Clinical Trials
Displaying 175 studies
The purpose of this study is to identify immune mediated diabetes in patients treated with PD 1 inhibitors, and characterizing its clinical course, laboratory features and possible risk factors.
The purpose of this study is to determine the longitudinal effect of diabetes-associated variation in TCF7L2 on a-cell function and the contribution of a-cell function to longitudinal glucose tolerance and EGP in non-diabetic subjects.
The purpose of this study is to identify potentially modifiable barriers to hyperglycemia management in hospitalized diabetic patients. Both general hospitalized diabetic patients and first time renal transplant patients will be studied.
The purpose of this study is to establish a biobank of blood samples to study the relationship between diabetes mellitus and other pancreatic conditions.
The objective of this study is to gain understanding of how patients with diabetes mellitus (DM) dispose of hazardous waste items (e.g., needles, used glucometer strips, unused insulin) with the goal of providing education regarding safe practices
The goal of this study is to understand how and why insulin resistant individuals respond differently to exercise as compared with insulin sensitive individuals at the skeletal muscle and gene expression level.
The purpose of this study is to compare the effectiveness and safety of an automated insulin delivery (AID) study system using a Model Predictive Control (MPC) algorithm versus Sensor Augmented Pump (SAP) (which may or may not include PLGS; to be referred to as SAP) therapy in people with type 1 diabetes. A Pilot Phase involving up to 7 participants using the study system for 10-14 days will be conducted prior to the crossover trial.
The purpose of this study is to determine if a 6 month supply (1 meal//day) of healthy food choices readily available in the patients home and self management training including understanding how foods impact diabetes, improved food choices and how to prepare those foods, will improve glucose control, and if there will be lasting behavior change modification after the program.
The objectives of this study are to determine whether the InPen® alters the glycemic control and variability in adolescents and emerging adults with type 1 diabetes, and to determine if InPen® use alters the perceived burden of diabetes cares, diabetes distress scores, transition readiness scores, and parental experience of child illness scale (11-13).
The goal of this study is to determine the role of postprandial glucagon suppression and insulin secretion in the progression of glucose intolerance in people with diabetes-associated variation in TCF7L2.
The purpose of this study is to use the well-characterized Diabetes Control and Complications Trial (DCCT) cohort of 1,400 patients to determine the long-term effects of prior separation of glycemic levels on micro- and macrovascular outcomes.
The purpose of this study is to establish a cohort of new onset diabetes patients.
Patients age 25 to 75 who are in the care of one of the primary care physicians at Mayo Clinic in Jacksonville, Florida or Montage Health in Monterey, California and have a recent HbA1c in the range of 7.5% to 13% will be prospectively identified and eligible for participation in this randomized, crossover clinical trial examining the effect of medically tailored meal delivery on glycemic control. Eligible patients who sign informed consent will be randomized in a 1:1 fashion to treatment sequence AB or treatment sequence BA. In the first study phase, participants randomized to sequence AB will receive 3 ...
The purpose of this study is to determine if patient’s own Continuous Glucose Monitoring (CGMs) worn in the non-ICU hospital setting have adequate accuracy for blood glucose monitoring when compared to point-of-care capillary glucose measurement, and to determine if alerts given by CGMs worn in the non-ICU hospital would prevent episodes of hyperglycemia and hypoglycemia.
The obectives of this study are to identify insulin resistance (IR)-specific chromatin signatures in mature adipocytes and myotubes, and to identify IR-specific chromatin signatures in progenitor cells from adipose tissue (AT) and skeletal muscle (SM).
The purpose of this study is to demonstrate that a morning injection of Toujeo compared to Lantus will provide better glycemic control, as shown by Continuous Glucose Monitoring (CGM), in adult patients with type 1 diabetes mellitus.
The purpose of this study is to identify changes to the metabolome (range of chemicals produced in the body) and microbiome (intestine microbe environment) that are unique to Roux-en-Y gastric bypass surgery and assess the associated effect on the metabolism of patients with type 2 diabetes.
The primary aim of this study is to compare the outcome measures of adult ECH type 2 diabetes patients who were referred to onsite pharmacist services for management of their diabetes to similar patients who were not referred for pharmacy service management of their diabetes. A secondary aim of the study is to assess the Kasson providers’ satisfaction level and estimated pharmacy service referral frequency to their patients. A tertiary aim of the study is to compare the hospitalization rates of type 2 diabetes rates who were referred to onsite pharmacist services for management of their diabetes to similar patients ...
To explore the feasibility of conducting a family centered wellness coaching program for patients at high risk for developing diabetes, in a primary care setting.
To determine engagement patterns.
To describe characteristics of families who are likely to participate.
To identify barriers/limitations to family centered wellness coaching.
To assess whether a family centered 8 week wellness coaching intervention for primary care patients at high risk for diabetes will improve self-care behaviors as measured by self-reported changes in physical activity level and food choices.
This study is being done to understand metformin's mechanisms of action regarding glucose production, protein metabolism, and mitochondrial function.
The purpose of this study is to assess the effectiveness of Revita® DMR for improving HbA1c to ≤ 7% without the need of insulin in subjects with T2D compared to sham and to assess the effectiveness of DMR versus Sham on improvement in Glycemic, Hepatic and Cardiovascular endpoints.
The purpose of this study is to identify risk factors for ICI associated diabetes mellitus and to assess the severity and natural course of this immune related adverse effect.
The purpose of this study is to evaluate the impact of a digital storytelling intervention derived through a community-based participatory research (CBPR) approach on type 2 diabetes mellitus (T2D) outcomes among Hispanic adults with poorly controlled type 2 diabetes mellitus (T2D) in primary care settings through a randomized clinical trial.
The purpose of this study is to assess the impact of a whole food plant-based diet on blood sugar control in diabetic patients versus a control group on the American Diabetics Association diet before having a total hip, knee, or shoulder replacement surgery.
The purpose of this study is to learn more about if the medication, Entresto, could help the function of the heart and kidneys.
The purpose of this study is to evaluate 6 weeks of home use of the Control-IQ automated insulin delivery system in individuals with type 2 diabetes.
This study will evaluate whether bile acids are able to increase insulin sensitivity and enhance glycemic control in T2DM patients, as well as exploring the mechanisms that enhance glycemic control. These observations will provide the preliminary data for proposing future therapeutic as well as further mechanistic studies of the role of bile acids in the control of glycemia in T2DM.
The purpose of this study is to determine if Inpatient Stress Hyperglycemia is an indicator of future risk of developing type 2 Diabetes Mellitus.
The purpose of this study is to collect blood samples for biomarker assessment in type 1 diabetes prior to and at specific time points during closed loop control.
The purpose of this study is to assess the effectiveness of a digital storytelling intervention derived through a community based participatory research (CBPR) approach on self-management of type 2 diabetes (T2D) among Somali adults.
The GRADE Study is a pragmatic, unmasked clinical trial that will compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
Hypothesis: Increased contact with the diabetes care team throughout pregnancy will lead to improved glucose control during pregnancy.
The overall goal of this proposal is to determine the effects of acute hyperglycemia and its modulation by Glucagon-like Peptide-1 (GLP-1) on myocardial perfusion in type 2 diabetes (DM). This study plan utilizes myocardial contrast echocardiography (MCE) to explore a) the effects of acute hyperglycemia on myocardial perfusion and coronary flow reserve in individuals with and without DM; and b) the effects of GLP-1 on myocardial perfusion and coronary flow reserve during euglycemia and hyperglycemia in DM. The investigators will recruit individuals with and without DM matched for age, gender and degree of obesity. The investigators will measure myocardial perfusion ...
The purpose of this study is to test the hypothesis that patients with T2DM will have greater deterioration in BMSi and in cortical porosity over 3 yrs as compared to sex- and age-matched non-diabetic controls; and identify the circulating hormonal (e.g., estradiol [E2], testosterone [T]) and biochemical (e.g., bone turnover markers, AGEs) determinants of changes in these key parameters of bone quality, and evaluate the possible relationship between existing diabetic complications and skeletal deterioration over time in the T2DM patients.
The purpose of this study is to serve as a comparator group to a group of patients that will be managed with AP for varying periods of time during pregnancy.
The purpose of this study is to determine the effect of endogenous GLP-1 secretion on islet function in people with Typr 2 Diabetes Mellitus (T2DM).
GLP-1 is a hormone made by the body that promotes the production of insulin in response to eating. However, there is increasing evidence that this hormone might help support the body’s ability to produce insulin when diabetes develops.
The purpose of this study is to assess whether psyllium is more effective in lowering fasting blood sugar and HbA1c, and to evaluate the effect of psyllium compared to wheat dextrin on the following laboratory markers: LDL-C, inflammatory markers such as ceramides and hsCRP, and branch chain amino acids which predict Diabetes Mellitus (DM).
The purpose of this study is to evaluate glucose variability in patients with type 1 diabetes (T1D) and insulin antibodies, to evaluate the clinical significance of insulin antibodies, and to establish an in vitro assay that would detect antibodies to insulin and insulin analogs.
This clinical trial will identify exercise-related and emotional stress related effects on glycemic control in patients with type 1 diabetes using sensor-augmented pump (SAP) therapy.
This study will test the efficacy of BKR-017 (colon-targeted 500 mg butyrate tablets) on insulin sensitivity, glucose control and triglycerides in type-1 diabetes subjects.
The purpose of this research is to test the safety and effectiveness of the interoperable Artificial Pancreas System Smartphone App (iAPS) in managing blood sugars in pregnant patients with type 1 diabetes.
This mixed methods study aims to answer the question: "What is the work of being a patient with type 2 diabetes mellitus?" .
The objective of this study is to evaluate the EWIS in patients with type 1 diabetes on insulin pump therapy.
This study is a multi-center, non-randomized, prospective single arm study with type 1 patients with diabetes on insulin pump therapy with Continuous Glucose Monitoring (CGM).
A total of up to 300 subjects will be enrolled at up to 20 investigational centers in the US in order to have 240 subjects meeting eligibility criteria. Each subject will wear their own MiniMed™ 670G insulin system. Each subject will be given 12 infusion sets to wear (each infusion set for at least 174 hours, or ...
The purpose of this study is to use the USS Virginia Closed-Loop system for overnight insulin delivery in adults with Type 1 Diabetes (T1DM) in an outpatient setting to evaluate the system's ability to significantly improve blood glucose levels. This protocol will test the feasibility of "bedside" closed-loop control - an approach comprised of standard sensor-augmented pump therapy during the day using off-the-shelf devices and overnight closed-loop control using experimental devices in an outpatient setting. The rationale for this study is as follows: we anticipate that closed-loop control may ultimately be adopted by patients with T1DM in a selective manner. ...
The purpose of this study is to assess penile length pre- and post-completion of RestoreX® traction therapy compared to control groups (no treatment) among men with type II diabetes.
This observational study is conducted to determine how the duodenal layer thicknesses (mucosa, submucosa, and muscularis) vary with several factors in patients with and without type 2 diabetes.
The overall objective of this study is to perform baseline and repeat assessments over time of the metabolic and immunologic status of individuals at risk for type 1 diabetes (T1D) to:
- characterize their risk for developing T1D and identify subjects eligible for prevention trials;
- describe the pathogenic evolution of T1D; and
- increase the understanding of the pathogenic factors involved in the development of T1D.
Our goal in this pilot study is to test and develop a novel method that will accurately measure, in vivo, glucagon kinetics in healthy humans and generate preliminary data in type 1 diabetes (T1DM) subjects under overnight fasted conditions.
This trial is a multi-center, adaptive, randomized, double-blind, placebo- and active- controlled, parallel group, phase 2 study in subjects with Type 2 Diabetes Mellitus to evaluate the effect of TTP399 on HbA1c following administration for 6 months.
The purpose of this study is to find the inheritable changes in genetic makeup that are related to the development of type 2 diabetes in Latino families.
The objective of this early feasibility study is to assess the feasibility and preliminary safety of the Endogenex Divice for endoscopic duodenal mucosal regeneration in patients with type 2 diabetes (T2D) inadequately controlled on 2-3 non-insulin glucose-lowering medications.
The purpose of this study is to evaluate if breathing pure oxygen overnight affects insulin sensitivity in participants with diabetes.
The purpose of this study is to determine the impact of patient decision aids compared to usual care on measures of patient involvement in decision-making, diabetes care processes, medication adherence, glycemic and cardiovascular risk factor control, and use of resources in nonurban practices in the Midwestern United States.
The purpose of this study is to assess a novel informatics approach that incorporates the use of patient’s diabetes self-care data into the design and delivery of individualized education interventions to improve diabetes control.
The purpose of this study is to assess the glycemic variability in patients with complex diabetes admitted in the hospital using a glycemic sensor.
The purpose of this research is to create a single registry for type 1 and type 2 diabetes at Mayo Rochester and affiliated Mayo sites.
The study purpose is to understand patients’ with the diagnosis of Diabetes Mellitus type 1 or 2 perception of the care they receive in the Diabetes clinic or Diabetes technology clinic at Mayo Clinic and to explore and to identify the healthcare system components patients consider important to be part of the comprehensive regenerative care in the clinical setting.
However, before we can implement structural changes or design interventions to promote comprehensive regenerative care in clinical practice, we first need to characterize those regenerative practices occurring today, patients expectations, perceptions and experiences about comprehensive regenerative care and determine the ...
The purpose of this study is to estimate the risk of diabetes related complications after total pancreatectomy. We will contact long term survivors after total pancreatectomy to obtain data regarding diabetes related end organ complications.
The multi-purpose of this study is to examine the effectiveness of “InsulisiteGuider” in patients with type 1 diabetes (T1D) through a two-group randomized controlled trial, to characterize the RNA biomarkers in skin epithelial cells isolated from the continuous subcutaneous insulin infusion (CSII) cannulas from T1D patients, and to characterize RNA biomarkers in the blood and saliva of TID patients.
The purpose of this study is to understand nighttime glucose regulation in humans and find if the pattern is different in people with Type 2 diabetes
Can QBSAfe be implemented in a clinical practice setting and improve quality of life, reduce treatment burden and hypoglycemia among older, complex patients with type 2 diabetes?
Questionnaire administered to diabetic patients in primary care practice (La Crosse Mayo Family Medicine Residency /Family Health Clinic) to assess patient’s diabetic knowledge. Retrospective chart review will also be done to assess objective diabetic control based on most recent hemoglobin A1c.
Exendin-(9,39) has been shown to have effects on beta-cell function, and after gastric bypass, to accelerate gastrointestinal transit. - infused at rates of 300pmol/kg/min. Given that gastrointestinal transit is typically delayed by Glucagon-Like Peptide-1 (GLP-1) and also that this hormone causes decreased food intake through increased satiation, it is reasonable to expect an effect of Exendin-9,39 on appetite. This may help explain the effects of gastric bypass on food intake. To examine the effect of Exendin on food intake we propose a dose-response study to determine whether the compound has effects in a dose-dependent fashion. We will examine the presence ...
The purpose of this study is to evaluate the dose-dependent effects of TAK-954 on gastric emptying time of solids in participants with diabetic or idiopathic gastroparesis assessed by scintigraphy.
The primary goal of this study protocol is to determine the candidate ratio of pramlintide and insulin co-infusion in individuals with type 1 diabetes (T1DM) to enable stable glucose control during the overnight post-absorptive and in the postprandial periods.
The purpose of this trial is to assess the performance of an Artificial Pancreas (AP) device using the Portable Artificial Pancreas System (pAPS) platform for subjects with type 1 diabetes using an insulin pump and rapid acting insulin. This proposed study is designed to compare closed-loop control with or without optimization of initialization parameters related to basal insulin infusion rates and insulin to carbohydrate (I:C) ratios for meals and snacks. The study consists of an evaluation of the Artificial Pancreas device system during two 24-27.5-hour closed-loop phases in an outpatient/hotel environment. Prior to the closed-loop phases, each subject will undergo ...
The study is being done to find out if low blood sugar (hypoglycemia) can be reduced in people with type 1 diabetes (T1D) 65 years and older with use of automated insulin delivery (AID) system.
The device systems used in this study are approved by the Food and Drug Administration (FDA) for diabetes management. We will be collecting data about how they are used, how well they work, and how safe they are.
This study aims to identify an early stage biomarker for type 1 diabetes. In vitro evidence identified a significant enrichment of the chemokine CXCL10 in β-cell derived EXO upon exposure to diabetogenic pro-inflammatory cytokines. The study also aims to test protocols for efficient isolation of plasma-derived EXO from small volumes of sample, develop an assay for the sensitive detection of CXCL10 in plasma-derived EXO, and characterization of plasma-derived EXO through assessment of concentration, size, and content (proteomics).
The study is designed to understand the confidence and competence level of patients with type 1 diabetes mellitus in their ability to make changes to their insulin pump.
The investigators will determine whether people with high muscle mitochondrial capacity produce higher amount of reactive oxygen species (ROS) on consuming high fat /high glycemic diet and thus exhibit elevated cellular oxidative damage. The investigators previously found that Asian Indian immigrants have high mitochondrial capacity in spite of severe insulin resistance. Somalians are another new immigrant population with rapidly increasing prevalence of diabetes. Both of these groups traditionally consume low caloric density diets, and the investigators hypothesize that when these groups are exposed to high-calorie Western diets, they exhibit increased oxidative stress, oxidative damage, and insulin resistance. The investigators will ...
The purpose of this study is to gather preliminary data to better understand acute effects of exercise on glucose metabolism. We will address if subjects with Type 1 Diabetes (T1D) are more insulin sensitive during and following a short bout of exercise compared to healthy controls. We will also determine insulin dependent and insulin independent effects on exercise in people with and without type 1 diabetes.
The purpose of this study is to retrospectively and prospectively compare maternal and fetal/newborn clinical outcomes in age-matched pregnant patients with T1D and healthy controls and to assess the relationship between glycemic variability and pregnancy outcomes in the current era.
The objective for thisstudy is to characterize the impact of glycemic excursions on cognition in Type 1 Diabetes (T1D) and determine mediators and moderators of this relationship. This study will allow us to determine how glycemic excursions impact cognition, as well as to identify mediators and moderators of this relationship that could lead to novel interventions.
The purpose of this research is to find out how genetic variations in GLP1R, alters insulin secretion, in the fasting state and when blood sugars levels are elevated. Results from this study may help us identify therapies to prevent or reverse type 2 diabetes mellitus.
The purpose of this study is to compare the effectiveness and safety of an automated insulin delivery (AID) system using a model predictive control (MPC) algorithm versus Sensor-Augmented Pump/Predictive Low Glucose Suspend (SAP/PLGS) therapy with different stress assessments over a 4-week period.
This study is being done to determine the roles that several molecules play in the repair of injured cells that line your blood vessels.
This purpose of this study is to determine if activation of a person's immune system in the small intestine could be a contributing cause of Type 1 Diabetes.
The purpose of this project is to collect data over the first year of clinical use of the FDA approved 670G closed loop insulin delivery system among patients with type 1 diabetes. The goal is to evaluate how this newly approved system impacts both clinical and patient-reported outcomes.
It is unknown how patient preferences and values impact the comparative effectiveness of second-line medications for Type 2 diabetes (T2D). The purpose of this study is to elicit patient preferences toward various treatment outcomes (e.g., hospitalization, kidney disease) using a participatory ranking exercise, use these rankings to generate individually weighted composite outcomes, and estimate patient-centered treatment effects of four different second-line T2D medications that reflect the patient's value for each outcome.
The purpose of this mixed-methods study is to deploy the tenets of Health and Wellness Coaching (HWC) through a program called BeWell360 model , tailored to the needs of Healthcare Workers (HCWs) as patients living with poorly-controlled Type 2 Diabetes (T2D). The objective of this study is to pilot-test this novel, scalable, and sustainable BeWell360 model that is embedded and integrated as part of primary care for Mayo Clinic Employees within Mayo Clinic Florida who are identified as patients li)ving with poorly-controlled T2D.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM). It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking is responsible for muscle insulin resistance, although it has been shown that raising FFA with Intralipid can cause muscle insulin resistance within 4 hours. We do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. We propose to alter the profile and concentrations of FFA of healthy, non-obese adults using an overnight, intra-duodenal palm oil infusion vs. ...
The objectives of this study are to identify circulating extracellular vesicle (EV)-derived protein and RNA signatures associated with Type 2 Diabetes (T2D), and to identify changes in circulating EV cargo in patients whose T2D resolves after sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB).
This research study is being done to develop educational materials that will help patients and clinicians talk about diabetes treatment and management options.
The purpose of this study is to assess the effectiveness and safety of treatment with various dose levels of TAK-906 in adult participants with gastroparesis compared with placebo during 12 weeks of treatment.
The purpose of this study is to evaluate whether or not a 6 month supply (1 meal//day) of healthy food choices readily available in the patient's home and self management training including understanding of how foods impact diabetes, improved food choices and how to prepare those foods, improve glucose control. In addition, it will evaluate whether or not there will be lasting behavior change modification after the program.
To understand why patients with indigestion with or without diabetes have gastrointestinal symptoms and in particular to understand where the symptoms are related to increased sensitivity to nutrients.
To determine if the EndoBarrier safely and effectively improves glycemic control in obese subjects with type 2 diabetes.
The primary objective of this study is to determine if continuous glucose monitoring (CGM) can reduce hypoglycemia and improve quality of life in older adults with type 1 diabetes (T1D).
The purpose of this study is to compare the rate of progression from prediabetes at 4 months to frank diabetes at 12 months (as defined by increase in HbA1C or fasting BS to diabetic range based on the ADA criteria) after transplantation in kidney transplant recipients on Exenatide SR + SOC vs. standard-of-care alone.
The purpose of this study evaluates a subset of people with isolated Impaired Fasting Glucose with Normal Glucose Tolerance (i.e., IFG/NGT) believed to have normal β-cell function in response to a glucose challenge, suggesting that – at least in this subset of prediabetes – fasting glucose is regulated independently of glucose in the postprandial period. To some extent this is borne out by genetic association studies which have identified loci that affect fasting glucose but not glucose tolerance and vice-versa.
Increased accumulation of fat into the muscles is associated with what is called insulin-resistant state, which is a pre-diabetic state. The purpose of this research is to find out how fat circulating in the blood following fat consumption is taken up by the muscles in healthy people as well as people that are insulin-resistant. The investigators are specifically interested in how a hormone called insulin is involved in this process. Findings from this research will contribute to our understanding of why insulin-resistant people have increased accumulation of fat in their muscles, and ultimately help to design appropriate interventions to prevent ...
The purpose of this study is to learn more about how the body stores dietary fat. Medical research has shown that fat stored in different parts of the body can affect the risk for diabetes, heart disease and other major health conditions.
The purpose of this study is to see why the ability of fat cells to respond to insulin is different depending on body shape and how fat tissue inflammation is involved.
The purpose of this study is to determine the mechanism(s) by which common bariatric surgical procedures alter carbohydrate metabolism. Understanding these mechanisms may ultimately lead to the development of new interventions for the prevention and treatment of type 2 diabetes and obesity.
The purpose of this study is to determine the metabolic effects of Colesevelam, particularly for the ability to lower blood sugar after a meal in type 2 diabetics, in order to develop a better understanding of it's potential role in the treatment of obesity.
The purpose of this study is to test whether markers of cellular aging and the SASP are elevated in subjects with obesity and further increased in patients with obesity and Type 2 Diabetes Mellitus (T2DM) and to relate markers of cellular aging (senescence) and the SASP to skeletal parameters (DXA, HRpQCT, bone turnover markers) in each of these groups.
Integration of Diabetes Prevention Program (DPP) and Diabetes Self Management Program (DSMP) into WellConnect.
The purpose of this study is to investigate if a blood test measuring copeptin within 24 hours following pituitary surgery could predict development of diabetes insipidus (increased urination and thirst with fluid balance problems) as opposed to the clinical methods we currently use.
This is a study to evaluate a new Point of Care test for blood glucose monitoring.
Women with gestational diabetes mellitus (GDM) are likely to have insulin resistance that persists long after pregnancy, resulting in greater risk of developing type 2 diabetes mellitus (T2DM). The study will compare women with and without a previous diagnosis of GDM to determine if women with a history of GDM have abnormal fatty acid metabolism, specifically impaired adipose tissue lipolysis. The study will aim to determine whether women with a history of GDM have impaired pancreatic β-cell function. The study will determine whether women with a history of GDM have tissue specific defects in insulin action, and also identify the effect of a ...
The Early Detection Initiative for pancreatic cancer is a multi-center randomized controlled trial to determine if algorithm-based screening in patients with new onset hyperglycemia and diabetes can result in earlier detection of pancreatic ductal adenocarcinoma.
The purpose of this study is to determine the changes in tissue function that occur in the first year postpartum in women with and without gestational diabetes mellitus.
The purpose of this study is assess the feasibility, effectiveness, and acceptability of Diabetes-REM (Rescue, Engagement, and Management), a comprehensive community paramedic (CP) program to improve diabetes self-management among adults in Southeast Minnesota (SEMN) treated for servere hypoglycemia by the Mayo Clinic Ambulance Services (MCAS).
The objective of the study is to assess efficacy and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The purpose of this study is to improve our understanding of why gastrointestinal symptoms occur in diabetes mellitus patients and identify new treatment(s) in the future.
These symptoms are often distressing and may impair glycemic control. We do not understand how diabetes mellitus affects the GI tracy. In 45 patients undergoing sleeve gastrectomy, we plan to compare the cellular composition of circulating peripheral mononuclear cells, stomach immune cells, and interstitial cells of Cajal in the stomach.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM), whereas lower body obesity (LBO) is characterized by near-normal insulin sensitivity. It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking differs between different obesity phenotypes. Likewise, we do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. By measuring muscle FFA storage into intramyocellular triglyceride, intramyocellular fatty acid trafficking, activation of the insulin signaling pathway and glucose disposal rates we will provide the first integrated examination ...
The goal of this study is to evaluate the presence of podocytes (special cells in the kidney that prevent protein loss) in the urine in patients with diabetes or glomerulonephritis (inflammation in the kidneys). Loss of podocyte in the urine may be an earlier sign of kidney injury (before protein loss) and the goal of this study is to evaluate the association between protein in the urine and podocytes in the urine.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM). It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking is responsible for the abnormal response to insulin. Likewise, we do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. We will measure muscle FFA storage into intramyocellular triglyceride, intramyocellular fatty acid trafficking, activation of the insulin signaling pathway and glucose disposal rates under both saline control (high overnight FFA) and after an overnight infusion of intravenous ...
Using stem cell derived intestinal epithelial cultures (enteroids) derived from obese (BMI> 30) patients and non-obese and metabolically normal patients (either post-bariatric surgery (BS) or BS-naïve with BMI < 25), dietary glucose absorption was measured. We identified that enteroids from obese patients were characterized by glucose hyper-absorption (~ 5 fold) compared to non-obese patients. Significant upregulation of major intestinal sugar transporters, including SGLT1, GLU2 and GLUT5 was responsible for hyper-absorptive phenotype and their pharmacologic inhibition significantly decreased glucose absorption. Importantly, we observed that enteroids from post-BS non-obese patients exhibited low dietary glucose absorption, indicating that altered glucose absorption ...
The purpose of this study is to evaluate the effectiveness and safety of brolucizumab vs. aflibercept in the treatment of patients with visual impairment due to diabetic macular edema (DME).
The purpose of this study is to determine if a blood test called "pancreatic polypeptide" can help distinguish between patients with diabetes mellitus with and without pancreatic cancer.
The purpose of this study is to create a prospective cohort of subjects with increased probability of being diagnosed with pancreatic cancer and then screen this cohort for pancreatic cancer
The purpose of this study is to develop a better blood test to diagnose early kidney injury in type 1 diabetes.
Although vitreous hemorrhage (VH) from proliferative diabetic retinopathy (PDR) can cause acute and dramatic vision loss for patients with diabetes, there is no current, evidence-based clinical guidance as to what treatment method is most likely to provide the best visual outcomes once intervention is desired. Intravitreous anti-vascular endothelial growth factor (anti-VEGF) therapy alone or vitrectomy combined with intraoperative PRP each provide the opportunity to stabilize or regress retinal neovascularization. However, clinical trials are lacking to elucidate the relative time frame of visual recovery or final visual outcome in prompt vitrectomy compared with initial anti-VEGF treatment. The Diabetic Retinopathy Clinical Research ...
The purpose of this study is to demonstrate feasibility of dynamic 11C-ER176 PET imaging to identify macrophage-driven immune dysregulation in gastric muscle of patients with DG. Non-invasive quantitative assessment with PET can significantly add to our diagnostic armamentarium for patients with diabetic gastroenteropathy.
What are the effects of transient insulin deprivation on brain structure, blood flow, mitochondrial function, and cognitive function in T1DM patients? What are the effects of transient insulin deprivation on circulating exosomes and metabolites in T1DM patients?
The purpose of this study is to evaluate the effects of multiple dose regimens of RM-131 on vomiting episodes, stomach emptying and stomach paralysis symptoms in patients with Type 1 and Type 2 diabetes and gastroparesis.
The purpose of this study is to demonstrate the safety and effectiveness of the Hybrid Closed Loop system (HCL) in adult and pediatric patients with type 1 diabetes in the home setting. A diverse population of patients with type 1 diabetes will be studied. The study population will have a large range for duration of diabetes and glycemic control, as measured by glycosylated hemoglobin (A1C). They will be enrolled in the study regardless of their prior diabetes regimen, including using Multiple Daily Injections (MDI), Continuous Subcutaneous Insulin Infusion (CSII) or Sensor-Augmented Pump therapy (SAP)
The purpose of this study is to identify novel genetic variants that predispose to Type 1 Diabetes.
The purpose of this study is to evaluate the safety of utilizing insulin lispro-aabc in the MiniMed™ 780G System to support product and system labeling.
The objective of the study is to assess the efficacy and safety of home use of a Control-to-Range (CTR) closed-loop (CL) system.
