a) basic needs of an ethical committee within an institution; b) a typical ethical approval process (with examples from three different universities); and c) the ways to obtain informed consent with some examples. These are summarised in this paper with some example comparisons of ethical approval processes from different universities. We believe this paper will provide guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Introduction
Ethics and ethical behaviour (often linked to “responsible practice”) are the fundamental pillars of a civilised society. Ethical behaviour with integrity is important to maintain academic and research activities. It affects everything we do, and gets precedence with anything that would include/affect, transform, or impact upon individuals, communities or any living creatures. In other words, ethics would help us improve our living standards (LaFollette, 2007 ). The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law, but is also gaining recognition in all disciplines engaged in research. Therefore, institutions are expected to develop ethical guidelines in research to maintain quality, initiate/own integrity and above all be transparent to be successful by limiting any allegation of misconduct (Flite and Harman, 2013 ). This is especially true for higher education organisations that promote research and scholarly activities. Many European institutions have developed their own regulations for ethics by incorporating international codes (Getz, 1990 ). The lesser developed countries are trying to set up these committees to govern their academia and research. World Health Organization has stated that adhering to “ ethical principles … [is central and important]... in order to protect the dignity, rights and welfare of research participants ” (WHO, 2021 ). Ethical guidelines taught to students can help develop ethical researchers and members of society who uphold values of ethical principles in practice.
As the first European-wide consortium established to assist academic integrity (European Network for Academic Integrity – ENAI), we felt the importance of guiding those institutions and communities that are trying to teach, research, and include ethical principles by providing overarching understanding of ethical guidelines that may influence policy. Therefore, we set up an advisory working group within ENAI in 2018 to support matters related to ethics, ethical committees and assisting on ethics related teaching activities.
Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications among peers. This became the premise for this research paper. We first carried out a literature survey to review and summarise existing ethical governance (with historical perspectives) and procedures that are already in place to guide researchers in different discipline areas. By doing so, we attempted to consolidate, document and provide important steps in a typical ethical application process with example procedures from different universities. Finally, we attempted to provide insights and findings from practical workshops carried out at the 5th International Conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019, focussing on:
• highlighting the basic needs of an ethical committee within an institution,
• discussing and sharing examples of a typical ethical approval process,
• providing guidelines on the ways to teach research ethics with some examples.
We believe this paper provides guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Background literature survey
Responsible research practice (RRP) is scrutinised by the aspects of ethical principles and professional standards (WHO’s Code of Conduct for responsible Research, 2017). The Singapore statement on research integrity (The Singapore Statement on Research integrity, 2010) has provided an internationally acceptable guidance for RRP. The statement is based on maintaining honesty, accountability, professional courtesy in all aspects of research and maintaining fairness during collaborations. In other words, it does not simply focus on the procedural part of the research, instead covers wider aspects of “integrity” beyond the operational aspects (Israel and Drenth, 2016 ).
Institutions should focus on providing ethical guidance based on principles and values reflecting upon all aspects/stages of research (from the funding application/project development stage upto or beyond project closing stage). Figure 1 summarizes the different aspects/stages of a typical research and highlights the needs of RRP in compliance with ethical governance at each stage with examples (the figure is based on Resnik, 2020 ; Žukauskas et al., 2018 ; Anderson, 2011 ; Fouka and Mantzorou, 2011 ).
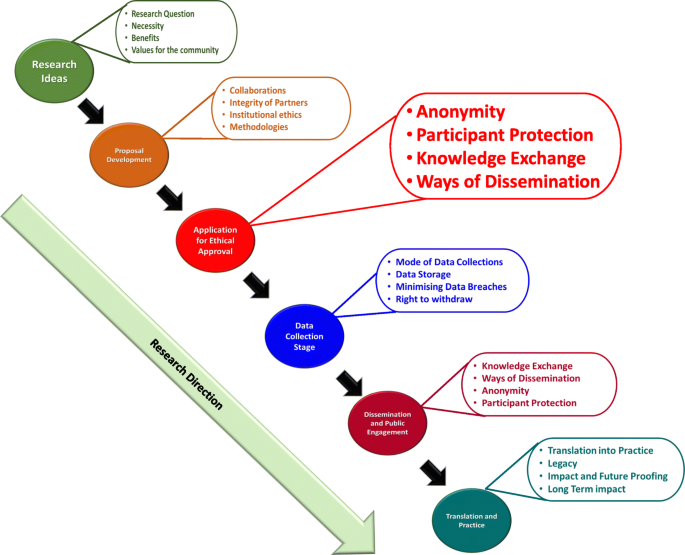
Summary of the enabling ethical governance at different stages of research. Note that it is imperative for researchers to proactively consider the ethical implications before, during and after the actual research process. The summary shows that RRP should be in line with ethical considerations even long before the ethical approval stage
Individual responsibilities to enhance RRP
As explained in Fig. 1 , a successfully governed research should consider ethics at the planning stages prior to research. Many international guidance are compatible in enforcing/recommending 14 different “responsibilities” that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP. In order to understand the purpose and the expectation of these ethical guidelines, we have carried out an initial literature survey on expected individual responsibilities. These are summarised in Table 1 .
By following these directives, researchers can carry out accountable research by maximising ethical self-governance whilst minimising misconducts. In our own experiences of working with many researchers, their focus usually revolves around ethical “clearance” rather than behaviour. In other words, they perceive this as a paper exercise rather than trying to “own” ethical behaviour in everything they do. Although the ethical principles and responsibilities are explicitly highlighted in the majority of international guidelines [such as UK’s Research Governance Policy (NICE, 2018 ), Australian Government’s National Statement on Ethical Conduct in Human Research (Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR), 2018 ), the Singapore Statement (2010) etc.]; and the importance of holistic approach has been argued in ethical decision making, many researchers and/or institutions only focus on ethics linked to the procedural aspects.
Studies in the past have also highlighted inconsistencies in institutional guidelines pointing to the fact that these inconsistencies may hinder the predicted research progress (Desmond & Dierickx 2021 ; Alba et al., 2020 ; Dellaportas et al., 2014 ; Speight 2016 ). It may also be possible that these were and still are linked to the institutional perceptions/expectations or the pre-empting contextual conditions that are imposed by individual countries. In fact, it is interesting to note many research organisations and HE institutions establish their own policies based on these directives.
Research governance - origins, expectations and practices
Ethical governance in clinical medicine helps us by providing a structure for analysis and decision-making. By providing workable definitions of benefits and risks as well as the guidance for evaluating/balancing benefits over risks, it supports the researchers to protect the participants and the general population.
According to the definition given by National Institute of Clinical care Excellence, UK (NICE 2018 ), “ research governance can be defined as the broad range of regulations, principles and standards of good practice that ensure high quality research ”. As stated above, our literature-based research survey showed that most of the ethical definitions are basically evolved from the medical field and other disciplines have utilised these principles to develop their own ethical guidance. Interestingly, historical data show that the medical research has been “self-governed” or in other words implicated by the moral behaviour of individual researchers (Fox 2017 ; Shaw et al., 2005 ; Getz, 1990 ). For example, early human vaccination trials conducted in 1700s used the immediate family members as test subjects (Fox, 2017 ). Here the moral justification might have been the fact that the subjects who would have been at risk were either the scientists themselves or their immediate families but those who would reap the benefits from the vaccination were the general public/wider communities. However, according to the current ethical principles, this assumption is entirely not acceptable.
Historically, ambiguous decision-making and resultant incidences of research misconduct have led to the need for ethical research governance in as early as the 1940’s. For instance, the importance of an international governance was realised only after the World War II, when people were astonished to note the unethical research practices carried out by Nazi scientists. As a result of this, in 1947 the Nuremberg code was published. The code mainly focussed on the following:
Informed consent and further insisted the research involving humans should be based on prior animal work,
The anticipated benefits should outweigh the risk,
Research should be carried out only by qualified scientists must conduct research,
Avoiding physical and mental suffering and.
Avoiding human research that would result in which death or disability.
(Weindling, 2001 ).
Unfortunately, it was reported that many researchers in the USA and elsewhere considered the Nuremberg code as a document condemning the Nazi atrocities, rather than a code for ethical governance and therefore ignored these directives (Ghooi, 2011 ). It was only in 1964 that the World Medical Association published the Helsinki Declaration, which set the stage for ethical governance and the implementation of the Institutional Review Board (IRB) process (Shamoo and Irving, 1993 ). This declaration was based on Nuremberg code. In addition, the declaration also paved the way for enforcing research being conducted in accordance with these guidelines.
Incidentally, the focus on research/ethical governance gained its momentum in 1974. As a result of this, a report on ethical principles and guidelines for the protection of human subjects of research was published in 1979 (The Belmont Report, 1979 ). This report paved the way to the current forms of ethical governance in biomedical and behavioural research by providing guidance.
Since 1994, the WHO itself has been providing several guidance to health care policy-makers, researchers and other stakeholders detailing the key concepts in medical ethics. These are specific to applying ethical principles in global public health.
Likewise, World Organization for Animal Health (WOAH), and International Convention for the Protection of Animals (ICPA) provide guidance on animal welfare in research. Due to this continuous guidance, together with accepted practices, there are internationally established ethical guidelines to carry out medical research. Our literature survey further identified freely available guidance from independent organisations such as COPE (Committee of Publication Ethics) and ALLEA (All European Academics) which provide support for maintaining research ethics in other fields such as education, sociology, psychology etc. In reality, ethical governance is practiced differently in different countries. In the UK, there is a clinical excellence research governance, which oversees all NHS related medical research (Mulholland and Bell, 2005 ). Although, the governance in other disciplines is not entirely centralised, many research funding councils and organisations [such as UKRI (UK-Research and Innovation; BBSC (Biotechnology and Biological Sciences Research Council; MRC (Medical Research Council); EPSRC (Economic and Social Research Council)] provide ethical governance and expect institutional adherence and monitoring. They expect local institutional (i.e. university/institutional) research governance for day-to-day monitoring of the research conducted within the organisation and report back to these funding bodies, monthly or annually (Department of Health, 2005). Likewise, there are nationally coordinated/regulated ethics governing bodies such as the US Office for Human Research Protections (US-OHRP), National Institute of Health (NIH) and the Canadian Institutes for Health Research (CIHR) in the USA and Canada respectively (Mulholland and Bell, 2005 ). The OHRP in the USA formally reviews all research activities involving human subjects. On the other hand, in Canada, CIHR works with the Natural Sciences and Engineering Research Council (NSERC), and the Social Sciences and Humanities Research Council (SSHRC). They together have produced a Tri-Council Policy Statement (TCPS) (Stephenson et al., 2020 ) as ethical governance. All Canadian institutions are expected to adhere to this policy for conducting research. As for Australia, the research is governed by the Australian code for the responsible conduct of research (2008). It identifies the responsibilities of institutions and researchers in all areas of research. The code has been jointly developed by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Universities Australia (UA). This information is summarized in Table 2 .
Basic structure of an institutional ethical advisory committee (EAC)
The WHO published an article defining the basic concepts of an ethical advisory committee in 2009 (WHO, 2009 - see above). According to this, many countries have established research governance and monitor the ethical practice in research via national and/or regional review committees. The main aims of research ethics committees include reviewing the study proposals, trying to understand the justifications for human/animal use, weighing the merits and demerits of the usage (linking to risks vs. potential benefits) and ensuring the local, ethical guidelines are followed Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research, 2020 ; Guide for Research Ethics - Council of Europe, 2014 ). Once the research has started, the committee needs to carry out periodic surveillance to ensure the institutional ethical norms are followed during and beyond the study. They may also be involved in setting up and/or reviewing the institutional policies.
For these aspects, IRB (or institutional ethical advisory committee - IEAC) is essential for local governance to enhance best practices. The advantage of an IRB/EEAC is that they understand the institutional conditions and can closely monitor the ongoing research, including any changes in research directions. On the other hand, the IRB may be overly supportive to accept applications, influenced by the local agenda for achieving research excellence, disregarding ethical issues (Kotecha et al., 2011 ; Kayser-Jones, 2003 ) or, they may be influenced by the financial interests in attracting external funding. In this respect, regional and national ethics committees are advantageous to ensure ethical practice. Due to their impartiality, they would provide greater consistency and legitimacy to the research (WHO, 2009 ). However, the ethical approval process of regional and national ethics committees would be time consuming, as they do not have the local knowledge.
As for membership in the IRBs, most of the guidelines [WHO, NICE, Council of Europe, (2012), European Commission - Facilitating Research Excellence in FP7 ( 2013 ) and OHRP] insist on having a variety of representations including experts in different fields of research, and non-experts with the understanding of local, national/international conflicts of interest. The former would be able to understand/clarify the procedural elements of the research in different fields; whilst the latter would help to make neutral and impartial decisions. These non-experts are usually not affiliated to the institution and consist of individuals representing the broader community (particularly those related to social, legal or cultural considerations). IRBs consisting of these varieties of representation would not only be in a position to understand the study procedures and their potential direct or indirect consequences for participants, but also be able to identify any community, cultural or religious implications of the study.
Understanding the subtle differences between ethics and morals
Interestingly, many ethical guidelines are based on society’s moral “beliefs” in such a way that the words “ethics”‘and “morals” are reciprocally used to define each other. However, there are several subtle differences between them and we have attempted to compare and contrast them herein. In the past, many authors have interchangeably used the words “morals”‘and “ethics”‘(Warwick, 2003 ; Kant, 2018 ; Hazard, GC (Jr)., 1994 , Larry, 1982 ). However, ethics is linked to rules governed by an external source such as codes of conduct in workplaces (Kuyare et al., 2014 ). In contrast, morals refer to an individual’s own principles regarding right and wrong. Quinn ( 2011 ) defines morality as “ rules of conduct describing what people ought and ought not to do in various situations … ” while ethics is “... the philosophical study of morality, a rational examination into people’s moral beliefs and behaviours ”. For instance, in a case of parents demanding that schools overturn a ban on use of corporal punishment of children by schools and teachers (Children’s Rights Alliance for England, 2005 ), the parents believed that teachers should assume the role of parent in schools and use corporal or physical punishment for children who misbehaved. This stemmed from their beliefs and what they felt were motivated by “beliefs of individuals or groups”. For example, recent media highlights about some parents opposing LGBT (Lesbian, Gay, Bisexual, and Transgender) education to their children (BBC News, 2019 ). One parent argued, “Teaching young children about LGBT at a very early stage is ‘morally’ wrong”. She argued “let them learn by themselves as they grow”. This behaviour is linked to and governed by the morals of an ethnic community. Thus, morals are linked to the “beliefs of individuals or group”. However, when it comes to the LGBT rights these are based on ethical principles of that society and governed by law of the land. However, the rights of children to be protected from “inhuman and degrading” treatment is based on the ethical principles of the society and governed by law of the land. Individuals, especially those who are working in medical or judicial professions have to follow an ethical code laid down by their profession, regardless of their own feelings, time or preferences. For instance, a lawyer is expected to follow the professional ethics and represent a defendant, despite the fact that his morals indicate the defendant is guilty.
In fact, we as a group could not find many scholarly articles clearly comparing or contrasting ethics with morals. However, a table presented by Surbhi ( 2015 ) (Difn website c ) tries to differentiate these two terms (see Table 3 ).
Although Table 3 gives some insight on the differences between these two terms, in practice many use these terms as loosely as possible mainly because of their ambiguity. As a group focussed on the application of these principles, we would recommend to use the term “ethics” and avoid “morals” in research and academia.
Based on the literature survey carried out, we were able to identify the following gaps:
there is some disparity in existing literature on the importance of ethical guidelines in research
there is a lack of consensus on what code of conduct should be followed, where it should be derived from and how it should be implemented
The mission of ENAI’s ethical advisory working group
The Ethical Advisory Working Group of ENAI was established in 2018 to promote ethical code of conduct/practice amongst higher educational organisations within Europe and beyond (European Network for Academic Integrity, 2018 ). We aim to provide unbiased advice and consultancy on embedding ethical principles within all types of academic, research and public engagement activities. Our main objective is to promote ethical principles and share good practice in this field. This advisory group aims to standardise ethical norms and to offer strategic support to activities including (but not exclusive to):
● rendering advice and assistance to develop institutional ethical committees and their regulations in member institutions,
● sharing good practice in research and academic ethics,
● acting as a critical guide to institutional review processes, assisting them to maintain/achieve ethical standards,
● collaborating with similar bodies in establishing collegiate partnerships to enhance awareness and practice in this field,
● providing support within and outside ENAI to develop materials to enhance teaching activities in this field,
● organising training for students and early-career researchers about ethical behaviours in form of lectures, seminars, debates and webinars,
● enhancing research and dissemination of the findings in matters and topics related to ethics.
The following sections focus on our suggestions based on collective experiences, review of literature provided in earlier sections and workshop feedback collected:
a) basic needs of an ethical committee within an institution;
b) a typical ethical approval process (with examples from three different universities); and
c) the ways to obtain informed consent with some examples. This would give advice on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Setting up an institutional ethical committee (ECs)
Institutional Ethical Committees (ECs) are essential to govern every aspect of the activities undertaken by that institute. With regards to higher educational organisations, this is vital to establish ethical behaviour for students and staff to impart research, education and scholarly activities (or everything) they do. These committees should be knowledgeable about international laws relating to different fields of studies (such as science, medicine, business, finance, law, and social sciences). The advantages and disadvantages of institutional, subject specific or common (statutory) ECs are summarised in Fig. 2 . Some institutions have developed individual ECs linked to specific fields (or subject areas) whilst others have one institutional committee that overlooks the entire ethical behaviour and approval process. There is no clear preference between the two as both have their own advantages and disadvantages (see Fig. 2 ). Subject specific ECs are attractive to medical, law and business provisions, as it is perceived the members within respective committees would be able to understand the subject and therefore comprehend the need of the proposed research/activity (Kadam, 2012 ; Schnyder et al., 2018 ). However, others argue, due to this “ specificity ”, the committee would fail to forecast the wider implications of that application. On the other hand, university-wide ECs would look into the wider implications. Yet they find it difficult to understand the purpose and the specific applications of that research. Not everyone understands dynamics of all types of research methodologies, data collection, etc., and therefore there might be a chance of a proposal being rejected merely because the EC could not understand the research applications (Getz, 1990 ).
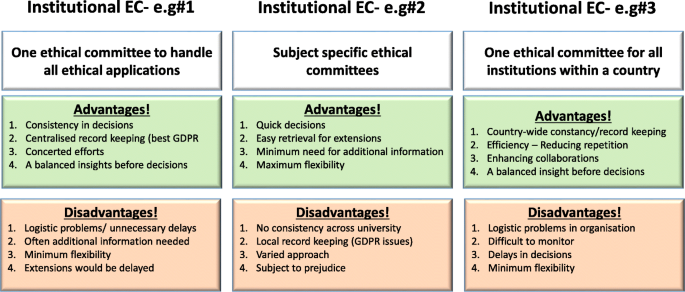
Summary of advantages and disadvantages of three different forms of ethical committees
[N/B for Fig. 2 : Examples of different types of ethical application procedures and forms used were discussed with the workshop attendees to enhance their understanding of the differences. GDPR = General Data Protection Regulation].
Although we recommend a designated EC with relevant professional, academic and ethical expertise to deal with particular types of applications, the membership (of any EC) should include some non-experts who would represent the wider community (see above). Having some non-experts in EC would not only help the researchers to consider explaining their research in layperson’s terms (by thinking outside the box) but also would ensure efficiency without compromising participants/animal safety. They may even help to address the common ethical issues outside research culture. Some UK universities usually offer this membership to a clergy, councillor or a parliamentarian who does not have any links to the institutions. Most importantly, it is vital for any EC members to undertake further training in addition to previous experience in the relevant field of research ethics.
Another issue that raises concerns is multi-centre research, involving several institutions, where institutionalised ethical approvals are needed from each partner. In some cases, such as clinical research within the UK, a common statutory EC called National Health Services (NHS) Research Ethics Committee (NREC) is in place to cover research ethics involving all partner institutions (NHS, 2018 ). The process of obtaining approval from this type of EC takes time, therefore advanced planning is needed.
Ethics approval forms and process
During the workshop, we discussed some anonymised application forms obtained from open-access sources for qualitative and quantitative research as examples. Considering research ethics, for the purpose of understanding, we arbitrarily divided this in two categories; research based on (a) quantitative and (b) qualitative methodologies. As their name suggests their research approach is extremely different from each other. The discussion elicited how ECs devise different types of ethical application form/questions. As for qualitative research, these are often conducted as “face-to-face” interviews, which would have implications on volunteer anonymity.
Furthermore, discussions posited when the interviews are replaced by on-line surveys, they have to be administered through registered university staff to maintain confidentiality. This becomes difficult when the research is a multi-centre study. These types of issues are also common in medical research regarding participants’ anonymity, confidentially, and above all their right to withdraw consent to be involved in research.
Storing and protecting data collected in the process of the study is also a point of consideration when applying for approval.
Finally, the ethical processes of invasive (involving human/animals) and non-invasive research (questionnaire based) may slightly differ from one another. Following research areas are considered as investigations that need ethical approval:
research that involves human participants (see below)
use of the ‘products’ of human participants (see below)
work that potentially impacts on humans (see below)
research that involves animals
In addition, it is important to provide a disclaimer even if an ethical approval is deemed unnecessary. Following word cloud (Fig. 3 ) shows the important variables that need to be considered at the brainstorming stage before an ethical application. It is worth noting the importance of proactive planning predicting the “unexpected” during different phases of a research project (such as planning, execution, publication, and future directions). Some applications (such as working with vulnerable individuals or children) will require safety protection clearance (such as DBS - Disclosure and Barring Service, commonly obtained from the local police). Please see section on Research involving Humans - Informed consents for further discussions.
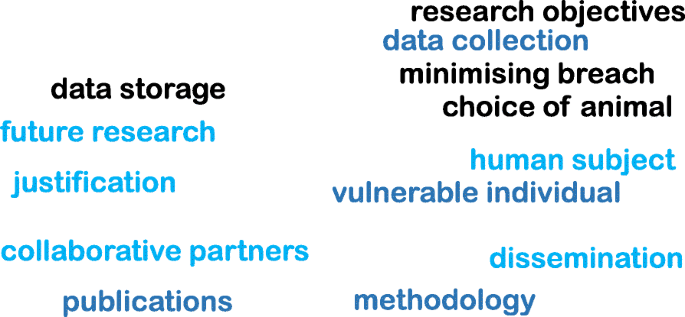
Examples of important variables that need to be considered for an ethical approval
It is also imperative to report or re-apply for ethical approval for any minor or major post-approval changes to original proposals made. In case of methodological changes, evidence of risk assessments for changes and/or COSHH (Control of Substances Hazardous to Health Regulations) should also be given. Likewise, any new collaborative partners or removal of researchers should also be notified to the IEAC.
Other findings include:
in case of complete changes in the project, the research must be stopped and new approval should be seeked,
in case of noticing any adverse effects to project participants (human or non-human), these should also be notified to the committee for appropriate clearance to continue the work, and
the completion of the project must also be notified with the indication whether the researchers may restart the project at a later stage.
Research involving humans - informed consents
While discussing research involving humans and based on literature review, findings highlight the human subjects/volunteers must willingly participate in research after being adequately informed about the project. Therefore, research involving humans and animals takes precedence in obtaining ethical clearance and its strict adherence, one of which is providing a participant information sheet/leaflet. This sheet should contain a full explanation about the research that is being carried out and be given out in lay-person’s terms in writing (Manti and Licari 2018 ; Hardicre 2014 ). Measures should also be in place to explain and clarify any doubts from the participants. In addition, there should be a clear statement on how the participants’ anonymity is protected. We provide below some example questions below to help the researchers to write this participant information sheet:
What is the purpose of the study?
Why have they been chosen?
What will happen if they take part?
What do they have to do?
What happens when the research stops?
What if something goes wrong?
What will happen to the results of the research study?
Will taking part be kept confidential?
How to handle “vulnerable” participants?
How to mitigate risks to participants?
Many institutional ethics committees expect the researchers to produce a FAQ (frequently asked questions) in addition to the information about research. Most importantly, the researchers also need to provide an informed consent form, which should be signed by each human participant. The five elements identified that are needed to be considered for an informed consent statement are summarized in Fig. 4 below (slightly modified from the Federal Policy for the Protection of Human Subjects ( 2018 ) - Diffn website c ).
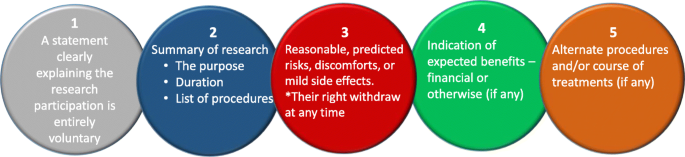
Five basic elements to consider for an informed consent [figure adapted from Diffn website c ]
The informed consent form should always contain a clause for the participant to withdraw their consent at any time. Should this happen all the data from that participant should be eliminated from the study without affecting their anonymity.
Typical research ethics approval process
In this section, we provide an example flow chart explaining how researchers may choose the appropriate application and process, as highlighted in Fig. 5 . However, it is imperative to note here that these are examples only and some institutions may have one unified application with separate sections to demarcate qualitative and quantitative research criteria.
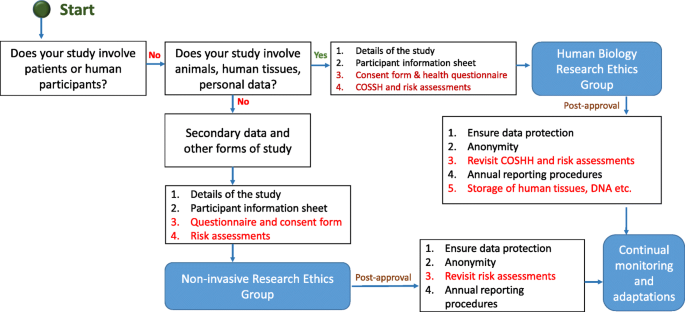
Typical ethical approval processes for quantitative and qualitative research. [N/B for Fig. 5 - This simplified flow chart shows that fundamental process for invasive and non-invasive EC application is same, the routes and the requirements for additional information are slightly different]
Once the ethical application is submitted, the EC should ensure a clear approval procedure with distinctly defined timeline. An example flow chart showing the procedure for an ethical approval was obtained from University of Leicester as open-access. This is presented in Fig. 6 . Further examples of the ethical approval process and governance were discussed in the workshop.
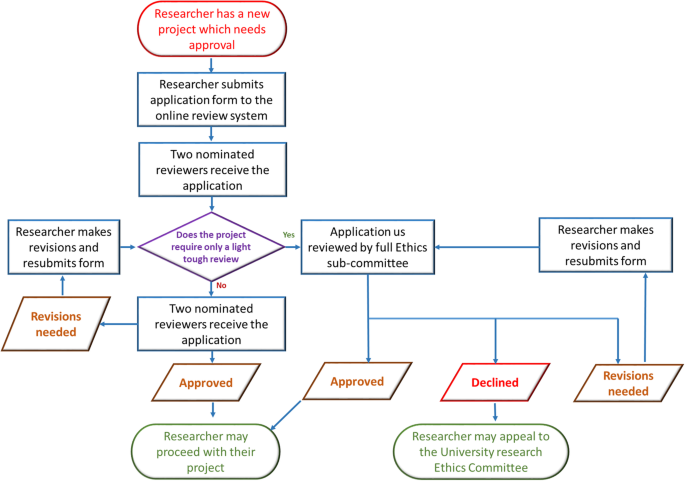
An example ethical approval procedures conducted within University of Leicester (Figure obtained from the University of Leicester research pages - Difn website d - open access)
Strategies for ethics educations for students
Student education on the importance of ethics and ethical behaviour in research and scholarly activities is extremely essential. Literature posits in the area of medical research that many universities are incorporating ethics in post-graduate degrees but when it comes to undergraduate degrees, there is less appetite to deliver modules or even lectures focussing on research ethics (Seymour et al., 2004 ; Willison and O’Regan, 2007 ). This may be due to the fact that undergraduate degree structure does not really focus on research (DePasse et al., 2016 ). However, as Orr ( 2018 ) suggested, institutions should focus more on educating all students about ethics/ethical behaviour and their importance in research, than enforcing punitive measures for unethical behaviour. Therefore, as an advisory committee, and based on our preliminary literature survey and workshop results, we strongly recommend incorporating ethical education within undergraduate curriculum. Looking at those institutions which focus on ethical education for both under-and postgraduate courses, their approaches are either (a) a lecture-based delivery, (b) case study based approach or (c) a combined delivery starting with a lecture on basic principles of ethics followed by generating a debate based discussion using interesting case studies. The combined method seems much more effective than the other two as per our findings as explained next.
As many academics who have been involved in teaching ethics and/or research ethics agree, the underlying principles of ethics is often perceived as a boring subject. Therefore, lecture-based delivery may not be suitable. On the other hand, a debate based approach, though attractive and instantly generates student interest, cannot be effective without students understanding the underlying basic principles. In addition, when selecting case studies, it would be advisable to choose cases addressing all different types of ethical dilemmas. As an advisory group within ENAI, we are in the process of collating supporting materials to help to develop institutional policies, creating advisory documents to help in obtaining ethical approvals, and teaching materials to enhance debate-based lesson plans that can be used by the member and other institutions.
Concluding remarks
In summary, our literature survey and workshop findings highlight that researchers should accept that ethics underpins everything we do, especially in research. Although ethical approval is tedious, it is an imperative process in which proactive thinking is essential to identify ethical issues that might affect the project. Our findings further lead us to state that the ethical approval process differs from institution to institution and we strongly recommend the researchers to follow the institutional guidelines and their underlying ethical principles. The ENAI workshop in Vilnius highlighted the importance of ethical governance by establishing ECs, discussed different types of ECs and procedures with some examples and highlighted the importance of student education to impart ethical culture within research communities, an area that needs further study as future scope.
Declarations
The manuscript was entirely written by the corresponding author with contributions from co-authors who have also taken part in the delivery of the workshop. Authors confirm that the data supporting the findings of this study are available within the article. We can also confirm that there are no potential competing interests with other organisations.
Availability of data and materials
Authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
ALL European academics
Australian research council
Biotechnology and biological sciences research council
Canadian institutes for health research
Committee of publication ethics
Ethical committee
European network of academic integrity
Economic and social research council
International convention for the protection of animals
institutional ethical advisory committee
Institutional review board
Immaculata university of Pennsylvania
Lesbian, gay, bisexual, and transgender
Medical research council)
National health services
National health services nih national institute of health (NIH)
National institute of clinical care excellence
National health and medical research council
Natural sciences and engineering research council
National research ethics committee
National statement on ethical conduct in human research
Responsible research practice
Social sciences and humanities research council
Tri-council policy statement
World Organization for animal health
Universities Australia
UK-research and innovation
US office for human research protections
Alba S, Lenglet A, Verdonck K, Roth J, Patil R, Mendoza W, Juvekar S, Rumisha SF (2020) Bridging research integrity and global health epidemiology (BRIDGE) guidelines: explanation and elaboration. BMJ Glob Health 5(10):e003237. https://doi.org/10.1136/bmjgh-2020-003237
Article Google Scholar
Anderson MS (2011) Research misconduct and misbehaviour. In: Bertram Gallant T (ed) Creating the ethical academy: a systems approach to understanding misconduct and empowering change in higher education. Routledge, pp 83–96
BBC News. (2019). Birmingham school LGBT LESSONS PROTEST investigated. March 8, 2019. Retrieved February 14, 2021, available online. URL: https://www.bbc.com/news/uk-england-birmingham-47498446
Children’s Rights Alliance for England. (2005). R (Williamson and others) v Secretary of State for Education and Employment. Session 2004–05. [2005] UKHL 15. Available Online. URL: http://www.crae.org.uk/media/33624/R-Williamson-and-others-v-Secretary-of-State-for-Education-and-Employment.pdf
Council of Europe. (2014). Texts of the Council of Europe on bioethical matters. Available Online. https://www.coe.int/t/dg3/healthbioethic/Texts_and_documents/INF_2014_5_vol_II_textes_%20CoE_%20bio%C3%A9thique_E%20(2).pdf
Dellaportas S, Kanapathippillai S, Khan, A and Leung, P. (2014). Ethics education in the Australian accounting curriculum: a longitudinal study examining barriers and enablers. 362–382. Available Online. URL: https://doi.org/10.1080/09639284.2014.930694 , 23, 4, 362, 382
DePasse JM, Palumbo MA, Eberson CP, Daniels AH (2016) Academic characteristics of orthopaedic surgery residency applicants from 2007 to 2014. JBJS 98(9):788–795. https://doi.org/10.2106/JBJS.15.00222
Desmond H, Dierickx K (2021) Research integrity codes of conduct in Europe: understanding the divergences. https://doi.org/10.1111/bioe.12851
Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR). (2018). Available Online. URL: https://www.nhmrc.gov.au/about-us/publications/australian-code-responsible-conduct-research-2018
Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research (2020, October 26). Available online. URL: https://www.enago.com/academy/importance-of-ethics-committees-in-scholarly-research/
Difn website c - Ethics vs Morals - Difference and Comparison. Retrieved July 14, 2020. Available online. URL: https://www.diffen.com/difference/Ethics_vs_Morals
Difn website d - University of Leicester. (2015). Staff ethics approval flowchart. May 1, 2015. Retrieved July 14, 2020. Available Online. URL: https://www2.le.ac.uk/institution/ethics/images/ethics-approval-flowchart/view
European Commission - Facilitating Research Excellence in FP7 (2013) https://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf
European Network for Academic Integrity. (2018). Ethical advisory group. Retrieved February 14, 2021. Available online. URL: http://www.academicintegrity.eu/wp/wg-ethical/
Federal Policy for the Protection of Human Subjects. (2018). Retrieved February 14, 2021. Available Online. URL: https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects#p-855
Flite, CA and Harman, LB. (2013). Code of ethics: principles for ethical leadership Perspect Health Inf Mana; 10(winter): 1d. PMID: 23346028
Fouka G, Mantzorou M (2011) What are the major ethical issues in conducting research? Is there a conflict between the research ethics and the nature of nursing. Health Sci J 5(1) Available Online. URL: https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
Fox G (2017) History and ethical principles. The University of Miami and the Collaborative Institutional Training Initiative (CITI) Program URL https://silo.tips/download/chapter-1-history-and-ethical-principles # (Available Online)
Getz KA (1990) International codes of conduct: An analysis of ethical reasoning. J Bus Ethics 9(7):567–577
Ghooi RB (2011) The nuremberg code–a critique. Perspect Clin Res 2(2):72–76. https://doi.org/10.4103/2229-3485.80371
Hardicre, J. (2014) Valid informed consent in research: an introduction Br J Nurs 23(11). https://doi.org/10.12968/bjon.2014.23.11.564 , 567
Hazard, GC (Jr). (1994). Law, morals, and ethics. Yale law school legal scholarship repository. Faculty Scholarship Series. Yale University. Available Online. URL: https://digitalcommons.law.yale.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3322&context=fss_papers
Israel, M., & Drenth, P. (2016). Research integrity: perspectives from Australia and Netherlands. In T. Bretag (Ed.), Handbook of academic integrity (pp. 789–808). Springer, Singapore. https://doi.org/10.1007/978-981-287-098-8_64
Kadam R (2012) Proactive role for ethics committees. Indian J Med Ethics 9(3):216. https://doi.org/10.20529/IJME.2012.072
Kant I (2018) The metaphysics of morals. Cambridge University Press, UK https://doi.org/10.1017/9781316091388
Kayser-Jones J (2003) Continuing to conduct research in nursing homes despite controversial findings: reflections by a research scientist. Qual Health Res 13(1):114–128. https://doi.org/10.1177/1049732302239414
Kotecha JA, Manca D, Lambert-Lanning A, Keshavjee K, Drummond N, Godwin M, Greiver M, Putnam W, Lussier M-T, Birtwhistle R (2011) Ethics and privacy issues of a practice-based surveillance system: need for a national-level institutional research ethics board and consent standards. Can Fam physician 57(10):1165–1173. https://europepmc.org/article/pmc/pmc3192088
Kuyare, MS., Taur, SR., Thatte, U. (2014). Establishing institutional ethics committees: challenges and solutions–a review of the literature. Indian J Med Ethics. https://doi.org/10.20529/IJME.2014.047
LaFollette, H. (2007). Ethics in practice (3rd edition). Blackwell
Larry RC (1982) The teaching of ethics and moral values in teaching. J High Educ 53(3):296–306. https://doi.org/10.1080/00221546.1982.11780455
Manti S, Licari A (2018) How to obtain informed consent for research. Breathe (Sheff) 14(2):145–152. https://doi.org/10.1183/20734735.001918
Mulholland MW, Bell J (2005) Research Governance and Research Funding in the USA: What the academic surgeon needs to know. J R Soc Med 98(11):496–502. https://doi.org/10.1258/jrsm.98.11.496
National Institute of Health (NIH) Ethics in Clinical Research. n.d. Available Online. URL: https://clinicalcenter.nih.gov/recruit/ethics.html
NHS (2018) Flagged Research Ethics Committees. Retrieved February 14, 2021. Available online. URL: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/flagged-research-ethics-committees/
NICE (2018) Research governance policy. Retrieved February 14, 2021. Available online. URL: https://www.nice.org.uk/Media/Default/About/what-we-do/science-policy-and-research/research-governance-policy.pdf
Orr, J. (2018). Developing a campus academic integrity education seminar. J Acad Ethics 16(3), 195–209. https://doi.org/10.1007/s10805-018-9304-7
Quinn, M. (2011). Introduction to Ethics. Ethics for an Information Age. 4th Ed. Ch 2. 53–108. Pearson. UK
Resnik. (2020). What is ethics in Research & why is it Important? Available Online. URL: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Schnyder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V (2018) The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online 23(1):1. https://doi.org/10.1080/10872981.2018.1424449
Seymour E, Hunter AB, Laursen SL, DeAntoni T (2004) Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci Educ 88(4):493–534. https://doi.org/10.1002/sce.10131
Shamoo AE, Irving DN (1993) Accountability in research using persons with mental illness. Account Res 3(1):1–17. https://doi.org/10.1080/08989629308573826
Shaw, S., Boynton, PM., and Greenhalgh, T. (2005). Research governance: where did it come from, what does it mean? Research governance framework for health and social care, 2nd ed. London: Department of Health. https://doi.org/10.1258/jrsm.98.11.496 , 98, 11, 496, 502
Book Google Scholar
Speight, JG. (2016) Ethics in the university |DOI: https://doi.org/10.1002/9781119346449 scrivener publishing LLC
Stephenson GK, Jones GA, Fick E, Begin-Caouette O, Taiyeb A, Metcalfe A (2020) What’s the protocol? Canadian university research ethics boards and variations in implementing tri-Council policy. Can J Higher Educ 50(1)1): 68–81
Surbhi, S. (2015). Difference between morals and ethics [weblog]. March 25, 2015. Retrieved February 14, 2021. Available Online. URL: http://keydifferences.com/difference-between-morals-and-ethics.html
The Belmont Report (1979). Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Retrieved February 14, 2021. Available online. URL: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
The Singapore Statement on Research Integrity. (2020). Nicholas Steneck and Tony Mayer, Co-chairs, 2nd World Conference on Research Integrity; Melissa Anderson, Chair, Organizing Committee, 3rd World Conference on Research Integrity. Retrieved February 14, 2021. Available online. URL: https://wcrif.org/documents/327-singapore-statement-a4size/file
Warwick K (2003) Cyborg morals, cyborg values, cyborg ethics. Ethics Inf Technol 5(3):131–137. https://doi.org/10.1023/B:ETIN.0000006870.65865.cf
Weindling P (2001) The origins of informed consent: the international scientific commission on medical war crimes, and the Nuremberg code. Bull Hist Med 75(1):37–71. https://doi.org/10.1353/bhm.2001.0049
WHO. (2009). Research ethics committees Basic concepts for capacity-building. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
WHO. (2021). Chronological list of publications. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/publications/year/en/
Willison, J. and O’Regan, K. (2007). Commonly known, commonly not known, totally unknown: a framework for students becoming researchers. High Educ Res Dev 26(4). 393–409. https://doi.org/10.1080/07294360701658609
Žukauskas P, Vveinhardt J, and Andriukaitienė R. (2018). Research Ethics In book: Management Culture and Corporate Social Responsibility Eds Jolita Vveinhardt IntechOpenEditors DOI: https://doi.org/10.5772/intechopen.70629 , 2018
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference.
Not applicable as this is an independent study, which is not funded by any internal or external bodies.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, DE22 1, Derby, GB, UK
Shivadas Sivasubramaniam
Department of Informatics, Mendel University in Brno, Zemědělská, 1665, Brno, Czechia
Dita Henek Dlabolová
Centre for Academic Integrity in the UAE, Faculty of Engineering & Information Sciences, University of Wollongong in Dubai, Dubai, UAE
Veronika Kralikova & Zeenath Reza Khan
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was entirely written by the corresponding author with contributions from co-authors who have equally contributed to presentation of this paper in the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania. Authors have equally contributed for the information collection, which were then summarised as narrative explanations by the Corresponding author and Dr. Zeenath Reza Khan. Then checked and verified by Dr. Dlabolova and Ms. Králíková. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Shivadas Sivasubramaniam .
Ethics declarations
Competing interests.
We can also confirm that there are no potential competing interest with other organisations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S., Dlabolová, D.H., Kralikova, V. et al. Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures. Int J Educ Integr 17 , 14 (2021). https://doi.org/10.1007/s40979-021-00078-6
Download citation
Received : 17 July 2020
Accepted : 25 April 2021
Published : 13 July 2021
DOI : https://doi.org/10.1007/s40979-021-00078-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Ethical codes
- Ethics committee
- Post-secondary education
- Institutional policies
- Research ethics
International Journal for Educational Integrity
ISSN: 1833-2595
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

The Importance of Ethics in Research Essay
Introduction.
In science and medical research, ethics is essential in enhancing the safety and well-being of the subjects or participants. Different studies globally expose vulnerable populations or subjects to abuse, affecting their overall health. In the same case, researchers are employing diverse strategies to enhance ethics and reduce subjects’ vulnerabilities to negative implications of studies and abuse. For these reasons, it is essential to examine the factors that enhance subjects’ vulnerability to abuse and maltreatment during scientific studies. These reasons are economic and financial problems, impractical hope, improper patient advocacy, as well as non-compliance to research ethics. Contrarily, encouraging compliance with ethical principles during research would reduce subjects’ susceptibility to abuse and negative research implications.
Factors that Increase Vulnerability of Subjects to Research Abuse
Economic and financial issues are among the factors that increase subjects’ vulnerability to abuse during research. Kelly (2013) indicates that “people struggling to put food on the table and a roof over their heads” are vulnerable to abuse during clinical trials and pharmaceutical studies. Washington (2008) also notes that “jobless white men turned their noses at the disgusting work and partly pay” when referring to a perilous clinical experiment conducted in 1935 by the University of Pennsylvania. The sentiment indicates that the university conducted unethical and dangerous medical research on subjects with economic and financial problems (Washington, 2008). The phrase “when you see an opportunity to feed your starving family, […], or get treatment for a terminal disease?” also indicates how financial issues make subjects partake in unsafe clinical trials (Kelly, 2013). Thus, these studies increase the health risks, burden, disparity, and complications among subjects from developing and low-income countries as well as communities.
Unrealistic hope is another factor that increases the risks of abuse among subjects of research activities. According to Washington (2008), patients desperate for healing from diseases, surviving, living longer, and stressed about their health conditions are vulnerable to abuse during medical studies. The phrase “was described to him as his last chance at a meaningful life” shows that James Quinn was a victim of research abuse because of hoped to live a productive life after artificial heart implantation (Washington, 2008). The quote, “Do you have a choice about participating when you see an opportunity to (…) get treatment for a terminal disease,” indicates that subjects are always hopeful of improving their health after clinical studies (Kelly, 2008). This makes patients accept risky treatments or clinical trials that are mentally or physically abusive to their health, hoping to enhance their lifespan.
Improper patient advocacy and education are also major factors that increase the dangers of abusing subjects during clinical research. Washington (2008) questions how medical researchers and providers empower patients about the risks and benefits of clinical trials before treatments. Washington (2008) asks, “But are such warnings offered in a fair and intelligible manner?” The quote proves that researchers obtain patients’ consent for treatments when they are mentally, emotionally, and cognitively incapable of making informed decisions about these interventions. Kelly (2013) also agrees that healthcare researchers fail to provide accurate and quality patient information before clinical trials. The sentence “one of the most commonly cited ethical qualms with clinical trials tends to be misinformation” indicates that patients’ advocacy teams misinform subjects before clinical trials (Kelly, 2013). The wrong information affects the ability of patients to make informed decisions about participating or not partaking in medical studies.
Finally, non-compliance to research ethics and regulations among researchers also makes subjects vulnerable to abuse during studies. Washington (2008) indicates that “the informed consent process consists of much more than obtaining a patient signature on a piece of paper.” The quote implies that medical researchers are violating the informed consent ethics of research that requires patients’ participation only after knowing all the risks and benefits of a treatment. In the statement, “one of the most commonly cited ethical qualms with clinical trials tends to be misinformation,” Kelly (2013) supports Washington (2008) about the voluntary and involuntary deception of subjects to participate in their studies. Generally, violation of ethics makes vulnerable subjects partake in medical or scientific studies that harm their health and those around them.
The Solution to Reduce Vulnerability of Subjects to Research Abuse
To reduce the susceptibility of patients or subjects to abusive medical and scientific studies, adherence or compliance to research ethics is essential. For instance, Kelly (2013) suggests that continuous investigation by ethics boards on researchers violating ethical requirements would enhance compliance with research regulations, integrity, and morals. The statement “informed consent is an ongoing process of patient notification and education” also implies that continuous patient advocacy, edification, and communication before treatments and clinical trials is essential in reducing non-compliance to research ethics (Washington, 2008). Moreover, when seeing patient permission for research inclusion, Washington (2008) proposes that researchers should make patients aware of treatment risks through the phrase “the scientist must […] knows all the known risks and must inform the subject proxy.” This will make subjects decide to participate in research after knowing the benefits and negative implications on them and the people around them.
Conclusively, the lack of compliance with ethical research principles among scientists and the economic issues of patients make them susceptible to abuse during studies. Unrealistic hope, desperation, and inappropriate patient advocacy and education make subjects vulnerable to research abuse. Based on these, developing, implementing, and complying with research ethics is a feasible approach to reducing the vulnerability of research subjects to abuse. Therefore, patient abuse is a systemic issue in medical and scientific studies. This means that this problem requires universal or systemic solutions to achieve the desired outcome of protecting patients’ interests and well-being during and after research.
Kelly, S. (2013). Testing drugs on the developing world . The Atlantic. Web.
Washington, H. (2008). Medical apartheid: The dark history of medical experimentation on Black Americans from colonial times to the present. Psychiatric Services , 58 (10), 1380-1381.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, April 1). The Importance of Ethics in Research. https://ivypanda.com/essays/the-importance-of-ethics-in-research/
"The Importance of Ethics in Research." IvyPanda , 1 Apr. 2024, ivypanda.com/essays/the-importance-of-ethics-in-research/.
IvyPanda . (2024) 'The Importance of Ethics in Research'. 1 April.
IvyPanda . 2024. "The Importance of Ethics in Research." April 1, 2024. https://ivypanda.com/essays/the-importance-of-ethics-in-research/.
1. IvyPanda . "The Importance of Ethics in Research." April 1, 2024. https://ivypanda.com/essays/the-importance-of-ethics-in-research/.
Bibliography
IvyPanda . "The Importance of Ethics in Research." April 1, 2024. https://ivypanda.com/essays/the-importance-of-ethics-in-research/.
- Ned Kelly as an Iconic Figure
- Kelly’s Business: Planning for Growth
- Non-Compliance in Diabetic Patients as a Nursing Issue
- Kelly Sandwich Stop Company's Growth Planning
- The History of the Kelly Gang: Realistic and Artistic Value
- Rhetoric in “The Land Before Time” by M. T. Kelly
- Kelly’s cognitive theory
- Donnie Darko by Richard Kelly Review
- Employment Non-Compliance in Australia in 2008-12
- Feldman and Kelly's Views on Disagreement
- Practice-Informed Research: Case Analysis
- Ethical Issues Associated with Online Research
- Ethical Principles in Research
- Discussion: Ethics and Fashion
- Abortion Rights: The Ethical Issues
Public Health Notes
Your partner for better health, research ethics: definition, principles and advantages.
October 13, 2020 Kusum Wagle Epidemiology 0