The purpose of this 3-month extension study (DCLP3 Extension) following a primary trial (DCLP3 or NCT03563313) to assess effectiveness and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The goal of this work is to identify an early stage biomarker for type 1 diabetes. In vitro evidence using rodent models has identified a significant enrichment of the chemokine CXCL10 in β-cell derived sEV upon exposure to diabetogenic pro-inflammatory cytokines. The aims of this project will focus on 1) testing protocols for efficient isolation of plasma-derived sEV from small volumes of sample, 2) development of an assay for the sensitive detection of CXCL10 in plasma-derived sEV, and 3) characterization of plasma-derived sEV through assessment of concentration, size, and content (proteomics). The study plans to include children that ...
The purpose of this study is to look at how participants' daily life is affected by their heart failure. The study will also look at the change in participants' body weight. This study will compare the effect of semaglutide (a new medicine) compared to "dummy" medicine on body weight and heart failure symptoms. Participants will either get semaglutide or "dummy" medicine, which treatment participants get is decided by chance. Participants will need to take 1 injection once a week.
This study aims to measure the percentage of time spent in hyperglycemia in patients on insulin therapy and evaluate diabetes related patient reported outcomes in kidney transplant recipients with type 2 diabetes. It also aimes to evaluate immunosuppression related patient reported outcomes in kidney transplant recipients with type 2 diabetes.
The purpose of this study is to assess key characteristics of bone quality, specifically material strength and porosity, in patients who have type 2 diabetes. These patients are at an unexplained increased risk for fractures and there is an urgent need to refine clinical assessment for this risk.
The objectives of this study are to evaluate the safety of IW-9179 in patients with diabetic gastroparesis (DGP) and the effect of treatment on the cardinal symptoms of DGP.
The purpose of this study is to understand why patients with indigestion, with or without diabetes, have gastrointestinal symptoms and, in particular, to understand where the symptoms are related to increased sensitivity to nutrients.Subsequently, look at the effects of Ondansetron on these patients' symptoms.
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics, and exploratory effectiveness of nimacimab in patients with diabetic gastroparesis.
The purpose of this study is to assess the safety and tolerability of intra-arterially delivered mesenchymal stem/stromal cells (MSC) to a single kidney in one of two fixed doses at two time points in patients with progressive diabetic kidney disease.
Diabetic kidney disease, also known as diabetic nephropathy, is the most common cause of chronic kidney disease and end-stage kidney failure requiring dialysis or kidney transplantation. Regenerative, cell-based therapy applying MSCs holds promise to delay the progression of kidney disease in individuals with diabetes mellitus. Our clinical trial will use MSCs processed from each study participant to test the ...
The purpose of this study is to prospectively assemble a cohort of subjects >50 and ≤85 years of age with New-onset Diabetes (NOD):
- Estimate the probability of pancreatic ductal adenocarcinoma (PDAC) in the NOD Cohort;
- Establish a biobank of clinically annotated biospecimens including a reference set of biospecimens from pre-symptomatic PDAC and control new-onset type 2 diabetes mellitus (DM) subjects;
- Facilitate validation of emerging tests for identifying NOD subjects at high risk for having PDAC using the reference set; and
- Provide a platform for development of an interventional protocol for early detection of sporadic PDAC ...
The purpose of this study is to evaluate whether or not semaglutide can slow down the growth and worsening of chronic kidney disease in people with type 2 diabetes. Participants will receive semaglutide (active medicine) or placebo ('dummy medicine'). This is known as participants' study medicine - which treatment participants get is decided by chance. Semaglutide is a medicine, doctors can prescribe in some countries for the treatment of type 2 diabetes. Participants will get the study medicine in a pen. Participants will use the pen to inject the medicine in a skin fold once a week. The study will close when ...
The study is being undertaken to understand how a gastric bypass can affect a subject's diabetes even prior to their losing significant amounts of weight. The hypothesis of this study is that increased glucagon-like peptide-1 (GLP-1) secretion explains the amelioration in insulin secretion after Roux-en-Y Gastric Bypass (RYGB) surgery.
The purpose of this study is to assess the effectiveness and safety of D-PLEX administered concomitantly over a period of 90 days (3 months)with the standard of care (SOC) IV prophylactic antibiotic treatment vs. SOC in prevention of post-cardiac surgery sternal infections.
The primary purpose of this study is to prospectively assess symptoms of bloating (severity, prevalence) in patients with diabetic gastroparesis.
The purpose of this study is to track the treatment burden experienced by patients living with Type 2 Diabetes Mellitus (T2DM) experience as they work to manage their illness in the context of social distancing measures.
To promote social distancing during the COVID-19 pandemic, health care institutions around the world have rapidly expanded their use of telemedicine to replace in-office appointments where possible.1 For patients with diabetes, who spend considerable time and energy engaging with various components of the health care system,2,3 this unexpected and abrupt transition to virtual health care may signal significant changes to ...
Assessment of glucose metabolism and liver fat after 12 week dietary intervention in pre diabetes subjects. Subjects will be randomized to either high fat (olive oil supplemented),high carb/high fiber (beans supplemented) and high carb/low fiber diets. Glucose metabolism will be assessed by labeled oral glucose tolerance test and liver fat by magnetic resonance spectroscopy pre randomization and at 8 and 12 week after starting dietary intervention.
To study the effect of an ileocolonic formulation of ox bile extract on insulin sensitivity, postprandial glycemia and incretin levels, gastric emptying, body weight and fasting serum FGF-19 (fibroblast growth factor) levels in overweight or obese type 2 diabetic subjects on therapy with DPP4 (dipeptidyl peptidase-4) inhibitors (e.g. sitagliptin) alone or in combination with metformin.
Diabetics are at risk for invasive pneumococcal infections and are more likely to have severe outcomes with infection compared to the general population. The pneumococcal (PPSV23) vaccination is recommended for all people with type 1 diabetes, but whether the vaccine is beneficial for this population has not been established. The purpose of this study is to determine if children with type 1 diabetes have adequate immune response to the PPSV23 vaccination and to assess factors affecting immune response through a pre and post vaccination blood sample.
The purpose of this study is to collect device data to assist in the development of a Personalized Closed Loop (PCL) system.
The purpose of this study is to demonstrate the performance of the Guardian™ Sensor (3) with an advanced algorithm in subjects age 2 - 80 years, for the span of 170 hours (7 days).
The objectives of this study are to evaluate the effectiveness and safety of PB in the treatment of patients with hereditary nephrogenic diabetes insipidus, to evaluate the effectiveness and safety of PB in polyuric patients with autosomal dominant polycystic kidney disease treated with tolvaptan, and to evaluate the effectiveness and safety of PB in polyuric patients previously treated with lithium.
The primary purpose of this study is to evaluate the impact of dapagliflozin, as compared with placebo, on heart failure, disease specific biomarkers, symptoms, health status and quality of life in patients with type 2 diabetes or prediabetes and chronic heart failure with preserved systolic function.
The purpose of this study is to look at the relationship of patient-centered education, the Electronic Medical Record (patient portal) and the use of digital photography to improve the practice of routine foot care and reduce the number of foot ulcers/wounds in patients with diabetes.
Diabetes mellitus is a common condition which is defined by persistently high blood sugar levels. This is a frequent problem that is most commonly due to type 2 diabetes. However, it is now recognized that a small portion of the population with diabetes have an underlying problem with their pancreas, such as chronic pancreatitis or pancreatic cancer, as the cause of their diabetes. Currently, there is no test to identify the small number of patients who have diabetes caused by a primary problem with their pancreas.
The goal of this study is to develop a test to distinguish these ...
A research study to enhance clinical discussion between patients and pharmacists using a shared decision making tool for type 2 diabetes or usual care.
While the potential clinical uses of pulsed electromagnetic field therapy (PEMF) are extensive, we are focusing on the potential benefits of PEMF on vascular health. We are targeting, the pre diabetic - metabolic syndrome population, a group with high prevalence in the American population. This population tends to be overweight, low fitness, high blood pressure, high triglycerides and borderline high blood glucose.
The purpose of this study is to compare incidence rates of complete hard-to-heal diabetic foot ulcer healing in Medicare beneficiaries following application of the 3C Patch® plus usual care (i.e., care consistent with the International Working Group on the Diabetic Foot guidelines), tested against a historical control group of similar patients that received usual care during a randomized controlled trial.
The purpose of this study is to evaluate the safety and efficacy of oral Pyridorin 300 mg BID in reducing the rate of progression of nephropathy due to type 2 diabetes mellitus.
The purpose of this study is to evaluate the effect of Aramchol as compared to placebo on NASH resolution, fibrosis improvement and clinical outcomes related to progression of liver disease (fibrosis stages 2-3 who are overweight or obese and have prediabetes or type 2 diabetes).
To compare the effect of senolytic drugs on cellular senescence, physical ability or frailty, and adipose tissue-derived MSC functionality in patients with chronic kidney disease. Primary Objectives: To assess the efficacy of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on clearing senescent adipose-derived MSC in patients with CKD. To assess the efficacy of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on improving adipose-derived MSC functionality in patients with CKD. Secondary Objective: To assess the short-term effect of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on ...
This protocol is being conducted to determine the mechanisms responsible for insulin resistance, obesity and type 2 diabetes.
The purpose of this study is to assess the effects of a nighttime rise in cortisol on the body's glucose production in type 2 diabetes.
As the global epidemic of obesity and diabetes mellitus spreads, an exponential rise in incident chronic kidney disease (CKD) complicated by end stage renal disease (ESRD) is predicted, leaving healthcare systems overwhelmed worldwide. Hence, there is urgent need for novel therapies to slow the progression of DKD and optimize the health of this patient population. The purpose of this study is to examine the effect of a supplement on mesenchymal stem cells, physical body function (or frailty), kidney function, and total clearance of senescent cells in individuals with CKD. At present, we are enrolling participants with CKD, with a subset ...
The goal of this study is to evaluate a new format for delivery of a culturally tailored digital storytelling intervention by incorporating a facilitated group discussion following the videos, for management of type II diabetes in Latino communities.
The purpose of this study is to evaluate the ability of appropriately-trained family physicians to screen for and identify Diabetic Retinopathy using retinal camera and, secondarily, to describe patients’ perception of the convenience and cost-effectiveness of retinal imaging.
The purpose of this study is to develop a blood test to distinguish various causes of diabetes by evaluating patients who have developed diabetes within the last 3 years, but we will also enroll a small number of patients with long-term diabetes and normal blood sugars for comparison.
Diabetes mellitus is a common condition which is defined by persistently high blood sugar levels. This is a frequent problem that is most commonly due to type 2 diabetes. However, it is now recognized that a small portion of the population with diabetes have an underlying problem with their pancreas, such as ...
The primary purpose of this study is to evaluate the impact of dapagliflozin, as compared with placebo, on heart failure disease-specific biomarkers, symptoms, health status, and quality of life in patients who have type 2 diabetes and chronic heart failure with reduced systolic function.
Hypothesis: We hypothesize that patients from the Family Medicine Department at Mayo Clinic Florida who participate in RPM will have significantly reduced emergency room visits, hospitalizations, and hospital contacts.
Aims, purpose, or objectives: In this study, we will compare the RPM group to a control group that does not receive RPM. The primary objective is to determine if there are significant group differences in emergency room visits, hospitalizations, outpatient primary care visits, outpatient specialty care visits, and hospital contacts (inbound patient portal messages and phone calls). The secondary objective is to determine if there are ...
The purpose of this research is to determine if CGM (continuous glucose monitors) used in the hospital in patients with COVID-19 and diabetes treated with insulin will be as accurate as POC (point of care) glucose monitors. Also if found to be accurate, CGM reading data will be used together with POC glucometers to dose insulin therapy.
The purpose of this study is to evaluate the effect of fenofibrate compared with placebo for prevention of diabetic retinopathy (DR) worsening or center-involved diabetic macular edema (CI-DME) with vision loss through 4 years of follow-up in participants with mild to moderately severe non-proliferative DR (NPDR) and no CI-DME at baseline.
The purpose of this study is gain the adolescent perspective on living with type 1 diabetes.
The purpose of this study is to use multiple devices to measure blood sugar changes and the reasons for these changes in healthy and diabetic children.
The purpose of this study is to assess painful diabetic peripheral neuropathy after high-frequency spinal cord stimulation.
The purpose of this study is to see if there is a connection between bad experiences in the patient's childhood, either by the patient or the parent, and poor blood sugar control, obesity, poor blood lipid levels, and depression in patients with type 1 diabetes.
The purpose of this study is to examine the evolution of diabetic kindey injury over an extended period in a group of subjects who previously completed a clinical trial which assessed the ability of losartan to protect the kidney from injury in early diabetic kidney disease. We will also explore the relationship between diabetic kidney disease and other diabetes complications, including neuropathy and retinopathy.
The objectives of this study are to determine if the 1-year graft success rate following DMEK performed with corneas from donors without diabetes is superior to the graft success rate with cornea donors with diabetes, to determine if the 1-year central endothelial cell loss (ECL) following DMEK performed with corneas from donors without diabetes is superior to the central ECL when corneas from donors with diabetes are used, nd to explore the relationship of severity of diabetes in the donor, as measured by eye bank-determined diabetes risk categorization scores, post-mortem hemoglobin A1c (HbA1c), and skin advanced glycation endproducts (AGE) and ...
The purpose of this study is to understand the day-to-day variability in stomach emptying and gastrointestinal (GI) transit in patients with digestive symptoms. This information will be useful for interpreting the results of stomach emptying studies in future.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
The purpose of this study is to determine whether short-term treatment with Fisetin reduces the rate of death and long term complications related to COVID-19.
This study (SE2030) will establish a platform of data to build the perfect stress echo test, suitable for all patients, anywhere, anytime, also quantitative and operator independent.

Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 November 2023
Label-free quantitative proteomics analysis for type 2 diabetes mellitus early diagnostic marker discovery using data-independent acquisition mass spectrometry (DIA-MS)
- Refat M. Nimer 1 ,
- Mahmoud A. Alfaqih 2 , 3 ,
- Eman R. Shehabat 1 ,
- Muhammad Mujammami 4 , 5 &
- Anas M. Abdel Rahman 6
Scientific Reports volume 13 , Article number: 20880 ( 2023 ) Cite this article
1330 Accesses
2 Altmetric
Metrics details
- Biochemistry
Type-2 diabetes mellitus (T2DM) therapy requires early diagnosis and complication avoidance. Unfortunately, current diagnostic markers do not meet these needs. Data-independent acquisition mass spectrometry (DIA-MS) offers a solution for clinical diagnosis, providing reliable and precise sample quantification. This study utilized DIA-MS to investigate proteomic differential expression in the serum of recently diagnosed T2DM patients. The study conducted a comparative protein expression analysis between healthy and recently diagnosed T2DM groups (discovery cohort). A candidate protein was then validated using enzyme-linked immune assay (ELISA) on serum samples collected from T2DM patients (n = 87) and healthy control (n = 60) (validation cohort). A total of 1074 proteins were identified, and 90 were significantly dysregulated between the two groups, including 32 newly associated with T2DM. Among these proteins, the expression of S100 calcium-binding protein A6 (S100A6) was validated by ELISA. It showed a significant increase in T2DM samples compared to the control group. It was evaluated as a biomarker using the receiver operating characteristic (ROC) curve, consistent with the DIA-MS results. Novel proteins are reported to be involved in the development and progression of T2DM. Further studies are required to investigate the differential expression of candidate marker proteins in a larger population of T2DM patients.
Similar content being viewed by others
Proteomic signatures for identification of impaired glucose tolerance
Pre-diagnostic biomarkers of type 2 diabetes identified in the UAE’s obese national population using targeted metabolomics
Quantifiable peptide library bridges the gap for proteomics based biomarker discovery and validation on breast cancer
Introduction.
The current opinion refers to Diabetes mellitus (DM) as a range of metabolic diseases characterized by elevated blood glucose levels. DM is a disease of pandemic proportions, and despite worldwide measures to control DM, disease prevalence is still rising. For example, recent estimates published by the International Diabetes Federation demonstrated that the number of individuals with DM is predicted to increase from 537 million in 2021 to 738 million in 2045 1 .
The classification of DM categorizes the disease into type-1 DM (T1DM), caused by the near complete absence of blood insulin, and type-2 DM (T2DM), predominantly affecting obese adults. Although hereditary factors appear to play a stronger role in T2DM etiology than T1DM, recent data demonstrate that the cause of T2DM is complex and multifactorial, with a range of presenting phenotypes 2 .
The complexity of DM could be attributed to several factors. Initially, although an increase in blood glucose is characteristic of all phenotypic presentations of T2DM, it is generally accepted that patients with T2DM also have dysregulation in their lipid and protein metabolism 3 , 4 . Furthermore, patients with T2DM could have normal blood insulin levels, low blood insulin levels, or insulin resistance accompanied by elevated blood insulin 5 . Moreover, in the late stages of disease progression, beta cell failure could lead to insulin resistance and reduced blood insulin levels. These hormonal and metabolic cues lead to a wide spectrum of disease presentation and phenotype variation, complicating clinical decision-making 6 .
Despite rapid and significant development in DM research over the last few decades, several clinical problems remain to be addressed, especially in biomarker discovery 7 . For instance, currently, available biomarkers do not provide enough power for the early diagnosis or the identification of seemingly healthy individuals at a higher risk of disease development in the future. Moreover, current research indicates several areas for improvement over relying solely on glucose measurements for clinical decision-making in patients with T2DM. Most importantly, using glucose readings alone does not fit well with personalized medicine, where using an algorithm of several variables is more powerful than one 7 .
Considering the above discussion, using an “omics” analytic platform that allows for analyzing many markers could be useful to mend the gaps mentioned above in DM biomarker discovery. In this context, mass-spectrometry (MS) based proteomics is a strong candidate for DM biomarker discovery since it allows for biomarker identification, including the precise and reproducible quantification of their levels in different biological niches 8 . Traditional specific experimental approaches in bottom-up proteomics include data-dependent acquisition (DDA) and data-independent acquisition (DIA) 9 . In a typical DDA-MS experiment, all precursor peptide ions are scanned during the survey scan (MS1) before a predetermined number of precursor ions are selected for further fragmentation (MS2) 10 . While DIA-MS relies on accumulating fragment ions in a defined number of broad isolation windows covering the whole mass-to-charge ratio (m/z) range, allowing for a more comprehensive sample analysis 11 .
DIA-MS analysis is often used due to its high depth of analysis, which yields consistent quantification and extensive proteome coverage 7 , 12 , 13 . However, limited studies have used the DIA-MS method for T2DM biomarker discovery in serum samples 4 , 14 , 15 .
This investigation utilized the DIA-MS approach to identify and quantitate serum proteins differentially expressed in recently diagnosed T2DM compared with healthy individuals. The findings on how the identified markers highlight differences in biological pathways and processes between the two groups are further discussed.
Materials and methods
Clinical sample collection and preparation.
Before recruiting patients, the study was approved by King Abdullah University Hospital (KAUH) Institutional Review Board (IRB) (Ref.:9/123/2019). All methods were performed following the KAUH guidelines and regulations. A written informed consent according to the Declaration of Helsinki and institutional approval was obtained from all participants involved in this study. Fasting blood samples were collected from patients recently diagnosed with T2DM (< 3 years) ( n = 87) who attended the endocrinology and diabetes clinics at KAUH, a tertiary hospital located in the Northern part of Jordan. All patients with T2DM had been diagnosed according to American Diabetes Association guidelines. Patients diagnosed with chronic diseases other than T2DM or with major complications of diabetes were excluded from this study. The Control group involved sixty non-diabetic subjects who were volunteers from Jordan University of Science and Technology (JUST) and their relatives. The age, gender, body mass index (BMI), and ethnic background were matched between the control and T2DM groups, as summarized in Table 1 .
10–12 h following an overnight fast, a venous blood sample was collected from each participant, placed in plain tubes, centrifuged at 3000 × g for 10 min, and the serum stored at – 80 °C until further analysis.
Biochemical measurements
Fasting Blood glucose, total cholesterol, triglycerides, High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), and HbA1c were all measured using a chemical analyzer (Roche Diagnostics, Mannheim, Germany) (Table 1 ).
Protein extraction
Serum samples from the T2DM ( n = 7) and control ( n = 7) groups (discovery cohort) matched with age, gender, and BMI were used for DIA-MS analysis. SDS-free lysate buffer (7 M urea, 2 M thiourea, and 20 mM Tris–HCl pH) (BGI, China) was added to 100 μL serum sample, and finally, to make up a total volume of 1 mL. The lysate was centrifuged, and the supernatant was collected for protein quantification using a Bradford assay 16 . Quality control of protein extraction and quantification was confirmed by SDS-PAGE (Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis) (Supplementary Fig. S1 ).
The reducing agent dithiothreitol (Amresco, Solon, OH, USA) was added to a final concentration of 10 mM and incubated at 37 °C for 30 min, followed by alkylation using iodoacetamide (Sigma, St. Louis, MO, USA) at a final concentration of 55 mM in the dark at room temperature for 45 min. Finally, the mixture was centrifuged at 25,000 g for 20 min at 4 °C. The mixture of proteins would be passed through a solid phase extraction (SPE) C18s (Agela Technologies, China) column for protein enrichment. Finally, 75% ACN was used to elute lower-abundance proteins 17 .
In-solution protein tryptic digestion
Enzymatic hydrolysis of proteins in solution was performed by mixing 100 µg of proteins with 50 mM NH 4 HCO 3 by 4 times volumes. A 2.5 μg trypsin (Hualishi Scientific, China) at a 40:1 ratio was added to samples and then incubated for 4 h at 37 °C. Finally, the resulting peptides were desalted with a Strata × column (Phenomenex, USA) and vacuumed till dryness.
DDA and DIA analysis by nano-LC–MS/MS
The dried peptide samples were reconstituted with mobile phase A (2% ACN, 0.1% FA), centrifuged at 20,000 g for 10 min, and the supernatant was taken for injection. Separation was carried out by a nano C18 column (150 μm internal diameter, 1.8 μm particle size, 35 cm column length) coupled in Thermo UltiMate 3000 UHPLC liquid chromatograph (Thermo Scientific, USA) at a flow rate of 500 nL/min by the following effective gradient: 0–5 min, 5% mobile phase B (98% ACN, 0.1% FA); 5–120 min, mobile phase B linearly increased from 5 to 25%; 120–160 min, mobile phase B rose from 25 to 35%; 160–170 min, mobile phase B rose from 35 to 80%; 170–175 min, 80% mobile phase B; 175–180 min, 5% mobile phase B. The nanoliter liquid phase separation end was directly connected to the mass spectrometer in the following settings.
For DDA (data-dependent acquisition) analysis, LC-separated peptides were ionized by nanoESI. They injected into tandem mass spectrometer Q-Exactive HF X (Thermo Fisher Scientific, San Jose, CA) with DDA detection mode. The main settings were ion source voltage 1.9 kV; MS scan range 350–1500 m/z; MS resolution 120,000, maximal injection time (MIT) 100ms; MS/MS collision type HCD, collision energy NCE 28; MS/MS resolution 30,000, MIT 100ms, dynamic exclusion duration 30 s. The start m/z for MS/MS was fixed to 100. Precursor for MS/MS scan satisfied: charge range 2+ to 6+, top 20 precursors with intensity over 5E4.AGC was: MS 3E6, MS/MS 1E 5 .
For DIA (data independent analysis), the main settings were ion source voltage 1.9 kV; MS scan range 400–1250 m/z; MS resolution 120,000, MIT 50 ms; 400–1250 m/z was equally divided to 45 continuous windows MS/MS scan. MS/MS collision type HCD, MIT was auto mode. Fragment ions were scanned in Orbitrap, MS/MS resolution 30,000. The collision energy was distributed mode: 22.5, 25, 27.5, AGC was 1E 6 .
Data analysis
The DDA sample data generated by the Q Exactive HF mass spectrometer was processed using the MaxQuant software (v. 1.5.3.30, Max Planck Institutes, GER), incorporating the Andromeda search engine. This enabled us to analyze and identify the spectra. To generate a spectral library, we utilized Spectronaut software (v. 13.12.200217.43655, Biogonosys, USA) in conjunction with the processed data 18 , 19 .
Several parameters were employed during the MaxQuant data analysis. The enzyme used for digestion was trypsin, and peptides with a minimum length of 7 amino acids were considered. To ensure accurate identification, a minimum of 1 unique peptide was required. The false discovery rate (FDR) at the peptide-spectrum match (PSM) level and protein level was set at 0.01. Additionally, fixed modifications included carbamidomethyl (cysteine), while variable modifications encompassed oxidation (methionine) and acetylation (protein N-terminus). The database used for protein sequence matching was UniProt homo_ (172419 sequences). The protein sequences retrieved were obtained from the UniProt database, accessible at https://www.uniprot.org/ . To ensure accurate retention time calibration of the DIA data, iRT peptides (Biognosys, Switzerland) were utilized. Subsequently, employing the target-decoy model for Sequential Window Acquisition of all Theoretical Mass Spectra (SWATH)-MS, a false positive control was implemented with a 1% false discovery rate (FDR), yielding reliable quantitative outcomes. The subsequent steps encompassed protein quantification, data preprocessing, and significant differential analysis, which were carried out using MSstate software 20 .
The differential analysis relied on a linear mixed-effect model to calculate fold change values. The data was preprocessed according to the predefined comparison group, and significance testing was conducted based on the established model. Following this, differential protein screening was performed using a fold change threshold of > 1.5 and a significance criterion of a P value < 0.05. These parameters were employed to identify proteins displaying significant differences in expression levels.
In order to determine potential biomarkers multivariate analysis, partial least-square discrimination analysis (PLS-DA) was conducted in MetaboAnalyst Software V5 (Montreal, QC, Canada) ( http://www.metaboanalyst.ca ). Additionally, potential biomarkers were evaluated by performing the receiver operating characteristic ROC curve analysis.
The functional classification of differentially expressed proteins (DEPs) and functional enrichment analysis were performed using Gene Ontology (GO) ( http://www.geneontology.org ) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases ( http://www.genome.jp/kegg/ ), respectively. Moreover, EuKaryotic Orthologous Groups (KOG) were applied to classify protein orthologs. Protein–protein interaction (PPI) and subcellular localization analysis of the DEPs were performed using the Search Tool for the Retrieval of Interacting Genes (STRING) v11.5 database ( https://string-db.org/ ) and Blast2go software ( www.blast2go.com ), respectively 21 , 22 .
Enzyme-linked immunosorbent assay (ELISA)
A protein S100A6 identified by LC–MS/MS was selected for further analysis by ELISA.
The levels of S100A6 in blood samples from the validation cohort group (control group, n = 60; and the T2DM group, n = 87) were determined quantitatively using the S100A6 ELISA Kit (CSB-E13089h, Cusabio, PRC); Cambridge, UK), following the instructions provided by the manufacturer.
Statistical analysis
The student’s t-test was used to compare the two groups and establish the statistical significance of the results. The threshold for statistical significance was set at P 0.05. The receiver operating characteristic curves (ROC) were generated using GraphPad Prism program v 8.0.
Characteristics of patients and healthy control (HC)
The baseline characteristics of the study group are presented in Table 1 . The mean age of the T2DM and HC groups was 52.1 ± 9.4, and 51.1 ± 9.7 years, respectively. The two study groups had no significant differences in age, BMI, and total cholesterol ( P > 0.05). However, LDL cholesterol and HDL cholesterol were significantly ( P < 0.05) higher in HC (3.4 ± 0.9 and 1.6 ± 0.5, respectively) than T2DM group (2.4 ± 1.0 and 1.2 ± 0.3, respectively).
As expected, FBS and HbA1c were significantly higher in patients with T2DM (8.0 ± 3.4 and 7.5 ± 1.2, respectively) than HC group (5.2 ± 0.4 and 5.2 ± 0.3, respectively) ( P < 0.05). Moreover, triglyceride was significantly ( P < 0.05) higher in T2DM (2.5 ± 1.3) than in the HC group (1.8 ± 0.9).
Identification of differentially expressed proteins in T2DM compared with normal serum
In this project, Q-Exactive HF X (Thermo Fisher Scientific, San Jose, CA) was used to acquire mass spectrometry (MS) data for 14 samples (7 patients and 7 controls) in DIA mode, 1074 proteins were identified, of which 90 DEPs were detected. Supplementary Table S1 shows 41 DEPs were upregulated, and 49 DEPs were downregulated in the serum from the T2DM group compared with the HC group.
A PLS-DA model was constructed to investigate and analyze the separation of the T2DM and healthy groups. As shown in Fig. 1 A, the T2DM and healthy groups were separated. The first and second principal components (PC1 and PC2) explained 39.4 and 9.1% of the variation in samples in the PLS-DA score plot.