Table of Contents
What is Research Ethics?
- Ethics are the set of rules that govern our expectations of our own and others’ behavior.
- Research ethics are the set of ethical guidelines that guides us on how scientific research should be conducted and disseminated.
- Research ethics govern the standards of conduct for scientific researchers It is the guideline for responsibly conducting the research.
- Research that implicates human subjects or contributors rears distinctive and multifaceted ethical, legitimate, communal and administrative concerns.
- Research ethics is unambiguously concerned in the examination of ethical issues that are upraised when individuals are involved as participants in the study.
- Research ethics committee/Institutional Review Board (IRB) reviews whether the research is ethical enough or not to protect the rights, dignity and welfare of the respondents.
Objectives of Research Ethics:
- The first and comprehensive objective – to guard/protect human participants, their dignity, rights and welfare .
- The second objective – to make sure that research is directed in a manner that assists welfares of persons, groups and/or civilization as a whole.
- The third objective – to inspect particular research events and schemes for their ethical reliability, considering issues such as the controlling risk, protection of privacy and the progression of informed consent.
Principles of Research Ethics:
The general principles of research ethics are:
Broad categorization of principles of research ethics:.
Broadly categorizing, there are mainly five principles of research ethics:
1. MINIMIZING THE RISK OF HARM
It is necessary to minimize any sort of harm to the participants. There are a number of forms of harm that participants can be exposed to. They are:
- Bodily harm to contributors.
- Psychological agony and embarrassment.
- Social drawback.
- Violation of participant’s confidentiality and privacy.
In order to minimize the risk of harm, the researcher/data collector should:
- Obtain informed consent from participants.
- Protecting anonymity and confidentiality of participants.
- Avoiding misleading practices when planning research.
- Providing participants with the right to withdraw .
2. OBTAINING INFORMED CONSENT
One of the fundamentals of research ethics is the notion of informed consent .
Informed consent means that a person knowingly, voluntarily and intelligently gives consent to participate in a research.
Informed consent means that the participants should be well-informed about the:
- Introduction and objective of the research
- Purpose of the discussion
- Anticipated advantages, benefits/harm from the research (if any)
- Use of research
- Their role in research
- Methods which will be used to protect anonymity and confidentiality of the participant
- Freedom to not answer any question/withdraw from the research
- Who to contact if the participant need additional information about the research
3. PROTECTING ANONYMITY AND CONFIDENTIALITY
Protecting the anonymity and confidentiality of research participants is an additionally applied constituent of research ethics.
Protecting anonymity: It means keeping the participant anonymous. It involves not revealing the name, caste or any other information about the participants that may reveal his/her identity.
Maintaining confidentiality: It refers to ensuring that the information given by the participant are confidential and not shared with anyone, except the research team. It is also about keeping the information secretly from other people.
4. AVOIDING MISLEADING PRACTICES
- The researcher should avoid all the deceptive and misleading practices that might misinform the respondent.
- It includes avoiding all the activities like communicating wrong messages, giving false assurance, giving false information etc.
5. PROVIDING THE RIGHT TO WITHDRAW
- Participants have to have the right to withdraw at any point of the research.
- When any respondent decides on to withdraw from the research, they should not be stressed or forced in any manner to try to discontinue them from withdrawing.
Apart from the above-mentioned ethics, other ethical aspects things that must be considered while doing research are:
Protection of vulnerable groups of people:
- Vulnerability is one distinctive feature of people incapable to protect their moralities and wellbeing. Vulnerable groups comprise captive populations (detainees, established, students, etc.), mentally ill persons, and aged people, children, critically ill or dying, poor, with learning incapacities, sedated or insensible.
- Their participation in research can be endorsed to their incapability to give an informed consent and to the need for their further safety and sensitivity from the research/researcher as they are in a greater risk of being betrayed, exposed or forced to participate.
Skills of the researcher:
- Researchers should have the basic skills and familiarity for the specific study to be carried out and be conscious of the bounds of personal competence in research.
- Any lack of knowledge in the area under research must be clearly specified.
- Inexperienced researchers should work under qualified supervision that has to be revised by an ethics commission.
Advantages of Research Ethics:
- Research ethics promote the aims of research.
- It increases trust among the researcher and the respondent.
- It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants.
- Researchers can be held accountable and answerable for their actions.
- Ethics promote social and moral values.
- Promote s the ambitions of research, such as understanding, veracity, and dodging of error.
- Ethical standards uphold the values that are vital to cooperative work , such as belief, answerability, mutual respect, and impartiality.
- Ethical norms in research also aid to construct public upkeep for research. People are more likely to trust a research project if they can trust the worth and reliability of research.
Limitations of Research Ethics:
For subjects:
- Possibilities to physical integrity, containing those linked with experimental drugs and dealings and with other involvements that will be used in the study (e.g. measures used to observe research participants, such as blood sampling, X-rays or lumbar punctures).
- Psychological risks: for example, a questionnaire may perhaps signify a risk if it fears traumatic events or happenings that are especially traumatic.
- Social, legal and economic risks : for example, if personal information collected during a study is unintentionally released, participants might face a threat of judgment and stigmatization.
- Certain tribal or inhabitant groups may possibly suffer from discrimination or stigmatization, burdens because of research, typically if associates of those groups are recognized as having a greater-than-usual risk of devouring a specific disease.
- The research may perhaps have an influence on the prevailing health system: for example, human and financial capitals dedicated to research may distract attention from other demanding health care necessities in the community.
How can we ensure ethics at different steps of research?
The following process helps to ensure ethics at different steps of research:
- Collect the facts and talk over intellectual belongings openly
- Outline the ethical matters
- Detect the affected parties (stakeholders)
- Ascertain the forfeits
- Recognize the responsibilities (principles, rights, justice)
- Contemplate your personality and truthfulness
- Deliberate innovatively about possible actions
- Respect privacy and confidentiality
- Resolve on the appropriate ethical action and be willing to deal with divergent point of view.
References and For More Information:
http://dissertation.laerd.com/principles-of-research-ethics.php
https://researchethics.ca/what-is-research-ethics/
https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
https://research.ku.edu/sites/research.ku.edu/files/docs/EESE_EthicalDecisionmakingFramework.pdf
https://www.who.int/ethics/research/en/
https://www.ufrgs.br/bioetica/cioms2008.pdf
https://www.who.int/ethics/research/en/#:~:text=WHO%20Research%20Ethics%20Review%20Committee,financially%20or%20technically%20by%20WHO .
https://www.who.int/reproductivehealth/topics/ethics/review_bodies_guide_serg/en/
https://www.who.int/ethics/indigenous_peoples/en/index13.html
https://www.who.int/bulletin/archives/80(2)114.pdf
https://www.who.int/about/ethics
https://www.slideshare.net/uqudent/introduction-to-research-ethics
https://libguides.library.cityu.edu.hk/researchmethods/ethics#:~:text=Methods%20by%20Subject-,What%20is%20Research%20Ethics%3F,ensure%20a%20high%20ethical%20standard .
https://www.apa.org/monitor/jan03/principles
https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
https://www.skillsyouneed.com/learn/research-ethics.html
- advantages of research ethics
- difference between confidentiality and anonymity in research
- minimizing the risk of harm in research
- obtaining informed consent in research
- principles of research ethics
- PROTECTING ANONYMITY AND CONFIDENTIALITY
- what are the advantages of research ethics
- what are the limitations of research ethics
- what are the principles of research ethics
- what is obtaining informed consent in research
- what is research ethics
- what is right to withdraw in research
- what is ROTECTING ANONYMITY AND CONFIDENTIALITY in research
Copyright © 2024 | WordPress Theme by MH Themes
Writing Ethical Papers: Top Tips to Ace Your Assignment
17 August, 2021
13 minutes read
Author: Kate Smith
Writing a complex essay paper can be a tough task for any student, especially for those who do not have their skills developed well or do not have enough time for lengthy assignments. At the same time, the majority of college students need to keep their grades high to maintain their right to receive merit-based scholarships and continue their studies the next year. To help you with your ethical papers writing, we created this guide. Below, you will find out what an ethical paper is, how to structure it and write it efficiently.

What is an Ethical Paper?
An ethics paper is a type of an argumentative assignment that deals with a certain ethical problem that a student has to describe and solve. Also, it can be an essay where a certain controversial event or concept is elaborated through an ethical lens (e.g. moral rules and principles), or a certain ethical dilemma is explained. Since ethics is connected to moral concepts and choices, a student needs to have a fair knowledge of philosophy and get ready to answer questions related to relationships, justice, professional and social duties, the origin of good and evil, etc., to write a quality paper. Also, writing an ethics paper implies that a student should process a great amount of information regarding their topic and analyze it according to paper terms.
General Aspects of Writing an Ethics Paper
Understanding the ethical papers’ features.
Every essay has differences and features that make it unique. Writing ethical papers implies that a student will use their knowledge of morality and philosophy to resolve a certain ethical dilemma or solve a situation. It can also be a paper in which a student needs to provide their reasoning on ethical or legal circumstances that follow a social issue. Finally, it can be an assignment in which an ethical concept and its application are described. On the contrary, a history essay deals with events that took place somewhen earlier, while a narrative essay is a paper where students demonstrate their storytelling skills, etc.
Defining What Type of Essay Should Be Written
Most of the time, ethical paper topics imply that a student will write an argumentative essay; however, ethics essays can also be descriptive and expository. Each of these essay types has different guidelines for writing, so be sure you know them before you start writing your papers on ethics. In case you missed this step in your ethical paper preparation stage, you would end up writing a paper that misses many important points.
Studying the Ethical Paper Guidelines
Once you get your ethical paper assignment, look through the guidelines that your instructor provided to you. If you receive them during the class, don’t hesitate to pose any questions immediately to remove any misunderstanding before writing an ethics paper outline, or ask for references that you need to use. When you are about to write your first draft, don’t rush: read the paper instructions once again to make sure you understand what is needed from you.
Paying Attention to the Paper Topic
The next thing you need to pay attention to is the ethical paper topic: once you are given one, make sure it falls into the scope of your educational course. After that, consider what additional knowledge may be needed to elaborate on your topic and think about what courses of your program could be helpful for it. Once you are done, read through your topic again to recheck whether you understand your assignment right.
Understanding the Notions of Ethical Arguments, Ethical and Legal Implications, and Ethical Dilemma
Last but not least, another important factor is that a student has to understand the basic terms of the assignment to write a high-quality paper. Ethical arguments are a set of moral rules that are used to defend your position on an ethical issue stated in your essay topic. We refer to ethical versus legal implications when we think about the compensation for certain ethical dilemma outcomes and whether it should be a moral punishment or legal judgment. An ethical dilemma itself refers to a problem or situation which makes an individual doubt what position to take: e.g, abortion, bribery, corruption, etc.
Writing Outline and Structure of an Ethics Paper
Every essay has a structure that makes it a solid piece of writing with straight reasoning and argumentation, and an ethics paper is not an exclusion. This paper has an introduction, body paragraphs, and conclusion. Below, we will describe how each part of ethical papers should be organized and what information they should contain.
First comes the introduction. It is the opening part of your paper which helps a reader to get familiar with your topic and understand what your paper will be about. Therefore, it should contain some information on your ethics paper topics and a thesis statement, which is a central statement of your paper.
The essay body is the most substantive part of your essay where all the reasoning and arguments should be presented. Each paragraph should contain an argument that supports or contradicts your thesis statement and pieces of evidence to support your position. Pick at least three arguments to make your position clear in your essay, and then your paper will be considered well-structured.
The third part of an ethics paper outline is a conclusion, which is a finishing essay part. Its goal is to wrap up the whole essay and make the author’s position clear for the last time. The thoughtful formulation in this essay part should be especially clear and concise to demonstrate the writer’s ability to make conclusions and persuade readers.
Also, don’t forget to include the works cited page after your writing. It should mention all the reference materials that you used in your paper in the order of appearance or in the alphabetical one. This page should be formatted according to the assigned formatting style. Most often, the most frequently used format for ethical papers is APA.
20 Examples of Ethical Paper Topics
- Are there any issues in the 21st century that we can consider immoral and why?
- What is corporate ethics?
- Why is being selfish no longer an issue in 2023?
- Euthanasia: pros and cons
- Marijuana legalization: should it be allowed all over the world?
- Is abortion an ethical issue nowadays?
- Can we invent a universal religion appropriate for all?
- Is the church necessary to pray to God?
- Can we forgive infidelity and should we do it?
- How to react if you are witnessing high school bullying?
- What are the ways to respond to a family abusing individual?
- How to demand your privacy protection in a digital world?
- The history of the American ethical thought
- Can war be ethical and what should the conflicting sides do to make it possible?
- Ethical issues of keeping a zoo in 2023
- Who is in charge of controlling the world’s population?
- How to achieve equality in the world’s rich and poor gap?
- Is science ethical?
- How ethical is genetic engineering?
- Why many countries refuse to go back to carrying out the death penalty?
Ethical Papers Examples
If you still have no idea about how to write an ethics paper, looking through other students’ successful examples is always a good idea. Below, you can find a relevant ethics paper example that you can skim through and see how to build your reasoning and argumentation in your own paper.
https://www.currentschoolnews.com/education-news/ethics-essay-examples/
https://sites.psu.edu/academy/2014/11/18/essay-2-personal-ethics-and-decision-making/
Ethical Papers Writing Tips
Choose a topic that falls into the ethics course program.
In case you were not given the ethics paper topic, consider choosing it yourself. To do that, brainstorm the ethical issues that fascinate you enough to do research. List all these issues on a paper sheet and then cross out those that are too broad or require expertise that you don’t have. The next step you need to take is to choose three or four ethical topics for papers from the list and try to do a quick search online to find out whether these topics are elaborated enough to find sources and reference materials on them. Last, choose one topic that you like the most and find the most relevant one in terms of available data for reference.
Do your research
Once the topic is chosen and organized, dive deeper into it to find the most credible, reliable, and trusted service. Use your university library, online scientific journals, documentaries, and other sources to get the information from. Remember to take notes while working with every new piece of reference material to not forget the ideas that you will base your argumentation on.
Follow the guidelines for a paper outline
During the preparation for your ethical paper and the process of writing it, remember to follow your professor’s instructions (e.g. font, size, spacing, citation style, etc.). If you neglect them, your grade for the paper will decrease significantly.
Write the essay body first
Do not rush to start writing your ethics papers from the very beginning; to write a good essay, you need to have your outline and thesis statement first. Then, go to writing body paragraphs to demonstrate your expertise on the issue you are writing about. Remember that one supporting idea should be covered in one paragraph and should be followed by the piece of evidence that confirms it.
Make sure your introduction and conclusion translate the same message
After your essay body is done, write a conclusion and an introduction for your paper. The main tip regarding these ethics paper parts is that you should make them interrelated: your conclusion has to restate your introduction but not repeat it. Also, a conclusion should wrap up your writing and make it credible for the audience.
Add citations
Every top-quality paper has the works cited page and citations to demonstrate that the research on the topic has been carried out. Therefore, do not omit this point when formatting your paper: add all the sources to the works cited page and pay attention to citing throughout the text. The latter should be done according to the formatting style indicated in your instructions.
Edit your paper
Last but not least is the editing and proofreading stage that you need to carry out before you submit your paper to your instructor. Consider keeping your first draft away from sight for a day or two to have a rest, and then go back to check it for errors and redundant phrases. Don’t rush to change anything immediately after finishing your writing since you are already tired and less focused, so some mistakes may be missed.
Writing Help by Handmadewriting
If you feel that you need help with writing an ethics paper in view of its chellnging nature, you can contact us and send an order through a respective button. You can add your paper details by following all steps of the order placing process that you will find on the website. Once your order is placed, we will get back to you as soon as possible. You will be able to contact your essay writer and let them know all your wishes regarding your ethical paper.
Our writers have expertise in writing ethical papers including, so you don’t need to worry about the quality of the essay that you will receive. Your assignment will be delivered on time and at a reasonable price. Note that urgent papers will cost slightly more than assignments with a postponed deadline, so do not wait too long to make your order. We will be glad to assist you with your writing and guarantee 24/7 support until you receive your paper.
Lastly, remember that no paper can be written overnight, so if you intend to complete your paper in a few hours, you can end up writing only a first draft with imperfections. If you have only half a day before your task is due, feel free to place an urgent order, and we will deliver it in just three hours.