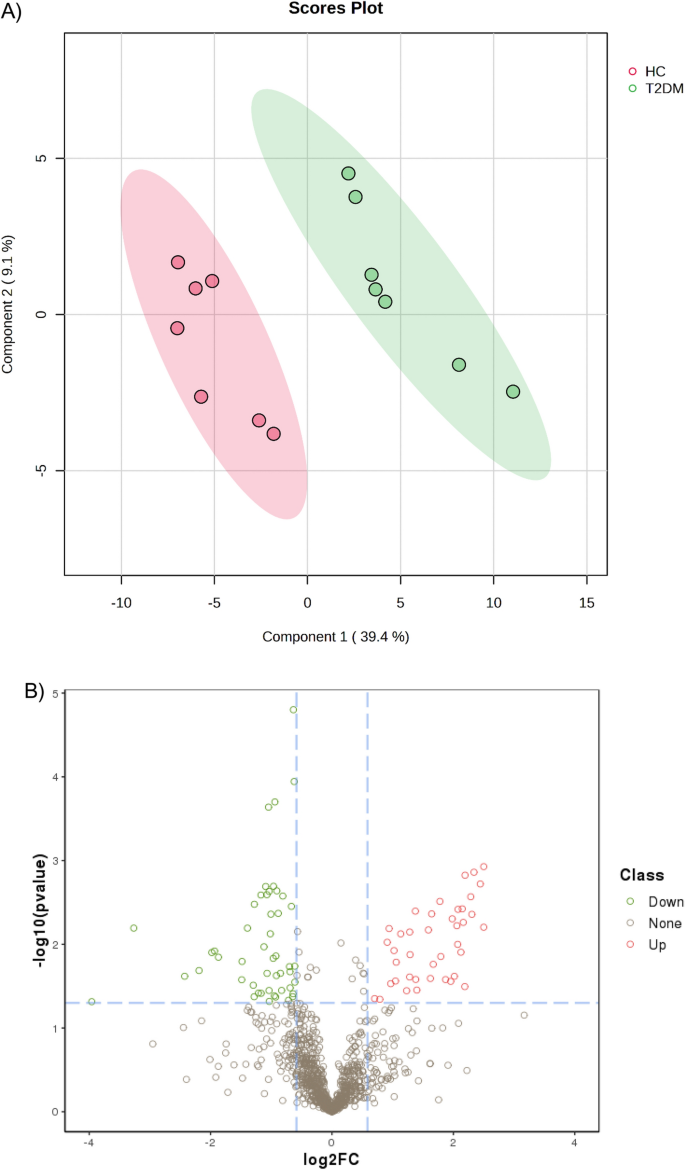
( A ) Partial Least-Squares Discriminant Analysis (PLS-DA) of T2DM and healthy serum proteomics data. ( B ) Volcano plot of differentially expressed proteins (DEPs) in serum of patients with T2DM and control. In this volcano plot, red dots represent proteins with a significant fold change (FC) > 1.5; green dots proteins with a significant FC < 0.67; grey dots proteins with no significant change.
The volcano plot in Fig. 1 B depicted differential abundances (T2DM versus control), with the log2 ratio on the x-axis representing the fold change and the − log10 (p-value) on the y-axis depicting significance. A horizontal line represents the position of a P -value of 0.05, and the positions of the upper right (Fold change > 1.5) and upper left (fold change < 0.67) are represented by two vertical lines. The red and green dots indicate up-regulated and down-regulated proteins, respectively. Ninety serum proteins were significantly altered, with 41 being upregulated and 49 being downregulated.
Evaluation of biomarkers between T2DM and HC
Based on the significant DEPs in the T2DM and HC groups, a multivariate exploratory ROC analysis was conducted utilizing PLS-DA as a classifier and feature ranker (Fig. 2 A). For the most important 10 proteins (alpha-2-HS-glycoprotein, epididymis luminal protein 213, Ig heavy chain variable region, anti-thrombopoietin receptor single-chain variable fragment, IBM-A1 heavy chain variable region, glutaminyl-peptide cyclotransferase, 10E8 heavy chain variable region, olfactory receptor 4D6, alpha-globin, and leucine-rich repeat-containing protein 4C) the AUC of the exploratory ROC curve was 0.99 (Fig. 2 B).
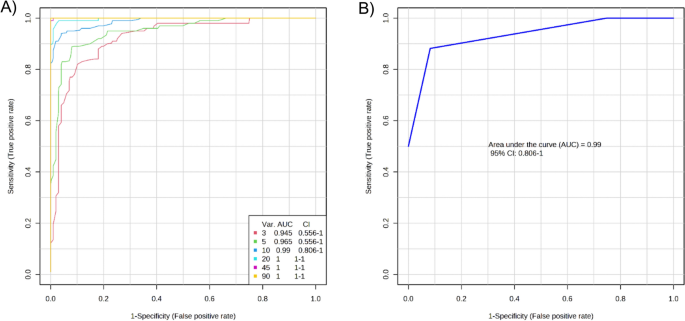
Biomarker prediction by Multivariate ROC curve based exploratory analysis. ( A ) An Overview of all ROC curves created by MetaboAnalyst 5.0 from 6 different biomarker models considering the different number of features (3, 5,10, 20, 45, and 90) with their corresponding AUC value and confidence interval. ( B ) ROC curve for selected biomarker model 3.
Functional classification and annotation of DEPs
Go enrichment analysis.
A GO annotation study was carried out using the Blast2GO software to determine the functional significance of all identified proteins in the serum of T2DM. Figure 3 A includes protein information and results visualization. The most enriched biological processes (out of 28 GO terms) were: ‘cellular process,’ ‘biological regulation,’ ‘response to stimulus,’ ‘metabolic process,’ and ‘regulation of the biological process,’ and. The most enriched cell components (out of 17 GO terms) were: ‘organelle,’ ‘cell,’ and ‘cell part.’ The most enriched molecular functions (out of 12 GO items) were: ‘binding,’ ‘catalytic activity, and ‘molecular function regulator.’
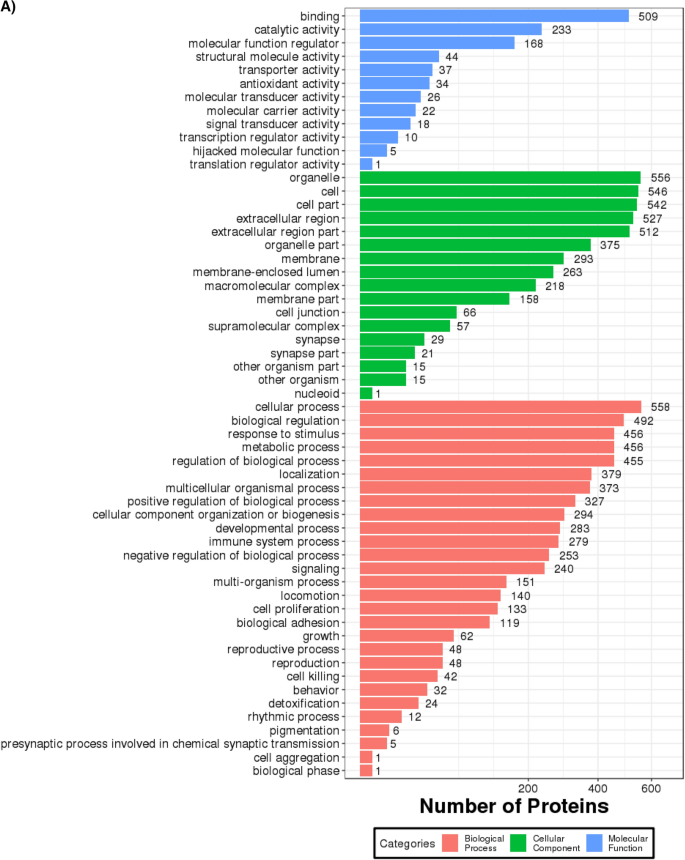
( A ) Functional GO classification of all the identified serum proteins in T2DM. ( B ) Differential protein function classification. The X-axis represents the number of differential proteins, and the Y-axis represents the GO annotation entry. ( C ) Up or down-regulation of differential proteins in GO function classification. The X-axis represents the GO annotation entry, and the Y-axis represents the number of differential proteins with up or downregulation.
Next, DEPs were subjected to GO enrichment analysis (Fig. 3 B). GO analysis and annotation classified DEPs into biological processes, cellular components, and molecular functions (Fig. 3 B). Cells, parts, and organelles were the most abundant in the cellular component category (Fig. 3 B). The top 5 biological processes were cellular process, biological regulation, regulation of the biological process, response to stimulus, and metabolic process (Fig. 3 B). The top 5 molecular functions were binding, catalytic activity, carrier activity, antioxidant activity, and molecular function regulator (Fig. 3 B).
The GO functional annotation results of the DEPs between patients of T2DM and control are shown in Fig. 3 C. For the cellular component domain, 56 DEPs were mainly concentrated in the cell part, among which the top upregulated and downregulated proteins were hemoglobin subunit alpha, and hemoglobin beta chain, respectively. For the molecular function domain, 51 DEPs were mainly in binding; among the top, upregulated proteins were the S100A6 protein. For the biological process domain, the DEPS were primarily involved in cellular processes (53 DEPs) and biological regulation (30 DEPs), and notably, the S100A6 protein was among the most upregulated proteins.
KOG annotation of DEPs
For DEPs, their annotated KOG terms were extracted and represented as bar plots in Fig. 4 . According to the KOG study, most annotated DEPs may impact “cell processes and signaling.” In this regard, most DEPs were associated with post-translational modification, protein turnover, chaperone activity, cytoskeleton, and signal transduction mechanisms.
KOG annotation of DEPs. X-axis displays the DEPs count; Y-axis displays KOG terms.
Pathway enrichment analysis of DEPs
It is possible to get a deeper understanding of the biological roles of the DEPs via analyses based on metabolic pathways. Therefore, in the present study, the KEGG pathway annotated the DEPs identified in the serum samples from patients with T2DM and control.
The KEGG-enriched pathways DEPs up- and down-regulated clusters are presented in Fig. 3 . The most enriched pathways were as follows: infectious diseases (32), immune system (26), transport and catabolism (22), and signal transduction (19) (Fig. 5 ).
Differential protein pathway classification. The X-axis represents the number of differential proteins, and the Y-axis represents the pathway annotation entry.
Subcellular localization and protein–protein interaction network analyses of DEPs
Analysis of subcellular localization of the identified DEPs using WoLF PSORT software ( https://wolfpsort.hgc.jp/ ) 23 showed that most proteins (32) were localized in the cytoplasm. The next most prominent localization was extracellular proteins (22), followed by the nucleus (19) (Fig. 6 ).
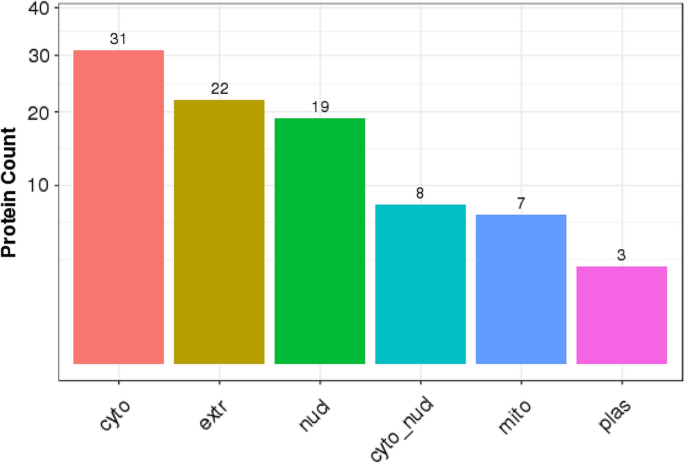
Bar chart of subcellular localization. X-axis represents the subcellular structure term; Y-axis represents protein count.
DEPs were widely distributed in the nucleus, cytoplasm, plasma membrane, and extracellular space (Fig. 6 ).
To better understand the possible protein–protein interaction (PPI) of the DEPs, we performed PPI proteomics network analysis utilizing the STRING) database (Fig. 7 ). The PPI network was produced when the median confidence level of 0.4 was used.
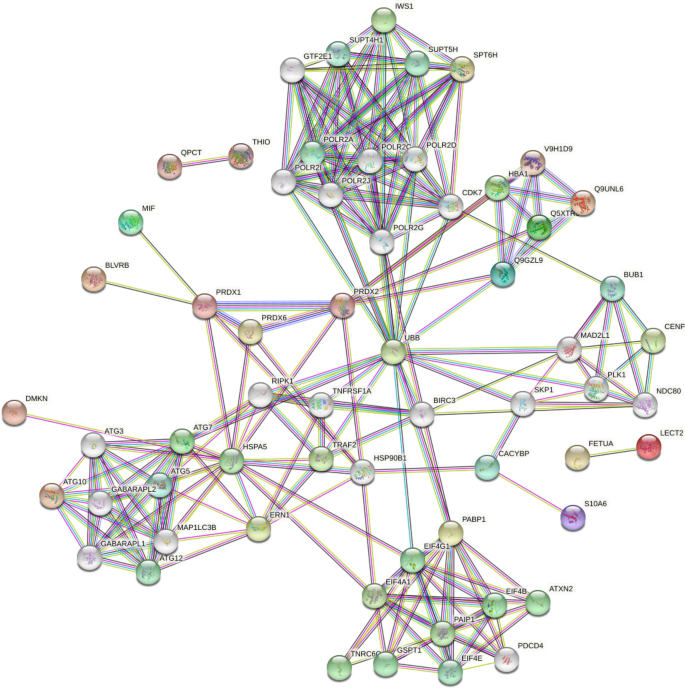
Protein–Protein Interaction (PPI) network of DEPs. Colored balls represent individual proteins, while lines show interactions between proteins.
Serum levels of S100A6 by validation experiment
To validate the DEPs in the LC–MS/MS experiment, S100A6 was selected for validation by ELISA due to the novelty of this candidate biomarker identified during proteomics screening.
Our data also showed a significant increase in the S100A6 levels in the T2DM group compared to the control group (Fig. 8 A). Therefore, the result is consistent with the LC–MS/MS experiment data. Furthermore, sensitivity and specificity for S100A6 as a biomarker were accessed by receiver operating characteristic (ROC) analysis. The area under the curve (AUC) for S100A6 (AUC = 0.7487, 95% confidence interval (CI): 0.6668 to 0.8305, P < 0.0001) (Fig. 8 B).
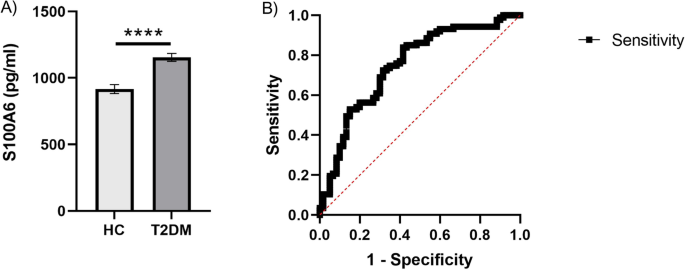
( A ) S100A6 protein concentration in serum. The horizontal axis represents the control group (n = 60) and the T2DM group (n = 87). The y-axis indicates the concentration of S100A6. Values are shown as the mean ± SEM; * P < 0.05. ( B ) ROC curve analysis of validated differentially expressed protein (S100A6). ROC‑AUC of S100A6 was 0.7487 (0.6668 to 0.8305, P < 0.0001).
Molecular biomarkers for T2DM are still desperately needed because of the multifactorial and multigenetic nature of the disease, as well as several clinical challenges that remain to be solved. Finding novel diagnostic biomarkers with the use of mass spectrometry (MS)-based proteomics could provide a solution to the clinical issues. In the present study, we aimed to demonstrate alterations in the serum proteome in patients recently diagnosed with T2DM using DIA-MS-based proteomics.
Several proteomic studies have been conducted in the context of T2DM research 24 , 25 . However, very few studies use DIA-MS to comprehensively analyze the proteome in the serum of T2DM patients 4 .
We identified a significant decrease in the level of abundance of 49 proteins in the serum of T2DM compared to the healthy group, while 41 proteins were increased significantly. Among 49 downregulated proteins, 15 proteins (Centrosomal protein of 290 kDa, zinc finger BED domain-containing protein 1, polyadenylate-binding protein 1, centromere protein F, 40S ribosomal protein S8, defensin-5, leiomodin-3, glutaminyl-peptide cyclotransferase, beta-taxilin, secretoglobin family 3A member 1, ubiquitin-like-conjugating enzyme ATG10, zinc finger SWIM domain-containing protein 6, protein kinase C and casein kinase substrate in neurons protein 2, integral membrane protein 2B, and UHRF1-binding protein 1) have not been previously reported in direct association with T2DM. Whereas in up-regulated proteins, 17 proteins (NEDD4-binding protein 3, serine/threonine-protein kinase/endoribonuclease IRE1, hemoglobin subunit delta, protein S100-A6, polyubiquitin-B, protein S100-A4, D-dopachrome decarboxylase, neurotensin/neuromedin N, chronic lymphocytic leukemia up-regulated 1 opposite strand Dermokine, Coiled-coil domain-containing protein 80, Transcription elongation factor SPT6, Olfactory receptor 4D6, Beta-globin Showa Yakushiji variant, Hemoglobin subunit delta, Beta-globin, Hemoglobin delta-beta fusion protein, and HCG1745306, isoform CRA_a) were considered novel observation in the context of T2DM.
Some DEPs, such as peroxiredoxin isoforms (1, 2, and 6), natriuretic peptides A, and complement protein C4B were previously studied in patients with T2DM 26 , 27 , 28 . Moreover, various isozymes of carbonic anhydrase play a role in T2DM, which fits well with our findings 29 .
Proteomic analysis has shown upregulated macrophage migration inhibitory factor (MIF) expression levels in T2DM patients. MIF is an inflammatory cytokine derived from T-cells 30 . Several studies have demonstrated that serum levels of MIF were elevated in T2DM and its complications 31 , 32 .
Furthermore, thioredoxin was upregulated in the serum of patients with T2DM. Thioredoxin is a marker of oxidative stress, and glucose intolerance was associated with high levels of thioredoxin 33 .
One of our findings was that the immunological class of proteins was extensively represented and significantly up or down-regulated in T2DM. Most of these proteins include the Ig heavy and light chains (κ, λ). Since immunity may contribute to T2DM pathogenesis 34 and, on the other hand, reduction of immunity is one of the principal consequences of T2DM, the function of immunity in T2DM is heterogeneous 35 .
Furthermore, Hb chains (Hb subunits α, β, γ, and δ) were upregulated in T2DM compared with the control. Only the Hb subunit α had been previously identified as a biomarker for T2DM among the aforementioned Hb-associated proteins 36 .
Analysis of subcellular locations showed that the DEPs were mainly in the cytoplasm, extracellular, and nucleus. As expected, the proteins that have a role in the pathogenesis of T2DM are localized primarily in the nucleus and cytoplasm 37 . Additionally, the extracellular localization is consistent with that increased extracellular matrix (ECM) protein synthesis is a hallmark of all long-term diabetes problems 38 .
Among these, an interesting protein identified in our study was the S100A6 protein. It belongs to the S100 family of calcium-binding proteins, which function in Ca2+-dependent protein–protein interactions (PPIs) that mediate intracellular and intercellular cellular regulation 39 . However, the actual biological or cellular function of S100A6 is not fully known, and conflicting functions have been hypothesized 40 .
The direct association of circulatory S100A6 protein with T2DM was not reported before in the literature to the best of our knowledge. Therefore, the S100A6 protein was examined by ELISA in a relatively larger sample size from the same sample setting to verify the LC–MS/MS findings. The findings revealed a significant association between increased S100A6 protein and T2DM. According to the ROC analysis, S100A6 can serve as a potential diagnostic biomarker for T2DM.
The experiments at the genome level showed that high glucose-stimulated S100A6 transcription by binding of c-Myc to its promoter to control S100A6 expression directly 41 . Using cumulus cells from mice, Jiang et al. found that S100A6 was significantly increased in the induced diabetes animals compared to the control group 42 . The S100A6 is a hepatocyte that contributes to hepato-pancreatic communication to reduce insulin production and promote the progression of T2DM in individuals with nonalcoholic fatty liver disease 43 . S100-A6 may be involved in the signal transduction of Ca2+-induced insulin release from pancreatic B cells, suggesting that it plays a role in the insulin release process 44 .
Despite the small number of patients, the study's strength lies in the fact our study is one of the few papers to identify proteomics changes in the serum of recently diagnosed T2DM using DIA-MS. Additionally, some DEPs may potentially elucidate underlying disease mechanisms' diagnostic and prognostic indicators; however, no further verification study was undertaken to verify the link between clinical features and the proteomics data, which should be clarified for the development and implementation of clinical practice. Notably, the other novel proteins will be investigated in the future using a high sample size. Certainly, our results represent the starting point for more in‐depth studies.
In conclusion, we applied a proteomic technique with label-free DIA-MS analysis to find alterations in protein profiles in the serum of recently diagnosed T2DM patients and healthy controls. We found proteins whose expression levels were altered in the serum of recently diagnosed T2DM patients compared to HCs. To our knowledge, our data showed the overexpression levels of S100A6 protein in the serum of recently diagnosed T2DM patients for the first time. This suggests that S100A6 may have a critical role in the pathogenesis of T2DM. Furthermore, our results revealed other DEPs identified herein had not previously been reported in T2DM, such as NEDD4-binding protein 3, neurotensin/neuromedin N, and beta-taxilin. As a result, this proteomics study provides reference proteins for future research on T2DM and may help develop treatments and meet the goals in early diagnosis and complication avoidance of T2DM. However, these findings need to be further studied and validated in future studies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author, Refat Nimer, on reasonable request.
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 157 , 107843 (2019).
Article PubMed Google Scholar
Philipson, L. H. Harnessing heterogeneity in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 16 , 79–80 (2020).
Article PubMed PubMed Central CAS Google Scholar
DeFronzo, R. A. et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers. 1 , 1–22 (2015).
Article Google Scholar
Abdulwahab, R. A., Alaiya, A., Shinwari, Z., Allaith, A. A. A. & Giha, H. A. LC-MS/MS proteomic analysis revealed novel associations of 37 proteins with T2DM and notable upregulation of immunoglobulins. Int. J. Mol. Med. 43 , 2118–2132. https://doi.org/10.3892/ijmm.2019.4127 (2019).
Kahn, C. R. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43 , 1066–1085 (1994).
Article PubMed CAS Google Scholar
Doria, A., Patti, M.-E. & Kahn, C. R. The emerging genetic architecture of type 2 diabetes. Cell Metabol. 8 , 186–200 (2008).
Article CAS Google Scholar
Shao, S., Guo, T. & Aebersold, R. Mass spectrometry-based proteomic quest for diabetes biomarkers. Biochim. Biophys. Acta BBA Proteins Proteom. 1854 , 519–527 (2015).
Neilson, K. A. et al. Less label, more free: Approaches in label-free quantitative mass spectrometry. Proteomics 11 , 535–553 (2011).
Guan, S., Taylor, P. P., Han, Z., Moran, M. F. & Ma, B. Data dependent–independent acquisition (DDIA) proteomics. J. Proteome Res. 19 , 3230–3237 (2020).
Krasny, L. & Huang, P. H. Data-independent acquisition mass spectrometry (DIA-MS) for proteomic applications in oncology. Mol. Omics 17 , 29–42 (2021).
Gillet, L. C. et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 11 , 6 (2012).
Hubbard, S. J. & Jones, A. R. Proteome Bioinformatics Vol. 604 (Springer, 2010).
Book Google Scholar
Hu, A., Noble, W. S. & Wolf-Yadlin, A. Technical advances in proteomics: New developments in data-independent acquisition. F1000Research 5 , 419 (2016).
Abdulwahab, R. A., Allaith, A. A. A., Shinwari, Z., Alaiya, A. & Giha, H. A. Association of TATA box-binding protein-associated factor RNA polymerase I subunit C (TAF1C) with T2DM. Gene 706 , 43–51. https://doi.org/10.1016/j.gene.2019.04.076 (2019).
Jia, S. Y., Zhang, Y. L., Sun, X. Y., Yuan, C. & Zheng, S. G. Impact of the glycemic level on the salivary proteome of middle-aged and elderly people with type 2 diabetes mellitus: An observational study. Front. Mol. Biosci. 8 , 790091. https://doi.org/10.3389/fmolb.2021.790091 (2021).
Article ADS PubMed PubMed Central CAS Google Scholar
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 , 248–254. https://doi.org/10.1006/abio.1976.9999 (1976).
Lin, Z. et al. Alternative strategy to explore missing proteins with low molecular weight. J. Proteome Res. 18 , 4180–4188 (2019).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26 , 1367–1372 (2008).
Searle, B. C. et al. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat. Commun. 9 , 1–12 (2018).
Article MathSciNet CAS Google Scholar
Choi, M. et al. MSstats: An R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30 , 2524–2526 (2014).
Von Mering, C. et al. STRING: Known and predicted protein–protein associations, integrated and transferred across organisms. Nucl. Acids Res. 33 , D433–D437 (2005).
Article ADS Google Scholar
Götz, S. et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucl. Acids Res. 36 , 3420–3435 (2008).
Article PubMed PubMed Central Google Scholar
Horton, P. et al. WoLF PSORT: Protein localization predictor. Nucl. Acids Res. 35 , W585–W587 (2007).
Nowak, C. et al. Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes 65 , 276–284. https://doi.org/10.2337/db15-0881 (2016).
Kim, S. W. et al. Proteomics approach to identify serum biomarkers associated with the progression of diabetes in Korean patients with abdominal obesity. PLoS One 14 , e0222032 (2019).
El Eter, E. & Al-Masri, A. A. Peroxiredoxin isoforms are associated with cardiovascular risk factors in type 2 diabetes mellitus. Braz. J. Med. Biol. Res. 48 , 465–469. https://doi.org/10.1590/1414-431x20144142 (2015).
Moin, A. S. M. et al. Hypoglycemia-induced changes in complement pathways in type 2 diabetes. Atheroscler. Plus 46 , 35–45. https://doi.org/10.1016/j.athplu.2021.11.002 (2021).
Sujana, C. et al. Natriuretic peptides and risk of type 2 diabetes: Results from the biomarkers for cardiovascular risk assessment in Europe (BiomarCaRE) consortium. Diabetes Care 44 , 2527–2535. https://doi.org/10.2337/dc21-0811 (2021).
Piumngam, K., Siriprungpong, P. & Roytrakul, S. Serum carbonic anhydrase combined with adiponectin as biomarkers of insulin resistance. ScienceAsia 47 , 287–292 (2021).
Sánchez-Zamora, Y. I. & Rodriguez-Sosa, M. The role of MIF in type 1 and type 2 diabetes mellitus. J. Diabetes Res. 2014 , 804519. https://doi.org/10.1155/2014/804519 (2014).
Toso, C., Emamaullee, J., Merani, S. & Shapiro, A. The role of macrophage migration inhibitory factor on glucose metabolism and diabetes. Diabetologia 51 , 1937–1946 (2008).
Mitamura, Y. et al. Macrophage migration inhibitory factor levels in the vitreous of patients with proliferative diabetic retinopathy. Br. J. Ophthalmol. 84 , 636–639 (2000).
Miyamoto, S. et al. Increased plasma levels of thioredoxin in patients with glucose intolerance. Intern. Med. 44 , 1127–1132 (2005).
Brooks-Worrell, B. & Palmer, J. Immunology in the Clinic Review Series; focus on metabolic diseases: Development of islet autoimmune disease in type 2 diabetes patients: potential sequelae of chronic inflammation. Clin. Exp. Immunol. 167 , 40–46 (2012).
Frydrych, L. M., Bian, G., O’Lone, D. E., Ward, P. A. & Delano, M. J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 104 , 525–534. https://doi.org/10.1002/jlb.5vmr0118-021rr (2018).
Kolberg, J. A., Gerwien, R. W., Watkins, S. M., Wuestehube, L. J. & Urdea, M. Biomarkers in Type 2 diabetes: Improving risk stratification with the PreDx® Diabetes Risk Score. Expert Rev. Mol. Diagn. 11 , 775–792 (2011).
Lu, Y., Li, Y., Li, G. & Lu, H. Identification of potential markers for type 2 diabetes mellitus via bioinformatics analysis. Mol. Med. Rep. 22 , 1868–1882. https://doi.org/10.3892/mmr.2020.11281 (2020).
Biswas, S. & Chakrabarti, S. Increased extracellular matrix protein production in chronic diabetic complications: Implications of non-coding RNAs. Noncoding RNA 5 , 30. https://doi.org/10.3390/ncrna5010030 (2019).
Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 33 , 637–668 (2001).
Bao, L. et al. The S 100A6 calcium-binding protein regulates endothelial cell-cycle progression and senescence. FEBS J. 279 , 4576–4588 (2012).
Zhang, J. et al. c-Myc Upregulated by High Glucose Inhibits HaCaT Differentiation by S100A6 Transcriptional Activation. Front. Endocrinol. Lausanne 12 , 676403. https://doi.org/10.3389/fendo.2021.676403 (2021).
Jiang, G. J. et al. Protein profiling the differences between diabetic and normal mouse cumulus cells. Mol. Reprod. Dev. 81 , 1080–1085. https://doi.org/10.1002/mrd.22419 (2014).
Dogra, S. et al. Liver derived S100A6 propels β cell dysfunction in NAFLD. Diabetes https://doi.org/10.2337/db22-0056 (2022).
Dogra, S. et al. Liver-derived S100A6 propels β-cell dysfunction in NAFLD. Diabetes 71 , 2284–2296. https://doi.org/10.2337/db22-0056 (2022).
Download references
Acknowledgements
This research was funded by Jordan University of Science and Technology, grant number 20190337. Also, the authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through project No. (IFKSUOR3-032-1)”.
This research was funded by Jordan University of Science and Technology, grant number 20190337 and by the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through project No. (IFKSUOR3-032-1)”.
Author information
Authors and affiliations.