A life lesson in Romeo and Juliet taught by death
Due to human nature, we draw conclusions only when life gives us a lesson since the experience of others is not so effective and powerful. Therefore, when analyzing and sorting out common problems we face, we may trace a parallel with well-known book characters or real historical figures. Moreover, we often compare our situations with […]

Ethical Research Paper Topics
Writing a research paper on ethics is not an easy task, especially if you do not possess excellent writing skills and do not like to contemplate controversial questions. But an ethics course is obligatory in all higher education institutions, and students have to look for a way out and be creative. When you find an […]

Art Research Paper Topics
Students obtaining degrees in fine art and art & design programs most commonly need to write a paper on art topics. However, this subject is becoming more popular in educational institutions for expanding students’ horizons. Thus, both groups of receivers of education: those who are into arts and those who only get acquainted with art […]

ESSAY SAUCE
FOR STUDENTS : ALL THE INGREDIENTS OF A GOOD ESSAY
Essay: Research ethics
Essay details and download:.
- Subject area(s): Education essays
- Reading time: 5 minutes
- Price: Free download
- Published: 16 February 2021*
- File format: Text
- Words: 1,298 (approx)
- Number of pages: 6 (approx)
Text preview of this essay:
This page of the essay has 1,298 words. Download the full version above.
Ethics is a system of moral principles or the moral values that influence the proper conduct of an individual or group. The term originated from the Greek word ‘ethos’ meaning habit or character, and it speaks to how we ought to live, that is, how we ought to treat others. Any research which involves human subjects or participants is bound to raise challenging ethical, social, legal and political considerations. Ethics in the context of research is particularly interested in the analysis of ethical issues that arise in research with people as participants. The primary concern of any research project is to protect the participants involved in the study and to ensure that the research is carried out in order to serve the interest of individuals, groups or the society as a whole. Another objective in research ethics is to analyse specific research projects and activities to decipher its ethical soundness. Ethical issues involving the protection of confidentiality, the management of risks, the process of obtaining informed consent, physical or legal harm, deception, the protection of privacy and anonymity should be properly addressed in any research project. A study involving human subjects’ especially vulnerable people as participants raises unique issues in any research context. In light of this, the sensitive nature of my research project which is on “police brutality in Nigeria: a human rights perspective”, raises questions on how ethical issues are to be addressed as it involves victims of police brutality and prisoners as participants in the study. The research seeks to identify the nature and causes of police brutality in Nigeria as well as proffer possible solutions to the problem. Also, through research this study is looking to discover the potential victims of this form of brutality, the human rights issues involved and the extent to which the international community or international human rights groups are aware of police brutality in Nigeria and what steps have or have not been taken to curb it. Furthermore, the methodology of this research project is via a qualitative approach as it acknowledges ethical issues, finds meaning through the eyes of the participants, and it’s ideal to explore and understand people’s experiences, attitudes, behaviour and interactions. The use of focus groups, personal experiences of victims and telephone interviews with international human rights groups like ‘Human Rights watch’ and ‘Amnesty International’ would be pertinent to carry out this research. Reports from credible human rights organisations would also be considered. Ethical issues are bound to arise within this research project considering its sensitivity. The ethical responsibility of the researcher is necessary throughout all the stages of the research process, from recruiting participants to the treatment of participants and to the consequences of their participation. The issue of informed consent is an ethical issue that could arise within this project. According to Miller and Brewer (2003), it is important that clear and accurate information regarding the research is communicated to the participants, including its possible benefits and risk. Information pertaining to the aims and objectives of the research, its methodology and intended outcomes should be given, presented in lay terms to enhance easy understanding. In addressing the issue of consent, the researcher should ensure that the participant is adequately informed and the consent is explicitly and voluntarily given. The researcher should never coerce anyone to participate in a research study and should make participants aware of their rights which include the fact that they could withdraw at anytime without penalty (Endacott, 2004). Confidentiality and anonymity are other ethical issues that could come up in this research project as the process can divulge sensitive and confidential information such as the disclosure of names, addresses, location and occupational details. The researcher should ensure that information shared by the participants is protected from unauthorized observation. It is not enough for the researcher to say he/she will ensure confidentiality, rather the researcher should demonstrate to the participants how. To do this, the researcher should present data publicly only in form of an aggregate e.g. as statistics or percentages (Neuman, 2009). Referring to the research study in question, the researcher should ensure that information concerning the mistreatment and abuse of inmates by police officers should be held in strict confidentiality which cannot be traced to a particular person. Another ethical issue is the protection of participants from harm, although obviously evident in medical research, social research can also cause great psychological or emotional distress. Qualitative interviews on sensitive topics could trigger powerful emotional responses from a participant and as such, the researcher should anticipate risks e.g. screening out high risk participants like people with heart conditions, mental problems or seizures. (Neuman, 2009). Research should not cause the participants harm whether physical or mental. The problem of access, which is how to get hold of participants in any research study, is of immense importance to the success of the study; participants could be in terms of an organisation or individual. To gain access to an institution or organisation, it is important to negotiate with gatekeepers who could deny access to the organisation, ration it or impose certain Conditions (Burnham et al, 2004). Access when denied, restricts the research process. There are certain ethical issues related to access, a research study could be viewed as an invasion of privacy or an intrusion to the institution to be studied as it interrupts routines and schedules causing inconveniences and disturbances; it is highly unethical to invade other people’s privacy and as such gaining access could be a really slow process. Furthermore, the fact that research could disclose the limitations of the activities of an institution or individual could further impede access (Flick, 2009). To gain access within the context of this research project, the researcher should be competent in establishing relationships and gaining the trust of its participants and institutions as much as possible to forge a working alliance in which research becomes possible. Qualitative research in cross- cultural studies is difficult, problematic, challenging and time consuming. Ethical issues in cross-cultural research include issues relating to values and world views which involve the misunderstanding of participants by researchers from a different culture. It is important for the researcher to respect the cultural views and belief systems of the participants and not to impose one’s values in the research process. The researcher should be sensitive to cultural and social differences. Ethical considerations should be in place when representing or in the dissemination of results in a cross-cultural research. According to Marshall and Batten (2003), the researcher should portray findings so that it does not damage the reputation of a community or group of people. Sue & Sue (1990) states that research procedures can be ethically sound by acknowledging and incorporating the cultural practices of participants and their larger communities. In the course of the research, there are ethical guidelines the researcher should adhere to while implementing the project. The researcher must responsibly conduct research morally and legally while conforming to ethical standards, the researcher should be informed and not knowingly contravene the legislation of a country in cross-cultural research. The researcher should not use deception to gain information from participants and should pursue objectivity while upholding their integrity without fear or favour. The researcher should be informative and descriptive rather than rigidly prescriptive or authoritative. The researcher should avoid undue intrusion, obtain informed consent and be confidential. The safety of both the researcher and participants in a study should be ensured and the risk of harm minimised. In conclusion, ethics in research should be concerned with finding a balance between benefits and risk for harm (Boeije, 2010). The results of findings based on data gathered unethically could lead to harm, possible conflicts and enormous dilemmas. As such, it is considered good practice for a research project to fully comply with ethical standards. Bakare Ibironke Helen 09281206 Page 1
...(download the rest of the essay above)
About this essay:
If you use part of this page in your own work, you need to provide a citation, as follows:
Essay Sauce, Research ethics . Available from:<https://www.essaysauce.com/education-essays/research-ethics/> [Accessed 03-05-24].
These Education essays have been submitted to us by students in order to help you with your studies.
* This essay may have been previously published on Essay.uk.com at an earlier date.
Essay Categories:
- Accounting essays
- Architecture essays
- Business essays
- Computer science essays
- Criminology essays
- Economics essays
- Education essays
- Engineering essays
- English language essays
- Environmental studies essays
- Essay examples
- Finance essays
- Geography essays
- Health essays
- History essays
- Hospitality and tourism essays
- Human rights essays
- Information technology essays
- International relations
- Leadership essays
- Linguistics essays
- Literature essays
- Management essays
- Marketing essays
- Mathematics essays
- Media essays
- Medicine essays
- Military essays
- Miscellaneous essays
- Music Essays
- Nursing essays
- Philosophy essays
- Photography and arts essays
- Politics essays
- Project management essays
- Psychology essays
- Religious studies and theology essays
- Sample essays
- Science essays
- Social work essays
- Sociology essays
- Sports essays
- Types of essay
- Zoology essays
- Share full article
Advertisement
Supported by
Guest Essay
In Medicine, the Morally Unthinkable Too Easily Comes to Seem Normal

By Carl Elliott
Dr. Elliott teaches medical ethics at the University of Minnesota. He is the author of the forthcoming book “The Occasional Human Sacrifice: Medical Experimentation and the Price of Saying No,” from which this essay is adapted.
Here is the way I remember it: The year is 1985, and a few medical students are gathered around an operating table where an anesthetized woman has been prepared for surgery. The attending physician, a gynecologist, asks the group: “Has everyone felt a cervix? Here’s your chance.” One after another, we take turns inserting two gloved fingers into the unconscious woman’s vagina.
Had the woman consented to a pelvic exam? Did she understand that when the lights went dim she would be treated like a clinical practice dummy, her genitalia palpated by a succession of untrained hands? I don’t know. Like most medical students, I just did as I was told.
Last month the Department of Health and Human Services issued new guidance requiring written informed consent for pelvic exams and other intimate procedures performed under anesthesia. Much of the force behind the new requirement came from distressed medical students who saw these pelvic exams as wrong and summoned the courage to speak out.
Whether the guidance will actually change clinical practice I don’t know. Medical traditions are notoriously difficult to uproot, and academic medicine does not easily tolerate ethical dissent. I doubt the medical profession can be trusted to reform itself.
What is it that leads a rare individual to say no to practices that are deceptive, exploitative or harmful when everyone else thinks they are fine? For a long time I assumed that saying no was mainly an issue of moral courage. The relevant question was: If you are a witness to wrongdoing, will you be brave enough to speak out?
But then I started talking to insiders who had blown the whistle on abusive medical research. Soon I realized that I had overlooked the importance of moral perception. Before you decide to speak out about wrongdoing, you have to recognize it for what it is.
This is not as simple as it seems. Part of what makes medical training so unsettling is how often you are thrust into situations in which you don’t really know how to behave. Nothing in your life up to that point has prepared you to dissect a cadaver, perform a rectal exam or deliver a baby. Never before have you seen a psychotic patient involuntarily sedated and strapped to a bed or a brain-dead body wheeled out of a hospital room to have its organs harvested for transplantation. Your initial reaction is often a combination of revulsion, anxiety and self-consciousness.
To embark on a career in medicine is like moving to a foreign country where you do not understand the customs, rituals, manners or language. Your main concern on arrival is how to fit in and avoid causing offense. This is true even if the local customs seem backward or cruel. What’s more, this particular country has an authoritarian government and a rigid status hierarchy where dissent is not just discouraged but also punished. Living happily in this country requires convincing yourself that whatever discomfort you feel comes from your own ignorance and lack of experience. Over time, you learn how to assimilate. You may even come to laugh at how naïve you were when you first arrived.
A rare few people hang onto that discomfort and learn from it. When Michael Wilkins and William Bronston started working at the Willowbrook State School in Staten Island as young doctors in the early 1970s, they found thousands of mentally disabled children condemned to the most horrific conditions imaginable: naked children rocking and moaning on concrete floors in puddles of their own urine; an overpowering stench of illness and filth; a research unit where children were deliberately infected with hepatitis A and B.
“It was truly an American concentration camp,” Dr. Bronston told me. Yet when he and Dr. Wilkins tried to enlist Willowbrook doctors and nurses to reform the institution, they were met with indifference or hostility. It seemed as if no one else on the medical staff could see what they saw. It was only when Dr. Wilkins went to a reporter and showed the world what was happening behind the Willowbrook walls that anything began to change.
When I asked Dr. Bronston how it was possible for doctors and nurses to work at Willowbrook without seeing it as a crime scene, he told me it began with the way the institution was structured and organized. “Medically secured, medically managed, doctor-validated,” he said. Medical professionals just accommodated themselves to the status quo. “You get with the program because that’s what you’re being hired to do,” he said.
One of the great mysteries of human behavior is how institutions create social worlds where unthinkable practices come to seem normal. This is as true of academic medical centers as it is of prisons and military units. When we are told about a horrific medical research scandal, we assume that we would see it just as the whistle-blower Peter Buxtun saw the Tuskegee syphilis study : an abuse so shocking that only a sociopath could fail to perceive it.
Yet it rarely happens this way. It took Mr. Buxtun seven years to convince others to see the abuses for what they were. It has taken other whistle-blowers even longer. Even when the outside world condemns a practice, medical institutions typically insist that the outsiders don’t really understand.
According to Irving Janis, a Yale psychologist who popularized the notion of groupthink, the forces of social conformity are especially powerful in organizations that are driven by a deep sense of moral purpose. If the aims of the organization are righteous, its members feel, it is wrong to put barriers in the way.
This observation helps explain why academic medicine not only defends researchers accused of wrongdoing but also sometimes rewards them. Many of the researchers responsible for the most notorious abuses in recent medical history — the Tuskegee syphilis study, the Willowbrook hepatitis studies, the Cincinnati radiation studies , the Holmesburg prison studies — were celebrated with professional accolades even after the abuses were first called out.
The culture of medicine is notoriously resistant to change. During the 1970s, it was thought that the solution to medical misconduct was formal education in ethics. Major academic medical centers began establishing bioethics centers and programs throughout the 1980s and ’90s, and today virtually every medical school in the country requires ethics training.
Yet it is debatable whether that training has had any effect. Many of the most egregious ethical abuses in recent decades have taken place in medical centers with prominent bioethics programs, such as the University of Pennsylvania , Duke University , Columbia University and Johns Hopkins University , as well as my own institution, the University of Minnesota .
One could be forgiven for concluding that the only way the culture of medicine will change is if changes are forced on it from the outside — by oversight bodies, legislators or litigators. For example, many states have responded to the controversy over pelvic exams by passing laws banning the practice unless the patient has explicitly given consent.
You may find it hard to understand how pelvic exams on unconscious women without their consent could seem like anything but a terrible invasion. Yet a central aim of medical training is to transform your sensibility. You are taught to steel yourself against your natural emotional reactions to death and disfigurement; to set aside your customary views about privacy and shame; to see the human body as a thing to be examined, tested and studied.
One danger of this transformation is that you will see your colleagues and superiors do horrible things and be afraid to speak up. But the more subtle danger is that you will no longer see what they are doing as horrible. You will just think: This is the way it is done.
Carl Elliott ( @FearLoathingBTX ) teaches medical ethics at the University of Minnesota. He is the author of the forthcoming book “The Occasional Human Sacrifice: Medical Experimentation and the Price of Saying No,” from which this essay is adapted.
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .

Ethics and Morality
Scientists possess inflated views of their own ethics, scientists are many things. being unbiased isn’t one of them..
Posted May 6, 2024 | Reviewed by Michelle Quirk
- Recent research calls into question researchers' biased perceptions of their own ethical practices.
- This, though, should be unsurprising, given that researchers are subject to the same biases as everyone else.
- Ample evidence exists to point toward bounded ethicality and motivated reasoning as culprits.

A recent Psychology Today post by Miller (2024) discussed the results of a research study 1 that included a sample of more than 10,000 researchers from Sweden. Respondents were provided with a description of ethical research practices (Figure 1) and asked to rate (1) how well they applied ethical research practices relative to others in their field and (2) how well researchers in their field applied ethical research practices relative to those in other fields.
The study itself was not overly complex (in fact, each rating was just a single item). When it came to rating their own application of research ethics, 55 percent rated themselves as equal to their peers, close to 45 percent rated themselves as better, and less than 1 percent rated themselves as worse. When it came to assessing others in their field, 63 percent rated their field as similar to others, 29 percent rated their field as better, and close to 8 percent rated their field as worse.

It seems unlikely that slightly less than half of researchers apply ethical research practices better than their peers while less than 1 percent apply them worse. 2 So, there’s likely a disconnect between researchers’ actual research practices and how they perceive their own and their field’s research practices. 3 Miller (2024) argues these results are “especially surprising since scientists are regularly thought to be objective.”
I would respectfully disagree with Miller’s conclusion. These results are right in line with what we know about human decision-making , especially when we’re talking about decision-making that involves people’s sense of self and their own morality .
Better-Than-Average Effect
Many people are aware of the better-than-average (BTA) effect, with the prototypical example used being the fact that more than 80 percent of drivers consider themselves to be better than average. 4 Although Saxena (2020), 5 Campbell (2020), 6 and others have explained how these results could be accurate (and probably are), there are scores of other studies that demonstrate the BTA effect for traits, abilities, and skills that we know are more equally distributed (e.g., intelligence , mathematical ability, attractiveness ). To synthesize a lot of this prior research, Zell et al. (2020) conducted a meta-analysis and found that for many traits, abilities, and skills, there is evidence for a BTE effect, with the size of that effect varying based on which constructs are included, the composition of the sample, and some other factors. 7 One of the interesting results was that a BTA effect was apparent for what they operationalized as easy skills, but a worse-than-average (WTA) effect was found for skills they operationalized as difficult skills.
It seems unlikely that researchers would consider the practices used to operationalize ethical research to be difficult to apply, so it stands to reason most researchers would consider themselves better than average at applying them, regardless of whether they objectively are. But there’s even further reason to conclude that the results Miller (2024) discussed are unsurprising.
Bounded Ethicality
Herbert Simon first introduced the expression bounded rationality back in 1957 ( Wheeler, 2018 ), which was used as a principal basis for understanding human rationality, including why humans pursue satisficing outcomes. 8 Chugh and Kern (2016) later applied the idea of boundedness to the concept of ethicality. 9 They defined bounded ethicality as “the systematic and ordinary psychological processes of enhancing and protecting our ethical self-view, which automatically, dynamically, and cyclically influence the ethicality of decision-making.” 10
Their definition confounds the idea of boundedness (i.e., that we’re constrained by what we can consider from an ethical perspective) with motivated reasoning (i.e., our ability to reason our way into an ethically questionable decision). And both can play a role in the choice to behave in ethically questionable ways.
Actual boundedness comes into play when we fail to recognize and use ethically relevant information that should (but ultimately does not) guide our decision. The frame of reference we bring to a situation can cause us to hone in on some specific details while ignoring others, even if some of those other details might be relevant to making an ethically defensible decision. In the case of researchers, the psychological context they bring to a decision situation will affect which details they focus on. It’s the details they may filter out that, if relevant, might affect the ethical defensibility of decisions they make.
In such cases, though, we typically aren’t aware that we’ve made some sort of ethical transgression. As Bazerman and Sezer (2016) argued, in cases where bounded awareness leads to ethically questionable behavior, the questionability of said behavior may only become evident if it produces some adverse effect that can be linked to the choices that were made. For example, unknowingly designing a study that conflicts with some element of ethical research practices might only become apparent if (a) an ethics review board flags it or (b) it results in some adverse consequence that is brought to the attention of the researcher. In such situations, our ethical assessment tends to be heavily grounded in outcome bias . If we perceive our decision to have produced no adverse effect, then we’re likely to conclude we made ethically defensible choices. And when it comes to the list of practices in Figure 1, bounded awareness could easily affect the ratings researchers provided.

But motivated reasoning can add an additional wrinkle to ethical decision-making. There is an abundance of evidence that most people consider themselves to be good people, and they’re motivated to maintain those views. And most people probably are goodish people, in the sense that they tend to act within ethical constraints (at least insofar as they are aware) much more often than they deviate from them.
There are times, though, when conflict can occur between our self-interests and the ethical pursuit of those self-interests, as I wrote about when discussing hypocrisy . And sometimes we reason our way into the pursuit of those self-interests at the expense of adherence to relational values or ethical rules (i.e., engage in motivated reasoning). But to do so, we need a mechanism to justify the pursuit of those self-interests. For that, we can turn to moral disengagement theory.
Moral Disengagement Theory
Albert Bandura originally introduced moral disengagement theory to explain the social-cognitive mechanisms that allow people to set aside internalized moral values to justify ethically questionable (and even indefensible) behavior. These mechanisms essentially provide the means to give ourselves a moral hall pass. Seven mechanisms were identified, ranging from moral justification (i.e., reasoning that our action somehow fulfills some moral obligation) to displacement of responsibility (i.e., blaming someone else for causing the behavior, such as a supervisor) to dehumanization (i.e., using dehumanizing language to justify unethical behavior toward some person or group). 11
All eight of the practices listed in Figure 1 can lend themselves to the various mechanisms that lead to moral disengagement, allowing researchers to maintain a sense of moral goodness by excusing situations where they may not have consistently applied those practices. 12 Additionally, although Bandura originally argued that moral disengagement was generally an a priori (before the act) requirement (at least for adults), these same mechanisms can be employed a posteriori (i.e., after the act) to help deflect responsibility for having missed or ignored ethically relevant information that produced an adverse effect (e.g., “It’s not my fault”).
Final Thoughts
To wrap things up, I want to return to Miller’s inference that the results are “especially surprising since scientists are regularly thought to be objective.” Although the belief that scientists are thought to be objective is certainly widely held and appears intuitively logical, as Reiss and Sprenger (2020) argued, “several conceptions of the ideal of objectivity are either questionable or unattainable.”
Now, there’s a difference, as Zaruk (2024) 13 argued, between activist scientists—who “[start] with conclusions and adapt the evidence”—and credible scientists—who “[start] with evidence and adapt the conclusions.“ But that doesn’t mean credible scientists are objective. As Reiss and Sprenger (and many others) have argued, scientists bring values and biases into the scientific process. Although science provides methodologies that offer some modicum of control over a specific scientist’s ability to rig the research studies to achieve desired conclusions, these controls are imperfect and only useful to the degree to which scientists choose to apply them.
And when it comes to self-assessments, researchers aren’t any different than other people. They have the same self-serving biases as everyone else. And, so, it comes as no real surprise that, when evaluating their own and their field’s work, these same researchers will show the same biases we would expect average people to show.
1. The study was conducted by Lindkvist et al. (2024) , for those who might have an interest in reading the actual study.
2. Although it may be unlikely, it is not mathematically impossible.
3. It’s also possible a lot of folks are lying, but that also seems highly unlikely. And with no evidence to support that conclusion, it’s best to make the inference I am making.
4. These results were originally produced by Svenson (1981) . I know they’ve been replicated at least once (I think in 1983, but I can’t say for sure).
5. Ambuj Saxena. Are you "better than average" driver? LinkedIn. August 10, 2020.
6. Adam J. Campbell, Ph.D. Most Drivers are Better than Average . July 20, 2020.
7. The Zell et al. study is available on ResearchGate, so interested readers can check out the full results.
8. Something I wrote about back in 2020 .
9. These were not the first authors to do so, but they were the first to offer a model of it.
10. This is actually a revision of the definition presented by Chugh et al. (2005) . I personally find the revision to be a huge departure from the idea of boundedness, as it confounds the actual ethicality of behavior with people’s motivation to maintain belief in their general ethicality. The original definition was truer to Simon’s original claims about decision-making.
11. The website I linked to discusses all seven and in more depth than I can here, so feel free to take a deeper dive there.
12. Not to mention that some of them, such as numbers 6 and 8, are quite subjective.
13. David Zaruk. Defining Activist Science . Firebreak. May 1, 2024.

Matt Grawitch, Ph.D. , is a professor at Saint Louis University (SLU), serving within the School for Professional Studies (SPS).
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS 2024
Conference Dates: (In person) 9 December - 15 December, 2024
Homepage: https://neurips.cc/Conferences/2024/
Call For Papers
Author notification: Sep 25, 2024
Camera-ready, poster, and video submission: Oct 30, 2024 AOE
Submit at: https://openreview.net/group?id=NeurIPS.cc/2024/Conference
The site will start accepting submissions on Apr 22, 2024
Subscribe to these and other dates on the 2024 dates page .
The Thirty-Eighth Annual Conference on Neural Information Processing Systems (NeurIPS 2024) is an interdisciplinary conference that brings together researchers in machine learning, neuroscience, statistics, optimization, computer vision, natural language processing, life sciences, natural sciences, social sciences, and other adjacent fields. We invite submissions presenting new and original research on topics including but not limited to the following:
- Applications (e.g., vision, language, speech and audio, Creative AI)
- Deep learning (e.g., architectures, generative models, optimization for deep networks, foundation models, LLMs)
- Evaluation (e.g., methodology, meta studies, replicability and validity, human-in-the-loop)
- General machine learning (supervised, unsupervised, online, active, etc.)
- Infrastructure (e.g., libraries, improved implementation and scalability, distributed solutions)
- Machine learning for sciences (e.g. climate, health, life sciences, physics, social sciences)
- Neuroscience and cognitive science (e.g., neural coding, brain-computer interfaces)
- Optimization (e.g., convex and non-convex, stochastic, robust)
- Probabilistic methods (e.g., variational inference, causal inference, Gaussian processes)
- Reinforcement learning (e.g., decision and control, planning, hierarchical RL, robotics)
- Social and economic aspects of machine learning (e.g., fairness, interpretability, human-AI interaction, privacy, safety, strategic behavior)
- Theory (e.g., control theory, learning theory, algorithmic game theory)
Machine learning is a rapidly evolving field, and so we welcome interdisciplinary submissions that do not fit neatly into existing categories.
Authors are asked to confirm that their submissions accord with the NeurIPS code of conduct .
Formatting instructions: All submissions must be in PDF format, and in a single PDF file include, in this order:
- The submitted paper
- Technical appendices that support the paper with additional proofs, derivations, or results
- The NeurIPS paper checklist
Other supplementary materials such as data and code can be uploaded as a ZIP file
The main text of a submitted paper is limited to nine content pages , including all figures and tables. Additional pages containing references don’t count as content pages. If your submission is accepted, you will be allowed an additional content page for the camera-ready version.
The main text and references may be followed by technical appendices, for which there is no page limit.
The maximum file size for a full submission, which includes technical appendices, is 50MB.
Authors are encouraged to submit a separate ZIP file that contains further supplementary material like data or source code, when applicable.
You must format your submission using the NeurIPS 2024 LaTeX style file which includes a “preprint” option for non-anonymous preprints posted online. Submissions that violate the NeurIPS style (e.g., by decreasing margins or font sizes) or page limits may be rejected without further review. Papers may be rejected without consideration of their merits if they fail to meet the submission requirements, as described in this document.
Paper checklist: In order to improve the rigor and transparency of research submitted to and published at NeurIPS, authors are required to complete a paper checklist . The paper checklist is intended to help authors reflect on a wide variety of issues relating to responsible machine learning research, including reproducibility, transparency, research ethics, and societal impact. The checklist forms part of the paper submission, but does not count towards the page limit.
Supplementary material: While all technical appendices should be included as part of the main paper submission PDF, authors may submit up to 100MB of supplementary material, such as data, or source code in a ZIP format. Supplementary material should be material created by the authors that directly supports the submission content. Like submissions, supplementary material must be anonymized. Looking at supplementary material is at the discretion of the reviewers.
We encourage authors to upload their code and data as part of their supplementary material in order to help reviewers assess the quality of the work. Check the policy as well as code submission guidelines and templates for further details.
Use of Large Language Models (LLMs): We welcome authors to use any tool that is suitable for preparing high-quality papers and research. However, we ask authors to keep in mind two important criteria. First, we expect papers to fully describe their methodology, and any tool that is important to that methodology, including the use of LLMs, should be described also. For example, authors should mention tools (including LLMs) that were used for data processing or filtering, visualization, facilitating or running experiments, and proving theorems. It may also be advisable to describe the use of LLMs in implementing the method (if this corresponds to an important, original, or non-standard component of the approach). Second, authors are responsible for the entire content of the paper, including all text and figures, so while authors are welcome to use any tool they wish for writing the paper, they must ensure that all text is correct and original.
Double-blind reviewing: All submissions must be anonymized and may not contain any identifying information that may violate the double-blind reviewing policy. This policy applies to any supplementary or linked material as well, including code. If you are including links to any external material, it is your responsibility to guarantee anonymous browsing. Please do not include acknowledgements at submission time. If you need to cite one of your own papers, you should do so with adequate anonymization to preserve double-blind reviewing. For instance, write “In the previous work of Smith et al. [1]…” rather than “In our previous work [1]...”). If you need to cite one of your own papers that is in submission to NeurIPS and not available as a non-anonymous preprint, then include a copy of the cited anonymized submission in the supplementary material and write “Anonymous et al. [1] concurrently show...”). Any papers found to be violating this policy will be rejected.
OpenReview: We are using OpenReview to manage submissions. The reviews and author responses will not be public initially (but may be made public later, see below). As in previous years, submissions under review will be visible only to their assigned program committee. We will not be soliciting comments from the general public during the reviewing process. Anyone who plans to submit a paper as an author or a co-author will need to create (or update) their OpenReview profile by the full paper submission deadline. Your OpenReview profile can be edited by logging in and clicking on your name in https://openreview.net/ . This takes you to a URL "https://openreview.net/profile?id=~[Firstname]_[Lastname][n]" where the last part is your profile name, e.g., ~Wei_Zhang1. The OpenReview profiles must be up to date, with all publications by the authors, and their current affiliations. The easiest way to import publications is through DBLP but it is not required, see FAQ . Submissions without updated OpenReview profiles will be desk rejected. The information entered in the profile is critical for ensuring that conflicts of interest and reviewer matching are handled properly. Because of the rapid growth of NeurIPS, we request that all authors help with reviewing papers, if asked to do so. We need everyone’s help in maintaining the high scientific quality of NeurIPS.
Please be aware that OpenReview has a moderation policy for newly created profiles: New profiles created without an institutional email will go through a moderation process that can take up to two weeks. New profiles created with an institutional email will be activated automatically.
Venue home page: https://openreview.net/group?id=NeurIPS.cc/2024/Conference
If you have any questions, please refer to the FAQ: https://openreview.net/faq
Ethics review: Reviewers and ACs may flag submissions for ethics review . Flagged submissions will be sent to an ethics review committee for comments. Comments from ethics reviewers will be considered by the primary reviewers and AC as part of their deliberation. They will also be visible to authors, who will have an opportunity to respond. Ethics reviewers do not have the authority to reject papers, but in extreme cases papers may be rejected by the program chairs on ethical grounds, regardless of scientific quality or contribution.
Preprints: The existence of non-anonymous preprints (on arXiv or other online repositories, personal websites, social media) will not result in rejection. If you choose to use the NeurIPS style for the preprint version, you must use the “preprint” option rather than the “final” option. Reviewers will be instructed not to actively look for such preprints, but encountering them will not constitute a conflict of interest. Authors may submit anonymized work to NeurIPS that is already available as a preprint (e.g., on arXiv) without citing it. Note that public versions of the submission should not say "Under review at NeurIPS" or similar.
Dual submissions: Submissions that are substantially similar to papers that the authors have previously published or submitted in parallel to other peer-reviewed venues with proceedings or journals may not be submitted to NeurIPS. Papers previously presented at workshops are permitted, so long as they did not appear in a conference proceedings (e.g., CVPRW proceedings), a journal or a book. NeurIPS coordinates with other conferences to identify dual submissions. The NeurIPS policy on dual submissions applies for the entire duration of the reviewing process. Slicing contributions too thinly is discouraged. The reviewing process will treat any other submission by an overlapping set of authors as prior work. If publishing one would render the other too incremental, both may be rejected.
Anti-collusion: NeurIPS does not tolerate any collusion whereby authors secretly cooperate with reviewers, ACs or SACs to obtain favorable reviews.
Author responses: Authors will have one week to view and respond to initial reviews. Author responses may not contain any identifying information that may violate the double-blind reviewing policy. Authors may not submit revisions of their paper or supplemental material, but may post their responses as a discussion in OpenReview. This is to reduce the burden on authors to have to revise their paper in a rush during the short rebuttal period.
After the initial response period, authors will be able to respond to any further reviewer/AC questions and comments by posting on the submission’s forum page. The program chairs reserve the right to solicit additional reviews after the initial author response period. These reviews will become visible to the authors as they are added to OpenReview, and authors will have a chance to respond to them.
After the notification deadline, accepted and opted-in rejected papers will be made public and open for non-anonymous public commenting. Their anonymous reviews, meta-reviews, author responses and reviewer responses will also be made public. Authors of rejected papers will have two weeks after the notification deadline to opt in to make their deanonymized rejected papers public in OpenReview. These papers are not counted as NeurIPS publications and will be shown as rejected in OpenReview.
Publication of accepted submissions: Reviews, meta-reviews, and any discussion with the authors will be made public for accepted papers (but reviewer, area chair, and senior area chair identities will remain anonymous). Camera-ready papers will be due in advance of the conference. All camera-ready papers must include a funding disclosure . We strongly encourage accompanying code and data to be submitted with accepted papers when appropriate, as per the code submission policy . Authors will be allowed to make minor changes for a short period of time after the conference.
Contemporaneous Work: For the purpose of the reviewing process, papers that appeared online within two months of a submission will generally be considered "contemporaneous" in the sense that the submission will not be rejected on the basis of the comparison to contemporaneous work. Authors are still expected to cite and discuss contemporaneous work and perform empirical comparisons to the degree feasible. Any paper that influenced the submission is considered prior work and must be cited and discussed as such. Submissions that are very similar to contemporaneous work will undergo additional scrutiny to prevent cases of plagiarism and missing credit to prior work.
Plagiarism is prohibited by the NeurIPS Code of Conduct .
Other Tracks: Similarly to earlier years, we will host multiple tracks, such as datasets, competitions, tutorials as well as workshops, in addition to the main track for which this call for papers is intended. See the conference homepage for updates and calls for participation in these tracks.
Experiments: As in past years, the program chairs will be measuring the quality and effectiveness of the review process via randomized controlled experiments. All experiments are independently reviewed and approved by an Institutional Review Board (IRB).
Financial Aid: Each paper may designate up to one (1) NeurIPS.cc account email address of a corresponding student author who confirms that they would need the support to attend the conference, and agrees to volunteer if they get selected. To be considered for Financial the student will also need to fill out the Financial Aid application when it becomes available.
SOC101: Introduction to Sociology (2020.A.01)
What is ethics in research and why is it important.
Read the article on the importance of ethics in research.
The ideas and opinions expressed in this essay are the author's own and do not necessarily represent those of the NIH, NIEHS, or US government.