Department of Medical Laboratory Sciences, Jordan University of Science and Technology, Irbid, 22110, Jordan
Refat M. Nimer & Eman R. Shehabat
Department of Physiology and Biochemistry, Faculty of Medicine, Jordan University of Science and Technology, Irbid, 22110, Jordan
Mahmoud A. Alfaqih
Department of Biochemistry, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, 15503, Bahrain
Department of Medicine, College of Medicine, King Saud University, 12372, Riyadh, Saudi Arabia
Muhammad Mujammami
University Diabetes Center, King Saud University Medical City, King Saud University, 12372, Riyadh, Saudi Arabia
Department of Chemistry, Memorial University of Newfoundland, St. John’s, NL, A1B 3X7, Canada
Anas M. Abdel Rahman
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, R.M.N.; methodology, R.M.N., M.A.A., and E.R.S.; validation, R.M.N., M.A.A., M.M; A.M.A. and E.R.S.; formal analysis, R.M.N. and E.R.S.; investigation, R.M.N., and E.R.S.; resources, R.M.N, and M.A.A.; data curation, R.M.N, and E.R.S.; writing original draft preparation, R.M.N.; writing—review and editing, M.A.A., M.M, A.M.A and E.R.S.; visualization, R.M.N, and E.R.S.; supervision, R.M.N. and M.A.A.; project administration, R.M.N.; funding acquisition, R.M.N, and M.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Refat M. Nimer .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Nimer, R.M., Alfaqih, M.A., Shehabat, E.R. et al. Label-free quantitative proteomics analysis for type 2 diabetes mellitus early diagnostic marker discovery using data-independent acquisition mass spectrometry (DIA-MS). Sci Rep 13 , 20880 (2023). https://doi.org/10.1038/s41598-023-48185-3
Download citation
Received : 24 March 2023
Accepted : 23 November 2023
Published : 27 November 2023
DOI : https://doi.org/10.1038/s41598-023-48185-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Previous Article
- Next Article
Introduction
When are srmas useless or even counterproductive, when should a meta-analysis be conducted within a systematic review, meta-analyzing more than randomized controlled trials, key components of an srma, recent developments in srmas, summary and conclusions, article information, a primer on systematic review and meta-analysis in diabetes research.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Deirdre K. Tobias , Stefania Papatheodorou , Jennifer M. Yamamoto , Frank B. Hu; A Primer on Systematic Review and Meta-analysis in Diabetes Research. Diabetes Care 1 November 2023; 46 (11): 1882–1893. https://doi.org/10.2337/dci23-0031
Download citation file:
- Ris (Zotero)
- Reference Manager
A systematic review is a rigorous process that involves identifying, selecting, and synthesizing available evidence pertaining to an a priori–defined research question. The resulting evidence base may be summarized qualitatively or through a quantitative analytic approach known as meta-analysis. Systematic review and meta-analysis (SRMAs) have risen in popularity across the scientific realm including diabetes research. Although well-conducted SRMAs are an indispensable tool in informing evidence-based medicine, the proliferation of SRMAs has led to many reviews of questionable quality and misleading conclusions. The objective of this article is to provide up-to-date knowledge and a comprehensive understanding of strengths and limitations of SRMAs. We first provide an overview of the SRMA process and offer ways to identify common pitfalls at key steps. We then describe best practices as well as evolving approaches to mitigate biases, improve transparency, and enhance rigor. We discuss several recent developments in SRMAs including individual-level meta-analyses, network meta-analyses, umbrella reviews, and prospective meta-analyses. Additionally, we outline several strategies that can be used to enhance quality of SRMAs and present key questions that authors, editors, and readers should consider in preparing or critically reviewing SRMAs.
Graphical Abstract

Systematic review and meta-analysis (SRMA) research has risen in popularity across the scientific realm, even securing a place atop “evidence pyramids” and study design hierarchies ( 1 , 2 ). A “systematic review” refers to the process of identifying all research meeting the scope and eligibility criteria for a specific scientific question defined a priori. The collation of this evidence is then summarized qualitatively and/or quantitatively, with the quantitative analytic approaches referred to as “meta-analysis.”
Well-conducted SRMAs are an indispensable tool that provide a comprehensive synthesis of available evidence ( 3 ). A fundamental strength is the standardized approach that serves to minimize selective reporting and other author biases and has generally been a welcome replacement for the often cherry-picked narrative reviews and expert opinion articles. As such, SRMAs are routinely used to inform clinical care guidelines such as the Standards of Care in Diabetes recommendations put forth by the American Diabetes Association ( 4 ). Policymakers also heavily rely on data synthesized through SRMA processes to develop their recommendations and monitor implementation effectiveness ( 5 , 6 ). Another advantage of SRMAs is that combining effect estimates from multiple studies can improve statistical power, and therefore precision, for an exposure-outcome association. An example is the case of dipeptidyl peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists and pancreatic cancer risk, where in initial retrospective case-control studies, a study design with high potential for selection bias and reverse causation but an advantage of greater statistical power for rare outcomes, investigators observed signals for a positive association ( 7 ). Prospective cohort studies are less prone to biases but were largely underpowered individually; however, SRMAs combining the estimates of multiple cohort studies or randomized clinical trials did not substantiate the early concerns observed in retrospective studies ( 7 , 8 ).
There is a misconception, however, that the standardized infrastructure to guide implementation means that SRMAs inherently arrive at unbiased and even definitive conclusions. Within the procedurally systematic framework, there are several subjective decision points and methodological considerations in conducting SRMAs, each with the potential to influence the authors’ findings, interpretations, and conclusions. A recent systematic review showed generally poor methodological and reporting quality of published SRMAs in diabetes research ( 9 ). The objective of this article is to provide up-to-date knowledge and a comprehensive understanding of strengths and limitations of SRMAs. We first provide an overview of the SRMA process and then offer ways to identify and overcome common pitfalls at key steps. We also present key questions that authors, editors, and readers should consider in preparing or critically reviewing SRMAs ( Table 1 ).
Key questions to consider in reviewing or developing an SRMA
The proliferation of SRMAs has also ushered in redundant reviews and reviews of questionable quality. A common misuse of the technique has led some to question the value of SRMAs altogether ( 10 ). As for any scientific endeavor, the investigator should first identify whether their investigation will address a gap in the evidence base. If a substantial amount of original research has accumulated, an SRMA may indeed be warranted. If there are already SRMAs addressing the same hypothesis, conducting an updated review could be justifiable if a critical mass of studies has been published since. However, sparse or heterogeneous evidence often precludes the ability to draw meaningful conclusions, leading the SRMA to be uninformative and add to uncertainty and confusion. There are many useful roadmaps to guide investigators in this decision-making process ( 11 – 13 ), which we have summarized in Fig. 1 .

Deciding whether a systematic review of your hypothesis is warranted.
Even when an SRMA is justified and suitable to advance a particular hypothesis, it is critical to recognize that this approach remains fraught with potential pitfalls. For example, in a recent study investigators examined the 20 most cited meta-analyses in the field of strength and conditioning and found that most of them (85%) suffered one or more common statistical errors such as mixing up SEs and SDs of estimates and double counting the same studies ( 14 ). These common errors highlight the importance of quality control by authors and readers in preparing or critically reviewing SRMAs.
A meta-analysis is simply the calculation of a weighted average of the individual studies’ effect estimates to generate a single summary statistic pertaining to an exposure or intervention and outcome relationship. There is no universally accepted decision tree or threshold to indicate the point at which a meta-analysis is warranted. Conducting a meta-analysis to statistically summarize findings in a systematic review is tempting, and technically, only two data points and their measures of variance are needed. One algorithm broadly outlines major decisions to aid quantitative synthesis decision-making, with consideration of compatibility of study hypotheses and designs, structure of the exposure and outcome data, and units of measurement ( 15 ). However, the question of “whether data should be statistically combined to begin with” supersedes any downstream considerations, and this relies heavily on having a well-defined hypothesis, a clearly specified SRMA protocol, and subject matter expertise.
If the investigator anticipates the possibility of synthesizing the results of their literature search and data extraction with a meta-analysis, the analytic plan and rationale should be specified in the protocol, including steps for harmonizing across units of exposure and outcome, effect estimate scales, and measures of variance. The protocol should also provide the rationale for the weighing scheme (e.g., fixed effects vs. random effects) and, where appropriate, methods for dose-response analysis and meta-regression. There are existing comprehensive resources for conducting these analyses that we refer the reader to ( 16 ). However, it may be the case that a meta-analysis should ultimately not be performed. There are several reasons why a meta-analysis is not appropriate, such studies using different methods for assessment of the exposure (e.g., daily dietary vitamin D intake vs. serum vitamin D concentrations) or having different end points (e.g., change in fasting blood glucose concentration vs. HbA 1c levels) or study designs (e.g., cross-sectional studies vs. prospective cohort studies vs. randomized intervention trials). Low-quality protocols with vague definitions of exposures and outcomes can lead to the inclusion of poorly aligned studies that may not be directly relevant or matched to the research question or objective of a systematic review.
As meta-analyses were originally developed for placebo-controlled randomized controlled trials (RCTs), conducting a meta-analysis is often more complicated for other types of research, such as in the case of observational data. A meta-analysis of exposure/outcome effect estimates derived from observational data should include consideration of the study populations likely have different exposure distributions, exposure and outcome ascertainment methods (e.g., self-report, registry database, blood biomarker, etc.), and analytic approaches. Further, it is common in observational research for investigators to conduct several multivariable-adjusted models for varying degrees of plausible confounders. The SRMA investigator who is familiar with the literature should anticipate this, and a priori specify in their protocol what constitutes appropriate inclusion and exclusion criteria for the literature search. Inappropriate inclusion and exclusion criteria without consideration of the analytic methods of the original studies can also lead to biased results. For example, some meta-analyses arrived at conclusions that those with a higher BMI have a lower risk of all-cause mortality than those with normal weight ( 17 ). However, these meta-analyses implicitly included studies conducted in patients with prevalent chronic conditions where gradual declines in body weight precede death, such as cancer or neurodegenerative diseases, resulting in “reverse causation” bias.
We will not provide an exhaustive guide on conducting an SRMA, as there are already numerous resources available to the research community ( 11 – 13 , 16 , 18 ). Generally, the process can be distilled to four main components as shown in Fig. 2 : 1 ) developing a clear research hypothesis and protocol, 2 ) implementing the protocol to identify and characterize the evidence base, 3 ) evidence synthesis and statistical analysis, and 4 ) formulating a conclusion informed from both the results and the quality of the evidence.

Key steps in conducting an SRMA.
Begin With a Clear Hypothesis
The SRMA process begins with articulating a clear research hypothesis and developing an appropriate protocol to address the question. There are several frameworks available that guide researchers through the process of formulating a well-defined hypothesis, with PICO (population, intervention, comparator, outcome) and its variants among the most commonly used ( 19 ). Briefly, elements of a well-defined research question include specifying the target patient population, intervention (or exposure) and comparator, and outcome definition. Other considerations include defining the relevant time frame or duration that would be reasonable for development of the outcome, optimal study designs and analytic approaches, and to be as detailed as if the investigators were planning to conduct an original study themselves.
Protocol, Protocol, Protocol
A well-developed protocol is critical for conducting a high-quality SRMA as it serves as the investigators’ roadmap for the systematic review process. The 17-item extension of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for protocols (PRISMA-P) checklist and several extensions for different types of reviews have been used to improve the quality of developing SRMA protocols ( 20 , 21 ). However, most published SRMAs related to diabetes research did not have available protocols and, even among those with available protocols, the adherence to the PRISMA-P checklist was poor ( 9 ). The absence or low quality of a prospective protocol may raise concerns about the rigor of the SRMA but does not necessarily mean it should be dismissed entirely.
Composing an SRMA protocol is seemingly straightforward and standardized tools such as PRISMA-P foster “systematic” and replicable results. However, this stage is arguably one of the major determinants of overall quality and bias. An ill-defined research question, such as in the case of failing to specify a comparator exposure, can lead to inappropriate inclusion of studies misaligned with the investigators’ original intent, result in substantial heterogeneity, and undermine the quality and certainty of the evidence base and the ability to draw meaningful conclusions. Similarly, vague protocol criteria introduce unnecessary subjectivity during screening and data extraction. The subject matter expertise required to formulate a research hypothesis and translate it into a protocol is also often underestimated. It is not uncommon for SRMAs to combine results from observational cohorts and randomized intervention trials, which can lead to misleading findings, given differences in study designs, duration of follow-up, exposure and comparator types, participant criteria, and outcome measurements. Alternatively, some SRMAs had available and seemingly eligible studies omitted without explanation.
In requiring authors to prospectively register SRMA protocols one seeks to improve transparency and reproducibility of the literature search process and potentially decrease some biases; at the very least, these records will serve as a resource for understanding discrepancies should similar SRMAs arrive at different conclusions. Scientific journals increasingly require authors of SRMAs to have prospectively filed their protocol (i.e., before initiating the literature search), and registries such as International prospective register of systematic reviews (PROSPERO) facilitate this ( 22 – 24 ). Preregistered SRMAs were found to have higher overall methodological quality compared with nonregistered reviews ( 25 ). Readers, journal editors, and reviewers should be encouraged to examine registered protocols while reviewing SRMAs to ensure the SRMA adheres to the predefined methods in the protocol.
Literature Search and Data Extraction
A well-developed protocol should include a comprehensive literature search strategy that is unbiased and reproducible. In general, inclusion and exclusion criteria should be clearly stated based on the research question before the literature search is started. Multiple databases such as PubMed, Embase, Web of Science, APA PsychInfo, and Cochrane Library should be searched, and unpublished studies and non–English language studies included, to ensure that all potential eligible studies are identified. Title and abstract screening, and full-text review of the retrieved studies and data extraction of eligible studies, should be conducted by two authors independently, and a third researcher may be called to resolve any disagreements. Newer systematic review platforms, such as Covidence ( https://www.covidence.org/ ), have made the duplicate screening and consensus processes efficient and easy to track. Often, the authors of original articles need to be contacted for missing or partially reported data. The Peer Review of Electronic Search Strategies (PRESS) checklist is a useful tool to guide and improve the quality of literature search strategies, and a PRISMA flow diagram is used to display the detailed search process and results ( 26 ). The PRISMA 2020 statement had a revised flow diagram with inclusion of the number of studies from previous reviews and those identified through other search strategies ( 27 ). Figure 3 is an example of a thorough PRISMA flowchart that describes the study selection process from a meta-analysis on the association between weight status and risk of diabetes in adults ( 28 ). As shown here, the flowchart should include the results of the initial search and any updated search of databases from inception until the start of data analysis. It should also describe the results from database search and other sources, e.g., hand searching reference lists of the included studies. It is important for the authors to provide as much detailed information as possible about the reasons for excluding studies in every step. The diagram can be modified to accommodate updated or continually updated (“living”) systematic reviews. The final set of manuscripts included in the SRMA are then described in a table, which provides high-level summaries of data extracted on the study design, study population, interventions or exposure, and outcomes for each manuscript.

PRISMA 2020 flow diagram example for systematic reviews. Cochrane, Cochrane Library. Reprinted with permission from Yu et al. ( 28 ). OB, obesity; NW, normal weight; OW, overweight.
Quantifying and Interpreting Heterogeneity
A critical step of data synthesis is assessing heterogeneity, which refers to clinically and/or statistically different effect estimates among the eligible studies. Despite a well-defined hypothesis and SRMA protocol, two or more seemingly similar studies may estimate statistically different effects due to random chance or factors the investigator did not control for in the protocol development and screening process. In a qualitative synthesis of evidence, results of the individual studies should be summarized in a table, with enough information for the reader to interpret the effect estimate, such as the units of the outcome and scale of the effect estimate and variance.
In a quantitative synthesis, forest plots are emblematic of meta-analyses as they provide a visual representation of the individual studies’ estimates and contain important information about the evidence base and a qualitative glimpse at consistency in the results, or lack thereof, between studies. The plots are useful in inspecting implausibly large effect sizes, implausibly narrow 95% CIs, outlier studies, and large between-study heterogeneity.
The heterogeneity among studies in a meta-analysis is quantified by the Cochran Q test and I 2 statistic. The I 2 describes the percentage of total variation in the summary estimates that can be attributed to between-study heterogeneity. The χ 2 test is for addressing the null hypothesis that the individual study effect estimates are similar and any differences are due to chance alone. A higher I 2 value and a significant χ 2 P value would indicate the presence of statistical between-study heterogeneity. I 2 values of 25%, 50%, and 75% are generally considered indicative of low, moderate, and high heterogeneity, respectively. However, these cut points are somewhat arbitrary. Of note, because I 2 is a relative rather than an absolute measure of statistical heterogeneity, it tends to be inflated in meta-analyses of large observational studies where the variability due to sampling error is relatively low ( 29 ). Therefore, it is common to observe a high I 2 value in a meta-analysis of large cohort studies, even if the estimates across individual studies are relatively consistent. In addition, meta-analyses of continuous outcomes often exhibit substantially higher I 2 values compared with meta-analyses of binary outcomes ( 30 ).
There are important considerations for interpreting a meta-analysis summary statistic in the presence of between-study heterogeneity. First, it is crucial to assess what features of study design, population characteristics, and intervention/exposure, among others, might have led to different results. The SRMA protocol should include a list of possible factors the investigator anticipates examining, mainly based on prior knowledge or biological plausibility. Sensitivity analyses where justified outliers are excluded can help with assessment of their impact on the meta-analysis. Meta-regression analyses may also be useful in assessing whether a specific study-level factor explains between-study differences, keeping in mind that inferences of study-level differences cannot be attributed to individual-level effects (i.e., ecological fallacy) ( 16 ). It is possible that significant effect modification by a study-level factor would warrant presenting a stratified meta-analysis. This decision should be clearly documented and justification should be included if it deviates from the original analysis plan, usually driven by biological plausibility and/or strong prior evidence ( 31 ). While significant between-study heterogeneity is often considered as a negative aspect in assessing the degree of certainty of evidence, as it may suggest inconsistency of the literature, it may suggest true etiological differences that should be explored rather than dismissed outright ( 32 ). As discussed above, it is important to interpret a high I 2 value from meta-analyses of large cohort studies with caution and not automatically assume it to be an indicator of inconsistency or a justification for downgrading the certainty of the meta-evidence ( 29 ).
In a series of SRMAs on diet and lifestyle exposures with incident chronic disease risk from observational data, the Global Burden of Disease group used a statistical method to model and account for between-study heterogeneity ( 33 ). With this method they calculated the estimated uncertainty intervals (UIs) that were several times wider than the 95% CIs generated by conventional random-effects models. As an example, although there was a highly statistically significant and approximately linear positive association between red meat intake and risk of type 2 diabetes, their lower 95% boundary of the UIs included 1 due to an ∼2.5-fold inflation of the width of the conventional CIs, resulting in a rating of weak evidence (two out of five stars) for the association ( 33 ). In their analyses of smoking and health outcomes ( 34 ), the evidence on smoking and heart disease was rated “moderate” (three-star) despite overwhelming evidence from multiple sources of data to support a strong causal relationship ( 35 ). The UIs were calculated with similar methods to estimate prediction intervals for the random-effects summary estimates ( 36 , 37 ). Because UIs incorporate the uncertainty in both the mean effect from a random-effects model and the heterogeneity parameter, they are much wider than 95% CIs. UIs are intended to account for additional variability arising from the wide range of effects observed in individual studies and therefore are useful in predicting the range of effects that may be observed in a new study. However, it is important to note that UIs should not be used to draw conclusions about the overall impact of an exposure or treatment based on existing evidence ( 38 ). It is more appropriate to use the pooled effect estimates and corresponding 95% CIs from SRMAs in estimating and interpreting population average effects, which are crucial for making public health or clinical recommendations.
Fixed-Effects Versus Random-Effects Meta-analysis
There has been a long-standing debate regarding whether fixed-effects or random-effects meta-analysis is the preferred model, especially in the presence of significant heterogeneity and small study effects. While the fixed-effects model assumes a common true treatment effect across studies, the random-effects model assumes a distribution of true treatment effects across studies. Although inverse variance weighting is used in both fixed-effects and random-effects models, the former includes consideration of only within-study variability and the latter incorporates both within-study and between-study variability. The DerSimonian and Laird method is the most commonly used method to combine data from individual studies using random-effects models ( 39 ). As shown in Fig. 4 , small studies contribute more weight to the overall estimate in applying a random-effects model, while their weights are more uniformly distributed in comparison with the fixed-effects model. In this example, the largest study contributes 17.7% of the estimate in the random-effects model as opposed to 92.8% in the fixed-effects model. In the case of very limited or absent between-study heterogeneity, random-effects and fixed-effect weights are very similar or the same.

Random-effects and fixed-effect meta-analyses comparing the effect of intravenous magnesium with placebo on overall mortality in patients with acute myocardial infarction. A risk ratio (RR) <1 indicates that intravenous magnesium is better than placebo. Reprinted with permission from da Costa and Juni ( 12 ).
When small study effects (small studies demonstrate extreme effects) exist, the results of fixed and random effects can be substantially different. A classic example is a meta-analysis of the effect of intravenous magnesium on mortality following myocardial infarction, in which beneficial effects of intervention were found in a meta-analysis of small studies, with these findings subsequently challenged when the very large ISIS-4 (Fourth International Study of Infarct Survival) had null results ( 40 ). Because there was substantial between-trial heterogeneity, the studies were weighted much more uniformly in the random-effects analysis than in the fixed-effects analysis, with small studies contributing more to the pooled estimate. In the fixed-effects analysis, ISIS-4 contributes >90% of the weight and so the pooled estimate shows no beneficial intervention effect. In the random-effects analysis, the small studies contributed most of the weights and there appeared to be strong evidence of a beneficial effect of intervention ( Fig. 4 ). Of note, the proportion of events contributed by ISIS-4 was 92% (4,319 of 4,696). In interpreting the evidence, it is crucial to make a judgement about the validity of the combined estimates from the smaller studies in comparison with that from ISIS-4.
A common practice is to use random-effects meta-analysis when tests of heterogeneity are statistically significant; otherwise, a fixed-effects meta-analysis is conducted. One caveat with this approach is that the heterogeneity test largely depends on the sample sizes of the included studies. Another consideration is that random-effects models give disproportionate weights to the small studies, thus penalizing the large studies, which results in wider CIs of the pooled estimates and greater uncertainty of the findings ( 41 ). For these reasons, some have urged that fixed-effects models are more appropriate than random-effects models regardless of between-study heterogeneity ( 42 ). In practice, it might be useful to report both fixed-effects and random-effects summary estimates and their 95% CIs to gauge the robustness of the findings. The determination of which of the two primarily depends on several factors including the possibility of small study effects, and the expected clinical heterogeneity regarding population characteristics, and differences in study designs and intervention doses or types.
There are several strategies to address the presence of a large degree of heterogeneity. One option is not to perform a meta-analysis when heterogeneity is so severe where there is clear inconsistency in the direction and the magnitude of the effects, which may mean that the studies are not comparable enough to be meta-analyzed. If a decision to meta-analyze the data is reasonable, then exploring the potential causes of heterogeneity is mandatory. Implementing a random-effects model takes into account between-study heterogeneity, but it does not substitute a thorough investigation of heterogeneity, which is typically performed through subgroup analysis and meta-regression. Characteristics of the studies that may be associated with heterogeneity should be prespecified in the review protocol. Also, we need to note that lack of power to meaningfully explore heterogeneity is common when a few studies are synthesized.
Grading the Degree of Certainty of the Evidence
A meta-analysis summary statistic does not signify the completion of the SRMA evidence synthesis process and reporting. Once the evidence base has been summarized qualitatively or quantitatively, the next critical step is to assess the overall validity and certainty of the synthesized evidence. This begins with evaluating the individual studies for their quality and transparency in design, conduct, analysis, and reporting of the research, including careful consideration for potential biases undermining the validity of their effect estimates. In general, it is not advisable to draw conclusions about the validity of evidence based solely on the type of study designs, for example, automatically favoring RCTs over observational studies. There is no one-size-fits-all approach to systematically evaluating these, and an appropriate appraisal will require an understanding of the study designs’ strengths and limitations, statistical approaches, and substantive knowledge.
Next, a critical appraisal of the totality of the evidence included in the review is conducted to determine the level of confidence that the investigator puts in the SRMA’s analytic findings. The appraisal involves the assessment of individual study quality and the consistency of existing evidence, biological plausibility, and level of evidence certainty, often from multiple lines of evidence. In Table 2 , we recommend several quality, bias, and certainty appraisal tools that can assist authors to enhance the overall quality of SRMA. The appraisal of evidence levels is crucial to formulating the SRMA’s final conclusions. In a recent assessment of the epidemiological characteristics and the overall methodological quality of SRMAs of diabetes treatment, less than half of the SRMAs (45.2%) included assessment and documented scientific quality of included studies, and only 34.5% SRMAs considered it when formulating conclusions ( 43 ). Failing to properly appraise and apply validity and certainty in an SRMA’s conclusions may lead a falsely inflated or diminished level of confidence in the underlying evidence base, with the potential to negatively influence clinical and public health recommendations.
Good practice tools for assessment of study-level quality and risk of bias and improvement of overall SRMA reporting
An example highlighting complexities of evidence appraisal is the series of SRMAs evaluating intake of red and processed meats with risks of major chronic disease incidence and mortality. As would be anticipated, the evidence base consisted almost exclusively of observational cohort studies, given the long-term health outcomes of interest. The authors applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for evaluating the certainty of evidence, which is a well-established and validated tool for evaluating strengths of evidence from RCTs of pharmacological or other medical interventions. Its direct application to evaluating observational evidence, however, can be problematic ( 32 ). The authors assigned the evidence to the level of “very low and/or low certainty” owing to lack of randomization and substantial between-study heterogeneity ( 44 ) and as such concluded with recommendations for individuals to continue their red and processed meat consumption habits. In contrast, using a certainty rating system tailored specifically for nutritional exposure research, a separate group rated the same body of literature as “moderate quality” and “high quality” on the associations of red and processed meat intakes with mortality and type 2 diabetes, respectively ( 45 , 46 ). A key difference between the GRADE and NutriGRADE approach is that NutriGRADE does not automatically assign a low rating to observational evidence as a default starting point. Instead, it is tailored to specific characteristics, strengths and limitations, and potential biases of nutrition research ( 32 ).
The risk of bias in nonrandomized studies of interventions (ROBINS-I) tool has been used to assess the risk of bias across multiple domains in observational studies ( 47 ), where an effect size consistent with the plausible biological exposure/outcome relationship informed by other lines of evidence and the presence of a dose-response relationship can serve as justifications for upgrading the certainty of observational evidence. The incorporation of ROBINS-I with GRADE offers a more suitable method for evaluating the certainty of observational evidence related to nutritional, lifestyle, and environmental exposures ( 48 ). This integration ensures that GRADE does not assign an automatic low rating to observational evidence as a starting point. It is also important that a high I 2 value from meta-analyses of large cohort studies does not lead to an automatic downgrade of the certainty of observational evidence ( 29 ).
It is common for an SRMA protocol to incorporate rationale for the inclusion of multiple study designs that address complementary hypotheses concerning the same exposure/outcome association, such as efficacy intervention trials alongside epidemiologic studies assessing long-term habitual exposure and the same outcomes. In such cases, evidence synthesis may be best performed separately for each study design, and quality and certainty assessments should be performed with tools that are optimized to specific types of research being evaluated.
Assessing Publication and Selective Reporting Biases
Understanding of the mechanisms by which publication bias and other forms of selective reporting bias might arise is a prerequisite for minimizing their impact on our interpretation of the literature, and correspondingly these mechanisms need to be thoroughly examined by the diabetes research community. Such mechanisms include confirmation bias (selective preference for new results that agree with prior evidence), improper study parameters (e.g., lack of power, improper specification of the population for the intervention), hypothesis testing practice (discontinuation of the manuscript development due to negative results in the analysis), lack of appropriate avenues for reporting negative studies beyond “grey literature” (ideally, the probability of publication of study findings should be independent of statistical significance), and selective outcomes reporting bias (reporting only outcomes with nonnull findings).
In a recent assessment of the methodological quality of SRMAs on diabetes treatment investigators concluded that <40% included assessment of the potential for publication bias ( 43 ). A thorough and comprehensive search for relevant records is extremely important for minimizing publication bias. Searching for so-called “grey literature,” including dissertations, preprints, and reports from the government or industry or conference proceedings, as well as preregistration databases with additional study information and outcome data, such as ClinicalTrials.gov, may be worth the effort ( 49 ). Although we can examine the presence of publication bias through statistical tests, we cannot provide proof of publication bias directly with use of any of these methods. Nonetheless, with these methods we can examine certain properties of the data that may be indicative of proof of publication bias.
The most used graphical method to explore publication bias is the funnel plot ( Fig. 5 ). In the absence of publication bias, the scatter will be due to the sampling variation only, with corresponding number of studies in each bottom side of the funnel plot. In the presence of publication bias, studies from either side of the bottom of the funnel plot will be missing. However, funnel plot asymmetry can also be due to true study heterogeneity, other reporting biases, or chance ( 50 ). Eyeballing a funnel plot for evidence of publication bias is subjective and therefore can be misleading. It is important to note that funnel plots can be symmetrical even in the presence of publication and other reporting biases ( 51 , 52 ).

Asymmetric funnel plot with evidence of publication bias (left) and symmetric funnel plot with no evidence of publication bias (right). The graphs were created with simulated data. A funnel plot is a scatter plot of effect sizes on the x -axis and a measure of their SEs on the y -axis. The y -axis is inverted in the funnel plot, with studies with a small SE (typically, larger studies have more precision) to occupy the top of the funnel.
The role of chance is of particular importance in the interpretation of a funnel plot because most meta-analyses only have a few studies and therefore may be underpowered. In evaluating the role of chance, statistical tests for funnel plot asymmetry (small study effects) are used to examine whether the association between published effect estimates and measures of study size is greater just by chance alone. These tests such as the Begg rank test ( 53 ) and Egger regression test ( 54 ), and its extensions ( 55 – 57 ), are often underpowered. Thus, even if their results are null, publication and other reporting biases cannot be excluded.
Adhering to Recognized Standards for Reporting SRMAs
It is essential for authors to follow recognized standards for reporting and publishing SRMAs so that all of the steps are reported in detail and reproducible by an independent researcher. The PRISMA 2020 statement, which was updated from the 2009 statement, provides guidance for reporting and assessment of the quality of SRMAs ( 27 ). The 27-item checklist covers the study inclusion and exclusion criteria, databases and search terms to be used, literature screening and extraction procedures, statistical methods for meta-analysis, methodsfor individual study quality and bias assessment, and methods for assessing certainty level of the meta-evidence. The checklist is useful for improving the transparency and methodological standards of systematic reviews.