When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius. This is the most common way of defining "ethics": norms for conduct that distinguish between acceptable and unacceptable behavior. Most people learn ethical norms at home, at school, in church, or in other social settings. Although most people acquire their sense of right and wrong during childhood, moral development occurs throughout life and human beings pass through different stages of growth as they mature. Ethical norms are so ubiquitous that one might be tempted to regard them as simple commonsense. On the other hand, if morality were nothing more than commonsense, then why are there so many ethical disputes and issues in our society? One plausible explanation of these disagreements is that all people recognize some common ethical norms but interpret, apply, and balance them in different ways in light of their own values and life experiences. For example, two people could agree that murder is wrong but disagree about the morality of abortion because they have different understandings of what it means to be a human being. Most societies also have legal rules that govern behavior, but ethical norms tend to be broader and more informal than laws. Although most societies use laws to enforce widely accepted moral standards and ethical and legal rules use similar concepts, ethics and law are not the same. An action may be legal but unethical or illegal but ethical. We can also use ethical concepts and principles to criticize, evaluate, propose, or interpret laws. Indeed, in the last century, many social reformers have urged citizens to disobey laws they regarded as immoral or unjust laws. Peaceful civil disobedience is an ethical way of protesting laws or expressing political viewpoints. Another way of defining 'ethics' focuses on the disciplines that study standards of conduct, such as philosophy, theology, law, psychology, or sociology. For example, a "medical ethicist" is someone who studies ethical standards in medicine. One may also define ethics as a method, procedure, or perspective for deciding how to act and for analyzing complex problems and issues. For instance, in considering a complex issue like global warming, one may take an economic, ecological, political, or ethical perspective on the problem. While an economist might examine the cost and benefits of various policies related to global warming, an environmental ethicist could examine the ethical values and principles at stake. Many different disciplines, institutions, and professions have standards for behavior that suit their particular aims and goals. These standards also help members of the discipline to coordinate their actions or activities and to establish the public's trust of the discipline. For instance, ethical standards govern conduct in medicine, law, engineering, and business. Ethical norms also serve the aims or goals of research and apply to people who conduct scientific research or other scholarly or creative activities. There is even a specialized discipline, research ethics, which studies these norms. See Glossary of Commonly Used Terms in Research Ethics. There are several reasons why it is important to adhere to ethical norms in research. First, norms promote the aims of research, such as knowledge, truth, and avoidance of error. For example, prohibitions against fabricating, falsifying, or misrepresenting research data promote the truth and minimize error. Second, since research often involves a great deal of cooperation and coordination among many different people in different disciplines and institutions, ethical standards promote the values that are essential to collaborative work , such as trust, accountability, mutual respect, and fairness. For example, many ethical norms in research, such as guidelines for authorship, copyright and patenting policies, data sharing policies, and confidentiality rules in peer review, are designed to protect intellectual property interests while encouraging collaboration. Most researchers want to receive credit for their contributions and do not want to have their ideas stolen or disclosed prematurely. Third, many of the ethical norms help to ensure that researchers can be held accountable to the public . For instance, federal policies on research misconduct, conflicts of interest, the human subjects protections, and animal care and use are necessary in order to make sure that researchers who are funded by public money can be held accountable to the public. Fourth, ethical norms in research also help to build public support for research. People are more likely to fund a research project if they can trust the quality and integrity of research. Finally, many of the norms of research promote a variety of other important moral and social values , such as social responsibility, human rights, animal welfare, compliance with the law, and public health and safety. Ethical lapses in research can significantly harm human and animal subjects, students, and the public. For example, a researcher who fabricates data in a clinical trial may harm or even kill patients, and a researcher who fails to abide by regulations and guidelines relating to radiation or biological safety may jeopardize his health and safety or the health and safety of staff and students.
Codes and Policies for Research Ethics
Given the importance of ethics for the conduct of research, it should come as no surprise that many different professional associations, government agencies, and universities have adopted specific codes, rules, and policies relating to research ethics. Many government agencies have ethics rules for funded researchers.
- National Institutes of Health (NIH)
- National Science Foundation (NSF)
- Food and Drug Administration (FDA)
- Environmental Protection Agency (EPA)
- US Department of Agriculture (USDA)
- Singapore Statement on Research Integrity
- American Chemical Society, The Chemist Professional's Code of Conduct
- Code of Ethics (American Society for Clinical Laboratory Science)
- American Psychological Association, Ethical Principles of Psychologists and Code of Conduct
- Statement on Professional Ethics (American Association of University Professors)
- Nuremberg Code
- World Medical Association's Declaration of Helsinki
Ethical Principles
The following is a rough and general summary of some ethical principles that various codes address*:

Strive for honesty in all scientific communications. Honestly report data, results, methods and procedures, and publication status. Do not fabricate, falsify, or misrepresent data. Do not deceive colleagues, research sponsors, or the public.
Objectivity

Strive to avoid bias in experimental design, data analysis, data interpretation, peer review, personnel decisions, grant writing, expert testimony, and other aspects of research where objectivity is expected or required. Avoid or minimize bias or self-deception. Disclose personal or financial interests that may affect research.

Keep your promises and agreements; act with sincerity; strive for consistency of thought and action.
Carefulness

Avoid careless errors and negligence; carefully and critically examine your own work and the work of your peers. Keep good records of research activities, such as data collection, research design, and correspondence with agencies or journals.

Share data, results, ideas, tools, resources. Be open to criticism and new ideas.
Transparency

Disclose methods, materials, assumptions, analyses, and other information needed to evaluate your research.
Accountability

Take responsibility for your part in research and be prepared to give an account (i.e. an explanation or justification) of what you did on a research project and why.
Intellectual Property

Honor patents, copyrights, and other forms of intellectual property. Do not use unpublished data, methods, or results without permission. Give proper acknowledgement or credit for all contributions to research. Never plagiarize.
Confidentiality

Protect confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient records.
Responsible Publication

Publish in order to advance research and scholarship, not to advance just your own career. Avoid wasteful and duplicative publication.
Responsible Mentoring

Help to educate, mentor, and advise students. Promote their welfare and allow them to make their own decisions.
Respect for Colleagues

Respect your colleagues and treat them fairly.
Social Responsibility

Strive to promote social good and prevent or mitigate social harms through research, public education, and advocacy.
Non-Discrimination

Avoid discrimination against colleagues or students on the basis of sex, race, ethnicity, or other factors not related to scientific competence and integrity.

Maintain and improve your own professional competence and expertise through lifelong education and learning; take steps to promote competence in science as a whole.

Know and obey relevant laws and institutional and governmental policies.
Animal Care

Show proper respect and care for animals when using them in research. Do not conduct unnecessary or poorly designed animal experiments.
Human Subjects protection

When conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy; take special precautions with vulnerable populations; and strive to distribute the benefits and burdens of research fairly.
Ethical Decision Making in Research
Although codes, policies, and principles are very important and useful, like any set of rules, they do not cover every situation, they often conflict, and they require considerable interpretation. It is therefore important for researchers to learn how to interpret, assess, and apply various research rules and how to make decisions and to act ethically in various situations. The vast majority of decisions involve the straightforward application of ethical rules. For example, consider the following case,
The research protocol for a study of a drug on hypertension requires the administration of the drug at different doses to 50 laboratory mice, with chemical and behavioral tests to determine toxic effects. Tom has almost finished the experiment for Dr. Q. He has only 5 mice left to test. However, he really wants to finish his work in time to go to Florida on spring break with his friends, who are leaving tonight. He has injected the drug in all 50 mice but has not completed all of the tests. He therefore decides to extrapolate from the 45 completed results to produce the 5 additional results. Many different research ethics policies would hold that Tom has acted unethically by fabricating data. If this study were sponsored by a federal agency, such as the NIH, his actions would constitute a form of research misconduct, which the government defines as "fabrication, falsification, or plagiarism" (or FFP). Actions that nearly all researchers classify as unethical are viewed as misconduct. It is important to remember, however, that misconduct occurs only when researchers intend to deceive : honest errors related to sloppiness, poor record keeping, miscalculations, bias, self-deception, and even negligence do not constitute misconduct. Also, reasonable disagreements about research methods, procedures, and interpretations do not constitute research misconduct. Consider the following case:
Dr. T has just discovered a mathematical error in his paper that has been accepted for publication in a journal. The error does not affect the overall results of his research, but it is potentially misleading. The journal has just gone to press, so it is too late to catch the error before it appears in print. In order to avoid embarrassment, Dr. T decides to ignore the error. Dr. T's error is not misconduct nor is his decision to take no action to correct the error. Most researchers, as well as many different policies and codes would say that Dr. T should tell the journal (and any coauthors) about the error and consider publishing a correction or errata. Failing to publish a correction would be unethical because it would violate norms relating to honesty and objectivity in research. There are many other activities that the government does not define as "misconduct" but which are still regarded by most researchers as unethical. These are sometimes referred to as "other deviations" from acceptable research practices and include:
- Publishing the same paper in two different journals without telling the editors
- Submitting the same paper to different journals without telling the editors
- Not informing a collaborator of your intent to file a patent in order to make sure that you are the sole inventor
- Including a colleague as an author on a paper in return for a favor even though the colleague did not make a serious contribution to the paper
- Discussing with your colleagues confidential data from a paper that you are reviewing for a journal
- Using data, ideas, or methods you learn about while reviewing a grant or a papers without permission
- Trimming outliers from a data set without discussing your reasons in paper
- Using an inappropriate statistical technique in order to enhance the significance of your research
- Bypassing the peer review process and announcing your results through a press conference without giving peers adequate information to review your work
- Conducting a review of the literature that fails to acknowledge the contributions of other people in the field or relevant prior work
- Stretching the truth on a grant application in order to convince reviewers that your project will make a significant contribution to the field
- Stretching the truth on a job application or curriculum vita
- Giving the same research project to two graduate students in order to see who can do it the fastest
- Overworking, neglecting, or exploiting graduate or post-doctoral students
- Failing to keep good research records
- Failing to maintain research data for a reasonable period of time
- Making derogatory comments and personal attacks in your review of author's submission
- Promising a student a better grade for sexual favors
- Using a racist epithet in the laboratory
- Making significant deviations from the research protocol approved by your institution's Animal Care and Use Committee or Institutional Review Board for Human Subjects Research without telling the committee or the board
- Not reporting an adverse event in a human research experiment
- Wasting animals in research
- Exposing students and staff to biological risks in violation of your institution's biosafety rules
- Sabotaging someone's work
- Stealing supplies, books, or data
- Rigging an experiment so you know how it will turn out
- Making unauthorized copies of data, papers, or computer programs
- Owning over $10,000 in stock in a company that sponsors your research and not disclosing this financial interest
- Deliberately overestimating the clinical significance of a new drug in order to obtain economic benefits
These actions would be regarded as unethical by most scientists and some might even be illegal in some cases. Most of these would also violate different professional ethics codes or institutional policies. However, they do not fall into the narrow category of actions that the government classifies as research misconduct. Indeed, there has been considerable debate about the definition of "research misconduct" and many researchers and policy makers are not satisfied with the government's narrow definition that focuses on FFP. However, given the huge list of potential offenses that might fall into the category "other serious deviations," and the practical problems with defining and policing these other deviations, it is understandable why government officials have chosen to limit their focus. Finally, situations frequently arise in research in which different people disagree about the proper course of action and there is no broad consensus about what should be done. In these situations, there may be good arguments on both sides of the issue and different ethical principles may conflict. These situations create difficult decisions for research known as ethical or moral dilemmas. Consider the following case:
Dr. Wexford is the principal investigator of a large, epidemiological study on the health of 10,000 agricultural workers. She has an impressive dataset that includes information on demographics, environmental exposures, diet, genetics, and various disease outcomes such as cancer, Parkinson's disease (PD), and ALS. She has just published a paper on the relationship between pesticide exposure and PD in a prestigious journal. She is planning to publish many other papers from her dataset. She receives a request from another research team that wants access to her complete dataset. They are interested in examining the relationship between pesticide exposures and skin cancer. Dr. Wexford was planning to conduct a study on this topic. Dr. Wexford faces a difficult choice. On the one hand, the ethical norm of openness obliges her to share data with the other research team. Her funding agency may also have rules that obligate her to share data. On the other hand, if she shares data with the other team, they may publish results that she was planning to publish, thus depriving her (and her team) of recognition and priority. It seems that there are good arguments on both sides of this issue and Dr. Wexford needs to take some time to think about what she should do. One possible option is to share data, provided that the investigators sign a data use agreement. The agreement could define allowable uses of the data, publication plans, authorship, etc. Another option would be to offer to collaborate with the researchers. The following are some step that researchers, such as Dr. Wexford, can take to deal with ethical dilemmas in research:
What is the problem or issue? It is always important to get a clear statement of the problem. In this case, the issue is whether to share information with the other research team.
What is the relevant information? Many bad decisions are made as a result of poor information. To know what to do, Dr. Wexford needs to have more information concerning such matters as university or funding agency or journal policies that may apply to this situation, the team's intellectual property interests, the possibility of negotiating some kind of agreement with the other team, whether the other team also has some information it is willing to share, the impact of the potential publications, etc.
What are the different options? People may fail to see different options due to a limited imagination, bias, ignorance, or fear. In this case, there may be other choices besides 'share' or 'don't share,' such as 'negotiate an agreement' or 'offer to collaborate with the researchers.' How do ethical codes or policies as well as legal rules apply to these different options? The university or funding agency may have policies on data management that apply to this case. Broader ethical rules, such as openness and respect for credit and intellectual property, may also apply to this case. Laws relating to intellectual property may be relevant.
Are there any people who can offer ethical advice? It may be useful to seek advice from a colleague, a senior researcher, your department chair, an ethics or compliance officer, or anyone else you can trust. In the case, Dr. Wexford might want to talk to her supervisor and research team before making a decision. After considering these questions, a person facing an ethical dilemma may decide to ask more questions, gather more information, explore different options, or consider other ethical rules. However, at some point he or she will have to make a decision and then take action. Ideally, a person who makes a decision in an ethical dilemma should be able to justify his or her decision to himself or herself, as well as colleagues, administrators, and other people who might be affected by the decision. He or she should be able to articulate reasons for his or her conduct and should consider the following questions in order to explain how he or she arrived at his or her decision: .
- Which choice will probably have the best overall consequences for science and society?
- Which choice could stand up to further publicity and scrutiny?
- Which choice could you not live with?
- Think of the wisest person you know. What would he or she do in this situation?
- Which choice would be the most just, fair, or responsible?
After considering all of these questions, one still might find it difficult to decide what to do. If this is the case, then it may be appropriate to consider others ways of making the decision, such as going with a gut feeling or intuition, seeking guidance through prayer or meditation, or even flipping a coin. Endorsing these methods in this context need not imply that ethical decisions are irrational, however. The main point is that human reasoning plays a pivotal role in ethical decision-making but there are limits to its ability to solve all ethical dilemmas in a finite amount of time.
Promoting Ethical Conduct in Science

Most academic institutions in the US require undergraduate, graduate, or postgraduate students to have some education in the responsible conduct of research (RCR). The NIH and NSF have both mandated training in research ethics for students and trainees. Many academic institutions outside of the US have also developed educational curricula in research ethics Those of you who are taking or have taken courses in research ethics may be wondering why you are required to have education in research ethics. You may believe that you are highly ethical and know the difference between right and wrong. You would never fabricate or falsify data or plagiarize. Indeed, you also may believe that most of your colleagues are highly ethical and that there is no ethics problem in research.. If you feel this way, relax. No one is accusing you of acting unethically. Indeed, the evidence produced so far shows that misconduct is a very rare occurrence in research, although there is considerable variation among various estimates. The rate of misconduct has been estimated to be as low as 0.01% of researchers per year (based on confirmed cases of misconduct in federally funded research) to as high as 1% of researchers per year (based on self-reports of misconduct on anonymous surveys).
Clearly, it would be useful to have more data on this topic, but so far there is no evidence that science has become ethically corrupt, despite some highly publicized scandals. Even if misconduct is only a rare occurrence, it can still have a tremendous impact on science and society because it can compromise the integrity of research, erode the public's trust in science, and waste time and resources. Will education in research ethics help reduce the rate of misconduct in science? It is too early to tell. The answer to this question depends, in part, on how one understands the causes of misconduct. There are two main theories about why researchers commit misconduct. According to the "bad apple" theory, most scientists are highly ethical. Only researchers who are morally corrupt, economically desperate, or psychologically disturbed commit misconduct. Moreover, only a fool would commit misconduct because science's peer review system and self-correcting mechanisms will eventually catch those who try to cheat the system. In any case, a course in research ethics will have little impact on "bad apples," one might argue. According to the "stressful" or "imperfect" environment theory, misconduct occurs because various institutional pressures, incentives, and constraints encourage people to commit misconduct, such as pressures to publish or obtain grants or contracts, career ambitions, the pursuit of profit or fame, poor supervision of students and trainees, and poor oversight of researchers. Moreover, defenders of the stressful environment theory point out that science's peer review system is far from perfect and that it is relatively easy to cheat the system. Erroneous or fraudulent research often enters the public record without being detected for years. Misconduct probably results from environmental and individual causes, i.e. when people who are morally weak, ignorant, or insensitive are placed in stressful or imperfect environments. In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For example, some unethical authorship practices probably reflect traditions and practices that have not been questioned seriously until recently. If the director of a lab is named as an author on every paper that comes from his lab, even if he does not make a significant contribution, what could be wrong with that? That's just the way it's done, one might argue. Another example where there may be some ignorance or mistaken traditions is conflicts of interest in research. A researcher may think that a "normal" or "traditional" financial relationship, such as accepting stock or a consulting fee from a drug company that sponsors her research, raises no serious ethical issues. Or perhaps a university administrator sees no ethical problem in taking a large gift with strings attached from a pharmaceutical company. Maybe a physician thinks that it is perfectly appropriate to receive a $300 finder's fee for referring patients into a clinical trial. If "deviations" from ethical conduct occur in research as a result of ignorance or a failure to reflect critically on problematic traditions, then a course in research ethics may help reduce the rate of serious deviations by improving the researcher's understanding of ethics and by sensitizing him or her to the issues. Finally, education in research ethics should be able to help researchers grapple with the ethical dilemmas they are likely to encounter by introducing them to important concepts, tools, principles, and methods that can be useful in resolving these dilemmas. Scientists must deal with a number of different controversial topics, such as human embryonic stem cell research, cloning, genetic engineering, and research involving animal or human subjects, which require ethical reflection and deliberation.
Find anything you save across the site in your account