Do Not Overinterpret the Findings From SRMAs
Because well-conducted SRMAs sit atop the evidence hierarchy, there is a tendency for authors to draw overly confident conclusions beyond the strength of the data. For example, some authors may portray their findings from SRMAs as definitive or causal. It should be noted that an SRMA per se is not a tool for establishing causality, although the findings from well-conducted SRMAs quantify the strength, consistency, and dose-response relationship of evidence to inform causal inference ( 58 , 59 ). Often, authors translate their SRMA findings directly into public health recommendations, without examining other important considerations such as biological plausibility, implementation and scalability, cost-effectiveness, environmental impact, side effects or safety, and more. For example, the previously mentioned SRMAs on red meat and health outcomes were published alongside the authors’ proposed revised dietary guidelines for individuals to continue their current meat consumption habits, which has led to a great deal of public confusion ( 44 ). Similarly, in the Burden of Proof studies investigators converted the SRMA findings of epidemiological studies on lifestyle factors and chronic diseases into a simplified one- to five-star rating system for policy recommendations without considering other lines of evidence ( 60 ). These examples highlight the importance of exercising caution in drawing conclusions with policy implications based on evidence from SRMAs.
There are several newer variants in meta-analysis with expanded scope and capabilities in comparison with more conventional methods of research synthesis. Examples include individual-level meta-analyses, network meta-analyses, prospective meta-analyses, and umbrella reviews. Here we briefly describe the strengths and limitations of these approaches.
Individual-Level Data Meta-analysis
A meta-analysis is classically performed through analysis of aggregate data; however, the quality of study reporting, different outcome definitions, and analyses performed may limit the validity of and ability to combine these data ( 16 ). The individual participant data meta-analysis addresses many of these concerns and could yield higher-quality meta-analyses in comparison with literature-based SRMAs. This type of SRMA is performed by collecting individual-level data from study investigators. This allows for consistent inclusion/exclusion criteria and outcome definitions, standardized analytic approaches and effect estimates, and analyses including unpublished data. Analyses with individual-level data also avoid ecological fallacy in examining sources of between-study heterogeneity and allow for intervention-interactions by patient-level characteristics and duration of follow-up. An important barrier to using the individual participant data meta-analysis is the higher cost, time, and coordination required, including data-sharing agreements and data transfers. Detailed protocols are needed to harmonize exposures, covariates, and outcomes and to pool and analyze data from diverse data resources. In a recent systematic review of individual participant data meta-analysis published from 1991 to 2019, investigators found that the methodologic quality of these was far from satisfactory ( 61 ); as for aggregate-level meta-analyses, the validity of the individual participant–level meta-analysis is contingent on high-quality methodology and reporting.
Network Meta-analysis
Since conventional SRMA only accommodates pairwise exposure comparisons, the network meta-analysis approach was developed to consider more than two exposures of interest. Network meta-analysis statistically contrasts any number of pairwise exposure effect estimates with a common outcome, using both direct and indirect comparisons ( 62 ). Thus, it offers a comparison of two interventions even if they have never been directly tested head-to-head. If used appropriately, this is a powerful tool to inform clinical decision-making when resources are limited for conducting multiple comparative effectiveness trials. As an example, in a recent network meta-analysis investigators examined 816 trials with 471,038 patients, together including evaluation of 13 different drug classes, with confirmation of the benefits of sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in reducing cardiovascular disease and end-stage kidney disease compared with standard care ( 63 ). Of note, the estimates of indirect comparisons between two treatments should be interpreted with caution when the treatment groups were not derived from the same study population and estimates were obtained under different study protocols ( 64 ). Further, inherent to the design, foundational steps such as risk of bias and assessment of heterogeneity may be more challenging to interpret ( 65 ). Since a network meta-analysis yields more than one effect estimate, bias from any single trial or heterogeneity between trials may affect several pooled effect estimates or impact multiple other comparisons.
Prospective Meta-analysis
As for all SRMAs, a protocol specifying the research question in a prospective meta-analysis is critical; however, the key feature that differentiates this type of SRMA from a conventional meta-analysis is that protocol development and the identification of studies for inclusion precede the reporting of individual study results ( 66 ). With this type of SRMA investigators seek to minimize the pitfalls of publication bias and selective reporting of outcomes after knowledge of the results. Additionally, with the prospective inclusion of studies, efforts are made to enhance consistency between study designs and analytic plans. Some drawbacks of prospective meta-analyses include the longer duration and higher cost, as well as the high level of coordination, planning, and collaboration required, as is seen with individual-patient data meta-analysis ( 66 ). While the publication of prospective meta-analyses has increased over time, they remain relatively rare in the literature. However, with the emergence of the COVID-19 pandemic there was a sudden and urgent need for evidence regarding the prognosis and treatment of COVID-19. The explosion of clinical trials and prospective cohort studies occurring across the globe offered an ideal opportunity for the prospective meta-analysis, and thus several RCTs and prospective cohorts were designed with harmonization of treatment protocols, exposure definitions, and data analysis plans ( 67 , 68 ). More work is required to develop evidence-based reporting tools for these reviews.
Umbrella Reviews
The proliferation of the SRMA has sparked the need for an additional methodology to summarize findings across multiple SRMAs, called an umbrella review. Also called an “overview of reviews,” the umbrella review includes identification and compilation of available systematic reviews in an area of research. This methodology may be particularly helpful to summarize evidence where there are multiple interventions for the same condition, to examine the same intervention across different populations, or to examine adverse events related to a given intervention in different populations ( 69 ). It is also useful in assessing risk factors for diseases, with the goal of identifying those with robust evidence for an association ( 70 ). While transparent reporting is a cornerstone in SRMAs, studies examining the quality of reporting of umbrella reviews have revealed that insufficient reporting is commonplace ( 71 – 73 ). It was only recently that an evidence-based reporting guideline for umbrella reviews was published ( 74 ). The preferred reporting items for overviews of reviews (PRIOR) statement includes a comprehensive 27-item checklist with 19 subitems recommended for the complete reporting of umbrella reviews ( 74 ).
High-quality SRMAs will remain an important and robust methodology to inform clinical practice and research. However, with the sheer number of published SRMAs it is not surprising that SRMAs of poor methodologic quality are all too frequent. A systematic review of diabetes-related SRMAs suggested several critical areas for quality improvement: adherence to guidelines for protocol development, more careful assessment of heterogeneity, and investigating risk of bias in individual studies and meta-analyses ( 9 ). For enhancement of the quality and trust of SRMAs, there are a number of key questions that authors, editors, and readers should ask in preparing or critically reviewing SRMAs ( Table 1 ).
There are several commonly used checklists/tools available that should accompany SRMA submissions, and individual choice in those used will depend on the type of review and studies included. These include reporting checklists (e.g., PRISMA and Meta-analysis of Observational Studies in Epidemiology [MOOSE]) and reporting standards/tools (e.g., A MeaSurement Tool to Assess systematic Reviews [AMSTAR]). A summary of some common checklists is included in the sample resources presented in Table 2 . Of note, these tools are still subjective with regard to reviewer experience, bias, and subject matter expertise, and many fail to adequately capture key sources of bias and uncertainty across diverse research areas.
Diabetes research and clinical practice will continue to rely on SRMAs to synthesize important and growing bodies of evidence, underscoring the importance of investigators, peer reviewers, and journal editors to upholding high standards of quality. Although guidelines of best practices for protocol development, literature search and screening tools, and tools for analysis and interpretation undoubtedly improve rigor and minimize biases, many aspects of the SRMA process are still susceptible to error, subjectivity, and bias. Therefore, continued scrutiny and vigilance are warranted for authors, editors, and readers in preparing or critically reviewing SRMAs to ensure reliability and integrity of the findings.
Funding. This work was supported by National Institutes of Health grants DK127601 and HL60712.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.K.T., S.P., J.M.Y., and F.B.H. wrote the manuscript, contributed to the discussion, and reviewed and edited the manuscript.
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 24 May 2022
The experiences of patients with diabetes and strategies for their management during the first COVID-19 lockdown: a qualitative study
- Mireia Vilafranca Cartagena ORCID: orcid.org/0000-0003-2953-3196 1 , 2 ,
- Glòria Tort-Nasarre ORCID: orcid.org/0000-0001-5270-821X 3 , 4 , 5 ,
- Maria Romeu-Labayen ORCID: orcid.org/0000-0001-9482-9474 5 , 6 &
- Josep Vidal-Alaball ORCID: orcid.org/0000-0002-3527-4242 7 , 8
BMC Nursing volume 21 , Article number: 124 ( 2022 ) Cite this article
3217 Accesses
5 Citations
3 Altmetric
Metrics details
During the pandemic, primary care systems prioritised attention to COVID-19 patients; chronically ill patients, such as people with Type 2 Diabetes were obliged to take more responsibility for their own care. We aimed to analyse the experiences of patients with Type 2 Diabetes Mellitus during the stay-at-home order that was in place during the first wave of the COVID-19 pandemic and identify the strategies and resources used in managing their care.
We conducted a qualitative descriptive study. The participants were ten patients with type 2 Diabetes Mellitus who experienced strict lockdown during the first wave of the COVID-19 pandemic in Catalonia, Spain, selected using intentional sampling. We recorded semi-structured interviews with the participants and conducted thematic analysis.
We identified 14 subthemes, which we then grouped into three overarching themes: 1) anxiety, fear, and vulnerability (anxiety, fear, vulnerability, rethinking life, loneliness, sadness), 2) insufficient diabetes monitoring by the health system (health care received, glycaemic control, view of treatment by health providers) and proactive self-care (changes in daily routine, diet, physical activity, medication, personal protective equipment & social distancing).
Despite the exceptional nature of the situation and the stress, worry, and changes in their daily lives, many respondents reported that they had successfully modified their lifestyles. Self-care was effective during confinement and was based on a process of adaptation using the resources available, without face-to-face contact with primary care health staff.
Relevance to clinical practice
These results can help to guide the design and implementation of self-care-focused strategies and also to explore new ways of empowering patients without access to health care personnel.
Peer Review reports
Introduction
Novel coronavirus disease (COVID-19) is a highly transmissible, rapidly spreading disease which has had a dramatic impact all over the globe. Although the overall mortality rate due to COVID-19 is relatively low [ 1 ], diabetes has emerged as a prominent comorbidity, associated with a severe and acute picture of respiratory distress and increased mortality. Thus, patients with chronic diseases such as type 2 diabetes (T2D) appear to be particularly vulnerable to the effects of the virus, and T2D is a major risk factor for poor prognosis in COVID-19 infection [ 2 ].
With the outbreak of the pandemic, governments imposed policies to reduce the transmission of the virus, including quarantine, isolation, social distancing and stay-at-home orders. These exceptional measures had a direct effect on the health behaviours of patients with chronic pathologies such as T2D [ 3 ]. Many patients with diabetes have encountered barriers to care due to the policies introduced to combat COVID-19, although maintaining good blood glucose control in these patients has proved to be an effective measure in preventing the transmission of the virus [ 4 ].
Type 2 diabetes cannot be cured, but lifestyle changes such as following a healthy diet, regular physical activity, and maintaining normal body weight can slow the progression of the disease and reverse its effects [ 5 ]. However, previous studies have shown that long-term maintenance of weight loss and complete adherence to diet and physical exercise recommendations is rare, especially in the adult population. Understandably, during the pandemic, many patients with T2D have found it particularly difficult to adhere to these lifestyle recommendations due to the restrictions on their access to health services and the problems in obtaining fresh food and in exercising [ 6 ].
In Catalonia (Spain), a stay-at-home order took place during the first wave of COVID-19, March 14 to May 2, 2020, at which time the measures were progressively relaxed. Stay-at-home orders (or “lockdown”) are implemented when quarantine for exposed patients and isolation for infected patients are insufficient to contain the spread of a disease [ 7 ]. During the seven weeks of strict lockdown in Spain, people were only allowed to leave home to receive medical treatment, buy food or work as an essential worker. Leaving home for exercise was prohibited, and non-essential businesses were shuttered.
Early research shows mixed effects of COVID-19 lockdowns on patients with diabetes. [ 8 ]) show that while glucose levels for type 1 diabetes patients improved significantly, those for T2D worsened in the short term. Makki et al. [ 9 ] show that patients with T2D had better glycaemic control during lockdown, but they do not specify whether the lockdown conditions were as strict as those in Spain.
Nursing professionals have a vital role to play in educating patients about the need to adapt their lifestyles and in helping them to modify their behaviour with respect to their health [ 10 ]. During the pandemic, primary care nurses have been obliged to prioritise care for COVID-19 patients [ 11 ], and as a result they have had to postpone the care of the chronically ill [ 12 ]. In this scenario, innovative strategies are needed to monitor and motivate diabetic patients who have had to take on more responsibility for their care [ 13 ].
Qualitative research on the experience of patients with COVID-19 has provided valuable information [ 4 ]. However, few qualitative studies have addressed the experiences of patients with chronic pathologies during the pandemic, and even fewer in patients with T2D. People with chronic conditions experienced a confluence of the COVID-19 pandemic and chronic diseases in the context of difficulty in accessing healthcare, sedentary lifestyle and increased stress and anxiety [ 14 ]. Shi et al. describes the perceived barriers to diabetes self-management of people with T2D during the pandemic: inadequate knowledge and behavioural beliefs, shortage of resources, health problems, negative emotions and lack of support [ 15 ]. A structured analysis of the experiences of these patients would provide a valuable tool for organising the community and human resources needed in similar situations.
The aim of the present study is to analyse the experiences of patients with T2D that were under a stay-at-home order during the first wave of the COVID-19 pandemic and to identify the strategies and resources used in the management of T2D in this new situation.
We conducted a qualitative descriptive study, a design that is suited to arriving at a deeper understanding of practice in applied disciplines and is especially pertinent when the goal is to understand participants’ perspective and experience [ 16 ]. We began with a deductive approach to develop the interview guide and then conducted an inductive analysis of the resulting data. The study is part of an ongoing project about diabetes and physical activity, which was underway when the pandemic began (Authors, in progress).
Participants
Sampling was intentional [ 17 ]. The participants were the ten patients with T2D from four different primary health centres in central Catalonia (Spain) that were participating in our ongoing study about diabetes and physical activity. The inclusion criteria were adults aged 55 to 79 years diagnosed with T2D at least two years previously. We chose this age range because 55 is the age at which the prevalence of T2D begins to increase rapidly in the population, and a cut-off at 79 allowed us to ensure that participants were young enough to conduct physical activity [ 18 ]). Additional inclusion criteria were having no complications associated with T2D, having good metabolic control (hbA1c < 7), and showing good adherence to T2D treatment (defined as adherence to prescribed medication for T2D, physical activity > 150 min/week, and healthy diet). The exclusion criteria were gestational diabetes or type 1 diabetes, cognitive impairment, or admission to hospital during confinement. All ten participants from our initial study agreed to a follow-up interview about their experiences of COVID-19. Data saturation [ 19 ] was reached by the tenth interview, when we detected that no relevant new information was emerging.
Data collection
Data were collected through a semi-structured interview. The research team developed a set of interview questions relevant to the study objectives, based on the researchers’ clinical experience and a review of the scarce existing literature about patients with chronic illness during the pandemic: How is the COVID-19 pandemic affecting you as a person with diabetes? Can you describe the effect of the stay-at-home order on you at a personal, family, and professional level? Describe to me the care you received for your T2D during the stay-at-home order. How did your lifestyle change (In what sense? Can you tell me?). During the interview, follow-up questions were asked to encourage participants to provide additional details about their perspective.
The interviews were conducted by the principal investigator (PI) between July 2020 and January 2021. In the initial interviews for the ongoing study about T2D and physical activity, the PI had conducted interviews with the participants lasting approximately 45 min. When the pandemic broke out, the team devised a second phase of the study, and the PI invited the participants to a follow-up interview about their COVID-19 experiences. All ten agreed to participate and gave their informed consent. We opted for telephone interviews because we thought it would be easier for participants than video conferencing. We suggested that participants conduct the interview from a quiet place in which they wouldn’t be interrupted. This second interview lasted between 15 and 35 min, meaning that for each participant we have a total of between 60 and 80 min of recorded data. Participants’ confidentiality was protected by giving them pseudonyms. The voice files and transcriptions were encrypted and stored on a computer protected with an encrypted password. The interviews were performed and transcribed in Catalan or Spanish, depending on the preference of the participant. Later, the transcribed interviews were returned to participants for their approval. All participants accepted their transcribed interviews without changes.
Data analysis
Data were analysed using thematic analysis [ 20 ] by ATLAS ti ®vs 9 support. We identified and reported patterns that emerged from the data and arranged them systematically to shed light on the research questions, while trying to keep faithful to the perspectives expressed by participants [ 16 ]. We conducted the analysis in the following phases:
Phase 1 Become familiar with the data by listening to recordings, transcribing them, and reading and rereading the transcripts. Entering transcripts into software Atlas-ti vs 9. Author 1 (MCV) participated in this phase.
Phase 2: Segmenting the meaning units in the transcripts and inductively grouped them to create subthemes and identify relationships among them. Author 1 participated in this phase.
Phase 3: Group the meaning units and abstracted the subthemes. Define the parameters of each subtopic. 14 subtopics have been tagged. Author 1 participated in this phase.
Phase 4: Group the subthemes into overarching themes (which became the primary structure for our analysis). Which in turn we grouped into three themes. Devise a glossary of themes. Author 1 participated in this phase.
Phase 5: Revised, discussed and agreed upon the subthemes and themes while returning to the data to verify the analysis. Authors 1, 2 (MRL) and 4 (GTN) participated in this phase.
Phase 6: Write the research report. Authors 1, 2 and 4 participated in this phase. Author 3 (JVA) examined both the processing and product of the research study.
Rigour, reflexivity and quality criteria
The trustworthiness of data was determined by Credibility, Dependability, Conformability, Transferability [ 21 ].
Credibility has been achieved thanks to the analyst triangulation, to undertook constant revisions of the themes, subthemes and units of analysis and evaluation, ensuring qualitative validity by authors 1, 2 and 4. Transferability has been achieved by describing a phenomenon in sufficient detail to transferable to other settings and people. Dependability was ensured in this study thanks to the review by the third researcher who examined both the processing and product of the research study. Confirmability was achieved through the reflective effort of each researcher to be aware of and try to limit the influence of their own positionality on their analysis. As well as a transparent description of the research steps taken from the start of a research project. All methods were carried out by relevant guidelines and regulations.
The research team have experience with qualitative research and resolved disagreements by consensus, and complied with the Consolidated Criteria for Reporting Qualitative Research [ 22 ].
Ten patients with T2D from four primary care centres in central Catalonia (Spain) participated in the study. Table 1 displays the participants’ main sociodemographic characteristics. Ages ranged from 58 to 79 years, and 60% of participants had had T2D for more than 10 years; most also had a past history of pathology other than T2D.
In our inductive analysis, we identified 14 subthemes, which we grouped into three themes: 1) anxiety, fear and vulnerability, 2) insufficient diabetes monitoring by the health system, and 3) proactive self-care. Table 2 shows an example of the final themes, the codes from which they are built, and an example of a meaning unit from each code.
Anxiety, fear and vulnerability
The context of pandemic and confinement had a strong emotional impact on participants, and the most-expressed emotions were anxiety, fear, and vulnerability. Participants described the lockdown during first wave of the pandemic as something that was totally abnormal and hard to believe; they were shocked to hear the news of the number of deaths in Spain every day:
I thought I was dreaming. I thought this shouldn’t be happening in this day and age 3: 1 (P3).
One issue that respondents mentioned was the fear of infecting others, despite all the protective measures they used. For example, one participant, a health centre worker, was afraid of contagion in spite of the measures she took with her family:
In fact, at first I was worried that I might pass it on to them; I was working, I think the worst time was before [the state of emergency] (…). I got a room ready in case I had to isolate 5:13 (P5).
They also reported negative emotions, such as anxiety and worry:
I have anxiety problems, what`s been getting me down is the fact that I’m feeling a little agoraphobic 8:10 (P8).
It was the anguish of being locked away, of thinking you couldn’t see my 5-year-old granddaughter. My brother …. the family … my daughter and my son… 4:3 (P4).
Others felt fear at seeing so many COVID-19 infections at close range:
We’re all a bit scared. My children have all been through it, three of my four grandchildren. My daughter-in-law has had some awful aftereffects 3: 8 (P3).
Or at living close to death:
Scared. Because you see that the people who started to fall ill were mainly over 55 years old and it really hits you … 6: 1 (P6).
It was made worse by the experience of the loss of friends and family, or by news of acquaintances being admitted to the ICU:
I felt very sad to think of all the people who … I have relatives who have died and … it affected me a lot … not being able to be there … not being able to be with them 3.3 (P3).
On the other hand, some of them managed to keep these feelings of sadness at bay, thanks to their contacts with family, mainly through social media and video conferencing.
I saw them on the phone … and that kept me happy 7:16 (P7).
This feeling of social isolation was extremely negative:
I took it badly because I couldn’t leave the house, I couldn’t see my friends… 9.1 (P9).
For some, it was a negative experience because it disrupted their everyday routines and their self-care.
I felt terrible, it disrupted everything for me. I go to the pool for my water aerobics class, and everything was closed (…). I felt really bad having to spend all day at home 1: 1 (P1).
I used to walk two hours a day, when I was confined because I stayed at home, and I started to put on weight again … 6.4 (P6).
On the other hand, some respondents reported that the confinement and the change in their daily routines was an opportunity for reflection and thinking about their lives:
Three months, locked up at home without singing, without walking, without exercising … I mean, it practically gives you a vision of yourself, the experience of being alone for so many days, it’s a bit like being in a monastery (…). From this point of view the confinement was quite interesting 10.5 (P10).
Insufficient diabetes monitoring by the health system
During the pandemic, health centres prioritised attention to COVID-19 patients, and on-site care of chronic diseases was postponed. Patients reported that their analyses and tests were cancelled:
During the pandemic no diabetes care was available. And even now, there are people who are being told over the phone that it isn’t important … they’re told not to come because no tests are being done 5.6 (P5).
Nonetheless, medication and supplies for diabetics were provided:
At the beginning of the pandemic, I went to look for supplies for diabetes and they gave me enough for three or four months 7.9 (P7).
Some respondents felt abandoned by the health staff who normally cared for them:
Abandoned… (silence) … The normal monthly check-up with the nurse to look at everything (…) didn’t happen. I also have blood tests every three or six months to check my sugar level… (…) but they didn’t happen either 8.2 (P8).
Some participants expressed not understanding the reason for the restriction:
Why can children go to school in a group, in a class, but a doctor can’t see you, they can only talk to you by phone … even though when you go for an appointment there’s a separation between you, the desk, you’re at least a yard away … and wearing their masks … and it turns out they can’t see you … well, a lack of personal protection … yes, you really notice it, because there has been a lot of neglect 8.5 (P8).
But others expressed more understanding of the situation even though they were not seen by health staff:
If you put yourself in their shoes, you realise they couldn't have done any more … 3.5 (P3).
Some patients realised that they had to take control of their disease, because no one else could help them; they ended up accepting the situation:
Well, you realise you’ve got to take care of yourself. And in all, a little self-discipline. Because I didn’t have anyone else to depend on, it was only me, there was no one else (P8).
Others stated that this situation did not affect them because they were already used to a patient-centred model and that the maximum responsibility for their care lay with them:
What sort of care do you expect? We have to care for ourselves … no matter how much they call me and ask me if I’m following my diet, if I’m eating properly, if I’m walking … no matter how much they call … it's up to you …. it's not an injury that you need someone to come and treat you, this is something that’s your own decision 4.5 (P4).
Most participants monitored their blood glucose:
Because I knew I had to check my glucose, I checked it every day and no problem 7.11 (P7).
Proactive self-care
In the management of their disease during lockdown, patients with T2D introduced changes in terms of their physical activity, diet, and medication. Given the impossibility of going outside to exercise, many adapted their physical activity to their home space:
Well, being at home, I coped quite well. I went out onto the rooftop, where I was able to move around and pass the time. I walked up the stairs two or three times. So, I coped quite well 2.2 (P2).
Many participants established routines and did their regular activities, at different levels of intensity:
Every day, every day, every day, and it started … first I started 15 min a day, and then went up to 45 min every day and more intense; I walked fast, then I ran, faster and faster until I got a sore back 4.4 (P4).
This change in physical activity was regarded as a problem by some, but not by others:
I would open all the doors of the apartment and go around until I got tired, and when I got tired, I stopped. It was very boring 1: 5 (P1).
As there was time for everything (…), establishing a routine of walking one hour in the morning and one in the afternoon was not too hard 10: 7 (P10).
However, others abruptly stopped taking exercise:
I didn't do any physical activity while the stay-at-home order lasted 6.6 (P6).
All participants had access to fresh food and their normal diet, since the food shops stayed open during lockdown.
The shops where I go have got everything, fish, meat, chicken, everything 11.9 (P1).
Most reported good adherence to their regular diet:
Well, I saw that I couldn’t … do anything else, or go out … well, it's better to take care of yourself a little, isn’t it? This is also unconscious because I don't think about being diabetic … it’s something I’ve just accepted …. 13.7 (P4).
Others ate between meals, out of stress or boredom:
When I’m nervous, when I’m anxious … there are people whose stomachs close up, but I’m the opposite. I have snacks even though I’m not hungry 12.2 (P3).
None of the respondents had trouble getting their usual medication, and they followed their prescriptions, although they stressed that they were taking the medication without any medical supervision:
What I did is what I always did, there was no change. I went to the pharmacy every month to get my medication 11.9 (P1).
Most participants complied with the recommendations regarding personal protective equipment, hand washing, disinfection, ventilation of the home, and social distancing.
I was careful with my mask, I washed my hands a lot, and cleaned the flat 3.11 (P3).
Some participants applied specific protective measures in their homes:
At the door everyone took off their shoes, and they left their coats in a separate room, they sprayed their hands continuously, and every other day I changed the bed linen, ventilated the flat, cleaned everything. (…). Every time I went to the bathroom I pulled the chain with the lid down, and then cleaned my hands with disinfectant and the toilet as well 4:14 (P4).
Others reported taking particularly strict protective measures, due to their condition:
I took much more care (than other people) because I’m diabetic 8:12 (P8).
Some participants reported that they kept their distance from others, due to their diabetes:
I kept away because I thought I was much more likely to infect them than they were to infect me … so to avoid contagion I kept away from them 8:17 (P8).
Or that their families imposed this distancing on them, in order to protect them:
I asked her [the participant’s granddaughter] to give me a kiss because I needed one, but she said, “No, grandma, I have to go to school, and I don't want to” … And I said, “I’ll just give you a little kiss on your head” and she said “No, no, no!” She wouldn’t let me … 3: 9 (P3).
We have analysed the experiences of patients with T2D in lockdown during the first wave of the COVID-19 pandemic in Catalonia, Spain, and their strategies for managing their disease. Patients with diabetes felt especially vulnerable to infection, and presented emotional difficulties similar to those recorded in patients with COVID-19 at home or with other chronic conditions [ 6 ]. However, despite the changes they experienced in their daily lives and the barriers to accessing chronic care follow-up in primary care centres, they were able to establish routines for self-care.
Fear, anxiety, and vulnerability
Global guidelines on containment measures for the prevention of COVID-19 place special emphasis on vulnerable populations, including people with diabetes [ 23 ]. Our results show that when patients were aware of the risk of contracting COVID-19 due to their T2D status they felt particularly vulnerable and fearful of falling ill. Our data are in line studies showing that having a chronic illness (including T2D), belonging to a risk group, or the death of a family member due to COVID-19 are positively associated with fear of COVID-19 [ 24 ]. The emotional impact of the pandemic was considerable, as the necessary lifestyle changes caused feelings of anxiety among many patients. Elsewhere, the pandemic has been associated with increased stress in general populations, and external stress may reduce physical activity and lead to a poorer diet [ 25 ].
The participants engaged in social distancing due to their fear of infecting others but found the experience to be emotionally challenging. Indeed, due to the high mortality related to COVID and the frequency of near-death experiences, an increased awareness of mortality has been reported during lockdown [ 25 ]. Not only diabetic patients have this perception: other patients with chronic and immunocompromised diseases such as cancer, rheumatoid arthritis, asthma, Crohn’s disease, hypertension, and cardiovascular disease also felt anxiety and fear during the pandemic [ 26 ].
Despite the negative emotional experience of most, for some participants, the suspension of everyday life routines represented an opportunity for reflecting on what was most important to them.
In contrast to reports in other countries [ 27 ], our participants had no difficulty accessing medication or blood glucose control equipment such as glucose strips, needles, or glucometers. However, all of them encountered barriers to accessing primary care. Although they expressed understanding of the pandemic situation, many felt abandoned by the health care system, as other researchers have reported [ 28 ].
Our data suggest that the use of telemedicine and an e-Health model could achieve satisfactory levels of self-care especially in patients with an hb1Ac greater than 6. The popularisation of the Internet and the use of smartphones and emerging fifth-generation networks have allowed patients to attend medical appointments remotely instead of coming to the hospital during the COVID-19 outbreak.
Our results also provide relevant data regarding blood glucose control during the COVID-19 pandemic. Although diabetes is a primary risk factor for the development of severe and septic pneumonia due to infection, patients do not generally intensify their metabolic controls [ 29 ]. This may be due to a lack of information received from professionals monitoring the chronicity of primary pare or due to the lack of protocols or clinical practice guidelines adapted to the situation. In the study by [ 30 ], medication intake was significantly reduced during the pandemic, although in our study compliance with medication intake remained good.
Changes in the provision of health care due to the pandemic have created the need for greater attention to emotional and psychosocial health of patients with T2D. [ 31 ].
The measures imposed by the authorities affected the daily life of the general population as well as that of patients with T2D. In general, this situation was experienced negatively, given that it caused social and family isolation.
The restrictions introduced by the authorities to prevent or reduce the risk of virus transmission led to significant changes in diabetes control. One of the nursing strategies applied to address the needs of patients with T2D in primary care was to promote self-care. Self-care-focused nursing interventions can achieve significant improvements in responsibility for health, physical activity, nutrition, and stress management [ 32 ].
All patients had access to fresh food, since food shops remained open during the lockdown and most patients continued with their usual diets. Most already had a good adherence to diet, although some reported eating between meals out of boredom. Although our participants had access to medication and food, the pandemic made it more difficult to manage their diabetes [ 31 ].
The results show that the stay-at-home order forced patients with T2D to limit their activities, including physical activity. Barone et al. [ 33 ] found that physical activity in diabetic patients fell by 59.5% during the COVID-19 pandemic and suggested that this variable be closely monitored due to its potential negative consequences on metabolic and cardiovascular health. As regards physical exercise, some patients reported decreased activity; others adapted their routines at home to be able to carry out physical activities recommended for a healthy lifestyle, such as walking, running, and going up and down stairs. A few participants performed no physical activity during lockdown, and some achieved optimal T2D risk prevention values, by brisk walking or by observing the current recommendation of 150 min/week of moderate aerobic activity or 30 min/day for 5 days/week [ 34 ]. Despite these changes in behaviour, however, the amount of time devoted to exercise was not optimal for preventing the risks caused by diabetes. The emotional and social impact on certain patients may also be related to the reduction in physical activity, as regular exercise is acknowledged to improve the mental and social health of patients with T2D [ 35 ].
Limitations
This study has several limitations. The first is that the results can only be extrapolated to similar clinical contexts and similar users. The sample is small, and therefore is not representative of all T2D patients with a similar profile. This study can be a launch point, useful for comparison with larger studies in other contexts, to identify best practices in caring for people with T2D during a health crisis.
Second, the context in which the study was carried out was limited to primary care centres in Catalonia with specific sociodemographic characteristics and with good adherence to their prescribed T2D care. Including other types of patients from other geographic areas could provide different results.
Finally, our study includes only the perspective of patients. A fuller picture would emerge if the perspective of nurses monitoring diabetic patients were also included.
Conclusions
This study has provided information on the experiences and emotional responses of patients with T2D during home confinement and on the adaptation of the management of their pathology in Catalonia, Spain. All participants were diabetic patients with good adherence to treatment prior to the pandemic. Due to their health status, patients reported feeling highly vulnerable and fearful of infection. Despite this, patients with T2D were able to establish self-care routines for physical activity and nutrition. In some cases, the lack of access to their normal care at primary care centres made them feel abandoned, although the fact that they were well and that their blood sugar levels were within the recommended levels meant that they did not feel particularly anxious; in general, they were sympathetic to the situation of the health workers. A silver lining of the pandemic may be the way it allowed patients to take control of their disease. This pro-activity on the part of patients should be considered in preparation for future health crises.
Availability of data and materials
The interviews were conducted by the lead author and she is the only researcher who knew the identity of the participants. Her record of interviewees’ names and other personal information will be deleted after publication. Data will be provided upon reasonable request.
Ioannidis JPA. OMS | Tasa de letalidad por la infección de la COVID-19 calculada a partir de los datos de seroprevalencia. WHO [Internet]. World Health Organization; 2021 [Cited 2021 May 26]; Available from: http://www.who.int/bulletin/volumes/99/1/20-265892-ab/es/
Bellido V, Pérez A. Consequences of COVID-19 on people with diabetes. Endocrinol Diabetes y Nutr [Internet]. Elsevier Doyma; 2020 [cited 2021 May 26];67:355–6. Available from: https://www.elsevier.es/es-revista-endocrinologia-diabetes-nutricion-13-articulo-consecuencias-covid-19-sobre-personas-con-S253001642030104X
International Diabetes Federation. COVID-19: Perspectives from people with diabetes. Diabetes Res Clin Pract. 2020;163:108201. https://doi.org/10.1016/j.diabres.2020.108201 .
Banerjee M, Chakraborty S, Rimesh P. Diabetes self-management amid COVID-19 pandemic | Elsevier Enhanced Reader. Diabetes Metab Syndr [Internet]. 2020;14:351–4. [Cited 2021 May 26]. Available from: https://reader.elsevier.com/reader/sd/pii/S1871402120300783?token=FB54D3A227BBCC2E2790E384834A32E17A24F9748CDB1F68563AA3F7178AD32A01D7F49F8B1A42EF80BD4D2E9DEB83B3&originRegion=eu-west-1&originCreation=20210526105736 .
Schmidt SK, Hemmestad L, Macdonald CS, Langberg H, Valentiner LS. Motivation and barriers to maintaining lifestyle changes in patients with type 2 diabetes after an intensive lifestyle intervention (The U-TURN trial): A longitudinal qualitative study. Int J Env Res Public Heal. 2020;17:1–16 MDPI AG.
Google Scholar
Musche V, Kohler H, Bäuerle A, Schweda A, Weismüller B, Fink M, et al. Covid-19-related fear, risk perception, and safety behavior in individuals with diabetes. Healthc [Internet]. 2021;9:480. MDPI AG; [Cited 2021 Jul 19]. Available from: https://doi.org/10.3390/healthcare9040480 .
Sánchez-Villena AR, de La Fuente-Figuerola V. COVID-19: cuarentena, aislamiento, distanciamiento social y confinamiento, ¿son lo mismo? An Pediatr [Internet]. 2020;93:73–4. Elsevier; [Cited 2022 Mar 1]. Available from: https://www.analesdepediatria.org/es-covid-19-cuarentena-aislamiento-distanciamiento-social-articulo-S1695403320301776 .
Eberle C, Stichling S. Impact of COVID-19 lockdown on glycemic control in patients with type 1 and type 2 diabetes mellitus: a systematic review. Diabetol Metab Syndr [Internet]. BioMed Central Ltd. 2021;13:1–8. [Cited 2022 Mar 5]. Available from: https://doi.org/10.1186/s13098-021-00705-9 .
Makki I, Alnoon N, Rahmani N, Almulla J, Alamiri A, Alfalasi A, et al. Impact of COVID 19 Lockdown on Glycemic Control in Patients with Type 2 Diabetes Mellitus in Dubai. Curr Diabetes Rev [Internet]. Curr Diabetes Rev; 2021 [cited 2022 Mar 5];18. Available from: https://pubmed.ncbi.nlm.nih.gov/34879807/
Kemppainen V, Tossavainen K, Turunen H. Nurses’ roles in health promotion practice: An integrative review. Heal Promot Int. 2013;28:490–501.
Article Google Scholar
Agencia de SalutPública de Catalunya. INFORME-TECNIC-1-COVID-19–19032020. 2020.
Ojeda Ibáñez MF. Telemedicina como estrategia para el control de los pacientes con diabetes mellitus tipo ll en el contexto de pandemia por la COVID-19. Estado del arte [Internet]. Universidad Peruana Cayetano Heredia; 2021. Available from: https://repositorio.upch.edu.pe/handle/20.500.12866/9262
Zhai YK, Zhu WJ, Cai YL, Sun DX, Zhao J. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltimore) [Internet]. Medicine (Baltimore); 2014 [cited 2022 Mar 1];93:e312. Available from: https://pubmed.ncbi.nlm.nih.gov/25526482/
Singh K, Kaushik A, Johnson L, Jaganathan S, Jarhyan P, Deepa M, et al. Patient experiences and perceptions of chronic disease care during the COVID-19 pandemic in India: a qualitative study. BMJ Open [Internet]. BMJ Open; 2021 [cited 2022 Mar 2];11. Available from: https://pubmed.ncbi.nlm.nih.gov/34145019/
Shi C, Zhu H, Liu J, Zhou J, Tang W. Barriers to self-management of type 2 diabetes during covid-19 medical isolation: A qualitative study. Diabetes Metab Syndr Obes [Internet]. 2020;13:3713–25. Dove Medical Press Ltd; [Cited 2021 Jul 22]. Available from: https://pubmed.ncbi.nlm.nih.gov/33116721/ .
Colorafi KJ, Evans B. Qualitative Descriptive Methods in Health Science Research. HERD [Internet]. 2016;9:16–25. HERD; [Cited 2022 Mar 7]. Available from: https://pubmed.ncbi.nlm.nih.gov/26791375/ .
Morse JM. Molding qualitative health research. Qual Heal Res [Internet]. SAGE PublicationsSage CA: Los Angeles, CA; 2011 [cited 2022 Mar 7];21:1019–21. Available from: https://journals.sagepub.com/doi/ https://doi.org/10.1177/1049732311404706
Tulloch H, Sweet S, Fortier M, Capstick G, Kenny G, Sigal R. Exercise facilitators and barriers from adoption to maintenance in the diabetes aerobic and resistance exercise trial. Can J Diabetes [Internet]. 2013;37:367–74. [Cited 2014 Dec 17]. Available from: http://www.sciencedirect.com/science/article/pii/S1499267113012744 .
Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant [Internet]. 2018;52:1893–907. Qual Quant; [Cited 2022 Mar 15]. Available from: https://pubmed.ncbi.nlm.nih.gov/29937585/ .
Braun V, Clarke V. What can ‘thematic analysis’ offer health and wellbeing researchers? Int J Qual Stud Health Well-being. 2014;9:20–2.
Guba E, Lincoln Y. Competing paradigms in qualitative research. In: Denzin N, Lincoln Y, editors. Handb Qual Res. Thousands Oaks, California: Sage; 1994. p. 105–17.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Heal Care. 2007;19:349–57.
Beran D, Aebischer Perone S, Castellsague Perolini M, Chappuis F, Chopard P, Haller DM, et al. Beyond the virus: Ensuring continuity of care for people with diabetes during COVID-19. Prim Care Diabetes. 2021;15:16–7.
TzurBitan D, Grossman-Giron A, Bloch Y, Mayer Y, Shiffman N, Mendlovic S. Fear of COVID-19 scale: Psychometric characteristics, reliability and validity in the Israeli population. Psychiatry Res. 2020;289:113100 Elsevier Ireland Ltd.
Article CAS Google Scholar
Munekawa C, Hosomi Y, Hashimoto Y, Okamura T, Takahashi F, Kawano R, et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: A cross-section and retrospective cohort study. Endocr J [Internet]. 2021;68:201–10. Japan Endocrine Society; [Cited 2021 Jul 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/32999133/ .
Al-Rahimi JS, Nass NM, Hassoubah SA, Wazqar DY, Alamoudi SA. Levels and predictors of fear and health anxiety during the current outbreak of COVID-19 in immunocompromised and chronic disease patients in Saudi Arabia: A cross-sectional correlational study. PLoS One [Internet]. Public Library of Science; 2021 [cited 2021 Jul 22];16. Available from: https://pubmed.ncbi.nlm.nih.gov/33901260/
Wicaksana AL, Hertanti NS, Ferdiana A, Pramono RB. Diabetes management and specific considerations for patients with diabetes during coronavirus diseases pandemic: A scoping review. Diabetes Metab Syndr. 2020;14(5):1109–20. https://doi.org/10.1016/j.dsx.2020.06.070 .
Jeong IK, Yoon KH, Lee MK. Diabetes and COVID-19: Global and regional perspectives. Diabetes Res Clin Pr. 2020;166:108303 Elsevier Ireland Ltd.
Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol Elsevier Ltd. 2020;8:546–50.
Alshareef R, Zahrani A Al, Alzahrani A, Ghandoura L. Impact of the COVID-19 lockdown on diabetes patients in Jeddah, Saudi Arabia. Diabetes Metab Syndr Clin Res Rev [Internet]. 2020;14:1583–7. Elsevier Ltd; [Cited 2021 May 26]. Available from: https://pubmed.ncbi.nlm.nih.gov/32947759/
Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complicat [Internet]. J Diabetes Complications; 2020 [cited 2022 Mar 15];34. Available from: https://pubmed.ncbi.nlm.nih.gov/33059981/
Spoorenberg S, Wynia K, Uittenbroek RJ, Kremer H, Reijneveld SA. Effects of a population-based, person-centred and integrated care service on health, wellbeing and self-management of community-living older adults: A randomised controlled trial on Embrace. PLoS One. 2018;13. https://doi.org/10.1371/journal.pone .
Barone MTU, Harnik SB, de Luca PV, Lima BL de S, Wieselberg RJP, Ngongo B, et al. The impact of COVID-19 on people with diabetes in Brazil. Diabetes Res Clin Pr. 2020;166:108304. https://doi.org/10.1016/j.diabres.2020.108304 .
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care. 2010;33(12):e147–67. https://doi.org/10.2337/dc10-9990 .
Wylie TAF, Shah C, Connor R, Farmer AJ, Ismail K, Millar B, et al. Transforming mental well-being for people with diabetes: research recommendations from Diabetes UK’s 2019 Diabetes and Mental Well-Being Workshop. Diabet Med. 2019;36:1532–8.
Download references
Acknowledgements
The research team would like to thank all participants for their collaboration. We would also like to thank the expert Dr. Susan Frekko.
This research received no external funding.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Nursing, Faculty of Health Science and Welfare, University of Vic-Central University of Catalonia (UVIC-UCC), Av. Universitaria 4-6, 08242, Manresa, Spain
Mireia Vilafranca Cartagena
Althaia Fundation, C/Dr Joan Soler 1-3, 08243, Manresa, Spain
Department of Nursing, Faculty of Nursing and Physiotherapy, University of Lleida, C/Montserrat Roig, 25198, Lleida, Spain
Glòria Tort-Nasarre
SAP ANOIA. Gerencia Territorial Catalunya Central. Institut Català de La Salut, 087272, Sant Fruitós del Bages, Spain
AFIN Research Group and Outreach Centre, Autonomous University of Barcelona. Campus Bellaterra, Cerdanyola del Vallès, Spain
Glòria Tort-Nasarre & Maria Romeu-Labayen
Department of Public Health, Mental Health and Mother-Infant Nursing, Faculty of Medicine and Health Sciences, University of Barcelona, L’Hospitalet del Llobregat, Spain
Maria Romeu-Labayen
Health Promotion in Rural Areas Research Group, Gerència Territorial de La Catalunya Central, Institut Català de La Salut, 08272, Sant Fruitós de Bages, Spain
Josep Vidal-Alaball
Unitat de Suport a La Recerca de La Catalunya Central, Fundació Institut Universitari Per a La Recerca a L’Atenció Primària de Salut Jordi Gol I Gurina, 08272, Sant Fruitós de Bages, Spain
You can also search for this author in PubMed Google Scholar
Contributions
The research team was formed by three nurses (authors 1, 2 and 4) and a medical doctor (author 3). All the authors have experience with qualitative research, but authors 2 and 4 have long experience. Author 4 proposed the study, contributed to its design and to data analysis and supervised the project. Author 2 contributed to analysis. Author 3 examined both the processing and product of the research study. Author 1, who is also the PI, conducted the interviews and contributed to the analysis and to writing the discussion. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Mireia Vilafranca Cartagena .

Ethics declarations
Ethics approval and consent to participate.
The study was approved by the ethics and research committee “Fundació Unió Catalana d'Hospitals” (code nº 19/45) and also complied with the principles of the Helsinki Declaration. Participants received verbal and written information explaining that their participation was voluntary and that they could withdraw from the project at any time. All participants provided informed consent. All interviews were anonymised by assigning an alphanumeric code in observance of the Spanish legislation on personal data protection of 2018.
Consent for publication
All the authors gave their consent for publication.
Competing interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vilafranca Cartagena , M., Tort-Nasarre, G., Romeu-Labayen, M. et al. The experiences of patients with diabetes and strategies for their management during the first COVID-19 lockdown: a qualitative study. BMC Nurs 21 , 124 (2022). https://doi.org/10.1186/s12912-022-00911-4
Download citation
Received : 09 November 2021
Accepted : 09 May 2022
Published : 24 May 2022
DOI : https://doi.org/10.1186/s12912-022-00911-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 2 diabetes mellitus
- Patient isolation
- Primary health care
- Qualitative Research
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]
- Open access
- Published: 09 December 2022
Acceptability and feasibility of continuous glucose monitoring in people with diabetes: protocol for a mixed-methods systematic review of quantitative and qualitative evidence
- Jennifer V. E. Brown ORCID: orcid.org/0000-0003-0943-5177 1 ,
- Ramzi Ajjan 2 ,
- Najma Siddiqi 1 , 3 , 4 &
- Peter A. Coventry 1 , 5 , 6
Systematic Reviews volume 11 , Article number: 263 ( 2022 ) Cite this article
2880 Accesses
1 Citations
6 Altmetric
Metrics details
Good glycaemic control is a crucial part of diabetes management. Traditional assessment methods, including HbA1c checks and self-monitoring of blood glucose, can be unreliable and inaccurate. Continuous glucose monitoring (CGM) offers a non-invasive and more detailed alternative. Availability of this technology is increasing worldwide. However, there is no current comprehensive evidence on the acceptability and feasibility of these devices. This is a protocol for a mixed-methods systematic review of qualitative and quantitative evidence about acceptability and feasibility of CGM in people with diabetes.
We will search MEDLINE, Embase, CINAHL, and CENTRAL for qualitative and quantitative evidence about the feasibility and acceptability of CGM in all populations with diabetes (any type) using search terms for “continuous glucose monitoring” and “diabetes”. We will not apply any study-type filters. Searches will be restricted to studies conducted in humans and those published from 2011 onwards. We will not restrict the search by language. Study selection and data extraction will be carried out by two reviewers independently using Rayyan and Eppi-Reviewer, respectively, with disagreements resolved by discussion. Data extraction will include key information about each study, as well as qualitative evidence in the form of participant quotes from primary studies and themes and subthemes based on the authors’ analysis. Quantitative data relating to acceptability and feasibility including data loss, adherence, and quantitative ratings of acceptability will be extracted as means and standard deviations or n/N as appropriate. Qualitative evidence will be analysed using framework analysis informed by the Theoretical Framework of Acceptability. Where possible, quantitative evidence will be combined using random-effects meta-analysis; otherwise, a narrative synthesis will be performed. The most appropriate method for integrating qualitative and quantitative findings will be selected based on the data available.
Ongoing assessment of the acceptability of interventions has been identified as crucially important to scale-up and implementation. This review will provide new knowledge with the potential to inform a programme theory of CGM as well as future roll-out to potentially vulnerable populations, including those with severe mental illness.
Systematic review registration
PROSPERO CRD42021255141.
Peer Review reports
For individuals living with diabetes, glucose control is an important part of self- and clinical management [ 1 , 2 , 3 , 4 ]. Inadequately controlled glucose levels can lead to serious microvascular and macrovascular complications, creating significant strain on the health system and impairing quality of life [ 5 ]. Traditionally, glycaemic control has been assessed using glycated haemoglobin (HbA1c) as a proxy measure of average blood glucose over the 8- to 12-week period prior to measurement [ 6 ]. However, due to the nature of HbA1c, this measurement does not detect hypoglycaemia and fluctuations in glucose levels, both of which are implicated in adverse clinical outcome [ 4 , 6 ]. As any given HbA1c measurement might correspond to a range of mean glucose levels, HbA1c may, for some patients, fail to reliably indicate how well their glucose is controlled [ 4 , 7 ]. Self-monitoring of blood glucose (SMBG) throughout the day using finger-prick tests has been used as an adjunct to regular HbA1c checks to support glycaemic control [ 4 , 8 ]. SMBG places the onus on the user, is often perceived as burdensome, and compliance can be low [ 3 , 4 , 9 , 10 , 11 ]. Furthermore, data are not collected continuously during a 24-h period, for example overnight or while the individual is working, driving, or otherwise occupied, leading to glucose levels throughout large parts of the day not being captured [ 3 , 4 , 12 ].
In recent years, continuous glucose monitoring (CGM), measuring glucose in interstitial tissue, has become increasingly used in diabetes care, particularly for individuals with type 1 or insulin-controlled type 2 diabetes [ 3 , 7 , 8 , 13 , 14 ]. CGM systems provide a more comprehensive assessment of glycaemia by measuring glucose levels every 5–15 min (i.e. 96–298 readings/day), which would be impossible with SMBG [ 4 ]. The burden on the individual is also reduced considerably.
CGM systems continue to be developed by a number of different manufacturers (see Lin et al. [ 15 ] and Bruttomesso et al. [ 16 ], for an overview). They can be categorised into blinded systems (also called professional CGM, where glucose readings are not immediately visible to the wearer), unblinded systems, and flash glucose monitors (also called intermittently scanned CGM or isCGM; see, for example, Wood et al., 2018) [ 12 ]. CGM systems work by giving individuals the means to know their blood glucose levels as part of diabetes self-management without controlling blood glucose directly.
While CGM devices have been around for decades, their use has been restricted due to low accuracy, high cost, and bulky devices. More recently, however, CGM sensors made a significant improvement in accuracy and became more affordable, allowing widespread use, particularly for individuals with type 1 diabetes. International guidelines suggest that CGM should be considered as an option to support the assessment of glucose profiles in people receiving insulin, particularly in those having difficulties controlling glucose levels [ 17 ]. Different commissioning and prescribing arrangements are in place internationally. For example, in the UK, new guidance from the National Institute for Health and Care Excellence (NICE) is expected to recommend access to CGM or isCGM for all adults with type 1 diabetes [ 18 ]. Currently, specific groups of individuals with type 2 diabetes can also access CGM technology, provided they meet the following criteria: insulin treatment plus a learning disability, receiving haemodialysis, or diabetes associated with cystic fibrosis [ 19 ]. NICE guidance under development suggests that isCGM be made available to a wider range of adults with type 2 diabetes [ 20 ]. Furthermore, there is an emerging body of evidence that CGM may have the potential to improve maternal and infant outcomes in pregnant women with diabetes [ 21 ].
Understanding the feasibility and acceptability of innovative health technologies, such as CGM systems, is an important step in assessing their scalability and potential implementation [ 22 , 23 ]. This also applies to interventions already rolled out, given that better understanding of contextual factors can help address key uncertainties about how and why an intervention does — or does not — work [ 24 ]. Continuous and iterative assessment of feasibility and acceptability is promoted by the new Medical Research Council (MRC) framework for the development and evaluation of complex interventions which highlights that uncovering contextual factors and change mechanisms of existing interventions is crucial to intervention improvement and scalability [ 24 ]. Importantly, if acceptability and feasibility can be confirmed, there is potential for wider use of CGM in populations who might otherwise struggle to (self-) manage diabetes, such as people with severe mental illness (SMI). An established body of evidence indicates that people with SMI have higher rates of diabetes and die 20 to 25 years younger than the general population [ 25 , 26 ]. Improved CGM access might improve their glucose profile reducing complication rates, thus addressing this health inequality and mortality gap.
However, there are no recent systematic reviews of the acceptability and feasibility of CGM among people with diabetes. Existing reviews are largely unsystematic without comprehensive searches, clearly defined eligibility criteria, or reproducible methods reported in line with PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidance [ 27 ]. While available reviews tend to report a positive user experience and possible improvements in quality of life [ 15 , 16 , 28 , 29 ], they do not provide robust evidence for acceptability or feasibility. Others have focused on technical parameters and/or effectiveness [ 12 , 30 , 31 , 32 ], rather than acceptability and feasibility, or included one type of CGM system only [ 28 , 29 ]. The one existing Cochrane review of CGM is a decade old and focused only on type 1 diabetes; none of the included primary studies reported patient satisfaction or acceptability data, while quality of life was inconsistently reported, and results were inconclusive [ 33 ].
This is a protocol for a mixed-methods systematic review of qualitative and quantitative evidence relating to the acceptability and feasibility of CGM. Findings from this review will have the potential to inform understanding of acceptability and feasibility as interlinked concepts that play an important role in the programme theory underpinning the use of CGM in diabetes care.
Review aim and objective
This systematic review aims to evaluate if CGM systems used to support self-management of diabetes are acceptable and feasible to individuals with diabetes, their carers, and the professionals involved in their care, why (not), and in what context(s).
To address this aim, we will systematically review and synthesise quantitative and qualitative evidence about the acceptability and feasibility of CGM systems to support the self-management of any type of diabetes across a range of populations.
This systematic review protocol (PROSPERO registration: CRD42021255141) [ 34 ] is reported in line with the PRISMA-P 2015 statement [ 35 ], including relevant elements from the adapted PRISMA for reporting systematic reviews of qualitative and quantitative evidence [ 36 ]. The completed PRISMA checklist can be found in the online supplementary material .
Definition of acceptability and feasibility
Within the context of this systematic review, we will broadly consider “acceptability” as a measure of whether people have a satisfactory experience using (service users/carers) or deploying (healthcare professionals) CGM systems (“Do they like it?”). In turn, “feasibility” will be an assessment of the logistical aspects of deploying CGM in clinical practice and/or research settings (“Can it be done?”).
The primary quantitative outcome of this review will be acceptability of CGM as measured in patient-reported scales. We will include any scale deemed appropriate by the study authors and will standardise means across different questionnaires where appropriate. Secondary outcomes will be feasibility as measured through data on wear time, uptake, and data loss, as well as attrition rates in included studies.
Acknowledging the iterative nature of mixed-methods systematic reviews, additional quantitative outcomes may be explored based on findings from the included qualitative studies. This will be clearly reported.
Literature searching
We will search MEDLINE, Embase, CINAHL, and CENTRAL for completed and ongoing studies exploring the acceptability and feasibility of CGM in diabetes. We will limit the search to studies conducted with humans. In the interest of producing a current yet comprehensive systematic review, we will limit the search to evidence published in 2011 or later. This will cover important technical developments and advances in accuracy and availability of CGM in the past decade. We will not apply any language or study-type restrictions. Search terms will capture “diabetes” and “continuous glucose monitoring” (including terms for “intermittently scanned CGM” and “flash glucose monitoring”). The full MEDLINE search strategy (developed in partnership with a subject librarian and peer reviewed by an information specialist) is included in Additional file 1 .
Thesis databases (Ethos, ProQuest) and conference proceedings of key international diabetes conferences, such as the International Diabetes Federation Annual Conference and the European Association for the Study of Diabetes Annual Meeting, will also be searched. Relevant systematic and narrative reviews will be used for reference searching to identify additional records. Forward citation tracking will be used to find further studies that have cited included papers.
References will be managed and deduplicated in EndNote [ 37 ]. The final list of unique search results will be exported into the systematic review app Rayyan [ 38 ].
Study selection
Supported by the machine learning algorithm in Rayyan [ 38 ], two reviewers will carry out title and abstract selection independently and in duplicate. Any disagreements will be resolved through discussion.
Full texts will then be sought for all potentially eligible titles and abstracts and imported into EPPI-Reviewer [ 39 ], where two reviewers will independently assess eligibility with disagreements again resolved in discussion.
During study selection, publications relating to the same study will be grouped and a main reference identified. If needed, authors will be contacted to confirm related publications.
Eligibility criteria
Both title and abstract and full-text selection of qualitative and quantitative studies will be based on the following criteria:
- a Studies involving children and/or adolescents and/or their parents, guardians, or other carers will be eligible for inclusion as we expect a considerable part of the existing research to have been conducted in this population. Restricting the eligibility criteria to studies of adults only would risk excluding evidence describing potentially important and relevant experiences of CGM.
Where mixed populations are reported, e.g. participants with and without a diagnosis of diabetes, papers will be included if either results for eligible participants can be extracted separately or the majority of participants (51% or more) were eligible as per the above criteria.
Intervention
Specifically, the following categories of CGM systems will be eligible as follows:
Blinded systems (also called professional CGM) , where the data are stored on the sensor until they are downloaded. No data are fed back to the user automatically. Blinded systems are used primarily in research and as a diagnostic tool in clinical practice [ 31 ].
Unblinded systems , where data are continuously sent to a reading device, such as a specialist reader or a smartphone, allowing the user to observe their blood glucose levels in near-real time. Some systems support the use of alarms or alerts when glucose levels are “out of range”.
Flash glucose monitors (or isCGM), where the data are collected blinded but can be accessed by the user by scanning the sensor with a smartphone or reader. Both flash and unblinded CGM systems are used primarily by individuals receiving insulin treatment and those with type 1 diabetes to support self-management [ 31 ]. Implantable systems fall into this category [ 40 ].
Outcomes/data reported
Study design, data extraction.
Data extraction for all included studies (both quantitative and qualitative) will be carried out in EPPI-Reviewer [ 39 ]. Descriptive information will include year of publication, study setting, sample size, participant details (including group [service users, carers/parents, or healthcare professionals], age [for service users], type of diabetes, medication, comorbidities), description of the CGM system used, and relevant outcomes reported.
Quantitative results will be extracted for any direct measures of acceptability such as questionnaires (means and standard deviation or n/N if data were dichotomised by study authors) as well as proxy measures of acceptability and measures of feasibility such as attrition rates and completeness of data collection. We will use an inclusive approach and extract any outcome data broadly relating to the concepts of acceptability and feasibility.
Qualitative data will be extracted in the form of participant quotes reported in included studies as well as interpretative text and overarching themes identified by study authors. To facilitate framework analysis (see below), extraction of qualitative data will be guided by the Theoretical Framework of Acceptability (TFA) [ 41 ] and organised into the seven domains of the framework: affective attitude, burden, ethicality, intervention coherence, opportunity costs, perceived effectiveness, and self-efficacy. We will also extract for each paper if the outcomes reported related to prospective, concurrent, or retrospective acceptability, as per the TFA.
All data extraction processes will be piloted on a small number of studies to ensure they are fit for purpose. Changes will be made as necessary. Quantitative data extraction will be carried out by one reviewer, and at least 10% will be checked by another. Qualitative data will be extracted and coded to the framework by one reviewer and then discussed with another.
Assessment of risk of bias and study quality
As suggested by Noyes et al. [ 42 ], the mixed-methods appraisal tool [ 43 ] (MMAT) will be used to assess the methodological quality of all included studies. The MMAT includes assessment criteria for qualitative as well as a range of quantitative study designs, offering the ease and convenience of using one tool across all included studies. The following domains are assessed by the MMAT as follows:
Qualitative studies: Appropriateness of approach used, adequacy of data collection methods, adequacy of findings derived from the data, interpretation of results sufficiently substantiated by data, and coherence between data sources, collection, analysis, and interpretation
Quantitative studies:
◦ RCTs: Appropriateness of randomisation, comparability of groups at baseline, completeness of outcome data, blinding of outcome assessors, and adherence of participants to assigned intervention
◦ Non-randomised studies: Representativeness of participants, appropriateness of measurements, completeness of outcome data, accounting for confounders, and intervention (or exposure) delivered as intended
◦ Descriptive studies: Relevancy of sampling strategy, representativeness of sample, appropriateness of measurements, risk of nonresponse bias, and appropriateness of analysis
Mixed-methods studies: Adequacy of rationale for mixed-methods design, effectiveness of integration of study components, adequacy of interpretation of integration, adequacy of addressing discrepancies between components, and methodological quality of study components
Quality assessment will be conducted by one reviewer with at least 10% checked by another.