In the campus protests over the war in Gaza, language and rhetoric are—as they have always been when it comes to Israel and Palestine—weapons of mass destruction.
By Zadie Smith
A philosophy without a politics is common enough. Aesthetes, ethicists, novelists—all may be easily critiqued and found wanting on this basis. But there is also the danger of a politics without a philosophy. A politics unmoored, unprincipled, which holds as its most fundamental commitment its own perpetuation. A Realpolitik that believes itself too subtle—or too pragmatic—to deal with such ethical platitudes as thou shalt not kill. Or: rape is a crime, everywhere and always. But sometimes ethical philosophy reënters the arena, as is happening right now on college campuses all over America. I understand the ethics underpinning the protests to be based on two widely recognized principles:
There is an ethical duty to express solidarity with the weak in any situation that involves oppressive power.
If the machinery of oppressive power is to be trained on the weak, then there is a duty to stop the gears by any means necessary.
The first principle sometimes takes the “weak” to mean “whoever has the least power,” and sometimes “whoever suffers most,” but most often a combination of both. The second principle, meanwhile, may be used to defend revolutionary violence, although this interpretation has just as often been repudiated by pacifistic radicals, among whom two of the most famous are, of course, Mahatma Gandhi and Martin Luther King, Jr . In the pacifist’s interpretation, the body that we must place between the gears is not that of our enemy but our own. In doing this, we may pay the ultimate price with our actual bodies, in the non-metaphorical sense. More usually, the risk is to our livelihoods, our reputations, our futures. Before these most recent campus protests began, we had an example of this kind of action in the climate movement. For several years now, many people have been protesting the economic and political machinery that perpetuates climate change, by blocking roads, throwing paint, interrupting plays, and committing many other arrestable offenses that can appear ridiculous to skeptics (or, at the very least, performative), but which in truth represent a level of personal sacrifice unimaginable to many of us.
I experienced this not long ago while participating in an XR climate rally in London. When it came to the point in the proceedings where I was asked by my fellow-protesters whether I’d be willing to commit an arrestable offense—one that would likely lead to a conviction and thus make travelling to the United States difficult or even impossible—I’m ashamed to say that I declined that offer. Turns out, I could not give up my relationship with New York City for the future of the planet. I’d just about managed to stop buying plastic bottles (except when very thirsty) and was trying to fly less. But never to see New York again? What pitiful ethical creatures we are (I am)! Falling at the first hurdle! Anyone who finds themselves rolling their eyes at any young person willing to put their own future into jeopardy for an ethical principle should ask themselves where the limits of their own commitments lie—also whether they’ve bought a plastic bottle or booked a flight recently. A humbling inquiry.
It is difficult to look at the recent Columbia University protests in particular without being reminded of the campus protests of the nineteen-sixties and seventies, some of which happened on the very same lawns. At that time, a cynical political class was forced to observe the spectacle of its own privileged youth standing in solidarity with the weakest historical actors of the moment, a group that included, but was not restricted to, African Americans and the Vietnamese. By placing such people within their ethical zone of interest, young Americans risked both their own academic and personal futures and—in the infamous case of Kent State—their lives. I imagine that the students at Columbia—and protesters on other campuses—fully intend this echo, and, in their unequivocal demand for both a ceasefire and financial divestment from this terrible war, to a certain extent they have achieved it.
But, when I open newspapers and see students dismissing the idea that some of their fellow-students feel, at this particular moment, unsafe on campus, or arguing that such a feeling is simply not worth attending to, given the magnitude of what is occurring in Gaza, I find such sentiments cynical and unworthy of this movement. For it may well be—within the ethical zone of interest that is a campus, which was not so long ago defined as a safe space, delineated by the boundary of a generation’s ethical ideas— it may well be that a Jewish student walking past the tents, who finds herself referred to as a Zionist, and then is warned to keep her distance, is, in that moment, the weakest participant in the zone. If the concept of safety is foundational to these students’ ethical philosophy (as I take it to be), and, if the protests are committed to reinserting ethical principles into a cynical and corrupt politics, it is not right to divest from these same ethics at the very moment they come into conflict with other imperatives. The point of a foundational ethics is that it is not contingent but foundational. That is precisely its challenge to a corrupt politics.
Practicing our ethics in the real world involves a constant testing of them, a recognition that our zones of ethical interest have no fixed boundaries and may need to widen and shrink moment by moment as the situation demands. (Those brave students who—in supporting the ethical necessity of a ceasefire—find themselves at painful odds with family, friends, faith, or community have already made this calculation.) This flexibility can also have the positive long-term political effect of allowing us to comprehend that, although our duty to the weakest is permanent, the role of “the weakest” is not an existential matter independent of time and space but, rather, a contingent situation, continually subject to change. By contrast, there is a dangerous rigidity to be found in the idea that concern for the dreadful situation of the hostages is somehow in opposition to, or incompatible with, the demand for a ceasefire. Surely a ceasefire—as well as being an ethical necessity—is also in the immediate absolute interest of the hostages, a fact that cannot be erased by tearing their posters off walls.
Part of the significance of a student protest is the ways in which it gives young people the opportunity to insist upon an ethical principle while still being, comparatively speaking, a more rational force than the supposed adults in the room, against whose crazed magical thinking they have been forced to define themselves. The equality of all human life was never a self-evident truth in racially segregated America. There was no way to “win” in Vietnam. Hamas will not be “eliminated.” The more than seven million Jewish human beings who live in the gap between the river and the sea will not simply vanish because you think that they should. All of that is just rhetoric. Words. Cathartic to chant, perhaps, but essentially meaningless. A ceasefire, meanwhile, is both a potential reality and an ethical necessity. The monstrous and brutal mass murder of more than eleven hundred people, the majority of them civilians, dozens of them children, on October 7th, has been followed by the monstrous and brutal mass murder (at the time of writing) of a reported fourteen thousand five hundred children. And many more human beings besides, but it’s impossible not to notice that the sort of people who take at face value phrases like “surgical strikes” and “controlled military operation” sometimes need to look at and/or think about dead children specifically in order to refocus their minds on reality.
To send the police in to arrest young people peacefully insisting upon a ceasefire represents a moral injury to us all. To do it with violence is a scandal. How could they do less than protest, in this moment? They are putting their own bodies into the machine. They deserve our support and praise. As to which postwar political arrangement any of these students may favor, and on what basis they favor it—that is all an argument for the day after a ceasefire. One state, two states, river to the sea—in my view, their views have no real weight in this particular moment, or very little weight next to the significance of their collective action, which (if I understand it correctly) is focussed on stopping the flow of money that is funding bloody murder, and calling for a ceasefire, the political euphemism that we use to mark the end of bloody murder. After a ceasefire, the criminal events of the past seven months should be tried and judged, and the infinitely difficult business of creating just, humane, and habitable political structures in the region must begin anew. Right now: ceasefire. And, as we make this demand, we might remind ourselves that a ceasefire is not, primarily, a political demand. Primarily, it is an ethical one.
But it is in the nature of the political that we cannot even attend to such ethical imperatives unless we first know the political position of whoever is speaking. (“Where do you stand on Israel/Palestine?”) In these constructed narratives, there are always a series of shibboleths, that is, phrases that can’t be said, or, conversely, phrases that must be said. Once these words or phrases have been spoken ( river to the sea, existential threat, right to defend, one state, two states, Zionist, colonialist, imperialist, terrorist ) and one’s positionality established, then and only then will the ethics of the question be attended to (or absolutely ignored). The objection may be raised at this point that I am behaving like a novelist, expressing a philosophy without a politics, or making some rarefied point about language and rhetoric while people commit bloody murder. This would normally be my own view, but, in the case of Israel/Palestine, language and rhetoric are and always have been weapons of mass destruction.
It is in fact perhaps the most acute example in the world of the use of words to justify bloody murder, to flatten and erase unbelievably labyrinthine histories, and to deliver the atavistic pleasure of violent simplicity to the many people who seem to believe that merely by saying something they make it so. It is no doubt a great relief to say the word “Hamas” as if it purely and solely described a terrorist entity. A great relief to say “There is no such thing as the Palestinian people” as they stand in front of you. A great relief to say “Zionist colonialist state” and accept those three words as a full and unimpeachable definition of the state of Israel, not only under the disastrous leadership of Benjamin Netanyahu but at every stage of its long and complex history, and also to hear them as a perfectly sufficient description of every man, woman, and child who has ever lived in Israel or happened to find themselves born within it. It is perhaps because we know these simplifications to be impossible that we insist upon them so passionately. They are shibboleths; they describe a people, by defining them against other people—but the people being described are ourselves. The person who says “We must eliminate Hamas” says this not necessarily because she thinks this is a possible outcome on this earth but because this sentence is the shibboleth that marks her membership in the community that says that. The person who uses the word “Zionist” as if that word were an unchanged and unchangeable monolith, meaning exactly the same thing in 2024 and 1948 as it meant in 1890 or 1901 or 1920—that person does not so much bring definitive clarity to the entangled history of Jews and Palestinians as they successfully and soothingly draw a line to mark their own zone of interest and where it ends. And while we all talk, carefully curating our shibboleths, presenting them to others and waiting for them to reveal themselves as with us or against us—while we do all that, bloody murder.
And now here we are, almost at the end of this little stream of words. We’ve arrived at the point at which I must state clearly “where I stand on the issue,” that is, which particular political settlement should, in my own, personal view, occur on the other side of a ceasefire. This is the point wherein—by my stating of a position—you are at once liberated into the simple pleasure of placing me firmly on one side or the other, putting me over there with those who lisp or those who don’t, with the Ephraimites, or with the people of Gilead. Yes, this is the point at which I stake my rhetorical flag in that fantastical, linguistical, conceptual, unreal place—built with words—where rapes are minimized as needs be, and the definition of genocide quibbled over, where the killing of babies is denied, and the precision of drones glorified, where histories are reconsidered or rewritten or analogized or simply ignored, and “Jew” and “colonialist” are synonymous, and “Palestinian” and “terrorist” are synonymous, and language is your accomplice and alibi in all of it. Language euphemized, instrumentalized, and abused, put to work for your cause and only for your cause, so that it does exactly and only what you want it to do. Let me make it easy for you. Put me wherever you want: misguided socialist, toothless humanist, naïve novelist, useful idiot, apologist, denier, ally, contrarian, collaborator, traitor, inexcusable coward. It is my view that my personal views have no more weight than an ear of corn in this particular essay. The only thing that has any weight in this particular essay is the dead. ♦
New Yorker Favorites
The day the dinosaurs died .
What if you started itching— and couldn’t stop ?
How a notorious gangster was exposed by his own sister .
Woodstock was overrated .
Diana Nyad’s hundred-and-eleven-mile swim .
Photo Booth: Deana Lawson’s hyper-staged portraits of Black love .
Fiction by Roald Dahl: “The Landlady”
Sign up for our daily newsletter to receive the best stories from The New Yorker .
By signing up, you agree to our User Agreement and Privacy Policy & Cookie Statement . This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

By Isaac Chotiner

By David Remnick

By Louis Menand

By Andrew Marantz

IMAGES
VIDEO
COMMENTS
The ideas and opinions expressed in this essay are the author's own and do not necessarily represent those of the NIH, NIEHS, or US government. ... Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. ...
Research ethics are a set of principles that guide your research designs and practices in both quantitative and qualitative research. In this article, you will learn about the types and examples of ethical considerations in research, such as informed consent, confidentiality, and avoiding plagiarism. You will also find out how to apply ethical principles to your own research projects with ...
Research Ethics is defined here to be the ethics of the planning, conduct, and reporting of research. It is clear that research ethics should include: Protections of human and animal subjects. However, not all researchers use human or animal subjects, nor are the ethical dimensions of research confined solely to protections for research ...
An ethics essay is a type of academic writing that explores ethical issues and dilemmas. Students should evaluates them in terms of moral principles and values. The purpose of an ethics essay is to examine the moral implications of a particular issue, and provide a reasoned argument in support of an ethical perspective.
question. Such alternative answers can appear in your essay as counterarguments. Each time you address a counterargument, your thesis becomes more plausible, since you have eliminated one of the possible alternative answers to your question. 2. Counterarguments lend tension and structure to your argument.-Counter
What is Ethics in Research & Why is it Important? W by David B. Resnik, J.D., Ph.D. December 1, 2015 The ideas and opinions expressed in this essay are the author's own and do not necessarily represent those of the NIH, NIEHS, or US government. hen most people think of ethics (or morals), they think of rules for distinguishing between right and
Ethics in research, writing, and publication are critical in medicine and nursing—decisions that affect human lives often are influenced by knowledge that is disseminated in healthcare journals. While it may seem less critical that healthcare design adhere to strong ethical principles in research, writing, and publication of findings, huge ...
2) Central Essay: Research Ethics: an Introduction by Tom Regan 3) Applied Ethics: Research Ethics as a Discipline. Four major ethical approaches; some key questions in the Research Ethics tradition. Resource: "Six domains of research ethics: A heuristic framework for the responsible conduct of research." by Ken Pimple.
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it's often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own ...
Research ethics are moral principles that guide researchers to conduct and report research without deception or intention to harm the participants of the study or members of the society as a whole, whether knowingly or unknowingly. Practising ethical guidelines while conducting and reporting research is essential to establish the validity of ...
ethics in research as the disclosure of methods applied during. the study, where the researcher must always maintain an. impartial position, being honest in his intentions and not. giving away the ...
Introduction. In science and medical research, ethics is essential in enhancing the safety and well-being of the subjects or participants. Different studies globally expose vulnerable populations or subjects to abuse, affecting their overall health. In the same case, researchers are employing diverse strategies to enhance ethics and reduce ...
Research ethics is unambiguously concerned in the examination of ethical issues that are upraised when individuals are involved as participants in the study. Research ethics committee/Institutional Review Board (IRB) reviews whether the research is ethical enough or not to protect the rights, dignity and welfare of the respondents. ...
An ethics paper is a type of an argumentative assignment that deals with a certain ethical problem that a student has to describe and solve. Also, it can be an essay where a certain controversial event or concept is elaborated through an ethical lens (e.g. moral rules and principles), or a certain ethical dilemma is explained.
Ethics of Science is a comprehensive and student-friendly introduction to the study of ethics in science and scientific research. The book covers: * Science and Ethics * Ethical Theory and ...
Ethics is a system of moral principles or the moral values that influence the proper conduct of an individual or group. The term originated from the Greek word 'ethos' meaning habit or character, and it speaks to how we ought to live, that is, how we ought to treat others. Any research which involves human subjects or participants is bound ...
Why do research ethics matter? Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe. ... In shorter scientific papers, where the aim is to report the findings of a specific study, you ...
Research ethics is a common phrase that I h ave met severally both in class and in the field. As. much a s it is a very popular phrase to me, as a young researcher my previous understanding of ...
Ethics is an essential part of any research project. One may assume ethics is just another stage of research, one that is tackled with filing out a standardized set of forms submitted to an ethics committee. (e.g. IRB) may not lend itself to effectively assessing ethical issues. Ethics has become a cornerstone for conducting effective and ...
Dr. Elliott teaches medical ethics at the University of Minnesota. He is the author of the forthcoming book "The Occasional Human Sacrifice: Medical Experimentation and the Price of Saying No ...
Key points. Recent research calls into question researchers' biased perceptions of their own ethical practices. This, though, should be unsurprising, given that researchers are subject to the same ...
The paper checklist is intended to help authors reflect on a wide variety of issues relating to responsible machine learning research, including reproducibility, transparency, research ethics, and societal impact. The checklist forms part of the paper submission, but does not count towards the page limit.
ocesses in HIV research in low- and middle-income countries (LMICs). Methods We conducted a digital crowdsourcing open call for ideas to improve AYA consent to HIV research in LMICs. Crowdsourcing involves engaging a group of people in problem-solving, then sharing emergent solutions. Submissions were evaluated by 3 independent judges using predefined criteria, with exceptional strategies ...
It is therefore important for researchers to learn how to interpret, assess, and apply various research rules and how to make decisions and to act in various situations. The vast majority of decisions involve the straightforward application of ethical rules. For example, consider the following case, Case 1:
One may also define ethics as a method, procedure, or perspective for deciding how to act and for analyzing complex problems and issues. For instance, in considering a complex issue like global warming, one may take an economic, ecological, political, or ethical perspective on the problem. While an economist might examine the cost and benefits ...
By Zadie Smith. May 5, 2024. Illustration by Nicholas Konrad / The New Yorker. A philosophy without a politics is common enough. Aesthetes, ethicists, novelists—all may be easily critiqued and ...
The guidance discusses seven topics that are likely to be considered key information, including: Use of videos, illustrations. The National Organization for Rare Disorders (NORD) applauded the draft guidance for allowing the use of innovative informed consent approaches, such as videos. "It is important to ensure that informed consent is ...
Readers respond to funding academic medical centers, the 'residency research arms race,' and more. By Patrick Skerrett. Reprints. Molly Ferguson for STAT. STAT now publishes selected Letters ...