Sampling of qualitative studies
Regardless of the number of eligible qualitative studies, we will employ a framework to sample studies for inclusion in the analysis. We will follow methods proposed by Ames et al. [ 44 ] which we have successfully deployed previously [ 45 ]. The aim will be to sample for maximum variation and data richness, considering study population and relevance to the review question.
Study population : We will sample all studies that include any of the following study populations:
Individuals with type 2 diabetes
Individuals with diabetes (any type) and reported comorbid mental and physical health conditions
Informal carers
Data richness : Using the criteria presented below, we will include all studies scoring 4 or higher for data richness. The pool of studies scoring 3 will be scrutinised for any studies offering a unique perspective which will then be sampled. All studies scoring 2 or lower, and not in the populations defined in 1 above, will be excluded from the analysis (Table 1 ).
Data richness will be assessed independently by two reviewers. Disagreements will be discussed until consensus is achieved. Details about eligible studies that are not sampled for inclusion in the analysis will be presented in a table along with an explanation of why the study was not sampled.
To avoid the risk of nuanced meaning being lost or biases introduced, we will not attempt translation of qualitative studies where the full text is not available in English and will exclude such papers from the analysis. Articles published in English where the original qualitative data collection was carried out in a non-English language will be eligible for sampling provided translation decisions are reported transparently and in line with the framework proposed by Abfalter et al. [ 46 ].
Certainty of the evidence
To date, no tool exists that specifically supports the assessment of the certainty of evidence in the context of a mixed-methods systematic review. Consequently, separate tools will be used to assess the certainty of the quantitative and qualitative evidence.
For quantitative evidence, we will use the five domains of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) [ 47 ] approach to determine if the certainty in the included evidence can be categorised as high, moderate, low, or very low: methodological quality/risk of bias, inconsistency, indirectness, imprecision, and reporting bias.
Correspondingly, CERQual (Confidence in the Evidence from Reviews of Qualitative research) [ 48 ] will be used to assess the certainty of the qualitative evidence, as recommended by the Cochrane Qualitative and Implementation Methods Group [ 49 ]. Mirroring the GRADE approach, the CERQual domains are as follows: methodological limitations, relevance, coherence, and adequacy of data. The assessment of confidence in the qualitative findings will use four levels (high, moderate, low, very low).
For both qualitative and quantitative evidence, summary of finding tables will be produced, and GRADE/CERQual findings will inform the integrative synthesis.
Given the complexity of “acceptability” as a construct as defined by Sekhon et al. [ 41 ], a mixed-methods approach that includes evidence from quantitative and qualitative studies is best suited to answer the research question. The combination and integration of both types of evidence will add value beyond what separate systematic reviews could offer.
We expect to find considerable clinical and methodological heterogeneity in the included studies and will take this into consideration in the analyses to ensure that only evidence from comparable studies is synthesised. We will group the included studies based on key characteristics such as population, type of diabetes, and type of CGM investigated and decide within the team which studies are similar enough to allow a synthesis of their findings.
Synthesis of quantitative studies
Where possible, we will synthesise quantitative data, including acceptability questionnaire scores, measures of data completeness, and attrition rates, using random-effects meta-analysis. Attrition rates (n/N of participants “dropped out” or withdrawn) will be summarised to calculate relative risk (and 95% confidence interval) of attrition. The I 2 statistic will be used to estimate statistical heterogeneity [ 50 ].
If a meta-analysis of attrition rates is possible, we will further explore the potential impact of study design characteristics on any observed differences in attrition between intervention and control groups. We will conduct sensitivity analyses on attrition outcomes to include only studies with a low risk of attrition bias and compare these findings with all included studies [ 51 ].
We will be guided by the Cochrane Handbook in addressing unit of analysis issues [ 52 ], for example in cluster-randomised or cross-over trials, as well as in our approach to dealing with missing data [ 53 ]. To assess the risk of publication bias within meta-analyses of RCTs, we will generate funnel plots and visually inspect them for asymmetry [ 54 ].
In line with recommendations made in the Cochrane Handbook [ 55 ], non-randomised studies will be meta-analysed assuming they are deemed to be sufficiently homogenous and at low risk of bias.
Where meta-analysis is not possible, data will be synthesised narratively following methods described by Popay et al. [ 56 ] and reported following the synthesis without meta-analysis (SWiM) guidance [ 57 ].
Synthesis of qualitative evidence
To synthesise data from qualitative studies, we will use the TFA [ 41 ] to inform a “best-fit” framework analysis [ 58 ], which has been described as “highly suitable for applied … clinical questions in a specific setting or context” [ 59 ].
Participant quotes from the primary studies as well as author-inferred themes and interpretations will be mapped against the seven domains of the TFA (affective attitude, burden, ethicality, intervention coherence, opportunity costs, perceived effectiveness, and self-efficacy) with the aim to gain insights into the experience of using or deploying CGM.
Any findings that do not fit the TFA will be analysed separately. We will then explore the potential for any additional concepts derived from the primary studies to further inform or enhance the TFA.
Subgroup and sensitivity analyses
Any subgroup analyses will be informed by the qualitative synthesis.
- Integrative synthesis
To maximise the value of this review of quantitative and qualitative evidence, a mixed-methods synthesis will be undertaken. Synthesis will either be conducted following a result-based convergent design, where quantitative and qualitative studies are synthesised separately but simultaneously and then combined in a third synthesis, or a sequential design, where one type of evidence is analysed first with the results from that synthesis and then used to inform the synthesis of the other type of studies [ 60 ]. Either synthesis design will allow for an integration of findings that will provide knowledge beyond that which could be gained from separate syntheses alone.
The TFA will be used as the guiding framework throughout all stages of the synthesis.
Service user and carer involvement
There is strong evidence supporting the involvement of service users and carers in research, and the importance of meaningful involvement is widely recognised [ 61 ], including in systematic reviews [ 62 ]. Based on findings from the qualitative synthesis, we will identify groups who may face particular challenges when using CGM, for example people with SMI or learning disabilities. Building on existing contacts and networks, we plan to arrange interactive workshops during the integrative synthesis phase to share emergent findings and discuss overlap and discrepancies between qualitative and quantitative evidence. These collaborative sessions will also present an opportunity to use service user perspectives to highlight which areas may need to be addressed in the future to improve the acceptability and feasibility of CGMs for groups who have not yet had a chance to use them.
The proposed review will address a gap in our understanding about acceptability and feasibility of an emerging health technology that has the potential to transform diabetes self-management, including among more vulnerable groups. Understanding if these devices are acceptable and feasible to a range of people, including users, their carers, and healthcare professionals, is a crucial step [ 24 ]. It will be the first comprehensive, systematic review in this area and comes at an important time as access to CGM technology is increasing worldwide, including in the UK [ 18 , 20 ].
By using a truly integrative mixed-methods design to combine qualitative and quantitative evidence, the review findings will offer an in-depth evaluation of the acceptability and feasibility of CGM systems. Data analysis will be informed by the TFA [ 41 ], and findings will contribute to our understanding about the overlap of the interrelated concepts of acceptability and feasibility which might have extensions to other applied health research contexts. Findings will have the potential to inform the development of programme theory about the implementation of CGM which may have relevance to individuals living with diabetes and other health conditions, for example SMI. Even though CGM has been in use for several decades, ongoing robust evaluation of feasibility and acceptability is crucial to support reach and scale-up in the context of populations that have hitherto not had access to this technology, including vulnerable adults with SMI. This is recognised and highlighted in the new MRC framework [ 24 ]; our systematic review will have the potential to contribute new knowledge to this process.
The inclusion of qualitative evidence of acceptability and feasibility of using CGM in particular will offer a chance to improve understanding about challenges or barriers faced by patient subgroups and whose, as such, experience might be different compared with the general population with diabetes. In particular, individuals with multimorbidity, i.e. several co-occurring mental or physical health problems, might have a uniquely different user experience. There is a body of evidence to suggest that people with SMI engage differently with healthcare systems than the general population [ 5 , 63 , 64 , 65 , 66 ]. A systematic review by Firth et al. [ 67 ] including data from over 3000 individuals with psychosis suggests that smartphone ownership is increasing in this population, and that technology-supported self-management is well received. In order to ensure that this vulnerable population with a particularly high burden of diabetes does not miss out on innovative technologies, it is crucial to highlight their lived experience explicitly, where it is appropriate to do so.
Furthermore, draft NICE guidance, due to be published in early 2022, recognises that a wide range of individuals with type 2 diabetes (as well as those with type 1 diabetes) can benefit from access to CGM technology [ 18 , 20 ]. While people with SMI are not explicitly included in the guidance, they may fall into a number of the categories that are likely to be eligible for CGM technology once the guidance is published: impaired hypoglycaemia awareness, inability to use SMBG, or needing assistance to monitor blood glucose. The draft guidance recognises the importance of education but fails to include specific recommendations for how this should be delivered. Understanding, qualitatively and quantitatively, what does — and does not — make CGM technology feasible and acceptable to use, will be crucial for developing fit-for-purpose education programmes, including for potentially vulnerable populations and those with lower health literacy.
The research question used to inform this draft NICE guidance only relates to effectiveness of CGM; acceptability is not considered [ 68 ]. As such, it seems pertinent to produce a robust and comprehensive evaluation of acceptability and feasibility of this technology in parallel with the accelerating roll-out in line with steps for intervention evaluation as recommended by the MRC framework [ 24 ].
Dissemination
The completed review will be submitted for publication in a peer-reviewed journal as well as prepared for presentation at relevant conferences. In addition, existing social media and dissemination channels will be used to reach and engage with a wider audience. A plain language summary will be made available online.
Availability of data and materials
Not applicable.
Abbreviations
Confidence in the Evidence from Reviews of Qualitative research
- Continuous glucose monitoring
Grading of Recommendations Assessment, Development and Evaluation
Glycated haemoglobin A1c
Intermittently scanned CGM
Mixed-methods appraisal tool
Medical Research Council
National Institute for Health and Care Excellence (UK)
Preferred Reporting Items for Systematic Review and Meta-Analysis
Randomised controlled trial
Self-monitoring of blood glucose
Severe mental illness
Theoretical Framework of Acceptability
Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet. 2004;364(9444):1523–37.
Article Google Scholar
Nobis S, Lehr D, Ebert DD, Baumeister H, Snoek F, Riper H, et al. Efficacy of a web-based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: a randomized controlled trial. Diabetes Care. 2015;38(5):776–83.
Funtanilla VD, Candidate P, Caliendo T, Hilas O. Continuous glucose monitoring: a review of available systems. Pharm Ther. 2019;44(9):550–3.
Google Scholar
Ajjan RA. How can we realize the clinical benefits of continuous glucose monitoring? Diabetes Technol Ther. 2017;19(S2):S-27–36.
Becker T, Hux J. Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada. Diabetes Care. 2011;34(2):398–402.
Johnson ML, Martens TW, Criego AB, Carlson AL, Simonson GD, Bergenstal RM. Utilizing the ambulatory glucose profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019;21(S2):S2-17–25.
Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–26.
Malandrucco I, Russo B, Picconi F, Menduni M, Frontoni S. Glycemic status assessment by the latest glucose monitoring technologies. Int J Mol Sci. 2020;21(21):8243.
Article CAS Google Scholar
Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence. 2014;8:237–46.
Polonsky WH, Fisher L, Hessler D, Edelman SV. What is so tough about self-monitoring of blood glucose? Perceived obstacles among patients with type 2 diabetes. Diabet Med. 2014;31(1):40–6.
Ward JEF, Stetson BA, Mokshagundam SPL. Patient perspectives on self-monitoring of blood glucose: perceived recommendations, behaviors and barriers in a clinic sample of adults with type 2 diabetes. J Diabetes Metab Disord. 2015;14(1):43.
Wood A, O'Neal D, Furler J, Ekinci EI. Continuous glucose monitoring: a review of the evidence, opportunities for future use and ongoing challenges. Intern Med J. 2018;48(5):499–508.
Chen C, Zhao X-L, Li Z-H, Zhu Z-G, Qian S-H, Flewitt AJ. Current and emerging technology for continuous glucose monitoring. Sensors. 2017;17(1):182.
Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019;43(4):383–97.
Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med. 2021;n/a(n/a):e14528.
Bruttomesso D, Laviola L, Avogaro A, Bonora E, Del Prato S, Frontoni S, et al. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: a consensus view of Italian diabetes experts using the Delphi method. Nutr Metab Cardiovasc Dis. 2019;29(5):421–31.
International Diabetes Federation. Global guidelines for type 2 diabetes. 2012.
National Institute for Health and Clinical Excellence (NICE). Guideline: type 1 diabetes in adults: diagnosis and management. Draft for consultation 2021 [Available from: https://www.nice.org.uk/guidance/GID-NG10265/documents/draft-guideline ]. Accessed 5 Dec 2021.
NHS England. Flash glucose monitoring: national arrangements for funding of relevant diabetes patients. 2020.
National Institute for Health and Clinical Excellence (NICE). Guideline: type 2 diabetes in adults: management. Draft for consultation 2021 [Available from: https://www.nice.org.uk/guidance/GID-NG10264/documents/draft-guideline ]. Accessed 5 Dec 2021.
García-Moreno R, Benítez-Valderrama P, Barquiel B, González Pérez-de-Villar N, Hillman N, Lora Pablos D, et al. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabet Med. 2022;39:e14703.
World Health Organization. Scaling up projects and initiatives for better health: from concepts to practice. 2016.
Centre for Epidemiology and Evidence. Increasing the scale of population health interventions: a guide. Sydney: NSW Ministry of Health; 2014.
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061.
Reilly S, Olier I, Planner C, Doran T, Reeves D, Ashcroft DM, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
Ward M, Druss B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry. 2015;2(5):431–51.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Ang E, Lee ZX, Moore S, Nana M. Flash glucose monitoring (FGM): a clinical review on glycaemic outcomes and impact on quality of life. J Diabetes Complications. 2020;34(6):107559.
Leelarathna L, Wilmot EG. Flash forward: a review of flash glucose monitoring. Diabet Med. 2018;35(4):472–82.
Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5(1):39.
Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017;31(1):280–7.
Kamusheva M, Tachkov K, Dimitrova M, Mitkova Z, García-Sáez G, Hernando ME, et al. A systematic review of collective evidences investigating the effect of diabetes monitoring systems and their application in health care. Front Endocrinol. 2021;12(178).
Langendam M, Luijf YM, Hooft L, DeVries JH, Mudde AH, Scholten R. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;1.
Brown JVE, Coventry P, Siddiqi N, Ajjan R. Acceptability and feasibility of continuous glucose monitoring in people with diabetes: protocol for a mixed-methods systematic review of quantitative and qualitative evidence 2021 [Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=255141 ]. Accessed 19 Nov 2021.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. BMC Syst Rev. 2015;4(1):1.
Pluye P, Hong Q, Vedel I. Toolkit for mixed studies reviews (V3). Quebec: Department of Family Medicine, McGill University, and Quebec-SPOR SUPPORT Unit; 2016. [Available from: http://toolkit4mixedstudiesreviews.pbworks.com/ ]
The EndNote Team. EndNote. EndNote X9 ed. Philadelphia: Clarivate; 2013.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. BMC Syst Rev. 2016;5(1):210.
Thomas J, Graziosi S, Brunton J, Ghouze Z, O'Driscoll P, Bond M. EPPI-Reviewer: advanced software for systematic reviews, maps, and ecidence synthesis: EPPI-Centre. London: UCL Social Research Institute, University College London; 2020.
Christiansen M, Klaff L, Bailey T, Brazg R, Carlson G, Tweden K. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: the PRECISION study. Diabetes Technol Ther. 2019;21(5):231–7.
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88.
Noyes J, Booth A, Moore G, Flemming K, Tunçalp Ö, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Glob Health. 2019;4(Suppl 1):e000893.
Hong Q, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT), version 2018. Registration of Copyright (#1148552), Canadian Intellectual Property Office, Industry Canada; 2018.
Ames H, Glenton C, Lewin S. Purposive sampling in a qualitative evidence synthesis: a worked example from a synthesis on parental perceptions of vaccination communication. BMC Med Res Methodol. 2019;19(1):26.
Balogun-Katung A, Carswell C, Brown JVE, Coventry P, Ajjan R, Alderson S, et al. Exploring the facilitators, barriers, and strategies for self-management in adults living with severe mental illness, with and without long-term conditions: a qualitative evidence synthesis. PLoS One. 2021;16(10):e0258937.
Abfalter D, Mueller-Seeger J, Raich M. Translation decisions in qualitative research: a systematic framework. Int J Soc Res Methodol. 2021;24(4):469–86.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gülmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med. 2015;12(10):e1001895.
Noyes J, Booth A, Flemming K, Garside R, Harden A, Lewin S, et al. Cochrane Qualitative and Implementation Methods Group guidance series—paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018;97:49–58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60.
Coventry PA, Meader N, Melton H, Temple M, Dale H, Wright K, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262.
Higgins J, Li H, Deeks J. Chapter 6: Choosing effect measures and computing estimates of effect. In: JPT H, J T, J C, M C, T L, MJ P, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 63: Cochrane; 2022.
Deeks J, Higgins J, Altman D. Chapter 10: Analysing data and undertaking meta-analyses. In: JPT H, J T, J C, M C, T L, MJ P, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 63: Cochrane; 2022.
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Reeves B, Deeks J, Higgins J, Shea B, Tugwell P, Wells G. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 62 (updated February 2021): Cochrane; 2021.
Popay J, Roberts H, Sowden A, Petticrew M, Rodgers M, Britten N. Guidance on the conduct of narrative synthesis in systematic reviews; 2006.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890.
Carroll C, Booth A, Leaviss J, Rick J. “Best fit” framework synthesis: refining the method. BMC Med Res Methodol. 2013;13(1):37.
Flemming K, Noyes J. Qualitative evidence synthesis: where are we at? Int J Qual Methods. 2021;20:1609406921993276.
Hong QN, Pluye P, Bujold M, Wassef M. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. BMC Syst Rev. 2017;6(1):61.
National Institute for Health Research (NIHR). NIHR Annual Report 2019/20. 2021.
Pollock A, Campbell P, Synnot A, Smith M, Morley R. Patient and public involvement in systematic reviews 2021 [Available from: https://g-i-n.net/chapter/systematic-reviews/ ]. Accessed 12 Oct 2021.
Chadwick A, Street C, McAndrew S, Deacon M. Minding our own bodies: reviewing the literature regarding the perceptions of service users diagnosed with serious mental illness on barriers to accessing physical health care. Int J Ment Health Nurs. 2012;21(3):211–9.
De Hert M, Cohen D, Bobes J, Cetkovich-Bakmas M, Leucht S, Ndetei DM, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138–51.
Karim MA, Al-Baz N, Ouanes S, Khalil A, Assar AH, Alsiddiqi A, et al. Quality of diabetes care in patients with schizophrenia: a case-control study in Qatar. BMC Psychiatry. 2021;21(1):149.
van Hasselt FM, Oud MJT, Loonen AJM. Improvement of care for the physical health of patients with severe mental illness: a qualitative study assessing the view of patients and families. BMC Health Serv Res. 2013;13(1):426.
Firth J, Cotter J, Torous J, Bucci S, Firth JA, Yung AR. Mobile phone ownership and endorsement of “mHealth” among people with psychosis: a meta-analysis of cross-sectional studies. Schizophr Bull. 2016;42(2):448–55.
National Institute for Health and Clinical Excellence (NICE). Diabetes, type 1 and type 2 - continuous glucose monitoring in adults. Review questions 2021 [Available from: https://www.nice.org.uk/guidance/gid-ng10264/documents/review-questions ]. Accessed 5 Dec 2021.
Download references
Acknowledgements
The authors would like to thank Judy Wright for peer reviewing the search strategy.
Author information
Authors and affiliations.
Department of Health Sciences, University of York, York, YO10 5DD, UK
Jennifer V. E. Brown, Najma Siddiqi & Peter A. Coventry
Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK
Ramzi Ajjan
Hull York Medical School, York, UK
Najma Siddiqi
Bradford District Care NHS Foundation Trust, Bradford, UK
York Environmental Sustainability Institute, University of York, York, UK
Peter A. Coventry
Leverhulme Centre for Anthropocene Biodiversity, University of York, York, UK
You can also search for this author in PubMed Google Scholar
Contributions
JVEB conceptualised and designed this review protocol and developed the methods. RA, NS, and PAC provided supervision and critical feedback on drafts. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jennifer V. E. Brown .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
RA received honoraria, consultancy fees and research funding from Abbott Diabetes Care. JVEB, NS, and PAC declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
MEDLINE search strategy.
Additional file 2.
PRISMA-P 2015 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Brown, J.V.E., Ajjan, R., Siddiqi, N. et al. Acceptability and feasibility of continuous glucose monitoring in people with diabetes: protocol for a mixed-methods systematic review of quantitative and qualitative evidence. Syst Rev 11 , 263 (2022). https://doi.org/10.1186/s13643-022-02126-9
Download citation
Received : 08 March 2022
Accepted : 08 November 2022
Published : 09 December 2022
DOI : https://doi.org/10.1186/s13643-022-02126-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mixed methods
- Qualitative
- Quantitative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 01 August 2022
Quantitative and qualitative analysis of the quality of life of Type 1 diabetes patients using insulin pumps and of those receiving multiple daily insulin injections
- Lilian Tzivian 1 ,
- Jelizaveta Sokolovska 1 ,
- Anna E. Grike 2 ,
- Agate Kalcenaua 3 , 4 ,
- Abraham Seidmann 5 , 6 ,
- Arriel Benis 7 , 8 ,
- Martins Mednis 4 ,
- Ieva Danovska 4 ,
- Ugis Berzins 4 ,
- Arnolds Bogdanovs 4 &
- Emil Syundyukov 4 , 9
Health and Quality of Life Outcomes volume 20 , Article number: 120 ( 2022 ) Cite this article
5192 Accesses
9 Citations
7 Altmetric
Metrics details
Introduction
Insulin pump therapy represents an alternative to multiple daily injections and can improve glycemic control and quality of life (QoL) in Type 1 diabetes mellitus (T1DM) patients. We aimed to explore the differences and factors related to the T1DM-specific QoL of such patients in Latvia.
Design and methods
A mixed-method cross-sectional study on 87 adult T1DM patients included 20 pump users and 67 users of injections who participated in the quantitative part of the study; 8 pump users and 13 injection users participated in the qualitative part. Patients were invited to participate using a dedicated digital platform. Their QoL and self-management habits were assessed using specially developed questionnaires adapted to Latvian conditions. Multiple logistic regression models were built to investigate the association between social and self-management factors and patients’ QoL. In addition, qualitative analysis of answers was performed.
Insulin pump users were younger, had higher incomes, and reported higher T1DM expenses than users of multiple daily injections. There were no differences in self-management between the groups; Total QoL differed at the 0.1 significance level. In fully adjusted multiple logistic regression models, the most important factor that increased Total QoL was lower T1DM-related expenses (odds ratio, OR 7.02 [95% confidence interval 1.29; 38.0]). Men and those with more years of living with T1DM had better QoL (OR 9.62 [2.20; 42.1] and OR 1.16 [1.05; 1.29], respectively), but the method of administration was not significantly associated with QoL (OR 7.38 [0.87; 62.9]). Qualitative data supported the results of quantitative analysis.
Conclusions
QoL was the main reason to use an insulin pump, while the expense was the main reason to avoid the use of it or to stop using it. Reimbursement policies thus should be considered to enable patients to choose the more convenient method for themselves.
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune characterized by hyperglycemia due to loss of insulin producing cells of the pancreas that can end in diabetic coma and eventually death [ 1 , 2 ]. T1DM incidence has increased on average 3–4% over the past 30 years [ 3 ], reaching an incidence of 15 people per 100,000 and a prevalence of 9.5 per 10,000 worldwide [ 4 ]. In Latvia, there were 4169 patients with T1DM in 2015 (prevalence of 211.7 per 100,000), and an incidence of 13.5 people per 100,000 [ 5 ].
The main therapy for T1DM patients is insulin regulation via multiple daily injections or continuous subcutaneous infusions using an insulin pump. Patients aim for glycated hemoglobin (HbA1c) levels below 7% [ 1 ] without an unacceptable incidence of hypoglycemia [ 6 ]. This process demands a certain amount of self-management, such as treatment diaries and recording and interpretation of blood sugar levels. Some patients, however, struggle with these tasks and fail to successfully continue a therapy, especially in the case of multiple daily injections. The use of an insulin pump as a technological solution can simplify efforts to manage the process and to maintain desired levels of blood glucose [ 7 ].
Administration of insulin via a pump improves glycemic control with fewer hypoglycemic episodes in T1DM subjects previously conventionally treated with multiple daily injections, achieving a significant reduction in HbA1c. Meta-analyses reveal that in patients treated with an insulin pump, Hb1A1c decreased more pronouncedly and reported insulin requirements were lower [ 8 ] than for injection patients, especially young children. Severe hypoglycemia episodes were rare, indicating better glycemic control and lower incidence of nocturnal hypoglycemia [ 8 , 9 , 10 ].
The quality of life (QoL) of patients with T1DM is affected by complications and fear of them and is lower than that of healthy peers [ 11 ]. Using an insulin pump reduces fear of severe hyperglycemia and diabetic coma [ 12 , 13 ]. Patients using a pump have more flexible possibilities regarding meals, diet, everyday activities, and socialization [ 14 ], as the pump supports improved self-management habits [ 15 , 16 , 17 ]. Some additional non-health-related benefits, such as reduced worry about supplies while traveling, can significantly improve patients’ QoL as well [ 12 ]. However, the pump itself and related physical restrictions can be mentioned as disadvantages [ 11 , 14 ]. The most prominent problem with an insulin pump is the expense. There is a large difference in cost between injections and a pump. Although studies show that there is a good value for money in the use of a pump, many adult patients may be unable to afford one [ 12 ].
According to the Latvian Diabetes Association, 3700 patients in Latvia currently have T1DM [ 18 ]. Insulin pumps are covered by the state until 18 years old [ 19 ], but adult patients must pay for the pump itself (approximatively 3500 EUR) and also cover the cost of pump-related disposables, amounting to more than 100 EUR per month. Considering the low-income level in Latvia (an average of 583 EUR per household member per month, in 2020) [ 20 ], insulin pump therapy is a huge financial burden. In these circumstances, the investigation of factors related to the QoL of patients using different methods of insulin administration can identify appropriate changes to reimbursement policies to improve such patients’ disease-related conditions.
The aim of this mixed-method cross-sectional study was to compare the QoL and T1DM-related self-management of two groups of patients residing in Latvia—insulin pump users and those who use multiple daily insulin injections, and to investigate factors associated with their QoL. Our main hypotheses were as follows:
The QoL of insulin pump users is better than that of injection users, and T1DM-related self-management is easier for pump users than injection users.
Easier T1DM-related self-management is associated with better QoL.
We investigated also specific reasons to use or not the pump or injections, including the reasons for changes between different methods of insulin administration, using both qualitative and quantitative methodologies. Our main hypothesis was that the major reason for using a specific method of administration and for changes in the method used is treatment-related expenses.
Research design and methods
Study design and population.
The mixed-method cross-sectional study was conducted in April and May 2021 and consisted of a quantitative part and a qualitative part. We chose a combined approach due to the small number of insulin users in Latvia that lead to imprecision in the quantitative results. All T1DM patients at least 18 years of age who signed informed consent forms were eligible to participate in the study. As the total number of adult insulin pump users in Latvia is very small (about 40 users to our knowledge), we invited all of them to participate in the quantitative part of this study. The number of multiple injection users was planned to be in a proportion of 1:2 according to the enrolled sample of insulin pump users, and the calculated power of the study in that case was 80%. For the qualitative part of the study, the number of participants depended on their agreement and on the saturation of interviews—a lack of new information collected during the additional interviews. The saturation was defined by the investigator during the interviewing process. Both groups of participants—those using insulin pumps and those receiving multiple insulin injections—were enrolled in the qualitative part of the study. The study was approved by the Scientific Research Ethic Commission of the Institute of Cardiology and Regenerative Medicine of the University of Latvia on February 2, 2021.
Methods of enrolment of the study participants
Patients learned about the study from e-mail materials received from doctors, patient organizations’ representatives, or diabetes nurses. Additionally, they could learn about the study from posts in the closed Facebook group “Diabetes in Latvia”. We also identified potential study participants using metadata from the longitudinal study “LatDiane: Latvian diabetic nephropathy study”, initiated in 2013 [ 21 ]. Currently, more than 355 well-characterized patients with Type 1 diabetes are in the LatDiane study. Invitations included a description of the study and its objectives, as well as a technical guide for onboarding on the digital platform developed for this study [ 22 ]. Patients were invited to participate using a digital engagement platform, equipped with a dynamic e-consent management tool (Fig. 1 ). The web-based and mobile-ready engagement platform was developed as a collaboration among clinicians, epidemiologists, and data protection and digital health specialists. The website of this study includes detailed instructions in Latvian and Russian, conditions for participation, information regarding the aims and organization of the study, and a contact section. Participants were asked to provide their consent (which could be dynamically managed on the platform, e.g., for opt-out) for data processing, in compliance with the General Data Protection Regulation (GDPR) [ 23 ].

Visual engagement material used in study invitations
Once they provided their informed consent, participants were invited to complete the online questionnaire and were informed about the time slots available for semi-structured interviews. After data collection, the system extrapolated a dataset that described the user survey input results, fully separated from the actual database (Fig. 2 ).
Electronic platform—research study metadata query view
The quantitative part of the study
The quantitative part of the study included self-reported socio-demographic information (age, gender, education, living conditions, financial needs, and income) and disease-related factors, including weight and height for calculation of the body-mass index (BMI) (Additional file 1 : Supplement 1), years of living with T1DM, number of hypoglycemia incidents per week, number of hypoglycemia incidents per half-year, HbA1c in the last medical check, number of HbA1c checks during the year, and T1DM expenses (Additional file 1 : Supplement 2).
We developed questionnaires for this study that consider conditions in Latvia. The QoL questionnaire comprised 35 questions divided into five blocks: Signs and symptoms (15 questions), Therapy (5 questions), Care (6 questions), Concerns (4 questions), and Communication (6 questions) (Additional file 1 : Supplement 3). The self-management questionnaire consisted of 19 questions divided into three blocks: General, Diet, Physical activities (Additional file 1 : Supplement 4).
All questionnaires were available in the two main languages in use in Latvia – Latvian and Russian. Translation and back translation of questionnaires were performed by two independent professional translators.
Statistical analysis of the quantitative part
The reliability of the questionnaires was checked using the alpha-Cronbach’s test (α) after the first 20 participants had responded (α > 0.75 for all blocks of the questionnaire). These participants were subsequently included in the study sample, and their answers were analyzed together with those of other participants. For both questionnaires, we transformed the answers into values between 0 and 100 and then calculated means for each block. We further calculated the Total QoL and Total SM (self-management) scores as the means of all questions in their respective surveys. Higher values mean better QoL or better self-management.
We next compared pump users and injection users for all demographic variables, using central and dispersion measures according to the type of each variable. We used the Mann–Whitney test to compare qualitative variables and Chi-squared or Cramer’s V tests to compare the quantitative ones. We investigate the correlation between individual subscales and Total QoL and Total SM using Spearman correlation. We considered a two-sided 0.1 significance level for this stage of analysis.
We built multiple logistic regression models for Total QoL, dividing the Total QoL variable at the median (‘worse’ ≤ 67.9’, ‘better’ > 67.9). Variables found univariately statistically significantly related to Total QoL at the 0.1 significance level were included in logistic regression models together with demographic and T1DM-related variables that were found significantly different between pump users and injection users. The full adjustment set included the method of administration, age, sex, education, income, T1DM expenses, years with T1DM, and Total SM. We choose the best model fit according to the − 2 Log-likelihood test. p value < 0.05 was considered statistically significant for this part of the study. Odds ratio (ORs) and 95% confidence intervals were presented for multiple logistic regression models. We used Statistical Package for Social Science (SPSS) software (26th version) for the statistical analysis [ 24 ].
Additional and sensitivity analyses
For additional insight, we asked pump users about the number of years they have used the pump and their reasons for using one (6 categories: QoL, insulin dosing, less pain, less hypoglycemia, just trying, and other). We asked injection users about reasons for not using a pump (6 categories: no trust, expensive, lack of appropriate model, lack of willingness, negative information, other) and reasons for ceasing to use a pump if it was used previously (5 categories: expensive, lack of trust, not comfortable, not resultative, and other). For the sensitivity analysis, we built multiple logistic regression models for two QoL blocks that significantly differed between the user groups (Therapy and Concerns), dividing the results for each block by the median value into ‘worse’ and ‘better’ and using the same set of covariates.
Qualitative part of the study
The qualitative part of the study consisted of analysis of semi-structured interviews performed face-to-face or via telephone or video chats. Interviews were recorded, coded, transcribed according to their major theme, and analyzed using Nvivo software (version 12) [ 25 ] to obtain subcategories of each major theme. Coding of interviews included changing participants’ names. In this paper, we provide part of the results of the qualitative analysis as support for interpreting the quantitative results.
Study participants
We enrolled 87 T1DM patients in the quantitative part of the study: 20 pump users and 67 injection users. Both groups included mostly women. Pump users generally had at least some secondary education and had higher incomes, while injection users mostly had just a high school education. Pump users were younger (mean age 21.5 years, standard deviation (SD) 4.4) than injection users (mean age 33.6 years, SD 11.0). The groups did not differ by other socio-demographic characteristics.
HbA1c values at the last medical check did not differ significantly between the groups. In both groups, most of the patients performed one medical check during 2020 and till May 2021. Only 10% of pump users and 15% of injection users mentioned four medical checks during this period. There were no differences between pump users and injection users in this parameter. T1DM-related expenses were statistically significantly higher for pump users: for 94.7% of them, these expenses were more than 100 EUR/month; just 29.2% of injection users had similarly high expenses (Table 1 ).
Quality of life and self-management
The reliability of all scales was high, ranging from α = 0.75 to α = 94 for all blocks for both questionnaires (excluding the SM Diet that had medial reliability; α = 0.63). Correlation between QoL and self-management was weak and partly insignificant. Self-management blocks correlated among themselves significantly, but not strongly (Additional file 1 : Table S1). No significant relations were found between the number of tests and three self-management blocks ( p = 0.12, p = 0.86, and p = 0.36, respectively).
Significant differences at the 0.1 significance level were observed between user groups in their Therapy and Concerns blocks, and in Total QoL. The highest values for both groups were found for Therapy and Communication blocks of QoL. There were no significant differences between groups in their self-management blocks (Table 2 ). Univariate relationships were found between Total QoL and sex ( p = 0.03).
In fully adjusted multiple regression models, pump users were seven times more likely to have a high Total QoL than injection users (OR 7.38; CI 0.87; 62.9). Factors that increased Total QoL were lower age, male sex, lower T1DM expenses (the most prominent association), more years living with T1DM, and better self-management. Most of the confidence intervals were wide, pointing to the low number of participants in the study (Table 3 ). However, the post hoc calculated power of analysis was 70.1% ( p = 0.01), indicating the study’s medial power.
For pump users, the main reason to use a pump was improved QoL; this was mentioned by 90% of them. For injection users, the median time they had been using insulin injections was eight years, and the main reason for not using a pump was its cost, as mentioned by almost half of these respondents. Of the 13 patients that previously used a pump, the main reason why they stopped was the cost (mentioned by 46.2% of those that stopped using a pump) (Additional file 1 : Table S2).
In the univariate analysis between the Therapy block of QoL and demographic and T1DM-related factors, significant relationships at the 0.1 significance level were found for years with T1DM ( p < 0.01) and T1DM expenses ( p = 0.08); for the Communication block, significant univariate relationships were found for the number of hypoglycemic episodes per week ( p = 0.09) and sex ( p = 0.07). Consistent with the main analysis, male sex, lower T1DM expenses, and years living with T1DM were associated with better Therapy and Communication blocks (Additional file 1 : Table S3).
Of those included in the quantitative part of the study, 8 pump users and 13 injection users also participated in the qualitative interviews; 15 of these were women. The men-women proportion in each study arm was similar to that in the quantitative part of the study.
The age of the interviewees ranged from 18 to 50 years, and years with T1DM ranged from 1 to 35. Eight participants did not have T1DM diaries, three had one only at the beginning of their treatment, two use them only for visits with a physician, and six regularly recode their activities in their diaries (two using an app to do so). One participant kept a diary when she used multiple insulin injections but stopped when she switched to an insulin pump (Table 4 ).
Analysis of 40 identified codes of the interviews revealed three major themes of answers: diagnosis-related, daily self-management, and life with T1DM. Each of the major themes was further divided into three to four subcategories (Table 5 ). Here we will present a part of the results related to one subcategory for each category of answers: perception of diagnosis (major theme: diagnosis-related), insulin administration (major theme: daily self-control), and T1DM-related costs (major theme: life with T1DM).
Perception of diagnosis
Before their diagnosis, most participants had had some symptoms that they had not related to T1DM, such as thirst, frequent urination, weight loss, and weakness. Therefore, for nearly all of them, the diagnosis was unexpected and shocking. For example, I, who was diagnosed at the age of 28 after being hospitalized due to T1DM:
I didn't know anything before, it seemed to me that diabetes could be born or not. I was so bad in that resuscitation because I was in a severe hypoglycemic condition … my head was dull … it was so hard to grasp.
This reaction was not related to the participant’s age at the time of diagnosis (Additional file 1 : Supplement 5).
Insulin administration
One of the main reasons to use an insulin pump was the QoL that it provides (Additional file 1 : Supplement 6). For example, M said:
I have much more control with the pump, because I can adjust insulin doses if necessary, and adjust the time for basal insulin. I can stop insulin if needed. with the syringe, you are injecting and then you can no longer control what is going. The pump gives much more control to both the doctor and the patient, if a person understands how the pump works. But that's what training is for.
However, some of the injection users saw positive aspects in their treatment method as well. For example, I, who uses the injections:
When using injections, it is nice to inject insulin once and that is .
F, who has used injections for 31 years, was categorically opposed to the idea of a pump:
No, never! It is not practical for me to have a foreign object that is always present at my waist area. I feel very uncomfortable. That limits me .
To summarize: although QoL was mentioned by most of the participants as the determining factor for use of the pump, some participants feel that a pump is less comfortable and even disturbing. This supports the quantitative result showing a lack of proper relations between the method of administration and QoL.
T1DM-related costs
Most pump users in our study mentioned the cost of this administration method (Additional file 1 : Supplement 7). For some participants, the decision whether to use a pump depends on the monthly costs. For example, K said:
It is an extra investment [talking about the pump] —now I have needles and insulin for free, I do not have to buy anything extra—just those test strips, because the glucometer is also free for me. Together it's pretty affordable .
D switched from the pump to injections several times because of financial problems:
I had already used it [pump] as a child, I was 13 years old. […] I used to have insulin pens, but then my mom saved money so I could have the pump. […] After that I had to switch back to insulin pens because I was in big financial trouble. However, I really wanted to get back to the pump.
In Latvia, state reimbursement for insulin pumps is possible until the age of 18. Thus, some people are forced to switch to injections at that point. For most of the participants who would like to use an insulin pump, treatment-related costs are too high, and some of them were forced to change to the cheaper injection method. This supports the quantitative result of the study on the relation between T1DM-related costs and QoL.
Discussion and conclusions
In this study, we investigated quantitatively and qualitatively factors related to the QoL of patients with T1DM according to their method of insulin administration: using an insulin pump or using multiple daily injections. The reported QoL was found to be associated with the method of insulin administration, the age and sex of the participants, the number of years the patient had lived with T1DM, self-management, and T1DM-related expenses. QoL was the main reason cited for using a pump, while the expense was the main reason to avoid its use or to stop using it.
An association between the method of insulin administration and the QoL of patients with T1DM has been shown previously both in qualitative and in quantitative studies [ 11 ]. However, until recently, most of the studies on insulin pumps were qualitative and were performed on populations of children [ 26 , 27 , 28 ]. In the last decade, quantitative evaluations of pump use had appeared as well, but studies combining these two methods of investigation are still scarce. However, similarity among their objectives allows us to combine the results of different studies to provide additional explanations of our observed results. For example, Alqambar et al. found higher scores for QoL for pump users than for injection users. The former had significantly higher satisfaction with their treatment and had a lower burden of disease (both with p < 0.01) [ 29 ]. These results are supported by the qualitative study by Mesbah et al., which described higher satisfaction among pump users in many areas [ 22 ]. In our study, although we did not observe statistically significant differences in QoL between pump and injections users, QoL was the main reason given for using the pump. Nevertheless, in our study some participants had a negative attitude toward the pump. Mesbah et al. likewise report the existence of negative feelings toward pumps, such as fear of being dependent on a machine or concern about sporadic mechanical problems [ 30 ].
As QoL is multidimensional, factors affecting it might differ according to study design and measures. For example, in our study we did not observe any association of QoL with the level of HbA1c. In contrast, in the study by Alavrado-Martel et al. performed in Spain, worse QoL was associated with increasing HbA1c [ 31 ]. This fact is extremely interesting, as in both studies the mean age of participants was 31 years and mean years living with T1DM were 14, and participants had similar levels of education. It is possible that the difference can in part be explained by the proportion of pump users: a third of our participants use a pump and therefore are in reduced risk of an increased level of blood sugar, but in the Spanish study only 5% of patients were pump users. Therefore, the association with the level of HbA1c was not prominent. In addition, in the Spanish study a better QoL was associated with the female sex, but in our study, it was associated with the male sex. As mentioned by Mesbah et al., lack of flexibility in clothing options can reduce the QoL of female pump users [ 30 ], and this may be reflected in our results. In another study [ 32 ] women with diabetes were found to evaluate their health status and diabetes-related care worse than men; they also had more diabetes-related worries related to higher levels of Hb1Ac, although their level of metabolic control did not differ from that of men.
Initiation of pump therapy in Latvia usually is not a choice, but a costly necessity due to problems in diabetes management (such as hypoglycemia) and discomfort associated with diabetes treatment (e.g., pain, fear of injections). Studies describe substantial clinical benefits of insulin pumps for such patients. For example, in the meta-analysis by Benkhadra et al. based on 25 randomized clinical trials, absolute HbA1c reduction was better managed in pump users than in injection users (difference of 37%, CI 0.24; 0.51), and this result was consistent across adults and children. In addition, pump users had a lower risk of hypoglycemia (relative risk, RR = 0.85, CI 0.6–1.2) [ 10 ]. These results were supported by another meta-analysis by Jeitler et al. that analyzed 33 studies and found a 43% difference (CI – 0.65; – 0.20) between groups with different methods of insulin administration [ 8 ]. In addition to clinical benefits, the patient’s ability to self-manage should be considered when choosing the method of administration. Our study did not observe any difference in self-management between pump users and injection users, but we do observe a slight but significant increase in QoL for those with better self-management. The main cause seems to be educational training provided for all patients with T1DM. Previous studies have described the effectiveness of such training on self-management. For example, in the structural analysis by Campbell et al. based on 18 studies, people who attended educational training gained clinical benefits by managing their lives according to the knowledge they received during these sessions. However, people were often tired and encountered difficulties in managing their everyday lives according to guidelines even during these educational trainings, which made additional follow-up by the physician essential [ 7 ]. For pump users less intensive follow-up is needed, thus removing a level of stress from both physician and patient. Overall, a personal approach when choosing the method of insulin administration seems to be the best in the case of T1DM patients.
Limitations of the study
The main limitation of our study is its cross-sectional nature, which does not allow us to evaluate causal relationships. The study included a small number of participants, especially in the pump-users group, and we did not divide participants into age groups. Further, the use of the Internet for enrollment limited the available pool of participants and may introduce a volunteer bias that can affect the validity of the results. Further limitations include self-evaluation of QoL and self-management and possible errors regarding the number of medical checks due to memory bias. Specific questions on installation, operation, troubleshooting, and handling of the insulin pumps (these factors could affect the quality of therapy in pump-users and have an impact on QoL) were not included in the study to avoid complexity. In addition, some limitations in the qualitative part of the study could be related to the language, as the native language of the interviewer was Latvian. Despite the good knowledge of Russian, some impreciseness could occur.
Although the reliability of all parts of the survey was high at the initial stage of their check, we observed the medial reliability of one of its parts after collecting the information about all study participants. As we did not see any difference between the insulin pump users and multiple injection users in other parts of the SM questionnaire, we assumed that the lower reliability of this part of the questionnaire will not affect the results of our study.
Another limitation of our study is the high proportion of insulin pump users. Before we start the study, we knew that the number of insulin pump users in the Latvian population is relatively small. Therefore, we decided to invite participants in the proportion of 1:3 (pump users versus injection users) to increase the overall power of analysis. We attempt to invite as many pump users as it was feasible. As a result of this strategy, we observed a disproportion between the pump users and injection users in their relation to the whole pump/injection users’ population in the country. This can affect the results of our study, especially the qualitative part of them.
Strength of the study
A major strength of our study is a mixed methodology that allows us to describe QoL-related parameters of T1DM patients from various sides. Further, although the sample size was small, it represented more than half of all pump users in Latvia, and regardless of the small sample size, the power of analysis was 70%.
QoL was the main reason to use an insulin pump, while the main reasons to avoid one were expenses related to its use. However, the expense related to diabetes treatment, not the method of insulin administration, was the strongest predictor of T1DM patients’ QoL. Reimbursement policies thus should not only consider the patient’s personal preference for treatment, but also be structured to alleviate ongoing maintenance costs, particularly as high costs drive reduced adherence to treatment regimens that in turn impose higher costs on the healthcare system in the form of additional disorders and comorbidities.
Development of national insurance policies is critical worldwide, but especially in countries like Latvia with overall weak health care and public health systems, supporting reimbursement for insulin pumps could help:
to reduce complications related to poor treatment adherence,
to avoid increased additional morbidity, and
to prevent an overload of the health system.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Longenesis Curator platform, accessible via following link: www.longenesis.com/curator .
Abbreviations
Type I diabetes mellitus
Glycated hemoglobin
- Quality of life
95% Confidence interval
Relative risk
Janež A, Guja C, Mitrakou A, et al. Insulin therapy in adults with type 1 diabetes mellitus: a narrative review. Diabetes Ther. 2020;11(2):387–409. https://doi.org/10.1007/s13300-019-00743-7 .
Article PubMed PubMed Central Google Scholar
Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3(1):17016. https://doi.org/10.1038/nrdp.2017.16 .
Article PubMed Google Scholar
Norris JM, Johnson RK, Stene LC. Type 1 diabetes—early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020;8(3):226–38. https://doi.org/10.1016/S2213-8587(19)30412-7 .
Article CAS PubMed PubMed Central Google Scholar
Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10(2):98–115. https://doi.org/10.34172/hpp.2020.18 .
Centre of Disease Prevention and Control https://www.spkc.gov.lv/lv/latvijas-veselibas-aprupes-statistikas-gadagramata-2015/3_sabiedribas_veseliba_2015_11.pdf (Last visited: 26 December 2021)
Pickup JC. Is insulin pump therapy effective in type 1 diabetes? Diabet Med. 2019;36(3):269–78. https://doi.org/10.1111/dme.13793 .
Article CAS PubMed Google Scholar
Campbell F, Lawton J, Rankin D, et al. Follow-Up Support for Effective type 1 Diabetes self-management (The FUSED Model): A systematic review and meta-ethnography of the barriers, facilitators and recommendations for sustaining self-management skills after attending a structured education programme. BMC Health Serv Res. 2018;18(1):898. https://doi.org/10.1186/s12913-018-3655-z .
Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941–51. https://doi.org/10.1007/s00125-008-0974-3 .
Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004;27(11):2590–6. https://doi.org/10.2337/diacare.27.11.2590 .
Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2017;55(1):77–84. https://doi.org/10.1007/s12020-016-1039-x .
Pouwer F, Hermanns N. Insulin therapy and quality of life. A review Diabetes Metab Res Rev. 2009;25(S1):S4–10. https://doi.org/10.1002/dmrr.981 .
Cummins E, Royle P, Snaith A, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess. 2010. https://doi.org/10.3310/hta14110 .
Rechenberg K, Whittemore R, Grey M. Anxiety in youth with type 1 diabetes. J Pediatr Nurs. 2017;32:64–71. https://doi.org/10.1016/j.pedn.2016.08.007 .
Alsaleh FM, Smith FJ, Taylor KM. Experiences of children/young people and their parents, using insulin pump therapy for the management of type 1 diabetes: qualitative review: experiences of using insulin pumps. J Clin Pharm Therap. 2012;37(2):140–7. https://doi.org/10.1111/j.1365-2710.2011.01283.x .
Article CAS Google Scholar
Abualula NA, Jacobsen KH, Milligan RA, Rodan MF, Conn VS. Evaluating diabetes educational interventions with a skill development component in adolescents with type 1 diabetes: a systematic review focusing on quality of life. Diabetes Educ. 2016;42(5):515–28. https://doi.org/10.1177/0145721716658356 .
Ross LJ, Neville KA. Continuous subcutaneous insulin infusion versus multiple daily injections for type 1 diabetes. J Paediatr Child Health. 2019;55(6):718–22. https://doi.org/10.1111/jpc.14480 .
Thabit H, Hovorka R. Continuous subcutaneous insulin infusion therapy and multiple daily insulin injections in type 1 diabetes mellitus: a comparative overview and future horizons. Expert Opin Drug Deliv. 2016;13(3):389–400. https://doi.org/10.1517/17425247.2016.1115013 .
http://www.diabets-asoc.lv/Anglu_valoda/izvelne.htm
http://diabetam.lv/2018/06/07/skaidrojums-par-kriterijiem-insulina-pumpja-terapijas-piemerosanai/ (Last visited: 26 December 2021)
Central Statistical Bureau (CSB) - https://lvportals.lv/dienaskartiba/323225-2019-gada-majsaimniecibu-riciba-esosie-ienakumi-pieauga-par-68-2020 (Last visited: 26 December 2021)
Salna I, Salna E, Pahirko L, Skrebinska S, Krikova R, Folkmane I, Pīrāgs V, Sokolovska JJ. Achievement of treatment targets predicts progression of vascular complications in type 1 diabetes. Diabetes Compl. 2021;35(12):108072.
Article Google Scholar
Digital Engagement Platforms, Longenesis Curator. Available: ttps:// www.longenesis.com/curator ; Longenesis Engage. Available: https://www.longenesis.com/engage
Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation), OJ 2016 L 119/1.
IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
QSR International Pty Ltd. 2018; NVivo (Version 12), https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home
Valenzuela JM, Patino AM, McCullough J, et al. Insulin pump therapy and health-related quality of life in children and adolescents with type 1 diabetes. J Pediatric Psychol. 2006;31(6):650–60. https://doi.org/10.1093/jpepsy/jsj088 .
Al Shaikh A, Al Zahrani AM, Qari YH, et al. Quality of life in children with diabetes treated with insulin pump compared with multiple daily injections in tertiary care center. Clin Med Insights Endocrinol Diabetes;2020;13:117955142095907. https://doi.org/10.1177/1179551420959077
Müller-Godeffroy E, Treichel S, Wagner VM, on behalf of the German Working Group for Paediatric Pump Therapy. Investigation of quality of life and family burden issues during insulin pump therapy in children with Type 1 diabetes mellitus-a large-scale multicentre pilot study. Diabetic Medicine. 2009;26(5):493–501. doi: https://doi.org/10.1111/j.1464-5491.2009.02707.x
Alqambar M. Impact of insulin pump use on disease control and quality of life in type I diabetes patients. Endocrinol Metab Int J. 2018. https://doi.org/10.15406/emij.2018.06.00169 .
Mesbah NI, Taha NAER, Rahme ZN, Sukkar FF, Omar DM. Experiences of adults using continuous subcutaneous insulin infusion: a qualitative study. Med Princ Pract. 2020;29(3):255–61. https://doi.org/10.1159/000503705 .
Alvarado-Martel D, Velasco R, Sánchez-Hernández RM, Carrillo A, Nóvoa FJ, Wägner AM. Quality of life and type 1 diabetes: a study assessing patients’ perceptions and self-management needs. Patient Preference and Adherence; 2015:1315. https://doi.org/10.2147/PPA.S87310
Undén A-L, Elofsson S, Andréasson A, Hillered E, Eriksson I, Brismar K. Gender differences in self-rated health, quality of life, quality of care, and metabolic control in patients with diabetes. Gender Med. 2008;5(2):162–80.
Download references
Acknowledgements
The authors acknowledge the help of the LatDiane cohort database, the Children’s Clinical University Hospital, the Latvian Association of Endocrinology, and Latvian diabetes organizations.
This study was partially financially supported by Medtronic B.V. Representative Office in Latvia.
Author information
Authors and affiliations.
Faculty of Medicine, University of Latvia, Jelgavas Str. 3, Riga, Latvia
Lilian Tzivian & Jelizaveta Sokolovska
Faculty of Humanities, University of Latvia, Riga, Latvia
Anna E. Grike
Faculty of Medicine, Riga Stardins University, Riga, Latvia
Agate Kalcenaua
Longenesis Ltd, Riga, Latvia
Agate Kalcenaua, Martins Mednis, Ieva Danovska, Ugis Berzins, Arnolds Bogdanovs & Emil Syundyukov
Questrom Business School, Boston University, Boston, MA, 02215, USA
Abraham Seidmann
Digital Business Institute, Health Analytics and Digital Health, Boston University, Boston, MA, 02215, USA
Faculty of Industrial Engineering and Technology Management, Holon Institute of Technology, 5810201, Holon, Israel
Arriel Benis
Faculty of Digital Technologies in Medicine, Holon Institute of Technology, 5810201, Holon, Israel
Faculty of Computing, University of Latvia, Raina boulevard 19, Riga, 1050, Latvia
Emil Syundyukov
You can also search for this author in PubMed Google Scholar
Contributions
LT, JS, ES and AK designed the study; AEG performed face-to-face interviews and analyzed a qualitative part of the study; LT analyzed a quantitative part of the study and wrote the main manuscript text; AS, AB and ES consulted the overall design of the study and wrote the main manuscript text; ID and ES prepared all figures and contributed to development of the digital platform; MM, UB, AB and ES developed a digital platform for the study. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Lilian Tzivian .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Scientific Research Ethic Commission of the Institute of Cardiology and Regenerative Medicine of the University of Latvia on February 2, 2021. Participants provide their consent (which could be dynamically managed on the platform, e.g., for opt-out) whilst entering the Digital Engagement platform.
Consent for publication
Not applicable.
Competing interests
Authors declare no conflict of interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Surveys by blocks, qualitative responses of participants, supplemental tables and figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tzivian, L., Sokolovska, J., Grike, A.E. et al. Quantitative and qualitative analysis of the quality of life of Type 1 diabetes patients using insulin pumps and of those receiving multiple daily insulin injections. Health Qual Life Outcomes 20 , 120 (2022). https://doi.org/10.1186/s12955-022-02029-2
Download citation
Received : 05 January 2022
Accepted : 18 July 2022
Published : 01 August 2022
DOI : https://doi.org/10.1186/s12955-022-02029-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 1 diabetes mellitus
- Insulin pump
- Multiple daily insulin injections
- Real World Data digital tool
- Diabetes-related expenses
- Comparative effectiveness research
- Health economics
Health and Quality of Life Outcomes
ISSN: 1477-7525
- Submission enquiries: [email protected]

COMMENTS
The primary quantitative objective of this study is to estimate associations between self-care maintenance, self-care monitoring, and self-care management at baseline with HbA1c in adults with T2DM, after a follow up of 12 months. ... Journal of Diabetes Research, 2016. 10.1155/2016/2109542 [PMC free article] [Google Scholar] Barbour RS (2001 ...
An estimated 79 million American adults are at risk for developing type 2 diabetes, based on a condition referred to as prediabetes. 1 Although there is currently no cure for type 2 diabetes, studies have definitively shown that the progression from prediabetes to diabetes can be delayed or prevented through lifestyle modifications and ...
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 ...
The hypothesis that type 2 diabetes is preventable 5,6 is supported by observational studies and two clinical trials of diet, exercise, or both in persons at high risk for the disease 7,8 but not ...
We then offer a brief, quantitative summary of our findings and provide details of exemplar studies to illustrate the unique value added by using MMR. We conclude the article with a discussion of implications and recommendations for the use of MMR in diabetes communication research.
Studies show that weight loss can produce remission of type 2 diabetes mellitus (T2DM) in a dose-dependent manner. In patients with T2DM and obesity, weight loss of ~15 kg, achieved by an ...
The purpose of this research is to create a single registry for type 1 and type 2 diabetes at Mayo Rochester and affiliated Mayo sites. Natural History of Diabetes after Total Pancreatectomy Rochester, MN. The purpose of this study is to estimate the risk of diabetes related complications after total pancreatectomy.
Scientific Reports - Label-free quantitative proteomics analysis for type 2 diabetes mellitus early diagnostic marker discovery using data-independent acquisition mass spectrometry (DIA-MS) Skip ...
Systematic review and meta-analysis (SRMA) research has risen in popularity across the scientific realm, even securing a place atop "evidence pyramids" and study design hierarchies (1,2).A "systematic review" refers to the process of identifying all research meeting the scope and eligibility criteria for a specific scientific question defined a priori.
An international study identified diabetes related distress as a major factor responsible for poor adherence to self-management ... The quantitative phase of the study involved a cross sectional survey and data analysis. ... A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med. 2010; 9 (45):112-8. [Google ...
Type 2 diabetes cannot be cured, but lifestyle changes such as following a healthy diet, regular physical activity, and maintaining normal body weight can slow the progression of the disease and reverse its effects [].However, previous studies have shown that long-term maintenance of weight loss and complete adherence to diet and physical exercise recommendations is rare, especially in the ...
- A follow-up study assessing mother and child health six years after a pregnancy diagnosed with gestational diabetes, Diabetes Research and Clinical Practice, 211, (111657), (2024). https://doi ...
This search generated 236 article abstracts that we read to determine whether each article included original qualitative data (a primary inclusion criterion), and identified 53 articles not eligible for inclusion (e.g. the paper was a 'qualitative' review of quantitative research or merely described a study protocol to collect 'qualitative' data).
This relationship between diabetes knowledge and poor self-management has been demonstrated in quantitative studies [Citation 22, Citation 23], including a positive association between diabetes education and self-management behaviours [Citation 24].
Good glycaemic control is a crucial part of diabetes management. Traditional assessment methods, including HbA1c checks and self-monitoring of blood glucose, can be unreliable and inaccurate. Continuous glucose monitoring (CGM) offers a non-invasive and more detailed alternative. Availability of this technology is increasing worldwide. However, there is no current comprehensive evidence on the ...
Insulin pump therapy represents an alternative to multiple daily injections and can improve glycemic control and quality of life (QoL) in Type 1 diabetes mellitus (T1DM) patients. We aimed to explore the differences and factors related to the T1DM-specific QoL of such patients in Latvia. A mixed-method cross-sectional study on 87 adult T1DM patients included 20 pump users and 67 users of ...
Natural products in diabetes research: quantitative literature analysis. Andy Wai Kan Yeung a Oral and Maxillofacial Radiology, Applied Oral Sciences and Community Dental Care, ... The current study aimed to identify which natural products and which research directions are related to the major contributors to academic journals for diabetes ...